Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
10-JGr-GKJrJYJ LO~Gl I'IHIfITJ 6 JhlUlKt i'JGIO -ftiJb YJI~I'Jl h'.1~~1~J1~
CA 02327169 2000-09-29
wo ms332~ rcrrcs9sro~o99
-1-
S()L1D PHASE TEST FOR ~NDOTOXIN
" This invention relates to a solid phase test for endotoxin, in particular.a
test such
as in a flat sheet or in capillary form for the detection of endotoxin in
aqueous
solutions. More particularly the invention relates to a direct solid phase
chromogenic assay, which permits a quick biochemical identification of
endotoxin
and is sensitive to pico~3ram endotoxin~amounts.
There is a high demand for testing for contamination of solutions and
biological
fluids in medicine and iriotechnologies; for instance, the quality of water
used for
the dilution of solute concentrates for hemodialysis. Specifically, there is a
need
for pyrogen free water to make dialysate, and far monitoring of the dialysate
in the
dialysis machine itself in centres or at home or other non-laboratory
settings. (n
this respect, the production of sterile and pyrogen free dialysate needs a
pyrogen
control method which will give a 'yes" or "contaminated" result. Endotoxin,~ a
potent pyrogen specifically from gram-negative bacterial cell wall components,
is
one of the mast known key contaminants. Measurements of endotoxin are today
used as the major safe-y parameter of infusions and fluids in general, drugs
and
devices, in the pharma:ology and food industry.. The most available method to
qualitatively and quanti-:atively detect endotoxin in biological or aqueous
solutions
is based on the limulus amoebocyte lysate (LAL) test known in the art.
The LAL test was dis~:overed in 196ø by Levin and Bang' . Horseshoe crab
(limulus) amoebocyte lysate provokes together with endotoxin from gram-
negative
bacteria a gelatination induced by a stepwise activation of several
coagulation
factors contained in tile lysate. An assay comprising the extract (lysate) of
horseshoe crab blood cell (amoebocyte) to induce this ge(atination in a
reagent
glass as a liquid phasE system is welt known as a method and it is called the
limulus test. The application of the LAL to measure endvtoxin involves the
gelatination of the sample (gelation method, turbidimetry assay) in the
reagent
glass or a more sen;,itive method based on a chromogenic substrate and
measurement by optics absorption. Today different LAL from horseshoe crab I
are
SUBSTrTUT>w SHEET (RUE~E 26)
sa-5tr-~t~ m: ~~ MH I HY5 7S ShIU 1 Kt d'~I~ ~rase dem r. tai
CA 02327169 2000-09-29
WD 99153322 , PCT1GB991Q1099
-2-
commercialized through the world: Limulus Polyplfemus, Tchypleus tridendatrrs,
Tachypleus gigas and Carcinoscorpius rotundicauda.
r.'
Al! commercialized LAL testing methods based on a chromogenic substrate are
liquid-phase assays. Th~. principle is that in a reagent glass containing a
lysate and
sample, bacterial sndo~toxin contained in the sample initiates activation of a
cascade of serine protease (Factor 8, Factor C, Proclotting enzyme, Coagulin)
enzymes in LAL that cl~:ave the end product coagulogen into a peptide coagulin
which produces clotting. The use of a synthetic chromogenic or fluorogenic
substrate increases the sensitivity of the testing and makes the
quantification of
endotoxin possible. As described by Iwanaga et al', a synthetic peptide is
used
having an amino acid sequence in common with the hydrolysis sites of
coagulogen,
namely the chromogenic substrate Boc-Leu-Gly-Arg-p-nitraanilide IpNA~ or the
fluoro-genie substrate Box-Leu-Gly-Arg-4-methylcoumaryf-7-amide. !n the
presence
of LAL and endotoxin, the colourless substrate is rapidly cleaved to form the
chromophore p-nitroaniline or the fiuorogenic 4-methylcoumaryl-7-amide.
