Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02339575 2001-02-05
WO 00/09018 PCT1US99/18095
COLLAGEN TYPE I AND TYPE III HEMOSTATIC COMPOSITIONS
FOR USE AS A VASCULAR SEALANT AND WOUND DRESSING
1o Inventors:
Chnnlin Yang, James W. Polarek, and Thomas B. Neff
CROSS REFERENCE TO RELATED APPLICATIONS
This application is a continuation-in-part of U.S. application provisional no.
1 s 60/095,977
FIELD OF THE INVENTION
The present invention is directed to polymerized recombinant type I and/or
type III collagen-based compositions and combinations thereof, including
gelatin
2o based compositions, for medical use as sealants and wound dressings. The
present
invention is further directed to the preparation of such compositions. These
compositions are useful as sealants in a variety of medical applications,
including
vascular plug type devices, wound closure devices, tendon wraps for preventing
the
formation of adhesion following surgical procedures, and dressings for use to
treat
25 incisions, seeping wounds, and the like, and as medical adhesives for
bonding tissues.
In a further aspect of the present invention, the compositions include agents
which
induce wound healing or provide additional beneficial characteristics desired
in a
tissue sealant. More particularly, the compositions of the present invention
are can be
used as vascular sealants.
BACKGROUND OF THE INVENTION
Mecl:apical, Chemical, Synthetic and Autologous Adhesion Technigues.
The ability to bond biological tissues is an important area of investigation
for
biomedical researchers. Attempts to provide desired adhesion through purely
-t-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
mechanical bonding have proven to be neither convenient nor permanent.
(Buonocore, M., Adhesion in Biological Systems, R. S. Manly, ed., Academic
Press,
New York, 1970, Chap. 15.) For example, the conventional methods of choice to
close incisions in soft tissue following surgery, injury, and the like have
been sutures
and staples. These techniques and methods are limited by, for example, tissue
io incompatibility with sutures or staples which may cause painful and
difficult to treat
fistulas granulomas and neuromas. Mechanical means can also be limited to
being
purely adhesive, and thus not fully satisfactory as compared to sealants,
applied to
close wounds that are bleeding or seeping, etc. Sutures and staples may also
tend to
cut through weak parenchymatous or poorly vascularized tissue. Sutures can
leave
behind a tract which can allow for leakage of fluids and organisms. Sutures
can be
further problematic in that the needle for any suture is larger than the
thread attached
to it and the needle tract is thus larger than can be filled by the thread
used to form the
sutures.
In addition, limits are imposed by the manual and visual dexterity required on
2o the part of the surgeon and the excessive amount of time needed for the use
of sutures
or staples in microsurgeries. Furthermore, the joints in the gaps between
staples or
sutures, even when properly applied, the staples or sutures are inherently
weak or may
structurally weaken over time and leak.
Several investigators have worked on laser closure of wounds. (See, e.g.,
2s Abergel, R.P. et al. (1986) J. Am. Acad. DenmatoI. 14(5):810-814; Cespanyi,
E. et al.
(1987) J. Surg. Res. 42(2):147-152; Oz, M.C. et al. (1989) Lasers Surg. Med.
9(3):248-253; Oz, M.C. et al. (1991) Am. Surg. 57(5):275-279; and Oz, M.C. et
al.
(1993) J. Clin. Laser Med. Surg. 11(3):123-126.) Early efforts concentrated on
welding tissues using lasers of different wavelengths applied directly to
wound edges
3o and investigating the microstructural basis of the tissue fusion thus
produced.
Researchers proposed that a homogenizing change in collagen with
interdigitation of
altered individual fibrils. (See, e.g., Schober, R. et al. (1986) Science
232(4756):1421-1422.) Investigators explored the idea of heating the collagen
fibrils
-2-
SUBSTITUTE SHEET (RULE 28)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
above a threshold level allowed for cross-linking. However, the heat necessary
to
allow this reaction causes collateral thermal damage. This is undesirable as
even a
slight distortion in, for example, ocular tissue may have functional
consequences.
Also, in the event of laser weld failure, the edges of the tissues may be
damaged by
the original treatment and cannot be re-exposed to laser energy.
1o Further research attempted to enhance heat-activated cross-linking by
placing
a dye in the wound. It was reported that matching the absorbance of the dye
with the
laser wavelength achieved an adhesive effect with less laser power output and
collateral thermal injury. (See, e.g., Chuck, R.S. et al. (1989) Lasers Surg.
Med.
9(5):471-477; and Oz, M.C. et al. (1990) J. Vasc. Surg. 11(5):718-725.)
Coupling the
dye with a protein to create a tissue "solder" was also investigated. The
protein
commonly used is fibrinogen, and, in particular, autologous fibrinogen, which
is used
to avoid problems of the transfer of viral diseases through the use of blood
components from pool donors. In previous applications, fibrinogen has been
obtained
as a fraction of whole blood, contains other blood elements, such as clotting
factors.
2o Application of such a protein-dye mixture in various animal models proved
to be an
improvement to dye alone. (See, e.g., Moazami, N. et al. (1990) Arch. Surg.
125(11):1452-1454; and Oz et al. (1990).) However, direct application in
humans
was prevented due to the need to isolate the necessary protein fibrinogen from
the
patient prior to the procedure to avoid risks of infection from donor plasma.
Other
proteins, for example, albumin, were unsatisfactory substitutes as welds of
comparable strength were not achieved.
Comparisons of protein-dye applications and sutured closures show that the
protein-dye applications produce less of an inflammatory response and result
in
greater collagen production, greater mean peak stress at rupture, and better
cosmesis.
(See, e.g., Wider, T.M. et al. (1991) Plast. Reconstr. Surg. 88(6):1018-1025.)
Ophthalmologic applications of such a tissue solder have included the sealing
of
conjunetival blebs, sclerostomy, closure of retinectomies, and
thermokeratoplasty.
-3-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
(See, e.g., Fink, A.J. et al. (1986) Am. J. Ophthalmol. 101(6}:695-699; and
Latina,
M.A. et al. ( 1990) Arch. Ohthalmol. 108( 12):1745-1750.)
Due to the deficiencies and limitations of mechanical means, such as the above
mentioned sutures, staples, and laser techniques, efforts were made to develop
synthetic polymers, such as, for example, cyanoacrylates, as biomedical
adhesives and
1o sealants. These plastic materials, however, induce inflammatory tissue
reactions. In
addition, the ability of these materials to establish permanent bonding under
physiological conditions has yet to be fully realized.
The known toxicity associated with synthetic adhesives has led to
investigators to the development of biologically-derived adhesives as bonding
materials. Fibrin-based glues, for example, have commanded considerable
attention.
(See, e.g., Epstein, G. H. et al. Ann. Otol. Rhinol. Laryngol. 95: 40-45
(1986); Kram,
H. B et al. Arch. Surg. 119: 1309-1311 (1984); Scheele, J. et al. Surgery 95:
6-12
(January 1984); and Siedentop, K. H. et al. Laryngoscooe 93: 1310-1313 (1983)
for
general discussion of fibrin adhesives.) Commercial fibrin tissue adhesives
are
2o derived from human plasma and thus pose potential health risks such as
adverse
immunogenic reactions and transmission of infectious agents, such as, for
example,
Hepatitis B virus. Moreover, the bond strength imparted by such adhesives are
relatively weak compared to collagen adhesives. (See, for example, De Toledo,
A. R.
et al. Assoc. for Res. in Vision and Ophthalmology, Annual Meeting Abstract,
Vol.
31, 317 (1990).) Accordingly, there is a need for safe and effective
biologically
compatible tissue adhesives for biomedical applications.
More recently, combination products have been devised for use as tissue
adhesives and sealants. The use of a combination of three separately prepared
substances, human fibrinogen cryoprecipitate, thrombin in the presence of
calcium
3o ion, and Factor XIII concentrate, to obtain a glue for application in skin
graft
applications, myringoplasty, repair of dural defects, hemeostatis after
tonsillectomy,
and tracheoplasty has been described. (See, Staindl (Ann. Otol (1979) 88:413-
418).)
In this same time frame, Immuno-AG, Vienna, Austria, began producing and
-4-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCTNS99/18095
commercializing a two-component "fibrin seal" system, wherein one component
contains highly concentrated human fibrinogen, Factor XIII, and other human
plasma
protein, prepared from pooled blood, and the other component supplies thrombin
and
calcium ion. The two components are added together in the presence of a
fibrinolysis
inhibitor. After application, coagulation and fibrin cross-linking occur.
