Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02317180 2000-08-31
1
The present invention relates to the surface treatment of
metals to activate them or prepare them for receiving coatings.
Among the objects of the present invention is the provision
of novel methods for effecting activation and preparation.
Additional objects of the present invention include the
provision of novel forms of activated metal.
The foregoing as well as further objects of the present
invention will be more fully understood from the following
description of several of its exemplifications, reference being
made to the accompanying drawing in which:
Fig. 1 is a somewhat diagrammatic illustration of an
activating treatment according to the present invention. The
activating treatment is described in detail in U.S. Patent
4,895,609 issued 1/23/90.
By way of additional illustration, a readily ignited metal
such as titanium or magnesium in foil or wire form can be given
a nickel coating, as by rolling, and the nickel coating then
activated to render it pyrophoric. To ignite the ignitable metal
the coating should be at least about 0.4 mil thick.
Activated powders containing boron, or even free of boron,
are stored out of contact with air or oxygen to preserve their
pyrophoricity. Water is not a suitable preserving liquid for the
activated boron-containing powders. Acetone preserves them for
at least three weeks, as does an azeotropic mixture of trifluoro-
trichloro ethane with methylene chloride, described in U.S.
Patent 2,999,817. Ordinary fluoro-chloro ethanes and urethanes
that are normally used as refrigerants or propellants are also
suitable and they can be used by themselves or mixed with each
other or with acetone. Mixtures of the activated powders with
preserving liquids that are also propellants, are particularly
desirable in that they can be packed in an aerosol-type container
from which they are readily discharged to provide a cloud of
pyrophorically oxidizing particles.
CA 02317180 2000-08-31
2
Titanium can also be aluminized and/or boronized by modified
treatment, to make it more resistant to corrosive attack. For
this purpose, the aluminum is not leached out as it is when
activating. It is also very desirable to diffusion coat titanium
in the absence of hydrogen and in the complete or substantially
complete absence of conventional halide activators like A1C13 and
NHqCl. Thus engine compressor blades made of Ti6A14V can be
embedded in a pack of 20% A1-Si alloy powder containing 12%
silicon, and 80% aluminum, completely free of energizer, and at
1300°F for 14 hours under argon, acquires a protective aluminide
case 0.5 to 1 mil thick. Using pure aluminum instead of the A1-
Si alloy reduces the case thickness by about half.
Adding 1/4o A1C13 or NH4C1 energizer to the pack causes the
titanium substrate to be significantly embrittled. However, the
aluminizing can be speeded by using a pack that bad been
pre-fired with such a halide energizer for a time long enough to
drive out essentially all the energizer --- at least one to two
hours at 700°F or higher. It appears that some energizer remains
or some change is caused, to make the pre-fired pack much more
effective in aluminizing the titanium. In any event the
resulting aluminizing produces thicker cases, and can be readily
effected at temperatures as low as 1000 ° F or in times as short as
hours.
The addition to the pack of about 1/10 0 of a titanium halide
such as TiCl2, preferably sealed in a polyethylene tube so as to
be protected against exposure to the atmosphere, is also helpful
to speed up the diffusion coating. The polyethylene tube melts
before the pack reaches diffusion temperature, releasing its
contents.
The aluminized titanium compressor blades can then be
boronized instead of or before applying a top coating as
described in connection with Canadian patent 779, 173. A suitable
boronizing pack for this purpose is a diluent-containing pack
using ammonium fluoborate as the energizer and with added
titanium powder in an amount about half the amount of boron
CA 02317180 2000-08-31
3
powder, by weight. A 0.3 mil boronized case is thus formed at
1075°F for 14 hours in argon. The titanium powder helps keep the
titanium substrate from being attacked by the halide in the pack,
and can also be added to the pack used for aluminizing titanium.
It can also be omitted, particularly when only a thin diffusion
coating is desired. When the powdered titanium is used, it can
range from about 1/5 the boron content to about equal the boron
content by weight. Boron being a very high melting material, it
can be used with little or no refractory diluent, particularly at
diffusion temperatures low enough to keep the workpiece surfaces
from reaching a sintering condition. On the other hand, the
boron content of a boronizing pack can be as low as 20, although
at least 4% is preferred. To be sure that no sintering takes
place, an inert refractory diluent like A1203, kaolin or Mg0 can
be present in the pack in a concentration of at least 300.
The re-use of packs containing sodium fluoborate energizer
can be complicated by the gradual build-up of sodium fluoride
with each use. This problem does not appear to arise when
ammonium fluoborate is the energizer.
Masking can also be used to localize the top coating of
metals like titanium that are to be protected as by aluminizing,
boronizing, nitriding or the like. Thus pack formulations can be
applied to those localized areas to prevent coating. For this
purpose, the titanium content in the pack should be at least
about 35% to assure the desired masking effect, and the thickness
of the masking layer at least about 3 millimeters. For best
results the masking formulation should contain about 43% titanium
diluted with an inert powder such as alumina or kaolin or
magnesia. Inasmuch as such high titanium concentrations can
cause alloying ingredients in the workpiece to diffuse out from
the workpiece to the mask, and to inhibit such loss, the mask
preferably contains such alloying ingredients in addition to the
titanium. The most preferred masking formulations thus contain
about 43% by weight titanium powder plus an aluminum metal
content equal to the aluminum content in the pre-alloyed
CA 02317180 2000-08-31
4
workpiece, and in addition a content of other metals equal to
about one-fifth their content in the pre-alloyed workpiece.
By way of example, for localized masking against the
boronizing or aluminizing of Ti-6A1-4V, a very effective
formulation is:
43o titanium
6o aluminum
0.8% vanadium
balance alumina
For similarly masking Ti-3A1-llCr-13V, the preferred
formulation is:
43o titanium
3o aluminum
2.2% chromium
2.6% vanadium
balance alumina
These highly effective formulations can have their
ingredient percentages varied plus or minus about loo without
significantly detracting from their effectiveness. The
formulations should also be broken in by a pre-heat to between
about 750 to about 1000°C for about 4 to about 15 hours with
about '~ to about 1% activator added. Suitable activators are
NH9C1 and NH9Br. A 980°C pre-heat for 10 hours is preferred.
The masking compositions can be applied to the workpieces as a
slurry in a vaporizable liquid like water or methylchloroform,
and the coated workpiece then packed in place in the pack of a
coating retort before the coating dries and loosens.
Alternatively a little binder such as poly(ethylmethacrylate)
resin can be added to the masking composition as a 1 to 30
solution in methylchloroform, for example, to hold the masking
coat in place during the coating heat. Where the heat is hot
enough to drive off such resin binder and loosen the mask, non-
fugitive binders such as bentonite can be used.
For some purposes it is helpful to have pyrophoric decoy
pieces that when discharged into the air from a rapidly moving
aircraft, do not immediately slow down and stop their discharge
movement. To this end the pieces can be contained in a wire
CA 02317180 2000-08-31
netting that permits them to spread out to only about 10 meters
when discharged. Alternatively or additionally, the decoy pieces
can be made so that their air resistance is small and inertia
high. Thus the carrier web can be tantalum, silver or lead foil
as much as 3/4 mil thick, and only about 1 square centimeter
discs. Silver webs also contribute very good-electrical
conductivity that provides the pieces with an electrical dipole
that helps decoy against radar signals. Copper has a similar
dipole effect.
The decoying action can be modified by arranging for a
succession of decoy charges to be expelled by a moving aircraft
in 20 to 30 second intervals, for example. This appears to a
heat-seeking missile as a series of hot clouds that move with the
aircraft, and thus becomes a more attractive decoy target.
The pyrophoric behavior of activated iron particles is
different when they are prepared in different ways. The most
vigorous behavior is obtained when the particles and the
precursor aluminides from which they are made, are kept from
melting during the preparation. The following is an example:
40 grams minus 325 mesh iron powder are mixed with 60 grams
similarly sized aluminum powder and 1 gram anhydrous aluminum
chloride powder, and the mixture placed in a steel retort, the
retort loosely covered and placed in a larger retort through
which a stream of argon flushes. The retort assembly is then
inserted in a furnace, heated to 1200°F and kept there for 1 '-~
hours. During the initial heat-up, a stream of hydrogen is
substituted for the stream of argon. After cooldown, the powder
particles have sintered together to a large degree, and the
resulting masses are ground, as with a chopper blade such as used
in a micro-mill type grinder, to very fine particle size, for
example 325 mesh. These particles can be screened out, if
desired and constitute particles that can be somewhat larger in
CA 02317180 2000-08-31
6
size than the original particles. These aluminized particles can
now be subjected to a caustic leach treatment to produce highly
pyrophoric iron powder, the particles of which are about the same
overall size as the aluminized particles. The grinding can be
controlled to provide activated particles of larger or smaller
size.