There are today increasing improvements in the accuracy and, sensitivity of
liquid
phase assays: in US patent 5 605 806, S. Tanaka and H. Tamura proposed a LAL
and an antibody to ne~~traiize the false positive results due to (1 -> 3)-Q-
glucan.
Shigenori et a! proposed the use of factor G activation to resolve the same
problem
(US patent 5.641,643?. Besides the liquid phase reaction systems, a method for
lysate immobilization tc~ wells of a polystyrene microplate has been
published. In
US patent 5,550,30, Tanaka disclosed a quantitative kit for endotoxin
determination which comprises an insoluble carrier vn which is immobilized an
endotoxin-sensitive factor derived from a limulus amoebocyte which
specifically
reacts with endotoxin Yr'rthout reacting with 13-glucan. The test system
involves the
indication of endotoxi~ when colouring occurs in the liquid phase.
Quick and simple testing for endotoxin is important in a number of
circumstances,
including when changilg the dialysis machine, or monitoring tap water. It is
also
desirable to determine endotoxin levels of the fluids in a laboratory-setting.
The
SUBSTITUTE SHEET (RULE 26)
1a-5tr-~~d lb: ~~ f9H ~ hirb 2~ 51~u1Kt nee rrx~e~ eiemi r. no.,~r~
CA 02327169 2000-09-29
w0 99I533Z2 PCTIGB99101099
-3-
degree of contaminati~~n of water and dialysate fluids falls into several
categories,
but in all cases it is de~.irable to monitor endotoxin present in the fluid on
a frequent
-' basis. A simple and quick method for determining qualitatively endotoxin in
a-test
solution is thus much sought in the art.
The present mventio.~ provides an analytical test strip for the detection of
endotoxin in aqueous .solutions in which the strip is wetted at the bottom
with the
sample. After a certain period of time, until the sample flows from the bottom
to
the top, an indicator is added at the top, to develop a red colour which is
indicative
for the presence or the absence of endotoxin in the sample.
Using a technique of a solid phase chromogenic substrate assay, the present
invention involves an improvement to a quick test strip for the detection of
endotoxin. The test strip provides a dry analytical element for endotoxin
which
comprises an absorbent carrier divided in four portions impregnated with: 1 ).
a
reagent system of lim~:lus factors; 2) synthetic chromogenic substrate
derivatized
with' p-nitroaniline; .3) .3 buffer system containing chloride acid and sodium
nitrite;
41 a buffer system containing chloride acid and ammonium. The test afro
includes
a solution containing 111-naphtylethylenediamine which .is added at the top of
the
_ test trip to develop a coloration by coupling the derivatized p-NA- to ~N1-
napthylethylenediamine as positive results.
T'he present invention provides apparatus and method as,defined in the claims,
in
particular an analysis method comprising applying a aqueous sample solution to
the
bottom of the described dry analytical element to react with endotoxin, and
determining coloratioi on the top of the dry element by coupling N1-
napthylethylenediamine. The present invention also provides an endotoxin kit
using
- the above described dry analytical element.
A further object of this; invention is to provide a method for preparing the
above
mentioned dry analytical element andlor the portions of the dry analytical
element
for preparing the above described endotoxin test strip.
SUBSTITUTl; SHEET (RULE 26)
18-SEP-2000 16~23 MHTHYS 8 SQUIRE 020 ?830 0001 P.0?/30
CA 02327169 2000-09-29
WO 99153322 - PCTIGB99101099
The method involved in the invention is a direct solid phase chromogenic
caiorimetrical detection. Four supports are arranged successively in the same
alignment to achieve << flow communi6ation with each other. The supports are '
-
mounted in a closed arid pyrogen free casting module, The test strip comprises
a
solid support having four portions (at least three portions) being in flow
communication with ~:ach other whereby reaction products can flow from one
portion to the next. The solid support is preferably shaped in the form of
strip,
with the first, second, third and fourth portions being arranged on the strip
in the
same plane. The size:; of the portion may be the same or different. The
portions
are manufactured to absorb the different chemicals and dried, and attached on
the
strip in a way that flow communication occurs from one to the next.
