Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02324630 2000-10-26
r
Complex salts for use in electrochemical cells
The invention relates to a process for preparing
complex salts and to their use in electrochemical
cells.
Lithium ion batteries are among the most
promising systems for mobile applications. The fields
of application extend from high-quality electronic
equipment (e.g. mobile telephones, camcorders) to
batteries for electrically driven vehicles.
Rechargeable lithium batteries have been commercially
available since the early 1990s.
These batteries consist of cathode, anode,
separator and a nonaqueous electrolyte. As cathode, use
is typically made of Li (MnMeZ) 204, Li (COMeZ) 02,
Li(CoNi.{Me~)OZ or other lithium intercalation and
insertion compounds. Anodes can consist of lithium
metal, carbon, graphite, graphitic carbons or other
lithium intercalation and insertion compounds or alloy
compounds. Electrolytes used are solutions of lithium
salts such as LiPF6, LiBFq, LiC104, LiAsF6, LiCF3S03,
LiN (CF3S0~) 2 or LiC (CF3S02) 3 and mixtures thereof in
aprotic solvents.
At present, the LiPF6 used as electrolyte salt
in many lithium ion batteries represents a very
hydrolysis-sensitive and thermally unstable substance.
Contact with atmospheric moisture and/or residual water
in the solvents immediately forms hydrofluoric acid HF.
Apart from its toxic properties, HF has an adverse
effect on the cycling behaviour and thus on the
performance of the lithium battery, since metals can be
leached from the electrodes.
US 4505997 describes lithium imides and
US 5273840 describes lithium methanides. Both salts
have a high anodic stability and in organic carbonates
form solutions having a high conductivity. Aluminium,
the caLhodic terminal lead in lithium ion batteries, is
not passivated to a sufficient extent, at least by
lithium imide. Lithium methanide, on the other hand, is
CA 02324630 2000-10-26
- 2 -
very costly to prepare and purify. In addition, the
electrochemical properties, e.g. oxidation stability
and passivation, of aluminium are very dependent on the
purity of the methanide.
WO 98 / 07729 therefore describes a new class of
electrolyte salts, namely lithium borate complexes. In
cycling experiments, these compounds have given
particularly good results and have proven to be
particularly stable. In combination with other salts,
these complexes display a synergistic stabilizing
effect against oxidation.
The lithium bis[5-fluoro-2-olatobenzene-
sulfonato(2-)0,0']borate(1-) described as electrolyte
salt is, owing tc its properties, a promising
electrolyte salt for use in lithium ion batteries.
However, the costly and complicated synthesis of the
precursors is problematical.
It is therefore an object of the present
invention to provide materials which passivate the
cathodic terminal leads and are stable to oxidation
processes, and to provide a simple process for
preparing these materials.
The object of the invention is achieved by
complex salts of the formula
M'+[EZ]~,f (I)
where:
x, y are 1, 2, 3, 4, 5, 6,
Mx+ is a metal ion,
E is a Lewis acid selected from the group consisting
of
BR1RZR3, AIR1RZR3, PR1RZR3R4R5, As R1 R2 R3RqR5, VR1RZR3RqR5,
R1 to R' are identical or different, may be joined
directly to one another by a single or double bond and
can each be, either individually or together,
CA 02324630 2000-10-26
a halogen (F, C1, Br),
an alkyl or alkoxy radical (C1 to Ce) which may be
partly or fully substituted by F, C1, Br,
an aromatic ring, which may be bound via oxygen,
selected from the group consisting of phenyl, naphthyl,
anthracenyl and phenanthrenyl, which may be
unsubstituted or monosubstituted or multiplesubstituted
by alkyl (C1 to C8) or F, C1, Br, or
an aromatic heterocyclic ring, which may be bound via
oxygen, selected from the group consisting of pyridyl,
pyrazyl and pyrimidyl, which may be unsubstituted or
monosubstituted to tetrasubstituted by alkyl (C1 to C8)
or F, C1, Br, and
Z is OR6, NR6R', CR6R'R8, OSOZR6, N ( SOZR6) ( SOZR' ) ,
2 0 C ( SOzR6 ) ( SOzR' ) ( SO2R8 ) , OCOR°, where
R6 to Rg are identical or different, may be joined
directly to one another by a single or double bond and
are each, either individually or together,
a hydrogen atom or as defined for R1 ~o RS.