To reduce the tendency for the particles to sinter together
during the diffusion coating, the diffusion temperature and/or
time can be lowered to as low as about 800 ° F to 900 ° F and/or
inert refractory particles such as alumina powder can be mixed
with the iron and aluminum powders . After such a mixture has
completed its diffusion coating treatment or the leaching
treatment, the inert alumina can be separated out magnetically.
The leached iron particles are magnetic, whereas the alumina
particles are nonmagnetic, so that pouring a stream of the
mixtures through a magnetic field causes the iron particles to be
deflected away from the alumina particles. There may be some
tendency for the fine alumina or other refractory particles to
physically adhere to the diffusion-coated iron particles, in
which event the diffusion-coated mixture can be forcefully
agitated in water preferably containing a little surface active
agent to wash the fine alumina or the like off the heavier iron
particles and permit those heavier particles to settle out.
Powdered pyrophoric metal or powdered precursor alloys can
also be separated from inert diluents or other ingredients in
diffusion coating packs by having different sizes for the
particles to be separated and sieving the mixture to effect the
separation. Thus cobalt or iron balls at least about 20 mils in
diameter can be diffusion coated in a pack whose particles are
all smaller than 2 mils thick.
The pyrophoric particles can be used to generate a hot
cloud, as for example to decoy heat-seeking missiles. Thus the
pyrophoric particles produced according to Example 1 can be
rinsed with water then with acetone and packed under argon in a
simple container or in a spray can, and about 100 grams of such
CA 02317180 2000-08-31
7
powder projected into the air. These particles promptly heat up
and oxidize. The resulting cloud of particles rises as a result
of the heating. It is only after several minutes that the
oxidized particles settle down to the ground.
The pyrophoric particles are conveniently discharged to form
the desired cloud, by loading them into a shotgun shell in place
of the shot and the shot-dispersing gunpowder. A relatively
short 12-gauge paper shell can thus be packed with about 50 grams
of the powder sealed airtight in a plastic enclosure around which
the shell is crimped. The usual primer charge will be enough,
when detonated, to expel the powder and create the desired cloud.
Larger quantities, up to a pound or more, can be poured into
a valued pressure-resistant container which is then pressurized
to about 200 to 600 pounds per square inch gauge with argon.
Upon opening the valve, the powder contents are propelled out
with the argon.
Modifying Example 1 by using particle size of about 30 to
100 microns for the iron powder and for the aluminum powder and
reducing the diffusion time to one hour after it reaches 900°F,
yields iron-aluminum alloy particles not heavily sintered
together; a light crushing in a mortar and pestle yields a powder
that can be somewhat coarser. A one-hour leaching of that powder
in 17% aqueous NaOH by weight while keeping the leaching
temperature no higher than 100°F leaves a pyrophoric powder that
when discharged produces a hot cloud that does not rise much
before eventually settling out.
An alloy in which before leaching the aluminum content is at
least about 40o by weight should be used to make the desired
cloud, but an aluminum content of at least about 50% by weight is
preferred. Cooling of the leaching reaction may be required,
depending on the quantity of reactants and the volume of the
leach solution. Because of the fineness of the particles, the
entire leaching step takes about one hour or less; shorter
leaching times (e. g., 30 minutes) give the best results. Caustic
potash can be used in place of caustic soda under the same
CA 02317180 2000-08-31
8
conditions. The addition of stannite or stannate to the leaching
caustic, as described in U.S. Patent 4,435,481, is desirable.
The foregoing leaching leaves the leached particles with
only a small aluminum content and highly pyrophoric. More
vigorous leaching leaves a smaller aluminum content in the
leached particles, but appears to attack the active iron sites
and also leaves them somewhat less pyrophoric and less effective.
Pyrophoricity is readily measured by exposing a 25 to 35
milligram sample of the activated powder to air and using a
two-mil platinum-platinum/rhodium wire thermocouple to measure
the temperature rise during the exposure. A temperature of 600°F
should be reached, but preferred temperatures are as high as
1100°F or higher.
Substituting nickel for the iron in the diffusion coated
particles, or using commercial Raney nickel powder, gives
somewhat better results in producing a rising cloud of
pyrophorically heated particles. Thus, nickel-aluminum alloys
containing as little as about 35 o aluminum before leaching can be
very effectively used.
Where, before leaching, the pyrophoric particles are made by
diffusing aluminum into iron or nickel, it is important to
conduct the diffusing operation at relatively low temperatures,
such as below 1200°F and preferably below 1000°F. Even at
temperatures as low as 850°F the time at temperature can be as
short as about 45 minutes when a diffusion activator such as
A1C13 is used.
The alloy particles can also contain other ingredients such
as boron, titanium, carbon, zirconium and magnesium that help
generate heat. Excluding the aluminum, the content of pyrophoric
metal in the leached alloy should be at least about half by
weight in order to have enough pyrophoricity to cause the
remaining ingredients to react and generate their reaction heat.
The pyrophoric particles used to make the hot cloud can be
mixed with other materials that increase or decrease the heat
generation and/or vertical cloud movement. Thus the activity of
CA 02317180 2000-08-31
9
the pyrophoric particles can be reduced as by too vigorous
leaching or by particularly light leaching, and the sizes of the
pyrophoric particles can be increased to 100 or more microns to
keep them from rising much in the hot cloud. Alloying the
particles with other ingredients such as silicon or chromium that
are not rendered pyrophoric, also reduces the upward movement of
the cloud they generate after activation.
Alternatively, the pyrophoric particles can be mixed with
non-pyrophoric particles such as unactivated iron powder or
carbon powder which act as diluents and also burn.
Another example is
EXAMPLE 2
PACK COMPOSITION
2108 minus 325 A1 powder
2108 minus 325 Fe powder
4g A1C13
The above is mixed and placed in a plain steel retort fitted
with an internal thermocouple . The retort so loaded is placed in
a furnace and fired at 1000°F under hydrogen. When the internal
temperature reached about 400°F, an exothermic reaction occured
as revealed by an instantaneous surge of the internal temperature
to 1735°F. The retort was maintained in the 1000°F furnace for
two hours . It was then cooled under hydrogen and purged with
argon after reaching room temperature.
The pack in the retort was hard but it could be unloaded and
ground with mortar and pestle to pass through a minus 100 mesh
sieve. The yield was 960. 2008 of the sieved FeAl2 product was
gradually added to a solution of 267g NaOH + 7g SnC12~2H20 in 2
liters of water at 140-160°F. The resulting leaching was carried
out under an argon atmosphere to insure no oxidation of the
activated iron particles. The activated iron now contains less
than about 2% Al, and it was rinsed in water to pH 8, then rinsed
in acetone to remove the water, and dried in argon.
The dried activated iron was very pyrophoric. Similarly
CA 02317180 2000-08-31
activated iron particles prepared by leaching an alloy having the
same proportion but supplied as a solidified melt from commercial
sources, was far less pyrophoric. Dispersing of the powders by
throwing out into the air 50 grams of the respective activated
products resulted in immediate incandescence of essentially all
unmelted particles, but only delayed or no incandescence from the
particles that had a history of melting. It was also noted that
the latter particles tended to become passivated and generated
less total heat. Subjecting the respective particles to the mild
acid after-treatment of U.S. Patent 4,927,798 further increases
their heat generation and reduces their response time.
Use by or against fast-moving aircraft can be greatly
assisted by the immediate incandescence. However, it is
sometimes desirable to have immediate heat-up of the particles,
but with the heat-up temperature sufficiently low that no
incandescence is visible. To this end, the particles are diluted
with inactive powder or provided with inactive coatings that
delay but do not completely block access to oxygen. Thus the
addition to the very reactive particles of about 10 to 28% of
minus 325 mesh oxide sharply reduces the incandescence of the
mixture. Other inert oxides such as MgO, Ti02 and Zr02 have a
similar effect. White additives are preferred because they also
lighten up the dark-colored smoke that is produced by the
pyrophoric reaction.
Alternatively the iron being activated can be alloyed with
non-pyrophoric metal such as chromium or silicon, and only about
5 to 20% of such alloying can
completely prevent incandescence.
Incandescence-inhibiting coatings can be of any type
including water-soluble and water-dispersible silicates and boro-
silicates. By way of example 10 grams of the highly active iron
particles can be stirred into 100 cc of a loo aqueous solution of
a soluble borosilicate containing 65% BZ 03, and filtered off
after 30 minutes standing, to show essentially no incandescence
after drying.
