Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-1-
Title: Apparatus and Method for Desolvating and Focussing
Ions for Introduction into a Mass Spectrometer
FIELD OF THE INVENTION
The present invention relates to an apparatus and method for
desolvating and selectively transmitting ions, based on the ion focussing
principles of high field asymmetric waveform ion mobility spectrometry,
for introduction into a mass spectrometer.
BACKGROUND OF THE INVENTION
High sensitivity and amenability to miniaturization for
field-portable applications have helped to make ion mobility spectrometry
an important technique for the detection of many compounds, including
narcotics, explosives, and chemical warfare agents (see, for example, G.
Eiceman and Z. Karpas, lon Mobility Spectrometry (CRC. Boca Raton, FL.
1994); and Plasma Chromatography, edited by T.W. Carr (Plenum, New
York, 1984)). In ion mobility spectrometry, gas-phase ion mobilities are
determined using a drift tube with a constant electric field. Ions are gated
into the drift tube and are subsequently separated based upon differences
in their drift velocity. The ion drift velocity is proportional to the
electric
field strength at low electric fields (e.g., 200 V / cm) and the mobility, K,
which is determined from experimentation, is independent of the applied
field. At high electric fields (e.g. 5000 or 10000 V/cm), the ion drift
velocity
may no longer be directly proportional to the applied field, and K becomes
dependent upon the applied electric field (see G. Eiceman and Z. Karpas,
lon Mobility Spectrometry (CRC. Boca Raton, FL. 1994); and E.A. Mason
and E.W. McDaniel, Transport Properties of Ions in Gases (Wiley, New
York, 1988)). At high electric fields, K is better represented by K~,, a
non-constant high field mobility term. The dependence of Kh on the
applied electric field has been the basis for the development of high field
asymmetric waveform ion mobility spectrometry (FAIMS), a term used by
the inventors throughout this disclosure, and also referred to as transverse
field compensation ion mobility spectrometry, or field ion spectrometry
CA 02339552 2001-02-05
13-11-2000 CA 009900715
-2-
(see I. Buryakov, E. Krylov, E. Nazarov, and U. Rasulev, Int. J. Mass
Specixom. Ion Proc. 128. 143 (1993); D. Riegner, C. Harden, B. Carnahan,
and S. Day, Proceedings of the 45th ASMS Conference on Mass
Spectrometry and Allied Topics, Palm Springs, California, 1-5 June 1997, p.
473; B. Carnahan, S. Day, V. Kouznetsov, M. Matyjaszczyk, and A.
Tarassov, Proceedings of the 41st ISA Analysis Division Symposium,
Framingham, MA, 21-24 April 1996, p. 85; and B. Carnahan and A.
Tarassov, U.S. Patent Number 5,420,424). Ions are separated in FAIMS on
the basis of the difference in the mobility of an ion at high field Kh
relative
to its mobility at low field K. That is, the ions are separated because of the
compound dependent behaviour of Kh as a function of the electric field.
This offers a new tool for atmospheric pressure gas-phase ion studies since
it is the change in ion mobility and not the absolute ion mobility that is
being monitored.
An instrument based on the FAIMS concept has been
designed and built by Mine Safety Appliances Company of Pittsburgh, Pa.
("MSA") for use in trace gas analysis. The MSA instrument is described in
U.S. Patent No. 5,420,424 and is available under the trade mark FIS (for
Field Ion Spectrometer). While the use of the MSA instrument (and
similar instruments based on the FAIMS concept) for trace gas analysis is
known, the inventors believe that they have identified certain heretofore
unrealized properties of these instruments which make them more
versatile.
The realization of these properties has resulted in the
development of an invention which is designed to extend the
functionality of the MSA instrument (and similar instruments based on
the FAIMS concept). A summary and detailed description of the present
invention is provided below.
SUMMARY OF THE INVENTION
In a first aspect of the present invention, there is provided an
apparatus for desolvating and selectively transmitting ions, comprising
AMENDED SHEET
13'11'200~ CA 02339552 2001-02-05
V CA 009900715
-3-
a) at least one ionization source for producing ions;
b) a high field asymmetric waveform ion mobility spectrometer,
comprising:
i) an analyzer region defined by a space between at least
first and second spaced apart electrodes, said analyzer
region being in communication with at least one of each
of a gas inlet, a gas outlet, an ion inlet and an ion outlet,
said ion inlet introducing a flow of said ions into said
analyzer region, and said ion outlet allowing extraction
of ions from said analyzer region;
ii) at least one source of gas for providing a gas flow into
said gas inlet, a gas flow through said analyzer region, a
gas flow out of said gas outlet, and a gas flow being
which is flowing counter-current to said flow of ions
being produced by said ion source so as to desolvate said
flow of ions entering said ion inlet; and
iii) an electrical controller connectable to said electrodes and
capable of applying an asymmetric waveform voltage
and a direct-current compensation voltage to selectively
transmit a type of ion in said analyzer region between
said electrodes at a given combination of asymmetric
waveform voltage and compensation voltage.
In another embodiment, the present invention provides an
apparatus , further comprising a mass spectrometer having a sampler
orifice, said sampler orifice being positioned proximate to said ion outlet to
receive said selectively transmitted ions for analysis within said mass
spectrometer.
In one embodiment, said first and second electrodes comprise
curved electrode bodies and provide a non-constant electric field
therebetween, said ions being selectively focussed in a focussing region
created between said curved electrode bodies in said analyzer region.
AMENDED SHEET
CA 02339552 2001-02-05
13-11-2000 CA 009900715
-4-
In another embodiment, said first and second electrodes
comprise outer and inner generally cylindrical coaxially aligned electrode
bodies with a generally annular space formed between them, said annular
space defining said analyzer region.
In yet another embodiment, said gas outlet and said ion outlet
are proximate to said focussing region at said second end.
In another embodiment, said sampler orifice is positioned
proximate to said ion outlet to receive said selectively focussed ions.
In another embodiment, said generally cylindrical inner
electrode body has a curved surface terminus proximate to said second
end, said ion outlet being axially aligned with said inner electrode body,
said asymmetric waveform voltage, compensation voltage, and said gas
flow being adjustable, whereby, said focussed ions tend to follow the
curved surface of said terminus and are directed towards said ion outlet.
In yet another embodiment, said outer electrode body forms a
curved surface which substantially follows the curved surface of said
terminus, so as to maintain a substantially constant distance between said
inner and outer electrodes at said second end.
In another embodiment, said ionization source is coaxially
aligned with said electrodes and positioned external to said inner electrode
body, whereby, in use, said flow of ions are evenly directed into said
generally annular shaped analyzer region in a radial fashion.
In another embodiment, said apparatus further comprises a
generally cylindrical ionization chamber housing said ionization source,
said ionization chamber being axially aligned with said inner electrode,
said ion inlet comprising a gap between said ionization chamber and said
inner electrode.
In another embodiment, said ion inlet is located in said outer
electrode wall for introduction of said ions into said analyzer region.
In another embodiment, said apparatus further comprises an
ionization chamber housing said ionization source, said ionization
chamber being provided with a second gas outlet for allowing said
AMENDED SHEET
CA 02339552 2001-02-05
13-11-2000 CA 009900715
-5-
counter-current gas flow to exit.
In another embodiment, said apparatus further comprises a
purge gas chamber positioned between said ionization source and said ion
inlet, said purge gas chamber providing a purge gas flow for desolvating
ions entering said ion inlet.
In another embodiment, said ionization source is an
electrospray ioruzer for producing ions from a sample in liquid phase,
whereby, in use, said counter-current gas flow reduces the level of
solvation of said flow of ions being introduced into said analyzer region.
In another aspect of the present invention, there is provided
a method for desolvating and selectively focussing ions produced by
electrospray ionization for introduction into a mass spectrometer,
comprising the steps of:
a) providing at least one electrospray ionization source for
producing ions from a sample in liquid phase;
b) providing an analyzer region defined by a space between at
least first and second spaced apart electrodes, said analyzer
region being in communication with at Ieast one of each of a
gas inlet, a gas outlet, an ion inlet and an ion outlet;
c) providing a gas flow into said gas inlet, and within said
analyzer region, and out of said gas outlet, at least some gas
flow being counter-current to ions being produced at said ion
source so as to desolvate said flow of ions entering said ion
inlet;
25. d) providing an electrical controller connectable to said
electrodes and capable of applying an asymmetric waveform
voltage and a direct-current compensation voltage, to at least
one of said electrodes;
e) adjusting said asymmetric waveform voltage and said
compensation voltage to selectively focus a type of ion; and
f) extracting said selectively transmitted ions from said analyzer
AMENDED SHEET
CA 02339552 2001-02-05
13-11-2000 CA 009900715
-6-
region at said ion outlet for introduction into a sampler
orifice of a mass spectrometer.
