Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02331934 2001-02-07
Protein and its encoding nucleotide sequences for diagnosis, prevention and
treatment of lung injuries and disorders
FIELD OF THE INVENTION
The present invention relates to a novel protein, named pneumo-secretoglobin
(P-SCGB), nucleic acid sequences encoding said protein and portions thereof.
The
invention further relates to the use of said nucleic acids or protein
sequences as a
marker of respiratory health status and its use in the prognosis, diagnosis,
prevention
and/or treatment of lung ~njuries and diseases, and of inflammation or
immunomodulation-related disorders. More particularly, the present invention
relates to
the use of said nucleic acids or proteins for diagnosis or treatment of
asthma. The
present invention also relates to the use of the spec'rfic marker in the
assessment of a
compound's impact on trachea and lung integrity.
BACKGROUND OF THE INVENTON
It is known that the lung interfaces with the environment access a continuous
and
heterogeneous epithelium. The proximal conducting airways are lined with a
pseudostratified epithelium that is progressively replaced by a simple
cuboidal cell layer
in the more distal airways and by a very thin epithelial lining coating more
than 95% cf
the lung's surface area in the alveoli. Numerous cell types, irxluding
ciliated, basal,
goblet and Clara cells, are present along the airways (Breeze R. G. et al.,
Am. Rev.
Respir. Dis. 116:705, 1977).
The primary function of the lung and of the alveolar epithelium in particular,
is to
provide an extensive and thin surface for gas exchange. The pulmonary
epithelium
serves a number of additional functions that basically act to preserve the
capacity for
such gas exchange. It provides a barrier that protects the host from the
outside
environment by segregating inhaled foreign agents, and it controls the
movement of
solutes and water, contributing 'o the maintenance of lung fluid balance. The
lung
epithelium also plays an active role in the metabolism of endogenous mediators
and
xenobiotic agents, and is capable of regeneration, allowing normal cell
turnover and
restoration of airway and alveolar functions after lung injury. Beyond this,
the lung
epithelium produces complex secretions, among which is the mucus blanket, a
surface-
CA 02331934 2001-02-07
2
active agent (surfactant), as well as several proteins important for host
defense (Shale S.
et al., In R.G. Crystal, J.B. West, E.R. Weibel and P.J. flames, ed. The
Lung:Sdentific
Foundations. Lippincotl-Raven, Philadelphia, 479, 1996).
Sampling of the epithelial lining fluid (ELF} by bronchoalveolar lavage (BAL)
is
the common means of studying the proteins secreted by the lung epithelium and
investigating their changes in lung diseases (Reynolds H. Y. and Newball H.H.,
J Lab.
Clin. Med. 84:559, 1974}. Recent studies have shown that some proteins
secreted by
airvvay or alveolar epithelial cells are present not only at the surface of
the respiratory
tract, but also and normally in small amounts in the bloodstream. Among these
proteins
to are the 16-kD Clara cell secretory protein (CC16, CC10), three surfactant-
associated
proteins (surfactant protein [SP]-A, SP-8, and SP-D) and mucin-associated
antigens, as
recognized by monoclonal antibodies (KL-6, 17-81, 17-D2). Because these
proteins are
mainly, if not exclusively, secreted within the respiratory tract, their
occurrence in the
vascular compartment is explained by their leakage from the lung into the
bloodstream.
The leakage of proteins from the lung into the circulation appears to be
governed by a
number of factors, among which the permeabiiityof the tight bronchoalveolar
epithelium,
and the molecular features of the proteins themselves (e.g., size and charge)
and their
exchangeable pool, appear to be critical (Hermans C. et al., Am. J. Respir.
Crit. Care
Med. 159(2):646, 1999). The significance of the levels of these proteins in
serum
2o depends markedly on whether the air-blood barrier is intact or damaged.
Moreover,
these proteins show variations in the serum of patients with d'rfferent lung
diseases and
subjects exposed to lung toxicants (Lindaht M. et al., Thorax 51:1028, 1996;
Lenz A. G.
et al., Electrophoresis 11:540, 1990; Lenz A.G. et aL, Electrophoresis 14:242,
199.
Patent application W099/09054 discloses the discovery of a new bronchoalveolar
protein, belonging to the family of peroxiredoxins. The inventors identified
proteins from
bronchoalveolar lavage fluids (BALF's) which were obtained by washing the
epithelial
lining fluid of the lungs with saline. A pool of BALFs from various patients
was analyzed
by 2D electrophoresis and was fcund to contain a total of 211 silver-stained
spots in the
range of pl 3.5-10 and molecular mass 5-100 kDa. Next, 182 spots were
identrfied by
microsequence analysis and by matching with human blood plasma and the
Macrophage Like Cell line reference 2-DE maps available from the SWISS-2DPAGE
database. The human bronchoalveolar lavage fluid was finally found to contain
61
different proteins or isoforms thereof. Most of the proteins identified in the
lavage were
known and either were produced locally or originate from plasma. However, one
CA 02331934 2001-02-07
3
particular peptide sequence did not correspond to a previously know protein
and thus
was subjected to further analysis to unravel the potentially new protein
associated. The
DNA coding sequence was cloned and sequenced and the protein was found to be a
new peroxiredoxin (Knoops B. ef al., J. Biol. Chem. 274(43):3045 t, 1999).
As mentioned above, the concentration of lung specrfic proteins in serum can
be
used in the evaluation of lung disorders with a similar utility as proteinuria
in kidney
diseases involving the glomeruli. Human and experimental data indicate that
comparable
structural and functional features govern the passage of proteins across the
lung
epitheliumJblood barrier and the glomerular filter. ft is known that the
concentrations in
1o serum of some lung-specific secretory proteins, such as the bronchiolar
Clara cell 16 kD
protein (CC16) and alveolar surfactant-associated proteins A and 8 (SP-A and
SP-B),
can be used to assess the integrity of the respiratory tract epithelium.
Therefore, newly identified lung-specific proteins could be used as diagnostic
tools for lung disorders in the same way as human Clara cell protein (CC16,
another
member of the uteroglobin/secretoglobin family). In addition, such newly
identified
molecules may help to develop and/or to improve several therapeutic
applications of
various lung injuries and disorders, and of inflammation- or immunomodulation-
related
disorders. Any protein spec'rfic for the lung and secreted in respiratory
airways is likely
to be used as peripheral lung marker especially if it is a small size protein
(molecular
2o weight less than 20 kD) readily diffusible from the lung into serum.
Therefore, it is a major goal of the present invention to provide nucleic acid
as
well as amino acid sequences encoding novel lung-specific proteins.
It is another major goal of the present invention to provide methods and kits
for
the diagnosis and detection of pulmonary injuries and disorders, as well as
inflammation
or immunomodulation-related disorders. Human health is determined by the
complex
interplay between genetic susceptibility and environmental exposure. It is a
further goal
of the invention to demonstrate the linkage of the new protein and sequences
to lung
diseases and disorders, as well as inflammation- or immunomodulation-related
disorders, and to provide tools for the analysis of such disorders. In
particular the
3o determination of an individual's allelic pattern for a gene related to
particular diseases
provides greater insight into the identification of individuals at risk for
such diseases,
early prevention and therapy, and understanding of the pathology. It is a
further goal of
the invention to provide methods for this application in the field of lung
diseases and
disorders, as well as inflammation- or immunomodulation-related disorders.
CA 02331934 2001-02-07
4
It is a further goal of the present invention to provide compos~fions for the
prevention andlor treatment of said lung injuries and the related disarde.~s
as metrsoned
above.
Another goal of the present invention is to provide methods a>tvw to screen
ccrnpounds used for the prevention or treatment of lung injuries and reed
deso~s.
All the goals of the present invention have been met by the emooc5rnertts at
set
a.t below in the description of the invention.
SJMMARY OF THE INVENTION
to
The essence of this invention is based on the identif'~ ~ a never low
molecular weight human bronchoalveolar protein and its encodes reudeic acid
sequence. Said protein was found by studying 2D electrophoresis ~oten reaps of
h;~man bronchoalveolar lavage fluids (BALF's) from individual patients w~ wNl-
defined
t5 interstitial lung diseases (sarcoidosis, idiopathic pulmonary fps (1PF] and
hypersensitivity pneumonitis (HP)). The protein spots were furtt~ analysEd by
r~icrosequence analysis and by matching with human blood ~asr-~a and the
t~!$crophage Like Cell Line reference 2-DE maps available from tte S~NtSS-2D
PAGE
database. This microsequencing gave the sequence of a short pence from which
20 o~gonucleotides were designed to deduce the sequence of the compi~ txxr~an
cDNA
~quence (SEQ ID N02) of a new bronchoalveolar protein (SEQ i0 ~t0 t), named
pneumo-secretoglobin (P-SCGB). In addition, the human genomic DNA seq;~ence
(SEo
ID NO 3), and the homologous mouse cDNA (SEA 10 NO 4) and its encoring amino
acid sequence (SEO ID NO 5) have been revealed. It was furthem~ap demonstrated
25 tt'~at the expression of said protein is very specific to the respiratory
tract, esa. l4ng and
t~achea.
DETAILED DESCRIPTION OF THE INVENTION
3o According to a first embodiment, the present invention relates to an ~aated
~~ucleic acid encoding a new lung marker protein or an immunologocaA~f acwe
andlor a
'~nctional fragment of said protein selected from the group consistasg of
;a) a nucleic acid comprising or consisting of at least a functional ,:art of
the DNA
sequence as given in SEQ ID NO 2, 3 or 4, or the complement thefxt.
