Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
TITLE OF THE INVENTION
ANTAGONISTS OF GONADOTROPIN RELEASING HORMONE
BACKGROUND OF THE INVENTION
The gonadotropin-releasing hormone (GnRH), also referred
to as luteinizing hormone-releasing hormone (LHRH), is a decapeptide
that plays a key role in human reproduction. The hormone is released
from the hypothalamus and acts on the pituitary gland to stimulate the
biosynthesis and secretion of luteinizing hormone (LH) and follicle-
stimulating hormone (FSH). LH released from the pituitary gland is
primarily responsible for the regulation of gonadal steroid production in
both sexes, whereas FSH regulates spermatogenesis in males and
follicular development in females. GnRH agonists and antagonists have
proven effective in the treatment of certain conditions which require
inhibition of LH/FSH release. In particular, GnRH-based therapies
have proven effective in the treatment of endometriosis, uterine fibroids,
polycystic ovarian disease, precocious puberty and several gonadal
steroid-dependent neoplasia, most notably cancers of the prostate, breast
and ovary. GnRH agonists and antagonists have also been utilized in
various assisted fertilization techniques and have been investigated as a
potential contraceptive in both men and women. They have also shown
possible utility in the treatment of pituitary gonadotrophe adenomas,
sleep disorders such as sleep apnea, irritable bowel syndrome,
premenstrual syndrome, benign prostatic hyperplasia, hirsutism, as
an adjunct to growth hormone therapy in growth hormone deficient
children, and in murine models of lupus. The compounds of the
invention may also be used in combination with bisphosphonates
(bisphosphonic acids) and other agents, such as growth hormone
secretagogues, for the treatment and the prevention of disturbances of
calcium, phosphate and bone metabolism, in particular, for the
prevention of bone loss during therapy with the GnRH antagonist, and
in combination with estrogens, progesterones, antiestrogens,
antiprogestins and/or androgens for the prevention or treatment of bone
-1-
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
loss or hypogonadal symptoms such as hot flashes during therapy with
the GnRH antagonist.
Additionally, a compound of the present invention may be
co-administered with a 5a-reductase 2 inhibitor, such as finasteride or
epristeride; a 5a-reductase 1 inhibitor such as 4,?b-dimethyl-4-aza-5a
cholestan-3-one, 3-oxo-4-aza-4,7b-dimethyl-16b-(4-chlorophenoxy)-5a-
androstane, and 3-oxo-4-aza-4,7b-dimethyl-16b-(phenoxy)-5a-androstane
as disclosed in WO 93/23420 and WO 95/11254; dual inhibitors of 5a-
reductase 1 and 5a-reductase 2 such as 3-oxo-4-aza-17b-(2,5-
trifluoromethylphenyl-carbamoyl)-5a-androstane as disclosed in WO
95/07927; antiandrogens such as flutamide, casodex and cyproterone
acetate,. and alpha-1 blockers such as prazosin, terazosin, doxazosin,
tamsulosin, and alfuzosin.
Further, a compound of the present invention may be
used in combination with growth hormone, growth hormone releasing
hormone or growth hormone secretagogues, to delay puberty in growth
hormone deficient children, which will allow them to continue to gain
height before fusion of the epiphyses and cessation of growth at puberty.
Further, a compound of the present invention may be used
in combination or co-administered with a compound having luteinizing
hormone releasing activity such as a peptide or natural hormone or .
analog thereof. Such peptide compounds include leuprorelin,
gonadorelin, buserelin, triptorelin, goserelin, nafarelin, histrelin,
deslorelin, meterlin and recirelin.
Additionally, a compound of the present invention may be
used as described in U.S. Patent No. 5,824,286 which discloses the
administration of peptide GnRH antagonists such as Antide and azaline
B to premenopausal women to enhance the readability of
mammographic film relative to a mammogram effected in the absence
of the administration.
Current GnRH antagonists are GnRH-like decapeptides
which are generally administered intravenously or subcutaneously
presumably because of negligible oral activity. These have amino
acid substitutions usually at positions one, two, three, six and ten.
-2-
CA 02326185 2000-09-26
WO 99/51233 PC1'/US99/06727
Non-peptide GnRH antagonists offer the possible
advantage of oral adminstration. Non-peptide GnRH antagonists have
been described in European Application 0 219 292 and in De, B. et al.,
J. Med. Chem., 32, 2036-2038 (1989), in WO 95/28405, WO 95/29900 and
EP 0679642 all to Takeda Chemical Industries, Ltd.
Substituted indoles known in the art include those
described in the following patents and patent applications. US Patent
No. 5,030,640 discloses alpha-heterocyclic ethanol aminoalkyl indoles
which are potent 13-agonists. US Patent No. 4,544,663 discloses
indolamine derivatives which are allegedly useful as male anti-fertility
agents. WO 90/05721 discloses alpha-amino-indole-3-acetic acids useful
as anti-diabetic, anti-obesity and anti-atherosclerotic agents. French
patent 2,181,559 discloses indole derivatives with sedative, neuroleptic,
analgesic; hypotensive, antiserotonin and adrenolytic activity. Belgian
patent 879381 discloses 3-aminoalkyl-1H-indole-5-thioamide and
carboxamide derivatives as cardiovascular agents used to treat
hypertension, Raynaud's disease and migraine. U.S. Patent Nos.
5,756,50?, 5,780,437 and 5,849,764 also disclose substituted arylindoles as
non-peptide antagonists of GnRH.
SUMMARY OF THE INVENTION
The present invention relates to compounds which are
non-peptide antagonists of GnRH which can be used to treat a variety of
sex-hormone related conditions in men and women, to methods for their
preparation, and to methods and pharmaceutical compositions
containing said compounds for use in mammals.
Because of their activity as antagonists of the hormone
GnRH, the compounds of the present invention are useful to treat a
variety of sex-hormone related conditions in both men and women.
These conditions include endometriosis, uterine fibroids, polycystic
ovarian disease, hirsutism, precocious puberty, gonadal steroid-
dependent neoplasias such as cancers of the prostate, breast and
ovary, gonadotrophe pituitary adenomas, sleep apnea, irritable bowel
syndrome, premenstrual syndrome and benign prostatic hypertophy.
-3-
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
They are also useful as an adjunct to treatment of growth hormone
deficiency and short stature, and for the treatment of systemic lupus
erythematosis. Further, the compounds of the invention may be useful
in in vitro fertilization and as contraceptives. The compounds may also
be useful in combination with androgens, estrogens, progesterones,
antiestrogens and antiprogestogens for the treatment of endometriosis,
fibroids and in contraception. They may also be useful in combination
with testosterone or other androgens or antiprogestogens in men as
a contraceptive. The compounds may also be used in combination
with an angiotensin-converting enzyme inhibitor such as Enalapril
or Captopril, an angiotensin II-receptor antagonist such as Losartan
or a renin inhibitor for the treatment of uterine fibroids. Additionally,
the compounds of the invention may also be used in combination with
bisphosphonates (bisphosphonic acids) and other agents, for the
treatment and the prevention of disturbances of calcium, phosphate and
bone metabolism, in particular, for the prevention of bone loss during
therapy with the GnRH antagonist, and in combination with estrogens,
progesterones and/or androgens for the prevention or treatment of bone
loss or hypogonadal symptoms such as hot flashes during therapy with
the GnRH antagonist.
Additionally, a compound of the present invention may be
co-administered with a 5a-reductase 2 inhibitor, such as finasteride or
epristeride; a 5a-reductase 1 inhibitor such as 4,7b-dimethyl-4-aza-5a-
cholestan-3-one, 3-oxo-4-aza-4,7b-dimethyl-16b-(4-chlorophenoxy)-5a-
androstane, and 3-oxo-4-aza-4,7b-dimethyl-16b-(phenoxy)-5a-androstane
as disclosed in WO 93/23420 and WO 95/11254; dual inhibitors of 5a-
reductase 1 and 5a-reductase 2 such as 3-oxo-4-aza-17b-(2,5-
trifluoromethylphenyl-carbamoyl)-5a-androstane as disclosed in
WO 95/07927; antiandrogens such as flutamide, casodex and cyproterone
acetate, and alpha-1 blockers such as prazosin, terazosin, doxazosin,
tamsulosin, and alfuzosin.
Further, a compound of the present invention may be
used in combination with growth hormone, growth hormone releasing
hormone or growth hormone secretagogues, to delay puberty in growth
-4-
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
hormone deficient children, which will allow them to continue to gain
height before fusion of the epiphyses and cessation of growth at puberty.
Further, a compound of the present invention may be used
in combination or co-administered with a compound having luteinizing
hormone releasing activity such as a peptide or natural hormone or
analog thereof. Such peptide compounds include leuprorelin,
gonadorelin, buserelin, triptorelin, goserelin, nafarelin, histrelin,
deslorelin, meterlin and recirelin.
Additionally, a compound of the present invention may be
used as described in U.S. Patent No. 5,824,286 which discloses the
administration of peptide GnRH antagonists such as Antide and azaline
B to premenopausal women to enhance the readability of
mammographic film relative to a mammogram effected in the absence
of the administration.
DETAILED DESCRIPTION OF THE INVENTION
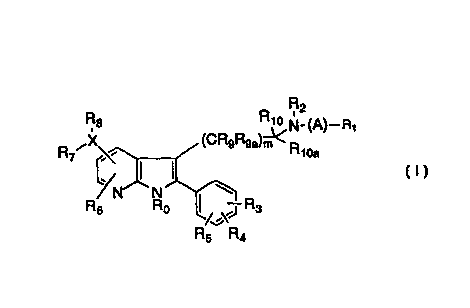
The present invention relates to compounds of the general
formula
R2
Rs R
' '° N-
R~~X~~ URsRsa)m'CR
ii ,
1
Rs Ro ~. 'i R3
R5 Ra (I)
wherein
A is C1-CS alkyl, substituted C1-Cg alkyl, Cg-C7 cycloalkyl,
substituted C3-C7 cycloalkyl, C3-C6 alkenyl, substituted
Cg-C6 alkenyl, C3-C6 alkynyl, substituted C3-C6 alkynyl,
C 1-Cg alkoxy, or Cp-C5 alkyl-S(O)n-CO-C5 alkyl, Cp-C5 alkyl-
O-CO-C5 alkyl, CO-C5 alkyl-NRlg-Cp-C5 alkyl where Rlg and
-5-
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
the Cp-C5 alkyl can be joined to form a ring,
N-(CHZ)p-
R16 , or a single bond.
Rp is hydrogen, C1-Cg alkyl, substituted C1-Cg alkyl, wherein
the substituents are as defined below; aryl, substituted aryl,
aralkyl or substituted aralkyl, wherein the substituents are
as defined for Rg, R4 and R~.