CA 02317180 2000-08-31
11
The pyrophoric product is also a very effective catalyst for
different kinds of chemical and electrochemical reaction, such as
electrolysis of water with very low over-voltages, ammonia
oxidation, CO oxidation and NOX removal. This catalytic quality
remains essentially unaffected after the pyrophoric product is
permitted to undergo its pyrophoric reaction with air. The
pyrophoric product can also be stabilized by exposure to small
concentrations of air under conditions that keep it from getting
warm enough to rapidly react, as described in U.S. Patent
4,820,362.
The pyrophoric action of pyrophoric members can be increased
by placing in intimate contact with the member, a solid or liquid
that undergoes an exothermic reaction when heated. Metals like
magnesium and titanium, and even boron can thus be adhered to a
pyrophoric foil as by placing a magnesium ribbon over an
activated foil and passing the assembly between a pair of
pressure rollers. Powdered materials such as magnesium,
titanium, manganese, zirconium, carbon, aluminum or boron can be
dispersed in a volatile liquid and the dispersion applied to an
activated member and dried. Commercially available boron powder
is very effective when dispersed in an alcohol such as ethanol.
Such pyrophoric combinations need not be in the form of very
thin coated foils, and can take other forms. Thus a 5-mil thick
felt of boron, or carbon fibers or ordinary paper or cotton cloth
can be coated on one or both sides with a paste of pyrophorically
activated iron or nickel powder, and the coated felt subjected to
a drying and if desired a sintering treatment to form a self-
supporting sheet in which the pyrophoric particles are embedded
in the inter-fiber spaces. A boron felt weighing about 0.1 gram
per square centimeter of gross surface (as measured with a
ruler), carrying 0.2 gram pyrophoric iron powder per square
centimeter of gross surface, generates a very large amount of
heat when exposed to the atmosphere. Ordinary steel wool also
makes a very effective porous substrate for impregnation.
CA 02317180 2000-08-31
12
As noted, the pyrophoric material can be prepared as a
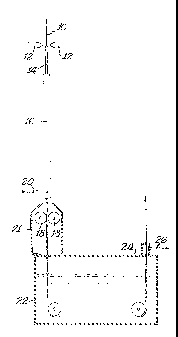
coating on a thin carrier web. Fig. 1 illustrates an improved
modification of this technique.
In Fig. 1, a carrier foil 10 of stainless steel or 1010
steel about 1/40 to about 1/20 millimeter thick is spray coated
on both its faces from spray nozzles 12,12 with a binder-
containing mixture of iron powder and aluminum powder in a
proportion that makes a Raney type alloy. The binder is a resin,
preferably an acrylate, dissolved in a solvent like methyl
chloroform. The powder particles are preferably no larger than
about 5 to 20 microns.
The thus coated web 14 is led through an externally fired
tube 16 of a tube furnace where it is rapidly heated to over
500°C. By that time the coating layers have been dried, the
resin binder volatilized off, and the metal powders have
interreacted to form the Raney alloy, FeAl3 for example. After
leaving the tube furnace but before the coatings have cooled
excessively, the coated web 14 is passed between reduction rolls
18,18 where the hot Raney alloy is compacted to essentially
eliminate voids.
A protective gas such as argon is preferably introduced, as
at 20, into the furnace tube 16. Some of that gas will rise in
tube 16 and thus protect the coated web from the oxidizing action
of the ambient air as it heats up. Portions of the gas will also
flow down from the exit end of tube 16 and similarly protect the
hot emerging web. A cylindrical shell 21 may be positioned about
the emerging web to help confine the downwardly-flowing
protective gases against the hot web.
The reduction rolls 18,18 should be sufficiently massive so
that they are not excessively heated by the hot web. If desired,
the rolls can be liquid cooled to help hold down their surface
temperature.
The compacted web is then led through a tank 22 containing
aqueous caustic soda which dissolves much of the aluminum from
the Raney particles on the web. The trip through the caustic
CA 02317180 2000-08-31
13
soda is preferably arranged to take two to 10 minutes, as by
adjusting the temperature and concentration of the caustic. The
web entering the caustic can be as hot as 600°C and the action of
the caustic generates heat, so it is simpler to keep the caustic
solution hot, at least about 80°C.
The caustic generates large quantities of hydrogen as it
acts on the web, so that a hood 24 can be placed over tank 22,
with a suction connection 26 to an aspirator that aspirates off
the evolved hydrogen. The web emerging from tank 22 is wet with
the caustic, and that caustic should be replaced.
The proportions of iron powder to aluminum powder can be
varied, nickel powder can be substituted for some or all of the
iron powder, and iron or nickel aluminides mixed with the
incoming powders. The aluminide formation can also be effected
in stages . The presence of a little copper in the sprayed-on
coating mixture helps the aluminide coating adhere to a stainless
steel web core. Instead of spraying the coating on the web, the
web can be dipped in a coating bath, or the coating applied by
any other technique. The amount of coating should be such as to
leave a compacted web not over about 1/5 millimeter thick.
The following sintering example is preferred:
A mixture of the following powders
6 parts by weight aluminum
4 parts by weight iron
1 part by weight copper, and
1 part by weight tin
is pressed in a 1~ inch diameter mold with a plunger powered at
30 tons to make a disc about 15 to 20 mils thick. The disc is
then removed from the mold and subjected to sintering at 1500°F
to provoke the reaction between the aluminum and the other
metals. After subsequent cooling, the sintered disc is leached
with loo aqueous NaOH at 160°F for 20 minutes, then rinsed and
dried in argon. Blowing air 6 feet per minute at the dried disc
causes pyrophoric reaction that carries the disc to 820°C in less
than one second and holds it above 750°C for twelve seconds.
This is a very strong response believed to be due to the manner
CA 02317180 2000-08-31
14
in which the activated product is produced. A similar
improvement in catalytic response is also noted.
The presence of the tin in the original powder mixture makes
it unnecessary to have dissolved tin in the leach solution to
improve the leaching action. About 5 to about 15 weight % tin
content in the powders is preferred.
Nickel powder can be substituted for the iron powder in the
compacts, but is preferably mixed with 1'-~ times its weight of
aluminum powder. A 50-mil thick disc press-sintered from such a
mixture and then leached 14 hours with hot 20% aqueous NaOH is
more pyrophoric than the leached iron-aluminum disc of the
foregoing Example but its pyrophoricity does not last as long.
Such a leached nickel-aluminum disc can have its pores
impregnated with inert particles such as the alumina and silica
mentioned supra, to stretch out its pyrophoric heat output. It
will for example ignite micron-sized boron particles without the
need for the low ignition-temperature zirconium.
The press-sintered masses can be made thicker or thinner
than 50 mils. Making them 10 mils thick for example, makes them
sufficiently light in weight so that they will fall through the
air relatively slowly, particularly if the discs are 1 ~ inches
or more in diameter. A group of such discs can then be
discharged in the air to act as an effective decoy for heat-
seeking missiles. Their effectiveness as a radar decoy is
improved if the discs are given a plating of copper or silver on
one or both faces. Not more than about 0.1 mil of such plating
is needed. Electroplating is preferred over electroless plating.
The foregoing discs are somewhat friable, particularly when
only about 20 mils thick or less. To provide a useful degree of
ruggedness, such thin discs can have one face of unactivated
metal that when sintered more securely holds the activated
particles together. That face need only have a depth about 1/10
the total disc thickness. The following is an illustration:
In a square mold having a '-~ inch by '-~ inch cross section,
there is first spread an 0.8 mil thick layer of minus 325 mesh
CA 02317180 2000-08-31
iron powder. This is tamped down and then over it is poured a
layer about 7.5 mils thick of a uniform mixture of the same size
iron powder with an equal weight of minus 325 mesh aluminum
powder. The combination is now subjected to pressing at 5 tons
per square inch to form a coherent disc about 8 mils thick and '~
inch by 1/2 inch in outline. It has a face about 1/10 of its
thickness, of only iron particles. That disc is easily pushed
out of the mold without being damaged.
The disc is now placed on a ceramic plate and so held is
passed through a tube furnace heated to about 600°C. After a few
seconds the disc becomes heated red hot by the inter-reaction of
the aluminum and the iron in its top layer. A protective stream
of nitrogen or argon can be passed through the furnace tube to
keep the disc from oxidizing. The disc is then pushed out of the
heated part of the furnace and permitted to cool for a few more
seconds.
The disc is at this point extremely rugged. It is now
dropped into a 20o NaOH solution in water heated to about 80°C,
to leach out much of the aluminum that has reacted with the iron.
After about 30 minutes to several hours the leached disc is
strongly pyrophoric, and can be removed under argon, washed and
dried. It is still somewhat rugged and will easily withstand the
handling needed to fully load a decoy shell with a quantity of
the discs.
