Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-1-
NATURAL RESIN FORMULATIONS
The present invention relates to the production and use of a natural resin,
derived from wood, bark, forest residues, wood industry residues and other
biomass
materials using fast pyrolysis, and its use as an adhesive in the manufacture
of
manufactured wood products.
BACKGROUND OF THE INVENTION
"Resin" is a generic term used to describe both natural and synthetic glues
which
derive their adhesive properties from their inherent ability to polymerize in
a consistent
and predictable fashion. The vast majority of modern industrial resins are
synthetic, and
are normally derived from petroleum feedstocks. Two of the most important
classes of
synthetic resins, in terms of production volume and total sales are phenol
formaldehyde
(P/F) and urea formaldehyde (LJ/F) resins. In both cases, the principal market
application
is for use as a glue binder in man-made wood products.
Phenol formaldehyde (P/F) resin, because of its resistance to moisture, has a
particular value in external (outdoor) or damp environments. It is therefore,
the leading
adhesive used for the manufacture of plywood, oriented strand board (OSB) and
wafer
board (Sellers, 1996). P/F resins are also widely used in laminates,
insulation, foundry
materials, moulding compounds, abrasives and friction materials for the
transportation
industry (ie., clutch facings, disk facings and transmission components). As
its name
suggests, the principal ingredients in P/F adhesives are phenol and
formaldehyde.
However, the finished product is actually a mixture of P/F, caustic, and
water,. Assorted
fillers, extenders and dispersion agents may then be added for specific
adhesive
applications:
The formaldehyde ingredient in P/F resin is derived from methanol, normally
produced from natural gas. The phenol ingredient is typically manufactured
from
benzene and propylene via a cumene intermediate. In addition to P/F adhesive
manufacture, phenol is used in the manufacture of other important products,
for example,
Bisphenol A and Caprolactam. Bisphenol A is a principal component in
polycarbonates
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-2-
used in automotive parts, compact discs and computer discs, and Caprolactam is
a raw
material for Nylon 6, used within stain resistant carpets.
When mixed together in water and with caustic added as a catalyst, phenol and
formaldehyde undergo a condensation reaction to form either ortho- or para-
methylolphenol. The resultant PF resin, as shipped to market, is a dark brown
liquid
which is polymerized and cross-linked to an intermediate degree. It is then
cured in the
final board, laminate or other product without catalyst simply with the
addition of heat
at which time the final polymerization and cross-linking take place via
condensation
10 reactions. The release of free formaldehyde during the resin manufacture
and resin use
stages is a concern from a health and safety perspective. Furthermore, the
costs
associated with formaldehyde production have increased and there is a need in
the art
for alternative materials for use as wood adhesives and binders.
15 One alternative for phenol that has been considered is lignin. Lignin and
P/F
formaldehyde resins are structurally very similar. Lignin is a random network
polymer
with a variety of linkages, based on phenyl propane units. Lignin-based
adhesive
formulations have been tested for use within plywood, particle board and fibre
board
manufacture. The addition of polymeric lignin to P/F formulations has been
found to
20 prematurely gel the P/F resin thereby reducing shelf life, limiting
permeation of the
lignin-P/F resin into the wood and producing an inferior mechanical bond
(Kelley
1997).
Pyrolysis of lignin has been considered as a potential approach to upgrading
25 lignin to more usable phenolic type resins. While relatively mild thermal
or thermo-
catalytic processing at low pressures can be used to break the lignin
macromolecules
into smaller macromolecules, lignin segments and monomeric chemicals, such
procedures may cause condensation reactions producing highly condensed
structures
such as char and tar, rather than depolymerized lignin fragments or monomeric
30 chemicals.
CA 02319416 2001-07-25
A further alternative for the production of phenolic compounds involves use of
pyrolvtic oils produced in the fast pyrolvsis of wood and other biomass. Fast
pyrolvsis
can be achieved by rapid heat transfer to the teed material, by rapid removal
of the
product via a vacuum, or t>v a combination of rapid heat transfer and
pyrolysis under
vacuum. These pyrolytic oils are comprised of a complex mixture of compounds
including phenol, guaiacel. svrin~ol and para substituted derivatives,
carbohydrate
fragments, polyols, organic acids, tormal~iehyde, acetaldehyde, furfuraldehyde
and
other oligomeric products (Pakdel et al 1.996). However, wood-derived lignin
and
Lignin-rich pyrolytic bio-oils have lacked consistency and have exhibited
inferior
properties when compared with phenol-formaldehyde resins (Chum et al. 1989;
Scott
1988; Himmelblau 1997; Kelley et al., 1997').
Due to the complexity of pyrolytically-derived bio-oils, further processing is
required in order to obtain suitable fractions useable as a replacement for
phenol, or
to be considered as an r;xtender for petroleum-derived phenol within P/F resin
formulations. 'Typically the phenolic derived from pyrolysis oils requires
separation
prior to use in order to remove impurities One such method involves water
extraction
of the whole-oil, followed by precipitation and centrifugation or filtration
and drying
of the non-aqueous Lracr_ian to prepare a "pyrolytic lignin" fraction (Scott
1988).
However, adhesive forrr~ulations prepared using pyrolytic lignin were found to
be
inferior to P/F resin fornzulations in both colour and odour, and required
long press
times in order to avoid de-lamination of waferboards. Tests indicated that
none of the
pyrolytic lignin samples meet the internal bond (IB) test requirement (Scott
1988, see
pp. 91-9?) .
In US 4,209,647 ;June 24, 1980) a fractionation method for the preparation of
a phenol- enriched pyrolytic oil is disclosed which involved a multistep
process that
selectively solubilized neutral phenols, and organic acids of the whole-oil
with NaOH
followed by extraction with methylene chloride. However, this multistep
process is
costly, labourious, time consuming and involves the use of volatile solvents
that are
known to be health threatenin;.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-4-
Another fractionation method involves adding ethyl acetate to whole-oil to
produce ethyl acetate soluble and insoluble fractions, followed by a water
wash and
NaHC03 extraction of the ethyl acetate soluble fraction, with evaporation of
the ethyl
acetate to produce a fraction containing phenolic and neutrals (P/N) derived
from the
pyrolytic oil (Chum et al. 1989, US Patents 4,942,269, July 17, 1990, and
5,235,021,
August 10, 1993). Preliminary results with the PIN fractions revealed that
fractionated
pyrolytic oils could be used within P/F resin compositions, as P/N containing
resins
exhibited equivalent gel times as noted for P/F resins. However, the
fractionation
protocol is not suitable for industrial scale production, nor is this process
cost effective
for the preparation of alternative components for use within P/F resins at a
commercial
scale (Kelley et al., 1997).
All of the process disclosed within the prior art as outlined above involve
the
extraction of a phenol-enhanced fraction from the whole pyrolytic oil product
using
solvents and alkali. None of the prior art discloses a method which is
directed at
producing a fraction of bio-oil suitable for adhesive use, yet that does not
require any
solvent extraction.
SUMMARY OF THE INVENTION
The present invention relates to the production and use of a natural resin,
derived from wood, bark and other biomass residues using fast pyrolysis.
Specifically,
the natural resins (NR) of this invention are obtained from the fast pyrolysis
of wood
products. The NR comprises a thermal fraction of the liquid product produced
from
fast pyrolysis of bio-mass
By the processes of the present invention, there is no need to extract a
phenol
enhanced portion using solvents and alkali. Rather the NR of this invention is
selected
as a thermal fraction from the whole bio-oil produced during the pyrolytic
process.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-5-
The natural resins of the present invention can be directly used as a
substitute
for some of the phenol in phenol/formaldehyde, phenol urea formaldehyde, and
phenol
melamine urea formaldehyde resins used as adhesives in the manufacture of wood
products or it can be used as a substitute of some of the phenol and some the
formaldehyde components of phenol-containing formaldehyde resins. Furthermore,
the NR of this invention can be used as a substitute within urea formaldehyde
resins,
and melamine urea formaldehyde, and related resins. With further processing to
remove organic acids, a natural resin can be produced which can be used as a
substitute
for either some of the phenol component of a phenol-containing formaldehyde
resin or
for both the phenol and formaldehyde components of the resin, or as a
substitute within
urea formaldehyde type resins..
The natural resins of the present invention exhibit high reactivity due to the
presence of a high number of active sites for binding and cross linking during
polymerization.
According to the present invention there is provided a method of preparing a
natural resin comprising:
i) liquefying a suitable biomass using fast pyrolysis in order to produce
product vapours and char;
ii) removing the char from the product vapours;
iii) removing a first set of components from the product vapours by rapid
quenching thereby producing a remaining selected product vapour;
iv) obtaining the natural resin from the remaining selected product vapour.