Rl is
N Z,
Z1 ~ I y ~ , i . ~N
I R13 ~/~~ R13 ~ ~ R
Z ~~~ 1 15
R; ~~R R14 R15 Ri4 Ri5 R13 R/ R
14 13
/Z ~ R15 ~Z,N (ZjRl4
~, .N
R , I 'I ~/ ~,,~-.rI,N ~r ~~ R 11
14 ,~ ~\ R 14 .'''~ ~ N .r,,,.',r
R13 R13 R13 R19 Ri3 R14
Z w
~~ ~,N-Ri 1
I N-N R19 -N N-nl IvFN
Rig R19 ~ Rii
Rii
R11
~~~N _~N~~R13
~~~~JRi$ I~NRl3 l/~~JR13 N
R15 R14 Ri~ R14 R15 R14 R15' 14 R19
/ ~N / ~~ ,.~.~. ~iRis
I ~~ R13 ~/~yJ R13 '/ I ~~J R13 R/ \R
R15 \R14 R15 R14 R15 R14 15 14
~~ R ~~ R1~/~
I ~ R13 14 LLN R13 'N
R15 N R14 R13
-6-
CA 02326185 2000-09-26
WO 99/51233 PCTNS99/06727
the nitrogen atoms contained in the R1 heteroaromatic rings may exist
either as drawn or, when chemically allowed, in their
oxidized (N-~O) state;
R2 is hydrogen, C1-Cg alkyl, substituted C1-Cg alkyl, aralkyl,
substituted aralkyl, aryl, substituted aryl, alkyl -OR11,
C1-C6(~11R12), C1-Cg(CONR11R12) or C(NR11R12)~;
R2 and A taken together form a ring of 5-7 atoms;
R3, R4 and R5 are independently hydrogen, C 1-C6 alkyl, substituted
C1-C6 alkyl, C2-Cg alkenyl, substituted C2-Cg alkenyl, CN,
nitro, C1-Cg perfluoroalkyl, C1-Cg perfluoroalkoxy,_aryl,
substituted aryl, aralkyl, substituted aralkyl, 8110{CH2~-,
R11C(O)O(CH2)p-, R110C(O)(CH2)p-, -(CH2)pS(O)nRl7~
-(CH2)pC(O)NR11R12 or halogen; wherein R17 is hydrogen,
C1-Cg alkyl, C1-C3 pex~luoroalkyl, aryl or substituted aryl;
R3 and R4 taken together form a carbocyclic ring of 3-7 carbon atoms or a
heterocyclic ring containing 1-3 heteroatoms selected from
N, O and S;
Rg is hydrogen, C 1-Cg alkyl, substituted C 1-Cg alkyl, aryl,
substituted aryl, C1-Cg perfluoroalkyl, CN, N02, halogen,
R110(CH2)p-, NR12C(O)R11, NR12C(O)NR11R12 or SOnRll;
R7 is hydrogen, C1-Cg alkyl, or substituted C1-Cg alkyl, unless X
is hydrogen or halogen, then R7 is absent;
Rg is hydrogen, C(O)ORg, C(O)NR11R12, NR11R,12~ C(O)Rll~
~12C(O)Rll~ NR12C(O)NR11R12~ NR12S(O)2R11~
NR12S(O)2~11R12~ OC{O)R11, OC(O)NR11R12~ ORlh
SOnRll, S(O)nNR11R12~ C1-C6 alkyl or substituted C1-Cg
alkyl, unless X is hydrogen or halogen, then Rg is absent; or
R7 and Rg taken together form a carbocyclic ring of 3-7 atoms;
Rg and Rga are independently hydrogen, C1-Cg alkyl, substituted C1-C6
alkyl; aryl or substituted aryl, aralkyl or substituted aralkyl
when m~0; or
O
Rg and Rga taken together form a carbocyclic ring of 3-7 atoms or ~
when m$0;
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
Rg and A taken together form a heterocyclic ring containing 3-7 carbon
atoms and one or more heteroatoms when m~0; or
Rlp and RlOa are independently hydrogen, C1-Cg alkyl, substituted
C1-Cg alkyl, aryl, substituted aryl, aralkyl or substituted
aralkyl; or
O
Rl0 and RlOa taken together form a carbocyclic ring of 3-7 atoms or I~ ,
Rg and Rlp taken together form a carbocyciic ring of 3-7 carbon atoms or
a heterocyclic ring containing one or more heteroatoms
when m~0; or
Rg and R2 taken together form a heterocyclic ring containing 3-7 carbon
atoms and one or more heteroatoms when m~0; or
Rl0 and R2 taken together form a heterocyclic ring containing 3-7 carbon
atoms and one or more heteroatoms;
Rl0 and A taken together form a heterocyclic ring containing 3-7 carbon
atoms and one or more heteroatoms; or
Rl1 and R12 are independently hydrogen , C 1-Cg alkyl, substituted
C 1-Cg alkyl, aryl, substituted aryl, aralkyl, substituted
aralkyl, a carbocyclic ring of 3-7 atoms or a substituted
carbocyclic ring containing 3-7 atoms;
Rll and R12 taken together can form an optionally substituted ring of 3-7
atoms;
R13 is hydrogen, OH, NR7Rg, NR11S02(C1-Cg alkyl),
~11502(substituted C1-Cg alkyl), NR11S02(aryl),
~11502(substituted aryl), NR11S02(C1-Cg perfluoroalkyl);
S02NR11(C1-C6 alkyl), S02NR11(substituted C1-Cg alkyl),
S02NR11(aryl), S02NR11(substituted aryl), S02NR11(C1-C3
perfluoroalkyl); S02NR11(C(O)C1-Cg alkyl); S02NR11(C(O)-
substituted C1-C6 alkyl); S02NR11(C(O)-aryl);
S02NR11(C(O)-substituted aryl); S(O)n(C1-Cg alkyl); S(O)n
( substituted C1-Cg alkyl), S(O)n(aryl), S(O)n(substituted
aryl), C1-Cg perfluoroalkyl, C1-Cg perfluoroalkoxy, C1-Cg
alkoxy, substituted C1-Cg alkoxy, COOH, halogen, N02 or
CN;
_g_
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
R14 and R15 are independently hydrogen, C 1-Cg alkyl, substituted C 1-Cg
alkyl, C2-CS alkenyl, substituted C2-Cg alkenyl,
CN, nitro,
C1-Cg perfluoroalkyl, C1-Cg perfluoroalkoxy, aryl,
substituted aryl, aralkyl, substituted aralkyl,
R110(CH2)p-,
R11C(O)O(CH2)p-, R110C(O)(CH2)p-, -(CH2)pS(O)nRl7~
-(CH2)pC(O)NR11R12 or halogen; wherein R17 is hydrogen,
C1-Cg alkyl, C1-Cg perfluoroalkyl, aryl or substituted
aryl;
R1g is hydrogen, C1-CS alkyl, substituted C1-Cg alkyl,
or
N(R11R12)~
R1g is hydrogen, C1-Cg alkyl, substituted C1-C6 alkyl,
C(O)ORg,
C(O)~11R12~ C(O)R11~ S(O)nRll~
R1g is either the definition of R13 or R14;
X is hydrogen, halogen, N, O, S(O)n, C(O), (CR11R12)p~
C2-C6
alkenyl, substituted C2-Cg alkenyl,C2-Cg alkynyl,
or
substituted C2-Cg alkynyl; when X is hydrogen or
halogen,
R7 and Rg are absent; when X is O, S(O)n, C(O),
or CR11R12
only R7 or Rg is possible;
Z is O, S, or NR11;
m is 0-3;
n is 0-2;
p is 0-4; and
the alkyl, alkenyl and alkynyl substituents are selected from
C1-Cg alkyl, Cg-C7 cycloalkyl, aryl, substituted aryl, aralkyl,
substituted aralkyl, hydroxy, oxo, cyano, C 1-Cg alkoxy,
fluoro, C(O)OR11~ aryl C1-C3 alkoxy, substituted aryl C1-C3
alkoxy, and the aryl substituents are as defined for R3, R4
and R5;
or a pharmaceutically acceptable addition salt and/or hydrate thereof, or
where applicable, a geometric or optical isomer or racemic mixture
thereof.
Unless otherwise stated or indicated, the following
definitions shall apply throughout the specification and claims.
When any variable (e.g., aryl, heterocycle, Rl, etc.) occurs
more than one time in any constituent or in formula I, its definition on
-9-
CA 02326185 2000-09-26
WO 99/51233 PCTNS99/06727
each occurrence is independent of its definition at every other
occurrence. Also, combinations of substituents and/or variables are
permissible only if such combinations result in stable compounds.
The term "alkyl" is intended to include both branched-
and straight-chain saturated aliphatic hydrocarbon groups having the
specified number of carbon atoms, e.g., methyl (Me), ethyl (Et), propyl,
butyl, pentyl, hexyl, heptyl, octyl, nonanyl, decyl, undecyl, dodecyl, and
the isomers thereof such as isopropyl (i-Pr), isobutyl (i-Bu), secbutyl
(s-Bu), tertbutyl (t-Bu), isopentane, isohexane, etc.
The term "aryl" includes phenyl and naphthyl. In a
preferred embodiment, aryl is phenyl.
The term "halogen" or "halo" is intended to include
fluorine, chlorine, bromine and iodine.
As used herein, the term "composition" is intended to
encompass a product comprising the specified ingredients in the
specified amounts, as well as any product which results, directly or
indirectly, from combination of the specified ingredients in the specified
amounts
It is intended that the connecting group A can be bonded to
any of the available carbon or heteroatoms of the heteroaromatic groups
R1, including both rings of the benzo-fused heterocyclic groups and,
likewise, R13, R14, and R15 can be bonded to any of the available carbon
atoms of the heteroaromatic groups R1.
In addition, it is well known to those skilled in the art that
many of the foregoing heterocyclic groups can exist in more than one
tautomeric form. It is intended that all such tautomers be included
within the ambit of this invention.
The optical isomeric forms, that is mixtures of enantiomers
or diasteromers, e.g., racemates, as well as individual enantiomers or
diastereomers of the instant compound are included. These individual
enantiomers are commonly designated according to the optical rotation
they effect by the symbols (+) and (-), (L) and (D), ( 1) and (d) or
combinations thereof. These isomers may also be designated according
- 10 -
CA 02326185 2000-09-26
WO 99/51233 PCTNS99/06727
to their absolute spatial configuration by (S) and (R), which stands for
sinister and rectus, respectively.
The individual optical isomers may be prepared using
conventional resolution procedures, e.g., treatment with an appropriate
optically active acid, separating the diastereomers and then recovering
the desired isomer. In addition, the individual optical isomers may be
prepared by asymmetric synthesis.
Additionally, a given chemical fomula or name shall
encompass pharmaceuticaly acceptable addition salts thereof and
solvates thereof, such as hydrates.
The compounds of the present invention, while effective
themselves, may be formulated and administered in the form of their
pharmaceutically acceptable addition salts for purposes of stability,
convenience of crystallization, increased solubility and other desirable
properties.
The compounds of the present invention may be
administered in the form of pharmaceutically acceptable salts. The
term "pharmaceutically acceptable salt" is intended to include all
acceptable salts Examples of acid salts are hydrochloric, nitric, sulfuric,
phosphoric, formic, acetic, trifluoroacetic, propionic, malefic, succinic,
malonic, methanesulfonic, benzenesulfonic and the like which can be
used as a dosage form for modifying the solubility or hydrolysis
characteristics or can be used in sustained release or pro-drug
formulations. Depending on the particular functionality of the
compound of the present invention, pharmaceutically acceptable salts of
the compounds of this invention include those formed from cations such
as sodium, potassium, aluminum, calcium, lithium, magnesium, zinc,
and from bases such as ammonia, ethylenediamine, N-methyl-
glutamine, lysine, arginine, ornithine, choline, N,N'-dibenzyl-
ethylenediamine, chloroprocaine, diethanolamine, procaine, N-
benzylphenethylamine, diethylamine, piperazine, tris(hydroxymethyl)
aminomethane, and tetramethylammonium hydroxide. These salts
may be prepared by standard procedures, e.g. by reacting a free acid
- 11 -
CA 02326185 2000-09-26
WO 99/51233 PCTNS99/06727
with a suitable organic or inorganic base, or alternatively by reacting a
free base with a suitable organic or inorganic acid.
Also, in the case of an acid (-COOH) or alcohol group being
present, pharmaceutically acceptable esters can be employed, e.g.
methyl, ethyl, butyl, acetate, maleate, pivaloyloxymethyl, and the like,
and those esters known in the art for modifying solubility or hydrolysis
characteristics for use as sustained release or prodrug formulations.
The compounds of the present invention may have chiral
centers other than those centers whose stereochemistry is depicted in
formula I, and therefore may occur as racemates, racemic mixtures
and as individual enantiomers or diastereomers, with all such
isomeric forms being included in the present invention as well as
mixtures thereof. Furthermore, some of the crystalline forms for
compounds of the present invention may exist as polymorphs and
as such are intended to be included in the present invention. In
addition, some of the compounds of the instant invention may form
solvates with water or common organic solvents. Such solvates are
encompassed within the scope of this invention.
The compounds of the invention are prepared according to
the following reaction schemes. All of the substituents are as defined
above unless indicated otherwise.