CA 02331934 2001-02-07
(b) a nucleic acid which selectively hybridizes with any of the sequences as
given in
SEO 10 NO 2, 3, 4, 7, 8, 9 or 10,
(c) a nucleic acid comprising at least a functional part of a sequence
encoding a protein
with an amino acid sequence which is at least 60 ~ homologous to the amino
acid
5 sequence as given in SEA ID NO 1 or 5,
(d) a nucleic acid encoding a protein comprising or consisting of the amino
acid
sequence as given in SECT ID NO 1 or 5,
(e) a nucleic acid sequence which is degenerated as a result of the genetic
code to a
nucleotide sequence encoding a protein as given in SEO NO 1 or 5 or to a
nucleic
1o acid as defined in (a) to (d), and,
(t) a nucleic acid sequence encoding a protein as defined in SEQ NO 1 or S or
as
defined in any one of (a) to (e) interrupted by intervening DNA sequences.
As illustrated in Example t , the present inventors were able to clone and
sequence a new gene encoding a new human bronchoalveolar protein, named pneumo
secretoglobin (P-SCGB). This new protein was found by sequencing proteins from
human bronchoalveolar fluids (BALF's) which were separated on a 2D-
electrophoresis
get. The present inventors surprisingly found that this until now unknown
protein was
differentially expressed in some lung pathologies, as it clearly appears in
bronchoalveolar lavage fluids (BALF) from patients suffering from idiopathic
pulmonary
fibrosis (1PF} and is almost undetectable in BALF from controls.
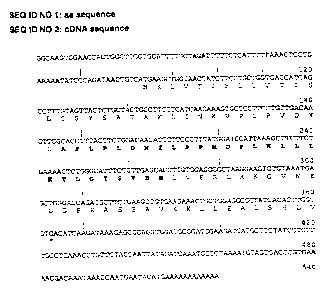
In Figure 1 the cDNA sequence given by SEA ID NO 2 and the corresponding
amino acid sequence given by SEA ID NO 1 is represented. Sequence similarity
searches on both nucleic acid (SEO ID NO 2) as protein level (SEQ ID NO 1 )
with
sequences available in public databases revealed that the cDNA sequence
including its
encoded protein sequence do not share significant homology with previously
known
proteins or cDNA's of human origin, and therefore can be defined as new.
According to the invention, the protein P-SCGB given by SEG1 ID NO 1 presents
a pl of 7.66 and a molecular weight of 10161 D. Other structural properties of
P-SCGB
are further defined in example 2. The sequence of this new protein given by
SEO ID NO
1 shows sign'rficant similarity with the consensus sequence of the
uteroglobins/secretoglobins, as illustrated in example 3. This finding
together with
additional functional properties of the protein as described in example 3
indicate that the
new protein P-SCGB can be classified within the "uteroglobin/secretoglobin"
family. The
inventors were also able to clone the homologous gene from mouse (example 5).
In
CA 02331934 2001-02-07
6
Figure 5 the mouse cDNA sequence given by SEO ID NO 4 and its corresponding
amino
acid sequence given by SEQ ID NO 5 are represented. Finally, the inventors
have also
identified the genomic sequence of P-SCGB (example 6). This genomic sequence
is
given by SEA ID NO 3 and illustrated in Fgure 7.
The term "nucleic acid" as used above refers to DNA or RNA, or ampl'rfied
versions thereof, or the complement thereof. DNA may refer to genomic,
synthetic DNA
or cONA.
The term "isolated" distinguishes the protein or nucleic aad according to the
invention from the naturally occuring versions thereof. A nucleic acid
accorcf~ng to the
to invention may also comprise any modified nucleotide known in the art.
The term "complement' refers to a nucleotide sequence which is complementary
to an indicated sequence and which is able to hybridize to the indicated
sequence.
The term "functional fragment" when referred to nucleic acids, refers to a
part of
the nucleic acid encoding substantially at least one biological function of
the protein
which said nucleic acid is encoding.
In the context of the present invention, and since P-SCGB is specifically
expressed in the airway tract, it is believed that this protein is very
important in the field
of lung and/or inflammation related disorders. A list of lung disorders which
may be
related to the expression of P-SCGB is given ~n the description of this
invention further
2o below.
Furthermore, the inventors could assign the gene coding for the protein P-SCGB
to chromosome 5 locus Sq31-33 (example 6). Therefore P-SCGB is likely to play
a role
in diseases linked to this chromosomal locus. Evidence for linkage of this
locus to the
immune response was provided by studies showing that it is a site of
regulation of
peripheral blood eosinophilia (Martinez F.D. ef al., Am. J. Respir. Crit. Care
Med.
158(6):1739, 1998) and the immunoglobulin E concentration in serum. These
parameters correlate with clinical expression of allergy, atopy and asthma.
More
precisely, this chromosomal locus has been associated with disorders of the
bronchial
responsiveness. Indeed, chromosome 5 is particularly studied in the field of
asthma
research. Asthma is known as a complex heritable disease. This means that
there are a
number of genes that contribute towards a person's susceptibility to that
disease, and in
the case of asthma, chromosomes 5, fi, 11, 14 and 12 have all been implicated.
The
relative roles of these genes in asthma predisposition are not clear, but one
of the most
promising sites for investigation is on chromosome ~. Although a gene for
asthma from
CA 02331934 2001-02-07
7
this site has not yet been specifically identified, it is known that this
region is rich in
genes coding for key molecules in the inflammatory response seen in asthma.
This is
consistent with the suspected anti-inflammatory properties of P-SCGB.
Loci associated with asthma have been more precisely located to 5q31-33 and
11 q13 (Palmer L.J. et al., Am. J. Respir. Crit. Care Med. 158(6):1825, 1998).
It is known
that 11q13 is the locus of CC16 (Zhang Z. et al., DNA Cell Biol. Jan 16(1):73.
199, a
protein definitely associated with asthma (Lensmar C. et al., Ce!! Mol. Life
Sci.
57(6):976, 2000), and it is known that a mutation in the CC16 gene is
associated with
asthma (Laing LA. et al., J. Med. Genet. 35(6):463, 1998).
to The fact that the chromosomal locus for P-SCGB is linked to asthma, like
the one
for CC16, the fact that P-SCGB is an uteroglobin-like, small MW, secreted and
lung-
specific protein like CC16, lead the inventors to suggest that P-SCGB is
associated with
asthma. Further evidence for linkage of the chromosomal linkage of the
chromosomal
locus of P-SCGB to asthma and disorders of responsiveness is provided by
numerous
studies (Los H. et al., Eur. E~espir. J. 14(5):1210, 1999; Mansur A.H. et aL,
Clin. Exp.
Allergy 28(2):141, 1998; Holgate S. T., J. Allergy Clin. ImmunoL 104(6):1139,
1999; Km
H. S. et al., Cun-. Opin. Pulm. Med. 4(1)'46, 1998; Mansur A.H. et al., CGn.
Exp. Allergy
28(2):141, 1998; Kawakami Y. et aL, Respirology 2(1):7, 1997).
It has also been shown that chromosome 5 aberrations are associated with an
Zo increased risk for lung cancer (Wu X. et al., Int. J. Cancer 79(5):490,
1998), or an
increase in prostate cancer aggressiveness (Sark T et al., Int. J. Cancer
81(2):219,
1999) the locus being once again 5q. This is consistent with some properties
already
demonstrated for CC16, i.e. that its overexpression reduces carcinogenesis and
has
tumor suppressor-like effects (Zhang Z. et al., Pros. Natl. Acad. Sci. USA
96(7):3963,
1999). Thus information provided in the present invention are useful in the
prognosis,
diagnosis and/or treatment of some forms of cancer, more particularly prostate
and lung
cancer.
The term "functional fragment" when referred to peptides or proteins, refers
to a
fragment having substantially at least one of the biological activities of the
polypeptide
from which it is derived. The term "functional fragment" of a protein relates
to a truncated
version of the original protein or polypeptide referred to. The truncated
protein sequence
can vary widely in length; the minimum size being a sequence of sufficient
size to
provide a sequence with at least a comparable function and/or activity of the
original
sequence referred to, while the maximum size is not critical. In some
applications, the
CA 02331934 2001-02-07
8
maximum size usually is not substantially greater than that required to
provide the
desired activity and/or functions) of the original sequence. A functional
fragment can
also relate to a subunit with similar function as said protein. Typically, the
truncated
amino acid sequence will range from about 5 to about 60 amino acids in length.
More
typically, however, the sequence will be a maximum of about 50 amino acids in
length,
preferably a maximum of about 60 amino acids. It is usually desirable to
select
sequences of at least about 10, 12 or 15 amino acids.
Functional fragments include those comprising an epitope or an amino acid
stretch which is specific or unique for the proteins according to the
invention. Epitopes
may be determined using, for example, peptide scanning techniques as described
in
Geysen et al. (1996). Preferred functional fragments have a length of at
least, for
example, 5, 10, 25 or 50 amino acids.
With "immunologically active' is meant that a molecule or specific fragments
thereof such as epitopes or haptens are recognized by, i.e. bind to
antibodies. This
i5 recognition is preferably a specific immunological recognition.
