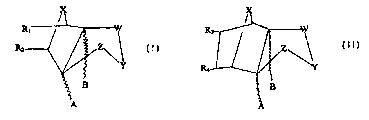Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
ANHYDRIDE MODIFIED CANTHARIDIN ANALOGUES USEFUL IN THE TREATMENT OF CANCER
TECHNICAL FIELD
This invention relates to compounds useful in the treatment of certain forms
of
cancer; processes for producing these compounds; methods of treatment using
these
compounds per se; methods of treatment using these compounds which methods
also
increase the sensitivity of cancer cells to other treatments; methods of
screening these
compounds for anti-cancer activity; and methods of screening these compounds
for
anti-cancer activity and/or ability to sensitise cancer cells to other methods
of treatment.
More particularly, the compounds are specific inhibitors of protein
phosphatases 1 and
I o 2A.
BACKGROUND ART
Protein phosphatase inhibitors and the abrogation of cell cycle checkpoints
The regulation of protein phosphatases is integral to the control of many cell
processes, including cell growth, transformation, tumour suppression, gene
transcription,
t 5 apoptosis, cellular signal transduction, asneurotransmission, muscle
contraction,
glycogen synthesis, and T-cell activation. The role of protein phosphatases in
many of
these processes is often mediated via alterations in the cell cycle. Cell
cycle progression
is tightly regulated to ensure the integrity of the genome. During cell
division it is
imperative that each stage of the cell cycle be completed before entry into
the next, and
2o this is achieved through a series of checkpoints. The cell cycle can be
broken down into
four phases, the first gap (G, ), is followed by a phase of DNA synthesis (S-
phase); this is
followed by a second gap (Gz) which in turn is followed by mitosis (M).which
produces
two daughter cells in G,. There are two major control points in the cell
cycle, one late in
G,. and the other at the G~/M boundary. Passage through these control points
is
CA 02337771 2001-O1-15
WO 00104023 PCT/AU99/00567
controlled by a universal protein kinase, cdkl. The kinase activity of cdkl is
dependant
on phosphorylation and the association with a regulatory subunit, cyclin B.
The periodic
association of different cyclins with different cyclin dependent kinases (cdk)
has been
shown to drive different phases of the cell cycle; thus cdk4-cyclin D 1 drives
cells
through mid G,, cdk2-cyclin E drives cells in late G,, cdk2-cyclin A controls
entry into
S-phase and cdc2-cyclin B drives the GZ/M transition (O'Connor, 1996, 1997).
Following DNA damage induced by chemotherapy or radiation treatment these
checkpoints are responsible for halting cell cycle progression in G,, S and/or
GZ phases
(O'Connor, 1996). The cell undergoes a cell cycle arrest so that the damaged
DNA can
t o be repaired before entry into S phase or mitosis. The phase at which the
cell cycle is
halted will depend upon the type of DNA damaging agent used and the point
during the
cell cycle that the damage was incurred (O'Connor, 1997). The cell cycle is
controlled
and regulated by an intricate phosphorylation network (Stein et al., 1998).
More
particularly, activation of cdk/cyclin complexes requires the phosphorylation
of a
t s conserved threonine residue, which are catalysed by CAK kinase, as well as
the removal
of inhibitory phosphorylations by the phosphatase cdc25. Cdc25 is only active
in its
phosphorylated form. Therefore, protein phosphatase 2A (PP2A) can inhibit the
activation of cdk/cyclin complexes by inhibiting CAK activity and by
dephosphorylating
cdc25. The G,/S checkpoint is predominantly regulated by the cdk/cyclin D/E
complex
20 that mediates its effects by phosphorylating and inactivating the tumour
suppressor
protein retinoblastoma (pRb). The phosphorylation of pRb prevents it from
interacting
with the S-phase transcription factor E2F. E2F controls the transcription of
proteins
needed for DNA synthesis and entry into S-phase including thymidylate
synthase.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
_;_
Accordingly, the inactivation of pRb by phosphorylation permits entry into the
S-phase
and vice versa. However, protein phosphatase 1 (PP 1 ) can dephosphorylate pRb
and
inhibit the cell cycle (Durfee et al., 1993). Thus, PP1 and PP2A are both
negative
regulators of the cell cycle. Inhibition of PP 1 and PP2A would abrogate these
checkpoints and prematurely force cells through the cell cycle.
Serine/threonine phosphatases, which are responsible for protein
dephosphorylation, comprise a unique class of enzymes consisting of four
primary
subclasses based on their differences in substrate specificity and
environmental
requirements. Of the serine/threonine phosphatases, protein phosphatases 1 and
2A (PP1
and PP2A, respectively) share sequence identity between both enzyme subunits
(50% for
residues 23-292; 43% overall), are present in all eukaryotic cells and are
together
responsible for 90% of all cellular dephosphorylation. Knowledge of structure
and
subsequent correlation of binding function for both PP 1 and PP2A would
therefore
provide a vital link toward understanding the biochemical role of these
enzymes. A goal
~ 5 of the medicinal chemist is the development of potent and selective
inhibitors of these
protein phosphatases.
The natural toxins, okadaic acid, calyculin A, microcystin-LR and tautomycin
are
representative of a structurally diverse group of compounds that are all
potent protein
phosphatase 1 (PP 1 ) and 2A (PP2A) inhibitors. Okadaic acid is more specific
for PP2A
20 (ICSO 1nM) than PP1 (IC;o 60nM), while calyculin is slightly more specific
for PP1 (ICSo
0.5-1. OnM) than PP2A (ICso 2nM). All of these phosphatase inhibitors are
known to
abrogate cell cycle checkpoints, particularly the G2 checkpoint of the cell
cycle and
induce cellular mitoses (Yamashita et al., 1990). Abrogation of the GZ
checkpoint means
that the cell does not have the capacity to detect DNA damage or malformation
of the
CA 02337771 2001-O1-15
WO 00/04023 PCTIAU99/00567
-4-
genome prior to entry into mitosis. Therefore, cells which have a deficient G~
checkpoint
are unstable. and incapable of detecting DNA damage, initiating GZ arrest, or
undergoing
DNA repair. Such cells enter the mitotic stage of the cell cycle prematurely
with
malformed spindles. The abrogation is of the GZ checkpoint in the cell cycle
by okadaic
acid is mediated via the activation of cdc2/H1 kinase, the major mitotic
inducer, and
results in a premature mitotic state (Yamashita et al., 1990). Although
okadaic acid is
known as a tumour promoter, in some cell types, it has been shown to revert
the
phenotype of oncogene-transformed cells to that of normal cells, and to
inhibit neoplastic
transformation of fibroblasts (Schonthal, 1991 ).
1 o Furthermore, okadaic acid has been shown to selectively enhance the
cytotoxicity
of vinblastine and the formation of apoptotic cells, in HL60 cells which are
p53 nul
(Kawamura, 1996). Interestingly, calyculin enhances irradiation killing in
fibroblast cells
at doses that are non toxic when given as a single treatment. (Nakamura and
Antoku,
1994). Data also shows that okadaic acid can abrogate the G,/S checkpoint of
the cell
~ 5 cycle. In this context, okadaic acid has been shown to overide the S-phase
checkpoint
and accelerate progression of GZ-phase to induce premature mitosis (Gosh et
al., 1996).
In addition, okadaic acid has been shown to significantly increase the
fraction of
quiescent cells entering the S-phase via modifications in the phosphorylation
state of
pRb (Lazzereschi et al., 1997). Other studies have shown that the
hyperphosphoryation
2o state of pRb forces cells prematurely into S-phase and pRb can be kept in a
phosphorylated state via protein phosphate inhibition (Herwig and Strauss,
1997). Cells
lacking functional pRb show increased apoptosis and cytotoxicity following 5-
fluorouracil and methotrexate treatment (Heiv~rig and
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-s-
Strauss, 1997). We propose that cell death would be substantially enhanced in
cells
forced to enter the S-phase prematurely (via G, checkpoint abrogation) and
which were
lacking key S-phase components such as dTMP (via TS inhibition).