The test strip would be submerged with the sample at the bottom to wet the
first
portion and allow liquid to flow from bottom to the top of the test strip. The
sample solution woulc start to flow by capillary action from one portion to
the next
higher portion. Thus, the reactants occurring in each portion would
be.transported
by capillary flow to th~: last portion to develop a red coloration as a
positive result.
The first portion contains a solid support and a (imulus -amaebocyte lysate or
substances containirn3 endotoxin sensitive factors which react with endotoxin
to
produce coagulin. ~he method of the immobilization of the lysate or of the
endotoxin sensitive f~sctor to the solid support are: 1 ) absorption: 2)
adsorption by
ionic forces orland hydrophobic interaction, 3y covalent binding. The
procedures
for binding the lysate in the solid support are generally known the art. The
lysate
may be the commercial available limulus amoebocyte fysate such as Limulus
Polyphemus, Tchyp~eus iridendafus, Tachypleus gigas and Carcinoscorpius
~otundicauda. Preferably a lysate without Factor G may used in order to avoid
a
positive false-reaction with i~-31-f3-WGlucan contained in the sample or in
the solid
support. A purified form of a mixture containing factors C. B and prociotting
enzymes, which react with- endotoxin to produce coagulin may also be used.
Preferably a lysate preparation known in the art without Factor G which
does.not
react with (1-3Lf3-D-Gtucan may also be used. The amount and biological
activity
SUBSTITUTE SHEEt (RUSE 25)
18-SEP-2000 16:23 MRTHYS & 5QU1RE bid 'ftiib bbdl h'.btii,5b
CA 02327169 2000-09-29
WO 99/53322 PCT/GB99I01099
-5-
of endotoxin reagents, and the material and size of the solid support
determine the
sensitivity of the test strip. Upon wetting of the solid support with the
sample,
sample.~houid react with immobilized lysate or substances containing endotoxin
factors to activate Factor and clotting enzymes. Depending upon the presence
of
endotoxin in the samples, endotoxin activates the bound lysate in the
following
reaction steps:
t1 f Endatoxin -> activated Factor C
t21 activated Factor C -~ Factor B -> activated Factor B
(3) activated Factor 8 -> Praclotting enzyme
The clotting enzyme produced through the cascade reaction by the action of
activated Factor C or proclotting enzymes is capable of hydrolysing coagu(in
or an
amide linkage of a synthetic peptide substrate at the specific sites
intermediate
between Arg and GIy ~~r intermediates between Arg and Thr.
The solid support which is- employed in this test strip is one which is
capable of
absorbing endotoxin i~rom the sample. When wetted, the sample containing
endotoxin reacts with reagents in the first portion and the reaction products
are
transported by flow to the dry region of the first support and thus to the
second
portion of the test strip. In addition, the solid support is one which is
capable of
absorbing both sample: and binder. In order to achieve a flow communication
between the portion, it is preferable to use an hydrophillic polymers solid
support.
Examples of suitable supports are chromatographic papers, nitrocellulose,
dextran
and glass fibres. Preverabiy, chromatographic hydrophobic polymers are used
though if using chrom:~tography papers, it is important to use reagents which
do
not react with -(3-gluca~, as described for example in US patent 5,550,030.
The second portion contains a solid support and a synthetic substrate. A
typical
substrate to be immobi ized in the second portion is t-butaxycarbanyl-
Ieucyl~glycyl-
SUBSTITUTE SHEET (RULE Z5j
ia-~r-~~d ib: ~4 MH I HY5 XWhIU 1 Kt. l~~b ~re,se dm r. ey~,s~o
CA 02327169 2000-09-29
WU 99153322 , PCTIGB99101099
arginine-paranitroanilide (8oc-Leu-Gly-Arg-pNA) to release paranitroani(ide.
Usable
synthetic peptides with a known detectable group are those described in EP-A-
0000063, EP-A-00113112, W079/00602, W082/02382 and US 4,188,264.