The term 'spec'rfically hybridizing" means hybridizing under conditions
wherein
sequences can be detected which are homologues of the sequences of the
invention,
but which are for instance derived from heterologo~s cells or organisms, and
wherein
said sequences do not hybridize with known sequences. It is well known to the
person
skilled in the art which methods for hybridization can be used and which
conditions are
necessary for selectively or specifically hybridizing. Hybridization
conditions are
essentially described in Sambrook (SambrookJ., Fritsch E.F. and Maniatis T.
Molecular
cloning: a laboratory manual. Cold Spring Harfwr Laboratory Press, blew York;
198,
and preferably specific or high stringently hybridization conditions are aimed
at. Stringent
conditions are sequence dependent and will be ditierent under different
circumstances.
Generally, stringent conditions are selected to be about 5°C lower than
the thermal
melting point Tm for the specific sequence at a defined ionic strength and pH.
The Tm is
the temperature (under defined ionic strength and pH) at which 50% of the
target
sequence hybridizes to a perfectly matched probe. Typically, stringent
conditions will be
3o those in which the salt concentration is about 0.02 molar at pH 7 and the
temperature is
at least about 60'C.
According to a more specific embodiment, the present invention relates to a
nucleic acid sequence which is at least 35 % identical to the nucleic acid
sequence as
given in SEC7 ID NO 2. Preferably said nucleic acid is 40 %, 45 % or 50 %
identical,
CA 02331934 2001-02-07
9
more preferably 55 °~, 60 %, 65 %, 70%, 75°~6, 80%, 85%, 90%,
most preferable 95
identical to the nucleic acid sequence as given in SEO ID NO 2.
According to another specific embodiment, the present invention relates to a
nucleic acid sequence which is at least 35 % identical to the nucleic acid
sequence as
given in SE(7 ID NO 3. Preferably said nucleic acid is 40 %, 45 % a 50 %
identical,
more preferably 55 %, 60 %, 65 %, 70%, 75%, 80%, 85%, 90°~6, most
preferable 95
identical to the nucleic acid sequence as given in SEO ID NO 3.
According to another specific embodiment, the present invention relates to a
nucleic acid sequence which is at least 35 % identical to the nucleic acid
sequence as
io given in SEA ID NO 4. Preferably said nucleic acid is 40 °~, 45 % of
50 % identical,
more preferably 55 %, 60 %, 65 %, 70%, 75%, 80%, 85%, 90%, most preferable 95
identical to the nucleic acid sequence as given in SEQ ID NO 4.
According to yet another embodiment the present invention relates to a nucleic
acid sequence comprising a sequence encoding a protein which is at least 60
i5 homologous, preferably 65%, 70 % or 75 % homologous, more preferable 80 %,
85 % or
90 % identical, most preferable 95 % homologous to the amino acid sequences as
given
in SEO tD NO 1.
According to yet another embodiment the present invention relates to a nucleic
acid sequence comprising a sequence encoding a protein which is at least 60
2o homologous, preferably 65%, 70 % or 75 % homologous, more preferable 80 %,
85 % or
90 % identical, most preferable 95 % homologous to the amino acid sequences as
given
in SEA ID NO 5.
The term "identityl' as used above means the degree of sequence relatedness
between two polypeptide or nucleic acid sequences as determined by the
identity of the
25 match between two strings of such sequence. The term "homology' between two
polypeptides is determined by comparing the amino acid sequence of one
polypeptide to
the sequence of a second polypeptide. Amino acids are considered homologous
when
they have the same basic properties in view of their hydrophobicity,
hydrophilicity,
hydrophobic moment, etc. (Vogr G. ef ai., J. Mol. Biol. 249(4):816, 1995).
30 As a practical matter, whether any particular amino acid or nucleic acid
sequence
is identical or homologous to, for instance, the amino acid or nucleic acid
sequence of
this invention, can be determined conventionally using known computer program
methods. Such computer program methods include, but are not limited to, GCG
CA 02331934 2001-02-07
program package (Oevereux J., Nucleic Acids Research 12, 387; 1984), BLASTP,
BLASTN and FASTA (Alfschul S.F., et al., J. Mol. Biol. 215, 403; 19907.
The present invention is also 'related to an antisense molecule comprising a
nucleic acid sequence capable of specifically hybridizing to a nucleic acid of
the present
invention as defined above. Such antisense molecule allows to block a nucleic
acid
sequence of the invention. Nucleic acids of the invention may be inserted into
vectors in
an antisense orientation to provide far the production of antisense RNA. In
addition,
antisense RNA or other antisense nucleic acids may also be produced by
synthetic
means.
to Said antisense molecules may be particularly advantageous for development
of
therapeutics for lung or related disorders. For example, an antisense nucleic
acid
capable of binding to the nucleic acid sequences of the invention may be used
to
selectively inhibit expression of the corresponding polypeptides, leading to
reduction of
associated lung disorders or related disorders.
IS According to further embodiments the present invention also relates to
probes or
primers containing a sequence of at least 15 contiguous nucleotides of a
nucleic acid as
defined above.
The probes will hybridize specifically with any of the ~ nucleic acids of the
invention.
zo The term "probe" according to the present invention refers to a single-
stranded
oligonucleotide sequence which is designed to seleCively hybridize to any of
the nucleic
acids of the invention. The probes used in the process of the invention can be
produced
by any method known in the art, such as cloning of recombinant plasmids
containing
inserts including the corresponding nucleotide sequences, rf need be, by
cleaving the
z5 latter out from the Boned plasmids upon using the appropriate nucleases and
recovering
them (e.g. by fractionation according to molecular weight). Preferably the
probe is about
5-50 nucleotides, more preferably 20, 25, 30, 35, 40 or 45 nucleotides.
The primers will specifically amplify any of the nucleic acids of the
invention.
The term "primer" refers to a single stranded nucleotide sequence capable of
3o acting as a point of initiation for synthesis of a primer extension product
which is
complementary to the nucleic acid strand to be copied. The length and the
sequence of
the primer must be such that they allow to prime the synthesis of the
extension products.
Preferably the primer is about 5-50 nucleotides, more preferably 20, 25, 30,
35, 40 or 45
nucleotides. Specific length and sequence wilt depend on the complexity or the
required
CA 02331934 2001-02-07
it
DNA or RNA targets, as well as on the conditions of primer use such as
temperature and
ionic strength. The fact that amplification primers do not have to match
exactly with
corresponding template sequence to warrant proper amplification is amply
documented
in the literature (Kwok et al., Nucl. Acids Res. 18:99, 1990).
The primers according to the invention may be used in polymerise chain
reaction (PCR) cloning mechanisms which generally involve making a pair of
primers,
which may be from approximately 10 to 50 nuGeotides to a region of the gene
which is
desired to be cloned, bringing the primers into contact with mRNA, cDNA or
genomic
DNA to be amplified, performing a polymerise chain reaction under conditions
which
1o bring about ampf'rfication of the desired region, isolating the amplified
region or fragment
and recovering the amplified DNA. Generally, such techniques as defined herein
are well
known in the art, such as described in Sambrook (Sambrook J., Frttsch E.F. and
Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor
Laboratory
Press, New York; 1989). These primers can also be used to clone homologues of
the
nucleic acid sequences of the invention in other organisms.
The probes and primers as defined above can also be synthesized chemically,
for instance, by the conventional phopho-triester method.
The probes and primers of the invention can optionally be labeler using any
canventional label. ~ his may include the use of labelled nucleotides
incorporated during
the polymerise step of the amplification or by any other method known to the
person
skilled in the art. Suitable labels include radioisotopes such as ~P, ~'P
or'~S, enryme
labels or other protein labels such as biotin or fluorescent markers. Such
labels may be
added to any nucleic acid or oligonucleotide of the invention and may be
detected using
techniques known in the art.
The present irivention is also related to a vector comprising a nucleic acid
sequence as defined above.
Additionally, this vector may be transformed, transfected or infected into a
host
cell.
According to a more preferred embodiment, said vector is an expression vector
3o comprising one or more adjacent regulatory or control sequences (such as
promoter(s),
secretion and termination signal sequence(s)) advantageously operably Inked to
the
nucleic acid sequence of the invention.
The vector according to the invention may advantageously be a plasmid, cosmid,
virus or other suitable vector which is known to those skilled in the art.
E~ression can
CA 02331934 2001-02-07
12
be done in both prokaryotic as eukaryotic host cells such as bacteria, yeast,
fungi, insect
or mammalian cells, which have been previously transformed or transfected by a
vector
coding for the polypeptides of the invention. It is expected that those of
skilled in the art
are knowledgeable in the numerous expression systems available for expression
in
these systems.
According to another embodiment, the present invention relates to an isolated
new lung marker protein comprising one of the polypeptides selected from the
group
consisting of
(a) a polypeptide as given in SEO ID NO 1 or 5,
(b) a polypeptide with an amino acid sequence which is at least 60 %
homologous to
the amino acid sequence as given in SEO ID NO 1 or 5,
(c) a polypeptide encoded by a nucleic acid as given in any of SEO ID NO 2,3
or 4,
(d) a polypeptide encoded by a nucleic acid as given in any of claims 1 or 2,
or a homologue or a derivative of said protein, or an immunologically active
andlor
functional fragment thereof.
The term "protein" in the context of the present invention is used
interohangeabty
with the terms 'polypeptide" and "peptide".
