Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02329350 2008-09-23
TITLE OF THE INVENTION
TOPICAL FORMULATION COMPRISING POLOXAMERS AND FURTHER
MICROBICIDES
FIELD OF THE INVENTION
This invention relates to formulations comprising film-forming components and
any
active ingredient, particularly to topical formulations. More particularly,
this invention relates to
topical formulations to prevent or to treat diseases associated with or
transmitted through mucosae
or skin, caused by any causative agent, particularly a pathogen. This
invention also relates to an
applicator for the uniform delivery of topical formulations to prevent or to
treat any disease
associated with or transmitted through mucosal cavity, or to prevent invasion
by an external agent
such as sperm or nucrobe.
BACKGROUND OF THE INVENTION
The spread of sexually transmitted diseases (STDs) caused by human
immunodeficiency
virus (HIV), herpes and other pathogens is going at a bewildering rhythm. The
global incidence,
morbidity, and mortality of STDs are very significant. Worldwide, it is
estimated that over 900
million individuals are infected with sexually transmitted pathogens. Each
year more than 12
million people in the United States are newly infected with a pathogen
responsible for STDs.
Herpes simplex virus type-1 (HSV-1) and type-2 (HSV-2), are the most common
causes of genital
ulceration in developed countries. Genital herpes infection is life-long and
may result in painful
and recurrent genital lesions, systemic complications, psychosocial morbidity
and also serious
neonatal disease following intrapartum transmission of HSV. The genital
transmission of this
pathogen is usually due to asymptomatic viral shedding by people who are
unaware that they are
infected. HSV-2 is now detectable in 1 out of 5 americans 12 years of age or
older. In addition, it
is estimated that over one-third of the world's population has recurrent HSV
infections and has
therefore the capability to transmit the virus during episodes of productive
infection. Neisseria
gonorrheae and Chlamydia trachomatis are recognized as two of the most
prevalent sexually
transmitted bacterial infections. Worldwide, there is an estimated annual
incidence of
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-2-
25 million cases of gonorrhea and 50 million cases of chlamydia. On the other
hand,
recent epidemiologic data indicate that the number of individuals infected
with HIV is
growing dramatically throughout the world. According to United Nations
officials,
epidemiologic data estimates suggest that as many as 16,000 individuals become
infected with HIV every day during 1997. Recent statistics (as of end 1997)
from the
World Health Organization (WHO) indicated that there are about 31 million
people
infected with HIV worldwide and this number is projected to reach 40 millions
by year
2000.
Globally, heterosexual transmissions may account for 85-90% of HIV infection.
As there is no vaccine against HIV, preventive measures are the only tools
that can
presently reduce the transmission of this retrovirus. The consistent and
careful use of
condoms represents an effective barrier against the sexual transmission of HIV
and
other sexually transmitted pathogens, but they should be used in all risky
sexual
intercourses to significantly reduce the probability of acquiring infection.
In Africa, the
most intensive prevention programs were only able to increase condom use to
approximately 70% of all sexual intercourses in female prostitutes.
Consequently,
doubts arise about the possibilities of condom promotion in controlling the
AIDS
epidemic in high risk groups. In situations where heterosexual transmission of
HIV is
important, preventive measures where women could prevent their risk of
contracting
STDs could be an additional tool to restrain the epidemic. Such a protective
tool may
also be used in male homosexual relations as it could provide additional
protection
under the control of the receptive partner. Therefore, it is important to
develop barrier
method that could be used as an alternative to condoms where the person could
protect themselves against infection without having to ask their sexual
partners.
Preventive measures aimed at blocking the initial transmission of pathogens
that are
the causative agents of AIDS, herpes and other STDs will lead, of course, to
enormous
benefits.
The development of safe topical microbicides is actually a very high priority
for
the Worfd Health Organization (WHO) and the National Institutes of Health
(NIH) in the
field of HIV prevention. A topical microbicide is often composed of an active
ingredient
and a vehicle. Active ingredients may act via a variety of mechanisms
including: i)
disrupting the organism cell membrane, envelope or capside lipid or protein
constituents (e.g. detergent-type spermicides/microbicides such as nonoxynol-
9), ii)
blocking the receptor-ligand interactions essential for infectivity (e.g.
microbial
SU6STITUTE SHEET (RULE 25)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-3-
adhesion inhibitors such as sulfated compounds), iii) inhibiting the
intracellular or
extracellular replication of the pathogen (e.g. antimicrobial drugs), iv)
altering the
vaginal environment and reducing susceptibility to infection (e.g. buffering
agents and
products that maintain normal vaginal flora and environment) or v) enhancing
local
immune responses (e.g. immune response modifiers). The overall efficacy of a
topical
microbicide against the sexual transmission of pathogens causing STDs depends
on
the efficacy of the active ingredient to be delivered and its ability to cover
the entire
vaginal/cervix area for maximal efficacy against pathogens. The capacity of
these
active agents to cover the entire vaginal cavity greatly depends of the type
of vehicle
used. Typical formulations of vehicles include gels, creams, foams,
suppositories,
sponges and films.
Most currently available vaginal formulations use the spermicide nonoxynol-9,
a nonionic surfactant, as a microbicide. In vitro, nonoxynol-9 inactivates
enveloped
viruses, such as HSV, HIV and other microorganisms including Chiamydia
trachomatis,
Neisseria gonon=hoeae. However, the potential efficacy of nonoxynol-9 against
HIV is
not yet clearly established and results of clinical trials are controversial.
A recent
controlled trial conducted among 1292 HIV-negative female sex-workers in
Cameroon
showed that the use of a vaginal film containing 70 mg nonoxynol-9 did not
reduce the
rate of new HIV, gonorrhea or chlamydia infection (Roddy et al., 1998, N.
Engl. J.
Med., 339:504-510). The failure of nonoxynol-9 film in reducing the
transmission of
infectious agents could be attributed to the incomplete coverage of the entire
vagina/cervix area with the drug delivery system for nonoxynol-9 or to the
occurrence
of mucosal toxicity favoring infection of microorganisms. Because of the
dramatic
increase in the number of individuals throughout the world who are infected
with HIV,
herpes, or other sexually transmitted pathogens, there is an urgent need to
develop
active products and/or appropriate delivery systems that can reduce the sexual
transmission of these pathogens with minimal mucosal irritation and minimal
effects
on the vaginal flora and pH.
Sodium lauryl sulfate (SLS) is a sulfated surfactant that denatures membrane
proteins of pathogens. It thus has a dual action as a detergent and as a
chaotropic
agent. With this notion, we have performed experiments to evaluate the
potential
microbicidal effect of SLS on HSV and HIV. Our preliminary studies clearly
demonstrated that SLS modifies in vitro the infectivities of both viruses.
More recently,
Howett et al. have confirmed our findings that SLS is also a potent
inactivator of HSV-
SU9STITUTE SHEET (RULE 26)
CA 02329350 2007-04-11
-4-
2, HIV-1 (Antimicrob. Agents Chemother. 43(2): 314-321, 1999). In addition,
they have
shown that SLS is effective against rabbit, bovine and human papillomaviruses
(non-
enveloped viruses) after brief treatment with low concentrations of this
product. However,
this reference does not teach the use of a vehicle to deliver this potential
microbicide. The
choice of vehicle is very important because it affects the concentration of
available drugs,
the duration of drug availability and the degree of mucosal coverage by the
formulation
which are key factors for offering protection against invading pathogens.
Another
interesting category of candidate microbicides is microbial adhesion
inhibitors, such as
sulfated compounds, which block the interaction between host cell receptor and
microbe. A
known example of microbial adhesion inhibitors is dextran sulfate (DS), a
polysulfated
carbohydrate, which has been shown to inhibit in vitro the infectivities of
HIV and
herpesviruses.
We have recently developed a gel formulation that could be applied to the
vaginal,
cervical or ano-rectal mucosae and which could be effective to prevent
sexually transmitted
pathogens. One paramount characteristic of this gel formulation is its
thermoreversible
property. The transition from the liquid state at room temperature to the gel
state at body
temperature is of prime importance because when applied on rough biological
surfaces such
as the vaginal or ano-rectal epithelia, the gel should penetrate into the
smallest irregularities
forming a good physical barrier against infectious agents. The gel formulation
has the
following key characteristics that both FDA and NIH consider important: i) it
is colorless,
odorless and non-staining, ii) it should cover the whole vagina/cervix because
it is applied
in liquid state, iii) it is compatible with male latex condom, iv) it resists
to elution by
aqueous flow, v) it has a pH similar to that of a healthy vagina (pH 4.0-4.5),
vi) it maintains
the desired rheological properties under extreme heat and cold conditions and
vii) it does
not affect, in vitro, the normal vaginal flora, especially Lactobacillus spp.
Our international publication (WO 97/42962) discloses the use of formulations
compi-ising film-forming components capable of forming per se a physical
barrier to
pathogens. Thermoreversible gels such as poloxamers are particularly preferred
for that use.
The film-forming formulations may further comprise microbicides, spermicides
or any other
drug, which choice is guided by the pathogen, organism or the disease to be
inactivated or
treated. The formulations are therefore efficient as a physical, and
optionally, as a chemical
or pharmacological barrier as well as usable as a sustained drug-release
system at the locus
of administration.
=.. . T- u_ v. i. = yV - 11 .~1'r Ja1 -tJO-!. TYJ O~
24-08-2000 111:14 De-GUUUFEAU iiAl~ UUBUC CA 02329350 2000-10-20I-514-387-4382
E-733 P-I CA 009900359
-5-
These formuiations are intended for use in the prevention of sexualiy
traAstnitted
diseases, as well as in the treatrnent of infections, caxtcer, inflammation or
any disease or
state wttich requires a pharmacological treatrnent. In addition, this
publication teaches that
ft formulation decreases the toxicity of potcnr spermicidcsimic.robicides such
as
uonoxynol-9. However, this publication does not specificalty reach the use of
SLS as a
chemical catulidats of choice incarporated into the topical formulations.
US patent publication number 5,275,805 describes an oral composition
comprising
an ariti.glaque agent, a eu:fsctant mixture which may comprise BI.S, a
polyoxyethylene/pol,yoxypropylene block polymer and a taurate salt. The
surfactant mixture
is said to be effective "to stabllize the oral composition to phase separation
without
comprotaise of the antibacterial efficacy of the [antiplaciue agent]". This
purpose is served
using rather low cancemtrations of each compor,ent of the surfactant mixture;
tbese low
concentrations are not believed to achiave antimicrobial activity and a
physieai barrier.