Eventually,
1o the seal may lyse in the process of healing of the wound or trauma which
accompanies
the reconstruction of the tissue. The development of an applicator device for
this
system which mixes and applies the two components of the system simultaneously
has been described. (Redl, H., et al., "Biomaterials 1980," Winter, G. D., et
al., eds.
( 1982), John Wiley & Sons, Ltd., at page 669-675.) These combination systems
and
their uses have been described widely. (See, e.g., Seelich, T., J Head and
Neck Pathol
( 1982) 3:65-69; O'Connor, A. F., et al., Otolaryngol Head Neck Surg ( 1982)
90:347-
348; Marquet, J., J Head and Neck Pathol (1982) 3:71-72; Thorson, G. K., et
al., J
Surg Oncol (1983) 24:221-223.) It has also been reported that the addition of
barium
ion to this fibrin glue system in the treatment of a bleeding duodenal sinus
facilitates
2o follow-up surveillance. (See, for example, McCarthy, P. M., et al., Mayo
Clin Pros
(1987) 62:317-319; Portmann M., J Head and Neck Pathol (1982) 3:96; Panis, R.,
ibid., 94-9S.)
Efforts have recently focused on methods which seek to avoid health issues
raised by the use of blood plasma-derived products in commercially available
tissue
2s adhesive products and systems. Attempts have been made to isolate an
autologous
counterpart of the fibrinogen-containing component. (See, for example,
Feldman, M.
C., et al., Arch Otolaryngol-Head and Neck Surg (1988) 114:182-185; Feldman,
M.
C., et al., Arch Ophthalmol (1987) lOS:963-967; Feldman, M. C., et. al., M J
Otolog
(1988) 9:302-305; Silberstein, L. E., et al., Transfusion (1988) 28:319-321.)
Use of
3o autologous fibrinogen preparations also have obvious limitations.
vascular Sealants. One critical aspect of tissue adhesion is the sealing
of wounds, and, in particular, vascular punctures and other vascular wounds
resulting
from, for example, surgery.
-5-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
For example, percutaneously accessing major vascular structures is a key step
in a variety of diagnostic and therapeutic procedures, including Percutaneous
Transluminal Coronary Angioplasty (PTCA), Percutaneous Coronary Angiography,
and Percutaneous Coronary Atherectomy. In a percutaneous intravascular
procedure,
access to the vascular space is generally obtained using the so-called
Seldinger
io technique where, first, a hollow needle is used to create a puncture wound
through the
skin, the underlying muscle tissue, and the wall of a selected blood vessel,
such as the
femoral artery. Next, a guidewire is inserted through the tubular needle until
its distal
end is located in the blood vessel, at which time the needle is stripped off
of the
guidewire and replaced with an introduces sheath and dilator. The introduces
sheath
typically includes a self sealing hemostatic valve on its proximal end for
sealing
around the guidewire. The guidewire is then advanced into the vascular space
through
the introduces and directed to a preselected area of the vascular system. Once
the
guidewire is positioned, a catheter is advanced over the guidewire to the
desired area.
Once the procedure has been completed and the catheter and the introduces
2o sheath are removed from the puncture site, there may be profuse bleeding,
especially
when the patient has been on anticoagulant therapy such as heparin, coumadin,
aspirin, or thrombolytic agents. The most common method used to prevent post-
procedure bleeding at the access site involves the application of direct
pressure to the
perforation site until normal physiologic pathways nave sealed the access
site. There
are several problems with this method. First, the pressure application
technique may
fail to prevent hemorrhage. Such a hemorrhage may be life-threatening or can
lead to
a large hematoma. A large hematorna in the groin, for instance, may compromise
the
major nerve supply to the anterior lower extremity.
Secondly, the pressure application technique extends the length of the in-
hospital stay. For example, a PTCA may be completed in 2 to 3 hours, but the
patient
will typically be hospitalized for several additional hours or overnight to
allow the
access site to seal physiologically. During this extended hospital stay the
patient is
-6-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
required to stay immobile, often with a sand bag taped to the patient's thigh,
such as
in the case of femoral artery access.
These and other complications are exacerbated where PTCA procedures are
performed in elderly patients who commonly have arteries with reduced natural
elasticity. The access perforation in a relatively inelastic artery does not
contract or
1o shrink upon itself to the same extent as in an artery of normal elasticity.
The resulting
undeflected perforation is typically two to three times larger than an access
perforation in a normal artery, further complicating the initiation of
hemostasis and
the normal physiologic sealing of the access site.
More than 500,000 PTCAs were performed worldwide in 1992 (Cowen
t 5 Report, March 1993), and several times that number of other procedures
requiring
accessing major vascular structures percutaneously and were also performed.
Thus,
the increased length of in-hospital stay necessitated by the pressure
application
technique considerably increases the expense of procedures requiring such
vascular
access.
20 A technique that would allow faster and safer sealing of a vascular access
site
would save a significant amount of health care resources. Medical literature
has
addressed the problem of achieving hemostasis following removal of a
percutaneously
applied intravascular introducer in such uses as angiography or angioplasty by
a
number of divergent means. U.S. Patent No. 5,290,310 describes a device for
25 delivering a collagen plug subcutaneously against a penetration site in a
wall of a
blood vessel. An instrument containing a toroidal-shaped collagen plug within
a
barrel thereof is made to surround the exterior of a tubular introducer. The
instrument
includes a pusher mechanism for ejecting the collagen plug into the puncture
wound
and against the exterior wall of the blood vessel at the site of the puncture.
This
30 device relies upon a collagen plug which is derived from animal sources and
is
therefore comprised primarily of heterotrimer collagen type I.
U.S. Patent No. 5,129,882 also discloses a surgical implement for injecting a
hemostatic agent in a puncture wound by routing the injection device through
the
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCTNS99/18095
s lumen of the introducer sheath after it has been retracted sufficiently so
that the distal
end thereof is no longer in the blood vessel. Deploying a plunger, the
hemostatic
agent is forced out of the instrument and against the exterior wall of the
artery
proximate the puncture wound.
U.S. Patent Nos. 4,744,364, 4,852,974, 4,890,612, 5,021,059, and 5,222,974
to each describe a method and apparatus for effecting hemostasis by inserting
an
anchoring device through the puncture wound and into the blood vessel while
using a
filament attached to the anchoring device to inject an appropriate sealant
into the
wound. The anchoring device prevents entrance of the sealing material into the
blood
vessel and serves as an anchor and guide for addressing selected vessels.
15 Still other devices for injecting a hemostatic agent into a puncture wound
following a vascular procedure are described in U.S. Patent Nos. 5,281,197,
4,838,280, 5,192,300, and 4,738,658 and in published European Patent
Application
0 476 178A 1.
CollagenlGelatin As A Biomaterial. Collagen, the major connective
2o tissue protein in animals, possesses numerous characteristics not seen in
synthetic
polymers. Characteristics of collagen include good compatibility with living
tissue,
promotion of cell growth, and absorption and assimilation of implantations.
(See,
e.g., Shimizu, R. et al. Biomat. Med. Dev. Art. Org., S(1): 49-66 (1977).)
These same
characteristics are also true of gelatins, derivation products of collagens.
25 Various applications of collagen as a biomaterial are being tested, for
example,
the use of collagens in dialysis membranes of artificial kidney, artificial
cornea,
vitreous body, artificial skin and blood vessels, hemostatic agents, soft
contact lesnes,
and in surgery. (Sterzel, K. H. et al. Ameri. Soc. Artif. Int. Organs 17: 293
(1971),
Rubin, A. L. et al. Nature 230: 120 (1971), and U.S. Pat. No. 4,581,030, Dunn,
M. et
3o al. Amer. Soc. Artif. Int. Organs 17: 421 (1971), Krajicek, M. et al. J.
Surg. Res. 4,
290 (1964), U.S. Pat. No. 4,215,200, U.S. Pat. Nos. 4,264,155; 4,264,493;
4,349,470;
4,388,428; 4,452,925, and 4,650,616, and Chvapil, M. et al. Int. Rev. Conn.
Tiss.
Res. 6: 1-61 (1973).)
