Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02334964 2010-02-04
P rOFIRECTlON
wo 9943883 rcr/11.98A06270
PHYSIOLOGICAL STRESS DETECTOR DEVICE AND METHOD
FIELD OF THE INVENTION
The present invention relates to instruments which operate on the
principle of pulse oximetry, in particular, to non-invasive hemoglobin
saturation
detectors and methods, and may be generally applied to other electro-optical
methods of measuring blood constituents.
BACKGROUND OF THE INVENTION
Electro-optical measurement of blood characteristics has been found to
be useful in many areas of blood oonstituent diagnostics, such as glucose
levels,
oxygen saturation, hematocrit, billirubin and others. This method is
advantageous
in that it can be perf=omied in a non-invasive fashion. In paracuiar, much
reseanh
has been done on oximetry, a way of ineasuring oxygen saturation in the blood,
as an early indicator of respiratory distress.
Infants during the first year of life are susceptible to breathing
disturbances (apnea) and respiratory distress. Sudden Infant Death Syndrome
(SIDS) is a medical condition in which an infant enters respiratory distress
and
stops breathing, leading to the death of the infant. Although the cause and
waming signs of SIDS are not clear, it has been shown that early detection of
respiratory distress can provide the time to administer the aid necessary to
2o prevent death.
Many types of baby monitors are currentiy available, from simple motion
detectors to complicated systems which stream oxygen enriched air into the
1
CA 02334964 2010-02-04
SEC'I~n'kl R GQRPECTION
Or-C $ FnTFFEOATE
Cc:;4.^:~ A n.~ICLE
is 8
WO 99/63883 PCT/IL98/00270
infant's environment. Some of the more accepted monitoring methods Include
chest motion monitors, carbon dioxide level monitors and heart rate (pulse)
monitors. Unfortunately these methods often do not give the advance waming
necessary for the caregivers to administer aid. In addition, these monitors
are
administered by attaching a series of straps and cords which are cumbersome to
use and present a strangulation risk.
The chest niotion monitor gives no warning when the breathing pattems
become irregular or when hyperventiiation is occurring, since the chest
continues
to move. Distress is only noted once the chest motion has ceased at which
point
Io there may only be a slight chance of resuscitation without brain damage. In
addition these devices are known to have a high level of 'false alarms" as
they
have no way to distinguish between the lapses in breathing which are normal
for
an infant (up to 20 seconds) and respiratory distress. These devices can cause
excessive anxiety for the caregivers or cause them to ignore a signal which is
true
after responding repeatedly to false aiarms.
Among other symptoms, SIDS causes an irregular heartbeat, resuiting
eventually in the cessation of heartbeat with the death of the infant. There
are
some instruments which use the EKG principle to monitor this clinical
phenomenon. This is a iimited method which has a very high rate of false
positives since the monitors have inadequate algorrthms to determine what is a
SIDS event. Obviously, this is not a convenient method, nor is it desirable to
have
the Infant constantly hooked up to an EKG monitor.
2
CA 02334964 2010-02-04
~" r7 7'9^~' R aECTION
~..__ .. .v
CA7E
w..~ o
P=
WO 99/63$83 PC,'I'/IL98/00270
In light of these disadvantages a better method to use is a form of
electro-optical measurement, such as pulse oximetry, which is a well-developed
art. This method uses the difference in the absorption properties of
oxyhemoglobin and deoxyhemoglobin to measure blood oxygen saturation in
arterial blood. The oximeter passes light, usually red and infrared, through
the
body tissue and uses a photodetector to sense the absorption of light by the
tissue. By measuring oxygen levels in the blood, one is able to detect
respiratory
distress at its onset giving sufficiently early waming to allow aid to be
administered
as necessary.
ao Two types of pulse oximetry are known. Until now, the more commonly
used type has been transmission oximetry in which two or more wavelengths of
light are transmitted through the tissue at a point where blood perfuses the
tissue
(i.e. a finger or earlobe) and a photodetector senses the absorption of light
from
the other side of the appendage. The light sources and sensors are mounted in
a
i5 clip which attaches to the appendage and delivers data by cable to a
processor.
These dips are uncomfortable to wear for extended periods of time, as they
must
be tight enough to exdude extemal light sources. Additionally, the tightness
of the
clips can cause hematomas. Use of these dips is limited to the extremities
where
the geometry of the appendages is such that they can accommodate a clip of
this
2o type. The dip must be designed specifically for one appendage and cannot be
used on a different one. Children are too active to wear these dips and
consequentty the accuracy of the reading suffers.
3
CA 02334964 2010-02-04
5~~?n**~~ R c'ORRECTION
3a=ic13rE
C'-:. A ;:;C ioi= 8
woMM
PGT/IL98/00270
In another form of transmission oximetry, the light source and detector
are placed on a ribbon, often made of -ubber, which is wrapped around the
appendage so that the source is on one side and the detector is on the other.
This
is commonly used with children. In this method error is high because movement
can cause the detector to become misaligned with the light source.
It would be preferable to be able to use the other type of pulse oximetry
known as reflective, or backscattering, oximetry, in which the light sources
and
light detector are placed side by side on the same tissue surface. When the
light
sources and detector can be placed on the tissue surface without necessitating
a
lo dip they can be applied to large surfaces such as the head, wrist or foot.
In cases
such as shock, when the blood is centralized away from the limbs, this is the
way
meaningful results can be obtained.
One difficuiiy in reflective oximetry is in adjusting the separation between
the light source and the detector such that the desired variable signal
component
(AC) received is strong, since it is in the altemating current that
information is
received. The challenge is to separate the shunted, or coupled, signal which
is
the direct current (DC) signal component representing infiitration of extemai
light
from the AC signal bearing the desired information. This DC signal does not
provide powerful information. If the DC signal component is not separated
completely, when the AC signal is amplified any remaining DC component will be
amplified with it, corrupting the results. Separating out the signal
components is
not a simple matter since the AC signal component is only 0.1 % to 1 k of the
total
4
CA 02334964 2010-02-04
SECY7ra'lR CORiRECT1CN
~ . __ . 6 -sc 4TE
. ..., . d" =.a t u ~
,~LE8
WO 99/63883 PCT/IL9810670 a ! ""CAT
reflected light received by the detector. Many complicated solutions to this
problem have been proposed.
If the light source and detector are moved further apart, this reduces the
shunting problem (DC), however, it also weakens the already weak AC signal
s component. If the tight source and detector are moved close together to
increase
the signal, the shunting (DC) will overpower the desired signal (AC).
Takatani et al., in US Pat. No. 4,867,557, Hirao et al., in US Pat. No.
5,057,695 and Mannheimer, in US Pat. No.5,524,617 all disclose reflecfive
oximeters which require multiple emitters or detectors in order to better
calculate
the signal.
A number of attempts have been made to filter out the DC electronically
(see Mendelson et al., in US Pat. No. 5,277,181). These methods are very
sensiflve to changes in signal level. The AC remaining after the filtering
often
contains a small pordon of DC, which upon amplification of the AC becomes
amplified as well, resulting in inaccurate readings. Therefore, this method is
only
useful in cases where the signal is strong and uniform.
Israeli patents 114082 and 114080 disclose a sensor designed to
overcome the shunting problem by using optical fibers to fllter out the
undesired
light. This is a complicated and expensive solution to the problem which
requires
2o a high level of technical skill to produce. In addition, it is ineffectual
when the AC
signal is relatively weak.