Instead of being square in outline, the discs can be
rectangular or circular or have any other shape that permits them
to be loaded with a minimum of unused space.
The final disc thickness can be increased to about 12 mils,
the aluminum to iron proportion is changed to 2.1, and the layer
of inactive powder is placed in the center of the disc and is
increased to about 1 1/4 mils. The final product has a layer of
unreacted metal on both faces of which are sintered pyrophoric
layers each a little less than 6 mils thick.
A 1 mil thick iron foil can be substituted for the inactive
layer, the aluminum to iron ratio is 2.5, and the pyrophoric
CA 02317180 2000-08-31
16
layers are each about 5 mils thick.
The iron foil can be replaced by a 60 by 60 iron wire screen
woven from 2 '-~ mil thick wire, the aluminum to iron atom ratio is
changed to 1.9 and the overall disc is made 7 mils thick.
Perforated metal having other forms can be used in place of
the wire gauze, and powdered nickel can be substituted for some
or all of the powdered iron.
The inert metal need not be identical to the powdered metal
that is mixed with the aluminum. Copper-plated iron or nickel
more effectively sinters to the active particles and can be
effectively used.
The foregoing discs are very good catalysts and can be used
in the catalytic process mentioned hereinabove. For catalytic
use it is generally safer to first eliminate the pyrophoricity,
as by treatment with HZOZ and water as described in parent Patent
4,443,557.
An active platinum disc made by the press-sintering
technique is also a very good catalyst after leaching, but
eliminating its pyrophoricity is best effected by contacting it
with dilute hydrochloric acid or other mineral acid. Catalytic
platinum prepared this way has the added advantage that its
content of contaminants such as carbon or iron can be kept
extremely low. When preparing such catalytic platinum with an
aluminum-diffusion step, the diffusion retort used to contain the
diffusion materials tends to diffuse some of its constituents
into the platinum. Carbon is thus frequently found in platinum
that has been given a diffusion treatment in a steel retort. The
use of a diffusion-coating retort made of nickel containing less
than 0.9o carbon, does prevent such carbon contamination.
However, the discs are particularly useful as decoy material
for heat-seeking missiles. Their pyrophoric character and their
compactness permit loading of the discs in a discharge shell for
example, with very little waste space and somewhat greater
pyrophoric material than the prior art pyrophoric foils.
CA 02317180 2000-08-31
17
Other metals, even copper, brass and bronze, can be
activated by the press-sintering technique. Not all such
activated metals are pyrophoric, but they are all good catalysts .
The thus-activated coppers, brasses and-bronzes are quite
effective in the alcohol synthesis of Fig. 1 of U.S. Patent
4,927,798 for example. The chromium, molybdenum, manganese,
vanadium, titanium, tungsten and tin alloy catalysts described
above for denitration, are also effectively prepared by the
press-sintering technique. Expensive alloys can be replaced by
sintered alloys of their constituent metals or only of their
essential constituent metals.
For some purposes it is desirable that the activated
material be carried by wires . Thus a 3-mil thick nickel wire can
be coated to a thickness of 10 mils with a suitable mixture of
iron and aluminum powders held in place with a resin binder, and
the coated wire then passed through a furnace to drive off the
binder and cause the iron and aluminum powders to interact. The
resulting aluminide-coated wire is then passed through a leach
bath to dissolve out much of the aluminum.
The final wire with its activated coating can then be coiled
up or wound on a form for catalytic use. For decoying heat-
seeking missiles, the wire can be wound up into a tight coil and
loaded into a discharge shell. Upon discharging the shell, the
pyrophoric wire uncoils and pyrophorically reacts with the air to
provide a decoy heat generator that does not scatter much.
Before loading, the wire can be passed between compacting
rollers that give the coating a rectangular cross-section to thus
make the coiling more compact.
Similar results are obtained when the wire carrier is
replaced by a strip of 1 mil thick foil about one to two
millimeters wide. In both cases the hot cloud developed by the
decoys are only about 2 meters wide or less, and more effective
in their decoy action. Cutting one radial slit from the coil
center to its edge does not increase the cloud size significantly
inasmuch as the cut lengths tend to entangle with each other as
CA 02317180 2000-08-31
18
they unpeel. However the cutting eases the unpeeling.
When the coating layers contain iron aluminide, the caustic
solution preferably contains a little dissolved tin, as described
in the prior art. Also as described in the prior art, a little
hydrogen peroxide can also be added to the caustic solution.
Potassium hydroxide can be substituted for all or part of the
sodium hydroxide.
The aluminide-forming reaction in tube 16 is generally
completed in a matter of seconds, so that the web can be passed
through the furnace at a rate of about 3 meters per minute, and
the furnace tube need be no longer than about 30 or 40
centimeters.
The above-described roll compacting carried out while the
web is hot, at least 500°C, is more effective than on a colder
web, and only requires a compacting force of about 30 kilograms
per centimeter width of the web. Compacting can also be effected
on the leached web, that is the web leaving the caustic solution,
but is not as effective. The aluminide particles on the leached
web are softer than those on the unleached web when the webs are
at room temperature, but heating a pyrophoric leached web in an
attempt to soften the leached particles, adversely affects their
pyrophoricity. The greater the compaction, the more pyrophoric
strips can be packed into a decoy shell used against heat-seeking
missiles.
By way of example, a one-meter-long induction coil furnace
can be provided for coated foil to move through at the rate of
two meters per hour, followed by a five-centimeter gap through
which the foil cools by radiating its heat to the surroundings,
and then by a ten-centimeter travel through a leaching bath held
at 190°F. Following that bath the foil moves through a falling
stream of rinse water that terminates the leaching, rinses off
the leachant, and cools the foil to about room temperature. The
water-wet foil can then be dried under argon and spooled.
There is generally no need to use a diffusion coating
energizer such as ammonium chloride in the atmosphere adjacent
CA 02317180 2000-08-31
19
the surface being diffusion coated by the foregoing technique,
but it can be used and will then help if the incoming coil has
not been completely cleaned. Blasting with a stream of blasting
grit generally does an adequate cleaning. With or without an
energizer in an inert or reducing diffusion atmosphere, a
continuous spray of aluminum on a continuously fed foil, or a
continuous passage of such a foil through or on a body of molten
aluminum, does not leave the objectionable roughness described
above for the dipping of individual workpieces in molten
aluminum.
However, the smoothness of the sprayed-on aluminum layer
does not assure suitable diffusion aluminizing when the diffusion
step is conducted at elevated temperatures, i.e., about 1300°F.
Thus a two-mil thick 1010 type steel when sprayed with molten
aluminum and rapidly heated to 1450 ° F where it is kept for 30
minutes and then cooled, becomes wrinkled and distorted. A
similar distortion problem is experienced by a 10-minute
diffusion heat at 1600°F. Such wrinkling and/or distortion makes
it awkward to manipulate the foils, but essentially entirely
disappears during the leaching, and so does not significantly
prevent the tight packing of such activated foils in containers
for use as thermal decoys. Sprayed-on aluminum coatings weighing
from about 5 to about 30 milligrams per square centimeter,
whether sprayed on as molten aluminum or as a slurry of aluminum
particles, lead to the wrinkling and distortion.
For activating only one face of the foil, the other face can
be protected or masked against diffusion, or the aluminum spray
coating can be confined to the one face to be diffusion coated.
This also leads to distortion and wrinkling.
Pre-alloying the aluminum with 5~ to 15% silicon by weight
reduces the wrinkling and distortion, but does not eliminate them
completely. However, the diffusing in of a pre-coated foil
yields after leaching an activated product having a thermal
output when exposed to air, somewhat less than that of foils
prepared by pack diffusion. Alloying with other materials such
CA 02317180 2000-08-31
as zinc, calcium, germanium, magnesium, nickel and boron, also
reduces the wrinkling and distortion but has less of an adverse
effect on the thermal output. Conducting the leaching at
elevated temperatures such as over 220°F, and particularly with
aqueous solutions of at least 20o caustic soda or potash by
weight also helps.
Wrinkling and distortion can also be reduced by conducting
the diffusion at lower temperatures. Below about 1000°F, the
wrinkling and distortion is completely prevented, but the
diffusion time is increased to as much as four hours or more.
Also at these low temperatures ammonium chloride is not a
preferred energizer and pack diffusion is simpler to conduct.