These complex salts are particularly suitable
as electrolyte salts in electrolytes for electro-
chemical cells.
It has surprisingly been found that the salts
of the invention can passivate transition metal
cathodes and terminal leads. The frequently used
aluminium terminal leads, in particular, can be
protected by passivation against the pit corrosion
which occurs in conventional systems.
It has been found that the complex salts of the
formula (I) have good electrochemical properties. Thus,
for example, good oxidation stability was observed.
CA 02324630 2000-10-26
_ 4 _
It has surprisingly been found that the salts
of the invention have greatly improved ion conductivity
compared with conventional electrolyte salts.
The complex salts of the formula (I) and their
mixtures can be used as electrolyte salts in
electrolytes for electrochemical cells. Likewise, they
can be used in proportions of from 1 to 99o in
combination with other electrolyte salts.
Particularly suitable complex salts of the
formula (I) are those in which M"+ - Li+ or tetraalkyl
ammonium and E - BRlaR2bF,, and PRldR2eR3 fR4gFh where a to h
- 0, l, 2, 3, 4 or 5, where a+b+c=3 and d+e+f+g+h=5.
The complex salts of the invention can be used
in customary electrolytes. Suitable electrolytes are,
for example, those containing electrolyte salts selec
ted from the group consisting of LiPF6, LiBF4, LiC104,
LiAs Fn, LiCF3S03, LiN ( CF3S02 ) 2 or LiC (CF3S02 ) 3 and mix-
tures thereof.
The electrolytes can further comprise organic
isocyanates (DE 199 44 603) to reduce the water
content. Likewise, organic alkali metal salts (DE
199 10 968) may be present as additives in the electro
lytes. Suitable alkali metal salts are alkali metal
borates of the formula
Li+B- ( OR1 ) m ( ORZ ) p
where
m and p are 0, 1, 2, 3 or 4 with m+p=4 and
R1 and Rz are identical or different,
may be joined directly to one another by a single or
double bond,
are each, either individually or together, an aromatic
or aliphatic carboxylic, dicarboxylic or sulfonic acid
radical, or
CA 02324630 2000-10-26
' _ 5 _
are each, either individually or together, an aromatic
ring selected from the group consisting of phenyl,
naphthyl, anthracenyl and phenanthrenyl, which may be
unsubstituted or monosubstituted to tetrasubstituted by
A or Hal, or
are each, either individually or together, a hetero-
cyclic aromatic ring selected from the group consisting
of pyridyl, pyrazyl and bipyridyl, which may be
unsubstituted or monosubstituted to trisubstituted by A
or Hal, or
are each, either individually or together, an aromatic
hydroxy acid selected from the group consisting of
aromatic hydroxycarboxylic acids and aromatic hydroxy-
sulfonic acids, which may be unsubstituted or mono-
substituted to tetrasubstituted by A or Hal,
and
Hal can be F, Cl or Br
and
A can be alkyl having from 1 to 6 carbon atoms,
which may be monohalogenated to trihalogenated.
Likewise suitable are alkali metal alkoxides
(DE 9910968) of the formula
Li+ OR-
where R
is an aromatic or aliphatic carboxylic, dicarboxylic or
sulfonic acid radical, or
is an aromatic ring selected from the group consisting
of phenyl, naphthyl, anthracenyl and phenanthrenyl,
which may be unsubstituted or monosubstituted to tetra-
substituted by A or Hal, or
CA 02324630 2000-10-26
- ' _
is a heterocyclic aromatic ring selected from the group
consisting of pyridyl, pyrazyl and bipyridyl, which may
be unsubstituted or monosubstituted to trisubstituted
by A or Hal, or
is an aromatic hydroxy acid selected from the group
consisting of aromatic hydroxycarboxylic acids and
aromatic hydroxysulfonic acids, which may be
unsubstituted or monosubstituted to tetrasubstituted by
A or Hal,
and
Hal is F, C1 or Br,
and
A is alkyl having from 1 to 6 carbon atoms, which may
be monohalogenated to trihalogenated.