The term "hcmologue" of a protein of the invention are those peptides,
oligopeptides, polypeptides, proteins and enzymes which contain amino acid
substitutions, deletions and/or additions relative tv the said protein with
respect to which
they are a homolog, without altering one or more of its funcfional properties,
in particular
without reducing the activity of the resulting. For example, a homolog of said
protein will
consist of a bioactive amino acid sequence variant of said protein. To produce
such
homologs, amino acids present in the said protein can be replaced by other
amino acids
z5 having similar properties, for example hydrophobicity, hydrophilicity,
hydrophobic
moment, antigenicity, propensity to form or break a-helical structures or ~-
sheet
structures, etc.
The term "derivative" of a protein of the invention are those peptides,
oligopeptides, polypeptides, proteins and enzymes which comprise at least
about five or
ten contiguous amino acid residues of said polypeptide but which retain the
biological
activity of said protein. A "derivative" may further comprise additional
naturally-occurring,
altered glycosylated, acylated or non-naturally occurring amino acid residues
compared
to the amino acid sequence of a naturally-occurring form of said polypeptide.
Alternatively or in addition, a derivative may comprise one or more non-amino
acid
CA 02331934 2001-02-07
t3
substituents compared to the amino acid sequence of a naturally-occurring form
of said
polypeptide, for example a reporter molecule or other ligand, covalently or
non-
covalently bound to the amino acid sequence such as, for example, a reporter
molecule
which is bound thereto to facilitate its detection.
Said polypeptide of the invention may be produced in a recombinant manner The
present invention therefore relates to a method for producing a polypeptide as
defined
above comprising culturing a host cell under conditions allowing the
expression of said
polypeptide and recovering said produced polypeptide from the culture.
Recombinant
host cells according to the present invention as described below may be used
for the
1o recombinant expression of said polypeptide.
The polypeptide may be expressed in a modified form, such as a fusion protein,
and may include not only secretion signals, but also additional heteroiogous
functional
regions. For instance, a region of additional amino acids, particularly
charged amino
acids, may be added to the N-terminus of the polypeptide to improve stability
and
persistance in the host cell, during purification, or during subsequent
handling and
storage. Also, peptide moieties may be added to the polypeptide to facilitate
purification.
Such regions may be removed prior to final preparation of the polypeptide. The
addition
of peptide moieties to polypeptides to engender secretion or excretion, to
improve
stability and facilitate purification, among others, are familiar and routine
techniques in
2o the art.
Said recombinant polypeptides may advantageously be used in diagnostic
methods or kits or the like.
According to yet another embodiment the present invention is related to an
antibody specifically recognizing a protein as defined above, or recognizing
's immunologically active parts or specific epitopes thereof.
The term "antibody" refers to both monoclonal or polycfonal antibodies,
capable
of specrfically binding to one or more epitopes of the proteins of the
invention.
The term "specifically recognizing' implies that there is substantially no
cross
reaction of the antibody with other proteins. The antibodies according to the
invention
3o may be produced according to techniques which are known to those skilled in
the art.
Monoclonal antibodies may be prepared using conventional hybridoma technology
as
described by Kohler and Milstein (Kohler F. and Milstein C. Nature 256, 495;
1975). This
classical method comprises producing any hybridoma formed by, on the one hand,
isolating splenic lymphocytes of an animal, particularly a mouse or a rat
immunized
CA 02331934 2001-02-07
I4
against a polypeptide of the present invention or a fragment as defined above,
and cells
of a myeloma cell line on the other hand, and selecting said hybridoma for the
ability to
produce the monbclonal antibodies recognizing the polypeptide which has been
initially
used for the immunization of the animals.
The antibodies involved in the invention can be labelled by an appropriate
label
of the enzymatic, fluorescent or radioactive type or any label known in the
art.
Alternative to preparing monoclonal antibody-secreting hybridomas, a
monoclonal antibody directed against a polypeptide of the invention can be
identified
and isolated by screening a recombinant combinatorial immunoglobuiin library
(e. g., an
to antibody phage display library) with the polypeptide of interest. flits for
generating and
screening phage display libraries are commercially available (e. g., the
Pharmacia
Recombinant Phage Antibody S~sie~rn, Catalog No. 27-9400-01; and the
Stratagene
SurJZAPPhage Display Krt, Catalog No. 240612). Additionally, examples of
methods
and reagents particularly amenable for use in generating and screening
antibody display
IS library can be found in, for example, U. S. Patent No. 5,223,409; PCT
Publication No.
WO 92/18619; PCT Publication No. WO 91/17271; PCT Publication No. WO 92/20791;
PCT Publication No. NlO 92/15679; PCT Publication No. WO 93/01288; PCT
Publication No. WO 92/01047; PCT Publication No. WO 9?J09690; PCT Publication
No. WO 90/02809; Fucks et al. BioITechnology 9: 1370, 1991; Hay et at.
20 Hum. Anfibod. Hybridomas 3: 81, 1992; Huse et al. Saence 246: 1275, 1989;
Griffiths
et al. EMBO J. 12: 725, 1993).
Additionally recombinant antibodies, such as chimeric and humanized
monoclonal antibodies, comprising both human and non-human portions, which can
be
made using standard recombinant DNA techniques, are within the scope of the
z5 invention.
A chimeric antibody is a molecule in which different portions are derived from
different animal species, such as those having a variable region derived from
a murine
mAb and a human immunoglobulin constant region. (see, e. g., Cabilly et al.,
U. S.
Patent No. 4,816,567; and Boss ef al., U. S. Pafenf No. 4,816397, which are
3o incorporated herein by reference in their entirety.)
Humanized antibodies are antibody molecules from non-human species having
one or more complementarily determining regions (CDRs) from the non-human
species
and a framework region from a human immunoglobulin molecule (see, e. g.,
queen, U.
S. Patent No. 5,585,089, which is incorporated herein by reference in its
entirety.) Such
CA 02331934 2001-02-07
IS
chimeric and humanized monoclonal antibodies can be produced by recombinant
DNA
techniques known in the art, for example, but not limited to, using methods
described in
PCT Publication No. WO 87/02671; European Patent Application 184,187; European
Patent Application 171,496; European Patent Application 173,494; PCT
Publication No.
WO 86/01533; U. S. Patent No. 4,816,567; European Patent Application 125,023;
Better
et al. Science 240: 1041, 1988; Liu et aL Proc. Natl. Acad. Sci. USA 84: 3439,
1987; Liu
et al. J Immunol. 139: 3521, 1987; Sun et al. Proc. NatL
Acad. Sci. USA 84: 214, 1987; Nishimura et al. Canc. Res. 4T 999, 1987; Wood
et aL
Nature 314: 446, 1985; Shaw et al. J. Natl. Cancer Inst.
80: 1553, 1988; Marrison Science 229: 1202, 1985; Oi et al. SiolTechniques 4:
214,
1986; U. S. Patent 5,225,539; Jones et al. Nature 32i: 552, 1986; Verhoeyan et
al.
Science 239: 1534, 1988; Beidler et al. J. lmmunol. 141: 4053, 1988; Holliger
et al.
Cancer Metastasis Rev. 18(4):411, f 999; and Kipriyanov et al., 12(2):173,
1999).
Completely human antibodies are particularly desirable for therapeutic
treatment
t5 of human patients. Such antibodies can be produced, for example, using
transgenic
mice which are incapable of expressing endogenous immunoglobulin heavy and
light
chains genes, but which can express human heavy and light chain genes. The
transgenic mice are immunized in the normal fashion with a selected antigen,
e. g., all or
a portion of a polypeptide of the invention. Monoclonal antibodies directed
against the
antigen can be obtained using conventional hybridoma technology. The human
immunoglobulin transgenes harbored by the transgenic mice rearrange during B
cell
differentiation, and subsequently undergo class switching and somatic
mutation. Thus,
using such a technique, it is possible to produce therapeutically useful IgG,
IgA and IgE
antibodies.
For an overview of this technology for producing human antibodies, see Lonberg
and Huszar (Int. Rev. Immunol. 13: 65-93, 1995). For a detailed discussion of
this
technology for producing human antibodies and protocols for producing such
antibodies,
see, e. g., U. S. Patent 5,625,126; U. S. Patent 5,633,425; U. S. Patent
5,569,825; U. S.
Patent 5,661,016; and U. S. Patent 5,545,806. In addition, companies such as
Abgenix,
Inc. (Fremont, CA), can be engaged to provide human antibodies directed
against a
selected antigen using technology similar to that described above. Completely
human
antibodies which recognize a selected epitope can be generated using a
technique
referred to as 'guided selection." In this approach a selected non-human
monoclonal
antibody, e. g., a mouse antibody, is used to guide the selection of a
completely human
CA 02331934 2001-02-07
l6
antibody recognizing the same epitope. (Jaspers ef al.
&otechnology 12: 899, 199.
Polyclonal antibodies may also be prepared using conventional technology well
known to those skilled in the art, and which comprises inoculating a host
animal, such as
a mouse, with a protein or epitope according to the invention and recovering
the immune
serum.
The present invention also includes fragmertis of whole antibodies which
maintain their binding activity, such as for example, Fv, F(ab') and F(ab')2
fragments as
well as single chain antibodies, and which are prepared according to methods
weU
l0 known in the art.
Antibodies according to the invention may be used in a method of detecting the
presence of a polypeptide according to the invention, which method comprises
reacting
the antibody with a sample from a body fluid (of a patient) and identifying
any protein
bound to said antibody. A diagnostic kit may also be provided for performing
said
method which comprises an antibody according to the invention and means for
reacting
the antibody with said sample.
The antibodies according ;o the invention may also be used as a medicament for
treating lung disorders, or may be ~mprised in a pharmaceutical composition.