The okadaic acids class of compounds, with the exceptions of okadaic acid,
s cantharidin (Honaken) and thyrisferyl 23-acetate (Matszawa et. al) (being
PP2A
selective) exhibit poor selectivity. Furthermore, the concentration of PPl and
PP2A
inside cells is such that high concentrations of these inhibitors are required
to generate a
response irc vivo resulting in the loss of effectiveness of any in vitro
selectivity (Wang).
Cantharidin (exo.exo-2,3-dimethyl-7-oxobicyclo[2.2.1]heptane-2,3-dicarboxylic
t o acid anhydride), is a major component of the Chinese blister beetles:
Mylabris phalerata or M. cichorii)(Yang; Cavill et. al). The dried body of
these beetles
has been used by the Chinese as a natural remedy for the past 2000 years.
Although
Western medicine decreed cantharidin to be too toxic in the early 1900's
(Goldfarb et.
al) its purported aphrodisiac qualities (the active ingredient of "Spanish
Fly"), and its
i s W despread occurrence in cattle feed still results in numerous human and
livestock
poisonings (Schmitz).
Li and Casida, and previous work in this laboratory (McCluskey et. al) (and
more recently Pombo-Villar, Sodeoka) has assisted in the delineation of
certain features
crucial for inhibition of PP2A by cantharidin analogues (Figure 1 ). However
the
20 corresponding picture for PPl is not so clear, the majority of data refers
to possible
interactions with the known crystal structures, and in some cases the
inhibition values
for PP 1 are not reported.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-6-
Involvement of Tumour Suppressor Gene p53
The most commonly mutated gene in human cancers is the tumour suppressor
gene p53, which is abnormally expressed in more than 50% of tumours. The
development of chemotherapeutic agents which selectively target cancer cells
with
mutant p53 is certainly desirable, for two main reasons. Firstly, cells that
have an
abnormal p53 status are inherently resistant to conventional chemotherapy and
produce
the more common, and more aggressive tumours such as colon carcinoma and non
small
cell lung cancer. Secondly, a chemotherapy regime that targeted only those
cells with a
mutant p53 phenotype would potentially produce fewer side effects since only
the
cancer cells would be killed and not the p53 proficient normal healthy cells.
DISCLOSURE OF THE INVENTION
In relation to the discussion above, the present inventors believed that the
replacement of the ether O atom of the anhydride with N or S (as N-H and N-R,
where
R = alkyl or aryl) would allow them to probe the H-bonding requirements of
this region
~ 5 of cantharidin analogues. Previous studies in their laboratory had shown
limited
tolerance for modification of the 7-oxa position. An ability to modify these
heteroatoms
is crucial to the development of selective inhibitors based on this simple
skeleton.
There is not, at present, an inhibitor with either absolute specificity or
high
enough selectivity which renders the inhibitor effectively specific in vivo.
2o It has surprisingly been found that anhydride modified cantharidin
analogues,
which are the subject of this invention, may possess one or more of the
properties of
being potent, selective, oxidatively stable, and cell permeable inhibitors of
protein
phosphatases 1 and 2A.
CA 02337771 2001-O1-15
WO 00/04023 - 7 - PCT/AU99/00567
Therefore, according to the first aspect of this invention there are provided
cell .
permeable inhibitors of protein phosphatases 1 and 2A, said inhibitors being
anhydride
modified cantharidin analogues.
According to a particular embodiment of the first aspect of this invention
there
are provided compounds of the formula:
Rt
R ~Z'Y
2 B
A
~ o wherein R, and RZ are H. aryl or alkyl; X is O, N or S; Y is O, S, SR, NH,
NR, CHZOH,
CHZOR; R is alkyl or aryl; A and B are H or CH3; W and Z are CHOH or C=0 and
Ri
and R~ can cyclise to form a ring as follows:
X
R I
w
i s ZI
AB
wherein R3 and R4 are H. aryl or alkyl.
The aryl group may suitably be phenyl or naphthyl for example, and may be
2o attached via a carbon spacer of between 6 and 10 carbon atoms. The alkyl
group may
suitably be C,-C,o.
According to the second aspect of this invention there is provided a process
for
producing anhydride modified cantharidin analogues. The process may include
the
steps of:
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
_g_
dissolving a diene in a suitable solvent and adding to the resultant solution
an
ene.
According to a third aspect of the invention there is provided a process for
producing anhydride modified cantharidin analogues, involving the step of
reacting a
dime with an ene.
The process may further involve hydrogenation of the adduct of the diene and
ene and/or optionally, ring opening of the adduct.
Generally, the reaction conditions for the production of the anhydride
modified
cantharidin analogues are dependent on the aromaticity of the starting dime.
Suitable
t o reaction conditions are exemplified below.
According to a fourth aspect of this invention there is provided a method of
treating a cancer which method comprises administering to a patient in need of
such
treatment, an effective amount of an anhydride modified cantharidin analogue
of the
first aspect of this invention, together with a pharmaceutically acceptable
carrier, diluent
and/or excipient.
The method may be carried out in conjunction with one or more further
treatments for treating the cancer.
According to a fifth aspect of this invention there is provided a method of
sensitising cancer cells to at least one method of treating cancer, which
method of
2o sensitising comprises administering to a patient in need of such treatment,
an effective
amount of an anhydride modified cantharidin analogue of the first aspect of
this
invention, together with a pharmaceutically acceptable carrier, diluent and/or
excipient.
According to a sixth aspect of the invention there is provided a method of
treating cancer which method comprises:
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-9-
administering to a patient in need of such treatment, an effective amount of
an -
anhydride modified cantharidin analogue to sensitise cancer cells of the
patient to one or
more cancer treatments; and utilising the one or more cancer treatments.
According to a seventh aspect of this invention there is provided a method of
screening a compound for anti-cancer activity.
According to an eighth aspect of this invention there is provided a method of
screening compounds for use in the fourth aspect of this invention, said
method
comprising screening for anti-cancer activity; and screening for ability to
abrogate either
the G, or the G~ checkpoint of the cancer cell cycle. The method may also
comprise
1o screening for the ability of said compounds to sensitise cancer cells to
one or more
cancer treatments.
The one or more cancer treatments mentioned above may be selected from
treatments involving cisplatin, irradiation, taxanes and antimetabolites.
The invention will hereinafter be described with reference to Examples and the
I5 accompanying figures.
Brief Description of the Figures
Figure 1 is a schematic representation of the structure activity data
generated for
inhibition by PP2A by cantharidin analogues;
Figure 2: New cantharidin analogues.
2o Figure 3: Cytotoxicity of cantharidin and the new cantharidin analogues.
Figure 4: Cell cycle analysis 12h following exposure to cantharidin, MK-2 or
MK-4.
Figure 5: Cell cycle analysis 18h after 6Gy of radiation and 12h after
exposure to
cantharidin, MK-2 or MK-4.
25 Figure 6 (a-c): Combination index versus fraction affected: HCT116 colon
cells
in simultaneous combination with cisplatin and MK-4.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
- 10-
Figure 7 (a-b): Combination index versus fraction affected: HT29 colon cells-
in
simultaneous combination with cisplatin and MK-4.
Figure 8 (a-c): Combination index versus fraction affected: HCT116 colon cells
in simultaneous combination with taxotere and MK-4
Figure 9 (a-c): Combination index versus fraction affected: HT29 colon cells
in
simultaneous combination with taxotere and MK-4.
Best and other Modes for Carrying Out the Invention
As mentioned above, the reaction conditions for producing anhydride modified
cantharidin analogues encompassed by the present invention generally depend on
the
t o aromaticity of the starting dime. This is illustrated by a description of
examples of the
methods wherein the starting materials are furan (Method 1 below); thiophene
(Method
2 below); and pyrrole (Method 3 below).