BRIEF_DESCRIPTTON OF THE DRAWINGS
For a better understanding of the present invention, and by
way of example, reference will now be made to the accompanying
drawings, which show preferred embodiments of the present invention in
which:
Figure 1 shows three possible examples of changes in ion
mobility as a function of the strength of an electric field;
Figure 2 illustrates the trajectory of an ion between two parallel
plate electrodes under the influence of the electrical potential V(t);
Figures 3A and 3B show schematically an embodiment of a
modified FAIMS device;
Figure 4 illustrates two opposite waveform modes which may
be used with the apparatus of Figures 3A and 3B;
Figures 4A and 4B show compensation voltage spectra obtained
under identical conditions except for the applied waveform being reversed
in polarity between P1 mode and P2 mode;
Figures 5A and 5B show schematically the coupling of the
FAIMS apparatus of Figures 3A and 3B together with a mass spectrometer;
Figures 6A and 6B shows schematically a FAIMS apparatus for
measuring the ion distribution in the analyzer region;
Figures 7 illustrates the high voltage, high frequency
AMENDED SHEET
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
_ 7-
asymmetric waveform applied to the FAIMS apparatus shown in Figures
6A and 6B;
Figure 8 illustrates varying ion arrival time profiles at the
innermost ion collector electrode of the FAIMS apparatus in Figures 6A
and 6B;
Figure SA(A) shows a compensation voltage spectrum for CsCI
in an ion filtering experiment;
Figures 8A(B) and 8A(C) show mass spectra obtained by setting
the compensation voltage at two points of interest indicated by the vertical
dashed lines in Figure 8A(A);
Figure 8B(A) shows an example of ions separated by FAIMS
using equine cytochrome C;
Figure 8B(B) shows a mass spectrum collected with the FAIMS
not functioning (i.e., DV=0);
Figures 8B(C) and 8B(D) show mass spectra collected under
different compensation voltage conditions for equine cytochrome C that
illustrate the ion focussing concept compared with Figure 8B(B);
Figures 9A and 9B show schematically a first embodiment of a
3-dimensional atmospheric pressure high field asymmetric waveform ion
trap, referred to as the FAIMS-R2-prototype;
Figure 10I shows the experimental result for extraction of ions
trapped using the FAIMS apparatus of Figure 9A with the extraction
voltage set at +30 volts;
Figures 11A-11C show a second embodiment of a 3-
dimensional atmospheric pressure high field asymmetric waveform ion
trap, referred to as the FAIMS-R3-prototype;
Figure 11D shows a timing diagram for a voltage applied to the
FAIMS apparatus of Figures 11A-11C;
Figure 12 shows an alternative embodiment of the FAIMS
apparatus of Figures 11A-11C, having a simplified electrospray ionization
chamber, and using the sampler cone as an extraction grid;
Figures 14A-14C show schematically an alternative
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
_g_
embodiment of a 3-dimensional atmospheric pressure high field
asymmetric waveform ion trap; and
Figures 19G-19I illustrate various ion trajectory calculations
near the curved terminus of an inner electrode.
DETAILED DESCRIPTION OF THE INVENTION
As an important preliminary note, although the discussion
below generally uses the term "ion" to mean a charged atomic or
molecular entity, the "ion" can be any electrically charged particle, solid or
liquid, of any size. The discussion always refers to the "ion" as positively
charged. However, all of the discussion in this disclosure is equally
applicable to negative ions, but with the polarity of applied voltages being
reversed.
Principles of FAIMS
The principles of operation of FAIMS have been described in
Buryakov et. al. (see I. Buryakov, E. Krylov, E. Nazarov, and U. Rasulev,
Int. J. Mass Spectrom. Ion Proc. 128. 143 (1993)) and are summarized here
briefly. The mobility of a given ion under the influence of an electric field
can be expressed by: Kh(E) = K(1+f(E)), where Kh is the mobility of an ion at
high field, K is the coefficient of ion mobility at low electric field and
"f(E)"
describes the functional dependence of the ion mobility on the electric
field (see E.A. Mason and E.W. McDaniel, Transport Properties of Ions in
Gases (Wiley, New York, 1988); and I. Buryakov, E. Krylov, E. Nazarov,
and U. Rasulev, Int. J. Mass Spectrom. Ion Proc. 128. 143 (1993)).
Referring to Figure 1, three examples of changes in ion
mobility as a function of the strength of an electric field are shown: the
mobility of type A ions increases with increasing electric field strength; the
mobility of type C ions decreases; and the mobility of type B ions increases
initially before decreasing at yet higher fields. The separation of ions in
FAIMS is based upon these changes in mobility at high electric fields.
Consider an ion 1, for example a type A ion shown in Figure 1, that is
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-9-
being carried by a gas stream 6 between two spaced apart parallel plate
electrodes 2, 4 as shown in Figure 2. The space between the plates 2, 4
defines an analyzer region 5 in which the separation of ions may take
place. The net motion or the ion i netween me p~azes ~, ~ 15 use gum m d
horizontal x-axis component due to a flowing stream of gas 6 and a
transverse y-axis component due to the electric field between the plates 2,
4. (The term "net" motion refers to the overall translation that the ion 1
experiences, even when this translational motion has a more rapid
oscillation superimposed upon it.) One of the plates is maintained at
ground potential (here, the lower plate 4) while the other (here, the upper
plate 2) has an asymmetric waveform, V(t), applied to it. The asymmetric
waveform V(t) is composed of a high voltage component, V1, lasting for a
short period of time t2 and a lower voltage component, V2, of opposite
polarity, lasting a longer period of time tl. The waveform is synthesized
such that the integrated voltage-time product (thus the field-time product)
applied to the plate during a complete cycle of the waveform is zero ( i.e.,
V 1 t2 + V 2 tl = 0 ); for example +2000 V for 10 ~s followed by -1000 V for
20
~.s. Figure 2 illustrates the ion trajectory 8 (as a dashed line) for a
portion
of the waveform shown as V(t). The peak voltage during the shorter, high
voltage portion of the waveform will be called the "dispersion voltage" or
DV in this disclosure. During the high voltage portion of the waveform,
the electric field will cause the ion 1 to move with a transverse velocity
component vl = KhEhigh, where Ehigh is the applied field, and Kh is the high
field mobility under ambient electric field, pressure and temperature
conditions. The distance travelled will be dl = vlt2 = KhEhight2~ where t2 is
the time period of the applied high voltage. During the longer duration,
opposite polarity, low voltage portion of the waveform, the velocity
component of the ion will be v2 = KEIoW, where K is the low field ion
mobility under ambient pressure and temperature conditions. The
distance travelled is d2 = v2t1= KEhWtl. Since the asymmetric waveform
ensures that (Vl t2) + (V2 tl) = 0, the field-time products Ehight2 and EloWtl
are equal in magnitude. Thus, if Kh and K are identical, dl and d2 are
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-10-
equal, and the ion 1 will be returned to its original position along the
y-axis during the negative cycle of the waveform (as would be expected if
both portions of the waveform were low voltage). If at Eh;gh the mobility
Kh > K, the ion 1 will experience a net displacement from its original
position relative to the y-axis. For example, positive ions of the type A
shown in Figure 1 will travel further during the positive portion of the
waveform (i.e., dl > d2) and the type A ion 1 will migrate away from the
upper plate 2 (as illustrated by the dashed line 8 in Figure 2). Similarly,
ions of type C will migrate towards the upper plate 2.
If an ion of type A is migrating away from the upper plate 2, a
constant negative do voltage can be applied to this plate 2 to reverse, or
"compensate" for this transverse drift. This do voltage, called the
"compensation voltage" or CV in this disclosure, prevents the ion 1 from
migrating towards either plate 2, 4. If ions derived from two compounds
respond differently to the applied high electric fields, the ratio of Kh to K
may be different for each compound. Consequently, the magnitude of the
compensation voltage CV necessary to prevent the drift of the ion toward
either plate 2, 4 may also be different for each compound. Under
conditions in which the compensation voltage CV is appropriate for
transmission of one compound, the other will drift towards one of the
plates 2, 4 and subsequently be lost. The speed at which the compound
will move to the wall of the plates 2, 4 depends on the degree to which its
high field mobility properties differ from those of the compound that will
be allowed to pass under the selected condition. A FAIMS instrument or
apparatus is an ion filter capable of selective transmission of only those
ions with the appropriate ratio of Kh to K.
The term FAIMS, as used in this disclosure, refers to any device
which can separate ions via the above described mechanism, whether or
not the device has focussing or trapping behaviour.
Improvements to FAIMS
The FAIMS concept was first shown by Buryakov et. al. using
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-11-
flat plates as described above. Later, Carnahan et. al. improved the sensor
design by replacing the flat plates used to separate the ions with concentric
cylinders (see B. Carnahan, S. Day, V. Kouznetsov, M. Matyjaszczyk, and
A. Tarassov, Proceedings of the 41st ISA Analysis Division Symposium,
Framingham, MA, 21-24 April 1996, p. 85; U.S. Patent No. 5,420,424 issued
to Carnahan et al.). The concentric cylinder design has several advantages
including higher sensitivity than the flat plate configuration (see R.W.
Purees, R. Guevremont, S. Day, C.W. Pipich, and M.S. Matyjaszczyk, Rev.
Sci. Instrum., 69, 4094 (1998)).
As mentioned earlier, an instrument based on the FAIMS
concept has been built by Mine Safety Appliances Company (MSA). The
MSA instrument uses the concentric cylinder design and is described
further below. (For the purposes of this disclosure, the MSA instrument is
referred to as FAIMS-E, where E refers to an electrometer or electric
current detection device.)
One previous limitation of the cylindrical FAIMS technology
(see D. Riegner, C. Harden, B. Carnahan, and S. Day, Proceedings of the
45th ASMS Conference on Mass Spectrometry and Allied Topics, Palm
Springs, California, 1-5 June 1997, p. 473; and B. Carnahan, S. Day, V.
Kouznetsov, M. Matyjaszczyk, and A. Tarassov, Proceedings of the 41st
ISA Analysis Division Symposium, Framingham, MA, 21-24 April 1996, p.
85) was that the identity of the peaks appearing in the FAIMS-E CV spectra
could not be unambiguously confirmed due to the unpredictable changes
in Kh at high electric fields.
Thus, one way to extend the capability of instruments based on
the FAIMS concept, such as the FAIMS-E instrument, is to provide a way
to determine the make-up of the FAIMS-E CV spectra more accurately, for
example, by introducing ions from the FAIMS-E device into a mass
spectrometer for mass-to-charge (m/z) analysis.
In addition, it has been found that a modified FAIMS
instrument, or any similar instrument, can be used in a new method of
separating isomers and different conformations of gaseous phase ions.
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-12-
The present invention is directed to a new method of separating isomers
and different conformations of ions and illustrates the method by several
examples. Details of the method of the present invention are described
below.
Electrospray Ionization
ESI is one of several related techniques that involves the
transfer of ions (which can be either positively or negatively charged) from
liquid phase into the gas-phase. Kebarle has described four major
processes that occur in electrospray ionization (intended for use in mass
spectrometry): (1) production of charged droplets, (2) shrinkage of charged
droplets by evaporation, (3) droplet disintegration (fission), and (4)
formation of gas-phase ions (Kebarle, P. and Tang, L. Analytical Chemistry,
65 (1993) pp. 972A-986A). In ESI, a liquid solution (e.g. 50/50 w/w
water/methanol) is passed through a metal capillary (e.g., 200 ~.m outer
diameter and 100 ~.m ID) which is maintained at a high voltage to generate
the charged droplets, say +2000 V {50 nA) for example. The liquid samples
can be pumped through at, say, 1~L/min. The high voltage creates a very
strong, non-constant electric field at the exit end of the capillary, which
nebulizes the liquid exiting from the capillary into small charged droplets
and electrically charged ions by mechanisms described by Kebarle and
many others. Several related methods also exist for creating gas-phase
ions from solution phase. Some examples of these methods include
ionspray, which uses mechanical energy from a high velocity gas to assist
in nebulization; thermospray, which applies heat instead of a voltage to
the capillary; and nanospray, which uses small ID capillaries. In this
disclosure, the term ESI is used to encompass any technique that creates
gas-phase ions from solution.