As can be seen from the above discussion, the prior art methods of
addressing the AC/DC signal separation problem in reflective oximetry
techniques
5
CA 02334964 2010-02-04
C71 n.~.. w P. 0 p R E C`n bN ART3Cl.E 8
WO 99153883 PCT/IL4~M90
are complicated and expensive. Therefore, it would be desirable to provide a
simple, low cost and effective method for achieving accurate reflective or
transmissive oximetry detection of respiratory stress.
6
CA 02334964 2010-02-04
SEC7INt C~n'PECT1014
8
WO 99/63883 PCT/II.9>kwi0' . r?'= ".yT
SUMMARY OF THE INVENTION
Accordingly, it is the broad object of the present invention to overcome
the probiems of separating the shunted light from the signal in order to
provide a
physiological stress detector which achieves accurate readings.
A general object of this invention is to overcome the problems of
separating the shunted light from the signal in order to provide a respiratory
stress
detector which achieves accurate pulse oximetry readings for respiratory
stress
applications.
The present invention discloses a small, independent, sensor, for
invasive and non-invasive appiications unencumbered by cables or wires, which
is
capable of being attached to different body parts, to comfortably and
accurately
monitor blood constituent levels and the pulse of an infant or any other
Iiving
organism. The apparatus may be applied to any part of the body without prior
calibration. Accurate readings of blood constituent levels are obtained using
the
inventive method in which a precise separation of the AC and DC signal
components has been achieved, allowing each signal component to be amplified
separate{y. In order to accomplish this precise separation, the signal
components
are separated by a novel signal processing technique.
The inventive sensor may be adapted for many health monitoring
2o situations including infant monitoring for SIDS, fetal monitoring, etc.
In a preferred embodiment adapted for SIDS, the sensor is designed to
apply reflective oximetry techniques, so as to comfortably and accurately
monitor
the arterial oxygen levels and the pulse of an infant or any other living
organism
7
CA 02334964 2010-02-04
sECI`UON A Gr~QqEGnOw
. ~. .., ,""",.e pS=f'C
Cc's
WO 99/63883 PGT/IL9"0370
prone to respiratory distress. This monitor is equipped with a processor
capable of
determining the need for an alarm and capable of signalling a distress signal
to
further alert to a crisis.
In another embodiment, in addition to the alarm being generated from
the sensor itseif, readings will be radio-transmitted to a base station,
possibly at a
nurse's station, to allow monitoring of the reading, and another alarm will be
activated from the base station when the readings are outside of the accepted
range.
In another preferred embodiment, the apparatus is mounted in a
sock-type mounting such that the apparatus is properly applied when the sock
is
put on in the usual fashion. In addition, the sock-type apparatus blocks
entrance
of extemal light to the area of the sensor apparatus.
In yet another preferred embodiment, the apparatus is mounted on a
ribbon-type mounting such that the apparatus is properly applied when the
ribbon
is tied around the head or other body part. In addition, the width of the
ribbon is
such that it will block entrance of extemal light to the area of the sensor
apparatus. Additionaiiy, the ribbon may be of dark color which also blocks
entrance of extemai light to the area of the sensor apparatus.
In yet another preferred embodiment, the apparatus is mounted on a
2o bracelet-type mounting such that the apparatus is properiy applied when the
bracelet is fastened to the wrist or other body part. In addition, the width
of the
bracelet is such that it blocks entrance of extemal light to the area of the
sensor
8
CA 02334964 2010-02-04
SECTIO~.) !a rn.r_ ~cGTION
s CATE
~
;C
W099/G3883
PClYIL98/00270
apparatus. Additionally, the bracelet may be of dark color which also blocks
entrance of extemal light to the area of the sensor apparatus.
There is therefore provided, in accordance with a preferred embodiment
of the present invention, A non-invasive device disposed proximate the surface
of an organ for measurement of a level of at least one blood constituent. The
device includes: at least one light source, providing light directed toward
the
surface of the organ, thellight being reflected from the organ, a light
detector
spaced apart from the at least one light source and being sensitive to
intensity
levels of the reflected light for producing intensity signals in accordance
therewith, and a processing unit for processing the intensity signals received
from the light detector. The processing unit includes: first and second
amplifiers
for amplifying the intensity signals, each in accordance with a respective
first
and second gain amplification factor, and a processor for automatically
determining the first and second gain amplification factors in adjustable
fashion.
During a first stage, the first and second amplifiers amplify a DC signal
component of the intensity signals in accordance with predetermined first and
second gain amplification factors, the DC signal component is subtracted from
the intensity signals at an input of the first amplifier, to isolate an AC
signal,
component of the intensity signals. During a second stage, the --tecond
amplifier amplifies the isolated AC signal component in accordance with the
adjustably-determined second gain amplification factor. The processing unit
produces output signals in accordance with the isolated AC signal component
9
CA 02334964 2010-02-04
SEC770" R Cn- P,!;,,ECTI N
cc,
`~ -
ATE
.,.,
WO 99/639e3 PCT/11.98J00270
and the DC signal component and calculates in accordance therewith, at least
one blood constituent level.
Furthermore, in accordance with another preferred embodiment of the
present invention, the light source and the light detector of the device are
held in
a spaced relationship while in contact with the surface of the organ so as to
substantially block entrance of extemal light therebetween.
Furthermore, in accordance with another preferred embodiment of the
present invention, the processing unit further comprises: means for
normalizing
the AC and DC output signal components to produce first and second
normalized signals, and means for forming a ratio of the first and second
normalized signals. The processor calculates the blood constituent level in
accordance with the ratio.
Furthermore, in accordance with another preferred embodiment of the
present invention, the organ is the skin and the device is arranged for
mounting
on a ribbon, a bracelet and the like for placement on a part of a human or an
animal body.
Furthermore, in accordance with another preferred embodiment of the
present invention, the organ is the skin and the device is arranged for
mounting
on a tightly-fitted garment to be wom over a part of the body.
Furthermore, in accordance with another preferred embodiment of the
present Invention, the device further includes a transmitter for transmitting
the
CA 02334964 2010-02-04
SECTiON P f_`O!? ?EGT101V
3'E
-rn1
OLa: s
,..~
wo 99/63883 PCT/IL98/00270 ` AT
output signals to a receiver at a remote location, allowing monitoring of the
at
least one blood constituent level from the remote location. The receiver is
equipped with an alarm unit for alerfing when the at least one blood
constituent
level falls outside of a predetermined range.
Furthermore, in accordance with another preferred embodiment of the
present invention, the processor develops a control signal when the
adjustably-determined sebond gain amplifir.ation factor is established in the
second stage, the signal is measured and the control signal shuts off the
light
source.
Furthermore, in accordance with another preferred embodiment of the
present invention, the control signal conserves energy by reducing the
operational duty cycie of the light source.
Furthermore, in accordance with another preferred embodiment of the
present invention, the first and second gain amplification factors are
determined
ss by the processor in an iterative process by adjustably setting a gain
amplification factor and measuring a'dynamic voltage range of the output
signals to determine if the voltage range falls within a predetermined window
established by the processor.