Method 1: Furan as the starting dime
A solution of furan (5 equivalents) is dissolved in a suitable solvent (about
5
t 5 times the volume of furan, the solvent can be for example, ether (for room
temperature
reactions); or benzene or xylene (the latter two for reactions at 80 and
130°C
respectively). To this solution is added one equivalent of the ene. The
reaction is then
heated (or stirred at room temperature), typically for 24 hours (2 days in.the
case of the
room temperature reaction). Upon cooling (or standing at room temperature) a
2o precipitate forms and is collected by vacuum filtration. The adduct is then
purified by
recrystalisation from for example, chloroform or ethanol. In the case of the
furan +
malefic anhydride compound care is exercised to minimise heating as this
causes a reto-
Diets-Alder reaction yielding only the starting materials.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-II-
Method 2: Thiophene as the startine dime
Thiophene (1.0168, 0.012 mol) and malefic anhydride (0.558Ø006 mol) are
mixed at room temperature in 2.5 mL of distilled dichloromethane. This mixture
is then
placed inside a high pressure reactor. They are compressed to a pressure of
l7kbax at
40°C for a period of 71 hours, after which the pressure is released and
the product
purified by chromatography.
Method 3: Pvrrole as the startin, diene
To [Os (NH3)SOs02 CF3 )] (CF3S03)2 , (0.3511 g, 0.4 mmol) and activated
magnesium (0.1511 g), pyrrole (0.45 mL, 0.6 mmol), DME (1 mL) and DMAc (0.3
mL)
I o are added in that order. The mixture is stirred for 1 hour, the
temperature gradually
rising to 40°C and then dropping. The brown slurry is filtered through
a thin pad of
celite, and the cake washed with DME in small portions (4 x 2 mL). The
filtrate is
added to dichloromethane ( 15 mL). Vigorous stirring results in the formation
of yellow
coloured precipitate which is collected by vacuum filtration, followed by an
ether wash
I 5 (2 x 2.5 mL). The product is dried under a stream of nitrogen yielding a
yellow-tan
solid (0.3438, 84%). To this pyrrole complex is added maleimide (0.058, 0.515
mmol)
(or any other ''ene", eg malefic anhydride, dimethyl maleate, etc) in
acetonitrile. The
mixture is allowed to stir at room temperature for 60 min. after which the
solvent is
removed by vacuum, yielding the exo isomer (0.3598, 64%). The crude material
is
?o purified by ion-exchange column (Sephadex-CM C-25, 2 x 10 cm), using NaCI
as the
mobile phase. The complexes axe precipitated by the addition of a saturated
sodium
tetraphenylborate solution.
The types of cancer which are amenable to treatment by these compounds
include those types of cancer which are inherently resistant to conventional
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
- l2-
chemotherapy. Typically. these types of cancer are represented by the more
common
and more aggressive tumour types such as, but not limited to, colon cancer and
non
small-cell lung cancer.
The compounds of this invention are suitably administered intravenously,
although other modes of administration are possible. Pharmaceutically
acceptable
diluents, adjuvants, carriers and/or excipients may be used in conjunction
with the
compounds of this invention.
Suitable such pharmaceutically acceptable substances are those within the
knowledge of the skilled person and include compounds, materials and
compositions
~ o deemed appropriate.
Actual dosage levels of the compounds of the invention may be varied so as to
obtain an amount of the active ingredient which is effective to achieve the
desired
response for a particular patient, composition and mode of administration.
The dosage level can be readily determined by the physician in accordance with
~ 5 conventional practices and will depend upon a variety of factors including
the activity of
the particular compound of the invention to the administered, the route of
administration, the time of administration, the rate of excretion of the
particular
compound employed, the age, sex, weight, condition, general health and prior
medical
history of the patient being treated, and like factors well known in the
medical arts.
2o The compounds of this invention may also sensitise cancer cells to other
methods of treatment. For example, typically these methods include irradiation
and
treatment with platinum anti-cancer agents, for example cisplatin.
In addition, sensitisation may also be brought about by, for example the use
of
the plant alkaloids vinblastine and vincristine, both of which interfere with
tubulin and
CA 02337771 2001-O1-15
WO 00!04023 PCT/AU99/00567
-13-
the formation of the mitotic spindle. as well as taxanes and antimetabolites,
including S-
fluorouracil, methotrexate and antifolates.
In particular, the compounds of this invention sensitise those cells with
deficient
p~3 activity.
When screening for anti-cancer activity as contemplated by the invention,
various cancer cell lines may be chosen. These are typically both
haematopoietic and
solid tumour cell lines with varying p53 status and include: L1210 (marine
leukaemia,
p53 wildtype), HL60 (human leukaemia, p53 nul), A2780 (human ovarian
carcinoma,
p53 wildtype), ADDP (cisplatin resistant A2780 cells, p53 mutant), SW480
(human
to colon carcinoma, p53 mutant), WiDr (human colon carcinoma, p53 mutant),
HT29
(human colon carcinoma. p53 mutant), HCT116 (human colon carcinoma, p53
wildtype) and 143B (human osteosarcoma, p53 mutant).
In addition to the methods for screening for anti-cancer activity, the
following
procedures may be suitably used in the remainder of the screening process. For
I 5 example, when screening for the ability to abrogate the G, and/or the GZ
checkpoint of
the cancer cell cycle, the following are suitably used:
Cell cvcle method
The cells are fixed in 70% ethanol and stored at - 20°C until
analysis is
performed (1-2 weeks). After fixing, the cells are pelleted and incubated in
PBS
3o containing propidium iodide (40mg/ml) and RNase A (200 mg/ml) for at least
30 min at
room temperature. The samples (2 X 104 events) are analysed using a Becton
Dickson
FACScan, fluorescence is collected in fluorescence detector 2 (FL2), filter
575/30 nm
band pass. Cell cycle distribution is assessed using Cell Quest software
(Becton
Dickson).
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
- 14-
Those protein phosphatase inhibitors which show abrogation of either the G~ or
G~ checkpoint will then be exploited in combination studies with either
radiation
exposure or chemotherapy drugs incubation. The MTT (3-[4,5-dimethylthiazol-2-
yl]2,5-diphenyl-tetrazolium bromide) assay is used to determine whether a
synergistic,
s antagonistic or additive effect is induced. The Median Effect method is
adopted to
mathematically determine the optimal combination index of the treatments
chosen
(Chou and Talalay, 1984). This method has been extensively used to investigate
the
cvtotoxicity of various drug combinations including cisplatin and D1694
(Ackland et al
1996; 1998). A combination index value less than I indicates synergism, a
value equal
I o to 1 indicates additivity and a value greater than one indicates
antagonism.
Cytotoxicit~y
When screening for the ability to sensitise cancer cells to conventional
chemotherapy and irradiation, the following methods are suitably used:
Cells in a subconfluent phase are transferred to 96- well microtitre plates. L
1210
I s cells are plated at a density of 1000 cells/well in 100p1 medium, while
all other cell
lines are plated at a density of 2000-25000 cells/well. The cells are left for
24h prior to
treatment to ensure exponential growth has been achieved, 24h after plating
(day 0),
1001 of phosphatase inhibitor is added to each well, control wells received
100p1 of
medium only. Drug exposure time is 72h (day 3). The effect of phosphatase
inhibition
2o is tested in triplicate over a concentration range of I x 10-3M - 1 x 10-8
M. Growth
inhibitory effects are evaluated using the MTT assay and absorbance read at
540 nm.
The ICSO is the drug concentration at which cell growth is 50% inhibited based
on the
difference of optical density on day 0 and day 3 of drug exposure.
Cytotoxicity is
evaluated using a spectrophotometric assay which determines the percentage of
cell
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
- IS-
growth following exposure of the cells to various concentrations of the
phosphatase
inhibitors for a period of 72 hours. The subsequent dose response curve is
used to
calculate ICso values (the drug concentration at which cell growth is 50%
inhibited).
Most drug discovery has focused on the development of new single agents.
However, in light of the success of combination chemotherapy it is
increasingly
apparent that successful anticancer treatment of the future will be based upon
the
discovery of agents which are synergistic in their action. In view of this,
the cytotoxicity
of phosphatase inhibitors in combination with either radiation, cisplatin,
taxanes,
antimetabolites or plant alkaloids is examined. As indicated above, calyculin
which by
I o itself is not cytotoxic, enhances irradiation induced cell death.