This invention also pertains to the above method wherein the first set of
components is removed from the product vapours within a first condenser.
Furthermore, this invention is directed to the method as defined above
wherein, the
natural resin is obtained from the selected product vapour using direct-liquid
contact
condensers; within a secondary condenser, and other down stream components
including a demister and a filter bed.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-6-
This invention is also directed to a natural resin (NR) characterized by
comprising a free phenol content from about 0.001 % to about 0.1 % (w/w), a
total
phenolic content from about 30 % to about 80 % (w/w), a pleasant smoky odour,
and
a pH greater than 2Ø
Furthermore, this invention relates an NR as defined above further
characterized by comprising a water content from about 10 to about 20 wt% , a
density
from about 1.0 to about 1.3 g/ml, a solids content from about 0.5 to about 1.0
wt% ,
an ash content from about 0.05 to about 0.5 (wt% ), and a viscosity at 75
°C greater
than 70 (cSt). The NR as defined may also further be characterized by
comprising a
net caloric value of about 4355 cal/g (18.22 MJ/kg), and a gross caloric value
of about
4690 cal/g (19.62 MJ/kg).
This invention is also directed to an adhesive composition that comprises the
NR as defined above. Furthermore, this invention is directed to an adhesive
composition comprising NR from about 1 % to about 40% (w/w) of the adhesive
composition.
This invention is also directed to an adhesive composition as defined above
comprising a second adhesive resin selected from a phenol-containing or urea
containing formaldehyde resin. Furthermore, this invention relates to an
adhesive
composition as defined above wherein the phenol-containing or urea-containing
formaldehyde resin is selected from the group consisting of phenol
formaldehyde, urea
formaldehyde, phenol melamine urea formaldehyde, melamine urea formaldehyde,
and
phenol urea formaldehyde.
This invention also relates to an adhesive composition as defined above
wherein
the NR comprises from about 20 to about 40 % (w/w) of the adhesive
composition.
Furthermore, the adhesive composition of this invention may further be
characterized
in that a portion of-the formaldehyde, within the formaldehyde-phenol resin is
replaced
with NR, and wherein the NR replaces up to about 50 % of the formaldehyde
content
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
of the resin. Preferably the adhesive composition comprises a
formaldehyde:phenol
ratio from about 1.5:1 to about 3:1. This invention is also directed to an
adhesive
composition wherein a portion of the formaldehyde within a urea-formaldehyde
resin
is replaced with NR.
This invention also relates to a natural resin that is washed, and to mixtures
of
natural resin, comprising the natural resin define above and washed natural
resin.
Furthermore, this invention is directed to adhesive compositions comprising
washed
natural resin and natural resin mixtures. This invention also includes phenol-
10 containing formaldehyde resins comprising washed natural resin that
replaces up to
100% of the phenol content of the phenol-containing resin.
This invention also embraces a wood product prepared using the adhesive
compositions as defined above. Preferably, the wood product is selected from
the
15 group consisting of laminated wood, plywood, particle board, high density
particle
board, oriented strand board, medium density fiber board, hardboard or wafer
board.
Furthermore, the wood product prepared using the adhesive composition of this
invention is used for exterior applications.
20 Use of a fast pyrolysis process to produce the bio-oil is beneficial in
that the
fast pyrolysis process depolymerizes and homogenizes the natural glue
component of
wood, that being lignin, while at the same time other constituents are also
depolymerized including cellulose and hemicellulose. The yield of NR,
depending
upon the biomass feedstock varies from 15-20 % of the feedstock and exhibits
25 properties that are useful within, for example, phenol-containing, or urea-
containing
formaldehyde resin compositions. The natural resin so produced can be
substituted for
some of the phenol, or some of the phenol and formaldehyde, content within
phenol-
containing formaldehyde resins, and such formulations meet or exceed current
phenol
formaldehyde resin industry specifications. Furthermore, NR can substitute for
some
30 of the formaldehyde within urea-containing formaldehyde resins. With
removal of the
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
_g-
organic acids, the NR can completely substitute for the phenol content in
phenol resins,
and can also be used within urea-containing formaldehyde resin formulations.
S BRIEF DESCRIPTION OF THE DRAWINGS
These and other features of the invention will become more apparent from the
following description in which reference is made to the appended drawings
wherein:
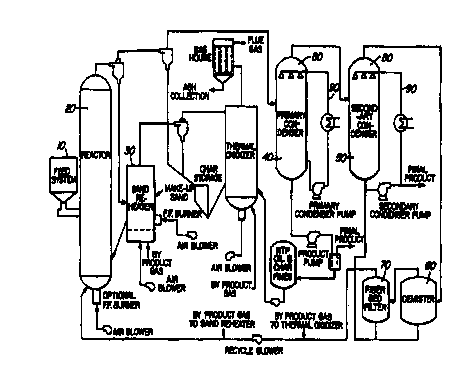
0 FIGURE 1 shows a schematic of a fast pyrolysis system.
FIGURE 2 shows the relationship between the viscosity of NR with increased
temperature.
FIGURE 3 shows the infrared spectra obtained from, FIGURE 3A: NR, and FIGURE
3B: alkali NR. The bands of the spectra are assigned as follows:
1. O-H stretchings of carboxilic acids, alcohols and phenols (this band is
not selective);
2. C-H stretchings of aromatic and aliphatic compounds;
:0 3. C = O stretchings of carboxylic acids ands aldehydes/ketones;
4, 4' . aromatic rings, in-plane skeletal bandings;
5. O-H phenolic banding;
6. C-O stretching of primary alcoholic groups.
'S DESCRIPTION OF PREFERRED EMBODI1VVIENT
The present invention relates to the production and use of a natural resin,
derived from wood bark and other biomass residues using fast pyrolysis.
30 ~ By "bio-oil" or "whole-oil" it is meant the whole liquid fraction
obtained
following the fast pyroiysis of wood or other biomass. The whole oil is
obtained from
CA 02319416 2001-07-25
the product vapour which is produced along with char following pyrolysis. Upon
removal of the char th~°_ prrduct vapour is condensed and collected
within one or more
condensers which are typically linked in series. Whole-oii. or bio-oil refers
to the
combination of the condensed products obtained from all of the condensers. Bio-
oil
is also reterred to in the fir:ld as light pitch.
By "phenolics ' it is meant polymeric phenols derived from lignin (lignin is a
phenolic polymer which holds wood and bark fibres together and which Gives
wood
its strength).
Bv "enhancers" it is meant carbonyl compounds, typically light aldehydes and
ketones.
By "selected product vapour" it is meant the product vapour that remains a
vapour following removal of char and the subsequent removal of other pyrolytic
products condensed by at least one rapid quenching step. The selected product
vapour
is typically characterized in having a lower acid content than a product
obtained as a
result of the earlier, at levast one quenching step. Furthermore, the selected
product
vapour comprises a higher molecular weight, viscosity, pH, phenolics content
when
compared with the earlier obtained product.
By "phenol-containing formaldehyde resin" it is meant adhesive compositions
that comprises phenol as one of its ingredients. Such resins include but are
not limited
to phenol formaldehyde ~'PF), phenolic melamine urea formaldehyde (PMUF), and
phenol urea formaldehyde: (PUF) resins. Similarly, by "urea-containing
formaldehyde
2~ resins" it is meant adhesive compositions ;.omprising urea as one of its
ingredients, for
example, but not limited to, urea formaldehyde (UF), phenol urea formaldehyde
(PUF), phenol melamine urea formaldehyde (PNIUF), and melamine urea
formaldehyde (MUF) resins. Without wishing to be bound by theory, it is
thought that
the addition of VR to urea-containing resins adds or complements the phenol
content
of these resins due to the high phenolic content of NR. Therefore, a OF resin
that is
partially replaced with NR may be considered a PUF-like resin.
CA 02319416 2001-07-25
1 ()
The natural r~siru (NR) of this invention is not a whole bio-oil product,
rather
it is a selected r°ined traction of the whole liquid product,
preferably produced from
the fast pyrolvsis of wood. However, other processes that are able to liquefy
wood
may also be used to prepare a prcoduct from which a NR may be obtained. The
refined
fraction is primarily comprised of depolymerised lignin and other reactive
components
including phenolics which provide an array= of active sites for binding and
cross linking
within NR. fotznulations Non-reactive components are removed during the
preparation
of the NR. The isolated NR traction is not subject to solvent or other
fractionation
processes used in the pr:c:r art, nor is it condensed (i.e. subject to
condensation
reactions) as would be typically done for conventional, or vacuum pyrolysis
liquid
products. Without wishing; to be bound by theory, it is possible that the
omission of
such condensation reactions during the production of the NR of this invention
is a
primary reason for the high reactivity of NR as a resin agent.
