Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02306728 2000-04-20
WO 99/Z0181 PCT/US98/22I14
APPARATUS AND METHOD FOR THE COLLECTION
OF INTERSTITIAL FLUIDS
s
BACKGROUND OF THE INVENTION
1. Field of the Invention
to
This invention relates to novel apparatus and methods for the collection of
bodily fluids, such as interstitial fluids, from the body of an animal, such
as a
mammal. The fluids so collected may then be analyzed for biological or medical
purposes, such as, for example, disease and health management activities.
is More particularly, this invention provides novel apparatus and methods for
the
collection of large quantities of interstitial fluids from areas of the skin
where the
stratum corneum has been breached.
2. Discussion of the Art
The stratum corneum is the outer horny layer of the skin comprising a
complex structure of compact keratinized cell remnants separated by lipid
domains. In humans, the stratum comeum typically has a thickness of about 10
Nm to about 30 Nm and overlays the epidermal Layer, which itself has a
2s thickness of on the order of about 100 Nm. The dermal layer, found below
the
epidermal layer, contains, among other things, capillary networks through
which
blood flows.
It has been proposed that interstitial fluids can be obtained from the
epidermal layer in a minimally invasive procedure by stripping away the
stratum
3o corneum to expose the epidermal layer and thereafter collecting
interstitial fluids
from the epidermis. Repeated application and removal of cellophane tape to the
same location can be used to strip away the stratum corneum to expose the
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
epidermal layer for the collection of interstitial fluids. Another technique
available
for the collection of interstitial fluids involves inserting a micro needle
into the
epidermal layer to allow fluids to be wicked up out of the body for deposit
onto a
membrane collection strip. This approach, however, requires precise insertion
of
s the micro needle, oftentimes by trained medical personnel, and also results
in
biohazardous "sharps".
Another series of techniques for collecting interstitial fluids are described
in PCT Patent Application, Serial No. PCT/US96/13865, published on March 6,
1997, International Publication No. W097/07734 and the prior art cited therein
to (hereinafter referred to as the "PCT application"). The PCT application
describes
the use of energies at various wavelengths and frequencies to form micropores
through the stratum corneum to a depth that exposes the epidermal layer.
Methods to form such micropores include laser, sonic energy, and thermal
energy, with or without the use of dyes or other energy absorbing materials to
~s assist in the ablation and removal of the stratum corneum. In the PCT
application, interstitial fluids are described as exuding from the epidermis
after
microporation of the stratum corneum. In addition, to induce fluid flow, a
vacuum (10 to 12 inches of Hg) can be applied to the microporation sites
(Examples 14 and 39 of the PCT application described above). Example 14
2o describes the use of the recovered fluids for analysis of biological
materials, such
as glucose levels. In Example 39, the use of a vacuum (i. e., a negative
pressure) and ultrasound was said to produce an increase in the quantity of
recovered interstitial fluid when compared with the use of vacuum alone.
In connection with the vacuum assist approach described in that PCT
2s application, the volume collected is a function of the number of
micropores, the
level of vacuum, and the length of time the vacuum is applied. However, the
techniques disclosed in the PCT application referred to above suffer from
several
disadvantages. First, even when all variables are optimized, the quantity of
interstitial fluids obtained from the micropores in a short time period may
not be
3o sufficient to utilize in various medically related testing procedures.
Second,
2
CA 02306728 2000-04-20
WO 99/20181 PGT/US98/22114
increasing the applied vacuum above about 13 inches Hg (about -6.5 psig) can
result in visible hematomas of the skin and patient discomfort. Moreover, the
use of vacuum assistance increases the evaporation of the fluids under
extraction and requires a substantially air-tight seal around the
microporation
s site, which can oftentimes be difficult to achieve, even in a clinical
setting.
Finally, this technique also requires vacuum pumps and attendant fixtures,
which
can be expensive to acquire and maintain.
These and other disadvantages of the prior art are overcome by the
apparatus and method of the present invention. In particular, the present
~o invention provides apparatus and methods that allow the collection of large
quantities of bodily fluids, such as interstitial fluids, from the epidermal
layer over
short periods of time, when compared with the amounts collectable through
prior
art techniques, without the need for vacuum assist devices. The apparatus and
methods are inexpensive to fabricate, easy to use, and present minimal
is discomfort to the patient.
SUMMARY OF THE INVENTION
In the present invention, it has been discovered that increased amounts of
2o interstitial fluids can be collected from micropores formed through the
stratum
corneum and extending into the epidermal layer by using a novel cup-shaped
pressure head applied to the area of the skin surrounding the micropores. The
pressure head is applied under a positive pressure, the force of which may
fall
within the broad range of about 1 to about 11 pounds, preferably from about 3
to
2s about 11 pounds, with about 4 to about 9 pounds being preferred. The
pressure
head includes an aperture of diameter sufficient to surround the micropores,
together with a reservoir volume in which the fluids may be collected and
maintained and from which the fluids may be sampled or removed. The positive
pressure may also be conveniently applied using the pressure head, with
3o collection of fluids being carried out with separate apparatus, such as a
capillary
3
CA 02306728 2000-04-20
WO 99/20181 PCTfUS98/22114
tube, an absorbent material, or other suitable device. The head may be housed
in a holder having an air ram or other mechanism to provide variable pressure
to
the head when the head is placed on a patient's skin. The method of the
present
invention includes forming a breach through the stratum corneum and into the
s epidermal layer, followed by the application of a positive pressure to the
area
surrounding the microporation site to cause interstitial fluids to exude
therefrom.
The interstitial fluids are then collected.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a typical microporation site having six micropores;
Figs. 2A, 2B, 2C, 2D, 2E, 2F, and 2G are cross-sectional views of
pressure heads of the present invention;
Fig. 3 is a graph of the data derived from Example 2;
is Fig. 4 is a graph of the data derived from Example 2;
Fig. 5 is a graph of the data derived from Example 2;
Fig. 6 depicts the holder and pressure head arrangement of the present
invention;
Figs. 7A through 7J are graphs of the data derived from Example 3;
2o Figs. 8A through 8C are graphs of the data derived from Example 4;
Figs. 9A through 9H are graphs of the data derived from Example 5; and
Figs. 10A and 10B are schematic views of an apparatus that can be used
to apply a force to the skin to aid in the collection of interstitial fluids
therefrom.
