Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
CONTROL SYSTEM FOR CONTINUOUS POLYAMIDATION PROCESS
BACKGROUND OF THE INVENTION
I. Field of the Invention
The present invention relates generally to processes for producing polyamides
from
s dicarboxylic acid monomers and diamine monomers. -_More particularly, the
invention relates to
an improved polymerization system and control system therefor which maintains
a desired mass
flow rate of molten reactants by measuring the molar ratio of the reactants
after the initialization
of polymerization and adjusting the flow rate of the molten reactant feed
stocks.
io II. Discussion of the Prior Art
A major challenge in the manufacture of polyamides is ensuring that reactants
combine
sufficient to balance the number of carboxylic and/or amine end groups in the
resulting
polyamide. If the reactants combine such that an uneven number of carboxylic
and/or amine end
groups remain, it can negatively influence certain characteristics of the
resulting polyamide. For
~s example, when producing nylon 6,6 from adipic acid and hexamethylenediamine
(HMD), it is
found that uneven end groups can adversely affect the dyeability of the nylon,
as well as curtail
the ability to make nylon 6,6 of high molecular weight. As a result,
manufacturers have placed
an emphasis on balancing the molar ratio of the reactants during
polymerization in the interest of
maximizing the quality of polyamides.
One prior art technique for producing polyamides involves a two-step process
in which a
dicarboxylic acid and a diamine are reacted in water to form a salt, and then
the salt is heated to
cause polymerization. This two-step polymerization process is disadvantageous,
however, in
that it requires the addition of water and the use of evaporation chambers to
remove the water
~s added when forming the salt. It is also difficult to control the process to
ensure proper molar
balance in the end polymer because the evaporation chambers are very
unpredictable and hard to
model.
A variety of control mechanisms have been employed in this two-step
polymerization
~o process. One known control mechanism involves physically removing a sample
of the in-
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
-2-
process material for end group analysis. This is disadvantageous, however, in
that it is time
consuming to perform and tends to introduce inaccuracy because the samples do
not always
adequately reflect the characteristics of the in-process materials.
s Other known control mechanisms involve conducting an in-line end group
analysis in an
effort to provide proper molar balance in the resulting polyamide. These in-
line control
mechanisms are helpful in terms of avoiding the need to physically sample the
in-process
materials. One technique involves an indirect assessment of molar balance by
measuring the pH
of the salt formed in the two-step polymerization process. This pH measuring
technique is
~o limited, however, because pH is not a particularly accurate indicator of
end group balance in the
resulting polyamide.
Another in-line end group analysis technique involves measuring the end-group
ratio of
molten reactants during polymerization and, based on this determination,
injecting an
is appropriate amount of reactant to the molten polymerization mixture to
provide a desired molar
balance in the resulting polyamide. This system is disadvantageous in that it
requires costly
additional metering devices and circuitry to inject additional reactant into
the molten
polymerization mixture. This system is also limited in that it requires
additional time for the
subsequently added reactants to combine with the molten polymerization
mixture.
zo
Attempts have been made to produce polyamides directly from the monomers
without
adding water. Controlling the degree to which the reactants combine has proven
to be quite
difficult, however, because an excess of one or the other will adversely
affect the molecular
weight and thus the physical properties of the product. Other problems with
such direct
2s polymerization processes include degradation of the monomers and/or the
polymer product as a
result of ( 1 ) being kept at high temperatures for lengthy periods of time
(e.g., several hours), (2)
contact of the molten monomer with oxygen, and (3) exposure to trace metal
impurities in the
materials from which the process equipment is made.
CA 02332937 2000-11-22
WO 99!61510 PCT/US99/11577
-3-
There is a long-standing need for an improved polymerization system and
control system
therefor which overcome the aforementioned drawbacks in the prior art.
SUMMARY OF THE INVENTION
s One aspect of the invention is an improved polymerization system for
producing a
polyamide from a dicarboxylic acid monomer and a diamine monomer. First
metering means
are provided for metering a supply of molten dicarboxylic acid monomer. Second
metering
means are provided for metering a supply of molten diamine monomer. The first
and second
metering means are coupled together such that the supply of molten
dicarboxylic acid monomer
io and the supply of molten diamine monomer combine to form a molten
polymerization mixture.
At least one unvented reaction vessel is provided for polymerizing the
polymerization mixture.
Means are provided for detecting the molar ratio of molten dicarboxylic acid
monomer and
molten diamine monomer in the polymerizing mixture. Control means are
communicatively
coupled to the means for detecting and the first and second metering means.