Other constituents which may be present are
compounds of the formula
~ ( ~R1 (CRZR3) k~ 1Ax) yKt~ + -NCCF3) 2
where
Kt is N, P, As, Sb, S, Se
A is N, P, P (0) , 0, S, S (0) , SO2, As, As (0) ,
Sb, Sb (0)
R', RZ and R3
are identical or different and are each
H, halogen, substituted and/or unsubstituted alkyl
CrH2n+1, substituted and/or unsubstituted alkenyl having
1-18 carbon atoms and one or more double bonds,
CA 02324630 2000-10-26
-
substituted and/or unsubstituted alkynyl having 1-18
carbon atoms and one or more triple bonds, substituted
and/or unsubstituted cycloalkyl CmH2~,_1, monosubstituted
or polysubstituted and/or unsubstituted phenyl, substi-
tuted and/or unsubstituted heteroaryl,
A can be included in various positions in R1, RZ
and/or R3,
Kt can be included in cyclic or heterocyclic
rings,
the groups bound to Kt can be identical or
different,
where
n is 1-18,
m is 3-7,
k is 0, 1-6,
1 is 1 or 2 when x=1 and 1 when x=0,
x is 0, 1,
y is 1-4
(DE 9941566). The process for preparing the compounds
is characterized in that an alkali metal salt of the
formula
D+ N (CF3) z.
where D+ is selected from the group consisting of
the alkali metals, is reacted in a polar organic
solvent with a salt of the formula
3 5 L ( L R1 ( CRzR3 ) x ] iAx ) yKt ] + -E
where
CA 02324630 2000-10-26
_ 8 _
- Kt, A, R1, RZ, R3, k, 1, x and y are as defined
above and
-E is F-, C1-, Br-, I , BF4 , C104-, As F6-, SbF6- or
PF6 .
The compounds of the invention may also be
present in electrolytes comprising compounds of the
formula
X- ( CYZ ) m-SO2N ( CR1RZR3 ) 2
where
X is H, F, C1, CnF~n+1. CnF2n-i. (SOz) xN (CR1RZR3) z,
Y is H, F, C1,
Z is H, F, C1,
R1, R2, R3 are H and/or alkyl, fluoroalkyl, cycloalkyl,
m is 0-9 and when X=H, m~0,
n is 1-9 and
k is 0 when m=0 and k=1 when m=1-9,
prepared by reacting partially fluorinated or perfluor-
inated alkylsulfonyl fluorides with dimethylamine in
organic solvents (DE 199 466 73).
Further constituents which may be present are
lithium complex salts of the formula
Rs
RS O~ S /0
Li ~ ~~OR ~
R° / O~B 2
OR
R3
where
R' and R? are identical or different, may be bound
directly to one another by a single or double bond and
are each, either individually or together, an aromatic
ring selected from the group consisting of phenyl,
CA 02324630 2000-10-26
- g -
. naphthyl, anthracenyl and phenanthryl, which may be
unsubstituted or monosubstituted to hexasubstituted by
alkyl (C1 to C6) , alkoxy groups (C1 to C6) or halogen
(F, C1, Br),
or are each, either individually or together, an aroma-
tic heterocyclic ring selected from the group consist-
ing of pyridyl, pyrazyl or pyrimidyl, which may be
unsubstituted or monosubstituted to tetrasubstituted by
alkyl (C1 to Cn) , alkoxy groups (C1 to C6) or halogen
(F, Cl, Br),
or are each, either individually or together, an
aromatic ring selected from the group consisting of
hydroxybenzenecarboxyl, hydroxynaphthalenecarboxyl,
hydroxybenzenesulfonyl and hydroxynaphthalenesulfonyl,
which may be unsubstituted or monosubstituted to
tetrasubstituted by alkyl (C1 to C6), alkoxy groups (C1
to C6) or halogen (F, Cl, Br),
R3-R° may each, either individually or in pairs,
possibly joined directly to one another by a single or
double bond, have the following meanings:
1. alkyl (C1 to C6) , alkyloxy (C: to C6) or halogen (F,
Cl, Br)
2. an aromatic ring selected from the groups consisting
of
phenyl, naphthyl, anthracenyl and phenanthrenyl, which
may be unsubstituted or monosubstituted to hexa-
substituted by alkyl (C1 to C6), alkoxy groups (C1 to
C6) or halogen (F, Cl, Br),
pyridyl, pyrazyl and primidyl, which may be
unsubstituted or monosubstituted to tetrasubstituted by
alkyl (C,_ to C5), alkoxy groups (C1 to C~) or halogen
(F, C1, Br) ,
CA 02324630 2000-10-26
- 10 -
which are prepared by the following method
(DE 199 32 317):
a) 3-, 4-, 5-, 6-substituted phenol is admixed in a
suitable solvent with chlorosulfonic acid,
b) the intermediate from a) is reacted with chloro-
trimethylsilane, filtered and fractionally distilled,
c) the intermediate from b) is reacted in a suitable
solvent with lithium tetramethoxyborate (1-) and the
end product is isolated therefrom, may also be present
in the electrolyte.
