Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
TITLE OF THE INVJ~NTION
TITANIUM CATALYZED PREPARATION OF CARBAPENEM INTERMEDIATES
BAC G~O~ OF THE INVENTION
The present invention relates to a process for synthesizing
1-~3-methyl-2- hydroxymethyl carbapenem intermediates. Generally the
carbapenems are substituted at the 2-position. The intermediate
compounds are included as well.
European applications 0330108, 0102239, 0212404, 0695753
and 0476649 disclose methods for synthesizing various antibiotic
derivatives.
Many of the carbapenems are useful against gram positive
microorganisms, especially methicillin resistant Staphylococcus aureus
(MRSA), methicillin resistant Staphylococcus epidermidis (MRSE), and
methicillin resistant coagulase negative Staphylococci (MRCNS). These
antibacterials thus comprise an important contribution to therapy for
treating infections caused by these difficult to control pathogens. There
is an increasing need for agents effective against such pathogens
(MRSA~NtRCNS) which are at the same time relatively free from
undesirable side effects.
~T TMMARY OF THE INVENTION
The invention describes a short and high yielding synthesis
of protected 1-~i-methyl-2-hydroxymethyl substituted carbapenems as key
intermediates for the synthesis of anti-MRSA carbapenem antibiotics.
The synthesis involves a highly diastereoselective addition of a titanium,
zirconium or hafnium enolate of a suitably protected 1-hydroxy-2-
butanone derivative with 4-acyl-2-azetidinone. Using this enolate, the
resulting derivatized 2-azetidinone product is obtained largely as a
single diastereomer rather than a mixture. Additionally, the two chiral
centers which are produced are of the correct absolute stereochemical
-1-
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
configuration for subsequent synthesis of 1-~i-methyl-2-hydroxymethyl
substituted carbapenems.
In one aspect of the invention, a process of synthesizing a
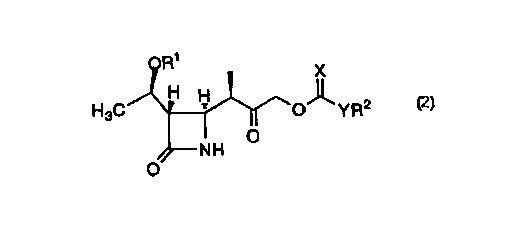
compound of formula 2:
R~
X
H ~
H3C H O- _YR2
O
NH
O
2
is disclosed wherein Rl represents H or a suitable protecting group for
an alcohol; R2 represents a benzyl, C1_s alkyl or aryl; Y represents C1_3
alkyl, O, NH or S; and X represents O, NH, or S
comprising reacting a compound of formula 1:
R1 O
H H O~ Ra
H3C
NH
O 1
wherein R' is described above and R4 represents C1_15 alkyl, aryl or C1_s
aralkyl;
with a compound of formula 3:
~~O~YR2
O ~'(X
wherein R2, X and Y are as previously defined in the presence of WZ4
and an amine to produce a compound of formula 2, wherein W is a
titanium, zirconium or hafnium metal and Z represents halo, sulfonate,
alkoxy, aryloxy or combination thereof.
-2-
CA 02328219 2000-10-12
WO 99/52908 PCTNS99/07956
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to a process for making
protected 1-p-methyl-2-hydroxymethyl substituted carbapenems which
are key intermediates in the synthesis of anti-MRSA carbapenem
antibiotics (such as those disclosed in USSN 08/825,786 filed on April 08,
1997, the teachings of which are hereby incorporated by reference). The
intermediates can be readily coupled to a wide range of functional
groups (see USSN 08/825,786).
The invention is described herein in detail using the terms
defined below unless otherwise specified.
The term "alkyl" refers to a monovalent alkane
(hydrocarbon) derived radical containing from 1 to 10 carbon
atoms unless otherwise defined. It may be straight, branched or cyclic.
Preferred alkyl groups include methyl, ethyl, propyl, isopropyl, butyl, t-
butyl, cyclopentyl and cyclohexyl. When substituted, alkyl groups may
be substituted with up to four substituent groups, selected from Rd and
Rl, as defined, at any available point of attachment. When the alkyl
group is said to be substituted with an alkyl group, this is used
interchangeably with "branched alkyl group".