Fast pyrolysis yde~structive distillation) of wood or other biomass residues
results in the preparation of product vapours and char. After removal of the
char
components from the product stream, the product vapours are condensed to
obtain a
whole-ail, or bio-oil product from pyrolysis. A suitable fast pyrolysis
process for
preparing such a bio-oil is described in WO 91/11499 (Freel and Graham,
published
August 8, 1991), and is diagrammatically presented in Figure 1. Briefly, the
system
includes a feed system x;10), a reactor (20), a particulate inorganic heat
carrier
repeating system (30). and for the purposes of the invention described herein,
primary
(40) and secondary (5()) condensers, through which the product vapours
produced
during pyrolysis are cooled and collected using a suitable condenser means
(80). The
NR of this invention is a product obtained from the secondary condenser, de-
mister
(60) and fiber filter bed (70), or a combination thereof. However, it is to be
understood that analogous fast pyrolysis systems, comprising different number
or size
of condensers, or different condensing means tnay be used for the selective
preparation
of the NR of this invention.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-11-
A portion of the product vapours produced during fast pyrolysis are rapidly
quenched within the primary condensation collection means outlined above. This
rapid
quenching enables the selective separation of a selected product vapour from
the
condensed liquid product which remains within the primary condenser. NR is
collected
from the selected product vapour, obtained from components down stream from
the
primary condenser, for example, but not limited to, the secondary condenser,
demister,
and fiber filter bed. It is to be understood that other fast pyrolysis
systems, with
various condenser means arrangements may be effectively used to partially
purify the
product vapour by removing a liquid fraction from the product vapour and
producing
10 a selected product vapour, from which a NR fraction, analogous to the NR of
this
invention, maybe obtained.
The condenser system used within the fast pyrolysis reactor system, outlined
in Figure 1, involves the use of direct-liquid contact condensers (80) to cool
the
15 pyrolytic oil product. In the preferred embodiment liquid, used within
these
condensers to cool the pyrolytic product, is obtained from the corresponding
cooled
primary or secondary condenser product {90; (Figure 1). However, as would be
evident to one of skill in the art, any other compatible liquid for cooling
the product
within the secondary condenser may also be used for this purpose. Furthermore,
it is
20 considered within the scope of this invention that other scrubber or
cooling means
including heat exchanges comprising solid surfaces and the like may also be
used for
cooling the product vapours, including the selected product vapour.
By thermal fraction it is meant the process of obtaining a selective fraction
of
25 the product vapour obtained from fast pyrolysis following removal of
products from
the product vapour stream that are rapidly quenched {e.g. from about
500°C to about
20°C within milliseconds). Surprisingly, the product that is left as a
selected product
vapour following the rapid quenching step can be directly utilized as a
replacement of
constituents within adhesive resins, such as phenol formaldehyde, urea
formaldehyde,
30 or related resins as defined above.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-12-
Without wishing to be bound by theory, it is thought that the rapid quenching
of product vapours within the first condenser removes compounds that interfere
with
the use of bio-oils within adhesive resin formulations. The products removed
from
the product vapour within the first condenser are those that are readily
soluble and
rapidly quenched. The less soluble compounds and those that form aerosols or
some
other form that assists in their remaining within the product vapour, are
transferred to
the secondary condenser. This transferred product vapour is then a selected
product
vapour, which is comprised of a predominantly phenolic fraction, and also
containing
aldehydes, and provides the NR with its desirable properties for use within
adhesive
formulations.
The NR of this invention may also be further processed to removed the organic
acid content of the resin. Any suitable method may be employed for this
process, for
example, and not wishing to be limited to this method, the NR of this
invention may
15 be washed in water by mixing the NR in water, allowing phase separation to
take
place, and recovering the oil fraction. Such a processed NR is, for the
purposes of
this invention referred to as "NRP" . The NRP, prepared in this manner,
comprises
the phenolic and aldehyde content of NR, with a dramatically reduced organic
acid
content when compared with NR, and is a more concentrate form of NR. As a
result
NRP contains up to about 80% (w/w) phenolics.
It has also been observed that NRP diluted with NR produces a product suitable
for use within adhesive resin formulations. For example NR50 refers to a 50-50
mix
of NRP and NR, however, as would be evident to one of skill in the art, other
mixture
ratios may also be obtained and used for the purposes disclosed herein.
The NR, or NRP so produced has been substituted for some of the phenol
content within PF resins, and such formulations meet or exceed current PF
resin
industry specifications. More specifically NR has been substituted up to about
60%
of the phenol content within PF resins, and NRP to about 100% of the phenol
content
within a PF resin. Resins so produced may comprise up to about 40% (w/w) of
NR.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-13-
Similarly, NR has also been used as replacement within PMUF and, PUF resins.
Furthermore, the NR of this invention has successfully replaced up to about 60
(w/w) of the urea formaldehyde within OF resins, and has been effectively used
within
PMUF and MUF resins.
As a result of selecting a specific thermal fraction for the preparation of
NR,
the recovery technique is more selective than solvent extraction-based
methods: For
example, the P/N fraction extracted using ethyl acetate (e.g. US 4,942,269; US
5,235,021), results in a fraction comprising any compound that is soluble in
this
10 solvent and that is co-extracted along with the desired-for resin
compounds. Several
of these co-extracted compounds are odorous (e.g. lactone, an acrid compound)
while
others dilute the P/N resin. The thermal recovery technique of this invention
is
selective in that essentially all of the desirable resin components (natural
phenolics
derived from lignin) are recovered, while other non-desired compounds are
removed
within other fractions. As a result, the NR of this invention exhibits many
beneficial
properties over prior art pyrolytic oil extractions and requires significantly
less
preparation. For example:
1. NR and NRP have a pleasant "smoky" odour, and lack the acrid smell
of solvent extracted fractions - when used within adhesive applications,
there is no residual odour. solvent extracted preparations, such as P/N
extractions, requires further processing to remove the acrid smell;
2. the fractionation of other pyrolytic oils, including P/N recovery,
requires add-on solvent extraction processes following the primary fast
pyrolysis process, while the isolation of NR is a thermal fraction
obtained during the primary fast pyrolysis process, and no further
processing is required. Furthermore, there is no extra "add-on"
equipment required for preparation of NR. The selected NR may be
modified by fine tuning of the fast pyrolysis process conditions. If
desired, the NR may be further processed (washed, extracted etc.) in
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-14-
order to optimize properties of the NR as required within different
applications;
3. in solvent extracted processes, including the process used to obtain P/N,
the solvent reacts with residuals in the fraction that is not used for P/N,
to form salts. These salts must be recovered using a recovery boiler
requiring additional costs, and the residual bio-oil is not available for
other commercial applications. The NR, on the other hand, is isolated
as a thermal cut which still permits the remaining bio-oil to be
processed as required for other commercial applications without
contamination;
4. the fast pyrolysis method used for the preparation of bio-oil, including
NR, has been successfully scaled up from bench-top trials to
industrial/commercial production levels (see W091/11499). Therefore,
NR preparations are easily produced on a commercial scale.
Characteristics of NR
The NR produced by the method of this invention has been found to be
consistent between batch to batch productions runs of NR (as tested when used
for OSB
production, see below), even when using different feedstocks including
hardwood and
softwood.
25 Molecular weight is typically used as an index for adhesive resin
reactivity and
viscosity, with higher molecular weights indicating higher viscosity, and a
corresponding lack of active sites necessary for cross-linking and binding
strength in
commercial resin formulations. However, with the NR product prepared following
the
method of this invention, the relationship between molecular weight, viscosity
and
reactivity is not valid.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-15
The free phenol content of a resin formulations is also used to determine the
suitability of alternative materials in PF resin formulations. The NR produced
following the method of this invention is characterised in having a very low
free phenol
content, from about 0.001 to about 0.05 % (w/w), yet the total phenolic
content is quite
high, from about 30 % to about 50 % (w/w) within NR, and up to about 80 % with
NRP. It is the phenolic content which is very reactive and provides an array
of active
sites for binding and cross linking within NR formulations.
Furthermore, the NR is characterized by the following parameters, however
these parameters even though typical are obtained from one sample and
variations in
these values are to be expected:
Water DensitySolid contentAsh contentViscosity
content at
pH
(glue) (~k) (~%) 75C (cSt)
NR 14 2.2 1.268 0.706 0.105 59.8
The viscosity of NR over a range of temperatures is presented in Figure 2.
The infrared spectra of NR and alkali NR are presented in Figure 3A and 3B,
respectively. Similar spectra are observed for both NR samples. The 1700 cm-'
band
that corresponds to the carbonylic group (band identified as 3) is reduced in
Figure 3B,
since R-COOH becomes R-COO' under the alkali conditions.
