Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/236~t3
APPLICATION OF NOBLE METALS TO INTERNAL SURFACES OF
OPERATING BOILING WATER REACTORS IN THE PRESENCE OF ZINC
IN REACTOR WATER
FIELD OF THE INVENTION
This invention relates generally to reducing the corrosion potential of
components exposed to high-temperature water. More particularly, the
invention relates to the application of noble metals onto operating reactor
surfaces in the presence of zinc to obtain adequate loading of reactor
surfaces
with noble metal and improved protection from corrosion and intergranular
stress corrosion cracking (IGSCC).
BACKGROUND OF THE INVENTION
Nuclear reactors are used in central-station electric power generation,
research and propulsion. A reactor pressure vessel contains the reactor
coolant, i.e. water, which removes heat from the nuclear core. Respective
to piping circuits carry the heated water or steam to the steam generators or
turbines and carry circulated water or feedwater back to the vessel.
Operating pressures and temperatures for the reactor pressure vessel are
about 7 MPa and 288 ~ C for a boiling water reactor (BWR), and about 15 MPa
and 320 ~ C for a pressurized water reactor (PWR). The materials used in both
t5 BWRs and PWRs must withstand various loading, environmental and
radiation conditions.
Some of the materials exposed to high-temperature water include
carbon steel, alloy steel, stainless steel, nickel-based, cobalt-based and
zirconium-based alloys. Despite careful selection and treatment of these
2o materials for use in water reactors, corrosion occurs in the materials
exposed
to the high-temperature water. Such corrosion contributes to a variety of
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCTNS99/23643
problems, e.g. , stress corrosion cracking, crevice corrosion, erosion
corrosion,
sticking of pressure relief valves and buildup of the gamma radiation-
emitting Co-60 isotope.
Stress corrosion cracking (SCC) is a known phenomenon occurring in
s reactor components, such as structural members, piping, fasteners and welds
exposed to high-temperature water. As used herein, SCC refers to cracking
propagated by static or dynamic tensile stressing in combination with
corrosion at the crack tip. The reactor components are subject to a variety of
stresses associated with, e.g., differences in thermal expansion, the
operating
to pressure needed for the containment of the reactor cooling water, and other
sources such as residual stress from welding, cold working and other
asymmetric metal treatments. In addition, water chemistry, welding, heat
treatment, and radiation can increase the susceptibility of metal in a
component to SCC.
1s It is well known that SCC occurs at higher rates when oxygen is
present in the reactor water in concentrations of about 5 ppb or greater. SCC
is further increased in a high radiation flux where oxidizing species, such as
oxygen, hydrogen peroxide, and short-lived radicals, are produced from
radiolytic decomposition of the reactor water. Such oxidizing species increase
2o the electrochemical corrosion- potential (ECP) of metals. Electrochemical
corrosion is caused by a flow of electrons from anodic to cathodic areas on
metallic surfaces. The ECP is a measure of the thermodynamic tendency for
corrosion phenomena to occur, and is a fundamental parameter in
determining rates of, e.g. , SCC, corrosion fatigue, corrosion film
thickening,
2s and general corrosion.
In a BWR, the radiolysis of the primary water coolant in the reactor
core causes the net decomposition of a small fraction of the water to the
chemical products Hz, HzOz, Oz and oxidizing and reducing radicals. For
2
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643
steady-state operating conditions, equilibrium concentrations of O2, H202,
and Hz are established in both the water which is recirculated and the steam
going to the turbine. The 02 and H202 generated are oxidizing and result in
conditions that can promote intergranular stress corrosion cracking (IGSCC)
of susceptible materials of construction. One method employed to mitigate
IGSCC of susceptible material is the application of hydrogen water chemistry
(HWC) , whereby the oxidizing nature of the BWR environment is modified
to a more reducing condition. This effect is achieved by adding hydrogen gas
to the reactor feedwater. When the hydrogen reaches the reactor vessel, it
to reacts with the radiolytically formed oxidizing species to reform water,
thereby lowering the concentration of dissolved oxidizing species in the water
in the vicinity of metal surfaces. The rate of these recombination reactions
is
dependent on local radiation fields, water flow rates and other variables.