Desolvation
In creating gas phase ions from a solution, some of the ions are
created directly from the liquid, some of the ions are produced out of small
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-13-
droplets containing both the ions and solvent, and it is probable that many
droplets are formed that do not produce any gas phase ions. Furthermore,
the gas in which this complex mixture of ions, charged droplets, and
non-charged droplets, are suspended also has a high concentration of
solvent molecules. If this gas is simply allowed to pass into a mass
spectrometer for analysis, the resulting spectra are extremely 'poor quality'
because of heavy solvation. In this context, poor quality means that a
particular charge state of an ion is represented in several places in the mass
spectrum (i.e. the mass to charge (m/z) scale). For example, if MH+ is the
solvent-free protonated ion, then this ion may also appear as MH+,
M(H20)H+, M(H20)2H+, M(H20)3H+ and so on. If the solution also
contains methanol (which is typically the case in ESI) a 'poor' spectrum
will contain, in addition to the series of hydrated ions noted above, yet
further such 'series' of ions containing methanol, and combinations of
water and methanol such as M(H20)n,(MeOH)nH~ (where m, n are integers
0 and higher). A 'good' quality spectrum will contain only MH+, i.e. m
and n will both be zero. A good quality spectrum is an important
requirement especially in experiments involving pharmaceutical and
biological applications where the sample often contains very large
numbers of different compounds, and compounds like proteins which
may appear in the mass spectrum at many charge/mass ratios. Solvated
ions that appear in these spectra add to the overall background in the
spectrum, decreases the capability of the instrumentation to detect small
quantities of specific compounds. Thus, high efficiency desolvation is
necessary in these types of applications.
Currently, two methods are commonly used to achieve ion
desolvation. The first method involves the use of what is referred to as a
"curtain gas" (developed by MDS Health Group Limited, of Etobicoke,
Ontario). In this method the orifice into the vacuum of a mass
spectrometer is protected by a curtain of gas which is travelling in a
direction different from that of the arriving ions. This curtain of gas has
the effect of removing water and solvent from the gas adjacent to the
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-14-
orifice leading into the vacuum. A second method called the "heated
capillary" method (used by Hewlett Packard Company, of Palo Alto,
California, and others), minimizes ion solvation by heating the gas and
ions as they pass into the vacuum of a mass spectrometer via a narrow
bore capillary tube.
Inventors' Experiments
Three important concepts that are referred to in this disclosure
are ion focussing, ion trapping and desolvation. These concepts are
explained below with reference to various experiments conducted by the
inventors. To set the background for the discussion a modified version of
the FAIMS-E device is first described.
A) Modified FAIMS-E
As a first step, the FAIMS-E device designed and built by Mine
Safety Appliances Company was modified to permit the introduction of
ions using ESI. The inventors believe that the coupling of an ESI source
together with a FAIMS-E device is not obvious as it is known that ions
produced by ESI have a high degree of solvation, and that a FAIMS-E
device may not function properly when exposed to high levels of solvent
vapour. The inventors have developed various practical embodiments of
an apparatus that combines an ESI source together with a FAIMS device to
show that such coupling is possible.
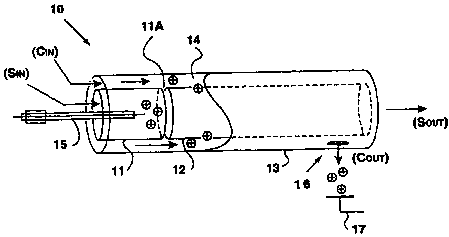
One example is the modified FAIMS-E device 10 shown
schematically in 3-dimensional view in Figure 3A and in cross section in
Figure 3B. The FAIMS-E apparatus 10 is composed of two short inner
cylinders or tubes 11, 12 which are axially aligned and positioned about 5
mm apart, and a long outer cylinder 13 which surrounds the two inner
cylinders 11, 12. The inner cylinders 11, 12 (12 rnm inner diameter, 14 mm
outer diameter), are about 30 mm and 90 mm long, respectively, while the
outer cylinder 13 (18 mm inner diameter, 20 mm outer diameter) is about
125 mm long. Ion separation takes place in the 2 mm annular space of
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/007I5
-15-
FAIMS analyzer region 14 between the long inner cylinder 12 and the
outer cylinder 13. To produce ions using electrospray ionization (ESI), for
introduction into the FAIMS analyzer region 14 of the FAIMS device, the
metal capillary of the ESI needle 15 was placed along the central axis of the
shorter inner cylinder 11, terminating about 5 mm short of the gap or ion
inlet between the two inner cylinders 11, 12. The positioning of the ESI
needle 15 shown in Figures 3(A) and 3(B) differs from the positioning of
the ionization source found in the MSA FAIMS-E device in that the ESI
needle 15 does not extend through the long inner cylinder 12 to which the
asymmetric waveform V(t) is typically applied. By introducing the ESI
needle 15 from the opposite end of the FAIMS-E, i.e. through the short
inner cylinder 11, and not positioning the tip of the ESI needle 15 too close
to the long inner cylinder 12, the performance of the ESI needle 15 is not
compromised by the asymmetric waveform V(t), which would be the case
if the ESI needle 15 was positioned within the long inner cylinder 12 (as
disclosed in U.S. Patent No. 5,420,424).
In an experiment conducted by the inventors, the solution was
pumped through the metal capillary of the ESI needle 15, which was held
between approximately +1500V and +2000V (e.g. 20 nA), at approximately
1 ~1/min. Solutions that were used in this work consisted of an analyte
which was dissolved in 0.1% acetic acid in a 1 to 1 (v/v) mixture of
water/methanol. Note that the inventors' experiments with ESI have not
been restricted to these chemicals/solvents alone; different solvents and
chemicals can also be used. Several examples have been described in the
literature.
As explained above, the FAIMS-E device 10 can be considered
as an ion "filter", with the capability of selectively transmitting one type
of
ion out of a mixture. If a mixture of ions is presented continuously to the
entrance of the FAIMS analyzer region 14, for example by an ESI needle 15,
and the ions are carried along the length of the analyzer 14 by a flowing gas
under conditions in which no voltages are applied to either the inner
cylinder 12 or outer cylinder 13 (i.e. the electrodes are grounded), some
CA 02339552 2001-02-05
WO 00/08455 PCTlCA99/00715
-16-
finite level of transmission for every ion is expected, albeit without any
separation.
It might be expected that the detected current of any selected
ion in this mixture should never exceed the current for that ion when it is
transmitted through the device 10 in the no-voltages condition. It might
also be expected that application of high voltages (i.e. application of
transverse fields, perpendicular to the gas flows) designed to yield ion
separation should not increase the ion transmission, but should decrease
transmission through collisions with the walls of the cylinders 12, 13.
That is, the asymmetric waveform might effectively narrow the "width" of
the FAIMS analyzer region 14, and therefore should decrease the ion
transmission. However, contrary to this prediction, experiments
conducted by the inventors and described in this disclosure have shown
that the sensitivity of ion detection in the cylindrical geometry FAIMS-E 10
increases as the voltage amplitude of the asymmetric waveform V(t) is
increased. As will be explained below, these unusual observations suggest
that atmospheric pressure ion focussing is occurring in the FAIMS
analyzer region 14.
The inventors believe that this phenomenon has many
practical applications in the manipulation of ions at atmospheric pressure.
For example, atmospheric pressure ion focussing could be used to improve
the ion sampling efficiency of mass spectrometers that require transport of
ions from atmospheric pressure to vacuum. These include atmospheric
pressure ionization (API) spectrometers and atmospheric pressure
sampling mass spectrometers, most notably those used for electrospray
ionization. Details are provided further below.
Still referring to Figures 3A and 3B, four gas connections
to the FAIMS-E apparatus 10 are shown. Compressed gas (e.g. air or
nitrogen) is passed through a charcoal/molecular sieve gas purification
cylinder (not shown) into the FAIMS-E 10 through carrier in (C;") and/or
sample in (S;n) ports. The gas exits the FAIMS-E 10 via the carrier out
(Co"t) and/or sample out (So"t) ports. All four gas flow rates can be
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-17-
adjusted. Non-volatile analytes are typically introduced into the FAIMS-E
using an ESI needle 15. Alternatively, volatile analytes may be
introduced into the FAIMS-E 10 through the S;n line, and a portion may be
ionized as the compounds) pass by a corona discharge needle.
5 In both cases, positively charged ions, formed in the short
inner cylinder 11 are driven radially outward by the electric field of the
ionization needle, whereas neutrals travel through the center of the long
inner cylinder 12 and exit via the Sout port. Neutrals are prevented from
entering the annular FAIMS analyzer region 14 by a portion of the C In flow
10 which is directed radially inward through the 5 mm gap or ion inlet
between the inner cylinders 11, 12, and exits via the Sout port. This portion
of the Cin gas flow that travels radially inward is counter-current to the
ions being driven radially outward and acts to reduce the solvation of the
ions. In addition, the inventors believe that further desolvation may be
occurring as certain solvated ions travel down the FAIMS analyzer region
14 and are made to oscillate rapidly by the application of V(t). This
desolvation capability of the FAIMS-E apparatus 10, as recognized by the
inventors, is very important since it permits the collection of high quality
electrospray CV spectra and mass spectra, without the use of other
desolvation techniques, such as the use of a curtain gas, or the use of a
heated capillary tube, both mentioned earlier.
Still referring to Figures 3A and 3B, the outer cylinder 13
of the FAIMS-E apparatus 10, and the shorter inner cylinder 11, are
typically held at an adjustable electrical potential (VpAIMS)~ VFAIMS is
usually ground potential in FAIMS-E. During operation, a high frequency
high voltage asymmetric waveform is applied to the long inner cylinder 12
to establish the electric fields between the inner and outer cylinders 12, 13.
