Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02309033 2002-O1-31
'1~
LACRIMAL GLAND SPECIFIC EMULSIONS FOR TOPICAL
APPLICATION TO OCULAR TISSUE
The present invention generally relates to novel
pharmaceutical compositions incorporating chemicals
ZO Which are poorly soluble in water and is more particu
larly related to a novel ophthalmic emulsion including
cyclosporin in admixture with castor oil and polysor
bate 80 with high comfort level and low irritation
. potential.
Cyclosporins are a group of nonpolar cyclic
oligopeptides with known immunosuppressant activity..
In addition, as set forth in U.S. Patent No.
4,839,342, cyclosporin (sometimes referred to in the
literature as "cyclosporine") has been found as
effective in treating immune medicated :keratoconjune-
tivitis sicca (KCS or dry eye disease) in a patient
suffering therefrom.
.As hereinabove noted, cyclosporin comprises a
group of cyclic oligopeptides and the major component
thereof is cyclosporin A (C~2H111N11012)Whzch has been
identified along with several other minor metabolites,
cyclosporin B through I. In addition, a number of
,30 synthetic analogs have been prepared.
In general, commercially available cyclosporins
. may contain a mixture of several individual cyclo-
sporins which all share a cyclic peptide structure
consisting of eleven amino acid residues with a total
molecular weight of about 1,200, but with different'
CA 02309033 2000-OS-30
WO 95/31211 PCTIUS95/06302
-2-
substituents or configurations of some of the amino
acids.
It should be appreciated that reference to the
term "cyclosporin" or "cyclosporins" is used through-
out the present specification in order to designate
the cyclosporin component in the composition of the
present invention.
However, this specific reference is intended to
include any individual member of the cyclosporin group
as well as admixtures of two or more individual cyclo-
sporins, whether natural or synthetic.
The activity of cyclosporins, as hereinabove
noted, is as an immunosuppressant and in the enhance-
ment or restoring of lacrimal gland tearing.
This activity can be enhanced if it is possible
to enhance the absorption of the cyclosporin in the
lacrimal gland. The present invention provides for a
formulation and method that produces optimal cyclo-
sporin A concentrations in the lacrimal gland and
other ocular surface tissues.
Unfortunately, the solubility of cyclosporin in
water is extremely low and as elaborated in U.S.
Patent No. 5,051,402, it has been considered not
merely difficult but practically impossible to prepare
-30 a pharmaceutical composition containing cyclosporin
dissolved in an aqueous medium.
As reported, the solubility of cyclosporin in
water is between about 20 ug/ml to 30 ~g/ml for
cyclosporin A. Hence, heretofore prepared formula-
tions incorporating cyclosporin have been prepared as
oily solutions containing ethanol. However, these
CA 02309033 2000-OS-30
WO 95131211 PCT/US95/06302
-3-
preparations limit the bioavailability to oral prep
arations and this is believed to be due to the separ
ation of cyclosporin as a solid immediately after it
comes into contact with water, such as in the mouth or
eye of a patient.
In the case of injectable preparations of cyclo-
sporin, they first must be diluted with physiological
saline before intravenous administration but this is
likely to result in the precipitation of cyclosporin
and therefore may be considered undesirable for
intravenous administration.
Surface active agents such as polyoxyethylated
castor oil have been utilized as solubilizers to in-
ject preparations in order to prevent cyclosporin from
separating. However, this also may give rise to
safety problems (see U.S: Patent No. 5,051,402).
The practical usefulness of cyclosporin would be
greatly enhanced if administration thereof could be
effective; for example, cyclosporin's effectiveness in
the treatment of ocular symptoms of Behcet's Syndrome.
However, if it is administered orally for the treat-
ment of these symptoms, the accompanying side effects
due to systemic circulation may cause adverse reac-
tions such as hypertrichosis or renal dysfunction.
On the other hand, if oily preparations contain-
- 30 ing cyclosporin are applied directly to the eyes, ir
ritation or a clouding of visual field may result.
This plus the difficulty in formulating cyclosporin
limits its use in formulations that would be useful
during keratoplasty as well in the treatment of
herpetic keratitis and spring catarrh.
CA 02309033 2000-OS-30
WO 95131211 PCT/US95/06302
-4-
Heretofore, as for example in U.S. Patent No.
5,051,402, attempts have been made to dissolve suffi-
cient cyclosporin in an aqueous solvent system so as
to reach an effective concentration for treatment.