The control means
~s selectively adjusts the mass flow rate of at least one of the supply of
molten dicarboxylic acid
monomer and the molten diamine monomer to balance the molar ratio of molten
dicarboxylic
acid monomer and molten diamine monomer in the polymerization mixture.
Another aspect of the present invention is a polymerization control system for
producing
zo a polyamide from dicarboxylic acid monomer and diamine monomer. First means
are provided
for metering a supply of molten dicarboxylic acid monomer. Second means are
provided for
metering a supply of molten diamine monomer into the supply of molten
dicarboxylic acid
monomer to form a molten polymerization mixture. Means are provided for
detecting the molar
ratio of the molten dicarboxylic acid monomer and molten diamine monomer in
the
zs polymerization mixture. A controller is provided communicatively coupled to
the means for
detecting and at least one of the first and second means for metering. The
controller controls at
least one of the first means for metering and the second means for metering
based on a molar
ratio input signal from the means for detecting to adjust the mass flow rate
of at least one of the
molten dicarboxylic acid monomer and the molten diamine monomer so as to
balance the molar
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
-4-
ratio of molten dicarboxylic acid monomer and molten diamine monomer in the
polymerization
mixture.
This control system operates continuously to ensure that the reactants
polymerize in a
s balanced fashion with equal numbers of carboxylic and amine end groups in
the resulting
polyamide. Any modification to the flow rate of the reactants is made prior to
the formation of
the molten polymerization mixture. That is, the first and second metering
means are adjusted to
vary the mass flow rate of at least one of the molten carboxylic monomer and
the molten
diamine monomer. No additional dicarboxylic acid monomer or diamine monomer
needs to be
i o added after the mixing.
The control system of the present invention is particularly suited for use
within a
polymerization system which produces polyamides directly from the monomers. In
this fashion,
there is no need to add water to the dicarboxylic acid, to the diamine, or to
the molten
is polymerization mixture.
The temperature of the polymerization mixture in the at least one unvented
reaction
vessel is between about 220 and about 300°C. Preferably the pressure in
the at least one
unvented reaction vessel is between about 0-500 psig, more preferably between
about 50-250
Zo psig, most preferably between about 120-180 psig. The residence time of the
polymerization
mixture in the at least one unvented reaction vessel is preferably between
about 0.01 minutes and
about 30 minutes, more preferably between about 0.5-30 minutes, most
preferably between
about 1-5 minutes. The polymerization mixture exiting the at least one
unvented reaction vessel
typically contains less than 40% by weight unpolymerized monomers, preferably
less than 10%
zs by weight unpolymerized monomers.
In certain embodiments, at least one vented reaction vessel may optionally be
employed
downstream of the at least one unvented reaction vessel for removing water
farmed during the
polymerization process and/or for further polymerization. When so employed,
the residence
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
-5-
time of the polymerization mixture in the at least one vented reaction vessel
is preferably from
about 1 minute to about 60 minutes.
An offgas recovery system may also be employed for recovering vaporized
diamine
s monomer and/or dicarboxylic acid monomer in the offgas produced by the at
least one vented
reaction vessel. This offgas stream typically comprises water vapor and
vaporized diamine
monomer. The offgas is contacted with molten dicarboxylic acid monomer in a
recovery
column, whereby at least a portion of the vaporized diamine monomer reacts
with the
dicarboxylic acid monomer to form polyamide. This serves to form a liquid
effluent stream
io within the recovery column that comprises polyamide and unreacted molten
dicarboxylic acid
monomer. The liquid effluent stream may be subsequently mixed with molten
diamine
monomer.
In one embodiment, the relative viscosity (RV) of the nylon 6,6 in the
polymerization
~s mixture exiting the unvented reaction vessel is between about 0 and about
3, and the relative
viscosity of the nylon 6,6 in the polymerization mixture exiting the vented
vessel is between
about 3 and about 15. Relative viscosity as used herein is the ratio of
viscosity (in centipoises)
at 25°C of 8.4% by weight solution of polyamide in 90% formic acid (90%
by weight formic
acid and 10% by weight water) to the viscosity (in centipoises) at 25°C
of 90% formic acid
2o alone.
The polyamidation process of the present invention can produce its end product
without
the need to add water to the reactants, and without the intermediate step of
forming a salt. In
addition, the process of the present invention can operate continuously and
with much shorter
z: residence times for the molten reactants and molten polymer in the high
temperature portions of
the process. This significantly reduces the water usage, waste water
production, and energy
consumption of the process. This also eliminates the need for or reduces the
required size of
some process equipment found in prior art processes, such as evaporators that
have been used to
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
-6-
remove the added process water. Further, excessive thermal exposure of the
reactants and
product is avoided.