In addition to this high frequency (e.g., 210 kHz) high voltage waveform a
do offset voltage (i.e. the compensation voltage CV added to FAIMS) is
applied to the long inner cylinder 12. This leads to the separation of ions
in the FAIMS analyzer region 14 in the manner discussed earlier.
Still referring to Figures 3A and 3B, some of the ions produced
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-18-
by the ionization source are carried by the gas stream along the length of
the annular space between the outer cylinder 13 and the long inner
cylinder 12, also referred to as the FAIMS analyzer region 14. If the
combination of DV and CV are appropriate, and the ion is not lost to the
tube walls, a series of openings or ion outlets 16 near the downstream end
of the outer cylinder 13 allow the ions to be extracted to an electrical
current detector 17 which is biased to about -100 V. (Note that here the
carrier gas also exits from the ion outlet 16.)
In practice, the simplified square wave version of V(t) shown
in Figure 2 cannot be used because of the electrical power demands that
such a wave would place on the waveform generator. The actual
waveforms V(t) appear in Figure 4. These waveforms are produced by the
electronic addition of a sine wave and its harmonic of twice the frequency.
As shown in Figure 4, the FAIMS-E apparatus 10 operates using one of the
two waveform modes (with the waveform applied to the inner cylinder).
These reversed polarity waveform modes do not yield "reversed polarity"
CV spectra as might be expected. This is because the reversal of polarity in
this manner also creates a mirror image effect of the ion focussing
behaviour of FAIMS. The result of such polarity reversal is that the ions
are not focussed, but rather collide with the walls of the cylinders 12, 13.
The mirror image of a focussing valley is a hill-shaped potential surface.
(This characteristic, and the various "modes" of operation of FAIMS, is
discussed further below.)
B) Ion Focussing
Referring now to Figures 6A and 6B, to demonstrate the
focussing effect referred to above, a special FAIMS instrument was
designed by the inventors and constructed to measure the ion distribution
between the two cylinders (outer and inner cylinders) of a FAIMS device.
This instrument will be referred to in this disclosure as the
FAIMS-R1-prototype 30 and is illustrated schematically in Figures 6A and
6B. Ions were generated inside of an electrically grounded cylinder 31
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-19-
approximately 35 mm long and 20 mm i.d.. The tip of an ionization
needle 15 was typically located near the center of this tube, and at least 15
mm from the end of the FAIMS analyzer region 34. The FAIMS analyzer
region 34 in this embodiment is composed of an outer tube 32 which is 70
mm long and 6 mm i.d., and which surrounds a 2 mm o.d. inner shield
electrode 33. The inner shield electrode 33 is an electrically grounded
stainless steel tube which is closed at the end that faces the ionization
needle 15. This inner electrode 33 surrounds, and shields, an electrically
isolated conductor 35 passing into its center. This innermost conductor 35
(i.e the ion collector electrode) is a collector for ions, and is connected to
a
fast current amplifier or electrometer 36 (e.g. Keithly model 428) and a
digital storage oscilloscope 37 (e.g. LeCroy model 9450).
In the system shown in Figures 6A and 6B, the ions which
surround the inner electrode 33 are forced inwards by a pulsed voltage.
These ions travel from the FAIMS analyzer region 34 to the innermost
conductor 35 through a series of 50 ~,m holes 38 drilled through the inner
shield electrode 33. The holes drilled in the inner shield electrode 33 are
positioned about 2 cm from the end facing the ionization needle 15, and
are spaced about 0.5 rnm apart for a distance of 10 mm on one side of the
inner shield electrode 33. The holes 38 drilled in the inner shield electrode
33 are located in this manner to minimize the variability in distance
between the inner shield electrode 33 and the outer cylinder 32 in the
vicinity of these holes 38. It was the inventors' objective to measure the
ion abundance radial profiles of the ions located in the annular space (i.e.
the FAIMS analyzer region 34) between the inner shield electrode 33 and
the outer electrode 32 by pulsing the ions toward the inner shield electrode
33 and through the holes 38 and against the innermost ion collector
electrode 35. The time-dependent distribution of ions arriving at the
innermost conductor 35 is related to the physical radial distribution of ions
around the inner electrode 33. Excessive variation in the distance
between the two cylinders 32, 33 would have increased the uncertainty of
the ion arrival times at the innermost conductor 35, thus decreasing the
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-20-
spatial resolution of the measurements made with this device.
Now referring to Figure 7, the high voltage, high frequency
asymmetric waveform V(t), applied to the FAIMS-Rl-prototype of Figures
6A and 6B, is shown. The waveform is divided into two parts, the
focussing period and the extraction period. The waveform was
synthesized by an arbitrary waveform generator (e.g. Stanford Research
Systems model DS340, not shown) and amplified by a pulse generator (e.g.
Directed Energy Inc., model GRX-3.OK-H, not shown). The frequency of
the waveform, and the relative duration of the high and low voltage
portions of the waveform could easily be modified. Because of the high
voltages, and steep rise-times of the square waves applied to this FAIMS
R1-prototype 30, the power consumption limits were severe, and
waveforms in excess of about 1330 pulses (16 ms at 83,000 Hz) could not be
delivered by this system without overheating electronic components of
the high voltage pulse generator.
Note that, in the case of the FAIMS-R1-prototype 30, the high
voltage, high frequency asymmetric waveform was applied to the outer
cylinder 32 of the FAIMS-R1-prototype 30 shown in Figures 6A and 6B.
Since all other forms of FAIMS discussed in this disclosure have the
waveform applied to the inner tube or electrode, confusion may arise from
the "polarity" of the waveform and the polarity of CV. In the
FAIMS-R1-prototype 30 shown in Figures 6A and 6B, ions of type A
(shown in Figure 1) are focussed during application of the opposite
polarity waveform and CV than that shown for the devices in Figures 3A,
3B, 5A and 5B. Nevertheless, for simplification, the polarity will be
written to be the same as if the device was constructed in the same way as
those of the more conventional configuration. In other words the ions
transmitted during application of waveform #1 will appear with DV
positive and with CV negative. (Please note, however, that the actual
voltages used on the device in Figures 6A and 6B are DV negative and CV
positive).
As was observed in the conventional parallel plate FAIMS
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-21-
apparatus described earlier (Figure 2), the application of a high voltage
asymmetric waveform V(t) will cause ions to migrate towards one of the
FAIMS electrodes 2, 4 because of the changes in ion mobility at high
electric fields (shown in Figures 1 and 2). This migration can be stopped
by applying an electric field or compensation voltage CV in a direction to
oppose the migration. For the FAIMS-R1-prototype 30 of Figures 6A and
6B, this CV was applied to the same electrode as the high voltage
asymmetric waveform (i.e. the outer electrode 32), and was added to the
waveform as a small do bias (up to ~ 50 V). At an appropriate
combination of DV, and compensation voltage CV, a given ion will pass
through the FAIMS device 30. The unit therefore acts like an ion filter. It
is possible to fix conditions such that a single type of ion is isolated in
the
FAIMS analyzer 34 although a mixture flows uniformly out of the exit of
the FAIMS device 30 although a mixture of ions are presented to the inlet
of the FAIMS analyzer region 34.
The second part of the waveform shown in Figure 7 (i.e. the
extraction period) was used to pulse the ions out of the FAIMS analyzer
region 34 between the outer electrode 32, and the inner shield electrode 33
(shown in Figures 6A and 6B). At the end of the focussing period, i.e. after
16 ms of waveform, the asymmetric waveform was replaced by a constant
do bias of approximately +30 V. This caused the ions from the annular
space 34 between the outer electrode 32 and the inner shield electrode 33 to
move in the direction of the inner shield electrode 33. A detector bias of -5
V, applied to innermost ion collector electrode 35, helped to carry the ions
from the vicinity of the holes 38 in the inner shield electrode 33, through
the holes 38 and into contact with the innermost ion collector electrode 35.
The +30 V bias created an electric field of approximately 150 V/cm across
the FAIMS analyzer region 34 and most ions located within this region 34
travelled across the 2 mm space in about 1 ms. The ion current due to the
arrival of ions at the center inner shield electrode 33 can be predicted. For
example, if only one type of ion, with mobility of 2.3 cm2/V-s, e.g.,
(H20)nH+ at ambient temperature and pressure conditions, was located in
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-22-
the FAIMS analyzer region 34, and if this ion was distributed evenly in the
space, an approximately square-topped signal lasting approximately 0.6 ms
should be observed. Deviation from this expected ion arrival profile
would suggest that the ions were distributed in non-uniform profile across
the FAIMS analyzer region 34 between the outer and inner cylinders of the
FAIMS device 30.
Still referring to Figures 6A, 6B, and 7, the FAIMS-R1-prototype
30 was operated as follows. A 2L/min flow of purified air, Carrier Gas In
(Cin), was passed into the cylinder 31 housing the ionization needle 15.
Approximately 2000 V was applied to the needle 15, and the voltage was
adjusted to produce a stable ionization current. The high voltage
asymmetric waveform V(t) was applied to the outer FAIMS cylinder 32 for
approximately 16 ms; this was followed by a 2 ms extraction pulse (Figure
7). The ion current striking the innermost ion collecting electrode 35 was
detected and displayed on a digital oscilloscope 37. A measurement would
typically consist of 100 averaged spectra, collected at a rate of
approximately
5 Hz. Many experimental parameters were varied, including gas flow
rates, the voltages of the asymmetric waveform V(t), the do voltage applied
to the outer electrode CV, and the extraction voltage.
Figure 8 illustrates the ion arrival times at the innermost ion
collector electrode 35 observed by conducting these experiments. Each
trace was recorded with 2500 V applied DV, but with variable CV voltages.