The injected hydrogen reduces the level of oxidizing species in the
~5 water, such as dissolved oxygen, and as a result lowers the ECP of metals
in
the water. However, factors such as variations in water flow rates and the
time or intensity of exposure to neutron or gamma radiation result in the
production of oxidizing species at different levels in different reactors.
Thus,
varying amounts of hydrogen have been required to reduce the level of
20 oxidizing species sufficiently to maintain the ECP below a critical
potential
required for mitigation from IGSCC in high-temperature water. As used
herein, the term "critical potential" means a corrosion potential at or below
a
range of values of about -0.230 to -0.300 V based on the standard hydrogen
electrode (SHE) scale. IGSCC proceeds at an accelerated rate in systems in
25 which the ECP is above the critical potential, and at a substantially lower
or
zero rate in systems in which the ECP is below the critical potential. Water
containing oxidizing species such as oxygen increases the ECP of metals
exposed to the water above the critical potential, whereas water with little
or
no oxidizing species results in an ECP below the critical potential.
3
SUBSTITUTE SHEET (RULE 26j
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643 ._
Corrosion potentials of stainless steels in contact with reactor water
containing oxidizing species can be reduced below the critical potential by
injection of hydrogen into the water so that the dissolved hydrogen
concentration is about 50 to 100 ppb or greater. For adequate feedwater
hydrogen addition rates, conditions necessary to inhibit IGSCC can be
established in certain locations of the reactor. Different locations in the
reactor system require different levels of hydrogen addition. Much higher
hydrogen injection levels are necessary to reduce the ECP within the high
radiation flux of the reactor core, or when oxidizing cationic impurities,
e.g.,
cupric ion, are present.
It has been shown that IGSCC of Type 304 stainless steel (e.g.,
composition in weight % 18.0-20.0 Cr, 8.0-10.0 Ni, 2.00 Mn, 1.0 Si, 0.08 C,
0.08
S, 0.045 P) used in BWRs can be mitigated by reducing the ECP of the stainless
steel to values below -0.230 V(SHE). An effective method of achieving this
~5 objective is to use HWC. However, high hydrogen additions, e.g., of about
100 ppb or greater, that may be required to reduce the ECP below the critical
potential, can result in a higher radiation level in the steam-driven turbine
section from incorporation of the short-lived N-16 species in the steam. For
most BWIZs, the amount of hydrogen addition required to provide mitigation
20 of IGSCC of pressure vessel internal components results in an increase in
the
main steam line radiation monitor by a factor of five from its background
level. This increase in main steam line radiation can cause high, even
unacceptable, environmental dose rates that can require expensive
investments in shielding and radiation exposure control. Thus, recent
25 investigations have focused on using minimum levels of hydrogen to achieve
the benefits of HWC with minimum increase in the main steam radiation dose
rates.
An effective approach to achieve this goal is to either coat or alloy the
stainless steel surface with palladium or any other platinum group metal. The
4
SUBSTITUTE SHEET (RULE 2B)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643
presence of palladium on the stainless steel surface reduces the hydrogen
demand to reach the required IGSCC critical potential of -0.230 V(SHE). T'he
techniques used to date for palladium coating include electroplating,
electroless plating, plasma deposition and related high-vacuum techniques.
s Palladium alloying has been carried out using standard alloy preparation
techniques. Both of these approaches are ex situ techniques in that they
cannot be practiced while the reactor is in operation.
U.S. Patent No. 5,135,709 to Andresen et al. discloses a method for
lowering the ECP on components formed from carbon steel, alloy steel,
io stainless steel, nickel-based alloys or cobalt-based alloys which are
exposed to
high-temperature water by forming the component to have a catalytic layer of
a platinum group metal. As used herein, the term "high temperature water"
means water having a temperature of about 150°C or greater, steam or
the
condensate thereof. As used therein, the term "catalytic layer" means a
15 coating on a substrate, or a solute in an alloy formed into the substrate,
the
coating or solute being sufficient to catalyze the recombination of oxidizing
and reducing species at the surface of the substrate. As used herein, the term
"platinum group metal" means metals from the group consisting of platinum,
palladium, osmium, ruthenium, iridium, rhodium, and mixtures thereof.