Borate salts (DE 199 59 722) of the formula
R4 R~ Y_
MX+ ~ B
R3 RZ "~Y
where:
M is a metal ion or tetraalkylammonium ion,
x, y are 1, 2, 3, 4, 5 or 6 and
R1 to R4 are identical or different and are alkoxy or
carboxy radicals (C1-Ce) which may be bound directly to
one another by a single or double bond, may also be
present. These borate salts are prepared by reacting
lithium tetraalkoxyborate or a 1:1 mixture of lithium
alkoxide and a boric ester in an aprotic solvent with a
suitable hydroxyl or carboxyl compound in a ratio of
2:1 or 4:1.
Additives such as silane compounds of the
formula
CA 02324630 2000-10-26
' - 11 -
SiR1R2R3R4
where R1 to R4 are H
CyFzy+i-ZHz
OCyFzy+1-ZHZ
OC ( 0 ) CyFzy+i-ZHz
OSOZCyFzy+i-ZHZ
and 1 <_ x < 6
1 <_ y <_ 8 and
0 <_ z _< 2y + 1
and
R1-R~ are identical or different and
are each an aromatic ring selected from the group
consisting of phenyl and naphthyl, which may be unsub-
stituted or monosubstituted or polysubstituted by F,
CyFzy+1-ZHz or OCYFzy+1_,HZ, OC (O) CyFzy+u-ZHz. OSOzCyFzy+i-zHz,
N ( CnF2n+1-zHz ) ?. Or
are each a heterocyclic aromatic ring selected from the
group consisting of pyridyl, pyrazyl and pyrimidyl,
which may each be monosubstituted or polysubstituted by
F, CyFzy+1-~,HZ or OCyFz:,+1-zHz, OC (0) CyFzy+1-,H:. _OSOzCyFzy+,-ZH-,,
N (CnFzn+i-zH~) z (DE 100 276 26) , may also be present.
The compounds of the invention can also be used
in electrolytes comprising lithium fluoroalkylphos-
phates of the following formula,
Li+[PF;~ (CyFzy+i-ZHz) s-X~
where
1 < x < 5,
3 < Y < 8.