As can be seen, during application of DV and CV, the radial distribution of
ions is not uniform across the annular space of the FAIMS analyzer region
34. For example, at CV near -11 V, the ions are focussed into a narrow
band near the inner electrode 33, and therefore are detected as a high
intensity pulse occurring very early after the extraction voltage has been
applied. At low CV, for example at -5.6 V, the ions are much more
uniformly distributed between the walls of the concentric cylinders 32 33
making up the FAIMS analyzer region 34. When no electrical voltages are
applied to the cylinders 32, 33, the radial distribution of ions should be
approximately uniform across the FAIMS analyzer region 34 (data for this
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-23-
no-voltage experimental condition is not shown in this document). The
experimental data shown in Figure 8 is evidence that the ion focussing is
indeed occurring in FAIMS instruments. This focussing results in the
ions being focussed in a uniform "sheet" or band around the inner
cylinder 33 within the FAIMS analyzer region 34. As mentioned
previously, to the inventors' knowledge, this focussing effect has never
been observed or explained previously.
C) 3-Dimensional Atmospheric Pressure lon Trap
Taking the focussing effect a step further, the inventors believe
they have developed a 3-dimensional atmospheric pressure ion trap,
which is the subject of a co-pending application [Attorney Docket No. 571-
538) filed by the inventors and which is explained briefly here.
The gas flows between the cylinders of the FAIMS devices
described above serve to carry the ions from one end of the device to the
other end. In every case the action of the electric fields is perpendicular to
the transporting motion of the gas flow. This is the reason the early
devices were referred to as "transverse field" compensation ion mobility
spectrometers. The inventors have carried out experiments in which the
2-dimensional ion focussing action of the FAIMS-E 10 and FAIMS-R1-
prototype 30 was utilized together with a gas flow to form a 3-dimensional
trap by ensuring that the ions are caught in a physical location in which
the gas flows and the electrical fields are not perpendicular, but rather act
in opposition to each other. This creates the situation in which the ion
cannot progress in any direction whatsoever. This is the 3-dimensional
atmospheric pressure ion trap. (An apparatus for trapping ions is shown
in Figures 9, 11, 12, 14A and 14B and is described in more detail below. As
shown in Figure 14B, ion trapping occurs near the curved or spherical
terminus of the inner electrode.)
Note that, in this disclosure, the term "ion focussing" is
restricted to a 2-dimensional configuration. That is, if the ions are
"focussed", they will be restricted to a sheet-like structure, and the thin,
flat
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-24-
sheet surrounds the inner cylinder. For example, if ions are "focussed"
around the external surface of a long metallic cylinder, this will mean that
they are restricted to be within a cylindrical space (composed of the ions)
which is coaxial to, or surrounding the metallic cylinder. This sheet of
ions will extend as far as the cylinder, and all around it continuously. On
the other hand, in this disclosure the term "ion trapping" is restricted to
the condition that an ion cannot move freely in any direction in
3-dimensional space. This is more restrictive than "focussing", in which
the ion is free to move anywhere in the 2-dimensions e.g. along the length
of the cylinder described in the example noted above or around the
cylinder at a fixed radius.
3-dimensional ion traps for operation in vacuum chambers of
mass spectrometers are well known, and several geometry's exist.
However, the mechanism and operation of these vacuum-ion-traps is
vastly different from that of the atmospheric pressure (760 torr) version of
the ion trap described in this disclosure. The physical geometry, the
layout of the hardware components, and the electrical voltages applied in
known 3-dimensional ion traps are in no way related to the present
atmospheric version of the ion trap. To the inventors' knowledge, an
atmospheric 3-dimensional ion trap has not been previously achieved.
It is also possible to operate a 3-dimensional ion trap in a
compromised, near trapping condition so that ions can be focussed into a
smaller region in space. This is described further below in reference to
Figures 14A-14C and 19G-19I.
D) Modes of Operation of FAIMS
The focussing and trapping of ions by the use of asymmetric
waveforms has been discussed above. For completeness, the behaviour of
those ions which are not focussed within the FAIMS analyzer region will
be described here. As explained earlier, the ions which do not have the
high field ion mobility properties suitable for focussing under a given set
of DV, CV and geometric conditions will drift toward one or another wall
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-25-
of the device, as shown in Figure 2. The rapidity with which they move
to the wall depends on the degree to which their Kh/K ratio differs from
that of the ion that might be focussed under the selected condition. At the
very extreme, ions of completely the wrong property i.e. type A ion versus
type C ion shown in Figure 1, will be lost to the walls very quickly.
The loss of ions should be considered one more way. If an ion
of type A (Figure 1) is focussed at DV 2500 volts, CV -11 volts in a given
geometry (for example, the FAIMS-E device of Figures 3A-3B), is it
reasonable to expect that the ion will also be focussed if the polarity of DV
and CV are reversed, i.e. DV of -2500 volts and CV of +11 volts (both
applied to the inner electrode). It would seem that the reversal of polarity
is a trivial exercise and the ion should be focussed, however, this is not
observed. Instead, the reversal of polarity in this manner creates the
mirror image effect of the ion focussing behaviour of FAIMS. The result
of such polarity reversal is that the ions are not focussed, but rather are
extremely rapidly rejected from the device, and collide with the walls of
the cylinders 12, 13. The mirror image of a trapping valley, is a hill-shaped
potential surface. The ions will slide to the center of the bottom of a
trapping potential valley (2 or 3-dimensions), but will slide off of the top
of
a hill-shaped surface, and hit the wall of an electrode. This apparently
anomalous behaviour is a consequence of the cylindrical geometry of the
FAIMS-E.
This is the reason for the existence, in the FAIMS, of the
independent "modes" called 1 and 2. In this disclosure, the FAIMS
instrument is operated in four modes: Pl, P2, N1, and N2. The "P" and
"N" describe the ion polarity, positive (P) and negative (N). The
waveform (Figure 4, wave #1) with positive DV (where DV describes the
peak voltage of the high voltage portion of the asymmetric waveform)
yields spectra of type P1 and N2, whereas the reversed polarity (Figure 4,
wave #2, negative DV) waveform yields P2 and Nl. The discussion thus
far has considered positive ions but, in general, the same principles can be
applied to the negative ions, as explained in the preliminary note to the
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-26-
Detailed Description.
Referring now to Figures 4A and 4B, CV spectra were collected
under identical conditions, but the applied waveform was reversed in
polarity (P1 and P2). The CV was scanned in both the negative and
positive polarity in each case. The ions of type A, Figure l, appear in
mode Pl in the negative CV portion of the Figure 4A, whereas ions with
type C behaviour, Figure 1, only appear in mode P2 and are seen in the
negative CV portion of Figure 4B. Mass spectrometry was used to
eliminate the possibility of incorrectly identified ions.
E) Spectra generated using ESI-FAIMS-MS
The FAIMS acts as an ion filter and can be used in the four
distinct modes described above. In this disclosure we discuss positive ions
(a similar argument can be made for negative ions). In particular, two
examples are discussed: one illustrates the desolvation capabilities of P1
mode (using CsCI), the other illustrates the desolvation capabilities of P2
mode (using equine cytochrome c). In general, small analytes (molecular
weight is ~ 300 or less) are observed in P1 spectra and larger analytes, such
as proteins, are observed in P2 mode. Both CV spectra and mass spectra
are shown for these two examples. We emphasize that several solutions
of analytes have been analyzed using this technique and that these are
only two examples to show the capabilities of desolvation.
Figure 8A(A) shows a total ion current CV-spectrum CsCI (m/z
range from 30 to 300 was monitored as a function of CV) when the DV was
set to 2500 V using P1 mode. Mass spectra of the two distinct peaks in
Figure 8A(A) were obtained. Figures 8A(B) and 8A(C) were collected by
setting the compensation voltage to -10.5 V and -7.5 V, respectively
(indicated by the vertical dashed lines in Figure 8A(A)). The mass
spectrum in Figure 8A(B) is dominated by the peak at m/z 65 which is
[CH30H]2H+ (from the solvent). Figure 8A(C) shows the mass spectrum
for the analyte of interest (Cs+) with relatively little residual solvation
(compared to spectra collected without any desolvation, not shown). The
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-27-
bare Cs+ ion is the most abundant peak (m/z 133} in this mass spectrum,
Cs[H20]+ (m/z 151) and Cs[CH30H]+ (m/z 165) are also present.
Figure 8B(A)-SB(D) illustrates by way of example the FAIMS
focussing concept described above using a protein, equine cytochrome c
(MW = 12360). Figure 8B(A) shows the CV-spectrum that is obtained by
selected ion monitoring the m/z ratio of the charge states of cytochrome c
from 5+ to 20+ (i.e., 2473.0 (z = 5), 2061.0 (z = 6), 1766.7 (z=7), ....,
619.0 (z = 20))
while scanning the CV. In obtaining this spectrum, the FAIMS was
operated in P2 mode with a DV of 3300 V. Mass spectra collected under
three different conditions are shown in Figure 8B(B), 8B(C) and 8B(D).
The mass spectrum shown in Figure 8B(B) was collected
without the application of the high voltage asymmetric waveform to the
FAIMS-MS (note that the IS-CV-spectrum in Figure 8B(A) was collected
with the waveform applied). Since the asymmetric waveform (and thus
DV) was not applied, the ions were not drifting toward either electrode,
and the compensation voltage (CV) was nearly zero (-0.2 volts) in order to
optimally transmit ions through the (non-functioning) FAIMS hardware.
This spectrum is only shown for comparison to the conditions shown in
Figure 8B(C) and 8B(D) in which the DV and CV were applied to the
FAIMS. The spectra shown in Figure 8B(C) and 8B(D) are of much higher
quality, and sensitivity and signal-to-noise ratio (S/N) than that shown in
Figure SB(B).
Figures 8B(C) and 8B(D) were collected immediately after
obtaining Figure 8B(B}; with the FAIMS "in operation", i.e. the DV was set
to 3300 and the CV was changed to -5.0 V (Figure 8B(C)) and -7.7 V (Figure
8B(D)). These CV values were selected since they corresponded to the
maximum values in the ion selective ("IS") CV spectra of the 16+ (CV =
-5.0 V) and 8+ (CV = -7.7 V) charge states (also corresponding to the
approximate peak maxima in the IS-CV-spectrum in Figure 8B(A)). When
the FAIMS was "turned off" in obtaining Figure 8B(B), these charge of
states of equine cytochrome c were still distinguishable. However, it is
clear that the S/N is greatly improved for these charge states as shown in
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99100715
-28-
Figures 8B{C) and Figure 8B(D) relative to those shown in Figure 8B(B).