The NR of this invention comprises from 10 to 20 % water, however, NR is
insoluble in water due to its low polarity and high content of non-polar
organics. By
increasing the pH of the NR (to about 10) and converting it into its phenoxide
ion form
it obtains a gum-like consistency, is water soluble and can be used within
formaldehyde-phenol formulations. The NR is soluble in organic solvents for
example
acetone, methanol, ethanol and isopropanol. Due to the hydrophobicity of NR,
it is
chemically compatible in the formulation of phenolic-based resins. NR is
soluble in
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-16-
a mixture of water/phenol, and when reacted with formaldehyde, gives methyol-
water
soluble derivatives.
Infrared analysis of NR, and alkali NR are exhibited in Figure 2A and Figure
S 2B. The same bands are detected between the two NR samples, however, in the
alkali
NR (Figure 3B), the 1700 cm-' band (relating to the carbonylic group #3; R-
COOH)
becomes R-COO- under the alkali conditions.
Calometric analysis indicates that NR has a net caloric value of 4355 cal/g
(18.22 MJ/kg), with a gros caloric value of 4690 cal/g (19.62 MJ/kg).
NR is stable and homogenous and has been stored in excess of 12 months
without loss of its properties.
NRP preparations exhibit similar properties to those of NR with the notable
exception that the phenolics content of NRP increases up to about 80 % (w/w),
and the
pH is more neutral. The exact pH of NRP is difficult to determine as water is
required
for this test, however, the acid content of NRP can be determined using gas
chromatography (GC). GC analysis indicate that up to 90% or more of the
organic
acid content of NR is removed as a result of the washing procedure. Due to the
removal of the organic acid content, NRP is a more concentrated form of NR.
The
content of phenolics, and the pH of NR-NRP mixtures, e.g. NR50, will vary
depending upon the proportions of NR or NRP within the mixture.
NR-50 is approximately 50% NRP and SO% raw NR. NR-50 is prepared by
blending two NR products, raw NR from the process and NRP.
NR60
NR-60 is a liquid which is approximately 60% NRP. However, the term "NR60"
is not representative of a single NR product. NR60 is not prepared by blending
raw NR
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-17-
and NRP as the case with NR-50. NR-60 is prepared from raw NR, after raw NR is
devolatilised thereby concentrating the raw NR to NR-60 (i.e., increasing the
NRP
concentration by driving of non-NRP volatiles). The non-NRP volatiles may be
driven
of using any suitable means, for example, but not limited to, heat, or
evaporation under
5 vacuum. It is to be understood that the term "NR60" represents an NR that
can comprise
a variety of NRP concentrations depending upon the NRP content initially used.
"NR60"
therefore includes an NR that comprises from about 40% to about 90% NRP. NR60
may
be obtained from a variety of lignocellulosic feedstock sources including
softwood,
hardwood, bark, white wood, or other lignocellulosic biomass feedstocks, for
example,
10 bagasse (sugar cane residue). The use of PF/NR60 resins are discussed in
Examples 7-
10, a specific NR60 resin formulation, obtained from bark, (NRB) is discussed
in
Example 10.
Unlike NR or NR-S0, the characteristic smoky odour is significantly reduced in
15 NR-60 during the devolatilisation/concentration process
NR-60 can be derived from NR or from selected NR fractions derived from the
fast pyrolysis of wood as described above (ie. from certain condensers,
filters, demisters,
etc.), or from the whole raw bio-oil as produced in the pyrolysis process. NR
fractions
20 and whole bio-oil can be processed and concentrated in several ways in
order to produce
NR-60; evaporation or distillation (for example, falling film, vacuum
distillation, wet
film evaporation, etc., or selective condensation of the raw NR, NR fractions
or whole
bio-oil), selective precipitation, or any other physical or chemical process
which removes,
evaporates, isolates or otherwise drives off certain acids, volatiles, water
and other light
25 components which are less effective in terms of resin properties and which
contain
odorous components.
With out intending to limit the present invention in any manner, NR-60 can be
prepared by heating NR material (ie., raw NR, NR fractions or whole bio-oil)
under
30 vacuum to a temperature which is sufficient to devolatilize odorous and non-
resin
components. The water content is monitored to determine the degree of
devolatilization
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-18-
so that a final water content of between about 1 and about 10% is obtained.
Preferably
the final water content is between about 3 and about 5%.
Once the desired degree of devolatilization has been reached, water is added
back
in order to reduce the NR-60 product viscosity to the desired spec. Typically,
sufficient
water is added to bring the water content to a level in the range of from
about 10 to about
25%. Preferably the final water content is about i 5 to about 18%.
Viscosity, acid content and NRP content determination for NR-60 comprising
60% NRP is characterized with acids (dry wt%) 2-4%; water content between 15-
18%;
NRP is about 60%, and viscosity at 70°C is in the range of 30 to
150cSt. An NR60
comprising a variety of NRP concentrations can be prepared in a similar
fashion to that
as indicated above.
15 A comparison of some of the characteristics of NR, NR50 and NRP are
provided below:
Component NR NR50 NRP NR60*
Acids (dry wt%) 10-12 5-10 0-5 2-4
Phenolics (dry wt%) 35-38 38-53 64-73 50-65
Enhancers (dry wt%) 12-15 15-19 22-27 -
pH 2.4-2.6 2.5-2.8 3 + -2.5
viscosity (70C)cSt 81-115 100-300 300+ 30-150
Water (wet wt%) 11-18 < 18 < 18 15-18%
average MW (Daltons) 500-1000 700-1200 1000-2000 -
*These values may differ in NR60 depending upon the final concentration of NRP
used
in the NR60 formulation.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-19-
NR-containing Phenol Formaldehyde (PF), or Urea Formaldehyde (UF) Resins
In order to formulate NR within phenol-containing formaldehyde, or urea-
containing formaldehyde resins, phenol or urea, water, paraformaldehyde, and
other
ingredients of the adhesive are mixed together and heated if required to
dissolve the
ingredients. If heated, the mixture is cooled prior to the addition of NR.
Caustic (for
example NaOH) is added to the mixture containing phenol or urea, formaldehyde
and
NR, to a desired pH. The addition of caustic ensures the solubilization of the
NR, and
initiates the reaction. This mixture may then be heated or cooled, and more
caustic
10 added during the preparation of the resin, as required. The resin is
typically maintained
at 10°C until use, and exhibits similar stability associated with
commercial PF resin
formulations. Phenolic melamine urea formaldehyde (PMUF), melamine urea
formaldehyde (MUF), phenol urea formaldehyde (PUF) resins are prepared in a
similar
manner.
NR can be added up to about 60% (w/w) of the phenol content of the resin, or
in the case of NRP, up to about 100 % (w/w) of the phenol content may be
substituted
by NRP. Furthermore, the formaldehyde content of phenol-containing or urea-
containing resins may be substituted with NR due to the natural aldehydes
present
within NR, for example NR can be used to replace up to about 50 % (w/w) of the
formaldehyde content of these resins. Similarly, up to about 60% (w/w) of the
urea-
formaldehyde content of a OF resin may be replaced using NR. Therefore, PF, OF
and related resins may be formulated that contain up to about 40 % (w/w) NR of
the
total resin composition.
Board manufacture suing NR-containing adhesives
The phenol-containing or urea-containing formaldehyde resins prepared above
may be used for the production of a range of board products, for example, but
not
limited to, laminate wood boards, plywood, particle board, high density
particle board;
oriented strand board, medium density fiber board, hardboard, or wafer board.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-20-
Preferably, NR-containing PF resins are used within boards to be subject to
exterior
use due to the excellent water repellency of the resin. Typically OF resins
are not
desired for outside use, however, NR-containing OF resins may have application
for
exterior use due to the reduced swelling observed in boards prepared with urea
formaldehyde adhesives comprising NR, compared with boards prepared using
commercial OF resin.
NR containing PF or OF resins can be used for the production of oriented
strand board (OSB) as outlined below. However, it is to be understood that
this
application of NR-containing resin is not to be considered limiting in any
manner, as
other wood derived products prepared using commercially available PF, UF, or
related
resins, which are commonly known within the art, may be prepared using resin
formulations comprising NR.
Oriented strand boards may prepared using standards methods that are known
to those of skill in the art. For example, but not to be considered limiting
in any
manner, the production of OSB may involve the following parameters:
wood matrix: particulate wood product, wood chips, wafers, veneer or
plywood etc.