Similarly abrogation of
the GZ checkpoint by either, caffeine or UCN-O1, also enhances the
cytotoxicity of y
irradiation in cells with mutant p53 (CA46 and HT-29 cells) (Powell et al.,
1995;
Russell et al., 1995; Wang et al., 1996). DNA damage induced by irradiation
causes
both a G, and Gz cell cycle arrest. In p53 mutant cells, the G~ checkpoint is
absent.
I5 However, following irradiation the cells will still arrest in the GZ phase,
and potentially
repair the damage. P53 mutant cells are generally more resistant to
conventional
chemotherapy and produce more aggressive tumours. Therefore,-in p53 deficient
cells,
DNA damage that is not detected by the G, checkpoint will be picked up by the
GZ
checkpoint. If the cells are deficient in both of these checkpoints then it is
believed that
2o the cells will be unable to initiate repair mechanisms and will be more
unstable and
increasingly susceptible to cell death induced by DNA damage.
Cisplatin is another commonly used anticancer treatment which binds to DNA
and produces DNA crosslinks and strand breaks. Cisplatin is particularly
useful in the
treatment of testicular carcinoma, small cell carcinoma of the lung. bladder
cancer, and
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
- 16-
ovarian cancer. Repair of cisplatin induced DNA damage is mediated via
nucleotide -
excision repair which is coordinated by p53 activation of Gadd45 (Smith et
al., 1994).
In this context, it has been suggested that cells that are p53 mutant are more
sensitive to
cisplatin treatment (Hawkins et al., 1996). A number of researchers have
investigated
this proposal in p53 mutant cell lines and in p53 mutant tumours, with mixed
results.
While it is apparent that cisplatin is more cytotoxic in cells lines that are
deficient in p53
(induced via papillomavirus) compared to the p53 proficient cells (Hawkins et
al.,
1996), it is harder to test this hypothesis in tumours and in cisplatin
resistant cells as
they may have several undefined mutations in their genome which would confound
such
studies {Herod et al., 1996). Nevertheless, the GZ abrogator UCN-O1 (7-
hydroxystaurosporine, a protein kinase inhibitor) has been shown to markedly
enhanced
the cell-killing activity of cisplatin in MCF-7 cells defective for p53
function (Wang et
al., 1996).
The development of chemotherapeutic agents which selectively target p53
~ 5 mutant cells is desirable since 50% of tumours have either a mutated or
deleted p53
gene. Many of these p~3 deficient cells and tumours are inherently resistant
to
conventional chemotherapy and represent the common more aggressive tumour
types
such as colon cancer, and non-small cell lung cancer. Thymidylate synthase
(TS)
inhibitors are another class of commonly used anticancer agents. TS catalyses
a critical
20 step in the pathway of DNA synthesis by converting dUMP to dTMP by
methylation
using the co-substrate NS,N10-methylene tetrahydrofolate (CHZ-THF) as a methyl
donor. This step is the only de novo source of dTMP, which is subsequently
metabolised
to dTTP exclusively for incorporation into DNA during synthesis and repair
(Jackman &
Calvert. 1995). Thus, TS is a key regulatory enzyme during the S-phase of the
cell cycle.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
. 17 _
Lack of dTTP results in DNA damage and ultimately cell death, but the
processes) by .
which cell death occurs is not clear. TS inhibitors such as fluorouracil,
raltitrexed, and
LY231514 play a pivotal role in anticancer treatment and are often the first
line
treatment of many cancers (Peters & Ackland, 1996). We propose that the TS
inhibitor
Thymitaq (Zarix, Ltd) be used in combination with cantharidin analogues.
Thymitaq is a
direct and specific TS inhibitor which does not require active transport into
the cell nor
does it require intracellular activation for its action.
The following examples are not to be construed as limiting on the scope of the
invention as indicated above.
l0 Example 1
Chemistry
Anhydride modified cantharidin analogues were synthesised by a variety of
modified literature procedures, as set out in schemes 1 and 2. These
modifications are
embodied in the three methods, which depend on the aromaticity of the starting
dienes,
~ 5 set out above. The dimethyl ester (3), which was prepared by the
application of high
pressure, l7kbar, 40°C, 61 hours, as shown in scheme 3.
° 0 0
a b
CO + O --~ I O O _-a O O
O O OH
° /o ° °
20 /
O O O O + O ~O
O ~OCH~ OCH~
Scheme 1. a. Furan:maleic anhydride (5:1), diethylether, 2d, RT, 96%; b. H~/
10% Pd-
C/ EtOH; c. p-TosOH, MeOH, chromatography; d. H, / 10% Pd-C/ Acetone; e. NaBH4
then HC 1.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
_18_
O O
CO + I NH ~g-~~ NH
1
p p
Scheme 2. Reagents and Conditions: f. Furan:maleimide (5:1 ), diethyl ether,
7d, in
dark, 75%, exo product; g. Furan:Maleimide (5:1), diethylether, sealed tube
12h, 90°C,
66%,endo product.
0
0
CO + I wOCH~ ~ I O ~H~
OCH~
O O
Scheme 3. Reagents and Conditions: h.
~ 5 Furan:dimethylmaleate (2:1 ), CHZC 12 ,17 Kbar, 40°C, 61 h, 56%.
Example 2
Development of potent, selective, oxidativelv stable and cell permeable
inhibitors of
protein Phosphatases 1 and 2A.
Crude natural product extracts have yielded isopalinurin and a series of
cantharidin analogues have been synthesised. In this context, the present
inventors have
developed the simple cantharidin analogue which is PP1 selective (ICso = 50mM,
with
0% inhibition of PP2A at concentrations > 1 OOOmM) representing the first
small
molecule to exhibit selectivity for PP 1. Results have indicated that a series
of simple
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
- 19-
synthetic modification of the cantharidin skeleton also allows the synthesis
of a PP2A .
selective compound (see Figure 1 ).
The present inventors have previously demonstrated that a facile ring opening
of
an anhydride is crucial to inhibition of PP2A. This is not possible with c
(previous
studies with the 7-0, and this analogue indicated considerable hydrolytic
stability of the
maleimide link). It is also interesting to note that endothal thioanhydride is
three fold
more potent than cantharidin, with the S atom being an important factor. It is
thus
envisaged that the 7-S group .presents itself to the active sites metals and
the N-H of the
maleimide occupies the hydrogen bond cavity normally reserved for the 7-O
substituent
to cantharidin.
Structure of cantharidin and selective analogues
0 0 o s o
00'..CH~
O
C O'CH~ OI~tH
!a) !b)
!C)
~ 5 (a) Shows structure of cantharidin;
(b) Shows PP 1 selective analogue; and
(c) Shows PP2A selective analogue. In the case of panel (c) IC;o ~ 25mM.
On the basis of these results and previous experience in our laboratory
(synthesis
and molecular modelling of cantharidin inhibitors at PP 1 and PP2A), we have
designed a
2o series of analogues which are more active and selective, whilst retaining
the desirable
properties of stability and cell permeability.
The synthetic pathways to these analogues are shown in schemes 1-3. Each
scheme allows for modification of the basic skeleton, and in some cases the
insertion of
beneficial feature that were present in the more complex natural toxins) (eg
okadaic
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-20
acid, calyculin, microcystin, etc). The inclusion of these features is
designed to provide
enhanced selectivity and potency.
0 0
O O y I O COiti
X ~' ~ X COzR
ii O O
O O
O o _,iii
X ~ X c0=R
z O z
O
t o Example 3
Synthetic development of a series of PP1 and PP2A analo~eues of cantharidin
(i) Diels-Alder addition (malefic anhydride) and subsequent manipulations of
X;
(ii) Diels-Alder addition (substituted malefic anhydrides), introduction and
manipulation
of Z (Z = hydrophobic tail; eg long chain nitrite: cf Calyculin A, long chain
terminating
I 5 in a spiro acetal: cf Tautomycin, Okadaic acid; long chain terminating in
an aromatic
ring: cf Adda in Microcystin-LR; (iii) stereospecific ring opening of the
anhydride
allowing further manipulations of the newly released functional groups (see
scheme 2).