The improvement of S/N for the 8+ charge state is greater than that for the
16+ because of a greater increase in the depth of the FAIMS trapping
potential well (FAIMS focussing) of the 8~ charge state relative to that of
the 16+ charge state. By setting the compensation voltage to the optimal
value for any charge state in the IS-CV-spectrum, the S/N ratio for that
individual charge state easily can be improved with the FAIMS is "in
operation" relative to conditions in which the applied voltages are
"turned off".
F) Other FAIMS Embodiments
Effective desolvation of the ESI ions is not limited to the
geometry of the FAIMS devices described above. Recall the term FAIMS
used herein globally refers to all the types of configurations of hardware
which will have the capability of separating ions and/or focussing ions at
atmospheric pressure using the high field ion mobility mechanism, and
asymmetric waveform discussed earlier. From the point of view of the
invention described in this disclosure, the effective 'desolvation' created by
the FAIMS is the same for all of the geometries of FAIMS described here.
The ion separation and/or ion focussing action of FAIMS only functions
properly (or at all in the case of contaminated gases) when the gas stream
in the FAIMS analyzer region, or FAIMS trapping region is substantially
free of solvents and neutral contaminant molecules (as distinct from the
normal components of the gas e.g. oxygen, nitrogen, argon, etc in purified
air). This means that if the FAIMS has functioned as described in this
disclosure, then it is assumed that the gases have been purified as required,
and that the ions have passed through the FAIMS analyzer in a clean
environment. This clean gas/ion mixture is exactly the prerequisite for
introduction of ions into the mass spectrometer. If the FAIMS has
functioned properly, the ions/gas mixture that leaves the FAIMS analyzer
region is ideally suited for immediate introduction into the entrance
(sampler) orifice of a mass spectrometer.
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-29-
1) FAIMS-MS
As discussed earlier, one way to extend the functionality
of FAIMS devices is to couple them together with a mass spectrometer.
The use of a mass spectrometer together with a FAIMS device is
advantageous because the mass spectrometer facilitates a mass-to-charge
(m/z) analysis to determine the make-up of CV spectra more accurately.
One possible FAIMS-MS embodiment is described here.
Referring to Figures 5A and 5B, the coupling of FAIMS and a
mass spectrometer (FAIMS-MS 20) is shown schematically. The
FAIMS-MS 20 of Figures 5A and 5B, and the FAIMS-E 10 shown in Figures
3A and 3B, differ significantly only at the detection end of the instrument.
In accordance with the invention, the electrometer 17 has been replaced by
a sampler cone 18, placed at the end of the FAIMS cylinders 12, 13 as is
shown in a simplified form in Figure 5B. The diameter of the orifice 19 in
the sampler cone 18 is approximately 250 ~.m. The gas flows in the
FAIMS-MS 20 are analogous to those in the FAIMS-E 10 except that the
Cout is divided into two components, namely the original Cout and the flow
through the orifice 19 into the mass spectrometer. The electrical
waveforms applied to the long inner cylinder 12 are identical to those used
in the FAIMS-E apparatus 10. The sampler cone 18 may be electrically
insulated from the other components so a separate voltage OR can be
applied to it. Furthermore, a voltage can be applied to the cylinders of the
entire FAIMS unit (VgAIMS) for the purpose of enhancing the sensitivity of
the FAIMS-MS.
Figure 5B shows the FAIMS cylinders 12, 13 at a 45 degree angle
in relation to the sampler cone 18 of the mass spectrometer. Figure 5A
showed the FAIMS cylinders 12, 13 at a 90 degree angle in relation to the
sampler cone 18. The way (i.e., the angle between the two tubes of the
FAIMS and the sampler cone 18) in which the ions are extracted from the
cylinders 12, 13 of the FAIMS-MS 20 into the mass spectrometer is not
limited to these angles. Furthermore, the location in which the ions are
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-30-
extracted from the two tubes can also be changed. That is, the ions can be
extracted anywhere along the separation region of the FAIMS.
2) Other Geometrical Considerations of the FAIMS-MS Interface
The FAIMS hardware described above represents only one
exampleof the FAIMS device; the geometry of the separation region can be
drastically changed. In this disclosure "FAIMS" has been used to describe
the class of devices which have one of these two properties: (1) separation
of ions at atmospheric pressure using the changes in ion mobility at high
electric field (Figure 1) and (2) focussing or trapping of ions at atmospheric
pressure by utilization of the changes in ion mobility at high electric field,
and/or a combination of utilization of the changes in ion mobility at high
electric field and at least one other force applied to the ions, including a
gas
flow, or an independently created electric field which acts to create a
location in space wherein the ions cannot escape. The term focussing has
been used to describe restriction of ions to a 2-dimensional, sheet-like
space (free to move along the surface of the sheet) and ion trapping is used
to describe the condition in which the ion cannot escape in any direction.
Because of diffusion, and space charge repulsion of ions, the actual
physical locations of the ions will generally be distributed over some space,
rather than strictly located in one infinitely small physical location. This
means that the 2-dimensional sheet described above has 'thickness'. This
means that within the 3-dimensional trapping zone, which might be
strictly speaking considered to be an infinitely small single point in space,
the ions actually occupy a region that surrounds this single point, and the
ions are in motion around the point. The ions will occupy a smaller
physical space if the trapping potential well is deeper.
In practice, the two functions described above are accomplished
by application of an asymmetric waveform in such a way that the ions are
at 'low electric field' for a time period of the waveform, and at 'high
electric field' for a shorter period of the waveform. This has been
discussed in detail above. The other requirement, in addition to the
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-31-
asymmetric waveform, is a non-constant electric field. The first function
described above i.e. ion separation can be achieved by use of a constant
electric field between flat parallel plates as shown in Figure 2. The ion
focussing and ion trapping requires a non-constant electric field, normally
occurring in a geometrical configuration in which the electrodes are
curved, and/or are not parallel to each other. These non-constant electric
fields can be created in a variety of ways. For example, a non-constant
electric field may be created using electrodes which are cylinders or a part
thereof; spheres or a part thereof; elliptical spheres or a part thereof;
conical or apart thereof, and so on. Combinations of these shapes may also
be used. Since the FAIMS technology is not well defined, some examples
of FAIMS using other possible electrode geometries will be discussed.
Several ESI-FAIMS-MS spectra have been obtained by
mounting the FAIMS-MS on a PE SCIEX Elan 5000 mass spectrometer
(single quadrupole). The sampler orifice plate of the FAIMS-MS was
threaded into the port typically used for the nickel "sampler cone" in an
ICP/MS experiment on the Elan 5000. The interface of the Elan 5000 was
modified to permit voltages to be applied to the sampler orifice cone (OR)
and to the "skimmer cone". The SCIEX Elan 5000 instrument is typically
used for elemental analysis by ICP/MS and is suited for high sensitivity
detection of low mass (atomic) ions.
For ESI-FAIMS-MS experiments that required a wider mass
range (e.g., proteins), an analogous interface was constructed for a PE
SCIEX API 300 triple quadrupole mass spectrometer. As described above, a
voltage was applied to the sampler cone 18A, but the skimmer cone 18B of
the API 300 remained at ground potential for the experiments described
herein. The small ring electrode located behind the orifice of the
conventional API 300 interface was not incorporated into the new
interface. The API 300 instrument permitted MS/MS experiments to
identify ions whose structures might be otherwise very difficult to
establish. Single ion monitoring experiments during which the
compensation voltage applied to the FAIMS was scanned produced "ion
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-32-
selected CV spectra" (IS-CV spectra). Scanned MS experiments displayed
as the sum of intensity of all detected ions are called "total ion current CV
spectra" (TIC-CV spectra). These spectra can be compared to CV spectra
collected with the electrometer-based instrument. The mass spectrum
collected at a fixed value of CV revealed the identity of any ions
transmitted through the FAIMS under fixed conditions of DV and CV.
Quadrupole mass analyzers were used simply because of their
availability in our laboratory. Note that other types of mass analyzers (e.g.,
ion trap, time-of-flight, fourier transform ion cyclotron resonance, etc.)
and hybrids thereof could also be used with the ESI-FAIMS-MS interface.
The FAIMS was placed at a 45 degree angle and also at a 90 degree angle
relative to the mass analyzers for various experiments described in this
disclosure. However, other angles can also be used.
3) FAIMS-R2-Prototype
Referring to Figures 9A and 9B, the device which will be
referred to as the FAIMS-R2-prototype 40 is shown. Here, the asymmetric
waveform V(t) and the compensation voltage CV are applied to the inner,
solid, electrode 42, having a diameter of about 2 mm. The outer,
electrically grounded electrode 43 has an inner diameter of about 6 mm,
thereby allowing an annular space of about 2 mm between the electrodes.
This annular space has been referred to as the FAIMS analyzer or FAIMS
analyzer region 14, 34, 44 in the discussion above, and for simplicity we
will continue to use this terminology. The ions are created by ionspray 15
in a closed cell (not shown) located adjacent to a 0.5 mm hole through the
wall of the outer cylinder. As shown in Figure 9A, ions are driven by the
high electric field generated by the ionization needle 15 (held at about +
1500 to 2000 V), through the 0.5 mm hole 45 (the ion inlet), and into the
FAIMS analyzer region 44 (only those ions travelling directly toward the
hole 45 are shown for simplicity). Inside the FAIMS analyzer region 44,
near this hole 45, the electric fields and the gas flow (shown to be flowing
from right to left in Figures 9A and 9B) are perpendicular to each other
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-33-
and the ions experience the 2-dimensional focussing effect described in the
earlier sections above. However the inner electrode 42 in the device
shown in Figure 9A, terminates about 2 to 4 mm from the end of the outer
electrode 43. The inner surface of the outer electrode 43 at the downstream
end is contoured in such a way as to maintain approximately the same
electric fields (i.e. created by the application of DV and CV) as would be
experienced along the length of the FAIMS analyzer region 44. The end of
the outer electrode has an exit grid 46 (the ion outlet) comprising a hole
(about 2 mm) which is covered with a fine, high transmission metallic
screen. The gas flowing through the device 40 also flows freely through
the grid 46 and exits from the space between the outer electrode 43 and a
collector plate 47. In the absence of any applied voltages (i.e. DV and CV)
the ions will travel through the device very much as shown in Figure 9A.