- 12 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
Reaction Scheme A
Re
R iX~ ~ (C~ ~O Pd(dpP~i2°CH2C1Z
a~ ~pGt LtCI, Na2C0~,
Rs '~ + Z / RtoRtoa ~ DMF, heat
N NHZ 2a Z = H
1 2b Z =Et3Si
2c Z =
R3
Rs 5v\R° Ra
\ (CR O
R7 r~ ~a~~ ~PGi R>~~ Z
RtoRtoa R5 r I I toRtoa
N ~ Z and 'N N 1~~ P
(CR
H 9R9e m
3b Z = Et3Si 4a Z = H
3c Z = ~ 4c Z =
R3 ~ -R3
/v~
R5 Ra
R5 Ra
A preferred method for the synthesis of the substituted
tryptamines described in this invention utilizes a palladium-catalyzed
cross coupling reaction as a key step as shown in Scheme A. This 7-
azaindole synthesis involves the reaction of a suitably fitnctionalized 3-
iodo-2-aminopyridine (1) with substituted acetylenes such as 2 in the
presence of a base like sodium carbonate, lithium chloride, and a
palladium catalyst such as (dppf)PdCl2~CHZC12. The reaction is
conducted in an inert organic solvent such as dimethylformamide at
elevated temperatures, for instance at 100°C, and the reaction is
conducted for a period of about 30 minutes to about 24 hours. A standard
I5 workup and isolation affords the substituted isomeric indole derivatives
3 and 4, and the isomer of general formula 3 is the preferred isomer.
The acetylene utilized in this reaction may be a terminal acetylene (2a)
or be optionally substituted on the terminal carbon atom with a
substituent Z (2b, 2c). The substituent abbreviated PGl indicates an
alcohol protecting group such as a benzyl ether, tert-butyl ether or the
- 13 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
like. The nature of the Z substituent determines the distribution of the 7-
azaindole isomers (3 and 4) produced in the reaction. For example, if the
substituent Z on the acetylene is a hydrogen atom then the isomer 4a is
the major product of the reaction. When the substituent Z is chosen to be
a substituted silyl group such as trimethylsilyl, triethylsilyl (as shown),
or the like, then isomer 3b is formed almost exclusively. When Z is a
substituted aryl group, then both isomers 3c and 4c may be formed and
the product mixture is separated using chromatographic or
crystallization techniques to afford the individual isomers.
If the synthesis is conducted with a silyl-substituted
acetylene 2b to produce a silyl-substituted 7-azaindole 3b, then the silyl
group is next converted to an aryl or substituted aryl group of general
formula 3c using the reactions described later in Scheme E. The 2-
arylsubstituted 7-azaindole derivatives 3c formed either directly from
arylacetylenes (2c) as shown in Scheme A or from 2-trialkylsilyl-7-
azaindoles using the method of Scheme E are then further elaborated as
described below to produce the novel 7-azaindole derivatives described in
this invention.
Reaction Scheme B
Rs Rs
/ w
7
R~ ~~ m-CPBA, R~ ~~ \ I N
Rs- I R~ I O \
N J H20-CHC13
C1CH2CH2C1,
- O 6 90°C, 3 days
Rs
R~ X\ HC1, 100°C Rs I2, Ag(CF3C0~, Re
O 16 h ~X MeOH, rt, 3 h.
ps-~ I '' R' r\ ~ R~
N N \ Rs_"' I R~
8
I / 9 N NH2 1 N NH2
~O
- 14 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
Scheme B illustrates the preparation of substituted
3-iodo-2-aminopyridines (1) which are utilized in the Larock 7-azaindole
synthesis described in Scheme A. The 3-iodo-2-aminopyridines (1) may
be prepared in several ways described in the literature of organic
synthesis. A preferred method involves the ortho-iodination of
substituted 2-aminopyridine derivatives of general formula 9 with an
electrophilic iodination reagent such as iodine, iodine monochloride,
N-iodosuccinimide or the like.. The ortho-iodination of 2-aminopyridine
derivatives (9) as illustrated in Scheme B employs iodine and silver
trifluoroacetate in a suitable organic solvent like methanol at room
temperature and 3-iodo-2-aminopyridines of general formula 1 are
produced in high yield.
In some cases, 2-aminopyridine derivatives such as 9 may
be commercially available or alternatively they may be prepared using
methodologies known in organic chemistry. For instance, application
of methodology reported by Wachi and Terada (Wachi, K.; Terada, A.
Chem. Pharm. Bull. 1980, 28, 465) allows the conversion of pyridines of
general formula 5 to 2-aminopyridines of general formula 9 as shown
at the top of Scheme B. In this synthetic transformation, a substituted
pyridine (5) is first converted to the corresponding N-oxide (6) with a
suitable oxidant such as meta-chloroperbenzoic acid. In the next step,
the substituted pyridine N-oxide 6 is reacted with 4-chloro-2,2-dimethyl-
2H-1,3-benzoxazine (7) in an inert high boiling solvent such as 1,2-
dichloroethane. An adduct initially formed in this reaction undergoes
a thermal rearrangement and the N-pyridyl substituted 4-oxo-4H-1,3-
benzoxazine 8 is produced. Finally, the substituted benzoxazine 8 is
hydrolyzed with concentrated hydrochloric acid at elevated temperature
to afford the substituted 2-aminopyridines of general formula 9.
- 15 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
Reaction Scheme C
NH
(CRgRg~m OH p~ O~ CC13 / 1 ) n-BuLi
CF SO H (CRgR9a) O \ ( THF, -78°C
3 3
2) Et3SiCl
CC)4-C6H~2 2a
'
(CR9Rga)m p
2b R~oRtoe
(~9R9a)m OH \ X Pd(OAc)2, PPh3, ~ (CR~9a~~OH
+ R5 l CuI, Er3N, ~ \ ~ R/to\Rtoe
R~oR~a Ro'~~~Ra 85°C Rs
10 11 R4~~R3 12
X = Br, I, OSOzCF3
H (CRgRgJm O \
Ph~O~CC13
\ RtoRtoa
CF3SO~I-I R ~
2c
CC)4-C6Ht2 ~ Ra
Acetylenic compounds of general structure 2 are prepared
5 using one of several methods depending upon the choice of the desired
substituents. When the substituents Re, Rea, Rlo and Rtoa are selected to
be hydrogen or lower alkyl groups, compounds of formula 2 may be
prepared from known acetylenic alcohols such as 3-butyn-1-ol, 4-pentyn-
2-0l or similar acetylenic alcohols reported in the chemical literature.
10 The conversion of acetylenic alcohols of general formula 10 to acetylene
derivatives of general formula 2 is shown in Scheme C. For clarity the
hydroxyl protecting group (PGl) illustrated in Scheme C is exemplified
as an O-benzyl ether. Thus, reaction of 10 with O-benzyl-2,2,2-
trichloroacetimidate in the presence of a catalytic amount of a strong
acid such as trifluoromethanesulfonic acid and in a suitable inert
organic solvent like carbon tetrachloride at room temperature affords
after 2 to 24 hours the protected acetylenic alcohol 2a. Compounds of
- 16 -
CA 02326185 2000-09-26
WO 99/51233 PGT/US99/06727
formula 2a may in turn be converted to acetylenes (2b) of general
formula 2 wherein Z is a trialkylsilyl group by deprotonation of the
acetylene with a base such as n-butyllithium in an inert organic solvent
like tetrahydrofuran followed by reaction with a trialkylsilyl chloride
such as triethylchlorosilane. The deprotonation and silylation reactions
are generally conducted at low temperatures, for instance between about
-78°C and room temperature, and after standard workup and
purification a silylacetylene of formula 2b is obtained.
As previously stated, acetylenes of general formula 2c
wherein Z is an aryl or substituted aryl group, are also useful in the 7-
azaindole synthesis illustrated in Scheme A. Arylacetylenes 2c may be
prepared using. a coupling reaction of cuprous acetylides derived from
acetylenic alcohols of formula 2a with various aryl halides or aryl
triflates (11). Such coupling reactions produce aryl acetylenes of general
formula 12 as shown at the bottom of Scheme C. These reactions are
generally carried out in a basic organic solvent like triethylamine at
elevated temperatures, typically between about 60°C and about
120°C,
and the coupling reaction is catalyzed by copper(I) salts such as
cuprous iodide and a palladium catalyst such as palladium acetate in
combination with triphenylphosphine. The hydroxyl group of the
arylacetylenes of general formula 12 can be protected with a suitable
protecting group such as the O-benzyl ether group shown in Scheme C,
to afford an arylacetylene (2c) of general formula 2 wherein Z is an aryl
or substituted aryl group. It is also recognized that in some cases it may
be preferable to reverse the order of the steps illustrated in Scheme C.
For instance, acetylenic alcohols (7) may be subjected to silylation or
arylation prior to the hydroxyl group protection step.
- 17-
CA 02326185 2000-09-26
WO 99/51233 PCTNS99/06727
Reaction Scheme D
H
Ph~O~CCb /
RO (CR9R9a)~OH
CF3SOgH RO (CRgRga)", O \ (
RtoRtoa
O CCI,~-C6H12 O RtoRtoa LiAIH.~, THF
13 14
1) DMSO, (COCI)Z, /
HO (C~a) O \ I CHzCl2, -50°C H (C~R9 ) O \
2) Et3N a~
RtoRtoa RtoRtoa
15 16
CBr4, PPh3, / I / I
CHZCh, rt H (CR~~ O~ CR O \
~ ( 9R9a)
I R~toa n-BuLi, THF, % R'oRtoa
B ~Br 17 -78°C to 0°C
Another useful approach for the preparation of acetylenic
compounds of general formula 2a employs an ethynylation reaction
sequence of aldehydes of general formula 16 as shown in Scheme D. The
aldehydes (16) used in the ethynylation sequence may be prepared using
various methods known in organic synthesis starting with hydroxy-
esters of general formula 13, from protected hydroxyesters of formula 14,
or from alcohols related to the mono-hydroxyl protected diols of formula
15. The choice of preferred starting material depends upon the nature of
the substituents R9, Rya, Rta, and R~oa selected. Scheme D illustrates this
strategy begining with the generalized hydroxy ester 13. Protection of
the hydroxyl group of 13, for instance as the O-benzylether shown,
affords a protected hydroxy ester of formula 14. The ester group of
compounds of formula 14 can then be converted to an aldehyde of
formula 16 either directly using a reagent like diisobutylaluminum
hydride in a solvent like toluene, or through a two step process. In the
two step process, reduction of the ester group with a reagent such as
lithium aluminum hydride in tetrahydrofuran affords alcohols of
- 18-
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
formula 15 which are then subjected to reoxidation, for instance using a
Swern-Moffatt oxidation, to afford the desired aldehydes of formula 16.
The ethynylation of aldehydes of formula 16 is accomplished
in two steps. First, aldehydes (16) are reacted with carbon tetrabromide
and triphenylphosphir~e in an inert organic solvent like dichloro-
methane to produce the dibromo olefins of formula 17. Next, the dibromo
olefins (17) are treated with two equivalents of a strong base such as
n-butyllithium in tetrahydrofuran at low temperature, for instance at
about -78°C. The strong base induces dehydrohalogenation and metal-
halogen exchange to afford lithium acetylides which upon quenching
and workup afford acetylenes of general formula 2a. Alternatively, the
intermediate lithium acetylides formed in the reaction may be treated
with a trialkylsilyl chloride, such as triethylchlorosilane, to afford
silylacetylenes of general formula 2b.
Reaction Scheme E
Ra Re
~CR9R9a~n~~~PGt Ri ~- ~~9R9a~~~~pGt
RB ~ I I RtoRtoa Rg ~ I I RtoRtoe
N p ~E~ ICI, AgBF4, N ~ ~ 18
3b MeOH-THF, 0'C
R~ ~, (CR9R9a)~o~~t
18 + I J R3 Rte' I I RtoRtoe
N ~N~
RS R4 (dPP~PdCl2~CHZCl2, H ~ ~ R3
toluene-EtOH8-O aq. NazC03, 3c
19 RS R4
The conversion of 2-silyl-substituted 7-azaindoles of general
formula 3b to 2-aryl-substituted 7-azaindoles of general formula 3c may
be accomplished in two steps as shown in Scheme E. The first step is a
halodesilylation reaction which converts silyl-substituted 7-azaindoles of
formula 3b into 2-halo-7-azaindoles of general formula 18. Scheme E
- 19 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
illustrates this process using iodine monochloride so that the product
obtained is a 2-iodoindole of general formula 18. Silver tetrafluoroborate
is also employed in this example to increase the reactivity of the halogen-
ating reagent. It is possible to effect the halodesilylation reaction with
other electrophilic halogenating reagents such as N-bromosuccinimide
in dichloromethane which affords a 2-bromo-7-azaindole derivative.