The European patent publication number 386,960 descn-bes a drug delivery
vehicle
comprising a thermosetting gel component and a filrn forming component. The
fiitn forming
component has for purpose to provide a firmer jelly. Spermicides may be
included thereto,
and chcy dso part of a list of drugs intended for vaginat drttg delivery.
There ic however no
demonstration whatroever that a spermicide, a thermosetting gel component and
fltn
forming canponent would form a pbysico-ehemical barrier against padzogens, or
thac a sub-
combination af these components would achieve t3te forumian of a physieo-
chemical
barrier, or else, that any product which can disrapt ft lipids of a call
membrane or the
conformation of a protein (detergents in general, or chaotrapic agents) won.ld
be effective
against patbagen.s, alone or in combination with a rherinosettiug gel.
Por an efficient delivery of seuu-solid or liquid formulations isito a body
cavity, an
applicator is a tool of choice. The ideal applicator should deliver
immediately, upon
actuation, the formulatiaa to the mucosa and this, without rxpulsing too much
air before the
formulation.
The LrS patent nurmber 2,683,456 descri-bes a rectal applicator which
comprises a
perforated elon.gated body so be inserted into a body cavity, and a proximal
enlarged
portion, which remains outside the body cavity. A tnedication tube is located
in close
proximity to the junction between the elongated body and ft enlarged portions,
so as to
AMENDED SHEET
.. rV:-~.. n nua_.:a~.aw.v . .__ _ v , a. = ia. . . ~ ~ ~
11 :15 Ae-GOUDREAU GAGE DUlUC i]-514-387-4332
E-T33 P.11J~ CA 009900359
24-08-2000 CA 02329350 2000-10-20
-5
discharge its corueats directly into the elongated body upon actaation. The
diffusion
channel, through which the medicadon travels before being expulsed to the body
cavity, is
sinlply forn-ed by the lumen of the elongatel body, which ailows the passage
and rhe
expulsion of quite a large air volume. There is no disclosure of a diffusion
channel of a
reduced volume, which would allow the medi.cation to be almost immediately
expulsed
without being preceded by such a large air volume.
B.ropean patent publicatxon nusnber 761246 desrribes an applicator comprising
a
reservoir. a pump having ari inlet port in thz reservoir. a btttton trigger to
actuate tle pump
and a tube to be introduced into a body cavity, containing a distribution head
at the tip of
the tube, that tube being connected ta an outlet port of the pump. The tube
section deftnes a
diffusion channel simply consisting of the lumen of the tube, through which a
medication
travels to the distribution head. The distribution head is made of an enlarged
section having
a filler portion and a plurality of perforations made through all the head
thickness. This
document does not describe any applicator which would have a diffusion channel
of a smau
volunte wriich would deliver a medication wishout delivering a substantially
high volume of
air.
The patent publication number pE 3513645 describes aaz agplica.toc which
comprises a sfon-perforated tip portion and a cylindrical body having an
ext.ernai perforated
wall. The lumen defined in the body is delineated simply by the external wall.
This lumen
constitutes a reservoir wherein a liquid or semi-solid formutation is placed.
A plunger
pushes the formuiation in the reservoir and, by compression against the non-
perforated tip
portion, the fornralation is forced out through the perforations. The lumen
and the plunger
may have a contplementary conical Sh,ape for delivering sma11 volumes of
forsnulation.
However, because the lumen has a relatively large volume, tbere may be a more
or less
high volume of air forced through the perforacaons bcfore the formulation
reaches the same.
RSV-1 and HSV-2 are neurotropic viruses which infect priacipaily the
neuroectocier*nal tissues iricluding the skin, the peripheral nerves and the
central nervous
system. Mucosal or skin surfaces are t3ze usual sites of primary infection.
Recurrent labialis
herpes and genital herpes represent the most common clinical manifestations
associated with
HSV-1 and HSV-2 infections, respectively. Recurren.es are spontaneous but are
associated
witli physical or emotional stress, fevcr, exposure to ldtraviolet light,
tissue damage and
immune suppression. Although it is a mild disease in iuununocompetent
individuals, HSV
AMENDED SHEET
24-08-2000 11 : I 5 De-60UGREAU GAtiE GUBU: CA 02329350 2000-10-2p}-514-381-
4381 E-133 P11
CA 009900359
-5 ter-
infections are troublesartte, especially far patients with frequent episodes.
Patients
compromised by either inuntute therapy or underlying disease have increased
risk to
develop HSV infecdons. Renal and cardiac aansplant recipients de,tuonstrated
an increased
severity of infection. In additioa, dc outbreak of AIDS has reinforced the
severity of HSV
cliraical disease in itnmunacornprcxnised hasts.
The current available topical antiviral trc:atmcnts have anly a Iiniited
efficacy
pardcularly against symptoma#ie recurrent herpes. The limited effmacy of these
topical
forrn4lationc on the developrnertt of hecpetic muc~tra*,~ous lesions may be
due to the poor
ability of thc drugs to penetrate into the skin. The siratum coraeam or horny
layer
consritutes the barrier for the penetration of most substanc.. s into the
skin. This layer
consists of corneocytes embedded in a dottble-layeted lipid matrix composod of
cbolesterol,
free f"auy acids and ceramides. Consequently, tbe nse of skin penetration
enbancers could
represent a coiivenient strategy to increase the penetration of topical drug
formulations into
the skin.
SLS is a surfactant which possesses skin penetration enhancer property by
increasing the fluidity of epidermal lipids. 'Fhe skin penetration enhancer
property of SLS
combined with its ability m modify viral infeetivity via itc detergent and
chaotropic
properties could further increase the efficacy of topical dtug formulations.
Furthet-tnorre,
because of its chaotropic properties, SLS may have a broader spectrum of
activity against
sperm, bacteria, fungi and vLruses than another simple detergent.
AMENDED SHEET
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-6-
Poloxamers are widely used in numerous pharmaceutical applications and their
non-toxic properties make them suitable for sustained drug delivery systems.
Poloxamers represent suitable matrices for dermatological applications.
Indeed, when
applied in liquid form, poloxamers allow a better surface coverage by
penetrating into
the smallest irregularities of the mucosa and/or skin . In addition, the
reticular array
formed by these poloxamers may act as a sustained drug release system
prolonging
drug action.
SUMMARY OF THE INVENTION
In accordance with the present invention, it is a first object to provide
formulations which comprise a film-forming component which is applied to the
surface
of mucosae or skin, preferably in the form of gel, cream or ointment. The gel
formulations are to be used for coating different types of mucosae such as
vaginal,
cervical, ano-rectal, eye, mouth, nose, or skin to prevent infection and/or
abnormal
conditions of mucosae and/or skin. Furthermore, the gel formulations can be
applied
topically to the eye for the treatment and/or prevention of infection of
ophthalmic
conditions. Preferably, a thermoreversible gel is used, which is applied in a
liquid form,
spreads on the surface and forms a semi-solid coating after it reaches the
temperature
of this body surface. More preferably, the thermoreversible gel is composed of
poloxamer 407. Similar polymers such as poloxamines can also be used. The
above
formulations also comprise an agent capable of interfering with the organism
cell
membrane, envelope or capside lipid or protein constituents in a target cell,
tissue or
microbe. The above combination of the film-forming component and the above
agent
may provide for formulations with improved efficacy and reduced toxicity.
In a specific embodiment, the agent is capable of interfering with the binding
of
a microbial outer protein to a host receptor. In a more specific embodiment,
the agent
is a microbial adhesion inhibitor, or is a detergent or a chaotropic agent
capable of
disrupting the integrity of said microbial outer protein. In an even a more
specific
embodiment, the microbial adhesion inhibitor is dextran sulfate; the detergent
is
selected from the group consisting of sodium lauryl sulfate, benzalkonium
chloride,
Iauroyl sarcosine, polyoxyethylene fatty acyl derivatives and polyoxyethylene
sorbitan
fatty acyl ester derivatives; and the chaotropic agent is sodium lauryl
sulfate or
guanidine. In the most specific embodiment, the agent is SLS, the latter being
a
chemical candidate of choice because of its numerous properties as a detergent
and
SU9STITUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-7-
a chaotropic agent and a putative microbial adhesion inhibitor. SLS alone is
efficient
against microbes. SLS efficacy is further improved when incorporated into the
present
formulations. Therefore, it is contemplated that SLS or any equivalent product
can be
used alone or in combination with the above film-forming component to prevent
microbial infection. SLS may be used alone or in combination with the above
formulations at any suitable concentration, preferably at a concentration of
about 0.1-
25% (w/v), and more preferably at a concentration of about 1-15% (w/v).
Poloxamer
407 concentration may be used at any suitable concentration, preferably at a
concentration of about 5-50% (w/v) and more preferably at a concentration of
about
15-35% (w/v) The physical properties of the final formulations largely depends
on the
drug to be incorporated in them, on the pH and solutes used in the making of
the
formulations and on the viscosity sought for a given purpose. The above
formulations
could further comprise a drug which is effective to prevent infection and/or
abnormal
conditions of the mucosae or skin. Vaginal formulations constitute a physical
and a
chemical barrier due to its film-forming and microbial disrupting components.
It goes
along that, with an activity against infective agents, these formulations may
also be
effective for preventing pregnancy. SLS will advantageously replace nonoxynol-
9 in the
formulations. SLS having a broader spectrum of activity against, inter alia,
sperm,
enveloped and non-enveloped viruses, it is a candidate of choice in the
present
formulation. The gel could contain a drug which is effective to prevent
infection and/or
abnormal conditions of mucosae and/or skin. For the purpose of the invention,
the term
"drug" is intended to cover any antimicrobial, bactericidal, virucidal,
chemotherapeutic,
antiinflammatory, antineoplastic, immunomodulator or any other agent or
combination
of them which is effective for the prevention of infection of mucosae and/or
skin. The
term "drug" also refers to cytokines or antigens that could stimulate an
immune
response that would protect against infection. The drugs could be incorporated
within
drug carriers such as gels, liposomes, nanoparticies or cyclodextrins, whose
encapsulation result in an improved prevention of infection.
It is further an object of the present invention to provide a unique
applicator that
can be used vaginally and/or ano-rectally to deliver topical formulations for
treatment
and/or prevention of infection and/or abnormal conditions of mucosae. The
applicator
can be designed in different ways to give the same required characteristics
specified
under detailed description of the invention. Examples of some different
concepts are
also discussed under the detailed description which are intended to describe
some of
SUBSTR'UTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-8-
the general design possibilities of the applicator, but are in no way intended
to limit the
scope thereof. It is important to mention that the final shape and appearance
of the
applicator can differ from the examples given herein.