2o In nuclear reactors, ECP is increased by higher levels of oxidizing
species, e.g., up to 200 ppb or greater of oxygen in the water measured in the
recirculation piping, from the radiolytic decomposition of water in the core
of
the nuclear reactor. The method disclosed in U.S. Patent No. 5,135,709 further
comprises providing a reducing species in the high-temperature water that
25 can combine with the oxidizing species. In accordance with this known
method, high concentrations of hydrogen, i.e., about 100 ppb or more, must
be added to the water to provide adequate mitigation to materials outside the
reactor core region, and still higher concentrations are needed to afford
mitigation to materials in the reactor core.
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCTNS99/23643
The formation of a catalytic layer of a platinum group metal on an
alloy from the aforementioned group catalyzes the recombination of reducing
species, such as hydrogen, with oxidizing species, such as oxygen or
hydrogen peroxide, that are present in the water of a BWR. Such catalytic
action at the surface of the alloy can lower the ECP of the alloy below the
critical potential where IGSCC is minimized. As a result, the efficacy of
hydrogen additions to high-temperature water in lowering the ECP of
components made from the alloy and exposed to the injected water is
increased many-fold. Furthermore, it is possible to provide catalytic activity
at metal alloy surfaces if the metal substrate of such surfaces contains a
catalytic layer of a platinum group metal. Relatively small amounts of the
platinum group metal are sufficient to provide the catalytic layer and
catalytic
activity at the surface of the metal substrate. For example, U.S. Patent No.
5,135,709 teaches that a solute in an alloy of at least about 0.01 wt. %,
~s preferably at least 0.1 wt. %, provides a catalytic layer sufficient to
lower the
ECP of the alloy below the critical potential. The solute of a platinum group
metal can be present up to an amount that does not substantially impair the
metallurgical properties, including strength, ductility, and toughness of the
alloy. The solute can be provided by methods known in the art, for example
2o by addition to a melt of the alloy or by surface alloying. In addition, a
coating
of the platinum group metal, or a coating of an alloy comprised of a solute of
the platinum group metal as described above, provides a catalytic layer and
catalytic activity at the surface of the metal. Suitable coatings can be
deposited by methods well known in the art for depositing substantially
2s continuous coatings on metal substrates, such as plasma spraying, flame
spraying, chemical vapor deposition, physical vapor deposition processes
such as sputtering, welding such as metal inert gas welding, electroless
plating, and electrolytic plating.
6
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643
Thus, lower amounts of reducing species such as hydrogen are
effective in reducing the ECP of the metal components below the critical
potential, because the efficiency of recombination of oxidizing and reducing
species is increased many-fold by the catalytic layer. Reducing species that
can combine with the oxidizing species in the high-temperature water are
provided by conventional means known in the art. In particular, reducing
species such as hydrogen, ammonia, or hydrazine are injected into the
feedwater of the nuclear reactor.
A need exists to provide for improved control over the deposition of
metals on the surface of components to protect them from corrosion and
intergranular stress corrosion cracking. The present invention seeks to
satisfy
that need.
SUMMARY OF THE INVENTION
In the majority of instances of BWR operation, zinc is added in the
form of zinc ions to control shut-down dose rates arising as a result of
is accumulation of cobalt-60 (~°Co) in the recirculation piping
associated with
the reactor. It has been discovered by the present inventors that the presence
of zinc ions in the water has a negative influence on the active noble metal
species available for the noble metal incorporation process. The presence of
zinc ions results in the formation of a zinc-containing spinet-type oxide film
20 on reactor internal surfaces and associated components where the zinc atoms
occupy sites that would otherwise have been occupied by the undesirable
~°Co isotope. Because of the higher solubility of zinc in water in the
temperature range used for noble metal chemical addition, i.e., about 120-
610°F or higher , release of zinc from reactor internal surfaces can
occur
2s during the noble metal incorporation process. The released zinc ions may
then react with the noble metal species present in anionic form to cause the
formation of a precipitate containing the noble metal species, thereby
7
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643
lowering the concentration of the noble metal species available for
incorporation.
It has been discovered, according to the present invention, that it is
possible to achieve improved noble metal loading on reactor metal surfaces
disposed in high temperature water containing zinc by introducing the noble
metal species into the high temperature water in positively charged cationic
form. In this way, precipitate formation with zinc cations is essentially
eliminated, thereby minimizing the negative effect the presence of zinc may
have on incorporation of noble metals species on the reactor surfaces.
