Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02306356 2000-04-19
-1-
A FASTENER HAVING A PROTECTIVE COATING
AND A METHOD OF PROVIDING SAME
Field Of The Invention
The present invention relates to a metal fastener with a protective coating
for
inhibiting corrosion of the fastener from occurring as a result of exposure to
environmental conditions. There is also disclosed a method for the manufacture
of the
fastener.
Background Of The Invention
1 o Corrosion of metal fasteners like nails in situ not only shortens their
life but may
also lead to staining of the material fastened. This can cause unsightly
marking of the
material and is particularly disadvantageous when the material is relatively
permeable to
water as metal oxides resulting from the corrosion can spread through the
material from
the fastener leading to more serious staining than may otherwise occur.
~ 5 It is known to treat such fasteners with a galvanising or like process to
provide
them with a protective coating to inhibit corrosion.
In United States Patent No. 5033181 there is disclosed a method of
manufacturing
nails from pre-galvanized steel rod. The method involves coating the nails
once formed
from the rod with a polymeric layer to cover exposed steel resulting from loss
of regions
20 of the zinc coating during the forming step. This restores corrosion
resistance to those
regions of the nail on which the zinc was lost and enhances resistance to
corrosion over
the remainder of the nail where the zinc coating remained in tact. However,
the patent
offers no solution to how corrosion resistance may be enhanced beyond that
provided by
CA 02306356 2000-04-19
-2-
the polymeric layer or any means of enhancing adherence of the polymeric layer
to the
zinc coating or the exposed steel of the nail.
A process for coating a metal fastener with an aliphatic polyurethane resin to
protect against corrosion is described in United States Patent No. 5178903.
The coating
s of the fastener is achieved by preferably dipping the fastener into a
aqueous dispersion of
the resin. Prior to coating the fastener with the resin, the fastener may be
galvanised or
otherwise precoated with a zinc layer over which a chromate conversion layer
is applied.
In Japanese Patent Publication No. 62-050480 there is disclosed a steel sheet
with a zinc
alloy coating and a protective layer of a carboxylated polyolefin resin. The
manufacturing process comprises firstly forming a chromate film over the zinc
alloy
layer and subsequently, coating the chromate film with the carboxylated
polyolefin resin.
The resulting protective coating again provides enhanced corrosion resistance.
However, as two coating steps are involved, such processes are inherently
inefficient.
A one-step process for coating a steel sheet is described in Japanese Patent
t 5 Publication No. 60-190572. In this process, a steel sheet is coated with a
composition
comprising a mixture of polyethylene resin powder, an aqueous dichromate
solution, an
organic resin emulsion and a reducing agent prior to the baking the steel
sheet in order to
form the protective coating thereon. A similar such process is described in
Japanese
Patent Publication No. 03-215683.
2o In United States Patent No. 4966634 there is disclosed a process for
treating
metallic surfaces in order to render them less susceptible to corrosion. The
process
involves applying an aqueous solution containing an acrylic polymer emulsion
and
hexavalent chromium ions to the metallic surface enabling chromating and
coating of the
CA 02306356 2000-04-19
-3-
surface with the before mentioned polymeric material to also be obtained in
the one step.
That disclosure is directed to the use of polyoxyethylene-polyoxypropylene
block
polymer in the provision of the acrylic polymer to improve miscibility with
chromic
acid.
Summary Of The Invention
Broadly stated, the present invention is based on the recognition that heating
a
metallic fastener prior to coating the fastener with a coating composition
containing a
passivating agent and a protective polymeric substance may provide the
resultant coating
when dry with enhanced desirable characteristics.
t o In one aspect of the present invention there is provided a method of
manufacturing
a fastener adapted to be driven into a material in use and having a protective
coating for
inhibiting corrosion of the fastener, wherein the method comprises the steps
of:
(a forming the fastener from a length of metal;
(b) heating the fastener to a desired temperature;
t 5 (c) immersing the heated said fastener in a solution containing a
passivating
agent and one or more polymeric substances;
(d) removing the fastener from the solution; and
(e) allowing remaining said solution on the fastener to dry to thereby form
the
protectrve coating.
2o The fastener will generally be a nail, screw, pin, tack or the like and the
metal will
usually be a ferrous metal. Most usually, the metal will be steel.
Preferably, the fastener will be formed from a length of metal such as coiled
metal
rod or wire feed, having an existing metallic coating resistant to corrosion.
The
CA 02306356 2000-04-19
-4-
corrosion resistant metallic coating will typically be a zinc coating and may
be formed
by a zinc plating, sheradizing, galvanising or other such process.