According to a further embodiment, the present invention also relates to an
arrfi-
idiotype antibody raised against the antibody as defined above.
In particular, an anti-idiotype antibody raised against an antibody as defned
above, refers to a monoclonal antibodies raised against the antigenic
determinants of
the variable region of the antibody raised against the polypeptides of the
present
inventron or against fragments thereof as defined above. These antigenic
determinants
of immunoglobins are known as idiotypes (set of idiotypes) and can therefore
be
considered to be the "fingerprint of an antibody. Monoclonal anti-idiotypic
antibodies
have the property of forming an immunological complex with the idiotype of the
monoclonal antibody against which they were raised.
In this respect the monoclonal antibody is referred to as Abl, and the anti
idiotypic antibody is referred to as Ab2. Ab2 or fragments thereof can thus
recognise the
interaction site or receptor of ;he polypeptide of the present invention, and
can
consequently block this interaction site. As a result Ab2 may prevent any lung
disorder
associated with an interaction between the polypeptide and its receptor. These
anti
idiotype antibodies can be prepared by methods well known in the art.
CA 02331934 2001-02-07
t~
Anti-idiotypic antibodies may also be used in c ats ,.sed for ~e
detection of lung and related disorders.
According to a next embodiment the present in~~: is .~eiat$d ~ a nor~-~s-man
transgenic animal transformed by a nucleic acid as defines above, or ~r a ~ ~
~e
present invention as defined above.
In a more preferred embodiment, the present investor rela;.es oc a me>~soa for
the production of a genetically modified non-human anrrat in wryd's ~ s
modficaZzon
results in overexpression, underexpression or no expresson of Lne nrdac aac~
or the
polypeptides of the invention as defined above.
1o Said anima( is preferably a mammal such as a ma.se or a ra: trar'sfom'ed by
a
vector according to the invention and overexpressing a protein ac~rding >3o
the
invention, or genetically modified by a partial or total ~ ~~ ~~~e
encoding the protein according to the invention (a knodc~u; non-hurrsar:
~~ar'~ct~1 and
obtained by methods well known by the person skilled in the ar. sr~ as ='ze
one
t5 described by Kahn et al. (Cell 92: 593596; 1998.
In particular, the present inventors have ider~e~d ~e h~-~obc;:.us «cuse
sequence of P-SCGB, as further illustrated in example i Ktcvwecge ~ d'ns
'''~cuse
sequence is the first and necessary step in the generation ~ ~ransges-~c a~
~'aa.
t7ther examples of genetically modified non-hcrar ar,mais :.r'~~d~ ~ fi''e
2o invention are for instance transgenic non-human arur~s comprssr~ ar a~sense
sequence as defined above and complementary to rie ~e~c a-=~ ~en~ces
according to the invention, and placed in such a way tha_ ~ is ~ns~'~ ~'I"~
3_~s~ense
mRNA which is complementary to the nucleic acid sequ~es assorting b ~e
in'~~~ti°n
and which hybridises to said nucleic acid sequences, the~Y ~~r~9 ~
b~'"°r'9 ~e'r
25 translation.
A further aspect of the present invention is relates ~n a com~o~r' 's~9 at
least one nucleic acid sequence, an antisense molecule, 3 pot~peptfce- ar,
artt~c~r, an
anti-idiotypic antibody as defined above, optionally in acr,-~x'u'e Win" a
;~arma~"~t tally
acceptable carrier.
3o A pharmaceutically acceptable carrier includes a°~ ca er ti~i yes
r:~t itself
induce the production of antibodies harmful to the indi~r~a.:a~ 'ece~Y~g ='~e
~r"ion.
Suitable carriers are typically large, slowly metabol~z:~g
rr'aa"..r'ra°~..les sLCi as
proteins, polysaccharides, polylactic acids, polyglycouc ands. ~YT"'~'~ ~'~
aids,
amino acid copolymers; and inactive virus particles. S..aw~~ers a,- weif to
CA 02331934 2001-02-07
I$
those of ordinary skilled in the art. Pharmaceutically acceptable carriers
vary accorang
to the mode of administration (intravenous, intramuscular, subcutaneous,
parental,
etc.), and may comprise also adjuvants well known by the person skilled in the
art to
increase, reduce andlor regulate humoral, local andlor cellular response of
the immune
system.
According to a further embodiment said composition may be used as a
medicament.
In a more preferred embodiment, said medicament is used for the diagnosis,
prevention andlor treatment of a patient, especially a human patient,
preferably affected
by lung disorders or any related disorder as defined hereunder. A sufficient
amount of
the pharmaceutical composition is administered to said patient in order to
detect, trzat,
avoid and/or reduce the symptoms of said injuries and/or diseases.
The various types of lung disorders which may be treated according to the
present invention can subdivided within 3 groups: (1 ) lung disorders caused
by altered
t5 air/blood (or alveolocapillary) permeability e.g. sarcoidosis, fibrosis,
acute respira;ory
distress syndrome CARDS), oedema, lung injury caused by toxic chemicals (omne,
particles, chlorine) or particles (crystalline silica, dust) and lung
inflammation (bronc~rtis,
pneumonia, LPS (lipopolysaccharides); (2) lung disorders caused by acute or
chronic
epithelium damage e.g. BPCO (bronchopulmonary chronic obstructive disease),
tobacco
smoking (active or passive), cancer, asthma, emphysema, fibrosis, lung
allergic
diseases, bronchial hyperresponsiveness and (3) lung disorders associated with
immature lung epithelium e.g. occurring in pre-term babies or babies at risk
for
bronchopulmonary dysplasia.
Other related disorders which can be prevented and/or treated within the scope
of this invention are injuries and/or diseases caused by oxidative stress,
inflammation
and/or aberrant responsiveness: e.g. BPD (bronchopulmonary dysplasia) in pre=
erm
babies, asthma, ARDS (acute respiratory distress syndrome), atopy, allergy,
fibrosis,
acute lung injury etc.
The composition and medicament in accordance with the present invention may
be provided to a patient by means well known in the art, i.e. orally,
intranasally,
subcutaneousiy, intramuscularly, intradermally, intravenously,
intraarterially, parenterally
or by means of catheterization.
CA 02331934 2001-02-07
l9
In a preferred embodiment, the invention relates to the use of a composition
as
described above for the preparation of a medicament for treating lung
disorders or
related disorders as defined above.
The present invention also relates to a method for the in vitro detection of
lung
disorders or other related disorders according to the invention as defined
above, which
method comprises the steps of:
(a) providing a sample from a body fluid of a patient,
(b) contacting said sample with an antibody according to the invention as
described
above,
to (c) optionally contacting said antibody with a secondary antibody, and
(d) detecting a reaction of a molecule in said sample with said antibody.
Said body fluid can be both pulmonary {bronchoalveolar lavage (BAL), sputum,
aspirate,
biopsy, condensate of exhaled air, etc.) or extrapulmonary materials (serum,
plasma,
urine, pleural fluid, etc.).
Said method may be based on one of the techniques known to the one skilled in
the art, such as for instance binding assays, especially ELISA's, RIA's, FAGS
analysis,
dot blot hybridisation, Western Slots: Line immuno-assay (Lia), BIAcore real
time
detection system, doutie immunodiffusion technique, counterelectrophoresis
technique,
agglutination assay, or a mixture thereof.
2o The antibodies used in said techniques may be labelled by a tag,
fluorescent
marker, an enzyme, a particle or a radioactive marker. Such labels are well
known to the
person skilled in the art and comprise, for example, horse radish peroxidase,
alkaline
peroxidase, alkaline phosphatase, ~-galactosidase, fluorochromes (tike
fluorescein,
rhodamine, Texas Red, etc.) colloidal metals (like gold particles), biotin,
digoxygenin and
chemi-or bioluminescent compounds. Any detection method may be assisted by
computer technology and detection methods can therefore be automated by
various
means.
A number of supports known in the art are suitable for use in the techniques
as
given above. Such supports may comprise membranes, plates, strips, wells of a
3o microtiter plate, microchips or containers. Suitable materials for such
supports or
materials for further coating of said supports include, but are not limited
to, glass,
polystyrene, polyvinyl chloride polypropylene, polyethylene, polycarbonate,
dextran,
nylon, amyloses, natural and modified celluloses, like nitrocellulose,
poiyacrylamide,
agaroses, magnetide and metals.
CA 02331934 2001-02-07
In a more preferred embodiment, said d~ection method is based on an ELISA
assay or a latex agglutination assay.
For example, an enzyme-linked immunosorbent assay (ELISA) can be used to
5 measure antigen concentrations, e.g. the concentration of P-SCGB in a body
fluid. This
ELISA method depends upon conjugation of an enzyme to an antibody, and uses
the
bound enzyme activity as a quantitative label. To measure said antigen
concentrations,
the test material containing antigen is fixed to a solid phase such as the
wells of a
microtiter plate, after an incubation the solid phase is washed and an enzyme-
labeled
io antibody, specific for said antigen, is added. Optionally, a first non-
labeled antibody can
be used, which is then incubated with a secondary enzyme-labeled antibody.
After
washing, substrate is added, and enzyme actNity is estimated colorimetricatty,
and
related to antigen concentration.
As an alternative an agglutination assay rnay be used. Said agglutination
assay
t5 may be based on cross(linking) one of the binding partners e.g. an antibody
specific for
said antigen P-SCGB, to a solid support such as a bead, preferably a latex
bead or a
colloidal gold particle. Said beads are incubated wrth the test material
containing antigen
and the interactionlagglutination of said beads is measured.