The ions enter the analyzer region 44, flow with the gas out through the
exit grid 46 of the outer electrode 43, and the few remaining ions are
attracted to an ion collector plate 47 biased at about -5 V. The collector
plate 47 was connected to a high gain current amplifier or electrometer 36
(e.g. Keithly 428) and an oscilloscope 37.
The application of an asymmetric waveform of the type shown
in Figure 7 resulted in the ion focussing behaviour described above except
that the focussing action extended around the generally spherically shaped
terminus 42T of the inner electrode 42, as shown in Figure 9B. This
means that the ions cannot escape from the region around the terminus
42T of the inner electrode 42. This will only occur if the voltages applied
to the inner electrode 42 are the appropriate combination of CV and DV as
described in the discussion above relating to 2-dimensional focussing. If
the CV and DV are suitable for the focussing of an ion in the FAIMS
analyzer region 44, and the physical geometry of the inner surface of the
outer electrode 43 and the curved terminus 42T in Figures 9A and 9B does
not disturb this balance, the ions will collect near the terminus 42T as
shown in Figure 9B. Several contradictory forces are acting on the ions in
this region near the terminus 42T of the inner electrode 42. The ion cloud
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-34-
shown near the terminus 42T of the inner electrode 42 in Figure 9B would
like to travel from right to left to the exit grid 46 in the manner shown in
Figure 9A, because of the force of the gas flow. This also means that the
ions cannot migrate back from left to right, toward the ion source 15. The
ions that get too close to the inner electrode 42 are pushed back away from
the electrode 42, arid those near the outer electrode 43 will migrate back
towards the inner electrode 42, because of the application of the negatively
polarized CV. The ions are captured in every direction, either by forces of
the flowing gas, or by the electric fields (electric potential well) of the
FAIMS mechanism.
Note that, while the above discussion refers to the ions as being
"captured", in fact, the ions (and neutrals) are subject to 'diffusion'.
Diffusion always acts contrary to focussing and trapping. The ions will
always require an electrical, or gas flow force to reverse the process of
diffusion. This means that although the ions may be focussed into an
imaginary cylindrical zone in space (with almost zero thickness), or within
a 3-dimensional ion trap, in reality it is well known that the ions will
actually be dispersed in the vicinity of this idealized zone in space because
of diffusion. This means that ions will always be "distributed" over some
region, rather than all precisely located in the same place. This is
important, and should be recognized as a global feature superimposed
upon all of the ion motions discussed in this disclosure. This means that.
for example, a 3-dimensional ion trap will actually have real spacial width,
and leak for several physical, and chemical reasons.
Expanding on the chemical effects in FAIMS, if an ion collides
with a neutral molecule and temporarily forms a stable complex, this
complex may drift out of the FAIMS focussing or trapping region because
this new complex has high field mobility properties which are different
from the original ion. This means that the complex may have behaviour
at high electric field (see Figure 1) which differs from the original simple
parent ion. For example (at the extreme) the original ion may be of type A,
and the new complex of the type C shown in Figure 1. If this is the case,
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-35-
the new complex will not be trapped at the prevailing DV and CV
conditions. The collision of any of these ions with the walls of the device
will soon result in loss of the ions from the trap. Although the original
ion itself may continue to be trapped, the removal of this ion via
"chemical" effects is entirely possible, and is the reason the FAIMS
analyzer will fail in the presence of significant water vapour or
contaminants in the gas flows. The FAIMS analyzer works best in very
clean conditions. During operation in P2 mode, the requirement for a
high purity gas is somewhat relaxed.
Figure 10I is an example of experimental results with the
FAIMS-R2-prototype. The dimensions of the electrodes were described
above, for Figures 9A and 9B. DV was 2090 volts, CV -12 volts, and the gas
flow through the device was 0.9 L/min. The DV and CV were applied for
about 16 msec, and these voltages replaced by an extraction voltage. The
trace in Figure 10I represents results for the ions extracted with a voltage
of
+30 volts. The extraction of trapped ions results in a positive pulse
recorded in the Figure. The negative pulse is the transient that occurs
when the DV and CV voltage are removed and replaced by the extraction
voltage. It is clear from the data shown in Figure l0I that the application
of a extraction voltage will yield a short, intense ion signal. This occurs
since the ions which were trapped at the tip of the inner electrode were
pulsed out of the trap by the +30 volts. The negative-going pulse shown in
Figure l0I appears even without ion introduction, but the positive-going
ion signal is absent if the ion production device (e.g. ionspray source in
Figure 12) is absent, or the device is turned off.
Although the diagram of FAIMS-R2-prototype in Figures 9A
and 9B, illustrates an ion collection plate 47, in accordance with the present
invention, the ion collector plate can be replaced with the sampling cone
18A of a mass spectrometer. As noted above for the other types of FAIMS,
the ion/gas stream from FAIMS-R2-prototype is ideally suited for
immediate introduction into a mass spectrometer because the ions have
been substantially desolvated, and the gas stream is largely free of
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-36-
contaminants and solvent vapours.
4) FAIMS-R3-Prototype
Now referring to Figures 11A through 11C, the
FAIMS-R3-prototype 50 is shown. This device is configured for detection
by mass spectrometry, and a sampler cone 18, through which gas and ions
are pulled into the vacuum chamber of a mass spectrometer is shown on
the left side of Figures 11A-11C. The right side of the vacuum housing,
and sampling cone 18, is at atmospheric pressure. The left side of those
components is labelled "Mass Spectrometric Vacuum Housing", and is
typically below 1 torr pressure. In most systems a second orifice (not
shown) leads the actual mass analyzer region of the mass spectrometer
which is usually below 10-5 torr pressure.
The FAIMS-R3-prototype analyzer 50 shown in Figure 11A
consists of an inner, solid, cylindrical electrode 52 of about 2 mm diameter,
and an outer electrode 53 which is about 6 mm inner diameter. The center
electrode 52 is powered, through an electrical connection, by an RF
asymmetric waveform generator power supply 55. Both DV and CV are
supplied by this generator 55. The waveforms, and the timing diagram
are shown in Figure 11D. As shown in Figure 11D, the asymmetric
waveform is applied continuously to the inner electrode 52. No other
variation of the voltage (other than manual selection of various CV and
DV settings) is applied to the inner electrode 52.
Referring back to Figure 11A, gas enters the FAIMS-R3
prototype 50 from the right side and flows along the annular space
comprising the FAIMS analyzer region 54, and out through the open end
of the outer electrode 53. Adjacent to the open end (left side) of the outer
cylinder 53 is an exit grid 56 comprising a fine, thin-wired metallic grid
which is electrically isolated from the outer electrode 53, and has an
electrical connection to a grid electric pulse generator power supply 57.
The voltage on the grid 56 can be changed stepwise using this power
supply. The grid voltage and timing diagram is shown in Figure 11D. The
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-37-
grid is typically maintained between -5 and +5 V during the ion storage
time shown in Figure 11D. The grid will then be stepped (100 ns
transition) to between -5 V and -50 V in order to extract the ions from the
3-dimensional atmospheric pressure trap which is located in front of the
spherical terminus of the inner electrode 52T. Figure 11B shows
schematically the approximate location of the ions during the storage
period. It should be kept in mind that the ions trapped here must have
the correct high field ion mobility (see Figure 1) so that their "net" motion
is zero at the combination of CV and DV being applied to the storage
device (the term "net" is used because the ion is constantly moving
back-and-forth due to the application of the asymmetric waveform: if the
ion returns to the same location repeatedly, then the "net" motion caused
by the application of DV and CV is zero). For example, the ions of type
(Hz0)nH+ will be stored in the geometry shown in Figure 11A-11C at a DV
of about +2000 V and a CV of approximately -10 V (typical of P1 mode ). At
conditions very different (e.g. at DV 2500 and CV -5 V) from this
combination of DV and CV the (H20)nH+ ions will not assemble into one
physical location as shown in Figure 11B. Instead, these ions will collide
with the walls in the FAIMS analyzer region 54. At a second set of DV and
CV conditions, such as the DV 2500 and CV -5 V noted above, another ion
(e.g.(Leucine)H+ ) may be able to collect at the tip 52T of the inner
electrode
52 as shown in Figure 11B.
As explained earlier, near the terminus 52T of the inner
electrode 52 shown in Figure 11B, the ions are restricted in motion because
of several contrary forces. The gas flowing along the FAIMS analyzer
region 54 applies a force which will prevent migration of ions from the left
to right (Figure 11B) back toward the ion source, and this force will also
tend to pull the ions out of the trap towards the exit grid shown at the left
end of the outer electrode. The electrical forces characteristic of FAIMS
maintain the ions at a fixed distance from the sides of the inner electrode
52: (1) the ions which are too distant from the inner electrode 52 are
attracted to the inner electrode 52 because of the negative polarity of the
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-38-
applied do offset, i.e. a negative CV; and (2) the ions close to the inner
electrode 52 are pushed away because of the increase of the ion mobility at
high field (see Figure 1) assuming the ions are of type P1.
Figure 11C illustrates the removal of ions from the
3-dimensional atmospheric pressure trap via a stepwise change to the
voltage applied to the grid electrode 56. If the voltage applied to the grid
56
is decreased from, say, 0 V to -15 V as shown in the timing diagram Figure
11D, the ion trap is reduced or eliminated, and the ions are free to escape
under the influence of the gas flow, or by the electric field which might
pull the ions toward the exit grid 56.
The FAIMS-R3-prototype 50 shown in Figures 11A-11C is
appropriate for detection of ions produced by electrospray ionization (ESI).
FAIMS is highly sensitive to moisture and contaminants in the gas
entering the analyzer region. It is usual that contaminants, or too much
water vapour, will result in complete loss of signal, and failure of the
FAIMS to function in the manner described in this disclosure. Since
electrospray ionization involves the high-voltage-assisted-atomization of
a solvent mixture, the amount of water and other volatile solvents is far
too high to be tolerated in the FAIMS. This will mean that the ESI-FAIMS
combination will always require a type of gas-isolation, curtain gas, or
counter-current gas flow, to prevent neutral solvent molecules from
entering the FAIMS analyzer. One method to accomplish this is shown in
Figures 11A-11C. The FAIMS is separated from the ESI chamber 60 by a
small chamber 61 which has provision for gas inlets 62 and gas outlets 63.