Cycloalkyl is a species of alkyl containing from 3 to
15 carbon atoms, without alternating or resonating double bonds
between carbon atoms. It may contain from 1 to 4 rings which are fused.
The term "alkenyl" refers to a hydrocarbon radical straight,
branched or cyclic containing from 2 to 10 carbon atoms and at least one
carbon to carbon double bond. Preferred alkenyl groups include ethenyl,
propenyl, butenyl and cyclohexenyl.
The term "alkynyl" refers to a hydrocarbon radical straight
or branched, containing from 2 to 10 carbon atoms and at least one
carbon to carbon triple bond. Preferred alkynyl groups include ethynyl,
propynyl and butynyl.
Aryl refers to aromatic rings e.g., phenyl, substituted
phenyl and the like as well as rings which are fused, e.g., naphthyl,
phenanthrenyl and the like. An aryl group thus contains at least one
-3-
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
ring having at least 5 atoms, with up to five such rings being present,
containing up to 22 atoms therein, with alternating (resonating) double
bonds between adjacent carbon atoms or suitable heteroatoms. The
preferred aryl groups are phenyl, naphthyl and phenanthrenyl. Aryl
groups may likewise be substituted as defined. Preferred substituted
aryls include phenyl and naphthyl.
Aryl also refer to heteroaryl, which is a monocyclic
aromatic hydrocarbon group having 5 or 6 ring atoms, or a polycyclic
aromatic group having 8 to 16 atoms, containing at least one heteroatom,
O, S, S(O), S02 or N, in which a carbon or nitrogen atom is the point of
attachment, and iri which one or two additional carbon atoms is
optionally replaced by a heteroatom selected from O or S, and in which
from 1 to 3 additional carbon atoms are optionally replaced by nitrogen
heteroatoms, said heteroaryl group being optionally substituted as
described herein. Examples of this type are pyrrole, pyridine, oxazole,
thiazole and oxazine. Additional nitrogen atoms may be present
together with the first nitrogen and oxygen or sulfur, giving, e.g.,
thiadiazole and the like.
As used herein, "aralkyl" is intended to mean an aryl or
heteroaralkyl moiety, as defined above, attached through a C1_s alkyl
linker, where alkyl is defined above. Examples of aralkyls include, but
are not limited to, benzyl, naphtylmethyl, phenylpropyl, 2-pyridylmethyl,
2-imidazolylethyl, 2-quinolinylmethy, 2-imidazolylmethyl and the like.
Examples of polycyclic heteroaromatics include
benzopyrans, benzofurans, benzopyrroles, benzimidazoles,
benzothiazoles, quinolines, purines, isoquinolines, benzopyrimidines,
dibenzofurans, dibenzothiophenes, 1,8-naphthosultams,
The term "heterocycle" (heterocyclyl) refers to a 5-16
membered cycloalkyl group (nonarornatic) with 1-4 rings, in which one
of the carbon atoms in the ring is replaced by a heteroatom selected from
O, S or N, and in which up to three additional carbon atoms may be
replaced by heteroatoms.
The term "heteroatom" means O, S, S(O), S(O)2 or N,
selected on an independent basis.
-4-
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
Halogen and "halo" refer to bromine, chlorine, fluorine and
iodine.
When a group is termed "protected", such as R', R5 and the
like, this means that the group is in modified form to preclude undesired
side reactions at the protected site. Suitable protecting groups for the
compounds of the present invention will be recognized from the present
application taking into account the level of skill in the art, and with
reference to standard textbooks, such as Greene, T. W. et al. Protective
C'=rows in Organic Synthesis Wiley, New York (1991). Examples of
suitable protecting groups are contained throughout the specification.
In some of the compounds of the present invention, Rl and
R6 represent alcohol and carboxyl protecting groups, respectively.