Although forming the fastener in this manner may result in a sparse covering
of the
metallic coating in some regions of the fastener such as the head and pointed
end of the
fastener as may be the case when the fastener is a nail, in these instances
there will
generally be sufficient such coating on those regions for facilitating
adherence of the
protective coat formed with further processing of the fastener in accordance
with the
instant method and for enabling adequate passivation by the passivating agent.
Alternatively, the corrosion resistant metallic coating may be coated on the
formed
fastener prior to the fastener being heated.
Of the above two options, the former is desirable as processes like
galvanising are
normally performed at processing facilities distant from where the forming of
the
fastener occurs, requiring the fasteners to be transported to and returned
from such a
processing facility before further treatment of the fasteners can proceed.
This involves
~ 5 significant time delays. In addition, it is much quicker and more
efficient to galvanise,
for example, a steel rod and then for the fasteners from the galvanised rod
compared to
galvanising fasteners in batches. Accordingly, the former option can provide a
number
of major benefits and more particularly, reduced costs, enhanced ease of
handling and far
shorter production times.
2o The term "passivating agent" is to be taken to mean an agent that acts to
render the
fastener less susceptible to corrosion and includes mixtures of substances.
Normally, the
passivating agent used in the present method will chemically react and bond
with the
CA 02306356 2000-04-19
-S-
surface of the metal from which the fastener is formed and/or the corrosion
resistant
coating on the fastener to thereby reduce the susceptibility of the fastener
to corrosion.
Preferably, the passivating agent will form a film of a transition metal over
the
fastener and be coated by the polymeric substance(s). Most preferably, the
passivating
agent will be a chromating agent.
The term "polymeric substance" is to be taken to mean a polymer or a mixture
of
polymers suitable for protecting the fastener against corrosion and capable of
fixedly
adhering to the passivating agent. Generally, the polymeric substance will
consist of one
or more synthetic thermoplastic organic polymers. Suitable such polymers
include
1 o acrylic resins, polyvinylidene resins, vinyl acetate resins and vinyl-
acrylic resins
including vinylidine-acrylic copolymers. Preferably, the polymeric substances)
will be
selected from the group comprising vinyl-acrylic resins.
Usually, the fastener will be immersed in a solution containing a passivating
agent
and more than one polymeric substance.
~ 5 It has been surprising found that heating the fastener prior to immersion
in the
solution allows excellent coating of the fastener by the solution and enables
the
subsequent formation of a strongly adherent protective coating with enhanced
corrosion
resistance to be achieved. Indeed, it is believed that the heating of the
fastener is
necessary for satisfactory adherence and formation of the coating to occur.
2o The coating of the fastener by the polymeric substances) taken together
with the
passivation provided by the passivating agent provides significantly greater
corrosion
resistance compared to that achieved by a metallic corrosion resistant coating
on the
fastener alone, like that provided by a galvanising or other such process.
CA 02306356 2000-04-19
-6-
Accordingly, the formation of the protective coating on the fastener by the
present
method may result in a significantly longer working life of the fastener and
reduce the
risk of the staining of material into which the fastener is driven in use
resulting from
corrosion of the fastener in situ.
Preferably, the fastener is subjected to accellerated drying such as by the
application of heat following the removal of the fastener from the solution
containing the
passivating agent and polymeric substance(s).
In a second aspect there is provided a fastener having a protective coating
and
manufactured by the inventive method as above described.
Unless the context clearly requires otherwise, throughout the description and
the
claims, the words 'comprise', 'comprising', and the like are to be construed
in an
inclusive sense as opposed to an exclusive or exhaustive sense; that is to
say, in the sense
of "including, but not limited to".
The invention will now be further described hereinafter with reference to a
number
~ 5 of preferred forms.
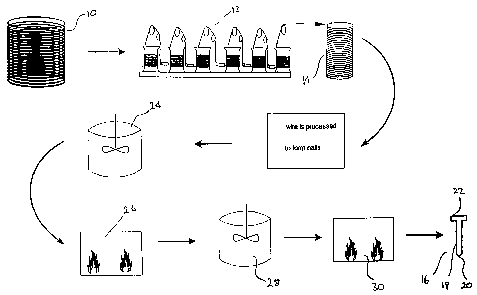
Brief Description Of The Accompanyi, ng F~ure
Figure 1 is a flow diagram illustrating a method for manufacturing a fastener
of the
invention.