Other assays than those described herein may be used, these assay formats
2o given herein are not a !imitation on the present irwention.
Said detection methods allow the diagnoss and/or monitoring of lung injuries
and
diseases or other related disorders such as oxidative stress related disorders
and
inflammation as defined above. More particularly said methods allow the
diagnosis
and/or monitoring of asthma.
Since the protein of the invention, P-SCGB is specifically expressed in the
respiratory tract, esp. lung and trachea, and Z has a small size which
facilitates its
transepithelial leakage from the lung into serum, it has all the features to
serve as a
peripheral lung marker, easily detectable in the serum by means of an
antibody. The
present inventors surprisingly found that this protein was differentially
expressed in
3o patients suffering from lung disorders, when compared to normal
individuals. In addition,
the assay of P-SCGB in urine might be used as test to detect proximal tubular
dysfunction in a variety of renal diseases (e.g. cadmium nephropathy, Fanconi
syndrome, diabetic nephropathy, etc.)
CA 02331934 2001-02-07
2t
According to another embodiment, the present invention relates to a method for
the detection of lung disorders or other related disorders of the invention,
which method
comprises the steps of:
(a) providing a sample containing nucleic acids from said patient,
(b) isolating and possibly purifying nucleic acids from said sample,
(c) amplifying said nucleic acids using a primer sequence as defined above,
and,
(d) detecting the presence of amplified DNA indicative for said lung disorders
or related
disorders.
According to the invention, the term "amplification' used in any method
described
may be performed by means of the polymerase chain reaction (PCR) and any of
the
primers of the invention. The amplification step is preferably done by the PCR
reaction
but can also be done by any other type of nucleic acid amplification, such as
6gase chain
reaction (LCR; Landgren et al., Science 241:1077, 1988; Wu and Wallace,
Genomics
4:560, 1989; Barany, Proc. Natl. Acad. Sci. USA 88: 189, 1991), nucleic acid
sequence
based amplification (NASBA; Guatelli ei aL, Proc. Natl. Acad Sd. USA 87: 1874,
1990;
Compton, Nature 350: 91, 1991), transcription-based amplification system (TAS;
Kwoh
et al., 1989), strand displacement amplificatin (SDA; Dudc, Biotechniques 9:
142, 1990;
Walker et al., Proc. Nafi. Acad. Sci. USA, 89: 392, 1992) or amplrfication by
means of
ass replicase (Lizardi et al., BicYTechnoiogy 6: 119? 1988; Lomeli ef al.,
Clin. Chem.
35: 1826, 1989) or any other suitable method to ampl'rfy nucleic acid
molecules. The
amplification reaction is preferably repeated between 20 and 70 times,
advantageously
between 25 and 45 times.
Also included in this invention are methods where the analysis of said
amplified
DNA as defined above and indicative for said lung disorders or related
disorders (more
particularly asthma) is done by direct sequencing or by micro array methods.
Also other methods can be used to analyze an amplified DNA or a mutation
characteristic for said lung disorders or related disorders of the invention,
including
methods such as STS-PCR, countourclamped homogeneous electric field (CHEF) gel
electrophoresis, restriction mapping, hybridization, Southern and Northern
blotting, FISH
3o analysis, mismatch cleavage, single strand conformation polymorhism (SSCP)
or any
other method known in the art. The diagnostic methods of the present invention
also
include segregation analysis, involving PCR-based genotyping and/or
haplotyping
methods. The diagnostic methods according to the present invention also
include
methods based on direct sequencing or CAS (coupled amplification and
sequencing)
CA 02331934 2001-02-07
22
optionally combined with additional analytic steps as known in the art, such
as ligation
analysis to detect and evaluate mutations.
According to yet another embodiment, the present invention relates to a method
for the detection of lung disorders or other related disorders which method
comprises the
s steps of:
(a) providing a sample containing nucleic acids from said patient,
{b) isolating and possibly purifying nucleic acids from said sample,
(c) contacting said nucleic acids with a DNA probe as defined above, and,
(d) detecting a hybridization product indicative for said lung disorders or
rated
disorders.
Thus the detection of said lung disorders or other related disorders of the
invention can also be done by means of a hybridisation reaction with any of
the probes
according to ~e invention. These tests generally comprise contacting the probe
with the
sample under hybridising conditions and detecting the presence of any duplex
or triplex
formation between the probe and any nucleic acid in the sample.
According to a following embodiment, the present invention relates to a method
for the detection of lung disorders or other related disorders which method
comprises the
steps of:
{a) providing a sample from a patient,
(b) contacting said sample with at ;east one of the polypeptides of as defined
above,
and,
(c) detecting a reaction between a molecule in said sample with said
polypeptide.
According to a further embodiment, the present invention relates to a method
for
identifying compounds for treating or preventing lung disorders or related
disorders,
more particularly asthma.
Such screening method may comprise the following steps of (a) contacting a
compound to be tested with at least one of the polypeptides of the invention
as defined
above, (b) detecting the complex formed between the compound to be tested and
said
polypeptide, (c) alternatively, examining the diminution of complex formation
between
3o said polypeptide and a receptor, caused by the addition of the compound
being tested,
(d) alternatively, examining the alteration in the functional activity of the
polypeptide.
caused by the addition of the compound being tested, and (e) identrfying saia
compound.
CA 02331934 2001-02-07
23
Said detection step in (b) is a quantitative detection step based on the
affinity of
said compound. A preferred example of said method is an in vitro assay based
on an
ELISA technique or any other method known in the art, and alkrnrs to test
possible drugs
which can be used in the field of lung disorders or related disorders as
defined above,
preferably via a high throughput method
High throughput screening methods are well known in the art. Any of the well
known assay formats, for example radioimmunoassays, competitive-binding
assays,
Western Blot analysis, antibody sandwich assays, antibody detection, ELISA
assays,
fluorescence polarization, fluorescence energy transfer including fluorescence
to resonance energy transfer (FRET) and homogenous time-resolved fluorescence
(HTRF), fluorescence intensity, fluorescence correlation spectroscopy,
sdntillation
proximity assay (SPA), flash plate assays, and assays which require biotin
incorporation
to provide a recognition event for binding or immobilization of one or more
components,
etc. can be used.
The invention also relates to the compounds identifiable by the above
described
methods.
According to a further embodiment, the present invention also relates to an
alternative compound screening method comprising the following steps of (a)
providing a
transgenic non-human animal as described above, in which knock-out,
underexpression
or overexpression of at least one of the poiypeptides of the invention results
in
symptoms indicative for lung disorders or related disorders, (b) administering
said
compound to said transgenic animal which allows said symptoms to be alleviated
or
cured, and (c) identifying said compound.
The invention thus relates to said non-human transgenic animals as a model
system far testing potential compounds or drugs for lung disorders or related
disorder,
more particularly asthma. Also the compounds identifiable by this method are
part of the
invention.
A good example of using a transgenic animal would be the study of asthma and
potential treatments of asthma using mice whose gene for P-SCGB is deleted or
mutated (known as knock-out mouse or transgenic mouse). The transgenic animal
may
be used for the study of diseases or compounds treating diseases in the field
of
inflammatory and immunomodulatory lung diseases, as defined elsewhere in the
patent.
Other examples describing the use of animal models as compound screening
method are incorporated by the following article references, but are not
considered as a
CA 02331934 2001-02-07
24
limitation on the present invention (Castro C.M. et al., Lab. Invest.
80(10):1533, 2000;
Mukherjee A.B. et aL, Am. J; Kidney l7is. 32(6):1106, 199a Nizielshi S.E. et
al.,
126(11):2697, 1996; Aguzzi A. et al., 74(3):111, 1996; Viney J.L 4(3):461,
1994;
Metsaranfa M. et al., Ann-Med 24(2):117, 1992).
The present invention also relates to methods for treating lung disorders or
related disorders (more particularly asthma) as defined above by administering
to a
patient a suitable nucleic acid delivery system for gene therapy. Said method
may
comprise administering to a patient in need a normal version of a nucleic add
or gene of
tt~e present invention or in the alternative switching off or krnrering the
possible
to overexpression of a nucleic acid or gene of the invention in a lung
disorder or related
disorder.
Known gene therapy protocols can consist of nucleic acid delivery systems,
such
as by means of expression vectors for transfection and expression of said
nucleic acids
as to reconstitute the function of the affected gene, or alternatively systems
whereby a
functional form of the affected gene or protein is delivered. Expression
constructs may
be administered in any biologicaly effective carrier as known in the art.
Refrovirus
vectors, adenovirus vectors and adeno-associated virus vectors are exemplary
nucleic
acid delivery carriers for the transfer of exogenous genes in vivo,
particularly into
humans.
In addition to viral transfer methods, non-viral methods can also be employed,
such as liposomal derived systems, poly-lysine conjugates, and artificial
viral envelopes.
In clinical settings, the gene delivery systems for therapeutic use can be
introduced into
a patient by any of a number of methods, each of which is familiar in the art.
The present invention also relates to a pharmaceutical preparation of the gene
therapy construct which can consist essentially of the gene delivery system in
an
acceptable diluent, or can comprise a slow release matrix in which the gene
delivery
vehicle is imbedded. Alternatively, where the complete gene delivery system
can be
produced intact from recombinant cells, e.g. retroviral vectors, the
pharmaceutical
preparation can comprise one or more cells which produce the gene delivery
system.