Likewise, Y may represent a protecting group for X, which in turn
represents O or N. These groups are generally removable, i.e., they can
be removed, if desired, by procedures which will not cause cleavage or
other disruption of the remaining portions of the molecule. Such
procedures include chemical and enzymatic hydrolysis, treatment with
chemical reducing or oxidizing agents under mild conditions, treatment
with a transition metal catalyst and a nucleophile and catalytic
hydrogenation.
Examples of carboxyl protecting groups Rb include
allyl, benzhydryl, 2-naphthylmethyl, benzyl, silyl groups such as
t-butyldimethylsilyl (TBDMS), trimethylsilyl, (TMS), triethylsilyl (TES),
phenacyl, p-methoxybenzyl, o-nitrobenzyl, p-methoxyphenyl, p-
nitrobenzyl (pNB), 4-pyridylmethyl and t-butyl, preferably pNB and
benzyl.
Examples of suitable alcohol protecting groups R' include
hydrogen, trialkylsilyl, diarylalkylsilyl, aryldialkylsilyl or trityl such as
TMS, TES, TBDMS, alkyl carbonates such as benzyl carbonate, allyl
carbonate, benzyl ether, diarylalkylsilyl, aryldialkylsilyl & trityl and the
like. Preferred R~ groups are trialkylsilyl or hydrogen.
Another aspect of the process that is of particular interest is
the synthesis of a compound of formula 5:
-5-
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
X
HOC O~ YR2
O O
O O- R5
wherein R' represents H or a suitable protecting group for an alcohol; RZ
represents a benzyl, C1_e alkyl or aryl; Y represents C,_3 alkyl, O, NH or
S; X represents O, NH, or S and R5 represents a carboxy protecting
5 group, comprising reacting a compound of formula 2:
R~
X
H
HsC H O~ YR2
NH O
O
2
wherein R1, R2, X and Y are as previously described, with an activated
oxalic acid agent in the presence of a base to produce a compound of
formula 5.
In another aspect of the invention a process for synthesizing
a compound of structural formula 6
nol
H H X
H3C
O~ YR2
0. C02R5
s
is disclosed wherein Rl represents H or a suitable protecting group for an
alcohol; RZ represents a benzyl, C1_s alkyl or aryl; Y represents C1_3 alkyl,
-6-
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
O, NH or S; X represents O, NH, or S and Rs represents a carboxy
protecting group, comprising reacting a compound of formula 5:
R1
X
H H
HsC O~ YR2
-- N _
O O
O O_ Rs
5 wherein Rl, R2, RS, X and Y axe as previously described with a phosphite
or phosphonite reagent to produce a compound of formula 6.
Another aspect of the process that is of interest is the
synthesis of a carbapenem compound of formula 6
r,r~1
H H X
H3C
O~ YR2
O_ 6 C02Rs
wherein R1 represents H or a suitable protecting group for an alcohol; R2
represents a benzyl, C1_s alkyl or aryl; Y represents C1_3 alkyl, O, NH or
S; X represents O, NH, or S and R5 represents a carboxy protecting
group, comprising reacting a compound of formula 2:
R1
X
H H
HaC O~ YR2
NH O
O
2
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
wherein Rl, R2, X and Y are as previously described, with an activated
oxalic acid agent in the presence of a base to produce a compound of
formula 5
X
H H
HsC O~ YR2
O
O N
O
O O_ Rs
5
and reacting a compound of formula 5, wherein Rl, R2, R6, X and Y are
as previously described with a phosphite or phosphonite reagent to
produce a compound of formula 6.