Such low-temperature pack diffusion is best conducted with extra
precautions to avoid side reactions such as etching, that are
caused by the presence of moisture. Thus anhydrous water-
insoluble CrCl3 can be used as the energizer or the diffusion-
coating pack can have embedded in it one or more packets of
anhydrous aluminum chloride in which that energizer is sealed in
an envelope made of plastic sheeting that melts and decomposes at
about 300°F to 600°F. Polyethylene and polyethylene
terephthalate plastics are suitable for this purpose. As the
packet-containing diffusion-coating pack is heated to diffusion-
coating temperature in a loosely covered retort contained in an
outer retort whose interior is kept flushed with argon or the
like, any moisture in the inner retort is first driven out and
flushed away, after which the packet walls melt and decompose to
liberate the anhydrous aluminum chloride so that it can
volatilize and effect the necessary energizing. If it is desired
to minimize the presence of carbonaceous material such as that
resulting from the plastic melting and decomposition, the packets
can be made of aluminum foil folded over and cemented together by
means of a very thin plastic coating.
Wrinkled and distorted foils can also be subjected to a
flattening treatment as by rolling between thickness-reducing
rollers. The pressure applied by these rollers should only be
CA 02317180 2000-08-31
21
enough to effect very little or no thickness reduction.
The leaching of aluminum from aluminized cases to provide an
activated substrate has been described in the art as conducted in
various manners. Reference is hereby made to U.S. Patents Nos.
3,637,437 (col. 5), 3,809,658 (cols. 1 and 2), 3,939,097 (cols.
4, 6 and 7), 4,206,081 (col. 2), 4,292,208 (col. 9 and the listed
references), 4,179,412 (cols. 3 and 4), and Japanese published
Application No. 55780/1976.
In some cases, it is desirable to conduct the leaching at
relatively low temperatures, at 140°F or 160°F, for example.
Thus, a cold leaching bath of 10% to 20o by weight NaOH in water
with or without a cold water jacket and/or agitation during
leaching of aluminized iron foils originally 1 mil thick, can
have its temperature rise limited so that it gets no hotter.
About 20 to 40 minutes of such low-temperature leaching yields
highly pyrophoric iron foils. Even lower leaching temperatures
can be used.
For the most vigorous pyrophoricity, an aluminized case
depth about 2 mils thick should be provided on an object in which
such case represents about 80% of the total thickness of the
aluminized object. The forming of an aluminized case increases
the overall thickness of the original object by about three-
fourths the case depth. For the above-noted thick case, leaching
with the 50% solution at boiling for one minute develops a
pyrophoricity that causes the substrate to heat up to over 1000°F
when it is subsequently dried and exposed to the air. To get hot
enough on a stainless steel to scorch paper, it is preferred to
provide a case depth at least one-sixth the total thickness of
the body of a stainless steel article, where the activated case
is only on one face, or at least one-twelfth that thickness where
both faces are activated.
Pyrophoric stainless steel screens and foils can be prepared
on a continuous basis, as described above for the plain steel
foils. Such continuous treatment can be used to prepare
pyrophoric or non-pyrophoric products depending upon whether the
CA 02317180 2000-08-31
22
foregoing control limitations are observed. Thus, very high
pyrophoricity is developed with type 430 stainless steel
screening woven from 20 mil thick wire aluminized with a 2-mil-
thick case having a surface aluminum content of 50 weight
percent, leached for only about '-~ minute with a 50o aqueous
solution of NaOH held at about 280°F. Leaching solutions as
strong as saturated aqueous caustic can be used at temperatures
as high as 300°F, even shorter leach times being then preferred,
but 40o NaOH solutions at 160°F are preferably used for as long
as 30 minutes to develop high pyrophoricity. It is not helpful
to have stannite tin present in leaching solutions that are used
to leach stainless steels.
Where the leaching solution is stronger than 500, as for
example when using solutions that are saturated with caustic at
the leaching temperatures, the leaching times are further
shortened by about 1% for every degree Fahrenheit increase in
leaching temperature above 290°F. A leaching solution that has
been used loses a little of its activity both because some of its
caustic is consumed and because soluble aluminate salts are
formed in it. A leaching bath originally having a 50% caustic
concentration but used to the point that its caustic
concentration has been reduced to 40% by weight, actually has a
leaching action more closely corresponding to a fresh 35 o caustic
concentration.
EXAMPLE 3
A type 430 stainless steel 50 X 50 wires per inch screen
woven from 10 mil thick wires is aluminized in a simple pack of
20% aluminum powder and 80% powdered alumina, with 0.5 A1C13
added as energizer. A 20-hour hold at 850°F under hydrogen,
yields a 2 mil thick case that is then leached with 30% aqueous
NaOH for 2'~ hours at 110 ° F to give a product that will scorch
paper after rinsing, drying and then exposed to air. After it
has completed its pyrophoric reaction with air it makes a very
effective catalyst for reducing NOX in internal combustion engine
CA 02317180 2000-08-31
23
exhausts and coal-burning furnace smokestacks by reacting with a
little NH3 at about 300° to 375°C. It also makes a good
catalyst for oxidizing ammonia to nitric oxide, for decomposing
ammonia into nitrogen and hydrogen, and for synthesizing ammonia
from nitrogen and hydrogen.
EXAMPLE 4
A 60 X 60 wires per inch screen of type 304 stainless steel
woven from wires 8 mils thick, is aluminized as in Example 3 but
with NHq C1 substituted for the A1C13, an aluminizing temperature
of 1650 ° F, and the time at that temperature two hours . After
cool-down the screen is removed from the coating pack and dropped
into 25% aqueous caustic by weight held at boiling for 30 to 60
seconds, then immediately rinsed. Upon drying it shows a
pyrophoricity even greater then the screens of Example 3.
Using a fine-wire thermocouple, pyrophoric temperature
increases to over 900°F have been measured with type 430
stainless steel screens aluminized as in Example 3 and leached as
in Example 4.
The foregoing pyrophoric activity is imparted by the same
process to other iron-chromium and iron-chromium-nickel alloys
containing over 5% and as much as 30% chromium. The
crystallagraphic structure of these alloys can be of any type,
including austenitic, martensitic and ferritic. Specific
stainless steel alloys suitable for such treatment include 25-12
stainless steels, as well as types 316, 321 and 247 and iron
containing 12 o chromium and the types 304 and 430 stainless steel
already noted. Although some of these stainless steels are not
true steels inasmuch as they contain little or no carbon, they
all come within the stainless steel category of the present
invention.
As pointed out above, the diffusion aluminizing conducted to
provide pyrophoric stainless steels is very effective when
carried out at temperatures of about 800°F to about 1650°F, and
is followed by a vigorous leaching with aqueous caustic having at
CA 02317180 2000-08-31
24
least 25~ NaOH by weight. If more than about 15 milligrams of
aluminum is leached out per square inch by vigorous leaching with
aqueous caustic having at least 45% caustic, the leached case
becomes loosened and can then be removed by rubbing, for
instance.
The pyrophoric stainless steels are strongly catalytic as
well as pyrophoric, and remain strongly catalytic when the
pyrophoricity is lost as by reacting with air or by dipping in 3
volume ~ Hz02 in water. Thus, the pyrophoric stainless steels are
particularly effective catalysts for reducing NOX emissions from
furnace stacks and the like with the help of NH3 or reducing
gases, as described in U.S. Patent 4,897,375. For such use,
these pyrophoric catalysts can be merely permitted to react with
air before, during or after they are installed in the stack.
However, they can alternatively have their pyrophoricity
eliminated by H202 treatment, if desired.
The pyrophoric stainless steels, like non-pyrophoric
stainless steels, show their maximum catalytic effectiveness for
NOX reduction at temperatures below about 350°C. They are
accordingly very desirable for such use.
The pyrophoric stainless steel is also very effective for
catalyzing the formation of ammonia as well as its decomposition,
and the oxidation of ammonia to nitric oxide. For such purposes
the catalysts are preferably in the form of wire screening as
described in U.S. Patent 4,897,325, or in the form of tubing as
described in Japanese Patent Application No. 55780/1976, or can
be fabricated or even cast in any other desired shape.
The maximum temperature attained by a pyrophoric metal foil
or screen when it undergoes its pyrophoric action, also depends
on the proportion of activated surface to non-activated core.
Inasmuch as the catalytic action of the activated surface is
essentially unaffected by how much unactivated core is under it,
an activated catalyst can have a very thick core and thus appear
not extremely pyrophoric as indicated by its temperature rise
upon exposure to air, yet be a highly effective catalyst.
CA 02317180 2000-08-31
However, for catalytic purposes it is preferred, particularly for
gaseous reactions, to have the catalyst surface undiluted as much
as practical by inactive core. This preference, considered with
the greater effectiveness of the surfaces that have been more
intensively pyrophorically activated, accordingly makes it
desirable to have the pyrophoricity as vigorous as practical,
even when the pyrophoricity is removed by an after treatment
after it is developed and before catalytic operation is started.