Figs. 11A and 11 B are schematic views of an apparatus that can be used
2s to apply a force to the skin to aid in the collection of interstitial
fluids therefrom.
DETAILED DESCRIPTION
The present invention makes use of a pressure head that is positioned on
3o the skin of an animal, such as a mammal, in a manner to encompass a site
that
4
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
has first been treated to breach the stratum corneum. Advantageously, the
pressure head can be used in instances in which the stratum corneum has been
removed by microporation techniques to expose the epidermal layer. Such
microporation techniques are described in detail in the PCT application
referred
s to above, which is incorporated herein by reference.
For example, the microporation technique may involve the use of focused
laser energy of a power and pulse width sufficient to ablate the stratum
corneum
to expose the epidermal layer without substantial exposure of the dermal
layer.
This technique may be used with dyes or other energy absorbing materials to
io assist in the transfer of energy to the stratum corneum, and hence ablation
of the
stratum corneum, or may be used without such absorbing materials and may be
applied to form one or more micropores, either sequentially or concurrently.
Such micropores may be of circular, elliptical, or other shape. As used
herein,
the term "micropore" means a small breach or pore formed in the stratum
is corneum in a selected area of the skin to lessen the barrier properties of
the
stratum corneum such that fluids, for example interstitial fluids, can exude
from
the epidermal layer. Such micropores include those described in the PCT
application referred to above and also include openings or breaches through
the
stratum corneum having diameters of on the order of up to 500 Nm, with about
20 100 Nm being preferred. For example, Fig. 1 shows a typical microporation
site
that includes six micropores 12, each having an elliptical shape of about 80
pm by 100 Nm in size. The overall size of the microporation site is about 1.5
mm
when measured from the outer edges of the micropores 12. The centers of
micropores 12 of Fig. 1 lie on a circle having a diameter of about 1 mm, with
the
2s centers of adjacent micropores being about 450 Nm apart.
Prior art techniques for collecting interstitial fluids from a microporation
site, such as site 10 of Fig. 1, involve either collecting the fluids as they
naturally
exude from the site or by providing a vacuum (i. e., negative pressure) to the
site
to cause more fluids to exude from the micropores 12. While these techniques
3o permit the collection of some quantities of interstitial fluids, it has
been
5
CA 02306728 2000-04-20
WO 99/20181 PCT/CTS98/22114
discovered that significantly larger quantities of fluids can be collected in
a
shorter amount of time using the apparatus and methods of the present
invention.
In particular, it has been discovered that the topical application of a
s positive pressure to the area surrounding the microporation site 10 permits
recovery of interstitial fluids in an amount that is from about three (3) to
about
thirty (30) times or more than the amounts collected using the vacuum assist
technique described above and in the PCT application referred to herein.
It has also been discovered that the positive pressure can advantageously
~o be applied by using a generally cup-shaped pressure head that may be
included
in a holder that permits the application of variable amounts of positive
pressure
to the microporation site.
Thus, the present invention described herein can be utilized for the
collection of interstitial fluids from a microporation site, irrespective of
the
is techniques used to form the breach. Although the examples which follow
below
describe the use of the present apparatus and methods to collect fluids from
micropores formed via laser energy, the invention is not so limited.
Referring now to Figs. 2A through 2G, wherein like reference numerals
refer to like components, various pressure heads of the present invention are
2o generally depicted. The heads may be made from any suitable polymeric
material, such as, for example acrylic, polypropylene, polyethylene, and
others,
including copolymeric and terpolymeric materials, as well as suitable metallic
materials such as stainless steel, or such other materials suitable for
formation of
the head and application of pressure to the skin.
2s Fig. 2D depicts a pressure head 14 having at one end thereof a threaded
end 16; preferably the threads are on the exterior wall 18 of the head 14,
although the threads may also be along the interior wall 20. The interior of
the
head 14 forms a reservoir 22. The head 14, at the end opposite threaded end
16, includes a bottom portion 24 which may be circular, elliptical, square,
3o rectangular or other shape. An aperture 26 is formed through the portion 24
to
6
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
form a communication channel to the reservoir 22. In the head 14 of Fig. 2D,
which is referred to in the examples that follow as "Head D", the radius of
curvature 28 of the exterior wall 18 near the bottom portion 24 is 0.45 inches
(11.43 mm), the bottom portion 24 being circular and having a diameter of 0.25
s inches (6.35 mm), and the aperture being circular and having a diameter of
0.10
inches (2.54 mm). As explained hereinafter, it has been discovered that the
radius of curvature of the exterior wall 18 near the bottom portion 24 has an
effect on the quantities of interstitial fluids that can be collected from a
microporation site.
to Fig. 2B depicts a pressure head 14, referred to below as "Head B", which
is similar to that shown in Fig. 2D; however, the bottom portion 30 of the
pressure head of Fig. 2B is concave and has a radius of curvature 32 of 0.50
inches (12.7 mm), with the concave portion having a diameter of 0.311 inches
(7.9 mm).
is Fig. 2C depicts a pressure head 14, referred to below as "Head C". The
exterior wall 18 of Head C has a radius of curvature 34 of 0.75 inches (19.05
mm) and the interior wall 30 of Head C has a corresponding radius of curvature
36 of 0.650 inches (16.51 mm). The bottom portion 24 of the head 14 of Fig. 2C
is circular and has a diameter of 0.25 inches (6.35 mm).
2o Fig. 2A depicts pressure head 14, referred to below as "Head A". The
exterior wall 18 of Head A has a radius of curvature 38 of 0.45 inches (11.43
mm) and the interior wall 20 of Head A has a radius of curvature 40 of 0.361
inches (9.17 mm). The bottom portion 24 of the head 14 of Fig. 2A has a
diameter of 0.377 inches (9.58 mm).