0 _< z <_ 2y + 1,
and the ligands (CyFzy+i-ZHZ) may be identical or differ-
ent, with the exception of the compounds of the
formula,
CA 02324630 2000-10-26
- 12 -
Li+[PFa(CHbFo(CF3)d)e]
in which a is an integer from 2 to 5, b = 0 or l, c = 0
or 1, d = 2 and
a is an integer from 1 to 4, with the provisos that b
and c are not simultaneously 0 and the sum of a + a is
6 and the ligands (CHbF~ (CF3) d) may be identical or
different (DE 100 089 55). The process for preparing
lithium fluoroalkylphosphates is characterized in that
at least one compound of the formula
HmP ( CnH2n+1 ) 3-m ( I
I I
) ,
OP (CnH2n+1) 3 ( IV)
,
C 1mP ( CnH2n+1 ) 3- m (
V )
r
FmP ( CnHzn+1 ) 3-m ( VI
) ,
Clop ( CnH2n+1 ) s-o ( VI
I )
,
FoP ( CnH2n+1 ) s-o ( VI
I I
) ,
in each of which
0 < m < 2, 3 < n < 8 and 0 < o < 4,
is fluorinated by electrolysis in hydrogen fluoride,
the resulting mixture of fluorination products is
fractionated by extraction, phase separation and/or
distillation and the fluorinated alkylphosphorane
obtained in this way is reacted in an aprotic solvent
or solvent mixture with lithium fluoride in the absence
of moisture, and the resulting salt is purified and
isolated by customary methods.
The compounds of the invention can also be used
in electrolytes comprising salts of the formula
Li ( P ( OR1 ) a ( CRZ ) b ( CR3 ) c ( CRS ) dFe ~
where 0 < a+b+c+d <_ 5 and a+b+c+d+e=6, and R1 to R4
are, independently of one another, alkyl, aryl or
CA 02324630 2000-10-26
- 13 -
heteroaryl radicals, where at least two of R1 to R4 may
be joined directly to one another by a single or double
bond (DE 100 16801). The compounds are prepared by
reacting phosphorus(V) compounds of the formula
P ( OR1 ) a ( ORZ ) b ( OR3 ) c ( OR4 ) dfe
where 0 < a+b+c+d _< 5 and a+b+c+d+e=5, and R1 to R4 are
as defined above, with lithium fluoride in the presence
of an organic solvent.
It is also possible for ionic liquids of the
formula
K+A-
where
K+ is a cation selected from the group
consisting of
R1 R1
\ R2 R6 \ R2
+ I
~5 N~R3 %N
R5 N
R4 R4
R1
R6 ~ R6 N\ R2
+~ ~ +
RS N R3 R5 N/ R3
I l
R4 R4
R5 R1 R5 ~R1
/+v~
R4'N N~R2 R4 g' _R2
R3
RS R1 R1
+v ~+~
R4 p ~ R2 R4 N RZ
I
R3
CA 02324630 2000-10-26
- 14 -
where R1 to RS are identical or different, may be joined
directly to one another by a single or double bond and
are each, either individually or together:
- H,
- halogen,
- an alkyl radical (C1 to CB) which may be partially or
fully substituted by further groups, preferably F, C1,
N ( CnF ( 2n+1-x ) Hx ) 2 r ~ ( CnF ( 2n+1-x ) Hx ) r S02 ( CnF ( 2n+1-x ) Hx
) r CnF-
(2n+i-x) Hx where 1<n<6 and 0<x<_13,
A- is an anion selected from the group consisting of
~B(0R1)n(OR2)m(0R3)o(~R4)p~
where 0<_n, m, o, p__<4 and
m+n+o+p=4
where R1 to R~ are different or identical in pairs, may
be joined directly to one another by a single or double
bond and are each, either individually or together,
an aromatic ring selected from the group consisting of
phenyl, naphthyl, anthracenyl or phenanthrenyl, which
may be unsubstituted or monosubstituted or
polysubstituted by CnF,2n+i-x~Hx where 1<n<6 and 0<x<_13 or
halogen (F, C1, Br),
an aromatic heterocyclic ring selected from the group
consisting of pyridyl, pyrazyl or pyrimidyl, which may
be unsubstituted or monosubstituted or polysubstituted
by CnF~zn+~-x~Hx where 1<n<6 and 0<x<_13 or halogen (F, Cl,
Br) , or
CA 02324630 2000-10-26
- 15 -
an alkyl radical (C1 to CB) which may be partially or
fully substituted by further groups, preferably F, Cl,
N (CnF(zn+1-x)Hx) 2r ~ (CnF(2n+1-xlHx) r S02 (CnF(2n+1-x)Hx) r
CnF-(zn+i-x)Hx where 1<n<6 and 0<x513,
or OR1 to ORq are each, either individually or together,
an aromatic or aliphatic carboxyl, dicarboxyl, oxy-
sulfonyl or oxycarboxyl radical which may be partially
or fully substituted by further groups, preferably F,
C1, N (Cnf(Zn+1-xJHx) 2r ~ (CnF(2n+1-x)Hx) r S~2 (CnF(2n+1-x)Hx) r
CaF-(zn+i-x)Hx where 1<n<6 and 0<x<_13 (DE 100 265 65) , to
be present in the electrolyte. Ionic liquids K+A- where
K+ is as defined above and
A- is an anion selected from the group consisting of
LP FK ( C, F~ f+'.-. H . ) 6-:~
where 1 <_ x < 6,
1 <_ y <_ 8 and
0 <_ z _< 2y + 1,
can also be present (DE 100 279 95).