For NOX reduction the stainless steels can, before
activation, contain helpful alloying metals such as vanadium,
manganese, and other metals referred to in U. S. Patent 4, 897, 375.
Thus, type 316 contains molybdenum, type 321 contains titanium,
and type 347 contains columbium. These alloying metals can be
present in the original stainless steel, or they can be placed or
diffused into the surface to be activated, or they can be alloyed
with the aluminum that is to be diffused in as the first step in
the activation. The content of any of these alloying metals is
preferably at least about 5% weight of the activated case.
The presence of about 10% manganese in the surface of those
stainless steel catalysts improves their effectiveness,
particularly in stack gases containing high sulfur dioxide
content, but the improvement is gradually lost over a span of
about a day or so. A typical diffusion coating with manganese is
conducted at 1725°F for five hours with a diffusion coating pack
of
53 g. manganese powder
212 g. A1203 powder
3 g. NHqCl powder
It can also be used to diffuse manganese into nickel as well as
nickel alloys other than stainless steels. On nickel it gives
a weight gain of about 21.9 milligrams per square centimeter, and
a case depth of about 1.4 mils.
The manganese-carrying nickel and other nickel alloys have
improved catalytic action, for example, in the oxidation of
formaldehyde to formic acid, as well as in the methanation of C0.
Nickel-cobalt-molybdenum alloys heretofore used in such
CA 02317180 2000-08-31
26
methanation are particularly improved.
A manganese content over about 20 weight percent in the
outer skin of any of the foregoing catalysts does not seem to be
worth the trouble.
For NOX reduction of internal combustion engine exhausts,
the foregoing catalysts, or any similarly reacting catalyst is
placed in the exhaust line, preferably close to the exhaust ports
of each engine cylinder of combustion discharge. Where the
exhaust line includes a converter that oxidizes carbonaceous
gases, the catalyst can be fitted to the converter outlet.
A small stream of ammonia is introduced into the exhaust so
that it can react with the NOX in the exhaust. Where there is an
engine-driven pump that injects air into the exhaust, the intake
of that pump can be connected to a source of ammonia such as a
container of ammonia-evolving material like ammonium carbamate or
ammonium carbonate or ammonium bicarbonate. Liquefied ammonia
can also be used, but must be kept under high pressure. The
container is kept closed when the engine is not operating, but
when operating its contents deliver a gradual stream of ammonia
or ammonia-containing gases which are pumped into the hot exhaust
with or without the air the pump delivers. A pump with an
operating speed controlled by the engine speed does a very good
job of metering the desired ammonia content into the hot exhaust.
The metering rate is easily adjusted so that the exhaust as
discharged into the atmosphere has no ammonia odor.
With either arrangement, the ammonia-supplying container is
conveniently vented to a supply of absorbent such as a
carbon-filled canister which can be the same or different from
the canister used to absorb gasoline vapors. The vent can be
controlled by a valve which is normally closed but opens to
relieve excess pressure as well as in response to intake suction.
The use of an ammonia feed reduces the need for exhaust gas
recirculation, which can then be diminished or entirely
eliminated. More power is then obtainable from the engine.
CA 02317180 2000-08-31
27
For ammonia oxidation to nitrogen oxides, the pyrophoric or
non-pyrophoric stainless steel screens activated by aluminizing
and leaching can merely be substituted for the platinum screens
used in the prior art reactors with a reduction in operating
temperature to 700°C or below. The pyrophorically activated
screens are preferred, particularly when their porous catalytic
surface is partially impregnated with potassium salts such as
potassium carbonate or vanadate.
The radar reflectivity associated with pyrophoric foils is
also increased by mixing them with standard aluminum radar chaff
or foils. Such aluminum chaff or foils only about '-~ mil thick or
thinner, mixed with two to five times as many pyrophoric one-inch
discs of iron foil, make an effective heat and radar decoy when
ejected as a mass from an exploding cartridge.
Regardless of how an activated leached iron or nickel
surface is obtained, it can-be used for many catalytic purposes
including the oxidation of methane, and such use is improved by
depositing on the surface a film of platinum or palladium, or a
thin layer of fine zirconium oxide powder. Metal films are
readily deposited by electroplating or in the case of
platinum-family metals by decomposing the chloride or other salt
of such metal. Powders can be applied by mixing them with
colloidal alumina or silica in suspension in water, then applying
the suspension and finally permitting the water to evaporate.
The Zr02 changes the wave length of the radiation emitted when
the activated surface pyropborically reacts.
According to another aspect of the present invention, there
are provided highly active-heat-generating compositions which
burn in air when heated, but are essentially inert at
temperatures below about 50°C to 100°C so they can be
conveniently stored for use when desired.
As shown, pyrophoric materials prepared by leaching FeAl3 or
NiAl3 for example, can be stabilized by subjecting them to a very
small quantity of oxygen in a manner that does not permit them to
appreciably heat up, the resulting stability tends to break down
CA 02317180 2000-08-31
28
at about 50°C or a little higher.
According to the present invention, there are provided
pyrotechnic compositions which ignite at temperatures above
100°C, e.g., at about 300°C, and have a substantial aluminum
content as well as a high thermal output . These compositions are
activated aluminides of metals like molybdenum, zirconium and
columbium that when not combined with aluminum ignite in air at
about 500°C to 700°C or higher at atmospheric pressure. These
metals are easily combined with aluminum to form alloys or
aluminides having two or more atoms of aluminum for each atom of
ignitable metal, and when so combined generally have ignition
temperatures not much different from that of the pure ignitable
metal as noted above. Incorporating about 2% to about loo boron
in such alloy, based on the weight of the ignitable metal,
generally lowers the ignition temperature by about 20°C or
somewhat more. However, the ignition temperature of the
aluminide with or without the boron, is lowered about 100°C to
about 150°C or more by activating the aluminide.
The activation of the present invention involves the
leaching out of some of the aluminum, as with aqueous caustic
soda or caustic potash. For this activation, the aluminum alloy
preferably has between about 2.5 and 3.5 atoms of aluminum for
every atom of alloyed ignitable metal, and the leaching
preferably removes all the aluminum in excess of two atoms per
atom of ignitable metal. This is illustrated by the following
example:
EXAMPLE 5
One kilogram of aluminum covered by a cryolite flux is
melted in a stainless steel retort under argon, and there is then
stirred into the melt 600 grams of powdered molybdenum. The
stirring is continued for about '-~ hour while the contents of the
retort are maintained at about 1200°C to about 1400°C to
complete
the alloying.
CA 02317180 2000-08-31
29
The melt is then permitted to cool and solidify, after which
the metal layer is crushed into small pieces and the pieces
ground to a maximum particle size of about 0.3 millimeter. The
ground product is now poured into an excess of 25s NaOH solution
in water. Bubbles are immediately evolved as the caustic attacks
the alloy particles, and the evolution begins to noticeably slow
down after several hours, reaching a very low level after about
six hours. The caustic is then poured off, and the residual
particles washed with water and dried.
Thus activated, the particles have a porous surface and are
stable in air at temperatures as high as 300°C. However, when a
mass of the particles is heated in air to above that temperature,
the particles ignite and vigorously react with the air. The
reaction is an oxidation and is completed on the smaller
particles before it is completed on the larger particles of the
ground mass, so that the pyrotechnic effect is more intense for
about the first minute, and drops off somewhat for about another
minute. On an asbestos pad the oxidizing particles become red
hot and gradually crumble to a powder. Such pyrotechnics
generate temperatures well over 1000°C and can accordingly be
used to supply heat for other purposes.
Zirconium and columbium behave very similarly when treated
as in the foregoing Example. After activation, they ignite at
about 350°C in air at atmospheric pressure, and the ignition
point of their aluminum alloy before activation is about 500°C,
being not much different from the ignition point of the pure
metals.
The activities of the activated alloys are lower when the
leaching is terminated earlier, and are also lower when the
alloys subjected to the activation have a less than 3:1
proportion of aluminum atoms to ignitible metal atoms. There are
also some reactivity increases when the leaching is made more
intense, as by starting with a hot or boiling caustic solution,
and by increasing the caustic concentration to saturation.
However, very good reactivity is obtained when the leaching is
CA 02317180 2000-08-31
effected at 20°C, although the leaching is slower at that
temperature. At boiling temperatures with saturated caustic, the
leaching can be completed in less than two minutes.