In one aspect, the invention provides a method for reducing corrosion
of alloy components in a water cooled nuclear reactor or associated
components in which the water of the reactor contains zinc cations,
comprising the step of injecting into the water of the reactor a noble metal
cation releasing compound which releases noble metal cations into the water
under operating reactor thermal conditions with essentially no precipitate
formation between the zinc and the released noble metal.
In another aspect, there is provided a method for reducing corrosion of
alloy components such as stainless steel components, in a water-cooled
nuclear reactor or associated components, wherein a solution of a noble metal
2o canon releasing compound containing a noble metal is injected into the
reactor water containing zinc ions at a temperature of about to 120° to
610°F.,
for example about 300° to 450°F, in an amount such that, upon
decomposition
of the noble metal compound under the operating reactor thermal conditions,
cations of the noble metal are released at a rate such that the concentration
of
the noble metal in the water is sufficient, once incorporated on the alloy
component's surface, to reduce the electrochemical corrosion potential of the
alloy components to a level below the critical potential, with essentially no
precipitate formation between the zinc and the noble metal.
8
SUBSTITUTE SHEET (RULE 2B)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643
In a further aspect, the invention provides a method for reducing
corrosion of alloy components in a water cooled nuclear reactor or associated
components, wherein zinc and a noble metal cation releasing compound are
added to the water of the rector such that the noble metal cation releasing
s compound releases noble metal canons into the water under operating reactor
thermal conditions. The zinc may be added prior to or subsequent to the
noble metal cation releasing compound.
As a result of the invention, it is possible to achieve good corrosion
resistance of metal surfaces disposed in high temperature water in the
io presence of zinc as a result of minimal precipitate formation between the
noble metal and the zinc.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a schematic showing a partially cutaway perspective view of a
conventional BWR; and
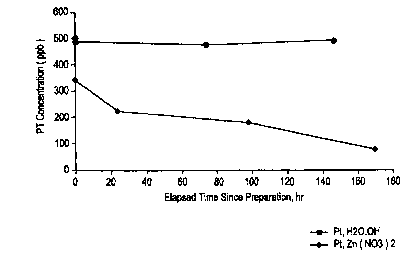
FIG. 2 shows the effect of zinc in sodium hexahydroxy platinate
~5 (NaPt(OH)6) solutions on platinum concentrations.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The fluid flow in a boiling water reactor will be generally described
with reference to FIG. 1. Feedwater is admitted into a reactor pressure vessel
(RPV) 10 via a feedwater inlet 12 and a feedwater sparger 14, which is a ring-
shaped pipe having suitable apertures for circumferentially distributing the
2o feedwater inside the RPV. A core spray inlet 11 supplies water to a core
spray
sparger 15 via core spray line 13. The feedwater from feedwater sparger 14
flows downwardly through the downcomer annulus 16, which is an annular
region between RPV 10 and core shroud 18. Core shroud 18 is a stainless steel
cylinder which surrounds the core 20 comprising numerous fuel assemblies
9
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643
22 (only two 2 x 2 arrays of which are depicted in FIG. 1). Each fuel assembly
is supported at the top by top guide 19 and at the bottom by core plate 21.
Water flowing through downcomer annulus 16 then flows to the core lower
plenum 24.
The water subsequently enters the fuel assemblies 22 disposed within
core 20, wherein a boiling boundary layer (not shown) is established. A
mixture of water and steam enters core upper plenum 26 under shroud head
28. Core upper plenum 26 provides standoff between the steam-water
mixture exiting core 20 and entering vertical standpipes 30, which are
disposed atop shroud head 28 and in fluid communication with core upper
plenum 26.
The steam-water mixture flows through standpipes 30 and enters
steam separators 32, which are of the axial-flow centrifugal type. The
separated liquid water then mixes with feedwater in the mixing plenum 33,
is which mixture then returns to the core via the downcomer annulus. The
steam passes through steam dryers 34 and enters steam dome 36. The steam
is withdrawn from the RPV via steam outlet 38.