The compounds of the invention can be used in
electrolytes for electrochemical cells which comprise
anode material consisting of coated metal nuclei
selected from the group consisting of Sb, Bi, Cd, In,
Pb, Ga and tin or their alloys (DE 100 16 024). The
process for preparing this anode material is
characterized in that
a) a suspension or a sol of the metal or alloy nucleus
in urotropin is prepared,
b) the suspension is emulsified with C5-Clz-hydro-
carbons,
c) the emulsion is precipitated onto the metal or alloy
nuclei and
CA 02324630 2000-10-26
- 16 -
d) the metal hydroxides or oxyhydroxides are converted
into the corresponding oxide by heat treatment of the
system.
The compounds of the inventian can also be used
in electrolytes for electrochemical cells having
cathodes of customary lithium intercalation and
insertion compounds or else comprising cathode
materials consisting of lithium mixed oxide particles
which are coated with one or more metal oxides
(DE 199 22 522) by suspending the particles in an
organic solvent, admixing the suspension with a
solution of a hydrolysable metal compound and a
hydrolysis solution and then filtering off, drying and,
if appropriate, calcining the coated particles. They
can also consist of lithium mixed oxide particles which
are coated with one or more polymers (DE 199 46 066),
obtained by a process in which the particles are
suspended in a solvent and the coated particles are
subsequently filtered off, dried and, if appropriate,
calcined. Likewise, the compounds of the invention can
be used in systems having cathodes consisting of
lithium mixed oxide particles which are coated with one
or more layers of alkali metal compounds and metal
oxides (DE 100 14 884). The process for preparing these
materials is characterized in that the particles are
suspended in an organic solvent, an alkali metal salt
compound suspended in an organic solvent is added,
metal oxides dissolved in an organic solvent are added,
the suspension is admixed with a hydrolysis solution
and the coated particles are subsequently filtered off,
dried and calcined. Likewise, the compounds of the
invention can be used in systems having cathodes
comprising anode materials with doped tin oxide
(DE 100 257 61). This anode material is prepared by
a) admixing a tin chloride solution with urea,
b) admixing the solution with urotropin and a
suitable dopant,
CA 02324630 2000-10-26
- 17 -
c) emulsifying the resulting sol in petroleum
ether,
d) washing the gel obtained and taking off the
solvent and
e) drying and heat-treating the gel.
The compounds of the invention can likewise be
used in systems having cathodes comprising anode
materials with reduced tin oxide (DE 100 257 62) . This
anode material is prepared by
a) admixing a tin chloride solution with urea,
b) admixing the solution with urotropin,
c) emulsifying the resulting sol in petroleum
ether,
d) washing the gel obtained and taking off the
solvent,
e) drying and heat-treating the gel and
f) exposing the Sn02 obtained to a reducing gas
stream in a furnace into which gas can be introduced.
The complex salts of the invention are thus
particularly suitable as electrolyte salts for lithium
ion batteries and supercapacitors.
In the following, a general example of the
invention will be described in more detail.