Importantly, this solvent system does not contain any
surface active agent such as polyoxyethylated castor
oil.
Conceptually, the purpose of dissolving the
cyclosporin in an aqueous solvent system is to enable
contact with body fluids which would merely constitute
dilution of the aqueous solvent system which hopefully
would eliminate the immediate precipitation of cyclo
sporin when contacted with the water content of the
body fluids.
For direct use in the eye, cyclosporin has been
formulated with a number- of pharmaceutically accept-
able excipients, for example, animal oil, vegetable
oil, an appropriate organic or aqueous solvent, an
artificial tear solution, a natural or synthetic poly-
mer or an appropriate membrane.
Specific examples of these pharmaceutically
acceptable excipients, which may be used solely or in
combination, are olive oil, arachis oil, castor oil,
mineral oil, petroleum jelly, dimethyl sulfoxide,
chremophor, liposomes, or liposome-like products or a
silicone fluid, among others.
- 30
In summary, a great deal of effort has been ex-
pended in order to prepare a pharmaceutical composi-
tion containing cyclosporin dissolved in an aqueous
medium or cyclosporin prepared as an oily solution.
However, successful formulations have yet to be accom-
plished as evidenced by the lack of commercial prod-
ucts.
CA 02309033 2000-OS-30
WO 95/31211 PCT/US95/06302
-5-
As hereinabove noted, it has been reported that
cyclosporin has demonstrated some solubility in oily
preparations containing higher fatty acid glycerides
such as olive oil, peanut oil, and/or castor oil.
These formulations frequently produce an unpleasant
sensation when applied to the eye because of stimula-
tion or the viscousness which is characteristic of
these oils.
Another drawback of these formulations is that
they contain a high concentration of oils, and oils
exacerbate the symptoms of certain ocular surface
diseases such as dry eyes, indicated by cyclosporin.
Therefore, these oily formulations may not be clini-
cally acceptable. Additionally, these formulations
often suffer from physical instability due to cyclo-
sporin°~ propensity to undergo conformational change
and cr;~~stallize out. The-crystallization problem has
been noticed in formulations containing corn oil or
medium chain triglycerides. Lastly, these formula-
tions often have a low thermodynamic activity (degree
of saturation) of cyclosporin which leads to a poorer
drug bioavailability.
It may be possible to minimize the problems
related to unpleasant sensation and syndrome exacerba-
tion by reducing the oil content and dispersing the
oil phase in water into an emulsion. However, it is
not an easy task to formulate an ophthalmic emulsion
because one indispensable class of ingredients in an
emulsion system is emulsifiers, and the majority of
emulsifiers is highly irritating to the eyes.
The present invention is directed to an emulsion
system which utilizes higher fatty acid glycerides but
in combination with polysorbate 80 which results in an
emulsion with a high comfort level and low irritation
CA 02309033 2000-OS-30
WO 95/31211 PCT/US95/06302
-6-
potential suitable for delivery of medications to sen-
sitive areas such as ocular tissues. Further, the
present invention provides a pharmaceutical composi-
tion and method for causing preferential absorption of
cyclosporin in the lacrimal gland. That is, for a
given instillation of the composition into an eye, a
greater amount of absorption occurs in the lacrimal
gland for formulations made in accordance with the
present invention than heretofore utilized formula
tions.
SUMMARY OF THE INVENTION
In accordance with the present invention, a non-
irritating pharmaceutical composition with high com-
fort level and low irritation potential suitable for
delivery to sensitive areas such as ocular tissues
comprises cyclosporin in admixture with an emulsifying
amount of a higher fatty acid glycerol and polysorbate
80. More particularly, the composition may comprise
cyclosporin A and the higher fatty acid glyceride may
comprise castor oil.
Preferably, the weight ratio of the castor oil to
the polysorbate 80 is between about 0.3 to about 30
and a weight ratio of the cyclosporin to castor oil is
below 0.16. More preferably, the weight ratio of
castor oil to polysorbate 80 is between 0.5 and 12.5,
and the weight ratio of cyclosporin to castor oil is
between 0.12 and 0.02.
When cyclosporin is dissolved in the oil phase in
accordance with the present invention, the emulsion is
found to be physically stable upon long term storage.
No crystallization of cyclosporin was noticed after
nine months at room temperature. Moreover, the
cyclosporin emulsion is formulated in such a way that
CA 02309033 2000-OS-30
WO 95/31211 PCTIUS95/06302
the drug has reasonably high thermodynamic activity,
yet without the crystallization problem.