2s Fig. 2E depicts pressure head 14, referred to below as "Head E". The
bottom portion 24 of the head 14 of Fig. 2A is circular and has a diameter of
9.5
mm.
Fig. 2F depicts pressure head 14, referred to below as "Head F". The
bottom portion 24 of the head 14 of Fig. 2F has a diameter of 9.5 mm.
CA 02306728 2000-04-20
WO 99/20181 PC1'/ITS98/22114
Fig. 2G depicts pressure head 14, referred to below as "Head G". The
bottom portion 24 of the head 14 of Fig. 2G has a diameter of 5.7 mm.
In the most general sense, the method of the present invention includes
the steps of forming a breach through the stratum corneum and into the
s epidermal layer, followed by the application of a positive pressure to the
area
surrounding the microporation site to cause interstitial fluids to exude
therefrom.
The interstitial fluids are then collected. The fluids can then be analyzed to
determine the concentration of an analyte, such as glucose. In operation, one
specific method for the collection of interstitial fluids from the body of an
animal
o comprises the steps of:
(a) forming a breach through the stratum corneum of the
animal, such that the breach extends at least into the epidermal layer of
the skin of the animal;
(b) placing a pressure head adjacent to the breach;
is (c) exerting a positive pressure on the pressure head in a
direction generally toward the skin of the animal; and
(d) collecting fluids from the breach.
In this specific method, it is preferred that the pressure head be positioned
such
that the fluids flow through the aperture in the pressure head and into the
2o reservoir.
Alternatively, another specific method for the collection of interstitial
fluids
from the body of an animal comprises the steps of:
(a) placing a pressure head against the skin of the animal;
(b) forming a breach through the stratum corneum of the animal
2s such that the breach extends at least into the epidermal layer of the skin
of the animal, the breach being adjacent to said pressure head;
(c) exerting a positive pressure on the pressure head in a
direction generally toward the skin of the animal; and
(d) collecting fluids from the breach.
8
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
A positive pressure can be exerted on the pressure head prior to forming the
breach in the stratum corneum. In this specific method, it is preferred that
the
breach be formed so that it is in register with the aperture in the pressure
head
so that the fluids flow through the aperture in the pressure head and into the
s reservoir. Variations of these specific procedures can also be used.
In order to examine the efficiency of interstitial fluids collection using the
pressure heads and methods of the present invention, a series of tests were
performed, as described below.
to Example 1
In this example, nine (9) human volunteers were used. The interior
forearms (between the elbow and the wrist) of each volunteer were subject to
laser microporation (wavelength of 810 nm, 20 millisecond pulse width,
is approximately 250 milliwatts, 20 to 30 pulses applied, black tape applied
to the
skin to act as an energy absorber) to form a microporation site similar to
microporation site 10 shown in Fig. 1. Two such sites were made on each arm
of each subject and were hydrated with a water droplet placed on the
microporation site for 10 to 15 seconds, followed by drying (using gentle
blotting)
2o prior to fluid extraction. Thus, a total of four microporation sites were
made on
each subject.
One site on the right arm and the corresponding site on the left arm of
each subject were treated in the following manner. Head D, Fig. 2D, was
manually placed over the microporation site so that the aperture 26
2s encompassed the site. Manual pressure was exerted on Head D in a direction
toward the microporation site for sixty (60) seconds. Interstitial fluids
flowed into
Head D and were collected by means of 1 NI capillary tubes, with the collected
volume recorded. By means of this technique, the volume of interstitial fluids
recovered ranged from 0.34 to 1 NI.
9
CA 02306728 2000-04-20
WO 99/201$1 PCT/US98/22114
The second site on the right arm and the corresponding second site on
the left arm of each subject were treated as follows. A vacuum system, -7.5
psig
(15 inches of Hg), was applied to each microporation site for 60 seconds.
Interstitial fluids were observed to flow from the microporation site and were
s collected by means of 1 NI capillary tubes, with the collected volume
recorded.
The interstitial fluids (hereinafter "ISF") collected by the application of
positive pressure were thereafter analyzed for glucose levels. It is to be
noted
that for each use of the vacuum assist technique, the volume of interstitial
fluids
recovered was in the range of 0.1 to 0.2 NI and no glucose determination was
io made. As a control, a finger stick was also performed on each volunteer and
approximately 50 NI of blood was withdrawn. The blood samples were
centrifuged and the plasma analyzed for glucose values. Table 1 below
presents the results of this example.
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
Table 1
Volunteer ISF Glucose
ID * collected (NI) in sample (m /dl)
. J K-1 0.73 153.29
J K-2 0.74 135.27
J K-3 -- 124.4
NL-1 0.77 147.01
N L-2 1. 36 150.00
N L-3 129.6
SW 1 0.36 260.56
SW 2 0.34 275.88
SW 3 270
DS-1 0.34 126.76
DS-2 0.50 136.60
DS-3 ----- 115.9
TM-1 0.63 124.60
TM-2 0.87 124.94
TM-3 126.1
ML-1 1.00 96.00
ML-2 0.45 111.33
ML-3 ----- 103.5
JG-1 0.89 126.97
JG-2 1.35 111.04
JG-3 --- 104.1
GH-1 0.92 90.43
GH-2 0.53 93.21
G H-3 ---- 91.1
KN-1 0.69 103.91
KN-2 0.68 113.24
KN-3 94.8
The number following each volunteer ID represents the following:
1 = left arm, pressure applied for 60 seconds;
2 = right arm, pressure applied for 60 seconds; and
3 = finger stick sample for comparison.
As can be seen from the foregoing table, the glucose values measured
to from the collected ISF are reasonably correlated to the glucose values
obtained
from the blood plasma. This example also demonstrates that the volume of ISF
CA 02306728 2000-04-20
WO 99/20181 PCTlUS98/22114
that can be obtained by the positive pressure technique disclosed herein is
significantly greater than that obtained when using the vacuum method.