Detailed Description Of Preferred Embodiments Of The Invention
2o The method illustrated in fig. 1 involves hard drawing a coiled galvanised
steel rod
having a diameter of about 7 mm using a conventional technique generally
indicated
by the numeral 12 to form a coil of drawn steel wire 14 having a diameter of
about 4
mm. The thickness of the zinc coating on the drawn wire is reduced to about
30pm from
CA 02306356 2000-04-19
_7_
a starting thickness on the steel rod of from between about 40pm to about
70~m. Steel
rod having a diameter of from between about 5.5 mm to about 9 mm is
particularly
suitable for use in the instant method. Generally, the diameter of the rod
will be drawn
down to a diameter of between about 1.85 mm to about 6 mm.
The drawn wire 14 is subsequently formed into individual nails 16 having a
shank
18 with a pointed end 20 and a head 22 on the opposite end of the shank. Any
known
nail forming process whereby the zinc coating substantially remains intact may
be used.
In the instant study, it has been found that the zinc coating on the pointed
end of the nails
can remain substantially intact following the formation of the nail and
indeed, some zinc
remains on the upper surface of the head of the nails although, that covering
may be
discontinuous.
Rather than using steel wire already having a zinc coating, the nails may be
galvanised after they have been formed. This may readily be achieved by
immersing the
nails into a bath of molten zinc ("hot dipping") or by any other suitable
method such as
15 an electrodeposition process.
As indicated in the flow diagram of fig. 1, the nails may be placed in a
stirred
solvent bath 24 in order to remove any foreign material such as dust, grease,
and residual
oil or wire drawing lubricant from the surface of the nails. Any suitable
solvent that
does not significantly affect the zinc coating on the nails may be used. If
desired, the
2o nails may be placed in a basket or similar holder to enable placement in
and removal of
the nails from the solvent bath 24, and to facilitate draining of the solvent
from the nails.
Following cleaning of the nails and the removal from the solvent bath 24, they
are
allowed to substantially dry prior to being placed in a furnace 26 and heated
to a
CA 02306356 2000-04-19
_g_
temperature in a range of from about 100°C to about 300°C. The
temperature to which
the nails are heated will vary depending on the diameter of the shank of the
nails.
Typically, the nails will be heated to a temperature within a range of from
about 150°C
to about 240°C.
While substantially at this temperature, the heated nails are briefly immersed
in a
stirred bath of a coating solution indicated by numeral 28, comprising a
mixture of
between about 15% w/w to about 25% w/w of Novacoat 600 part A [available from
Henkel Australia Pty Ltd, Kilsyth, Victoria, Australia] and about 10% w/w to
about 25%
w/w of Novacoat 600 part B [Henkel Australia Pty Ltd], with water making up
the
balance of the solution. Usually, the nails will be immersed in the solution
for a period
of from about 0.1 seconds to about 5 seconds or more. A period of from about 1
to 3
seconds is preferable to ensure complete wetting of the nails. The pH of the
coating
solution is maintained in a range of from about 1.5 to about 3.3.
The temperature of the solution is maintained in a range of from about
10°C to
t 5 about 40°C and more preferably, in a range of from about
10°C to about 30°C. It has
been found that excessive heating of the solution can cause the solution to
gel thereby
rendering the solution not suitable for coating purposes.
The nails are subsequently subjected to a blast of dry air at a temperature of
from
about 120°C to about 125°C in order to dry the nails following
their removal from the
2o coating solution. If desired, excess coating solution may be drained from
the nails
before they are subjected to forced drying. Rather than using hot air to dry
the nails,
they may alternatively be placed in a drying oven 30 for a predetermined
period of time
as indicated.
CA 02306356 2000-04-19
-9-
Novacoat 600 part A acts to passivate the nails and so renders the nails less
susceptible to corrosion by the formation of a zinc chromate film over the
nails. More
particularly, it comprises less than about 10% w/w phosphoric acid, less than
about 5%
w/w chromium trioxide, lithium salt in an amount less than about 10% w/w, zinc
phosphate in an amount of about the same as the lithium salt, and about 1 %
w/w of a
surfactant, with the remainder being water.
Novacoat 600 part B in contrast provides an organic polymeric coating for
further
inhibiting corrosion and more particularly comprises between about 10% w/w to
about
60% w/w vinylidine acrylic copolymer latex with the remainder again being
water.
Besides acting to passivate exposed surfaces of the nails, Novacoat 600 part A
acts
as a conversion coating providing a key for promoting adhesion of the
polymeric coat
provided by the part B solution to the nails.
