Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02339638 2001-02-05
13-07-2000 EP 009905459
1
MONOCYCLIC COMPOUNDS HAVING NK-2 ANTAGONIST ACTION AND
COMPOSITIONS CONTAINING THEM.
Field of the invention
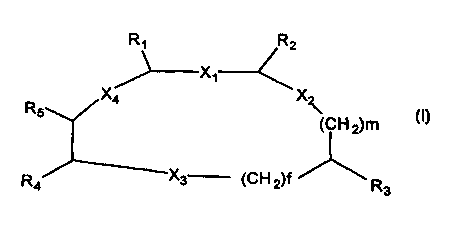
The present invention refers to compound of general formula (I)
s Ar Ar
f I
(CH2)r. (CH2)r
I I
X4 - CH - X 1 - CH
/ ~\
to CH2 X2
\ \
(CH2)m
R4 - CH - X3 - (CH2)f - CH
1
is (CH2)r
\ (I)
Ar1
wherein:
X1, X2, X3, Xq., same or different, are a group chosen among: -CONR-, -NRCO-,
-CH2-NR-, -NR-CH2- where R is H , C1_3 alkyl, benzyl;
2o f, m, same or different, are a number chosen among 0,1 and 2;
R1 and R2, same or different, are a group:
-(CH2)r -Ar where r = 0, 1, 2 and Ar is an aromatic group chosen among:
benzene, naphthalene, thiophene, benzothiophene, pyridine, quinoline, indole,
furan, benzofuran, thiazole, benzothiazole, imidazole, benzoimidazole,
possibly
2s substituted with up to 2 substituents chosen among C1_3 alkyl, haloalkyl,
C1-3
alkyloxy, C2..4 amino-alkyloxy, halogens, OH, NH2, CN, NRgR7, where Rg ed R7,
same or different, are H or C1_3 alkyl,
R3 is a group chosen among the following groups:
- (CH2)~Ar1 where r = 0, 1, 2 and Ar1 is an aromatic group chosen among:
3o benzene, naphtalene, thiophene, benzothiophene, pyridine, quinoline,
indole,
furan, benzofuran, thiazole, benzothiazole, imidazole, benzoimidazole,
possibly
substituted with up to 2 grog AMENDED SHEET C1-3 alkyl and haloalkyl, C1-3
CA 02339638 2001-02-05
13-07-2000 EP 009905459
la
alkyloxy and amino-alkyoxy, halogens, OH, NH2, NRgR7, where R6 and R7,
same or different, are H or C~_3 alkyl,
R4 is a group chosen among:
AMENDED SHEET
CA 02339638 2001-02-05
13-07-2000 EP 009905459
2
- NRgRg, where Rg is H or C1 _g alkyl and
Rg is
(i) a methanesulfonyl, tosyl, tetrahydropyranyl,
(ii) tetrahydrothiopyranyl possibly mono or di-substituted by oxygen on the S
s atom,
(iii) piperidyl possibly substituted on the N-atom by a C1_3 alkyl, C1-3 acyl,
aminosulfonyl, methanesulfonyl;
(iv) a group (CH2)g-R10 where g is 1,2,3 and R1p is chosen among
morpholine, furan, CN;
io or Rg and R9 together with the N atom to which they are linked form a
piperazine
possibly substituted on one of its nitrogen by C1-3 alkyl, C1-3 acyl or
methanesulfonyl;
- N(R11)CO(CH2)h-R12 where R11 is H, C1_3 alkyl; h is 0,1,2,3; and R12 is
chosen among: morpholine, pyrrolidine possibly substituted with an hydroxy or
an
is hydroxymethyl, piperidine possibly substituted with a group hydroxy,
carboxyamido or aminosulfonyl, piperazine possibly substituted on the N-atom
by
C1-3 alkyl, triazole, tetrazole, 5-mercapto-tetrazole, furan, thiophene,
thiomorpholine possibly mono or di-oxygenated on the S-atom, amino-
cyclohexane possibly substituted by an hydroxy group.
Zo -COR,3 wherein R,3 is morpholine or piperazine possibly substituted with a
C2~
alkyl containing one or more ether or hydroxy groups.
Since compounds of formula (I) present various chiral centers the present
invention obviously refers also to the single enantiomers and to the
diastereoisomers mixtures.
2s State of the art
The NK2 receptor of tachykinins is widely present in the peripheral nervous
system in mammals. One of the various effects of the selective stimulation of
the
NK2 receptor is the contraction of smooth muscles. Therefore the antagonists
of
the NK2 receptor are agents capable of controlling the excessive contraction
of
3o smooth muscles in all those pathologic condition where the release of
tachykinins
AMENDED SHEET
CA 02339638 2001-02-05
13-07-2000 EP 009905459
2a
contributes to the genesis of the corresponding pathological disorder.
More particularly the broncospastic component of asthma, cough, pulmonary
irritations, intestinal spasms or local spasms in bladder and ureter in the
case of
cystitis, infections and kidney colics can be considered conditions where the
s administration of NK2 antagonists is appropriated (E.M. Kudlacz et al. Eur.
J.
AMENDED SHEET
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
3
Pharmacol., 1993 36, 17-25).
Cyclic compounds, in particular cyclic hexapeptides, cyclic (A.T. McKnight et
al.
Br. J. Pharmacol. 1991, 104, 355 ) and bicyclic (V. Pavone et al. WO
93/212227),
or cyclic pseudopeptides (L. Quartara et al. J. Med. Chem., 1994, 37, 3630; S.
L.
s Harbeson et al. Peptides, Chemistry and Biology. Proceedings of Twelth
American
Peptide Symposium, 1992, 124) are known in literature for their strong
antagonistic activity on the NK-2 receptor of tachykinins.
In W09834949 it is described how compounds having lower molecular weight,
monoyclic, containing only four bi-functional residues linked among each other
by
io a peptide or pseudopeptide bond present pharmacological activity similar or
higher than that of known compounds and moreover show a high selectivity for
the
human NK2 receptor.
It is an object of the present invention to make available new monocyclic
compounds having four bi-functional residues and presenting new substituents
not
is described in W098/34949. These compounds are new interesting powerful
antagonists to NK2 receptor and therefore are useful for the treatment of
pathologies connected with such interaction moreover they show an in vitro and
in
vivo activity largely higher than that shown by the most similar compounds
described in W098/34949.
2o Detailed description of the invention
The present invention makes available new monocyclic compounds of general
formula (I) as above defined containing four residues linked to each other by
a
peptide or pseudopetide bond having an antagonistic action on the NK2
receptor.
The present invention refers also to the pharmaceutically acceptable salts of
the
2s above said compounds, to processes for their preparation and to
pharmaceutical
compositions containing them.
Since the compounds of formula (I) present chiral centers the present
invention
refers also to the corresponding enatiomers and the mixture of
diastereoisomers.
Preferred compounds according to the present invention are those wherein in
3o formula (I):
fist
m is 0
CA 02339638 2001-02-05
13-07-2000 EP 009905459
X1, X2, X3, Xq., same or different are a group -CONR- and -NRCO-,
R is H or methyl
R1 and R2 same or different, are::
-(CH2)-Ar wherein Ar is an aromatic group chosen among benzene, pyridine,
s indole, possibly substituted up to two residues with substituents chosen
among:
C1_3 alkyl and haloalkyl, C1_3 alkyloxy, C2~ amino alkyloxy, halogens, OH,
NH2,
CN, NR6R7, where R6 and R7, same or different, are H or C1_3 alkyl;
R3 is a group chosen among:
- CH2-Ar1 wherein Ar1 is an aromatic group chosen among: alfa naphthyl, beta
io naphthyl, phenyl, phenyl substituted up to two residues chosen among C~_3
alkyl
and haloalkyl, C1_3 alkyloxy, halogens, OH, NH2,
R4 is a group chosen among:
- NRgRg, where Rg is H or C1_3 alkyl and
Rg is chosen among: methanesulfonyl, tosyl, tetrahydropyranyl,
is tetrahydrothiopyranyl possibly mono or di-substituted by oxygen on the S
atom,
piperidyl possibly substituted on the N-atom by a C1_3 alkyl, C1-3 acyl,
aminosulfonyl, methanesulfonyl; or a group (CH2)g-R1p where g is 1,2,3 and R10
is chosen among morpholine, furan, CN;
or Rg and Rg together with the N atom to which they are linked form a
piperazine
zo possibly substituted on the N atom with a C1-3alkyl, C1-3 acyl or
methanesulfonyl;
- N(R11)CO(CH2)h-R12 where R11 is H, C1_3 alkyl; h is 0,1,2,3; and R12 is
chosen among: morpholine, pyrrolidine possibly substituted with an hydroxy or
hydroxymethyl, piperidine possibly substituted with a group hydroxy,
carboxyamido or aminosulfonyl, piperazine possibly substituted on the N-atom
by
2s C1_3 alkyl, triazole, tetrazole, 5-mercapto-tetrazole, furan, thiophene,
thiomorpholine possibly mono or di-oxygenated on the S-atom, amino- cylohexane
possibly substituted by an hydroxy group.
- COR13 wherein R13 is a group chosen among morpholine and piperazine
possibly substituted by a C2-g alkyl containing one or more ether or hydroxy
AMENDED SHEET
CA 02339638 2001-02-05
13-07-2000 EP 009905459
groups.
More preferred are the compounds of formula (I) wherein:
-X1, X2, X3, X4 are -CONR-,
-Rises;
s - R1 is the lateral chain of triptophane;
- R2 is the lateral chain of phenylalanine possibly substituted with up to two
residues chosen among: chlorine, fluorine, CF3, OH, CN i or a group 3-pyridyl-
methyl, 4-pyridyl-methyl;
- R3 is benzyl.
io and the other substituents are as above defined.
An even more preferred group of compounds according to the invention are those
wherein R, R1, R2, R3, f, m are as above defined and:
R4 is a group NR8R9 wherein: -
R8 is H or methyl;
is R9 is a group chosen among: : 4-tetrahydropyranyl, 4-tetraidrothiopyranyl,
1-oxo-
tetraidrothiopyran-4-yl, 1,1-dioxo-tetrahydrothiopyran-4-yl, N-methyl-4-
piperidinyl,
N-methansulfonyl-4-piperidinyl, N-aminosulfonyl-4-piperidinyl,
or R8 and R9 together with the N atom to which they are linked represent: N
methyl-piperazinyl, N-acetyl-piperazinyl, piperazinyl, N-methanesulfonyl
2o piperazinyl.