_g_
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCTNS99/18095
s Natural collagen fibers, however, are basically insoluble in mature tissues
because of covalent intermolecular cross-links that convert collagen into an
infinite
cross-linked network. Dispersal and solubilization of native collagen can be
achieved
by treatment with various proteolytic enzymes which disrupt the intermolecular
bonds
and remove immunogenic non-helical end regions without affecting the basic
rigid
1o triple-helical structure which imparts the desired characteristics of
collagen. (See,
e.g., U.S. Pat. Nos. 3,934,852; 3,121,049; 3,131,130; 3,314,861; 3,530,037;
3,949,073; 4,233,360, and 4,488,911 for general methods for preparing purified
soluble collagen.)
Various methods and materials have been proposed for modifying collagen to
~s render it more suitable as biomedical adhesives. (See, e.g., De Toledo, A.
R. et al.
Assoc. for Res. in Vision and Ophthalmology, Annual Meeting Abstract, Vol. 31,
317
( I 990); Lloyd et al., "Covalent Bonding of Collagen and Acrylic Polymers,"
American Chemical Society Symposium on Biomedical and Dental Applications of
Polymers, Polymer Science and Technology, VoI. 14, Plenum Press (Gebelein and
2o Koblitz eds.), New York, 1980, pp. 59-84; Shimizu et al., Biomat. Med. Dev.
Art.
Org., 5{1): 49-66 (1977); and Shimizu et al., Biomat. Med. Dev. Art. Org.,
6(4): 375-
391 (1978), for general discussion on collagen and synthetic polymers.) In
many
instances, the prior modified collagen-based adhesives suffer from various
deficiencies, including {1) cross-linking/polymerization reactions that
generate
2s exothermic heat, (2) long reaction times, and (3) reactions that are
inoperative in the
presence of oxygen and physiological pH ranges. (See, e.g., Lee M. L. et al.
Adhesion
in Biological Systems, R. S. Manly, ed., Academic Press, New York, 1970, Chap.
17.)
Moreover, many of the prior modified collagen-based adhesives contain toxic
materials, rendering them unsuitable for biomedical use. (See, for example,
30 Buonocore, M. G. (1970) and U.S. Pat. No. 3,453,222.)
Additionally, the use of collagen-based adhesives also presents immunological
concerns as such adhesives have been derived from animal sources and typically
bovine sources. Studies with respect to the use of such collagens as
injectible devices
-9-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCTNS99/18095
have reported minor inflammatory responses. More recently, potential issues
regarding the transmission of disorders to humans related to bovine spongiform
encephalopathy ("mad cow disease") have focused attention, especially in
Europe, to
limiting the use of animal, and particularly, bovine-sourced materials.
Notwithstanding these deficiencies, certain collagen-based adhesives,
to reportedly having appropriate adhesive strength and utility in many medical
applications, particularly involving soft tissues, have been described. (See,
e.g., U.S.
Patent Nos. 5,219,895, 5,614,587, 5,582,834, 5,575,997, 5,354,336, and
4,600,574.)
The literature identify the use of type I and type II in collagen-based
adhesives
wherein purified collagen types I and II are chemically modified to form
monomers
15 soluble at physiological conditions and polymerized to form compositions
having
adhesive and sealant properties. Notably, the reports are limited to collagen-
based
adhesives composed of collagens derived from natural sources which represent a
collagen mixture. For example, type I collagen as isolated from natural
sources
typically contains approximately 10-20% type III and other collagens,
depending
2o upon the tissue source used, and about 90-80% type I collagen. With respect
to the
"collagen type I" mixtures, the literature further teaches only the use of
collagen as an
adhesive as a consequence of its structural characteristics, or,
alternatively, the use of
predominantly collagen type I heterotrimers, as compared to collagen type I
homotrimers which have been implicated in the epithelial cell attachment.
(See, e.g.,
25 Ghersi, et al., 1989, Eur. J. Cell Biol. 50:279-84)).
Available reports do not refer to collagen type III, the unexpected hemostatic
characteristics of type III collagen, or the use of recombinant collagens
which would
allow the first chemical modification step, as described in the art, to be
avoided.
In summary, there is a need in the art for compositions useful as sealants and
3o wound dressings that permit faster and safer healing, that minimize the
risks of
infection from donor, including non-human, sources, that increase convenience
and
permanence, that minimize demands on surgical resources and time, and that
demonstrate superior biocompatibility. In addition, there is a need for
biologically-
-10-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PC'TNS99/18095
derived adhesives that offer improved convenience and permanence over
currently
available formulations, and that promote less-invasive treatment, resulting in
improved patient comfort and shorter time under medical supervision.
Additionally,
such compositions would preferably offer improved bond strength. There is also
a
need for non-adhesive compositions that provide the above-named advantages of
safer
i o and more effective healing and that are biologically compatible.
SUMMARY OF THE INVENTION
The present invention includes biologically compatible, collagen type III
and/or type I products with sealant properties which can be formed using
soluble
15 recombinantly derived collagen type III and/or type I monomers or gelatin
derived
from collagen type III and/or type I monomers (the collagen and gelatin
products are
collectively hereinafter referred to as "collagen") wherein said monomers are
polymerized to form a collagen type III and/or type I composition having
sealant
properties. Preferably, the collagen is human and is derived using recombinant
2o technology. Collagen type III was selected for its unexpectedly superior
hemostatic
characteristics, as compared to other collagen types. Collagen type I was
selected for
its structural characteristics, as well as for the hemostatic properties of
certain
collagen type I forms (e.g., collagen type I homotrimers). The polymerization
reaction may be initiated with an appropriate polymerization initiator such as
a
z5 chemical oxidant, ultraviolet irradiation, a suitable oxidative enzyme, or
atmospheric
oxygen. Additionally, cross-linking agents including glutaraldehyde, dye-
mediated
photooxidation , PEG and its derivatives, acyl azide, plyepoxy fixatives,
oxidized
starch (periodate) and water soluble carbodiimide ("WSC") may be used in the
polymerization process to form a collagen composition having sealant
properties.
3o For purposes of optimizing the sealant and adhesive properties of the
recombinant collagen product by optimizing the structural stability and the
hemostatic
characteristics of the product, the product is comprised of a combination of
pure
recombinant type I and type III collagen The ratio of pure recombinant
collagen type
-11-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCTNS99/18095
III to pure recombinant collagen type I (heterotrimer) is preferably about 30%
and
greater type III collagen to about 70% or less type I collagen (heterotrimer).
More
preferably, the ratio of pure recombinant type III collagen to pure
recombinant type I
collagen (heterotrimer) is about 30% to about 50% type III collagen to about
70% to
about 50% type I collagen (heterotrimer). Most preferably, the ratio of pure
1o recombinant type III collagen to pure recombinant type I collagen
(heterotrimer) is
about 30% to about 40% type III collagen to about 70% to about 60% type I
collagen
(heterotrimer).
With respect to compositions comprised of collagen type I homotrimer, the
ratio of pure recombinant type I homotrimer to a combination of recombinant
collagen
type I heterotrimer and recombinant collagen type III is about 90:10. More
preferably, the ratio of pure recombinant type I homotrimer to a combination
of
recombinant collagen type I heterotrimer and recombinant collagen type III is
about
75:25. Most preferably, the ratio of pure recombinant type I homotrimer to a
combination of recombinant collagen type I heterotrimer and recombinant
collagen
2o type III is about 50:50.
It is the object of this invention to provide for a pure recombinant collagen
type III tissue sealant, a pure recombinant type I tissue sealant, or a pure
recombinant
collagen type I and type III tissue sealant, free from other collagen types,
having at
least one of the following characteristics and capabilities:
(i) Hemostasis. The sealant acts as a hemostatic barrier and
reduces the risk of serum, lymph, and liquid leakage. As collagen type III
possesses
inherently hemostatic properties, its use in a hemostatic device provides an
improvement over known fibrin sealants. Collagen type I also possesses some
hemostatic properties.
(ii) Gluing. Due to its adhesive properties, the sealants of the
present invention connect tissues by forming a strong joint between them and
adapt
uneven wound surfaces. The glueing effect is increased by a combination of
agents,
such as those described below, and collagen type III and/or collagen type I.
-12-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
{iii) Wound healing. The sealant promotes the growth of fibroblasts
which, in combination with efficient hemostasis and adhesion between the wound
surfaces provides for an improved healing process. (See also, Ghersi, et al.,
1989,
Eur. J. Cell Biol. 50:279-284 (comparing characteristics of homotrimer and
heterotrimer collagen type I).) The use of the present compositions as anti-
1o adherence/wound healing compositions is expected to result in a normal
(regenerative) tissue rather than scar tissue, i.e. optimal wound healing.
Furthermore,
such compositions also reduce the inflammatory response.