The BWR also includes a coolant recirculation system which provides
the forced convection flow through the core necessary to attain the required
2o power density. A portion of the water is sucked from the lower end of the
downcomer annulus 16 via recirculation water outlet 43 and forced by a
centrifugal recirculation pump (not shown) into jet pump assemblies 42 (only
one of which is shown) via recirculation water inlets 45. The BWR has two
recirculation pumps, each of which provides the driving flow for a plurality
25 of jet pump assemblies. The pressurized driving water is supplied to each
jet
pump nozzle 44 via an inlet riser 47, an elbow 48 and an inlet mixer 46 in
flow
sequence. A typical BWR has 16 to 24 inlet mixers.
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
- WO 00/22627 PCT/US99/23643 .
In the following discussion, for convenience of description, reference
will be made to the use of platinum or mixtures of platinum and rhodium as ,
typical noble metals. It is understood, however, that the invention is not
limited to the use of platinum and rhodium, and other platinum group metals
may be used.
The term ~ platinum group metal", as used herein, means platinum,
palladium, osmium, ruthenium, iridium, rhodium and mixtures thereof.
Mixtures of platinum group compounds may also be used. Examples of
mixtures of the compounds which may be used are mixtures containing
1o platinum and iridium, and platinum and rhodium.
The presence of iridium or rhodium with the platinum gives good
long-term durability. It has been found that a combination of about 40-$0 ppb
Pt and 10-35 ppb Rh, for example concentrations of about 60 ppb Pt and about
20 ppb Rh in ionic form in water, provides good adherent properties of noble
t5 metal over extended periods of time. However, 1:1 ratios of Pt:Rh have also
been used with success.
The term "cation-releasing noble metal compound", as used herein,
means a noble metal-containing compound which releases noble metal as
cations (e.g. Ptz+) or as cationic species containing noble metal species with
2o attached ligands, such as for example Pt{OH)+, Pt(NHs)42+ into the reactor
water under operating reactor thermal conditions. Such compounds may be
organometallic, organic or inorganic, and may be soluble or insoluble in water
(i.e. may form solutions or suspensions in water and/or other media such
alcohols and/or acids). Examples of such compounds are platinum chloride,
25 palladium chloride, palladium acetyl acetonate, platinum acetyl acetonate,
palladium nitrate, platinum nitrate, palladium acetate, platinum acetate,
Pt(NHa)4(NOs)2 and Pt(NHs)2(NOz)2. Other examples are platinum(IV) oxide
(Pt(IV)OZ), platinum(IV) oxide-hydrate (Pt(IV)Oz.xHzO, where x is 1-10),
11
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643
rhodium(II) acetate (Rh(II)acz), IZh{III) nitrate (Rh{III)(NOs)a),
rhodium(III)
oxide (Rh(III)z03), rhodium(III) oxide-hydrate (Rh(III)zOs.xH20, where x is 1-
10), rhodium(II) phosphate (Rh(III)P04) and rhodium(III) sulphate
(Rh(III)z(S04)s). in general, any species of the form Pt(X)z+, Pt(X)4+,
Pd(X)z+,
s Rh(X)3+ where X is an organic or inorganic ligand may be used, but not
limited to Pt, Pd or Rh.
The metal compound may be injected in situ into the reactor in the
form of an aqueous solution or suspension. As used in the claims hereafter,
the term ~ solution ~ means solution or suspension. Solutions and
to suspensions may be formed using media well known to those skilled in the
art. Examples of suitable media in which solutions and/or suspensions are
formed, are water, alkanols such as ethanol, propanol, n-butanol, and acids
such as lower carboxylic acids, e.g. acetic acid, propionic acid and butyric
acid, or ketones such as acetone and acetylacetone.
~5 When the noble metal cation-releasing compound solution or
suspension enters the high-temperature water, the compound decomposes
very rapidly to release the cationic species (e.g. Ptz+) into the reactor
water
with or without the ligand. These species are then incorporated in the reactor
surface, usually by incorporation into the metal (typically stainless steel)
20 oxide film. Use of mixtures of noble metal canon-releasing compounds
results in release and deposition of both noble metals, usually by way of
incorporation of the noble metals on the oxided stainless steel surfaces.
While
not being bound to any theory, it is believed that they undergo conversion to
metal during the incorporation process.