However, synthetic p~;ptide substrates for activated Factor C as well as
synthetic
peptide substrates for activated clotting enzymes in which a carboxyl group of
the
C-terminated argininE~ -is substituted with the colour-developing residue p-
nitroaniiine, p-(N,N-di~ahylamine)aniline, p-(N-ethyl-N-f~-hydroxyethyl)
aniline can
also be used. Possible methods of immobilization of the chromogenic substrate
to
the solid support ors: 1 ) absorption; 2) adsorption by ionic forces or/and
hydrophobic interacti~3n, and 3) covalent binding. Preferably, the support is
absorbed with the substrate. The solid support is typically made with the same
material as the first s~:pport and it is one which is capable of absorbing the
liquid
and reactants flowing from the first support, and which when wetted is this
way,
provides for flow and analyte by capillary attraction from the first portion,
and
through the second portion into the third portion. Upon contact with the
liquid and
reactants flowing from the first support, solution should react with
immobilized
chromogenic substrate. Like the first portion, examples of suitable supports
are
chromatographic papers, nitrocellulose, dextran and glass fibres. Preferably,
chromatographic papers are used. The s4lid support absorbed with the
ohromogenic substratE is one which is capable o~absorbing reactants flowing
from
the first support. When wetted, the so-called reactants are capable of
reacting
with the second portic,n and the reactants with the new reaction products
being
transported to the dry regions and thus to the third portion of the test
strip. The
solid , support is so manufactured (with attention to the amount of absorbed
synthetic chromogenic substrate, size of the portion, material of the solid
support)
that activated clotting ~:nzyme andlor factor B flowing from the lower portion
reacts
with substrate in the second portion and the chromophore p-nitroaniline is
cleaved.
The cleaved p-nitroaniline is then transported to the next portion.
The third portion contains a solid support and a buffer containing 1 N NCI and
0.1 % (wlv) sodium nit~ite. Preferably, to make the support; it is impregnated
with
the buffer system, dried and is made with the same material as the second
support.
SUBSTiME SHEET (RULE ?.~)
11i-5th'-~I~b 1b~~4 IhHIHYS ZS SL,lUIKt 1~~'t~ '~ti,5l~ l:)b1~1 t'.11~~,310
CA 02327169 2000-09-29
WO 99153322 PC'T'IGB99101099
.7.
When dried, the~solid support is also one which is capable of absorbing the
liquid
and the chromophore p-nitroaniline flowing from the second support, and which
when wetted is this may, provides for flow from the second portion, and
through
the third portion into the fourth portion.
The fourth portion contains a solid support and a buffer containing N HCI
containing 0.5°6 ammonium. Preferably, to make the support, it is
impregnated
with the buffer system, end dried. When absorbed with the buffer system and
dried, the solid support is also one which is capable of absorbing the liquid
and the
chromaphore p-nitroa~iline flowing from the third support, and which when
wetted
is this way, provides for flow from the third portion, and through the dried
region,
The third and fourth portions are those through which when the flowing free p-
nittoaniiine released by the action of enzymes is derivatized to its diazanium
salt,
typically formed in th,: fourth portion.
The fifth portion is a neutral portion in which a drop of 0.05 % N 1-
naphtylethylenediamine in 40-50% (v/vf is added to form a highly visible red
colour
by cQUpling of the de-ivatized p-NA to N1-napthylethylenediamine.
The test strip described in this invention can suitably be prepared from any
matrix
material through whi~:h an aqueous solution can flow by capillarity. Suitable
materials for chromatographic strips are paper, nitrocellulose and
hydrophillic
polymers. because of the presence of f3-glucan in cellulosic material, paper
is riot
usually suitable without special treatment as it induces false positive
results. In
this invention, it is desirable to use hydrophillie polymers which allow
vertical
capillary flow excluding a bibulous lateral flow such as polyethylene sheet
material.
