Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02334332 2000-12-07
WO 00100478 PCT/US99/14395
SUBSTITUTED QUINOXALIN-2(1H)-ONES USEFUL AS HIV REVERSE
TRANSCRIPTASE INHIBITORS
FTELD OF THE INVENTTnu
This invention relates generally to substituted
quinoxalin-2(1H)-ones which are useful as in'.iibitors of HIV
reverse transcriptase, pharmaceutical compositions and
diagnostic kits comprising the same, and methods of using the
same for treating viral infection or as assay standards or
reagents.
BACKGROUND OF THE INVENT~Q~T
Two distinct retroviruses, human immunodeficiency virus
(HIV) type-1 (HIV-1) or type-2 (HIV-2), have been
etiologically linked to the immunosuppressive disease,
acquired immunodeficiency syndrome (AIDS). HIV seropositive
individuals are initially asymptomatic but typically develop
AIDS related complex (ARC) followed by AIDS. Affected
individuals exhibit severe immunosuppression which
predisposes them to debilitating and ultimately fatal
opportunistic infections.
The disease AIDS is the end result of an HIV-1 or HIV-2
virus following its own complex life cycle. The virion life
cycle begins with the virion attaching itself to the host
human T-4 lymphocyte immune cell through the bonding of a
glycoprotein on the surface of the virion's protective coat
with the CD4 glycoprotein on the lymphocyte cell. Once
attached, the virion sheds its glycoprotein coat, penetrates
into the membrane of the host cell, and uncoats its RNA. The
virion enzyme, reverse transcriptase, directs the process of
transcribing the RNA into single-stranded DNA. The viral RNA
is degraded and a second DNA strand is created. The now
double-stranded DNA is integrated into the human cell's genes
and those genes are used for virus reproduction.
At this point, RNA polymerase transcribes the integrated
DNA into viral RNA. The viral RNA is translated into the
precursor gag pot fusion polyprotein. The polyprotein is
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/1_4395
then cleaved by the HIV protease enzyme to yield the mature
viral proteins. Thus, HIV protease is responsible for
regulating a cascade of cleavage events that lead to the
virus particle's maturing into a virus that is capable of
full infectivity.
The typical human immune system response, killing the
invading virion, is taxed because the virus infects and kills
the immune system's T cells. In addition, viral reverse
transcriptase, the enzyme used in making a new virion
particle, is not very specific, and causes transcription
mistakes that result in continually changed glycoproteins on
the surface of the viral protective coat. This lack of
specificity decreases the immune system's effectiveness
because antibodies specifically produced against one
glycoprotein may be useless against another, hence reducing
the number of antibodies available to fight the virus. The
virus continues to reproduce while the immune response system
continues to weaken. Eventually, the HIV largely holds free
reign over the body's immune system, allowing opportunistic
infections to set in and without the administration of
antiviral agents, immunomodulators, or both, death may
result.
There are at least three critical points in the virus's
life cycle which have been identified as possible targets for
antiviral drugs: (1) the initial attachment of the virion to
the T-4 lymphocyte or macrophage site, (2) the transcription
of viral RNA to viral DNA (reverse transcriptase, RT), and
(3) the processing of gag-pol protein by HIV protease.
Inhibition of the virus at the second critical point,
the viral RNA to viral DNA transcription process, has
provided a number of the current therapies used in treating
AIDS. This transcription must occur for the virion to
reproduce because the virion's genes are encoded in RNA and
the host cell reads only DNA. By introducing drugs that
block the reverse transcriptase from completing the formation
of viral DNA, HIV-1 replication can be stopped.
A number of compounds that interfere with viral
replication have been developed to treat AIDS. For example,
-2-
CA 02334332 2000-12-07
WO 00/00478 PCTlUS99/14395
nucleoside analogs, such as 3'-azido-3'-deoxythymidine (A?T),
2',3'-dideoxycytidine (ddC), 2',3'-dideoxythymidinene (d4T),
2',3'-dideoxyinosine (ddI), and 2',3'-dideoxy-3'-thia-
cytidine (3TC) have been shown to be relatively effective in
halting HIV replication at the reverse transcriptase (RT)
stage.
Non-nucleoside HIV reverse transcriptase inhibitors have
also been discovered. As an example, it has been found that
certain benzoxazinones are useful in the inhibition of HIV
reverse transcriptase, the prevention or treatment of
infection by HIV and the treatment of AIDS. U. S. Patent
Number 5,519,021, the contents of which are hereby
incorporated herein by reference, describes reverse
transcriptase inhibitors which are benzoxazinones of the
formula:
X1
Z
wherein X is a halogen, Z may be O. However, benzoxazinones
are not part of the present invention.
U.S. Patent No. 5,693,641 depicts bicyclic pyrimidine
derivatives useful as anticoagulants of the formula:
R5
6
R1- \ Z' 'N N R~
N~~
RZ N O
~Z2 R5
R3 '~ \ Ra
wherein Z1 and Z2, independently, can be -0-, -NR5-, or
-OCH2-; RS is H, alkyl, aryl, or aralkyl; R6 and R7 can be a
variety of groups. Compounds of this sort are not within the
scope of the presently claimed invention.
EP 0,657,166 A1 illustrates quinoxalines of the formula:
-3-
CA 02334332 2000-12-07
WO 00/00478 PCT/I1S99/14395
R5
R4
N
R1 ~ R3
N X
~2
R
which in combination with at least one nucleoside exhibit an
antiviral effect. The application describes quinoxalines
generally, wherein X is O or S; R2 or RS can be a variety of
groups including H, alkyl, alkenyl, alkynyl, cycloalkyl,
substituted carbonyl, substituted oxycarbonyl, substituted
aminocarbonyl; and R3 or R4, can be a variety of groups
including H, alkyl, alkenyl, cycloalkyl, and aryl, but not
IO alkynyl. However, EP 0,657,166 A1 does not disclose by
exemplification compounds wherein R3 or R4 are -CF3, -CF2CF3,
-CF2CF2CF3 or cyclopropyl, compounds wherein R3 or R4 are
alkynyls or substituted alkynyls.
Even with the current success of reverse transcriptase
inhibitors, it has been found that HIV patients can become
resistant to a single inhibitor. Thus, it is desirable to
develop additional inhibitors to further combat HIV
infection.
It has unexpectedly been found that compounds of the
present invention, most preferably, 3-(perfluoroalkyl)-3,4-
dihydro-1,H-quinoxalin-2-ones, are useful as HIV reverse
transcriptase inhibitors.
SUMMARY OF THE INVENTION
Accordingly, one object of the present invention is to
provide novel reverse transcriptase inhibitors.
It is another object of the present invention to provide
a novel method for treating HIV infection which comprises
administering to a host in need of such treatment a
therapeutically effective amount of at least one of the
compounds of the present invention or a pharmaceutically
acceptable salt or prodrug form thereof.
It is another object of the present invention to provide
a novel method for treating HIV infection which comprises
-4-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/~4395
administering to a host in need thereof a therapeutically
effective combination of (a) one of the compounds of the
present invention and (b) one or more compounds selected form
the group consisting of HIV reverse transcriptase inhibitors
and HIV protease inhibitors.
It is another object of the present invention to provide
pharmaceutical compositions with reverse transcriptase
inhibiting activity comprising a pharmaceutically acceptable
carrier and a therapeutically effective amount of at least
one of the compounds of the present invention or a
pharmaceutically acceptable salt or prod rug form thereof.
It is another object of the present invention to provide
a method of inhibiting HIV present in a body fluid sample
which comprises treating the body fluid sample with an
effective amount of a compound of the present invention.
It is another object of the present invention to provide
a kit or container containing at least one of the compounds
of the present invention in an amount effective for use as a
standard or reagent in a test or assay for determining the
ability of a potential pharmaceutical to inhibit HIV reverse
transcriptase, HIV growth, or both.
These and other objects, which will become apparent
during the following detailed description, have been achieved
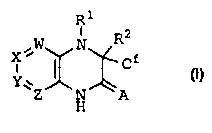
by the inventors' discovery that compounds of formula (I):
R1
X ~W N R2 f
Y.w ~ ~ C
Z N A
H
(I)
wherein A, W, X, Y, Z, R1, R2, and Cf are defined below,
stereoisomeric forms, mixtures of stereoisomeric forms, or
pharmaceutically acceptable salt forms thereof, are effective
reverse transcriptase inhibitors.
-5-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Thus, in a first embodiment, the present invention
provides a novel compound of Formula (I):
R1
R2
X ~W ( N C f
Y.
Z N A
H
(I)
or a stereoisomer or pharmaceutically acceptable salt form
thereof, wherein:
A is O or S;
W is N or CR3;
X is N or CR4;
Y is N or CRS;
Z is N or CR6;
Cf is cyclopropyl or C1_3 alkyl substituted with 3-7 halogen;
provided that the number of W, X, Y, and Z which are N, is
zero, one or two;
R1 is selected from:
-C02R12 , -COR12 . -S02R12 . -SOR12 , -CONHR12 ,
-(CHR7)pCHR?RB,
-(CHR7)pCH=CR7R8,
-(CHR7)pC=C-Re,
-C1_6 alkyl substituted with 0-3 R11,
-(CH2)pphenyl substituted with 0-3 R1~, and
-(CH2)p(C3-S cycloalkyl);
R2 is selected from:
-CH=CR7R8,
-6-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/1_4395
-C=C-RS,
-CH=CHCHR7R8,
-(CHR~)pCHR~R8,
-(CHR~)pCH=CR~RB,
-(CHR~)pC=-C-R8,
-C1_q alkyl substituted with 0-3 R11,
-(CHz)pphenyl substituted with 0-3 Rlo, and
-(CH2)p(C3_5 cycloalkyl);
R3 is selected from:
H, F, C1, Br, I, -OH, OCF3, -CN. N02, CHO, C(=O)CH3,
C (=O) CF3, C (=O)NH2, C (=0)NHCH3, NR~R7a,
NR~C (=O) OR7b, C (=O) OR7, SRS, S (=O) R~, S02R~, S02NHR~,
NR~S02R~b,
C1_3 alkyl substituted with 0-3 R11,
C2_3 alkenyl,
CZ_3 alkynyl,
C1_3 alkoxy,
phenyl substituted with 0-2 Rlo, and
5-6 membered aromatic heterocycle system containing from
1-4 heteroatoms selected from the group consisting
of N, O, and S and substituted With 0-2 Rlo;
R4 is selected from:
H, F, C1, Br, I, -OH, OCF3, -CN, N02, CHO, C(=O)CH3,
C(=O)CF3, C(=0)NH2, C(=O)NHCH3, NR7R78,
NR~C(=O)OR~b, C(=O)OR~, SRS, S(=O)R~, S02R~, S02NHR~,
NR7S02R~b,
C1_3 alkyl substituted with 0-3 R11,
C2_3 alkenyl,
C2_3 alkynyl,
Cl_3 alkoxy,
phenyl substituted with 0-2 Rlo, and
5-6 membered aromatic heterocycle system containing from
1-4 heteroatoms selected from the group consisting
of N, O, and S and substituted with 0-2 Rlo;
-?-
CA 02334332 2000-12-07
WO 00/00478 PCTlUS99/1_4395
alternatively, R3 and R4, when substituents on adjacent carbon
atoms, are taken together with the carbon atoms to which
they are attached to form a 5-7 membered carbocyclic
ring, said carbocyclic ring being aromatic or
nonaromatic, said carbocyclic ring being substituted
with 0-2 Rlo;
alternatively, R3 and R4, when substituents on adjacent carbon
atoms, are taken together with the carbon atoms to which
they are attached to form a 5-7 membered heterocyclic
ring containing 1, 2 or 3 heteroatoms atoms selected
from the group consisting of N, O, and S, said
heterocyclic ring being aromatic or nonaromatic, said
heterocyclic ring being substituted with 0-2 Rlo;
R5 is selected from H, F, C1, Br, I, -OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, and butoxy;
alternatively, R4 and R5, when substituents on adjacent carbon
atoms, are taken together with the carbon atoms to which
they are attached to form a 5-7 membered carbocyclic
ring, said carbocyclic ring being aromatic or
nonaromatic, said carbocyclic ring being substituted
with 0-2 Rlo;
alternatively, R4 and R5, when substituents on adjacent carbon
atoms, are taken together with the carbon atoms to which
they are attached to form a 5-7 membered heterocyclic
ring containing 1, 2 or 3 heteroatoms atoms selected
from the group consisting of N, O, and S, said
heterocyclic ring being aromatic or nonaromatic, said
heterocyclic ring being substituted with 0-2 Rlo;
R6 is selected from:
H. OH, F, C1, Br, I, OCF3, -CN, N02, CHO, C(=0)CH3,
C(=O)CF3, C(=0)NH2, C(=O)NHCFi3, NR~R7a~
NR~C(=O)OR~b, C(=O)OR~, SRS, S(=O)R~, S02R~, S02NHR~,
NR~S02R~b,
_g_
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
C1_3 alkyl substituted with 0-3 R11,
C2_3 alkenyl,
C2_3 alkynyl,
Cl_3 alkoxy,
phenyl substituted with 0-2 Rlo, and
5-6 membered aromatic heterocycle system containing from
1-4 heteroatoms selected from the group consisting
of N, O, and S and substituted with 0-2 Rlo;
R~, at each occurrence, is selected from H, methyl, ethyl,
propyl, and butyl;
Rya, at each occurrence, is selected from H, methyl, ethyl,
propyl, and butyl;;
Rib. at each occurrence, is methyl, ethyl, propyl, or butyl;
R8, at each occurrence, is selected from:
H, F, C1, Br, I, CH(-OCH2CH20-),
C1_4 haloalkyl,
c:,; 6 alkyl substituted with 0-3 R11,
C2_6 alkenyl,
C3-~ cycloalkyl substituted with 0-2 R9,
phenyl substituted with 0-2 Rlo, and
5-6 membered aromatic heterocycle system containing from
1-4 heteroatoms selected from the group consisting
of N, O, and S and substituted with 0-2 Rlo;
R9, at each occurrence, is selected from D, OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, buto
xy, and F;
Rlo, at each occurrence, is selected :from OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, butoxy, F, C1,
Br, I, CN, NR~R?a, arid C (=O) CH3;
R1I, at each occurrence, is selected from ORS, CN, F, C1, Br,
I. NOZ. NR~R~a, CHO, C(=O)CH3, C(=O)NH2;
-9-
CA 02334332 2000-12-07
WO 00!00478 PCT/US99/14395
R12, at each occurrence, is selected from
C1_6 alkyl,
C2_g alkenyl,
C2_4 alkynyl,
C3-7 cycloalkyl,
phenyl substituted with 0-2 Rlo, and
5-6 membered aromatic heterocycle system containing from
1-3 heteroatoms selected from the group consisting
of N, O, and S and substituted with 0-2 Rlo,
-(CH2)pphenyl substituted with 0-2 Rlo, and
-(CH2)p(C3-5 cycloalkyl); and
p, at each occurrence, is selected from 0, 1, 2, and 3;
provided, if, simultaneously, each of W, X, Y, and Z are
carbon, then R2 is not unsubstituted C1_4 alkyl.
In a preferred embodiment, the present invention
provides a novel compound of Formula (II), wherein:
wherein:
A is 0 or S;
R3 R1 ,
R4 N R2
Cf
R5 ~ N A
s H
R
(II)
Cf is -CF3, -CF2CF3, or -CFZCF2CF3;
R1 is selected from:
-C02R12, -COR12, -S02R12, -SOR12, -CONHR12,
-(CHR~)pCHR~Re,
-(CHR~)pCH=CR7Rg,
-(CHR~)pC~C-R8,
-C1-6 alkyl substituted with 0-3 R11,
-10-
CA 02334332 2000-12-07
WO 00/00478 PCT/US9911 _4395
-(CH2)pphenyl substituted with 0-3 Rlo, and
-(CH2)p(C3_g cycloalkyl);
R2 is selected from:
-CH=CR~R8,
-C=-C-Ra ,
-CH=CHCHR~R8,
-(CHR~)pCHR~R8,
-(CHR~)pCH=CR7R8,
-(CHR7)pC~C-Rg,
-(CH2)pphenyl substituted with 0-3 R1~, and
-(CH2)p(C3_5 cycloalkyl);
R3 is selected from:
H, F, C1, Br, I, -OH, OCF3, -CN, N02, CHO, C(=0)CH3,
C (=0) CF3, C (=O) NH2. C (=O) NHCH3, NR~R~a,
NR7C (=O) OR~b, C (=O) OR7, SR7, S (=O) R7, S02R?, S02NHR7,
NR~SOZR7b,
C1_3 alkyl substituted with 0-3 Rli,
C2_3 alkenyl,
C2_3 alkynyl,
Cl_3 alkoxy,
phenyl substituted with 0-2 RlO, and
5-6 membered aromatic heterocycle system containing from
1-4 heteroatoms selected from the group consisting
of N, O, and S and substituted with 0-2 RZ~;
R4 is selected from:
H, F, C1, Br, I, -OH, OCF3, -CN, N02, CHO, C(=0)CH3,
C(=0)CF3, C(=O)NH2, C(=O)NHCH3, NR7R7n~
NR7C(=O)OR7b, C(=O)OR~, SR7, S(=O)R~, S02R~, S02NHR7,
NR~S02R~b,
C1_3 alkyl substituted with 0-3 RZl,
C2_3 alkenyl,
C2_3 alkynyl,
C1_3 alkoxy,
phenyl substituted with 0-2 R10, and
-11-
CA 02334332 2000-12-07
WO 00/00478 PGT/US99/14395
5-6 membered aromatic heterocycle system containing from
1-4 heteroatoms selected from the group consisting
of N, O, and S and substituted with 0-2 Rlo;
alternatively, R3 and R4, when substituents on adjacent carbon
atoms, are taken together with the carbon atoms to which
they are attached to form -O-CH2-O-. -0-CH2-CH2-O-, or
-CH=CH-CH=CH-;
20 R5 is selected from H. F, C1, Br, I, -OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, and butoxy;
alternatively, R4 and R5, when substituents on adjacent carbon
atoms, are taken together with the carbon atoms to which
they are attached to form -O-CH2-O-, -O-CH2-CH2-O-, or
-CH=CH-CH=CH-;
R6 is selected from:
H, OH, F, C1, Br, I, OCF3, -CN, N02. CHO, C(=O)CH3,
C(=O)CF3, C(=O)NH2, C(=O)NHCH3, NR?R7a~
NR7C ( =O ) OR7b, C ( =O ) OR7 , SR' S ( =O ) R7 , S02R7 , S02NHR7 ,
NR~S02R~b.
C1_3 alkyl substituted with 0-3 R11,
C2_3 alkenyT,
C2-3 alkynyl,
Cl_3 alkoxy,
phenyl substituted with 0-2 Rlo, and
5-6 membered aromatic heterocycle system containing from
1-4 heteroatoms selected from the group consisting
of N, O, and S and substituted with 0-2 Rlo;
R~, at each occurrence, is selected from H, methyl, ethyl,
propyl, and butyl;
R7a, at each occurrence, is selected from H, methyl, ethyl,
propyl, and butyl;;
Rib, at each occurrence, is methyl, ethyl, propyl, or butyl;
-12-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
R8, at each occurrence, is selected from:
H, F, C1, Br, I, CH(-OCHZCHZO-),
C1_4 haloalkyl,
C1_6 alkyl substituted with 0-3 R11,
C2_6 alkenyl,
C3_~ cycloalkyl substituted v,ith 0-2 R9,
phenyl substituted with 0-2 Rlo, and
5-6 membered aromatic heterocycle system containing from
1-4 heteroatoms selected from the group consisting
of N, 0, and S and substituted with 0-2 Rlo;
R9, at each occurrence, is selected from D, OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, butoxy, and F;
R1~, at each occurrence, is selected from OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, butoxy, F, C1,
Br, I, CN, NR7R~a, and C(=0)CH3;
R11, at each occurrence, is selected from ORS, CN, F, C1, Br,
I, N02, NR?R~8, CHO, C(=O)CH3, Cu':7)NH2;
R12, at each occurrence, is selected from
C1_6 alkyl,
C2_4 alkenyl,
C2_4 alkynyl,
C3_~ cycloalkyl,
phenyl substituted with 0-2 Rlo, and
5-6 membered aromatic heterocycle system containing from
1-3 heteroatoms selected from the group consisting
of N, 0, and S and substituted with 0-2 Rlo
-(CH2)pphenyl substituted with 0-2 Rlo, and
-(CH2)p(C3_5 cycloalkyl); and
p, at each occurrence, is selected from 0, l, 2, and 3.
In a further preferred embodiment, the present invention
provides a novel compound of Formula (II), wherein:
-13-
CA 02334332 2000-12-07
WO 00!00478 PCT/US99l14395
A is 0 or S;
Cf is -CF3, -CF2CF3, or -CF2CF2CF3;
R1 is selected from:
-COZR12, -COR12. -S02R12~ -SOR12, -CONHR12,
-(CHR~)pCHR~R8,
-(CHR~)pCH=CR~RB,
-(CHR~)pC=C-Ra,
-C1-5 alkyl substituted with 0-3 R11,
-(CH2)pphenyl substituted with 0-3 Rla, and
_(CH2)p(C3_5 cycloalkyl);
R2 is selected from:
-CH=CR~R8,
-C=C-R8,
-CH=CHCHR~R8,
-(CHR~)pCHR~Rg,
-(CHR~)pCH=CR~RB,
-(CHR~)pC=C-R8,
-(CH2)pphenyl substituted with 0-3 R1~, and
-(CHZ)p(C3_5 cycloalkyl);
R3 is selected from:
H, F, C1, Br, I, -OH, -OCF3, -CN, -N02, -CHO, -C (=O) CH3,
-C(=O)CF3, -C(=O)NH2, -C(=0)NHCH3, -NH2, -NHCH3.
-N(CH3)2, -NHC(=O)OCH3, -C(=0)OCH3, -SCH3,
-S(=0)CH3, -S02CH3, -S02NHCH3, -NHSOZCH3,
C1_3 alkyl substituted with 0-3 R11,
C2_3 alkenyl,
C2_3 alkynyl,
C1_3 alkoxy,
R4 is selected from:
H, F, C1, Br, I, -OH, OH, -OCF3, -CN, -N02, -CHO,
-C(=O)CH3, -C(=O)CF3. -C(=0)NH2. -C(=0)NHCH3, -NH2.
-NHCH3, -NHCH2CH3, -N(CH3)2. -N(CH2~3)2.
-14-
CA 02334332 2000-12-07
WO 00100478 PCTIUS99/1.4395
-NHC(=O)OCH3, -NHC(=0)OCH2CH3, -C(=O)OCH3,
-C(=0)OCH2CH3, -SCH3, -SCH2CH3, -S(=O)CH3,
-S(=0)CHZCH3, -S02H, -SOzCHg, -SOZCH2CH3, -S02NHCH3,
-S02NHCH2CH3, -NHS02CH3, -NHS02CH2CH3,
C1_3 alkyl substituted with 0-3 R11,
C2_3 alkenyl,
C2_3 alkynyl,
C1_3 alkoxy,
alternatively, R3 and R4, when substituents on adjacent carbon
atoms, are taken together with the carbon atoms to which
they are attached to form -0-CH2-O-, -0-CH2-CH2-O-, or
-CH=CH-CH=CH-;
R5 is selected from H, F, C1, Br, I, -OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, and butoxy;
alternatively, R4 and R5, when substituents on adjacent carbon
atoms, are taken together with the carbon atoms to which
they are attached to form -0-CH2-O-, -O-CH2-CH2-O-, or
R6 is selected from:
H, F, C1, Br, I, -OH, -OCF3, -CN, -N02, -CHO, -C(=O)CH3,
2 5 -C ( =O ) CF 3 , -C ( =O ) NH2 , -C ( =0 ) NHCH3 , -NHZ , -NHCH3 ,
-N(CH3)2, -NHC(=O)OCH3, -C(=0)OCH3, -SCH3,
-S(=0)CH3, -S02CH3, -S02NHCH3, -NHS02CH3,
C1_3 alkyl substituted with 0-3 R11
C2_3 alkenyl,
C2-3 alkynyl,
C1_3 alkoxy,
R~, at each occurrence, is selected from H, methyl, ethyl,
propyl, and butyl;
Rya, at each occurrence, is selected from H, methyl, ethyl,
propyl, and butyl;;
-15-
CA 02334332 2000-12-07
WO 00/00478 PCTIUS9911_4395
R8, at each occurrence, is selected from:
H, F, C1, Br, I, CH(-OCHZCH20-),
C1_q haloalkyl,
C1_6 alkyl substituted with 0-3 R11,
C2_6 alkenyl,
C3_~ cycloalkyl substituted with 0-2 R9,
phenyl substituted with 0-2 Rlo, and
5-6 membered aromatic heterocycle system containing from
1-3 heteroatoms selected from the group consisting
of N, O, and S and substituted with 0-2 Rlo
R9, at each occurrence, is selected from D, OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, butoxy, and F;
R1~, at each-occurrence, is selected from OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, butoxy, F, C1,
Br, I, CN, NR~R~a, and C(=O)CH3
R11, at each occurrence, is selected from ORS, CN, F, C1, Br,
I, N02, NR~R7a, CHO, C(=O)CH3, C(=O)NH2;
R12, at each occurrence, is selected from
C1_6 alkyl,
C2_4 alkenyl,
C2_q alkynyl,
C3_~ cycloaikyl,
phenyl substituted with 0-2 Rlo, and
5-6 membered aromatic heterocycle system containing from
1-3 heteroatoms selected from the group consisting
of N, O, and S and substituted with 0-2 Rlo,
-(CH2)pphenyl substituted with 0-2 Rlo, and
-(CHZ)ptC3_5 cycloalkyl); and
p, at each occurrence, is selected from 0, 1, 2, and 3.
In a more further preferred ennbodiment, the present
invention provides a novel compound of Formula (II), wherein:
-16-
CA 02334332 2000-12-07
WO OOI00478 PGT/US99/14395
A is O;
Cf is -CF3 or -CF2CF3%
R1 is selected from:
-C02R12, -COR12, _g02R12~
-(CHR~)pCHR~Ra,
-(CHR~)pCH=CR~RB,
-(CHR~)pC=-C-R8,
-C1-5 alkyl substituted with 0-3 R~l,
-(CH2)pphenyl substituted with 0-3 Rl~, and
-(CH2)p(C3_5 cycloalkyl);
R2 is selected from:
-CH=CR~RB,
-C---C-R8 ,
-CH=CHCHR~R8,
-(CHR~)pCHR~RS.
-(CHR~)pCH=CR~RB,
-(CHR~)pC=C-R8,
-(CH2)pphenyl substituted with 0-3 Rl~, and
-(CH2)p(C3_5 cycloalkyl)%
R3 is selected from:
H, F, C1, Br, I, -OH, -OCF3, -CN, -N02, -CHO, -C(=O)CH3,
-C(=0)CF3, -NH2, -NHCH3, -N(CH3)2. -CF3, -CH3,
-CH2CH3, -OCH3. and -OCH2CH3,
R4 is selected from:
H, F, CI, Br, I, -OH, OH, -OCF3, -CN, -NOa, -CHO,
-C(=0)CH3. -C(=O)CF3, -C(=O)NH2, -C(=0)NHCH3, -NH2,
-NHCH3, -N(CH3)2. -NHC(=0)OCH3, -C(=0)OCH3, -CF3,
-CH3. -CH2CH3, -OCH3, and -OCH2CH3%
RS is selected from H, F, C1, Br, I, -OH, -CH3, -CH2GH3,
-OCH3, and -OCH2CH3;
R6 is selected from:
-17-
CA 02334332 2000-12-07
WO 00100478 PCT/US991~4395
H, F, C1, Br, I, -OH, -OCF3, -CN, -N02, -CHO, -C(=0)CH3,
-C(=O)CF3, -NH2, -NHCH3, -N(CH3)z, -CF3, -CH3,
-CH2CH3. -OCH3, and -OCH2CH3;
R~, at each occurrence, is selected from H, methyl, ethyl,
propyl, and butyl;
R8, at each occurrence, is selected from:
H, F, C1, Br, I, CH(-OCH2CH20-),
C1_4 haloalkyl,
C1_4 alkyl substituted with 0-3 R11,
C2_q alkenyl,
C3_6 cycloalkyl substituted with 0-2 R9,
phenyl substituted with 0-2 R1~, and
5-6 membered aromatic heterocycle system containing from
1-3 heteroatoms selected from the group consisting
of pyridinyl, furanyl, thienyl, pyrrolyl,
pyrazolyl, imidazolyl, and oxazolidinyl;
R9, at each occurrence, is selected from D, OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, butoxy, and F;
R1~, at each occurrence, is selected from OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, butoxy, F, C1,
Br, I, CN, -NH2. -rlHCH3. -NHCH2CHg, -N(CH3)2, -N(CH2CH3)2,
and C(=0)CH3;
R11, at each occurrence, is selected from ORS, CN, F, C1, Br,
I, N02, -NH2, -NHCH3, -NHCHZCH3, -N(CH3)2, -N(CH2CH3)2.
CHO, C(=O)CH3, C(=O)NH2;
R12, at each occurrence, is selected from
C1_6 alkyl,
C2_4 alkenyl,
C2_4 alkynyl,
C3_6 cycloalkyl,
phenyl substituted with 0-2 R10, and
-18-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
5-6 membered aromatic heterocycle system containing from
1-3 heteroatoms selected from the group consisting
pyridinyl, furanyl, thienyl, pyrrolyl, pyrazolyl,
imidazolyl, and oxazolidinyl,
-(CH2)pphenyl substituted with 0-2 R1~, and
-(CH2)p(C3_S cycloalkyl); and
p, at each occurrence, is selected from 0, 1, and 2.
In an even more further preferred embodiment, the
present invention provides a novel compound of Formula (III);
R3 R1
R4 N Rz
/ ~ CF3
RS ~ H O
R6
(III)
wherein:
R1 is selected from:
-CF3, -CF2H, -CH3, -CH2CH3, -CH2CH2CH3,
-CH2CH2CH2CH3, -CH(CH3)2, -CH2CH(CH3)Z, -CH2CH2C(CH3)3,
-CH2CH2CH(CH3)CH3,
-CH(=CH2)CH3, -CH2CH=CH2, -CH2-CH=C(CH3)2, -CHZ-C=CH,
-CHZ-C--_CCH3, -CH2Ph, -cycPr, -CH2cycPr, -CHZCH2cycPr,
-COZCH3, -C02CH2CH3, -C02CH2CH2CH3, -COZCH2CH2CH2CH3.
-COZCH(CH3)2, -C02CH2CH(CH3)2, -C02CH2Ph, -C02cycPr,
-C02CH2cycPr, -C02CHZCH=CHz, -S02CH2CH3, -SOZCH(CH3)2,
-COCH3, -COCH2CH3, -COCH2CH2CH3, -COCH(CH3)2, and
-COCH2cycPr;
R2 is selected from:
benzyl, phenethyl, -CH2CH2cycPr,
-CSC-CH3, -CSC-CFg, -CSC-Et, -C~-iPr, -C~-cycPr,
-19-
CA 02334332 2000-12-07
WO 00!004?8 PCT/US99/i_4395
-C=C-1-(CH3)cycPr, -C-~-CH=CH2, -C=C-C(=CH2)CH3,
-CH=CH-CH3, -CH=CH-CF3, -CH=CH-Et, -CH=CH-iPr,
-CH=CH-cycPr, -CH~H-CH=CH2, -CH2-C~-CH3,
-CH2-C=C-CF3, -CH2-C=C-Et, -CH2-C---C-iPr,
-CH2-C---C-cycPr, -CH2-C---C-CH=CH2, -CH2-CH=CH2,
-CH2-CH=CH-CH3, -CH2-CH=CH-CF3, -CHZ-CH=CH-Et,
-CH2-CH=CH-iPr, -CH2-CH=CH-cycPr, -CH2-CH=CH-CH=CH2,
-CH2-CH=C(CH3)2, and -CH=CH-CH2-cycPr;
R3 is selected from:
H, F, C1, Br, I, -OH, -OCF3, -CN, -NOz, -C(=O)CH3,
-C(=O)CF3, -NH2, -NHCH3, -N(CH3)2, -CF3, -CH3,
-CH2CH3, -OCH3, and -OCH2CH3,
R4 is selected from:
H, F, C1, Br, I, -OH, OH, -OCF3, -CN, -N02, -C(=0)CH3,
-C(=O)CF3, -C(=O)NH2, -C(=0)NHCH3, -NH2, -NHCH3,
-N(CH3)2, -NHC(=0)OCH3, -C(=O)OCH3, -CF3, -CH3,
W2~3 ~ -OCH3 , and -OCH2CH3 ;
25
R5 is selected from H, F, and C1; and
R6 is selected from:
H, F, Cl -OH, -OCF3, -CF3, -CH3, and -OCH3.
In a further preferred embodiment, a compound of the
present invention is selected from:
4-(cyclopropylmethyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(methyl)-3-(2-cyclopropylethynyl)-3-(trifluoromethyl)-3,4-
dihydro-quinoxalin-2(1H)-one;
3-(n-butyl)-3-(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-
one;
-20-
CA 02334332 2000-12-07
WO 00/00478 PGT/US99/14395
4-(methyl)-3-(n-butyl)-3-(trifluoromethyl)-3,4-dihydro-
quinoxalin-2(1H)-one;
3-(2-cyclopropylethynyl)-3-(trifluoromethyl)-3,4-dihydro-
quinoxalin-2(1H)-one;
3-(allyl)-3-(trifluoromethyl)-3,4-dihydro-guinoxalin-2(1H)-
one;
4-(allyl)-3-(2-cyclopropylethynyl)-3-(trifluoromethyl)-3,4-
dihydro-quinoxalin-2(1H)-one;
4-(benzyl)-3-(2-cyclopropylethynyl)-3-(trifluoromethyl)-3,4-
dihydro-quinoxalin-2(1H)-one;
4-(cyclopropylmethyl)-3-(allyl)-3-(trifluoromethyl)-3,4-
dihydro-quinoxalin-2(1H)-one;
4-(propargyl)-3-(2-cyclopropylethynyl)-3-(trifluoromethyl)-
3,4-dihydro-quinoxalin-2(1H)-one;
4-(cyclopropylethyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(isopropyl)-3-(2-cyclopropylethynyl)-3-(trifluoromethyl)-
3,4-dihydro-quinoxalin-2(1H)-one;
6-(fluoro)-4-(allyl)-3-(n-butyl)-3-(trifluoromethyl)-3,4-
dihydro-quinoxalin-2(1H)-one;
6-(fluoro)-4-(allyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(fluoro)-4-(cyclopropylmethyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(fluoro)-4-(cyclopropylmethyl)-3-(n-butyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
-21-
CA 02334332 2000-12-07
WO 00!00478 PCTNS99/14395
6-(chloro)-4-(cyclopropylmethyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(chloro)-4-(isobutyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(chloro)-4-(ally!)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(chloro)-4-(cyclopropylmethyl)-3-(phenethyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(chloro)-4-(ally!)-3-(phenethyl)-3-(trifluoromethyl)-3,4-
dihydro-quinoxalin-2(1H)-one;
6-(methoxy)-4-(cyclopropylmethyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(methoxy)-4-(ally!)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(cyclopropylmethyl)-3-(1-propynyl)-3-(trifluoromethyl)-3,4-
dihydro-quinoxalin-2(1H)-one;
4-(ally!)-3-(1-propynyl)-3-(trifluoromethyl)-3,4-dihydro-
quinoxalin-2(1H)-one;
4-(ethoxycarbonyl)-3-(2-cyclopropylethynyl)-3
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(ethoxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(isopropoxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
-22-
CA 02334332 2000-12-07
WO 00100478 PCT/US99/14395
4-(propen-2-yl-oxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(isobutoxycarbanyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(n-butoxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(allyloxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(benzyloxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(n-propylsulfonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(phenylcarbonyl)-3-(2-cyclopropylethynyl)-3
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(neopentyl-oxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(2-propynyl-oxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(isopropylcarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(cyclopropylcarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(iH)-one;
4-(ethylsulfonyl)-3-(2-cyclopropylethynyl)-3
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
4-(isopropylsulfonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
-23-
CA 02334332 2000-12-07
WO 00100478 PCT/US99/14395
4-(methoxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(chloro)-4-(ethoxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(chloro)-4-(isopropoxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(chloro)-4-(propen-2-yI-oxycarbonyl)-3-(2-
cyclopropylethynyl)-3-(trifluoromethyl)-3,4-dihydro-
quinoxalin-2(1H)-one;
6-(fluoro)-4-(ethoxycarbonyl)-3-(2-cyclopropylethynyl)-3
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one;
6-(fluoro)-4-(isopropoxycarbonyl)-3-(2-cyclopropylethynyl)-3-
(trifluoromethyl)-3,4-dihydro-guinoxalin-2(1H)-one; and
6-(fluoro)-4-(propen-2-yl-oxycarbonyl)-3-(2-cyclopropylethynyl;-3-
(trifluoromethyl)-3,4-dihydro-quinoxalin-2(1H)-one.