Example 2
In this example, the sequential application of pressure followed by
vacuum and vacuum followed by pressure was investigated. Two microporation
sites, similar to Fig. 1, were made on each arm (left and right) of seven (7)
human volunteers by means of the technique of Example 1. The microporation
to sites were hydrated as in Example 1 and were treated as follows. To one
site on
one arm (e. g., the right arm), Head D was applied, under manual pressure, for
60 seconds, iSF was collected, and the volume recorded. Thereafter, within two
to five minutes, a vacuum system was used to apply a vacuum (13 inches Hg) to
the same site for 60 seconds, ISF was collected, and the volume recorded. This
is pressure/vacuum technique was then applied to the corresponding site on the
volunteer's left arm. To the second site on the first arm (e. g., the right
arm} the
vacuum system was first applied (13 inches Hg) for 60 seconds, ISF was
collected, and the volume recorded. Thereafter, within two to five minutes,
Head
D was applied, under manual pressure, for 60 seconds to the same site, ISF was
2o collected, and the volume recorded. This vacuum/pressure technique was then
applied to the corresponding site on the volunteer's left arm in the same
manner.
Table 2 below presents the results of this example.
12
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/Z2114
Table 2
Volume ISF Volume ISF
(NI) collected(NI) collected
Volunteer Arm Condition under pressureunder vacuum
ID ''
MP Right PN 0.79 0.18
Left PN 1.25 0.11
Right V/P 0.61 0.03
Left VIP ~ 1.00 0.08
JG Right PN 1.09 0.21
Left PN 1.12 0.19
Right VIP 1.26 0.07
Left VIP 1.52 0.05
KN Right PN 0.45 0.34
Left PN 0.35 0.34
Right VIP 0.90 0.10
Left VIP 0.60 0.19
PB Right PN 0.53 0.30
Left PN 0.35 0.27
Right VIP 0.44 0.06
Left V/P 0.61 0.02
NL Right PN 0.75 0.30
Left PN 1.15 0.36
Right VIP 0.91 0.12
Left V/P 1.18 0.26
DS Right PN 0.48 0.25
Left PN 0.27 0.33
Right V/P 0.38 0.10
Left VIP 0.63 0.07
JB Right PN 0.71 0.38
Left PN 0.48 0.48
Right VIP 0.85 0.15
Left VIP 1.35 0.12
rw = rressure tonowed by vacuum; VIP = Vacuum followed by pressure
s As noted in Table 2, except for one instance involving volunteer DS, the
application of pressure gave a greater volume of !SF than did vacuum,
irrespective of whether the pressure was applied before or after the vacuum.
These data are presented graphically in Fig. 3.
13
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
Fig. 4 presents these data in a slightly different form. As there shown, for
example for volunteer MP, the average volume of ISF recovered from the right
arm through the application of pressure is shown in the left most bar as 0.70
NI.
This value is obtained from the foregoing table, where the ISF collected by
the
s pressure technique is 0.79 NI and 0.61 NI from the right arm; the average is
0.70
NI. The remaining data found in Fig. 4 is determined in the same manner. Fig.
4
thus highlights that the use of positive pressure to obtain ISF is superior to
vacuum techniques.
Fig. 5 is a further depiction of the data of Table 2 and again shows the
io distinct advantages of using positive pressure to obtain ISF. As there
shown, the
data are grouped by volunteer, according to the method first applied to
collect
ISF. For example, the left most bar in each data set represents the average
volume of ISF collected from both arms of each volunteer during the 60 seconds
of pressure application when pressure is applied first. For volunteer MP, this
is average value of 1.02 NI is obtained from the Table 2 data for the right
and left
arms (i. e., 0.79 girl and 1.25 NI respectively). The second bar represents
the
average value of ISF collected from both arms of volunteer MP during the 60
seconds of vacuum application when pressure is applied first. The third bar
represents the average value of ISF collected from both arms of volunteer MP
2o during the 60 seconds of vacuum application when vacuum is applied first,
followed by pressure. Finally, the fourth bar represents the average value of
ISF
collected from both arms of volunteer MP during the 60 seconds of pressure
application when vacuum is applied first. The data depicted in Fig. 5 for the
remaining volunteers is obtained in a similar manner.
2s The means of the data set forth above in Table 2 also show that the
application of positive pressure provides significant advantages to the
collection
of ISF over the vacuum technique. The following Table 3 sets forth the means
of
this data.
14
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
Table 3
Mean iSF volume collectedMean ISF volume collected
from both arms during from both arms during
Volunteer 60 60
ID seconds of pressure seconds of vacuum
application (NI) application (NI)
MP 0.91 0.10
JG 1.25 0.13
KN 0.57 0.24
PB 0.48 0.16
NL 1.00 0.26
DS 0.44 0.19
JB 0.85 0.28
s Thus, for each volunteer, the use of positive pressure provided
significantly higher ISF collection volumes than could be obtained from the
use of
vacuum. Indeed, across all volunteers, the mean collection volume of ISF was
0.78 NI by pressure but only 0.19 N1 by vacuum, a difference of over 300%.
to Example 3
As a follow-up to Examples 1 and 2, a further set of studies was
performed on five (5) human volunteers. In these studies, microporation sites,
similar to Fig. 1, were formed on the interior forearm of the volunteer by
means
~s of the technique of Example 1; the sites were hydrated as in Example 1.