,_ There now foNows description of specific embodiments of the present
invention,
illustrated by drawings in which:-
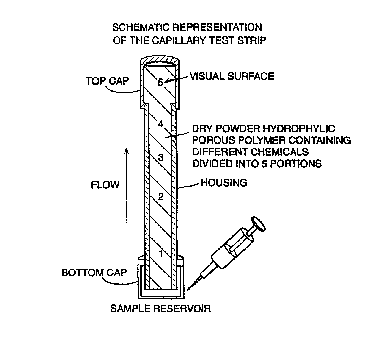
Fig. 1 shows a schematic representation of a capillary test strip;
SUBSTITUTE SHEET (RULE 26~
113-5th'-~t~b lb~ ~5 MH 1 HY5 X~ 5L,lUlKt 1~~4~ 'fti.5d dbl~l h'. l lii4~
CA 02327169 2000-09-29
wo 99rs33zz , rcrrc B99ro ~ 099
-e-
Fig. 2 shows .3 schematic representation of a paper test strip;
Fig. 3 shows mode of operation of a test strip;
Fig. 4 shows variation of substrate sensitivity and resulting lysate activity;
and
Fig. 5 shows ~~ quantitative test strip.
~xarnple 1: capillary test strip
As illustrated in figure 1, a glass howl capillary glass with a flat side, an
internal
dimension of 0.25 em and an external dimension of 0.2$ cm and a length of 4 cm
has been used as the casting module. For the bottom of the capillary there is
a cap
which is used as the ,;ample reservoir, whereas a second cap closes the top of
the
capillary and permits entry of chemicals for the colour reaction. The module
is
constructed in a w.~y that it remains pyrogen free. The howl module is
successively filled wi~:h dried hydrophillic porous spherical material (200-
500 ~nl,
The hyijrophillic por4~.~s polymer is in separate portions, impregnated as
follows:
Portion 1: hydrophillic polymeric powder was incubated under pyrogen free
conditions in 0.5 IU/ml (1 glml) of limulus amoebocyte lysate (Charles River-
Suizfel
Germany) until saturated absorption of the liquid was reached. After drying,
the
module has been filled to reach a height of 1 cm. 0.1 cm has then been filled
with
neutral polymeric povrder.
Portion 2; 10 ~rmo) of chromogemic substrate (t-butoxycarbonyl-ieucyl-glycyl-
arginme-paranitroanili~e) for endoxin (Pefachrome LAL Code.NR 11179-01 ISO
9001-1N29001 Penta~pharm AG BASEL CH) was diluted in 6.6 ml pyrogen free .
water. 1 g of hydrophillic polymeric material was also incubated with 1 m1
until
saturated absorption was reached. After drying the module has been filled to
reach
a height of 0.5 cm. C1.1 cm has then been filled with neutral polymeric
powder.
SUBSTITUTE SHEET (RULE 2B)
18-SEP-2000 16~25 MATHYS & SQUIRE 020 7830 0001 P.12i30
CA 02327169 2000-09-29
WO 99!53322 PCTIGB99I01099
-9-
Between portions 2 ;end 3: Tris-HC) is mixed with polymeric powder to obtain a
concentration of 0Ø5 mmol/I and inserted between portions 2 and 3 with a
height
of O.S cm. 0.1 cm has been filled ~evith neutral polymeric powder.
' ' Portion 3: Polymeric powder was submerged in a solution of 1 N HCI
containing
0.1 % (w/v) sodium nitrite, and dried. A quantity sufficient for 0.5 cm has
been
inserted into the module. 0.9 cm has been filled with neutral polymeric
powder.
Portion 4: 1 cm of polymeric powder was submerged with in solution of 1 N HCl
containing 0.5 °~ ih~'Ivl ammonium sulfate, dried and has been filled
into the
module. 0.1 cm has been filled with neutral polymeric powder.
Portion 5 : 2.5 mg of N1-naphthlethylenediamine in mixed with 0.5 cm polymeric
material.