Furthermore, in accordance with another preferred embodiment of the
2o present invention, the light source comprises a single light emitting unit
capable
of controllably providing light having a wavelength range selected from at
least a
first wavelength range and a second wavelength range. The first wavelength
11
CA 02334964 2010-02-04
SECTI0ta,q CO"IRECT9 N
~~:~~=
u_. ._ _ .. .. .. : ':"~~ E
CC3o'~ kn ;;^!E$
` r =. s
Vs'`~ . P ~ P t<.. s...~>oT
WO 99/63883 PCT/...98r00270
range is at least partially different from the second wavelength range. The
single light emit4ng unit can be switched from emitting light within the first
wavelength range to emitting light within the second wavelength range.
Furthermore, in accordance with another preferred embodiment of the
present invention, the light source includes at least a first light emitting
unit
capable of controllably emitting light having a first wavelength range and a
second light emitting unit capable of controllabiy emitting light having a
second
wavelength range. The first wavelength range is at least partially different
from
the second wavelength range.
Furthermore, in accordance with another preferred embodiment of the
present invention, the light source provides light having wavelengths in the
red
and infrared ranges.
Furthemnore, in accordance with another preferred embodiment of the
present invention, the organ is the skin, the blood constituent is hemoglobin,
and measurement of a level of oxygen saturation in the hemoglobin provides an
eariy indication of respiratory stress.
Furthermore, in accordance with another preferred embodiment of the
present invention, the respiratory stress is associated with Sudden Infant
Death
Syndrome.
Furthermore, in accordance with another preferred embodiment of the
present invention, the device produces an output signal sent by the processor
to
12
CA 02334964 2010-02-04
SECT!nN 8 CORftFGT'10M
~:+.... . .
AI'E
a=
C tE 8
T
WO 99/63883 PCT/IL98/00270
an alarm unit for alerdng when the at least one blood constituent level falls
outside of a predetermined range.
Furthemnore, in accordance with another preferred embodiment of the
present invention, the device is used to monitor the heart rate.
Furthermore, in accordance with another preferred embodiment of the
present invention, the device is used as an apnea monitor.
Furthermore, in accordance with another preferred embodiment of the
present invention, the device is a portable hand held reflective pulse
oximeter.
Furthermore, in accordance with another preferred embodiment of the
present invention, the device is adapted to determine blood billirubin levels.
Furthermore, in accordance with another preferred embodiment of the
present invention, the device is adapted for mapping the intensity of the AC
signal along the surface of the organ to detect regions of the organ having a
reduced blood flow.
There is further provided, in accordance with another preferred
embodiment of the present invention, a method for non-invasive measurement
of a level of at least one blood constituent. The method includes the .sfeps
of:
providing light from at least one light source disposed proximate the skin,
directing the light toward the skin surface, the light being reflected from
the skin,
providing a light detector spaced apart from the light source and being
sensitive
to intensity levels of the light reflected from the skin for producing
intensity
13
CA 02334964 2010-02-04
SECPnk! A COPRCCT10N
co-,
~~~
y . 3~'dCLE 8
.:..; m..d a~a~l
wo "i6sss pcTat.9siooz~o
signals in, accordance therewith, and processing the intensity signals
received
from the light detector. The processing step includes the steps of amplifying
the
intensity signals in first and second amplifiers, each in accordance with a
respective first and second gain amplification factor, and automatically
determining the first and second gain amplification factors in adjustable
fashion.
During a first stage, the first and second ampiifier amplify a DC signal
component of the intensity signals in accordance with predetermined first and
second gain amplification factors, the DC signal component being subtracted
from the intensity signals at an input of the first amplifier, thereby
isolating an
AC signal component of the intensity signals. During a second stage, the
second amplifier amplifies the isolated AC signal component in accordance with
the adjustably-determined second gain amplification factor. The processing
step produces output signals in accordance with the isolated AC signal
component and the DC signal component and calculates in accordance
therewith, the at least one blood constituent level.
Furthermore, In accordance with another preferred embodiment of the
present invention, the method further includes the step of transmitting the
output
signals to a receiver at a remote location, allowing monitoring of the at
least one
blood constituent level from the remote location. The receiver is equipped
with
an alarm unit for alerting when the at least one blood constituent level falls
outside of a predetermined range.
14
CA 02334964 2010-02-04
SECTt~A'! 770
Sz; `= t:: ~ ~ l~
u P 4 .y
WO 99/6390 PCT/1L98/00270
Furthermore, in accordance with another preferred embodiment of the
present invention, the step of processing further includes normalizing the AC
and DC output signal components to produce first and second normaiized
signals, forming a ratio of the first and second normalized signals, and
calculating the blood oonstituent level in accordance with the ratio.
Furthermore, in accordance with another preferred embodiment of the
present invention, the mefhod further includes the steps of developing a
control
signal when the adjustably-determined second gain amplification factor is
established in the second stage,
measuring the signal and shutting off the light source in response to the
controi signal.
Furthermore, in accordance with another preferred embodiment of the
present invention, the method further includes the steps of determining the
first
and second gain amplification factors by a processor in an iterative process
by
adjustably sett3ng a gain amplification factor, and measuring a dynamic
voltage
range of the output signals to determine if the voltage range falls within a
predetermined window established by the processor.
Furthermore, in accordance with another preferred embodiment of the
present invention, the blood constituent is hemoglobin, the method further
2o includes the step of ineasuring a level of oxygen saturation in the
hemoglobin
providing an earty indication of respiratory stress.
CA 02334964 2010-02-04
SECT7ON 8 M;?PECTtON
~~ ~.r_.~_,-9
~ __E ~. ,._:. ..':~ATE
C F'F':: C' 'u:,.; RTiCiõE 8
V -::; A T
WO "MIM PGT1IL98100270
Furthermore, in accordance with another preferred embodiment of the
present invention, the respiratory stress is associated with Sudden Infant
Death
Syndrome.
Furthermore, in accordance with another preferred embodiment of the
present invention, the method further includes the step of initiating an alarm
for
alerting when the blood constituent level falls outside of a predetermined
range.
Furthermore, in accordance with another preferred embodiment of the
present invention, the alarm is selected from an audible alarm, a visual
alarm, a
tactile alarm, dialing a telephone number and any combination thereof.
Furthermore, in accordance with another preferred embodiment of the
present invention, the light is altematingiy selected from at least a first
wavelength range and a second wavelength range. The first wavelength range
is at least partially different from the second wavelength range.
Furthermore, in accordance with another preferred embodiment of the
present invention, the first wavelength range includes wavelength of red light
and the second wavelength range inciudes wavelength of infra-red light, the
blood constituent is hemoglobin and the method determines the level of oxygen
saturation of the hemoglobin.
Furthermore, in accordance with another preferred embodiment of the
2o present invention, the method is used for monitoring the heart rate.
16
CA 02334964 2010-02-04
sECTrf~N g t;O~~ECTioN
~~
C ÃC M.,ATE
Ct,tE 8
WO 99I6388:i PCT/IL98/00270
Furthermore, in accordance with another preferred embodiment of the
present invention, the method is used for monitoring a condition of apnea.
Furthermore, in accordance with another preferred embodiment of the
present invention, the method is used for monitorfng the level of billirubin
in
blood.
Furthermore, in accordance with another preferred embodiment of the
present invention. The method further includes the step of repeating the steps
of
providing light, providing a light detector and processing at a plurality of
positions along the skin for mapping the levels of the AC signal component
along the surface of the skin to detect regions of reduced blood flow.