In other preferred embodiments, the present formulations are used to treat
viral
diseases and they further comprise as a drug an antiviral agent such as
acyclovir or
foscarnet, or any other antimicrobial agents, used alone or in combination, at
any
suitable concentration. In a most preferred embodiment, the formulation is
composed
of poloxamer 407 and contains foscarnet at a concentration ranging from 0.5 to
5%
(w/v). In another most preferred embodiment, the formulation is composed of
poloxamer 407 and contains acyclovir at a concentration ranging from 0.5 to 5%
(w/v).
In still another most preferred embodiment, the formulation is composed of
poloxamer
407 and contains SLS at a concentration ranging from 1 to 10% and foscarnet or
acyclovir at the above concentrations.
It is an object of the present invention to develop new topical formulations
to
prevent infection of mucosae and/or skin, more particularly those sexually
transmitted
infections and even more particularly those caused by HIV and herpes. The
microbicides or any other drug can be entrapped into the gel formulations
either as free
or encapsulated into drug carriers such as liposomes, nanoparticles or
cyclodextrins.
Such microbicidal gels could prolong the local microbicidal activity,
eliminate local
irritation and reduce systemic side effects of incorporated active agents.
It is also an objective of the invention to develop, for vaginal applications,
a
unique applicator which allows uniform distribution of the content to the
entire vagina
(delivery to sides) and cervix (delivery to front) for maximal protection
against the
sexual transmission of pathogens. Therefore, we have designed a unique
applicator
which allows about 360 distribution of its content into the vagina and far to
the cervix
which is a great improvement over existing conventional vaginal applicators
which
deliver contents only to front (cervix area only).
It is another object of the present invention to develop topical formulations
of
drugs which could improve the efficacy of chemically or pharmacologically
active
agents against mucocutaneous infections and more particularly those caused by
HSV
infections. The improved efficacy of drugs upon incorporation within suitable
matrices
and/or drug carriers could reduce the dosing interval and consequently improve
the
quality of life of patients. It is also an objective of the present invention
to develop
SUBSTI'TUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-9-
topical formulations for the treatment and/or healing of burn wounds as well
as to
prevent their potential infection.
DESCRIPTION OF THE SPECIFIC EMBODIMENTS OF THE PRESENT INVENTION
This invention will be described hereinbelow by referring to specific
embodiments and appended figures, which purpose is to illustrate the invention
rather
than to limit its scope.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 shows the infectivity of HSV-1 (strain F) to Vero cells following
pretreatment of the virus with different concentrations of SLS (Panel A) or DS
(Panel
B) for I h at 37 C (#) or following the addition of SLS or DS to viruses
without
pretreatment Q. Plaque forming units (PFU) are expressed as percentage of
control.
Results are mean + SD of 4 independent experiments.
Figure 2 shows the efficacy of different concentrations of SLS (Panel A) or DS
(Panel B) against HSV-1 (strain F) in Vero cells. Plaque forming units (PFU)
are
expressed as percentage of control. Results are mean SD of 4 independent
experiments.
Figure 3 illustrates the effect of pretreating HIV-1 (strain NL4-3) with 500
pM
of SLS for I h at 37 C on its infectivity to 1G5 cells. Values represent the
mean SD
of 3 determinations.
Figure 4 shows electron micrographs of Vero cells infected with HSV-1 (strain
F) pretreated for 1 h at 37 C with 50 ,uM (Panel B), 75 M (Panel C) and 100
M
(Panel D) of SLS. Cells infected with HSV-1 (strain F) in absence of SLS were
used
as control (Panel A). Magnification 70,000 X.
Figure 5 shows quantification of glycoprotein D of HSV-1 (strain F) pretreated
for 1 h at 37 C with 12.5, 25, 50, 75 and 100 M of SLS in Vero cells. Cells
infected
with HSV-1 (strain F) in EMEM + 2% FBS were used as control. Values are
expressed
as a percentage of the hybridization signal intensity compared to control.
Figure 6 shows the time evolution of survival of mice infected intranasally
with
HSV-2 (strain 22) pretreated for I h at 37 C with 6.25 (#), 25 Q and 100 (%)
M of SLS.
Mice infected with untreated virus were used as control (). Results are
expressed as
mean of 8 animals per group.
SUBSTfTUTE SHEET (RULE 25)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-10-
Figure 7 shows the time evolution of the mean lesion score of mice infected
cutaneously with HSV-1 (strain F) pretreated for 1 h at 37 C with different
concentrations (6.25 (#), 25 Q and 100 (%) M) of SLS (Panel A) or different
concentrations (0.25 (#), 1 () and 10 (%) nM) of DS (Panel B). Mice infected
with
untreated virus were used as control (.). Results are expressed as mean of 6
animals
per group.
Figure 8 shows the time evolution of mean lesion score of mice infected with
HSV-1 (strain F) following pretreatment of mice with the poloxamer formulation
alone
5 min Q or 1 h(~) prior to infection or with the poloxamer formulation
containing 5%
SLS also 5 min (,~) or 1 h (%) prior to infection. Infected untreated mice
were used as
control (.). Results are expressed as mean of 6 animals per group.
Figure 9 shows the time evolution of mean lesion score (Panel A) and survival
(Panel B) of mice infected intravaginally with HSV-2 (strain 333) pretreated
with the gel
alone (,,%,#) 5 min prior to infection. Infected untreated mice were used as
control
Results are mean of 8 animals per group.
Figure 10 shows the time evolution of survival of mice infected intravaginally
with HSV-2 (strain 333) pretreated with 2.5% SLS (4) or gel + 2.5% SLS (#) 5
min prior
to infection. Infected untreated mice were used as control (,). Results are
expressed
as mean of 8 animals per group.
Figure 11 shows the time evolution of survival of mice infected intravaginally
with HSV-2 (strain 333) pretreated with gel + 5% polyoxyethylene 40 stearate
(#), gel
+ 5% guanidine Q, gel + 2.5% lauroyl sarcosine (%), gel + 2.5% benzalkonium
chloride
(+) or gel + 5% tween 80 (õ) 5 min prior to infection. Infected untreated mice
were used
as control (.). Results are mean of 7 to 10 animals per group.
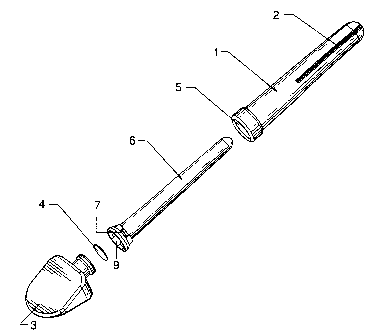
Figure 12a is a perspective view illustrating a first embodiment of an
applicator
according to an aspect of the present invention.
Figure 12b is a side elevational view showing the dimensions in inches of the
applicator of Figure 12a.
Figure 12c is an exploded view of the components of the applicator of Figure
12a.
Figure 12d is a perspective view illustrating the details of the external
surface
of the proximal end of the internal wall of the applicator of Figure 12a.
Figure 13a is a perspective view illustrating a second embodiment of an
applicator according to an aspect of the present invention.
SU9ST[TUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-11-
Figure 13b is a side elevational view illustrating the dimensions in inches of
the
applicator of Figure 13a in both insertion position and actuated position.
Figure 13c is an exploded view of the applicator of Figure 13a.
Figure 14a is a perspective view of a third embodiment of an applicator
according to an aspect of the present invention; the applicator being shown in
an
insertion position.
Figure 14b is a perspective view of the applicator of Figure 14a shown in an
actuated position.
Figure 14c is a side elevational view illustrating the internal details of the
applicator of Figure 14a in the insertion position.
Figure 14d is a side elevational view illustrating the internal details of the
applicator of Figure 14a in the actuated position.
Figure 15a is a perspective view of a fourth embodiment of the applicator
according to an aspect of the present invention.
Figure 15b is an exploded view of the applicator of Figure 15a.
Figure 15c is a side elevational view of the external wall of the applicator
of
Figure 15a where the dimensions are given in inches.
Figure 15d is a side elevational view of the piston/reservoir of the
applicator of
Figure 15a where the dimensions are given in inches.
Figure 15e is a sectional side elevational view of a portion of the applicator
of
Figure 15a illustrating the details of the arrangement of the piston/reservoir
with regard
to the internal and external walls of the body of the applicator.
Figure 16 shows the time evolution of mean lesion score (Panel A) and survival
(Panel B) of hairless mice infected cutaneously with HSV-1 and treated
topically with
the poloxamer alone (,), 0.5% foscamet in aqueous solution Q or poloxamer
containing
0.5% foscarnet (#). Infected untreated mice were used as control (). Treatment
started
24 h after infection and was repeated 3 times daily for 4 days. Values are
expressed
as mean of 4 animals per group.
Figure 17 shows the time evolution of the mean lesion score (Panel A) and
survival (Panel B) of hairless mice infected cutaneously with HSV-1 (strain F)
and
treated 24 h post-infection with a single application of either poloxamer
containing 5%
acyclovir (,,) or Zovirax ointment Q. Infected untreated mice () were used as
controls.
Values are expressed as mean of 7 to 10 animals per group.
SUBSTITUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 -12 PCT/CA99/00359
-
Figure 18 shows the time evolution of the mean lesion score (Panel A) and
survival (Panel B) of hairless mice infected cutaneously with HSV-1 (strain F)
and
treated with the poloxamer alone (,), poloxamer containing 5% acyclovir Q, or
with
Zovirax ointment Q. Infected untreated mice (.) were used as controls.
Treatment
started 5 days after the infection and was repeated 3 times daily for 4 days.
Values are
expressed as mean of 7 to 10 animals per group.
Figure 19 shows the distribution of foscarnet (õ%) and acyclovir in skin
tissues of uninfected (Panels A, C, E) and infected (Panels B, D, F) mice at
24 h after
their topical application, either in phosphate buffer (open symbols) or within
the
poloxamer (filled symbols). Panels A and B show the distribution of foscarnet
and
acyclovir in the stratum corneum strips. Panels C and D show the concentration
of
foscarnet and acyclovir in the epidermis whereas panels E and F show the
concentration of foscarnet and acyclovir in the dermis. Values are expressed
as mean
of 4 to 6 animals per group.
Figure 20 shows the concentration of acyclovir in plasma of uninfected and
infected mice at 24 h after its topical application, either in phosphate
buffer (open bars)
or in the poloxamer (filled bars). Values are expressed as mean of 4 to 6
animals per
group.
Figure 21 shows the time evolution of mean lesion score (Panel A) and survival
(Panel B) of hairless mice infected cutaneously with HSV-1 (strain F) treated
with the
poloxamer alone (,), poloxamer containing 3% foscarnet Q, poloxamer containing
5%
SLS (#) or poloxamer containing 3% foscarnet + 5% SLS Q. Infected untreated
mice
(.) were used as controls. Results are expressed as mean of 5 animals per
group.