Among this last group of compounds the following are especially preferred:
i) cyclo{Suc[1-(R}-(4-tetrahydropyranyl)amino]-Trp-Phe-[(R}-NH-CH(CH2-CgHS)-
CH2NH]}
ii) cyclo{Suc[1-(S)-(4-tetrahydropyranyl)amino]-Trp-Phe-[(R)-NH-CH(CH2-CgHS}-
2s CH2NH]}
iii) cyclo{Suc[1-(R)-(1-methyl-piperidin-4-yl)amino]-Trp-Phe-[(R)-NH-CH(CH2-
CgHS)-CH2NH]}
iv) cyclo{Suc[1-(R~(4-tetrahydrothiopyranyl)amino]-Trp-Phe-[(R~NH-CH(CH2-
CgH5~CH2NH]}
3o v) cyclo{Suc[1-(R)-(1-oxo-tetrahydrothiopyran-4-yl)amino]-Trp-Phe-[(RrNH-
CH(CH2-CgH5)-CH2NH]}
AMENDED SHEET
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
6
vi) cyclo{Suc[1-(R)-(1,1-dioxo- tetrahydrothiopyran-4-yl)amino]-Trp-Phe-[(R~NH-
CH(CH2-CgHS)-CH2NH]}
vii) cyclo{Suc[1-(R)-N-methyl-N-(4-tetrahydropyranyl)amino]-Trp-Phe-[(R~NH-
CH(CH2-CgHS)-CH2NH]}
s viii) cyclo{Suc[1-(R}-(4-tetrahydropyranyl)amino]-Trp-Tyr-[(R)-NH-CH(CH2-
CgHS)-
CH2NH]}
ix) cyclo{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Phe(4-F)-[(R)-NH-CH(CH2-
CgHS)-CH2NH]}
x) cyclo{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Phe(3,5-F)-[(R)-NH-CH(CH2-
to CgH5~CH2NH]}
xi) cyclo{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Phe(4-CN)-[(R~NH-CH(CH2-
CgH5~CH2NH]}
xii) cycio{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Phe(4-CF3)-[(R)-NH-CH
(CH2-CgHS)-CHZNH]}
is xiii) cyclo{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Ala(4-pyridyl)-[R)-NH-
CH(CH2-CgHS)-CH2NH]}
xiv) cyclo{Suc[1-(R~{4-tetrahydropyranyl)amino]-Trp-Ala(3-pyridyl)-[R)-NH-
CH(CH2-CgHS)-CH2NH]}
xv) cyclo{Suc[1-(R)-(1-methylsulfonyl-piperidin-4-yl)amino]-Trp-Phe-[(R~NH-
2o CH(CH2-C6H5)-CH2NH]}
xvi) cyclo{Suc[1-(R)-(1-aminosulfonyl-piperidin-4-yl)amino]-Trp-Phe-[(R)-NH-
CH(CH2-CgH5~CH2NH]}
xvii) cyclo{Suc[1-(R)-piperazin-1-yl]-Trp-Phe-[(R)-NH-CH(CH2-CgH5~CH2NH]}
xviii) cyclo{Suc[1-(R)-4-methyl-piperazin-1-yl]-Trp-Phe-[(R)-NH-CH(CH2-CgHS)-
2s CH2NH]}
xix) cyclo{Suc[1-(R)-4-acetyl-piperazin-1-yl]-Trp-Phe-[(R)-NH-CH(CH2-CgHS)-
CH2NH]}
xx) cyclo{Suc[1-(R)-4-methanesulfonyl-piperazin-1-yl]-Trp-Phe-[(R)-NH-CH(CH2-
CgHS}-CH2NH]}
CA 02339638 2001-02-05
13-07-2000 E P 009905459
Among the compounds of formula (I) wherein R, R1, R2, R3, f, m are as
hereabove defined preferred are also those wherein:
R4 represents a group NRgRg, where Rg is H and R9 is chosen among:
methanesulfonyl, tosyl, a group (CH2)g-R1 p wherein g is 1, 2 and R1 p is
chosen
s among: morpholine, furan, CN.
Among this last group of compounds particularly preferred are:
xxi) cyclo{Suc[1-(S)-4-methanesulfonylamino]-Trp-Phe-[(R)-NH-CH(CH2-CgHS)-
CH2NH]}
xxii) cyclo{Suc[1-(R)-4-methanesulfonylamino]-Trp-Phe-[(R)-NH-CH(CH2-CgH5)-
io CH2NH]}
xxiii) cyclo{Suc[1-(S)- (4-methylphenyl)sulfonylamino]-Trp-Phe-[(R)-NH-CH(CH2-
CgHS)-CH2NH]}
xxiv) cyclo{Suc[1-(R)- (4-methylphenyl)sulfonylamino]-Trp-Phe-[(R)-NH-CH(CH2-
CgHS)-CH2NH]}
is xxv) cyclo{Suc[1-(S)-2-(4-morpholino)ethylamino]-Trp-Phe-[(R)-NH-CH(CH2-
CgH5}-CH2NH]}
xxvi) cyclo{Suc[1-(R~2-(4- morpholino)ethylamino]-Trp-Phe-[(R)-NH-CH(CH2-
C6H5)-CH2NH]}
xxvii) cyclo{Suc[1-(R)-(2-furyl)methylamino]-Trp-Phe-[(R)-NH-CH(CH2-CgHS)_
2o CH2NH]}
xxviii) cyclo{Suc[1-(R)-cianomethylamino]-Trp-Phe-[(R)-NH-CH(CH2-CgHS)-
CH2NH]}
Another preferred selection of the compound of formula (I) wherein R, R1, R2,
R3,
f, rn are as previously defined, those wherein:
2s R4 represents a group - N(R11 )CO(CH2)h-R12 wherein R11 is H, h is 0 or 1,
and R12 is chosen among. : 1-tetrazolyl, 5-mercapto-tetrazol-1-yl, 1
triazolyl,
furanyl, thiophenyl, morpholine, 4-hydroxy-piperidine, 4-carboxyamido-
piperidine,
3-hydroxy-pyrrolidine, 2-hydroxymethylpyrrolidine, 4-methyl-piperazine, 4-
aminosulfonyl-piperazine, 1-oxo-thiomorpholine, 4-hydroxy-cyclohexan-1-yl-
amino
3o Among the compounds of this last group particularly preferred are:
AMENDED SHEET
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
8
xxix) cyclo{Suc[1-(Rr2-(4-morpholino)acetylamino]-Trp-Phe-[(R)-NH-CH(CH2-
CgHS}-CH2NH]}
xxx) cyclo{Suc[1-(S)-2-(4- morpholino)acetylamino]-Trp-Phe-[(R)-NH-CH(CH2-
CgHS)-CH2NH]}
s xxxi) cyclo{Suc[1-(S)-2-(tetrazol-1-yl)acetylamino]-Trp-Phe-[(R)-NH-CH(CH2-
CgH5rCH2NH]}
xxxii) cyclo{Suc[1-(R)-2-(tetrazol-1-yl)acetylamino)-Trp-Phe-[(R)-NH-CH(CH2-
CgH5)-CH2NH]}
xxxiii) cyclo{Suc[1-(S)-2-(5-mercapto-tetrazol-1-yl)acetylamino]-Trp-Phe-
[(R~NH-
io CH(CH2-CgHS)-CH2NH]}
xxxiv) cyclo{Suc[1-(R)-2-([1,2,4]triazol-1-yl)acetylamino]-Trp-Phe-[(R)-NH-
CH(CH2-CgH5}-CH2NH]}
xxxv) cyclo{Suc[1-(R) -(furan-2-yl)carbonylamino]-Trp-Phe-[(R)-NH-CH(CH2-
CgH5)-CH2NH]}
is xxxvi) cyclo{Suc[1-(R)-2-(thiophen-3-yl)acetyiamino]-Trp-Phe-[(R)-NH-CH(CH2-
CgHS)-CH2NH]}
xxxvii) cyclo{Suc[1-(R)-(4-morpholino)carbonylamino]-Trp-Phe-[(R)-NH-CH(CH2-
CgH5~CH2NH]}
xxxviii) cyclo{Suc[1-(R)-2-(4-hydroxy-piperidin-1-yl)acetylamino]-Trp-Phe-[(R)-
NH-
2o CH(CH2-CgH5)-CH2NH]}
xxxix) cyclo{Suc[1-(R)-2-(4-aminocarbonyl-piperidin-1-yl)acetylamino]-Trp-Phe-
[(R)-NH-CH(CH2-CgHS)-CH2NH]}
xl) cyclo{Suc[1-(R)-2-(3-hyroxy-pyrrolidin-1-yl)acetylamino]-Trp-Phe-[(R)-NH-
CH(CH2-CgHS)-CH2NH]}
2s xli) cyclo{Suc[1-(R)-2-(2-(S)-hydroxymethyl-pyrrolidin-1-yl)acetylamino]-
Trp-Phe-
[(R)-NH-CH(CH2-CgH5)-CH2NH]}
xlii) cyclo{Suc[1-(R)-2-(4-methyl-piperazin-1-yl)acetylamino]-Trp-Phe-[(R)-NH-
CH(CH2-CgHb)-CH2NH]}
xliii) cyclo{Suc[1-(R)-2-(4-methyl-piperazin-1-yl)carbonylamino]-Trp-Phe-[(R)-
NH-
3o CH(CH2-CgHS)-CH2NH]}
CA 02339638 2001-02-05
13-07-2000 EP 009905459
9=
xliv) cyclo{Suc(1-(R)-2-(4-aminosulfonyl-piperazin-1-yl)acetylaminoJ-Trp-Phe-
[(R}-
NH-CH(CH2-CgH5~CH2NH]}
xiv) cyclo{Suc[1-(R~2-(1-oxo-thiomorpholin-4.-yl)acetylamino)-Trp-Phe-[(R)-NH-
CH(CH2-CgHS}-CH2NH]}
s xlvi) cyclo{Suc[1-(Rr2-(frans-~-hydroxy-cyclohexan-1-yl-amino)acetylamino]-
Trp-
Phe-((R~NH-CH(CH2-CgHS)-CH2NH]}
Another preferred selection of compounds of formula (I) wherein R, R1, R2, R3,
f,
m are as above defined ace those wherein:
R4 is a group COR13 wherein R13 is a group chosen among: morpholine and 4-
io (hydroxyethyloxyethylrpiperazine.
Among this last group of compounds especially preferred are:
xlvii) cyclo{Suc[1-(4- morpholine)carbonyl]-Trp-Phe-[(R)-NH-CH(CH2-CgHS}-
CH2NHJ}
xlviii) cyclo{Suc[1-(4-hydroxyethyloxyethyl-piperazin-1-yl)carbonylJ-Trp-Phe-
[(R)-
~5 NH-CH(CH2-CgHS)-CH2NH]}
Phamaceuticaliy acceptable salts of compounds of formula (I) are for example
the
salts with inorganic acids (as hydrochloric, hydrobromic, hydroiodic,
sulphuric,
nitric, phosphoric) or organic acids (as acetic, propionic, succinic, malonic,
citric,
tartaric, methanesulfonic, p-toluensulfonic).
2o According to the invention the compounds of formula (I) containing peptide
or
pseudopeptide bonds can be obtained by the normal condensation reactions
according to known techniques. A general method of preparation of peptide
compounds (X1-X4 = -CONR-, -NRCO-) is for example to synthetise in a solution
the linear peptide chain using the appropriate aminoacids, carboxylic or
diamino
2s derivatives suitably protected, and after selective de-protection of the
terminal C-
and N- chains, to cyclise in polar organic solvents in a diluted solution. For
the
activation of the carboxylic group normally the methods using EDCLHCI and HOBt
or PyBOP and DiEA in DMF are preferred.