Head D
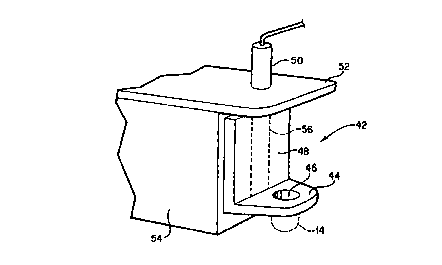
(see Fig. 2D) was used as the pressure head and was attached to the hotder 42
shown in Fig. 6. That holder includes a base plate 44 having a threaded
opening
46 for engagement with the threaded end 16 of head 14 (shown in dotted lines
in
Fig. 6). The holder 42 also includes a movable vertical plate 48 attached to
the
2o base plate 44. The movable plate 48 is connected to a ram 50. The ram 50,
which may be an air driven or hydraulic ram or a biased spring ram, operates
to
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
exert a force on the base plate 44, and hence to the threaded end 16 of the
head
14. The ram 50, as depicted in Fig. 6, is coupled to a top plate 52, which in
turn
is coupled to a stand 54. Of course, other ways of connecting the ram 50 to
the
movable plate 48 can be used. The stand 54 may also be provided with a
s tongue (or groove) or other suitable mechanism for engagement with a groove
(or tongue) or other suitable mechanism on the movable plate 48, as generally
depicted by dotted lines 56 in Fig. 6. Such arrangement permits the movable
plate 48 to travel in a repeatable manner when the holder 42 is used. The ram
may exert a known force to the head 14, which force may be varied from one use
io of the holder to another or during any single use thereof. In this Example
3, the
holder was operated such that a force of 4 through 11 pounds could be applied
to the head 14 at the threaded end 16 thereof.
During the course of Example 3, the force applied to the threaded end 16
of Head D was maintained constant during any single run, but was varied from
Is one run to the next. Thus, the following description of the tests performed
on
Subject 1 applies to the remaining subjects, unless otherwise noted.
After formation of the microporation site and hydration as in Example 1,
Head D, having a circular bottom portion 24 with a diameter of 2.5 mm, was
applied to the microporation site using a force of 5 pounds on the threaded
end
20 16. The ISF flux (in NI/minute) was then measured in 30 second increments
over
an elapsed time of 6 minutes. Thereafter, Head D was removed from the
microporation site. A new microporation site was formed and hydrated as in
Example 1 and Head D was applied to this new site using a force of 6 pounds on
the threaded end 16. The ISF flux (in Nl/minute) was measured as described,
2s after which Head D was again removed. Another microporation site was formed
and the above procedure was repeated using a force of 7 pounds applied to the
threaded end of Head D. The procedures were again repeated, as described,
with the application of 8 and 9 pounds of pressure to the threaded end of Head
D. Subjects 2 and 3 were treated as described above. Subject 4 was treated in
3o the same manner, except that a force of 11 pounds on the threaded end 16 of
16
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
Head D was also studied. Subject 5 was also treated in the same manner,
except that the forces applied to the threaded end 16 of Head D were 4, 5, 6,
7
and 8 pounds.
This example also investigated the effect on ISF recovery caused by
s increasing the diameter of the bottom portion 24 of Head D. Thus, the
procedures described above were used in conjunction with the Head D of Fig.
2D in which the diameter of the bottom portion 24 was 3.0 mm.
Figs. 7A through 7J depict the results of this example, in which the flux
rate of lSF is plotted against time (in minutes) for the applied forces and
where
to the figures represent the diameters of the bottom portion 24 of Head D as
described in the following Table 4.
Table 4
Subject2.5 mm diameter3.0 mm diameter
1 Fig. 7A Fig. 7B
2 Fi .7C Fig.7D
3 Fig. 7E Fig. 7F
4 Fig. 7G Fig. 7H*
Fig. 71 Fig. 7J
*Note: 11 pounds pressure not studied
Referring to Figs. 7A, 7C, 7E, 7G, and 71, it will be noted that, in most
instances for each force applied, the rate of ISF flow increases for the first
60
seconds that the force is applied and then tends to decrease thereafter.
2o However, there are some variations from subject to subject and, to a more
limited extent, within the subjects themselves. The same general observations
can be made from Figs. 7B, 7D, 7F, 7H, and 7J.
17
CA 02306728 2000-04-20
WO 99/20181 PC'T/US98/22114
Comparing the results obtained from using the 2.5 mm diameter Head D
to those from using the 3.0 mm diameter Head D, it can be seen that for all
subjects, except Subject 4, the initial rate of ISF flow was greater for the
2.5 mm
diameter Head D.
s
Example 4
In this example, Subjects 1, 3, and 6 of Example 3 were used to test the
recovery rate of the ISF using a vacuum followed by the application of
positive
to pressure. A microporation site was prepared and hydrated by means of the
technique of Example 1 and the volume of recovered ISF was measured. For
these subjects, ISF was collected for 120 seconds using vacuum (-12.73 psig),
immediately followed by vacuum removal for 60 seconds for site recovery. After
recovery, vacuum (-12.73 psig) was again applied for 120 seconds, followed by
is 60 seconds of site recovery (vacuum removed). This procedure was repeated
five times using vacuum assistance. Each subject was then allowed a five
minute recovery period, following which ISF was collected for 120 seconds
using
Head C with a force of 7 pounds applied to the threaded end 16 of Head C.
Thereafter, Head C was removed from the microporation site for 60 seconds to
2o allow for recovery. At the end of the collection period, ISF collection was
performed for 120 seconds using Head C with a force of 7 pounds applied to the
threaded end 16 of Head D. After this collection, Head C was removed for
another recovery period of 60 seconds. Collection in this manner using
positive
pressure was carried out five times over the period.
2s The results of this example are shown in Figs. 8A through 8C. From Figs.
8A through 8C, it is noted that the volume of ISF collected from each subject
using the vacuum approach remained substantially constant over the test
period,
although the volume collected from Subject 3 decreased at 8 minutes and 10
minutes.
CA 02306728 2000-04-20
WO 99/20181 PC'T/US98/22114
On the other hand, the volume collected from each subject upon the
application of positive pressure generally decreased over the entire test
period of
minutes. It is thus theorized that the ISF in the epidermis exists in
equilibrium
with fluids in the underlying dermal layer and the surrounding tissues.
Removing
s large quantities of ISF from the epidermis and dermis over a relatively
short
period of time, without providing a sufficient recovery period, upsets this
equilibrium and depletes the ISF residing in the epidermis and dermis of the
treated area. Indeed, in connection with the present invention, it has been
observed that when a recovery period of on the order of 3 to 8 minutes is
used,
to the next removal of ISF by application of positive pressure will be of a
high
volume. For example, with reference to Fig. 8A, had the recovery time between
the 6 minute and 8 minute positive pressure data been longer than the 60
seconds used, the volume of ISF recovered at 8 minutes would have been of the
magnitude shown for the 2 minute pressure data. This result has led to the
is theory that there two mechanisms affecting the quantity of ISF in the
epidermis.