Panel thickness: from about 1/16" tot"
Resin content: from about 0.5 to about 20.0%
Wax content: from about 0.5 to about 5
Mat moisture: from about 2 to about 10%
Press time: from about 2 min to 30 min
Press temperature: from about 150 °C to about 275°C
It is to be understood that these parameters may be adjusted as required in
order to
produce a suitable board product using NR-containing resins of this invention.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-21 -
Oriented strand boards, or other board types, as listed above, that are
prepared
using NR-containing PF resins are readily tested for suitability within the
industry.
For example, the OSB boards prepared above have been tested according to the
Canadian product standard for OSB (CSA 0437.1-93, April 1993). These tests
5 include; determination of density, internal bond (IB), modulus of rupture
(MOR), and
modulus of elasticity (MOE). Results of these tests indicate that phenol may
be
replaced by NR up to about 60 % (or up to 100 % in the case of NRP), and that
urea-
containing resins may also be replaced by up to 60% NR, and produce a OSB
product
that meets industrial standards, and that is equivalent to OSBs prepared using
commercially available phenol-containing, or urea-containing formaldehyde
resins.
Furthermore, OSB boards prepared with NR-containing resins require less
formaldehyde within resin formulations for equivalent cross-linking and
binding
properties as typically found with control resin formulations. Without wishing
to be
bound by theory, it is thought that the natural carbonyl components (such as
aldehydes
and ketones) within NR permits the use of less formaldehyde. In applications
which
require lower strength adhesive, the NR can be used alone without any addition
of
formaldehyde, but it is preferable to add formaldehyde to obtain a better
resin. These
carbonyl compounds have a molecular weight from about 30 to about 800 Daltons,
and
comprise about 23 % of the NR.
The NR produced following the method of this invention has a dark brown
colour, and when formulated into a resin, results in a dark reddish brown
colour.
However, during production runs using NR, OSB boards are lighter in colour
than PF
control boards. Furthermore, the NR has a mild, pleasant odour, yet OSB boards
25 prepaied using NR have no resultant odour. The odour can be reduced
following
heating of the NR, or through the removal of volatiles via flushing. The NR of
this
invention is also characterized by being acidic (pH -- 2.3), however, the NR
may be
further processed, if desired to raise the pH, for example by washing NR in
the
presence of water, and recovering an NRP fraction which exhibits a higher
content of
30 phenolics, a more neutral pH, and is a more concentrate form of NR.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-22-
Examples
Example 1: Method for obtaining, and the characteristics of, NR
5 Natural resin was obtained using red maple feedstock within a fast pyrolysis
reactor as described in WO 91/11499. Red maple feedstock is supplied to the
reactor
at a feedstock to heat carrier ratio of from about 5:1 to about 200:1. The
char is
rapidly separated from the product vapour/gas stream, and the product vapour
rapidly
quenched within the primary condenser using a direct liquid contact condenser.
The
compounds remaining within the product vapour are transferred to a secondary
condenser linked to the primary condenser in series. The product vapour is
then
quenched using a direct-liquid contact condenser within the secondary
condenser, and
the condensed product collected. Any remaining product within the product
vapour
is collected within the demister and filter bed (see Figure 1). The secondary
condenser
15 product, demister and filter bed products are pooled together to comprise
NR. The
yield of NR, using red maple as a feedstock, is 18 % .
The NR is characterized as exhibiting a low free phenol content ranging from
0.001
to 0.1 % (w/w); total phenolic content from about 35-80 % (w/w); a dark brown
colour
20 and a mild, pleasant smoky odour; a pH of about 2.0 to about 3.9;
insolubility in
water; and solubility in organic solvents including acetone, methanol, ethanol
and
isopropanol.
NR is readily washed with water to produce NRP which is characterized in
25 having a more neutral pH, and up to 90 % less organic acid content when
compared
with NR. Furthermore, the phenolic content of NRP is up to about 80 % (w/w) or
more, due to the removal of the organic acid component, and is a more
concentrate
form of NR.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 23 -
A comparison of the products collected from the primary condenser (1
°), NR
(obtained from the secondary condenser, demister, and fiber filter bed) are
presented
below. The values for NRP and NR50 are presented for comparison:
Component 1 NR NR50 NRP
Acids (dry wt% ) 15-20 10-12 5-10 0-5
Phenolics (dry wt% ) 18-21 35-38 38-53 64-73
Enhancers (dry wt% ) 6-8 12-15 15-19 22-27
pH 2.2-2.3 2.4-2.6 2.5-2.8 3 +
viscosity (70C) cSt 5-15 81-115 100-300 300+
Water (wet wt% ) 18-26 11-18 < 18 < 18
average MW 100-300 500-1000 700-1200 1000-2000
Example 2: Replacement of phenol within NR-containing PF resins
The NR produced according to the method of Example 1 was formulated into
a resin according to industry standards except that 40 % of the phenol content
was
replaced by the NR. The resultant formulation was termed E-L-1-6. E-L-1-6 was
20 compared with an commercially available PF resin (Tembec CL300), and E-K-4-
6, a
PF resin that prepared according to industry standards (Table 1) which
contained 100%
phenol, within OSB production.
Typical NR resin formulations involved loading phenol, water and
paraformaldehyde into a kettle and heating to 95°C to dissolve the
paraformaldehyde.
The mixture was cooled to 45°C and the NR added. Caustic (NaOH) was
then added
to the desired pH thereby solubilizing the NR and initiating the reaction.
During the
addition of caustic, the mixture is maintained at 45 °C for the first
caustic addition
(approximately 2/3 of the amount required). The nuxture is then slowly heated
to
90°C over a 30 min period over which time the resin is monitored for
viscosity and
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99I00051
subsequently cooled prior during which the remaining caustic is added. The
resin was
maintained at 10°C until use.
Table 1: comparison of properties of NR and phenol based resins
Resin Viscosity pH Caustic contentResin Solids
(cP)
NR: E-L-1-6 78.5 10.05 5.7 42.4
Control:E-K-4-6130 10.41 5.7 41.8
The OSB's were prepared following standard industrial procedures using either
E-L-1-
6 (NR), with the pH adjusted to 10.4, E-K-4.-6 (control) or CL300. The
parameters
for OSB production were as follows:
Strands: 3 inch poplar from an
OSB mill
Panel type: homogenous
Panel thickness: 7/16"
Panel size: 18" x18"
Resin content: 2.0%
Wax content: 1.5
Mat moisture: 5.5
Press time: 3 min or 4.5 min
Press temperature: 215 C
Replication: 4
The prepared OSB were tested for the following properties: density, IB
(internal bond),
MOR (modulus of rupture), and MOE (modulus of elasticity), according to the
Canadian product standard for OSB {CSA 0437.1-93, April 1993):
The test results are presented in Table 2
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/OOOSI
-25-
Table 2: Comparison of mechanical properties of panels prepared using
formulation with 0% and 40% phenol replacement with NR Oil no 1.
Resin Press DensityMOR IB MOE
Cycle MPa
S Code Phenol pH min Kg/m3 Dry Wet MPa MP
replacement
E-L-1-640% 10.40 3.0 660 31.0 14.8 0.368 SSS4
NR 4.S 660 33.6 16.0 0.445 SS13
E-K-4-60 10.41 3.0 662 32.5 14.8 0.427 5638
Lab 4.S 660 34.4 16.6 0.468 5723
Control
CL-300 0 10.34 3.0 664 36.5 15.6 O.S1S 5426
Ind. 4.S 66S 36.8 16.0 O.SOS 5776
Control
1S Note: The pH of E-L-1-6 was adjusted to 10.40 prior to panel preparation.
Panels produced using a resin composition comprising non-optimized NR
substituted
for 40% of phenol, exhibited properties equivalent to that of the industrial
PF resin
composition for press times of 4.S min. The OSB prepared using NR based resins
did
not exhibit any difference in appearance compared with OSB's prepared using PF
resins.
Without wishing to be bound by theory, it is possible that the organic acid
2S content of the NR based resin formulation may neutralize the caustic that
is normally
used to catalyse the condensation reaction used for the setting process of the
OSB
thereby requiring longer press times in order to ensure curing of the resin.
These
results indicate that a substantial proportion of phenol within PF resin
formulations
may be replaced with an NR fraction obtained from bio-oil.
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-26-
Example 3: NR-containing PF resin formulations - replacement of formaldehyde
Due to the natural aldehydes present within NR, a reduction in the
5 amount of formaldehyde within PF resin formulations comprising NR was
examined.
Results indicate that up to 20% of the formaldehyde can be replaced with NR
and the
OSB still maintains properties of control OSB's.
A control PF resin was formulated having a F/P molar ratio of 2.16:1.00.
