Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02336704 2001-O1-05
The invention relates to a preparation for transdermal
therapeutic application of glyceryl trinitrate through human
skin in an organism, containing, in a polyacrylate-based
self-adhesive layer provided with an active substance-
impermeable backing layer, a therapeutically active portion
of the active substance. The invention further relates to a
method of production of the said preparation, and to the use
thereof. The intention is to obtain with said preparation a
continuous and constantly dosable delivery of glyceryl
trinitrate to, respectively through, the skin, with the
preparation serving as active substance reservoir.
Patches for transdermal delivery of pharmaceutically active
substances have already been known for many years, and they
are successfully employed in medicine. Typically, when
employing the known preparations, the aim is to achieve a
slow and dosed delivery of the substances, with the
preparation serving as active substance depot. In this case
it is desirable for the active substance to be released over
a prolonged period of time. Transdermal application affords
the great advantage of avoiding the intestinal and the
hepatic first-pass effect. As a consequence, the
bioavailability of substances subjected to strong
metabilisation during the absorption process from the
intestine and during the first liver passage is increased.
Furthermore, it is possible to avoid that substances
exhibiting short elimination half-life are applied several
times a day, since the plasma curves obtained by a
transdermal system are equal to those of continuous infusion.
The active substances commonly used in therapy for
prophylaxis or treatment of coronary heart disease are
CA 02336704 2001-O1-05
2
organic esters of nitric acid. Preferably, glyceryl
trinitrate is used.
This active substance relaxes the smooth vascular muscles,
leads to a peripheral vasodilation and causes a reduction of
the cardiac preload and afterload.
As a consequence of this reduction and of the resultant
decrease in cardial work, there is a decrease of the oxygen
requirement of the heart. Further, glyceryl trinitrate causes
a reduction of the extravasal component of the coronary
resistance, and thereby an improvement in the supply of
oxygen.
Acute angina pectoris attacks can be treated quickly and
effectively by sublingual administration of glyceryl
trinitrate. This kind of application quickly leads to high
active substance concentration in the plasma. Since, however,
the plasma half-life of glyceryl trinitrate is only 1-3
minutes, the plasma concentration declines very rapidly, and
is not maintained in the therapeutic region over a prolonged
period. Sublingual administration, therefore, is not suited
for the prophylaxis of attacks.
A mode of administration better suitable for prophylaxis of
angina pectoris attacks is the transdermal administration.
Systemic glyceryl trinitrate absorption through the skin is
about 20 ug/cm'/h, dependent on the site of application. It
is of advantage in this context that bioavailability is not
seriously decreased due to the fact that the extensive
intestinal or hepatic first-pass effect is avoided. Compared
to intravenous application, a bioavailability of about 70%
results if glyceryl trinitrate is administered transdermally.
Firstly, it is only the stratum corneum of the skin and the
size of the application surface which are determining factors
for the amount of active substance circulating in the blood.
A relatively constant steady-state plasma concentration is
CA 02336704 2001-O1-05
3
achieved here over a prolonged period of time. The steady-
state plasma concentration is determined by the dosage of the
given patch and the corresponding absorption rate. For
example, if the absorption rate is 0.4 mg/h the plasma
concentration is on average 0.2 ul/1.
For the above-mentioned reasons, it is clear that the
transdermal administration of glyceryl trinitrate is the
means of choice for effective angina pectoris prophylaxis.
Numerous preparations for transdermal application of glyceryl
trinitrate are prior art. Patch-like systems are predominant.
Glyceryl trinitrate in this case is present either dissolved
or adsorbed to an auxiliary substance. Known systems are
simple matrix systems, for example according to US 4,751,087,
furthermore, reservoir systems having a complex structure,
for example according to US 4,725,272, as well as systems
containing the active substance within microcapsules, for
example according to (US 3,742,951 and US 4,336,243.
For a therapy to be successful it is important that the
active substance be released from the preparation in a
therapeutically effective degree and that it subsequently
permeate the skin. Whereas the release property is determined
by the active and auxiliary substances, the transdermal
active substance absorption is decisively influenced by the
stratum corneum. Absorption can be enhanced by using
permeation-enhancing additives. Thus, US patent 5,262,165
describes the use of N-methyl-2-pyrrolidone and oleic acid
for increasing glyceryl trinitrate absorption.
Synthetic acrylate polymers are frequently used as a
preparation base because of their non-allergenic character,
thus, for example, in US 4,505,891. It is a disadvantage that
most acrylate polymers are very good solvents for glyceryl
trinitrate. This good solubility is tantamount to low
CA 02336704 2001-O1-05
4
thermodynamic activity. To compensate for this, glyceryl
trinitrate must be incorporated at a very high concentration
in order to achieve a therapeutically satisfactory active
substance delivery.