Accordingly, by immersing the nails in a mixture of both Novacoat solutions
significantly enhanced adhesion to the nails of the polymeric substances in
the Novacoat
i 5 600 part B may be achieved. Although it is preferable to use Novacoat 600
solutions as
described above other coating solutions may also be utilised.
The temperature of the nails when immersed in the coating solution is
important in
ensuring that an adequate and adherent coating forms on the nails. In
particular, it is
believed that the retained heat in the nails effectively serves as a catalyst
in the coating
20 process promoting the rapid evaporation of water, passivation of the zinc
coating on the
fasteners and cross-linking of the polymeric substances provided by the
Novacoat 600
part B solution, resulting in enhanced coating durability and appearance of
the formed
coating when dry in addition to enhanced corrosion resistance.
CA 02306356 2000-04-19
- 10-
It will be appreciated that the coating solution in which the nails are
immersed
should have a low viscosity to facilitate draining excess solution from the
nails and/or
drying of the nails and furthermore, to achieve a substantially constant
thickness of the
resulting protective coating while at the same time avoiding sticking and
clumping of the
nails together during the drying step.
Advantageously, the resulting coating formed on the nails by the mixture of
the
Novacoat solutions may enhance the retention of the nails when driven into the
timber.
It is believed this results from the coat formed from the Novacoat solutions
bonding to
the timber.
1 o The invention will now further be described with reference to the
following
Examples.
Example 1
A comparison of the protective effect resulting from the passivation of the
zinc
coating of galvanised plasterboard nails by immersion in Novacoat part A
relative to
~ s plasterboard nails not so treated was carried out. All the nails used were
formed from
hard wire drawn from galvanised steel rod stock and so had an existing
protective zinc
coating.
The nails were divided into groups of 10 nails each. One test group was
immersed
in a 25°C stirred bath of 20% w/w solution of Novacoat part A while the
second test
2o group was immersed in the same solution but this time at a temperature of
150°C.
Following drying of the nails they were subjected to neutral salt spraying
(NSS) testing
in a cabinet as per Australian Standard AS 2331.3.1-1980. The test was halted
once
CA 02306356 2000-04-19
-11-
about 5% of the surface of the nails showed red rust. The time for the rust to
form was
recorded and the results are shown in Table 1.
Table 1: Corrosion Results For Nails Passivated With Novacoat 600 Part A
Sample Tested Immersion time (mins) Time to 5% Red Rust
in 20% (hours)
w/w Novacoat 600 Part
A
Plasterboard 0 180
Nail
30 (25C) 360
30 (150C) 340
As can be seen, immersion of the nails in the solution containg Novacoat 600
part
A improved the corrosion resistance of the galvanised nails by a factor of
about 2 or
more relative to a control group not immersed in the solution.
With later studies it was found that the immersion time of the nails could be
dramatically reduced while maintaining enhanced corrosion resistance.
Example 2
Samples of 3.75 mm x 75 mm and 2.8 mm x 50 mm bullet head nails formed from
hard drawn galvanised steel wire ("drawn nails") were evaluated for corrosion
resistance
compared to bullet head nails of corresponding size also having a zinc coating
that had
been formed by sheradising the nails ("plated nails").
t 5 Metallurgical investigations showed that the plated nails had a zinc
coating mass
ranging from about 650 g.rri 2 for the 50 mm nails up to about 850 g.rri Z for
the 75 mm
nails. The drawn nails were shown to have a zinc coating mass of around 150
g.rri 2 for
the 50 mm nails ranging up to about 250gm-2 for the 75 mm nails.
Micro-examination of the drawn and plated nails revealed the microstructures
of
2o all samples consisted of heavily elongated fine perlite and ferrite. For
the drawn samples
CA 02306356 2000-04-19
- 12-
this is indicative of the drawing of galvanised wire in the formation of those
nails while
in the plated samples it is indicative of mechanical plating after drawing
without any
intermediate heat treatment.
The mechanically plated galvanised nails were found to have an average coating
thickness on their shank of 100 ~m compared to the nails formed by the drawing
process
which had an average coating thickness on their shank of 30 ~.m, as determined
from
transverse sections of the nails.
To evaluate corrosion resistance, nails were placed in a cabinet and subjected
to
neutral salt spray testing (NSS) as per AS 2331.3.1-1980. For the purpose of
evaluation,
t o the nails were completely driven into timber prior to being subjected to
the salt spray
environment. The end point for testing purposes was taken as being the first
appearance
of red rust on the heads of the nails. The time taken for the appearance of
the red rust
was recorded for each group and the results are shown in Table 2.