Accordingly, it is an object of the present invention to provide polymerized
collagen type III and/or type I compositions as safe, effective biological
adhesives
~ 5 with appropriate adhesive strength for biomedical applications,
particularly those
involving soft tissues. More specifically, the present invention is directed
to
compositions useful in sealing punctures and incisions in large blood vessels
and the
heart. The polymerized materials may assume a number of sizes and shapes
consistent with their intended biomedical applications, which include use in
20 ophthalmology, plastic surgery, orthopedics, and cardiology. The vascular
sealant
compositions of the present invention, comprising collagen type III and/or I,
may be
used alone or in combination with a tissue sealant device, including, for
example, the
devices set forth in U.S. Patent Nos. 5,782,860 (issued July 21, 1998),
5,759,194
(issued June 2, 1998), and 5,728,132 (issued March 17, 1998).
25 In another object of the invention, the collagen type III and/or type I
composition is further comprised of agents which will confer additional
desirable
characteristics for a vascular sealant or wound dressing. For example, fibrin,
fibrinogen, thrombin, calcium ion, and Factor XIII may be included in the
composition to better effect the formation of a three-dimensional network of
3o polymerized collagen. In yet another object of the invention, the
recombinant
collagen type III composition incorporates a compound having wound healing
capabilities. In one embodiment, the compound is connective tissue growth
factor and
is incorporated in the composition to effect slow-release of the compound to
the
-13-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCTNS99/18095
wound. In a second embodiment of the invention, the drug improves
vascularization,
for example, tumour necrosis factor, as described in U.S. Patent No. 4,808,402
(issued
February 28, 1989).
BRIEF DESCRIPTION OF THE DRAWINGS
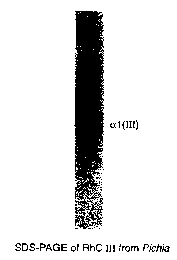
to Figure 1 shows SDS-PAGE analysis of recombinant type III collagen
produced by pichia pastoris.
Figure 2 shows data relating to the biocompatibility of recombinant type III
collagen and a commercially available collagen hemostat.
Figure 3 shows data relating to platelet aggregation experiments of
15 recombinant type III and bovine collagen type I.
Figure 4 shows data relating to the bleeding time of spleen treated with
recombinant collagen type III and bovine collagen type I.
Figure S shows a SDS-PAGE analysis of bovine collagen I cross-linked with
water soluble carbodiimide.
20 Figure 6 shows a SDS-Page analysis of recombinant collagen type III cross-
linked with water soluble carbodiirnide.
DETAILED DESCRIPTION OF THE INVENTION
It is understood that the present invention is not limited to the particular
25 methodology, protocols, cell lines, vectors, and reagents, etc., described
herein, as
these may vary. It is also to be understood that the terminology used herein
is used for
the purpose of describing particular embodiments only, and is not intended to
limit the
scope of the present invention. It must be noted that as used herein and in
the
appended claims, the singular forms "a," "an," and "the" include plural
reference
3o unless the context clearly dictates otherwise. Thus, for example, a
reference to "an
antibody" is a reference to one or more antibodies and equivalents thereof
known to
those skilled in the art, and so forth.
-14-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
Unless defined otherwise, all technical and scientific terms used herein have
the same meanings as commonly understood by one of ordinary skill in the art
to
which this invention belongs. Preferred methods, devices, and materials are
described,
although any methods and materials similar or equivalent to those described
herein
can be used in the practice or testing of the present invention. All
references cited
1 o herein are incorporated by reference herein in their entirety.
Definitions
As employed herein, the term "biologically compatible" refers to recombinant
collagen type III and/or type I modified in accordance with the present
invention (i.e.,
a polymerized collagen type III recombinant product) which is incorporated or
implanted into or placed adjacent to the biological tissue of a subject and
more
particularly, does not deteriorate appreciably over time or induce an immune
response
or deleterious tissue reaction after such incorporation or implantation or
placement.
As employed herein, the term "pure recombinant collagen type I" refers to
collagen type I manufactured by recombinant techniques which is substantially
free
2o from other collagen types. Unless otherwise specifically referenced, the
term pure
recombinant collagen type I includes both collagen type I homotrimer and
collagen
type I heterotrimer and mixtures thereof. The term includes any other forms of
recombinant collagen type I and any modifications made thereto that may be
categorized as a subset of collagen, such as gelatins. The term excludes
collagen type
I isolated from natural sources.
As employed herein, the term "pure recombinant collagen type III" refers to
human collagen type III manufactured by recombinant techniques which is
substantially free from other collagen types. The term includes any other
forms of
recombinant collagen type III and any modifications made thereto that may be
categorized as a subset of collagen, such as gelatins. The term excludes
collagen type
III isolated from natural sources.
-15-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCTNS99/18095
As employed herein, the term "substantially free" refers to a recombinant
collagen type that is substantially pure of any other collagen type or unmixed
with any
other collagen type, and is preferably at least 90% free from other collagen
types.
As employed herein, the term "vascular sealant" refers to any composition
useful in closing vascular wounds, including plugs, which possesses hemostatic
l0 properties.
As employed herein, the term "wound" refers to any opening in the skin,
mucosa or epithelial linings, most such openings generally being associated
with
exposed, raw or abraded tissue. There are no limitations as to the type of
wound or
other traumata that can be treated in accordance with this invention, such
wounds
15 including, but are not limited to, first, second, and third degree burns
(especially
second and third degree); surgical incisions, including those of cosmetic
surgery;
wounds, including lacerations, incisions, and penetrations; and ulcers,
including
decubital ulcers (bed-sores) and ulcers or wounds associated with diabetic,
dental,
haemophilic, malignant, and obese patients. Although the primary concern is
the
2o healing of major wounds by neovascularization, it is contemplated that the
present
invention may also be useful for minor wounds, and for cosmetic regeneration
of
epithelial cells. Preferably, the wounds to be treated are burns and surgical
incisions,
whether or not associated with viral infections or tumors
2s Preparation of Polymerized Recombinant Collagen Type I and III
Production of Collagen Type I and III Monomers. Types of collagen useful
in forming the biologically compatible collagen products of the invention with
adhesive and hemostatic properties are recombinant collagen type I and type
III.
Monomeric soluble collagen types I and III is obtained by recombinant
processes,
3o including processes involving the production of collagen type III in
transgenic
animals. Such recombinant processes are set forth, for example, in U.S. Patent
No.
5,593,859, which is incorporated herein by reference. Preferably, collagen
types I or
III will be recombinantly manufactured by culturing a cell which has been
transfected
-16-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
with at least one gene encoding the polypeptide comprising collagen type I or
III and
genes encoding the a and (3 subunits of the post-translational enzyme prolyl 4-
hydroxylase and purifying the resultant collagen monomer therefrom.
Preferably, the
monomeric soluble collagen type I and III material exhibits a viscous
consistency and
varying degrees of transparency and clarity.
Polymerization Of Collagen Type I ar:d III Monomers. The recombinant
collagen type I and III solution may be subsequently subjected to
polymerization or
cross-linking conditions to produce the polymerized collagen composition of
the
present invention. Polymerization may be carned out using irradiation, e.g.,
LJV,
gamma, or fluorescent light. UV irradiation may be accomplished in the short
wave
length range using a standard 254 nm source or using UV laser sources. With a
standard 254 nm source, 4-12 watts, polymerization occurs from 10 to 40
minutes,
preferably 20 to 30 minutes, at an exposure distance of from 2.5-10 cm,
preferably
from 2.5 to 5 cm distance. Excess LTV exposure will begin to depolymerize the
collagen polymers. Polymerization using gamma irradiation can be done using
from
0.5 to 2.5 Mrads. Excess gamma exposure will also depolymerize collage
polymers.
Polymerization in the presence of oxygen can be achieved by adding an
initiator to the
fluid prior to exposure. Non-limiting examples of initiators include sodium
persulfate,
sodium thiosulfate, ferrous chloride tetrahydrate, sodium bisulfate, and
oxidative
enzymes such as peroxidase or catechol oxidase. When initiators are employed,
polymerization occurs in 30 seconds to 5 minutes, usually from 1 to 3 minutes.
The polymerizing agent is preferably UV irradiation. However, the
polymerization or cross-linking of the monomeric substituents can be carned
out by
any of the methods will known in the art, including simply exposing the
material to
atmospheric oxygen, although the rate of polymerization is appreciably slower
than in
the case of LTV irradiation or chemical agents.
