Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02323721 2000-10-18
199-0258
HYDROGEN SEPARATOR
Background of the Invention
Field of the Invention
The present invention is directed to a hydrogen
separator and its method of manufacture. More
specifically, the invention is directed to a hydrogen
separator made from a thin foil secured to a support
structure having an undulating surface.
Descriytion of Related Art
Hydrogen separators are used in a number of
commercial applications, including the refining of
hydrocarbons, chemical processing, manufacture of
hydrogenated oils as well as fuel cells. The separators
work to refine a stream of relatively impure mixed gas
containing less than 100 hydrogen (typically in the
range of 20-80~) into very pure hydrogen (99.9990 . Most
common hydrogen separators use a thick palladium,
palladium alloy, or composite of a group Vb metal coated
with palladium or a palladium alloy. These common foils
usually have a thickness of greater than 25 microns. The
foil is produced by rolling or pressing ingots into
sequentially thinner sheets. The practical limit of the
rolling process is currently 25 microns. This is
considered thick foil. The thick foil is supported by a
method that allows the feed side pressure to be higher
than the permeate pressure since the 25 micron foil is
not capable of withstanding high pressure alone. One
example of this construction is illustrated in US patent
No. 5645626.
CA 02323721 2000-10-18
In systems that use a thick palladium or
palladium alloy, the performance is such that cost and
size are major barriers to automotive/commercial
separator design. For coated group Vb metals cost,
S performance and size are acceptable, however the coated
Group Vb metal substrates form hydrides that cause
embrittlement resulting in unacceptable cycle life. An
example of this construction is illustrated in US Patent
No. 5738708. Furthermore, the coatings (5000 Angstroms
palladium) will interdiffuse during even limited, <100
hours, operation at above 400° C causing failure of the
catalytic dissociation of HZ into H at the surface.
The hydrogen disassociates on the foil surface and forms
a metal hydride with the foil. The proton and electron
IS from the hydrogen atom migrate through the foil and
recombine on the opposite surface to form hydrogen gas.
This method is illustrated in U.S. Patent No. 5645626.
The foil generally expands up to 20~ when
exposed to hydrogen while the underlying support material
remains constant. Consequently, the foil must be made
relatively thick to provide the durability needed for
cyclic exposure to hydrogen gas. Unfortunately, the
ability to pass hydrogen through a foil is directly
proportional to the thickness of the foil while the cost
is exponentially proportional in the case of palladium
based foils. Increasing the foil thickness significantly
reduces hydrogen permeability; also known as flux
capacity. Increasing foil thickness also increases the
cost of the separator. Increasing the temperature or
pressure of the gas increases the flux capacity; however,
the increased temperatures and pressures will damage
thinner foils (<15 microns).
-2-
CA 02323721 2000-10-18
Another type of hydrogen separator uses very
thin layers of palladium between 0.1 and 0.5 microns
thick. Because these very thin layers cannot be made
self-supporting, they are plated onto a carrier. The
carrier, generally vanadium, niobium, or tantalum,
enables the disassociated hydrogen atom to pass through
the separator. Another coating containing palladium, on
the opposite surface of the separator, recombines the
disassociated hydrogen atoms into gaseous hydrogen. An
example of this construction is illustrated in U.S.
Patent Nos. 5738708 and 5149420.
This construction has the dual advantages of
providing a large flux capacity because the palladium
foil is very thin, and also relatively low cost because
very little palladium is used in the coating material.
Unfortunately, the current base metallic (e. g. vanadium)
carriers are susceptible to hydrogen embrittling. After
several cycles, the vanadium~intermediate layer suffers
internal fatigue and fractures which cause the separator
to fail when cycled to operating conditions. Increased
temperatures and gas pressures further exacerbate the
embrittling process and reduce the life of the separator.
Another separator construction uses a thin
coating of palladium on a ceramic substrate. The ceramic
substrate is made to be porous to hydrogen and to receive
the palladium coating. Because the coating is relatively
thin, it has a high flux capacity and relatively low
cost. Unfortunately, palladium coated ceramic substrates
suffer from the same durability problems as the vanadium
substrates. The ceramic substrate and palladium foil
have vastly different coefficients of thermal expansion.
-3-
CA 02323721 2000-10-18
Also, the ceramic cannot be made to have a uniform
porosity throughout the surface of the substrate. Those
areas having relatively larger porosity create a void
bridged by the palladium coating/foil. The ceramic
expands up to 50 percent more than the palladium foil.
This often causes the foil to crack or tear in areas of
coarser porosity. These small microtears in the
palladium foil reduce the separator's ability to filter
impurities from the source hydrogen stream.
In summary, the prior art systems for hydrogen
separation are too costly and large, or not reliable due
to cracking of the coated layers and/or substrates
to be viable for automotive/commercial separation of
large volumes of hydrogen.