There is still further provided, in accordance with another preferred
embodiment of the present invention, a method for measurement of a level of at
least one blood constituent. The method Includes the steps of providing light
from at least one light source disposed proximate the surface of an organ,
directing the light toward the surface of the organ, the light being reflected
from
the organ, providing a light detector spaced apart from the light source. The
light
detector is sensitive to intensity levels of the light reflected from the
organ for
producing intensity signals In accordance therewfth, and processing
the.iatensity
signals received from the light detector. The processing step includes the
steps
2o of amplifying the intensity signals in first and second amplifiers, each in
accordance with a respective first and second gain amplification factor, and
automatically deterrnining the first and second gain amplification factors in
17
CA 02334964 2010-02-04
SECT1C)Pf g CCpRFCT1D)4
(, . . . ,LE $
~:.!
WO 99/63883 PCT/1L98/00270
adjustable fashion. During a first stage, the first and second amplifier
ampl'ify a
DC signal component of the intensity signals in accordance with predetermined
first and second gain amplification factors, the DC signal component is
subtracted from the intensity signals at an input of the first amplifier,
thereby
isolating an AC signal component of the intensity signals. During a second
stage, the second amplifier amplifies the isolated AC signal component in
accordance with the adjustably-determined second gain amplification factor.
The processing step produces output signals in accordance with the isolated AC
signal component and the DC signal component, and calculating in accordance
therewith, the blood consatuent level.
Furthermore, in accordance with another preferred embodiment of the
present invention, the organ is an intemai organ and the method further
includes the step of repeating the steps of providing light, providing a light
detector, and processing, at a pluraiity of positions along the surface of the
intemaf organ for mapping the levels of the AC signal component along the
surface of the intemai organ to detect regions of reduced blood flow.
There is also provided, in accordance with another preferred embodiment
of the present invention, a method for non-invasively determining the blood
flow
velocity in a region of an organ. The method includes the steps of positioning
a
first pulse-oximetry device and a second pulse-oximetry device proximate the
surface of the region. The first and the second device are separated from each
other by a predetermined distance, simultaneously obtaining a first and a
18
CA 02334964 2010-02-04
SECTIO,Pq,9
CCFeEChO,
Co t AfE
! ~t 4~ 8
WO 99l63883 PCTlIL98/00270
second sets of data representing the pulsatile variation at the locations of
the
first and the second device, respectively, as a function of time, each of the
first
set and the second set of data includes at least one extremum data value, the
extremum data value of the first set of data corresponds to the extremum data
value of the second set of data, calculating the time interval between the
extremum data value of the first set of data and the extremum data value of
the
second set of data, dividing the value of the predetermined distance by the
value of the time interval to obtain a value representing the approximate
blood
flow velocity in the region of the organ, wherein each of the first device and
the
second device includes at least one light source, providing light directed
toward
the surface of the organ, the light being reflected from the organ, a light
detector
spaced apart from the at least one light source and being sensitive to
intensity
levels of the reflected light for producing intensity signals in accordance
therewith, and a processing unit for processing the intensity signals received
1s from the light detector. The processing unit includes first and second
ampiffiers
for ampiifying the intensity signals, each in accordance with a respective
first
and second gain amplification factor, and a processor for automatically
determining the first and second gain amplification factors in adjustable
fashion.
During a first stage, the first and second amplifiers amplify a DC signal
component of the intensity signals in accordance with predetermined flrst and
second gain ampiification factors, the amplified DC signal component being
subtracted from the intensity signals at an input of the first amplifier, to
isolate
an AC signal componenf of the intensity signals. During a second stage, the
19
CA 02334964 2010-02-04
SECT70t't R CORRECT10N
'i. ~+=...`C. . CY
SF'E ; 7 l~;~1TE
C~}tey' ..4~~,ti Ai TICl.E 8
WO 99/63883 PCT/IL98/00270
second amplifier amplifies the Isolated AC signal component in accordance with
the adjustably-determined second gain amplification factor. The processing
unit
produces output signals in accordance with the isolated AC signal component
and the DC signal component and calculates in accordance therewith.
Furthermore, in accordance with another preferred embodiment of the
present invention, the organ is the skin.
Finally, in accordance with another preferred embodiment of the present
invention, the extremum data value is selected from a minimum data value and
a maximum data value.
Other features and advantages of he invention will become apparent
from the following drawings and description.
CA 02334964 2010-02-04
SECTInN 8 CDqqECTtOIV'
:-tCLE 8
W0 99/63883 ~= _ .
PCT/IL98/OOZ7Q
BRIEF DESCRIPTION OF THE DRAWINGS
For a better understanding, the invention will now be described, by way
of example only, with reference to the accompanying drawings in which like
numerals designate like components throughout the application, and in which:
Fig. I is a schematic layout diagram of a physiological stress detector
device, constructed and operated in accordance with the principles of the
present
invention;
Fig. 2 is an electronic schematic diagram of a prior art signal processing
technique, for use with the device of Fig. 1;
Figs. 3a- 3b show, respectively, a prior art signal waveform representing
emitted and received light;
Figs. 4 and 5a-b show, respectively, arrangements for wearing the
device of Fig. 1 on the body of an infant on a leg, foot or head;
Fig. 6 is an electronic block diagram showing the signal processing
components of the device ofthe present invention;
Fig. 7 is an algorithm of a signal processing technique perFormed in
accordance with the principles of the present invention;
Figs. 8a-b are, respectively, signai waveforms representing emiited red
and infrared light used in the device of Fig. 1;
Fig. 9 is a timing diagram applied in an automatic gain adjustment
procedure during signal processing;
21
CA 02334964 2010-02-04 SEC TIONq CrPIRFCT10N
cP 'iC~TE
-> V- _ :~TICLE 8
WO ~/63"3 PCT/(L98/00270
Fig. 10 is a schematic illustration of a device for determining blood flow
velocity in accordance with another preferred embodiment of the. present
invention; and
Fig. 11 is a schematic graph useful in understanding the method of
s detemMning blood flow velocity used by the device of Fig. 10.
22
CA 02334964 2010-02-04
SEC7701q 8
SEE ~~ ~ , ~T! N
CDRp UE
ML~O~i V~ ,MB
WO 77~WOOJ rClM7wvv670
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The foilowing description presents a detailed construction of a
physiological stress detector device adapted for use in monitoring arterial
oxygen
levels. In this particular application, the reflective oximetry method uses
light
wavelengths in the red and infrared ranges, since these are most suitable for
detecting oxygen saturation in hemoglobin. As will be understood by those
skilled
in the art, particular design features used for this application can be varied
for
different applications. For example, in an application for monitoring jaundice
through bilirubin levels, other suitable, light wavelengths would be used.
Therefore, the light wavelengths discussed in the following description are
not
intended to limit the scope of the present invention, and are to be understood
as
pertaining to the subject example only.
Referring now to Fig. 1, there is shown a preferred embodiment of a
physiological stress detector device 10 constructed and operated in accordance
with the principles of the present invention. Device 10 comprises a housing 12
arranged for placement In dose proximity to a skin surface 14. Housing 12 may
be provided as a casing endosing a light source 16 emitting two wavelengths,
red
and infrared, and a photodetector 18 spaced apart from the light source 16...
Device 10 is designed to be operated such that when light source 16 emitEr
light of
a red or infrared wavelength, the light penetrates skin tissue (arrow A) and a
por6on of the light is reflected back to light detector 18, along a path
defined by
line 20.