Other agents may also be useful in the polymerization process. For example,
to improve the cohesive strength of adhesives formed from the compositions of
this
invention, difunctional monomeric cross-linking agents may be added to the
monomer
-17-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
compositions of this invention to effect polymerization. Such cross-linking
agents are
known in the art, for example, in U.S. Pat. No. 3,940,362 which is hereby
incorporated by reference herein.
Additionally, polymerization methods and cross-linking agents such as
glutaraldehyde, dye-mediated photooxidation , PEG and its derivatives, aryl
azide,
1o plyepoxy fixatives; oxidized starch (periodate) and water soluble
carbodiimide
("WSC") well known in the art may be used to produce the polymerized collagen
composition of the present invention. (See, e.g., U.S. Patent No. 4,615,794,
U.S.
Patent No. 5,444,154, U.S. Patent No. 4,500,453, U.S. Patent No. 5,702,818,
U.S.
Patent No. 5,415,938, U.S. Patent No.5,308,641, U.S. Patent No.5,264,551, U.S.
15 Patent No.5,258,501, U.S. Patent No.5,258,481, U.S. Patent No. 4,427,808,
U.S.
Patent No. 4,272,610.)
Moreover, the use of polyaldehyde compositions to effectuate polymerization
can be also utilized. (See, for example, PCT WO 97/29715 and EP 747,066 A2.)
Formation of Gelatin. The recombinant collagen protein of the present
20 invention may be further modified and processed into gelatin using
procedures known
in the art. (See, e.g., Veis, 1965, International Review of Connective Tissue
Research, "The Physical Chemistry of Gelatin", Academic Press, New York and
London.) For example, a common feature of all standard collagen to gelatin
conversion processes is the loss of the secondary structure of the collagen
protein, and
25 in the majority of instances, an alteration in either the primary or
tertiary structure of
the collagen. The collagens of the present invention can be processed using
different
procedures depending on the type of gelatin desired.
In one approach, modifications may occur to unpurified collagen or procollagen
present in the cell mass or in the culture medium or any further modifications
can be
30 made to the purified collagen as described above. For example, recombinant
collagen
or procollagen may be modified and processed into recombinant gelatin. Gelatin
may be
produced directly from the cell mass or the culture medium by taking advantage
of
gelatin's solubility at elevated temperatures and stability at conditions of
low or high pH,
-18-
SUBSTITUTE SHEET (RULE 2fi)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
low or high salt concentration, and high temperatures. For example, the cell
mass or
culture medium may further be treated to extract gelatin by denaturing the
triple helical
structure of collagen using detergents, heat or denaturing agents. (See, e.g.,
Vies inter
alia.) Operations well established for the manufacture of tissue-derived
gelatin can be
applied to the production of recombinant gelatin. This includes, but is not
limited to,
l0 treatments with strong alkali or strong acids, heat extraction in aqueous
solution, ion
exchange chromatography, cross-flow filtration, and heat drying.
Collagen Type I and III Compositions
The compositions of the present invention are comprised of polymerized type I
and III collagen wherein said composition is manufactured by a process
comprising
the steps of ( 1 ) production of collagen type I and III monomers by the
recombinant
methods described above; and (2) polymerization of such monomers. In addition,
where the final composition is a gelatin-based sealant or wound dressing, the
process
includes a step wherein the collagen is converted into gelatin.
For purposes of optimizing the sealant and adhesive properties of the
recombinant collagen product by optimizing the structural stability of the
product as
well as the hemostatic characteristics of the product, the product is
comprised
preferably of a combination of pure recombinant type I and type III collagen
The ratio
of pure recombinant collagen type III to pure recombinant type I
(heterotrimer) is
about 30% and greater type II1 collagen to about 70% or less type I collagen
(heterotrimer). More preferably, the ratio of pure recombinant type III
collagen to
pure recombinant type I collagen (heterotrimer) is about 30% to about 50% type
III
collagen to about 7U%to about SO% type I collagen (heterotrimer). Most
preferably,
the ratio of pure recombinant type III collagen to pure recombinant type I
collagen
(heterotrimer) is about 30% to about 40% type III collagen to about 70% to
about
60% type I collagen (heterotrimer).
The appropriate ranges of concentrations of components in the tissue sealants
and adhesives of the present invention can be determined by methods well-known
in
-19-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
the art. (See, e.g., Haraski, H. et al. (1999) Volume XXII, Society for
Biomaterials,
pages 158 through 159; Fasman, G. D., ed. (1989) Practical Handbook of
Biochemistry and Molecular Biology, Section 1, pages 126 through 130; U.S.
Patent
No. 5,834,232 (issued November 10, 1998); Sierra, D. H. et al. (1992) J. Appl.
Biomater. 3(2):147-151; Martinowitz, U. and R. Saltz (1996) Curr. Opin.
Hematol.
3(5):395-402; and Siriex, D. (1998) Ann. Vasc. Surg. 12(4):311-316.) The
actual
proportions of the collagen components of the compositions of the present
invention
will depend on the addition of other agents to the compositions and on the
desired use
of the compositions. The determination of suitable proportions for particular
compositions is within the level of skill in the art, and this invention
contemplates the
various combinations that can be reached.
The compositions of the present invention may be further comprised of other
agents useful in gluing or sealing vascular tissues; and more generally, soft
tissue. For
example, in addition to recombinant collagen type I and/or type III protein,
the
composition will preferably comprise transglutaminases such as Factor XIII
and/or
2o fibrin/ fibrinogen/fibronectin and/or plasminogen. The suitable
concentrations of
these components can be selected by methods-well known in the art. For
example,
fibrinogen can be present in plasma concentrations, such as from about 1.5 to
about
4.0 rng/ml, or higher. Fibrinogen can also be present in lower concentrations,
for
example, to monitor performance.
Preferably, the composition will also include clotting enzymes, i.e. thrombin,
especially in combination with bivalent calcium, such as calcium chloride. The
concentration of calcium chloride can vary, for example, from between 40 mM to
0.2
M, depending on the specific purpose of the tissue adhesive composition. High
concentrations of calcium chloride inhibit fibroblast growth and are therefore
3o preferred for anti-adherence applications {fibronectin, which stimulates
the growth of
fibroblasts, can be absent in such compositions). It may further be valuable
to include
a fibrinolysis inhibitor, such as a pIasmin inhibitor, e.g. aprotinin,
aprilotinin, alpha-2-
-20-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
antiplasmin, alpha-2-macroglobulin, alpha-1-antitrypsin, epsilon-aminocaproic
or
tranexamic acid, or a plasmin activator inhibitor, e.g., PAI-1 or PAI-2.
While the proportions of the previously known ingredients in the tissue
adhesive compositions of the invention may be selected according to methods
well-
known in the art, the necessary amount of the viscosity-enhancing polymer can
1o readily be determined by a person skilled in the art depending on the
particular
polymer and the intended use form. Thus, if the concentration and/or molecular
weight of the viscosity-enhancing polymer is too low, the viscosity increase
will be
insufficient, and a too high concentration and/or molecular weight will
inhibit the
fibrin polymerization and the adhesion to the tissue.
15 By increasing the thrombin concentration, the polymerization of composition
of the present invention may be quickened, reducing the time until the glue
sets. At
low thrombin concentrations, for example, the fibrin of the composition will
remain
more or less fluid for several minutes after application. A further beneficial
effect of
increasing the viscosity with a viscosity-enhancing polymer in accordance with
the
z0 invention is therefore that lower concentrations of thrombin, required in
situations
where the parts to be sealed require subsequent adaptation even on non-
horizontal
surfaces, can be used.
Likewise, the compositions of the present invention may, rather than including
a combination of the agents described herein, include a fusion protein wherein
the
25 collagen type I and/or type III and, for example, fibrin, are combined to
form one
molecule. Such fusion proteins may be manufactured according to recombinant
techniques described herein.
In a further embodiment of the invention, the composition of the present
invention includes agents useful in wound healing, either by inducing or
promoting
3o the formation of tissue, or, alternatively, by limiting the formation of
fibrotic
adhesions. Such agents include antibiotics, or growth factors, such as
connective
tissue growth factor, described in, for example, U.S. Patent No. 5,408,040 and
5,585,270, incorporated herein by reference. In another embodiment of the
invention,
-21-
SUBSTITUTE SHEET (RULE 26~
CA 02339575 2001-02-05
WO 00/09018 PGT/US99/I8095
the drug improves vascularization, for example, tumour necrosis factor, as
described
in U.S. Patent No. 4,808,402 (issued February 28, 1989).