The dicarboxylic precursors containing the R4 group and the diamino precursors
3o containing the R3 group were prepared according to the methods described in
literature.
AMENDED SHEET
CA 02339638 2001-02-05
13-07-2000 E P 009905459
io
In particular in the synhesis of derivatives wherein R4 = amino or carboxylic
group,
suitably protected aspartic or carbosuccinic acid were used respectively (E.
Perrotta et al, Synlett, 1999, 144-146). The synthesis of the ethylendiamine
derivatives containing the R3 groups was performed according to G. Kokotos et
s al., J. Chem. Research (S), 1992, 391.
The compounds of formula (I) as above described are powerful antagonists of
NK2 receptor of tachykinins and can be administered as agents capable of
controlling the excessive smooth muscular contraction in whatever pathological
condition where the release of tachykinins contributes to the pathology.
io In particular the broncospastic component of asthma, cough, pulmonary
irritation,
the intestinal spasms or local spasms of bladder and ureter during cystitis,
infections and kidneys colics, can be considered conditions where the
administration of compounds of formula (I) as NK2 antagonists, can be
appropriate.
is The compounds of formula (I) object of the present invention are useful for
the
administration to superior animals and humans by parenteral, oral, by
inhalation,
sublingual administration giving pharmacological effects thanks to their
properties.
For the parenteral administration (intravenous, intramuscular and intradermal)
sterile solutions or lyophilised preparations are used.
2o For nasal, by inhalation or sublingual administration aqueous solutions,
aerosol,
powders or capsules are used as appropriate.
The quantity of active principle administerd with the above said formulations
is
normally comprised between 0.1 and 10 mg/kg of patient body weight.
Hereinafter some specific examples of compounds according to the invention are
zs reported.
EXAMPLE 1: cyclo{Suc[1-(R~(4-tetrahydropyranyl)amino]-Trp-Phe-[(R)-NH-
CH(CH2-C6H6}-CH2NH]}
(compound of general formula I wherein X1 = X2 = X3 = X4 = -CO-NH-; R1 =
-CH2-(indol-3-yl); R2 = R3 = -CH2-CgH5; R4 = (4-tetrahydropyranyl)amino; m =
0,
3o f = 1; the carbon atoms C-R1 and C-R2 have configuration S, while C-R3 and
G
R4 have configuration R).
As starting compound AMENDED SHEET ~cI1-(R}-amino]-Trp-Phe-[(R~NH-
CA 02339638 2001-02-05
13-07-2000 EP 009905459
~i
CH(CH2C6H5)-CH2-NH]-) (Compound A).
(compound of formula (I) wherein: X1 = X2 = X3 = X4 = -CO-NH-; R1 = -CH2-
(indol-3-yl); R2 = R3 = -CH2-C6H5; R4 = -NH2; m = 0, f = 1; the carbon atoms C-
R1 and C-R2 have configuration S, while C-R3 and C-R4 have configuration R) is
s used . The compound A is prepared as follow:
a) Synthesis of dipeptide Boc-Trp-Phe-OH
To a solution of H-Trp-Phe-OH ( 5 g,) in dioxane (30 ml), H20 (15 ml) and NaOH
1 M ( 15.6 ml ), cooled at 0-5°C, under stirring, of tert-
butyldicarbonate (3.4 g) was
added. The reaction mixture was left under stirring for 2 h, concentrated, and
io extracted with pentane (2 x 20 ml). The aqueous phase was cooled with ice,
added wit AcOET (50 ml), acidified with KHS04 up to pH 2-3, separated and
extracted with AcOEt (2 x 50 ml). The organic phases pooled together were
washed with brine ( 50 ml ), dried and evaporated under vacuum at 30°C,
giving 6
g of the desired compound as a white semisolid residue.
is TLC: Rf 0.55 (chlorofom~Icyclohexane/AcOH/H20 = 45145/5/5), 0.52 (CHCI3/
MeOH = 9l1 )
b) Synthesis of (R)-1-benzyl-2-(N-benzyloxycarbonylamino)ethylamina
. (R)-1-benzyl-1-(N-tert butyloxycarbonylamino)ethylamina, prepared as
described
in G. Kokotos et al., J. Chem. Research (S), 1992, 391, was transformed into
the
2o corresponding (R)-benzyl-1-(N-Pert butyloxycarbonylamino)-2
(benzyloxycarbonylamino)ethylamina and this into (R)-1-benzyl-2-(N-
benzyloxycarbonylamino)ethylamina according to the usual methods of protection
and deprotection of aminoacids.
c) Synthesis of Boc-Trp-Phe-[(R~NH-CH(CH2-C6H5)-CH2-NH-Zj
2s To a solution of Boc-Trp-Phe-OH ( 1.19 g, 2.63 mmoli ) in anhydrous DMF (10
ml)
(R~1-benzyl-2-(benzyloxycarbonylamino)ethylamine ( 750 mg), PyBOP ( 1.37 g) a
DIEA ( 0.9 ml) were added under nitrogen. The reaction mixture was left under
stirring for a night at room, added with AcOEt ( 80 ml ), washed with HCi 1 N
( 3 x
30 ml ), Na2C03 5% ( 3 x 30 ml ) and H20 ( 30 ml ). The organic phase was
3o evaporated under vacuum at 30°C, giving 1.8 g of ivory colored solid
residue.
The crude was purified by washing in a warm AcOEt suspension followed by
AMENDED SHEET
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
12
MeOH washing at room temperature giving 1.15 g of the desired compound as a
white solid. MS (TS) : [MH+] = 718
d) Synthesis of H-Trp-Phe-[(R)-NH-CH(CH2-C6H5)-CH2-NH-Z]
To a suspension of the previously obtained compound ( 1.0 g) in CH2CI2 ( 25 ml
)
s TFA (15 ml) was added under stirring at 0°C. The reaction mixture was
left under
stirring for 30 minutes at 0°C and for 2 h at room temperature, the
formation of the
precursor is checked by HPLC.
After evaporating the solvent the residue was recovered with AcOET (100 ml),
washed with NaHC03 5% (2 x 30 ml} and brine (30 ml).
to The organic phase was dried with MgS04 and evaporated under vaccum at
30°C
giving 650 mg of the desired compound.
e) Synthesis of Boc-(D)-Asp{Trp-Phe-[(R)NH-CH(CH2-C6H5)-CH2-NH-Z}-OBzI
To a solution of Boc-(D)Asp-OBzI (690 mg), HOBt (850 mg) a EDCI.HCI (450 mg}
in anhydrous DMF (50 ml) a solution of the compound of Example 1(d} (1,3 g)
was
Is added under stirring at room temperature.
The reaction mixture was left under stirring at room temeprature for 4 h.After
evaporation of the solvent (under vacuum) the residue was treated with KHS04
aq. 5% giving a solid which was filtered, washed with NaHC03 aq. 5%, water,
and thereafter dried the product was crystallized from ethanol giving 850 mg
of the
2o desired compound as a white solid.
MS (ES+): [MH+] = 923; HPLC (Method A1 ): rt = 21.1 min.
f) Synthesis of Boc-(D)-Asp{Trp-Phe-[(R)NH-CH(CHZ-C6H5}-CH2-NH2]}-OH
The compound of example 1 a (800 mg) was solubilised in DMF (10m1) and diluted
with MeOH (40 ml), thereafter hydrogenated in the presence of Pd/C 10% (100
2s mg) at room pressure and temperature for 5 h. The catalyst was filtered and
washed with MeOH. After evaporation of the solvent 500 mg of the desired
product were obtained as a white solid.
MS (ES+): [MH+J = 663; HPLC (Method A2): rt=10.4 min.
Synthesis of cyclo{-Suc[1 (R)NHBoc]-Trp-Phe-[(R)NH-CH(CH2-C6H5)-CH2-NH]}
3o To a solution of the compound according to example 1 (f) (800 mg) in
anhydrous
DMF (200 ml) 465 mg of HOBt and 224 mg of EDCLHCI were added under stirring
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
13
and in nitrogen current. The reaction mixture was left under stirring for 5 h
and
after evaporation of the solvent the residue was solved in ethyl acetate and
the
organic phase was washed with an aqueous solution of KHS04 5%, NaHC03
5% and brine, thereafter was dried and evaporated, the recovered yellow solid
s (600 mg) was crystallized in isopropanol/water: 1/1 giving 450 mg of a white
solid.
MS (ES+): [MH+] = 681; HPLC (Method A2): rt=14.7 min..
Synthesis of cyclo{Suc(1 (R)NH2]-Trp-Phe-[(R)NH-CH(CH2-C6H5)-CH2-NH]} (_
Compound A)
To a suspension of the compound of EXAMPLE 1 g (400 mg) in CH2CI2 (40 ml),
to TFA ( 13 ml ) was added at 0°C under stirring. The reaction was
carried on for 2 h
at room temperature. The solvent was evaporated and the residue treated with
NaHC03 and water and extracted in ethyl acetate. The organic phase was
washed with brine, dried and evaporated giving 320 mg of a solid product.
MS (ES+): [MH+] = 581; HPLC (Method A2): rt=12.4 min.
is A sample of 20 mg was purified by preparative HPLC giving 15 mg of
trifluoroacetate: cyclo{-Suc[1 (S)NH2]-Trp-Phe-[(R)NH-CH(CH2-C6H5)-CH2-NH]-
).TFA
MS (ES+): [MH+] = 581; HPLC (Method A2}: rt=12.4 min; 1H-NMR 500 MHz
(DMSO): d 2.21 (dd, J = 6.1, 14.3 Hz) 2.68-2.82 (m, 6H), 2.95 (dd, J = 3.0,
14.4
2o Hz, 1 H), 3.08 (bd, J = 12.0 Hz, 1 H), 3.38 (dd J = 3.8, 14.2 Hz, 1 H),
3.48-3.56 (m,
2H), 3.98-4.08 (m, 1 H), 4.11-4.17 (m, 1 H), 4.20-4.28 (1 H, m), 6.71 (d, J =
9.1 Hz,
1 H), 6.98 (t, J = 9.1 Hz, 1 H), 7.04-7.09 (m, 1 H), (m, 2H), 7.15-7.21 (m,
4H,),
7.21-7.30 (m, 6H), 7.33 (d, J = 8.1 Hz, 1 H), 7.42 (d, J = 7.8 Hz), 7.67 (bs,
1 H),
7.82 (bs, 1 H), 8.63 (d, J = 5.2, 1 H), 10.81 (d, J = 1.3 Hz, 1 H).