Figure 22 shows the susceptibility of HSV-1 (strain F) to combinations of
different concentrations of foscarnet and SLS in Vero cells. Values are
expressed as
mean SD of 3 determinations.
Gel formulations
Poloxamer 407 is a block copolymer of polyoxyethylene and polyoxypropylene
in a 7:3 weight ratio with an average molecular weight of 12500. One important
characteristic of this block copolymer is its ability to form a
thermoreversible gel. The
transition from the liquid state at low temperature to the gel state at body
temperature
(the phase transition temperature being dependent, in part, on the
concentration of the
gel, the ionic strength and the incorporated solute) allows a number of
interesting
medical applications including topical applications. Such characteristic is of
prime
SUBSTITUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-13-
importance because when applied topically in its fluid state to the mucosa,
the gel
formulation should allow better penetration into the irregularities of the
skin and/or
mucosae during application and a longer persistence once the gel has reached
body
temperature. Because of the extremely low toxicity and irritancy of our gel
formulations,
they represent an attractive approach for topical drug delivery systems.
Details for the
preparation of the gel formulations are provided hereafter. This invention
covers gel
formulations of poloxamer 407 of any suitable concentration, and more
particularly
those between about 10 and 35 % w/w. This invention also covers any other fiim-
forming component, gel, cream, ointment or thermoreversible substance
including
other poloxamers, poloxamines or chemicals.
Drugs
Any antimicrobial, bactericidal, virucidal, chemotherapeutic,
antiinfiammatory,
antineoplastic, immunomodulator or combination of them which is effective to
prevent
or treat infection and/or abnormal conditions of mucosae and/or skin caused by
any
pathogen and/or any disease is under the scope of this invention. Any
detergent which
can disrupt the membrane of pathogens, any skin penetration enhancer that
increases
the penetration of drugs and/or drug carriers into the mucosae and/or skin,
any
microbial adsorption inhibitor which prevents pathogen's entry into a target
cell, any
cytokine or antigen that could stimulate an immune response that would protect
against pathogen's infection are also under the scope of this invention. This
invention
also covers any combination of topical formulations and/or drugs.
Examples invoiving our gel formuiations for prevention of Infection
The following examples are intended to demonstrate the preparation of gel
formulations that could be efficient to prevent infection and/or abnormal
conditions of
mucosae and/or skin caused by any pathogen and/or any disease, but are in no
way
intended to limit the scope of the present invention.
Prgparation of the gel formuiations
The gel formulations are prepared by adding an appropriate volume of distilled
water, buffer or any other suitable aqueous solution to the poloxamer 407 to
obtain the
desired concentration. An appropriate amount of drugs are then added either to
the
powder or solution of poloxamer to reach the desired concentration. The pH of
the gel
formulation can be adjusted to meet the requirements of each target tissue to
be
coated with the present formulations. For instance, if a formulation is to be
used to coat
vaginal mucosa, an acidic solution with pH of about 4.0-4.5 will be used. The
SU9S'TiTUTE SHEET (RULE ZS)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-14-
percentage of polymer may be adjusted accordingly to obtain an adequate
transition
temperature from liquid to solid state. These adjustments are well within the
knowledge
and ability of the skilled artisan.
Even though the description of this invention is limited to specific cases,
any
film-forming component and/or drug and/or liposomes (or other drug carriers)
or any
combination of the above are considered as potential candidates for the
development
of these topical presentations and are under the scope of this invention. The
formulations also include any film-forming component and/or drug and/or
liposomes
(or other drug carriers) or any combination of these products at any suitable
concentration.
In vitro infectivity of herpes viruses pretreated with SLS or DS
The effect of pretreating different strains of herpes viruses with SLS or DS
on
their viral infectivities to susceptible cells has been evaluated. In brief,
cells were
seeded in 24 well-plates (Costar, Montreal, QC, Canada). Prior to infection,
the virus
was either suspended in culture medium or phosphate buffered saline (PBS), or
incubated with different concentrations of SLS in PBS for 1 h at 37 C. At
confluency,
cells were incubated with viral suspensions by centrifuging the plates (750 x
g for 45
min at 20 C) to allow virus adsorption. Virus was removed and cell sheets were
then
overlaid with 0.5 ml of 0.6% agarose Seaplaque (Marine Colloids, Rockland, MA)
prepared in appropriate culture medium. The plates were incubated for 2 days
at 37 C.
Cells were then fixed with 10% formaldehyde in PBS for 20 min, washed with
deionized water and stained with 0.05% methylene blue. Viral infectivity was
evaluated
via the determination of Plaque Forming Units (PFU).
Table 1 shows that pretreatment of various HSV-1 and HSV-2 strains with SLS
for 1 h at 37 C decreased, in a concentration-dependent manner, their
infectivity on
Vero cells. HSV-1 (strain F) infectivity was reduced to 21 % when viral
particles were
pretreated with 25 pM SLS. The infectivities of all HSV-2 strains were between
50 to
70% following preincubation with 25 4M SLS. A complete loss of the infectivity
of all
strains tested were obtained following pretreatment of the viruses with 50 ,uM
SLS.
Preincubation of Vero cells for 1 h at 37 C with SLS concentrations ranging
from 6.25
to 100 pM prior to their infection with HSV-1 (strain F) did not result in a
loss of
infectivity of the virus (data not shown). These results suggest that SLS acts
directly
on the virus and not on cells.
SUBSTITUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-15-
Table 1: Infectivity of various HSV-1 and HSV-2 strains pretreated with
different
concentrations of SLS for 1 hour at 37 C.
PFU (% of control) for
SLS HSV-1 (F)a HSV-2 (333)e HSV-2 (22)a HSV-2 (6)b HSV-2 (15589)
concen-
tration
('UM)
6.25 101.1 t 7.0 102.9 23.5 128.0 18.5 105.3 12.4 108.7 22.2
12.5 79.2 36.4 115.4t17.0 103.4t14.9 82.1 t40.7 115.1 t17.5
25 21.2~18.0 72.9t9.1 63.8t11.9 51.1t30.1 59.0t24.0
50 0 0 0 0 0
awild-type strain
bacyclovir-resistant strain
foscarnet-resistant strain
Figure 1 shows the effect of pretreatment of HSV-1 (strain F) with different
concentrations of SLS or DS on its infectivity to Vero cells. When SLS was
immediately
added to Vero cells following their infection, the loss of viral infectivity
was less
dramatic compared to that obtained for virus pretreated for 1 h at 37 C with
the same
SLS concentrations. Following pretreatment, a loss of 50% of the viral
infectivity was
observed at a concentration of 20 uM compared to 75 ,uM when the virus was not
pretreated. Moreover, although a complete inhibition of viral infectivity was
obtained
following preincubation with 50 ,uM SLS, the inhibition was not complete even
at 100
/.cM without pretreatment. Similarly, pretreatment of the HSV-2 (strain 333)
with SLS
also influenced the infectivity of this strain (data not shown). On the other
hand, DS
reduces the infectivity of the virus independent of whether the virus was
pretreated with
DS. In this case, a loss of 50% of the viral infectivity was observed at a
concentration
of about 1 nM.
The viability of Vero cells exposed for I h at 37 C to SLS or DS
concentrations
similar to those used in Figure 1 and Table 1 was also tested using an MTS
test. No
signs of cytotoxicity could be demonstrated in the range of concentrations
used (data
not shown).
Figure 2 shows the efficacy of different concentrations of SLS (Panel A) or DS
(Panel B) against HSV-1 (strain F) in Vero cells. In brief, cells were
infected with the
SU6STITUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-16-
virus for 2 h at 37 C. Afterwards, supernatant was removed and cells were
overlaid
with 0.5 ml of EMEM + 2% FBS containing 0.6% agarose Seaplaque and SLS or DS
at the desired concentration. Plates were then incubated for 2 days at 37 C in
a 5%
CO2 atmosphere. Cells were fixed with 10% formaldehyde in PBS for 20 min,
washed
with deionized water and stained with 0.05% methylene blue. Viral infectivity
was
evaluated following the determination of PFU. Results show that both SLS and
DS
reduced in a concentration-dependent manner the viral repication in a similar
way with
complete efficacy at 100 M and 20 nM for SLS and DS, respectively. Without
bein.g
bound to any mechanism, the above results suggest that SLS may have a
microbial
adhesion inhibitor effect.
In vitro infectivitv of HIV-1 pretreated with $ S
The effect of pretreating HIV-1 (strain NL4-3) with SLS on its infectivity to
1 G5
cells, a Jurkat E6-1 derivative that harbors two stably integrated constructs
made up
of the luciferase gene under the control of the HIV-1SFZ LTR, has been
also.evaluated.
In brief, prior to infection, the virus was incubated with either culture
medium or 500 pM
SLS for 1 h at 37 C. Cells (1x105 cells/well) were then incubated with HIV-1
strain
NL4-3 (10 ng of p24) for 2 h at 37 C under a 5% COZ atmosphere. Afterwards,
cells
were washed, resuspended in 200 l of complete culture medium and transferred
in
a 96-well flat-bottomed tissue culture plate (Microtest III, Falcon; Becton
Dickinson,
Lincoln Park, NJ). After a 48 h incubation time at 37 C, cells were lysed,
subject to a
freeze-thaw cycle and luciferase activity was monitored using a microplate
luminometer (MLX; Dynex Technologies, Chantilly, VA). Results from this set of
experiments clearly show that pretreatment of HIV-1 (strain NL4-3) with 500 pM
SLS
for 1 h at 37 C almost completely inhibited HIV-1 infectivity to 1G5 cells
(figure 3).
Electron microscopy of Vero cells infected with HSV-1 (strain F) pretreated
with
SLS
The appearance of HSV-1 (strain F) pretreated with varying SLS concentrations
(50, 75 and 100 )uM) for 1 h at 37 C has been evaluated in Vero cells using
electron
microscopy. In brief, cells (80-90% confluent) were infected with the virus
(approximately 70 PFU/ml in 14 ml) for 48 h at 37 C in a 5% CO2 atmosphere.