Example 2: paper tes-: strip '
The strip can prepared from any matrix material through which the test fluid
can
vertically flow by capillarity; as illustrated in figure 2, a polystyrene
dipstick was
constructed to be fitted in a sterile battle. The active surface of the test
strip was
0.5 cm x 4.2 cm, separated every 0.5 cm between the first portion and the
third
portion with 0.5 cm x 0.2cm dry Sephadex. The active surface between the
sephadex strip was dated with Scotch adhesive transfer tape , 969 13M, USA,
5x144)
Portion 9: consisted cf 0.5cm x 0.5 cm ultra high molecular weight
polyethylene
sheet material tPorex Technologies) which was incubated for 10 min under
pyrogen
free conditions in 0.5 IUImI ( 1 glmll of limuius amoebocyte lysate (Charles
River-
Sulxfel Germany). At;er drying, it was applied to the bottom et the test
strip, A
i
neutral 0.5 cm x 0.7 :m-portion was added above the neutral sephadex.
Portion 2: consisted of 0.5cm x 0.5 cm ultra high molecular weight
polyethylene
' SUBSTITUTE SHEET (RULE 26)
18-SEP-2000 16~26 MRTHYS 8~ SQUIRE 020 7830 0001 P.13i30
' ~~ CA 02327169 2000-09-29
wo 99is33zZ
PCT~GS99lot 099
.10-
sheet material tPorex Technologies) which was spotted with 1.5 ,umollml of
Chrvmogenic substrate (t-butaxycarbonyl-leucyl-glycyl-arginine-pa-
ranitroanilide) for
~ndoxin (Pefachrome I.AL Code NR 11179-O1 ISQ 9001-7 N29001 Pentapharm AG
BASEL CH). The strip was applied lust above the neutral sephadex portion. , -
Between portions 2 and 3: consisted of 0.5em x 0.5 cm sephadex and Tris-HC( at
a concentration of 0.025 mmolh.
portion 3: consisted of a 0.5 cm x 0.5 em strip of filter paper (Schleicher &
Shuel
23 SL) which was submerged with solution of 1 N HCl containing 0.1 °~
(w!v)
sodium nitrite, dried and applied above the neutral sephadex portion.
Portion 4: consisted of a 0.5 cmx 0.5 cm strip of filter paper (Sehleicher &
Shue)
23 SL) which was submerged with in solution of 1 N HCI captaining 0.5 % (Wlv)
ammonium sulfate, dried and applied above the third portion.
Portion 5 : consisted of a 0.5 cmx 0.5 cm strip of filter paper (Schleicher &
Shuel
23 SL) applied above the fourth portion.
For both examples :he assay is carried out as follows:
1 ) add the simple to the first portion of the test strip or immerse the first
portion in the sample (example 2) or fill the reservoir with the sample
(example 1 )-
2) heat the test strip to 37 °C device to activate the reaction;
3) allow sufficient time (such as 4 min) for the sample to flow from the
bottom to tt-;e top:
4) dry the test strip by removing the sample reservoir from the bottom and
opening the bottom of the casting; and
5) for colour development, a drop of 47.5% ethanol is added, coloration
develops after drying in air.
The chemical reactions involved in each portion are described in Figure 3. In
__ ~ .........w lot tl ~ '~i~
18-5EP-2000 16~26 MRTHYS & Sf~UIRE 020 7830 0001 P.14~30
CA 02327169 2000-09-29
WO 99/53322 PGTlGB99101099
-11-
portion 1, depending upon the presence of endotoxin in the samples, clotting
enzyme is produced through the cascade reaction by the action of activated
Factor
C or prociotting enzymes. In portion 3 after the papillary flow through the
chromagenic substra~:e and in the presence of Tris-HCI, the activated clotting
enzyme andlor factor S flowing from the lower portion cleaves p-nitroaniline.
The
cleaved p-nitraanitine being transported to the third and fourth portion and
derivatized to its diazonium salt. In the fifth portion, the diazonium salt is
coupled
to N1-napthylethylenediamine to form a diazoamino of a red colour.
The assay can be performed with different sensitivities, according to the
grade of
contamination to be analyzed. (n the special application to test solutions
used in
hemadialysis therapy and according to Pharmacopoeia prescriptions, several
test
strips have been elaborated to read the minimal acceptable concentration of
endotoxin in aqueous solution (Table 1 ).