The aspect of the present invention relating to continuous melting of
dicarboxylic acid,
s such as adipic acid, provides a practical and economical method of
continuously supplying
molten dicarboxylic acid for use in a polyamidation process or for other uses.
The process
provides high quality molten acid without discoloration or other thermal
degradation. The
production of clear molten acid facilitates the production of high quality
polyamide.
io BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a block diagram illustrating an improved polyamidation system of
the present
invention;
Figure 2 is a block diagram illustrating a polymerization control system of
the present
invention;
~s Figure 3 is a second block diagram illustrating the polymerization control
system of the
present invention as shown in FIG. 2; and
Figure 4 is a block diagram illustrating another alternative polymerization
control system
of the present invention.
Zo DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
The polymerization system and polymerization control system of the present
invention
can be used to produce a variety of polyamides from diacid and diamine
monomers. These
systems are particularly useful for producing nylon 6,6 from adipic acid and
hexamethylenediamine.
?s
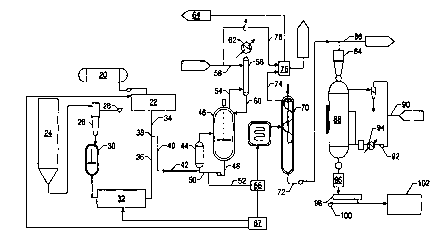
Figure 1 shows a process flow diagram for one embodiment of the process.
Molten
hexamethylenediamine (HMD) is provided from a molten HMD storage tank 20.
There are
several suitable ways of providing the molten HMD. One is to locate the
polyamidation process
equipment adjacent to a plant where HMD is produced, so that a molten HMD
stream can be
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
_7_
piped directly to the tank 20. Another way would be to provide an aqueous HMD
solution,
evaporate the water, and melt the HMD.
Heat optionally can be applied in this tank 20, for example by means of a heat
transfer
s jacket around the tank 20. The temperature in this tank is preferably about
70°C. The molten
HMD is then pumped through an HMD metering system 22 which precisely controls
the amount
of HMD fed to the downstream apparatus.
Adipic acid, typically in the form of dry crystals, is supplied from an adipic
acid storage
io silo 24. Adipic acid from the silo flows to a bulk oxygen eliminator tank
26. In this tank 26, air
is removed. Preferably, removal of air in the tank 26 is accomplished by
cycling vacuum with
nitrogen displacement in batch mode. The vacuum can be induced by means of a
vacuum pump
28. The frequency of cycling between vacuum and nitrogen pressure can be
adjusted to achieve
the desired level of oxygen removal.
~s
Preferably the bulk oxygen eliminator tank 26 comprises a pressure vessel
having a
bottom portion forming a hopper with a diminishing diameter towards its
bottom. The sides of
the hopper portion of the bulk oxygen eliminator tank preferably form an angle
with the
horizontal of at least 70° in order to facilitate flow out of the
bottom of the tank.
The adipic acid crystals, largely free of molecular oxygen, then flow
(preferably by
gravity, with a pressure assist by the nitrogen pressure in the bulk oxygen
eliminator tank) from
the bulk oxygen eliminator tank 26 to an adipic acid melter vessel 30. The
melter vessel 30
preferably is a continuously stirred jacketed vessel that operates slightly
pressurized with
2, nitrogen at a temperature slightly above the adipic acid melt point (i.e.,
above 153°C). Adipic
acid crystals entering this vessel through its top are quickly melted at the
surface of the molten
adipic acid therein. Thus the process can continuously melt adipic acid.
Preferably the melter
vessel 30 has a reversed conical entry nozzle to reduce flow resistance. It is
also preferred that
CA 02332937 2000-11-22
WO 99/61510 PCTNS99/11577
_g_
the melter vessel 30 be made of a metal alloy containing little or no
impurities that would
adversely affect the molten monomer. Hastolloy C and 316 stainless steel are
suitable materials.