Both 2-bromo and 2-iodo-7-azaindoles of formula 18 are useful in the
subsequent step.
The second step is a palladium-catalyzed cross coupling
reaction of the 2-halo-7-azaindole 18 with a suitable aryl or substituted
aryl organometallic reagent 19. Scheme E illustrates this process with
an aryl or substituted arylboronic acid as the organometallic reagent,
however, other organometallic reagents known to participate in
palladium-catalyzed cross-coupling reactions such as arylboronic esters
or arylstannanes may also be employed. In the example, a 2-iodo-7-
azaindole of general formula 18 is coupled with a generalized boronic
acid (19) using a catalyst such as [1,1'-bis(diphenylphosphino)ferrocene]
dichloropalladium(II) complex with dichloromethane (shown),
tetrakis(triphenylphosphine)-palladium(0) or the like. The reaction
is usually conducted at temperatures between room temperature and
about 100°C, for instance at about 80°C. This palladium
catalyzed cross-
coupling reaction may be effected using various combinations of
palladium catalysts and solvent compositions known in organic
chemistry, and the selection of the conditions is made depending upon
the type of organometallic reagent (19) used and the identity of the
substituent groups in the two starting materials. When the organo-
metallic reagent is a boronic acid or boronate ester then a preferred
solvent mixture consists of toluene, ethanol and an aqueous solution of
a base like cesium or sodium carbonate. If instead the organometallic
reagent 19 is an arylstannane, then no additional base is required, and a
polar aprotic solvent such as tetrahydrofuran or dimethylformamide is
employed.
- 20 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
Reaction Scheme F
~a
RR ~, (CRyR9a)~O~PGt RR ~ (CR9R9~~G~PGt
I RtoRtpa Protect ~ I ..,RtnoRtoa
7-azaindole N N
N
I , J Ra or introduce pG2 I ~ ~J R3
3c sv p4 Ro substituent 5 R4
Deprotect X PPh3, DEAD
alcohol Ri ~ (CR~R~)~ON Zn(N3~~2pyr,
RtoRtoa
imidazole, CH2CI2
N N ~ ~ R3 rt, 1-24 h
21
(CR~~ ~3
R~ I I R R a
~N N ~ to to
i
22 PGZ I~..~~3 10% Pd/C, H2,
R~ R. EtOH, rt 1-12 h
The next stage of the synthesis of the novel 7-azaindole
derivatives is illustrated in Scheme F. This sequence of reactions begins
with protection of the ?-azaindole with an amine protecting group (PG2)
to afford compounds of general formula 20. The protection step is
required to avoid competing side rections of the 2-aryltryptophol of
formula 21 (where PG2 is H) in the later conversion of compounds of
formula 21 to compounds of formula 22. The indole protection is followed
by removal of the hydroxyl protecting group (PGt) from the side chain at
the C-3 position of the 7-azaindole ring to afford compounds of general
formula 21. Finally, the hydroxyl group of 21 is converted to a primary
amine of general formula 23 which is then further functionalized as
shown below in the following schemes. The choice of an appropriate
amine protecting group (PG2) for the 7-azaindole is determined
primarily by which protecting group (PGl) is present on the hydroxyl
- 21 -
CA 02326185 2000-09-26
WO 99/St233 PCT/US99/06727
group in the C-3 sidechain, and by consideration of the chemical stability
of the amine protecting group (PGl) required in the remaining steps of
the synthesis. When the hydroxyl protecting group (PGl) is an0-benzyl
ether as illustrated previously in Schemes C and D, the 7-azaindole may
be protected as a carbamate derivative such as a tent-butylcarbamate
(BOC). In this case, the BOC-protected 7-azaindole is stable under the
hydrogenolysis conditions which are used to remove the O-benzyl ether
and it may be conveniently removed at the end of the synthesis using
acidic conditions. If it is desired to synthesize compounds of formula (I)
wherein Ro is alkyl, substituted alkyl or the like, then it is possible to
introduce that aubstituent at this point and the use of a protecting group
and its subsequent removal is not required.
An alcohol of general formula 21 may be converted to
a primary amine of general formula 23 using a variety of methods
known in the literature of organic chemistry. The bottom of Scheme F
illustrates a process where the alcohol 21 is first converted to an azide of
general formula 22, followed by reduction to afford the amine derivative
23. The synthesis of an azide of general formula 22 from alcohols like
21 is best accomplished by performing a Mitsunobu reaction in the
presence of an appropriate azide source such as diphenylphosphoryl
azide or zinc azide pyridine complex. Scheme F illustrates the reaction
of alcohol 21 with triphenylphosphine, diethylazodicarboxylate, zinc
azide pyridine complex and a proton source such as imidazole in an
inert solvent like methylene chloride or tetrahydrofuran. The reaction is
usually conducted at room temperature for periods between 1-24 hours,
typically overnight or about 15 hours, and affords the azide of general
formula 22 in good yield. Finally, an azide of formula 22 may then be
reduced to an amine of formula 23 using one of several methods
common in organic synthesis. One preferred method is catalytic
hydrogenation in a solvent like methanol or ethanol in the presence of a
catalyst such as 10% palladium on carbon. Alternatively, azides like 22
may be reacted with triphenylphosphine to form an iminophosphorane
which upon hydrolysis with water affords the amine of formula 23 and
triphenylphophine oxide.
- 22 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
Reaction Scheme G
NO"(A)R
t
O.
EDC, HOBt,
NMM, CH2C12 Re
(CR9R9a)m N~ I 1~~~~ R2 (A)
Rt0Rt08 ~ r ( ~a m t
R ~~. C )~~ R
- ~ RtpRto~ O
~N N ~ \ R9 X (A) R N N \
RO /v~J ~ ~ ~ '-R3
23 Rs Ro O 26 R° /..~J 25
Rs R4
Et3N, CHZC12
The final stage of the synthesis of the novel 7-azaindole
derivatives (I) involves elaboration of the sidechain at the C-3 position
of the 7-azaindole core. One method for the completion of the synthesis
is illustrated in Scheme G. As shown, the 2-aryltryptamine (23) may be
condensed with a carboxylic acid of type 24 using the coupling reagent
1-(3-dimethylamino-propyl)-3-ethylcarbodiimide hydrochloride (EDC),
1,3-dicyclohexyl-carbodiimide (DCC) or the like with or without 1-
hydroxybenzotriazole (HOBt) and a tertiary amine base such as N
methylmorpholine (NMM), triethylamine or the Iike in an inert organic
solvent such as methylene chloride, chloroform, dimethylformamide, or
mixtures thereof at or near room temperature for a period of 3-24 hours
to provide the corresponding amide derivative (25). Alternatively, 2-
aryltryptamine 23 can be treated with an active ester or acid chloride
of formula 26 in an inert organic solvent such as methylene chloride,
chloroform, tetrahydrofuran, diethyl ether, or the like and a tertiary
amine base such as triethylamine, diisopropylethylamine, pyridine or
the Iike at a temperature of 0°-25°C for 30 minutes to 4 hours
to give
compound 25.
- 23 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
Reaction Scheme H
R8 R2
RR ~. ~CR9R9~m~NyA)Rt
R toRtoa
N ~ ~ ~ R3
Ro ~v~J 27
LiAltiy, THF Rs R4
As shown in reaction Scheme H, the amide carbonyl of 25 can
be reduced by treatment with borane, lithium aluminum hydride, or
equivalent hydride sources in an inert organic solvent such as
tetrahydrofuran, diethyl ether, 1,4-dioxane or the like at about 25°C
to
about 100°C, preferably about 65°C, for a period of 1-8 hours to
give the
corresponding amine 27.
Reaction Scheme I
(CR9R9~~p~ 28
R
R2 O .LA) t
R toRtoe
Ro ~w~J H3 TFA, 3 f~ sieves
Rs R4 NaCNBH3, Me0
As shown in reaction Scheme I, the 2-aryltryptamine 23 can
be modified by treatment with an aldehyde or ketone of type 28 in the
presence of a weak acid such as trifluoroacetic acid (TFA), acetic acid
or the like, with or without a dessicant such as 3l~ molecular sieves or
magnesium sulfate, and a hydride source such as sodium borohydride
or sodium cyanoborohydride, in an inert organic solvent such as
methanol, ethanol, isopropanol, tetrahydrofuran, dichloromethane,
chloroform, or mixtures thereof at a temperature of about 0° to about
- 24 -
CA 02326185 2000-09-26
WO 99/51233 PCTNS99/06727
25°C for a period of 1-12 hours to give the corresponding secondary or
tertiary amine derivative 29.
Reaction Scheme J
OZ NO~
R~ / I
(CR9Ry~~NH~
---
R~~ I RtoRtoa ~ SOzCI
I ~N'
N
I J Rs 2,4,6-collidine,
23 CHzCIZ
/~%~"
Oa / N02
~e
Ri ~ (CR9R9a~aw Rt
cAi
(CR9R9~ NH Rs-
1. PPh3, DEAD, ~N I N l ~ Rto Rtoe
I I \ RtoRtoa benzene ~ ( R
N R° ~ ~ R HO~(A; Rt 31 32 R°
3
w
Rs R4 2. n-propylamine
As shown in reaction Scheme J, the tryptamine 23 can be
modified using the Fukuyama modification of the Mitsunobu reaction
(Fukuyama, T.; Jow, C.-K.; Cheung, M. Tetrachedron Lett. 1996, 36, 6373-
74). The tryptamine 23 may be reacted with an arylsufonyl chloride such
as 2-nitrobenzene-sulfonyl chloride, 4-nitrobenzenesulfonyl chloride or
2,4-dinitrobenzene-sulfonyl chloride and a hindered amine base such as
2,4,6-collidine, 2,6-lutidine or the like in an inert organic solvent such as
methylene chloride to provide the corresponding sulfonamide 30. The
sulfonamides can be further modified by reaction with an alcohol of type
31 in the presence of triphenylphosphine and an activating agent such
as diethylazodicarboxylate (DEAD), diisopropylazodicaboxylate or the
like in an inert organic solvent such as benzene, toluene, tetrahydro-
furan or mixtures thereof to give the dialkylsulfonamide adduct.
Removal of a dinitrobenzenesulfonyl group is accomplished by treatment
with a nucleophilic amine such as n-propylarnine or the like in an inert
-25-
CA 02326185 2000-09-26
WO 99!51233 PCT/US99/06727
organic solvent such as methyiene chloride to give secondary amines of
type 32. When a mono-nitrobenzenesulfonyl derivative is employed, the
removal of the sulfonamide is accomplished with a more nucleophilic
reagent such as thiophenol or mercaptoacetic acid in combination with
lithium hydroxide in DMF.
Reaction Scheme K
Ri ~. (CR9R9a~~OH pZN N02 1. PPh3, DEAD,
Re ~ I I R,o R,~a O ~ I benzene
N N -R + ~~ ~ 2. n-propylamine
3
21 PG2 5v~R4 HN~IAI.R, 33 3. Remove PG2
(CR~~~a~ R,
. ~ LA I
R I I R,o Rtoa
__
~_. R3
RO ~~~i~J
32(Rp=H) Q_ o_
Reaction Scheme K illustrates a method that is
complimentary to reaction Scheme J for completing the synthesis of the
novel compounds of formula (I). Scheme K also employs the Fukuyama
modification of the Mitsunobu reaction similar to that illustrated in
reaction Scheme J. However in this instance, the alcohol partner
employed is a 2-aryltryptophol of general formula 21 which has been
decribed previously in reaction Scheme F. The 2-aryltryptophol (21)
is reacted with a substituted sulfonamide of general formula 33,
triphenylphosphine and diethylazodicarboxylate in a suitable inert
organic solvent such as benzene, tetrahydrofuran, 1,4-dioxane or the
like. The reaction is generally conducted at room temperature for a
period of 2 to 24 hours, typically overnight or for about 12-16 hours. The
product is an N,N-disubstituted sulfonamide which is then separately
- 26 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
subjected to reaction with a base such as n-propylamine which removes
the sulfonamide substituent and furnishes a secondary amine related
to formula 32. The sulfonamides of formula 33 employed are readily
obtained from a primary amine and either 2-nitrobenzenesulfonyl
chloride, 4-nitrobenzenesulfonyl chloride or 2,4-dinitrobenzenesulfonyl
chloride (as shown) in the presence of a hindered amine base such as
2,4,6-collidine, 2,6-lutidine or the like in an inert organic solvent such
as methylene chloride. The final stage of the synthesis requires removal
of the protecting group on the 7-azaindole nitrogen atom (PG2) which
produces a compound of general formula 32 wherein Ro is a hydrogen
atom. It will be recognized by individuals skilled in the art of organic
synthesis that a preference for utilizing either the synthetic sequences
outlined in reaction Schemes J or K will be determined by the
substituents selected to be present in the compounds of formula (I).