It is not essential for the aluminides of the present
invention to be prepared by melting. Thus, iron, zirconium,
titanium, molybdenum and columbium can be effectively alloyed
with aluminum by a thermal diffusion. Fine powders of the
separate alloy ingredients can be uniformly mixed in a diffusion
coating retort and heated to about 600°C in an A1C13 atmosphere
for only about six hours to produce usable alloy when the
ignitable metal powder particles are no larger than about 10
microns. Larger particles take a little longer. Other halide
atmospheres, such as of anhydrous CrCl3, either in its water-
soluble or water-insoluble form, or elemental chlorine, bromine
or iodine, can be used in place of the A1C13 as the diffusion-
energizing atmosphere. Only about 1/2% to about 1% of such
energizer is mixed with the powders to be alloyed.
Tri-aluminides of some of the noted metals are also
available as articles of commerce.
The leaching of the present invention can also be effected
with inhibited hydrochloric acid such as that referred to ,
but the resulting activation is not as great as produced by
caustic leaches.
There can also be included in the foregoing alloys
ingredients that improve the pyrotechnic behavior.
Thus, about 2% to 200 of boron of magnesium or iron or
mixtures of these, are helpful in this respect, and can be added
to a melt or to a diffusion-alloying mixture. Also, these metals
can be introduced by diffusion into a pre-formed aluminide or by
diffusion with the aluminum. For example, 250 grams of powdered
ZrAl4 can be mixed with 10 grams of powdered boron and 5 grams of
solium fluoborate, and subjected to a diffusion heat as described
in U.S. Patent No. 3,801,357, but without using inert solid
diluent, for three hours at 1800°F in an argon-bathed atmosphere,
to diffuse the zirconium and boron into aluminized nickel powder
CA 02317180 2000-08-31
31
or NiAl3.
About to to about 10% of iron can be similarly introduced
into the aluminide with or without the boron, to provide
activatable alloys that after activation ignite at temperatures
of about 300°C or a little lower. Magnesium aluminum alloys can
also be activated by the foregoing techniques to provide
activated material having ignition temperatures below 300°C. The
magnesium-aluminum alloys preferably have, before activation, at
least two atoms of aluminum for every atom of magnesium, but can
be activated even when the aluminum-magnesium atom proportion is
as low as 1=~:1. The aluminum-to-zirconium atom ratio is
preferably at least 4:1 but can be as low as 2:1.
Including 2% to 20% boron in the magnesium-aluminum or
zirconium-aluminum alloys, based on the weight of the magnesium
or zirconium, also increases their pyrotechnic output.
The foregoing pyrotechnic improvements are also obtained
with alloys in the form of foils and sheets, as well as powders.
The boron additions of the present invention can also be
effected by the procedures described in U.S. Patent No.
4,536,215.
The foregoing low-ignition-point activated alloys are
readily ignited with an ordinary household match.
A stainless steel workpiece can be provided with a catalytic
pyrophoric surface, by first plating the stainless steel surface
with nickel or iron, then aluminizing the plated surface, and
finally leaching the aluminum out of the plating. Thus, a one to
two mil thick acid nickel electroplate on 304 stainless steel can
be aluminized at 750° to 800°F for twelve hours, as in U.S.
Patent No. 4,154,705, to provide an aluminum pick-up of 1.9 to 2
milligrams per square centimeter, after which the aluminized
surface can be leached in hot 20% aqueous NaOH to reduce the
pick-up to about 1.9 to 2 milligrams per square centimeter. This
leaves a stainless steel workpiece with a very active surface
highly suited for a water electrolyzing electrode, anode or
cathode, with reduced over-voltage. It is also suitable for use
CA 02317180 2000-08-31
32
as a fuel cell electrode, both anode and cathode.
The active nickel surface becomes warm when first exposed to
air, showing that it is pyrophorically reacting with the air.
Its best cathodic electrolyzing effects are provided if kept from
exposure to air or oxygen. These results are also obtained when
the aluminizing is conducted at other temperatures and for other
times and with other diffusion-coating packs. Similarly, the
nickel platings can be deposited by ion bombardment, gas plating
or other techniques, and the stainless steel can be of any other
type. The stainless steel support need not be more than about 10
mils thick, and can be a foil or screen.
The high diffusion temperatures suggested in U.S. Patent No.
4,116,804 are not desirable for aluminizing a stainless steel
supported thin nickel or iron layer, inasmuch as high diffusion
temperatures tend to cause some of the chromium from the
stainless steel to diffuse into the thin nickel or iron layer and
lower it's activity for electrolytic use. It is accordingly
desirable to keep the diffusion temperature below 1100°F and to
limit the dwell time at diffusion temperature to prevent chromium
from reaching the outer surface of the nickel or iron coating.
The nickel or iron top coating can be given a top flash
plating of silver or platinum about 0.05 mil to about 0.5 mil
thick, before the aluminizing. Such a flash coat of nickel over
an iron-plated stainless steel is also helpful.
Similar flash coatings can be applied over the activated
nickel or iron plated stainless steels after the activation is
completed by aluminizing and leaching.
Even without the stainless steel backings, a self-supporting
iron or nickel screen or foil having its surfaces activated as
noted, with or without the flash top coatings, make very good
fuel cell electrodes, much like the similar activated metals of
British Specification No. 1,289,751.
A stainless steel backed activated nickel or iron plating
also makes a good catalyst for NOX reduction.
CA 02317180 2000-08-31
33
When electrolyzing water in which an alkali like NaOH is
dissolved to increase its conductivity, a nickel anode previously
activated by aluminizing to give a 2-mil thick case followed by
a one-hour treatment in boiling 60% or 70% NaOH, provides a much
greater anodic current density than a corresponding anode in
which the leaching was only with hot 20% aqueous caustic.
Indeed, at low inter-electrode voltages as against an untreated
pure nickel cathode, the anodic current density is increased as
much as seven-fold. The foregoing anodes are preferably dipped
into dilute hydrogen peroxide after the leach is completed, with
or without an intervening rinse in hot or boiling water.
A somewhat smaller but still spectacular current density
increase is obtained from boiling 40% aqueous NaOH leach for one
hour.
In general the leaches with 50% or stronger caustic should
not be extended so as to remove much of the aluminum at the
interface between the aluminized case and the nickel core under
it. Too much removal at that location can reduce the adhesion
between the core and the leached case and cause the leached case
to spall off. A one-third hour leach at about 200°F is
appropriate for cases as thin as 0.5 mil, but boiling 70% caustic
should not be used for more than about 10 minutes unless the case
is thicker than two mils. Preferred leaching is with about 30%
to about 60% aqueous NaOH at temperatures from about 212°F to
about 300°F for at least a half-hour, but not long enough to
loosen the leached layer.
The foregoing vigorous leachings also improve the cathodic
current density when the leached nickel electrodes are used as
cathodes, but here the current density increases are only
effected at inter-electrode potentials greater than about 1.5
volts.
A 1.5 to 2 mil aluminized case applied on 430 stainless
steel by powder pack diffusion below 1000°F is preferably leached
for not over about 1-3/4 hours when the leaching is effected at
about 150 to about 190°F with 10% to 30% caustic. Most
CA 02317180 2000-08-31
34
preferably such leaching is for about 1 to about 1 '~ hours,
particularly for use as an NOX-reducing catalyst with NH3 in
furnace and internal combustion engine exhausts. Thinner cases
should be leached for proportional times. Leaching at lower
temperatures, e.g., at 90°F, can be extended to about 3 hours.
When leached aluminized stainless steel screening is used
for NOX removal, better results are obtained with the greatest
degree of leaching, but the aluminized case should not be
completely leached through.
As noted above, the leaching of aluminized ferrous metal is
greatly benefitted by the presence of dissolved tin in the
leaching liquor. Such leaching builds up a tin-containing sludge
that can be treated to recover and re-use the tin values. To
this end the sludge can be filtered off or separated by
centrifugation, and washed, as in a perforated barrel washer to
carry off most of the adhering caustic liquor. The washed
material is then dipped in aqueous acid such, as 1:1 dilution of
concentrated HC1 with water, to bring it to approximate
neutrality or slightly acid. A pH of about 3 to about 9 is
appropriate.
The approximately neutralized material is then retorted at
a temperature of about 275 to about 375°C in a non-oxidizing
atmosphere for about 5 to about 10 hours. The resulting material
analyzes to about 70 o tin and is completely soluble in strong HCl
or other acid. It can be used as a general source of tin, for
example by dissolving it in concentrated HC1 warmed to at least
about 60°C to yield a stannous chloride solution that can be
added to the caustic leach for the leaching of aluminized ferrous
metal.
The retorting is the key treatment, inasmuch as without the
retorting the sludge will not properly dissolve in the strong
acid. Hydrogen or inert gas atmospheres such as argon can be
used in the retorting. A 10-centimeter tall retort works
satisfactorily in that only about 5 retort hours is then needed.