In a most preferred embodiment, the present invention
provides a novel compound of Formula (I), Formula (II) or
Formula (III), or a stereoisomer or pharmaceutically
acceptable salt form thereof, wherein R1, Cf, A, W, X, Y, and
Z are as defined above; and R2 is -C=C-R8 or -(CHR~)pC=C-R8.
In another preferred embodiment, the present invention
provides a compound of Formula (IIb):
R1
R2
~W N
Cf
Z N A
H
(IIb)
wherein:
-24-
CA 02334332 2000-12-07
WO 00100478 PCT/US99/14395
A is O or S;
W is N or CR3 ;
X is N or CR4;
Y is N or CRS;
Z is N or CR6;
Cf is -CF3, -CF2CF3, or -CF2CF2CF3;
provided that one or two of W, X, Y, and Z are N;
R1 is selected from:
-C02R12, -COR12, -S02R12,
-(CHR~)pCHR~R8,
-(CHR~)pCH=CR~R8,
-(CHR~)pC~C-R8,
-C1_5 a~ yl substituted with 0-3 R11,
-(GH2)pphenyl substituted with 0-3 R1~, and
-(CH2)p(C3_5 cycloalkyl);
R2 is selected from:
-CH=CR~R8,
-C=-C-RS ,
-CH=CHCHR7R8 ,
-(CHR~)pCHR~R8,
-(CHR7)pCH=CR~RB,
-(CHR~)pC~C-R8,
-(CHZ)pphenyl substituted with 0-3 R10, and
-(CH2)p(C3_5 cycloalkyl);
R3 is selected from:
H, F, C1, Br, I, -OH, -OCF3, -CN, -N02, -CHO, -C(=0)CH3,
-C(=O)CF3, -NH2, -NHCH3, -N(CH3)2, -CF3, -CH3,
-CH2CH3, -OCH3, and -OCH2CH3,
-25-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
R4 is selected from:
H, F, C1, Br, I, -OH, OH, -OCF3, -CN, -N02, -CHO,
-C(=0)CH3, -C(=O)CF3, -C(=0)NH2, -C(=0)NHCH3, -NH2,
-NHCH3, -N(CH3)2, -NHC(=O)OCH3, -C(=O)OCH3, -CF3,
-CH3, -CHzCH3, -OCH3, and -OCH2CH3;
RS is selected from H, F, C1, Br, I, -OH, -CH3, -CH2CH3,
-OCH3, and -OCH2CH3;
15
R6 is selected from:
H, F, C1, Br, I, -OH, -OCF3, -CN, -NO2, -CHO, -C(=O)CH3,
-C(=0)CF3, -NH2, -NHCH3, -N(CH3)2, -CF3, -CH3,
-CH2CH3, -OCH3, and -OCH2CH3;
R~, at each occurrence, is selected from H, methyl, ethyl,
propyl, and butyl;
Re, at each occurrence, is selected from:
H, F, C1, Br, I, CH(-OCH2CH20-),
C1_4 haloalkyl,
C1_q alkyl substituted with 0-3 R11,
C2_4 alkenyl,
C3_6 cycloalkyl substituted with 0-2 R9,
phenyl substituted with 0-2 R1~, and
5-6 membered aromatic heterocycle system containing from
1-3 heteroatoms selected from the group consisting
of pyridinyl, furanyl, thienyl, pyrrolyl,
pyrazolyl, imidazolyl, and oxazolidinyl;
R9, at each occurrence, is selected from D, OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, butoxy, and F;
R1~, at each occurrence, is selected from OH, methyl, ethyl,
propyl, butyl, methoxy, ethoxy, propoxy, butoxy, F, C1,
Br, I, CN, -NH2, -NHCH3, -NHCH2CH3, -N(CH3)2, -N(CH2CH3)2,
and C(=O)CH3;
-26-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Rii, at each occurrence, is selected from ORS, CN, F, C1, Br,
I , N02 , -NH2 , -NHCH3 , -NHCH2CH3 , -N (CH3 } 2 . -N ( CH2CH3 ) 2 .
CHO, C ( =O ) CH3 , C ( =O ) NH2 ;
Riz, at each occurrence, is selected from
C1_6 alkyl,
C2_~ alkenyl,
C2_4 alkynyl,
C3_6 cycloalkyl,
phenyl substituted with 0-2 R1~, and
5-6 membered aromatic heterocycle system containing from
1-3 heteroatoms selected from the group consisting
pyridinyl, furanyl, thienyl, pyrrolyl, pyrazolyl,
imidazolyl, and oxazolidinyl,
-(CH2)pphenyl substituted with 0-2 R1~, and
-(CH2)p(C3-5 cycloalkyl); and
p, at each occurrence, is selected from 0, 1, and 2.
In more preferred embodiment, the present invention
provides a compound of Fort., ~_a (IIIa}
R1
Rz
X ~W N
I ~ ~CF3
Y~Z N O
H
(IIIb)
wherein:
R1 is selected from:
-CF3, -CF2H. -CH3, -CH2CH3, -CH2CH2CH3,
-CH2CH2CHZCH3, -CH(CH3)2. -CH2CH(CH3)2. -CH2CH2C(CH3)3,
-CH2CHZCH(CH3}CH3,
-CH(=CH2)CH3, -CH2CH=CH2, -CH2-CH~(CH3)2. -CH2-C=CH,
-CHZ-C=CCH3, -CH2Ph. -cycPr, -CHZCycPr. -CH2CH2cycPr,
-27-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
-C02CH3, -C02CH2CH3, -COZCH2CH2CH3; -C02CH2CH2CH2CH3,
-C02CH(CH3)2, -C02CH2CH(CH3)2, -COZCHZPh, -C02cycPr,
-C02CH2cycPr, -C02CH2CH=CH2, -S02CHZCH3, -S02CH(CH3)2,
-COCH3, -COCH2CH3. -COCHZCH2CH3, -COCH(CH3)2, and
-COCH2cycPr;
R2 is selected from:
benzyl, phenethyl, -CH2CHZCycPr,
-C=C-CH3, -C=C-CF3, -C=C-Et, -C~-iPr, -C~-cycPr,
-CSC-1-{CH3)cycPr, -CSC-CH=CHZ, -C=_C-C(=CH2)CH3,
-CH=CH-CH3, -CH~Fi-CF3, -CH=CH-Et, -CH=CH-iPr,
-CH=CH-cycPr, -CH~H-CH=CH2, -CHZ-C~-CH3.
-CH2-C=C-CF3, -CH2-C=C-Et, -CH2-C---C-iPr,
-CH2-C=C-cycPr, -CH2-C=C-CH=CH2, -CH2-CH--CH2,
-CHZ-CH=CH-CH3, -CH2-CH=CH-CF3, -CH2-CH=CH-Et,
-CH2-CH=CH-iPr, -CHZ-CH=CH-cycPr, -CHZ-CH=CH-CH=CH2,
-CH2-CH=C(CH3)2, and -CH=CH-CH2-cycPr;
R3 is selected from:
H, F, C1, Br, I, -OH, -OCF3. -CN, -N02, -C(=O)CH3,
-C ( =O ) CF3 , -NHZ . -NH~~Ei3 . -N ( CH3 ) 2 , -CF3 . -CH3 ,
-CH2CH3, -OCH3, and -OCHZCH3,
R4 is selected from:
H, F, C1, Br, I, -OH, OH, -OCF3, -CN, -NOZ, -C(=0)CH3,
-C(=O)CF3, -C(=O)NH2, -C(=O)NHCH3, -NH2, -NHCH3,
-N(CH3)2, -NHC(=0)OCH3, -C(=O)OCH3, -CF3, -CH3,
-CH2CH3. -OCH3, arid -OCHZCH3~
R5 is selected from H, F, and C1; and
R6 is selected from:
H, F, CI -OH, -OCF3, -CF3, -CH3, and -OCH3~
In another preferred embodiment, the present invention
provides a compound of Formula (Ia) or (Ib):
-28-
CA 02334332 2000-12-07
WO 00/00478 PC'T/US99/14395
RI R1
,W N R2 ,W N , RZ
X~ ~ ..~Cf X~ ~ ~Cf
Y~~ Yes.
Z H A Z H A
Ia Ib
or a stereoisomer or pharmaceutically acceptable salt form
thereof.
In a second embodiment, the present invention provides a
novel pharmaceutical composition comprising a
pharmaceutically acceptable carrier and a therapeutically
effective amount of a compound of formula (I) or
IO pharmaceutically acceptable salt form thereof.
In a third embodiment, the present invention provides a
novel method for treating HIV infection which comprises
administering to a host in need of such treatment a
therapeutically effective amount of a compound of formula (I)
or pharmaceutically acceptable salt form thereof.
In a fourth embodiment, the present inw~ntion provides a
novel method of treating HIV infection which comprises
administering, in combination, to a host in need thereof a
therapeutically effective amount of:
(a) a compound of Formula (I); and,
(b) at least one compound selected from the group
consisting of HIV reverse transcriptase inhibitors and HIV
protease inhibitors.
In another preferred embodiment, the reverse
transcriptase inhibitor is a nucleoside reverse transcriptase
inhibitor.
In another more preferred embodiment, the HIV reverse
transcriptase inhibitor is selected from AZT, 3TC,
rescriptor, ddI, ddC, efavirenz, and d4T and the protease
-29-
CA 02334332 2000-12-07
WO 00/00478 PCTlUS99/14395
inhibitor is selected from saquinavir, ritonavir, indinavir,
VX-478, nelfinavir, KNI-272, CGP-61755, and U-103017.
In an even more preferred embodiment, the HIV reverse
transcriptase inhibitor is selected from AZT, rescriptor,
efavirenz, and 3TC and the protease inhibitor is selected
from saquinavir, ritonavir, indinavir, and nelfinavir.
In a still further preferred embodiment, the nucleoside
reverse transcriptase inhibitor is AZT.
In another still further preferred embodiment, the HIV
reverse transcriptase inhibitor is efavirenz.
In another still further preferred embodiment, the
protease inhibitor is indinavir.
In a fifth embodiment, the present invention provides a
pharmaceutical kit useful for the treatment of HIV infection,
which comprises a therapeutically effective amount of:
(s) a comp~eund of Formula (I); and,
(b) at least one compound selected from the group
consisting of HIV reverse transcriptase inhibitors and HIV
protease inhibitors, in one or more sterile containers.
In a sixth embodiment, the present invention provides a
novel method of inhibiting HIV present in a body fluid sample
which comprises treating the body fluid sample with an
effective amount of a compound of Formula (I).
In a seventh embodiment, the present invention to
provides a novel a kit or container comprising a compound of
formula (I) in an amount effective for use as a standard or
reagent in a test or assay for determining the ability of a
potential pharmaceutical to inhibit HIV reverse
transcriptase, HIV growth, or both.
-30-
CA 02334332 2000-12-07
WO 00/00478 PC'TJUS99/14395
DEFINITIONS
As used herein, the following terms and expressions have
the indicated meanings. It will be appreciated that the
compounds of the present invention contain an asymmetrically
substituted carbon atom, and may be isolated iri optically
active or racemic forms. It is well known in the art how to
prepare optically active forms, such as by resolution of
racemic forms or by synthesis, from optically active starting
materials. All chiral, diastereomeric; racemic forms and all
geometric isomeric forms of a structure are intended, unless
the specific stereochemistry or isomer form is specifically
indicated.
The processes of the present invention are contemplated
to be practiced on at least a multigram scale. kilogram
scale, multikilogram scale, or industrial scale. Multigram
scale, as used herein, is preferably the scale wherein at
least one starting material is present in 10 grams or more,
more preferably at least 50 grams or more, even more
preferably at least 100 grams or more. Multikilogram scale,
as used herein, is intended to mean the scale wherein more
than one kilogram of at least one starting material is uses.
Industrial scale as used herein is intended to mean a scale
which is other than a laboratory scale and which is
sufficient to supply product sufficient for either clinical
tests or distribution to consumers.
The reactions of the synthetic methods claimed herein
may be, as noted herein, carried out in the presence of a
suitable base, said suitable base being any of a variety of
bases, the presence of which in the reaction facilitates the
synthesis of the desired product. Suitable bases may be
selected by one of skill in the art of organic synthesis.
Suitable bases include, but are not intended to be limited
to, inorganic bases such as alkali metal, alkali earth metal,
thallium, and ammonium hydroxides, alkoxides, phosphates, and
carbonates, such as sodium hydroxide, potassium hydroxide.
sodium carbonate, potassium carbonate, cesium carbonate,
thallium hydroxide, thallium carbonate, tetra-n-butylamanonium
carbonate, and ammonium hydroxide. Suitable bases also
-31-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
include organic bases, including but not limited to aromatic
and aliphatic amines, such as pyridine; trialkyl amines such
as triethylamine, N,N-diisopropylethylamine,
N,N-diethylcyclohexylamine, N,N-dimethylcyclohexyl~mine,
N,N,N'-triethylenediamine, N,N-dimethyloctylamine;
1,5-diazabicyclo[4.3.0]non-5-ene (DBN);
1,4-diazabicyclo(2.2.2]octane (DABCO);
1,8-diazabicyclo[5.4.0]undec-7-ene (DBU);
tetramethylethylenediamine (TMEDA); and substituted pyridines
such as N,N-dimethylaminopyridine (DMAP),
4-pyrrolidinopyridine, 4-piperidinopyridine.
Suitable halogenated solvents include: carbon
tetrachloride, bromodichloromethane, dibromochloromethane,
bromoform, chloroform, bromochloromethane, dibromomethane,
butyl chloride, dichloromethane, tetrachloroethylene,
trichloroethylene, 1,1,1-trichloroethane, 1,1,2-
trichloroethane, 1,1-dichloroethane, 2-chloropropane,
hexafluorobenzene, 1,2,4-trichlorobenzene, o-dichlorobenzene,
chlorobenzene, or fluorobenzene.
Suitable ether solvents include, but are not intended to
.~e limited to, dimethoxymethane, tetrahydrofuran, 1,3-
dioxane, 1,4-dioxane, furan, diethyl ether, ethylene glycol
dimethyl ether, ethylene glycol diethyl ether, diethylene
glycol dimethyl ether, diethylene glycol diethyl ether,
triethylene glycol dimethyl ether, or t-butyl methyl ether.
Suitable protic solvents may include, by way of example
and without limitation, water, methanol, ethanol, 2-
nitroethanol, 2-fluoroethanol, 2,2,2-trifluoroethanol,
ethylene glycol, 1-propanol, 2-propanol, 2-methoxyethanol, 1-
butanol, 2-butanol, i-butyl alcohol, t-butyl alcohol, 2-
ethoxyethanol, diethylene glycol, 1-, 2-, or 3- pentanol,
neo-pentyl alcohol, t-pentyl alcohol, diethylene glycol
monomethyl ether, diethylene glycol monoethyl ether,
cyclohexanol, anisole, benzyl alcohol, phenol, or glycerol.
Suitable aprotic solvents may include, by way of example
and without limitation, tetrahydrofuran (THF),
dimethylformamide (DNg'), dimethylacetamide (DMAC), 1,3-
dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU), 1,3-
-32-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99J14395
dimethyl-2-imidazolidinone (DMI), N-methylpyrrolidinone
(NMP), formamide, N-methylacetamide, N-methylformamide,
acetonitrile, dimethyl sulfoxide, propionitrile, ethyl
formate, methyl acetate, hexachloroacetone, acetone, ethyl
methyl ketone, ethyl acetate, sulfolane, N,N-
dimethylpropionamide, tetramethylurea, nitromethane,
nitrobenzene, or hexamethylphosphoramide.
Suitable hydrocarbon solvents include, but are not
intended to be limited to, benzene, cyclohexane, pentane,
hexane, toluene, cycloheptane, methylcyclohexane, heptane,
ethylbenzene, m-, o-, or p-xylene, octane, indane, nonane, or
naphthalene.
As used herein, the term "amine protecting group" (or
"N-protected") refers to any group known in the art of
organic synthesis for the protection of amine groups. As
used herein, the term "amine protecting group reagent" refers
to any reagent known in the art of organic synthesis for the
protection of amine groups which may be reacted with an amine
to provide an amine protected with an amine protecting group.
Such amine protecting groups include those listed in Greene
and Wuts, "Protective Groups in Organic Synthesis" John Wiley
& Sons, New York (1991) and "The Peptides: Analysis,
Synthesis, Biology, Vol. 3, Academic Press, New York (1981),
the disclosure of which is hereby incorporated by reference.
Examples of amine protecting groups include, but are not
limited to, the following: 1) acyl types such as formyl,
trifluoroacetyl, phthalyl, and p-toluenesulfonyl; 2) aromatic
carbamate types such as benzyloxycarbonyl (Cbz) and
substituted benzyloxycarbonyls, 1-(p-biphenyl)-1-
methylethoxycarbonyl, and 9-fluorenylmethyloxycarbonyl
(Fmoc); 3) aliphatic carbamate types such as tert-
butyloxycarbonyl (Boc), ethoxycarbonyl,
diisopropylmethoxycarbonyl, and allyloxycarbonyl; 4) cyclic
alkyl carbamate types such as cyclopentyloxycarbonyl and
adamantyloxycarbonyl; 5) alkyl types such as triphenylmethyl
(trityl) and benzyl; 6) trialkylsilane such as
trimethylsilane; and 7) thiol containing types such as
phenylthiocaxbonyl and dithiasuccinoyl.
-33-
CA 02334332 2000-12-07
WD 00/00478 PCT/US99114395
Amine protecting groups may include, but are not limited
to the following: 2,7-di-t-butyl-[9-(10,10-dioxo-10,10,10,10-
tetrahydrothio-xanthyl))methyloxycarbonyl; 2-trimethylsilyl-
ethyloxycarbonyl; 2-phenylethyloxycarbonyl; 1,1-dimethyl-2,2-
dibromoethyloxycarbonyl; 1-methyl-1-(4-biphenylyl)-
ethyloxycarbonyl; benzyloxycarbonyl; p-nitrobenzyl-
oxycarbonyl; 2-(p-toluenesulfonyl)ethyloxy-carbonyl;
m-chloro-p-acyloxybenzyloxycarbonyl; 5-benzyisoxazolyl-
methyloxycarbonyl; p-(dihydroxyboryl)benzyloxycarbonyl;
m-nitrophenyloxycarbonyl; o-nitrobenzyloxycarbonyl;
3,5-dimethoxybenzyloxycarbonyl; 3,4-dimethoxy-6-nitrobenzyl-
oxycarbonyl; N'-p-toluenesulfonylaminocarbonyl; t-amyloxy-
carbonyl; p-decyloxybenzyloxycarbonyl; diisopropylmethyloxy-
carbonyl; 2,2-dimethoxycarbonylvinyloxycarbonyl; di(2-
pyridyl)methyloxycarbonyl; 2-furanylmethyloxycarbonyl;
phthalimide; dithiasuccinimide; 2,5-dimethylpyrrole; benzyl;
5-dibenzylsuberyl; triphenylmethyl; benzylidene;
diphenylmethylene; or methanesulfonamide.
As used herein, "alkyl" is intended to include both
branched and straight-chain saturated aliphatic hydrocarbon
groups having the specified number of carbon atoms; for
example, "C1-6 alkyl" denotes alkyl having 1 to 6 carbon
atoms, ie. methyl, ethyl, propyl, butyl, pentyl, hexyl, and
branched isomers therin.. Examples of alkyls include, but
are not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, i-butyl, s-butyl, t-butyl, i-pentyl, n-pentyl, and
s-pentyl. "Haloalkyl" is intended to include both branched
and straight-chain saturated aliphatic hydrocarbon groups
having the specified number of carbon atoms, substituted with
1 or more halogen (for example -CvFW where v = 1 to 3 and w =
1 to (2v+1)). Examples of haloalkyl include, but are not
limited to, trifluoromethyl, trichloromethyl,
pentafluoroethyl, pentachloroethyl, 2,2,2-trifluoroethyl,
heptafluoropropyl, and heptachloropropyl. "Alkoxy~
represents an alkyl group as defined above with the indicated
number of carbon atoms attached through an oxygen bridge.
Examples of alkoxy include, but are not limited to, methoxy,
ethoxy, n-propoxy, i-propoxy, n-butoxy, s-butoxy, t-butoxy,
-34-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
n-pentoxy, and s-pentoxy. "Cycloalkyl" is intended to
include saturated ring groups, such as cyclopropyl,
cyclobutyl, cyclopentyl, or cyclohexyl. "Alkenyl" is
intended to include hydrocarbon chains of either a straight
or branched configuration and one or more unsaturated
carbon-carbon bonds which may occur in any stable point along
the chain, such as ethenyl, propenyl, butenyl and the like.
"Alkynyl" is intended to include hydrocarbon chains of either
a straight or branched configuration and one or more triple
carbon-carbon bonds which may occur in any stable point along
the chain, such as ethynyl, propynyl, butynyl and the like.
"Halo" or "halogen" as used herein refers to fluoro,
chloro, bromo and iodo. "Counterion" is used to represent a
small, negatively charged species such as chloride, bromide,
hydroxide, acetate, sulfate and the like.
As used herein, "aryl" or "aromatic residue" is intended
to mean an aromatic moiety containing the specified number of
carbon atoms, such as phenyl or naphthyl. As used herein,
"carbocycle" or "carbocyclic residue" is intended to mean any
stable 3- to 7- membered monacyclic or bicyclic or 7- to 14-
membered bicyclic or tricyclic carbon ring, which may be
saturated or partially unsaturated. Examples of such
carbocyles include, but are not limited to, cyclopropyl,
cyclopentyl, cyclohexyl, phenyl, biphenyl, naphthyl, indanyl,
adamantyl, or tetrahydronaphthyl (tetralin).
As used herein, the term "heterocycle" or "heterocyclic
system" is intended to mean a stable 5- to 6- membered
monocyclic heterocyclic ring which is saturated partially
unsaturated or unsatuxated (aromatic), and which consists of
carbon atoms and from 1 to 3 heteroatoms independently
selected from the group consisting of N. O and S. The
nitrogen and sulfur heteroatoms may optionally be oxidized.
The heterocyclic ring may be attached to its pendant group at
any heteroatom or carbon atom which results in a stable
structure. The heterocyclic rings described herein may be
substituted on carbon or on a nitrogen atom if the resulting
compound is stable. If specifically noted, a nitrogen in the
heterocycle may optionally be quaternized. It is preferred
-35-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
that when the total number of S and O atoms in the
heterocycle exceeds one, then these heteroatoms are not
adjacent to one another. It is preferred that the total
number of S and O atoms in the heterocycle is not more than
one.
As used herein, the term "aromatic heterocyclic system"
is intended to mean a stable 5- to 6- membered monocyclic
heterocyclic aromatic ring which consists of carbon atoms and
from 1 to 3 heterotams independently selected from the group
consisting of N, O and S. It is preferred that the total
number of S and 0 atoms in the aromatic heterocycle is not
more than one.
Examples of heterocycles include, but are not limited
to, 2-pyrrolidonyl, 2H-pyrrolyl, 4-piperidonyl, 6H-1,2,5-
thiadiazinyl, 2H,6H-1,5,2-dithiazinyl, furanyl, furazanyl,
imidazolidinyl, imidazolinyl, imidazolyl, isoxazolyl,
morpholinyl, oxadiazolyl, 1,2,3-oxadiazolyl, 1,2,4-
oxadiazolyl, 1,2,5-oxadiazolyl, 1,3,4-oxadiazolyl,
oxazolidinyl., oxazolyl, piperazinyl, piperidinyl,
pteridinyl, piperidonyl, 4-piperidonyl, piperonyl,
pteridinyl, purinyl, pyranyl, pyrazinyl, pyrazolidinyl,
pyrazolinyl, pyrazolyl, pyridazinyl, pyridinyl, pyridyl,
pyrimidinyl, pyrrolidinyl, pyrrolinyl, pyrrolyl,
tetrahydrofuranyl, 6H-1,2,5-thiadiazinyl, 1,2,3-thiadiazolyl,
1,2,4-thiadiazolyl, 1,2,5-thiadiazolyl, 1,3,4-thiadiazolyl,
thiazolyl, thienyl, thienothiazolyl, thienooxazolyl,
thienoimidazolyl, thiophenyl, triazinyl, 1,2,3-triazolyl,
1,2,4-triazolyl, 1,2,5-triazolyl, and 1,3,4-triazolyl.
Preferred heterocycles include, but are not limited to,
pyridinyl, furanyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl,
and oxazolidinyi. Also included are fused ring and spiro
compounds containing, for example, the above heterocycles.
As used herein, "HIV reverse transcriptase
inhibitor" is intended to refer to both nucleoside and non-
nucleoside inhibitors of HIV reverse transcriptase (RT).
Examples of nucleoside RT inhibitors include, but are not
limited to, AZT, ddC, ddI, d4T, and 3TC. Examples of non-
nucleoside RT inhibitors include, but are not limited to,
-36-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
efavirenz (DuPont Merck), rescriptor (delavirdine, Pharmacia
and Upjohn), viviradine (Pharmacia and Upjohn U901525),
PNU142721 (Pharmacia and Upjohn), TIHO derivatives, BI-RG-
587, nevirapine, L-697,661, LY 73497, and Ro 18,893 (Roche).
As used herein, "HIV protease inhibitor" is intended to
refer to compounds Which inhibit HIV protease. Examples
include, but are not limited, saquinavir (Roche, Ro31-8959),
ritonavir (Abbott, ABT-538), indinavir (Merck, MK-639), VX-
478 (Vertex/Glaxo Wellcome), nelfinavir (Agouron, AG-1343),
I~tI-272 (Japan Energy), CGP-61755 (Ciba-Geigy), DMP450
(DuPont Merck), and U-103017 (Pharmacia and Upjohn).
Additional examples include the cyclic protease inhibitors
disclosed in W093/07128, W094/19329, W094/22840, and PCT
Application Number US96/03426 and the protease inhibitors
disclosed in W094/04993, W095/33464, W096/28,418, and
W096/28,464.
As used herein, "pharmaceutically acceptable salts"
refer to derivatives of the disclosed compounds wherein the
parent compound is modified by making acid or base salts
thereof. Examples of pharmaceutically acceptable salts
include, but are not limited to, mineral or organic acid
salts of basic residues such as amines; alkali or organic
salts of acidic residues such as carboxylic acids; and the
like. The pharmaceutically acceptable salts include the
conventional non-toxic salts or the quaternary amanonium salts
of the parent compound formed, for example, from non-toxic
inorganic or organic acids. For example, such conventional
non-toxic salts include those derived from inorganic acids
such as hydrochloric, hydrobromic, sulfuric, sulfamic,
phosphoric, nitric and the like; and the salts prepared from
organic acids such as acetic, propionic, succinic, glycolic,
stearic, lactic, malic, tartaric, citric, ascorbic, pamoic,
malefic, hydroxymaleic, phenylacetic, glutamic, benzoic,
salicylic, sulfanilic, 2-acetoxybenzoic, fumaric,
toluenesulfonic, methanesulfonic, ethane disulfonic, oxalic,
isethionic, and the like.
The pharmaceutically acceptable salts of the present
invention can be synthesized from the parent compound which
-37-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
contains a basic or acidic moiety by conventional chemical
methods. Generally, such salts can be prepared by reacting
the free acid or base forms of these compounds with a
stoichiometric amount of the appropriate base or acid in
water or in an organic solvent, or in a mixture of the two;
generally, nonaqueous media like ether, ethyl acetate,
ethanol, isopropanol, or acetonitrile are preferred. Lists
of suitable salts are found in Remi.ngton's Pharmaceutical
Sciences, 17th ed., Mack Publishing Company, Easton, PA,
1985, p. 1418, the disclosure of which is hereby incorporated
by reference.
The phrase "pharmaceutically acceptable" is employed
herein to refer to those compounds, materials, compositions,
and/or dosage forms which are, within the scope of sound
medical judgment, suitable for use in contact with the
tissues of human beings and animals without excessive
toxicity, irritation, allergic response, or other problem or
complication commensurate with a reasonable benefit/risk
ratio.
"Prodrugs" are intended to include any covalently bonded
carriers which release the active parent drug according to
formula (I) or other formulas or compounds of the present
invention in vivo when such prodrug is administered to a
mammalian subject. Prodrugs of a compound of the present
invention, for example formula (I), are prepared by modifying
functional groups present in the compound in such a way that
the modifications are cleaved, either in routine manipulation
or in vivo, to the parent compound. Prodrugs include
compounds of the present invention wherein the hydroxy or
amino group is bonded to any group that, when the prodrug is
administered to a mammalian subject, cleaves to form a free
hydroxyl or free amino, respectively. Examples of prodrugs
include, but are not limited to, acetate, formate and
benzoate derivatives of alcohol and amine functional groups
in the compounds of the present invention, and the like.
"Stable compound" and "stable structure" are meant to
indicate a compound that is sufficiently robust to survive
isolation to a useful degree of purity from a reaction
-38-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
mixture, and formulation into an efficacious therapeutic
agent. Only stable compounds are contempleted by the present
invention.
"Substituted" is intended to indicate that one or more
hydrogens on the atom indicated in the expression using
"substituted" is replaced with a selection from the indicated
group(s), provided that the indicated atom's normal valency
is not exceeded, and that the substitution results in a
stable compound. When a substituent is keto (i.e., =O)
group, then 2 hydrogens on the atom are replaced.
"Therapeutically effective amount" is intended to
include an amount of a compound of the present invention or
an amount of the combination of compounds claimed effective
to inhibit HIV infection or treat the symptoms of HIV
infection in a host. The combination of compounds is
preferably a synergistic combination. Synergy, as described
for example by Chou and Talalay, Adv. Enzyme Regul. 22:27-55
(1984), occurs when the effect (in this case, inhibition of
HIV replication) of the compounds when administered in
c~znbination is greater than the additive effect of the
co~:,pounds when administered alone as a single agent. In
general, a synergistic effect is most clearly demonstrated at
suboptimal concentrations of the compounds. Synergy can be
in terms of lower cytotoxicity, increased antiviral effect,
or some other beneficial effect of the combination compared
with the individual components.
$:C7~TBF~.~
The compounds of the present invention can be prepared
in a number of ways well known to one skilled in the art of
organic synthesis. The compounds of the present invention
can be synthesized using the methods described below,
together with synthetic methods known in the art of synthetic
organic chemistry, or variations thereon as appreciated by
those skilled in the art. Preferred methods include but are
not limited to those methods described below. Each of the
references cited below are hereby incorporated herein by
reference.
-39-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
The following abbreviations are used herein:
cycPr cyclopropyl
ACN acetonitrile
AcOH acetic acid
CAN ceric ammonium nitrate
DCE dichloroethane
DIBAL-H diisobutylaluminum hydride
10DIPEA diisopropylethylamine
DMAP 4-dimethylaminopyridine
DMF N,N-dimethylformamide
EtOAc ethyl acetate
EtOH ethyl alcohol
15MCPBA m-chloroperoxybenzoic acid
PMBC1 p-methoxybenzyl chloride
pyr pyridine
SEMC1 2,-(trimethylsilyl>ethoxymethyl chloride
TEA triethyl amine
20TFA trifluoroacetic acid
THF tetrahydrofuran -
In the Schemes which follow: Cf is shown as a CF3 group,
but could be any one of the presently described R1 groups; G
25 represents R3, R3a, R3b, or R3~ or any combination of these
groups.
Schemed illustrates a method for making 3,3-
disubstituted-3,4-dihydroquinoxalin-2-ones starting from an
30 appropriately substituted ortho-phenylenediamine. The
phenylenediamine is stirred with condensed hexafluoro-
propylene oxide to form compounds of formula ~, after which
the cyclic amide moiety of ~ is protected, for example with
SEM, to form compounds of formula 2_. Addition of
35 appropriately substituted organometallics, RZM, provide the
3,3-disubstituted compounds ,~. Treatment with base is
followed by the addition of an appropriately substituted
alkyl halide, RlBr, to form compounds of formula 4_. The
-40-
CA 02334332 2000-12-07
WO 00/00498 PCTIUS99/14395
product ~ are deprotected to give compounds of the present
invention.
BCH~ 1
G \ ~z Hexafluoropropylene oxide G \ N~CF3
/ NaHC03, ether
NH2 H O
SEMC1, DIPEA, DMF G I \ N~ CF3 RZM, THF
N O
SEM
H R2 R1 2
G ~ \ N~CF3 RlBr, tBuOK, THF G \ N R
/ ~CF3
SEM O ~~ O
4
Rl 2
BFg.EtZO, CHZC12 G \ R
/ ~CF3
~N
O
H
SCE la
Y'W~ ~2 Hexafluoropropylene oxide X~W~~ CF3
..
Z ~ NaHC03 , ether Y' Z ~ 0
2
Scheme la illustrates a method, analogous to Scheme 1,
of making derivatives to tetrahydroquinoxalinone compounds of
formula ;Z wherein W, X, Y, and/or Z are nitrogen.
-41-
CA 02334332 2000-12-07
WO 00/00478 PCT1US99/14395
sc~ a
0~ OR12
G ~ N R2 Ri2C02C1, nBuLi, THF G ~ N CF3
~ / ~CF3 I /
~N O N
S~ SEM
_3
O~ OR12
2
LiBF4, CH2Clz G ~ N~[ R
[ _CF3
/ NCO
H
7
Scheme 2 illustrates the acylation of 3,4-dihydro-
quinoxalin-2-ones. Treatment of compounds of formula 3_, as
can be prepared by Scheme 1, with base is followed by the
addition of an appropriately substituted chloroformate,
R12C02C1 to form compounds of formula ~. The product 6_ is
deprotected to give compounds of formula ~.