Another aspect of the process that is of interest is the
synthesis of a carbapenem compound of formula 6
X
H3C ~
O' -YR2
6 C02Rs
wherein Rl represents H or a suitable protecting group for an alcohol; R2
represents a benzyl, C1_s alkyl or aryl; Y represents C1_3 alkyl, O, NH or
S; X represents O, NH, or S and R5 represents a carboxy protecting
group, comprising reacting a compound of formula 1:
_g_
CA 02328219 2000-10-12
WO 99/52908 PCTNS99/07956
R1 O
H H O~ R4
H3C
NH
O 1
wherein R1 is described above and R4 represents C1_~s alkyl, aryl or C,_s
aralkyl;
with a compound of formula 3:
'~'O~YR2
IOI X
3
wherein R2, X and Y are as previously defined in the presence of WZ4
and an amine to produce a compound of formula 2:
R~
X
H
HaC H O~YR2
NH O
O
2
wherein W is a titanium, zirconium or hafnium metal and Z represents
halo, sulfonate, alkoxy, aryloxy or combination thereof, and R1, R2, X
and Y are as previously described, reacting a compound of formula 2
with an activated oxalic acid agent in the presence of a base to produce a
compound of formula 5
-9-
CA 02328219 2000-10-12
WO 99/52908 PCTNS99/07956
R1
X
HsC H H O~YR2
-N _
O O
O O- R5
and reacting a compound of formula 5, wherein Rl, R2, R5, X and Y are
as previously described with a phosphate or phosphonite reagent to
5 produce a compound of formula 6.
Another aspect of the process that is of particular interest is
the synthesis of a compound of formula 5:
X
HsC O~ YR2
U O
5 O O_ Rs
wherein Rl represents H or a suitable protecting group for an alcohol; R2
represents a benzyl, C1_e alkyl or aryl; Y represents C1.3 alkyl, O, NH or
S; X represents O, NH, or S and R5 represents a carboxy protecting
group, comprising reacting a compound of formula 1:
R~ O
H H O~ Ra
H3C
NH
O
- 10-
CA 02328219 2000-10-12
WO 99/52908 PCTNS99/07956
wherein Rl is described above and R4 represents Cl.lb alkyl, aryl or C1_s
aralkyl;
with a compound of formula 3:
~~'O~YR2
'OI X
3
wherein R2, X and Y are as previously defined in the presence of WZ4
and an amine to produce a compound of formula 2:
R~
X
H
HsC H O~ YR2
NH O
O
2
wherein W is a titanium, zirconium or hafnium metal and Z represents
halo, sulfonate, alkoxy, aryloxy or combination thereof, and R1, R2, X
and Y are as previously described, and reacting a compound of formula
2 with an oxalimide forming agent in the presence of a base to produce a
compound of formula 5.
Suitable amines includes trialkylamines such as
triethylamine, tributylamine, trimethylamine, ethyldimethylamine, tri-
n-propylamine, di-isopropylethylamine, aniline, N,N-di-C1_s-
alkylanilines such as N,N-diethylaniline and the like.
Suitable bases include trialkylamines such as
triethylamine, trimethylamine, ethyldimethylamine, tri-n-propylamine
and the like, 1,8-diazabicyclo[5.4Ø]undec-7-ene (DBU), pyridine,
imidazole, lutidine, coliidine, 4-dimethylaminomethylpyridine,
inorganic carbonates and bicarbonates such as sodium carbonate,
sodium bicarbonate, potassium bicarbonate, potassium carbonate, and
the like and tartrates such as potassium sodium tartrate, potassium
tartrate, potassium bitartrate, sodium tartrate, sodium bitartrate and
the like, preferably pyridine, lutidine or collidine.
-11-
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
Suitable phosphates include P(ORa)(ORb)(OR');
P(ORa)(ORb)(NR'R°); P{Re)(Rb)(R'); catechol phosphates or catechol
dimer
phosphates, wherein Re, Rb, R' and Ra may be the same or different and
represent a straight or branched chain C1_s alkyl or a phenyl, both of
which may be optionally substituted with, for example, a C1_3 alkyl.
Preferable phosphates are trialkylphosphites such as triethyl phosphate,
tributyl phosphate, triisopropyl phosphate, trimethyl phosphate and the
like, most preferably triethylphosphite.
Suitable phosphonites include P(ORe)(OR~)(Rg), wherein Re
and Rf independently represent C1_4 alkyl, allyl, benzyl or phenyl,
optionally substituted with C1_3 alkyl or C1_3 alkoxy and Rg presents C1_4
alkyl, trifluoromethyl or phenyl, which is optionally substituted with C1_3
alkyl or C 1_3 alkoxy.