With respect particularly to the vascular sealant aspect of the present
invention, vascular sealant compositions comprising collagen type III and/or I
may be
used alone or in combination with a tissue sealant device, including, for
example, the
to devices set forth in U.S. Patent Nos. 5,782,860 (issued July 21, I998),
5,759,194
(issued June 2, 1998) and 5,728,132 (issued March 17, 1998).
Fields Of Use
The polymerized collagen type III and/or type I products of the present
invention may be useful to produce mechanical sealants and adhesive systems.
15 Vascular Adhesive Systems. Fields of application include, but are not
limited
to, general surgery, dentistry, neurosurgery, plastic surgery, thorax and
vascular
surgery, abdominal surgery, orthopaedics, accident surgery, gynaecology,
urology,
and opthalmology. The collagen sealants of the present invention have also
been used
for local application of drugs, such as antibiotics, growth factors, and
cytostatics.
2o Sealant Films and Wound Dressings. In one aspect of the invention, the
polymerized collagen products can be made in the form of a sealant film. A
collagen-
based film will be flexible and elastic with the consistency and feel of
plastic film, but
can exhibit high biological compatibility. Uses of sealant films include, but
are not
limited to, prevention of adhesion formation following tendon surgery (i.e.,
use as a
25 wrap around tendons), use as a synthetic tympanic membrane, and uses as
substitute
facial tissue and wound dressing components. Additional examples of potential
uses
of sealant films include, treatment of corneal abrasions, wound closure,
coating of
catheters and instruments, and use as a material to prevent adhesion formation
in
tissues and tendons (e.g., peritoneal cavity).
3o Further embodiments of the present invention include sealant and adhesive
formulations which can be used in systems specific for delivery of numerous
drugs
and pharmaceutical compositions, including growth factors, antibiotics, and
other
-22-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
s biologically beneficial compounds. Such materials can be added to the
collagen
adhesive or sealant to promote cell migration, cell adhesion, and wound
healing.
Angioplasty and Angiography. Angiography is a diagnostic procedure
whereby dye is injected into an artery, preferably the femoral artery, to
detect the
presence or absence of coronary disease. Angioplasty, also known as PCTA, is a
1o therapeutic procedure which involves the inflation of a balloon in an
artery, such as
the coronary artery, for the purpose of relieving arterial blockages. After
puncturing
the femoral artery, a balloon-catheter is introduced through the femoral
artery and
navigated through to the coronary artery blocked by atherosclerosis (plaque).
Once in
position, the balloon is inflated and deflated several times in an effort to
open the
1 S artery by pushing the fatty material against the vessel walls, allowing
for blood to
circulate to the affected regions of the heart muscle. Various types of
balloon
catheters are commonly used in angioplasty and angiography, including over-the-
wire
catheters which utilize an independent guidewire to the site of the disease;
2) fixed-
wire catheters, which combine a balloon catheter with a guidewire into one
device; 3)
2o rapid-exchange or single-operator exchange catheters, which are over-the-
wire
catheters that can be exchanged more conveniently than standard over-the-wire
catheters; and 4) perfusion catheters, which allow blood flow during the
procedure. A
rotational tip catheter removes plaque buildup on arterial walls. These
devices utilize
a technique called differential cutting. Calcified material is rendered into
microscopic
25 particles without damaging the artery due to the elastic nature of the
arterial walls.
Angioplasty is a more invasive and complicated procedure than angiography,
requiring the insertion of a larger sheath than that used in angiography. The
sheath is
used as a vehicle for introducing the catheter into the artery. Additionally,
angioplasty also requires the use of blood thinners, such as heparin, to
prevent clotting
3o during and after the surgical procedure. The anti-clotting agent prevents
the body's
natural sealing/clotting mechanism and, thus, sealing punctures requires a
significant
length of time.
-23-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
According to the present invention, after withdrawing the catheter and other
invasive devices from the artery, an adhesive applicator may optionally be
inserted
into the sheath and placed into a position near to or contacting the puncture
in the
artery. During the procedure, manual or mechanical pressure is applied to the
artery
to reduce the flow of blood at the puncture site. If possible, excess
blood/fluid is
to removed from the puncture site. Subsequently, recombinant collagen type III
and/or
type I monomer of the present invention may be applied to the puncture on the
external surface of the artery and/or within the puncture track. The monomer
then is
polymerized and/or cross-linked by the techniques described herein, for
example, UV
irradiation, such that polymerization takes place within 0 to 300 seconds,
preferably
within 0 to 120 seconds, more preferably within 0 to 30 seconds, and most
preferably
3 to 10 seconds. By applying the collagen monomer composition on the outside
of
the artery, the incidence of embolism (blockage of the artery or circulatory
system) is
virtually eliminated. Alternatively, polymerization may be achieved according
to the
methods set forth in PCT WO 97/29715 and EP 747,066 A2, incorporated herein by
2o reference.
Alternatively, a polymerized collagen type III and/or type I may be used and
the polymerization step may be avoided. Because of the bonding strength of the
adhesive of the present invention, only small amounts of the adhesive are
required to
seal a punctured artery. Moreover, because the surgical adhesive according to
the
present invention can polymerize almost immediately, the adhesive can
polymerize on
the surface and/or along the puncture track of the artery without penetrating
the
interior of the artery. Accordingly, large pieces or particles of material
will not enter
the circulatory system, thereby substantially reducing risk of embolism. Due
to the
fast and strong bonding of preferred adhesives of the invention, the patient
will need
3o to be immobilized for only a minimal period of time.
Administration
Formulations. The tissue treatment composition of the present invention may
be presented in the same type of preparations as prior art fibrin sealants.
The
-24-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
components may be provided in deep frozen solution form or as lyophilized
powders,
to be diluted prior to use with appropriate aqueous solutions, e.g. containing
aprotinin
and calcium ions, respectively. Additionally, the vascular sealants of the
present
invention may be formulated and shaped in the form of collagen plugs, as
described in
the art and known to one of ordinary skill in the art.
to The compositions of the present invention can additionally comprise
pharmaceutical agents, such as, for example, an antibiotic or a growth factor,
by
incorporating the agent into the tissue adhesive so as to be enclosed in the
collagen
network formed upon application of the tissue adhesive. The agent is thus kept
at the
site of application while being controllably released from the composition,
such as
15 when the composition is used as ocular drops, or a wound healing
preparation, ete. As
also mentioned above, the pharmaceutically active substance to be released
from the
present tissue adhesive composition may be the viscosity-enhancing polymer in
itself
or a substance coupled thereto. A specific example of such a viscosity-
enhancing
polymer fulfilling the viscosity enhancing requirement as well as having
therapeutical
20 and pharmaceutical utility, and in which it may be desired to sustain
bioavailability, is
hyaluronic acid and salts and derivatives thereof which are easily soluble in
water and
have an extremely short biological half life. Thus, in one aspect,
compositions of the
present invention constitute an advantageous slow-release preparation for
proteoglycans such as hyaluronic acid and its salts and derivatives, which
25 considerably increases the bioavailability thereof.
Notably, the compositions of the present invention are not restricted to those
having adhesive properties. Non-adhesive compositions are also included,
especially
when these compositions are primarily intended for wound healing. These
compositions may in particular include non-adhesive proteins such as albumin
and/or
3o growth factors. Substantially non-adhesive compositions may also be
obtained when
the polymer part of the composition inhibits the adhesive properties of the
protein
part. It should in this context be emphasized that the invention comprises
both
-25-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
adhesive and substantially non-adhesive compositions, although it has for
simplicity
reasons often has been referred to as an "adhesive" in this specification.
Application Of Compositions. The compositions of the present invention may
be applied using a variety of dispensing devices. For example, the surgical
adhesive
may be applied using the devices set forth in U.S. Patent Nos. 4,900,303
(Lemelson)
1o and 5,372,585 (Tiesenbrun) while monitoring the application process through
an
optical viewing system. The composition of the present invention may also be
applied by the devices set forth in U.S. Patent No. 5,129,$82 (Weldon et al.),
or the
other devices referenced above, or other devices as well known in the art.
Compositions according to the present invention may also be applied in
~5 conjunction with other sealing means. For example, adhesive compositions
may be
applied to puncture sites which have been closed using surgical suture or
tape, such as
in the sealing of a puncture or incision in vascular tissues, including the
heart. The
adhesive in this instance will provide a complete seal, thereby reducing the
risk of
body fluid leakage from the organ or vessel, e.g., leakage from artery
puncture sites.