Importantly, the composition of the present in-
vention provides for enhanced absorption of the cyclo-
sporin in the lacrimal gland of the eye. In this
manner, the activity of the cyclosporin in restoring
lacrimal gland tearing is increased. That is, since
a greater amount of cyclosporin is absorbed into the
lacrimal gland, more of the cyclosporin is effective
in producing lacrimal gland tearing than heretofore
possible.
BRIEF DESCRIPTION OF THE DRAWINGS
The advantages and features of the present inven-
tion will be better understood by the following
description when consideYed in conjunction with the
accompanying drawings in which:
Figure 1 is a bar chart of conjunctival concen-
tration of cyclosporin A after a single topical
instillation of various formulations in a rabbit eye;
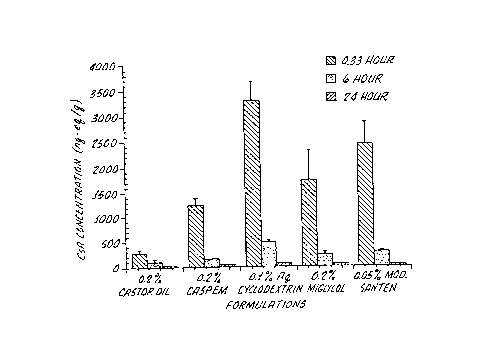
Figure 2 is a bar chart of cornea concentration
of cyclosporin A after a single topical instillation
of various formulations in a rabbit eye;
Figure 3 is a bar chart of ciliary body concen-
tration of cyclosporin A after a single topical
instillation of various formulations in a rabbit eye;
and
Figure 4 is a bar chart of lacrimal gland concen-
tration of cyclosporin A after a single topical
instillation of various formulations in a rabbit eye.
DETAILED DESCRIPTION
As hereinabove noted, cyclosporin is available as
a mixture in which the principal ingredient is cyclo-
CA 02309033 2000-OS-30
WO 95131211 PCT/US95/06302
_g_
sporin A with significant, but smaller, quantities of
other cyclosporins such as cyclosporin B through I.
However, as also hereinabove noted, the present inven
tion may be applied to either a pure cyclosporin or to
a mixture of individual cyclosporins.
The discovery on which the present invention is
founded relates to a combination of a higher fatty
acid glyceride and an emulsifier and dispersing agent,
polysorbate 80. The selection of these components
could not have been anticipated on the basis of con-
ventional thinking.
For example, although it is well known that
cyclosporin may be used in combination with castor
oil, this combination is irritating to sensitive
tissues such as the eye. Thus, conventional teaching
in the art is away from a formulation which utilizes
a higher fatty acid glyceride, such as castor oil, and
cyclosporin.
Stated another way, there is no way of deducing
that the use of an emulsifier and dispersing agent
such as polysorbate 80 will reduce the irritation po-
tential of an emulsion utilizing castor oil. There
are no examples of polysorbate in combination with
castor oil which, when admixed to cyclosporin, pro-
duces an emulsion with a high comfort level and low
irritation potential suitable for the delivery of
-30 medication to sensitive areas such as ocular tissues.
The present invention achieves a stable solution
state of cyclosporin. This stable solution state is
another important performance characteristic differ-
entiating the present invention from the conventional
oil systems. Cyclosporin is notorious for its ten-
CA 02309033 2002-O1-31
WO 95131211 P~.TfUS95/06302
_g_
dency to precipitate out in conventional oil systems
in which it is fully dissolved initially.
In accordance with the present invention, the
emulsions can be further stabilized using a polyelec-
trolyte, or polyelectrolytes if more than one, from
the family of cross-linked p.olyacrylates, such as
carbomers and Pemulen~.
Pemulen~ is a polymeric emulsifier having a CTFA
name of Acryl~tes/C10-30 Alkyl Acrylate Cross-Polymer
and is described in the "Garbomer 1342" monograph in the United
States Pharmacopeial National Formuiary, United States
Pharmacopeial Gonvention inc., 2002. ._
In addition, the tonicity of the emulsions can be
further adjusted usincr a.lvcerine, mannitol, or sorbi-
tol if desired. The pH of the emulsions can be ad-
justed in a conventional manner using sodium hydroxide
to a near physiological pH level and while buffering
agents are not required, suitable buffers may include
phosphates, citrates, acetates and borates.