The compounds of the present invention are useful in
the treatment of various sex-hormone related conditions in men and
women. This utility is manifested in their ability to act as antagonists
of the neuropeptide hormone GnR.H as demonstrated by activity in the
following in vitro assays.
GnR,H receptor binding assay-,
The ligand binding assay is conducted with the human
GnRH receptor (hGnRHR) obtained from CHO-K1 cells stably expressing
the cloned receptor. Crude membrane suspensions are prepared from
large batches of hGnR,HR-CHO-Kl cells and stored as aliquots at -80°C.
The radioactive peptide ligand (5-(lasIodo-Tyr)-Buserelin] is obtained
from Woods Assays (Portland, Oregon) and has a radioactive specific
activity of 1000 Ci/mmol. The membranes and the radioligand are
diluted in assay buffer which consists of 50 mM Tris-HCl (pH 7.5), 2 mM
MgCl2 and 0.1% bovine serum albumin. The ligand binding assay is
performed at 22°C for 1 hour in 96-well polypropylene plates in a final
volume of 200 ul. Each well in the assay plate contains 0.1 nM l2sl-
buserelin, 10-15 ug of hGnR,H receptor-membrane protein and the test
compound. Test compounds are examined over a range of
-27-
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
concentrations from 0.01 to 10,000 nM. The incubation is terminated by
vacuum filtration onto 96-well Packard GF/C Unifilter plates (pretreated
with 0.1% polyethyleneimine) and then washed with 2 mL of cold
phosphate buffered saline (pH 7.5). The Unifilter plates are dried prior
to addition of scintillation fluid and counting in a Packard TopCount
detector
Phosnhoinositide (PI) turnover assay
Chinese hamster ovary cell lines expressing the human
GnRH receptor functionally coupled to phospholipase C were estal~Iished
and seeded at a concentration of 60,000 cells/mlJwell in inositol-free F12
medium containing 10% dialyzed fetal bovine serum, 1% Pen/Strep, 2
mM glutamine, 500 ~.g/mL 6418 and 1 ~,Ci (~H)inositol in 24-well tray.
Forty-eight hours after seeding, cells were washed with 3 x 1 mL of PBS
containing 10 mM LiCl and treated with various concentrations of
GnRH antagonist for 2 hrs at 37°C before the addition of 0.5 nM
GnRH.
After incubation at 37°C for an additional 60 min, the medium was
removed and the cells were lysed with 1 mL of 0.1 M formic acid. The
trays were freeze-thawed once and the cell extract was applied onto a
Dowex AGl-X8 column. The column was washed with 2 x 1 mL H20 to
remove free (gH)inositol and (3H)inositol phosphates were eluted with 3 x
1 mL 2 M ammonium formate in 1M formic acid. The eluate was then
counted in a scintillation counter.
The compounds of formula I are useful in a number of
areas affected by GnRH. They may be useful in sex-hormone related
conditions, sex-hormone dependent cancers, benign prostatic
hypertrophy or myoma of the uterus. Sex-hormone dependent cancers
which may benefit from the administration of the compounds of this
invention include prostatic cancer, uterine cancer, breast cancer and
pituitary gonadotrophe adenomas. Other sex-hormone dependent
conditions which may benefit from the administration of the compounds
of this invention include endometriosis, polycystic ovarian disease,
uterine fibroids and precocious puberty. The compounds may also be
used in combination with an angiotensin-converting enzyme inhibitor
such as Enalapril or Captopril, an angiotensin II-receptor antagonist
- 2$ -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
such as Losartan or a renin inhibitor for the treatment of uterine
fibroids.
The compounds of the invention may also be useful for
controlling pregnancy, as a contraceptive in both men and women, for
in vitro fertilization, in the treatment of premenstrual syndrome, in the
treatment of lupus erythematosis, in the treatment of hirsutism, in the
treatment of irritable bowel syndrome and for the treatment of sleep
disorders such as sleep apnea.
A further use of the compounds of this invention is as
an adjunct to growth hormone therapy in growth hormone deficient
children. The compounds may be administered with growth hormone
or a compound which increases the endogenous production or release
of growth hormone. Certain compounds have been developed which
stimulate the release of endogenous growth hormone. Peptides which
are known to stimulate the release of endogenous growth hormone
include growth hormone releasing hormone, the growth hormone
releasing peptides GHRP-6 and GHRP-1 (described in U.S. Patent
No. 4,411,890, PCT Patent Pub. No. WO 89/07110, and PCT Patent Pub.
No. WO 89/07111) and GHRP-2 (described in PCT Patent Pub. No.
WO 93/04081), as well as hexarelin (~T. Endocrinol Invest., 1.5(Suppl 4),
45 (1992)). Other compounds which stimulate the release of endogenous
growth hormone are disclosed, for example, in the following: U.S.
Patent No. 3,239,345; U.S. Patent No. 4,036,979; U.S. Patent No. 4,411,890;
U.S. Patent No. 5,206,235; U.S. Patent No. 5,283,241; U.S. Patent No.
5,284,841; U.S. Patent No. 5,310,737; U.S. Patent No. 5,317,017; U.S.
Patent No. 5,374,721; U.S. Patent No. 5,430,144; U.S. Patent No. 5,434,261;
U.S. Patent No. 5,438,136; EPO Patent Pub. No. 0,144,230; EPO Patent
Pub. No. 0,513,974; PCT Patent Pub. No. WO 94/07486; PCT Patent Pub.
No. WO 94/08583; PCT Patent Pub. No. WO 94/11012; PCT Patent Pub.
No. WO 94/13696; PCT Patent Pub. No. WO 94/19367; PCT Patent Pub. No.
WO 95/03289; PCT Patent Pub. No. WO 95/03290; PCT Patent Pub. No.
WO 95/09633; PCT Patent Pub. No. WO 95/11029; PCT Patent Pub. No.
WO 95/12598; PCT Patent Pub. No. WO 95/13069; PCT Patent Pub. No.
WO 95/14666; PCT Patent Pub. No. WO 95/16675; PCT Patent Pub. No.
- 29 -
CA 02326185 2000-09-26
WO 99/51233 PCTNS99/06727
WO 95/16692; PCT Patent Pub. No. WO 95/17422; PCT Patent Pub. No.
WO 95/17423; ' en ~0, 1640-1643 (June 11, 1993); Ann. Ren Med.
Chem., x,177-1$6 (1993); Bioorg. Med. Chem. Ltrs., 4_(22), 2709-2714
(1994); and Proc. Natl. Acad. Sci. USA ~, 7001-7005 (July 1995).
Representative preferred growth hormone secretagoues
employed in the present combination include the following:
1) N-[1(R)-((1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-( 1H-indol-3-yl)ethyl]-2-amino-2-methyl-
propanamide;
2) N-[1(R)-[(1,2-Dihydro-1-methanecarbonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-(1H-indol-3-yl)ethyl]-2-amino-2-methyl-
propanamide;
3) N-[1(R)-[(1,2-Dihydro-1-benzenesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-(1H-indol-3-yl)ethyl]-2-amino-2-methyl-
propanamide;
4) N-[1(R)-[(3,4-Dihydro-spiro[2H-1-benzopyran-2,4'-piperidin]-1'-yl)
carbonyl]-2-{ 1H-indol-3-yl)ethyl]-2-amino-2-methylpropanamide;
5) N-[1(R)-[(2-Acetyl-1,2,3,4-tetrahydrospiro[isoquinolin-4,4'-piperidin]-
1'-yl)carbonyl]-2-(indol-3-yl)ethyl]-2-amino-2-methyl-propanamide;
6) N-[1(R)-((1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-(phenylmethyloxy)ethyl]-2-amino-2-
methylpropanamide;
7) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-{phenylmethyloxy)ethyl]-2-amino-2-
methylpropanamide methanesulfonate;
- 30 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
8) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-(2',6'-difluorophenylmethyloxy)ethyl]-2-
amino-2-methylpropanamide;
9) N-[1(R)-[(1,2-Dihydro-l-methanesulfonyl-5-fluorospiro[3H-indole-3,4'-
piperidin] -1'-yl)carbonyl] -2-(phenylmethyloxy)ethyl]-2-amino-2-
methylpropanamide;
10) N-[1(S)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl) carbonyl]-2-(phenylmethylthio)ethyl]-2-amino-2-
methylpropanamide;
11) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-3-phenylpropyl]-2-amino-2-methyl-
propanamide;
12) N-[1(R)-[(1,2-Dihydro-l-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-3-cyclohexylpropyl]-2-amino-2-methyl-
propanamide;
13) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-4-phenylbutyl]-2-amino-2-methyl-
propanamide;
14) N-[1(R)-[(1,2-Dihydro-1-methanesulfonylspiro[3H-indole-3,4'-
piperidin]-1'-yl)carbonyl]-2-(5-fluoro-1H-indol-3-yl)ethyl]-2-amino-2-
methylpropanamide;
15) N-[1(R)-[(1,2-Dihydro-I-methanesulfonyl-5-fluorospiro[3H-indole-
3,4'-piperidin]-1'-yl)carbonyl]-2-(5-fluoro-1H-indol-3-yl)ethylJ-2-amino-2-
methylpropanamide;
- 31 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
16) N-[1(R)-[(1,2-Dihydro-1-(2-ethoxycarbonyl)methylsulfonylspiro-[3H-
indole-3,4'-piperidin]-1'-yl)carbonyl]-2-(1H-indol-3-yl)ethyl]-2-amino-2-
methylpropanamide;
17) N-[1(R)-[(1,2-Dihydro-1,1-dioxospiro[3H-benzothiophene-3,4'-
piperidin] -1'-yl)carbonyl] -2-(phenylmethyloxy)ethyl] -2-amino-2-
methylpropanamide;
and pharmaceutically acceptable salts thereof.
The compounds of the invention may also be used in
combination with bisphosphonates (bisphosphonic acids) and other
agents, such as growth hormone secretagogues, for the treatment and
the prevention of disturbances of calcium, phosphate and bone
metabolism, in particular, for the prevention of bone loss during therapy
with the GnRH antagonist, and in combination with estrogens,
progesterones and or androgens for the prevention or treatment of bone
loss or hypogonadal symptoms such as hot flashes during therapy with
the GnRH antagonist.
Bisphosphonates (bisphosphonic acids) are known to inhibit
bone resorption and are useful for the treatment of bone lithiasis as
disclosed in U.S. Patent 4,621,077 to Rosini, et al.
The literature discloses a variety of bisphosphonic acids
which are useful in the treatment and prevention of diseases involving
bone resorption. Representative examples may be found in the
following: U.S. Patent No. 3,251,907; U.S. Patent No. 3,422,137; U.S.
Patent No. 3,584,125; U.S. Patent No. 3,940,436; U.S. Patent No. 3,944,599;
U.S. Patent No. 3,962,432; U.S. Patent No. 4,054,598; U.S. Patent No.
4,267,108; U.S. Patent No. 4,327,039; U.S. Patent No. 4,407,761; U.S.
Patent No. 4,578,376; U.S. Patent No. 4,621,077; U.S. Patent No. 4,624,947;
U.S. Patent No. 4,746,654; U.S. Patent No. 4,761,406; U.S. Patent No.
4,922,007; U.S. Patent No. 4,942,157; U.S. Patent No. 5,227,506; U.S.