The corrosion resistance of the above nails following being coated with a
mixture
of 20% w/w Novacoat part A and 20% w/w Novacoat part B red, blue or green
solutions
[all available from Henkel Australia Pty Ltd] in water was also evaluated by
NSS testing
using the same procedure as indicated above. The results are also set out in
Table 2.
CA 02306356 2000-04-19
-13-
Table 2: Corrosion Results For Drawn Nails Compared To Plated Nails
Bullet Head Hrs to first
Nail Red Rust
on Nail
Head
(Test Terminated
at 1900
Hrs)
Untreated Red Blue Green
2.8 x 50mm 170 1900 1900 1900
Plated
215 1900 1900 1900
240 1900 1900 1900
335 1900 1900
335 1900 1900
2.8 x 50mm 240 1010 504 1010
Drawn
265 1900 1900 1010
335 1900 1900 1900
504 1900 1900 1900
3.75 x 75mm 240 600 335
Plated
240 1346 670
240 1900 1010
240 1900 1900
240 1900 1900
3.75 x 75mm 240 840 432
Drawn
240 1900 672
335 1900 1010
335 1900 1010
380 1900 1900
CA 02306356 2000-04-19
- 14-
Despite the substantially heavier zinc coating mass of the plated nails
compared to
the drawn nails, and the assumed advantage of the presence of the significant
coating of
zinc on the head of those nails, the plated nails performed similarly in the
NSS test to the
drawn nails.
The table further shows that coating the nails with a mixture of both Novacoat
600
part A and part B, substantially increases the corrosion resistance of the
nails relative to
corresponding groups of nails not so treated. In addition, the table shows
that
comparable corrosion inhibition results were obtained for the plated and drawn
nails
coated with the Novacoat mixture.
t o Example 3
A study was undertaken using plasterboard nails formed from wire hard drawn
from galvanised steel wire rod, to determine the effect of applying a mixture
of Novacoat
600 parts A and B in inhibiting corrosion of the nails.
Test group 1 comprised plasterboard nails that had been immersed in an
solution of
t 5 20% w/w Novacoat 600 Part A at ambient temperature for a half hour period.
Test
group 2 comprised nails preheated to 150°C prior to immersion in a 20%
w/w solution of
Novacoat 600 part A at ambient temperature, while test groups 3 to 8 comprised
nails
preheated to 150°C prior to being immersed in a solution of 20% w/w
Novacoat 600 part
A and 20% w/w Novacoat part B of different colours respectively, namely green,
2o evergreen, orange, red, black and clear [all available from Henkel
Australia Pty Ltd].
All test groups in which the nails had been preheated were immersed in the
corresponding solutions for the same period of time. The same number of nails
were
used in each test group. Corrosion was again assessed by neutral salt spray
testing
CA 02306356 2000-04-19
- 1$ -
(NSS) as per Australian Standard AS 23313.1-1980. Each group were removed from
the
salt spray environment when it was estimated that red rust had formed over 5%
of the
surface area of the nails in the group. The time taken for the formation of
the rust was
recorded and the results are set out in Table 3.
Table 3: Corrosion Results For Nails With A Coating Formed Form Novacoat 600
Part
A And Novacoat 600 Part B
Test Groups Hours to 5% Red
Rust
Untreated 180
1 360
2 340
3 2600
4 >2600
5 1200
6 1400
7 2600
8 1200
As can been seen, treatment of the nails with only a 20% w/w solution of the
Novacoat 600 part A appears to approximately double the time for corrosion to
appear in
NSS testing compared to the untreated control group. It is further apparent
that when the
Novacoat 600 part A and part B solutions were mixed, the protection against
corrosion
increased by a factor of from about 6.5 when clear part B solution was used up
to a
factor of 14 or more when the green or evergreen part B solutions were
utilised.
CA 02306356 2000-04-19
- 16-
Table 3 further shows that significantly greater corrosion protection was
afforded
to nails preheated to 150°C and treated with both the part A and part B
Novacoat 600
solutions relative to nails that were preheated and treated with only Novacoat
600 part A.
Again, later studies have shown that immersion of the nails in both part A and
part
B Novacoat 600 solutions can be substantially reduced with retention of
enhanced
corrosion resistance.
Accordingly, although the present invention has been described hereinbefore
with
reference to preferred embodiments and a number of the examples, the skilled
addressee
will appreciate that numerous variations and modifications are possible with
out
departing from the scope of the invention.