2s k) 50 mg of Compound A prepared as described in EXAMPLE 1 a-1 h, were
solved
in 5 ml methanol. Acetic acid (0.1 ml), tetrahydro-4H-pyran-4-one (18 mg
solved in
1 ml of methanol) and sodium cianoborohydride (12 mg) are added in the given
order. The mixture is kept for one night under stirring, acidified with HCI 1
N up to
pH=1-2, diluted with water; the methanol is evaporated, NaHC03 is added and
3o the solution is extracted with ethyl acetate, washing with brine and drying
on
sodium sulfate. The solution is concentrated and purified by preparative HPLC
(Method P1 ).
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
14
1 H-NMR (DMSO-d6, 500 MHz): d 1.57 (2H, bs); 1.90-2.04 (2H, m); 2.38-2.47 (1
H,
m); 2.67-2.98 (SH,m); 3.06-3.25 (4H, m); 3.25-3.42 (m, sovrapposto al segnale
delfacqua); 3.72 (1 H, bs); 3.82-3.95 (2H, m); 3.95-4.11 (2H, m); 4.25 (1 H,
bs);
4.33 (1 H, m); 6.86 (1 H, d, J = 8.4 Hz); 6.97- 7.03 (1 H, m); 7.04-7.31 (12H,
m);
s 7.35 (1 H, d, J = 8.1 Hz); 7.41-7.51 (1 H, bs); 7.43 (1 H, d, J = 7.9 Hz);
8.82-9.11
(3H, m); 10.85 (1 H, d, J = 1.0 Hz).
MS: mlz : 665.4 {MH+).
By similar procedure the following compounds were obtained:
EXAMPLE 2: cyclo{Suc[1-(S)-(4-tetrahydropyranyl)amino]-Trp-Phe-[(R)-NH-
io CH(CH2-C6H5)-CH2NH]}
(compound of general formula (I) wherein C-R4 has S configuartion, R4 is (4-
tetrahydropyranyl)amino and the other substituents are as described for
Compound A).
The compound is obtained according to the procedure of Example 1 but the
Is starting product is the isomer of Compound A having S configuration at the
C-R4.
HPLC (Method A2): rt = 12.8 min
MS: m/z : 665.4 (MH+).
EXAMPLE 3: cyclo{Suc[1-(R)-(1-methyl-piperidin-4-yl)amino]-Trp-Phe-[(R)-NH-
CH(CH2-C6H5)-CH2NH]}
20 (compound of general formula I wherein R4 is {1-methyl-piperidin-4-yl)amino
and
the other substituents are as described for Compound A.
The compound is prepared as in example 1 but using as reagent 1-methyl-4-
piperidone.
1 H-NMR (DMSO-d6, 500 MHz): d 1.75 (2H, bs); 2.17 (1 H, bs); 2.25 (1 H, bs);
2s 2.34-2.38 {1 H, m); 2.69-3.05 (m overlapped at bs); 2.75 (s); 3.05-3.58 (m,
overlapped to the water signal); 3.70 (1 H, bs); 3.93-4.10 (2H, bs); 4.10-4.39
(2H,
bs); 6.85 (1 H, d, J = 8.4 Hz); 7.00 (1 H, m); 7.05-7.36 (12H, m); 7.36 {1 H,
d, J = 8.1
Hz); 7.43 (1 H, bs); 7.49 (1 H, d, J = 8.0 Hz); 8.94 (1 H, bs); 9.26 (1 H,
bs); 9.72 (1 H,
bs); 10.90 (1 H, s).
3o MS: mlz = 678, MH+.
EXAMPLE 4: cyclo{Suc[1-(R)-(4-tetraidrotiopyranil)amino]-Trp-Phe-[(R)-NH-
CA 02339638 2001-02-05
WO 00/0804b PCT/EP99/05459
is
CH(CH2-CgHS)-CH2NH]}
(compound of formula I wherein R4 is (4-tetrahydrothiopyranyl)amino and the
othe substituents are as described for compound A).
The compound is prepared according to Example 1 but using as reagent
s tetrahydro-thiopyran-4-one.
MS: mlz = 681, MH+.
EXAMPLE 5: cyclo{Suc[1-(R~(1-oxo-tetrahydrothiopyran-4-yl)amino]-Trp-Phe-
[(R)-NH-CH(CH2-CgH5~CH2NH]}
(compound of general formula I wherein R4 is (1-oxo-4.
io tetrahydrothiopyranyl)amino and the other substituents are the same of
Compound A).
The compound is prepared as in example 1 but using as reagent 1-oxo-
tetrahydro-thiopyran-4-one.
HPLC (Method A2): rt = 12.7 min.
is MS: m/z = 697.3 (MH+).
EXAMPLE 6: cyclo{Suc[1-(R)-(1,1-dioxo-tetrahydrothiopyran-4-yl)amino]-Trp-Phe-
[(R)-NH-CH(CH2-CgHS)-CH2NH]}
(compound of general formula I wherein R4 is (1,1-dioxo-4
tetrahydrothiopyranil}amino and the other substituents are the same of
Compound
2o A).
The compound is prepared as in example 1 but using as reagent 1,1-dioxo-
tetrahydro-thiopyran-4-one.
HPLC (Method A2): rt = 13.7 min.
MS: m/z = 713.2 (MH+).
2s EXAMPLE 7: cyclo{Suc[1-(R)-N-methyl-N-(4-tetrahydropyranyl)amino]-Trp-Phe-
[(R)-NH-CH(CH2-CgH5~CH2NH]}
(compound of general formula I wherein R4 is N-methyl-N-(4-
tetrahydropyranyl)amino and the other substituents are the same of Compound
A).
30 50 mg of the compound described in Example 1 are solved in 5 ml of
anhydrous
methanol. Acetic acid (0.1 ml), paraformaldheyde (60 mg) and sodium
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
16
cianoboroidride (40 mg) are added in the given sequence. The mixture is left
under stirring for a night, acidified with HCI 1 N up to pH=1-2, diluted with
water
and the methanol is evaporated; NaHC03 is added and then the solution is
extracted with ethyl acetate, the extracted is dried on sodium sulfate. The
soluton
s is concentrated and purified by preparative HPLC (Method P2).
HPLC (Method A2): rt = 13.7 min.
MS: m/z = 679.3 (MH+).
EXAMPLE 8: cyclo{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Tyr-[(R)-NH-
CH(CH2-CgHS}-CH2NH]}
to (compound of general formula I wherein cui R2 = 4-hydroxybenzyl, R4 = (4-
tetrahydropyranyl)amino and the other substituents are as defined for Compound
A).
The compound is prepared according to Example 1 (b)-1 (k) but Boc-Trp-
Tyr(OBzI)-
OH is used instead of Boc-Trp-Phe-OH.
is HPLC (Method A2): rt = 11.0 min.
MS: m/z = 681.3 (MH+).
EXAMPLE 9: cyclo{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Phe(4-F}-[(R)-NH-
CH(CH2-CgH5~CH2NH]}
(compound of general formula I wherein cui R2 =4-fluorobenzyl, R4 = {4-
2o tetrahydropyranyl)amino and the other substituents are as defined for
Compound
A).
The compound is prepared according to Example 1 (b)-1 (k) but Boc-Trp-Phe(4-F)-
OH is used instead of Boc-Trp-Phe-OH.
HPLC (Method A2): rt = 13.7 min.
Zs MS: mlz = 683.3 (MH+).
EXAMPLE 10: cyclo(Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Phe(3,5-Fr[(R)-
NH-CH(CH2-CgH5rCH2NH]}
(compound of general formula I wherein cui R2 = 3,5-difluorobenzyl, R4 = (4-
tetrahydropyranyl)amino and the other substituents are as defined for Compound
3o A).
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
1?
The compound is prepared according to Example 1 (b~1 (k) but Boc-Trp-Phe(3,5-
F)-OH is used instead of Boc-Trp-Phe-OH.
HPLC (Method A2): rt = 14.3 min.
MS: m/z = 701.2 (MH+).
s EXAMPLE 11: cyclo{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Phe(4-CND[(R}-
NH-CH(CH2-C6H5)-CH2NH]}
To 377 mg of Boc-(S)-4-ciano-phenylalanine, solved in 8 ml of DMF, HOBt (470
mg), EDCI.HCI (330 mg) and 630 mg of (R~1-benzyl-2-(N-
fluorenylmethyloxycarbonylamino)ethylamina trifluoroacetate (prepared
according
io to Example 1(b)), solved in 8 ml of DMF are added in the given order. DIEA
(0.38
ml) is added drop by drop maintaining under stirring for 3 h. The solution is
dried
and the residue is treated with citric acid 105 and water; the precipitated
solid is
filtered, washed with water, NaHC03 5%, water and dried. The obtained solid
(790 mg) is suspended in dichlorometane (6.5 ml).
is The suspension is cooled at 0°C, (3.5 ml) is added and the
tempearure is raised at
room temperature maintaining under stirring for 1 h. The solution is
concentrated
to dryness and the residue is treated with ethyl ether, under stirring, the
formed
solid is filtered and washed with ether.
After drying the obtained solid (550 mg) is solved in 8 ml of DMF are added to
a
2o solution of DMF (6 ml), Boc-Trp-OH (250 mg), HOBt (216 mg), EDCI.HCI (200
mg). DIEA (0.23 ml) is added drop by drop and the solution is stirred for 1 h.
The
solution is concentrated to dryness and the residues treated with water and
citric
acid, under stirring; the formed solid is filtered and washed with water,
NaHC03
5%, water; 623 mg of a solid compound are obtained.
2s The obtained solid is solved in DMF (15 ml); diethylamine (1.5 ml) is added
and
the solution is stirred for 2 h. The solvent is evaporated and the residue is
treated
with diethylether under stirring, the formed solid is filtered and washed with
diethylether obtaining 220 mg of a solid product.
The product is solved in 4 ml of DMF and added drop by drop to a solution of
3o Fmoc-D-Asp-(OtBu)-OH (150 mg), HOBt (115 mg), EDCI.HCI (84 mg) in DMF (4
ml).
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
18
The solution is maintained 2 h under stirring, concentrated to dryness and the
residue is treated with citric acid 10% and water; the formed solid is
filtered,
washed with water, NaHC03 al 5%, water and dried, 340 mg of a solid product
are obtained.
s The obtained product is suspended in dichloromethane, ethanediol (0.035 ml)
and, at 0°C, TFA (4 ml). The temperature is brought to room temperature
under
stirring for 1 h. the solution is dried and the residue is treated with
diethylether
under stirring, the formed solid is filtered and washed with diethylether.
After drying 280 mg of solid product are obtained.
to The product is solved in 30 ml of DMF, HOBt (185 mg) and EDCI.HCI (160 mg)
are added and the solution is maintained under stirring for 5 h and then left
staying for one night. The solution is concentrated and the residue is treated
with
citric acid 10% and water, the formed solid is filtered. Washed with water,
NaHC03 5%, water and dried giving 220 mg of a solid product.
is The obtained solid is solved in DMF (10 ml); added with diethylamine (1.0
diethylether under stirring, the formed solid is filtered, washed with
diethylether
giving 157 mg of a solid product.