Patent No. 5,270,365; EPO Patent Pub. No. 0,252,504; and J.J. OrE. hem.,
~, 3843 (1971).
- 32 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
The preparation of bisphosphonic acids and halo-
bisphosphonic acids is well known in the art. Representative examples
may be found in the above mentioned references which disclose the
compounds as being useful for the treatment of disturbances of calcium
or phosphate metabolism, in particular, as inhibitors of bone resorption.
Preferred bisphosphonates are selected from the group of
the following compounds: alendronic acid, etidrononic acid, clodronic
acid, pamidronic acid, tiludronic acid, risedronic acid,
6-amino-1-hydroxy-hexylidene-bisphosphonic acid, and 1-hydroxy-
3(methylpentylamino)-propylidene-bisphosphonic acid;
or any pharmaceutically acceptable salt thereof. A particularly
preferred bisphosphonate is alendronic acid (alendronate), or a
pharmaceutically acceptable salt thereof.' An especially preferred
bisphosphonate is alendronate sodium, including alendronate sodium
trihydrate. Alendronate sodium has received regulatory approval for
marketing in the United States under the trademark FOSAMA~~.
Additionally, a compound of the present invention
may be co-administered with a 5a-reductase 2 inhibitor, such as
finasteride or epristeride; a 5a-reductase 1 inhibitor such as 4,7x-
dimethyl-4-aza-5a-cholestan-3-one, 3-oxo-4-aza-4,7~i-dimethyl-16(3-(4-
chlorophenoxy)-5a-androstane, and 3-oxo-4-aza-4,7(3-dimethyl-16(3-
(phenoxy)-5a-androstane as disclosed in WO 93/23420 and WO 95/11254;
dual inhibitors of 5a-reductase 1 and 5a-reductase 2 such as 3-oxo-4-aza-
17(3-(2,5-trifluoromethylphenyl-carbamoyl)-5a-androstane as disclosed in
WO 95/0?927; antiandrogens such as flutamide, casodex and cyproterone
acetate, and alpha-1 blockers such as prazosin, terazosin, doxazosin,
tamsulosin, and alfuzosin.
Further, a compound of the present invention may be
used in combination with growth hormone, growth hormone releasing
hormone or growth hormone secretagogues, to delay puberty in growth
hormone deficient children, which will allow them to continue to gain
height before fusion of the epiphyses and cessation of growth at puberty.
Further, a compound of the present invention may be
- 33 -
CA 02326185 2000-09-26
WO 99/51233 PCTNS99/06727
used in combination or co-administered with a compound having
luteinizing hormone releasing activity such as a peptide or natural
hormone or analog thereof. Such peptide compounds include
leuprorelin, gonadorelin, buserelin, triptorelin, goserelin, nafarelin,
histrelin, deslorelin, meterlin and recirelin.
For combination treatment with more than one active
agent, where the active agents are in separate dosage formulations, the
active agents may be administered separately or in conjunction. In
addition, the administration of one element may be prior to, concurrent
to, or subsequent to the administration of the other agent.
The pharmaceutical compositions containing the active
ingredient may be in a form suitable for oral use, for example, as tablets,
troches, lozenges, aqueous or oily suspensions, dispersible powders or
granules, emulsions, hard or soft capsules, or syrups or elixirs.
Compositions intended for oral use may be prepared according to any
method known to the art for the manufacture of pharmaceutical
compositions and such compositions may contain one or more agents
selected from the group consisting of sweetening agents, flavoring
agents, coloring agents and preserving agents in order to provide
pharmaceutically elegant and palatable preparations. Tablets contain
the active ingredient in admixture with non-toxic pharmaceutically
acceptable excipients which are suitable for the manufacture of tablets.
These excipients may be for example, inert diluents, such as calcium
carbonate, sodium carbonate, lactose, calcium phosphate or sodium
phosphate; granulating and disintegrating agents, for example, corn
starch, or alginic acid; binding agents, for example starch, gelatin or
acacia, and lubricating agents, for example, magnesium stearate,
stearic acid or talc. The tablets may be uncoated or they may be coated
by known techniques to delay disintegration and absorption in the
gastrointestinal tract and thereby provide a sustained action over a
longer period. For example, a time delay material such as glyceryl
monostearate or glyceryl distearate may be employed. They may also be
coated by the technique described in the U.S. Patent 4,256,108; 4,166,452;
and 4;265,874 to form osmotic therapeutic tablets for control release.
- 34 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
Formulations for oral use may also be presented as hard
gelatin capsules wherein the active ingredient is mixed with an inert
solid diluent, for example, calcium carbonate, calcium phosphate or
kaolin, or as soft gelatin capsules wherein the active ingredient is mixed
with water or an oil medium, for example peanut oil, liquid paraffin, or
olive oil.
Aqueous suspensions contain the active material in
admixture with excipients suitable for the manufacture of aqueous
suspensions. Such excipients are suspending agents, for example
sodium carboxymethyl-cellulose, methylcellulose, hydroxy-propylmethy-
cellulose, sodium alginate, polyvinyl-pyrrolidone, gum tragacanth and
gum acacia; dispersing or wetting agents may be a naturally-occurring
phosphatide, for example lecithin, or condensation products of an
alkylene oxide with fatty acids, for example polyoxyethylene stearate,
or condensation products of ethylene oxide with long chain aliphatic
alcohols, for example heptadecaethylene-oxycetanol, or condensation
products of ethylene oxide with partial esters derived from fatty acids
and a hexitol such as polyoxyethylene sorbitol monooleate, or
condensation products of ethylene oxide with partial esters derived from
fatty acids and hexitol anhydrides, for example polyethylene sorbitan
monooleate. The aqueous suspensions may also contain one or more
preservatives, for example ethyl, or n-propyl, p-hydroxybenzoate, one or
more coloring agents, one or more flavoring agents, and one or more
sweetening agents, such as sucrose, saccharin or aspartame.
Oily suspensions may be formulated by suspending the
active ingredient in a vegetable oil, for example arachis oil, olive oil,
sesame oil or coconut oil, or in mineral oil such as liquid paraffin. The
oily suspensions may contain a thickening agent, for example beeswax,
hard paraffin or cetyl alcohol. Sweetening agents such as those set forth
above, and flavoring agents may be added to provide a palatable oral
preparation. These compositions may be preserved by the addition of an
anti-oxidant such as ascorbic acid.
Dispersible powders and granules suitable for preparation
of an aqueous suspension by the addition of water provide the active
- 35 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
ingredient in admixture with a dispersing or wetting agent, suspending
agent and one or more preservatives. Suitable dispersing or wetting
agents and suspending agents are exemplified by those already
mentioned above. Additional excipients, for example sweetening,
flavoring and coloring agents, may also be present.
The pharmaceutical compositions of the invention may also
be in the form of an oil-in-water emulsions. The oily phase may be a
vegetable oil, for example olive oil or arachis oil, or a mineral oil, for
example liquid paraffin or mixtures of these. Suitable emulsifying
agents may be naturally-occurring phosphatides, for example soy beans,
lecithin, and esters or partial esters derived from fatty acids and hexitol
anhydrides, for example sorbitan monooleate, and condensation
products of the said partial esters with ethylene oxide, for example
polyoxyethylene sorbitan monooleate. The emuisions may also contain
sweetening and flavouring agents.
Syrups and elixirs may be formulated with sweetening
agents, for example glycerol, propylene glycol, sorbitol or sucrose.
Such formulations may also contain a demulcent, a preservative and
flavoring and coloring agents. The pharmaceutical compositions may
be in the form of a sterile injectable aqueous or oleagenous suspension.
This suspension may be formulated according to the known art using
those suitable dispersing or wetting agents and suspending agents
which have been mentioned above. The sterile in~jectable preparation
may also be a sterile injectable solution or suspension in a non-toxic
parenterally-acceptable diluent or solvent, for example as a solution in
1,3-butane diol. Among the acceptable vehicles and solvents that may
be employed are water, Ringer's solution and isotonic sodium chloride
solution. In addition, sterile, fixed oils are conventionally employed as
a solvent or suspending medium. For this purpose any bland fixed oil
may be employed including synthetic mono- or diglycerides. In addition,
fatty acids such as oleic acid find use in the preparation of injectables.
Compounds of Formula I may also be administered in the
form of a suppositories for rectal administration of the drug. These
compositions can be prepared by mixing the drug with a suitable non-
- 36 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
irritating excipient which is solid at ordinary temperatures but liquid at
the rectal temperature and will therefore melt in the rectum to release
the drug. Such materials are cocoa butter and polyethylene glycols.
For topical use, creams, ointments, jellies, solutions or
suspensions, etc., containing the compound of Formula I are employed.
(For purposes of this application, topical application shall include mouth
washes and gargles.)
The compounds for the present invention can be
administered in intranasal form via topical use of suitable intranasal
vehicles, or via transdermal routes, using those forms of transdermal
skin patches well known to those of ordinary skill in the art. To be
administered in the form of a transdermal delivery system, the dosage
administration will, of course, be continuous rather than intermittent
throughout the dosage regimen. Compounds of the present invention
may also be delivered as a suppository employing bases such as cocoa
butter, glycerinated gelatin, hydrogenated vegetable oils, mixtures of
polyethylene glycols of various molecular weights and fatty acid esters of
polyethylene glycol.
The dosage regimen utilizing the compounds of the
present invention is selected in accordance with a variety of factors
including type, species, age, weight, sex and medical condition of
the patient; the severity of the condition to be treated; the route of
administration; the renal and hepatic function of the patient; and the
particular compound thereof employed. A physician or veterinarian
of ordinary skill can readily determine and prescribe the elective
amount of the drug required to prevent, counter, arrest or reverse
the progress of the condition. Optimal precision in achieving
concentration of drug within the range that yields efficacy without
toxicity requires a regimen based on the kinetics of the drug's
availability to target sites. This involves a consideration of the
distribution, equilibrium, and elimination of a drug. Preferably,
doses of the compound of structural formula I useful in the method
of the present invention range from 0.01 to 1000 mg per adult human
per day. Most preferably, dosages range from 0.1 to 500 mg/day. For
- 37 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
oral administration, the compositions are preferably provided in the
form of tablets containing 0.01 to 1000 milligrams of the active
ingredient, particularly 0.01, 0.05, 0.1, 0.5, 1.0, 2.5, 5.0, 10.0, 15.0,
25.0, 50.0, 100 and 500 milligrams of the active ingredient for the
symptomatic adjustment of the dosage to the patient to be treated. An
effective amount of the drug is ordinarily supplied at a dosage level of
from about 0.0002 mg/kg to about 50 mg/kg of body weight per day.
The range is more particularly from about 0.001 mg/kg to 1 mg/kg of
body weight per day.
Advantageously, the active agent of the present invention
may be administered in a single daily dose, or the total daily dosage
may be administered in dividend doses of two, three or four times
daily.
The amount of active ingredient that may be combined
with the carrier materials to produce a single dosage form will vary
depending upon the host treated and the particular mode of
administration.
It will be understood, however, that the specific dose level
for any particular patient will depend upon a variety of factors including
the age, body weight, general health, sex, diet, time of administration,
route of administration, rate of excretion, drug combination and the
severity of the particular disease undergoing therapy.
The following examples illustrate the preparation of some of
the compounds of the invention and are not to be construed as limiting
the invention disclosed herein.
~ -S~ 1;(N,N-Diisobutvlamino)-2-(2-(3~5- imet ~l~henyl)-3
fl-methyl-2-(4-wridin-4-yl-butvlamino)eth 1~1- "~~ olof2"3-blp idin-
5-yll-2-methylpropan-1-one
- 38 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
CH3
Hs ~ H3 CH3 ~H3
/ \
H CH ~N~ ~ \ CH3 ~ ~ N
3 3
CH3
Step lA: Methyl (S)-2-(3-(2-benzyloxy-1-methylethyl)-2-triethylsilyl-
l~-pyrrolo f 2, 3-bl Ryr~' din-5-yll -2-met~luronanoate
A vigorously stirred suspension of methyl 2-(6-amino-5-
iodopyridin-3-yl)-2-methylpropanoate (4.61 g, 14.4 mmol), (S)-(4-
benzyloxy-3-methylbut-1-ynyl)triethylsilane (4.98 g, 17.3 mmol),
Pd(dppf)C12~CH2C12 (0.59 g, 0.72 mmol) LiCl (0.61 g, 14.4 mmol), Na2C03
(3.82 g, 36.0 mmol) and N,N dimethylformamide (60 mL) was degassed
via three vacuum/nitrogen ingress cycles and the resulting mixture was
heated at approximately 90°C overnight. After cooling to ambient
temperature, the reaction mixture was diluted with ethyl acetate and
filtered through celite~ washing copiously with ethyl acetate. The
filtrate was poured into water and extracted with ethyl acetate (x3).