25 Reaction of the noble metal with cationic zinc ions (Znz+) present in the
water is minimized due to the noble metal being in cationic form. Typically,
zinc is present in the reactor water in an amount of about 10 to 500 ppb, but
this amount may be more or less, depending on the conditions present in the
12
SUBSTIME SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643 ._
reactor. The inventors have found that no precipitate is observed with
solutions of 500 ppb zinc nitrate when platinum is added, for example as
platinum chloride or tetrammineplatinum nitrate. Improved noble metal
loading is accordingly obtained over that where the noble metal is in a form
(e.g., anion) which reacts with zinc ions to form a precipitate.
The solution or suspension of the noble metal compound is typically
introduced into the high-temperature water initially. No further agents, such
as hydrogen or other reducing agents, needed to be introduced into the high-
temperature water when the noble metal cation-releasing compound solution
Io or suspension is injected into and decomposes in the high-temperature
reactor
water.
The process of the present invention is distinguished from the
processes of U.S. patents 5,130,080 and 5,130,181 to Niedrach. The Niedrach
patents teach that it is possible to electrolessly plate oxide films using
~s conventional electroless plating techniques. Conventional electroless
plating
is carried out at relatively low temperatures, typically in the region of 50
to
80~ C., possibly lower, and requires the presence of an added reducing agent,
typically sodium hypophosphite, to supply electrons for reduction of the
noble metal ions to the metal. The reaction takes place only on a catalytic
2o surface which has been sensitized/activated beforehand, for example with
stannous chloride, and the process results in a build-up of metal coating on
the surface which eventually coats the entire surface with deposited metal.
The electroless plating bath typically contains high ionic concentrations, of
the
order of thousands of ppm, of chemicals, including, for example, palladium
2s (II) chloride, ammonium hydroxide, ammonium chloride, disodium EDTA
and hydrazine, as well as a reducing agent (e.g. sodium hypophosphite). The
pH of the electroless bath is usually in the region of 9.0 to 10.5 in view of
the
presence of base (ammonium hydroxide and ammonium chloride).
13
SUBSTnUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643 .
The process of the present invention does not rely on the use of
electroless plating techniques or other techniques which result in the metal
being plated on the oxide surface. In the present process, the metal
compound or mixture of metal compounds is introduced into the high-
s temperature water containing zinc in an amount such that the concentration
of the metals) in the water is very low, i.e. in the ppb range, but is
sufficient
such that when present on the metal component after incorporation, the ECP
is lowered below the critical potential required for mitigation from stress
corrosion cracking with very low levels of hydrogen.
The compound solution or suspension may be injected into the high-
temperature water while the reactor is operating and generating nuclear heat
(full power operation), or during cool down, during outage, during heat-up,
during hot standby, or during low power operation. The noble metal may be
introduced into residual heat removal (RHR) piping, recirculation piping,
~s feedwater line, core delta P line, jet pump instrumentation line, control
rod
drive cooling water lines, water level control points, reactor water clean-up
(RWCU) system (discussed in more detail below) which may or may not be in
operation during the application period, or any other location which provides
introduction of the noble metal into the reactor water and good mixing with
2o the water. High temperature water can be found in a variety of known
apparatus, such as water deaerators, nuclear reactors (BWR's and PWR's), and
steam-driven power plants.
Typically, the metal compound is added in such an amount to produce
a metal concentration in the reactor water of no higher than 2000 ppb, for
25 example 0.1 to 1000 ppb. More typically, the concentration in the water is
0.1
to 1 ppm, for example 1 to 500 ppb, more usually 5 to 100 ppb.
The temperature of the water when noble metal is added to the reactor
water is typically in the range of 120 - 550°F (BWR), 120 -
610°F (PWR). The
14
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCTNS99/23643
temperature is generally in the range of 212 - 350°F, more usually
about 340° -
360°F. If noble metal addition is performed at full power operation,
the
temperature will be about 550°F.
At the very low levels of metal{s) introduced into the reactor, the
s stainless steel oxide surface is not covered completely with metal.
Typically,
the oxide surface has metal present in an amount of about 0.1-15 atomic%, for
example 0.5-10 atomic%, more usually 2-5 atomic%.
It is important to monitor the noble metal concentration during the
application process so that the desired concentration can be maintained
within the reactor water. The concentration is desirably continuously
monitored, typically by taking samples from any sampling location and
analyzing for the noble metal concentration.