SCE 3
R12
S'
H Rz ' z
G ' ~ N~CF3 RlzgOzCl, nBuLi, THF G ~ ~ N~CF3
~N O
SEM O SEM
_3
R12
0=S'
BF3 . Et20, CHZClz _ G ~ N~ Rz
CF3
/ NCO
H
-42-
CA 02334332 2000-12-07
WO OOI00478 PCT/US99/14395
In analogous fashion to Scheme 2, treatment of a
compound of formula 3_ with base followed by an appropriately
substituted sulfonyl chloride, R12S02C1, provide protected
compounds ,$, as shown in Scheme 3. The product is
deprotected to give compounds of formula ~.
Analogous to Schemes 2 acid 3, Scheme 4 describes the
preparation of amides, ,Z,Q, from acid chlorides R12COC1.
In an alternative route to the synthesis of 3,4-
IO dihydroquinoxalin-2-ones, as shown in Scheme 5, a substituted
quinoxalin-2-one, ~, can be O-protected to form a compound of
formula ~. The addition of an organometallic reagent R2M
followed by the quenching of the resulting anion with a
chloroformate can produce compounds of formula ~. The
deprotection of a compound ~ will result in compounds of
formula 7.
SCE 4
O'' R12
H 2 G ~N z
G ~ ~ N~C 3 RIZCOC1, nF , T~i, TH ~ ( ~ CF3
_N
SEM O S~ O
O Rlz
R2
BF3.Et20, CH2C12 G I ~ N~CF3
N O
H
-43-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
SCE 5
~ N~CF3 PMBC1, Ag2C03, DMF G ' ~ N~CF3
N O N OPMB
H
COOR2
(1) nBuLi, THF, RZM G~N R
f ~ CF3
(2) NaI, EtCOOCl, THF ~ N OPMB
COOR
CAN, AcCN:HzO G ~ N~ R2
\~~ I'CF3
N O
H
7
Scheme 6 illustrates yet another route for the
preparation of compounds of the present invention. N-oxide
compound ~. can provide a substrate for the addition of
organometallic species R2::~~, followed by the reductive cleavage
of the resulting N-hydroxy compound to form compounds of
formula ~. Subsequent substitution at the 4-position by R1
radicals is performed as previously described.
Compounds the present invention that are thioamides can
be prepared as illustrated in Sceme 7 by treating the
corresponding amides with either Lawesson's reagent (2,4-
bis(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane-2,4-
disulfide] or phosphorous pentasulfide.
-44-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
SC's 6
H
G ~ N~CF3 NaCNBH3, AcOH, MeOH G I ~ N~CF3
~
O
SEM SEM
O
a ~4
0
mCPBA, CH2C12 G ( ~ N~ CF3 R2M, THF
N 0
SEM
OH 2 H 2
G I ~ N~CF3 Zn, AcOH ~ G ~ ~ N~CF3
,,,,~~ O ~ O
N N
SEM SEM
R1
2
RlBr, tBuOK, THF G ( ~ N~ CF3 BF3.Et20, CH2Cl2~
N O
SEM
Ri
G N~ R2
CF3
NCO
H
SCE 7
R1 R1
I R2 I R2
X'W ~ N Cf Lawesson's Reagent X%W ~ N
Z H O Z H S
One isomer of a compound of Formula (I) may display
superior activity compared with the other. Thus; both of the
following stereochemistries, (Ia) and (Ib), are considered to
be a part of the present invention.
-95-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
R1 R1
t 2 I 2
X ~W N R f X~W N R
C I ~ ~ '~~~Cf
Y~~ ~ ~' Y~.
Z H A Z H A
(Ia) (Ib)
When required, separation of the racemic material can be
achieved by HPLC using a chiral column or by a resolution
using a resolving agent such as camphonic chloride as in
Steven D. Young, et al, Ant.imicrobial Agents and
Chemotheraphy, 1995, 2602-2605. A chiral compound of Formula
(I) may also be directly synthesized using a chiral catalyst
or a chiral ligand, e.g. Andrew S. Thompson, et al, Tet.
lett. 1995, 36, 8937-8940. In addition, separation may be
achieved by selective cystallization, optionally in the
presence of a chiral acid or base thereby forming a chiral
salt.
Other features of the invention will become apparent in
the course of the following descriptions of exemplary
embodiments which are given for illustration of the invention
and are nc,:. intended to be limiting thereof .
Abbreviations used in the Examples are defined as
follows: anal. for combustion analysis, "g" for gram or
grams, HRMS for high resolution mass spectrometry, "mg" for
milligram or milligrams, "mL" for milliliter or milliliters,
"mmol" for millimole or millimoles, "h" for hour or hours,
"HPLC" for high performance liquid chromatography, "M" for
molar, "min" for minute or minutes, "MHz" for megahertz, "MS"
for mass spectroscopy, "TLC" for thin layer chromatography.
For further clarification of the stereochemistry, in
compounds with stereochemistry designated as "ref-(3S,5S)"
the 3-substituent is cis to the 5-trifluoromethyl group while
in compounds with stereochemistry designated as "ref-(3R,5S)"
the 3-substituent is traps to the 5-trifluoromethyl group.
-46-
CA 02334332 2000-12-07
W O 00/00478 PCT/US99/14395
Preparation of 4-(cyclopropylmstbyl)-3-(a-cyclopro~ylmthyayl)
-3- ( trifluorcsnethyl ) -3, 4-dihydro-quiacacalia-a ( 1H) -oae .
N "'
CF3
N O
Fi5
Sten AA: Preparation of compound of formula ~ wherein G = H
To a slurry of 1,2-phenylenediamine (10.8 g, 100 mmol) in
ether (200 mL) at room temperature was added sodium
bicarbonate (25.4 g, 300 mmol) followed by the condensation
of hexafluoropropylene oxide (21 g, 120 mmol) and the
resulting reaction mixture was allowed to stir at room
temperature for 3 hours. The reaction mixture is diluted
with water (500 mL) and extracted with EtOAc (3x200 mL). The
combined EtOAc extracts were dried over anhydrous Na2S04 and
concentrated in vacuo to provide 19.3 g of compound of
formula ~ (21.4 g theoretical, 90%). 1H NMR (300 MHz,
CD3COCD3) S 11.67(br s, 1H), 7.93(m, 1H), 7.75(m, 1H), 7.46(m,
2H). 19F NMR (282 MHz, CD3COCD3) 8 -70.93(s, 3F). High
resolution mass spec: calculated for C9H6NZOF3 (M+H)+:
215.0423; found: 215.0432.
Step B: Preparation of compound of formula ~ wherein G = H
To a solution of quinoxalin-2-one of formula ~ (5.64 g, 26.3
mmol) in DMf (120 mL) at room temperature was added
diisopropylethylamine (18.32 mL, 105.2 mmol) followed by
SEMC1 (9.28 mL, 52.6 m~nol) and the resulting reaction mixture
was allowed to stir at room temperature for 14 hours. The
reaction mixture is poured onto 1N HC1 and extracted with
ether (3x100 mL). The combined ether extracts were dried
over anhydrous Na2S04 and concentrated in vacuo.
Chromatography (Si02, 10% EtOAc-hexanes eluant) provided 8.15
-47-
CA 02334332 2000-12-07
WO 00/00478 PGT/US99/14395
g of compound of formula ~ (9.05 g theoretical, 90%). 1H NMR
(300 MHz, CDC13) 8 8.02(m, 1H), 7.74(m, 2H), 7.48(m, 1H),
5.77(s, 2H), 3.74(t, J = 8Hz, 2H), 0.98(t, J = BHz, 2H),
0.01(s, 9H). 19F I~ (282 MHz, CDC13) b -61.53 s, 3F). Mass
spec. (NH3-CI): 345(M+H)* (54.6%), 317 (100%).
Sten CC: Preparation of compound of formula ~ wherein G = H,
R2 = cyclopropylacetylene
To a solution of cyclopropylacetylene (23.4 mL, 106.2 mmol)
in THF (150 mL) at 0°C was added nBuLi (59 mL, 94.4 mmol) and
the resulting reaction mixture was allowed to stir at 0°C for
30 minutes. Thereafter the reaction mixture was cannulated
to stirred solution of quinoxalinone of formula 2_ (8.15 g,
23.6 mmol) in THF (300 mL) at -78°C. The dry ice bath is
removed and the reaction mixture is stirred for an additional
minutes. The reaction mixture is poured onto saturated
NH4C1 and extracted with ether (3x100 mL) and the combined
ether extracts were dried over anhydrous Na2S04 and
20 concentrated in vacuo. Chromatography (Si02, 10% EtOAc-
hexanes eluant) provided 8.95 g of compound of formula ~,
(9.68 g theoretical, 92%). 1H NMR (300 MHz, CDC13) S 7.36m,
1H), 7.26(m, 1H), 7.08(m, 2H), 6.91(m, 1H), 5.52(d, J = llHz,
1H), 5.30(d, J = llHz, 1H), 3.61(t, J = 8Hz, 2H), 1.38(m,
1H), 0.93(t, J = 8Hz, 2H), 0.85(m, 2H), 0.54(m, 2H). 1gF NMR
(282 MHz, CDC13) 8 -75.22(s, 3F). Mass spec. (NH3-CI):
411(M+H)*, 5.2%, 383 (100%).
Sten DD: Preparation of compound of formula ø wherein G = H,
R2 = cyclopropylacetylene and R1 = cyclopropylmethyl
To a solution of protected quinoxalinone of formula 3_ (123
mg, 0.3 mmol) in DMF' (4 mL) at room temperature was added
tBuOK in THF (1.5 mL, 1.5 mmol) was added cyclopropylmethyl
bromide (290 E1.1, 3.0 mmol) and the resulting reaction mixture
was allowed to stir at 80°C for 14 hours. The reaction
mixture is poured onto water and extracted with ether (3x50
mL) and the combined ether extracts were dried over anhydrous
-48-
CA 02334332 2000-12-07
WO 00100478 PCTlUS99/14395
Na2S04 and concentrated in vacuo. Chromatography (Si02, 10~
EtOAc-hexanes eluant) provided 69 mg of compound of formula
ø, (139 mg theoretical, 50$). 1H NMR (300 MHz, CDC13) 8
7.42(m, IH), 7.12(m, 1H), 7.02(m, 1H), 6.94(m, 1H), 5.94(d, J
- llHz, 1H), 5.05(d, J = llHz, 1H), 3.9(m, 1H), 3.68(t, J =
8Hz, 2H), 3.45(m, 1H), 1.42(m, 1H), 1.2(m, 1H), 0.9(m, 6H),
0.6(m, 1H), 0.45(m, 1H), 0.35(m, 2H), 0.01(s, 9H). Mass
spec. (NH3-CI): 465(M+H)+, 50~, 437 (90~), 335(M-SEM+H+,
1000 .
S t~j, E
To a solution of the alkylated quinoxalinone of formula 4_ (69
mg, 0.15 mmol) in CH2C12 (1 mL) at room temperature was added
BF3.Et20 (95 ).~L, 0.75 mmol) and the resulting reaction mixture
was allowed to stir at room temperature for 20 minutes. The
reaction mixture was poured onto saturated NaHC03 and
extracted with CHZC12 (3x25 mL) and the combined CH2C12
extracts were dried over anhydrous Na2S04 and concentrated in
vacuo. The residue was taken up in MeOH (1 mL) and 15~ NaOH
(lmL) was added to the reaction and the resulting reaction
mixture was allowed to stir at room temperature for 10
minutes. The reaction mixture was poured onto water and
extracted with CH2C12 (3x25 mL) and the combined CH2C12
extracts were dried over anhydrous Na2S04 and concentrated in
vacuo. Chromatography (Si02, 10~ EtOAc-hexanes eluant)
provided 41 mg of the title compound, (50 mg theoretical,
82~). IH NMR (300 MHz, CDC13) 8 9.46(br s, 1H), 7.1(m, 1H),
6.95(m, 1H), 6.85(m, 2H), 3.87(dd, J = 4, lSHz, 1H), 3.35(dd,
J = 8, lSHz), 1.4(m, 1H), 1.2(m, 1H), 0.9(m, 4H), 0.6(m, 1H),
0.4(m, 3H). 19F NMR (282 MHz, CDC13) S -73.38(s, 3F). High
resolution mass spec: calculated for ClgH1gN20F3 (M+H)+:
335.1371; found: 335.1371.
$~
Preparation of 4-(aoath~yl)-3-(2-cyclopropylsthyayl)-3-
(trif lnoroemethyl ) -3, 4-di~dro-quinoocs7.ia-a ( 1H) -one .
-49-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
~H3
i
CF3
N O
H
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step D methyl iodide
was used instead of cyclopropylmethyl bromide: 1H iVMR (300
MHz, CDC13) S 8.75(br s, 1H), 7.1(m, 1H), 6.85(m, 3H), 3.25(s,
3H), 1.4(m, 1H), 0.85(m, 4H). High resolution mass spec:
calculated for C15H14N20F3 (M+H)+: 295.1058; found: 295.1073.
~CAMpLE ~
Preparation of 3-(a-butyl)-3-(trifluora~etl~yl)-3,4-dihydro-
quinoxalin-2(iH)-one.
H
~~nBu
II I'CF3
~~ NCO
H
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step C n-butyl
magnesium chloride was used instead of lithium cyclopropyl
acetylide: 1H NMR (300 l~iz, CDC13) S 8.8(br s, 1H), 6.9(m,
1H), 6.75(m, 3H), 4.05(s, 1H), 2.2(m, 1H), 1.85(m, 2H),
1.35(m, 2H), 0.9(m, 3H). High resolution mass spec:
calculated for C13H16N20F3 (M+H)+: 273.1214; found: 273.1210.
Preparation of 4-(methyl)-3-(n-butyl)-3-(trifluoro~ethyl)-
3,4-dihydm-quinoxalin-2(18)-one.
~H3
~nBu
['CF3
NCO
H
-50-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step C n-butyl
magnesium chloride was used instead of lithium cyclopropyl
acetylide and in Step D methyl iodide was used instead of
cyclopropylmethyl bromide: 1H NMR (300 MHz, CDC13) S 8.85(br
s, 1H), 7.05(m, 1H), 6.8(m, 3H), 2.95(s, 3H), 2.65(m, 2H),
2.1(m, 1H), 1.4(m, 4H), 0.95(m, 3H). High resolution mass
spec: calculated for C14H1gN20F5 (M+H)*: 287.1371; found:
287.1362.
'"
Preparation of 3-(2-cyclopropylsthyayl)-3-(trifluorcm~sthyl)-
3,4-dibydro-quinoxalia-Z(1S)-ous.
H
CF3
N O
H
The title compound was prepared in a manner similar to
the product of Example 1, Step C: 1H NMR (300 MHz, CDC13) 8
9.0(br s, 1H), 7.0(m, 1H), 6.85(m, 2H), 6.8(m, 1H), 4.45(br
s, 1H), 1.4(m, 1H), 0.8-0.6(m, 4H). 19F NMR (282 MHz, CDC13)
S -77.13(s, 3F). High resolution mass spec: calculated for
C14H11N20F5 (M)*: 280.0823; found: 280.0828.
E7CA~Lg 6
Prsparation of 3-(allyl)-3-(trifluoranethyl)-3,4-dihydro-
qusnoxali.a-2(18)-oas.
H
CF3
N O
H
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step C allyl
magnesium bromide was used instead of lithium cyclopropyl
acetylide: 1H NMR (300 MHz, CDC13) 8 8.25(br s, lH), 6.95(m,
1H), 6.75(m, 3H), 5.85(m, 1H), 5.25(m, 2H), 4.2(br s, 1H),
-51-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
3.1(m, 1H), 2.65(m, 1H). 19F NMR (282 MHz, CDC13) 8 -71.16(s,
3F). High resolution mass spec: calculated for C12H12N20F3
(M+H)+: 257.0901; found: 257.0898.
ALE 7
Preparation of 4-(allyl)-3-(2-cyclopropylethyayl)-3-
(trifluoroanat~rl ) -3, 4-831~y~dro-quiaoxalia-Z ( iH) -oas.
i
~ CF3
'N O
H
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step D allyl iodide
was used instead of cyclopropylmethyl bromide: 1H NMR (300
MHz, CDC13) 8 9.4(br s, 1H), 7.0(m, 1H), 6.8(m, 3H), 5.8(m,
1H), 5.2(m, 2H), 4.6(m, 1H), 4.2(m, 1H), 1.4(m, 1H), 0.9(m,
4H). 19F NMR (282 MHz, CDC13) S -74.49(s, 3F). High
resolution mass spec: calculated for C1~H16N20F3 (M+H)*:
321.1214; found: 321.1198.
2 0 87C111~L13 8
Preparation of 4-(beazyl)-3-(2-cyclopropyletl~nyl)-3-
(trifluoramaethyl)-3,4-dihydro-quiaoxalin-2(1H)-one.
~Hz Ph
i
CF3
N O
H
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step D benzyl
bromide was used instead of cyclopropylmethyl bromide: 1H NMR
(300 MHz, CDC13) 8 8.85(br s, 1H), ?.3(m, SH), 7.25(m, 1H),
6.8(m, 3H), 5.3(d, J = llHz, 1H), 4.6(d, J = llHz, 1H),
1.35(m, 1H), 0.8(m, 2H), 0.6(m, 2H). 1gF NMR (282 MHz, CDC13)
-52-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
8 -78.08(s, 3F). High resolution mass spec: calculated for
C21H1sN20F5 (M+H)+: 371.1371; found: 371.1365.
Preparation of 4-(cyclopropylmathyl)-3-(allyl)-3-
(trifluoromstl~yl)-3,4-dihydro-quinoualia-2(1H)-one.
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step C allyl
magnesium bromide was used instead of lithium cyclopropyl
acetylide: 1H NMR (300 MHz, CDC13) S 8.35(br s, 1H), 7.1(M,
2H), 6.85(m, 1H), 6.75(m, 1H), 5.9(m, 1H), 5.25(m, 2H),
3.45(M, 2H), 3.2(m, 1H), 2.8(M, 1H), 1.0(m, 1H), 0.7(m, 1H),
0.55(m, 1H), 0.3(m, 2H). 19F NMR (282 MHz, CDC13) b -70.22(s,
3F). High resolution mass spec: calculated for C16H1gN20F3
(M+H)+: 311.1371; found: 311.1325.
2 0 E7C~L8~ 10
Preparation of 4-(proparQyl)-3-(2-cyclopropyletbyayl)-3-
(trifluorometbyl)-3,4-dihydro-qufaoxalin-2(iH)-one.
N
CF3
0
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step D propargyl
bromide was used instead of cyclopropylmethyl bromide: 1H NMR
(300 MHz, CDC13) S 9.35(br s, 1H), 7.15(m, 2H), 6.95(m, 2H),
4.6(dd, J = 2,28Hz, 1H), 4.4(dd, J = 2,18Hz, 1H), 2.25(t, J =
2Hz, 1H), 1.4(m, 1H), 0.9(m, 4H). Anal. (C1~H13N20F3) Calcd:
-53-
CA 02334332 2000-12-07
WO 00/00478 PCTlUS99/14395
C, 64.15; H, 4.126; N, 8.80; Found: C, 64.23; H, 4.00; N,
8.61.
$~CAIIFLE I1
Preparation of 4-(cycloprapyl~thyl)-3-(a-cyclopropylett~ynyl)-
3-(trifluorcanethyl)-3,4-dihydro-quinoxalin-a(1H)-oaw.
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step D
cyclopropylethyl bromide was used instead of
cyclopropylmethyl bromide: 1H NMR (300 MHz, CDC13) S 9.2(br s,
1H), 7.0(m, 1H), 6.8(M, 3H), 4.0(m, 1H), 3.65(m, 1H), 1.6-
1.35(m, 3H), 0.9(m, 3H), 0.7(m, 1H), 0.45(m, 1H), 0.1(m, 1H).
Anal. (C1gH19N20F3) Calcd: C, 65.51; H, 5.507; N, 8.04; F,,r
16.36; Found: C, 65.23; H, 5.51; N, 8.05; F, 15.97.
2 0 EXiI~LE 12
Preparation of 4-(isopropyl)-3-(a-cyclopropylet~ynyl)-3-
(trifluoraanethyl)-3,4-dihydro-quinoxalin-a(18)-one.
N i
CF3
N O
H
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step D isopropyl
iodide was used instead of cyclopropylmethyl bromide: 1H NMR
(300 MHz, CDC13) 8 8.4(br s, 1H), 7.05(m, 1H), 7.0(m, 1H),
6.9(m, 1H), 6.8(m, 1H), 4.6(p, J = 7Hz, 1H), 1.45(d, J 7Hz,
3H), 1.4(m, 1H), 1.2(d, J = 7Hz, 3H), 0.9(m, 4H). High
-54-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
resolution mass spec: calculated for C17H18N20F3 (M+H)+:
323.1371; found: 323.1364.
BXAMyl~B 13
preparation of 6-(fluoro)-4-(allyl)-3-(n-butyl)-3-
(trifluoramethyl)-3,4-dihydro-quinoxalin-2(iFt)-tee.
~nBu
F~N~CF3
~~,,,,~~~ NCO
H
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step A 4-fluoro-1,2-
phenylenediamine was used instead of 1,2-phenylenediamine, in
Step C nbutyl magnesium bromide was used instead of lithium
cyclopropylmethyl acetylide and in Step D allyl iodide was
used instead of cyclopropylmethyl bromide: 1H NMR (300 MHz,
CDC13) S 9.2(br s, 1H), 6.65(m, 1H), 6.5(m, 2H), 5.8(m, 1H),
5.35(m, 'H), 4.0(m, 2H), 2.65(m, 1H), 2.0(m, 1H), 1.4(m, 4H),
0.95(m, .iH). 19F NMR (282 MHz, CDC13) b -73.60(s, 3F),
-147.85(s, 1F). High resolution mass spec: calculated for
C16H18NOF4 (M)+: 330.1335; found: 330.1332.
R7~'1~ 14
Preparatioa of 6-(fluoro)-4-(allyl)-3-(a-cyclopropyletl~y~yl)-
3- (trif luoroaaetl~yl ) -3, 4-dibydro-qniaoacalin-Z ( 18) -one .
F ~ N ii
CF3
0
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step D allyl iodide
was used instead of cyclopropylmethyl bromide: 1H NMR (300
MHz, CDC13) 8 9.65(br s, 1H), 6.6(m, 1H), 6.5(m, 2H), 5.8(m,
-55-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
1H), 5.2(m, 2H), 4.6(m, 1H), 4.1(m, 1H), 1.4(m, 1H), 0.9(m,
4H). 19F NMR (282 MHz, CDC13) 8 -74.62(s, 3F), -117.46(s,
1F). High resolution mass spec: calculated for C1~H15N20F4
(M+H)+: 339.1120; found: 339.1143.
10
L~ 15
Preparation of 6-(fluoro)-4-(cyclopro~rylmatbyl)-3-(2-
cyclopropylathyayl)-3-(trifluorcm~sthyl)-3,4-dihydro-
quiaoxalia-2(1H)-oae.
F ~ N i
CF3
N O
H
The title compound was prepared in a manner similar to
the product of Example 1: 1H I~t (300 I~Iz, CDC13) 8 9. 0 (br s,
1H), 6.75(m, 2H), 6.55(m, 1H), 3.8(m, 1H), 3.35(m, 1H),
1.4(m, 1H), 1.15(m, 1H), 0.9(m, 4H), 0.6(m, 1H), 0.5(m, 1H),
0.35 (m, 2H) . 19F lit (282 l~iz, CDC13) S -74.34 (s, '~) ,
-117.47(s, 1F). Anal. (C18H16NZOF4 1/2H20) Calcd: C, 59.83; H,
4.74; N, 7.75; Found: C, 59.56; H, 4.61; N, 7.37.
25
L8 16
Preparation of 6-(fluoro)-4-(cyclopropylamethyl)-3-(a-butyl)-
3-(trifluorcm~athyl)-3,4-dihydro-quiaoxalin-2(1H)-one.
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step C nbutyl
magnesium bromide was used instead of lithium
cylcopropylmethyl acetylide: 1H Nl~t (300 l~iz, CDC13) 8 96.6(br
s, 1H), 6.7(m, 2H), 6.5(m, 1H), 3.45(m, 1H), 3.15(m, 1H),
-56-
CA 02334332 2000-12-07
WO 00100478 PCT/US99/14395
2.75(m, 1H), 1.9(m, 1H), 1.75(m, 1H), 1.4(m, 3H), 1.05(m,
1H), 0.95(m, 3H), 0.65(m, 2H), 0.35(m, 2H). 19F Nl~t (282
N~Iz, CDC13) b -73.36(s, 3F), -117.79(s, 1F). Anal.
(C17H2pN20F4) Calcd: C, 59.30; H, 5.85; N, 8.145; Found: C,
58.98; H, 5.73; N, 7.90.
$ul~l~r.E i7
Preparation of 6-(ahloro)-4-(cycloprapylmetl~yl)-3-(Z-
cyclopropyletvynyl)-3-(trifluora~thyl)-3,4-dil~ydro-
quinoxalin-a(18)-o~n..
C1 ,~ N
1~ CF3
v 'N O
H
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step A 4-chloro-1,2-
phenylenediamine was used instead of 1,2-phenylenediamine:
1H Nit (300 l~Iz, CDC13) 8 9 .5 (br s, 1H) , 6.9 (m, 1H) , 6.8 (m,
2H), 1.4(m, 1H), 1.2(m, iH), 0.95(m, 4H), 0.6(m, 1H), 0.5(m,
1H), 0.35(m, 2H). 19F Nt~t (282 I~iz, CDC13) 8 -71.80is, 3F).
Anal. (C1aH16N2OC1F3) Calcd: C, 58.62; H, 4.37; N, 7.606; F,
15.45; C1, 9.61; Found: C, 58.27; H, 4.39; N, 7.46; F, 15.83;
C1, 9.62.
Preparation of 6-(chloro)-4-(isobutyl)-3-(a-
cyclopropyletl~yqyl ) -3- ( trif luoraausthyl ) -3, 4-diby~dro-
quiaoxalia-a(iH)-o~.
c
-57-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step D isoamyl
bromide was used instead of cyclopropylmethyl bromide: 1H NMR
(300 MHz, CDC13) 8 9.5(br s, 1H), 6.8(m, 2H), 6.7(rn, 1H),
3.9(m, 1H), 3.6(m, 1H), 1.7(m, 1H), 1.6(M, 1H), 1.4(m, 2H),
0.95(d, J = 7Hz, 3H), 0.9(d, J = 7Hz, 3H), 0.9-0.8(m, 4H).
19F ~ (282 MHz, CDC13) S -71.67(s, 3F). Anal.
(ClgH2pN20C1F3) Calcd: C, 59.30; H, 5.248; N, 7.289; F, 14.81;
C1, 9.21; Found: C, 59.12; H, 5.19; N, 7.04; F, 15.09; f1,
9.22.
ALE 19
Preparation of 6-(chloro)-4-(allyl)-3-(a-ayclopropylethynyl)-
3-(trifluoraanethyl)-3,4-di~ydro-qufaoxalia-2(1H)-one.
C1 ~ N i
CF3
N O
H
The title compound was prepared in a manner similar to
the product of Example l, except that in Step D allyl iodide
was used instead of cyclopropylmethyl bromide: 1H NMR (300
MHz, CDC13) S 9.65(br s, 1H), 6.8(m, 2H), 6.75(m, 1H), 5.8(m,
1H), 5.3(m, 2H), 4.6(m, 1H), 4.1(m, 1H), 1.4(m, 1H), 0.9(m,
4H). 19F NMR (282 MHz, CDC13) S -71.88(s, 3F). Anal.
(C17H14N20C1F3) Calcd: C, 57.56; H, 3.987; N, 7.906; F, 16.07;
C1, 9.99; Found: C, 57.87; H, 4.25; N, 7.61; F, 15.93; C1,
9.82.
ALE ZO
Prsparatioa of 6-(chloro)-4-(cyclopropylan~atb~yl)-3-
(phenet~yl)-3-(trifluoro~etl~,yl)-3,4-dihydro-quiaoxalia-2(1H)-
one.
-58-
CA 02334332 2000-12-07
WO 00/OOd78 PCT/US99/14395
C1 ~ N~ CH2CH2Ph
['CF3
NCO
H
The title compound was prepar;~d in a manner similar to
the product of Example 1, except that in Step C phenethyl
magnesium bromide was used instead of lithium
cyclopropylmethyl acetylide: 1H NMR (300 MHz, CDC13) b 8.9(br
s, 1H), 7.25(m, 5H), 7.)(m, 1H), 6.8(m, 1H), 6.65(m, 1H),
3.5(m, 1H), 3.3(m, 1H), 3.0(m,2H), 2.75(m, 1H), 2.3(m, 1H),
1.1(m, 1H), 0.8(m, 2H), 0.4(m, 2H). High resolution mass
spec: calculated for C21H2oN20F3C1 (M)+: 408.1216; found:
408.1197.
praparatian of 6-(chloro)-4-(allyl)-3-(pl~sasthyl)-3-
(trifluora~stbyl)-3,4-dihydro-quiaoxalia-2(1H)-one.
C 1 N~CHZCH2 Ph
\~ CF3
v 'N 0
H
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step C phenethyl
magnesium bromide was used instead of lithium
cyclopropylmethyl acetylide and in Step D allyl iodide was
used instead of cyclopropylmethyl bromide: 1H NMR (300 MHz,
CDC13) 8 9.5(br s, 1H), 7.25(m, 2H), 6.8(m, 1H), 5.9(m, 1H),
5.3(m, 1H), 5.)(m, 1H), 4.3(m, 1H), 4.1(m, 1H), 3.1(m, 1H),
2.9-2.8(m, 2H), 2.3(m, 1H). Anal. (C2oH1aN20C1F3) Calcd: C,
60.84; H, 4.605; N, 7.105; F, 14.44; C1, 8.989; Found: C,
61.39; H, 4.83; N, 6.68; F, 14.25; C1, 8.89.
-59-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
ALE 22
PraDaration of 6-(methoxy)-4-(cyclopropylmathyl)-3-(2-
cyclopropylethynyl)-3-(trifluorc~ethyl)-3,4-dihydro-
quinoxalin-2(1H)-one.
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step A 4-methoxy-
1,2-phenylenediamine was used instead of 1,2-
phenylenediamine: 1H NMR (300 MHz, CDC13) 8 8.95(br s, 1H),
6.8(m, lH), 6.6(m, 1H), 6.4(M, 1H), 3.9(m, 1H), 3.8(m, 3H),
3.4(m, 1H), 1.4(m, 1H), 1.2(m, 1H), 0.9(m, 4H), 0.6(m, 1H),
0.45(m, 1H), 0.35(m, 2H). 19F NMR (282 MHz, CDC13) b
-73.19(s, 3F). Anal. (C19Hi9N202F3) Calcd: C, 62.63; H,
5.266; N, 7.698; F, 15.64; Found: C, 62.17; H, 5.36; N, 7.20;
F, 14.79. .
$~h8 a3
Preparation of 6-(methoxy)-4-(allyl)-3-(2-
cyclopropylethynyl)-3-(trifluorom~etl~yl)-3,4-dihydro-
quiaoxalin-2(1H)-one.
Me ~ N i.
CF3
N O
H
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step D allyl iodide
was used instead of cyclopropylmethyl bromide: 1H NMR (300
MHz, CDC13) 8 9.0(br s, 1H), 6.7(m, 1H), 6.35(m, 2H), 5.8(m,
1H), 5.2(m, 2H), 4.6(m, 1H), 4.1(M, 1H),3.8(s, 3H), 1.4(m,
1H), 0.95(m, 4H). 19F NMR (282 MHz, CDC13) b -73.44(s, 3F).
-60-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Anal. (C18H17N202F3) Calcd: C, 61.71; H, 4.89; N, 8.006; F,
16.27; Found: C, 62.34; H, 4.94; N, 7.81; F, 15.00.
$~~LE 24
Preparation of 4-(cyclopropylmethyl)-3-(1-propyayl)-3
( trif luosomathyl ) -3, 4-8il~dxo-qtiiacxalia-a ( 1H) -o~ae .
The title compound was prepared in a manner similar to
the product of Example 1, except that in Step C lithium
propyne was used instead of lithium cylocpropylmethyl
acetylide: 1H NMR (300 MHz, CDC13) S 8.1(br s, 1H), 7.1(m,
1H), 6.9(m, 1H), 6.8(m, 1H), 6.75(m, 1H), 3.85(m, 1H), 3.4(m,
1H), 2.)(s, 3H), 1.4(m, 1H), 0.6(m, 1H), 0.45(m, 1H), 0.35(m,
2H). 19F NMR (282 MHz, CDC13) S -71.16(s, 3F). High
resolution mass spec: calculated for C16H16NZOF3
(M+H)+:309.1214; found:309.1224.
Preparation of 4-(allyl)-3-(1-propy~yl)-3-(trifluorc~athyl)-
3,4-dibydro-qufaoxalin-Z(1H)-oae.
The title compound was prepared in a manner similar to
the product of Example l, except that in Step C lithium
propyne was used instead of lithium cyclopropylmethyl
acetylide and in Step D allyl iodide was used instead of
cyclopropylmethyl bromide: 1H NMR (300 MHz, CDC13) 8 8.4(br s,
-61-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
1H), 7.0(m, 1H), 6.8(m, 3H), 5.8(m, 1H), 5.2(m, 2H), 4.6(m,
1H), 4.2(m, 1H0, 2.0(s, 3H). 19F NMR (282 MHz, CDC13) 8
-71.79(s, 3F). High resolution mass spec: calculated for
C15H14N20F3 (M+H)+: 295.1058; found: 295.1056.
Preparation of 4-(ethoaqrcarbor~yl)-3-(a-cyclopropyletb~yl)
-3-(trifluoromethyl)-3,4-dihydro-Quinoxalia-a(iH)-one.
O~ OEt
N
~ CF3
_N O
H
Step A: Preparation of compound of formula ø wherein G = H,
R2 = cyClopropylacetylene and R1 = COOEt
To a solution of protected quinoxalinone of formula 3_ as
prepared in step C in Example 1 (147 mg, 0.42 mmol) in THF
(1.5 mL) at -78°C was added nBuLi (0.31 mL, 0.5 mmol) and
stir~:ad for 5 minutes. Thereafter ethyl chloroformate (80
N.L, 0.84 mmol) was added to the reaction mixture which was
allowed to warm to room temperature and stir for an hour.
The reaction mixture was poured onto saturated ammonium
chloride and extracted with ether (3x25 mL) and the combined
ether extracts were dried over anhydrous Na2S04 and
concentrated in vacuo. Chromatography (Si02, 10$ EtOAc-
hexanes eluant) provided 122 mg of compound of formula ø,
(202 mg theoretical, 60~). 1H NMR (300 MHz, CDC13) $ 7.46(m,
1H), 7.30(m, 1H), 7.15(m, 2H), 5.82(d, J = llHz, 1H), 5.15(d,
J = llHz, 1H), 4.4(m, 2H), 3.7(m, 2H), 1.4(m, 4H), 0.9(m,
6H), 0.01(s, 9H). 19F NMR (282 MHz, CDC13) 8 -73.06(s, 3F).
Mass spec. (NH3-CI): 483(M+H+, 1000 .