The combined organic extract was washed with brine, dried (MgS04)
and concentrated in vaccuo. The residue was purified by flash
chromatography on silica gel (gradient elution; 20-25% ethyl
acetate/hexanes as eluent) to give the title compound as a viscous pale
yellow oil (6.11 g).
Step 1B: Methyl (S)-2-[3-(2-benzyloxy-1-methylethyl)-2-iodo-1H
pvrrolo(2.3-blRvridin-5-vll-2-meth" l~Dropanoate
A solution of iodine monochloride (2.83 g, 17.4 mmol) in
MeOH (25 mL) was added over approximately 0.5 h via pressure
equalizing addition funnel to a vigorously stirred mixture of methyl
(S)-2-(3-(2-benzyloxy-1-methylethyl)-2-triethylsilyl-1H pyrrolo[2,3-
b]pyridin-5-yI]-2-methylpropanoate (6.00 g, 13.4 mmol) and silver
tetrafluoroborate (3.39 g, 17.4 mmol) in MeOHPTHF (1:2; 75 mL) at 0°C.
- 39 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
After an additional 0.5h, the reaction was quenched with 1M Na2S203,
warmed to room temperature and filtered through celite~ washing
copiously with ethyl acetate. The filtrate was concentrated under
reduced pressure, poured into 1M NazS203 and extracted with ethyl
acetate (x3). The combined organic extract was washed with brine,
dried (MgS04) and concentrated in vacuo. The residue was purified by
flash chromatography on silica gel (25% ethyl acetate/hexanes as eluent)
to give the title compound as a colourless solid (5.54 g).
Step 1C: Methyl (S)-2-[3-(2-benzyloxy-1-methylethyl)-2-(3,5-dimethyl-
~vl)-1H p3~olof2,3-blp3rridin-5-vll-2-meth l~nropanoate
A vigorously stirred suspension of methyl (S)-2-[3-(2-
benzyloxy-1-methylethyl)-2-iodo-1H-pyrrolo[2,3-b]pyridin-5-yl]-2-
methylpropanoate (4.00 g, 8.12 mmol), 2,5-dimethylphenylboronic acid
(1.83 g, 12.2 mmol) and Pd(dppf)CI2~CH2Cl2 (0.33 g, 0.406 mmol) in
toluene/MeOH (5:2; 140 mL) was degassed via three vacuum/nitrogen
ingress cycles and the resulting mixture was heated to approximately
80°C. 1M Na2C03 (20.3 mL, 20.3 mmol) was added dropwise via syringe
and the resulting mixture maintained at reflux overnight. After cooling
to ambient temperature, the reaction mixture was diluted with ethyl
acetate and filtered through celite~ washing copiously with ethyl acetate.
The filtrate was poured into water and extracted with ethyl acetate (x3).
The combined organic extract was washed with brine, dried (MgS04)
and concentrated in vacuo. The residue was purified by flash
chromatography on silica gel (25% ethyl acetate/hexanes as eluent) to
afford the title compound as a colourless foam (3.$2 g).
Step 1D: (S)-2-[3-(2-Benzyloxy-1-methylethyl)-2-(3,5-dimethylphenyl)-
1H pyrrolof2,3-blRvridin-5~ill-2-meth~pro a~noic acid
A vigorously stirred suspension of methyl (S)-2-[3-(2-
benzyloxy-1-methylethyl)-2-(3,5-dimethyl-phenyl)-1H-pyrrolo [2,3-
b]pyridin-5-yl]-2-methylpropanoate (3.82 g, 8.12 mmol) and 2N KOH (40.6
mL, 0.081 mol) in MeOH (40 mL) was heated at 80°C for approximately 4
h. After cooling to room temperature, the reaction mixture was acidified
- 40 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
to pH 6 with 2N HCl and extracted with ethyl acetate (x3). The combined
organic extract was washed with brine, dried (MgS04) and concentrated
in vacuo. The crude residue was used without further purification in
the subsequent reaction.
Step lE: (S)-1-(N,lV Diisobutylamino)-2-[3-(2-benzyloxy-1-methyl-
ethyl)-2-(3,5-dimethylphenyl)-1H-pyrrolo[2,3-b]-pyridin-5-yl]-
2-methvlnropan-1-one
PyBOP is added to a stirred mixture of (S)-2-[3-(2-benzyloxy-
1-methylethyl)-2-(3,5-dimethylphenyl)-1H pyrrolo-[2,3-b]pyridin-5-yl]-2-
methylpropanoic acid, diisobutylamine and triethylamine in CH2Cl2 at
ambient temperature. After approximately 12h, the reaction mixture
is poured into water/brine (1:1) and extracted with ethyl acetate. The
combined organic extract is washed with brine, dried (MgS04) and
concentrated in vacuo. The residue is purified by flash chromatography
on silica gel (gradient elution; 60-75% ethyl acetate/hexanes as eluent) to
give the title compound.
Step 1F: (S)-1-(N,N Diisobutylamino)-2-[3-(2-benzyloxy-1-methyl-
ethyl)-2-(3,5-dimethylphenyl)-1H-1-(tent-butoxycarbonyl)-
pvrrolof 2,3-bl Ryridin-5-vll-2-meth~prox~an-1-one
Di-tert-butyldicarbonate is added to stirred suspension
of (S)-1-(N,N diisobutylamino)-2-[3-(2-benzyloxy-1-methylethyl)-2-(3,5-
dimethylphenyl)-1H-pyrrolo[2,3-b]pyridin-5-yl]-2-methylpropan-1-one,
DMAP, and potassium carbonate in CH2C12 at room temperature.
After approximately 3h, the reaction mixture is poured into water and
extracted with ethyl acetate. The combined organic extract is washed
with brine, dried (MgS04) and concentrated in vacuo. The residue is
purified by flash chromatography on silica gel (60% ethyl
acetate/hexanes as eluent) to give the title compound.
Step 1G: (S)-1-(N,N Diisobutylamino)-2-[3-(2-hydroxy-1-methyl-
ethyl)-2-(3,5-dimethylphenyl)-1H-1-(tent-butoxycarbonyl)-
pvrrolof2.3-blRyridin-5 yll-2-meth~pronan-1-one
- 41 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
A mixture of (S)-1-(N,N diisobutylamino)-2-[3-(2-benzyloxy-
1-methylethyl)-2-(3,5-dimethylphenyl)-1H-1-(tert-butoxycarbonyl)-
pyrrolo(2,3-b]-pyridin-5-yl]-2-methylpropan-1-one and Pd(OH)2 (Pd-20%)
in glacial acetic acid/EtOH (1:1) is hydrogenated at 50 psi for about 4
days. The resulting mixture is filtered through celite~ washing
copiously with EtOH, the filtrate is evaporated in vacuo and the residue
purified by flash chromatography on silica gel (gradient elution; 75-90%
ethyl acetate/hexanes) to give the title compound.
Step 1H: (S)-1-(N,N-Diisobutylamino)-2-[3-(2-azido-1-methylethyl)-2-
(3,5-dimethylphenyl)-1H-1-(tert-butoxycarbonyl)pyrrolo-
2,3-bl pyridin-5=yll-2-methylpropan-1-one
DEAD is added dropwise via syringe to a stirred solution
of (S)-1-(N,N diisobutylamino)-2-(3-(2-hydroxy-1-methylethyl)-2-(3,5-
dimethylphenyl)-1FI-1-(tert-butoxycarbonyl)pyrrolo[2,3-b]-pyridin-5-yl]-
2-methylpropan-1-one, ZnNs~2py, PPh3 and imidazole in CH2Cl2 at
approximately 0°C. After allowing to warm to ambient temperature
overnight, the reaction mixture is filtered through celite~ washing
copiously with CH2C12, the filtrate is poured into water and extracted
with CH2C12. The combined organic extract is washed with brine, dried
(MgS04) and concentrated in uacuo. The residue is purified by flash
chromatography on silica gel (gradient elution; 25-40% ethyl
acetate/hexanes as eluent) to give the title compound.
Step lI: (S)-1-(N,lV Diisobutylamino)-2-[3-(2-amino-1-methyl-
ethyl)-2-(3,5-dimethylphenyl)-1H-1-(tert-butoxycarbonyl)-
p, o~lof2.3-bl-Rvridin-5-yll-2-methylnropan-1-one
A mixture of (S)-1-(N,N diisobutylamino)-2-[3-(2-azido-
1-methylethyl)-2-(3,5-dimethylphenyl)-1H-1-(tert-butoxycarbonyl)
pyrrolo[2,3-b]pyridin-5-yl]-2-methylpropan-1-one and Pd/C (Pd-10%) in
MeOH/EtOH is hydrogenated at 50 psi overnight. The resulting mixture
is filtered through celite~ washing copiously with EtOH, the filtrate is
evaporated in uacuo and the residue purified by preparative thin layer
- 42 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
chromatography on silica gel (double elution; 100%ethyl acetate as
eluent) to give the title compound.
Step 1J: (S)-1-(N,1V Diisobutylylamino)-2-{2-(3,5-dimethylphenyl)-3-
[1-methyl-2-(4-pyridin-4-yl-butylamino)ethyl]-1H-1-(tert-
butoxycarbonyl)pyrrolo [2, 3-b] pyridin-5-yl }-2-methyl-
propan-1-one
A solution of 4-(pyridin-4-yl)butanal in chloroform is added
via syringe to a stirred suspension of (S)-1-(N,N diisobutylamino)-2-[3-(2
amino-1-methylethyl)-2-(3,5-dimethylphenyl)-1H-1-(tert-butoxycarbonyl)
pyrrolo[2,3-b]pyridin-5-yl]-2-methylpropan-1-one and magnesium sulfate
in chloroform at 0°C. After approximately 1 h, sodium borohydride
followed by MeOH is added and the resulting mixture allowed to warm
to room temperature. After 2 h, the reaction mixture is quenched with
saturated aqueous NaHC03, poured into water and extracted with ethyl
acetate. The combined organic extract is washed with brine, dried
(MgS04) and concentrated in vacuo. The residue is purified by
preparative thin layer chromatography on silica gel (100% ethyl
acetate as eluent) to give the title compound.
Step 1K: (S)-1-(N,lV Diisobutylamino)-2-{2-(3,5-dimethylphenyl)-
3-[1-methyl-2-(4-pyridin-4-yl-butylamino)ethyl]-1H
Rvrrolo f 2.3-blpyridin-5-yll-2-methylpropan-1-one
A solution of (S)-1-(N,N diisobutylamino)-2-{2-(3,5-
dimethylphenyl)-3-[1-methyl-2-(4-pyridin-4-yl-butylamino)ethyl]-1
H-1-(tert-butoxycarbonyl)pyrrolo[2,3-b]pyridin-5-yl}-2-methylpropan-
1-one in trifluoroacetic acid/dichloromethane (1:2) is aged for
approximately 3 h. The resulting mixture is concentrated in vacuo and
the residue partitioned between saturated aqueous NaHC03 and CH2C12.
The organic phase is separated and the aqueous phase re-extracted with
CH2C12. The combined organic extract is washed with brine, dried
(MgS04) and concentrated in vacuo. The residue is purified by flash
chromatography on silica gel [NH40H/MeOH/CHZC12 (1:4:95) as eluent]
to give the title compound.