The obtained product is solved in methanol (13 ml) and added with acetic acid
(0.26 ml), tetrahydro-4H-pyran-4-one (80 mg) and sodium cianoborohydride (55
2o mg) in the given order. The solution is kept under stirring overnight,
acidified with
HCI 1N up to pH=1-2, stirred for 1 h, methanol is evaporated and NaHC03 is
added, the solution is extracted with ethylacetate and dried on sodium
sulfate.
The solution is concentrated and purified by preparative HPLC (Method P3).
MS: m/z = 690.2 (MH+}.
2s HPLC (Method A2): rt = 12.7 min.
EXAMPLE 12: cyclo{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Phe(4-CF3)-[(R)-
NH-CH(CH2-C6H5~CH2NH]}
(compound of formula I wherein R2 - (4-trifluoromethyl)benzyl, R4 - (4-
tetrahydropyranyl)amino and the other substituents are as in Compound A.
3o The compound is prepared according to Example 1 (b}-1 (k) but using Boc-Trp-
Phe(4-CF3~OH instead of Boc-Trp-Phe-OH.
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
19
HPLC (Method A2): rt = 15.4 min.
MS: m/z = 733.2 (MH+).
EXAMPLE 13: cyclo{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Ala(4-pyridyl)-
[(R)-
NH-CH(CH2-C6H5~CH2NH]}
s (compound of formula I wherein R2 - 4-pyridylmethyl, R4 - (4-
tetrahydropyranyl)amino and the other substituents are as in Compound A.
The compound is prepared according to Example 11 but using Boc-(S)-3-(4-
pyridyl)alanine instead of Boc-(S~4-ciano-phenylalanine.
HPLC (Method A2): rt = 6.9 min.
to MS: mlz = 666.3 (MH+).
EXAMPLE 14: cyclo{Suc[1-(R)-(4-tetrahydropyranyl)amino]-Trp-Ala(3-pyridyl~[(R)-
NH-CH(CH2-C6H5}-CH2NH]}
(compound of general formula I wherein R2 - 3-pyridylmethyl, R4 - (4-
tetrahydropyranyl) and the other substituents are as in Compound A.
is The compound is prepared according to Example 11 but using Boo-(S)-3-(3-
pyridyl)alanine instead of Boc-(S)-4-ciano-phenylalanine.
HPLC (Method A2): rt = 7.3 min.
MS: m/z = 666.3 (MH+).
EXAMPLE 15: cyclo{Suc[1-(R)-(1-methylsulfonyl-piperidin-4-yl)amino]-Trp-Phe-
20 [(R~NH-CH(CH2-C6H5rCH2NH]}
(compound of formula I wherein R4 = (1-methylsulfonyl)piperidin-4-yl)amino
and the other substituents are as in Compound A).
The compound is prepared according to Example 11 but using as reagent (1-
methylsulfonyl)piperidin-4-one).
2s HPLC (Method A2): rt =14.0 min.
MS: m/z = 742.2 (MH+).
EXAMPLE 16: cyclo{Suc[1-(R}-(1-aminosulfonyl-piperidin-4-yl)amino]-Trp-Phe-
[(R)-NH-CH(CH2-C6H5)-CH2NH]}
(compound of general formula l wherein R4 - (1-aminosulfonyl)piperidin-4-
3o yl)amino and the other substituents are as in Compound A).
CA 02339638 2001-02-05
13-07-2000 EP 009905459
The compound is prepared according to Example 1 but using as reagent (1-
aminosulfonyl)piperidin-4-one.
HPLC (Method A2): rt =13.5 min.
MS: mlz = 743.2 (MH+).
s EXAMPLE 17: cyclo{Suc[1-(R~(piperazin-1-yl)]-Trp-Phe-[(R)-NH-CH(CH2-C6H5)-
CH2NH]}
(compound of formula l wherein R4 = piperazin-1-yl and the other substituents
are
as in Compound A.
The compound is prepared according to Example 1 but using as reagent N-Boc
io iminodiacetaldheyde, carrying on the reaction for 16 h and removing the
protective
group N-Boc with TFA in dichloromethane. The so obtained product is purified
by
preparative HPLC (Method P2).
1 H-NMR (DMSO-d6, 500 MHz): d 2.39 (1 H, dd, J = 10.2, 12.4 Hz); 2.65-2.79
(5H,
m); 2.79-2.91 (3H, m); 2.99-3.15 (6H, m); 3.22-3.48 (m, overlapping the water
is signal); 3.51 (1 H, dd, J = 4.4, 10.1 Hz); 3.95-4.04 (1 H, m); 4.08-4.18
(2H, m); 6.92
(1 H, d, J = 8.7 Hz); fi.98 (1 H, m); 7.04-7.11 (2H, m); 7.11-7.28 (10H, m);
7.33 (1 H,
d, J = 8.1 Hz); 7.32-7.37 (1 H, m); 7.44 (1 H, d, J = 7.9 Hz); 8.32 (1 H, d, J
= 7.4 Hz);
8.40 (1 H, bs); 8.71 (1 H, d, J = 5.0 Hz); 10.82 (1 H, d, J = 2.1 Hz).
MS: mlz = 650, MH+.
2o EXAMPLE 18: cyclo{Suc[1-(R)-4-methyl-piperazin-1-ylj-Trp-Phe-[(R)-NH-CH(CH2-
C6H5)-CH2NH]}
(compound of formula I wherein R4 = 4-methyl-piperazin-1-yl and the other
substituents are as described in Compound A)
To 50 mg of the compound described in example 17, solved in 2 ml methanol, 10
2s mg paraformaldeide, 25 mg of sodium cianoborohydride, and 50 NI actic acid
are
added. The solution is stirred for one night, thereafter the solvent is
evaporated,
the residue is treated with HCI 0.1 N, potassium carbonate up to basic pH and
extracted with ethyle acetate, washed with brine and dried on magnesium
sulfate. The solvent is evaporated giving 34 mg of crude product which are
3a purified by preparativeHPLC (Method P3).
MS: mlz = 664.5 (MH+).
AMENDED SHEET
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
21
HPLC (Method A2): rt =12.4 min.
EXAMPLE 19: cyclo{Suc[1-(Rr4-acetyl-piperazin-1-yl]-Trp-Phe-[(R)-NH-CH(CH2-
C6H5)-CH2NH]}
(compound of general formula I wherein R4 = 4-acetyl-piperazin-1-yl and the
other
s substituents are as described in Compound A)
To 40 mg of the compound described in Example 17, solved in 2 ml acetonitrile
and 0.5 ml DMF, 50 NI of acetic anhydride are added; the mixture is stirred
for one
night, concentrated, poured into water, left under stirring for 30 minutes,
added
with potassium carbonate up to basic pH; the solution is extracted with ethyle
to acetate, washed with brine and dried on magnesium sulfate. The solvent is
evaporated giving 16 mg of a crude product which is purified by preparative
HPLC
(Method P4).
MS: mlz = 692.5 (MH+).
HPLC (Method A2): rt =12.8 min.
is EXAMPLE 20: cyclo{Suc[1-(R)-(4-methanesulfonyl-piperazin-1-yl)]-Trp-Phe-
[(Rr
NH-CH(CH2-C6H5)-CH2NH]}
(compound of general formula I wherein R4 = 4-methanesulfonyl-piperazin-1-yl
and the other substituents are as described in Compound A).
The compound described in Example 17 was solved in anhydrous DMF treated
2o with TEA and methanesulfonyl chloride. After 3 h under stirring at room
temperature the mixture is purified by preparative HPLC (Method P6).
1 H-NMR (DMSO-d6, 500 MHz): d 2.41 (1 H,t, J = 11.1 Hz); 2.66-2.81 (3H, m);
2.81-3.00 (5H, m); 2.92 (3H, s); 3.00-3.61 (m, overlapping the signal of
water);
3.96-4.07 (1 H, m); 4.12 (1 H, bs); 4.19 (1 H, bs); 6.92 (1 H, d, J = 8.6 Hz);
6.98 (1 H,
2s t, J = 7.4 Hz); 7.03-7.30 (12H, m); 7.45 (1 H, d, J = 7.9 Hz); 7.50 (1 H,
bs); 8.00-
8.60 (1 H, bs); 8.75 (1 H, bs); 10.82 (1 H, s).
MS: mlz = 728 (MH+).
EXAMPLE 21: cyclo{-Suc[1-(S)-methanesulfonylamino]-Trp-Phe-[(R)-NH-
CH(CH2C6H5)-CH2-NH]}
30 (compound of general formula I wherein C-R4 has S-configuration, R4 is
methanesulfonylamino and the other substituens are as described in compound A)
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
22
To a solution of 60 mg of the isomer of Compound A having S-configuration at
the C-R4, prepared as described in Example 1 (a~1 (h), in 1 ml DMF, at
0°C, 24 ml
of N-methylmorpholine and 10 ml of methanesulfonylchloride are added; the
solution is left under stirring for 2 and half h. The reaction mixture is
concentrated
s under vacuum, diluted with ethylacetate and washed with an aqueous solution
of
citric acid (10%), water, saturated solution of NaHC03 and water in the given
order. After drying on Na2S04 and evaporation of the solvent the product is
isolated by preparative HPLC.
1 H-NMR (DMSO-d6, 500 MHz): d 10.80 (d, J = 1.6, 1 H); 8.54 (s broad, 1 H);
8.34
io (dd, J= 3.8, 8.6, 1 H); 7.61 (d, J = 7.6, 1 H); 6.90-7.40 (m, 16H); 6.64
(d, J = 9.5,
1 H) 4.30-4,38 (m, 1 H); 4.25-4.30 (m,1 H); 4.00-4.10 (m, 2H); 3.65-3.77 (m, 1
H);
3.30-3.35 (m, 1 H); 2.97 (s, 3H); 2.58-2.95 (m, 8H).
MS: mlz = 659, MH+.
Following the same procedure reported above, the following products are
is obtained.
EXAMPLE 22: cyclo{Suc[1-(R)-methanesulfonylamino]-Trp-Phe-[(RrNH-CH(CH2-
C6H5~CH2NH]}
(compound of general formula I wherein R4 is methanesulfonylamino and the
other substituents are as described for Compound A)
20 1 H-NMR (DMSO-d6, 500 MHz): d 10.83 (d, J = 1.6, 1 H); 8.82 (d, J= 4.7, 1
H);
8.12 (s broad, 1 H); 7.44 (d, J = 7.9, 1 H); 6.92-7.42 (m, 16H); 6.82 (d, J =
8.8, 1 H)
4.11-4,23 (m, 3H); 4.02 (m, 1 H); 3.35 (m, 2H); 2.95 (s, 3H); 2.70-2.95 (m,
6H);
2.34 (dd, J = 9.3, 13.5, 1 H).
MS: m/z = 659, MH+.
2s EXAMPLE 23: cyclo{Suc[1-(S)-(4-methylbenzen)sulfonylamino]-Trp-Phe-[(R)-NH-
CH(CH2-C6H5)-CH2NH]}
(compound of general formula I wherein C-R4 has S-configuration, R4 is (4-
methylbenzen)sulfonylamino and the other substituents are as described for
Compound A)
3o As starting compound the isomer of Compound A having S-configuration at the
C-
R4 is used.