Sten B:
To a solution of the acylated quinoxalinone of formula ø (84
mg, 0.17 mmol) in CH2C12 (1 mL) at room temperature was added
LiBF4 (1M in ACN, 0.85 mL, 0.85 mmol) and the resulting
-62-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99li 4395
reaction mixture was heated to reflex for 14 hours. The
reaction mixture was poured onto saturated water and
extracted with ether (3x25 mL) and the combined ether
extracts were dried over anhydrous Na2S04 and concentrated in
vacuo. Chromatography (Si02, 20% EtOAc-hexanes eluant)
followed by a PTLC (Si02, 5% EtOAc-CH2C12 eluant) provided 15
mg of the title compound, (60 mg theoretical, 25%). 1H NMR
(300 MHz, CDC13) 8 8.06(br s, 1H), 7.35(m, 1H), 7.05(m, 2H),
6.8(m, 1H), 4.37(m, 2H), 1.4(m, 4H), 0.9(m, 4H). 19F NMR
(282 MHz, CDC13) 8 -73.55(s, 3F). High resolution mass spec:
calculated for C1~H15N203F3 (M+H)+: 353.1113; found: 353.1093.
ale Z6A
Preparation of 4-(ethaaqrcarbonyl)-3-(2-cyclopropyletI~yayl)
-3-(trifluoromethyl)-3,4-dibydro-quinoxalin-Z(18)-oue.
ii
CF3 _
0
H
Sten AA: Preparation of compound of formula ~ wherein G = H.
To a solution of the quinoxalinone of formula ~ as prepared
in step A in Example 1 (3.55 g, 16.59 mmol) in DMf (35 mL) at
room temperature was added silver carbonate (13.74 g, 49.7
mmol) follwed by PNJBC1 (2.48 mL, 18.25 mmol) and the
resulting reaction mixture was allowed to stir at room
temperature for 14 hours protected from light by aluminum
foil. The reaction mixture was filtered through Celite and
the filterate washed with water. The organic layers were
dried over anhydrous Na2SOQ and concentrated in vacuo.
Chromatography (Si02, 5% EtOAc-hexanes) provided 1.28 g of
compound of formula ~, (5.54 g theoretical, 23%). 1H NMR
(300 MHz, CDC13) 8 8.2(m, 1H), 7.9(m, 1H), 7.8(m, 1H), 7.46(d,
J = 9Hz, 2H), 6.93(d, J = 9Hz, 2H), 5.59(s, 2H), 3.81(s, 3H).
19F ~ (282 MHz, CDC13) b -68.38(s, 3F). High resolution
-63-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
mass spec: calculated for C1~H14N202F3 (M+H)+: 335.1007; found:
335.1012.
Step B: Preparation of compound of formula ~ wherein G = H,
R2 = cyclopropylacetylene and R1 = COOEt
To a solution of cyclopropylacetylene (297 ~t.L, 2.25 mmol) in
THF (5 mL) at 0°C was added nBuLi (1.25 mL, 2 mmol) and the
resulting reaction mixture was allowed to stir at 0°C for 30
minutes. Thereafter the reaction mixture was cannulated to
stirred solution of quinoxalinone of formula ~ (167 mg, 0.5
mmol) in THF (2.5 mL) at -78°C. The dry ice bath is removed
and the reaction mixture is allowed to warm up as it stirred
for an hour. NaI (300 mg, 2 mmol) was added to the reaction
mixture and the resulting reaction mixture was allowed to
stir at room temperature for 10 minutes. Thereafter ethyl
chloroformate (478 ~,L, 5 mmol) was added to the reaction
mixture was stirred for an additional 10 minutes. The
reaction mixture is poured onto saturated NH4C1 and extracted
with ether (3x50 mL) and the combined ether extracts were
dried over anhydrous Na2S04 and concentrated in vacuo.
Chromatography (Si02, 10% EtOAc-hexanes eluant) provided 78 mg
of compound of formula 1~, (236 mg theoretical, 33%) 1H NMR
(300 MHz, CDC13) 8 7.37(d, J = 9Hz, 2H), 7.35(m, 1H), 7.2(m,
1H), 7.15(m, 2H), 6.9(d, J = 9Hz, 2H), 6.90(d, J = l2Hz, 1H),
5.26(d, J = l2Hz, 1H), 4.35(m, 2H), 3.81(s, 1H), 1.37(t, J =
7Hz, 3H), 1.25(m, 1H), 0.8(m, 2H), 0.6(m, 2H). Mass spec.
(NH3-CI): 473(M+H)+ (20%), 353 (M-PMB+H+~100%).
Stey~ C:
To a stirred solution of the PMB protected quinoxalinone of
formula 1_~, (28 mg, 0.06 nanol) in CH3CN:H20 (9:1) at room
temperature was added CAN (162 mg, 0.30 manol) and the
resulting reaction mixture was allowed to stir at room
temperature for one hour. The reaction mixture was poured
onto water and extracted with EtOAc (3x25 mL) and the
combined EtOAc extracts were dried over anhydrous Na2S04 and
concentrated in vacuo. Chromatography (Si02, 20% EtOAc-
-64-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
hexanes eluant) provided 16 mg of the title compound, (21 mg
theoretical, 76~). 1H NMR (300 MHz, CDC13) 8 8.06(br s, 1H),
7.35(m, 1H), 7.05(m, 2H), 6.8(m, 1H), 4.37(m, 2H), 1.4(m,
4H), 0.9(m, 4H). 19F NMR (282 MHz, CDC13) 8 -73.55(s, 3F).
High resolution mass spec: calculated for C1~H15N203F3 (M+H)+;
353.1113; found: 353.1093.
S~,IZ
Preparation of 4-(iaopropo~ycarbonyl)-3-(2-
cycloyropylethyayl)-3-(trifluoraarethyl)-3,4-dil~ydro-
duinoxalin-a(1H)-one.
CF3
0
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A isopropyl
chloroformate was used instead of ethyl chloroformate: 1H NMR
(300 MHz, CDC13) b 8.4(br s, 1H), 7.35(m,lH), 7.15(m, 1H),
6.8(m, 1H), 5.15(p, J = 7Hz, 1H), 1.45(m, 1H), 1.4(d, J =
7Hz, 3Hz, 3H), 1.35(d, J = 7Hz, 3H), 0.85(m, 4H). 19F NMR
(282 MHz, CDC13) S -73.46(s, 3F). High resolution mass spec:
calculated for ClgH1gN203F3 (M+H)+: 367.1269; found: 367.1286.
susaa~s a s
Preparation of 4-(propea-2-yl-oo~ycarboayl)-3-(Z-
cyclonroDylethys~l) -3-(triflnorcm~stbyl)-3,4-dihy~dro-
quino~alia-Z(18)-ooe.
i
CF3
N O
H
-65-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99I14395
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A isopropenyl
chloroformate was used instead of ethyl chloroformate: 1H NMR
(300 MHz, CDC13) S 8.6(br s, 1H), 7.4(m, 1H), 7.15(m, 2H),
6.85(m, 1H), 4.85(4.87(d, J = 2Hz, 2H), 4.78(d, J = 2Hz, 1H),
2.05(s, 3H), 1.4(m, 1H), 0.85(m, 4H). 19F NMR (282 MHz,
CDC13) b -73.60(s, 3F). High resolution mass spec: calculated
for C1gH16N203F3 (M+H)+: 365.1113; found: 365.1100.
8~1~'L~ 29
Preparation of 4-(isobutoxycarboayl)-3-(2-cyclopropylet~ynyl)
-3-(trifluoroanethyl)-3,4-dihydro-quinoxalin-2(y.S)-one.
O
!.
'CF3
W
O
H
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A isobutyl
chloroformate was used instead of ethyl chloroformate: 1H NMR
(300 MHz, CDC13) b 8.6(br s, 1H), 7.3(m, 1H), 7.15(m, 1H),
6.85(m, 1H), 4.2(dd, J = 7,3Hz, 1H), 3.95(dd, J = 7,3Hz, 1H),
2.1(p, J = 7Hz, 1H), 1.4(m, 1H), 0.95(d, J = 3Hz, 3H), 0.9(d,
J = 3Hz, 3H), 0.85(m, 4H). 19F NMR (282 MHz, CDC13) S
-73.49(s, 3F). High resolution mass spec: calculated for
ClgHZpN203F3 (M+H)+: 381.1426; found: 381.1445.
L8 30
Preparation of 4-(n-buto~r~carbonyl)-3-(2-cycloprapylethyayl)
-3 - ( trif luoraaneti~yl ) -3, 4-dihydro-quinaoralia-a ( 1H) -one .
i
CF3
N O
H
-66-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/t4395
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A nbutyl
chloroformate was used instead of ethyl chloroformate: 1H NMR
(300 MHz, CDC13) S 8.65(br s, 1H), 7.3(m, 1H), 7.1(m, 2H),
6.85(m, 1H), 4.4(m, 1H), 4.2(m, 1H), 1.65(m, 2H), 1.45(m,
2H), 1.4(m, 1H), 0.95(t, J = SHz, 3H), 0.85(m, 4H). 19F NMR
(282 MHz, CDC13) 8 -73.53(s, 3F). High resolution mass spec:
calculated for C19H2oN2O3F3 (M+H)*: 381.1426; found: 381.1421.
LlIP ~ 31
Preparation of 4-(allylo~carbooyl)-3-(a-cyclopropylet~rr~l)
-3- (trif luoraerathyl ) -3, 4-dil~ydro-quinoacalia-a ( iH) -one .
i
CF3
H O
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A allyl
chloroformate was used instead of ethyl chloroformate: 1H NMR
(300 MHz, CDC13) 8 8.95(br s, 1H), 7.3(m, 1H), 7.15(m, 2H),
6.85(m, 1H), 6.0(m, 1H), 5.45-5.3(m, 2H), 4.9-4.7(m, 1H),
1.4(m, 1H), 0.85(M, 4H). 19F NMR (282 MHz, CDC13) & -73.57(s,
3F). High resolution mass spec: calculated for C1gH16NZ03F3
(M+H)+: 365.1113; found: 365.1119.
~1
preparatioa of 4- (ben~yrlo~carbooo~yl) -3- (Z-ayclopropylethyayl)
-3-(trifluoromethyl)-3,4-dil~yaro-quiaoacalia-a(iS)-one.
p, _pr~Ph
\ i
CFg
/ N O
H
-67-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99I14395
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A benzyl
chloroformate was used instead of ethyl chloroformate: 1H NMR
(300 MHz, CDC13) 8 8.6(br s, 1H), 7.4(m, 5H), 7.3(m, 1H),
7.15(m, 2H), 6.85(, 1H), 5.45-5.2(m, 3H), 1.35(m, 1H),
0.75(m, 4H). 19F NMR (282 MHz, CDC13) 8 -73.54(s, 3F). High
resolution mass spec: calculated for C22H1gNz03F3 (M+H)+:
415.1284; found: 415.1269.
~f.711~L8 33
Preparation of 4-(a-propylsulfonyl)-3-(2-cyclopropylethyayl)
-3-(trifluorcm~et~yl)-3,4-dihydro-quinoxalia-2(18)-oae.
~S02nPr
~ N
~ CF3
'N O
H
The title compound was prepared in a manner similar to
the product of Example 40, except that in Step A
n-propylsulfonyl chloride was used instead of
isopropylsulfonyl chloride: 1H NMR (300 MHz, CDC13) 8 8.1(br
s, 1H), 7.4(m, 1H), 7.2(m, 1H), 7.15(m, 1H), 6.85(M, 1H),
3 .65 (m, 1H) , 3.3 (m, 1H) , 2.0 (m, 2H) , 1.45 (m, 1H) , 1.1 (t, J =
7Hz, 3H), 0.9(m, 4H). 19F NMR (282 MHz, CDC13) b -73.17(s,
3F). High resolution mass spec: calculated for C17H1gN203F3S
(M+H)+: 387.0990; found: 387.0996.
BXA1~LE 34
Preparation of 4-(pheaylcarbo~l)-3-(2-cyclopropylethynyl)-3-
( trif luorom~ethyl ) -3, 4-dibydro-quinoacalia-2 ( 18) -oae .
O~ Ph
N i
CF3
N O
H
-68-
CA 02334332 2000-12-07
WO 00/00478 PCTlUS99/14395
The title compound was prepared in a manner similar to
the product of Example 37, except that in Step A benzoyl
chloride was used instead of isobutyzyl chloride: 1H NMR (300
MHz, CDC13) b 8.2(br s, 1H), 7.55(m, 2H), 7.45(m, 1H), 7.3(m,
2H), 7.)(m, 1H), 6.85(M, 1H), 6.75(m, 1H), 6.9(m, 1H),
1.35(m, 1H), 0.8(m, 4H). 1gF NMR (282 MHz, CDC13) 8 -72.16(s,
3F). High resolution mass spec: calculated for C21H16N202F3
(M+H)+: 385.1163; found: 385.1184.
Preparation of 4-(neopentyl-oxycarboayl)-3-(Z-
cyclopropylethynyl) -3-(trifluoro~sth~l)-3,4-dihydro-
quinoxalia-2 (lii) -one.
N
CF3
N O
H
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A neopentyl
chloroformate was used instead of ethyl chloroformate: 1H NMR
(300 MHz, CDC13) S 8.55(br s, 1H), 7.3(m, 1H), 7.15(m, 2H),
6.85(m, 1H), 4.3(d, J = llHz, 1H), 3.8(d, J = llHz, 1H),
1.4(m, 1H), 1.0(s, 9H), 0.85(m, 4H). 19F NMR (282 MHz, CDC13)
S -73.42(s, 3F). High resolution mass spec: calculated for
C20H22N203F3 (M+H)+: 395.1582; found: 395.1587.
ALE 36
prsparati~ of 4-(2-propy~l-oaqr~aarbo~yl)-3-(Z-
cyclopropyle~thynyl) -3-(triiluorom~ethyl)-3,4-dil~ydro-
quinoxalia-2(1x)-onm.
-69-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
N
CF3
N O
H
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A propargyl
chloroformate was used instead of ethyl chloroformate: 1H Nl~t
(300 I~iz, CDC13) 8 9.0 (br s, 1H) , ?.35 (m, 1H) , 7.15 (m, 2H) ,
6.9(m, 1H), 4.95(dd, J - 2,13Hz, 1H), 4.85(dd, J = 2,13Hz,
1H), 2.95(t, J = 2Hz, 1H), 1.4(m, 1H), O.BS(m, 4H). 19F lit
(282 l~iz, CDC13) S -73.62(s, 3F). Anal. (C17H13N203F3) Calcd:
C, 59.637; H, 3.626; N, 7.73; F, 15.76; Found: C, 60.18; N,
3.84, N, 7.38; F, 15.66.
PST 39
Preparation of 4-(isopropylcarbonyl)-3-(2-
cyclopropylethynyl)-3-(trifluosromethyl)-3,4-dibydm-
quiao~calin-2 ( 7.a) -one.
O
N i
CF3
N O
H
Step Preparation of compound of formula ~ wherein G = H,
R2 = cyclopropylacetylene and R1 = COiPr
To a solution of protected quinoxalinone of formula 3_ as
prepared in step C in Example 1 (250 mg, 0.61 mmol) in THF
(2.5 mL) at -78°C was added nBuLi (0.53 mL, 0.85 mmol)
followed by isobutyryl chloride (0.15 mL, 1.46 mmol) and the
resulting reaction mixture was allowed to stir for an hour
with warming to room temperature. The reaction mixture is
poured onto saturated NHqcl and extracted with ether (3x50mL)
-70-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
and the combined ether extracts were dried over anhydrous
Na2SOq and concentrated in vacuo. Chromatography (Si02, 5%
EtOAc-hexanes eluant} provided 189 mg of compound of formula
,~Q, (293 mg theoretical, 64%). 1H NMR (300 MHz, CDC13) 8
7.5(m, 1H), 7.2(m, 2H}, 6.9(m, 1H), 5.85(d, J = llHz, 1H),
5.29d, J = llHz, 1H), 3.7(m, 2H), 3.15(m, 1H), 1.4(m, 1H),
1.31(d, ..T = 7Hz, 3H), 1.13(d, J = 7Hz, 3H), 0.95(m, 2H),
0.85(M, 4H). Mass spec. (NH3-CI): 481(M+H+, 100%).
Step B:
To a solution of the acylated quinoxalinone of formula ~Q
(189 mg, 0.39 mmol) in CH2C12 (2 mL) at 0°C was added BF3.Et20
(110 ~,L, 0.87 nunol} and the resulting reaction mixture was
allowed to stir at 0°C for 30 minutes and stirred for an
additional hour with warniing to room temperature.. To the
reaction mixture was added MeOH (1 mL) and 15% NaOH (1 mL)
and the resulting reaction mixture was allowed to stir at
room temperature for 10 minutes. The reaction mixture was
poured onto water and extracted with CH2C12 (3x25 mL) and the
combined CH2C12 extracts were dried over anhydrous Na2S04 and
concentrates ..n vacuo. Chromatography (Si02, 25% acetone-
hexanes eluant) followed by PTLC (Si02, CH2C12 eluant)
provided 10.5 mg of the title compound, (136.5 mg
theoretical, 7.7%). 1H NMR (300 MHz, CDC13) $ 8.65(br s, 1H),
7.15(m, 2H), 6.95(m, 2H), 3.15(m, 1H), 1.4(m, 1H), 1.28(d, J
- 7Hz, 3H), 1.11(d, J = 7Hz, 3H), 0.8(m, 4H). 19F NMR (282
MHz, CDC13) $ -72.78(s, 3F). High resolution mass spec:
calculated for C18H18N202F3 (M+H}+: 351.1320; found: 351. 1299.
~L8 38
8rsparation of 4-(cy~clo~ro~ylcarboayl)-3-(Z-
cy~clopropyl~thyayl ) -3 - ( trif luosbm~tl~rl ) -3, 4 -dibydro-
quinoxalia-Z(18)-one.
-71-
CA 02334332 2000-12-07
WO 00/00478 PCT/ITS99114395
N i
~ CF3
'N O
H
The title compound was prepared in a manner similar to
the product of Example 37, except that in Step A cyclopropane
carbonyl chloride was used instead of isobutyryl chloride: iH
NMR (300 MHz, CDC13) 8 8.6(br s, 1H), 7.35(m, iH), 7.2-7.0(m,
2H), 6.9(m, 1H), 1.95(m, 1H), 1.35(m, 2H), 1.2(m, 1H), 1.0(m,
1H), 0.9(m, 1H), 0.85(m, 4H). High resolution mass spec:
calculated for Ci8H16N202F3 (M+H)*: 349.1163; found: 349.1153.
BXAHPLE 39
Preparation of 4-(athylsulfo~yl)-3-(2-cyclopropylethyayl) -3-
( tri f luoraanethyl ) -3 , 4 -dil~ydro-Quinoxalia-Z ( iH ) -one .
~02Et
N i
~I CF3
v 'N O
H
The title compound was prepared in a manner similar to
the product of Example 40, except that in Step A
ethylsulfonyl chloride was used instead of isopropylsulfonyl
chloride: 1H NMR (300 MHz, CDC13) S 8.8(br s, 1H), 7.4(m, 1H),
7.25(m, 1H), 7.15(m, 1H), 6.9(m, 1H), 3.75(p, J = 7Hz, 1H),
3.45(p, J = 7Hz, 1H), 1.5(t, J = 7Hz, 3H), 1.4(m, 1H), 0.9(m,
4H). 1SF NMR (282 MHz, CDC13) 8 -73.13(s, 3F). High
resolution mass spec: calculated for C16H16N203F3S (M+H)+:
373.0833; found: 373.0829.
~L8 40
preparation of 4-(isopropylsulfonyl)-3-(a-cyclopropylethyayl)
-3-(trifluoram~ethyl)-3,4-dihyaio-quiaoxalin-2(1H)-one.
-72-
CA 02334332 2000-12-07
WO 00100498 PCT/US99/14395
S02 i Pr
N
CF3
N O
H
Sten A:A: Preparation of compound of formula ~ wherein G = H,
R2 = cyclopropylacetyle::~e and R1 = SOOiPr
To a solution of protected quinoxalinone of formula ~ as
prepared in step C in Example 1 (250 mg, 0.61 mmol) in THF
(2.5 mL) at -78°C was added nBuLi (0.53 mL, 0.85 mmol)
followed by isopropylsulfonyl chloride (164 N,L, 1.46 mmol)
and the reaction mixture was allowed to warm to room
temperature and stir for an hour. The reaction mixture was
poured onto saturated NaHC03 and extracted with ether (3x25
mL) and the combined ether extracts were dried over anhydrous
Na2S04 and concentrated in vacuo. Chromatography (Si02, 10%
EtOAc-hexanes eluant) provided 51 mg of compound of formula
(315 mg theoretical, 16%). 1H NMR (300 MHz, CDC13) 8
7.5(m, 1H), 7.35(m, 2H), 7.2(m, 1H), 5.8(d, J = llHz, 1H),
5.15(d, J = llHz, 1H), 4.25 1H), 3.7(m, 2H), 1.65(m, 3H),
1.45(m, 4H), 0.95(m, 5H), 0.01(s, 9H). Mass spec. (NH3-CI):
534(M+NH4+, 100%).
Step B:
To a solution of the sulfonamide-quinoxalinone of formula ,$
(51 mg, 0.099 mmol) in CH2C12 (1 mL) at 0°C was added BF3.Et20
(27 ~tL, 0.22 mmol) and the resulting reaction mixture was
allowed to stir at 0°C for 30 minutes, and stirred for an
additional 1 hour with warming to room temperature. To the
reaction mixture was added MeOH (1 mL) and 15% NaOH (1 mL)
and the resulting reaction mixture was allowed to stir at
room temperature for 10 minutes. The reaction mixture was
poured onto water and extracted with CH2C12 (3x25 mL) and the
combined CH2C12 extracts were dried over anhydrous Na2S04 and
concentrated in vacuo. Chromatography/PTLC (Si02, 25%
acetone-hexanes eluant) provided 14 mg of the title compound,
(38 mg theoretical, 37%). 1H NMR (300 MHz, CDC13) S 8.63(br
-73-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99l14395
s, 1H), 7.4(m, 1H), 7.25(m, 1H), 7.15(m, 1H), 6.85(m, 1H),
4.2(m, 1H), 1.6(d, J = 7Hz, 3H), 1.45(m, 1H), 1.39(d, J =
7Hz, 3H), 0.9(m, 4H). 19F NMR (282 MHz, CDC13) 8 -73.05(x,
3F). High resolution mass spec: calculated for Cl~H1gN203F3S
(M+H)+: 387.0990; found: .387.1002.
$3C~LPLE 41
Preparation of 4-(matho~qrcarbonyl)-3-(2-cyclapropylethyayl)
-3- (trif luorcm~ethyl ) -3, 4-dihy~dro-quiaoxalia-2 ( 18) -one.
i
~ CF3
_N O
H
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A methyl
chloroformate was used instead of ethyl chloroformate: 1H NMR
(300 MHz, CDC13) s 8.45(br s, 1H), 7.25(m, 1H), 7.05(m, 2H),
6.85(m, 1H), 8.85(x, #J), 1.4(m, 1H), 0.85(m, 4H). 19F NMR
(282 MHz, CDC13) 8 -73.65(x, 3v). High resolution mass spec:
calculated for C16H14N203F3 (M+H)*: 339.0956; found: 339.0932.
25
87CA~~LI: 42
Preparation of 6-(chloro)-4-(ethoxycarbonyl)-3-(2-
cyclopropylethyayl)-3-(trifluora~etl~yl)-3,4-dib~8ro-
quiaoxalin-2(18)-one.
of
( CF3
O
The title compound was prepared in a manner similar to
the product of Example 26: 1H NMR (300 MHz, CDC13) S 8.65(br
s, 1H), 7.35(m, 1H), 7.1(m, 1H), 6.8(m, 1H), 4.45-4.3(m, 2H),
1.4(t, J = 7Hz, 3H), 1.35(m, 1H), 0.85(m, 4H). 19F NMR (282
-74-
CA 02334332 2000-12-07
WO 00/00478 PCTIUS99/14395
MHz, CDC13) b -73.54(s, 3F). High resolution mass spec:
calculated for C17H13N203F3C1 (M-H)+: 385.0566; found:
385.0570.
~AD~LE 43
8raparatioa of 6-(chloro)-4-(iaopropasycsrboayl)-3-(Z-
cyclopropylat~yayl)-3-(trifluoramatbyl)-3~4-dibydro-
quiaoxalin-2(iH)-oae.
C
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A isopropyl
chloroformate was used instead of ethyl chloroformate: iH NMR
(300 MHz, CDC13) 8 8.65(br s, 1H), 7.45(m, 1H), 7.35(m, 1H),
15(m, 1H), 6.8(m, 1H), 5.15(p, J = 7Hz, 1H), 1.4(d, J = 7Hz,
3H), 1.38(d, J = 7Hz, 3H), 1.35(m, 1H), 0.~.(m, 4H). 19F NMR
(282 MHz, CDC13) 8 -73.47(s, 3F). High resolution mass spec:
calculated for C1gH15N203F3C1 (M-H)+: 399.0723; found:
399.0719.
B'~L~~..44
Preparation of 6-(chloro)-4-(propsa-a-yl-o~eyaarbonyl)-3-(2-
cycloprapyl.thyr~yl ) -3- (trifluosro~tl~yl ) -3, 4-d,i~y8ro-
quincxalia-2(iH)-ons.
C
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A isopropenyl
-75-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
chloroformate was used instead of ethyl chloroformate: 1H NMR
(300 MHz, CDC13) 8 8.8(br s, 1H), 7.4(m, 1H), 7.15(m, 1H),
6.8(m, 1H), 4.9(m, 1H), 4.8(m, 1H), 2.05(s, 3H), 1.4(m, 1H),
0.85(m, 4H). 19F NMR (282 MHz, CDC13) S -73.60(s, 3F). High
resolution mass spec: calculated for C1gH13N203F3C1 (M-H)*:
397.0566; found: 397.0563.
ALE 45
Preparation of 6-(fluoro)-4-(othoxycarbo~yl)-3-(2-
cyclopropylathyayl)-3-(trifluoramethyl)-3,4-dihydro-
quinoxalia-a(18)-one.
The title compound was prepared in a manner similar to
the product of Example 26: 1H NMR (300 MHz, CDC13) 8 8.7(br s,
1H), 7.1(m, 1H'" 6.8(m, 2H), 4.4(m, 2H), 1.42(t, J = 7Hz,
3H), 1.4(m, 1H), 0.85(m, 4H). 1gF NMR (282 MHz, CDC13) 8
-73.54(s, 3F), -117.47(s, 1F). High resolution mass spec:
calculated for C1~H13N203F4 (M-H)*: 369.0862; found: 369.0852.
~~LS 46
Preparation of 6- ( f luoro) -4- ( isopropo~qtcari~onyl ) -3- ( 2-
cyclopropylethya~l)-3-(trifluoromethyl)-3,4-dihydro-
quinoxalia-2(18)-one.
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A isopropyl
-76-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
chloroformate was used instead of ethyl chloroformate: 1H NMFt
(300 MHz, CDC13) S 8.85(br s, 1H), 7.15(m, 1H), 6.8(m, 2H),
5.15(p, J = 7Hz, 1H), 1.45(d, J = 7Hz, 3H), 1.42(d, J = 7Hz,
3H), 1.4(m, 1H), 0.85(m, 4H). 19F lit (282 MHz, CDC13) b
-73.45(s, 3F), -117.63(s, 1F). High resolution mass spec:
calculated for C1gH15N203F4 (M-H)+: 385.1018; found: 383.1045.
ALE 47
Preparatioa of 6-(fluoro)-4-(Drapes-Z-~1-oo~carl~onyl)-3-(a-
cyclopropylet~yayl)-3-(trifluoraorethyl)-3,4-dibydro-
quiaoacalia-a ( la) -aaa.
The title compound was prepared in a manner similar to
the product of Example 26, except that in Step A isopropenyl
chloroformate was used instead of ethyl chloroformate: 1H 1w~'c,t
(300 MHz, CDC13) S 9.0(br s, 1H), 7.2(m, 1H), 6.85(m, 2H),
4.9(m, 1H), 4.8(m, 1H), 2.05(s, 3H), 1.4(m, 1H), 0.85(m, 4H).
19F NMR (2B2 MHz, CDC13) S -73.61(s, 3F), -117.10(s, 1F).
High resolution mass spec: calculated for C1gH15N203F4 (M+H)+:
383.1018; found: 383.1018.
_77-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Table 1
R
G \ N1 Rz
CF3
N 0
H
Ex. G R 1 R2 mass spec
#
1 H CH2cycPr ~C_cycpr 280.0828
2 H Me ~C~ycpl. 295.1073
3 H H nButyl 273.1211
4 H Me nButyl 287.1363
5 H H C-=C-cycPr 335.1371
6 H H allyl 257.0898
7 H allyl ~C_cycPr 321.1199
8 H benzyl ~C-cycPr 371.1365
9 H CHZcycPr allyl 311.1353
10 H propargyl ~C~ycpr 319.1057
11 H CH2CHZcycPr ~C_cycPr 349.1555
12 H isopropyl ~C_cycPr 323.1365
13 6-F allyl nButyl 330.1332
14 6-F allyl ~C_cycPi. 339.1143
15 6-F CH2cycPr ~C_cycPr 353.1265
16 6-F CH2cycPr nButyl 344.1520
17 6-CI CH2cycPr ~C_cycPr 369.0995
18 6-CI isobutyl ~C_cycPr 385.1298
19 6-Cl allyl ~C_cycPr 355.0839
20 6-CI CH2cycPr phenethyl 408.1198
21 6-Cl allyl phenethyl 395.1111
22 6-OMe CH2cycPr ~C~ycPr 365.1463
23 6-OMe allyl ~C-cycPr 351.1212
24 H CH2cycPr ~C_Me 309.1224
25 H allyl (~C_Me 295.1057
26 H COOEt ~C~ycpi, 353.1093
_78_
CA 02334332 2000-12-07
WO 00/00478 PCTNS99I14395
27 H ~COOiPr C~C_cycPr 367.1286
28 H COOC(CH2)Me C~C_cycPr 365.1011
29 H COOiBu C=C_cycPr 381.1445
30 H COOnBu C~C_cycPr 381.1422
31 H COOCH2CHCH2 CSC-cycpr 365.1120
32 H COOBn C~C~ycPr 415.1285
33 H S02nPr C$C_cycPr 387.0997
34 H COPh (~C_cycPr 385.1184
35 H COOCH2iBu C~C_cycPr 395.1288
36 H COOCH2CCCH3 C~C~ycPI. 363.0950
37 H COiPr C~C~ycpt. 351.1299
38 H COcycPr C~C~ycPr 349.11
39 H S02Et C~C~ycpl. 373.0829
40 H S02iPr ~C_cycPr 387.1002
41 H COOCH3 C=C~ycPr 339.0932
42 6-Cl COOEt C~C~ycp=, 385.0570
43 6-CI COOiPr C=C_cycPr 399.0719
44 6-Cl COOC(CH2)CH3C~C_cycpr 397.0563
-.
45 6-F COOEt ~C_cycPr 369.0852
46 6-F COOiPr C=C_cycpi. 383.1045
47 6-F COOC(CH2)CH3CsC_cycpr 383.1019
*Unless othezvuise noted, stereochemistry is (+/-).
Tables 2 and 3 show representative compounds of the
present invention. Each formula shown at the start of Table
2 and 3 is intended to be paired with each entry in the table
which follows.
_79_
CA 02334332 2000-12-07
WO 00/00478 PCTIUS99/14395
Table 2
C1 R
C1 N1 Rz C1 N1 Rz C1 N1 Rz
/ ~ CF3 ~ / ~ CF3 ( / ~ CF3
H 0 H 0 H 0
a b c
F R
F N1 Rz F N1 Rz F N1 Rz
/ ~ CF3 I / ~ CF3 ~ / ~ CF3
H O H O H O
d a f
C1 R F R R1
F Nl Rz C1 Nl Rz Me0 N R2
I / ~ CF3 I / ~ CF3 I / ~ CF3
H O H 0 H O
g h i
C1 R F R Me0 R1
Me0 Nl Rz Me0 Nl Rz Cl N R2
/ ~ CF3 ~ / ~ CF3 ~ / ~ CF3
H O H O H 0
3 ~ Z
Rl ~ O R
Me0 R2 Me Nl Rz O Nl Rz
F ( / N~ CF3 ( / ~ CF3 I / ~ CF3
H O H 0 H O
m n g
O 1
~~,, CCRz N1~~,,CCRz
HzN ~ / ~CF3 I / ~CF3
H O H O
r s
-80-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Table 2 cont
Ex. gl g2 Ex. gl g2
# #
201 -CH3 n-butyl 228 -CH -CH=CH-3-Fur
202 -CH3 benzyl 229 -CH3 -CH=CH-2-Imid
203 -CH3 phenethyl 230 -CH3 -CH=CH-5-Imid
204 -CH3 -CH2CH2-cycPr231 -CH3 -CH2C~C-CH3
205 -CH3 -CSC-CH3 232 -CH3 -CH2CfC-CF3
206 -CH3 -C~-CF3 233 -CH3 -CH2C~C-Et
207 -CH3 -C:C-Et 234 -CH3 -CH2C~C-iPr
208 -CH3 -CaC-iPr 235 -CH3 -CH2C~-cycPr
209 -CH3 -CSC-cycPr 236 -CH3 -CH2CsC-CH=CH2
210 -CH3 -CSC-1-(Me)cycPr237 -CH3 -CH2C~C-2-Fur
211 -CH3 -CSC-CH=CH2 238 -CH3 -CH2C~C-3-Fur
212 -CH3 -CSC-C(=CH2)CH3239 -CH3 -CH2C~C-2-Imid
213 -CH3 -CEC-2-pyridyl240 -CH3 -CH2C~C-5-Imid
xl4 -CH3 -CSC-3-pyridyl241 -CH3 -CH2CH=CH2
215 -CH3 -CSC-2-Fur 242 -CH3 -CH2CH=CH-CH3
216 -CH3 -CSC-3-Fur 243 -CH3 -CH2CH=CH-CF3
217 -CH3 -C3C-2-Imid 244 -CH3 -CH2CH=CH-Et
218 -CH3 -C$C-5-Imid 245 -CH3 -CH2CH=CH-iPr
219 -CH3 -CH=CH-CH3 246 -CH3 -CH2CH=CH-cycPr
220 -CH3 -CH=CH-CF3 247 -CH3 -CH2CH=CHCH=CH2
221 -CH3 -CH=CH-Et 248 -CH3 -CH2CH=C(CH3)2
222 -CH3 -CH=CH-iPr 249 -CH3 -CH2CH=CH-2-Fur
223 -CH3 -CH=CH-cycPr 250 -CH3 -CH2CH=CH-3-Fur
224 -CH3 -CH=CH-CH=CH2251 -CH3 -CH2CH=CH-2-Imid
225 -CH3 -CH=CH-2-pyridyl252 -CH3 -CH2CH=CH-5-Imid
226 -CH3 -CH=CH-3-pyridyl253 -CH3 -CH=CHCH2-cycPr
227 -CH3 -CH=CH-2-Fur 254 -CH3 -CH=CHCH2-2-Fur
a-rur szanas =or turan-Z-yl
* 3-Fur stands for furan-3-yl
* 2-Imid stands for imidazol-2-yl
* 5-Imid stands for imidazol-5-yl
-81-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Table 2 cont.