The depth of metal in the oxide surface is generally in the range of 100
to 1000 Angstroms, more usually 200 to 500 Angstroms. The external
~s appearance of the oxided alloy treated according to the present process
does
not differ from the appearance of untreated stainless steel oxide. The noble
metal containing surface does not have a bright metallic luster as is
generally
obtained with electroplating or electroless coating processes, nor is the
surface
100% covered with noble metal.
2o In the present process, only very dilute compound solution or
suspension is injected into the high-temperature water. No reducing agents
(including hydrogen), acids and bases, are added. As a result, the typical pH
of the water at ambient temperature is in the region of 6.5 to 7.5, and at
higher
operating temperatures is lower, generally in the region of about 5.5 - 5.8,
for
2s example 5.65. This is due to increased dissociation of the water at the
higher
temperatures. During noble metal application, the pH may be 4.5 to 9.5,
depending on other ionic species in the water.
SU8ST1TUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643
An operating BWR has very stringent coolant water conductivity levels
which must be observed. Typically, the conductivity of the coolant water
must not exceed 0.3 ~ S/cm, and more usually must be less than 0.1 ~ S/cm.
Such conductivity levels are adversely impacted by high concentrations of
ionic species, and every effort is made in the present process to ensure that
reactor ionic concentrations are maintained as low as possible after clean-up,
preferably less than 5 ppb. The process in particular maintains chloride ion
at
a very low level in view of its corrosive nature.
The time period over which the process of noble metal application is
to conducted will depend on the concentration and temperature conditions.
Usually, the noble application is carried out over a period ranging from 4
hours to one week, more typically 4 to 48 hours, provided that any limits set
on pH and conductivity are maintained.
A typical plant application process may be performed at a temperature
~5 of 280 ~ 20°F over a period of about 24 to 48 hours. The noble metal
concentration during the application process should be maintained at a level
of about 40 to 1000 ppb while not allowing the conductivity of the reactor to
exceed undesirable levels, typically not to exceed 30 :S/cm, over this limited
period of time.
2o The present process does not involve any catalytic
activation/sensitization of the stainless steel oxide surface. The use of
stannous chloride to achieve such activation would be incompatible with
operation of the BWR and the stringent conductivity limits on the coolant
water referred to above.
25 After completion of the application process, the plant will proceed to
its normal outage related activities, while the RWCU system is still in
operation in order to clean up residual noble metal before plant start-up.
16
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
' WO 00/22627 PC'T/US99/Z3643 .
Confirmation of deposition of noble metal on the internal surface of the
reactor and components thereof is accomplished by (a) mass balance
calculations during application, (b) surface analysis of accessible regions
and
analyzing for noble metal content during subsequent outages and (c) by
s hydrogen benchmark Cramping) after plant start-up.
While not being bound by theory, it is understood that the metal in
ionic form, for example platinum and/or rhodium, is deposited on the reactor
surface, typically by way of incorporation into the stainless steel oxide
film.
Thermal decomposition of the compound forms metal ions which apparently
1o replace iron, nickel and/or chromium atoms in reactor/component surface. It
is believed that the metal ions are incorporated into the oxide film,
resulting
in a metal-doped oxide film. The metal, such as platinum either alone or with
rhodium, may for example be incorporated within or on the surface of the
oxide film. The oxide film is believed to include mixed nickel, iron and
is chromium oxides containing zinc, if the plant has operated with natural or
added zinc for some time.
The ECPs of the stainless steel components all drop by approximately
0.5 to 0.6 V after injection of the noble metal and subsequent addition of low
levels of hydrogen. It is possible to reduce the ECP of Type 304 stainless
steel
2o to IGSCC mitigation values without injecting hydrogen when an organic or
an organometallic compound has been injected into the water. The catalytic
oxidation of organics on noble metal-doped surfaces consumes oxygen,
thereby lowering the dissolved oxygen content in the high temperature water.
Good results are also obtained when an inorganic metal compounds) is used.
2s Moreover, clean-up of the water is easier when inorganic(s) such as
nitrates
are used as compared to organics such as formates and acetates. For this
reason, inorganic compounds, particularly inorganic platinum group metal
compounds (e.g. noble metal nitrates and nitrites), are typically used.
17
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
- ' WO 00/22627 PCTNS99/23643 .