Use is made of Lewis acid-solvent adducts,
preferably selected from the group consisting of
BR1RZR3, AlRIRZR3, PR1RZR3R~R5, As RIRzR3RqR5, VR'RZR3R4R'.
where
R1 to RS are identical or different, may be joined
directly to one another by a single or double bond and
are each, either individually or together,
a halogen (F, Cl, Br),
an alkyl or alkoxy radical (C1 to CB) which may be
partially or fully substituted by halogen (F, C1, Br),
CA 02324630 2000-10-26
- 18 -
an aromatic ring, which may be bound via oxygen,
selected from the group consisting of phenyl, naphthyl,
anthracenyl and phenanthrenyl, which may be
unsubstituted or monosubstituted or multiplesubstituted
by alkyl (C1 to Cg) or F, Cl, Br,
an aromatic heterocyclic ring, which may be bound via
oxygen, selected from the group consisting of pyridyl,
pyrazyl or pyrimidyl, which may be unsubstituted or
monosubstituted to tetrasubstituted by alkyl (C1 to Ce)
or F, C1, Br.
These adducts are dissolved in suitable battery
solvents, preferably selected from the group consisting
of dimethyl carbonate, diethyl carbonate, propylene
carbonate, ethylene carbonate, ethyl methyl carbonate,
methyl propyl carbonate, y-butyrolactone, methyl
acetate, ethyl acetate, methyl propionate, ethyl
propionate, methyl butyrate, ethyl butyrate, dimethyl
sulfoxide, dioxolane, sulfolane, acetonitrile, acrylo-
nitrite, tetrahydrofuran, 2-methyltetrahydrofuran and
mixtures thereof.
Addition of metal salts in which the anion is
selected from the group consisting of
OR°, NR6R' or CR6R'R8,
OS02R6, N ( SO2R6 ) ( SOzR' ) , C ( SOZR6 ) ( SOZR' ) ( S02R8 ) or
OCOR°,
where
R6 to Re are identical or different, may be joined
directly to one another by a single or double bond and
are each, either individually or together,
a hydrogen atom or as defined for R1 to R5, gives
compounds of the formula (I).
Particular preference is given to preparing
compounds of the formula (I) where M''+ - Li+ or a tetra-
alkylammonium ion and E=BRlaRzbF~ and PRldR2eR3fR4gFh, where
a to h - 0, 1, 2, 3, 4 or 5, where a+b+c=3 and
CA 02324630 2000-10-26
- 19 -
d+e+f+g+h=5, by reacting a corresponding boron or
phosphorus Lewis acid-solvent adduct with a lithium or
tetraalkylammonium-imide, -methanide or -triflate.
The following examples are intended to
illustrate the invention without restricting it.
Examples
Example 1
Preparation of the complex salt Li [BF3 ~ N (SOZCF3) z]
Diethyl carbonate is treated at room
temperature with boron trifluoride gas for 20 minutes.
Here, the reaction temperature is maintained at 40°C by
external cooling. On cooling, a colourless, crystalline
BF3 ~ diethyl carbonate precipitates. The solid is
filtered off under protective gas and dried under
reduced pressure at room temperature.
A mixture of 29.7 g of ethylene carbonate and
26.22 g of diethyl carbonate is placed in a PTFE
reaction vessel. While cooling, 5.5 g (0.03 mol) of BF3
diethyl carbonate and 8.5 g (0.03 mol) of lithium
imide Li [N (SOZCF3) 2] are added. The solution obtained is
used directly as battery electrolyte comprising Li[BF3
~ N (SOZCF3) 2] as electrolyte salt.
Concentration of the electrolyte salt: 0.5 mol/kgSoiVent
19F-NMR (282 MHz, CD3CN)
ppm: -151 s (3F), 3 B-F
-80 s ( 6 F) , 2 SOzC-F3
Example 2
Preparation of the complex salt Li [BF3 ~ S03CF3]
Diethyl carbonate is treated at room
temperature with boron trifluoride gas for 20 minutes.
Here, the reaction temperature is maintained at 40°C by
external cooling. On cooling, a colourless, crystalline
CA 02324630 2000-10-26
' - 20 -
BF3 ~ diethyl carbonate precipitates. The solid is
filtered off under protective gas and dried under
reduced pressure at room temperature.