In this instance we have developed synthetic protocols in our laboratory that
allow the facile assembly of these analogues. Biological evaluation and
molecular
2o modelling of the most active molecules will allow compounds to be
evaluated.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-2t-
Additional modification to the basic structure can be obtained as exemplified
.
below.
x x
0
,",~_ o ,.,~o . 'coNoH cooH
C UO COiPHB ~ O~C~Ph
X . CH2. S, O
Example 4
A specific example of one class of cantharidin analogue that shows promise as
a
selective inhibitor of protein phosphatases l and 2A.
t 0 OMs H
N O O
MeOtC ~ OTBDMS R O
NHi R H X
R
Example 5
Stereospecific route towards 7-azabicyclo [2.2.11 heptanes
~ 5 We have shov~n that the introduction of the bridgehead nitrogen improves
the
potency, selectivity and stability of similar analogues, the above pathway has
been
developed to further improve the bio-activity of these analogues. The
synthetic routes
alluded to herein may allow the rapid assembly of the target molecules.
Those agents which meet the requirements of being stable. specific, potent,
and
2o membrane permeable protein phosphatase inhibitors are screened for their
anti-cancer
activity.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-zz-
Example 6
Biochemistry
All synthesised compounds were tested for their ability to inhibit protein
phosphatases l and 2A. Initial investigations were carned out at 100 mM.
Promising
s analogues were then assayed in triplicate for estimation of ICso values.
Protein phosphatase 1 and 2A were partially purified from chicken skeletal
muscle essentially as described by Cohen Protein phosphatase activity was
measured at
37°C in 50 mM Tris-HCl buffer (pH 7.4), 0.1 mM EDTA, 5 mM caffeine,
0.1% 2-
mercaptoethanol and 1 mg/ml bovine serum albumin using 30 mg [32P] -
phosphorylase
to as substrate. The total assay volume was 30 ml. The assay conditions were
restricted to
20% dephosphorylation to ensure linearity and inhibition of protein
phosphatase activity
was determined by including cantharidin or its analogues at the required
concentrations
in the reaction buffer. Reactions were terminated by the addition of 0.1 ml
ice cold 20%
trichloroacetic acid. Precipitated protein was pelleted by centrifugation and
the
~ 5 radioactivity in the supernatant measured by liquid scintillation
counting. Data is
expressed as the percentage inhibition with respect to a control (absence of a
competing
compound) incubation.
Example 7
Screening various PP1 and PP2A inhibitors for anti-cancer activity
20 (a) Cytotoxicity of protein phosphatase inhibition:
Those PP1 and PP2A inhibitors which fulfil the requirements detailed above
were tested in various cancer cell lines. The cells lines chosen for study
included both
haematopoietic and solid tumour cell lines with varying p53 status and
include:
CA 02337771 2001-O1-15
WO 00/04023 PCTIAU99/00567
_ ?; _
L 1210 (marine leukaemia, p53 wildtype),
HL60 {human leukaemia, p53 nul),
A2780 (human ovarian carcinoma, p53 wildtype),
ADDP (cisplatin resistant A2780 cells, p53 mutant),
SW480 (human colon carcinoma, p53 mutant),
WiDr (human colon carcinoma, p53 mutant).
HT29 (human colon carcinoma, p53 mutant)
HCT116 {human colon carcinoma, p53 wildtype)
143B (human osteosarcoma, p53 mutant)
Anti-cancer screening of the protein phosphatase inhibitors is assessed using
the
MTT assay. This assay determines cell viability by the ability of
mitochondria)
dehydrogenase to produce formazan crystals from 3-(4,5-dimethylthiazol-2-yl) -
2, 5-
diphenyltetrazolium bromide. The viable cell number/well is directly
proportional to the
production of formazan, which following solubilization, can be measured
~ 5 spectrophotometrically (540nm). This technique is also used by the
National Cancer
Institute to screen for new anticancer agents.
As described herein a number of cantharidin analogues have been synthesised
and tested for their anticancer activity in nine cancer cell lines using the
MTT assay after
72 h exposure. These new analogues are shown in Figure 2 and have been
designated
2o iViK-1 through to MK-9. The cytotoxicity (ICso) of these cantharidin
analogues is shown
in Table 1 and Figure 3. In summary, the MK-I analogue did not show any
significant
cwotoxicity in any of the cell lines tested (ICSO >1000uM). Only marginal
cvtotoxicity
across all cell lines tested was observed for MK-3 {ICSO 247 to > 1000pM), MK-
7 (ICso
I 80-367pM) and MK-8 (ICSo 173-385p,M). Greater cytotoxicity was obsen-ed with
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-24-
to eh e! N ~ ~ a
O tf~ o~ a1 N a
W tl +1 +1 tl al tl +1 +1
N M 1~ !'9 M P. tff O 1~
~ N N ~ N ~
ar~~ c'~~ ~ ~ N ~ ~ N
+1 +1tl+i+I+I+1+I+1
f~ t~M CDt!'!CoerfI~M
eh 01r O CDf~i~fDIn
M N l~N ch~-cnM t'~
N
~ ~ N ~ N N
t~ M f~ ~O
al +1+I+)*1+I+I+I+1
~ ~ Q N M
m c~ y n N C C~ c
"~ O N c~ n
~- c'~
M N <
N
X O r ~ ~ O N ~ r
y In ~ tl+I+I +Ir +1+I
N +I
Y n C~f~O n M 00r-P.
N tDto Q N M v
ch~ N N
3 C
M M
~ ~ ~ ~
v 1 ~ n.o cwn
C
+I +1+I+~+1a~y +I*1
C Lr O C7 ~7 aD
0 O N ~ ~ 00
b ~ t c'
0 '~
l0
~
ate-ltje~-O N O ~.-M N
+I +1+18 +I~ tl+I+I
I~ t~Iw.n tnj~O O
c ~ ~ o v
C
v
~ tc ~ ~ ~ N 1Mf! Of
~1
[ _ N 7 e Of+Ial+I+I+I+1
-s +I ! +I
+I
un ~ N c9aoO c~aow
~
a W 1~~nO N COO O Wn
O
'- ~ O O O
~ ~ ~
Y O o 0 0 O
c. ~ n n n n n n n n n
x
a~
CON P.t~
O ~- C ~- O O
+1 altl+Ialaitl+i+I
a0 M N N N Q ~ N
C U .- ~ r d r cCfp
0
U
L
A 3 c '~3E E 3 E E c
a
H
O
O O d r CO
Os v ,~
U
a
o ~ E
.~3! ~ a
m E
o ~do
' _
~ ~ O ~ O U c3U V
~ A ~ ~ 3
V ~ o ~ ~ > > > > > a
F- :E Z Z Z Z Z Z Z Z
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-25-
MK-2 (ICSO 157-248pM) and MK-9 (ICso 107-233pM) which was also consistent
across
the nine cell lines. The greatest cytotoxicity was observed with the MK-4 and
MK-S
analogues. however, the magnitude of this response was cell line dependent. In
this
context, MK-4 and MK-5 were selectively more cytotoxic in the human colon
cancer
cell lines (ICso 14-88pM; 28-247pM) compared with leukaemia (ICSO 393-680~M;
323-
>1000p.M) ovarian (ICS 275-333pM; 260-567pM), and osteosarcoma (IC~o 450pM;
>1000p,M) cells respectively.
(b) Abrogation of cell cycle checkpoints:
The ability of the protein phosphatase inhibitors to abrogate the G1 or G2
checkpoint of the cell cycle may be determined by cell cycle analysis using
flow
cvtometry. Briefly, asynchronous cell cultures are harvested 18h after 6Gy
irradiation
and/or 12h incubation with the protein phosphatase inhibitor. Depending upon
the p53
status of the cell line, radiation treatment alone will induce arrest in
either G, and/or G2
phase of the cell cycle.