23
CA 02334964 2010-02-04 SEGPP,,A!,, ~^!~
. : ~?qE~ ~'TlOia
~"F s P:w ~j
....1.jf'tl6:A~
GOf~i it;E 8
}' .: .. .'i~;~-T
WO 99/63883 PCT/IL98/00270
The light source 16 may be implemented as a single component which
can controllably emits red or infrared light. A non limiting example of the
light
source 16 is the selectable wavelength light emitting diode (LED) component
model L122R61R880, or for pediatric or prematurely born baby applications the
s component model SML12R6IR880, both components are commercially available
from Ledtronics, CA, U.S.A. However, The light source 16 may also include two
separate suitable light sources. For example, the light source 16 may include
two
separate light sources (not shown) such as an LED emitting red light and
another
different LED emitting infrared light.
It is noted that, while, preferably; the light source 16 includes one or
more LEDs emitbng In the suitable red and infrared ranges, other light sources
may be used such as incandescent lamps in combination with suitable optical
filters, various types of gas discharge or arc lamps, with or without optical
filters,
diode laser deviCes, or any other.
For the pulse-oximetry application the light detector 18 may be a
photodiode, such as the model BPW34 photodiode, or for pediatric and premature
bom babies the model BPW34S photodiode, both commercially available from
Siemens Semiconductor Group, Gemiany. However, many other types of
photo-detecting devices may be used such as resistive photocells, or any other
2o type of photodetector which has the required sensitivity at the wavelengths
used
for the specPfic application of the device 10.
24
CA 02334964 2010-02-04
SECfi1CM g
C~PRFCTi N
...~ATE
CO~~ ~ ~ ~ A= ::u! E ~
V
WO 99/63883 PCT/1L98100270
It is noted that the device 10 of Fig. I also includes further electronic
components (not shown in Fig. 1) which are disclosed in detail hereinbelow (
as
best seen in Fig. 6).
As described in the background of the invention, the device 10 empioys
non-invasive reflecfive oximetry techniques to provide measurement of blood
characteristics useful in diagnostic procedures and detec6on of physioiogical
stress. As mentioned, one difficulty in reflective oximetry is in adjusting
the
separation between light source 16 and detector 18 such that the desired
signal
received by light detector 18 is strong and not affected by shunted, or
coupled,
light from source 16. Figs. 2 and 3a-3b illustrate this problem and the prior
art
techniques currentiy available for its solution.
In Fig. 2 there is shown an electronic schematic diagram of a signal
processing filter 22 used to separate the variabie signal (AC) component of
received light from the shunted (DC), or coupled, light. The separatlon is
achieved by a blocking capacitor 24 on the input of an operational amplifier
26
used to amplify the variable signal portion. The DC signal component of the
received light, which does not pass through blocking capacitor 24, forms the
input
of, and is ampiified by operationai amplifier 28.
As illustrated in Figs 3a-3b, the signal waveform representing the
2o emitted light, (Fig. 3a) is substantiially reproduced as a received signal
waveform
(Fig. 3b). Even after filtering by signal processing filter 22, the AC signal
component remaining oSIG is only a small portion of a larger signal which has
been amplified by operational amplifrer 26, and therefore dominates the
variabie
CA 02334964 2010-02-04
SECTIQM 8 CORPECTlON
SEF 07 :d^6CATi=
~,..8
WO 99/63883 PCT/IL98/00270
signal portion. Thus, this method of signal separation resufts in inaccurate
readings of reflected light, and cannot provide accurate information in
oximetry
nieasurements.
In Figs. 4 and 5a-b there are shown altemative configurations of device
10, respectively, provided in a foot bracelet 30, a sock 32 worn around the
ankle,
and a ribbon 34 wom around the head. In each arrangement, casing 12 is
designed to be held tightiy against skin surface 14 to reduce the amount of
stray
light entering into the optical path between light source 16 and detector 18.
Preferably, the casing 12 is made from a material opaque to light in the
relevant spectral range to which the detector 18 is sensitive, such as an
opaque
plastic material, metal or the like. The foot bracelet 30, the sock 32 and the
ribbon
34 may be made of a material which allows the casing 12 to be tightly pressed
against the skin. This material may be a flexible material such as a flexible
fabric.
The material may also be a porous or woven material to prevent excessive
perspiration of the skin thereunder.
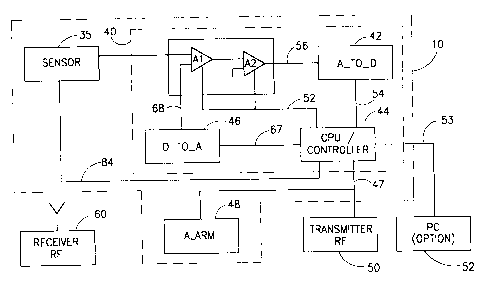
Referring now to Fig. 6 , there is shown an electronic schematic block
diagram of device 10. Device 10 comprises a sensor 35 incorporating light
source
16 and detector 18. The sensor 35 may also include a preamplifier circuit (not
shown) for ampl'ifying the output signals of the detector 18 and feeding the
ampiified signals to the processing unit 40. It will be appreciated by those
skilled
in the art that the numbers of light sources and detectors can be varied while
keeping the same processing method. In addition, device 10 comprises a signal
processing unit 40 induding a pair of operational amplifiers Al and A2, an
analog
26
CA 02334964 2010-02-04
SECTION 8 C0p'7ECTIOK
~~=~ ~r_P~~, '.{'A
a LE 8
:wõT
WO "/~ PCT/IL98/00270
to digital converter 42, a central processing unit (CPU)lcontroller 44, and a
digital
to analog converter 46. In critical applications, such as SIDS, when there
exists a
need for emergency first aid availability, when CPU 44 has determined that the
value obtained is not within the acceptable range an output signal 47 is fed
to an
alarm unit 48 causing an alarm to be activated. Optional connections to an RF
transmitter 50 and PC computer 52 are available. Sensor 35 is designed to be
(not shown).
powered by a small battery
According to another embodiment of the present invention, processing
unit 40 with or without alarm 48, RF transmittor 50 and/or PC 52 are connected
to
the sensor 35 via a cable or by wireless transition. In this case sensor 35
does not
require a battery.
It is noted that, the alarm unit 48 may activate a visual alarm, an audio
alarm, a tactile alarm (such as a vibratory signal), or an audio-visual alarm.
The
alarm unit 48 may also initiate the automatic dialing of a telephone number
and
may also activate any combination of any of the above types of alarms, or of
other
types of alarms.
The coupling of operationai amplifiers Al and A2 is between the output
of amplifier Al and the input of amplifier A2. The gain amplification factor
of each
amplifier is set by the central processing unit 44 via a signal in accordance
with an
automatic adjustable gain technique described further herein. Analog to
digital
converter 42 provides a digital input signal 54 based on the level af output
signal
56 from amplifier A2. The central processing unit 44 is programmed to process
the information contained in input signal 54, and thereby determine blood
oxygen
27
CA 02334964 2010-02-04
SECT7C),N $ COPRECTIaN
5EG C'd:'~i "T
CDfa~~' ~'n.,
~;,.,ds.'ra-"..io0t.E8
v
C:
wo M63s83 PCrnL9Srooz7o
saturation levels detected by sensor 35. The output signal 47 from CPU 44 may
be used to trigger alarm 48, or its information can be transmitted by an RF
transmitter 50 to a receiver 60 for remote station processing. Data analysis
can
be performed by PC 52 based on a data output signal 53.