The present invention attempts to provide a
separator that has the high flux capacity and low cost of
the thin foil devices together with the high temperature
and high pressure durability of thick, non-embrittling
foils. These and other disadvantages of the related art
are overcome by the construction described herein.
Summary of the Invention
The present invention is directed to a hydrogen
separator that includes a thin hydrogen permeable foil
having a thickness between 3 and 15 microns. The foil is
bonded to a support structure. The support structure is
formed to have an undulating surface. The surface
includes contact areas on which are secured the foil.
The foil generally conforms to the undulating surface.
The foil is only secured to the contact areas of the
support structure; this enables the foil to move in three
-4-
CA 02323721 2000-10-18
directions as it expands and contracts when exposed to
the hydrogen source stream. The contact areas are spaced
relatively closely in comparison to the foil thickness
and enable the thin foil to withstand high pressures.
The foil, once integrated with the support,
becomes thick enough to then reliable secure it to
mounting surfaces used in keeping separate the high and
low pressure sides. If a thin foil were just rested on a
support it would usually tear at this securing point if
not fully integrated with the support.
The foil generally takes on a convoluted,
repeatable shape, matching the undulating surface of the
separator. Common separators include mesh or wire
screens. The convoluted surface of the foil is generally
between 20 and 50~ larger than the plan view area of the
foil. Screens having a mesh between 200 and 635 squares
per inch were found to be generally suitable for use as
hydrogen separators.
The separators are manufactured by rolling a
thin palladium containing foil between 3 and 15 microns.
The foil is then secured to the support structure. A
rolling or pressing process is found suitable to
mechanically fasten the foil to the support structure
along the contact areas. The foil generally conforms to
the undulating surface of the screen. The screen imparts
convolutions onto the foil and increases its surface area
by 20~ to 50~. The foil remains secured to the screen
without tearing or folding.
-5-
CA 02323721 2000-10-18
The use and other desired objects of the
present invention will become more apparent in the course
of the following detailed description and the appended
claims. The invention may best be understood with
reference to the accompanying drawings, wherein
illustrative embodiments are shown.
Brief Description of the Drawings
Figure 1 is a cross-sectional view of a
separator assembly being joined in a press.
Figure 2 is a cross-sectional view of a
separator using a standard mesh screen.
Figure 3 is a cross-sectional view of a
separator assembly being joined by a pair of rollers.
Figure 4a are various views of a standard
screen.
Figure 4b are various views of a "dutch weave"
screen.
Figure 5 is a graph of thermal expansion for
palladium foil plotted against hydrogen flow.
Detailed Descriytion and Best Mode
The invention will be illustrated and described
as a hydrogen separator for use with a fuel cell. The
invention is useful for any situation where a mixed gas
stream containing hydrogen is separated into a relatively
pure hydrogen stream. These and other devices and
methods of operation are included within. the invention
described herein. The following items are a word list of
the components described in the drawings and are
reproduced to aid in understanding the invention:
- 6-
CA 02323721 2000-10-18
word list
10,10' press
12,14 press surfaces
13,15 rollers
16 foil
18 support structure
20 contact areas
22 separator
24 foil surface
26 mesh openings
28 deformation amplitude
30 aluminum cloth
The invention is designed to provide a low cost
hydrogen separator that can withstand the rigors of
automotive applications. The separator has an operating
temperature range from between -40 to 600°C and is
capable of withstanding pressures up to 25 bar. The
invention utilizes a lower cost, thin palladium or
palladium alloy foil as a separator material. The thin
foil has the dual advantages of increasing the capacity
of the separator while reducing the material cost. The
foil and its method of manufacture are described in a
commonly assigned patent application titled "METHOD OF
MANUFACTURING THIN METAL ALLOY FOILS", filed on even date
herewith and incorporated herein by reference. The foil
is imparted with a convoluted shape to increase the
surface area and to provide a unique structure that is
capable of expanding and contracting without tearing. A
support structure made of non-embrittling material
imparts the convoluted shape to the foil and serves to
support the foil during operation.
Illustrated in Figure 1 is a press 10 that is
used to fasten the foil and support structure together.
The press 10 may be either hydraulically, pneumatically,
or mechanically driven.
_7_
CA 02323721 2000-10-18
The press 10 includes two press surfaces 12, 14
that join the foil and substrate. A palladium foil 16 is
placed between the press surfaces 12, 14. The foil is
composed of palladium, palladium alloys that have been
shown to not embrittle, or palladium coated non-
embrittling metals such as a body centered cubic alloy.
The foil has a thickness of 3-15 microns, with the
preferred thickness in the range of 5-7 microns.