~s Data shown in Table 2 and Figure 4 show the cell cycle response of L1210,
HL60, HT29 and HCT116 cells to cantharidin and the new cantharidin analogues
MK-2
and MK-4 after 12h exposure. In summary, cantharidin and MK-2 produced a
similar
response and induced GZ arrest in all four cell lines tested. MK-4 also
induced G2 arrest
but only in L1210, HL60 and HCT116 cells. In HT29 cells, MK-2 induced G~ cell
cycle
2o arrest. The magnitude of the cell cycle arrest induced by these drugs
directly correlated
with their cytotoxicity in the respective cell lines. The ability of the
parent compound
cantharidin to inhibit cell growth is also shown (ICso 6.1-1$pM). The
cytotoxicity of the
cantharidin is greater than for its analogues. Interestingly, cantharidin also
showed slight
selectivity towards the colon cancer cells.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/ 00567
- ~- n
26
-
A ~ m
m
C9
~ ~
C~ t U ,
o r ao o ~ - ~n ~o,q eo~ eo
.~ ~a
, , , .-ofr~
M ~ M Qi
~
M M ~f N M ! ~ M N N N
~ f
O GOM f~ M ~ON ~ d M 1f7
t0 N
(/~ 0D P.Of 'C 01 N.ap 1!fN. N N
- ~C r "~ r-r
".
c9 N st O ~ cha0 vl!t~.d M N Iw
I~ vt
, ~ ~ ~~ a
C ~ O O - O vTt0 N tn ~ O 4 '~
00 N
Of~' 1Cicp Cse~f ~7 N efe0I~
N ~! M
.' r r
N
~
_ _
C 47
U ~ e'p
~' ~ C~
O c~
C ~ ~ v~ao - ao .yn in q r,~ an
o r~
' f/! ~ v Q ~ ~ M ~ ~ ~'
N ~
_C7 ~ e v t e v C
e 7
t0 N CD a0 h. 00O l''~Ll7~ <D~ 1~
O r-
~ ~ H:c0 r. of P7~D ~ N ~-e'f~ t~f
V r ~ r r- r ~ v~ e- e~r r r
~ r r
~ r-
C
M CfOf ~- N N N a O C~~ N O
r p W ,
M tn O
Cf~
00
b.st M ~f ~Q'd C
sT thM N ~ '7
t~7 N
(~ yn N o .- n. r~q u~ ~ u~o o~
N ~ ca
CD !VN ~ ~ ~ , N C' e!!Va~J
tC r r- Of
~
H
N
~ ~ o W '
c c o
u o
N N
C V~ U' U C~ U U'
N 1~~O H- V~ Q '~ ~ ~ M ~OO st
~ ~T
V ~, ap CfO ~ M M ~ N c~c0'70'7
N N O c0~ i
t'7
M
ur M 7
-_ ao .-n. ee o e9co ~a eo ceee
,n 00
(/ W ~ h r r tD~y ~: r N CVN!
C ~ f> N is C~ N N N
N N N N N
N
, M N N N N N
H ~ ~ O ~l! O ~ N tG ~ C~ QO Of fr1r7O ~
iD 1~
~ N ~ Q , tl' ~ !'~~~ 'Oa'~ ~ ~ N
~ ~ d'
_
of 1!!f~ eel~ a00D EC C'!07N
Is N,
=
.-. p~ nj ~ . O N ~ C~IO tp
.. erf
N
E
..
a a~ a
o ;v
H H ._
c~ c~
~ v ~ a~ cc ...r.q o ~ o w ~n
o~ r. o~
O ~ ~- C N ~-N C
~ N CD ~l7 t0
N r
-
,~ N ~ M M M tD M
~ e
f
~ 1~t0 00 at N ~ ~ Os C~d'to
O t~'! tp
c~~fM Me~ N , , ~~ ~ ~ N N N
N ~
G) O ~ c0b O ~ fsN r7 O O N r~
- ~ tt7
o E ~,~'e ~ ~~ ~ g Q ~~ ~
0
_ ~ u o o~ v coce o~ ao o et
W .r
O O C r- O C O P')C O O p r
G !V
~o
O r tf! ~ O ~ ~ ~ O
0 ~
C .O C
~
U U E . c
c
_ ca
a~
~ ~
U U
U ~ g
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-27-
If the protein phosphatase inhibitor abrogates the GZ checkpoint then the
cells -
will not arrest in the G~ phase of the cell cycle and the cells will continue
through the
cell cycle and accumulate in the G, phase of the cell cycle only. Similarity
if the protein
phosphatase inhibitors abrogates the G~ checkpoint then the cells will not
arrest in the G,
phase of the cell cycle and accumulate in the GZ phase of the cell cycle only.
Cell cycle
analysis using propidium iodide labelling of DNA has been used extensively in
our
laboratory to assess the effect of specific anticancer agents that induce S-
phase cell cycle
arrest and apoptotic cell death (Sakoff, Ackland and Stewart, 1998).
Experiments were
performed on a Becton Dickinson FACScan and using Cell Quest software.
Data shown in Table 3 and Figure 5 show the cell cycle response of L1210,
HL60, HT29 and HCT116 cells. The cells were treated with 6Gy of radiation and
then
treated with cantharidin 6h later. The ability to abrogate cell cycle arrest
was assessed
12h after the addition of the drugs. Cantharidin and MK-2 both abrogated
radiation
induced G, arrest in all cell lines. MK-4 also abrogated G, arrest in L 1210,
HL60 and
~ 5 HCT116 cells. In HT29 cells, MK-4 induced abrogation of the G2 checkpoint.
It is
important to note that the exposure of HT29 cells to MK-4 induced the greatest
cytotoxicity (IC;o 14~M) as determined by the MTT assay. Not surprisingly, the
ability
to abrogate the GZ checkpoint was more lethal than the ability to abrogate the
G,
checkpoint.
(c) Combination studies:
The cell lines listed above are exposed continuously to cisplatin and the
phosphatase inhibitor in various drug ratio combinations for 72h and then
assayed for
cvtotoxicity. Similarly, the cells are exposed to 8 Gy of radiation and
incubated with the
phosphatase inhibitor and assessed for cytotoxicity at 72 h.
CA 02337771 2001-O1-15
WO PC T/AU99/0056~
00/04023
-28-
O
G
G ' ~ 1~ !'~! ~
N ~ ~
M 01 ~!
4! '~ tt!
~ ~ 1~
. ~T ~ 1 !!
O Qf
!C tn
O
lh
a Q Q
M tf~
~
t0
a
N 1~ t~! ~ ~ ~ ~ H
t0 ~
ttf ~
lrf O
H.
G'~ CO , O ~ ,~ ~
C e0 O
O of
N ~
~ N
N ~n -- m .r - c~ e~ ev,
o ~ r,
o~ ~
~ f
o t~7 a0 ~ tf! ~fiO O
~ M O as r
Q N e'f e~!
sr M
M M N r! M Pf
N tt
N
N
9 as cp e~ cpsen o ao c~ cn
y- u~ o0
r.
ao
~e1 ~D u7 Is C I~ ~O cD O
t0 r erf t~f
N
s0
r
N
_g
rr
N
G
N
.- N N7 N N M ~O ~ M C9 ~
i''7 ~T C!
O
C 00 M N 1~.h. Os CV r 07 h
~ iti N t0 t0 t0
eh t0
, tD ~n to O u7 vrf
tp <D
P
()~ r aD Q ~f O~ M N eh N m n. N
t0 O~ t0
~ ; M O C~ H. tp A ~' tD u7 Cf o0
CD ~ ef' C
CD .- .-
"
M r-
OD '~ ~D 00 Q~ C~ Q 1~
f'~ N O> ~
st
T CO m CD
N N " - M
O M
N r-
L ~ N N ~ ~ r
~
U (~C7 N O N f~ M ~ N ch ~'~7 C'7
O ~ N, ~
~T , ef !'~!!~! O e0 (rf
et ~ eh tp m d
~C!