2o The surgical adhesive of the present invention may additionally be used in
conjunction with other sealing means, such as plugs, and the like. Such
techniques are
set forth in, for example, U.S. Patent Nos. 4,852,568 (Kensey), 4,890,612
(Kensey),
5,053,046 (Janese), 5,061,274 (Kensey), 5,108,421 (Fowler), 4,832,688 (Sagae
et al),
5,192,300 (Fowler), 5,222,974 (Kensey et al.), 5,275,616 (Fowler), 5,282,827
25 (Kensey et al.), 5,292,332 (Lee), 5,324,306 (Makower et al.), 5,370,660
(Weinstein et
al.), and 5,021,059 (Kensey et al.). The subject matter of these patents is
incorporated
herein by reference.
Notably, the compositions of this invention can be used to join together two
surfaces by applying the particular composition to at least one of the
surfaces.
30 Depending on the particular requirements of the user, the adhesive
compositions of
this invention can be applied by known means, such as with, for example, a
glass
stirring rod, sterile brush, or medicine dropper, in many situations,, a
pressurized
aerosol dispensing package is preferred in which the adhesive composition is
in
-26-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCTNS99/18095
solution with a compatible anhydrous propellant. Aerosol application of the
monomers is particularly advantageous for use in hemostasis. Mechanisms for
aerosol applications axe well known in the art.
EXAMPLES
to The following examples are provided solely to illustrate the claimed
invention,
and are not intended to limit the scope of the invention.
Purification of recombinant collagen type III from yeast expression system.
The following protocol was used to purify recombinant human collagen type
III ("rhc III" or "Rhc III") from Pichia.
1. Resuspend 1 volume of cell pellets with 7 volume O.1N HCL;
2. Fill Bead-Beater chamber half full with glass bead just taken from -
20C freezer;
3. Fill the chamber with cell suspension;
4. Assemble the chamber with Ice-water Jacket;
5. Fill out the jacket with ice water with some sodium chloride;
6. Homogenize the cell pellets for 5 X 1 mins with 5 min. of interval
between each 1 min. homogenization;
7. Recover the homogenate by filter through a Buchner Funnel without
filter paper;
8. Add pepsin solution to final concentration of 0.2 mg/ml and incubate
for 8 hours at 4°C;
9. Centrifuge for 30 min. at 10,000 rpm and collect the supernant (fraction
S 1 ) and pellet (fraction P 1 );
10. Adjust the pH to 7.4 with l OM NaOH, Incubate overnight at 4°C;
11. Add SM NaCI and HAC to 1M NaCI, O.SM HAC;
12. Incubate at 4°C for 1 hour;
13. Collect the pellets by centrifugation (fraction P2);
-27-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
14. Dissolve the pellet in 3 volume O.1M HCl (depending on the collagen
amount, adjust the collagen concentration to about 0.3mg/ml);
1 S. Add 1.5 volume of 3M urea, 0.3M NaCI, O.15M Tris, pH 7.4 and adjust
the pH to 7.4 with NaOH;
16. Run through DEAF-cellulose column (1.6 X 15 cm) at a flow rate of 0.1-
0.2m1/min;
17. Collect the flowthrough;
18. Concentrate the collagen by precipitation in 1M NaCI, O.SM HAC;
19. Redissolve the pellet in l OmM HCl (fraction rhcIII);
20. Dialyze the collagen solution against l OmM HCl if necessary;
Characterization of recombinant human collagen type III
As defined above, purified rhc III was tested by SDS-PAGE as shown in Figure
1 and amino acid analysis was performed and amino acid composition of purified
rhc III
is shown as below at TABLE 1.
TABLE 1
Amino Acid Composition of rhc III Purified from Plcltla PastOris
Amino acid ~ RhC III from Picl:ia PastorisHuman Collagen III
Asp 46 42
Glu 68 71
Hyp 132 125
Ser 36 39
Gly 349 350
His - - 6
~'g 42 _. 46
T~ 21 13
-28-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/I8095
Ala 92 g(
Pro 1 OS 107
Val 14 14
Met 7 - g
TYr - 3
Ile 14 13
Leu 20 22
Hyl ~ _
Lys 44 _ 30
Phe 9 _ 8
Hyp/(Hyp+Pro) 0.557 0.538
Biocompatibility and Tissue Response Tests
Biocompatibility and tissue response of rhc III and a commeric was tested in a
rat subcutaneous model. Rhc III and commercial available Collagen Hemostat
were
to formulated into injectable paste/gel under sterile condition. The rhc III
samples was
tested to insure the endotoxin level is below the limit. The gel was injected
subcutaneously into rats. The preliminary data indicates that Rhc III does not
cause
any erythema and edema. The gel was dissected in day 2, 7 and 28 after
injection and
examined histologically with H&E staining. The commercial available collagen
hemostat has a much stronger tissue response than rhc. A comparison of rhc III
with
the commercial available collagen hemostat harvested from rats on day 7 is
shown in
Figure 2.
2o Platelet Aggregation Test
Methods. The fibrillogenesis of above described recombinant human collagen
type III ("rchIII") was tested according to the following method: First rhc
III solution
was diluted with l OmM HCl to 1 mg/ml, next, 1/10 volume of 200mM Na2HP04, pH
-29-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
11.2 was added. The solution was then mixed well and incubated at room
temperature
(20-22° C) overnight. Following incubation, the fibril slurry was
vortexed before
usage.
Human Platelet Rich Plasma (PRP) was then prepared from fresh blood of an
apparently healthy donor. The PRP was then adjusted to 200K/pl and the
collagen
fibril slurry was added into the PRP. Following addition of the fibril slurry,
the
platelet aggregation profile with an aggregometer was detected. The minimal
amount
of collagen to induce complete platelet aggregation is estimated from the
amount of
collagen to induce a fall in optical density of at least 30% occurred within 5
minutes.
Experimental Results. The platelet aggregation capacity of recombinant
human collagen III was compared to the platelet aggregation capacity of bovine
skin
derived collagen according to the methods set forth above and as more fully
described
in Balleisen, et al., 1975, Klin. Wschr x:903-905, incorporated herein by
reference.
As set forth below in TABLE 2, fibrils generated from recombinant human
collagen
III has a lower minimal amount in inducing human platelet aggregation than
fibrils
2o generated from bovine skin collagen. Recombinant human collagen III also
has a
shorter onset time to induce platelet aggregation. These results indicate that
recombinant human collagen is a more hemostatic than tissue derived collagen.
TABLE 2
Collagen samples Minimal Amount for Time to Onset (Second)
induction of platelet
aggregation (fig)
Bovine Skin Collagen 10 48
Recombinant human <5 36
Collagen III from
Pichia
In a subsequent experiment, platelets were obtained from three healthy donors
and were adjusted to 200K/ml in plasma. Collagen fibril slurry was added to
the
platelet suspension and the aggregation was measured with an Aggregometer. The
minimal amount of collagen capable of inducing complete platelet aggregation
was
-30-
SUBSTITUTE SHEET (RULE 26j
CA 02339575 2001-02-05
WO 00/09018 PCTNS99/I8095
s determined by stepwise decreasing the amount of collagen added. It was
assumed
that complete aggregation had occurred when a fall in OD of at least 30%
occurred
within 5 minutes. It was shown that rhc III has a lower minimal amount to
induce
complete aggregation of platelet, and indicates that rhc III is more
hemostatic than
bovine collagen I. This results depicted in Figure 1 demonstrate that collagen
III
to fibrils are more hemostatic than collagen I fibrils.
Hemostatic Effects of Rhc III Sponge
Rhc III was prepared as follows:
Rhc III was expressed in pichia pastoris. A selected expression clone was
15 cultivated in a bioreactor under defined conditions. Rhe III was purified
from the
harvested cell pellets by limited pepsin digestion and differential salt
precipitation.
Purified rhc III was formulated into fibrils at first by neutralization with
phosphate
buffer and incubation at room temperature overnight. The fibrils were
collected
by centrifugation and then resuspended in water. After homogenization, the rhc
2o III gel was transferred into a mould and lyophilized into a sponge. This
type of
sponge has very poor water absorption.