While the preferable medications in accordance
with the present invention include cyclosporin; other
chemicals which are poorly soluble in water such as
indomethacin and steroids such as androgens, predniso
.lone, prednisolone acetate, fluorometholone, and
dexamethasones, may be emulsified with castor oil and
polysorbate 80 resulting in a composition with similar
-30 low irritation potential.
The invention is further illustrated by the
following =xamples with all parts and percentages
expressed .by weight. The cyclosporin used in the
examples was supplied by Sandoz.
CA 02309033 2000-OS-30
WO 95/31211 PCTlUS95106302
-10-
Example 1
A B C D E
Cyclosporin 0.40% 0.20% 0.20% 0.10% 0.05%
A
Castor oil 5.00% 5.00% 2.50% 1.25% 0.625%
Polysorbate 1.00% 1.00% 1.00% 1.00% 1.00%
80
Pemulenm 0.05% 0.05% 0.05% 0.05% 0.05%
G1 cerine 2.20% 2.20% 2.20% 2.20% 2.20%
NaOH qs qs s s qs
Purified waterqs qs qs qs qs
pH 7.2-7.67.2-7.67.2-7.6 7.2-7.67.2-7.6'.
Example 2
A B C D
Castor oil 5.00% 2.50% 1.25% 0.625%
Pol sorbate 1.00% 1.00% 1.00% 1.00%
80
Pemulenm 0.05% 0.05% 0.05% 0.05%
Glycerine 2.20 2.20% 2.20% 2.20%
NaOH s s s s
2 0 Purified waters s s s
pH 7.2-7.67.2-7.67.2-7.67.2-?.6'
Example 3
A
Castor oil 2.50%
Polysorbate 80 0.75%
Carbomer 1382 0.05%
Glycerine 2.20%
3 0 NaOH qs
Purified water qs
pH 7.2-7.6
CA 02309033 2000-OS-30
WO 95/31211 PC'TIUS95/06302
-11-
Example 4
A
Castor oil 5.00%
Polysorbate 80 0.75%
Carbomer 981 0.05%
G1 cerine 2.20%
NaOH s
Purified water qs
pH 7.2-7.6
The formulations set forth in Examples 1-4 were
made for treatment of keratoconjunctivitis sicca (dry
eye) syndrome with Examples 2, 3 and 4 without the
active ingredient cyclosporin utilized to determine
the toxicity of the emulsified components.
The formulations in Examples 1-4 were applied to
rabbit eyes eight times a day for seven days and were
found to cause only slight to mild discomfort and
slight hyperemia in the rabbit eyes. Slit lamp exam-
ination revealed no changes in the surface tissue. In
addition, the cyclosporin containing castor oil emul-
sion, as hereinabove set forth in Examples lA-1D, was
also tested for ocular bioavailability in rabbits; and
the therapeutic level of cyclosporin was found in the
tissues of interest after dosage. This substantiates
that cyclosporin in an ophthalmic delivery system is
useful for treating dry eye as set forth in U.S.
'30 Patent No. 4,839,342.
In addition, no difference in toxicity was found
between formulations with cyclosporin (Examples lA-1D)
and formulations without cyclosporin (Examples 2-4).
The formulations set forth in Examples 1-4 were
found to be physically stable upon long term storage.
CA 02309033 2000-OS-30
WO 95131211
-12-
pGTIUS95/06302
With regard to formulations lA-1D, no crystallization
of cyclosporin was noticed after nine months at room
temperature.
Further, other higher fatty acid glycerides such
as olive oil, peanut oil and the like may also be
utilized with the polysorbate 80 with similar results
regarding biotoxicity.
The following examples demonstrate the activity
of the composition in accordance with the present
invention for enhanced absorption of cyclosporin A in
the lacrimal gland.
Materials
The [Mebmt-3H]-cyclosporin-A (lot #TRQ6553) was
prepared by Amersham International (Buckinghamshire,
England) with radiochemical purity of -98% (by
reversed phase HPLC) and specific activity of 2.6
Ci/mmol (2.16 mCi/mg). The 3H-label is a metabolic-
ally stable position as shown by the asterisk. The
radiolabeled CsA was supplied as an ethanol solution
(1 mCi/ml). All organic solvents used in the
procedures described in this study were "HPLC grade".
all other chemicals and reagents were analytical grade
unless otherwise noted.
The compositions of the six formulations tested
- - 30 are listed in Table A.