EP 0 622 075 A1 describes a preparation containing glyceryl
trinitrate in a concentration of 50 - 65%-wt. The drawback of
such high glycerol trinitrate amounts has its base in the
properties thereof. Glyceryl trinitrate shows a disadvan-
tageous reaction to thermal and mechanical stress in the form
of explosions, or it leads to unwanted changes in the
adhesive properties, for example to a reduction in tack,
adhesion and cohesion.
For obtaining preparations with acceptable adhesive
properties, US 5,474,783 describes the possibility of
modifying the thermodynamic activity of acrylate polymers by
admixture of a polysiloxane. Polysiloxanes have poor
solubility for glyceryl trinitrate, thereby reducing the
total solubility in the preparation. The reduced saturation
solubility results in an increased release rate. It a.s
possible to control the release kinetics by varying the
amount of polysiloxane admixed. Since the system described is
a multi-phase system, demixing and, thereby, inhomogeneity
may occur during the manufacture thereof.
It is the object of the present invention to provide a
preparation for the transdermal therapeutic application of
glyceryl trinitrate through human skin, of the kind as
described in the introductory portion of claim 1, and to
provide a method for the production thereof, which
preparation develops a high thermodynamic activity while
overcoming the above-described difficulties and technical
limitations, in order to eliminate, avoiding comparatively
high portions of the active substance glyceryl trinitrate,
the problems caused by such high concentrations, and in order
to enable effective therapeutic therapy with long-lasting and
CA 02336704 2001-O1-05
j
constantly controllable active substance release while
significantly increasing bioavailability, which preparation
can moreover be economically produced and affords user-
friendly application.
To achieve this object, a preparation of the kind mentioned
above is proposed which is characterized in that
- for the active substance-containing layer a
polyacrylate-based self-adhesive mass is used,
- the concentration of vinyl acetate in the mass is
maximally 6%-wt., and
- the concentration of active substance in the mass is
maximally 25%-wt.
Further advantageous embodiments of the invention are
provided in accordance with the subclaims.
The preparation has high thermodynamic activity, thereby
ensuring a high release rate while employing low amounts of
active substance. This high thermodynamic activity is
achieved by reducing the saturation solubility of the
respective active substance. This can be achieved by using a
base material for the preparation which as such has a low
active substance solubility, and by avoiding additives that
lead to an increase in active substance solubility.
For the preparation according to the invention, a self-
adhesive polyacrylate-based mass is used. According to the
invention, this mass is characterized in that its vinyl
acetate content is at most 6%-wt. The reason for this
reduction of the vinyl acetate portion .is that - as will be
shown in the following - increasing concentration of vinyl
acetate leads to a decline in the release rate of glyceryl
trinitrate. It must be taken into account that vinyl acetate,
both as copolymer of the acrylate polymer and as an added
homopolymer, reduces the release rate. Hy reason of the
CA 02336704 2001-O1-05
6
measure according to the invention described herein, the
glyceryl trinitrate content is typically within a range of 10
to 30%-wt., preferably below 25 %-wt.
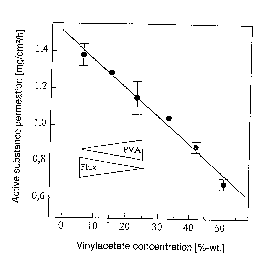
In FIG. 1 the permeation rate of glyceryl trinitrate through
an artificial membrane is shown as a function of the vinyl
acetate concentration of a polyacrylate mass. Vinyl acetate,
in the form of a homopolymer (polyvinyl acetate), was added
to a polyacrylate-based self-adhesive mass. It a.s shown in
the figure that with decreasing portion of vinyl acetate the
permeation rate, respectively the release rate, is raised at
an increasing degree.
FIG. 2 describes the permeation of glycerol trinitrate
through human full-thickness skin. For preparing the
preparation, a vinyl acetate-free polyacrylate mass, shown as
filled squares, or a polyacrylate-vinyl acetate copolymer,
shown as empty circles, was used. As it turns out, the extent
of the permeation through human full-thickness skin is
increased by the use of vinyl acetate-free polyacrylate.
The lowering of the permeation caused by vinyl acetate can be
explained by an increase in the solubility of glyceryl
trinitrate in the self-adhesive polyacrylate mass. Vinyl
acetate, here, acts as a solubilizer. Owing to the increased
solubility, the migration of the active substance from the
mass into a membrane is reduced, since a substance will
become distributed between two phases in correspondence with
saturation solubility. As.a consequence, permeation is also
decreased since the active substance flow through a membrane
is in proportional relationship to the distribution
coefficient.