It may be useful to include additional measures for further oxygen removal
from this
s melter vessel, to minimize the potential for thermal degradation. One way of
doing this is to
supply vibrational energy to the molten adipic acid in the melter vessel 30,
for example by
means of an ultrasonic device. The vibrational energy can facilitate the
escape of entrained air
from the molten acid, causing air bubbles to rise to the surface of the molten
acid.
io The residence time of the molten adipic acid in the melter vessel 30
preferably is
minimized to reduce the thermal exposure of that reactant. Preferably the
residence time is less
than three hours, more preferably between about 1-2 hours. The molten adipic
acid exits the
bottom of the melter vessel 30 and is pumped to a molten adipic acid metering
system 32 which
precisely controls the amount of adipic acid fed to the downstream apparatus.
is
The combination of the bulk oxygen eliminator tank 26 and the adipic acid
melter vessel
30 permits the continuous melting of adipic acid crystals without thermal
degradation or
discoloration.
zo The HMD metering system 22 and the adipic acid metering system 32 supply
the molten
monomers in stoichiometric amounts such that the molten HMD and molten adipic
acid are
combined at a Y junction 38 to form what will hereinafter be referred to as a
"polymerization
mixture." Stated another way, the molten monomers combine and co-mingle at the
Y junction
to initiate the polymerization process. The polymerization mixture progresses
through the next
zs segment 40 of piping and into an unvented mixer 42, which is preferably an
inline static mixer.
In a preferred embodiment, the molten adipic acid stream 36 is at a
temperature of about
170°C and the molten HMD stream 34 is at about 70°C, and the
pressure at the Y-junction 38 is
about 1 SO psig. The inline static mixer is preferably a Kenics static mixer
with 24 elements.
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
-9-
The walls of the Y junction and the inline mixer 42 are preferably kept at
about 268°C. The
residence time of the monomers in the mixer 42 is preferably between about 1-
30 seconds, more
preferably about 3 seconds. The polymerization mixture leaving the mixer 42
passes into an
unvented pipe, allowing for example an additional 10-60 seconds of reaction
time at 260°C and
s 150 psig.
Although the process of the present invention can operate without the
inclusion of water
in the reactants, it is not required that the reactants be entirely anhydrous.
For example, the
HMD feed stream could contain as much as about 5% water by weight, and the
adipic acid
io stream could contain as much as about 2% water by weight, and the process
should still function
properly. Reactant streams having such low concentrations of water are
referred to herein as
"essentially dry."
Some reaction of the HMD and adipic acid occurs from the time they contact
each other
is at the Y junction 38 continuing through the time they enter the heat
exchanger 44. The
temperature and residence time employed in this portion of the process can be
selected to cause
complete polymerization by this point, or to prevent compete polymerization
from occurring by
this point. In the latter situation, the partial reaction product generated by
the contacting of the
monomers is referred to herein as the ''prepolymer." The prepolymer mass in
the pipe
zo downstream of the mixer 42 will typically be 60-90% converted to nylon 6,6.
No plugging
should occur because the conditions employed prevent crystallization of low
melting
intermediates. It is important to optimum operation of the process that the
piping 40 and mixer
42 be unvented, and that the pressure therein be relatively low, for example
between about 0-500
prig, most preferably about 150 psig.
zs
In the embodiment shown in Figure 1, the prepolymer next passes through a heat
exchanger 44 and into a vented prepolymer reactor 46. It is not critical that
a heat exchanger be
used here. Any required heat could instead be provided by internal heating
coils within the
reactor 46. or by jacket around the reactor. The heated prepolymer exiting the
heat exchanger 44
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
- 10-
preferably enters the reactor 46 at a point below the surface of the liquid
material therein.
Further polymerization can occur in this reactor 46, which is preferably a
continuously stirred
tank reactor. The reactor bottoms stream 48 optionally can be split into a
recycle stream 50 and
a second stream 52 that is routed for further processing. If recycle is used,
the recycle stream 50
s flowrate is preferably at least 15 times larger than the flowrate of fresh
prepolymer feed to the
reactor 46. The reactor 46 is preferably operated about 50% full of liquid
material in order to
provide a large vapor/liquid disengagement surface.
It is highly desirable in this process to provide backmixing of polymer
endgroups, high
~o surface area interface generation which facilitates devolitilization of the
molten material, and
high heat transfer rates which can rapidly increase the temperature of the
melted material. These
advantages can be achieved, for example, either by use of a continuously
stirred tank reactor, or
by use of a plug flow reactor together with recycle of the product stream.
is The overhead stream 54 from the reactor 46 is vapor including steam (i.e.,
vaporized
water produced by the polycondensation reaction) and typically some HMD. The
overhead 54
passes into an HMD recovery column 56, into which is also fed water 58.
Condensate stream
60, containing some HMD and water, is recycled to the reactor 46, while the
remaining vapor is
cooled by a heat exchanger 62 and removed as part of an offgas stream 64.