Suitable activated oxylic acid agents include acid and
carbodiimide moieties such as oxalyl chloride and benzyl oxalyl chloride.
In particular, processes of interest are those described
above wherein Rl represents an alcohol protecting group selected from
the group consisting of H, TES, TMS, TBDMS, pNB, p-
nitrobenzyloxycarbonyl, allyl and allyloxycarbonyl.
Other processes that are of particular interest are those
described above wherein R5 represents an carboxylic acid protecting
group selected from the group consisting of p-nitrobenzyl (pNB),
trimethylsilyl (TMS), triethylsilyl (TES), tert-butyldimethylsilyl
(TBDMS), allyl, p-methoxybenzyl, benzyl, trichloroethyl, 2-
trimethylsilylethyl, and the like.
Still other processes that are of particular interest are those
described above wherein X represents O.
Still other processes that are of particular interest are those
described above wherein Y represents O or CH2.
Still other processes that are of particular interest are those
described above wherein Y represents O.
Still other processes that are of particular interest are those
described above wherein W represents zirconium metal.
-12-
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
Still other processes that are of particular interest are those
described above wherein W represents titanium metal.
Still other processes that are of particular interest are those
described above wherein W represents hafnium metal.
Still other processes that are of particular interest are those
described above wherein Z represents a halogen, most preferably
chloride.
The process of the present invention is illustrated by the
following generic scheme:
SCHEME A
TBDMS
1.~O O RZ H OII
C H H OAc O O H3C H O~O R2
N H Metaltetrahalide
O Tria~~~ O TESCI
2. Acid
OII
TES O H 3C ~O R2
H
H3C ~ ~O p R2 p-nitrobenzyloxalyl chloride
1 0
NH
O
Trialkylphosphite reagent H3C
O R2
1-Hydroxy-2-butanone is readily available and can be
suitably protected by a number of synthetic methods. (3R,4R)-4-acetoxy-
3-[(R)-(tertbutylmethylsilyloxy)ethyl]-2-azetidinone and (3R,4R)-4-
acetoxy-3-[(R)-(hydroxyethyl]-2-azetidinone are both readily available and
undergo the addition reaction with high diastereoselectivity and in high
yield.
-13-
CA 02328219 2000-10-12
WO 99/52908 PCTNS99/0795b
Typical conditions for the reaction involve generation of the
titanium, zirconium or hafnium enolate of a suitably protected
derivative of 1-hydroxybutanone such as an alkyl or aryl carbonate,
preferably ethyl carbonate or isobutylcarbonate. This can be achieved by
the addition of the corresponding metal tetrahalide to the derivative of 1-
hydroxybutanone followed by addition of a trialkylamine. The
stoichiometry of the enolate formation requires at about 0.5 to 3.0
equivalents, preferably 1 to 2.0 equivalents of metal tetrahalide. About
0.5 to about 5 equivalents, preferably about 1 to about 3 equivalents and
most preferably about 1 to about 2.0 equivalents of trialkyl amine is used.
The enolate generation is generally carried out at a temperature of about
-80°C to about 60°C, preferably about -~0°C to about
30°C.
Generally, the azetidinone is added to the enolate and the
reaction temperature warmed to about 0°C - 30°C. The
stoichiometry of
the reaction requires about 1.0 to about 5 equivalents, preferably about 1
to about 2.0 equivalents of the enolate of the alkyl or aryl carbonate of 1-
hydroxybutanone or its synthetic equivalent.
Suitable solvents for the reaction include aromatic solvents
such as benzene, toluene, xylene and the like, ethereal solvents such as
tetrahydrofuran (THF), diethyl ether, dioxane and the like and haloalkyl
solvents such as 1,2 dichloroethane, dichloromethane, chloroform,and
the like, preferably the aromatic solvents.
In a typical reaction, the azetidinone is reacted with, for
example, a titanium enolate of the ethyl or isobutyl carbonate of 1-
hydroxy-2-butanone, preferably the isobutyl carbonate moiety. The
protecting group (e.g. TBDMS) is then preferably removed by the addition
of an acid such as hydrofluoric acid (HF), HCI, or fluorosilicic acid
(H2SiFs ) and subsequently reprotected with another alcohol protecting
group (e.g. TES derivative, typically using TESCI, benzyl ethers or allyl
ethers), in the presence of a base such as imidazole or pyridine.