If a flow of gas enters this intermediate chamber 61, and a portion of the
gas flows toward the ESI chamber, then the neutral solvent molecules will
exit via the port on the ESI chamber, and will be prevented from entering
the vicinity of the entrance to the FAIMS. The electrospray needle 15,
shown in Figures 11A-11C is more likely to be in a horizontal plane or
lower than the FAIMS analyzer region 54, rather than the higher, vertical
position shown. This minimizes the tendency for very large droplets to
fall via gravity, into the FAIMS analyzer region 54. In a horizontal or
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-39-
lower configuration the large droplets will fall into the bottom of the ESI
chamber 60, which could (optionally) have a drain for removal of excess
solvent.
The counter-current of gas can be achieved in a second way
shown in Figure 12 (gas flows are emphasized, and most of the ions are
omitted). If the FAIMS analyzer gas flow is adjusted so that some of the
gas will exit the FAIMS analyzer region 54 into the ESI chamber 60, the
entrance of neutral contaminants can be avoided. This may result in
higher ion transmission than that for the device shown in Figures 11A-
11C, but the device may not be user-friendly since accidental gas flow
adjustment such that the gas from the ESI chamber 60 is passed into the
FAIMS analyzer region 54 may compromise FAIMS performance for some
period of time (hours) after the accident. Note also that the exit grid
electrode 56 (Figures 11A-11C) has not been shown in Figure 12. In this
embodiment the 'extraction' pulse that destroys the ion trap is applied to
the mass spectrometer sampling cone 18.
5) FAIMS-R4-prototype
Now referring to an alternative embodiment shown in Figures
14A-14C, referred to as FAIMS-R4-prototype 80, a FAIMS 3-dimensional
atmospheric pressure ion trap is shown in which the electrospray (or other
ionization) occurs within the radius of the inner electrode 82. In general,
ions may be introduced to the FAIMS analyzer region 84 either from
outside (external) to the outer electrode 83, or from inside (internal) the
inner electrode 82. The latter is less convenient because the dimensions
are small, and the radius of the inner electrode 82 must be much larger
than can be used in devices using the external ion source. Moreover, the
ionization source (e.g. ESI needle) may be susceptible to the influence of
the high voltages applied in the asymmetric waveform. The electrode
immediately surrounding the ionization source is electrically grounded in
the FAIMS shown schematically in Figure 3A and 3B.
In the device shown in Figures 14A-14C, the inner electrode 82
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-40-
would be about 14 mm outer diameter, and the outer electrode 83 about 18
mm inner diameter, with about 2 mm annular space (FAIMS analyzer
region 84) between these two concentric cylinders 82, 83. The end of the
inner cylinder 82T (left end in Figures 14A-14C) is closed, and shaped
either spherically, or cone shaped as appropriate to maintain the electric
fields suitable for FAIMS ion trapping in all locations near the end of the
electrode 82T. The inside of the outer cylinder electrode is shown to be
uniform in diameter in Figures 14A-14C, but with wide diameter inner
electrodes 82 such as shown in Figure 14A-14C, it is very likely that the
FAIMS analysis conditions will be better maintained if the inner surface of
the outer electrode is contoured very much like that shown in Figures 9A
and 9B. This will maintain substantially constant distance between the
inner electrode, and the outer electrode near the spherically shaped (or
conical etc.), closed end 82T of the inner electrode 82.
Gas flows enter the end of the FAIMS analyzer region 84
shown in Figures 14A-14C (right hand side of the FAIMS in the figure),
and flow toward the closed end or terminus 82T of the inner electrode 82.
Beyond the terminus 82T of the inner electrode 82 the gas flow passes
through an exit grid 85 comprising a high transparency, fine-wire grid, and
exits through the space between the mass spectrometer sampler cone 18
and the exit grid 85. A portion of the gas flows into the sampler cone
orifice 18, drawn by the vacuum of the mass spectrometer. Some of the
ions which have passed through the exit grid 85 during the extraction time
period will also be drawn into the mass spectrometer, by gas flows and by
electrical fields.
Some of the gas entering the FAIMS analyzer region 84 shown
in Figures 14A-14C must be permitted to flow inwards (i.e. the counter
current gas flow) from the analyzer region 84 into the ionization region 86,
thereby preventing neutral molecules, large liquid droplets and other
unwanted non-charged components from passing info the FAIMS
analyzer region 84. These components would contaminate the gas in the
analyzer 84, and the ion focussing and trapping described elsewhere in this
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-41-
disclosure will be degraded. The device therefore may fail if the gas flow
from the FAIMS analyzer into the ionization region is reversed during
electrospray experiments. If the ionization occurs in a very clean
non-contaminated gas, then this restriction on the gas flow direction may
be relaxed ( e.g. ionization of clean gas with radioactive 63Ni foil, corona
discharge ionization, ionization by UV light radiation etc.). During
operation in P2 mode the requirement for high purity gas is somewhat
relaxed.
The device shown in Figures 14A-14C operates in a manner
analogous to that described previously. The ions pass radially out of the
ionization region 86, transported by electric fields against the radially
inward flowing gas. Having passed into the FAIMS analyzer region 84 the
electric fields will either confine the ion inside the analyzer region 84
(focussing or trapping), or the ion will collide with the walls of the device
because of application of DV and CV which are not appropriate.
Assuming that the DV and CV are appropriate for one of the ions in the
sample, that ion will be focussed in the FAIMS analyzer region, and flow
with the gas (since in the analyzer region the gas and electric fields act
perpendicularly to each other) toward the closed, dome-shaped terminus
82T of the inner electrode 82. If the trapping fields {electrical potential
well) remain appropriate, the ions will assemble near the terminus 82T of
the inner electrode 82 as shown in Figure 14B. This will occur because the
ions cannot return toward the ion source against the flow of gas, and the
ions cannot flow with the gas out of the grid 85 because of the confining
action of the electric fields near the terminus 82T of the inner electrode.
As long as the following conditions are maintained, this trap will exist: (1)
the DV and CV must be applied, and the voltages remain appropriate for
the ion being trapped; (2) the voltages on the outer electrode and the grid
remain fixed, e.g. near 0 V, as appropriate for the ion being trapped; and (3)
the gas flow is maintained. If any condition changes the ions may leave
the trap. If it is desired to have the ions travel to the sampler cone 18 of
the mass spectrometer after passing out of the trapping region, and
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-42-
through the grid 85 as shown in Figure 14C, then one of the above
conditions may be optionally changed to achieve this result. This could
occur in a number of ways:
(1) The grid 85 voltage may be dropped (from its value during trapping)
relative to the inner electrode 82, and relative to the outer electrode
83. This will have the effect of attracting (positively charged ions)
away from the FAIMS trapping region (near the terminus 82T), and
thereby breaking the hold of the trap. The ions will leave the trap,
and travel toward the grid 85. Some ions will strike the grid wires,
and some will travel through (assisted by the gas flow). Since all of
the voltages in the device must be considered relative to each other,
somewhat the same effect can be achieved by changes in the voltages
applied to the outer electrode 83, and to the inner electrode 82. For
example, an increase in voltage applied to both the outer electrode 83
and to the inner electrode 82, will have the same effect as a decrease
in the voltage applied to the grid 85.
(2) The DV or CV can be changed in many ways which alter the ion
motion in the vicinity of the FAIMS trapping region. If the CV is
made more negative the ions (positive ions) will tend to collide with
the inner electrode 82, and if the CV is more positive the ions will be
positioned farther frum the inner electrode 82, and at some voltage
the FAIMS trap will no longer exist for this ion and the ion will
travel with the gas flow and under the influence of the average do
electric field, to the grid, as noted in (1) above. If DV is removed the
trap will no longer function. If CV is altered, e.g. more positive, and
DV is removed, (positively charged) ions will be repelled from the
inner electrode 82, and may travel to the grid.
(3) The gas flow can be changed. If the gas flow is sufficiently high to
overcome the trapping action of the electric fields near the closed end
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
-43-
of the inner electrode 82T, the ions will be pushed out of the trap and
toward the grid 85, as described above. If the gas flow is decreased, or
stopped, the ions will move via diffusion, and via chemical changes.
The diffusion will permit the ions to return back toward the ion
source, thereby de-populating the FAIMS trapping region near the
terminus 82T of the inner electrode 82. Even in the presence of gas
flows the ions may soon de-populate the trap because of chemical
effects. If the ion collides with a neutral molecule and temporarily
forms a stable complex, this complex may drift out of the FAIMS
trapping region because this new complex has high field mobility
properties which were different from the original ion. This means
that the complex may have behaviour at high electric field (see Figure
1) which differs from the original simple parent ion. For example (at
the extreme) the original ion may be of type A, and the new complex
of the type C shown in Figure 1. If this is the case, the new complex
will not be trapped at the prevailing DV and CV conditions. The
collision of any of these ions with the walls of the device will soon
result in loss of the ions from the trap. Although the original ion
itself may continue to be trapped, the removal of this ion via
"chemical" effects is entirely possible, and is the reason the FAIMS
analyzer will fail in the presence of water vapour or contaminants in
the gas flows. The FAIMS analyzer works best in very clean
conditions.
Still referring to Figures 14A-14C, when conditions are set
such that an apparatus for 3-dimensional ion trapping is operated in a
compromised condition, i.e., very near trapping conditions, the ions
shown in Figure 14B will not be trapped near the curved surface of the
spherical end 52T of the electrode, but will still tend to move toward the
center axis as they move from right to left in the figure. This is shown in
Figures 19G-19I where the flow of ions progressively widen around the
center axis of the FAIMS device as conditions progressively change from
the very near trapping conditions shown in Figure 19G. Advantageously,
CA 02339552 2001-02-05
WO 00/08455 PCT/CA99/00715
the focusing action shown in Figures 19G-19I, and particularly in Figure
19G, acts to enhance the efficiency of transporting ions from the analyzer
region of the FAIMS device into the sampling orifice of a mass
spectrometer.
Although the invention has been described and illustrated
with reference to specific embodiments, those skilled in the art will
recognize that the invention may be otherwise embodied within the scope
of the following claims.