In one embodiment, the prepolymer is heated to about 260°C in the heat
exchanger 44,
and the reactor 46 operates at about 260°C and 150 psig. As an example
of suitable relative
flowrates, if the fresh prepolymer is fed to the reactor 46 at a rate of 100
Ibs. per hour, the reactor
bottoms recycle flowrate is preferably about 2,000 lbs. per hour. A reactor 46
operated under
2> these conditions can yield greater than 95% conversion of monomers to nylon
6,6 with a three
weight percent water concentration after 20 min. residence time in the reactor
46.
In accordance with the present invention, a control system is provided for
adjusting the
feed rate or mass flow rate of at least one of the molten dicarboxylic acid
monomer and the
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
-11-
molten diamine monomer to ensure a proper molar ratio. In a preferred
embodiment, the mass
flow rate of at least one of the reactants is adjusted depending upon the
balance of the carboxylic
end groups and amine end groups of the reactants within the polymerization
mixture. This in-
line end group measurement can be performed at any point downstream of the Y
junction 38. In
s the embodiment shown, this end group balance measurement is performed in the
stream 52
leaving the reactor 46. Photospectrometry is the preferred methodology for
measuring the
balance of carboxyl and amine end groups of the molten monomers within the
polymerization
mixture. In the preferred embodiment, a near-infrared (NIR) analyzer 66
detects the number of
carboxyl and amine end groups in the polymerization mixture by assessing the
spectral
~ o photometric content of the monomers therein.
In one embodiment, the control system of the present invention includes the
near-infrared
(NIR) device 66, a controller 67 receiving an input from the NIR analyzer 66,
the HMD metering
system 22, and the molten adipic acid metering system 32.
~s
The NIR analyzer 66 is provided by way of example and not limitation as a
device for
determining the molar ratio of the molten dicarboxylic acid monomer and molten
diamine
monomer within the polymerization mixture. The NIR analyzer 66 achieves this
by
continuously detecting the number of carboxyl and amine end groups in the
partially
zo polymerized material leaving the reactor 46. Although employing the NIR
analyzer 66 is
preferred, it is contemplated control system of the present invention may be
employed with any
number of means for determining the molar ratio or molar balance of the molten
dicarboxylic
acid and molten diamine during polymerization
zs The NIR analyzer 66 generates an input signal to the controller 67
indicative of the
balance of the carboxyl and amine end groups of the molten dicarboxylic acid
monomer and the
molten diamine monomer in the polymerization mixture. Using this input signal.
the controller
67 can adjust the mass flow rate of the molten diamine monomer and/or the
molten diamine
monomer such that the polyamide formed by polymerizing the polymerization
mixture will have
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
-I2-
a desired molar ratio. In a preferred embodiment, the controller 67 employs a
feed forward
control algorithm which varies the feed rate of the molten diamine monomer
depending upon the
input signal from the NIR analyzer 66. Using this feed forward control
algorithm, the ratio of
molten dicarboxylic acid monomer and molten diamine monomer may be controlled
at the input
s to produce a polymerized end product having a predetermined molar ratio. In
a preferred
embodiment, this is accomplished by trimming the feed rate of the molten
diamine monomer via
the HMD metering system 22. The control and operation of the HMD metering
system 22 and
the molten adipic acid metering system 32 will be discussed in greater detail
below with
reference to FIGS 2-4.
~o
It should be noted that, although shown disposed along the stream 52, it is
contemplated
that the NIR analyzer 66 may be located at any point downstream from the Y
junction 38. For
example, the NIR analyzer 66 may be located within the reactor 46 at a point
below the liquid
level, between the static mixer 42 and the reactor 46, within the static mixer
42, or between the
~s static mixer 42 and the Y junction 38.
Although the material at this point in the process is polymerized, in some
embodiments
of the process the extent of the polymerization, and therefore the molecular
weight and relative
viscosity (RV) of the polymer, will not be as high as is desired for the final
product. Therefore,
Zo the partially polymerized material can be passed through a flasher 68 to
supply additional heat,
and then into a second reactor 70. The purpose of the second reactor 70 is to
permit further
polymerization and thus to increase the molecular weight and RV of the
product. The polymer
product in the bottoms stream 72 from the second reactor should have the
desired molecular
weight for the end product. Preferably the temperature in the second reactor
70 is between about
zs 260 and about 280 °C, and the pressure is atmospheric.