3o The present invention also relates to a method for identifying compounds
which
selectively modulate the expression/production of a polypeptide of the
invention, or
alternatively which inhibit, activate or interfere with the functionality of
said polypeptide of
the invention, or more specific compounds which selectively inhibit, induce or
interfere
with one of the metabolic pathways in which said polypeptide is involved.
CA 02331934 2001-02-07
Such a screening method may comprise the following steps (a) providing a hose
cell comprising at least part of a nucleic acid sequence of the invention in a
expressible
format, or transformed, transfected or infected with a vector according to the
invention as
described above, (b) contacting a compound to be tested with said host cell,
(c)
5 monitoring the increased or decreased expression of said potypeptide caused
by said
compound, and, {d) identifying said compound.
The present invention further relates to a method for preparing a composition
comprising a compound of the invention as detined above, comprising admixing
said
compound with a suitable adjuvant.
1o According to a further embodiment, the present invention relates to a
diagnostic
kit ~mprising an element selected from the group consisting of the nucleic
adds,
arrisense molecules, potypeptides, antibodies and anti-idiotypic antibodies of
the
invention as defined above.
Said diagnostic kit may comprise also necessary reactants and media far the
diagnosis
15 according to the invention. Said diagnostic kit can be based upon a
technique selected
frcrn the group of techniques consisting of ELISA's, RIA's, FACS analysis, dot
blot
hybridisation, Western Blots, Line immuno-assay (Lia), BIAcore real time
detection
system, double immunadiffusion technique, countereiectrophoresis technique,
agglutination assay, or additionally selected from the group consisting of .n
s;tu
2o hybridisation, Northern blot hybridisation, Southern blot hybridisation,
isotopic or non
isotopic labelling (by immunofluorescence or biodnylated probes), genetic
amplification
(especially by PCR or LCR), STS-PCR, countourclamped homogeneous electric
field
(CHEF) gel electrophoresis, restriction mapping, FISH analysis, mismatch
deavage,
single strand conformation poiymorhism (SSCP) or any other method known in the
art,
25 or a mixture thereat.
Said diagnostic kit thus allows the diagnosis and/or monitoring of lung
injuries
arid diseases or related disorders, such as inflammation- or immunomodulation-
related
d'sorders. More particularly, said diagnostic kit allows the diagnosis andlor
monitor;ng of
asth ma.
3o According to yet another embodiment, the present invention relates to a
nucleic
acid sequence encoding a promotor sequence comprising at least part of the
sequence
as defined in SEQ ID NO 3 allowing gene expression restricted to organs of the
respiratory tract.
CA 02331934 2001-02-07
26
When analyzing the genomic sequence (SEG7 ID NO 3 and illustrated in Fgure
7), the inventors identified a promotor sequence upstream from the
transcription start.
Said sequence presented all the features of an eukaryotic promotor and
contains a
characteristic sequence as given in SEO 1D NO 11. It was shown that expression
of P-
SCGB is very specific of the respiratory tract, especially lung and trachea.
Use of this
promotor thus would allow expression of a gene restricted to lung and trachea.
This is a
very useful tool, not only for expression studies in animals, but also in the
prospect of
gene therapy for lung disorders as set out further below.
Also included within the scope of this invention is a vector comprising said
promotor sequence as defined above. Said vector can be an expression vector,
as
defined elsewhere in this patent application. In addition to the promotor
sequence, it may
be desirable to add other regulatory sequences which allow for regulation of
expression.
Regulatory sequences are known to those of skill in the art, and examples
include those
which cause the expression of a gene to be turned on or off in response to a
chemical
stimulus, including the presence of a regulatory compound. Other types of
regulatory
elements may also be present in the vector, for example, enhancer sequences.
A host cell containing said promotor sequence or, transformed, transfected or
infected with a vector comprising said sequence is also included in this
invention.
Said promotor sequence of the invention as defined above can be used in the
2o generation of non-human transgenic animals. Said promotor will allow
expression of
genes specifically in lung tissue or other tissues of the respiratory tract.
In the transgenic
animal genes may be overexpressed, underexpressed or knocked-out in said
tissues by
the aid of said promotor.
Said promotor sequence of the invention as defined above can also be used in
nucleic acid delivery systems for gene therapy as described elsewhere in this
patent
application. In particular, it can be usetul in the treatment of patients with
lung disorders
or other related disorders (more particularly asthma), as described before in
this
application. In these patients, said promotor can be used for the expression
of a gene
restricted to the respiratory tract, especially lung and trachea. The promotor
may allow
3o the expression of a normal version of a gene in the respiratory tract of
said patients or
alternatively, it may allow switching off or lowering a possible
overexpression of a gene
restricted to the respiratory tract.
CA 02331934 2001-02-07
2~
Another embodiment of the present invention is related to a host cell
containing a
nucleic acid of the invention as defined above, or transformed, transfected or
infected
with a vector of the invention.
According to a further embodiment, said host ceii is selected from the group
comprising bacterial, fungal, insect or mammal cells.
Several documents are cited throughout this text. Each of the documents cited
herein are hereby incorporated by reference, however thefe is no admission
that any
document cited is indeed prior art of the present invention.
Further aspects of the present invention will be described in the enclosed non-
to limiting examples in reference to the following Figures.
CA 02331934 2001-02-07
28
LIST OF FIGURES
Figure 1. cDNA sequence (SEA ID NO 2) and corresponding amino acid sequence
(SEA ID NO 1) of the newly identified new human branchoatveoiar protein, P-
SCGB.
The peptide sequence (SEG7 ID NO 6) is given in bold.
Figure 2. Sequence alignment between P-SCGB and the consensus sequence of the
family of uteroglobinslsecretoglobins.
1o Figure 3. (A) A radioactive probe was designed based on the cDNA sequence
of P-
SCGB (SEA 1D NO 2). The probe was used to check the expression of P-SCGB in 8
different human tissues in a Human RNA Blot (Clontech # 7760-1 ). This
experiment
shows that expression of P-SCGB is restricted to lung tissue.
(B) Control hybridization of the prcbe to a ubiquitos gene ((i-actin).
t5
Figure 4. Hybridization of a radioactive probe (prepared as described in
F'~gure 3) to a
Human Multiple Expression Array (Clontech # 7775) containing poly A+ RNA from
76
different human tissues (+ 8 control RNAs and DNAs). This experiment confirms
that the
expression of P-SCGB is restricted to trachea, lung and fetal lung. The
sutoradiography
2o shown in (A) must be compared to the array diagram of the different
analyzed RNAs
given in (B).
Figure 5. Homologuous cDNA sequence from mouse {SEA ID NO 4) and its
corresponding amino acid sequence (SEQ ID NO 5).
Figure 6. Alignment of the human P-SCGB amino acid sequence (SEf~ ID NO 1 )
with
the homologous mouse sequence (SEO ID NO 5).
Figure 7. Genomic sequence of the new protein P-SCGB (SEO ID NO 3). Capital
letters
3o indicate bases retrieved on the cDNA given by SEO ID NO 2. Italic letters
represent the
promotor sequence given by SEO ID NO 1 t .
Figure 8. Schematic representation of the gene organization of P-SCGB.
CA 02331934 2001-02-07
29
EXAMPLES
EXAMPLE 1: Cloning and sequencing of a gene encoding a new human
branchoalveolar protein, P-SCGB.
A new protein was identified by studying 2D-electrophoresis (2-DE) protein
maps of human bronchoalveolar lavage fluids (BALFs) using a pool of BALFs from
individual patients with well-defined intestitial lung diseases such as
sarcoidosis,
idiopathic pulmonary fibrosis and hypersensitivity pneumonitis. On these 2-DE
gels
to about 600-1000 stained protein spots could be identfied by microsequence
analysis and
by matching with human blood plasma and the Macrophage Like Celt Line
reference 2-
DE maps available from the SWISS-2D PAGE database. This microsequencing gave
the
sequence of a short peptide (SEA 10 NO 6: APLPLDNILPFMDPLKLLLKTLGISVEH).
Frorn the peptide sequence homologies were searched in published databases.
t5 No signrficant homologies were found, indicating that this amino acid
sequence does not
appear in known peptide databases.
From the peptide sequence oligonudeotide primers were designed
corresponding to the sequence (SEO ID NO 7: 5'-ATATCCCAGATAACTGTCATGAAGC
and SEQ ID NO 8: 5'-CCAAGTGTGATAGCGCCTCCAGCAG). Using these two primers,
2o and following a strategy known as "5' an 3' Rapid Amplification of cONA
Ends', cDNA of
human origin was amplified. Amplification was done on a cDNA library from
human lung
(Clontech #7408), and allowed to clone two DNA fragments, which were next
sequenced. This allowed to deduce the complete sequence of a cDNA (SEGl ID NO
2)
expressed in human lung (Figure 1 ). This cDNA sequence is new. Comparison of
the
25 sequence with all the sequences available in public databases (Genbank)
shows that the
sequence does not share significant homology with a previously characterized
human
cDNA sequence.
Analysis of the sequence and particularly translation simulations showed that
this
sequence is actually the coding sequence of a gene for a new protein harboring
the
3o peptide sequence given by SEO ID NO 6. The amino acid sequence of the
encoded
protein sequence is given in SEO ID NO 1 (Figure t). Comparison of the
sequence with
all the sequences available in public da:abases of showed that the sequence
does not
share significant homology with a previously known polypeptide of human
origin.