Ex. gl g2 Ex. gl g2
# #
301 -CH(CH3)2 n-butyl 328 -CH(CH3)2 -CH=CH-3-Fur
302 -CH(CH3)2 benzyl 329 -CH(CH3)2 -CH=CH-2-Imid
303 -CH(CH3)2 phenethyl 330 -CH(CH3)2 -CH=CH-5-Imid
304 -CH(CH3)2 -CH2CH2-cycPr331 -CH(CH3)2 -CH2C~-CH3
305 -CH(CH3)2 -C'd-CH3 332 -CH(CH3)2 -CH2C~-CF3
306 -CH(CH3)2 -C~-CF3 333 -CH(CH3)2 -CH2C~-Et
307 -CH(CH3)2 -CSC-Et 334 -CH(CH3)2 -CH2C~-iPr
308 -CH(CH3)2 -C---C-iPr 335 -CH(CH3)2 -CH2C~C-cycPr
309 -CH(CH3)2 -C=-C-cycPr 336 -CH(CH3)2 -CH2C=C-CH=CH2
310 -CH(CH3)2 -C=C-1-(Me)cycPr337 -CH(CH3)2 -CH2CsC-2-Fur
311 -CH(CH3)2 -C--_C-CH=CH2338 -CH(CH3)2 -CH2C~-3-Fur
312 -CH(CH3)2 -C~-C(=CH2)CH3339 -CH(CH3)2 -CH2C$C-2-Imid
313 -CH(CH3)2 -Cx-2-pyridyl340 -CH(CH3)2 -CH2C~-5-Imid
314 -CH(CH3)2 -C-C-3-pyridyl341 -CH(CH3)2 -CH2CH=CH2
315 -CH(CH3)2 -C~-2-Fur 342 -CH(CH3)2 -CH2CH=CH-CH3
316 -CH(CH3)2 -C$C-3-Fur 343 -CH(CH3)2 -CH2CH=CH-CF3
317 -CH(CH3)2 -C~-2-Imid 344 -CH(CH3)2 -CH2CH=CH-Et
318 -CH(CH3)2 -C~-5-Imid 345 -CH(CH3)2 -CH2CH=CH-iPr
319 -CH(CH3)2 -CH=CH-CH3 346 -CH(CH3)2 -CH2CH=CH-cycPr
320 -CH(CH3)2 -CH=CH-CF3 347 -CH(CH3)2 -CH2CH=CHCH=CH2
321 -CH(CH3)2 -CH=CH-Et 348 -CH(CH3)2 -CH2CH=C(CH3)2
322 -CH(CH3)2 -CH=CH-iPr 349 -CH(CH3)2 -CH2CH=CH-2-Fur
323 -CH(CH3)2 -CH=CH-cycPr350 -CH(CH3)2 -CH2CH=CH-3-Fur
324 -CH(CH3)2 -CH=CH-CH=CH2351 -CH(CH3)2 -CH2CH=CH-2-Imid
325 -CH(CH3)2 -CH=CH-2-pyridyl352 -CH(CH3)2 -CH2CH=CH-5-Imid
326 -CH(CH3)2 -CH=CH-3-pyridyl353 -CH(CH3)2 -CH=CHCH2-cycPr
327 -CH(CH3)2 -CH=CH-2-Fur354 -CH(CH3)2 -CH=CHCH2-2-Fur
-82-
CA 02334332 2000-12-07
WO 00/004?8 PCT/US99/14395
Table 2 cont.
Ex.# gl g2 Ex.# gl g2
401 -CH2CH(CH3)2n-butyl 428 -CH2CH(CH3)2-CH=CH-3-Fur
402 -CH2CH(CH3)2benzyl 429 -CH2CH(CH3)2-CH=CH-2-Imid
403 -CH2CH(CH3)2henethyl 430 -CH2CH(CH3)2-CH=CH-5-Imid
404 -CH2CH(CH3)2-CH2CH2-cycPr431 -CH2CH(CH3)2-CH2C~C-CH3
405 -CH2CH(CH3)2-CSC-CH3 432 -CH2CH(CH3)2-CH2CeC-CF3
406 -CH2CH(CH3)2-CaC-CF3 433 -CH2CH(CH3)2-CH2C~-Et
407 -CH2CH(CH3)2-C~-Et 434 -CH2CH(CH3)2-CH2C~C-iPr
408 -CH2CH(CH3)2-CSC-iPr 435 -CH2CH(CH3)2-CH2CaC-cycPr
409 -CH2CH(CH3)2-CeC-cycPr 436 -CH2CH(CH3)2-CH2CaC-CH=CH2
410 -CH2CH(CH3)2-C$C-1-(Me)cycPr437 -CH2CH(CH3)2-CH2C~C-2-Fur
411 -CH2CH(CH3)2-CSC-CH=CH2 438 -CH2CH(CH3)2-CH2C~-3-Fur
412 -CH2CH(CH3)2-CsC-C(=CH2)CH3439 -CH2CH(CH3)2-CH2C~-2-Imid
413 -CH2CH(CH3)2-CSC-2-pyridyl440 -CH2CH(CH3)2-CH2CeC-5-Imid
414 -CH2CH(CH3)2-CeC-3-pyridyl441 -CH2CH(CH3)2-CH2CH=CH2
415 -CH2CH(CH3)2-CSC-2-Fur 442 -CH2CH(CH3)2-CH2CH=CH-CH3
416 -CH2CH(CH3)2-C~-3-Fur 443 -CH2CH(CH3)2-CH2CH=CH-CF3
417 -CH2CH(CH3)2-CSC-2-Imid 444 -CH2CH(CH3)2-CH2CH=CH-Et
418 -CH2CH(CH3)2-C~-5-Imid 445 -CH2CH(CH3)2-CH2CH=CH-iPr
419 -CH2CH(CH3)2-CH=CH-CH3 446 -CH2CH(CH3)2-CH2CH=CH-cycPr
420 -CH2CH(CH3)2-CH=CH-CF3 447 -CH2CH(CH3)2-CH2CH=CHCH=CH2
421 -CH2CH(CH3)2-CH=CH-Et 448 -CH2CH(CH3)2-CH2CH=C(CH3)2
422 -CH2CH(CH3)2-CH=CH-iPr 449 -CH2CH(CH3)2-CH2CH=CH-2-Fur
423 -CH2CH(CH3)2-CH=CH-cycPr450 -CH2CH(CH3)2-CH2CH=CH-3-Fur
424 -CH2CH(CH3)2-CH=CH-CH=CH2451 -CH2CH(CH3)2-CH2CH=CH-2-Imid
425 -CH2CH(CH3)2-CH=CH-2-pyridyl452 -CH2CH(CH3)2-CH2CH=CH-5-Imid
426 -CH2CH(CH3)2-CH=CH-3-pyridyl453 -CH2CH(CH3)2-CH=CHCH2-cycPr
427 -CH2CH(CH3)2-CH=CH-2-Fur454 -CH2CH(CH3)2-CH=CHCH2-2-Fur
-83-
CA 02334332 2000-12-07
WO 00/00478 PCTNS99/14395
Table 2 cont.
Ex.#gl g2 Ex.# gl g2
SO1 -CH2CH2CH3>2n-butyl 52B -CH2CH2~t~312-CH=CH-3-Fur
502 -CH2CH2~~CH3>2benzyl 529 -~2CH2CH~CH3~2-CH=CH-2-Imid
503 -CH2CH2~3)2phenethyl 530 -CH2CH2CH(CH3)2-CH=CH-5-Imid
504 -CH2~2~~cH3)2-CH2CH2-cycPr531 -CH2~2~H3)2-CH2CgC-CH3
505 -~2CH2~H3)2-C~-CH3 532 -CH2CH2CH(~3)2-CH2C-~-CF3
-CH2~2~ -C~C-CF3 53 -CH2CH2CH -CH2 C~-Et
0 H3 ~ 2 3 W3 ) 2
6
507 -CH2CH2CH(CH3~2-C=C-Et 534 -CH2CH2Cti(CH3)2-CH2C~-iPr
5 -~2CH2~ -C=C- i Pr 5 -~2CH2CH -CH2 CSC-cyc
0 H3 ~ 2 3 3 > 2 Pr
8 5
509 -CH2CH2cH -C=-C-cYcPr 53 -CH2CH2~ -CH2C=C-CH=CH2
3 > 2 6 ~CH3 ~
2
510 -CH2~2~~cH3>2-CeC-1-(Me)cycPr537 -CH2CH2~~CH3>2-CH2CC-2-Fur
511 -CH2~2~ -C~-CH=CH2 53 -CH2CH2CH -CH2C~-3 -Fur
3 > 2 8 (CH3 )
2
512 -~2~T2~~CH3)2-CeC-C(=CH2)CH3539 -CH2CH2CH~CH3)2-CH2C~-2-Imid
513 -~2~2~~CH312-CC-2-PYridyl540 -~2CH2~CH3~2-CH2C~-5-Imid
514 -CH2Cfi2CH~CH3)2-C-=C-3-PYridyl541 -CH2~2~3)2-CH2CH=CH2
515 -~2CH2CH~CH3O2-C-C-2-Fur 542 -CH2~2CH~CH3~2-CH2CH=CH-CH3
516 -~2CH2CH~CH3>2-C=C-3-Fur 543 -~2~2~~CH3~2-CH2CH=CH-CF3
517 -CH2~2~3~2 -C=C-2-Imid 544 -~2CH2CH~CH3)2-CH2CH=CH-Et
518 -CH2CH2~~CH3)2-CSC-5-Imid 545 -CH2CH2CH3>2-CH2CH=CH-iPr
519 -CH2CH2~3)2-CH=CH-CH3 546 -~2CH2~312-CH2CH=CH-cycPr
520 -CH2CH2CH~CH3~2-CH=CH-CF3 547 -CH2CH2CH(CH3>2-CH2CH=CHCH=CH2
521 -CH2CH2~~CH3)2-CH=CH-Et 548 -~2CH2~~CH3~2-CH2CH=C(CH3)2
522 -CH2CH2CH~CH3)2-CH=CH-iPr 549 -~2CH2~t~3)2-CH2CH=CH-2-Fur
523 -~2~2~~cH3)2-CH=CH-cycPr550 -CE;2CH2~3)2-CH2CH=CH-3-Fur
524 -cH2CH2CfilCH3)2-CH=CH-CH=CH2551 -~2~2CH3)2-CH2CH=CH-2-Imid
525 -CH2CH2CH~CH3)2-CH=CH-2-pyridyl552 -CH2~2CH~CH3)2-CH2CH=CH-5-Imid
526 -CH2CH2~3)2-CH=CH-3-pyridyl553 -CH2CH2CHt~3)2-CH=CHCH2-cycPr
527 -~2CH2CH~CH3)2-CH=CH-2-Fur554 -~2CH2~312-CH=CHCH2-2-Fur
-84-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Table 2 cont.
Ex.~gl g2 Ex.~gl g2
601 -~2~2C(~3)3n-butyl 628 -~2~2C(~3)3-CH=CH-3-Fur
602 -~2~2C benzyl 629 -Cf32CFI2C -CH=CH-2-Imid
(~3 ) (CH3 )
3 3
603 -~2~2C(~3)3henethyl 630 -~2~2C(~3)3-CH=CH-5-Imid
604 -~2~2C -CH2CH2-cycPr631 -CHZCH2C -CH2C~-CH3
(~3 ) (CH3 )
3 3
60 -~2~2C -C~C-CH3 632 -CHaCH2C -CH2C=C-CF3
5 (~3 ) (CH3 )
3 3
606 -CH2CHZC(CH3)3-Cry-CF3 633 -CHZCH2C(CH3)3-CH2CeC-Et
607 -CH2CHZC(CH3>3-CSC-Et 634 -CH2CFI2C(CH3)3-CH2C~C-iPr
608 -~2~2C(~3)3-C'~-iPr 635 -~2~2C(~3)3-CH2C-=C-cycPr
609 -CH2~2C(~3)3-CSC-cycPr 636 -CHZCH2C(CH3)3-CH2C~C-CH=CH2
610 -CH2CH2C(c~i3)3-CaC-1-(Me)cycPr637 -~2~2C(~3)3-CH2C~-2-Fur
611 -CH2CHZC(CH3)3-C-sC-CH=CH2 638 -cH2CH2C(CH3)3-CH2CC-3-Fur
612 -CH2Cx2C -~C-C (=CH2 63 -~2~2C (~3 -CH2C$C-2-Imid
(Cx3 ) ) CH3 9 ) 3
3
613 -~2~2C(~3)3-CSC-2-pyridyl640 -CH2CH2C(CH3)3-CH2CeC-5-Imid
614 -CH2Cx2c -~-3-pyridyl 641 -~2~2C (~3 -CH2CH=CH2
(c:~i3 ) 3
) 3
615 -~2~2C(~3)3-CSC-2-Fur 642 -CH2CH2C(CH3)3-CH2CH=CH-CH3
616 -CHZCHZC -CSC-3-Fur 643 -~2~2C (~3 -CH2CH=CH-CF3
(CH3 > ) 3
3
617 -CHZCHZC(CH3)3-C;C-2-Imid 644 -CH2CH2C(CH3)3-CH2CH=CH-Et
618 -~2~2C(~3)3-C~C-5-Imid 645 -CH2CH2C(CA3)3-CH2CH=CH-iPr
619 -~2~2C -CH=CH-CH3 646 -CH2CH2C -CH2CH=CH-cycPr
(~3 ) (CH3 )
3 3
620 -~2~2C(~3)3-CH=CH-CF3 647 -CHZCH2C(CH3)3-CH2CH=CHCH=CH2
621 -~2~2C(~3)3-CH=CH-Et 648 -CH2CHZC(CH3)3-CH2CH=C(CH3)2
622 -CH2~2C(~3)3-CH=CH-iPr 649 -CH2CH2C(CH3)3-CH2CH=CH-2-Fur
623 -~2~2C(~3)3-CH=CH-cycPr 650 -~2~2C(~3)3-CH2CH=CH-3-Fur
624 -~2~2C -CH=CH-CH=CH2651 -CHyCtI2C -CH2CH=CH-2-Imid
(~3 ) (CH3 )
3 3
625 -~2~2C -CH=CH-2-pyridyl652 -CH2CH2C -CH2CH=CH-5-Imid
(~3 ) (CS3 )
3 3
626 -~2~2C(~3)3-CH=CH-3-pyridyl653 -CH2CH2C(CEi3)3-CH=CHCH2-cycPr
627 -CHZCH2C(CH3)3-CH=CH-2-Fur 654 -~2~2C(~3)3-CH=CHCH2-2-Fur
-85-
CA 02334332 2000-12-07
WO 00/00478 PCTNS99/14395
Table 2 cont.
Ex.#gl g2 Ex.#gl g2
701 -CH2cycPr n-butyl 728 -CH2cycPr -CH=CH-3-Fur
702 -CH2cycPr benzyl 729 -CH2cycPr -CH=CH-Z-Imid
703 -CH2cycPr henethyl 730 -CH2cycPr -CH=CH-5-Imid
704 -CH2cycPr -CH2CH2-cycPr731 -CH2cycPr -CH2C~C-CH3
705 -CH2cycPr -CsC-CH3 732 -CH2cycPr -CH2C~C-CF3
706 -CH2cycPr -C~-CF3 733 -CH2cycPr -CH2CEC-Et
707 -CH2cycPr -C$C-Et 734 -CH2cycPr -CH2C~-iPr
708 -CH2cycPr -CeC-iPr 735 -CH2cycPr -CH2C~-cycPr
709 -CH2cycPr -CSC-cycPr 736 -CH2cycPr -CH2C~-CH=CH2
710 -CH2cycPr -CSC-1-(Me)cycPr737 -CH2cycPr -CH2C~-2-Fur
711 -CH2cycPr -CeC-CH=CH2 738 -CH2cycPr -CH2C~-3-Fur
712 -CH2cycPr -CSC-C(=CH2)CH3739 -CH2cycPr -CH2C~-2-Imid
713 -CH2cycPr -C~-2-pyridyl740 -CH2cycPr -CH2C~C-5-Imid
714 -CH2cycPr -CEC-3-pyridyl741 -CH2cycPr -CH2CH=CH2
715 -CH2cycPr -CgC-2-Fur 742 -CH2cycPr -CH2CH=CH-CH3
716 -CH2cycPr -CSC-3-Fur 743 -CH2cycPr -CH2CH=CH-CF3
717 -CH2cycPr -C=C-2-Imid 744 -CH2cycPr -CH2CH=CH-Et
718 -CH2cycPr -CSC-5-Imid 745 -CH2cycPr -CH2CH=CH-iPr
719 -CH2cycPr -CH=CH-CH3 746 -CH2cycPr -CH2CH=CH-cycPr
720 -CH2cycPr -CH=CH-CF3 747 -CH2cycPr -CH2CH=CHCH=CH2
721 -CH2cycPr -CH=CH-Et 74B -CH2cycPr -CH2CH=C(CH3)2
722 -CH2cycPr -CH=CH-iPr 749 -CH2cycPr -CH2CH=CH-2-Fur
723 -CH2cycPr -CH=CH-cycPr 750 -CH2cycPr -CH2CH=CH-3-Fur
724 -CH2cycPr -CH=CH-CH=CH2751 -CH2cycPr -CH2CH=CH-2-Imid
725 -CH2cycPr -CH=CH-2-pyridyl752 -CH2cycPr -CH2CH=CH-5-Imid
726 -CH2cycPr -CH=CH-3-pyridyl753 -CH2cycPr -CH=CHCH2-cycPr
727 -CH2cycPr -CH=CH-2-Fur 754 -CH2cycPr -CH=CHCH2-2-Fur
~ ~ r n n
~
-86-
CA 02334332 2000-12-07
WO 00/00478 PCTIUS99lI4395
Table 2 cont.
Ex.~gl g2 Ex.~gl g2
II
801 -CH2CH2cycPrn-butyl 828 -CH2CH2cycPr-CH=CH-3-Fur
802 -CH2CH2cycPrbenzyl 829 -CH2CH2cycPr-CH=CH-2-Imid
803 -CH2CH2cycPrphenethyl 830 -CH2CH2cycPr-CH=CH-5-Imid
804 -CH2CH2cycPr-CH2CH2-cycPr831 -CH2CH2cycPr-CH2CsC-CH3
805 -CH2CH2cycPr-C~-CH3 832 -CH2CH2cycPr-CH2CsC-CF3
806 -CH2CH2cycPr-CSC-CF3 833 -CH2CH2cycPr-CH2C~C-Et
807 -CH2CH2cycPr-CSC-Et 834 -CH CH
2 2cYcPr -CHZC$C-iPr
808 -CH2CH2cycPr-CSC-iPr 835 -CH2CH2cycPr-CH2C~eC-cycPr
809 -CH2CH2cycPr-C=C-cycPr 836 -CH2CH2cycPr-CH2C~C-CH=CH2
810 -CH2CH2cycPr-C~-1-(Me)cycPr837 -CH2CH2cycPr-CH2C$C-2-Fur
811 -CH2CH2cycPr-CSC-CH=CH2 838 -CH2CH2cycPr-CH2C~C-3-Fur
812 -CH2CH2cycPr-CSC-C(=CH2)CH3B39 -CH2CH2cycPr-CH2CsC-2-Imid
813 -CH2CH2cycPr-CSC-2-pyridyl840 -CH2CH2cycPr-CH2CsC-5-Imid
814 -CH2CH2cycPr-C~-3-pyridyl841 -CH CH
2 2cYcpr -CH2CH=CH2
815 -CH2CH2cycPr-C=C-2-Fur 842 -CH2CH2cycPr-CH2CH=CH-CH3
816 -CH2CH2cycPr-CSC-3-Fur 843 -CH2CH2cycPr-CH2CH=CH-CF3
817 -CH2CH2cycPr-C=C-2-Imid 844 -CH2CH2cycPr-CH2CH=CH-Et
818 -CH2CH2cycPr-CSC-5-Imid 845 -CH2CH2cycPr-CH2CH=CH-iPr
B19 -CH2CH2cycPr-CH=CH-CH3 846 -CH2CH2cycPr-CH2CH=CH-cycPr
820 -CH2CH2cycPr-CH=CH-CF3 847 -CH2CH2cycPr-CH2CH=CHCH=CH2
821 -CH2CH2cycPr-CH=CH-Et B48 -CH2CH2cycPr-CH2CH=C(CH3)2
822 -CH2CH2cycPr-CH=CH-iPr 849 -CH2CH2cycPr-CH2CH=CH-2-Fur
823 -CH2CH2cycPr-CH=CH-cycPr 850 -CH2CHZcycPr-CH2CH=CH-3-Pur
824 -CH2CH2cycPr-CH=CH-CH=CH2851 -CH2CH2cycPr-CH2CH=CH-2-Imid
825 -CH2CH2cycPr-CH=CH-2-pyridyl852 -CH CH
2 2cYcpr -CH2CH=CH-5-Imid
826 -CH2CH2cycPr-CH=CH-3-pyridyl853 -CH2CH2cycPr-CH=CHCH2-cycPr
827 -CH2CH2cycPr-CH=CH-2-Fur 854 -CH2CH2cycPr-CH=CHCH2-2-Fur
-87-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Table 2 cont.
Ex.# g1 g2 Ex.#gl g2
901 -CH2CH~H2 n-butyl 928 -CH2CH=CH2-CH=CH-3-Fur
902 -CH2CH~H2 benzyl 929 -CH2CH~H2 -CH=CH-2-/mid
903 -CH2CH=CH2henethyl 930 -CH2CH~H2 -CH=CH-5-/mid
904 -CH2CH~H2 -CH2CH2-cycPr931 -CH2CH~H2 -CH2C~-CH3
905 -CH2CH~H2 -CSC-CH3 932 -CH2CH~H2 -CH2C~C-CF3
906 -CH2CH~H2 -C~-CF3 933 -CH2CH~H2 -CH2C~-Et
907 -CH2CH~H2 -C~-Et 934 -CH2CH~H2 -CH2C$C-iPr
908 -CH2CH~H2 -C-~-iPr 935 -CH2CH~H2 -CH2C~C-cycPr
909 -CH2CH~H2 -CSC-cycPr 936 -CH2CH~H2 -CH2C~C-CH=CH2
910 -CH2CH~H2 -CSC-1-(Me)cycPr937 -CH2CHxH2 -CH2C~-2-Fur
911 -CH2CH~H2 -C=C-CH=CH2 938 -CH2CH=CH2-CH2CasC-3-Fur
912 -CH2CH~H2 -CSC-C(=CH2)CH3939 -CH2CH~H2 -CH2C-~C-2-Imid
913 -CH2CH=CH2-C=-C-2-pyridyl940 -CH2CH~H2 -CH2C=C-5-Zmid
914 -CH2CH=CH2-CC-3-pyridyl941 -CH2CH~H2 -CH2CH=CH2
915 -CH2CH=CH2-CC-2-Fur 942 -CH2CH~H2 -CH2CH=CH-CH3
916 -CH2CH~H2 -C=C-3-Fur 943 -CH2CH~H2 -CH2CH=CH-CF3
917 -CH2CH~H2 -C-~-2-Imid 944 -CH2CH~H2 -CH2CH=CH-Et
918 -CH2CH=CH2-C~-5-Imid 945 -CH2CH~H2 -CH2CH=CH-iPr
919 -CH2CHxH2 -CH=CH-CH3 946 -CH2CH=CH2-CH2CH=CH-cycPr
920 -CH2CH~H2 -CH=CH-CF3 947 -CH2CH~H2 -CH2CH=CHCH=CH2
921 -CH2CH~H2 -CH=CH-Et 948 -CH2CH~Fi2-CH2CH=C (CH3
) 2
922 -CH2CH~H2 -CH=CH-iPr 949 -CH2CH~H2 -CH2CH=CH-2-Fur
923 -CH2CH~H2 -CH=CH-cycPr 950 -CH2CH~H2 -CH2CH=CH-3-Fur
924 -CH2CH~H2 -CH=CH-CH=CH2951 -CH2CH~H2 -CH2CH=CH-2-Imid
925 -CH2CH--CH2-CH=CH-2-pyridyl952 -CH2CH=CH2-CH2CH=CH-5-Imid
926 -CH2CH~H2 -CH=CH-3-pyridyl953 -CH2CH~H2 -CH=CHCH2-cycPr
927 -CH2CH~H2 -CH=CH-2-Fur 954 -CH2CH~H2 -CH=CHCH2-2-Fur
-88-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Table 2 cont.
Ex.#R1 g2 Ex.~ Rl R2
1001-C(~H2)CH3n-butyl 1028 -C(xH2)CH3-CH=CH-3-Fur
1002-C(~H2)CH3benzyl 1029 -C(~H2)CH3-CH=CH-2-Imid
1003-C(-CH2)CHgphenethyl 1030 'C(~H2)CH3-CH=CH-5-Imid
1004-C(~H2)CH3-CH2CH2-cycPr1031 -C(~H2)CH3-CH2CaC-CH3
1005-C (~H2 -C?~C-CH3 1032 -C (~H2 -CH2C~C-CF3
) CH3 ) CH3
1006-C (xH2 -C~-CF3 1033 -C (~H2 -CH2CcC-Et
) CH3 ) CH3
1007-C(~H2)CH3-C;C-Et 1034 -C(=CH2)CH3-CH2C~-iPr
1008-C(=CH2)CH3-C=C-iPr 1035 -C(~H2)CH3-CH2C~C-cycPr
1009-C(=CH2)CH3-C~-cycPr 1036 -C(~H2)CH3-CH2C~-CH=CH2
1010-C(=CH2)CH3-C~-1-(Me)cycPr1037 -C(~H2)CH3-CH2CgC-2-Fur
1011-C(xH2)CH3-C=C-CH=CH2 1038 -C(xH2)CH3-CHZC~C-3-Fur
1012-C(=CH2)CH3-CfC-C(=CH2)CH31039 -C(~H2)CH3-CH2C~C-2-Imid
1013-C(=CH2)CH3-CSC-2-pyridyl1040 -C(xH2)CH3-CH2C~C-5-Imid
1014-C(~H2)CH3-C~-3-pyridyl1041 -C(~H2)CH3-CH2CH=CH2
1015-C (= ~e~>-CSC-2-Fur 1042 -C (~H2 -CH2CH=CH-CH3
) CH3 ) CH3
1016-C(=CH2)CHg-CiC-3-Fur 1043 -C(~H2)CH3-CH2CH=CH-CF3
1017-C(~H2)CH3-CSC-2-Imid 1044 -C(=CH2)CH3-CH2CH=CH-Et
1018-C(=CH2)CH3-CEC-5-Imid 1045 -C(~H2)CH3-CH2CH=CH-iPr
1019-C(=CH2)CH3-CH=CH-CH3 1046 -C(~H2)CH3-CH2CH=CH-cycPr
1020-C(=CH2)CH3-CH=CH-CF3 104? -C(=CH2)CHg-CH2CH=CHCH=CH2
1021-C(~H2)CH3-CH=CH-Et 1048 -C(xH2)CH3-CH2CH=C(CH3)2
1022-C(=CH2)CH3-CH=CH-iPr 1049 -C(~H2)CH3-CH2CH=CH-2-Fur
1023-C(~H2)CH3-CH=CH-cycPr 1050 -C(=CH2)CH3-CH2CH=CH-3-Fur
1024-C(~H2)CH3-CH=CH-CH=CH21051 -C(aCH2)CH3-CH2CH=CH-2-Imid
1025-C(=CH2)CH3-CH=CH-2-pyridyl1052 -C(~:H2)CH3-CH2CH=CH-5-Imid
1026-C(~H2)CHg-CH=CH-3-pyridyl1053 -C(~H2)CH3-CH=CHCH2-cycPr
1027-C(~H2)CH3-CH=CH-2-Fur 1054 -C(~H2)CH3-CH=CHCH2-2-Fur
-89-
CA 02334332 2000-12-07
WO 00/00478 PCTYUS99/14395
Table 2 cont.
Ex.#gl g2 Ex.#gl g2
1101CH2CH=C(Me)2n-butyl 1128CH2CH=C(Me)2-CH=CH-3-Fur
1102CH2CH=C(Me)2benzyl 1129CH2CH=C(Me)2-CH=CH-2-Imid
1103CH2CH=C(Me)2henethyl 1130CH2CH~(Me)2-CH=CH-5-Imid
1104CH2CH=C(Me)2-CH2CH2-cycPr1131CH2CH~(Me)2-CH2C~-CH3
1105CH2CH~ -C~-CH3 1132CH2CH~ (Me)-CH2C~-CF3
(Me) 2 2
1106CH2CH~(Me)2-CSC-CP3 1133CH2CH~(Me)2-CH2C~-Et
1107CH2CH~(Me)2-CSC-Et 1134CH2CH~(Me)2-CH2C~-iPr
1108CH2CH~(Me)2-CeC-iPr 1135CH2CH=C(Me)2-CH2C=C-cycPr
1109CH2CH~(Me)2-CeC-cycPr 1136CH2CHx (Me)2-CH2C~-CH=CH2
1110CH2CH~(Me)2-CaC-1-(Me)cycPr1137CH2CH=C(Me)2-CH2C~-2-Fur
1111CH2CH~(Me)2-CeC-CH=CH2 1138CH2CH=C(Me)2-CH2C=C-3-Fur
1112CH2CH~(Me)2-C$C-C(=CH2)CH31139CH2CH=C(Me)2-CH2C=C-2-Imid
1113CH2CHx -C~-2-pyridyl1140CH2CH=C(Me)2-CH2C-~-5-Imid
(Me)2
1114CH2CH~(Me)2-C~-3-pyridyl1141CH2CH=C(Me)2-CH2CH=CH2
1115CH2CH~ -C~-2-Fur 1142CH'2CH=C -,:H2CH=CH-CH3
(Me) 2 (Me) 2
1116CH2CH~(Me)2-C~-3-Fur 1143CH2CH~(Me)2-CH2CH=CH-CF3
1117CH2CH~(Me)2-CSC-2-Imid 1144CH2CH~(Me)2-CH2CH=CH-Et
1118CH2CH~(Me)2-C~-5-Imid 1145CH2CH=C(Me)2-CH2CH=CH-iPr
1119CH2CH~(Me)2-CH=CH-CH3 1146CH2CH=C(Me)2-CH2CH=CH-cycPr
1120CH2CH=C(Me)2-CH=CH-CF3 1147CH2CH~(Me)2-CH2CH=CHCH=CH2
1121CH2CH~(Me)2-CH=CH-Et 1148CH2CHx (Me)2-CH2CH=C(CH3)2
1122CH2CH=CIMe)2-CH=CH-iPr 1149CH2CH~(Me)2-CH2CH=CH-2-Fur
1123CH2CH~(Me)2-CH=CH-cycPr 1150CH2CHx (Me)2-CH2CH=CH-3-Fur
1124CH2CH~(Me)2-CH=CH-CH=CH21151CH2CH~(Me)2-CH2CH=CH-2-Imid
1125CH2CH~(Me)2-CH=CH-2-pyridyl1152CH2CH~(Me)2-CH2CH=CH-S-Imid
1126CH2CH=C(Me)2-CH=CH-3-pyridyl1153CH2CH~(Me)2-CH=CHCH2-cycPr
1127CH2CH~(Me)2-CH=CH-2-Fur 1154CH2CH~(Me)2-CH=CHCH2-2-Fur
_gp_
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Table 2 cont.
Ex.4 gl g2 Ex.~ gl g2
1101 benzyl n-butyl 1128 benzyl -CH=CH-3-Fur
1102 benzyl benzyl 1129 benzyl -CH=CH-2-Imid
1103 benzyl phene~hyl 1130 benzyl -CH=CH-5-Imid
1104 benzyl -CH2CH2-cycPr1131 benzyl -CH2C-..C-CH3
1105 benzyl -CSC-CH3 1132 benzyl -CH2C~C-CF3
1106 benzyl -C~-CF3 1133 benzyl -CH2C~-Et
1107 benzyl -CSC-Et 1134 benzyl -CH2CeC-iPr
1108 benzyl -CeC-iPr 1135 benzyl -CH2C~C-cycPr
1109 benzyl -C'eC-cycPr 1136 benzyl -CH2CfC-CH=CH2
1110 benzyl -CSC-1-(Me)cycPr1137 benzyl -CH2CeC-2-Fur
1111 benzyl -CeC-CH=CH2 1138 benzyl -CH2C~-3-Fur
1112 benzyl -C$C-C(=CH2)CH31139 benzyl -CH2C~-2-Imid
1113 benzyl -C'C-2-pyridyl1140 benzyl -CH2C~C-5-Imid
1114 benzyl -CSC-3-pyridyl1141 benzyl -CH2CH=CH2
1115 benzyl -C~-2-Fu-r 1142 benzyl -CH2CH=CH-CH3
1116 benzyl -CeC-3-Fur 1143 benzyl -CH2CH=CH-CF3
1117 benzyl -CSC-2-Imid 1144 benzyl -CH2CH=CH-Et
1118 benzyl -CeC-5-Imid 1145 benzyl -CH2CH=CH-iPr
1119 benzyl -CH=CH-CH3 1146 benzyl -CH2CH=CH-cycPr
1120 benzyl -CH=CH-CF3 1147 benzyl -CH2CH=CHCH=CH2
1121 benzyl -CH=CH-Et 1148 benzyl -CH2CH=C(CH3)2
1122 benzyl -CH=CH-iPr 1149 benzyl -CH2CH=CH-2-Fur
1123 benzyl -CH=CH-cycPr1150 benzyl -CH2CH=CH-3-Fur
1124 benzyl -CH=CH-CH=CH21151 benzyl -CH2CH=CH-2-Imid
1125 benzyl -CH=CH-2-pyridyl1152 benzyl -CH2CH=CH-5-Imid
1126 benzyl -CH=CH-3-pyridyl1153 benzyl -CH=CHCH2-cycPr
1127 benzyl -CH=CH-2-Fur1154 benzyl -CH=CHCH2-2-Fur
-91-
CA 02334332 2000-12-07
WO 00100478 PCT/US99114395
Table 2 cont.
Ex.#R1 R2 Ex.#R1 g2
1201-CH2-C=CH n-butyl 1228-CH2-C~CH -CH=CH-3-Fur
1202-CH2-C~H benzyl 1229-CH2-C~H -CH=CH-2-Imid
1203-CH2-C=CH phenethyl 1230-CH2-C~H -CH=CH-5-Imid
1204-CH2-C-CH -CH2CH2-cycPr1231-CH2-C~CH -CH2C-C-CH3
1205-CH2-C~CH -CSC-CH3 1232-CH2-CsCH -CHZC~-CF3
1206-CH2-C-CH -CSC-CF3 1233-CH2-C~H -CH2C~C-Et
1207-CH2-G~CH -CSC-Et 1234-CH2-C~H -CHZC~-iPr
1208-CH2-C=-CH-C~-iPr 1235-CH2-C~CH -CH2C~-cycPr
1209-CH2-C=-CH-C~-cycPr 1236-CH2-C~H -CH2C~C-CH=CH2
1210-CH2-C~CH -CffC-1-(Me)cycPr1237-CH2-C=CH -CH2C~-2-Fur
1211-CH2-C~H -C-=C-CH=CH2 1238-CH2-C~H -CH2C~-3-Fur
1212-CH2-C-CH -C=C-C(=CH2)CH31239-CH2-C~H -CH2C~-2-Imid
1213-CH2-C=CH -C=C-2-pyridyl1240-CH2-C=-CH -CH2C~-5-Imid
1214-CH2-C=CH -C=C-3-pyridyl1241-CH2-C=-CH -CH2CH=CH2
1215-CH2-C=CH -C~-2-Fur ..'.42-CH2-C=CH -CH2CH=CH-CH3
1216-CH2-C~H -CSC-3-Fur 1243-CH2-C-~H -CH2CH=CH-CF3
1217-CH2-C=CH -C~-2-Imid 1244-CH2-C~CH -CH2CH=CH-Et
1218-CH2-C~H -CSC-5-Imid 1245-CH2-C~CH -CH2CH=CH-iPr
1219-CH2-CeCH -CH=CH-CH3 1246-CH2-CfCH -CH2CH=CH-cycPr
1220-CH2-C~H -CH=CH-CF3 1247-CH2-C~H -CH2CH=CHCH=CH2
1221-CH2-C~H -CH=CH-Et 1248-CH2-C~H -CH2CH=C(CH3)2
1222-CH2-C~H -CH=CH-iPr 1249-CH2-C~H -CH2CH=CH-2-Fur
1223-CH2-CcCH -CH=CH-cycPr 1250-CH2-C~H -CH2CH=CH-3-Fur
1224-CH2-C~CH -CH=CH-CH=CH21251-CH2-C~CH -CH2CH=CH-2-Imid
1225-CH2-C~CH -CH=CH-2-pyridyl1252-CH2-C~H -CH2CH=CH-5-Imid
1226-CH2-~H -CH=CH-3-pyridyl1253-CH2-C~CH -CH=CHCH2-cycPr
1227-CH2-C~H -CH=CH-2-Fur 1254-CH2-C~H -CH=CHCH2-2-Fur
-92-
CA 02334332 2000-12-07
WO 00/00478 PGT/US99114395
Table 2 cont.