First, ISF naturally resides in the epidermal and dermal layers and is
available to
be removed upon the application of pressure. Second, there is a steady influx
of
ISF consisting of blood plasma filtrate from the capillaries, through the
dermal
layer, and into the epidermis. Although this influx occurs at a finite rate,
this
observation establishes the ability to continuously monitor ISF for fluid
analysis
and other purposes.
Example 5
2s In this example, Heads A through D (see Figs. 2A-2D), each having an
aperture diameter of 2.5 mm, were used to collect ISF from Subject 1 of
Example
3. A microporation site was prepared by means of the technique of Example 1
and (SF was collected by means of the technique described in Example 3. The
rate of ISF removal and the volume of ISF removed was measured. Figs. 9A
3o through 9H set forth the flux rate and cumulative recovered volume data
19
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
obtained in this example. As seen from a comparison of Figs. 9A, 9C, 9E, and
9G, the Head C generally provides the greatest initial flow {i. e., slope) of
ISF
over the first 60 seconds as compared to Heads A, B and D. As observed from
Figs. 9B, 9D, 9F, and 9H, the Head C also provides the greatest volume of ISF
s collected over all applied pressures as compared to the other heads.
These data thus indicate that the head shape, particularly the radius of
curvature, has an effect on the flow rate and volume of ISF recovered. In
particular, as the shape of the exterior wall 18 of the pressure head 14
approaches that of a cylinder (i. e., no curvature along the longitudinal axis
of the
~o head when viewed from the threaded end 16 to the bottom portion 24), the
rate
of ISF flow and the volume of ISF recovered increases.
Examlale fi
is In this example, six different head configurations were used to extract
ISF.
Twelve microporation sites, similar to those of Fig.1, were made on the
interior
forearms of five human volunteers by means of the technique of Example 1.
Heads were attached to the holder 42 shown in Fig. 6. Head A was attached to
the holder and was used to apply four (4) pounds force to the microporation
site
2o in the same manner as in Example 3. Another microporation site was then
formed and head A was used to apply six (6) pounds of force to the
microporation site. This process was repeated in this fashion until all heads,
A
B, C, D, E, and F were used on each subject. In each case, the ram fixture was
used as in Example 3. ISF flux (in NI/minute) was then measured in 30-second
2s increments over a five-minute period.
Table 5 shows the results of this experiment, including the average
amount of ISF collected for all five subjects, for the times of 30 seconds and
60
seconds. Table 5 also shows the percentage of the total amount of 1SF
collected
in 60 seconds that was collected in the first 30 seconds. This percentage
3o indicates how quickly the rate of collection increases to its maximum and
is
CA 02306728 2000-04-20
WO 99/20181 PCTNS98/22114
pertinent because it is desirable for the instrument to collect the fluid in a
short
amount of time.
Table 5
s
Head Force Average Std. RSD' Median PercentPercentPercent
(pounds)volume Dev. (%) volume of of of
collected(NI) collectedfluid collectionscollections
collected>1NI >0
5NI
(NI) (NI) in first .
30
seconds
A 4 0.23 0.22 96 0.16 12 0 20
A 6 0.41 0.37 89 0.45 31 0 40
B 4 0.15 0.18 115 0.08 29 0 0
B 6 0.11 0.13 112 0.08 6 0 0
C 4 0.52 0.40 77 0.35 35 20 40
C 6 0.93 0.29 32 1.10 39 60 80
D 4 0.38 0.40 103 0.23 28 20 20
D 6 0.48 0.25 51 0.44 34 0 40
E 4 1.03 0.24 23 1.02 44 60 100
E 6 1.44 0.48 34 1.42 44 80 100
F 4 1.12 0.43 38 0.98 43 40 100
F 6 1.92 0.43 23 2.07 43 100 100
" RSD means standard deviation (Std. Dev.) divided by average volume collected
times 100%.
Total amount of fluid collected (NI):
Subject 1: 10.72
to Subject 2: 9.39
Subject 3: 8.45
Subject 4: 4.49
Subject 11: 10.66
21
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/ZZ114
Examele 7
In this example, 18 microporation sites, as in Example 1, were made on
the interior forearms of six human volunteers. In this example, the micropores
were arranged (a) singly, (b) in a straight line separated by 1 mm, or (c) in
a
triangle with each micropore forming a vertex of an equilateral triangle 1 mm
on
each side. Heads C, E, and G were used. The force was either four (4) pounds
or seven (7) pounds for each combination of pore number and head. A fixture
similar to the ram fixture was used, but instead of compressed air, this
fixture
to utilized a system of weights applied to the top of the ram to deliver the
force.
This change was made to increase the accuracy of the force delivery and to
reduce friction in the force delivery device. Fluid was collected for one
minute at
intervals of 30 second (if possible) and the volume collected was calculated.
Table 6 below shows the results of this example, including the average
is amount of ISF collected for all five subjects, for the times of 30 seconds
and 60
seconds. Table 6 also shows the percentage of the total amount of ISF
collected
in 60 seconds that was collected in the first 30 seconds. This percentage
indicates how quickly the rate of collection increases to its maximum and is
pertinent because it is desirable for the instrument to collect the fluid in a
short
2o amount of time. The final column shows the increase in volume of fluid
collected
in one minute in the presence of additional micropores relative to volume of
fluid
collected in the presence of a single micropore. In general terms, the
percentage increase in going from one micropore to two micropores was greater
than the percentage increase in going from two micropores to three micropores.
z5 This was especially true of the more aggressive heads (head E and head G).