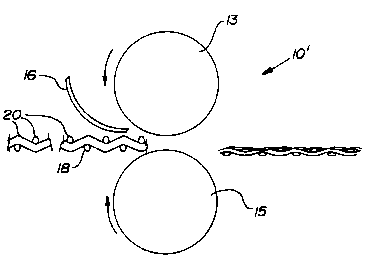
Illustrated in Figure 2 is a roll press 10'. Rollers 13,
15 press the foil onto the support structure 18.
A support structure 18 is placed juxtaposed to
the foil 16. The support structure has an undulating
surface to provide a variety of contact areas 20 for
attachment to the foil 16. The undulating surface of the
foil forms a mechanical lock with the support. The
contact areas 20 have a space therebetween so that the
foil surface contacting adjacent contact areas 20 forms
convolutions. A readily available material that has the
needed undulating surface is a wire or mesh screen (wire
cloth) having a mesh opening between 2 and 20 microns
before compaction and 1 to 12 microns after compaction.
The screen may be fabricated from a material that is
insensitive to hydrogen exposure such as stainless steel,
hasteloy, monel, nickle, or other suitable material.
Stainless steel was found to be particularly well suited
for use as a support structure because it was non-
embrittling, readily available, high strength, and low
cost. The support structure 18 can be coated with a
3o material to further isolate metallic interdiffusion
between the screen and the foil such as alumina or
titanium.
_g_
CA 02323721 2000-10-18
The foil 16 and the support structure 18 are
placed between the press surfaces 12, 14 or rollers 13,
15 and the press 10 is moved to the closed position or
the rollers 13, 15 are indexed. The press 10, 10'
applies between 20 and 70 tons of force per square inch
for a period of 1-5 seconds. The foil 16 is mechanically
fastened to the support structure 18. When the pressure
exceeds 60,000 psi, the palladium foil becomes coined and
forms a mechanical bond to the support structure by
interlocking with the screen gaps as they close during
the deformation process.
The foil 16 and support structure 18 form a
separator 22, as illustrated in Figure 3. The separator
22 includes the foil 16 and the support structure 18.
The foil 16 and the support structure 18 are mechanically
fastened at the contact areas 20 by the foregoing
pressing process. The pressing process also causes a
deformation in the foil surface 24. The foil surface 24
is pushed into the mesh openings 26. The deformation
amplitude 28 creates a series of convolutions on the foil
surface 24. The foil surface 24 increases between 20 and
50~ as compared to the plan view area of the foil before
the joining operation. The foil 16 is supported over the
contact areas 20 to span the mesh openings. This span
distance is approximately equal to the screen mesh size.
The screen mesh size is selected to support the foil 16
for a given operating pressure, temperature and foil
thickness. Thinner foils and higher operating pressures
or temperatures generally require a smaller mesh sizes.
The separator 22 is placed within a passage or
chamber that receives a source gas stream that contains
-9-
CA 02323721 2000-10-18
impure hydrogen. The source gas contacts the foil
surface 24. Hydrogen within the source gas disassociates
on the foil surface 24 and forms atomic hydrogen. The
atomic hydrogen forms a metallic bond with the host ~Pd or
Pd Alloy and passes through the foil 16 and reassociates
on the opposite foil surface to form gaseous hydrogen.
The gaseous hydrogen passes freely through an underlying
support structure 18. Separators of this construction
are capable of purifying a source stream containing 15-
99~ hydrogen to greater than 99.999 pure hydrogen.
The source stream is generally heated to
between 200 and 600°C, preferably 450°C to facilitate
catalysis and hydration of the hydrogen into the foil 16.
Both the hydration and elevated temperature cause the
foil 16 to expand and elongate between 10 and 30~. This
expansion and elongation causes the deformation amplitude
28 to increase. The expansion and elongation of the foil
16 is accommodated within the mesh opening 26. The
contact areas 20 remain relatively constant. The foil 16
is free to expand and contract without tearing or
folding.
A wide variety of screens having different mesh
openings and mesh configurations exist. An example of
this construction is illustrated in Figure 4a and b. The
screen in Figure 4b uses a "dutch weave" construction
where vertical threads interlock pairs of horizontal
thread. This provides a coarser mesh having a
rectangular opening.
Illustrated in Figure 5 is a graph of the
hydrogen flux capacity vs. the palladium foil thickness.
- 10-
CA 02323721 2000-10-18
Foils made by the present invention have a hydrogen flux
capacity more than five times that of prior art thick
foils.
While particular embodiments of the invention
have been illustrated and described, it will be clear to
those skilled in the art that various changes and
modifications may be made thereto without departing from
the scope of the invention and it is intended to cover in
l0 the appended claims all such modifications and
equivalents as fall within the true spirit and scope of
the invention.
-11-