Cells
were scrapped off from the dishes and resuspended in culture medium. Cells
were
centrifuged (515 x g for 10 min at 4 C) and the supernatant was decanted and
cells
were resuspended in approximately 500 l medium. Cells were transferred in an
eppendorf tube and centrifuged at (10,000 x g for 5 min at 4 C). The pellet
was
SU6STiTUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-17-
resuspended in approximately 200 l of 20% bovine serum albumin (BSA). Few
drops
of 25% glutaraldehyde were added to the mixture and samples were immediately
put
in an ice bath to allow BSA polymerization. The pellet was then cut in 1 mm3
samples
which were then fixed in 2% glutaraldehyde in PBS for I h, 1% Os04 in PBS for
1 h
and then with 0.1 % tannic acid in PBS for 30 min. Samples were rinsed 3 times
in PBS
for 5 min between each step. Samples were stained with 2% uranyl acetate in
10%
ethanol for 30 min. Samples were dehydrated and embedded in Epon following
routine
procedures. Sections (approximately 75 nm thickness) were mounted on copper
grid
(200 mesh). Specimens were stained with uranyl acetate, counterstained with
lead
citrate and observed with a JEOL 1010 electron microscope (JEOL Canada Inc.,
St-
Hubert, QC, Canada).
Figure 4 (Panel A) shows the normal appearence of the virus in the nuclei of
Vero cells. Viral particles were composed of a capsid, hexagonal in shape and,
containing an electron-dense DNA core. Complete viral particles formed by a
nucleocapsid surrounded by an envelope were also found in the cytoplasm of
most
cells. In Vero cells infected with viruses pretreated with 50 (Panel B), 75
(Panel C) and
100 (Panel D) MM SLS, viral particles could be recovered in the nuclei but not
in the
cytoplasm of cells. No mature nucleocapsid could be observed in the nuclei but
viral
particles were constituted by capsids containing a discrete accumulation of
electron-
dense material. The number of empty capsids found in nuclei of cells infected
with
viruses pretreated with SLS decreased with the increased concentrations of
drug used
for the pretreatment. In cells infected with viruses pretreated with 100 MM
SLS, only
a few cells with empty capsids in the nuclei could be detected. Taken
together, thes
results could explain the loss of infectivity of herpes viruses in presence of
SLS.
Quantification of HSV glycoqrotein D gene
The quantification of the glycoprotein D gene of HSV-1 (strain F) pretreated
with
SLS was also evaluated in Vero cells in order to determine the presence of
viral DNA
in the infected cells. In brief, HSV-1 (strain F) was pretreated with varying
SLS
concentrations (12.5, 25, 50, 75 and 100 M) in EMEM + 2% FBS for 1 h at 37 C.
Vero cells (80-90% confluent) were infected with the virus (100 PFU/ml in 20
ml) for
48 h at 37 C in a 5% CO2 atmosphere. The culture medium was removed and cell
sheet was washed twice with 1X HBSS. Cells were scrapped off from the dishes
and
resuspended in EMEM + 2% FBS. Total DNA was extracted using a standard
phenoUchloroform procedure. Quantitation of total DNA was achieved using the
Burton
SU9ST'iTUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 - 18 - PCT/CA99/00359
procedure. The probe used for this study corresponds to a part of glycoprotein
D of
HSV-2 (strain 333), generated by PCR using the following primers:
P1 (5'-GCCACCATGGGGCGTTTGACC-3') and
P2 (5'-AAACTCAGTTATCTAGTCCTCGGGGTC-3')
and was [32P]-labeled by random priming. Hybridization was performed at 65 C
in 0.25
M Na2HPO4 (pH 6.8 with orthophosphoric acid) and 7% SDS. Washes were done in
40 mM Na2HPO4 (pH 6.8 with orthophosphoric acid) and 1% SDS for 20 min at 65 C
followed by 20 min at 25 C.
Figure 5 (Panel A) shows the quantification of the glycoprotein D gene of HSV-
1
(strain F) pretreated with varying concentrations of SLS in Vero cells.
Following a 48
h incubation, cells were collected and total DNA was extracted. Panel A shows
Bglll-
fragmented DNA aliquots (325 ng) applied to a 0.8% agarose gel, transferred to
a
nylon membrane, and hybridized with the glycoprotein D probe. Panel B shows
the
quantitative measurements of HSV-1 DNA levels obtained by scanning
densitometry
of the autoradiogram using an Alphalmager. No major modification in the
expression
of the glycoprotein D gene of the virus could be observed in cells infected
with HSV-1
(strain F) pretreated with 12.5, 25 and 50 M SLS compared to control.
Quantitative
measurements of HSV-1 DNA levels obtained by scanning densitometry of the
autoradiogram were similar (Panel B). However, when the virus was pretreated
with
higher concentrations of SLS (75 and 100 M), a marked reduction in the
expression
of the glycoprotein D gene was observed with a reduction in the DNA levels to
65.1 %
and 34.9% of control values, respectively. These data suggest that SLS could
interfere
with the maturation of viral nucleocapsids either by reducing their rate of
maturation
or by interfering with the encapsidation of DNA into the capsid shell.
In vivo Infectivity of herpes viruses aretreated with SLS (intranasal model)
The effect of pretreating HSV-2 (strain 22) with SLS on viral infectivity has
also
been evaluated in a murine intranasal infection model. In brief, female Balb/c
mice
(Charles River Breeding Laboratories Inc., St-Constant, QC, Canada) 4 weeks-
old
were used throughout this study. Prior to the infection, HSV-2 (strain 22) was
incubated for 1 h at 37 C with PBS or with different concentrations of SLS
(6.25, 25
or 100 ~zM) to reach a final viral inoculum of 2,000 PFU/20 ,ul. Mice were
slightly
anesthetized using Aerrane (Isoflurane, USP; Janssen, North York, ON, Canada)
and
viral suspension (20 mi total volume) was applied into the external left nare
of mice.
Mice were then returned to their cages and survival was evaluated daily.
SUBSTITUTE SHEET (RULE 25)
CA 02329350 2000-10-20
WO 99/53897 - 19 - PCT/CA99/00359
Figure 6 shows that all mice infected with untreated virus died of
encephalitis
between day 9 and day 11. In contrast, 67% of mice infected with the viral
inoculum
pretreated with 6.25 and 25 ,uM SLS survived the infection. Of prime interest,
all mice
infected with a viral suspension pretreated with 100,uM SLS survived the
infection and
did not demonstrate any sign of illness.
1rLyjvo infectivity of herpes viruses nretreated with SLS or DS (cutaneous
model)
The effect of pretreating HSV-1 (strain F) with SLS on viral infectivity has
also
been evaluated in a murine cutaneous infection model. Female hairless mice
(SKH1;
Charles River Breeding Laboratories Inc., St-Constant, QC, Canada), 5-6 weeks
old
were used throughout this study. Prior to infection, HSV-1 (strain F) was
incubated for
1 h at 37 C with PBS, with 6.25, 25 or 100 M SLS or with 0.25, 1 or 10 nM DS
to
obtain a viral inoculum of 3x105 PFU/50,u1. Mice were anesthetized by
intraperitoneal
injection of a mixture containing 70 mg/kg ketamine hydrochloride (Rogarsetic'
injection USP; Rogar/STB Inc. Montreal, QC, Canada) and 11.5 mg/kg xylazine
(Rompun ; Miles Canada Inc., Etobicoke, ON, Canada). The virus was inoculated
on
the lateral side of the body in the left lumbar skin area. The skin was
scratched six
times in a crossed-hatched pattern with a 27-gauge needle held vertically.
Viral
suspension (50 I) was deposited onto the scarified area and rubbed for 10 to
15 sec
with a cotton tipped applicator saturated with EMEM + 2% FBS or SLS or DS
solutions.
The scarified area was protected with a corn cushion which was maintained on
the
mice body with surgical tape. The porous inner wall of the aperture of the
corn cushion
was impermeabilized with tissue adhesive prior to use to prevent absorption of
the
drug. The aperture of the corn cushion was also closed with surgical tape.
Mice were
then returned to their cages and observed twice daily.
Figure 7 shows the time evolution of the mean lesion score of hairless mice
infected cutaneously with HSV-1 (strain F) pretreated with different
concentrations of
SLS or DS for 1 h at 37 C. The evaluation of the lesion score was performed
according to the criteria presented in Table 2. In infected untreated mice, no
pathological signs of cutaneous infection were visible during the first four
days
following infection and only the scarified area remained appearent. On day 5,
herpetic
skin lesions began to appear in some mice in the form of small vesicles
distant from
the inoculation site. On day 6, almost all untreated mice developed herpetic
skin
lesions in the form of a 4-5 mm wide band extending from the spine to the
ventral
SU6STiTUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-20-
midline of the infected dermatome similar to zoster-like infections. Maximal
mean
lesion score was observed on day 8. Mean lesion score decreased thereafter
from day
11 to day 15 because of spontaneous regression of cutaneous lesions in some
mice.
Mice infected with the virus pretreated with 6.25 and 25 M SLS did not
demonstrate
a significant reduction of the mean lesion score. However, mice infected with
the virus
pretreated with 100 kzM SLS did not demonstrate any signs of cutaneous
lesions. Of
prime importance, all mice infected with the virus pretreated with 100 AcM SLS
survived
the infection (data not shown). On the other hand, mice infected with the
virus
pretreated with 0.25 nM DS showed a partial reduction of the mean lesion score
whereas mice infected with the virus pretreated with either 1 or 10 nM DS gave
better
protection against the development of herpetic lesions.
Table 2: Criteria used for the evaluation of herpetic cutaneous lesions
Score Appearence of the lesion
0 No visible infection
1 Infection visible only at inoculation site, scarification area
2 Infection at inoculation site only, with swelling, crust and erythema
3 Infection at inoculation site with discrete lesions forming away from
inoculation site
4 Rash visible around half of body but not yet confluent
5 Rash confluent but not yet necrotic or ulcerated
6 Complete rash with necrosis or ulceration, hind limb paralysis, bloating,
death
In vivo prophylactic effect of poloxamer formulations containing or not SLS
(cutaneous model)
The efficacy of the poloxamer alone and of the poloxamer containing 5% SLS
to prevent the development of cutaneous lesions in mice has also been
evaluated.
Female hairless mice (5-6 weeks old) were used throughout this study. In
brief, mice
were anesthetized by intraperitoneal injection of a mixture containing 70
mg/kg
ketamine hydrochloride and 11.5 mg/kg xylazine. The formulations were applied
topically on the lateral side of the body in the left lumbar skin area. Five
minutes and
1 hour after the application, one drop of viral inoculum (3.15 x 108 PFU/ml)
was
SUBSTTTUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 - 21 - PCT/CA99/00359
deposited onto the skin and a scarification was made with a 27G needle
throughout
the drop to mimic an accident that may occur to health care workers. In this
model, the
viral inoculum needs to be higher to obtain a complete zosteriform rash in
almost all
mice. However, the mortality associated to infection was low and could not be
used as
a criteria to evaluate the efficacy of treatments. The scarified area was
protected with
a corn cushion which was maintained on the mice body with surgical tape. The
aperture of the corn cushion was also closed with surgical tape. Mice were
then
returned to their cages and observed twice daily.