Reaction with p-nitrobenzyloxalyl chloride affords the oxalimide, the
precursor to the cyclization step. The cyclization step typically involves
reacting the oxalimide in the presence of a phosphite or phosphonite
reagent, preferably a trialkylphosphite agent. The stoichiometry of the
- 14-
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
cyclization requires from about 2 to about 6 equivalents, preferably about
2.5 to about 5 equivalents of the phosphite or phosphonite. The
cyclization is generally carried out at a temperature of about 25°C to
about 200°C, depending on the nature of the phosphorus reagent used.
When using a trialkylphosphite reagent the temperature is generally
about 90°C to about 160°C.
The carbapenem produced in the cyclization is a key
intermediate in the synthesis of anti-MRSA carbapenem antibiotics and
can be readily coupled to a wide range of functional groups in via
methods taught in USSN 08/825,786.
The final product may be characterized structurally by
techniques such as NMR, IR, MS, and W. For ease of handling, the
final product, if not crystalline, may be lyophilized from water to afford
an amorphous, easily handled solid.
The compounds of the present invention are valuable
intermediates for antibacterial agents that are active against various
Gram-positive and to a lesser extent Gram-negative bacteria, and
accordingly find utility in human and veterinary medicine.
Many of the compounds that can be made in accordance
with the present invention are biologically active against
MRSAlMRCNS. In vitro antibacterial activity is predictive of in vivo
activity when the compounds are administered to a mammal infected
with a susceptible bacterial organism.
The invention is further described in connection with the
following non-limiting examples.
EXAMPLE 1
TBDMS TBDMS O
H ~O~OiBu H H
H3C H OAc p IOI H3C O OiBu
I ~ NH O
N H TiCl4 O
O Bu3N
PhMe
- 1S -
CA 02328219 2000-10-12
WO 99/52908 PGT/US99/07956
(3R,4R)-4-acetoxy-3-[(R)-tertbutyldimethyl- 32.Og (0.11 mol)
silyloxy)ethyl]-2-azetidinone
isobutyl 1-(2-oxobutane)carbonate 29.4g (0.156 mol)
titanium tetrachloride (1M in toluene) 156 mL
tributylamine 44 mL
toluene 400 mL
Titanium tetrachloride solution was added to a solution of the isobutyl
carbonate in toluene at -40°C. Tributylamine was added. The acetoxy
azetidinone was then added and the reaction stirred at room
temperature. After 3 hours the reaction was quenched with dilute
hydrochloric acid. The toluene layer was washed with dilute HCI. The
toluene layer was used in the subsequent step.
Isolated prod, 13C NMR (CDC13) b -5.0, -4.3, 11.7, 17.9, 18.8, 22.5, 25.8,
27.8, 44.6, 51.0, 61.7, 65.4, 69.8, 74.8, 154.8, 168.3, 205.65
EXAMPLE 2
TBDMS H O
O'I H H
H
HsC H O~OiBu HF H3C O OiBu
" ~ O
NH O MeCN / PhM~ O N H
O
TBDMS azetidinone isobutyl carbonate
in toluene solution from above Examplel 40g in 450 mL
HF (48% aqueous) 20 mL
Acetonitrile 400 mL
To the toluene solution from Example 1 was added acetonitrile and the
HF solution. After 6 hours the reaction was quenched with aq. Rochelles
salt. The toluene layer was dried and the solvent was removed. The
crystalline product was swished with hexanes and filtered to yield 4-[3-
((1-oxy-2-oxobutane)isobutyl carbonate)]-2-azetidinone (23.3g) as a white
solid.