A mixture of 31.50 g of ethylene carbonate and
27.82 g of diethyl carbonate is placed in a PTFE
reaction vessel. While cooling, 8.80 g (0.03 mol) of
BF3 ~ diethyl carbonate and 4.80 g (0.03 mol) of
lithium triflate Li[S03CF3] are added. The solution
obtained is used directly as battery electrolyte
comprising Li [BF3 ~ S03CF3] as electrolyte salt.
Concentration of the electrolyte salt: 0.5 mol/kgsoivent
19F-NMR (282 MHz, CD3CN)
ppm: -149 s (3F), 3 B-F
-79 s (3F) , 1 S03C-F3
Example 3
Electrochemical stability of the electrolytes
A plurality of cyclovoltammmograms were in each
case recorded in succession in a measurement cell
having a platinum electrode, a lithium counterelectrode
and a lithium reference electrode. For this purpose,
the potential was firstly increased from the rest
potential to 6 V against Li/Li' at a rate of 20 mV/s
and subsequently brought back to the rest potential. As
electrolytes, the solutions indicated in Examples 1 and
2 were used.
The characteristic curve shown in Figures 1 and
2 is obtained. The electrolytes are thus suitable for
use in lithium ion batteries having a transition metal
cathode.
CA 02324630 2000-10-26
- 21 -
Example 4
Ion conductivity of the electrolytes
The ion conductivity of the electrolyte salts
was measured in a solvent mixture of EC/DEC ( 1: 1 ) at a
concentration of 0.5 mol/kg and a temperature of 25°C.
Electrolyte salt Conductivity [mS/cm]
Li [BF3 ' N (SOzCF3)4. 8
2]
Li [N (SOzCF3) 2] 4 . 5
Ll [BF3 ' S03CF3] 3. 7
Li [ S03CF3 ] 1 . 9
The sometimes greatly improved conductivities
of the complex salts compared with the comparison
compounds indicate that they are good electrolyte salts
for electrochemical cells.
Example 5
Passivation of aluminium
A plurality of cyclovoltammmograms were in each
case recorded in succession in a measurement cell
having an aluminium electrode, a lithium counter-
electrode and a lithium reference electrode. For this
purpose, the potential was firstly increased from the
rest potential to 5 V against Li/Li+ at a rate of
20 mV/s and subsequently brought back to the rest
potential. As electrolytes, the solutions indicated in
Examples 1 and 2 were used.
The characteristic curve shown in Figure 4
(Li [BF3 N (SO2CF3) 2] ) and Figure 5 (Li [BF3 S03CF3] ) is
obtained. The decrease in the current with increasing
number of cycles indicates passivation of the alumin-
ium. After the experiment, no corrosion of the
aluminium can be seen. The electrolyte is thus suitable
for use in lithium ion batteries having a transition
metal cathode.
CA 02324630 2000-10-26
' - 22 -
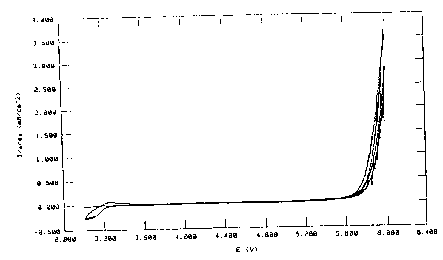
Comparative Example 1
Corrosion of aluminium
A plurality of cyclovoltammmograms were in each
case recorded in succession in a measurement cell
having an aluminium electrode, a lithium counter-
electrode and a lithium reference electrode. For this
purpose, the potential was firstly increased from the
rest potential to 5 V against Li/Li+ at a rate of
20 mV/s and subsequently brought back to the rest
potential. Solutions of lithium imide Li [N (SOZCF3) 2] or
lithium triflate Li[S03CF3] in EC/DEC 1:1 were used as
electrolyte.
Both electrodes display the same,
characteristic current-voltage curve. The increase in
current with increasing number of cycles indicates
corrosion of the aluminium. After the experiments,
clear signs of corrosion (pit corrosion) can be seen.
Figure 3 shows, by way of example, the curve in the
lithium imide electrolyte. The electrolytes are thus
not suitable for use in lithium ion batteries having a
transition metal cathode and aluminium terminal leads.