Excess retorting does not help or hurt.
CA 02317180 2000-08-31
The leaching of aluminized iron foils or powder is improved
when conducted with a little hydrogen peroxide in the leaching
liquid. As little as 0.1% H202 by weight of the leachant is
enough to show such results, and from about 0.2% to about 0.5% is
preferred. Concentrations of 3% or higher tend to darken the
work and diminish its pyrophoric activity.
By way of example, to a liter of 10 weight percent solution
of NaOH in water there is added 5 grams of SnCl2 2H20 and 5 cc of
30o aqueous H202 and the resulting mixture at room temperature is
used to leach 2 mil thick iron foil that has been aluminized to
a depth of ~ mil on both its faces. The leaching generates a
very small amount of gas as compared to corresponding leaching
without the H202 and is completed in about 30 minutes even if the
leach solution warms up to about 50°C during the leaching.
Essentially no sludge precipitates from the leach solution, even
though a large quantity of tin-containing sludge precipitates if
the HzOz is omitted.
About the same results are obtained when the leachant is KOH
and starting leach solution is at any temperature from about zero
to about 50°C, although leachant that starts at 50°C can heat up
to about 60°C . The foregoing leachings are conducted with about
one hundred times as much leach solution as substrates being
leached, by weight. Preferred caustic concentrations are from,
about 8% to about 20°s NaOH or KOH in water, by weight.
Instead of pouring H20z into a leach solution, peroxides such
as sodium peroxide, potassium peroxide and calcium peroxide can
be added to the leach solution to form H202 in situ.
The foil activated in accordance with the foregoing example
also shows a small gain in pyrophoric heat output as compared to
the corresponding foil-activated without the help of the H202.
Iron powders also give about the same results as the foils do.
Leaching of aluminized iron, either foil or powder, is best
conducted with some tin or stannite ion dissolved in the
leachant, but the concentration of the tin can be reduced to as
little as about %~ gram dissolved tin per liter of leachant when
CA 02317180 2000-08-31
36
the H202 of the present invention is present in the leachant.
Preferably the dissolved tin content is as high as 1 to 3 grams
per liter.
Not only is less tin needed in the leachant, but the iron
activated with the H202 in the leachant contains less tin than is
contained in iron activated pursuant to the prior art. Such tin
is introduced as metal into the pores of the activated iron and
even in very small amounts helps preserve the activation.
It is noted that the presence of the H202 in the leachant
according to the present invention, has an effect opposite to
that of the H20z when it is applied to the activated metal after
the leaching is completed. Such later application causes the
activated metal to lose some or all of its pyrophoricity, and to
turn black.
The small increase in heat output caused by the presence of
the H202 in the leachant, can be heightened by subsequently
subj ecting the activated metal to the short treatment with dilute
acid, with or without the folding, as described above.
The foregoing leaching, with or without the H20z, is also
very effective for leaching the rapidly formed sintered aluminum
alloys. Those alloys are of the Raney type formed by placing the
alloy precursor metals on a carrier and then rapidly heating the
combination to a temperature high enough to trigger the inter-
reaction of the precursor metals.
A particularly desirable technique uses as a carrier
inexpensive steel such as type 1010, in the form of a foil about
25 to 50 microns thick. Such a foil can be seriously embrittled
by the inter-reacting metals at the high inter-reaction
temperatures generated, and to avoid excessive damage, the
precursor metals are applied in at least two stages, with the
first stage arranged to generate relatively low inter-reaction
temperatures. Thus the atomic proportion of the aluminum to the
iron, nickel or cobalt precursors should be no greater than about
2, or the metal alloying with the aluminum should be mostly iron,
or the precursors can be diluted with preformed alloy, or
CA 02317180 2000-08-31
37
combinations of these techniques used. One desirable arrangement
uses as a first stage a mixture of:
50 grams iron powder
68.4 grams aluminum powder
14.8 grams nickel powder
This mixture can be poured onto the steel foil in a layer about
74 microns thick, or suspended in a binder solution that is
applied to the foil or into which the foil is dipped. A typical
binder solution is a 6% solution of polyethylacrylate in methyl
chloroform. The dried combination is then heated to about 700°C,
setting off the reaction between the aluminum and the other
metals. After about seven seconds the reaction is completed and
the alloy particles formed are sintered to each other and to the
foil, with the thus-coated foil retaining some of its
malleability. It can for example be bent at least about 20
degrees around a mandrel 3 centimeters in diameter.
Over the reacted coating there is then applied another
precursor layer, this time a mixture of
108.8 grams aluminum
51.2 grams nickel
40.0 grams preformed NiAl2
2.0 grams boron
and the heat treatment repeated. The resulting coated foil can
still be bent at least about 20 degrees around a mandrel 3
centimeters in diameter.
The final product, where both the precursor layers are about
equal in thickness, is essentially a mixture of iron and nickel
trialuminides, with a very small content of boron, sintered to
aluminized iron foil. It is noted that when the precursor layers
are applied by dipping, the resulting coatings are on both faces
of the foil. Either way, the aluminide-coated foil is still
strong enough to hold itself together and hold the aluminide
particles in place. It is now leached as for example in 10%
aqueous NaOH at 50 to 65°C for two to thirty minutes, preferably
with tin dissolved in the leachant.
CA 02317180 2000-08-31
38
After rinsing, the leached product can be compacted between
compacting rolls, and will then be about 125 to 200 microns
thick. It is highly pyrophoric and for maximum pyrophoric heat
output, the heat treatments are conducted in a protective
atmosphere. Conducting them in air for a total time less than
about 30 seconds does not materially detract from the pyrophoric
heat output. It will be noted that the reacted precursor metals
as well as the carrier foil are rendered pyrophoric by the
leaching. Also some of the carrier foil will oxidize during the
pyrophoric heat generation, even when the foil is not rendered
pyrophoric.
The foil can also be kept from excessive deterioration
during the sintering operation by holding it in contact with a
heat absorber that keeps it from getting too hot. Thus the foil
with a coating on only one face can be fed through the sintering
step while its other face is pressed against the rim of a
stainless steel wheel that rotates to effect the feeding. The
rim should be fairly thin to permit adequate sintering.
The use of very thin foils, e.g., less than 25 microns
thick, preferably less than 15 microns thick, makes possible a
decoy action with incandescence that starts immediately upon
exposure of the activated material to the air. When carrier
foils are over about 50 microns thick, the incandescence is
delayed a fraction of a second or more, whether the foil has the
sintered-on layer on one or both of its surfaces. In any event
the sintered-on layers should be at least about 25 microns thick,
before or after compacting.
Such thin foils are preferably stainless steels, type 302
for example, inasmuch as they better resist the sintering
operation. By way of example
A coating binder is prepared by dissolving 10 parts by
weight of acrylate resin in 90 parts of acetone. Into 100 grams
CA 02317180 2000-08-31
39
of this solution is stirred the following powders:
220 grams A1 (1-3 micron particle size)
207 grams Fe (minus 325 mesh)
75 grams Cu (minus 325 mesh)
The resulting dispersion is applied by dipping onto both
faces of a 302 steel foil about 12.5 microns thick, to build up
coatings that air dry to a thickness of about 5.0 mils (125
microns). The coated foil, before or after drying, is subjected
to the above-described sintering and leaching treatment and is
preferably compacted before the leach. Under argon or under
water it is then cut into 2 cm x 2 cm squares that when dry
immediately become incandescent upon exposure to air.
The addition of 5 grams amorphous boron to the powders
sharply increases the intensity of the pyrophoric action. On the
other hand, the addition of 5 grams A1z03 or Mg0 powder reduces
its intensity and delays its incandescence onset. Reducing or
eliminating the copper reduces somewhat the adhesion of the
sprayed-on coatings, and is undesirable.
Type 302 steel is sufficiently heat-resistant that a 15
micron thick foil can be perforated, for example, with openings
1 millimeter wide spaced 1 millimeter from each other, and still
provide the foregoing results.
The pyrophoric material should be kept away from oxygen, as
by being thoroughly wet with water or other protective liquid, to
preserve it against changes. It can be cut to short lengths for
loading into a decoy shell used for protecting aircraft against
heat-seeking missiles. It can also be stabilized by contacting
it with very low concentrations of oxygen, as described in Patent
4,820,362, to render it non-pyrophoric unless heated to above
50°C.
Obviously, many modifications and variations of the present
invention are possible in the light of the above teachings. It
is, therefore, to be understood that within the scope of the
appended claims the invention may be practiced otherwise than as
specifically described.