HMD vapor and steam generated in the second reactor 70 are removed in an
overhead
stream 74 which enters a scrubber 76. A water stream 78 is also fed to this
scrubber, so that the
CA 02332937 2000-11-22
WO 99/61510 PCTNS99/11577
-13-
steam will be condensed and can be removed as a sewer water stream 80.
Remaining vapor
leaves the scrubber 76 in an overhead stream 82 and becomes part of the offgas
stream 64.
The polymer product can either be sent through a pelletizer 84 or routed
through a bypass
s line 86. If it is run through the pelletizer, the polymer pellets are then
passed into a dryer 88. A
nitrogen gas feed 90, a nitrogen blower 92, and a nitrogen heater 94 are used
to supply nitrogen
gas to the vessel 88, which dries the polymer pellets. The dried pellets
passing out the bottom of
the dryer 88 pass through a water spray cooler 96, a screener 98, and are
moved by a blower 100
to a product storage area 102.
~o
Refernng now to FIG. 2, shown is a block diagram of a control system of a
preferred
embodiment of the present invention, designated generally at 120, for use in
the improved
polymerization system shown in FIG. 1. The control system 120 includes the
molten diamine
(HMD) metering system 22, the molten adipic acid metering system 32, the
controller 67, and
> > the NIR analyzer 66. The control system 120 serves to control the amount
of molten adipic acid
which is combined with the molten diamine at the Y junction 38 to form the
polymerization
mixture which enters the static mixer 42 in route to the prepolymer reaction
vessel 46.
The molten diamine metering system 22 includes a diamine meter pump 124 and a
zo diamine flow meter assembly 126. In a preferred embodiment, the diamine
meter pump 124 is a
positive displacement pump having a main drive motor 128, a plurality of main
pumping heads
130-134, and a trim head 136. The main drive motor 128 includes a drive shaft
138 which
extends into each of the main pumping heads 130-134 and the trim head i 36.
Individual pistons
(not shown) are disposed within the main pumping heads 130-134 and trim head
136. The
zs pistons (not shown) are coupled to the drive shaft 138 to provide the
positive displacement of
molten diamine monomer from the molten diamine vessel 20 past the flow meter
assembly 126
and onward to the Y junction 38 for passage into the static mixer 42.
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
- 14-
The main drive motor 128 also includes a speed encoder 140 and a speed
controller 142
which collectively form a closed loop feedback system for controlling the
speed of the main
drive motor 128. The speed encoder 140 monitors the speed of the main drive
motor 128 and
transmits a signal indicative of motor speed to the controller 67. The speed
controller 142
> receives an input signal from the controller 67 to control the speed of the
main drive motor 128.
The main pumping heads 130-134 are equipped with servo motors 144-148, stroke
position encoders 152-156, and stroke position controllers 160-164. The servo
motors 144-148
are coupled to the pistons (not shown) disposed within the respective main
pumping heads 130-
io 134. The stroke position encoders 152-156 monitor the shaft position of
each servo motor 144-
148 and transmit signals indicative of stroke volume (0-100%) to the
controller 67. The stroke
position controllers 160-164 receive input signals from the controller 67 to
control the shaft
position of the servo motors 144-148 to produce a predetermined stroke volume
(0-100%) within
the main pumping heads 130-134. The main pumping heads 130-134 are preferably
capable of
~ s providing flow rates suitable to provide a sufficient capacity of molten
diamine to the system
depending upon the size of the application.
The trim head 136 is similarly equipped with a servo motor 150, a stroke
position
encoder 158, and a stroke position controller 166. The servo motor I50, stroke
position encoder
20 158, and stroke position controller 166 cooperate in the same fashion as
those found in the main
pumping heads 130-134. The main distinction is that the trim head 136 has a
substantially
smaller flow rate capability than the main pumping heads 130-134. This is
because the trim
head 136 is employed to supply relatively small flow rate of molten diamine to
the larger flow
rate from the main pumping heads 130-134 in the interest of fine-tuning the
overall supply of
molten diamine to the static mixer 42. As will be explained in greater detail
below. this feature
is significant in that it allows the control system 120 of the present
invention alter the ratio of the
initial reactants (molten adipic acid and molten diamine) prior to mixing such
that the resulting
polyamide has a stoichiometrically balanced molar ratio.
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
-15-
The flow meter assembly 126 of the molten diamine metering system 22 includes
a flow
meter 168 and a flow transmitter 170. The flow transmitter 170 is configured
to monitor the
flow rate of the molten diamine monomer as detected by the flow meter 168 and
to
simultaneously transmit an output signal to the controller 67 representing the
diamine flow rate
s into the static mixer 42. The flow meter 168 and flow transmitter 170 may
comprise any of a
variety of commercially available flow meters and flow transmitters.