Table 1
AppGoatians SubstitutionitemodialysisDialysateWater
for on-
solution water solutionfine HDF
for
Hamvdiafiiuatlon
Lowest LPS concentration
'n l:Ulml
0.05 0.25 0.5 t
In respect to the sensitivity of the test, numerous modification can be
performed,
such as by varying the: titration of the LAL-concentration and the matrix
material
used in the first portion. By the application of high molecular weight
polyethylene
sheet material (Pore;; Technologies) and using several titrations of limulus
amoebocyte lysate varying from 0.1 to 0.5 lUlml and several matrices, for
examples 1 and 2, it is possible to prepare 3 classes of lysate
concentrations. The
method used to prepa~e the desired lysate concentration is first based on a
liquid
.I chramagenic subsuate assay in a microplate according to the LAL-test
Endochrome-K~'"' (US. License No ~ 073) except that the I.AL-concentration is
not
related to the protocol. Figure 4 illustrates the resulting absorbance due to
the
SUBS'ffTUTE SHEET (RULE 26)
18-SEP-2000 16=2? MRTHYS 8 S(~UIRE 020 9830 0001 P.15i30
CA 02327169 2000-09-29
WO 99/53322 PC"TIG 899/0!099
- 12-
variation of the f_A!_ which allow 4 classes of test-strips. Applied to the
test-strip
of example 2, it is pv~sible to prepare a test-strip especially for the
detection of
endotoxin concentration. in diaiysate-solution, for which the acceptable
firnit is ~-
prescribed to be beiour a.5 EU/m!. Detection spots for this prepared test
strip are
ihustrated in Figure 5.
suesTtn~ sHEF-r (pum zs)
lti-5th'-~lJdd 1b ~ ~'f num t a a ~u a r~~ ~~... . ........ ....... . .
CA 02327169 2000-09-29
WO 99/S33ZZ PCTfG899101099
-13-
References:
" 7 J. Levin and F.3. Bang Bult. Johns Hopkins Hospital, 115, 265.274 (1964)
2 Progress in Clinical and Biological Research, Volume 93; 'Endotoxin and
their petection with the Limulus Amoebocyte Lysate Test"; edited by Stanly
W. Watson, Jack Levin and Thomas J. Novitsky (1982); pages 7-24
3 lwanaga et al. -temostasis, Z, 1$3-199 (18781
4 J. Clin Microbio. 17, 1050-1053 11983)
Related Application Data:
1 Solid phase chromatographic immuno assay- Rosenstein-US patent
5,591,fi45 (Jar4 7, 1997)
2 Method for the detection of protein urine- Cahil et ai: US patent 5,593,895
(Jan 14, 1997)
3 Reagent for en~otoxin-specific assay- Tanak et al, US patent 5,550,030
(Aug 27 1996)
4 Test strip for blood glucose testing- Kelvin J. Philipps- US patent
5,556,761
tSep 17, 1996)
- 5 Quantitative de°_ection of analytes on immunochromatographic strip-
Ronald
G Sommer- US patent 5,569,608 (Oct 29, 1996)
6 Horses Crab arroebocyte lysate factor G activation inhibitor- Tanaka S.- US
patent 5,641,63 (Jun, 24, 1997)
SUBS'1'1TUTE SHEET (RULE Z6)
18-SEP-2000 16~27 MRTHYS & SQUIRE 020 7830 0001 P.17/30
CA 02327169 2000-09-29
WO 99153322 . PC'1'IGB99101099
-14-
7 Reagent for assaying endotoxin. Tanak S- US patent 5,605.806 (Feb 25,
1997)
8 Test articles fvr performing dry reagent prothrombin time assays Stephen E.
Zweig et al. US patent 5,418,141 (May 23, 1995)
9 Composite celil:lose nitrate membrane on polyester support- Beer et a!- US
patent 5,628,9130 (May 13, 1997)
One step-test d~:vice. Philip W. Sayies. US patent 5,591,401 Clan 7, 1997)
SU8ST1TUTE SHEET (RULE 26)