Ex gl g2 Ex gi g2
.1~ .
~k
1301-C02CH3 n-butyl 1328-C02CH3 -CH=CH-3-Fur
1302-C02CH3 benzyl 1329-C02CH3 -CH=CH-2-Imid
1303-C02CH3 phenethyl 1330-C02CH3 -CH=CH-5-Imid
1304-C02CH3 -CH2CH2-cycPr1331-C02CH3 -CH2CgC-CH3
1305-C02CH3 -CSC-CH3 1332-C02CH3 -CH2C~C-CF3
1306-C02CH3 -C~-CF3 1333-C02CH3 -CH2C~C-Et
1307-C02CH3 -C~-Et 1334-C02CH3 -CH2CsC-iPr
1308-C02CH3 -C:C-iPr 1335-C02CH3 -CH2CmC-cycPr
1309-C02CH3 -CSC-cycPr 1336-C02CH3 -CH2CC-CH=CH2
1310-C02CH3 -C$C-1-(Me)cycPr1337-C02CH3 -CH2C~C-2-Fur
1311-C02CH3 -C$C-CH=CH2 1338-C02CH3 -CH2C~C-3-Fur
1312-C02CH3 -CSC-C(=CH2)CH31339-C02CH3 -CH2C~-2-Imid
1313-C02CH3 -CSC-2-pyridyl1340-C02CH3 -CH2C~C-5-Imid
1314-C02CH3 -C~-3-pyridyl1341-C02CH3 -CH2CH=CH2
1315-C02CH3 -CSC-2-Fur 1342-C~.;~'H3 -CH2CH=CH-CH3
1316-C02CH3 -CSC-3-Fur 1343-C02CH3 -CH2CH=CH-CF3
1317-C02CH3 -C~-2-Imid 1344-C02CH3 -CH2CH=CH-Et
1318-C02CH3 -CSC-5-Imid 1345-C02CH3 -CH2CH=CH-iPr
1319-C02CH3 -CH=CH-CH3 1346-C02CH3 -CH2CH=CH-cycPr
1320-C02CH3 -CH=CH-CF3 1347-C02CH3 -CH2CH=CHCH=CH2
1321-C02CH3 -CH=CH-Et 1348-C02CH3 -CH2CH=C(CH3)2
,
1322-C02CH3 -CH=CH-iPr 1349-C02CH3 -CH2CH=CH-2-Fur
1323-C02CH3 -CH=CH-cycPr 1350-C02CH3 -CH2CH=CH-3-Fur
1324-C02CH3 -CH=CH-CH=CH21351-C02CH3 -CH2CH=CH-2-Imid
1325-C02CH3 -CH=CH-2-pyYidyl1352-C02CH3 -CH2CH=CH-5-Imid
1326-CO2CH3 -CH=CH-3-pyridyl1353-C02CH3 -CH=CHCH2-cycPr
1327-C02CH3 -CH=CH-2-Fur 1354-C02CH3 -CH=CHCH2-2-Fur
i ~ a
~
-93-
CA 02334332 2000-12-07
WO 00100478 PCTNS99/14395
Table 2 cont.
Ex.#R1 R2 Ex.# R1 R2
1401-C02CH2CH3n-butyl 1428 -C02CH2CH3-CH=CH-3-Fur
1402-C02CH2CH3benzyl 1429 -C02CH2CH3-CH=CH-2-Imid
140 -C02CH2CH3phenethyl 1430 -C02CH2CH3-CH=CH-5-Imid
3
1404-C02CH2CH3-CH2CH2-cycPr1431 -C02CH2CH3-CH2C~C-CH3
1405-C02CH2CH3-CEC-CH3 1432 -C02CH2CH3-CH2C~-CF3
1406-C02CH2CH3-C~-CF3 1433 -C02CH2CH3-CH2C~C-Et
1407-C02CH2CH3-C-=C-Et 1434 -C02CH2CH3-CH2C~C-iPr
1408-C02CH2CH3-C=-C-iPr 1435 -C02CHZCH3-CH2C~-cycPr
1409-C02CH2CH3-C~-cycPr 1436 -C02CH2CH3-CH2C~-CH=CH2
1410-C02CH2CH3-C~-1-(Me)cycPr1437 -C02CH2CH3-CH2C~C-2-Fur
1411-C02CH2CH3-C=C-CH=CH2 1438 -C02CH2CH3-CH2C=C-3-Fur
1412-CO2CH2CH3-C~-C(=CH2)CH31439 -C02CH2CH3-CH2C~-2-Imid
1413-C02CH2CH3-C~-2-pyridyl1440 -C02CH2CH3-CH2C~-5-Imid
1414-C02CH2CH3-C~-3-pyridyl1441 -C02CH2CH3-CH2CH=CH2
1415-C02CH2CH3-CSC-2-Fur 1442 -C02CH2CH3-CH2CH=CH-CH3
1416-C02CH2CH3-CsC-3-Fur 1443 -C02CH2CH3-CH2CH=CH-CF3
1417-C02CH2CH3-CSC-2-Imid 1444 -C02CH2CH3-CH2CH=CH-Et
1418-C02CH2CH3-C~C-5-Imid 1445 -C02CH2CH3-CH2CH=CH-iPr
1419-C02CH2CH3-CH=CH-CH3 1446 -C02CH2CH3-CH2CH=CH-cycPr
1420-C02CH2CH3-CH=CH-CF3 1447 -C02CH2CH3-CH2CH=CHCH=CH2
1421-C02CH2CH3-CH=CH-Et 1448 -C02CH2CH3-CH2CH=C(CH3)2
1422-C02CH2CH3-CH=CH-iPr 1449 -C02CH2CH3-CH2CH=CH-2-Fur
1423-C02CH2CH3-CH=CH-cycPr 1450 -C02CH2CH3-CH2CH=CH-3-Fur
1424-C02CH2CH3-CH=CH-CH=CH21451 -C02CH2CH3-CH2CH=CH-2-Imid
1425-C02CH2CH3-CH=CH-2-pyridyl1452 -C02CH2CH3-CH2CH=CH-5-Imid
1426-C02CH2CH3-CH=CH-3-pyridyl1453 -C02CH2CH3-CH=CHCH2-cycPr
1427-C02CH2CH3-CH=CH-2-Fur 1454 -C02CH2CH3-CH=CHCH2-2-Fur
-94-
CA 02334332 2000-12-07
WO 00/00478 PCTNS99/14395
Table 2 cont.
Ex.# gl g2 Ex.# gl g2
1501 -C02CH(CH3)2n-butyl 1528 -C02CH(CH3)2-CH=CH-3-Fur
1502 -C02CH(CH3)2benzyl 1529 -C02CH(CH3)2-CH=CH-2-Imid
1503 -C02CH(CH3)2phenethyl 1530 -C02CH(CH3)2-CH=CH~ i-Imid
1504 -C02CH(CH3)2-CH2CH2-cycPr1531 -C02CH(CH3)2-CH2C~C-CH3
1505 -C02CH(CH3)2-C=C-CH3 1532 -C02CH(CH3)2-CH2C~C-CF3
1506 -C02CHtCH3)2-C~-CF3 1533 -C02CH(CH3)2-CH2C~C-Et
1507 -C02CH(CH3)2-CSC-Et 1534 -C02CH(CH3)2-CH2CsC-iPr
1508 -C02CH(CH3)2-CSC-iPr 1535 -C02CH(CH3)2-CH2C~C-cycPr
1509 -C02CH(CH3)2-CSC-cycPr 1536 -C02CH(CH3)2-CH2CsC-CH=CH2
1510 -C02CH(CH3)2-C~-1-(Me)cycPr1537 -C02CH(CH3)2-CH2CfC-2-Fur
1511 -C02CH(CH3)2-C=-C-CH=CH21538 -CD2CH(CH3)2-CH2C~C-3-Fur
1512 -C02CH(CH3)2-CSC-C(=CH2)CH31539 -C02CH(CH3)2-CH2CsC-2-Imid
1513 -C02CH(CH3)2-C$C-2-pyridyl1540 -C02CH(CH3)2-CH2CaC-5-Imid
1514 -C02CH(CH3)2-C-~-3-pyridyl1541 -C02CH(CH3)2-CH2CH=CH2
1515 -C02CH(CH3)2-C~-2-Fur 1542 -C02CH(CH3)2-CH2CH=CH-(.
,,
1516 -C02CH(CH3)2-CSC-3-Fur 1543 -C02CH(CH3)2-CH2CH=CH-CF3
1517 -C02CH(CH3)2-Cx-2-Imid 1544 -C02CH(CH3)2-~2CH=CH-Et
1518 -C02CH(CH3)2-C~-5-Imid 1545 -C02CH(CH3)2-CH2CH=CH-iPr
1519 -C02CH(CH3)2-CH=CH-CH3 1546 -C02CH(CH3)2-CH2CH=CH-cycPr
1520 -C02CH(CH3)2-CH=CH-CF3 1547 -co2cH(cH3)2-CH2CH=CHCH=CH2
1521 -C02CH(CH3)2-CH=CH-Et 1548 -C02CH(CH3)2-CH2CH=C(CH3)2
.
1522 -C02CH(CH3)2-CH=CH-iPr 1549 -C02CH(CH3)2-CH2CH=CH-2-Fur
1523 -C02CH(CH3)2-CH=CH-cycPr1550 -C02CH(CH3)2-CH2CH=CH-3-Fur
1524 -C02CH(CH3)2-CH=CH-CH=CH21551 -C02CH(CH3)2-CH2CH=CH-2-Imid
1525 -C02CH(CH3)2-CH=CH-2-pyridyl1552 -C02CH(CH3)2-CH2CH=CH-5-Imid
1526 -C02CH(CH3)2-CH=CH-3-pyridyl1553 -C02CH(CH3)2-CH=CHCH2-cycPr
1527 -C02CH(CH3)2-CH=CH-2-Fur1554 -C02CH(CH3)2-CH=CHCH2-2-Fur
-95-
CA 02334332 2000-12-07
WO 00/00478 PCTIUS99/14395
Table 2 cont.
Ex.# R1 R2 Ex.# Rl R2
1601 C02CH2CH2CH3n-butyl 1628 C02CH2CH2CH3-CH=CH-3-Fur
1602 C02CH2CH2CH3benzyl 1629 C02CH2CH2CH3-CH=CH-2-Imid
1603 C02CH2CH2CH3henethyl 1630 C02CH2CH2CH3-CH=CH-5-Imid
1604 C02CH2CH2CH3-CH2CH2-cycPr1631 C02CH2CH2CH3-CH2C~-CH3
1605 C02CH2CH2CH3-C~-CH3 1632 C02CH2CH2CH3-CH2C~-CF3
1606 C02CH2CH2CH3-C~-CF3 1633 C02CH2CH2CH3-CH2Cs~-Et
1607 C02CH2CH2CH3-C=-C-Et 1634 C02CH2CH2CH3-CH2CsC-iPr
1608 C02CH2CH2CH3-C-=C-iPr 1635 C02CH2CH2CH3-CH2C~C-cycPr
1609 C02CH2CH2CH3-C---C-cycPr1636 C02CH2CH2CH3-CH2C~-CH=CH2
1610 C02CH2CH2CH3-C~-1-(Me)cycPr1637 C02CH2CH2CH3-CH2C-C-2-Fur
1611 C02CH2CH2CH3-C=C-CH=CH2 1638 C02CH2CH2CH3-CH2C~C-3-Fur
1612 C02CH2CH2CH3-C=C-C(=CH2)CH31639 C02CH2CH2CH3-CH2C~C-2-Imid
1613 C02CH2CH2CH3-C~-2-pyridyl1640 C02CH2CH2CH3-CH2C~-5-Imid
1614 C02CH2CH2CH3-C=C-3-pyridyl1641 C02CH2CH2CH3-CH2CH=CH2
_~15 C02CH2CH2CH3-CEC-2-Fur 1642 C02CH2CH2CH3-CH2CH=CH-CH3
1616 C02CH2CH2CH3-C~-3-Fur 1643 C02CH2CH2CH3-CH2CH=CH-CF3
1617 C02CH2CH2CH3-CSC-2-Imid 1644 C02CH2CH2CH3-CH2CH=CH-Et
1618 C02CH2CH2CH3-CSC-5-Imid 1645 C02CH2CH2CH3-CH2CH=CH-iPr
1619 C02CH2CH2CH3-CH=CH-CH3 1646 C02CH2CH2CH3-CH2CH=CH-cycPr
1620 C02CH2CH2CH3-CH=CH-CF3 1647 C02CH2CH2CH3-CH2CH=CHCH=CH2
1621 C02CH2CH2CH3-CH=CH-Et 1648 C02CH2CH2CH3-CH2CH=C(CH3)2
1622 C02CH2CH2CH3-CH=CH-iPr 1649 C02CH2CH2CH3-CH2CH=CH-2-Fur
1623 C02CH2CH2CH3-CH=CH-cycPr1650 C02CH2CH2CH3-CH2CH=CH-3-Fur
1624 C02CH2CH2CH3-CH=CH-CH=CH21651 C02CH2CH2CH3-CH2CH=CH-2-Imid
1625 C02CH2CH2CH3-CH=CH-2-pyridyl1652 C02CH2CH2CH3-CH2CH=CH-5-Imid
1626 C02CH2CH2CH3-CH=CH-3-pyridyl1653 C02CH2CH2CH3-CH=CHCH2-cycPr
1627 C02CH2CH2CH3-CH=CH-2-Fur1654 C02CH2CH2CH3-CH=CHCH2-2-Fur
-96-
CA 02334332 2000-12-07
WO 00/00478 PCTNS99/14395
Table 2 cont.
Ex.#R1 g2 Ex.#Ri 1I R2 --
1701-C02~2~(~3)2n-butyl 1728-C02CH2CHtCH3)2-CH=CH-3-Fur
1702-~2~2~(~3)2benzyl 1729-~CH2CH(CEI3)2-CH=CH-2-Imid
1703-C02~2~(~3)2phenethyl 1730-C02CH2c~ItCH3)2-CH=CH-5-Imid
1704-o02CH2CH(CH3)2-CH2CH2-cycPr1731-C02Cii2CHtt~t3)2-CH2C~C-CH3
1705-C02CH2CFi(CEt3)2-CeC-CH3 1732-CO2CH2CH(CH3)2-CH2C~C-CF3
1706-coZCH2cH(cx3)2-c~c-cF3 1733-co2cHacHtcH3)a-cH2c~c-Et
1707-COZCH2CH(CH3)2-CSC-Et 1734-C02CH2CH(CH3)2-CH2C~C-iPr
1708-~2~2CH(~3)2-C;C-iPr 1735-C02CH2CH(CH3)2-CH2C~-cycPr
1709-C02Cx2CH(CH3)2-CSC-cycPr 1736-C02CH2CH(CH3)2-CH2C~-CH=CH2
1710-CO2CH2CH(CH3)2-CsC-1-(Me)cycPr1737-CO2CH2CH(CH3)2-CH2C=C-2-Fur
1711-CO2CH2CH(CH3)2-C-~-CH=CH2 1738-C02~2~(~3)2-CH2C~C-3-Fur
1712-C02CH2CH(CH3)2-~-C(=CH2)CH31739-C02CF12CH(CH3)2-CH2C;C-2-Imid
1713-C02CH2CH(CH3)2-CeC-2-pyridyl1740-C02CH2CH(CH3)2-CH2C$C-5-Imid
1714-C02CH2CH(CH3)2-CSC-3-pyridyl1741-C02CH2CtilCH3)2-cH2CH=cH2
1715-CO2cx2CFi(cH3)2-(~-2-Fur 1742-CO2CH2CH(CH3)2-CH2CH=CH-CH3
1716-C02CH2cH(CH3)2-CSC-3-Pur 1743-C02cH2CH(c~i3)2-CH2CH=CH-CF3
1717-~CH2CH(CH3)2-CSC-2-Imid 1744-C02CH2CH(CH3)2-CH2CH=CH-Et
1718-C02CH2CH(CH3)2-C~-5-Imid 1745-CO2CH2CH(CH3)2-CH2CH=CH-iPr
1719-C02~2~(~3)2-CH=CH-CH3 1746-C02~2~f~T3)2-CH2CH=CH-cycPr
1720-C02CH2CH(CH3)2-CH=CH-CF3 1747-C02CH2CH(CH3)2-CH2CH=CHCH=CH2
1721-C02CH2CH(CH3)2-CH=CH-Et 1749-C02CFI2CH(CH3)2-CH2CH=C(CH3)2
1722-C02CH2CH(CH3)2-CH=CH-iPr 1749-COaCH2CH(CH3)2-CH2CH=CH-2-Fur
1723-~CH2CHfCH3)2-CH=CH-cycPr 1750-~2CH(Cii3)2-CH2CH=CH-3-Fur
1724-CO2CH2C7i(CH3)2-CH=CH-CH=CH21751-~02CH2CH(Qi3)2-CH2CH=CH-2-Imid
1725-C02CH2~(~3)2-CH=CH-2-pyridyl1752-C02CH2CEt(CH3)2-cH2CH=cH-5-Imid
1726-C02CH2CH(CH3)2-CH=CH-3-pyridyl1753-CO2Cfi2CH(CH3>2-CH=CHCH2-cycPr
1727-CO2CH2CH(CH3)2-CH=CH-2-Fur 1754-COaCH2CH(CH3)2-CH=CHCH2-2-Fur
-97-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
Table 2 cont.
Ex.# gl R2 Ex.# R1 R2
1801 -C02(CH2)3CH3n-butyl 1828 -C02(CH2)3CH3-CH=CH-3-Fur
1802 -C02(CH2)3CH3benzyl 1829 -C02(CH2)3CH3-CH=CH-2-Imid
1803 -C02(CH2)3CH3phenethyl 1830 -C02(CH2)3CH3-CH=CH-5-Imid
1804 -C02(CH2)3CH3-CH2CH2-cycPr1831 -C02(CH2)3CH3-CH2C=C-CH3
1805 -C02(CH2)3CH3-CSC-CH3 1832 -C02(CH2)3CH3-CH2C~-CF3
1806 -C02(CH2)3CH3-C~-CF3 1833 -C02(CH2)3CH3-CH2C~C-Et
1807 -C02(CH2)3CH3-C~-Et 1834 -C02(CH2)3CH3-CH2C=C-iPr
1808 -C02(CH2)3CH3-CSC-iPr 1835 -C02(CH2)3CH3-CH2C~-cycPr
1809 -C02(CH2)3CH3-C=-C-cycPr 1836 -C02(CH2)3CH3-CH2C~-CH=CH2
1810 -C02(CH2)3CH3-C~-1-(Me)cycPr1837 -C02(CH2)3CH3-CH2C~-2-Fur
1811 -C02(CH2)3CH3-C-=C-CH=CH21838 -C02(CH2)3CHg-CH2C~C-3-Fur
1812 -C02(CH2)3CH3-C~-C(=CH2)CH31839 -C02(CH2)3CH3-CH2C=C-2-Imid
1813 -C02(CH2)3CH3-C~-2-pyridyl1840 -C02(CH2)3CH3-CH2C~-5-Imid
1814 -C02(CH2)3CH3-C$C-3-pyridyl1841 -C02(CH2)3CH3-CH2CH=CH2
1815 -C02(CH2)3CH3-CSC-2-Fur 1842 -C02(CH2)3CH3-CH2CH=CH-CH3
1816 -C02(CH2)3CH3-CSC-3-Fur 1843 -C02(CH2)3CH3-CH2CH=CH-CF3
1817 -C02(CH2)3CH3-C~-2-Imid 1844 -C02(CH2)3CH3-CH2CH=CH-Et
1818 -C02(CH2)3CH3-CW 5-Imid 1845 -C02(CH2)3CH3-CH2CH=CH-iPr
1819 -C02(CH2)3CH3-CH=CH-CH3 1846 -C02(CH2)3CH3-CH2CH=CH-cycPr
1820 -C02(CH2)3CH3-CH=CH-CF3 1847 -C02(CH2)3CH3-CH2CH=CHCH=CH2
1821 -C02(CH2)3CH3-CH=CH-Et 1848 -C02(CH2)3CH3-CH2CH=C(CH3)2
1822 -C02(CH2)3CH3-CH=CH-iPr 1849 -C02(CH2)3CH3-CH2CH=CH-2-Fur
1823 -C02(CH2)3CH3-CH=CH-cycPr1850 -C02(CH2)3CH3-CH2CH=CH-3-Fur
1824 -C02(CH2)3CH3-CH=CH-CH=CH21851 -C02(CH2)3CH3-CH2CH=CH-2-Imid
1825 -C02(CH2)3CH3-CH=CH-2-pyridyl1852 -C02(CH2)3CH3-CH2CH=CH-5-Imid
1826 -C02(CH2)3CH3-CH=CH-3-pyridyl1853 -C02(CH2)3CH3-CH=CHCH2-cycPr
1827 -COZ(CH2)3CH3-CH=CH-2-Fur1854 -C02(CH2)3CH3-CH=CHCH2-2-Fur
-98-
CA 02334332 2000-12-07
Date: 12/1/00 29 : LEONZIEE Time: 10:32:17 Al~
L~~~IJ~JCSIJ~~
\\server~name
PSCRIPT Page Separator
CA 02334332 2000-12-07
WO 00/00478 PCT/US99I14395
Table 2 cont.
Ex.# R1 R2 Ex.#R1 R2
1901 -C02CH2CH=CHZn-butyl 1928-C02CH2CH=CH2-CH=CH-3-Fur
1902 -C02CH2CH=CH2benzyl 1929-C02CH2CH=CH2-CH=CH-2-Imid
1903 -C02CHZCH=CHZphenethyl 1930-C02CH2CH=CH2-CH=CH-5-Imid
1904 -C02CH2CH=CH2-CH2CH2-cycPr1931-C02CH2CH=CH2-CH2C=C-CH3
1905 -C02CH2CH=CHZ-C~-CH3 1932-C02CH2CH=CH2-CH2C~-CF3
1906 -C02CH2CH=CHZ-Cx-CF3 1933-COZCH2CH=CH2-CH2C~-Et
1907 -COZCH2CH=CHa-C=C-Et 1934-C02CH2CH=CHZ-CH2C~-iPr
1'908-C02CH2CH=CHZ-C---C-iPr 1935-C02CH2CH=CH2-CH2C~-cycPr
1909 -C02CH2CH=CH2-C=-C-cycPr 1936-C02CH2CH=CH2-CH2C~C-CH=CH2
1910 -C02CH2CH=CH2-C-=C-1-(Me)cycPr1937-COZCH2CH=CH2-CH2C~C-2-Fur
1911 -COZCH2CH=CHZ-C~-CH=CH2 1938-C02CH2CH=CH2-CH2C-~-3-Fur
1912 -COZCH2CH=CH2-C=-C-C(=CH2)CH31939-C02CH2CH=CH2-CH2C~-2-Imid
1913 -C02CH2CH=CHZ-C=-C-2-pyridyl1940-C02CH2CH=CH2-CH2C=C-5-Imid
1914 -COZCH2CH=CHZ-C---C-3-pyridyl1941-C02CH2CH=CH2-CH2CH=CH2
1915 -C02CHZCH=CHZ-C~-2-Fur 1942-C02CH2CH=CH2-CH2CH=CH-CH3
1916 -C02CHZCH=CH2-CSC-3-Fur 1943-C02CH2CH=CH2-CH2CH=CH-CF3
1917 -COZCH2CH=CH2-CSC-2-Imid 1944-C02CH2CH=CH2-CH2CH=CH-Et
1918 -C02CHZCH=CH2-C-=C-5-Imid 1945-COZCH2CH=CH2-CH2CH=CH-iPr
1919 -C02CH2CH=CHZ-CH=CH-CH3 1946-COZCHZCH=CHZ-CH2CH=CH-cycPr
1920 -COZCHZCH=CH2-CH=CH-CF3 1947-C02CH2CH=CH2-CH2CH=CHCH=CH2
1921 -C02CH2CH=CH2-CH=CH-Et 1948-C02CH2CH=CH2-CH2CH=C(CH3)2
1922 -C02CH2CH=CH2-CH=CH-iPr 1949-C02CH2CH=CH2-CH2CH=CH-2-Fur
1923 -C02CH2CH=CH2-CH=CH-cycPr 1950-C02CH2CH=CH2-CH2CH=CH-3-Fur
1924 -C02CH2CH=CH2-CH=CH-CH=CH21951-C02CH2CH=CH2-CH2CH=CH-2-Imid
1925 -C02CH2CH=CH2-CH=CH-2-pyridyl1952-C02CH2CH=CHZ-CH2CH=CH-5-Imid
1926 -C02CH2CH=CH2-CH=CH-3-pyridyl1953-C02CH2CH=CH2-CH=CHCH2-cycPr
1927 -C02CH2CH=CH2-CH=CH-2-Fur 1954-C02CH2CH=CH2-CH=CHCH2-2-Fur
-99-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Table 2 cont.
Ex.~gl g2 Ex.# I gl g2
2001-C02CH2(C6H5)n-butyl 0 -C02CH2(C6H5)-CH=CH-3-Fur
8
2002-C02CH2(C6H5)benzyl 2029 -C02CHg(C6H5)-CH=CH-2 -Imid
2003-C02CH2(CgHS)phenethyl 2030 -COZCHZ(C6H5)-CH=CH-5-Imid
2004-C02CH2(C6H5)-CH2CH2-cycPr2031 -C02CH2(C6H5)-CH2C~-CH3
2005-C02CH2(C6H5)-C~-CH3 2032 -C02CH2(C6H5)-CH2C~C-CF3
2006-C02CH2(C6H5)-CSC-CF3 2033 -C02CH2(C6H5)-CH2C~C-Et
2007-C02CH2(C6H5)-CiC-Et 2034 -COZCHZ(C6H5)-CH2C~C-iPr
2008-COZCH2(CgHS)-C~-iPr 2035 -C02CHa(C6H5)-CH2CeC-cycPr
2009-C02CH2(C6H5)-CfC-cycPr 2036 -COZCH2(C6H5)-CH2C3C-CH=CH2
2010-COZCH2(C6H5)-C;C-1-(Me)cycPr2037 -C02CH2(C6H5)-CH2CeC-2-Fur
2011-C02CH2(C6H5)-CSC-CH=CH2 2038 -C02CHZ(C6H5)-CH2C~C-3-Fur
2012-C02CH2(C6H5)-CSC-C(=CH2)CH32039 -C02CHZ(C6H5)-CH2C~C-2-Imid
2013-C02CH2(C5H5)-C~-2-pyridyl2040 -C02CH2(C6H5)-CH2C=C-5-Imid
2014-COzCH2(C6H5)-CSC-3-pyridyl2041 -C02CH2(C6H5)-CH2CH=CH2
2015-C02CH2(CgHS)-CSC-2-Fur 2042 -C02CHZ(C6H5)-CH2CH=CH-CH3
2016-C02CH2(C6H5)-C$C-3-Fur 2043 -COZCH2(CgHg}-CH2CH=CH-CF3
2017-COyCH2(C6H5)-CeC-2-amid 2044 -G02CH2(C6H5)-CH2CH=CH-Et
2018-COZCHZ(C6H5)-CSC-5-Imid 2045 -COZCH2(C6H5)-CH2CH=CH-iPr
2019-C02CH2(C5H5)-CH=CH-CH3 2046 -C02CH2(C5H5)-CH2CH=CH-cycPr
2020-C02CH2(C6H5)-CH=CH-CF3 2047 -C02CH2(C6H5)-CH2CH=CHCH=CH2
2021-C02CH2(C6H5)-CH=CH-Et 2048 -C02CH2(C6H5)-CH2CH=C(CH3)2
2022-C02CH2(C6H5)-CH=CH-iPr 2049 -C02CH2(C6Hg)-CH2CH=CH-2-Fur
2023-COZCH2(C6H5)-CH=CH-cycPr 2050 -C02CH2(C6H5}-CH2CH=CH-3-Fur
2024-C02CH2(C6H5)-CH=CH-CH=CH22051 -C02CH2(CgHS)-CH2CH=CH-2-Imid
2025-COZCH2(C6H5)-CH=CH-2-pyridyl2052 -C02CH2(C6H5)-CH2CH=CH-5-Imid
2026-C02CH2(C6H5)-CH=CH-3-pyridyl2053 -COZCH2(C6H5)-CH=CHCH2-cycPr
2027-C02CH2(C6H5)-CH=CH-2-Fur 2054 -C02CH2(C6H5)-CH=CHCH2-2-Fur
-100-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99I14395
Table 2 cont.
Ex.# Rl R2 Ex.#Rl H2
2101 -COZcycPr n-butyl 2128-C02cycPr -CH=CH-3-Fur
2102 -COZcycPr benzyl 2129-C02cycPr -CH=CH-2-Imid
2103 -COZcycPr phenethyl 2130-C02cycPr -CH=CH-5-Imid
2104 -C02cycPr -CH2CH2-cycPr2131-C02cycPr -CH2CgC-CH3
2105 -C02cycPr -C~-CH3 2132-COZcycPr -CH2C;C-CF3
2106 -C02cycPr -CC-CF3 2133-COZcycPr -CH2C~-Et
2107 -COZcycPr -C~-Et 2134-COZcycPr -CH2C~C-iPr
2108 -COZcycPr -CSC-iPr 2135-C02cycPr -CH2C~-cycPr
2109 -C02cycPr -CSC-cycPr 2136-COZcycPr -CH2C~-CH=CH2
2110 -C02cycPr -C-~C-1-(Me)cycPr2137-CO~cycPr -CH2C=C-2-Fur
2111 -C02cycPr -CSC-CH=CH2 2138-C02cycPr -CH2C~C-3-Fur
2112 -C02cycPr -C=-C-C(=CH2)CH32139-C02cycPr -CH2C~C-2-Imid
2113 -COZcycPr -C=C-2-pyridyl2140-C02cycPr -CH2C~C-5-Imid
2114 -C02cycPr -C=C-3-pyridyl2141-C02cycPr -CH2CH=CH2
2115 -C02cycPr -CSC-2-Fur 2142-C02cycPr -CH2CH=CH-CH3
2116 -C02cycPr -C=C-3-Fur 2143-C02cycPr -CH2CH=CH-CF3
2117 -COZcycPr -CSC-2-Imid 2144-COZcycPr -CH2CH=CH-Et
2118 -C02cycPr -C=C-5-Imid 2145-C02cycPr -CH2CH=CH-iPr
2119 -C02cycPr -CH=CH-CH3 2146-C02cycPr -CH2CH=CH-cycPr
2120 -COZcycPr -CH=CH-CF3 2147-C02cycPr -CH2CH=CHCH=CH2
2121 -C02cycPr -CH=CH-Et 2148-C02cycPr -CH2CH=C(CH3)2
2122 -C02cycPr -CH=CH-iPr 2149-COgcycPr -CH2CH=CH-2-Fur
2123 -C02cycPr -CH=CH-cycPr 2150-C02cycPr -CH2CH=CH-3-Fur
2124 -C02cycPr -CH=CH-CH=CH22151-COycycPr -CH2CH=CH-2-Imid
2125 -C02cycPr -CH=CH-2-pyridyl2152-C02cycPr -CH2CH=CH-5-Imid
2126 -C02cycPr -CH=CH-3-pyridyl2153-C02cycPr -CH=CHCH2-cycPr
2127 -COZcycPr -CH=CH-2-Fur 2154-C02cycPr -CH=CHCH2-2-Fur
-101-
s,.
CA 02334332 2000-12-07
WO 00100478 PCT/US99/14395
Table 2 cont.
Ex.~ gl g2 Ex.~gl g2
2201 -C02CHZcycPrn-buty 2228-C02CH2cycPr-CH=CH-3-Fur
2202 -C02CH2cycPrbenzyl 2229-COZCHZcycPr-CH=CH-2-7snid
2203 -C02CH2cycPrphenethyl 2230-C02CH2cycPr-CH=CH-5-Imid
2204 -C02CH2cycPr-CH2CH2-cycPr2231-C02CH2cycPr-CH2C=C-CH3
2205 -C02CHZcycPr-CSC-CH3 2232-C02CHZcycPr-CH2CiC-CF3
2206 -C02CH2cycPr-C~-CF3 2233-C02CH2cycPr-CH2C~C-Et
2207 -C02CHZcycPr-CSC-Et 2234-COgCH2cycPr-CH2C~C-iPr
2208 -COZCH2cycPr-CSC-iPr 2235-COZCH2cycPr-CH2C3C-cycPr
2209 -C02CH2cycPr-CfC-cycPr 2236-COZCH2cycPr-CH2C~C-CH=CH2
2210 -C02CH2cycPr-CSC-1-(Me)cycPr2237-C02CH2cycPr-CH2C~-2-Fur
2211 -COZCH2cycPr-CSC-CH=CH2 2238-C02CH2cycPr-CH2~-3-Fur
2212 -COZCHZcycPr-CSC-C(=CH2)CH32239-COZCH2cycPr-CH2C~C-2-Imid
2213 -COZCH2cycPr-CSC-2-pyridyl2240-C02CHZcycPr-CH2C$C-S-Imid
2214 -COZCH2cycPr-CSC-3-pyridyl2241-COZCH2cycPr-CH2CH=CH2
'215 -COZCHgcycPr-CSC-2-Fur 2242-C02CHZcycPr-CH2CH=CH-CH3
2216 -COZCH2cycPr-CSC-3-Fur 2243-COgCH2cycPr-CH2CH=CH-CF3
2217 -C02CH2cycPr-CSC-2-Imid 2244-C02CHZcycPr-CH2CH=CH-Et
2218 -C02CHZcycPr-CSC-5-Imid 2245-C02CH2cycPr-CH2CH=CH-iPr
2219 -COZCH2cycPr-CH=CH-CH3 2246-C02CHZCycPr-CH2CH=CH-cycPr
2220 -COZCH2cycPr-CH=CH-CF3 2247-COZCH2cycPr-CH2CH=CHCH=CH2
2221 -C02CH2cycPr-CH=CH-Et 2248-COZCH2cycPr-CH2CH=C(CH3)2
2222 -C02CH2cycPr-CH=CH-iPr 2249-C02CH2cycPr-CH2CH=CH-2-Fur
2223 -COZCH2cycPr-CH=CH-cycPr 2250-C02CH2cycPr-CH2CH=CH-3-Fur
2224 -C02CHZcycPr-CH=CH-CH=CH22251-COZCH2cycPr-CH2CH=CH-2-Imid
2225 -C02CH2cycPr-CH=CH-2-pyridyl2252-C02CH2cycPr-CH2CH=CH-5-Imid
2226 -C02CH2cycPr-CH=CH-3-pyridyl2253-C02CH2cycPr-CH=CHCH2-cycPr
2227 -COZCH2cycPr-CH=CH-2-Fur 2254-C02CHZcycPr-CH=CHCH2-2-Fur
-102-
CA 02334332 2000-12-07
WO 00/00478 PGT/iJS99/14395
Table 2 cont.