Several NR based resins were prepared by replacing 40% of the phenol (w/w) and
having a F/P molar ratio of 2.16:1.00, 1.8:1.00 or 1.50:1.00. The properties
of the
different resins used are listed in Table 3, where the following NR's were
used:
NR - standard hardwood-derived NR
NRP - washed NR
PO - softwood-derived NR
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-27-
Table 3: Physical and Chemical Characteristics of Resins
MaterialViscositySolid CausticpH FinalGel Free Free Molar
After
S Code (cP) ContentContentCausticpH Time PhenotHCHO Ratio
(sec)(%) (Ro) (HCHO:
Phenol)
PF 98.5 41.9 5.7 9.25 10.36609 0.64 1.68 2.16:1.00
Control
NR 22b.5 43.5 8.02 9.1 10.28507 non 7.18 2.16:1.00
detects
d
NRP 73 41.4 5.7 8.85 9.92 485 non 10.17 2.16:1.00
detects
d
NR 73 41.2 8.02 9.1 10.27560 * 2.17 1.80:1.00
PO 79 42.6 8.02 * 10.19873 * 0.86 1.50:1.00
*=Data not available
15 OSB panels were prepared and tested as defined in Example 2 using CL300
(industry control), control, NR, NRP or PO based PF resin formulations defined
in
Table 3. Except that only a 3 min press time was used. The results of the
tests are
presented in Tables 4 and 5.
CA 02319416 2000-07-28
WO 99/38935 PC'f/CA99/00051
-28-
0
C
a~
C. o
..
o .. o o o,
~ ~
O
0
a o .~ a N N
x C1. z
~
~
ca
D.
~t in N
.3 Z
C3 0~0N
O N ~t N M
~.o ' p.,
W r ,..., ~ 'd~M ~
a ,Q O O O O
C
CC
O''O y ~C7CT h 00
O "L7 0 a ~ !f'
3
N ~D 01 N
O' A M M N N
C
Q.O
M
C~ A N M
1
a
E
w
O ~,
a~
M M M M
.., U
N
E
it
CL y,,
E C
O U
U c
v
y w ~ ~ z z
H x
O o
w O ,.,z z
'v v
v-, O
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 _29 _ PCT/CA99/00051
a~
~o
x
U
x
~. o
o g g g 8
0 0
N N ..,oo ~n
.lr N .-w .--r
~r
O
N
V iv ~f' ~ N 00
E"' C/~ ~ N ~ ~ N
Q.
C~ G=1 ~ ~O N O~ OW
M VN1~ N N
z V~'1
s
w
...
3 ..
w
d' M "~ ~ vt
O O O O CD
x
0
M
O y C Cy .'~N
M N M N N
...
it
C. ,~~'' ~.
Q" .~ ~ ~n N ~n M
00
a
...
C
CC
O O O O O
~ U ~ M M M M M
4r
O y,
C C
O
H
L U
o o .:.
V w cx b: ~
'n ~c -- o
y o Z ~ z ~
E'' ~ ~ U 0. A
U
0
SUBSTITUTE SHEET { rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 30-
From the results it can be seen that OSB's prepared using a F/P molar
ratio of 1.80:1.00 gave equivalent results to that produced using PF resins
with
3 min press times. Also, NRP-containing resins exhibit similar properties to
that of commercially available PF resins. These results also indicate that
softwood-derived non-optimized NR may also be used within PF resin
formulations .
Example 4: PF resin formulations comprising NR
A series of PF resins were prepared with the compositions listed in
Table 6. The base phenol-formaldehyde resin composition was a resol-type
resin pH 11 with a P:F ratio of 2.5:1. The NR used in the sample preparations
was obtained as described in Example 1.
Table 6: PF resin compositions
Sample Phenolic Quebracho tannin*NR Triacetine**P:F
resin
(w/w) % (w/w) % (w/w)% (w/w) ratio
A 100 0 0 2 2.5
B 81 0 19 2 2.5
C 62 0 38 2 2.5
D 40.5 40.5 19 2 1.6
* quebracho tannin (273 g/mol monomeric molecular equivalent) was used as
a natural phenolic additive.
** triacetine was used as an accelerator of the resin.
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 31-
These samples were used to prepare particle boards under the following
conditions:
press temperature: 175C
maximum pressure: 30
Bar
thickness: 9.0
mm
press time: 15
s/mm
degassing time: 30
sec.
The prepared panels exhibited the characteristics as disclosed in Table 7:
Table 7: Characteristics of particle board panels prepared using the
resins of Table 6.
Sample internal Bending StrengthSwelling Density (Kglm3)
bond *
(N/mm2) (N/mm2) ( % )
A 1.30 22.5 15 830
B 1.30 22.1 26 830
C 1.15 21.0 45 825
D 1.25 22.0 32 825
*measured after 24 hours soaking at 20°C
These results indicate that NR can be substituted for phenol, up to 40 % ,
within PF resin compositions with no significant effect on internal bond or
bending strength.
Example 5: Urea-formaldehyde resins formulated with NR.
A commercially available OF resin (L2600) produced by Rescol (Italy)
was used having a 63 % dry content, and a formaldehyde:urea ratio of 1.2:1.
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 32
This resin was used as a base resin for a series of OF formulations comprising
NR, obtained as outlined in Example 1. Particle boards were prepared as
defined in Example 6, with a total of 12 % dry glue on dry wood. The resin
compositions and test results are disclosed in Table 8. The amounts were
calculated for dry resin and dry wood. The glue mixtures were all hardened
with 1.5 % ammonium sulphate:
Table 8: OF Adhesive mixtures used, and mechanical characteristics of
particle board prepared with the defined adhesive compositions.
Sample OF NR InternalBonding Swelling** Density
* % Bond strength % (Kg/m3)
*
(~~2)
A 12 0 1.30 21.2 24 720
B 6 0 0.60 9.3 SO 725
C 7.2 4.8 1.15 15.7 35 720
D 6 6 0.70 13.2 48 710
* (w/w) -
**measured after
24 hours soaking
at 20C
The results of Table 8 demonstrate that with reduced amounts of OF
(note: no added NR), several of the particle board characteristics deteriorate
(compare A and B). However, the addition of NR (see D) enhances the
characteristics observed within the UF-prepared panel. Furthermore,
replacement of 40% of the OF within the resin formulation produces a similar
particle board product as that prepared with OF resin.
Example 6: Testing of different NR's within PF adhesives at various
formaldehyde mole ratios.
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/3$935 PCT/CA99/00051
- 33-
Several different NR preparations were tested within PF resin
formulations. The NR's were obtained as outlined in Example 1, except that
pine was used as the feedstock.
The formaldehyde:phenol molar ratio tested were 1.5:1 and 1.8:1, with 40%
of the phenol replaced by NR. The characteristics of the PF resin formulations
comprising NR are defined in Table 9.
Table 9: Physical and chemical characteristics of resins
Viscosity Solid Caustic final gel time Molar
(cP) content Content pH (sec) ratio
(%) (%) P:F
Control 126 42.1 5.7 10.31 518 2.16
E-H-2-7
E-1-2-7 120 41.7 8.18 10.11 530 1.8
E-1-3-7 113 42.2 7.9 10.05 568 1.5
E-1-4-7 220 41.7 8.32 10.19 621 1.5
E-1-5-7 3260 41.6 7.49 10.22 335 I.8
The resin formulations
of Table 9 were used for
the production of
OSB's under the following
conditions:
Replications: 4 per press cycle
strands: 3 in poplar
support: cauls
panel type: homogeneous
thickness: 11.1 mm
resin content: 2.0%
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 34-
MC%: 5.0%
press cycle: 3.0 min (includes 30 sec closing and 30 sec
opening)
press temp: 215 °C
wax: 1.5
The boards were tested for internal bond strength (IB), modulus of rupture
(MOR), modulus of elasticity (MOE), torsion shear (TS) and some panels were
tested for thickness swelling (ThS). The results of these test are presented
in
Table 10.
Table 10: effect of formaldehyde:phenol molar ratio on mechanical
properties for a softwood NR (pine), and "conditioned NR".
PhenolDensityMOR IB MOE TS ThS
(MPa)
(%) (Kg/m') (MPa) (MPa) (in.lb)(%)
dry wet
Control 100 685 35.4 17.8 0.429 5483 30.5 n.d.
E-H-2-7
E-1-2-7 60 680 34.3 16.1 0.387 5413 21.9 n.d.
E-1-3-7 60 680 42.8 15.1 0.340 6101 18.7 n.d.
E-1-4-7 60 680 35.4 14.4 0.378 5618 16.2 n.d.
E-1-5-7 60 680 27.7 11.5 0.325 4720 8.8 n.d.
2S The results of Table 10 demonstrate that PF resins, containing 40 % NR
derived from softwood (pine), at a formaldehyde:phenol ratio of I.8:1 or
1.5:1, produces OSB's with comparable properties to OSB's prepared using
commercial PF resin formulations with a formaldehyde:phenol ratio of 2.16:1.