~
L t rJ
O N
r
p O
U U
U
C
c~ 00 GO ~- O '~ O M GC N
.'. (r G1 cy
O
M
N vCI tt~ 'at elf tn t'~N C N 0D
~ ~ ~ t'M 00 CO G3 OD
~ 1~
~ a0 t0 CO 00
CO DO
V 'A N O In eh N C0,M OD O O 'Q
~ M ~ i
C7fnc~J N7 M v fV N n'7 N N ch ta
~ N O
'd
C V ''
Q c~f N r. cp Of r ~ Q I~ N, N o~
N ~ N
(p~ ui , vritD cD In to ~C of
tn Ki W In
W C v
UrcD N et t7 O O tt) 1~ 01 GD M
eh ~- t0
0~
0 -~ ? tG c0 if I~ lt!Ilf !n CD C~ C~
~ ~f m ~"
~
~ ~ N
~ O
y O _
t.
. N
.r. ~ O ~ O
..
T~ ' A
N
y,r
~r% a ep ~l7 tn ~ CD O M fw (~;
h. sT Cf 00,
~
C C a ~' Q ~ ~ 0 ~ O
M
r" V7C9 V rf N.
l
_ 00 ID ~ !r ~ GD O O 1~ Ij7 ~ fD
~ ~ O
N ~ ~' ~ fAN to .- r- P t"7 ~ 00 f~ ~
of tf~ C t0
N N N r'
Q, wr l N M N M N N ~ N
~ 7 ~-
C ~ ~ ~ O _ c'~ N CD C~ ~t C, d N, N N O N N
~ r- d
~ C~tl~ f~. O tp Of aD G ~ N - aD
d st r-
eh
r:rO ~ 0 N N N N N ~- ~ ~ ~- N N N N r-
co c0 t; t0 D tf> Of CO st r'
J of OI Of O
'~
.~ U ~'- ~' N ch ~ ~' !~'! .- r N M !C7
~ ~ !O
t!!
s~ ~ 3 ~
c pc ~ o r~og o ,~ 8~8 0 ~8~8
~
~
>' c
~
V = t
ca
a~ _ ..-
~ ... cy
C
U U v ~ Y Y
~
Q U
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-29-
Data shown in Figures 6-9 shows the results of combination studies utilising
the
Median Effect Method in HT29 and HCT116 human colon cells. This method tests
the
cytotoxicity of various drug combinations from which a combination index can
be
calculated. A value of greater than one indicated antagonism, a value equal to
1 indicates
additivity, while a value less than one indicates synergism. The HT29 and
HCT116 cell
lines were chosen as they have differing p53 status and they represent the
tumour types
that responded the greatest to cantharidin and its analogues.
The data show that the simultaneous combination of cisplatin and MK-4 in both
HCT116 and HT29 cells was additive and not synergistic using drug molar ratios
of 1:1,
i o 10:1 and 1:10. An additive response indicated that the drugs were
mediating their effects
via two separate biochemical pathways. The simultaneous combination of
taxotere and
MK-4 in HT29 cells was also additive using drug molar ratios of 1:10, 1:100,
1:1000
(Taxotere: MK-4). However, this drug combination of taxotere and MK-4 induced
a
synergistic response in HCT116 cells. A synergistic response indicates that
the two
t 5 drugs were interacting in such a way as to enhance the overall cytotoxic
response and to
induce "more than the additive" response of each individual agent.
Consequently, the
addition of subtoxic levels of MK-4 clearly enhanced the cytotoxicity of
taxotere.
Example 8
Results and Discussion
2o Anhydrides and simple analogues were synthesised according to literature
procedures {Eggelte et. al: 1973), and then subjected to a PPl and PP2A bio-
assay (see
biochemistry) to determine their ability to inhibit these enzymes. The results
of initial
screening at 100 mMs are shown in Table 4, along with ICSO values in some
instances.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-30-
Table 4. The inhibition of protein phosphatase 1 and 2A by
.anhydride modified cantharidin analocues
Compound Inhibition of Inhibition of Selectivity
PP1 (%) PP2A (%) PP2A/PP1
0 0 90 97 0.875
o IC,a 2 . 4 ),!M IC,o 2 .1 E1M
G
0
1
o ~ _ g5
~o
0
a
0 0
ICso 50ELM ICso>10.000)LM >200
ocH,
0
3
0 0 13 11
0
I
CHI
4
0 0 15 8
0
N'
HOC
5
11
0
OH
6
o ND 21
0
7
0 o ND 15
~~~~,'sl~NH
O
a
_~ __
o I
~N
O
9
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
_;1 _
Of the compounds listed in Table 4, only l and 2 show any significant
inhibition
of PP2A, at 97% and 95% respectively (with little selectivity apparent for
either
enzyme). Interestingly the bioisoseteric replacement of the anhydride oxygen
atom of 1
results in a complete loss of inhibition. Indeed no modification of the cyclic
anhydride,
is tolerated, and consequently results in no inhibition of PP2A.
Previously we have shown that analog 2 undergoes a rapid conversion to the
dicarboxylic acid under assay conditions. We thus examined the stability of
the non-
active analogues (in Table 4) and found that they were stable under assay
conditions
showing no decomposition. in fact 5 can be synthesised via the Diels-Alder
reaction in
I o water (Eggelte et al; i 973).
In all instances, the corresponding dicarboxylic acid derivatives display
lower
inhibitory values at PP2A (Tables 5 and 6). Even though the anhydrides undergo
a facile
ring opening to the dicarboxylic acids, the original conformation presented at
the active
site must also play a role in determining the overall level of inhibition.
Consequently,
t 5 we believe that the conformation of anhydride carbonyl groups is more
favourable for
inhibition (essentially only one conformation presented at the active site),
than that of the
dicarboxylic acid (four possible minimum energy conformations, data not
shown).
In an attempt to determine the feasibility of anhydride opening via
nucleophilic
attack from Tyr272, we conducted a series of model experiments in which 2 was
allowed
2o to stand in a chloroform solution of phenol. This mixture was examined
periodically by
~H NMR spectroscopy and showed the growth of a new species over a period of
time (ca
days). Further analysis indicated the presence of a phenolate ester of
norcantharidin
(scheme 4). Consequently, a metal assisted or nucleophilic attack under
physiological
conditions represents a possible mode of assisted ring opening with the
anhydride held in
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-32-
Table 5. Effects of anhydride to dicarboxylic acid on the
inhibition of PP2A.
Entry Anhydride Inhibition Carboxylic Inhibition
(%) acid (%)
0 0 0
1
H SO
~ H
,o (This work) o
0 0
2 92-95 H 92-95
0 0 ' O H
H 17
1 O ~ OH
O O
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-33-
TABLE 6 Inhibition of PPl and PP2A by selected cantharidin
analogues.
Entry Compound Inhibition Inhibition o~ Selectivity
of PPl(%) PP2A(%) PP2A/PPl
a
97 0.875
( ICso2 . 4~1M) ( ICso2 . l~iM)
' 46 6 >200
(ICsoSO/,iM) (ICso>10000)LM)
3 Not
o~. determined
15 69 Not
determined
CA 02337771 2001-O1-15
WO 00/04023 - 34 - PCT/AU99/00567
a favourable conformation within the active site. In turn the resultant diacid
rapidly .
binds in a more favourable manner.
oe
0 0 ~' o 0
0
0
CdlH
Scheme 4
The results presented herein indicate that cantharidin analogues, via
anhydride
opening are more potent inhibitors of PP2A. Analogues in which the anhydride
moiety
has been modified preventing a facile ring opening (except where otherwise
indicated)
are extremely poor inhibitors of PP2A (Tables ~ and 6).
t o However, the most interesting result reported herein (see table 4) is the
selective
inhibition of PP 1 by the dimethyl ester (3). Simple diesterification of 2 has
completely
reversed the previously reported PP2A selectivity (ca 10 fold) of
norcantharidin for
PP2A to yield selective small synthetic molecule for the inhibition of either
PP 1 or
PP2A. Again this suggests that presentation of a diacid moiety to the active
site is
~ 5 crucial for the inhibition of PP2A. No such restrictions are apparent with
the limited
structure activity data for PP 1.