- 16-
CA 02328219 2000-10-12
WO 99/52908 PC'f/US99/07956
1H NMR 8 0.95 (d, 6H), 1.25 (d, 3H), 1.3 (d, 3H), 2.0 (m, 1H), 2.9 (m, 2H),
3.85 (m, 1H), 3.9 (d, 2H), 4.1 (m, 1H), 4.75 (m, 2H), 6.3 (s, 1H)
EXAMPLE 3
H O TES O
HsC H H O~OiBu TESCI H3C H H O~OiBu
iH O '- IH O
O N MeCN O N
4-[3-((1-oxy-2-oxobutane)isobutyl carbonate)]- 8.04g (0.027 mol)
2-azetidinone
triethylsilyl chloride 4.7 mL (0.028 mol)
imidazole 2.Og (0.029 mol)
acetonitrile 60 mL
To a slurry of the azetidinone in acetonitrile was added imidazole. The
reaction became homogeneous and triethylsilyl chloride was added.
After 2 hours the reaction was given an aqueous work up and the
organics were concentrated in vacuo to afford ll.Og of TES azetidinone
isobutyl carbonate.
1H NMR b 0.5 (q, 9H), 0.9 (t, 6H), 0.9 (d, 6H), 1.2 ( 2 doublets, 6H), 2.0 (m,
1H), 2.9 (m, 2H), 3.8 (m, 1H), 3.9 (d, 2H), 4.1 (m, 1H), 4.7 (m, 2H), 6.4 (s,
1H)
EXAMPLE 4
0
TES ~ O"CI H S O
H ~
H ~ ~ , HaC O- 'OiBu
HaC H O- _OiBu O
O 2N ~ N O
N H Pyridine O O
O MeCN
O O-pNB
-17-
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
TES azetidinone isobutyl carbonate ll.Og (0.027 mol)
p-NB oxalylchloride 7.15g (0.029 mol)
pyridine 4 mL
acetonitrile 150 mL
Pyridine was added to a solution of pNB oxalylchloride in acetonitrile.
After 20 minutes the TES azetidinone isobutyl carbonate was added. The
reaction was given an aqueous work up, the organics were concentrated
in vacuo to afford TES oxalimide isobutyl carbonate ( 15.2 g) as a white
solid.
13C NMR 8 4.8, 6.7, 14.0, 18.8, 22.5, 27.8, 40.9, 54.4, 61.3, 64.7, 66.7,
69.9,
74.8, 123.8, 129.0, 141.2, 148.0, 154.7, 156.0, 159.4, 164.9, 204.6.
EXAMPLE 5
TES
HaC H H 0I 'OiBu TES
p H H O
O' P(OEt)3 H3C
O O~OiBu
p-xylene N
O
O O-pNB ~02pNB
TES oxalimide isobutyl carbonate 15.2g (0.024 mol)
triethyl phosphate 8.8 mL
xylene 200 mL
Triethyl phosphate was added to a solution of TES oxalimide isobutyl
carbonate in xylene. The reaction was heated to 135°C for 3 hours. The
reaction was given several aqueous washes, dried and the solvent
removed in vacuo to afford the desired compound (12.2g).
1H NMR (399.87 MHz, CDC13) d 8.22 (m, 2 H), 7.66 (m, 2 H), 5.57 (d,
J=14.5, 1 H), 5.46 (d, J=13.7, 1 H), 5.27 (d, J=13.7, 1 H), 4.83 (dd, J=14.5,
1.2, 1 H), 4.26 (overlapping m, 2 H), 3.95 (d, J=6.8, 2 H), 3.33 (m, 1 H),
3.28
-18-
CA 02328219 2000-10-12
WO 99/52908 PCT/US99/07956
(dd, J=5.6, 3.2, 1 H), 1.99 (m, 1 H), 1.26 (d, J=6.0, 3 H), 1.20 (d, J=7.2, 3
H),
0.95 (t, J=8.0, 9 H), 0.60 (m, 6 H)
13C ~R (100.55 MHz, CDCl3) d 174.8, 160.4, 155.0, 147.7, 145.5, 142.6,
128.4, 128.1, 123.7, 74.5, 65.7, 65.5, 61.6, 60.7, 55.9, 40.3, 27.8, 22.5,
18.8,
15.3, 6.7, 4.9
- 19-