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 35-
Example 7 - Testing of NR60 with PF Adhesives
Eleven 3' x 3' x 0.5" plywood panels were manufactured in order to evaluate
the effects of varying concentrations NR60 substitution for phenol in PF
resin.
1.0 Plywood Panel Manufacture
1.1 Blending and Forming
Three different resin compositions were applied to pine veneers (Table
9). This resulted in three groups with a minimum of three panels per
group. All applciations were made at a 35 1b/1000 ftz loading rate. All
resins were applied using a plywood glue spreader and applied on a
single glue line.
Billet lay-up for each panel consisted of four plies. The face plies were
laid-up parallel to the machine direction and the core plies were laid-up
perpendicular to machine direction. Three control panels control four
PF/NR60, at 10% panels (Group NR60-10%), and four PF/NR60 at
% (Group NR60 - 20 % ) panels were manufactured in the trial.
1.2 Pressing and Testing;
Before pressing, the billets were pre-pressed (cold) at 150 psi for four
minutes in a 4' x 8' press. The panels were then transferred for hot
pressing to a 3' x 3' press. The panels were pressed under constant
pressure control for 300 seconds at 300°F. Pressing was monitored and
controlled with a PressMAN~ Press Monitoring System.
After pressing, the panels were trimmed to 28" x 28" dimensions and
hot stacked. Once cooled, the panels were evaluated. The panels were
tested for plywood glue bond and flexural creep (CSA 0151-M1978).
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99100051
- 36-
1.3 Observations
No resin quality differences were noted visually during panel
manufacture. The control and NR substituted resins behaved in the
same manner with equal spreadability. The shear data suggests the NR
substituted resin performed as well as the control (Table 10). The
NR60 - 10 % and NR60 - 20 % resins both performed comparably to the
control, under both test conditions with respect to shear strength. The
resins showed exemplary strength characteristics with the ply only
failing on the glue bond a maximum of 12 % (PG2-88 % average wood
failure) under both test conditions. The strength of the NR-resin data
is further supported by the fact not one sample demonstrated less than
60 % , or less than 30#, wood failure under both test conditions .
Table 9
Group No. Resin Type Resin Loading Pressing
ID of Time
Panels (sec)
Control 3 GP PF Resin 35 lbs/1000ftZ 300
single
(Control) glue line
NR60-10% 4 GP PF/NR 35 lbs/1000ft2single300
10
Resin glue line
NR60-20% 4 GP PF/NR 35 lbs/1000ft' 300
20 single
glue line
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 37
Table 10A SUMMARY OF GEORGIA PACIFIC CONTROL AND GEORGIA
PACIFIC NR RESIN PLYWOOD SHEAR TESTS: NR I O RESIN &
NR20 RESIN
(Average values for ten specimens per panel from 3 panels per group)
Test Property CSA 0151 UnitsControlNR NR60
Condition Requirement 60 20%
10!0
Vacuum-
Pressure Shear StrengthNo. Req. psi 89 102 88
Soak:
Percent 80 % 95 90 88
Wood
Failure
Average
Percent 90 % 100 100 100
Wood
Failure
> =60
Percent 95 % 100 100 100
Wood
Failure
> =30
Boil-Dry Shear StrengthNo. Req. psi 79 80 69
Boil:
Percent 80 % 91 90 91
Wood
Failure
-
Average
Percent 90 % 93 I00 100
Wood
Failure
> =60
Percent 95 % 100 100 100
Wood
Failure
> =30
Introduction
Example 8. NR-60 used at 25% for the Preparation of Plywood and OSB Panels
A total of seventeen 3' x 3' x 0.50" OSB, and fifteen 3' x 3' x 0.50" plywood
panels were manufactured to evaluate the effects of 25 % substitution of NR60
for phenol in PF resin, for both OSB and plywood.
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99138935 PCT/CA99/00051
- 3~
1.0 OSB Panel Manufacture
1.1 Blending and Forming:
The resins were supplied by Neste in the following formats:
Neste PF face control # 1, Neste PF core control #2 and Neste
PF/NR-60 - 25 % (experimental). Three groups of panels were
manufactured as indicated in Table 11. The control group
{SNC) consisted of the Neste face control #1 resin applied to the
strands along with commercial E-wax; the strands were then
formed into random homogenous mats. The first experimental
group (SNE) consisted for the substitution of the Neste
PF/NR60 - 25 % resin for the face control resin in the same
manufacturing methodology. The final experimental OSB group
{SN) utilized Neste PF/NR 60-25 % on the panel face strands
and the Neste core control #2 on the panel core strands. The
SN mats were of 50/50 face-core random construction.
TABLE 11. PF AND PF-NR60 RESIN OSB TESTS
Group No. Resln PANEL SPECIFICATIONS
of
ID PanelsContent ConstructionThicknessDensityComments
(in.) (lb/ft')
SNC 8 Neste PF Homogenous0.5 39 OSB
Face resin, control
3.5%
(Control
#1)
SNE* 6 Neste PF/NRHomogenous0.5 39 OSB Trial
25, 3.5%
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 39-
SN** 3 Face: Neste50/50 face-0.5 39 Face
NR
PF/NR 25, core Substitute
3.5% Core
Core: Neste Control
PF core on OSB
resin,
3.5%
(Control
a2)
* NR/IZF resin used on the surface and core of the OSB
** NR/PF resin used on surface only
AlI resins were applied at a 3.5 % solids basis. The commercial
e-wax was applied at a 1.0% solids basis. All billets were hand
formed to yield a density of 39 lb/ft3 when pressed to a thickness
of 0.5".
1.2 Pressing and Testing:
After formation, the mats were then pressed utilizing a standard
OSB pressing cycle. The total pressing time was set to a
conservative 400-second cycle to ensure complete cure of the
applied resin. Pressing was monitored and controlled with a
PressMAN~ Press Monitoring System.
After pressing, the panels were removed, trimmed to 28" x 28"
dimensions, then measured for out-of press thickness and
density.
After the measurements were made, the panels were hot-stacked.
Upon cooling, the panels were tested to CSA of 0437.2 - 93 for:
MOR/MOE, IB, bond durability (2hr and 6hr cycles), thickness
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99100051
- 40-
swell (24hr soak), and linear expansion (ODVPS) as well as
flexural creep.
2.0 Plywood Panel Manufactwe
2. i Glue Spreading and Veneer Lay Up
The plywood portion of the study involved the gluing and lay up
of commercial pine veneers. Two plywood resins were used for
the study. The first resin was identified as the Neste PF
(plywood control) while the second was identified as Neste
' PF/NR 25 (plywood experimental).
The resins were applied to the veneers using a glue spreader.
A rate of 35 lbs.per 1000ft2, applied on a single glue line was
utilized. The lay up consisted of two face veneers, parallel to
machine direction, and two core veneers, perpendicular to
machine direction, for each panel. Eleven control (Group PNC)
and four experimental (Group PNE) panels, were manufactured
(Please refer to Table 12).
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 '41' PCT/CA99/00051
a~
c
a
a~
w
w
E b
E
0 0 0
o -N N
U
Q N
z
o '
0 0 3
U _'' '' _~
,..
E
a
A C
z
U
p.i V b
N N
w ~ ~ O O E
d
w
a .Q.N .u.N
0 ~
~
3 . ~ ~ E
U o w
w > >
a. E
.r
z
N
w c a w a w b C~
n
U ~ ~ ran~ 0 0
o ~ o ~
x 'N -, ~ 3 ~ ~ 3
z
z M z
d
=
w a
w a
z
N
cd
O
p z o z ~ z A
a. v a.
t!7 O N
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
4z
2.2 Pressing and Testing
After pre-pressing at four minutes and 150 psi, the billets were
placed in a press for final cure and pressing. The first seven control panels
(PNC 1-7) were used to establish the pressing time. This resulted in the
establishment of 300 seconds as the required pressing time. Pressing was
monitored and controlled via a PressMAN~ Press Monitoring System.
After pressing, the panels were then trimmed to 28" x 28"
dimensions then hot stacked. Upon cooling, the panels were
evaluated. Testing consisted of glue-bond shear and flexural
creep evaluation.
3.0 Observations and Results
Virtually no difference was observed between the control and NR
substitution resins. Color, viscosity and spreadability for all resins seemed
equal. A slightly different odor was noted in the NR resins versus the
controls.
This odor did not prove to be pervasive or intrusive however. Otherwise, all
resins appeared to behave equally in a manufacturing situation.
A comparison of the NR substituted resins versus the control (SN, SNE,
vs. SNC) show bending and bond properties to be equal between the three
groups (Table 13). The results indicate, especially with group SN, a drop in
bond durability and linear expansion versus the control. Group SN showed a
value of water swell well within the maximum requirement.