Ex.#R1 R2 Ex.# R1 R2
2301-S02CH2CH3n-butyl 2328 -S02CH2CH3-CH=CH-3-Fur
2302-S02CH2CH3benzyl 2329 -S02CH2CH3-CH=CH-2-Imid
2303-S02CH2CH3phenethyl 2330 -S02CH2CH3-CH=CH-5-Imid
2304-S02CH2CH3-CH2CH2-cycPr2331 -S02CH2CH3-CH2C~C-CH3
2305-S02CH2CH3-CSC-CH3 2332 -S02CH2CH3-CH2C~-CF3
2306-S02CH2CH3-CSC-CF3 2333 -S02CH2CH3-CH2C~-Et
2307-S02CH2CH3-C~-Et 2334 -S02CH2CH3-CH2C~-iPr
2308-S02CH2CH3-CSC-iPr 2335 -S02CH2CH3-CH2Cx-cycPr
2309-S02CH2CH3-CeC-cycPr 2336 -S02CH2CH3-CH2C=C-CH=CH2
2310-S02CH2CH3-CSC-1-(Me)cycPr2337 -S02CH2CH3-CH2CC-2-Fur
2311-S02CH2CH3-C=C-CH=CH2 2338 -S02CH2CH3-CH2C~C-3-Fur
2312-S02CH2CH3-C~-C(=CH2)CH32339 -S02CH2CH3-CH2CaC-2-Imid
2313-S02CH2CH3-C~-2-pyridyl2340 -S02CH2CH3-CH2C~-5-Imid
2314-S02CH2CH3-CC-3-pyridyl2341 -S02CH2CH3-CH2CH=CH2
2315-S02CH2CH3-CSC-2-Fur 2342 -S02CH2CH3-t"~32CH=CH-CE:
2316-S02CH2CH3-C~-3-Fur 2343 -S02CH2CH3-CH2CH=CH-CF3
2317-S02CH2CH3-CSC-2-/mid 2344 -S02CH2CH3-CH2CH=CH-Et
2318-S02CH2CH3-C=-C-5-Imid 2345 -S02CH2CH3-CH2CH=CH-iPr
2319-S02CH2CH3-CH=CH-CH3 2346 -S02CH2CH3-CH2CH=CH-cycPr
2320-S02CH2CH3-CH=CH-CF3 2347 -S02CH2CH3-CH2CH=CHCH=CH2
2321-S02CH2CH3-CH=CH-Et 2348 -S02CH2CH3-CH2CH=C(CH3)2
2322-S02CH2CH3-CH=CH-iPr 2349 -S02CH2CH3-CH2CH=CH-2-Fur
2323-S02CH2CH3-CH=CH-cycPr 2350 -S02CH2CH3-CH2CH=CH-3-Fur
2324-S02CH2CH3-CH=CH-CH=CH22351 -S02CH2CH3-CH2CH=CH-2-Imid
2325-S02CH2CH3-CH=CH-2-pyridyl2352 -S02CH2CH3-CH2CH=CH-5-Imid
2326-S02CH2CH3-CH=CH-3-pyridyl2353 -S02CH2CH3-CH=CHCH2-cycfr
2327-S02CH2CH3-CH=CH-2-Fur 2354 -S02CH2CH3-CH=CHCH2-2-Fur
-103-
i ti
CA 02334332 2000-12-07
", f
WO 00/00478 PCT/US99/14395
Table 2 cont.
Ex gl g2 Ex. gl I g2
. )k
#
2401 -S02CH(CH3)2n-butyl 2428-S02CH(CH3)2-CH=CH-3-Fur
2402 -S02CH(CH3)2benzyl 2429-S02CH(CH3)2-CH=CH-2-Imid
2403 -S02CH(G~i3)2phenethyl 2430-S02CH(CH3)2-CH=CH-5-Imid
2404 -S02CH(CH3)2-CH2CH2-cycPr2431-S02CH(CH3)2-CH2C~-CH3
2405 -S02CH(CH3)2-CSC-CH3 2432-S02CH(CH3)2-CH2C~C-CF3
2406 -S02CH(CH3)2-C~-CF3 2433-S02CH(CH3)2-CH2C~C-Et
2407 -S02CH(CH3)2-C~-Et 2434-S02CH(CH3)2-CH2CeC-iPr
2408 -S02CH(CH3)2-C~-iPr 2435-S02CH(CH3)2-CHZC~C-cycPr
2409 -S02CH(CH3)2-CSC-cycPr 2436-S02CH(CH3)2-CH2C~C-CH=CH2
2410 -S02CH(CH3)2-C~-1-(Me)cycPr2437-S02CH(CH3)2-CH2C~-2-Fur
2411 -S02CH(CH3)2-C~-CH=CH2 2438-S02CH(CH3)2-CH2C~-3-Fur
2412 -S02CH(CH3)2-C~-C(=CH2)CH32439-S02CH(CH3)2-CH2C~-2-Imid
2413 -S02CH(CH3)2-CSC-2-pyridyl2440-S02CH(CH3)2-CH2C~C-5-Imid
2414 -S02CH(CH3)2-C$C-3-pyridyl2441-S02CH(CH3)2-CH2CH=CH2
2415 -S02CH(CH3)i'eC-2-Fur 2442-S02CH(CH3)2-CH2CH=CH-CH3
;
2416 -S02CH(CH3)2-CgC-3-Fur 2443-S02CH(CH3)2-CH2CH=CH-CF3
2417 -S02CH(CH3)2-C~-2-Imid 2444-S02CH(CH3)2-CH2CH=CH-Et
2418 -S02CH(CH3)2-CaC-5-Imid 2445-S02CH(CH3)2-CH2CH=CH-iPr
2419 -S02CH(CH3)2-CH=CH-CH3 2446-S02CH(CH3)2-CH2CH=CH-cycPr
2420 -S02CH(CH3)2-CH=CH-CF3 2447-S02CH(CH3)2-CH2CH=CHCH=CH2
2421 -S02CH(CH3)2-CH=CH-Et 2448-S02CH(CH3)2-CH2CH=C(CH3)2
2422 -S02CH(CH3)2-CH=CH-iPr 2449-S02CH(CH3)2-CH2CH=CH-2-Fur
2423 -S02CH(CH3)2-CH=CH-cycPr 2450-S02CH(CH3)2-CH2CH=CH-3-Fur
2424 -S02CH(CH3)2-CH=CH-CH=CH22451-S02CH(CH3)2-CH2CH=CH-2-Imid
2425 -S02CH(CH3)2-CH=CH-2-pyridyl2452-S02CH(CH3)2-CH2CH=CH-5-Imid
2426 -S02CH(CH3)2-CH=CH-3-pyridyl2453-S02CH(CH3)2-CH=CHCH2-cycPr
24271-S02CH(CH3)2-CH=CH-2-Fur 2454-S02CH(CH3)2-CH=CHCH2-2-Fur
I ,
-104-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Table 2 cont.
Ex.#gl g2 Ex.# gl g2
2501-C(=O)CH3 n-butyl 2528 -C(=O)CH3 -CH=CH-3-Fur
2502-C(=O)CH3 benzyl 2529 -C(=0)CH3 -CH=CH-2-Imid
2503-C(=0)CH3 phenethyl 2530 -C(=O)CH3 -CH=CH-5-Imid
2504-C(=0)CH3 -CH2CH2-cycPr2531 -C(=0)CH3 -CH2C~-CH3
2505-C(=O)CH3 -CSC-CH3 2532 -C(=0)CH3 -CH2C~-CF3
2506-C(=O)CH3 -CSC-CF3 2533 -C(=O)CH3 -CH2C~-Et
2507-C(=O)CH3 -C~-Et 2534 -C(=0)CH3 -CH2C~-iPr
2508-C(=O)CH3 -CSC-iPr 2535 -C(=O)CH3 -CH2C=-C-cycPr
2509-C(=O)CH3 -C~-cycPr 2536 -C(=O)CH3 -CH2C~-CH=CH2
2510-C(=O)CH3 -CSC-1-(Me)cycPr2537 -C(=O)CH3 -CH2C~C-2-Fur
2511-C(=O)CH3 -C$C-CH=CH2 2538 -C(=O)CH3 -CH2C~-3-Fur
2512-C(=O)CH3 -CSC-C(=CH2)CH32539 -C(=O)CH3 -CH2C~C-2-Imid
2513-C(=O)CH3 -~-2-pyridyl 2540 -C(=O)CH3 -CH2C=-C-5-Imid
2514-C(=O)CH3 -C$C-3-pyridyl2541 -C(=O)CH3 -CH2CH=CH2
2515-C(=O)CH3 -CSC-2-Fur 2542 -C(=O)~-~3-CH2CH=CH-CH3
2516-C(=O)CH3 -C~-3-Fur 2543 -C(=0)CH3 -CH2CH=CH-CF3
2517-C(=O)CH3 -CaC-2-Imid 2544 -C(=O)CH3 -CH2CH=CH-Et
2518-C(=O)CH3 -C~-5-Imid 2545 -C(=O)CH3 -CH2CH=CH-iPr
2519-C(=O)CH3 -CH=CH-CH3 2546 -C(=O)CH3 -CH2CH=CH-cycPr
2520-C(=0)CH3 -CH=CH-CF3 2547 -C(=O)CH3 -CH2CH=CHCH=CH2
2521-C(=0)CH3 -CH=CH-Et 2548 -C(=O)CH3 -CH2CH=C(CH3)2
2522-C(=0)CH3 -CH=CH-iPr 2549 -C(=O)CH3 -CH2CH=CH-2-Fur
2523-C(=O)CH3 -CH=CH-cycPr 2550 -C(=O)CH3 -CH2CH=CH-3-Fur
2524-C(=0)CH3 -CH=CH-CH=CH22551 -C(=O)CH3 -CH2CH=CH-2-Imid
2525-C(=O)CH3 -CH=CH-2-pyridyl2552 -C(=O)CH3 -CH2CH=CH-5-Imid
2526-Cf=O)CH3 -CH=CH-3-pyridyl2553 -C(=O)CH3 -CH=CHCH2-cycPr
2527!-C(=0)CH3 -CH=CH-2-Fur 2554 -C(=O)CH3 -CH=CHCH2-2-Fur
~ ~ ~ I
-105-
f
CA 02334332 2000-12-07
WO 00100478 PCT/US99/14395
Table 2 cont.
Ex.# R1 'R2 Ex.#R1 R2
2601 -C(=0)CH2CH3n-butyl 2628-C(=O)CH2CH3-CH=CH-3-Fur
2602 -C(=0)CH2CH3benzyl 2629-C(=O)CH2CH3-CH=CH-2-Imid
2603 -C(=O)CH2CH3phenethyl 2630-C(=O)CH2CH3-CH=CH-S-Imid
2604 -C(=0)CH2CH3-CH2CH2-cycPr2631-C(=O)CH2CH3-CH2C~C-CH3
2605 -C(=0)CH2CH3-C~-CH3 2632-C(=O)CH2CH3-CH2C~-CF3
2606 -C(=0)CH2CH3-CSC-CF3 2633-C(=O)CH2CH3-CH2C~-Et
2607 -C(=O)CH2CH3-CaC-Et 2634-C(=O)CH2CH3-CH2CteC-iPr
2608 -C(=O)CH2CH3-C~-iPr 2635-C(=O)CH2CH3-CH2C~-cycPr
2609 -C(=O)CH2CH3-CSC-cycPr 2636-C(=O)CH2CH3-CH2C~C-CH=CH2
2610 -C(=O)CH2CH3-Cf=C-1-(Me)cycPr2637-C(=O)CH2CH3-CH2C~C-2-Fur
2611 -C(=O)CH2CH3-CffgC-CH=CH22638-C(=O)CH2CH3-CH2C$C-3-Fur
2612 -C(=O)CH2CH3-C~-C(=CH2)CH32639-C(=OICH2CH3-CH2CaC-2-Imid
2613 -C(=0)CH2CH3-CSC-2-pyridyl2640-C(=O)CH2CH3-CH2C~C-5-Imid
2614 -c(=O)CH2cH3-Cf~C-3-pyridyi2641-C(=o)cH2cH3-CH2cH=cH2
2615 -C(=0)CH2CH3-C$C-2-Fur '?r42-C(=O)CH2CH3-CH2CH=CH-CH3
2616 -C(=0)CH2CH3-CSC-3-Fur 2643-C(=O)CH2CH3-CH2CH=CH-CF3
2617 -C(=O)CH2CHg-CSC-2-Imid 2644-C(=O)CH2CH3-CH2CH=CH-Et
2618 -C(=0)CH2CH3-CsC-5-Imid 2645-C(=O)CH2CH3-CH2CH=CH-iPr
2619 -C(=0)CH2CH3-CH=CH-CH3 2646-C(=O)CH2CH3-CH2CH=CH-cycPr
2620 -C(=O)CH2CH3-CH=CH-CF3 2647-C(=0)CH2CH3-CH2CH=CHCH=CH2
2621 -C(=0)CH2CH3-CH=CH-Et 2648-C(=O)CH2CH3-CH2CH=C(CH3)2
2622 -C(=O)CH2CH3-CH=CH-iPr 2649-C(=O)CH2CH3-CH2CH=CH-2-Fur
2623 -C(=O)CH2CH3-CH=CH-cycPr 2650-C(=0)CH2CH3-CH2CH=CH-3-Fur
2624 -C(=O)CH2CH3-CH=CH-CH=CH22651-C(=O)CH2CH3-CH2CH=CH-2-Imid
2625 -C(=0)CH2CH3-CH=CH-2-pyridyl2652-C(=O)CH2CH3-CH2CH=CH-5-Imid
2626 -C(=0)CH2CH3-CH=CH-3-pyridyl2653-C(=O)CH2CH3-CH=CHCH2-cycPr
2627 -C(=0)CH2CH3-CH=CH-2-Fur 2654-C(=O)CH2CH3-CH=CHCH2-2-Fur
-106-
CA 02334332 2000-12-07
~yC 00~7g PCTNS99/14395
Table 2 cont.
Ex.# gl g2 Ex.# gl g2
2701 -C(=O)CH2cH2CH3n-butyl 2728 -C(~)CH2CH2CH3-CH=CH-3-Fur
2702 -C(~)CH2CH2CH3benzyl 2729 -C(=O)CH2CH2CH3-CH=CH-2-Imid
2703 -C(~)Cxacx2ctt3henethyl 2730 -C(~>C7i2CH2CH3-CH=CH-5-Imid
2704 -C(=o>a~2CH2CH3-CH2CH2-cycPr2731 -C(=O)CH2CH2cx3=CH2CgC-CH3
2705 -C(~)CHZCH2CH3-CSC-CH3 2732 -c(~l~igCH2CH3-CH2CsC-CF3
2706 -C(=O)c~2cx2cx3-~C-CF3 2733 -C(~)CEIZCH2CH3-CH2C~-Et
2707 -C(=o)c~IZCHZCHg-~C-Et 2734 -c(~)CH2c~I2CH3-CH2CsC-iPr
2708 -C(~)CH2CH2CH3-C~-iPr 2735 -C(~)CH2CH2c~3_CH2~_cycPr
2709 -C(~)CEI2CFt2CH3-CSC-cycPr 2736 -C(=0)CH2csi2C~i3-CH2C=C-CH=CH2
2710 -C(~)CH2CH2CH3-CeC-1-(Me)cYcPr2737 -C(~)CHZCH2CH3-CH2C~C-2-Fur
2711 -C(=O)CH2CFi2Ctt3-CSC-CH=CH2 2738 -C(~)cx2CH2CH3-CH2C=C-3-Fur
2712 -C(=olcxZCx2cx3-C!~-C(=CH2)CH32739 -c(~)CHaCH2CH3-CH2CC-2-amid
2713 -C(=O)CHZCH2CH3-CSC-2-pyridyl2740 -C(~)CH2Cf32CH3-CH2C~C-5-Imid
2714 -c(=o)CH2CH2CH3-C=C-3-pyridyl2741 -C(~)CH2CH2CH3-CH2CH=CH2
2715 -C(=O)CH2CH2CH3-C~-2-Fur 2742 -C(~>CHZCH2C~I3-CH2CH=CH-CH3
2716 -C(~)CH2CH2CH3-C~-3-Fur 2743 -C(~)CH2CH2CH3-CH2CH=CH-CF3
2717 -c(=o)cH2cH2cx3-C~-2-Imid 2744 -C(=O)CHgCS2cci3-CH2CH=CH-Et
2718 -C(=O)CH2CH2c~3-C=C-5-Imid 2745 -C(=O)CHZCH2CH3-CH2CH=CH-iPr
2719 -C(=O)CHZCH2CH3-CH=CH-CH3 2746 -C(=O)CHaCH2CH3-CH2CH=CH-cycPr
2720 -C(=O)CHZCH2CH3-CH=CH-CF3 2747 -C(~)CH2CH2CH3-CH2CH=CHCH=CH2
2721 -C(=O)CH2CH2CH3-CH=CH-Et 2748 -C(~)CH2Cii2CH3-CH2CH=C(CH3)2
2722 -C(~)CHZCH2CH3-CH=CH-iPr 2749 -C(~)CHaCH2CH3-CH2CH=CH-2-Fur
2723 -C(~)CH2CH2Qi3-CH=CH-cycPr2750 -C(~)CHyCH2CEi3-CH2CH=CH-3-Fur
2724 -C(~)Cli2Cti2CH3-CH=CH-CH=CH22751 -C(~)CH2CH2CH3-CH2CH=CH-2-Imid
2725 -C(~)CHZC~i2CH3-CH=CH-2-pyridyl2752 -C(~)CH2CH2CH3-CH2CH=CH-5-Imid
2726 -C(~)CH2CH2Cti3-CH=CH-3-pyridyl2753 -C(~)CH2CH2CA3-CH=CHCH2-cycPr
2727 -C(~)CH2CH2CH3-CH=CH-2-Fur2754 -C(~)CH2CH2CH3-CH=CHCH2-2-Fur
-107-
CA 02334332 2000-12-07
WO 00/00478 PCTNS99/14395
Table 2 cont.
Ex.# Rl R2 Ex.~ I Rl ~- R2
2801 -C(=o)CH(cH3)2n-butyl 2828 -C(=0)CH(Cti3)2-CH=CH-3-Fur
2802 -C(~)CHICH3>2benzyl 2829 -C(~)CH(Cx3)2-CH=CH-2-Imid
2803 -C(~)CH(C1~3)2phenethyl 2830 -C(=o)CH(c~3)2-CH=CH-5-Imid
2804 -C(~)CH(CH;)2-CH2CH2-cycPr2831 -C(~)CH(CS3>2-CH2C~-CH3
2805 -C(=o)cH(cx3)2-CSC-cH3 2832 -C(=o)CH(Cx3>2-CH2C~-CF3
2806 -C(~)CH(CH3)2-CSC-CF3 2833 -C(~)c3i1CH3)2-CH2C~-Et
2807 -C(~)CH(Cx3)2-CaC-Et 2834 -C(~)CH(CH3)2-CH2C;C-iPr
2808 -C(=o)CH(cx3)2-C~-iPr 2835 -c(~)CH(CH;)2-CH2C~C-cycPr
2809 -C(~)CH(Cti3)2-C~-cycPr 2836 -C(=O)Cx(CH3)2-CH2C~C-CH=CH2
2810 -c(~)CH(CH3)2-CSC-1-(Me)cycPr2837 -C(=O)CH(CH3)2-CH2CsC-2-Fur
2811 -CI=0)CH(CH3)2-C~-CH=CH2 2838 -C(~)CH(CH3)2-CH2C~-3-Fur
2812 -C(=O)CH(CH3)2-CeC-C(=CH2)CH32839 -C(=O)CIi(CH3)2-CH2CC-2-Imid
2813 -C(~)CH(CH3)2-CaC-2-pyridyl2840 -C(~)CH(CH3)g-CH2C=C-5-Imid
2814 -C1~)CH(CH3)2-C~-3-pyridyl2841 -C(~)CH(Cti3)2-CH2CH=CH2
2815 -C(~)C8(CH3)2-CSC-2-Fur 2842 -C(~)CH(CH3)2
--~H2CH=CH-CH3
2816 -C(~)cxlc~i3)2-C~-3-Fur 2843 -C(~)CH(Cx3)2-CH2CH=CH-CF3
2817 -c(=0)CH(CH3)2-CSC-2-Imid 2844 -C(~)CH(CH3)2-CH2CH=CH-Et
2818 -C(~)CH(CHg)2-CaC-5-Imid 2845 -C(~)CH(CH3)2-CH2CH=CH-iPr
2819 -C(~)CH(CH3)2-CH=CH-CH3 2846 -C(~)CfiiCH3)2-CH2CH=CH-cycPr
2820 -C(=0)CH(CH3)2-CH=CH-CF3 2847 -C(~)CH(CH3)2-CH2CH=CHCH=CH2
2821 -C(~)CH(CH3)2-CH=CH-Et 2848 -C1~)C~IlCH3)2-CH2CH=C(CH3)2
2822 -c(~)CHICx3)2-CH=CH-iPr 2849 -C(~)Cx(C83)2-CH2CH=CH-2-Fur
2823 -C(~)CFI(CHg)2-CH=CH-cycPr2850 -C(~)CH(CH3)2-CH2CH=CH-3-Fur
2824 -C(~)CH(CH3)2-CH=CH-CH=CH22851 -CLO)CEi(CEI3)2-CH2CH=CH-2-Imid
2825 -C(=O)CH(CH3)2-CH=CH-2-pyridyl2852 -C(~)CFi(C~t312-CH2CH=CH-5-Imid
2926 -C(s0)CH(CH3)2-CH=CH-3-pyridyl2853 -C(~)CH(CH3)2-CH=CHCH2-cycPr
2827 -C(=O)Cx(CH3)2-CH=CH-2-Fur2854 -C(~)CH(CH3)2-CH=CHCH2-2-Fur
-108-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Table 2 cont.
Ex.#R1 R2 Ex.# R1 R2
2901-C(=0)cycPrn-butyl 2928 -C(=O)cycPr-CH=CH-3-Fur
2902-C(=O)cycPrbenzyl 2929 -C(=0)cycPr-CH=CH-2-Imid
2903-C(=O)cycPrphenethyl 2930 -C(=O)cycPr-CH=CH-5-Imid
2904-C(=0)cycPr-CH2CH2-cycPr2931 -C(=O)cycPr-CH2C=-C-CH3
2905-C(=0)cycPr-C=-C-CH3 2932 -C(=O)cycPr-CH2C~C-CF3
2906-C(=O)cycPr-CSC-CF3 2933 -C(=O)cycPr-CH2C~-Et
2907-C(=O)cycPr-C-~-Et 2934 -C(=O)cycPr-CH2C~-iPr
2908-C(=0)cycPr-C'=C-iPr 2935 -C(=O)cycPr-CH2C~-cycPr
2909-C(=O)cycPr-C~-cycPr 2936 -C(=O)cycPr-CH2C~-CH=CH2
2910-C(=O)cycPr-C=C-1-(Me)cycPr2937 -C(=O)cycPr-CH2C~-2-Fur
2911-C(=0)cycPr-C=-C-CH=CH22938 -C(=O)cycPr-CH2C~-3-Fur
2912-C(=O)cycPr-CSC-C(=CH2)CH32939 -C(=O)cycPr-CH2C$C-2-Imid
2913-C(=O)cycPr-C-=C-2-pyridyl2940 -C(=O)cycPr-CH2C~C-5-Imid
2914.-C(=O)cycPr-CSC-3-pyridyl2941 -C(=O)cycPr-CH2CH=CH2
2915-C(=O)c;-r.Pr-C=C-2-Fur 2942 -C(=0)cycPr-CH2CH=CH-CH3
2916-C(=0)cycPr-C-=C-3-Fur 2943 -C(=O)cycPr-CH2CH=CH-CF3
2917-C(=0)cycPr-C=C-2-Imid 2944 -C(=0)cycPr-CH2CH=CH-Et
2918-C(=0)cycPr-C=C-5-/mid 2945 -C(=O)cycPr-CH2CH=CH-iPr
2919-C(=0)cycPr-CH=CH-CH3 2946 -C(=0)cycPr-CH2CH=CH-cycPr
2920-C(=O)cycPr-CH=CH-CF3 2947 -C(=O)cycPr-CH2CH=CHCH=CH2
2921-C(=O)cycPr-CH=CH-Et 2948 -C(=O)cycPr-CH2CH=C(CH3)2
2922-C(=O)cycPr-CH=CH-iPr 2949 -C(=O)cycPr-CH2CH=CH-2-Fur
2923-C(=O)cycPr-CH=CH-cycPr2950 -C(=O)cycPr-CH2CH=CH-3-Fur
2924-C(=O)cycPr-CH=CH-CH=CH22951 -C(=0)cycPr-CH2CH=CH-2-Imid
2925-C(=OcycPr -CH=CH-2-pyridyl2952 -C(=O)cycPr-CH2CH=CH-5-Imid
2926-C(=O)cycPr-CH=CH-3-pyridyl2953 -C(=0)cycPr-CH=CHCH2-cycPr
2927-C(=0)cycPr-CH=CH-2-Fur2954 -C(=O)cycPr-CH=CHCH2-2-Fur
*Unless otherwise noted, stereochemistry is (+/-) and in R2
all double bonds are traps.
-109-
CA 02334332 2000-12-07
PCT/US99/14395
Table 3
i ~2 1 R2 Me i R2 1 R
Cl N ~ F N ~ O N ~ N
/ ~ CF3 ~ / ~ CF3 ~ / ~ CF3 ~ / ~ CF3
O ~ O ~ O ~ 0
a b c d
C1 '"1 RZ F "1 R2 Oe a'1 RZ "1 F2
\ ~ CF3 ( \ ~ CF3 ~~i ~ ~ CF3 I '~ ~O F3
N ~ O N ~T O N ~ O N
f Q h
C1 R1 R2 F Ri R2 Me C1 Ri R2 Me F R1 R2
N N a 1J No
~CF3 ! \ ~CF3 i ~ ~CF3 I ~CF3
N H O N ~,,~0 N ~ O N # O
to ~ j k 1
C1 Ri RZ F i R2 C1 R1 RZ F R1 RZ
C1 N C1 ~ F FF
\ ~ CF3 I ~ CF3 I ~ ~ CF3 I \ ~ CF3
N ~ 0 N ~T O N ~ 0 N ~ O
m a p Q
C1 Ni RZ F "'1 R2 oe Ni R2 "1 R
N / ~ CF3 N / ~ CF3 lN.~~ ~0 F3 N / ~O F3
~ ° ~ °
r s t a
C1 Ri R2 F R1 R2 Me C1 Ri RZ Me F R1 R2
N N 0 00
CF3 CF3 CF3 CF3
N / ~ N \ ~ N / ~ N / ~O
O # O ~ O
v w x y
C1 ~i R2 F R1 RZ ~1 Ra
N / ~ CP; N ~ ~ CF3 N~\~ ~ CFg
0 ~ O ~ ~ O
z as bb
-110-
CA 02334332 2000-12-07
WO 00100478 PCTNS99/14395
Table 3 cont.
Ex.# R1 R2
4001 -CHZ-CH=CH2 n-butyl
4002 -CH2-CH=CH2 benzyl
4003 -CHz-CH=CH2 phenethyl
4004 -CHZ-CH=CH2 -CH2CH2-cycPr
4005 -CH2-CH=CH2 -CaC-CH3
4006 -CHZ-CH=CH2 -C---C-CF3
4007 -CH2-CH=CHZ -C-=C-Et
4008 -CH2-CH=CH2 -C=C-iPr
4009 -CH2-CH=CH2 -C=C-cycPr
4010 -CH2-CH=CH2 -C-=C-1-(Me)cycPr
4011 -CHZ-CH=CH2 -C---C-CH=CH2
4012 -CH2-CH=CH2 -CH=CH-CH3
4013 -CH2-CH=CH2 -CH=CH-CF3
4014 -CH2-CH=CH2 -CH=CH-Et
4015 -CH2-CH=CHZ -CH=CH-iPr
4016 -CH2-CH=CH2 -CH=CH-cycPr
4017 -Cii2-CH=CH2 -CH=CH-CH=CH2
4018 -CH2-CH=CH2 -CH2-C-_-C-CHg
4019 -CHZ-CH=CH2 -CHZ-C=C-CF3
4020 -CH2-CH=CHZ -CH2-C-C-Et
4021 -CH2-CH=CH2 -CH2-CSC-iPr
4022 -CH2-CH=CHZ -CH2-C---C-cycPr
4023 -CH2-CH=CH2 -CH2-C=C-CH=CH2
4024 -CH2-CH=CH2 -CH2-CH=CH2
4025 -CHZ_CH=CHZ -CHZ-CH=CH-CH3
4026 -CH2-CH CH2 -CH2-CH~Fi-CF3
4027 -CH2-CH=CH2 -CH2-CH=CH-Et
4028 -CH2-CH=CH2 -CH2-CH~:H-iPr
4029 -CH2-CH--CHZ -CH2-CH=CH-cycPr
4030 -CH2-CH=CH2 -CH2-CH=CH-CH=CH2
403 1 -CHZ-CH=CH2 -CH2-CH=C(CH3)2
-111-
CA 02334332 2000-12-07
WO 00!00478 PC'TNS99/14395
4032 -CH2-CH=CH2 -CH=CH-CH2-cycPr
4033 -CH2-CH=CH2 n-butyl
4034 -CH2-cycPr benzyl
4035 -CH2-cycPr phenethyl
4036 -CH2-cycPr -CH2CH2-cycPr
4037 -CH2-cycPr -CSC-CH3
4038 -CH2-cycPr -C---C-CF3
4039 -CH2-cycPr -CSC-Et
4040 -CH2-cycPr -C-~-ipr
4041 -CH2-cycPr -CSC-cycPr
4042 -CH2-cycPr -C--C-1-(Me)cycPr
4043 -CH2-cycPr -C--=C-CH=CH2
4044 -CHZ-cycPr -CH=CH-CH3
4045 -CH2-cycPr -CH=CH-CF3
4046 -CH2-cycPr -CH=CH-Et
4047 -CHZ-cycPr -CH=CH-iPr
4048 -CH2-cycPr -CH=CH-cycPr
4049 -CH2-cycPr _
4050 -CH2-cycPr -CH2_C~C-CH3
4051 -CH2-cycPr -CH2-C=C-CF3
4052 -CH2-cycPr -CH2-CSC-Et
4053 -CH2-cycPr -CH2-C=C-iPr
4054 -CH2-cycPr -CH2-C-~C-cycPr
4055 -CHZ-cycPr -CHZ-CSC-CH=CH2
4056 -CH2-cycPr -CH2-CH=CHZ
4057 -CH2-cycPr -CH2-CH=CH-CH3
4058 -CH2-cycPr -CH2-CH=CH-CF3
4059 -CH2-cycPr -CH2-CH=CH-Et
4060 -CH2-cycPr -CH2-CH--CH-iPr
4061 -CHZ-cycPr -CH2-CH=CH-cycPr
4062 -CH2-cycPr -CH2-CHI-CH=CH2
4063 -CH2-cycPr -CH2-CH=C(CH3)2
4064 -CH2-cycPr -CH=CH-CH2-cycPr
4065 -C02CH2CH3 n-butyl
-112-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14398
4066 -C02CH2CH3 benzyl
4067 -C02CH2CH3 phenethyl
4068 -C02CH2CH3 -CHZCH2-cycPr
4069 -COZCHZCH3 -C=C-CH3
4070 -C02CH2CH3 -C-=C-CF3
4071 -C02CH2CH3 -~C-Et
4072 -C02CH2CH3 -~C-iPr
4073 -COZCH2CH3 -C~-cycPr
4074 -C02CH2CH3 -C~-1-(Me)cycPr
4075 -C02CH2CH3 -C.C-CH=CH2
4076 -COZCH2CH3 -CH=CH-CH3
4077 -C02CH2CH3 -CH=CH-CF3
4078 -C02CH2CH3 -CH=CH-Et
4079 -C02CH2CH3 -CH=CH-iPr
4080 -C02CH2CH3 -CH=CH-cycPr
4081 -C02CH2CH3 -CH=CH-CH=CH2
4082 -C02CH2CH3 -CH2-C-=C-CH3
4083 -C02CH2CH3 -CH2-CSC-CF3
4084 -COzCH2CH3 -CHZ-C=C-Et
4085 -C02CH2CH3 -CH2-C-d-iPr
4086 -C02CHZCH3 -CHZ-C~-cycPr
4087 -C02CHZCH3 -CH2-C=-C-CH=CH2
4088 -C02CH2CH3 -CH2-CH--CH2
4089 -C02CH2CH3 -CH2-CH=CH-CH3
4090 -C02CH2CH3 -CHZ-CH=CH-CF3
4091 -C02CHZCH3 -CHZ-CH--CH-Et
4092 -C02CH2CH3 -CH2_~~H-iPr
4093 -C02CH2CH3 -CH2_~~-cycPr
4094 -C02CH2CH3 -CH2-CH=CH-CH=CHZ
4095 -COZCH2CH3 -CH2-CH--C(CH3)2
4096 -COZCH2CH3 -CH=CH-CH2-cycPr
4097 -C02CH(CH3)2 n-butyl
4098 -C02CH(CH3)2 benzyl
4099 -C02CH(CH3)2 phenethyl
-113-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
4101 -C02CH(CH3)2 -CH2CH2-cycPr
4102 -C02CH(CH3)2 -CSC-CH3
4103 -CO2CH(CH3)2 -C$C-CF3
4104 -C02CH(CH3)2 -C=-C-Et
4105 -C02CH(CH3)2 -~-iPr
4106 -C02CH(CH3)2 -~-cycPr
4107 -CO2CH(CH3)2 -C=C-1-(Me)cycPr
4108 -C02CH ( CH3 ) -~-~=~2
2
4109 -C02CH(CH3)2 -CH=CH-CH3
4110 -C02CH(CH3)2 -CH=CH-CF3
4111 -C02CH(CHg)2 -CH=CH-Et
4112 -C02CH(CH3)2 -CH=CH-iPr
4113 -C02CH(CH3)2 -CH=CH-cycPr
4114 -C02CH(CH3)2 -CH=CH-CH=CH2
4115 -C02CH(CH3)2 -CH2-C=C-CH3
4116 -C02CH(CH3)2 -CH2-C$C-CF3
4117 -C02CH(CH3)2 -CH2-C=C-Et
4118 -C02CH(CH3)2 -CH2-C~C-iPr
4119 -CO2CH(CH3)2 -CH2-C=C-cycPr
4120 -C02CH(CH3)2 -CH2-CSC-CH=CH2
4121 -C02CH(CH3)2 -CH2-CH=CH2
4122 -CO2CH(CH3)2 -CH2-CH=CH-CH3
4123 -C02CH (CH3 ) 2 -CH2-CH~Fi-CF3
4124 -C02CH(CH3)2 -CH2-CH=CH-Et
4125 -C02CH(CH3)2 -CH2-CH=CH-iPr
4126 -CO2CH(CH3)2 -CH2-CH=CH-cycPr
4127 -C02CH (CH3 ) 2 -CH2-CH~FI-CH=CH2
4128 -CO2CH(CH3)2 -CH2-CH=C(CH3)2
4129 -C02CH(CH3)2 -CH=CH-CH2-cycPr
4130 -CO2C(=CH2)CH3 n-butyl
4131 -C02C(=CH2)CH3 benzyl
4132 -CO2C(=CH2)CH3 phenethyl
4133 -C02C(=CH2)CH3 -CH2CH2-cycPr
4134 -C02C(=CH2)CH3
-114-
CA 02334332 2000-12-07
WO 00/00478 PCTNS99/14395
4135 -C02C(=CH2)CH3 -C=C-CF3
4136 -C02C(=CH2)CH3 -C=C-Et
4137 -C02C(=CH2)CH3 -~C-iPr
4138 -C02C(=CH2)CH3 -C._C-cycPr
4139 -C02C(=CHZ)CH3 -C~-1-(Me)cycPr
4140 -C02C(=CH2)CH3 -~C-CH=CH2
4141 -C02C(=CH2)CH3 -CH--CH-CH3
4142 -COZC(=CH2)CH3 -CH=CH-CF3
4143 -COZC(=CH2)CH3 -CH=CH-Et
4144 -C02C(=CH2)CH3 -CH=CH-iPr
4145 -C02C(=CHZ)CH3 -CH--CH-cycPr
4146 -COZC(=CH2)CH3 -CH=CH-CH=CHZ
4147 -C02C(=CH2)CH3 -CH2-CSC-CH3
4148 -COZC(=CH2)CH3 -CH2-~C-CF3
4149 -C02C(=CH2)CH3 -CHZ_CsC-Et
4150 -C02C(=CHZ)CH3 -CH2-CSC-iPr
4151 -C02C(=CH2)CH3 -CHZ-~-cycPr
4152 -C02C(=CH2)CH3 -CH2-C-~-CH=CH2
4153 -C02C(=CH2)CH3 -CH2-CH=CHZ
4154 -C02C(=CH2)CH3 -CH2-CH=CH-CH3
4155 -C02C(=CH2)CH3 -CH2-CH=CH-CF3
4156 -C02C(=CH2)CH3 -CH2-CH=CH-Et
4157 -C02C(=CH2)CH3 -CH2-CH=CH-iPr
4158 -C02C(=CH2)CH3 -CH2-CH=CH-cycPr
4159 -COzC(=CHZ)CH3 -CHZ-CH--CH-CH=CH2
4160 -C02C(=CH2)CH3 -~2-CH=C(CH3)2
4161 -C02C(=CH2)CH3 -CH=CH-CHZ-cycPr
4162 -C(=0)-cycPr n-butyl
4163 -C(=O)-cycPr benzyl
4164 -C(=O)-cycPr phenethyl
4165 -C(=O)-cycPr -CH2CH2-cycPr
4166 -C(=O)-cycPr _
4167 -C(=O)-cycPr -~-CF3
4168 -C (=O) -cycPr -~C-Et
-115-
CA 02334332 2000-12-07
WO 00/00478 PC'f/US99/14395
4169 -C(=0)-cycPr -CSC-iPr
4170 -C(=0)-cycPr -C._C-cycPr
4171 -C(=O)-cycPr -C=-C-1-(Me)cycPr
4172 -C(=0)-cycPr -CSC-CH=GH2
4173 -C(=0)-cycPr -CH=CH-CH3
4174 -C (=O) -cycPr -CH~Ii-CF3
4175 -C (=O) -cycPr -~=~-Et
4176 -C(=O)-cycPr -CH=CH-iPr
4177 -C(=O)-cycPr -~~-cycpr
4178 -C(=0)-cycPr -~~-CH=CH2
4179 -C(=0)-cycPr -CH2_~C_CH3
4180 -C(=O)-cycPr -CH2-C._C-CF3
4181 -C(=0)-cycPr -CHz-CSC-Et
4182 -C(=O)-cycPr -CH2-C=_C-iPr
4183 -C(=O)-cycPr -CH2-CSC-cycPr
4184 -C(=O)-cycPr -CH2-CSC-CH=CH2
4185 -C(=O)-cycPr -CH2-CH=CH2
4286 -C(=O)-cycPr -CH2-CH=CH-CH3
4187 -C(=O)-cycPr -CH2-CH=CH-CF3
4188 -C(=O)-cycPr -CH2-CH=CH-Et
4189 -C(=0)-cycPr -CH2-CH=CH-iPr
4190 -C(=0)-cycPr -CH2-CH=CH-cycPr
4191 -C(=0)-cycPr -CH2-CH=CH-CH=CH2
4192 -C(=0)-cycPr -CH2-CH=C(CH3)2
4193 -C(=0)-cycPr -CH=CH-CH2-cycPr
*Unlessotherwise noted, stereochemistry
is (+/-) and in R2,
all
double
bonds
are
trans.