The self-adhesive polyacrylate-based mass used for preparing
a preparation according to the invention is characterized in
that, for its production, acrylic acid and/or alkylacrylic
acid, especially methacrylic acid or the derivatives thereof,
CA 02336704 2001-O1-05
7
especially the alkyl esters, are used. Preferred alkyl esters
are those having 1 to 18 carbon atoms in the alkyl residue,
in particular methyl, ethyl, n-butyl, isobutyl, pentyl, 2-
ethylbutyl, n-hexyl, heptyl, n-octyl, isooctyl, 2-ethylhexyl,
n-decyl, isodecyl, n-dodecyl and stearyl acrylate or
methacrylate, respectively. Apart from these, further
comonomers may participate in the constitution of the
polymer/copolymer. Examples are acrylamide and/or
methacrylamide, hydroxyalkyl ester and polyalkylene glycol
ester or acrylic and/or methacrylic acid, nitrogen-containing
monomers of acrylic and/or methacrylic acid or the salts
thereof, ethylene, vinyl acetate, vinyl propionate, vinyl
butyrate, vinyl pyrrolidone, vinyl chloride, vinyl toluol,
acrylonitril, styrene and the like.
Further, a backing layer connected with the self-adhesive
mass is contained. Said backing layer is impermeable to the
active substance and has occlusive character. Any of the
materials employed for common preparations may be used.
Examples for such materials are cellulose acetate, ethyl
cellulose, polyethylene terephthalate, plasticized vinyl
acetate-vinyl chloride copolymer, nylon, ethylene-vinyl
acetate copolymer, plasticized polyvinyl chloride,
polyurethane, polyvinylidene chloride, polypropylene,
polyethylene, polyamide or aluminium.
The preparation may further contain: tackifier, penetration
enhancer or agents for alleviating skin irritation, as well
as metal ions, e.g. aluminium or titanium. To increase
cohesion, plasticizers, paraffins, cyclic hydrocarbons or
vegetable oils may be used.
As agents for increasing tack, colophony resins, polyterpene
resins, petroleum resins, coumaron-indene resins, terpene
phenol resins, hydrocarbon resins or liquid polybutene resins
may be used.
CA 02336704 2001-O1-05
g
Examples for agents enhancing the penetration of the active
substance are: pyrrolidone derivatives, fatty acids, fatty
alcohols, fatty acid esters, fatty ethers, paraffin
derivatives, terpenes, ethylene glykol monoalkyl ethers,
polyoxyethylene alkyl ethers, polyoxyethylene aryl ethers,
polyoxyethylene alkyl esters, polyoxypropylene alkyl ethers,
propylene glycol fatty acid derivatives, glycerol fatty acid
asters, polysorbates, poloxamers, dialkyl sulfoxides, urea
and urea derivatives, glycerol, native oils, laurocaprames,
phospholipides, amides, amino acids, N,N-dimethyl formamide,
N-methyl formamide, acetonides, calcium thioglycolate,
propylene glycol, polyethylene glycol, alkyl sulfate, sodium
lauryl sulfate, tetrahydrofurfuryl alcohol, N,N-diethyl-m-
toluamide, anticholinergics, macrocyclic compounds, polar
solvents such as isosorbitol or panthenal.
The preparation according to the present invention may
furthermore contain agents for alleviating skin irritations.
Examples for such alleviating agents are: bisabolol,
chamomile oil, allantoin, glycerol or dipanthenol
The invention will be explained in the following by means of
an example:
163 g of acrylate adhesive (e.g. Durotak~ 387-2353), 19 g of
dioctyl cyclohexane (Cetiol S), 19 g of hydrogenated
colophony resin (e. g. Stabelite Ester 3E), 50 g of glycerol
trinitrate (22.1%-wt. in ethyl acetate), 50 g of ethyl
acetate, 15 g of acetyl acetone and 0.1 g of aluminiumacetyl
acetonate (4%-wt. in ethyl acetate) were mixed. The solution
was coated, with the aid of a doctor knife, onto a silicon-
ized polyester film (e. g. Hostaphan~) at a wet-layer thickn-
ess of 350 dun. The moist film was dried for 30 minutes at
40 °C and subsequently laminated with a polyester film (e. g.
Hostaphan~). The weight per unit area of an adhesive film
prepared in this manner was 67 g/ms. From the laminate,
CA 02336704 2001-O1-05
9
patches of the desired size were punched out by means of a
punch, and the in-vitro pernneation through isolated human
skin was measured. The results of the skin permeation are
shown in FIG. 2 (filled squares). By the use of the vinyl
acetate-free composition, permeation was increased by 65%.