CA 02309033 2002-O1-31
WO 95/31211 . PCTfi3S9510G302
-I3-
TABLE A
Castor Miglyol
Aqueous- Polyoxyl
ngredients astor Oil-in- ~ Cyclo-Oil-in.-olyoxyl40 with
Oil Water dextrinWater 40 Edetate
j Emulsion Emulsion
C clos orin-A 0.20 0.20 0.10 0.20 0.05 0.05
C clodextrin 14
Castor Oil 99.8 1.25
Miglyoi* OiI
20
Pluronic* i_121 0.75
~- P123
Tween* 80 I . 00
Gl cerin 2.20 2.20
Pemulen~ TR-2 0.05
Carbopol* 981 0 . 05'
Polyoxyl 40 20 20
Steavate (mg)
FiPMC 0.3 0.3
Butylated
H drox toluene 0.001 0.001
Ethanol(9200 rood) 0.1
Sodium Chloride 0.73 0.73
Sodium
Mono hos hate 0.2 0.2
Disodium Edetate 0.1
~~
Water QS QS QS QS QS
Batch Size 1 g 5 g 1 g 5 g 1 g 1 g
The radiolabeled formulations were formulated to
ensure that the radioactivity was homogeneous
throughout the vehicle. The, expected radioactivity
concentrations of the radiolabeled drug formulations
were 1-2 mCi/ml. The expected specific activity of
radiolabeled cyclosporin A (CsA) formulations was 0.5-
2 mCi/mg. All test articles were stored at ambient
temperature.
* Trade mark
CA 02309033 2000-OS-30
WO 95/31211 PCT/US95/06302
-14-
Analysis of Test Drug Formulations
The test formulations were analyzed in triplicate
by HPLC to determine the concentration of CsA and
radiochemical purity of the CsA dosing solutions
(>93%) before dosing. The radioactive concentrations
of the test formulations were quantified by liquid
scintillation counting (LSC).
l0 Chromatographic Conditions
Pump: Beckman Model 126 (Beckman
Instruments, San Ramon, CA)
Mobile phase: Acetonitrile: 0.03a H3POq in water,
pH 3 (65:35 v/v)
Flow rate: 1.0 ml/min
Column: Supercosil C8, 7.5 cm x 4.6 mm,
3 ~cm (Supelco, Bellefonte, PA)
Superguard LC-8 (Supelco)
Column heater (Bio-rad, Richmond,
CA) at 60-70C
Injector: WISP 712B (Waters Associates,
Milford, MA)
1aC detector: Radio Isotope 171 Detector
(Beckman Instruments)
Scintillant: Ready Flow III (Beckman
Instruments), Flow Rate of
'4 ml/min
W detector: Model 166 (Beckman Instruments),
2 0 2 nm
- Data processor: Beckman System Gold (Beckman
Instruments)
Run Time: 15 min
Retention Time: 6 min (cyclosporin A)
CA 02309033 2002-O1-31
-15-
Animals
Female New Zealand albino rabbits were
obtained and quarantined for at least five days before
procedures. Animals were housed in temperature- and
humidity-controlled rooms. Food and tap water were
provided ad libitum. Fifty-eight rabbits (2-3 kg)
were selected Pram the colony to minimize bias. They
were individually identified by ear tags and appeared
to be healthy.
Dos inct
The animals were divided into six groups of nine
I5 rabbits; each group was treated with one of the six
CsA formulations. During dosing, the lower eyelid of
each rabbit was gently pulled away from the eye and
35 ~cl of the formulation were administered in the
lower conjunctival cul-de-sac of each eye. After
dosing, the upper and lower eyelid were handheld
closed for '5 seconds and released. The animals were
observed visually for any signs of tearing or ocular
discomfort.
Samr~li,ng
Tissues were collected at 20-min., 6-hr. and
24-hr. post-dose for each group. Three rabbits (six
eyes) were used at each time point. At the specific
sampling times, the animals were euthanized by an
intravenous injection of 0.5-1 ml Eutha-6 (Western
Supply Co., Arcadia, California) via marginal ear
vein. Each eye was then rinsed with normal saline.
The aqueous humor ('200 ~1) was removed by means of a
0.5~m1 tuberculin syringe. The orbital lacrimal gland
('400 mg) , upper and lower bulbar conjunctivae ('S0 mg
each), corneal ('50 mg) and iris-ciliary body ('S0 mg)
CA 02309033 2000-OS-30
WO 95/31211 PGTIUS95/06302
-16-
were dissected. The tissues dissected were blotted
dry and weighed. Ocular tissue and aqueous humor
samples from both eyes were collected from four
untreated animals to be used as blank samples.