Following injection and incorporation of the metals) in the oxided
stainless steel surfaces, the water is subjected to a conventional clean-up
process to remove ionic materials such as nitrate ions present in the water.
This clean-up process is usually carried out by passing a fraction of the
water
s removed from the bottom head of the reactor and recirculanon piping
through an ion exchange resin bed, and the treated water is then returned to
the reactor via the feedwater system. Hydrogen may subsequently be
introduced into the water some time after the doping reaction, for example 1
to 72 hours after injection and incorporation of the metal atoms in the oxided
surface, to catalyze recombination of hydrogen and oxygen on the metal
doped surfaces. As hydrogen is added, the electrochemical corrosion potential
of the metal-doped oxide film on the stainless steel components is reduced to
values which are much more negative than when low levels of hydrogen are
injected into a BWR having stainless steel components which are not doped
~5 with the noble metal.
The noble metal compound is usually injected at a point downstream
of the recirculation water outlet 43 (see FIG. 1) . The high temperatures as
well
as the gamma and neutron radiation in the reactor core act to decompose the
compound, thereby releasing noble metal canons or species for incorporation
20 in the oxide film of the reactor surfaces.
The noble metal injection solution may be prepared for example by
dissolving the noble metal compound in ethanol. The ethanol solution is then
diluted with water. Alternatively, a water-based suspension can be formed,
without using ethanol, by mixing the noble metal compound in water. The
25 widely used approach is to use a solution of noble metal compound in water.
The method of the invention may also be carried out by adding zinc
and a noble metal canon releasing compound to the water of the rector such
that the noble metal cation releasing compound releases noble metal cations
18
SUBSTITUTE SHEET (RULE 26)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643
into the water under operating reactor thermal conditions. The order of
addition is not critical, The zinc may be added prior to or subsequent to the
noble metal cation releasing compound.
The noble metal either deposits or is incorporated onto the reactor
s surface, typically into the (stainless steel) oxide film via a thermal
decomposition process of the noble metal compound. As a result of that
decomposition, noble metal caHons (species) become available to replace
atoms, e.g., iron atoms, in the oxide film, thereby producing a noble metal-
doped oxide film on stainless steel. As used herein, the term "atoms" means
atoms, ions or uncharged species.
The present invention offers the advantage that steel surfaces can be
doped with noble metal to good loading levels while zinc is present in the
reactor water using an in situ technique (while the reactor is operating)
which
is simple in application and also relatively inexpensive. The technique can be
is applied to operating BWRs and PWRs and their associated components, such
as steam generators.
The invention will now be further illustrated with reference to the
following working example.
EXAMPLE
Figure 2 shows the effect of zinc in sodium hexahydroxy platinate
20 (NaPt(OH)6) solutions on platinum concentrations. Sodium hexahydroxy
platinate (>50 ppm Pt) was mixed with zinc nitrate (500 ppm) to yield a
visible precipitate. While no precipitate was visible with Pt concentrations
of
100 - 500 ppb, a decrease in the Pt concentration was observed over time
when sodium hexahydroxy platinate was mixed with zinc nitrate (see
25 Figure 2).
19
SUBSTITUTE SHEET (RULE 2B)
CA 02333072 2000-11-23
WO 00/22627 PCT/US99/23643 ,
The overall effect of this zinc interference is the reduction of noble
metal loading on reactor internal surfaces. This results either in poor ECP
response in the presence of H2 and/or poor durability of the catalytic
surfaces. The present invention minimizes zinc interference by reducing
precipitate formation between zinc and noble metal, thereby improving noble
metal loading.
The foregoing method has been disclosed for the purpose of
illustration. Variations and modifications of the disclosed method will be
readily apparent to practitioners skilled in the art of hydrogen water
chemistry. For example, metals other than platinum/rhodium can be applied
using this technique, e.g., other platinum group metals. A platinum group
metal can be injected in the form of an organic, organometallic or inorganic
compound which produces noble metal canons or species in the high
temperature reactor water, to reduce the electrochemical corrosion potential
of stainless steel reactor components even in the absence of hydrogen
injection. It may also be possible to incorporate non-platinum group metals in
oxide films on stainless steel components, e.g., zirconium and titanium, using
the technique of the invention. All such variations and modifications are
intended to be encompassed by the claims set forth hereinafter.
SUBSTITUTE SHEET (RULE 26)