Figure 8 shows the time evolution of the mean lesion score of infected
untreated mice and of mice pretreated with the poloxamer alone or poloxamer
containing 5% SLS 5 min or 1 h prior to their cutaneous infection with HSV-1
(strain
F). Results show that mice pretreated with the gel alone 5 min or 1 h prior to
infection
give only a modest protection against the development of cutaneous lesions. Of
prime
interest, in mice pretreated both 5 min or 1 h with the poloxamer containing
5% SLS,
a complete protection against the development of cutaneous lesions was
observed.
These results show the great potential of our formulations as a prophylactic
approach
to prevent infection with pathogens. Such a tool could indeed protect against
accidental infection of health care workers.
In v1yQgfficagy of gel formulations to arotect against infection caused by
herpes
viruses (intravaginal model)
The efficacy of gel formulations to prevent the genital transmission of HSV-2
has been evaluated in a murine intravaginal infection model. In brief, female
Balb/c
mice aged 4 weeks were used for this study. To increase susceptibility of mice
to
herpes, 2.5 mg of progesterone (Depo-Provera) was administered subcutaneously
to
each mouse 7 days prior to and one day prior to inoculation with HSV-2.
Anesthetized
mice were inoculated with 5 NI of 2.4x10' pfu/ml of HSV-2 (strain 333) after
swabbing
the vagina with a calcium alginate thin tipped swab. To determine the efficacy
of the
gel formulations to block herpes infection, 15 NI of the gel was delivered
with a pipette
tip into the vagina a few minutes prior to the inoculation. The pipette tip
was moved in
and out four times to simulate stirring action of sexual intercourse while
being cautious
not to cause any bleeding.
Figure 9 shows the mean lesion score and survival rate of infected untreated
mice and of mice pretreated intravaginally with the gel alone prior to
infection with
HSV-2 (strain 333). Four days post-infection, infected untreated animals
demonstrated
SUBSTiTUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-22-
perineal oedema and redness and by 6 to 12 days, most of them died of
encephalitis.
Of prime importance, all mice pretreated with the gel alone survived the
infection and
did not demonstrate any sign of illness up to 16 days post-infection. The
presence of
the gel alone could thus abolish HSV-2 infection.
Figure 10 shows the survival rate of infected untreated mice and of mice
pretreated intravaginally with 2.5% SLS or gel containing 2.5% SLS prior to
infection
with HSV-2 (strain 333). Four days post-infection, infected untreated animals
demonstrated perineal oedema and redness and by 6 to 12 days, most of them
died
of encephalitis. Of prime importance, all mice pretreated with either 2.5% SLS
alone
or gel containing 2.5% SLS survived the infection and did not demonstrate any
sign of
illness up to 16 days post-infection. Taken together, these results clearly
indicate that
the use of our gel preparation could represent an innovative preventive
measure to
reduce the sexual transmission of herpes, HIV and other pathogens causing
STDs.
Figure 11 shows the survival rate of infected untreated mice and of mice
pretreated intravaginally with gel containing various compounds prior to
infection with
HSV-2 (strain 333). Those compounds were selected to represent other sulfated
and
non-sulfated compounds having or not detergent properties. They also represent
various ionic (anionic and cationic) and non-ionic compounds. This screening
approach
was aimed to find other potential candidate microbicides. Results showed that
the gel
formulation containing 2.5% lauroyl sarcosine gave complete protection against
infection (100% survival). On the other hand, the gel formulations containing
2.5%
benzalkonium chloride, 5% polyoxyethylene 40 stearate and 5% guanidine gave
60,
60 and 30% survival, respectively. Our preliminary results showed that lauroyl
sarcosine has good potential as a candidate microbicide that we are actually
exploring
now. However, other compounds such as benzalkonium chloride, polyoxyethylene
40
stearate and guanidine that showed partial microbicidal potential can also be
explored
by optimizing their concentration for better efficacy. Alternatively,
combinations of
these compounds may also provide optimal efficacy, if compatible. Without
being
bound to any theory, it is envisageable that the combination of a detergent
with a
chaotropic agent may provide for an efficacy as good as or even better than
SLS.
These are specific examples of potential microbicides, but are in no way
intended to
limit the scope thereof.
SU6STITUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-23-
Design of applicator for vaginal/ano-rectal deliverv of formulations
As mentioned above, it is an object of the present invention to provide
formulations to prevent infection and/or abnormal conditions of mucosae and/or
skin
caused by any pathogen and/or any disease. For vaginal applications, any
topical
formulations should be administered using an applicator which allows uniform
distribution of the content to the entire vagina (delivery to sides) and
cervix (delivery
to front) for maximal efficacy. Therefore, we have designed a unique
applicator which
allows about 3600 distribution of its content into the vagina and far to the
cervix which
is a great improvement over existing conventional vaginal applicators which
deliver
contents only to front (cervix area). The different objectives to achieve and
the main
characteristics that our unique applicator should have to deliver topical
formulations
include:
a) Uniform distribution of topical formulations as liquid or gel to the entire
vagina/cervix
b) Efficient and rapid delivery of its content
c) Resistance to temperature variations (-40 to 60 C)
d) Compatibility of the polymer of the applicator with the gel formulations
e) Ease of sterilization
f) No leakage
g) Ease of manipulation and insertion
h) Resistance to breakage, to expansion of content and to vibrations due to
transport
i) Compatibility with agents and/or conditions present in the surrounding
environment
Technical background and strategy
The efficacy of a formulation to block the sexual transmission of pathogens
causing STDs depends i) on the nature of the formulation to be delivered and
ii) on its
ability to cover the entire vaginal/cervix area. Unlike other products, we
have a unique
formulation with thermoreversible property which is delivered in liquid form
assuring a
good penetration of the formulation into the smallest irregularities of the
vaginal/cervical mucosae. For maximum protection, such a formulation should
cover
the entire vagina/cervix. However, the existing conventional vaginal
applicators have
a unique hole at the tip so that the content is delivered only to the cervix
area excluding
the vagina, limiting therefore its efficacy. Our unique vaginal applicator
will have
multiple holes and/or slots (at the tip and on the sides) to deliver our
formulation or any
other film-forming component, gel, cream, ointment and/or antimicrobial,
bactericidal,
virucidal, chemotherapeutic, antiinflammatory, antineoplastic, or
immunomodulatory
SUBST'i'TUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-24-
agent, detergents, microbial adsorption inhibitor, skin penetration enhancing
agent,
cytokine, antigen, vaccines, or combination of them thereof to treat or
prevent STDs,
cancer or any other disease, to uniformly cover both the vagina and cervix for
maximal
protection. Literature searches revealed that there is no applicators or
similar products
on the market having such a design which allow delivery of their content to
the entire
vagina/cervix.
Characteristics of our applicator
All of the existing vaginal applicators deliver formulations in a form of
gel/cream
which has the disadvantage of not covering the whole vagina/cervix area. On
the other
hand, our formulation has an important thermoreversible property being liquid
at room
temperature and gelifying at body temperature. When delivered as liquid, our
formulation would cover the whole vagina/cervix and it would penetrate through
the
smallest irregularities of vaginal and cervical mucosae. For our unique
formulation or
any other film-forming component, gel, cream, ointment and/or antimicrobial,
bactericidal, virucidal, chemotherapeutic, antiinflammatory, antineoplastic,
or
immunomodulatory agent, detergents, microbial adsorption inhibitor, skin
penetration
enhancing agent, cytokine, antigen, vaccines, or combination of them thereof
to treat
or prevent STDs, cancer or any other disease, we need a unique applicator to
deliver
from the very end as well as sides to cover the whole vagina/cervix which is
the key
factor for offering maximal protection against pathogens causing STDs. The
major
characteristics of the applicator are discussed below (see also Table 3):
a) Uniform distribution of topical formulations as liquid or gel to the entire
vagina/cervix
The applicator must deliver the formulation uniformly and must cover the whole
vagina/cervix area by delivering through apical and lateral holes.
Furthermore, the
applicator should deliver sufficient amount to cover both cervix and vagina.
This will
allow maximal protection of individuals against pathogens causing STDs.
b) Efficient and ra ip d delivej~t of its content
Most existing vaginal applicators deliver only a fraction of its content
limiting the
efficacy of the formulation. Therefore, the applicator must deliver either all
of its
content without leaving residual material in the reservoir or deliver the
quantity required
for sufficient coverage of all target mucosae. This will be achieved through
the design
of the reservoir and calculating the average force of the fingers pressing on
it to
release its content. The time of delivery will vary depending on whether the
content is
SUBSTiTUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-25-
delivered as a liquid, semi-viscous or gel. However, the delivery of
appticator's content
must be rapid.
c) Resistance to temperature variations (-40 tQ60
The applicator must resist temperature variations because storage and
transport environments will vary greatly from one country to another. It
should be
designed so that the applicator and the formulation remain unchanged under
temperature conditions ranging from -40 to 60 C.
d) Compatibility of the polymer of the applicator with the gel
The polymer used for the development of the vaginal applicator should not
affect the properties of the gel formulation (stability, viscosity parameters,
non-
cytotoxicity, efficacy to block pathogens, etc.).
e) Ease of sterilization
The applicator design and material must ensure that it can be sterilized using
a suitable method and should not result in changes in the characteristics of
it or its
content.
f) No leakage
The applicator must be leak-proof under storage and transport conditions. If
boxes are stacked on top of each other, the applicator should not leak its
content.
g) Ease of manipulation and insertion
The applicator must be user friendly, easy to manipulate and easy to insert
without causing any discomfort to its user. Furthermore, it should be
appealing to
users.
h) Resistance to breakage. to expansion of content and vibrations due to
transport
The applicator should resist breakage if it falls from the user's hand or when
it
is handled during transport. It should also resist expansion of its content.
Furthermore,
the applicator should be stable and resist tb vibrations during transport.
i) Compatibility with aaents and/or conditions present in the surrounding
environment
The applicator should resist to the agents and/or various conditions present
in
the surrounding environment. For example, it should not be affected by vaginal
acidic
pH, vaginal discharges or other similar conditions.