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
23
MS: mlz = 735, MH+.
EXAMPLE 24: cyclo{Suc[1-(R)-(4-methylbenzen)solfonylamino]-Trp-Phe-[(R}-NH-
CH(CH2-C6H5rCH2NH]}
(compound of formula I wherein R4 is (4-methylbenzen)sulfonyiamino and the
s other substituents are as described for Compound A)
1 H-NMR (DMSO-d6, 500 MHz): d 10.81 (d, J = 1.5, 1 H); 8.68 (d, J = 4.5, 1 H);
7.95 (s broad, 1 H); 7.90 (d, J = 8.8, 1 H); 6.95-7.75 (m, 20H); 6.78 (d, J =
8.9, 1 H)
4.17(m, 1 H); 4.10 (m,1 H); 4.05 (m, 1 H); 3.94 (m, 1 H); 3.17 (m, 1 H); 2.97
(m, 1 H);
2.65-2.85 (m, 7H); 2.36 (s, 3H); 2.09 (dd, J = 9.1, 13.5, 1H).
MS: m/z = 735, MH+.
EXAMPLE 25: cyclo{Suc[1-(S~(2-(4-morpholino)ethylamino]-Trp-Phe-[(R)-NH-
CH(CH2-C6H5)-CH2NH]}
(compound of general formula I wherein C-R4 has S-configuration, R4 is 2-(4-
morpholino)ethylamino and the other substituents are as described for Compound
i s A)
The compound is obtained following the procedure of example 1, but using as
starting product the isomer of Compound A having S-configuration at C-R4, and
2-
(4-morpholino}acetaldheyde as reagent
1 H-NMR (DMSO-d6, 500 MHz): d 2.61-3.87 (15H, m); 3.14 (1 H, dd, J = 4.6, 13.9
2o Hz); 3.19-3.90 (m, overlapping the signal of water); 3.98-4.06 (1 H, m};
4.08-4.16
(2H, m); 4.30-4.37 (1 H, m); 6.95 (1 H, s); 6.99 (1 H, m); 7.03-7.10 (2H, m);
7.14-
7.31 (11 H, m); 7.33 (1 H, d, J = 8.1 Hz); 7.37 (1 H, d, J = 8.9 Hz); 7.42 (1
H, d, J =
7.9 Hz); 8.25 (1 H, d, J = 5.2 Hz); 8.52 (1 H, d, J = 5.2 Hz); 10.83 (1 H, d,
J = 2.1
Hz).
2s MS: m/z = 694, MH+.
EXAMPLE 26: cyclo{Suc[1-(R)-(2-(4-morpholino)ethylamino]-Trp-Phe-[(R)-NH-
CH(CH2-C6H5)-CH2NH]}
(compound of general formula I wherein R4 is 2-(4-morpholino)ethylamino and
the other substituents are as described for Compound A)
3o The compound is prepared according to the procedure of example 1 but using
as
reagent 2-(4-morpholino)acetaldehyde.
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
24
MS: m/z = 694, MH+.
EXAMPLE 27: cyclo{Suc[1-(R)-(2-furylmethyl)amino]-Trp-Phe-[(R)-NH-CH(CH2-
C6H5~CH2NH]}
(compound of formula I wherein R4 is (2-furylmethyl)amino and the other
s substituents are as described for Compound A)
The compound is prepared according to the procedure of Example 1 but using as
reagent 2-furaldheyde. The so obtained crude was purified by prepartive HPLC
(Method P2).
1 H-NMR (DMSO-d6, 500 MHz): d 2.39-2.46 (1 H, m); 2.69-2.96 (SH,m); 3.02-3.22
io (2H, bs); 3.57-3.82 (1 H, bs); 4.04, 4.16 a 4.30 (5H, bs); 6.50 (1 H, bs);
6.59 (1 H,
bs); 6.84 (1 H, d, J = 7.1 Hz); 6,99 (1 H. m); 7.04-7.28 (14H, m); 7.35 (1 H,
d, J = 8.1
Hz); 7.48 (1 H, d, J = 7.8 Hz); 7.74 (1 H, bs); 8.81 (1 H, bs); 9.22-9.69 (1
H, bs);
10.88 (1 H, s).
MS: m/z = 661, MH+.
is EXAMPLE 28: cyclo{Suc[1-(R~cianomethylamino]-Trp-Phe-[(R)-NH-CH(CH2-
C6H5)-CH2NH]}
(compound of general formula I wherein R4 is cianomethylamino and the other
substituents are as described for Compound A)
To 50 mg of Compound A, prepared as described in EXAMPLE 1 (a}-(h), solved in
20 1 ml of DMF, 12 NI of TEA and 6.5 NI of chloroacetonitrile are added;
thereafter 15
mg of Nal are added and the mixture is stirred for about 16 h at room
temperature.
The solution is filtered and purified by preparative HPLC (Method P2).1 H-NMR
(DMSO-d6, 500 MHz): d 2.34 (1 H, dd, J = 7.4, 13.6 Hz); 2.71-2.84 (5H, m);
2.91
(1 H, dd, J = 4.3, 13.6 Hz); 3.16-3.27 (2H, m); 3.27-3.60 (m, overlapping the
signal
2s of water); 3.66 a 3.74 (2H, ABq, J = 17.5 Hz); 3.96-4.11 (1 H, m); 4.11-
4.27 (2H,
m); 6.77 (1 H, d, J = 9.0 Hz); 6.98 (1 H, m); 7.03-7.10 (2H, m); 7.14-7.21
(3H, m}
7.21-7.30 (5H, m); 7.34 (1 H, d, J = 8.1 Hz); 7.44 (1 H, d, J = 7.9 Hz); 7.64
(1 H, bs);
7.88 (1 H, bs); 8.75 (1 H, d, J = 4.9 Hz); 10.83 (1 H, d, J = 1.6 Hz).MS: mlz
= 620,
MH+.
3o EXAMPLE 29: cyclo{Suc[1-(R}-2-(4-morpholinoacetyl)amino]-Trp-Phe-[(R~NH-
CH(CH2-C6H5)-CH2NH]}
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
2s
(compound of general formula I wherein R4 is 2-(4-morpholinoacetyl)amino and
the other substituents are as described for Compound A)
To 21 mg of acid 4-morpholineacetic, solved in 5 ml DMF, 40 mg of 1-hydroxy-
benzotriazole and 20 mg of EDCI.HCI are added. The solution is left under
stirring
s for 10' and 60 mg of Compound A are added. After 4 h the solvent is
evaporated
and the residue is purified by preparative HPLC (Method P2).
1 H-NMR (DMSO-d6, 500 MHz): d 2.34 (1 H, dd, J = 8.3, 14.2 Hz); 2.71-2.90 (5H,
m); 2.97 (1H, dd, J = 4.1, 14.2 Hz); 3.00-3.24 (4H, bs); 3.26-3.53 (m,
overlapping
the signal of water); 3.79 (6H, bs); 4.00-4.10 (1 H,m); 4.13-4.20 (1 H, m);
4.20-4.27
io (1 H, m); 4.59-4.68 (1 H, m); 6.79 (1 H, d, J = 8.1 Hz); 6.95-?.01 (1 H,
m); 7.05-7.10
(1 H, m); 7.15-7.20 (4H, m); 7.23-7.29 (7H, m); 7.35 (1 H, d, J = 8.1 Hz);
7.4? (1 H,
d, J = 7.8 Hz); 8.04 (1 H, bs); 8.60 (1 H, d, J = 5.2 Hz); 8.53-8.70 (1 H,
bs); 10.70
(1 H, s).
MS: mlz = 708, MH+.
is According to the same procedure the following compounds are obtained.
EXAMPLE 30: cyclo{Suc[1-(S)-2-(4-morpholinoacetyl)amino]-Trp-Phe-[(R~NH-
CH(CH2-C6H5)-CH2NH]}
(compound of general formula I wherein R4 is 2-(4-morpholinoacetyl)amino, C
R4 has S-configuration and the other substituents are as described for
Compound
2o A)
1 H-NMR (DMSO-d6, 500 MHz): d 2.57 (1 H,dd, J = 4.4; 15.7 Hz); 2.66-2.85 (7H,
m); 2.98-3.59 (bs, overlapping the signal of water); 3.26 (dd, J = 4.4; 14.3
Hz);
3.59-4.03 (6H, m); 4.03-4.15 (2H,m); 4.36 (1 H, m); 4.77 (1 H, bs); 6.84 (1 H,
bs);
6.94 (1 H, d, J = 2.0 Hz); 6.98 (1 H, t, J = 7.2 Hz); 7.07 (1 H, t, J = 7.2
Hz); 7.13-7.31
2s (9H, m); 7.33 (1 H, d, J = 8.1 Hz); 7.41 (1 H, d, J = 7.8Hz); 8.32 (1 H,
bs); 8.49 (1 H,
d, J = 4.8 Hz); 8.86-9.10 (1 H, bs); 10.10-10.30 (1 H, bs); 10.81 (1 H, d, J =
1.7 Hz).
MS: m/z = 708, MH+.
EXAMPLE 31: cyclo{Suc[1-(S)-(2-tetrazol-1-yl)acetylamino]-Trp-Phe-[(R)-NH-
CH(CH2-C6H5~CH2NH]}
30 (compound of general formula I wherein C-R4 has S-configuration, R4 is (2-
tetrazol-1-yl)acetylamino and the other substituents are as described for
Compound A)
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
26
As starting compound the isomer of compound A having S-configuration at C-R4
is used.
1 H-NMR (DMSO-d6, 500 MHz): d 10.80 (d, J = 2.0, 1 H); 9.32 (s, 1 H); 8.87 (d,
J =
8.0, 1 H); 8.52 (d, J= 5.3, 1 H); 8.38 (dd, J = 4.0, 8.5 1 H); 6.93-7.42 (m,
17H); 6.78
s (d, J = 9.3, 1 H); 5.27 a 5.30 (spectrum AB, J = 16.6, 2H); 4.76 (m, 1 H);
4.35
(m,1 H); 4.01-4.13 (m, 2H); 3.73 (m, 1 H); 3.25-3.35 (m, 1 H); 2.54-2.86 (m,
8H).
MS: m/z = 691, MH+.
EXAMPLE 32: cyclo{Suc[1-(R)-(2-tetrazol-1-yl)acetylamino]-Trp-Phe-[(R)-NH-
CH(CH2-C6H5rCH2NH]}
io (compound of general formula I wherein R4 is (2-tetrazol-1-yl)acetylamino
and
the other substituents are as described for Compound A)
MS: mlz = 691, MH+.
EXAMPLE 33: cyclo{Suc[1-(S)-(2-(5-mercapto-tetrazol-1-yl)acetylamino]-Trp-Phe-
[(R)-NH-CH(CH2-C6H5)-CH2NH]}
is (compound of general formula I wherein C-R4 has S-configuration, R4 is (2-
(5-
mercapto-tetrazol-1-yl)acetylamino and the other substituents are as described
for
Compound A)
As starting compound the isomer of compound A having S-configuration at C-R4
is used.