Based on the block diagram of Fig. 6, device 10 can be constructed in
accordance with state of the art electronic design techniques employing, for
example a 8051 micro-controller, commercially available from Intel Corp,
U.S.A.,
or any other suitable processor or controller to implement the CPU/controller
44.
The properties of amplifiers Al and A2 are selected in accordance with
electronic design rules well known in the art. In a non-limiting example,
amplifier
Al is the model PGA205AU programmable gain instrumentation amplifier, and
amplifier A2 is the model PGA204AU programmable gain instrumentation
amplifier, commercially available from Burr-Brown, AZ, U.S.A. However, the
amplifiers Al and A2 may be any other suitable type of amplifier. For example,
while in the preferred embodiment disclosed hereinabove each of the amplifiers
Al and A2 is shown as an operational amplifier unit, each of the amplifiers Al
and
A2 may be implemented as a multi-stage amplifier device containing more than
one amplification stages.
As mentioned in the background of the invention, problems with prior art
reflective oximetry techniques are related to the measurement of the AC signal
component which forms a small part of the larger DC signal component provided
by light sensor 35. Whereas the previous techniques involved use of a blocking
capacitor 24 as described in Figs. 2 and 3a-3b, the present invention provides
a
28
CA 02334964 2010-02-04
SECT7ICJIV 8 COPPFCnoN
CDRFt LE 8
VC . , , .,.. 'y.
v1~ w. naia a\-':"f1
WO ~/63883
PCT/II,98/00270
novel soiution to the signal amplification problem such that more accurate
oximetry measurement may be obtained.
It is noted that, depending on the specific detector used, the AC and DC
signal components generated by the detector 18 may be current or voitage AC
and DC signal components, and that the terms AC signal component and DC
signal component throughout the specification and claims define AC and DC
components of the output signal of the detector 18 and may indude voitage
signal
components and current signal components. However, the AC and DC signal
components may also include any other type of eiectrical or photonic (optical)
signal which may be the output of any suitabie detector type useful with the
device of the present invention.
In aocordance with the principies of the present invention, processing
unit 40 applies a novel technique for sepaneting the AC signal component from
the DC signal component. The steps carried out by CPU 44 in this technique are
illustrated in the flow chart of Fig T.
In start bioclc 62, CPU 44 begins its operation by initializing the gain of
analog amplifiers Al and A2 automaticaily. In block 64 the detected signal
from
sensor 35 is measured, and this is performed by providing output signal 56
from
signal processing unit 40 to the analog to digital converter 42, so that it is
converted to a digitai input signal 54 for input to CPU 44. In block 66, CPU
44
calculates the DC signal component of the detected signal. This is achieved by
a
two-stage process.
29
CA 02334964 2010-02-04
SECT1Oa 8 COPP,FCTION
S rF C' r"T iF;C. ~ ~ E
CORRE I `s.,~. ;T L6 ~
V;' =
wo 99J638M pc-rin.9&W270 In the first stage, output signal 56 is treated as a
pure DC signal, such
that CPU 44 takes the average of this signal level, and generates a digital
output
signal 67 which is converted by the digital to analog converter 46 to an
analog
reference shift signal 68. In block 70, reference shift signal 68 is fed into
the
negative input of amplifier Al and amplifier Al effectively neutraiizes the DC
component by applying reference shift signal 68 against the detected signal
from
sensor 35. This produces a null output for input to ampiifier A2.
In the second stage, in block 72, amplifier A2 receives the AC signal
component of the detected signal and amplifies it, thereby producing an output
signal 56 containing information based on the reflective oximetry technique.
This
information, when converted to a digital signal in analog to digital converter
42,
provides digital input signal 54 to CPU 44. In block 74, the oximetry
calculation is
performed by the CPU/controller 44 based on measurements derived from sensor
35, in accordance with the information provided by digital input signal 54.
The
results of the oximetry calculation are provided as output signal 47 or in the
form
of a data signal 35 fed to a PC computer 52. Output signal 47 may be used to
activate an alarm 48 or it may be provided as the signal for transmission via
RF
transmitter 50 to a remote receiver 60, to allow base station monitoring of
the
reading.
Referring now to Figs. 8a-b, there are shown respectively, pulse signal
waveforms representing light received in the red and infrared ranges by light
detector 18 in sensor 35. Light is provided by light source 16 in pulses each
having, for example, a duration of 1.6 milliseconds and a period of 15.6
.30
CA 02334964 2010-02-04
BECTiON 8 CQRR,ECTlON
~C~ TM ~y E 8
WO " 893 PCT/IL98l00270
milliseconds. The analysis of a typical light pulse is provided in Fig. 9,
showing
the time scale division of the 1.6 millisecond pulse into two cyclical gain
adjustment periods 76 and 78, respectively. The red and infrared pulses are
staggered so as to minimize interference between them.
In Fig. 9, a time division scale is developed in which each of the pulsed
light waveforms is divided into two periods 76 and 78, each having, for
example, a
maximum duration of 800 microseconds, during which the gain amplification
factor
is set for each of operational amplifiers Al and A2. The first period is used
to set
the gain for and measure the DC signal component, and the second period is
io used to set the gain for and measure the AC signal component.
The gain amplification factor is automaticaify adjusted in an iterative
process. After a predetermined delay, for example 50 microseconds, the gain
ampiification factor Is set during interval 80, and the output signal 56 of
signal
processing unit 40 is measured to determine if it falls within the window
defined by
CPU 44. For example, a dynamic vottage range of between 0.4-4 volts is
established by CPU 44, and output signal 56 is measured during interval 82, to
see if it falls within this window. If it does, the gain amplification factor
is fixed at
its current value. If, on the other hand, output signal 56 does not fall
within this
window, another setting is provided by CPU 44 and again the output signal 56
Is
2o measured. This process is repeated, in Iterative fashion, within the first
period of
the cyclical gain adjustment procedure until the output signal 56 fails within
the
desired window.
31
CA 02334964 2010-02-04
SEC71OAi P COPPECTION
..Y.._...
C ATE
COg; grLE $
WO 99/63883 PGT/1L98/00270
If the desired window for the DC signal component is obtained before
the 800 microseconds of the first period has elapsed, the first period is
shortened
accordingly, and the second period is commenced, during which the same
procedure is performed for the AC signal component. Once a desirable window Is
attained for the AC signal component, the second period may be shortened
accordingly, and CPU 44 sends a control signal 84 to sensor 35, to shut off
the
light source for that pulse. In this fashion, an energy savings is achieved by
reducing the duty cycle of light source 16, and reducing the current drain
from the
battery and extending its useful life. Control signal 84 is provided for each
individual light pulse, so that the maximum energy savings is achieved. tf the
800
microseconds has elapsed without establishing the gain ampiification factor,
the
signal is ignored.
It Is noted that, the values disclosed hereinabove for the pulse duration
and pulse interval of Figs. 8a and 8b and for the two periods 76 and 78 of
Fig. 9
are given as a non-limiting example only and may be replaced by other suitable
values depending, inter alia, on the available electronic component speed, the
processing speed of the processor/controller 44 and the specific application
type.
For example, the pulse duration and pulse interval of Figs. 8a and 8b can have
the values of 0.6 milliseconds and 15.6 milliseconds, respectively, and the
two
periods 76 and 78 of Fig. 9 may each have the value of 300 microseconds.