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-43-
~O N O ~D M .-.
O ~ M ~
v~ ~ ~ ~ Q O
d d
.4~O it ..r
~ ~ O O N V1
W ~ ~ Y, ~t;N
JJ ~ ~ M ~' .M-. ~ N O O
z
..
V ~-"~ U ~ ov cn $ o~ .a M c~o
. 0
e~Nn ~' v~, ~ cNV' G7 O
C4
o
~ ~ r '~. 'a,
N
O
O ~ O V01V1 ~ N
d N ~ h ~ ~ N O O w
C ~ ~G X O
d C C ~ C
~ ~ ~ ~ ~ ~ ~ ~ V
C N ~ U
Qi~ ~ ~ ~ V
3 ~ x : x o
~ N .d v0 ~ v 'n
w ~a
. a. :d ~ N a _v o ~ a
y .r.,~,~ w N n
W ~ ~ N wr ~ H
N ~3ia 3 O D
a O O . by O 'O
I . N . ~ N ~ ~
L ~ ~ ._,~ ~ ~ a
cV ~r ~ ,t-'", G 'r'n
0 ~ ~ O A N C.
~ ',.~ _~ ~ u,
_ f~ 4.J W G_i
O b ~ .b V 'd
U ~ U r.'r"rC~ '~'"'~ Qi
Z
Z
N
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 44-
With respect to the plywood shear testing the results were favourable both
against the standard and the control Group (Table 14). A strong bond was
indicated by the shear strength performance under both test conditions.
Under both conditions 11 % or less failure could be attributed to the glue
while the maximum allowable is 20 % (89 % wood failure for Group PNE
under boil-dry-boil). A further indicator in the strength of the data was that
not one PNE sample showed wood failure values of less than b0 % or 30
under both test conditions (100% pass for both requirements on both test
regimens).
Table 14 SUMMfARY OF PF AND PF/NR60 - 25°lo RESIN PLYWOOD
SHEAR TESTS
Test Property CSA 0151 Units Control Neste
Condition Requirement Group NRIPF
(PNC) (PNE)
Vacuum- Shear StrengthNo. Req. psi 82 110
Pressure
Soak:
Percent Wood 80 % 87 93
Failure Average
Percent Wood 90 % 93 100
Failure >
=60
Percent Wood 95 % 100 100
Failure >
=30
Boll-Dry Shear StrengthNo. Req. psi 74 83 .
Boil:
Percent Wood 80 % 89 89
Failure Average
Percent Wood 90 % 100 100
Failure >
=60
Percent Wood 95 % 100 100
Failure >
=30
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 45-
Example 10. Use of NR60 at 40% in the production of OSB panels
An experimental resin formulation was used to prepare four 40 % phenol
substitution resins using NR60 (formaldehyde to phenol ratio 1.6:1) from four
different samples (labelled NR60-D, NRB 166, 1 °CT, and CALCT). A lab
control was also prepared and used as reference for the evaluation of all
experimental resins. NRB is an NR60 obtained from bark.
The performance of the formulated resins has been evaluated by testing the
mechanical properties such as internal bond strength (IB), modulus of
elasticity
(MOE), modulus of rupture (MOR, dry and wet), torsion shear, thickness
swelling and water absorption from 20 OSB panels manufactured with the five
resins. All the resins were characterised using several standard best methods
such as solids content, viscosity, pH, gel time and free formaldehyde.
Panel general parameters:
Replicates: 4
Strands: 3-in, poplar
Support: screen
Panel type: homogenous
Thickness: 11.1 mm
Target density: 640 kg/m3
Press temp: 215°C
Press cycle: 3.0 min
Moisture content: 5.0
Wax content: 1.5 %
Resin content: 2.0%
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
w0 99/38935 PCT/CA99/00051
- 46~
Test Results
Table 15 shows the physical and chemical characteristics of the resins
formulated from the different NR60 - 40% samples (NR60-D, NRB 166, 1
°CT
and CALCT). The results obtained with the phenol/formaldehyde control are
included for comparison. In every case, the resin formulated with NR60
needed 1.5 to 2.2 % more caustic catalysts to produce a resin with an
equivalent
pH to the control. The pH and viscosity values of E-F-4-8 and E-F-5-8 resins
are similar to the control but show less advanced characteristics for E-F-2-8,
and E-F-3-8. The same factor applies to the gel time obtained with the four
experimental resins even if it takes longer than the lab control value.
Table 16 summarizes the OSB test results for E-F-2-8 (NR60-D), E-F-3-8
(1 °CT), E-F-4-8 (CALCT) and E-F-S-8 (NRB166), compared to E-F-1-8 (lab
control). Results regarding the internal bond and the torsion shear are lower
than the control. MOE and MOR values (dry and wet) are similar to the
control. For thickness swell and water absorption, except E-F-4-8 (CALCT)
that gives better values, results were also lower than for the control.
Conclusion
Of all the resins E-F-4-8 (CALCT oil) gave the best results. The other three
had lower results in almost every test.
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
ao
W
W
~o
C
00
W
. 0 0 0
0o y '...o o vo ~o ~c
M
GZIO N ~D
w
O M V1 ~O v0 00
N "~ M et ~-! ~O ~1
~ U ~ ~ ~ rr
y
w w x ..
00
w
c~ H
'~ :~ ~ o
~. ~ x o a o 0 0
a~ u. c, ..,
o. o
~
a. Q U
a~
a
~ w Y ~ ~
t by
-.
c~
"", - .
u
~"
~ O O M !1'
V
N
N
p 'r ~' ~t et ~t
v U
~
b
c ~,
e~s
V N~ ~o ~ av ooo
... ,
A,
00 0 0oG1 00 0o Ero0
.~.N ~ M 'j'.-~V'
E" C~1
fi.~ ti. ti. u, ci.
U
LzlU tilZ Li7 fitU ti7
.... Z
~n o ~n
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
-48-
c ~.
o g~
~ O N M n O
~ v
00 I l~ ~' C
M M M M M
3aN
N O~ O ~O CO
01 O~ ~-~ 00 O
N.C "' ~' N ""' N
E'" v? N
00
N
0
M ~ N N
a~
E~ ~ ~.
00
- o
W p O
~Lj ~ O O O
pp ..~ .r
i
00 tt ~n ~n ~D
W --~ N M t~ O
~ h ue' ~ v'~1
A"
00 ~ d
N
W
...
W ~ I~ ~. V1 ~ M
M ~' ~1
00 .~. .-~ .-
t OG i.
v
~
W v ~ Cv o0 N ~1 et
C ~ N N N N N
.r
H
N
y 0 Ov N N O
vp
y r
.r -
G~ H v .r, O O O O O
O ~ >, ~ ~"~ M M M M
i
. U ..
c
co
c~ ai
v ~r v
c
~.
' a ~
~N
OO 00 00 00 OO
~ A E"
N ~ N M ~
~ ~ E~' u ~
w
b u, u; U . r
~ Q .
~
U ri1 W w ~ w
U z ~ U z
N
~n o ~n
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 4~
The present invention has been described with regard to preferred
embodiments. However, it will be obvious to persons skilled in the art that a
number of variations and modifications can be made without departing from the
scope of the invention as described herein.
SUBSTITUTE SHEET ( rule 26 )
CA 02319416 2000-07-28
WO 99/38935 PCT/CA99/00051
- 50~
References:
Chum et al., 1989, ACS Symposium Series No. 385, Adhesives from
Renewable Resm_,r~PC, Hemingway R.W. Conner A.H. eds, American
Chemical Society, pp. 135-151.
Forss K.G., Fuhrmami, A. 1979 Finnish plywood, particle board, and
fibreboard made with a lignin-based adhesive. Forest Prod. J, voI 29, pp. 39-
43.
Himmelblau D. A., Grozdits G.A. 1997, Production of wood composite
adhesives with air-blown, fluidized-bed pyrolysis oil.
Kelley et al., 1997, Use of Biomass pyrolysis oils for preparation of modified
phenol formaldehyde resins, Vol 1 pp. 557-172
Pakdel, H., Amen-Chen, C., Zhang, J., Roy, C. 1996, Phenolic compounds
from vacuum pyrolysis of biomass, pp. 124-131, CPL press
Scott 1988, Chemicals and fuels from biomass flash pyrolysis - part of the
bioenergy development program, Renewable Energy ~ranch, Energy Mines and
Resources Canada, Ottawa, Canada, DSS Contrac: File No. 38ST 23216-6-
65164;
Sellers 1996; Adhesives Age vol 39: pp. 6-9
White 1995; Forest Prod J. vol 45, pp.21-28
SUBSTITUTE SHEET ( rule 26 )