Collectively, the flow
meter assembly 126 and the diamine pump 124 cooperate with the controller 67
to form a closed
loop feedback arrangement for selectively adjusting the flow rate of the
molten diamine into the
static mixer 42.
~o
The molten adipic metering system 32 includes an adipic meter pump 172 and a
flow
meter assembly 174. In a preferred embodiment, the adipic meter pump 172 is a
positive
displacement pump having a main drive motor 176 and plurality of heads 178-
182. The main
drive motor 176 has a single drive shaft I 84 extending into each of the heads
178-182.
is Individual pistons (not shown) are disposed within the heads 178-182 and
coupled to the drive
shaft 184 to provide the positive displacement of molten adipic acid from the
adipic acid melter
vessel 30 past the flow meter assembly 174 and onward to the Y junction 38 for
passage into the
static mixer 42.
Zo The main drive motor 176 also includes a speed encoder 186 and a speed
controller 188
which collectively form a closed loop feedback system for controlling the
speed of the main
drive motor 176. The speed encoder 186 monitors the speed of the main drive
motor 176 and
transmits a signal indicative of motor speed to the controller 67. The speed
controller 188
receives an input signal from the controller 67 to control the speed of the
main drive motor 176.
is Manual stroke controllers 190-194 are provided for adjusting the stroke
volume of the pistons
within the heads 178-182, preferably between 0-100%.
The flow meter assembly 174 of the molten adipic acid metering system 32
includes a
flow meter 196 and a flow transmitter I 98. The flow transmitter 198 is
configured to monitor
CA 02332937 2000-11-22
WO 99/61510 PCTNS99/11577
- 16-
the flow rate of the molten adipic acid monomer as detected by the flow meter
196 and to
simultaneously transmit an output signal to the controller 67 representing the
adipic acid flow
rate into the static mixer 42. The flow meter 196 and flow transmitter 198 may
comprise any of
a variety of commercially available flow meters and flow transmitters.
The controller 67 is provided for receiving various input signals and
outputting various
control signals to coordinate the operation of the control system 120. The
controller 67 should
preferably be programmed to run according to a feed forward control algorithm.
Under such a
control scheme, the number of carboxyl and amine end groups are measured to
determine the
io end group balance of the unreacted molten monomers within the
polymerization mixture. From
this measurement, the controller 67 can employ a look-up table to determine to
what extent the
ratio of the initial reactants must be altered prior to mixing such that the
resulting polyamide is
provided having a stoichiometrically balanced molar ratio.
i s The NIR analyzer 66 includes an analyzer element 200 and an analyzer
transmitter 202.
The analyzer element 200 is coupled directly to the prepolymer reactor 46 for
detecting the
number of carboxyl and diamine end groups of the unreacted monomers within the
polymerization mixture as it exits from the prepolymer reactor 46. The
analyzer transmitter 202
is coupled between the analyzer element 200 and the controller 67 for
transmitting the output of
zo the analyzer element 200 to the controller 67. The NIR analyzer 66 may
comprise any number
of commercially available near-infrared analyzers capable of assessing diamine
end groups in the
resulting polyamide.
The NIR analyzer 66 transmits an output signal to the controller 67 indicative
of the
zs molar balance of the unreacted monomers within the polymerization mixture.
The controller 67
employs a feed forward control algorithm which varies the feed rate of the
molten diamine
monomer depending upon the output signal from the NIR analyzer 66. Using this
feed forward
control algorithm, the ratio of molten dicarboxylic acid monomer and molten
diamine monomer
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
-17-
may be controlled at the input to produce a polymerized end product having a
stoichiometrically
balanced molar ratio.
In one embodiment, this is accomplished by trimming the feed rate of the
molten diamine
s monomer via the diamine metering system 22. The control and operation of the
diamine
metering system 22 and the molten adipic acid metering system 32 will be
discussed in greater
detail below with reference to FIGS 2-4.
Referring to FIG. 3, the controller 67 may comprise any number of commercially
~o available programmable controllers, including but not limited to a
distributed control system
(DCS), a programmable logic unit, or a microprocessor-based personal computer.
The feed
forward control arrangement employed in the controller 67 is possible because
the components
of the improved polyamidation system shown in FIG. 1 are predictable in terms
of their
characteristics and effect upon the polyamidation process. Namely, by
eliminating the step of
~ s forming a salt via the addition of water, the present invention eliminates
the need for evaporators
in the polyamidation process. Evaporation vessels are unpredictable in terms
of their effect on
polyamidation and may exhibit wide variations during processing.