20 1 H-NMR (DMSO-d6, 500 MHz): d 10.79 (d, J = 1.8, 1 H); 8.79 (d, J = 7.9, 1
H);
8.54 (d, J= 5.2, 1 H); 8.39 (dd, J = 5.4, 8.2 1 H); 7.40 (d, J= 7.8, 1 H);
6.96-7.34 (m,
15H); 6.95 (s, 1 H); 6.77 (d, J= 9.3, 1 H); 4.98 a 5.01 (spectrum Ag, J =
16.7, 2H);
4.75 (m, 1 H); 4.35 (m,1 H); 4.01-4.12 (m, 2H); 3.74 (m, 1 H); 3.32-3.35 (m, 1
H);
2.63-2.85 (m, 7H); 2.58 (dd, J = 4.8, 15.5, 1 H).
2s MS: m/z = 723, MH+.
EXAMPLE 34: cyclo{Suc[1-(R~2=([1,2,4]triazol-1-yl)acetylamino]-Trp-Phe-[(R)-NH-
CH(CH2-C6H5)-CH2NH]}
(compound of general formula I wherein R4 is 2-([1,2,4]triazol-1-
yl)acetylamino
and the other substituents are as described for Compound A)
3o HPLC (Method A2): rt =13.8 min.
MS: mlz = 690.2 (MH+).
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
27
EXAMPLE 35: cyclo{Suc[1-(R)- (furan-2-yl)carbonylamino]-Trp-Phe-[(R)-NH-
CH(CH2-C6H5)-CH2NH]}
(compound of general formula I wherein R4 is (furan-2-yl)carbonylamino and the
other substituents are as described for Compound A)
s To 50 mg of Compound A solved in 1 ml DMF, 8.5 pl of 2-furanoyl chloride and
12
NI of TEA are added. The solution is stirred 30'. The product is purified by
preparative HPLC (Method P6), giving 30 mg of pure compound.
HPLC {Method A2): rt =16.6 min.
MS: m/z = 675.3 (MH+).
io EXAMPLE 36: cyclo{Suc[1-(Rr2-(thiophen-3-yl)acetylamino]-Trp-Phe-[(R}-NH-
CH(CH2-C6H5rCH2NH]}
(compound of general formula I wherein R4 is 2-(thiophen-3-yl)acetylamino and
the other substituents are as described for Compound A)
The compound was prepared according to the procedure of Example but using as
~s reagent 2-(thiophen-3-yl)acetic acid.
HPLC (Method A2): rt =17.5 min.
MS: mlz = 705.3 (MH+).
EXAMPLE 37: cyclo{Suc[1-(R~{4-morpholino)carbonylamino]-Trp-Phe-[(R)-NH-
CH(CH2-C6H5)-CH2NH]}
20 (compound of general formula I wherein R4 is (4-morpholino)carbonyfamino
and
the other substituents are as described for Compound A)
To a solution of 77 mg of compound A, obtained as described in example 1 (a}-
1 (h), in acetonitrile (2 ml}, 36 NI of TEA and, at room temperature, under
nitrogen,
16 NI of morpholin-4-carbonylchloride are added. The reaction is carried on
for 18
2s h, the solution is concentrated, and purified by preparative HPLC (Method
P6).
37 mg of solid product are obtained.
HPLC (Method A2): rt =14.9 min.
MS (ES+): 694.4 [MH+]
EXAMPLE 38: cyclo{Suc[1-(R)-2-(4-hydroxy-piperidin-1-yl)acetylamino]-Trp-Phe-
30 [(R}-NH-CH(CH2-C6H5)-CH2NH]}
(compound of general formula I wherein R4 is 2-{4-hydroxy-piperidin-1-
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
28
yl)acetylamino and the other substituents are as described for Compound A)
The compound was prepared according to example 29 but using as reagent 2-(4-
hydroxy-piperidin-1-yl)acetic acid.
HPLC (Method A2): rt =11.8 min.
s MS: m/z = 722.3 (MH+).
EXAMPLE 39: cyclo{Suc[1-(R)-2-(4-aminocarbonyl-piperidin-1-yl)acetylamino]-
Trp-Phe-[(R)-NH-CH(CH2-CgHS)-CH2NH]}
[(R)-NH-CH(CH2-CgHS)-CH2NH]}
(compound of general formula I wherein R4 is 2-(4-aminocarboniy-piperidin-1-
io yl)acetylamino and the other substituents are as described for Compound A)
The compound was prepared using the procedure of example 29 but using as
reagent 2-(4-aminocarbonyl-piperidin-1-yl)acetic acid.
HPLC (Method A2): rt =11.7 min.
MS: m/z = 749.4 (MH+).
is EXAMPLE 40: cyclo{Suc[1-(R)-2-(3-hydroxy-pyrrolidin-1-yl)acetylamino]-Trp-
Phe-
[(R)-NH-CH(CH2-CgH5~CH2NH]}
(compound of general formula I wherein R4 is 2-(3-hydroxy-pyrrolidin-1-
yl)acetylamino and the other substituents are as described for Compound A)
The compound was prepared according to example 29 but using as ragent 2-(3-
2o hydroxy-pyrrolidin-1-yl)acetic acid.
HPLC (Method A2): rt =11.9 min.
MS: m/z = 708.4 (MHO).
EXAMPLE 41: cyclo{Suc[1-(R)-2-(2-(S)-hydroxymethyl-pyrrolidin-1-
yl)acetylamino]-Trp-Phe-[(R)-NH-CH(CH2-CgH5)-CH2NH]}
2s (compound of general formula I wherein R4 is 2-(2-(S)-hydroxymethyl-
pyrrolidin-
1-yl)acetylamino and the other substituents are as described for Compound A)
The compound was prepared according to example 29 but using as reagent 2-(2-
(S)-hydroxymethyl-pyrrolidin-1-yl)acetic acid.
HPLC (Method A2): rt =12.2 min.
3o MS: mlz = 722.3 (MH+).
EXAMPLE 42: cyclo{Suc[1-(R)-2-(4-methyl-piperazin-1-yl)acetylamino]-Trp-Phe-
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
29
[(R}-NH-CH(CH2-C6H5)-CH2NH]}
(compound of general formula I wherein R4 is 2-(4-methyl-piperazin-1-
yl)acetylamino and the other substituents are as described for Compound A)
The compound was prepared according to example 29 but using as reagent 2-(4-
s methyl-piperazin-1-yl)acetic acid.
HPLC (Method A2): rt =11.4 min.
MS: m/z = 721.5 (MH+).
EXAMPLE 43: cyclo{Suc[1-(R~2-(4-methyl-piperazin-1-yl)carbonylamino]-Trp-
Phe-[(R)-NH-CH(CH2-C6H5rCH2NH]}
io (compound of general formula I wherein R4 is 2-(4-methyl-piperazin-1
yl)carbonylamino and the other substituents are as described for Compound A)
A .solution of 40 mg of compound A, obtained as described in EXAMPLE 1 (a}-
1 (h), and 400 Nl of DIPEA in THF (0.5 ml), is added, under nitrogen, to a
solution
of 27 mg of 4-methyl-1-piperazinocarbonyl chloride (prepared as described in
C.
is Jorand-Lebrun et al., Synth. Commun. (1998), 28, 1189) in 0.5 ml of
dichloromethane. The solution is stirred for 2 h at room temperature, dried
and
purified by HPLC (Method P7).
HPLC (Method A2): rt =11.8 min.
MS: m/z = 707.2 (MHO)
2o EXAMPLE 44: cyclo{Suc[1-(R)-2-(4-aminosulfonyl-piperazin-1-yl)acetylamino]-
Trp-
Phe-[(R~NH-CH(CH2-C6H5)-CH2NH]}
(compound of general formula I wherein R4 is 2-(4-aminosulfonyl-piperazin-1-
yl)acetylamino and the other substituents are as described for Compound A)
The compound was prepared according to EXAMPLE 29 but using as reagent 2-
2s (4-aminosulfonyl-piperazin-1-yl)acetic acid.
HPLC (Method A2): rt =12.5 min.
MS: mlz = 786.3 (MH+)
EXAMPLE 45: cyclo{Suc[1-(R~2-(1-oxo-thiomorpholin-4-yl)acetylamino]-Trp-Phe-
[(R)-NH-CH(CH2-C6H5~CH2NH]}
30 (compound of general formula I wherein R4 is 2-(1-oxo-thiomorpholin-4
yl)acetylamino and the other substituents are as described for Compound A)
CA 02339638 2001-02-05
13-07-2000 EP 009905459
The compound was prepared according to EXAMPLE 29 but using as reagent 2-
(1-oxo-thiomorpholin-4-yl)acetic acid.
HPLC (Method A2): rt =11.7 min.
MS: mlz = 740.4 (MH+)
s EXAMPLE .. 46: cyclofSuc[1-(Rr2-(traps-4-hydroxy-cyclohexan-1-yl-
amino)acetylamino]-Trp-Phe-[(R)-NH-CH(CH2-CgH5~CH2NH]}
(compound of general formula I wherein R4 is 2-(traps-4-hydroxy-cyclohexan-1-
yl-amino)acetylamino and the other substituents are as described for Compound
A).
io The compound was prepared according to EXAMPLE 29 but using as reagent 2-
(traps-4-hyroxy-cyclohexan-1-yl-amino)acetic acid.
HPLC (Method A2): rt =11.6 min.
MS: mlz = 736. 3 (MH+)
EXAMPLE 47: cyclo{Suc[1-(4-morpholino)carbonyl]-Trp-Phe-[(R~NH-CH(CH2-
is C6H5)-CH2NH]}
(compound of general formula I wherein : X1 = X2 = X3 = X4 = -CO-NH-; R1 =
-CH2-(indol-3-yl); R2 = R3 = -CH2-C6H5; R4 = (4-morpholino)carbonyl; m = 0, f
=
1; the C-R1 and C-R2 carbon atoms have S-configuration, while GR3 has R-
configuration)
2o a) Synthesis of Boc-Trp-Phe-[(R~NH-CH(CH2-C6H5}-CH2-NH2]
To a solution of Boc-Trp-Phe-[(R)-NH-CH(CH2-C6H5)-CH2-NH-Z] (1.20 g) in
methanol (36 ml) and DMF (14 ml), Pd/C 10% (120 mg) was added. The mixture
was stirred and hydrogenated at room temperature and pressure for 2 h. The
mixture was filtered and the solid washed with methanoLThe leuated were pooled
Zs together and evaporated giving a viscous oil which was solubilised in
ethylacetate.
The resulting solution was washed with water and brine and dried on anhydrous
sodium sulfate. By evaporating the organic phase 870 mg of a white solid were
obtained.
HPLC (Method A3): rt =11.8 min.