It is further noted that, while in the embodiment disdosed hereinabove
(Figs. 8a, 8b and 9) a DC gain correction procedure is performed for each
first
time period 76 as disclosed in detail hereinabove, it was found that the DC
32
CA 02334964 2010-02-04
SECTIpN 8 COPe~ECTIQN
SEE C"'r=vf.rr.. ,'E
t ~~f CQRRCC s 0.m10 ;~r -CL4 $
Ct~ ~'Ve AT
WO 99/6303 PGT/IL98/00270
correction can be performed much less often with no deterioration of the
devices
performance and in some cases with a resulting improvement of measurement
stability. For example, if a typical measurement cycle lasts approximately 4-5
seconds, in order to indude a few heart pulse cydes, and includes 256 infrared
and red light measurement periods (each of the light measurement periods
comprising the time periods 76 and 78), performing the DC correction procedure
only once for every 256 measurement periods (i.e once for each measurement
cycle) results with a better stability. Thus, the number of times of
performing the
DC correction procedure of the present invention per measurement cycle may be
io varied for optimizing the stability and accuracy of the measurements. The
optimal
number of times of performing the DC correcaon procedure of the present
invention per measurement cycle may depend, inter alia, on the optical
parameters of the light source 16 and the detector 18 of the device 10 and on
the
spedfic wavelengths implemented in the specific application.
An advantage of reducing the number of DC corrections per
measurement cyde is that it reduces the computational load of the CPU 44,
enabling increasing the number of light measurement time periods within each
given measurement cycle or, altematively, using a less powerful CPU 44 to
reduce the overall cost of the device 10 while conserving or even improving
the
2o accuracy and stability of the measurements.
The gain ampiiflcation factors are selected from a set of preselected
values. Amplifier Al, which acts to ampiify the DC signal component, can have
33
CA 02334964 2010-02-04 $EC77 W 8 cor"x7b'C ?,6(?'N
SEE CE{Tj7v,
CORFtECT"6, 3: - KL~ 8
VOIR CCRTir 4-AT
WO ~16M PCT/[L98/00270
gain amplification factors of 1, 2, 4 or 8. Amplifier A2, which amptifies the
AC
signal component, operates in the amplification ranges of 1, 10, 100 or 1000.
An advantage of the ability to automatically switch between the gain
amplification factors based on the iterative process performed by CPU 44, is
that
it allows the device 10 to obtain oximetry measurements in different parts of
the
body without recalibrating the gain amplification factor for each area.
The separated AC and DC signals are calibrated using the formulas:
(Vw) K
VõC _
AAC *ADC
Voc= (Va/d) K
VDC =
AAC ADC
where V,, is the signal from the analog to digital converter and A,C and
ADc represent the gain of the A2 and Al ampifiers, respectively. Using these
calibration equations it is possible to calculate a value for each of the
signal
components (V,,,c and Voc) which is substantially separated from the other
signal
component.
34
CA 02334964 2010-02-04 SE'CTt~
~~~~P C?
3r.~ ~~_;-'__, x}~ECTIpN
CORR,-7~;t~
liO1R r~t~LE a
WO 99/63883 PCT/IL98N0Z70
Once the AC and DC signal components are calibrated, calculations for
purposes of determining oxygen saturation are performed by taking the AC and
DC values for each wavelength and forming a ratio:
V(AC)mN(DC).,
s G=
V(AC)r,,,0N(DC);,,,.d
This ratio is used } to calculate the oxygen saturation in the formula:
SatO2= B- A t G
where B and A are constants. CPU 44 determines whether or not this
value falls within the desired window, and in cases where the value is
unacceptable and stress is detected, an output signal 47 is sent to alarm 48
and
the alarm wilitum on. Aitematively, or in addition, the output signal 47 can
be
sent to RF transm9tter 50 for transmission to receiver 60. Additional
informa#ion,
such as a log of all readings, may be sent from CPU 44 as a data output signal
53
is to PC 52.
In summary, the physiological stress detector device of the present
invention provides a non-invasive method for more accurately measuring blood
constituents in a compact, easily utilized design. It is especially useful for
application in StDS monitoring systems due to its compact light weight design
2o which is provided with no cumbersome, dangerous cable connections.
An advantage of the devices and methods of the present invention is
that the sensitivity and improved signal to noise ratio of the present method
enables use of transmissive methods of pulse oximetry under conditions where
CA 02334964 2010-02-04
SECTION 8 CORRECTi 6d
sFF r?:`; ^!',rF
CORRECT;1",~.;- r.771CivE 8
VGt
R G.a
WO 99/63883 PC71IL98/00270
the signals are of low amplitude relative to the noises. In a non-limiting
example,
the method and devices may be particulariy useful for transmissive oximetry
under conditions of low blood perfusion such as in systemically shocked
patients
or in cases of severe hypothermia.
A major advantage of the present invention is in its apptication to
reflective oximetry where the signals are usually of a relatively low
amplitude. In
particular, the sensitivity of the method and the devices may enable
performing
reflective pulse oximetry on regions of the body which exhibit pardcularly low
amplitude signals such as the wrist region, or the ankle region of adults and
babies.
Reference is now made to Fig. 10 which is a schematic illustration of a
device 90 for determining blood flow velocity in accordance with another
preferred
embodiment of the present invention.
The device 90 includes a housing 92 and two pulse oximetry devices
10a and 10b attached thereto. The devices 10a and 10b are constructed as the
device 10 disclosed hereinabove and are simultaneously operated to provide an
amplified pulse oximetry AC signal as disclosed in detail for the device 10
hereinabove. The fixed distance D between the device 10a and the device 10b is
represented by the double headed arrow labeled D. The device 90 is placed on a
region of skin A and the pulse oximetry AC signal is simultaneously determined
for each of the devices 10a and 10b.
Reference Is now made to Fig. 11 which is a schematic graph useful in
understanding the method of determining blood flow velocity used by the device
36
CA 02334964 2010-02-04
SECTIOM 8 Cnr?RFCTiON
SEE~~ac~"r .-
CORRi:CTaV `-.g~-
ii.LE i3
VOgR
WO 99/63883 PC7/IL98/~0270
90 of Fig. 10. The horizontal axis represents time and the vertical axis
represents
the amplitude of the reflective oximetry AC signals. The curve 94A represents
the
AC signal output from the device 10a and the curve 94B represents the AC
signal
output from the device 10b. The minima 96A and 96B of the curves 94A and
94B, respectively represent the minima of the reflected AC signal due to the
pulsation of the blood flow. The time delay AT between the reflection minima
96A and 96B represent the time delay between the registration of a minimum
reflectance by the device 10a and its registration by the device 10b. The
delay
resuits from the finite blood velocity and the distance D separating the
devices.
Since the distance D between the devices 10a and 10b is known, the
approximate blood flow vetocify V can be determined by calculating the value
V=D/OT.
The processing unit 40. of one of the devices 10a or 10b thus acquires
two data sets. The first data set represents the AC signal component of the
1s device 10a and the second data set represents the AC signal component of
the
device 10b. Preferably, both of the data sets are digital data sets and are
sampled simultaneously. The data sets are sampled such that each data set
Includes at least one extremum data value corresponding to a minimum or a
maximum value of the AC signai component, the processing unit 40 detects the
extremum point for each of the data sets using any method known in the art for
detecting an extremum point. The processing unit then calculates the time
interval OT between the corresponding extremum points of the first and the
second data sets and calculates the blood flow veiocity from the ratio AT/D.