SUBSTITUTE SHEET (RULE 25)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-26-
Table 3: Desired functions and target values of the applicator
No Function Description Target value
1 Distributes formulation as Once introduced, proceed to Quantity about 3-5 ml
liquid, semi-viscous, gel, expulsion and distribution of
cream, ointment or any formulation
film-forming component
2 Distributes formulation Distributes formulation to Distributes over about
uniformly cover the whole vagina/cervix 360 in vagina and over
about 360 in cervix
3 Contains formulation as Applicator has reservoir Minimal content of
liquid, semi-viscous, gel, injected volume
cream, ointment or any
film-forming component
4 Leak-proof No leakage from package and 0 mi
after initial manipulation
Easy to manipulate Applicator can be held easily Favourable opinion of
and is user friendly volunteers (7/10)
6 Easy to insert Applicator inserted without Average diameter of
pain and minimal resistance about 0.5 inch (12.5 mm)
7 Delivers to vagina/ceniix The applicator length allows it Average length of
about
to reach cervix 4.5 inch (115 mm)
induding reser-voir and
holding
8 Resists to fall The applicator should not Fall of about 60 inch (1.5
break and content should not m)
leak if it falls from user's hands
9 Resists to surrounding The applicator should not be Data from manufacture
environmental conditions affected by its content, vaginal of thermoplastic
resin
secretions or packaging
material
Not toxic and does not Does not affect the composi- Data from manufacture
affect surrounding envi- tion or quality of formulation; it of thermoplastic
resin
ronmental conditions should also not affect the and topical formulation
surrounding environment owner
11 Resists to vibration during The applicator and reservoir Standards to be
verified
transport should not be damaged and
should operate normally after
transport
12 Be efficient Be operational (delivers Favourable opinion of
content and distributes evenly volunteers (9/10)
without failure)
13 Delivers fast Content is rapidly ejected from About 5 sec
applicator
14 Resists to temperature The applicator should not be - 40 C to + 60 C
variation affected by temperature
variations
Can be rinsed under water Can be rinsed if drops from Data from manufacture
user's hands of thermoplastic resin
16 Sterilizable Suitable method to be selected Standards to be verified
SUBSTiTUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
- 27 -
The following are examples of some different concepts which are intended to
describe some of the general design possibilities of the applicator, but are
in no way
intended to limit the scope thereof. It is important to mention that the final
shape of the
applicator can differ from the examples given herein. It is deemed that such
designs
can be modified to suit ano-rectal application.
Figures 12-15 illustrate specific examples of applicators according to an
aspect
of the present invention. The fotlowing disclosure describes four embodiments
of
applicators illustrated in these figures.
Generally stated, the present applicator is designed to uniformly deliver any
formulation as liquid, semi-viscous, gel, cream, ointment or any other film-
forming
component described herein above into a mucosal cavity, with the smallest
residual
amount thereof left within the applicator. The present applicator comprises a
longitudinally extending body which has proximal and distal ends. The proximal
end
is located close to the external site of the mucosal cavity accessible to the
patient. The
body has external perforations, made as a series of slots or holes, for
uniform
distribution of any formulation as described above to be delivered to the
patient's
mucosal cavity. Upon insertion of the applicator and expulsion of the
formulation in the
mucosal cavity, the formulation which is contained in a reservoir, should
advantageously travel through a diffusion channel having a small volume, prior
to being
expelled through the perforations. Indeed, this allows both the rapid
expulsion of the
formulation and the minimization of the quantity of formulation left in the
applicator after
expulsion.
The diffusion channel is created by a free space between two walls defining
the
body. The first wall is an external wall of the body and includes apertures.
The second,
non perforated, internal wall is provided inside the first wall to create the
diffusion
channel. The internal wall is so configured and sized that it can be slidably
inserted into
the first wall. Alternatively, the internal wall, sized to be smaller than the
first one, may
be integrally molded with the external wall of the body.
The internal wall has a proximal end which is an inlet end for the formulation
into the diffusion channel. A directing element may also be provided to direct
the
formulation into the inlet end of the diffusion channel. The directing element
therefore
prevents entry of the formulation into another compartment than the diffusion
channel.
A reservoir capable of receiving the formulation is also part of the
applicator.
The reservoir can be located near the body of the applicator or inside the
body. The
SUBST'1'TUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 - 28 - PCT/CA99/00359
reservoir is operatively connected to an expulsion element. The expulsion
element is
itself connected to the proximal end of the body through a connector element.
The
expulsion element is actuated by the patient. Upon application of compression,
pull or
push movements, the expulsion element releases the content of the reservoir,
which
is contacted with the proximal entry end of the diffusion channel. The
formulation
therefore travels into the diffusion channel to the mucosal cavity, being
expulsed
through the perforations.
Turning now to Figures 12a-12d of the appended drawings, a first embodiment
of an applicator according to an aspect of the present invention will be
described.
Figure 12b shows an exploded view of this first applicator. The external wall
(1) of the
body of the applicator shows perforations (2) (only one shown) made as one
single slot
extending from one side of the body through the opposite side with no
interruption at
the distal end of the external wall (1). The longitudinal slot therefore
defines lateral and
distal perforations. In this embodiment, the reservoir and the expulsion
element are
one single element (3) made of a compressible material. The formulation is
contained
in the reservoir which ejects its content by pressing it with fingers. The
reservoir is
terminated by a membrane of low resistance to compression (4). The reservoir
being
the expulsion element, it is connected to the proximal end of the body through
a
connector element (5) represented by a screwable or snap-in connector element.
In
this particular embodiment, the internal wall (6) of the body is provided as a
separated
element dimensioned to be smaller than the external wall. The proximal end of
the
internal wall terminates with a protruding collar that sits onto the connector
element
formed at the proximal end of the external wall. The proximal part of the
internal wall
comprises a closing element (7) which closes the internal lumen formed by the
internal
wall. The closing element may have the shape of a disc. Alternatively, the
proximal end
of the internal wall may be integrally molded with the latter to be simply
closed.
Concentric to this closing element, there is an open concentric element (8)
located at
the periphery of the closing element. These elements provide for a generally
called
directing element, which directs the formulation into the diffusion channel
formed
between the internal and the external walls and away from the internal surface
of the
internal wall (6). Figure 12c also shows a tapered element (9), located at the
centre of
the directing means, provided to break the membrane (4) when adequate pressure
is
applied.
SU9STITt1TE SHEET (RULE 25)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-29-
A second embodiment of the applicator is illustrated in Figure 13. The same
peripheral and internal walls as in Figure 12 are used in this applicator.
However, a
plurality of slots regularly spaced from each other are provided in the
external wall. In
this specific version, the expulsion element and the reservoir are also one
single
element. However, the expulsion element is not a compressible reservoir. It is
rather
a piston-like structure (10) which comprises the formulation provided in a
pouch (11).
In this embodiment, the connector element (5) is telescopically insertable in
the piston-
like structure (10). The pouch is made of a material of low resistance to
compression.
To break this membrane, a tapered element is provided at the proximal end of
the
internal wall. Figure 13 shows this tapered element (9) as a disc provided
with a
pointed portion. The disc sits on the proximal end of the intemal wall, the
pointed
portion facing the pouch (11). In use, the piston-like structure (10) is
pressed by the
user, the membrane is thus pierced by the pointed portion, and the formulation
is thus
forced through the diffusion channel, and expelled through the perforations.
Figure 14 illustrates a third embodiment of the present applicator. While the
two
previous embodiments show a reservoir located near the proximal end of the
diffusion
channel, this third embodiment shows a reservoir (12) provided away from the
proximal
end of the diffusion channel. In this case, a seat (13) located away from the
reservoir
is provided. The seat is operatively connected to the piston (14) located
proximally to
the reservoir (12). The user pulls the piston and therefore compresses the
reservoir,
the content of which is engaged into the proximal inlet end of the diffusion
channel.
The formulation is expulsed through perforations made in the external wall of
the body
of the applicator, shown in Figure 14 as a plurality of holes (2). The holes
are spaced
in such a way that the formulation is uniformly distributed into the mucosal
cavity. The
holes are located in the longitudinal section of the external wall as well as
to the distal
end thereof. Figure 14 further shows that the internal and extemal walls of
the body of
the applicator may be integrally formed. Alternatively, the internal wall may
also take
the shape of the one shown in Figures 12 and 13, without the need of a tapered
element. The reservoir may include a membrane of low resistance to compression
in
such a way that, when compressed by the pull movement of the piston (14), the
membrane breaks and discharges its content into the diffusion channel. In this
embodiment of the applicator, the directing element is formed by the proximal
entry
end of the diffusion channel and a closing element located this time at the
proximal end
of the body (not shown).
SUBSTITUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-30-
Figure 15 shows a fourth embodiment of the applicator according to an aspect
of the present invention. In this embodiment, the reservoir and expulsion
element are
a single element. A membrane (4) of low resistance is located close to the
proximal
end of the body (1). The external wall of the applicator comprises slots that
are
practised as a plurality of grooves. The internal wall (6) is integrally
formed with the
outer wall. The internal wall terminates at its proximal end with a tapered
element (15).
The reservoir/piston (16) has a diameter which is slightly larger than the
external
diameter of the internal wall, but smaller than the internal diameter of the
external wall
of the body of the applicator. In use, the reservoir is slidably engaged
between the two
walls, the membrane is pierced and its contents are forced in to the diffusion
channel
and in the perforations located on the sides and at the distal end of the
external wall.
It is to be noted that in all the above described embodiments, the directing
element may be integrally formed with the proximal end of the internal wall of
the body
or be provided as a closing element or disc to block the passage of the
formulation into
the internal lumen formed by the internal wall and to direct the flow of the
formulation
into the diffusion channel.
Further, for ease of use, grasping elements may be provided in some
embodiments to help the user maintain the applicator in place while actuating
the
expulsion element. More specifically, in the second embodiment, the grasping
element
is defined by the annular collar (17) formed at the outer periphery of the
connector
element (5). The annular collar has an external thickness such that the user
has
enough space to grasp the distal end of the collar between fingers and push
the piston
with another finger. In the third embodiment, the grasping element is provided
at the
proximal end of the piston (see numeral 18). The extemal wall of the body
being of a
larger section than the piston, the user can hold the body of the applicator
by its
proximal end with one hand and pull the piston with another. Finally, in the
fourth
embodiment, the grasping element is provided as an elliptic handle (19)
located at the
proximal end of the body of the applicator and surrounding the connector
element. This
handle may be held between two fingers, while the piston is pushed with
another
finger.
Examples involving our poloxamer formulations for treatment of infection
For the purpose of testing the efficacy of our gel formulations in a murine
model
of cutaneous HSV-1 infection, the solutions were prepared within a phosphate
buffer
(0.2 M, pH 6) to be compatible with the pH of the skin.
SUBSTITUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
-31 -
Comparative efficacy of topical formulations of foscarnet. acyciovir* and of
Zovirax ointment against HSV-1 cutaneous lesions in mice
The efficacy of our different topical formulations has been evaluated in a
murine
model of cutaneous HSV-1 infection. In brief, female hairless mice (SKHI;
Charles
River Breeding Laboratories Inc., St-Constant, QC, Canada), 5-7 weeks old were
anesthetized by intraperitoneal injection of a mixture containing 70 mg/kg
ketamine
hydrochloride and 11.5 mg/kg xylazine. The virus was inoculated on the
lateral,side of
the body in the left lumbar skin area. The skin was scratched six times with a
27 gauge
needle held vertically in a crossed-hatched pattern. Fifty kii of viral
suspension (HSV-1
strain F, 1.5x10g plaque forming units (PFU)/ml) was rubbed for 10 to 15 sec
on the
scarified skin area with a cotton tipped applicator saturated with culture
medium
[minimum essential medium (MEM) supplemented with 100 U/mI of penicillin-
streptomycin, 2 mM L-glutamine and 2% fetal bovine serum (MEM-E + 2% FBS)].
The
scarified area was protected with a corn cushion which was maintained on the
mice
body with surgical tape. The porous inner wall of the aperture of the corn
cushion was
impermeabilized with tissue adhesive prior to use to prevent absorption of the
drug.
The aperture of the corn cushion was also closed with surgical tape. Mice were
then
returned to their cages and observed twice daily.
Different treatment regimens were evaluated in this study. Briefly, the tape
closing the aperture of the corn cushion was removed and the scarified area
was
cleaned with a cotton tipped applicator saturated with cold water. Fifteen l
of the
different formulations was applied onto the scarified area. The aperture of
the corn
cushion was closed with surgical tape to avoid rapid removal of the drug by
the mice.
This procedure also prevents accidental systemic treatment that could occur
due to
potential licking of the treated lesions. The efficacy of the different
formulations was
evaluated using lesion scores and survival.
Figure 16 (Panel A) shows the time evolution of mean lesion score of infected
untreated mice or mice treated with foscarnet in solution or incorporated into
poloxamer. Treatment was started 24 h after infection and was repeated 3 times
daily
for 4 days. In mice treated with the poloxamer alone, we observed a pattern
largely
similar to that seen with untreated mice except that the regression of
cutaneous lesions
seemed to go faster in the latter group. In mice treated with a solution of
0.5%
foscarnet, we observed a large reduction of mean lesion score which was more
pronounced when the drug was associated to the poioxamer formulation. Figure
16
SU6STiTUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
- 32 -
(Panel B) shows the corresponding survival for infected untreated mice and
mice
treated with the drug formulations. Death by encephalitis occured in 75% of
untreated
infected mice between day 7 and day 8. The mortality was similar in mice
receiving the
poloxamer alone and occured between day 8 and 10. Half of the mice treated
with
foscarnet in solution survived the infection. Of prime interest, 75% of mice
treated with
the poloxamer formulation of foscarnet survived the infection (p<0.05).
Figure 17 (Panel A) shows the time evolution of the mean lesion score of
infected untreated mice and of mice treated with a single application at 24 h
post-
infection of the poloxamer containing 5% acyclovir or the Zovirax ointment.
Of prime
interest, the poloxamer formulation containing 5% acyclovir demonstrated a
good
efficacy against the development of cutaneous lesions in mice, whereas the
Zovirax
ointment exerted only a modest effect. However, acyclovir incorporated into
the
poloxamer significantly reduced the lethality (p < 0.05), but not the Zovirax
ointment
(Panel B). The higher efficacy of the poloxamer formulation of acyclovir over
the
commercial Zovirax ointment highly suggests that the poloxamer could be a
better
vehicle for the topical delivery of this drug.
Figure 18 (Panel A) shows the time evolution of the mean lesion score of
control mice and of mice treated 3 times daily during 4 days and initiated 5
days post-
infection with the poloxamer alone, poloxamer containing 5% acyclovir or the
Zovirax
ointment. In mice receiving the poloxamer alone, a reduction in the mean
lesion score
compared to infected untreated mice was observed. Treatment with the Zovirax
ointment exerted only a modest effect. However, a marked reduction of the mean
lesion score was observed for mice treated with the poloxamer formulation
containing
5% acyclovir when compared to untreated infected animals. Of prime interest,
all mice
treated with the poloxamer containing 5% acyclovir survived the infection (p <
0.001)
(Figure 18, Panel B). Treatment with Zovirax ointment increase to a lesser
extent the
survival of infected mice (p < 0.05).
In vivo skin penetratiQo of antivirals
Figure 19 shows the distribution of foscarnet and- acyclovir in skin tissues
of
uninfected (Panels A, C, E) and infected (Panels B, D, F) mice at 24 h after
their
topical application, either in phosphate buffer or in the poloxamer matrix.
The
distribution of both formulations of foscarnet and of the buffered solution of
acyclovir
was similar in the stratum corneum tape strips of uninfected and infected
mice. In
contrast, the incorporation of acyclovir into the poloxamer markedly increased
the
SUBST'iTUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 -33 PCT/CA99/00359
-
amount of drug recovered in the stratum corneum of both uninfected and
infected
mice; the increased drug penetration being more pronounced in infected mice.
No or
negligible amounts of foscarnet were found in the underlying epidermis and
dermis of
uninfected mice irrespective of the carrier used for the drug application. The
concentration of foscarnet in the epidermis and dermis of infected mice was
significantly higher when the drug was incorporated within the poloxamer. The
concentration of acyclovir was higher than that of foscarnet in the epidermis
and
dermis of both uninfected and infected mice irrespective of the carrier used.
The
concentration of acyclovir incorporated within the poloxamer in the epidermis
of
uninfected mice was 6.1-fold greater than that of the drug in the buffered
solution.
Infection of mice did not significantly increase the amount of acyclovir in
the epidermis.
The concentration of acyclovir in the dermis of infected mice was 7.9-fold
greater than
that in uninfected mice when the drug was administered in the poloxamer
matrix.
Figure 20 shows the concentration of acyclovir in plasma of uninfected and
infected mice at 24 h after its topical application, either in phosphate
buffer or in the
poloxamer matrix. Similar concentrations of acyclovir were found in plasma of
uninfected mice for both formulations. Infection of mice markedly increased
the
concentration of acyclovir in plasma, especially when the drug was
incorporated within
the poloxamer matrix for which a 4-fold increased concentration was reached.
The
concentration of acyclovir in the plasma of infected mice was 2.1 fold greater
when the
drug was incorporated into the poloxamer matrix.
Effect of SLS on the efficacv of poloxamer formulations containing foscamet or
acyclovir against HSV-1 cutaneous lesions in mice
The influence of SLS on the efficacy of poloxamer formulations containing
foscarnet against HSV-1 infection has also been evaluated in mice. Figure 21
(Panel
A) shows the time evolution of the mean lesion score of untreated infected
mice and
of infected mice treated with a single application (given 24 h after the
infection) of the
poloxamer alone, poloxamer containing 3% foscarnet, poloxamer containing 5%
SLS,
or poloxamer containing 3% foscarnet + 5% SLS. Poloxamer alone did not give
any
protection against infection. Furthermore, a modest decrease in the mean
lesion score
was observed in mice treated with poloxamer containing either 5% SLS or 3%
foscarnet when compared to untreated infected mice. Of prime interest, in mice
treated
with the poloxamer containing 3% foscarnet and 5% SLS, we observed a marked
and
significant reduction (p < 0.05) in the mean lesion score compared to that of
untreated
SUBS'TITUTE SHEET (RULE 26)
CA 02329350 2000-10-20
WO 99/53897 PCT/CA99/00359
- 34 -
infected mice. The corresponding survival rates for the same treatment groups
are
given in Panel B which support the results of mean lesion scores. The skin
penetration
enhancer property of SLS combined with its ability to modify viral infectivity
could
explain the enhanced efficacy of the foscarnet formulation.
In vitro susceptibility of HSV-1 to combination of foscarnet and SLS
The effect of SLS on the efficacy of foscarnet against HSV-1 (strain F) was
investigated in Vero cells. In brief, cells were seeded in 24 well-plates
(Costar,
Montreal, QC, Canada) and were incubated with HSV-1 strain F (approximately
100
PFU/mi) for 2 h at 37 C to allow virus adsorption. Afterwards, virus was
removed and
cells overlaid with 0.5 ml of 0.6% agarose Seaplaque (Marine Colloids,
Rockland, MA)
containing different concentrations of foscarnet, SLS or combination of both
compounds. The plates were incubated for 2 days at 37 C. Cells were then fixed
with
10% formaldehyde in PBS for 20 min, washed with deionized water and stained
with
0.05% methylene blue. Virus susceptibility was evaluated via the determination
of
PFU. Figure 22 shows the susceptibility of HSV-1 strain F to combination of
different
concentrations of foscarnet and SLS on Vero cells. Results show that the
presence of
SLS enhanced the efficacy of foscarnet against HSV-1 (strain F) in Vero cells.
Potential applications
The following examples desc(bed herein below are specific potential
applications of our topical formulations, but are in no way intended to limit
the scope
thereof. As demonstrated in the above results, our gel formulations could be
used for
the prevention of infection of skin and/or mucosae and more particularly for
the
prevention of HSV and HIV. In addition, our results showed that our gel
formulations
can serve as a prophylactic agent to prevent accidental infection of health
care
workers. As also demonstrated in the above results, our gel formulations could
be used
for the treatment and prevention of infection of conditions of skin and/or
mucosae and
more particularly for the treatment and prevention of herpetic lesions. Beside
the above
applications, further potential applications are to use our gel formulations
i) for the
healing and/or treatment of burn wounds and prevention of further infection
and ii) for
the treatment and/or prevention of infection of ophthalmic conditions. In the
above
examples, our gel formulations may contain any antimicrobial, bactericidal,
virucidal,
chemotherapeutic, antiinflammatory, antineoplastic, immunomodulator or any
other
agent or combination of them which is effective for the treatment and/or
prevention of
SUBSITTUTE SHEET (RULE 26)
CA 02329350 2007-04-11
-35-
infection and/or abnormal conditions of mucosae and/or skin caused by any
pathogen and/or any disease.
The examples described herein are specific potential uses of our unique
applicator, but are in no way intended to limit the scope thereof. As
described above,
our applicator could be used for the delivery of any topical formulations used
to cover
cervical/vaginal/ano-rectal mucosae for the treatment and/or prevention of
infection
and/or abnormal conditions of mucosae. Our applicator could also be used to
deliver i)
any topical formulations that can prevent the sexual transmission of pathogens
causing
STDs, ii) vaginal contraceptive formulations, iii) topical microbicidal
formulations
against specific diseases and iv) any antimicrobial, bactericidal, virucidal,
chemotherapeutic, antiinflammatory, antineoplastic, or immunomodulatory agent,
detergents, microbial adsorption inhibitor, skin penetration enhancing agent,
cytokine,
antigen, vaccines, radioactive agents or combination of them thereof.