Analysis of Radioactivity
An aliquot of aqueous humor (50-175 ~.1) was
counted directly in 10 ml of Ready-Solv HP by LSC.
Tissue and blood samples were weighed into combustion
cones prior to combustion in a Model 307 Packard
Tissue Oxuduzer (Packard Co., Downers Grove,
Illinois). After combustion of the tissue samples,
3H20 was trapped in the Monophase-S solution (Packard)
and the radioactivity of the samples was determined by
LSC in a Beckman Model 1801 or 3801 scintillation
counter (Beckman Instruments, San Ramon, California).
Data Analyses
Excel software (version 4.0, Microsoft Corp,
Redmond, Washington) was used for data analysis.
concentrations of total radioactivity in the tissue
samples were expressed as dpm/g or dpm/ml and
converted to ng equivalents (eq) of CsA/g or ml, using
the specific activity of the dosing formulations.
Mean, standard deviation (SD) or standard error of the
mean (SEM) was calculated according to standard
methods. Radioactivity levels were not considered
- -30 significant unless the dpm was greater than twice that
of background b=(blanks).
Comparisons of tissue drug concentrations at each
time point for the formulations were determined by
one-factor ANOVA. All statistical comparisons were
made using StatView~ (version 1.03, Abacus Concepts,
Inc., Berkeley, California). the Fisher and Scheffe
CA 02309033 2002-O1-31
-17-
F tests were used to determine significant differences
between formulations at the 95% level (a = 0.05). The
rejection criteria for excluding any outlier data was
based on standard outlier tests. No mere than one
outlier was eliminated from any data set.
results aid ~i~scuss~on
The radioactivity concentrations in ocular
tissues at 20 minutes, 6 hours, and 24 hours after a
single topical application of various formulations are
depicted in Figures 1-4. In general, the concentra-
tions in the ocular tissues were greatest at the earl-
iest time point of 20 minutes as reported in~previous
single dose studies, The radioactivity concen
tration was highest in the conjunctiva and cornea for
each formulation. The relatively low aqueous humor
and iris-ciliary body concentrations suggest low in
traocular absorption of CsA, consistent with the low
2o CsA corneal permeability of -1.0 x 10'6 cm/sec.,
The decline of radioactivity concentrations from the
cornea was slower than those from the conjunctiva,
lacrimal gland, and aqueous humor. The obse~~-ved blood
radioactivity concentrations (<3 ng-eq/ml) were much ,
lower than trough plasma CsA concentrations of
80-250 ng/ml observed after oral dosing to humans,
The dependence of CsA corneal and conjunctival
penetration on the formulation was interpreted in
-30 terms of CsA concentration in formulation and the
release rate of CsA from formulation into tear film.
The aqueous formulations demonstrated a greater
propensity to release CsA for diffusion across. the
surface tissue epithelia. The 0.2% straight castor
oil was formulated below the CsA solubility and
therefore the release rate could be hampered by the
less than maximal CsA thermodynamic activity,
CA 02309033 2002-O1-31
- 1 8 -
The ocular surface tissues contained a higher
fraction of the CsA dose than the other tissues and
was used to discriminate among the aqueous, emulsion
and the straight castor oil formulations. The poly-
oxyl 4o formulation produced higher ocular surface
tissue concentrations than the emulsions and straight
castor oil. The emulsions were also effective in
delivery of CsA to the tissues of interest, lacrimal
gland, cornea, and conjunctiva. The castor oil emul-
lo sion showed higher lacrimal gland concentrations than
the modified Santen and the miglyol* ~ emulsion. The
straight castor oil showed the lowest concentrations
in surface ocular tissues. Apparently, the factors
influencing CsA penetration into the lacrimal gland
and the surface tissues are different.
Although there has been hereinabove described a
particular pharmaceutical composition in the form of
a nonirritating emulsion for the purpose of illustrat-
ing the manner in which the invention may be used to
advantage, it should be appreciated that the invention
is not limited thereto. Accordingly, any and all mod-
ifications, variations, or equivalent arrangements,
which may occur,to those skilled in the art, should be
considered to be within the scope of the present in-
vention as defined in the appended claims.
* Trade mark