-116-
CA 02334332 2000-12-07
WO 00/00478 PCTNS99/14395
Utilitv
The compounds of this invention possess reverse
transcriptase inhibitory activity, in particular, HIV
inhibitory efficacy. The compounds of formula (I) possess
HIV reverse transcriptase inhibitory activity and are
therefore useful as antiviral agents for the treatment of HIV
infection and associated diseases. The compounds of formula
(I) possess HIV reverse transcriptase inhibitory activity and
are effective as inhibitors of HIV growth. The ability of
the compounds of the present invention to inhibit viral
growth or infectivity is demonstrated in standard assay of
viral growth or infectivity, for example, using the assay
described below.
The compounds of formula (I) of the present invention
are also useful for the inhibition of HIV in an ex vivo
sample containing HIV or expected to be exposed to HIV.
Thus, the compounds of the present invention may be used to
inhibit HIV present in a body fluid sample (for example, a
serum or semen sample) which contains or is suspected to
contain or be exposed to HIV.
The compounds provided by this invention are also useful
as standard or reference compounds for use in tests or assays
for determining the ability of an agent to inhibit viral
clone replication and/or HN reverse transcriptase, for
example in a pharmaceutical research program. Thus, the
compounds of the present invention may be used as a control
or reference compound in such assays and as a quality control
standard. The compounds of the present invention may be
provided in a commercial kit or container for use as such
standard or reference compound.
Since the compounds of the present invention exhibit
specificity for HIV reverse transcriptase, the compounds of
the present invention may also be useful as diagnostic
reagents in diagnostic assays for the detection of HIV
reverse transcriptase. Thus, inhibition of the reverse
transcriptase activity in an assay (such as the assays
described herein) by a compound of the present invention
-117-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
would be indicative of the presence of HIV reverse
transcriptase and HIV virus.
As used herein "ug" denotes microgram, "mg" denotes
milligram, "g" denotes gram, "uL" denotes microliter, "mL"
denotes milliliter, "L" denotes liter, "nM" denotes
nanomolar, "uM" denotes micromolar, "mM" denotes millimolar,
"r" denotes molar and "nm" denotes nanometer. "Sigma" stands
for the Sigma-Aldrich Corp. of St. Louis, MO.
HIV RNA Assav
DNA Plasmids and in vitro RNA transcriptss
Plasmid pDAB 72 containing both gag and pol sequences of
BH10 (bp 113-1816) cloned into PTZ 19R was prepared according
to Erickson-Viitanen et al. AIDS Research and Human
Retroviruses 2989, 5, 577. The plasmid was linearized with
Bam HI prior to the generation of in vitro RNA transcripts
using the Riboprobe Gemini system II kit (Promega) with T7
RNA polymerase. Synthesized RNA was purified by treatment
with RNase free DNAse (Promega), phenol-chloroform
extrac.:ion, and ethanol precipitation. RNA transcripts were
dissolved in water, and stored at -70°C. The concentration
of RNA was determined from the A26o.
Biotinylated capture probes were purified by HPLC after
synthesis on an Applied Biosystems (Foster City, CA) DNA
synthesizer by addition of biotin to the 5' terminal end of
the oligonucleotide, using the biotin-phosphoramidite reagent
of Cocuzza, Tet. Lett. 1989, 30, 6287. The gag biotinylated
capture probe (5-biotin-CTAGCTCCCTGCTTGCCCATACTA 3') was
complementary to nucleotides 889-912 of HXB2 and the pol
biotinylated capture probe (5'-biotin -CCCTATCATTTTTGGTTTCCAT
3' ) was complementary to nucleotides 2374-2395 of H~2.
Alkaline phosphatase conjugated oligonucleotides used as
reporter probes were prepared by Syngene (San Diego, CA.).
The pol reporter probe (5' CTGTCTTACTTTGATAAAACCTC 3') was
complementary to nucleotides 2403-2425 of HX82. The gag
-118-
CA 02334332 2000-12-07
WO 00!00478 PCT/US99/14395
reporter probe (5' CCCAGTATTTGTCTACAGCCTTCT 3') was
complementary to nucleotides 950-973 of H~2. All nucleotide
positions are those of the GenBank Genetic Sequence Data Bank
as accessed through the Genetics Computer Group Sequence
Analysis Software Package (Devereau Nucleic Acids Research
1984, 12, 387). The reporter probes were prepared as 0.5 ~.iM
stocks in 2 x SSC (0.3 M NaCl, 0.03 M sodium citrate), 0.05 M
Tris pH 8.8, 1 mg/mL BSA. The biotinylated capture probes
were prepared as 100 uM stocks in water.
Streptavidin coated nla-PS:
Streptavidin coated plates were obtained from Du Pont
Biotechnology Systems (Boston, MA).
Cells and virus stocks:
MT-2 and MT-4 cells were maintained in RPMI 1640
supplemented with 5% fetal calf serum (FCS) for MT-2 cells or
10% FC5 for MT-4 cells, 2 mM z-glutamine and 50 ug/mL
gentamycin, all from Gibco. HIV-1 RF was propagated in MT-4
cells in the same medium. Virus stocks were prepared
approximately 10 days after acute infection of MT-4 ~..alls and
stored as aliquots at -70°C. Infectious titers of HIV-1(RF)
stocks were 1-3 x 107 PFU (plaque forming units)/mL as
measured by plaque assay on MT-2 cells (see below). Each
aliquot of virus stock used for infection was thawed only
once.
For evaluation of antiviral efficacy, cells to be
infected were subcultured one day prior to infection. On the
day of infection, cells were resuspended at 5 x 105 cells/mL
in RPMI 1640, 5% FCS for bulk infections or at 2 x 106/mL in
Dulbecco's modified Eagles medium with 5% FCS for infection
in microtiter plates. Virus was added and culture continued
for 3 days at 37°C.
HIV Rt~lA assay:
Cell lysates or purified RNA in 3 M or 5 M GED were
mixed with 5 M GED and capture probe to a final guanidinium
isothiocyanate concentration of 3 M and a final biotin
-119-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
oligonucleotide concentration of 30 nM. Hybridization was
carried out in sealed U bottom 96 well tissue culture plates
(Nunc or Costar) for 16-20 hours at 37°C. RNA hybridization
reactions were diluted three-fold with deionized water to a
final guanidinium isothiocyanate concentration of 1 M and
aliquots (150 uL) were transferred to streptavidin coated
microtiter plates wells. Binding of capture probe aad
capture probe-RNA hybrid to the immobilized streptavidin was
allowed to proceed for 2 hours at room temperature, after
which the plates were washed 6 times with DuPont ELISA plate
wash buffer (phosphate buffered saline(PBS), 0.05% Tween 20.)
A second hybridization of reporter probe to the immobilized
complex of capture probe and hybridized target RNA Was
carried out in the washed streptavidin coated well by
addition of 120 ul of a hybridization cocktail containing 4 X
SSC, 0.66% Triton X 100, 6.66% deionized formamide, 1 mg/mL
BSA and 5 nM reporter probe. After hybridization for one
hour at 37°C, the plate was again washed 6 times.
Immobilized alkaline phosphatase activity was detected by
addition of 100 uL of 0.2 mM 4-methylumbelliferyl phosphate
(MUBP, JBL Scientific, in buffer 8(2.5 M diethanolamine pH 8.9
(JBL Scientific), 10 mM MgCl2, 5 mM zinc acetate dihydrate and
5 mM N-hydroxyethyl-ethylene-diamine-triacetic acid). The
plates were incubated at 37°C. Fluorescence at 450 nM was
measured using a microplate fluorometer (Dynateck) exciting
at 365 nM.
~~»~~rP based compound evaluation in HIV-l infected MT-2
Compounds to be evaluated were dissolved in DMSO and
diluted in culture medium to twice the highest concentration
to be tested and a maximum I7~fS0 concentration of 2%. Further
three-fold serial dilutions of the compound in culture medium
were performed directly in U bottom microtiter plates (Nunc).
After compound dilution, MT-2 cells (50 uL) were added to a
final concentration of 5 x 105 per mL (1 x 105 per well).
Cells were incubated with compounds for 30 minutes at 37°C in
a COz incubator. For evaluation of antiviral potency, an
-120-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
appropriate dilution of HIV-1 (RF) virus stock (50 uL) was
added to culture wells containing cells and dilutions of the
test compounds. The final volume in each well was 200 uL.
Eight wells per plate were left uninfected with 50 uL of
medium added in place of virus, while eight wells were
infected in the absence of any antiviral compound. For
evaluation of compound toxicity, parallel plates were
cultured without virus infection.
After 3 days of culture at 37°C in a humidified chamber
inside a C02 incubator, all but 25 uL of medium/well was
removed from the HIV infected plates. Thirty seven uL of 5 M
GED containing biotinylated capture probe was added to the
settled cells and remaining medium in each well to a final
concentration of 3 M GED and 30 nM capture probe.
Hybridization of the capture probe to HIV RNA in the cell
lysate was carried out in the same microplate well used for
virus culture by sealing the plate with a plate sealer
(Costar), and incubating for 16-20 hrs in a 37°C incubator.
Distilled water was then added to each well to dilute the
hybridization reaction three-fold and 150 uL of this diluted
mixture was transferred to~a strept;,ridin coated microtiter
plate. HIV RNA was quantitated as described above. A
standard curve, prepared by adding known amounts of pDAB 72
in vitro RNA transcript to wells containing lysed uninfected
cells, was run on each microtiter plate in order to determine
the amount of viral RNA made during the infection.
In order to standardize the virus inoculum used in the
evaluation of compounds for antiviral activity, dilutions of
virus were selected which resulted in an ICgp value
(concentration of compound required to reduce the HIV RNA
level by 90~) for dideoxycytidine (ddC) of 0.2 ug/mL. ICgp
values of other antiviral compounds, both more and less
potent than ddC, were reproducible using several stocks of
HIV-1 (RF) when this procedure was followed. This
concentration of virus corresponded to ~3 x 105 PFU (measured
by plaque assay on MT-2 cells) per assay well and typically
produced approximately 75~ of the maximum viral RNA level
achievable at any virus inoculum. For the HIV RNA assay, ICgo
-121-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
values were determined from the percent reduction of net
signal (signal from infected cell samples minus signal from
uninfected cell samples) in the RNA assay relative to the net
signal from infected, untreated cells on the same culture
plate (average of eight wells). Valid performance of
individual infection and RNA assay tests was judged according
to three criteria. It was required that the virus infection
should result in an RNA assay signal equal to or greater than
the signal generated from 2 ng of pDAB 72 in vitro RNA
transcript. The IC9o for ddC, determined in each assay run,
should be between 0.1 and 0.3 ug/mL. Finally, the plateau
level of viral RNA produced by an effective reverse
transcriptase inhibitor should be less than 10~ of the level
achieved in an uninhibited infection. A compound was
considered active if its IC9p was found to be less than 20~.iM.
Compounds of the present invention have been found to have an
ICgp less than 20uPt.
For antiviral potency tests, all manipulations in
microtiter plates, following the initial addition of 2X
concentrated compound solution to a single row of wells, were
performed using a Perkin Elmer/Cetus w~oPette.
HTV-1 RT Assay Materials and Methods
This assay measures HIV-1 RT RNA dependent DNA
polymerase activity by the incorporation of 3H dTMP onto the
template primer Poly (rA) oligo (dT)12-18. The template
primer containing the incorporated radioactivity was
separated from unincorporated label by one of two methods:
Method 1. The template primer was precipitated with TCA,
collected on glass fiber filters and counted for
radioactivity with a scintillation counter.
Method 2. The currently used method is more rapid and
convenient. The template primer is captured on an diethyl
amino ethyl (DEAF) ion exchange membrane which is then
counted for radioactivity after washing off the free
nucleotide.
-122-
CA 02334332 2000-12-07
WO 00/00478 PCTNS99/14395
Materials and Read:
The template primer Poly (rA) oligo (dT)12-18 and dTTP
were purchased from Pharmacia Biotech. The template primer
and nucleotide were dissolved in diethyl pyrocarbonate water
to a concentration of 1 mg/ml and 5.8 mM respectively. The
substrates were aliquoted (template primer at 20 ul/aliquot,
dTTP at 9 ul/aliquot) and frozen at -20 C.
The 3H dTTP (2.5 mCi/ml in 10 mM Tricine at pH 7.6;
specific activity of 90-120 Ci/mmol) and the recombinant HIV-
1 Reverse Transcriptase (HxB2 background; 100 U/10 ul in 100
mM potassium phosphate at pH 7.1, 1 mM dithiothreitol and 50~
glycerol) were purchased from DuPont NEN. 1 Unit of enzyme
is defined by DuPont NEN as the amount required to
incorporate 1 nmol of labelled dTTP into acid-insoluble
material in 10 minutes at 37 C. The 3H dTTP was aliquoted at
23.2 ul/microfuge tube (58 uCi) and frozen at -20 C. The
HIV-1 Reverse Transcriptase (RT) was diluted 10 fold with RT
buffer (80 mM KC1, 50 mM Tris HC1, 12 mM MgCl2, 1 mM DTT, 50
pM EGTA, 5 mg/ml BSA, 0.01 Triton-X 100, pH 8.2) and
aliquoted at 10 ul/microfuge tube (10 Units/10 ul). One
aliquot (enough for a assays) was diluted further to 10
Units/100 ul and aliquoted into 8 tubes (1.25 Units/12.5 ul).
All aliquots were frozen at -70 C.
The Millipore Multiscreen DE 96 well filter plates,
multiscreen plate adaptors, and microplate press-on adhesive
sealing film were purchased from Millipore. The filter plate
containing 0.65 Eun pore size diethyl amino ethyl cellulose
(DEAF) paper disks was pretreated with 0.3 M ammonium formate
and 10 mM sodium pyrophosphate (2 times 200 ul /well) at
pH 8.0 prior to use. A Skatron 96 well cell harvester and
glass fiber filter mats were purchased from Skatron
Instruments. Microscint 20 scintillation cocktail was
purchased from Packard. Beckman Ready Flow III scintillation
cocktail was purchased from Beckman.
HIV-1 RT Assav:
The enzyme and substrate mixture were freshly prepared
from the above stock solutions. 1.25 Units of enzyme was
-123-
CA 02334332 2000-12-07
WO 00/004'78 PCT/US99/14395
diluted with RT buffer (containing 5 mg/ml BSA) to a
concentration of 0.05 Units/10 ul or 0.7 nM. Final enzyme
and BSA concentrations in the assay were 0.01 Units or 0.14
nM and 1 mg/ml respectively. The inhibitor and substrate
mixture were diluted With RT buffer containing no HSA. All
inhibitors were dissolved in dimethyl sulfoxide (DMSO) at a
stock concentration of 3 mM and stored at -20 C after use. A
Biomek robot was used to dilute the inhibitors in a 96 well
plate. Inhibitors were initially diluted 96 fold from stock
and then serially diluted two times (10 fold/dilution) from
31.25 uM to 3125 nM and 312.5 nM. Depending on the potency
of the inhibitor, one of the three dilutions was further
diluted. Typically the highest concentration (31.25 uM) was
serially diluted three times at 5 fold/dilution to 6.25,
1.25, and 0.25 uM. Final inhibitor concentrations in the
assay were 12.5, 2.5, 0.5, and 0.1 ~,iM. For potent inhibitors
of HIV-1 RT, the final inhibitor concentrations used were 0.1
or 0.01 that stated above. The substrate mixture contained
6.25 ug/ml of Poly (rA) oligo (dT)12-18 and 12.5 ~tM of dTTP
(58 pCi 3H dTTP). The final substrate concentrations were
2.5 ug/ml and 5 P.M respectively.
Using the Beckman Instruments Biomek robot, 10 ul of
HIV-1 RT was combined with 20 ul of inhibitor in a 96 well U
bottom plate. The enzyme and inhibitor were preincubated at
ambient temperature for 6 minutes. 20 ul of the substrate
mixture was added to each well to initiate the reaction
(total volume was 50 ul). The reactions were incubated at 37
C and terminated after 45 minutes.
For method 1, 200 ul of an ice-cold solution of 13%
trichloroacetic acid (TCA) and 10 mM sodium pyrophosphate was
added to each of the 96 wells. The 96 well plate was then
placed in an ice-water bath for 30 minutes. Using A Skatron
96 well cell harvester, the acid precipitable material was
collected on a glass fiber filter mat that had been presoaked
in 13% TCA and 10 mM sodium pyrophosphate. The filter disks
were washed 3 times (2.0 ml/wash) with 1 N HC1 and 10 mM
sodium pyrophosphate. The filter disks were punched out into
scintillation vials, 2.0 ml of Beckman Ready Flow III
-124-
CA 02334332 2000-12-07
WO 00/00478 PCTNS99/14395
scintillant was added, and the vials were counted for
radioactivity for 1 minute.
For method 2, the assay was terminated with the addition
of 175 ul/well of 50 mM EDTA at pH 8Ø Then 180 ul of the
mixture was transferred to a pretreated Millipore DE 96 well
filter plate. Vacuum was applied to the filter plate to
aspirate away the liquid and immobilize the template primer
on the DEAF filter disks. Each well was washed 3 times with
200 ul of 0.3 M ammonium formate and 10 mM sodium
pyrophosphate at pH 8Ø 50 ul of microscint 20
scintillation cocktail was added to each well and the plate
was counted for radioactivity on a Packard Topcount at 1
minute/well.
The ICSp values are calculated with the equation:
ICSp = [Inh]/(1/fractional activity - 1);
where the fractional activity = RT activity (dpms) in the
presence of inhibitor/RT activity (dpms) in the absence of
inhibitor. For a given inhibitor, the ICSp values were
calculated for the inhibitor concentrations that range
between 0.1-0.8 fractional activity. The ICSp values in this
range (generally 2 values) were averaged. A compound was
considered active if its ICSp was found to be less than 60u1K.
Compounds of the present invention have been found to have an
ICSO less than 60uM.
~roteinBindina and Mutant Resistance
In order to characterize NNRTI analogs for their
clinical efficacy potential the effect of plasma proteins on
antiviral potency and measurements of antiviral potency
against wild type and mutant variants of HIV which carry
amino acid changes in the known binding site for l~IRTIs were
examined. The rationale for this testing strategy is two
fold:
1. Many drugs are extensively bound to plasma proteins.
Although the binding affinity for most drugs for the major
components of human plasma, namely, human serum albumin (HSA)
or alpha-1-acid glycoprotein (AAG), is low, these major
components are present in high concentration in the blood.
-125-
CA 02334332 2000-12-07
WO 00/00478 PCTIUS99/14395
Only free or unbound drug is available to cross the infected
cell membrane for interaction with the target site (i.e.,
HIV-1 reverse transcriptase, HIV-1 RT). Therefore, the
effect of added HSA+AAG on the antiviral potency in tissue
culture more closely reflects the potency of a given compound
in the clinical setting. The concentration of compound
required for 90% inhibition of virus replication as measured
in a sensitive viral RNA-based detection method is designated
the IC90. The fold increase in apparent IC90 for test
compounds in the presence or added levels of HSA and AAG that
reflect in vivo concentrations (45 mg/ml HSA, 1 mg/ml AAG)
was then calculated. The lower the fold increase, the more
compound will be available to interact with the target site.
2. The combination of the high rate of virus
replication in the infected individual and the poor fidelity
of the viral RT results in the production of a quasi-species
or mixtures of HIV species in the infected individual. These
species will include a majority wild type species, but also
mutant variants of HIV and the proportion of a given mutant.
will reflect its relative fitness and replication rate.
Because mutant variants including mutants with changes in the
amino acid sequence of the viral RT likely pre-exist in the
infected individual's quasi-species, the overall potency
observed in the clinical setting will reflect the ability of
a drug to inhibit not only wild type HIV-1, but mutant
variants as well. We thus have constructed, in a known
genetic background, mutant variants of HIV-1 which carry
amino acid substitutions at positions thought to be involved
in NNRTI binding, and measured the ability of test compounds
to inhibit replication of these mutant viruses. The
concentration of compound required for 90% inhibition of
virus replication as measured in a sensitive viral RNA-based
detection method is designated the IC90. It is desirable to
have a compound which has high activity against a variety of
mutants.
-126-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99I14395
Dosaae and Formulation
The antiviral compounds of this invention can be
administered as treatment for viral infections by any means
that produces contact of the active agent with the agent's
site of action, i.e., the viral reverse transcriptase, in the
body of a mammal. They can be administered by any
conventional means available for use in conjunction with
pharmaceuticals, either as individual therapeutic agents or
in a combination of therapeutic agents. They can be
administered alone, but preferably are administered with a
pharmaceutical carrier selected on the basis of the chosen
route of administration and standard pharmaceutical practice.
The dosage administered will, of course, vary depending
upon known factors, such as the pharmacodynamic
Z5 characteristics of the particular agent and its mode and
route of administration; the age, health and weight of the
recipient; the nature and extent of the symptoms; the kind of
concurrent treatment; the frequency of treatment; and the
effect desired. A daily dosage of active ingredient can be
expected to be about 0.001 to about 1000 milligrams per
kilogram of body weight, with the preferred dose being about
0.1 to about 30 mg/kg.
Dosage forms of compositions suitable for administration
contain from about 1 mg to about 100 mg of active ingredient
per unit. In these pharmaceutical compositions the active
ingredient will ordinarily be present in an amount of about
0.5-95~ by weight based on the total weight of the
composition. The active ingredient can be administered
orally in solid dosage forms, such as capsules, tablets and
powders, or in liquid dosage forms, such as elixirs, syrups
and suspensions. It can also be administered parenterally,
in sterile liquid dosage forms.
Gelatin capsules contain the active ingredient and
powdered carriers, such as lactose, starch, cellulose
derivatives, magnesium stearate, stearic acid, and the like.
Similar diluents can be used to make compressed tablets.
Both tablets and capsules can be manufactured as sustained
release products to provide for continuous release of
-127-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99114395
medication over a period of hours. Compressed tablets can be
sugar coated or film coated to mask any unpleasant taste and
protect the tablet from the atmosphere, or enteric coated for
selective disintegration in the gastrointestinal tract.
Liquid dosage forms for oral administration can contain
coloring and flavoring to increase patient acceptance.
In general, water, a suitable oil, saline, aqueous
dextrose (glucose), and related sugar solutions and glycols
such as propylene glycol or polyethylene glycols are suitable
carriers for parenteral solutions. Solutions for parenteral
administration preferably contain a water soluble salt of the
active ingredient, suitable stabilizing agents, and if
necessary, buffer substances. Antioxidizing agents such as
sodium bisulfite, sodium sulfite, or ascorbic acid, either
alone or combined, are suitable stabilizing agents. Also
used are citric acid and its salts, and sodium EDTA. In
addition, parenteral solutions can contain preservatives,
such as benzalkonium chloride, methyl- or propyl-paraben and
chlorobutanol. Suitable pharmaceutical carriers are
described in Remington's Pharmaceutical Sciences, supra, a
standard reference text in this field.
Useful pharmaceutical dosage-fozins for administration of
the compounds of this invention can be illustrated as
follows
Capsules
A large number of unit capsules can be prepared by
filling standard two-piece hard gelatin capsules each with
100 mg of powdered active ingredient, 150 mg of lactose, 50
mg of cellulose, and 6 mg magnesium stearic.
.loft Gelatin Cag~g~
A mixture of active ingredient in a digestible oil such
as soybean oil, cottonseed oil or olive oil can be prepared
and injected by means of a positive displacement pump into
gelatin to form soft gelatin capsules containing 100 mg of
the active ingredient. The capsules should then be washed
and dried.
-128-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
Tablets
A large number of tablets can be prepared by
conventional procedures so that the dosage unit is 100 mg of
active ingredient, 0.2 mg of colloidal silicon dioxide, 5
milligrams of magnesium stearate, 275 mg of microcrystalline
cellulose, 11 mg of starch and 98.8 mg of lactose.
Appropriate coatings may be applied to increase palatability
or delay absorption.
Suspension
An aqueous suspension can be prepared for oral
administration so that each 5 mL contain 25 mg of finely
divided active ingredient, 200 mg of sodium carboxymethyl
cellulose, 5 mg of sodium benzoate, 1.0 g of sorbitol
solution, U.S.P., and 0.025 mg of vanillin.
Injectable
A parenteral composition suitable for administration by
injection can be prepared by stirring 1.5% by weight of
active ingredient in 10% by volume propylene glycol and
water. The solution is sterilized by conanonly used
techniques.
Combination of comDOnen (a) and (b)
Each therapeutic agent component of this invention can
independently be in any dosage form, such as those described
above, and can also be administered in various ways, as
described above. In the following description component (b)
is to be understood to represent one or more agents as
described previously. Thus, if components (a) and (b) are to
be treated the same or independently, each agent of component
(b) may also be treated the same or independently.
Components (a) and (b) of the present invention may be
formulated together, in a single dosage unit (that is,
combined together in one capsule, tablet, powder, or liquid,
etc.) as a combination product. When component (a) and (b)
are not formulated together in a single dosage unit, the
-129-
CA 02334332 2000-12-07
PCT/US99114395
component (a) may be administered at the same time as
component (b) or in any order; for example component (a) of
this invention may be administered first, followed by
administration of component (b), or they may be administered
in the revserse order. If component (b) contains more that
one agent, e.g:, one RT inhibitor and one protease inhibitor,
these agents may be administered together or in any order.
When not administered at the same time, preferably the
administration of component (a) and (b) occurs less than
about one hour apart. Preferably, the route of
administration of component (a) and (b) is oral. The terms
oral agent, oral inhibitor, oral compound, or the like, as
used herein, denote compounds which may be orally
administered. Although it is preferable that component (a)
and component (b) both be administered by the same route
(that is, for example, both orally) or dosage form, if
desired, they may each be administered by different routes
(that is, for example, one component of the combination
product may be administered orally, and another component may
be administered intravenously) or dosage forms.
As is appreciated by a medical practitioner skilled in
the art, the dosage of the combination therapy of the
invention may vary depending upon various factors such as the
pharmacodynamic characteristics of the particular agent and
its mode and route of administration, the age, health and
weight of the recipient, the nature and extent of the
symptoms, the kind of concurrent treatment, the frequency of
treatment, and the effect desired, as described above.
The proper dosage of components (a) and (b) of the
present invention will be readily ascertainable by a medical
practitioner skilled in the art, based upon the present
disclosure. By way of general guidance, typically a daily
dosage may be about 100 milligrams to about 1.5 grams of each
component. If component (b) represents more than one
compound, then typically a daily dosage may be about 100
milligrams to about 1.5 grams of each agent of component (b).
By way of general guidance, when the compounds of component
(a) and component (b) are administered in combination, the
-130-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
dosage amount of each component may be reduced by about 70-
80$ relative to the usual dosage of the component when it is
administered alone as a single agent for the treatment of HIV
infection, in view of the synergistic effect of the
combination.
The combination products of this invention may be
formulated such that, although the active ingredients are
combined in a single dosage unit, the physical contact
between the active ingredients is minimized. In order to
minimize contact, for example, where the product is orally
administered, one active ingredient may be enteric coated.
By enteric coating one of the active ingredients, it is
possible not only to minimize the contact between the
combined active ingredients, but also, it is possible to
control the release of one of these components in the
gastrointestinal tract such that one of these components is
not released in the stomach but rather is released in the
intestines. Another embodiment of this invention where oral
administration is desired provides for a combination product
wherein one of the active ingredients is coated with a
sustained-release material which effects a sustained-release
throughout the gastrointestinal tract and also serves to
minimize physical contact between the combined active
ingredients. Furthermore, the sustained-released component
can be additionally enteric coated such that the release of
this component occurs only in the intestine. Still another
approach would involve the formulation of a combination
product in which the one component is coated with a sustained
and/or enteric release polymer, and the other component is
also coated with a polymer such as a lowviscosity grade of
hydroxypropyl methylcellulose or other appropriate materials
as known in the art, in order to further separate the active
components. The polymer coating serves to form an additional
barrier to interaction with the other component. In each
formulation wherein contact is prevented between components
(a) and (b) via a coating or some other material, contact may
also be prevented between the individual agents of component
(b) .
-131-
CA 02334332 2000-12-07
WO 00/00478 PCTNS99/14395
Dosage forms of the combination products of the present
invention wherein one active ingredient is enteric coated can
be in the form of tablets such that the enteric coated
component and the other active ingredient are blended
together and then compressed into a tablet or such that the
enteric coated component is compressed into one tablet layer
and the other active ingredient is compressed into an
additional layer. Optionally, in order to further separate
the two layers, one or more placebo layers may be present
such that the placebo layer is between the layers of active
ingredients. In addition, dosage forms of the present
invention can be in the force of capsules wherein one active
ingredient is compressed into a tablet or in the form of a
plurality of microtablets, particles, granules or non-perils,
which are then enteric coated. These enteric coated
microtablets, particles, granules or non-perils are then
placed into a capsule or compressed into a capsule along with
a granulation of the other active ingredient.
These as well as other ways of minimizing contact
between the components of combination products of the present
invention, whether administered in a single dosage form or
administered in separate forms but at the same time or
concurrently by the same manner, will be readily apparent to
those skilled in the art, based on the present disclosure.
Pharmaceutical kits useful for the treatment of HIV
infection, which comprise a therapeutically effective amount
of a pharmaceutical composition comprising a compound of
component (a) and one or more compounds of component (b), in
one or more sterile containers, are also within the ambit of
the present invention. Sterilization of the container may be
carried out using conventional sterilization methodology well
known to those skilled in the art. Component (a) and
component (b) may be in the same sterile container or in
separate sterile containers. The sterile containers of
materials may comprise separate containers, or one or more
multi-part containers, as desired. Component (a) and
component (b), may be separate, or physically combined into a
single dosage form or unit as described above. Such kits may
-132-
CA 02334332 2000-12-07
WO 00/00478 PCT/US99/14395
further include, if desired, one or more of various
conventional pharmaceutical kit components, such as for
example, one or more pharmaceutically acceptable carriers,
additional vials for mixing the components, etc., as will be
readily apparent to those skilled in the art. Instructions,
either as inserts or as labels, indicating quantities of the
components to be administered, guidelines for administration,
and/or guidelines for mixing the components, may also be
included in the kit.
Obviously, numerous modifications and variations of the
present invention are possible in light of the above
teachings. It is therefore to be understood that within the
scope of the appended claims, the invention may be practiced
otherwise than as specifically described herein.
-133-