22
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
Table 6
Head Force NumberAverageStd. RSD" PercentPercentPercentRatio
(pounds)of volumeDev. (%) of of of of
pores collected(NI) fluid collectionscollectionsamount
(NI) collected> 1 >0.5NI collected
in NI compared
first to amount
30 collected
seconds with
1
pore
C 4 1 0.47 0.24 51 45 0 50 1.00
C 4 2 0.80 0.31 39 38 20 80 1.70
C 4 3 1.00 0.43 44 42 60 80 2.10
C 7 1 0.88 0.42 48 47 20 80 1.00
C 7 2 1.06 0.23 21 47 50 100 1.20
C 7 3 1.57 0.47 30 52 80 100 1.80
E 4 1 0.79 0.22 28 44 10 90 1.00
E 4 2 1.27 0.47 37 40 60 100 1.60
E 4 3 1.86 0.58 31 43 100 100 2.30
E 7 1 1.22 0.22 18 53 90 100 1.00
E 7 2 2.29 0.51 22 54 100 100 1.90
E 7 3 2.99 0.57 19 48 100 100 2.50
G 4 1 1.75 0.41 23 100 100 1.00
G 4 2 2.50 0.38 15 100 100 1.40
G 4 3 2.58 0.47 18 100 100 1.50
G 7 1 2.59 0.34 13 100 100 1.00
G 7 2 3.04 0.59 19 100 100 1.20
G 7 3 3.51 0.62 18 100 100 1.40
" RSD means standard deviation (Std. Dev.) divided by average volume collected
times 100%.
s Total amount of fluid collected (NI):
Subject 1: 40
Subject 2: 55
Subject 4: 46
Subject 8: 44
io Subject 11: 45
23
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
Exam~~le 8
This example shows the effect of aperture diameter on the amount of fluid
s collected and the rate at which [the greatest percentage of fluid] is
recovered. In
this example, 11 head configurations were tested on the interior forearm of
five
subjects. The configurations were as follows:
Head Diameter
of
aperture
(mm)
C 1.5
C 2.5
C 3.0
C 4.0
E 1.5
E 2.5
E 3.0
E 4.0
G 1.5
G 2.5
G 3.0
io Three micropores were arranged in a triangle, with each micropore
forming a vertex of an equilateral triangle 1 mm on each side. Four pounds of
force was used for each extraction. Each extraction had a duration of 60
seconds, with samples being collected at 30 and fi0 seconds.
Table 7 shows the results cf this experiment, including the average
~s amount of ISF collected for all five subjects, for the times of 30 seconds
and 60
seconds. Table 7 also shows the percentage of the total amount of ISF
collected
24
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
in 60 seconds that was collected in the first 30 seconds. This percentage
indicates how quickly the rate of collection increases to its maximum and is
pertinent because it is desirable for the instrument to collect the fluid in a
short
amount of time. This example shows that varying the diameter of the aperture
at
s the center of the head can result in significant changes in the volume of
fluid
collected and flux rates. The smaller the aperture, the faster the ISF is
collected,
but a lower total volume is collected. At the largest aperture tested (4 mm),
the
fluid flux rate had significantly decreased, and total volume of ISF collected
differed sign~cantly from that when optimum size was used. The optimum size
to was found to be 2.5 mm to 3.0 mm with these head configurations.
The conditions for forming the micropores in the skin and applying the
force to the skin were as follows:
is 1. Dye #5, ICI 2 mil w/carbon, removed after potation
2. Umbilical porator, 30 pulses, 250 mw
3. 30 ms pulse, 60 ms delay
4. "direct" RAM with 4 Ibs. of weight
CA 02306728 2000-04-20
WO 99/20181 PCT/US98122114
Table 7
Head Inside Average RSD" AverageRSD' PercentPercentPercent
diametervolume (%) volume (%) of of of
of collected, collected, fluid collectionscollections
aperture0-30 0-60 collected>1 NI >0.5
sec sec in first NI
(NI) ( I) 30
seconds
C 1.5 0.1033 74 0.2809 49 37 0 0
C 2.5 0.1828 70 0.5363 62 33 10 30
C 3.0 0.1606 70 0.5106 48 30 10 40
C 4.0 0.1225 50 0.4416 32 27 0 30
E 1.5 0.2729 54 0.6906 37 37 10 80
E 2.5 0.4131 54 1.0747 43 35 70 80
E 3.0 0.4369 41 1.2369 33 34 70 100
E 4.0 0.3803 47 1.2038 31 31 70 100
G 1.5 1.2344 30 1.9556 18 63 100 100
G 2.5 1.6919 14 2.5638 11 66 100 100
G ~ 3.0 1.7703 20 ~ 2.6272 17 ~ 67 ~ 100 100
~ ~ ~ ~ -.
RSD means standard deviation (Std. Dev.) divided by average volume collected
times 100%.
s Total amount of fluid collected (NI):
Subject 1: 21
Subject 4: 25
Subject 6: 26
Subject 8: 28
to Subject 11: 31
26
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
Pressure can be applied to the skin by means of apparatus other than the
pressure head and ram previously described. Figs. 10A and 10B show an
apparatus that employs a vacuum to cause atmospheric pressure to act upon a
piston in a cylinder and cause it to apply a force to the skin. The apparatus
s allows the user, i. e., the patient, to apply force to a body part, such as
a
forearm, without the need for providing an opposing force to inhibit motion.
Typically, when pressure is employed to force interstitial fluids to exude
from the skin, a stopping mechanism is required to oppose the applied force
and
keep the body part stationary. An apparatus that exerts a force on the skin of
io the forearm normally requires a means for supporting the backside of the
arm,
typically through the use of a mechanical clamp or an immovable object, such
as
a table. These means are large and uncomfortable for the user, or they require
proper technique to provide consistent results. The apparatus shown in Figs.
10A and 10B can be made in small sizes. It is less constraining than a clamp
or
is a strap or a band because it does not need to surround the site of interest
on the
body part. This apparatus is more comfortable than other apparatus currently
used to apply force to the skin. Unlike a clamp or a strap or a band, this
apparatus will not cause blood vessels to collapse. Because the apparatus
requires access to only one surface of a body part of a subject, it can be
applied
2o to virtually any site for obtaining samples of interstitial fluids, such as
the arm,
thigh, or waist, without any modifications.