Water absorptive capacity is critical for applications of rhc III as a
hemostat
and vascular sealant. To ensure the compositions of the present invention
could
satisfy this requirement, a process to formulate water absorptive rhc III
sponge
25 was developed. Essentially, a sponge was cross-linked, first with UV
irradiation
and then with 1 % WSC. The residual cross-linking reagent was removed by
incubation in PBS and the sponge was washed with water. Cross-linked sponges
are lyophilized for animal testing and formulation of tissue sealant. This
process
not only enhanced the water absorption of rhc III sponge, but also
significantly
3o increased the mechanical strength of rhc III sponges. In a control study,
bovine
collagen I was also formulated into sponges following the same procedures.
Acclimatized New Zealand White Rabbits were deeply anesthetized and
laparotomies were performed to expose the spleens. Using a scapel blade,
uniform
-31-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
s incisions about 1.0 cm long and 0.3cm deep were made into the spleens. The
incisions were then treated with rhc III sponges or with bovine collagen I
sponges.
The time interval from the application of the test article or positive control
material until the bleeding ceases was recorded. Statistical analysis of the
mean
time to hemostasis was performed using Anova and Student's two sample T-test.
It was observed that the bleeding time of spleens treated with rhc III sponges
was
significantly shorter than that of those treated with bovine collagen I. The
results
are shown in Figure 4.
Formulation of RhC III Sealant
To prepare a rhc III sealant, a three-step experimental approach was pursued
involving: 1 ) preparing a cross-linked rhc III sponge; 2) coating the sponge
with
human thrombin; and 3) subsequently coating the sponge with human fibrinogen.
A collagen sponge cross-linked with water soluble carbodiimide was
rinsed with 100% ethanol and placed in a filtration funnel. Human thrombin
suspended in ethanol (25U/ml) was used to coat the sponge by filtration. The
2o amount of thrombin on the sponge was about 1 OU/crnz. Human fibrinogen
dissolved in water at concentration of 4mg/ml was precipitated by mixing with
3
volume of ethanol. The precipitated fibrinogen was filtered through the sponge
by
vacuum. The amount of fibrinogen on the sponge was about 3mg/ cm'' . The
coated sponge was lyophilized and used for animal tests.
Acclimatized New Zealand White Rabbits were deeply anesthetized and
laparotomies were performed to expose kidneys and spleens. Using a scapel
blade,
incisions about 1.0 cm long and 0.3cm deep were made into the kidneys and/or
spleens. The incision was treated with commercial collagen sponge INSTAT or
rhc III sealant. The time interval from the application of the test article
until the
bleeding ceased was recorded. The adhesive capacity of testing articles was
estimated by peeling the articles from the test site after hemostasis had been
achieved. The results are shown in Table 3 and Table 4.
-32-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
TABLE 3
Hemostatic effect and adhesiveness of rhc III Sealant
in Kidney Injury Model
_Non-treatmentInstat '" lRhc III Sealant
Animal Numbers 7 4 4
Bleeding Time 384 +/- 169 67 +/-10 <I0 seconds*
( Seconds)
Adhesiveness** ND +
meeamg s~oppea mstanuy upon appucat~on of the test articles
** + weak, ++ medium, +++ strong.
TABLE 4
Hemostatic Effect and adhesiveness of rhc III Sealant
in Spleen Injury Model
Non-treatment Instat ~' Rhc III Sealant
Animal Numbers 7 3 4
Bleeding Time 30.85+/- 9.62 4.27+/-0.47 <IO seconds*
(minutes) (minutes)
Adhesiveness** ND -+ +-f-
n~~~u~~~g ~wppCU ms~anuy upon appucanon of tree test articles
** + weak, ++ medium, +++ strong.
The results of this study demonstrated that rhc III fibrils are able to induce
human platelet aggregation at a lower concentration than bovine collagen I,
indicating
that an Rhc III sponge stops bleeding in spleen injury models within a shorter
time
period than bovine collagen sponges. A prototype of rhc III enhanced fibrin
sealant
showed a superior hemostatic potential in vivo over commercial collagen
sponges.
Cross-linking Experiments
Bovine Collagen Type I
A feasibility cross-linking test was conducted with soluble bovine collagen I.
Bovine collagen I in l OmM HCl was neutralized with 1/10 volume of 0.2M
Na2HP04, pH I 1.2 and incubated overnight at room temperature. The fibrils
were washed with water and concentrated to 50 mg/ml by centrifugation. The
-33-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
homogenized gel was transferred to a small cell culture dish and lyophilized
to
form a sponge.
To cross-link the collagen, the bovine collagen sponge was incubated
in a solution of water soluble carbodiimide ("WSC") at room temperature
overnight,
then washed with PBS and water. The cross-linked sponge was then dried and
tested
1o for solubility and water absorption, and compared to a commercially
available
collagen sponge (collagen sponge for Angioseal T""). The data is shown in
Table 5.
TABLE 5
Samples Color Solubility in Water AbsorptionWetting
l OmM (mg/mg) Time
HCl (seconds)
of Control
Collagen White 1.9% 1 g.2
Sponge
for
Angioseal
TM
WSC Treated
Vitrogen
Sponge
0% White 100% 14.5 >120
0.1 % White 11.7% 18.3 >120
0.25% White 2.5% 19.3 100
0.5% White 0% 19.0 40
1 % White 0% 18.3 22
2% White 0% 19.8 18
As shown in Table 5 and Figure 5, the cross-linked sponges appeared to be
intact after incubation in water for 24 hours at room temperature, while the
non-
cross-linked collagen sponges were not intact. Using one's fingers,the cross-
linked
collagen sponges were split to test their mechanical strength. The collagen
sponges
2o cross-linked with WSC at a concentration higher than 0.5% demonstrated
reasonable
mechanical strength.
-34-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
Recombinant Collagen Type III
Rhc III collagen fibrils were prepared by neutralizing rhc III in l OmM HCl
with 1/10 volume of 0.2M Na2HP04, pH 11.2, and incubated overnight at room
temperature. Rhc III fibrils were harvested by centrifugation and washed with
water.
The fibrils were concentrated to 60 mg/ml and homogenized into a gel. The gel
was
1o transferred into a mold and lyophilized into a sponge with a thickness of
about
2.Smm.
The collagen sponge was cross-linked with 1 % WSC in 25% ethanol at room
temperature for 16 hours. Ethanol was added to accelerate the wetting of
collagen
sponges in the reaction solution. The cross-linked sponge was then washed with
PBS
for 2 hours, and subsequently washed three times with water. After
lyophilization,
collagen sponges of about 1.4 cmz in size were used to test for water
reabsorption.
In addition, 2 mg of the collagen sponge was used to test for solubility. As
shown in
Table 6, cross-linking of the rhc III sponge improved its water absorptive
capacity.
The wetting time of the cross-linked rhcIII sponge was reduced to about 10
seconds,
2o and water uptake increased from 76mg to 433mg. The cross-linked rhc III
sponge
remained intact after incubation in water for 24 hours at room temperature,
while the
non-cross-linked rhc III did not remain intact.
TABLE 6
Samples Color Solubility in Water Wetting
lOmM
HCl Absorption Time
mg
(% of Control) in 90 seconds(seconds)
Collagen SpongeWhite 1.9% 450 $
for TM Angioseal
Rhc III SpongeWhite 100% 76 >120
Cross-linked White 5.1 % 433 10
rhc
III sponge
The collagen sponges were also tested with SDS-PAGE. As shown in
Figure 6, lane 1 and lane 6 represent rhc III; lane 2 represents the collagen
sponge for
-35-
SUBSTITUTE SHEET (RULE 26)
CA 02339575 2001-02-05
WO 00/09018 PCT/US99/18095
AngiosealT"'; lane 3 represents cross-linked bovine collagen I sponge; lane 4
represents cross-linked rhc III sponge; and lane 5 represents non-cross-linked
rhc III
sponge. The collagen sponge AngiosealT"", cross-linked bovine collagen I
sponge,
and cross-linked rhc III sponge is not soluble in SDS-PAGE buffer.
Various modifications and variations of the described methods and systems of
l0 the invention will be apparent to those skilled in the art without
departing from the
scope and spirit of the invention. Although the invention has been described
in
connection with specific preferred embodiments, it should be understood that
the
invention as claimed should not be unduly limited to such specific
embodiments.
Indeed, various modifications of the described modes for carrying out the
invention
15 which are obvious to those skilled in molecular biology or related fields
are intended
to be within the scope of the following claims. All references cited herein
are
incorporated by reference herein in their entirety.
-36-
SUBSTITUTE SHEET (RUL.E 26)