Operation of the control system 120 will now be described with combined
reference to
Zo FIGS. 2 and 3. Referring first to FIG. 3, the initial step in the control
system 120 involves
having an operator input a set point (capacity SP) into the controller 67. For
convenience,
controller 67 is shown in FIG. 3 as including a terminal 67a for inputting the
set point and a
forward modeling controller 67b for managing the operation of the control
system 120. After
the user has input a desired set point (capacity SP), the computer terminal
67a transmits this
z, information to the forward modeling controller 67b.
The forward modeling controller 67b then sets the motor speed control for the
speed
controller 188 for the main drive motor 176 of the adipic meter pump 172. In a
preferred
embodiment, the flow rate of the adipic acid metering system 32 is further
controlled by
CA 02332937 2000-11-22
WO 99/b1510 PCTNS99/11577
-18-
manually adjusting the stroke controllers 190-194 such that molten adipic acid
progresses to the
static mixer 42 at a predetermined flow rate.corresponding to the set point as
selected by the
operator. Once set, the flow rate of the molten adipic acid should preferably
not be altered by
the changing the control signal from the controller 67 or by adjusting the
manual stroke
controllers 190-194.
The forward modeling controller 67b also sets the motor speed control for the
speed
controller 142 associated with the main drive motor 128 of the diamine meter
pump 124. The
forward modeling controller 67b continuously monitors the output signal from
the NIR analyzer
~0 66 to obtain an assessment of the molar ratio of the molten dicarboxylic
acid monomer and the
molten diamine monomer within the polymerization mixture. In an important
aspect, the
forward modeling controller 67b uses this NIR output signal to generate and
transmit a trim set
point signal to the stroke position controller 166 of the servo motor 150
associated with the trim
head 136. The trim set point signal transmitted to the stroke position
controller 166 is generated
is based on a feed forward control algorithm found in the controller 67b. Such
a feed forward
control algorithm may take the form of a memory look-up table containing data
representing the
degree to which the ratio of reactants (molten adipic acid and molten diamine)
must be varied to
obtain the desired capacity set point selected by the operator based on the
input from the NIR
analyzer 66.
In the preferred embodiment, the flow rate of the diamine metering system 22
will be
continually adjusted to provide the appropriate ratio of reactants (molten
adipic acid and molten
diamine) prior to mixing such that the resulting polyamide is provided having
a
stoichiometrically balanced molar ratio.
Referring to FIG. 2, the controller 67 accomplishes this by first controlling
the main
drive motor 128 and at least one of the servo motors 144-148 such that molten
diamine is
transmitted from the main pump heads 130-134 toward the static mixer 42. The
controller 67
will then adjust the trim head 136 to fine tune the ratio of reactants being
transferred into the
CA 02332937 2000-11-22
WO 99/61510 PCT/US99/11577
-19-
static mixer 42. The trimming adjustment is based on the flow rate of the
molten adipic acid (as
measured by the flow meter assembly 174), the flow rate of the molten diamine
(as measured by
the flow meter assembly 126), the measurement of percentage weight of each
reactant in the
partially polymerized mixture within or following the reactor 46 {as measured
by the NIR
s analyzer 66), and the stroke volume and motor speed information within the
diamine meter
pump 124 (as measured by the position encoders 152-158, the position
controllers 160-166, the
speed encoder 140, and speed controller 142).
It is within the scope of the invention to employ the feed forward control
algorithm
~o where the mass flow rate of the molten bicarboxylic is adjusted based on
the end group balance
measurement. With reference to FIG. 4, the flow rate of the molten diamine
monomer is
maintained at a constant rate, while the bicarboxylic monomer is fine-tuned to
produce the
appropriate ratio of reactants to produce a desired capacity set point. As
will be appreciated, the
control circuitry shown in FIG. 4 is reversed from that shown in FIG. 2 such
that a full
is discussion of the operation of the embodiment of FIG. 4 is not necessary.
The preceding description of specific embodiments of the present invention is
not
intended to be a complete list of every possible embodiment of the invention.
Persons skilled in
this field will recognize that modifications can be made to the specific
embodiments described
Zo here that would be within the scope of the present invention. For example,
although the detailed
embodiments described herein react adipic acid and hexamethylenediamine to
produce nylon
6,6, other monomers known to those skilled in this field could be used to
produce other
polyamides.
2~