A synthetic inhibitor such as 3 represents a significant advance on the
currently
widespread inhibitors of PP 1 and PP2A.
In conclusion, the present inventors have demonstrated that a facile ring
opening
20 of the anhydride moiety is relevant for inhibition at PP2A. Also, that
modification of the
dicarboxylic acid moiety gives rise to a PP 1 selective compound.
The above describes some embodiments of the present invention. Modifications
obvious to those skilled in the art can be made without departing from the
scope of this
invention.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-35-
Industrial Applicability
It should be clear that the present invention will find light applicability,
especially in the medical and veterinary fields.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-36-
Walsh A H, Chen A T, Honaken R E, FEES Lett., 1997, 416,
230
Roberge M, Tudan C, Hung SMF, Harder KW, Jirik FR, and
Anderson H Cancer Research 1994, 54, 6115-6121.
McCluskey, A; Taylor, C; Quinn, R J; Suganuma, M; Fujiki,
H; Bioorg. Med. Chem. Lett., 1996, 6, 1025.
McCluskey A, Keane M A, Sim A T R, J. Med. Chem. 1998,
subini t t ed .
Enz, A; Zenke, G; Pombo-Villar; Bioorg. Med. Chem. Lett.,
1997, 7, 2513; and Sodeoka, M; Baba, Y; Kobayashi, S;
Hirukawa, N; Bioorg. Med. Chem. Lett., 1997, 7, 1833.
"Cohen, P; Cohen, P T w; J. Biol. Chem., 1989, 264, 21435.
Cohen, P; "The Structure and Regulation of Protein
Phosphatases" Raven Press: New York, 1990, p230-235.
Shenolinkar, S; Nairn, A C; Adv. Second Messenger
Phosphoprotein Res., 1991b, 23, 1.
Krebs, E G; Angew. Chem. , Int. Ed. Engl. , 1993, 32, 1122 .
Fischer, E; Angew. Chem., Int. Ed. Engl., 1992, 32, 1130.
Tachibana, K; Scheuer, P J; Tsukitani, Y; Kikuchi, H; Van
Engen, D; Clardy, J; Gopichand, Y; Schmitz, F J; J. Am.
Chem. Soc., 1981, 103, 2469.
Cheng, X-C; Kihara, T; Kusakabe, H; Magae, J; Kobayashi,
Y; Fang, R-P; Ni, Z-F; Shen, Y-C; Ko, K; Yamaguchi, I;
Isono, K; J. Ant.ibiot. , 1987, 40, 907.
Botes, D P; Tuinman, A A; Wessels, P L; Viljoen, C C;
Kruger, H; Williams, D H; Santikarn, S; Smith, K J;
Hammond, S J; J. Chem. Soc. Perkin Trans I, 1984, 2311.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-37-
Harada, K-I; Matsuura, R; Suzuki, M; Oka, H; Watanabe, M
F; Oishi, S; Dahlem, A M; Beasley, V R; Carmichael, W W;
J. Chromatog., 1988, 448, 275.
Honaken, R E; FEES Lett, 1993, 330, 283.
Matszawa, S; Suzuki, T; Suzuki, M; Matsuda, A; FENS Lett.,
1994, 356, 272.
Wang, G-S; J. Ethnoparmacol., 1989, 26, 147.
Cavill, G W K; Clark, D V; Naturally Occuring Insecticides
(M:Jacobson and D G Crosby, Eds), Marcel Dekker, New York,
1971, 271.
Oaks, W W; DiTunno, J F; Magnani, T; Levey, H A; Mills, L
C ; A.M.A. Arch. Int. Med., 1960, 105, 106.
~Goldfarb, M T; Gupta, A K ; Sawchuk, W S; Dermatologic
Clinics, 1991, 9, 287.
a. Schmitz, D G; J. Vet. Intern. Med., 1989, 3, 208.
Polettini, A; Crippa, U; Ravagii, A; Saragoni, A.;
Forensic. Sci. Int., 1992, 56(I), 37.
Li, Y-M; Casida, J E; Proc. Natl. Acad. Sci. USA, 1992,
89, 11867.
McCluskey, A; Taylor, C; Quinn, R J; Suganuma, M; Fujiki,
H; eioorg. Med. Chem. Lett., 1996, 6, 1025.
Eggelte, T A; de Koning, H; Huisman, H O; Tetrahedron,
1973, 29, 2445; 3 see: Tamura, Y; Imanishi, K; Miki, Y;
Ikeda, M; Synthesis, 1977, 559; 5 & 9 see: Kwart, H;
Burchuk, I; J. Am. Chem. Soc., 1952, 74, 3094.
Goldberg, J; Huang, H-B; Kwon, Y-G; Greengard, P; Nairn, A
C; Kuriayan, J; Nature, 1995,376, 745.
Quinn, R J; Volter, K; Embury, K J, unpublished results.
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
_;8 _
Cohen, P; Alemany, S. ;Hemmings, B A; Resink, T J ; Stralfors, P and Tung, H Y
L in
Methods in Enzvmology (Ed. J D Corbin and R A Johnson) 1988, I59, 390,
Academic
Press (London).
Hardie, DG; Haystead TAJ; Sim ATR; Protein Phosphorylation, in methods in
enymology, in press. Enz, A; Zenke, G; Pombo-Villar; Bioorg. Med. Chem. Lett.,
1997, 7, 2513.
Sodeoka, M; Baba, Y; Kobayashi, S; Hirukawa, N; Bioorg. Med. Chem. Lett.,
1997, 7,
1833.
Durfee T., et al., (1993). The retinoblastoma protein associates with the
protein
to phosphatase type 1 catalytic subunit. Genes Dev. 7, S55-569.
Ghosh, S. et al. (1996). Okadaic acid overrides the S-phase checkpoint and
accelerates
progression of G2-phase to induce premature mitosis in Hela cells. Exp. Cell
Res. 227:165-
169.
Herwig, S., and Strauss, M. (1997). The retinoblastoma protein: a master
regulator of
is cell cycle, differentiation and apoptosis. Eur.J.Biochem. 246:581-601.
Jackman, AL and Calvert. AH (1995). Folate-based thymidylate synthase
inhibitors as
anticancer drugs. Ann.Oncol. 6:871-881, 1995.
Kawamura K-I. Grabowski D, Weizer K, Bukowski R, and Ganapathi R (1996).
Modulation of vinblastine cytotoxicity by dilantin (phentoin) or the protein
phosphatase
2o inhibitor okadaic acid involves the potentiation of anti-mitotic effects
and induction of
apoptosis in human tumour cells. Br. J. Cancer 73: 183-188.
Lazzereschi, D. et al. ( 1997). The phosphatase inhibitor okadaic acid
stimualtes the
CA 02337771 2001-O1-15
WO 00/04023 PCT/AU99/00567
-39-
TSH-induced GI-S phase transition in thyroid cells. Exp. Cell Res. 234:425-
433.
Nakamura K. and Antoku S. ( 1994). Enhancement of X-ray cell killing in
cultured
mammalian cells by the protein phosphatase inhibitor calyculin A. Cancer Res
54:2088-
20990.
O'Connor PM ( 1996). Cell cycle checkpoints: Targets for anti-cancer therapy.
Anti-Cancer
Drugs 7(sup 3): 135-41.
O'Connor PM (1997). Mammalian G1 and G2 phase chekpoints. Cancer Surveys
29:151-
I 82.
Peters GJ and Ackland SP ( 1996). New antimetabolites in preclinical and
clinical
development. Exp.Opin.Invest.Drugs S, 637-679.
Schsnthal A (1992). Okadaic acid - a valuable new tool for the study of signal
transduction and cell cycle regulation? New Biol. 4, 16.
Stein, G.S. et al. (1998). The molecular basis of cell cycle and growth
control. Wiley-Liss
Publishers, New York.
~ 5 Yamashita K., et al ( 1990). Okadaic acid, a potent inhbitor of type 1 and
type 2A protein
phosphatases, activates cdc2/H1 kinase and transiently induces a premature
mitosis-like
state in BHK21 cells. EMBO J. 9: 4331-4338.