Turning now to Figs. 10A and 10B, the apparatus 100 comprises a
cylinder 102 and a piston 104. The piston 7 04 comprises a seal 106 and a
pressure head 108. The pressure head 108 has a bottom portion 109, which
2s has a small aperture 110 at the lowermost point thereof. The pressure head
108
also contains a reservoir 112. The cylinder 102 has a vacuum port 114. The
purpose of the cylinder 102 is to position the apparatus over the site from
which
interstitial fluids are to be collected. The purpose of the piston 104 is to
apply
sufficient force to the skin to cause interstitial fluids to emerge therefrom.
The
3o purpose of the seal 106 is to maintain the vacuum at a level sufficient for
causing
27
CA 02306728 2000-04-20
WO 99/20181 PCT/I3S98/22114
the piston 104 to apply sufficient pressure to the skin. The purpose of the
pressure head 108 is to provide contact with the skin at the point of
application of
force. In addition, the pressure head 108 has a small aperture 110, through
which the interstitial fluid can flow for collection in the reservoir 112.
s In operation, a breach is formed in the stratum corneum by one of the
techniques described previously. The apparatus 100 is placed over the breach,
with the cylinder 102 being in contact with the skin so that the aperture 110
is in
register with the breach in the stratum corneum. The vacuum is applied via a
pump or the like (not shown) through the vacuum port 114. Under the influence
io of vacuum, the piston 104 is caused to travel downwards against the skin
because of atmospheric pressure acting on the upper surface 116 of the piston
104. See Fig. 10B. The positive pressure exerted on the skin by the pressure
head 108 causes interstitial fluids to flow through the breach in the stratum
corneum and through the aperture 110 and collect in the reservoir 112. The
fluid
is can then be analyzed determine the concentration of analyte. Alternatively,
the
apparatus 100 is placed over the skin, with the cylinder 102 being in contact
with
the skin. A breach is then formed in the stratum corneum so that the aperture
110 is in register with the breach in the stratum corneum. If desired,
pressure
can be applied to the skin prior to forming the breach in the stratum corneum.
2o The vacuum is applied via a pump or the like (not shown) through the vacuum
port 114. Under the influence of vacuum, the piston 104 is caused to travel
downwards against the skin because of atmospheric pressure acting on the
upper surface 116 of the piston 104. See Fig. 10B. The positive pressure
exerted on the skin by the pressure head 108 causes interstitial fluids to
flow
2s through the breach in the stratum corneum and through the aperture 110 and
collect in the reservoir 112. The fluid can then be analyzed determine the
concentration of analyte. Variations of these specific procedures can also be
used.
A pressure cuff can be used to apply force and pressure to a body part in
3o which a breach of the stratum corneum has been formed so that interstitial
fluids
28
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
can be collected from the breach. In appearance, the pressure cuff is
substantially similar to the pressure cuffs used to measure a person's blood
pressure. In other words, the pressure cuff comprises a strap or band that is
designed to surround the site of interest on the body part. Referring now to
Figs.
s 11A and 11 B, a pressure cuff 200 comprises a band 202 to which is attached
a
pressure head 204. The purpose of the pressure head 204 is to provide contact
with the skin at the point of application of force. At one end of the pressure
head
204 are means 206 for attaching the pressure head 204 to the band 202. Such
means 206 may include threads; preferably the threads are on the exterior wall
to 208 of the pressure head 204, although the threads may also be along the
interior wall 210 of the pressure head 204. The band 202 comprises a means
211 for securing the pressure head 204. If the pressure head 204 utilizes
threads, the securing means 211 preferably also uses threads. The interior of
the pressure head 204 forms a reservoir 212. At the end opposite the means
~s 206 for attaching the head 204 to the band 202 is a bottom portion 214
which
may be circular, elliptical, square, rectangular or other shape. An aperture
216 is
formed through the bottom portion 214 to form a communication channel to the
reservoir 212.
In operation, a breach is formed in the stratum corneum of the body part,
2o preferably the forearm, by one of the techniques described previously. The
band
202 is placed around the body part so that the pressure head 204 is directly
over
the breach, so that the aperture 216 is in register with the breach in the
stratum
corneum. The band 202 has an end 218, which is inserted through a buckle
220. The end 218 of the band 202 can be pulled to tighten the band 202 around
2s the site of interest on the body part. The band 202 can be tightened
further by
increasing the pressure within a bladder 222, located on the band 202. The
pressure can be increased in the bladder 222 by supplying air from a pump 224.
The increase in pressure can be monitored by a pressure gauge 226. The band
202 should be tightened sufficiently so that the pressure head 204 applies a
3o force to the skin suffcient to cause interstitial fluids to flow through
the breach in
29
CA 02306728 2000-04-20
WO 99/20181 PCT/US98/22114
the stratum corneum and through the aperture 216 and collect in the reservoir
212. The fluid can then be analyzed determine the concentration of an analyte.
Alternatively, the band 202 is placed around the body part. A breach is
formed in the stratum corneum of the body part, preferably the forearm, so
that
s the aperture 216 is in register with the breach in the stratum corneum. If
desired,
pressure can be applied to the skin prior to forming the breach in the stratum
corneum. The end 218 of the band 202 can be pulled to tighten the band 202
around the site of interest on the body part. The band 202 can be tightened
further by increasing the pressure within a bladder 222, located on the band
202.
to The pressure can be increased in the bladder 222 by supplying air from a
pump
224. The increase in pressure can be monitored by a pressure gauge 226. The
band 202 should be tightened sufficiently so that the pressure head 204
applies
a force to the skin sufficient to cause interstitial fluids to flow through
the breach
in the stratum corneum and through the aperture 216 and collect in the
reservoir
~s 212. The fluid can then be analyzed determine the concentration of analyte.
Variations of these specific procedures can also be used.
Upon reading and understanding the invention disclosed herein, it should
be apparent to those of skill in the art that modifications and changes to the
apparatus and methods disclosed herein can be made while still falling within
the
2o scope and spirit of the present invention. All such modifications and
changes
are included herein and the invention should be considered limited only by the
claims which follow hereafter.