CA 02331934 2001-02-07
EXAMPLE 2. Structural properties of P-SCGB.
Computer simulation indicated that the new protein P-SCGB given by SEA ID
NO 1 should theoretically have a length of 93 aa, a molecular weight of 10161
Da and
5 an isoelectric point of 7.66.
However it is well known that the protein actually expressed from genes can
have very drtferent characteristics, because proteins are subject to a lot of
post-
transtational processing such as glycosyiation, N-terminal truncation or other
intracellular
processing. Also, isoforms of the proteins may exist and co-exist in a living
cell.
to Particular features of the protein sequence SEA ID NO 1, as well as
experimental data, provide a probability that part of the sequence is cleaved
after
synthesis of the polypeptide and that the mature protein is shorter than 93
aa. In
particular, the N-terminal part of the sequence exhibits the properties of a
signal
sequence, and it is likely that this part of the sequence is cleaved after or
during
15 synthesis of the polypeptide. The point at which such a cleavage could
occur is either
between amino acids 18 and 19 or between amino acids 21 and 22, according to
computer simulations.
The peptide (SEQ ID NO 6) which was isolated after 2D electrophoresis had an
N-terminal sequence beginning at aminoacid 35, an apparent MW of 8500 Da and
an
2o isoelectric point of 5.4. This can be due to degradation of the protein in
the biological
extract or to the further processing of the polypeptide (cleavage of a pre-
sequence) to
yield the mature P-SCGB.
This result indicates also that P-SCGB is probably decorated with haptens such
as sugars or miristyl groups because the theoretical MW of a peptide beginning
at as 35
25 is only 6.4 kDa and its pl 6.0, thus slightly different from observations
(MW 8.5 kDa and
pl 5.4). Recent data suggest that cleavage of a signal peptide occurs at or
upstream
from as 16 since a peptide whose N-terminal is AFLINK was identified in the 2D
electrophoresis of BASF.
3o EXAMPLE 3: Functional properties of P-SCGB indicating that it might be
classified
within the "uteroglobin/secretoglobin" family.
Other features of the protein suggest its classification in the family of
"uteroglobinslsecretoglobins". Indeed, alignment of the P-SCGB protein with
the
CA 02331934 2001-02-07
31
consensus sequence of uteroglobins/secretoglobins shows signrficant similarity
as
illustrated in figure 2.
In the fo4lowing paragraph and related references a brief literature overview
related to the 'uteroglobin/secretoglobin" family is given. For a putative
biological
function of P-SCGB the inventors assume properties similar to the properties
of other
members of the "uteroglobin/secretoglobin" family, such as anti-inflammatory,
immunomodulatory, inhibition of phospholipase A2, binding of polychlorinated
biphenyls
(PCB's), tumor suppressor-like properties and inhibition of cancer
invasiveness.
~ Uteroglobin/secretoglobin is a secreted, steroid-inducibie, progesterone
binding
to protein. It is also a potent immunomodulatory/antiinflammatory agent.
Indeed it is an
inhibitor of phospholipase A2. (Phospholipases A2 (PLA2s; E.C.3.1.1.4) are a
family of
esterases that are involved in the pathogenesis of several human inflammatory
diseases).
Dierynck I. et al., Mutt-Scler. t (6): 385, 1996; Mukherjee A.B. et al., DNA
Cell 8iol.
11 (3):233, 1992; Miele L et aL, Adv. Exp. Med. t3ioL 279:137, 1990; Mukherjee
A.8. ef
al., 32(6):1106, 1998.
~ Uteroglobinlsecretoglobin is evolutionarily conserved and secreted by the
mucosal
epithelial of virtually all mammals. Initially, uteroglobin/secretoglobin was
identified as
the major protein of rabbit uterine secretion. Counterparts of the rabbit
zo uterogfobin/secretoglobin or its gene are described in rat, mouse, hamster,
hare, pig,
horse and human.
~ Uteroglobinlsecretoglobin is present in the blood and in other body fluids
including
urine, which is important for its use as a peripheral marker.
Recently, human uteroglobin/secretoglobin was shown to reverse the transformed
phenotype of cancer cells and consequentiy, may have tumor suppressor-like
effects.
Zhang Z. et al., Proc. Natl. Acad. Sci U S A 30;96(7):3963, 1999.
~ It has been shown that metabolites of polychlorinated biphenyls (PCBs) bind
with
high affinity to uteroglobin/secretoglobin, thus often called PCB-binding
proteins.
Hard T. et al., Nat. Struct. Biol. 2(11):983, 1995.
~ Finally, mechanisms of uteroglobinlsecretoglobin action are likely to be
even more
complex as it also functions via a putative receptor-mediated pathway that has
not
yet been clearly defined.
CA 02331934 2001-02-07
32
EXAMPLE 4: Distribution of P-SCGB and organ specificity
From the cDNA sequence SEA ID NO 2 previously determined new
oligonucleotides to amplify, Gone and sequence a DNA fragment corresponding to
the
complete cDNA were designed. This fragment was then radioactiveiy labelled and
used
to probe expression of the gene in eight different human tissues using a Human
RNA
Blot (Clontech #7760-1 ). The probe was hybridized to approximately 2 Ng
polyA+ RNA
from the tissues. It was found that the gene is expressed in the lung, but not
(or
undetectable) in heart, brain, placenta, liver, skeletal muscle, kidney or
pancreas (Figure
to 3-A). Control hybridization of the probe to a ubiquitos gene (p-actin) is
given in Figure 3-
B.
In addition, to confirm the lung specifidty of the expression of P-SCGB, a
Human
Multiple Tissue Expression Array (Clontech #7775-1 ) was used. A radioactive
probe was
prepared as described above and hybridized to potyA+ RNA from 76 different
human
tissues and 8 different control RNAs and DNAs. In Figure 4, the
autoradiography shown
in (A) must be compared to the array diagram of the different analyzed RNAs
given in
(B).
This result shows that the protein is expressed exclusively in trachea, lung
and
fetal lung. It is undetectable in any other tissues tested. The tissues used
for preparation
of RNAs were normal, under non-pathological conditions. Cause of death was
sudden
death/trauma.
EXAMPLE 5: Cloning of the homologous gene from mouse.
Two oligonucleotide primers (SEO ID NO 9: 5'- CAGATA,ACTGTCATGAAGCTGGTA
and SEo ID NO 10: 5'-CCAAGTGTGATAGCGCCTCCAGCA) were designed and used
to amplify cDNA from mouse lung. This allowed to amplify, clone and sequence a
cDNA
fragment (given by SEO ID NO 4) which is expressed in mouse lung. The protein
encoded by this sequence is given by SEQ ID NO 5. Both SEO ID NO 4 and SEO 1D
3o NO 5 are represented in Figure 5.
In Figure 6 alignment of the human P-SCGB peptide sequence (SECT ID NO 1 )
with the homologous mouse sequence (SEA VD NO 5) is given. As can be seen on
this
alignment, the protein encoded by the mouse cDNA is quite similar to the one
encoded
CA 02331934 2001-02-07
33
by the human cDNA, and thus can be considered as the mouse homologue of P-
SCGB.
The identity is 79 % and the similarity is 94 %.
EXAMPLE 6: Genomic sequence of P-SCGB and chromosomal localization.
Human genome databases were searched for information relevant to the
sequence of the new cDNA sequence as given by SEO ID NO 2 and revealed
signrficant
similarities with two large fragments of chromosome 5. This allowed to
identify the
genomic sequence coding for P-SCGB, as well as regulatory sequences,
infom~ation for
to RNA splicing, and so on. The genomic sequence given by SEO ID NO 3 is
represented
in Figure 7. Capital letters in Figure 7 indicate bases retrieved on the cDNA
sequence
given by SEQ ID NO 2. In Fgure 8 a scheme of the gene organization of P-SCGB
is
represented.
It is known that the sequence upstream from the transcription start contains a
promoter and/or regulatory sequence for the expression of the gene. As it was
demonstrated in the present invention that expression of P-SCGB is very
specific of the
respiratory tract, esp. lung and trachea, the use of this promotor andlor
regulatory
sequence thus will allow expression of a gene restricted to lung and trachea;
this will be
a very useful tool, not only for expression studies in animals, but also in
the prospect of
gene therapy far lung diseases, for example. The present invention also
relates to the
use of nucleic acid sequence derived from those given in Figure 7 and more
precisely to
the sequence given in SEo ID NO 11, which presents ail features of an
eukaryotic
promotor.
The chromosomal assignment of P-SCGB coding information by refined by the
method of Gene Radiation Hybrids. A portion of the gene for P-SCGB was
amplified by
PCR using 2 oligonucleotides 5'-CCTCTGGTCCCAGCTCATTTACACAG3' and 5'-
TGACTATGGCCATTGCAGGCTTCTCC-3'. The Genebridge4 radiation hybrid panel
was used according to Research Genetics, Inc. This panel of 93 Radiation
Hybrid clones
of the whole human genorne is a subset of the 199-clone panel by reported by
3o Goodfellow and Weissenbach (Walter M.A. et al., Net Genet. 7(1):22, 1994;
Gyapay G.
ef al. Hum. Mot. Genet. 5(3):339, 1996.
The obtained PCR scoring was transmitted to the "Whitehead Institute/MIT
Center for Genome Research. The locus for P-SCGB was determined as being on
CA 02331934 2001-02-07
34
chromosome S, between markers D5S436 and 055470. it places 2.43 cR from W I-
6763
and 0.50 cR from WI-2452.