- 43 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
PREPARATION OF SYNTHETIC INTERMEDIATES
Methvl 2-(6-amino-5-iodo,pvridin-3-vl)-2-methylpronanoate
Step A: EthyL2-met yl-2-( 'din- ~1)uropanoate
A solution of ethyl-3-pyridylacetate ( 10.0 g, 60.5 mmol) in
THF (50 mL) was added over approximately 0.5 h via pressure equalizing
addition funnel to a stirred mixture of potassium hexamethyldisilazane
(48.3 g, 0.242 mmol) in THF (250 mL) at -20°C. After 0.5 h, MeI was
added dropwise so as to maintain the internal temperature between
-15°C to -20°C. After warming to approximately 0°C over
12h, the
reaction was quenched with saturated aqueous NH4C1 and concentrated
under reduced pressure. The residue was partitioned between ethyl
acetate and water, the organic phase separated and the aqueous phase
re-extracted with ethyl acetate (x3). The combined organic extract was
washed with brine, dried (MgS04) and concentrated in vacuo. The
residue was purified by flash chromatography on silica gel (30% ethyl
acetate/hexanes as eluent) to give the title compound as a pale yellow oil
(10.1 g, 86%).
Step B: Ethyl 2-methyl-2-(Ryridin-3-yl)RroDanoate-N-oxide
Meta-chloroperoxybenzoic acid (55%; 21.3 g, 67,9 mmol)
was added in one portion to a vigorously stirred emulsion of ethyl 2-
methyl-2-(pyridin-3-yl)propanoate and NaHC03 (17.5 g, 0.209 mmol)
in water/chloroform ( 1:1; 500 mL) at ambient temperature. After
approximately 12 h, the organic phase was separated and the aqueous
phase extracted with chloroform (x3). The combined organic extract
was washed with brine, dried (MgS04) and concentrated in vacuo. The
residue ( 10.9 g, 100%) was judged pure by a combination of TLC and 'H
nmr analysis and was used without further purification in the
subsequent reaction.
CA 02326185 2000-09-26
WO 99/51233 PCTNS99/06727
Step C: Ethyl 2-[6-(2,2-dimethyl-4-oxo-4H-1,3-benzoxazin-3-yl)-
pvridin-3-vll-2-methyl~panoate
A solution of 4-chloro-2,2-dimethyl-2H-1,3-benzoxazine
(10.1 g, 51.6 mmol) and ethyl 2-methyl-2-(pyridin-3-yl)propanoate-
N oxide (9.0 g, 43.0 mmol) in 1,2-dichloroethane (100 mL) was heated
at approximately 90°C for 3 d. After cooling to room temperature, the
solvent was evaporated under reduced pressure and the residue purified
by flash chromatography on silica gel (gradient elution; 20-25% ethyl
acetate/hexanes as eluent) to give the title compound as a pale yellow
viscous oil ( 14.3 g, 90%).
Step D: 2-(6-Aminopyridin-3-yl)-2-methylpropanoic acid
hydrochloride
A stirred mixture of ethyl 2-[6-(2,2-dimethyl-4-oxo-4H-1,3-
benzoxazin-3-yl)-pyridin-3-yl]-2-methylpropanoate (13.3 g, 36.1 mmol) in
concentrated HCl (50 mL) was heated at 100°C overnight. After cooling
to ambient temperature, the reaction mixture was concentrated in vacuo
and the residue washed exhaustively with chloroform to give the title
compound as a colourless solid (6.90 g, 88%).
Step E: Methvl 2-(6-aminoRyridin-3-vl)-2-methyl_propanoate
A solution of 2-(6-aminopyridin-3-yl)-2-methylpropanoic
acid hydrochloride (35.0 g, O.I62 mmol) and concentrated H2S04 (5 mL)
in MeOH (250 mL) was heated at reflux overnight. After cooling to
ambient temperature, the reaction was quenched cautiously with
saturated aqueous NaHC03 and concentrated in vacuo. The residue was
partitioned between ethyl acetate and water, the organic phase separated
and the aqueous phase re-extracted with ethyl acetate (x3). The
combined organic extract was washed with brine, dried (MgS04) and
concentrated in vacuo. The residue (31.6 g, 100%) was judged pure by a
combination of TLC and 1H nmr analysis and was used without further
purification in the subsequent reaction.
Step F: Met vl 2-l6-amino-5-iodo~vridin-3-vl)-2-methylnronanoate
- 45 -
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
A solution of methyl 2-(6-aminopyridin-3-yl)-2-methyl-
propanoate (10.0 g, 51.5 mmol) in MeOH (50 mL) was added over
approximately 0.5 h via pressure equalizing addition funnel to a
vigorously stirred suspension of iodine (17.0 g, 67.0 mmol) and
silver trifluoroacetate (14.8 g, 67.0 mmol) in MeOH (200 mL) at room
temperature. After 3 h, the reaction was quenched sequentially with 1M
Na2S203 followed by saturated aqueous NaHCOs. The resulting mixture
was filtered through celite~ washing copiously with ethyl acetate, the
filtrate concentrated in vacuo and the residue partitioned between ethyl
acetate and water. The organic phase was separated and the aqueous
phase was re-extracted with ethyl acetate (x3). The combined organic
extract was washed with brine, dried (MgS04) and concentrated in
vacuo. The residue was purified by flash chromatography on silica gel
(gradient elution; 35-40% ethyl acetate/hexanes as eluent) to give the title
compound as an off white solid (10.3 g, 62%).
(S'7-(4-Benzvloxv-3-methylbut-1-ynyl_)triethvlsilane
Step AA: Methyl (S7-3-benzvloxy-2-methv~,propano~te
An oven dried 1 L single-necked round bottom flask was
equipped with a magnetic stir bar and then charged sequentially with
15.948 g (0.135 mol) of (R)-(-)-methyl-3-hydroxy-2-methylpropanoate,
carbon tetrachloride (150 mL), cyclohexane (300 mL), and 35.796 g (0.142
mol) of benzyl 2,2,2-trichloroacetimidate. Trifluoromethanesulfonic acid
(0.8 mL; 9.0 mmol) was added to the solution and the resulting mixture
was stirred for 16 h at room temperature under an NZ atmosphere. The
reaction mixture was then filtered and the filtrate concentrated in
vacuo. The residue was redissolved in 150 mL EtOAc and extracted
with saturated aqueous NaHC09 (1x100 mL). The organic layer was
washed with saturated NaCI, dried (MgS04), filtered and evaporated.
The residual oil was purified on a silica gel flash chromatography
column eluted with 10% EtOAc-hexane. The purified fractions were
combined and evaporated in vacuo to afford 20.787 (74%) of the title
compound as a colorless oil.
-46-
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
MS (CI): mle = 209 (M+1).
Step BB: (S'7-3-Benzvloxv-2-methyhropan-1-of
An oven dried three-necked 2 L round bottom flask was
equipped with a mechanical stirrer, a reflux condenser and a 500 mL
constant pressure addition funnel. The flask was charged with a
solution of 62.252 g (0.299 mol) of methyl (S)-3-benzyloxy-2-methyl-
propanoate in 600 mL of anhydrous THF, and a 1.0 M solution of lithium
aluminum hydride (300 mL; 0.3 mol) was transferred into the dropping
funnel via a cannula. Stirring was started and the lithium aluminum
hydride solution was added over 45 minutes to the reaction under
an N2 atmosphere while the temperature of the reaction mixture was
maintained between 25-30°C using an external ice-water bath. After
the addition was complete, the reaction was stirred an additional 6 h at
room temperature at which point TLC analysis (20% EtOAc-hexane)
indicated complete reaction. The reaction mixture was then cooled with
an external ice-water bath and quenched by serial addition of 11.4 mL
water, 11.4 mL of 15% aqueous NaOH, and 34.2 mL water. The reaction
mixture was then filtered, the solids were washed with EtOAc, the
filtrate and washings were combined and evaporated in vacuo. The
residue was redissolved in EtOAc, washed with 10% aqueous NaHS04,
saturated NaCI, dried (MgS04), filtered and evaporated. The residue
was purified by Kugelrohr distillation to afford 47.67 g (89%) of the title
compound as a colorless oil.
MS (CI): mle = 181 (M+1).
Step CC: (S)-3-Benzyloxv-2-methyl~,r ~ .~an_a 1
An oven dried three-necked 2 L round bottom flask was
equipped with a mechanical stirrer, a thermometer, an NZ inlet, and
a septum. The flask was charged with 24.050 g (0.189 mol) of oxalyl
chloride and 425 mL CH2Cl2. The reaction mixture was stirred under an
N2 atmosphere and cooled to -78°C with an external dry ice-acetone
bath.
A solution of methyl sulfoxide (29.607 g; 0.379 mol) in 85 mL CH2C12 was
then added over 5 min to the reaction mixture via cannula. After the
-47-
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
adition, the reaction was stirred an additional 5 min and then a solution
of 31.048 g (0.172 mol) of (S)-3-benzyloxy-2-methylpropan-1-of in 170 mL
CH2C12 was added via cannula. When the second addition was
completed the reaction mixture was stirred for 15 min at -78°C then
111.32 g (0.861 mol) of N,N diisopropylethylamine was added via syringe.
The reaction mixture was stirred an addition 15 min at -78°C, the
cooling
bath was removed and the reaction was allowed to warm. When the
internal temperature had reached -15°C, 350 mL of a 10% aqueous
NaHS04 solution was slowly added and the mixture was transferred
to a separatory funnel. The organic layer was separated, washed with
aqueous NaHS04 (2x250 mL), saturated NaCI, dried (MgS04), filtered
and evaporated. The residue was used immediately in the next step
without further purification.
Step DD: (S)-4-Benzyloxy-1,1-dibromo-3-methylbutene
An oven dried three-necked 2 L round bottom flask was
equipped with a mechanical stirrer, a thermometer, an N2 inlet,
and a septum. The flask was charged with 180.71 g (0.689 mol) of
triphenylphosphine and 925 mL of CHZC12. The reaction mixture was
stirred under an N2 atmosphere and cooled to 0-5°C with an external ice-
water bath. The septum was then removed and 114.25 g (0.344 mol) of
carbon tetrabromide was added in portions through the open neck of the
flask at a rate that maintained the temperature of the reaction mixture
below 20°C. After the addition was complete the reaction was stirred
for
1 h and then a solution of the (S)-3-benzyloxy-2-methylpropanal from the
previous step dissolved in 150 mL of CH2C12 was added via cannula over
a 5 min period. The reaction mixture was stirred under NZ for an
additional 1 h and allowed to warm to room temperature. A separate
10 L three-necked round bottom flask was equipped with a mechanical
stirrer and charged with 4 L of hexane. The stirrer was started and the
crude reaction mixture was introduced as a slow stream which resulted
in formation of a granular precipitate. After the transfer was complete
the reaction mixture was filtered and the solids were carefully washed
with hexane. The filtrate was evaporated in vacuo and additional solids
- 48 -
CA 02326185 2000-09-26
WO 99/51233 , PCT/US99/06727
were deposited. The residue was resuspended in hexane, filtered and
the filtrate reevaporated. The resulting oii was purified by Kugelrohr
distillation to afford 46.54 g (81% for two steps) of the title compound as a
colorless oil.
Step EE: (S'7-(4-benzvloxy-3-methvlbut-1-~~yl)triethylsilane
An oven dried 100 mL single-necked round bottom flask was
equipped with a magnetic stir bar and a septum then charged with 5.171
g (15.5 mmol) of (S)-4-benzyloxy-1,1-dibromo-3-methylbutene and 20 mL
of anhydrous THF. The reaction mixture was stirred at -78°C under an
N2 atmosphere and 12.4 mL of a 2.5 M solution of n-butyllithium (31.0
mmol) was added dropwise via syringe over 15 min. The reaction
mixture was stirred at -78°C for an additional 1 h, then quenched with
10% aqueous NaHS04 and extracted into EtOAc. The organic layer was
washed with water (3x25 mL), sturated NaCI, then dried (MgS04),
filtered, and evaporated. The residue was purified by Kugelrohr
distillation to afford 3.999 g (90%) of the title compound as a colorless oil:
Following procedures similar to those described above, the
following compounds are prepared:
CH3
H3 ~ ___
N -(A)-R~
CH3
H3 C
CH3
Ex. # -(A)-R 1
lA
,N
-49-
CA 02326185 2000-09-26
WO 99/51233 PCT/US99/06727
I1
,N
1C I \ Me
~N~
O
\ Me
I
/N\
O
IE I \ N,N
N
H
1F I \
N
N
H
1G / N.Me
O
- 50 -