37
CA 02334964 2010-02-04
SEC77OiV 8 COPl4ECTION
SE` Cr,TN :Cs'.: E
C RR-rt;'";";, E 8
VC '? ..._.,..,. .~;"
WO 99/63883 PGT/1L98/00270
Preferably, for devices using reflective pulse oximetry of the present
invention, the extremum data values used are minimum values representing
minimal values of reflected light due to maximal absorption of the light from
the
light sources 16 of the devices 10a and 10b. However, the extremum values
may also be maxima. For example, in an embodiment where transmissive pulse
oximetry devices are used, the extremum values may be maxima.
It is noted that, while each of the devices 10a and 10b may have a CPU
44 as disclosed hereinabove, in accordance with another preferred embodiment
of the present invention, the device 90 may include a single CPU unit (not
shown)
io which may be shared for performing all the calcuiations and control
functions
disclosed hereinabove for the operation of each of the devices 10a and 10b and
for additionaily performing the determination of AT and the calculation of the
approximate blood flow velocity therefrom.
It will be appreciated by those skilled in the art that suitable methods for
detecting and timing the reflection minima 96A and 96B are well known in the
art
and are not included in the subject matter of the present invention, and will
therefore not be described herein in detail.
It is noted that while the device and method for determining blood flow
velocity disclosed hereinabove is adapted for use with a pair of devices 10a
and
10b, a larger number of devices (not shown) may be used together either as a
multiplicity of device pairs or in any other geometrical configuration for
improving
the accuracy of the measurement by averaging the results of multiple pair
determinations or by any other suitable computational method known in the art.
38
CA 02334964 2010-02-04
SEC710M 8 COPc?FCTpON
SEE cbf~~~7.c._."' LE 8
WO 99163883 PCT/1L99/00270
It is noted that, while the preferred embodiments of the present invention
are particulariy adapted for reflective pulse oximetry applications, if may be
also
implemented in many other appiications. For example, the method and the
device of the present invention may be adapted to the monitor bdirubin levels
for
s the detection and monitoring of jaundice, by suitably selecting a light
source which
emits wavelengths of light in the range selectively absorbed by bilirubin,
(approximately between 400 - 600 nanometer).
In another example, the present invention may also be used to detect
and monitor blood constituents which have distinct absorbance peaks in the
visible range, the near ultraviolet (UV) range or in both the visible and the
near UV
range. For this type of applications one or more of the light wavelengths used
may be obtained from a gas discharge lamp or from any another suitable source
of light in the near UV range.
Another application of the present inventfon is the application of the
method for the determination and mapping of areas of organs suspected of a
reduced blood flow due to chronic or temporary clinical condition. For example
if
an intemai or extemai organ is suspected to have developed gangrene the device
10 of the present invention may be used to map areas having low or reduced
blood flow by moving the device 10 along the organ and in contact therewith
and
mapping areas of reduced blood flow by recording and mapping the amplitude of
the minima of the pulse oximetry AC component as disclosed hereinabove along
the surface of the organ. This method may be particuiarly useful in mapping of
such reduced flow areas in cases where regular transmissive pulse oximetry is
39
CA 02334964 2010-02-04 SECS~(~~ir~4.~ 8,C n~
,w C'D`iQN
CORREci'',z;iCLE $
voiR T
WO "163883 PCT/lL98/00270
not applicable due to inaccessibility problems or due to very noisy signal
conditions.
One exemplary application is mapping the extemal surface of the
intestines using a small pre-sterilized reflective oximetry device such as the
device
10 of the present invention. In such a case transmissive oximetry devices
cannot
be used because it is not possible to positivn a light source and a light
detector on
opposite sides of the intestinal wall. The device 10 is particularly
advantageous
here because it can be simply moved along the extemal surface of the suspected
intestinal part and because of its improved sensitivity and reduced noise
level.
The above mapping method may be applied to many other organs such
as limbs suspected of blood flow disturbances due to a gangrene condition or
other diseases.
It is noted that the devices of the present invention may be implemented
in a variety of different configurations. The devices 10 or 90 of Figs. I and
10,
respectively may be connected to a computer (not shown) or a monitor (not
shown). The computer or monitor may include a display device (not shown).
An altemative configuration may include the device 10, connected to a
housing(not shown) wirelessly or by suitable wires. The housing may also
include
a liquid crystal display device (LCD), such as the LCD display model
G1216001N000-3DOE, commercially available from Seiko Instruments Inc.,
Japan, suitably connected to the CPU 44 for displaying alphanumeric symbols
representative of one ore more parameters of the pulse oximetry signal such as
CA 02334964 2010-02-04
BECnoN 8 CORRECTION
vF:F C;b_:UXtCATE
CORPRw ,
~ ~'~~R~iCI.~ 8
at= :',~ Ã 4i EE.~b~
wo 99163883 PCT/IL98100270
the pulse frequency, or amplitude or any other data. The LCD display may also
display the AC signal graphically with or without the alphanumeric data.
In a third configuration of the device of the present invention the pulse
oximetry device indudes all the optical and electronic components within one
single device shaped as a wrist watch like device to be wom as a self
contained
unit. One non-limiting example (not shown) is a device worn on the wrist and
shaped like a wrist watch. All the components of the device 10 are integrated
within the device such that the light source 16 and the detector 18 are
attached to
the device so as to be in contact with the skin when the device is worn. All
the
necessary electronic components disclosed hereinabove are also integrated in
the device including a power source such as a battery. The device may thus
monitor signals, may or may not collect and store data and may or may not
activate an alarm unit or transmit a distress signal as disclosed hereinabove
in
detail. It is noted that this self contained integrated device configuration
may also
be shaped to be placed in contact with the skin on the limbs, forehead or any
other organ of the patient by suitable means such as strips bands of flexible
material, adhesives or any other suitable attachment means known in the art.
The self contained integrated device configurations may be used for a
variety of applications. For example, in a preferred embodiment of the present
2o invention, the device may determine the pulse rate of the wearer. It is
known that
during a meal the pulse rate increases. The pulse rate may thus be used for
diet
control by reporting to the user when the pulse rate reaches a predetermined
value or when the Increase in the pulse rate following the beginning of a meal
is
41
CA 02334964 2010-02-04 SECIION g CORRECTION
tivh1TE
COPr RTBCi_E 8
CCRTWiCAT
WO 99/63883 PCT/1L98/00270
within a predetermined rate. The user may thus use the device for obtaining an
indication of when to stop consuming food.
The device may also be used for radial pulse measurement in cardiac
measurements and for various bio-feedback application.
In all of the above applications of the self contained integrated device
configurations, such as a bracelet-like device or the like the device has an
advantage of being a compact, lightweight and convenient wearable device while
still providing the high sensitiv'ity, accuracy and relative immunity to
movement
artifacts of the present invention.
40 It is noted that the devices of the present invention, as used in the
various applications disclosed herein above, may also be configured and used
as
monitoring devices in a hospital environment, as well as for domestic use.
It is further noted that the devices and methods of the present invention
may be adapted for use of humans and animals.
Having described the invention with regard to certain specific
embodiments thereof, it is to be understood that the description is not meant
as a
limitation, since further modifications will now become apparent to those
skilled in
the art, and it is intended to cover such modifications as fall within the
scope of
the appended claims.
42