3o MS (ES+): [MH+] = 584
b) Synthesis of Boc-Tro-Phe-t(R~NH-CH(CH2-C6H5~CH2-NH-[2-(4-nitro-
AMENDED SHEET
CA 02339638 2001-02-05
VSO 00/08046 PCT/EP99/05459
31
benzyloxycarbonyl)-4-Pert-butyl)-succin-1-yl]}.
To a solution of [2-(4-vitro-benzyloxycarbonyl)-succinic acid 4-fert-butyl
ester (424
mg) in DMF (20 ml), at 0°C, HOBt (490 mg), EDCI.HCI (250 mg) and Boc-
Trp-
Phe-[(R)-NH-CH(CH2-CgHS)-CH2-NH2] {700 mg) were added. The mixture was
s reacted for 2 h at room temperature. The solvent was eliminated by
evaporation
under vacuum an the resulting residue was treated with KHS04 aq. 5% to give a
solid which was filtered, washed with NaHC03 aq. 5%, water and dried under
vacuum on CaCl2 giving 1.05 g of a solid product.
MS (ES+): [MH+J = 919.
to HPLC (Method A4): rt =20.3 min.
c) Synthesis of cyclo{Suc[1-(4-vitro-benzyloxycarbonyl)-Trp-Phe-[(R)-NH-CH(CH2-
CgHS)-CH2-NH)-}
In 20 ml of TFA cooled at 0°C, 1.0 g of Boc-Trp-Phe-{(R~NH-CH{CH2-
CgHS~
CH2-NH-[2-(4-vitro-benzyloxycarbonyl)-4-tent-butyl)-succin-1-yl]} was added in
is small portions.
The mixture was reacted for 30' at 0°C, concentrated under vacuum and
diluted
with DMF, thereafter evaporated giving an oil which was treated with
diethylether
giving a solid. The solid was filtered and washed with diethylether giving a
yellow
amorphous solid which was H-Trp-Phe-{(R)-NH-CH(CH2-CgHS)-CH2-NH-[2-(4-
ao vitro-benzyloxycarbonyl)]-1-succinic acid. 710 mg of product were obtained.
To a solution of 200 mg of H-Trp-Phe-{(R~NH-CH(CH2-CgHS)-CH2-NH-[2-(4-
nitro-benzyloxycarbonyl)]-1-succinic acid in DMF (10 ml), under nitrogen at
0°C,
PyBOP (160 mg} and TEA (108 NI) were added; the solution was left under
stirring
at room temperature for 2 hours and thereafter sampled by HPLC. The solvent
2s was evaporated and the residue was solved in ethyiacetate. The organic
phase
was washed with KHS04 aq. 5%, NaHC03 aq. 5%, brine and was dried on
anhydrous sodium sulfate. After filtration and evaporation of the solvent 180
mg of
a residue were obtained.
This crude was purified by preparative HPLC (Method P8). Two products were
30 obtained (diastereisomers) which were indicated as "fast moving" (fm) and
"slow
moving" (sm). Obtained 62 mg (fm) and 15 mg (sm}.
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
32
MS (ES+): [MH+j(fm) _ [MH+](sm) = 745
HPLC (Method A3): rt(fm) =15.1 min, rt(sm} =15.6 min.
d) Compound cyclo{Suc[1-(carboxy]-Trp-Phe-[(R}-NH-CH(CH2C6H5}-CH2-NH]}
The compound cyclo{Suc[1-(4-vitro-benzyloxycarbonylrTrp-Phe-[(R~NH-
s CH(CH2-C6H5)-CH2-NHJ} "fast moving" (100 mg) was added to a mixture 1:1 of
waterlisopropanole (3 ml) containing K2C03 (34 mg). The reaction mixture was
reacted for 18 h at room temperature, concentrated, diluted with water and
extracted with ethylacetate to eliminate the unreacted product.
The aqueous phase was acidified with HCI 1 N up to the formation of a white
io suspension and extracted with ethylacetate. The organic phase of the second
extraction was dried on anhydrous sodium sulfate and evaporated to give 55 mg
of a white solid. The product was purified by preparative HPLC (Method P8).
Two products (diastereoisomers) were obtained having a different retention
time
by HPLC they were defined "fast' moving" (fm') and "slow' moving" (sm').
is Obtained 16 mg {fm') a 7 mg (sm').
MS (ES+): [MH+](fm') _ [MH+](sm') = 610
HPLC (Method A2): rt(fm') =13.7 min, rt(sm') =15.1 min
d') Compound cyclo{Suc[1-(carboxy]-Trp-Phe-[(RrNH-CH(CH2C6H5)-CH2-NH]}
The compound cyclo{Suc(1-(4-nitro-benzyloxycarbonylrTrp-Phe-[(R)NH-CH(CH2-
2o C6H5)-CH2-NH]} "slow moving" (50 mg) was added to a mixture 1:1 of
water/isopropanole (2 ml) containing K2C03 (17 mg). The reaction mixture was
reacted for 24 h at room temperature, concentrated, diluted with water and
extracted with ethylacetate to eliminate the unreacted product. The aqueous
phase was acidified with HCI 1 N up to the formation of a white suspension and
2s extracted with ethylcetate. The organic phase of the second extraction was
dried
on anhydrous sodium sulfate and evaporated to give 18 mg of a white solid. The
product was purified by preparative HPLC (Method P8}.
Two products (diastereoisomers) were obtained having different retention time
by
HPLC , they were defined 'fast' moving" (fm') and "slow' moving" (sm').
3o Obtained 7 mg (fm') a 6 mg (sm').
MS (ES+): [MH+](fm') _ [MH+](sm') = 610
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
33
HPLC (Method A2): rt(fm') =13.7 min, rt(sm') =15.1 min
Compound cyclo{Suc[1-(4-morpholino)carbonyl]-Trp-Phe-[(R)-NH-CH(CH2-
C6H5~CH2NH]
To a solution of cyclo{Suc[1-(carboxy]-Trp-Phe-((R)-NH-CH(CH2C6H5rCH2-NH]}
s (product "fast'moving", 20 mg) in DMF (1 ml), HOBT (24 mg), EDCI.HCI (12 mg)
and morpholine (10 NI) were added in the given order. After 24 h stirring the
reaction mixture was diluted with 3 ml of a mixture water/acetonitrile 80:20
containing 0.1 % of TFA and purified by preparative HPLC (Method P5). 7 mg of
a
white solid were obtained.
io MS (ES+): [MH+] = 679
HPLC (Method A2): rt =14.8 min.
With the same procedure the following compound was obtained
EXAMPLE 48: cyclo{Suc(1-(4-hydroxyethyloxyethyl-piperazin-1-yl)carbonyl]-Trp-
Phe-[(R)-NH-CH(CH2-C6H5)-CH2NH))
is (compound of general formula I wherein R4 is (4-hydroxyethyloxyethyl-
piperazin-
1-yl)carbonyl and the other substituents are as described in EXAMPLE 47)
HPLC (Method A2): rt =11.9 min.
MS: m/z = 766.2 (MH+)
Preparative HPLC Methods
2o Mobile phase: A = H20 + 0.1 % TFA; B = CH3CN + 0.1 % TFA
Method P1:
Column: Deltapak RP18 10 N, 100 A, 19 x 300 mm
Gradient from A:B = 75:25 to A:B = 15:85 in 120 min
Flow rate: 15 mllmin
2s I = 220, 270 nm
Method P2:
Column: Symmetry RP18 7 N 100 A, 19 x 300 mm
Gradient from A:B = 75:25 to A:B = 15:85 in 120 min
Flow rate: 15 ml/min
3o I = 220, 270 nm
Method P3:
CA 02339638 2001-02-05
WO 00/08046 PC'f/EP99/05459
34
Column: Vydac RP18 20 N, 22 x 250 mm
Gradient from A:B = 90:10 to A:B = 30:70 in 120 min
Flow rate: 15 ml/min
I = 220, 270 nm
s Method P4:
Column: Symmetry RP18 7 N 100 A, 19 x 300 mm
Gradient from A:B = 85:15 to A:B = 25:75 in 60 min
Flow rate: 15 ml/min
I = 220, 270 nm
io Method P5:
Column: Vydac RP18 20 N, 22 x 250 mm
Gradient from A:B = 80:20 to A:B = 20:80 in 120 min
Flow rate: 20 mllmin
I=240nm
is Method P6:
. Column: Symmetry RP18 7 N 100 A, 19 x 300 mm
Gradient from A:B = 80:20 to A:B = 50:50 in 60 min, then from A:B = 50:50 to
A:B
= 20:80 in 120 min.
Flow rate: 15 ml/min
2o i = 220, 270 nm
Method P7:
Column: Symmetry RP18 7 N 100 A, 19 x 300 mm
Gradient from A:B = 83:17 to A:B = 23:77 in 120 min
Flow rate: 15 ml/min
2s 1= 220, 270 nm
Method P8:
Column: Delta PakTM, C18, 10 N, 100 A, 19 x 300 mm
Gradient from A:B = 75:25 to A:B = 20:80 in 120 min
Flow rate: 15 mllmin
3o I = 220, 270 nm
Analytical HPLC Methods
Mobile phase: A = H20 + 0.1 % TFA; B = CH3CN + 0.1 % TFA
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
Method A1:
Column: Symmetry C1 g 5m, 100 A, 3.9 x 150 mm
Gradient from A:B = 80:20 to A:B = 14:86 in 20 min followed by A:B = 14:86 for
6
min
s Flow rate: 1 ml/min
I = 220 nm
Method A2
Column: tuna 5N, C8(2), 100A, 4.6 x 250 mm
Gradient from A:B = 80:20 to A:B = 20:80 in 20 min
io Fiow rate: 1 ml/min
I = 220, 270 nm
Method A3:
Column: Symmetry Cg 5m, 100 A, 3.9 x 150 mm
Gradient from A:B = 80:20 to A:B = 20:80 in 20 min
is Flow rate: 1 ml/min
I = 220, 270 nm
Method A4:
Column: Symmetry Cg 5m, 100 A, 3.9 x 150 mm
Gradient from A:B = 80:20 to A:B = 20:80 in 20 min followed by A:B = 20:80 for
6
2o min
Flow rate: 1 ml/min
I = 220, 270 nm
Abbreviations: For the nomenclature of the amino acids and corresponding
abreviations reference is made to IUPAC-IUB Joint Commission on Biochemical
2s Nomenclature( Eur. J. Biochem. 1984, 138, 9 ); if not otherwise specified
the
aminoacids are in the S-configuration. The other abbreviation used are: aq. -
aqueous solution; Bzl - benzyl; DMF - dimethylformamide; EDCI - 1-(3-
dimethylaminopropyl)3-ethylcarbodiimide; Fmoc - fluorenylmethyloxycarbonyl;
PyBOP = benzotriazol-1-yl-oxy-tris-pyrrolidino-phosphonium
hexafluorophosphate;
3o TEA = triethylamine; TFA = trifluoroacetic acid; Z = Cbz = N-
benzyloxycarbonyl,
Boc = tert-butoxycarbonyl; -Suc- = succinyl; DIEA = N,N-diisopropylethylamine;
DMF - N,N-dimethylformamide; NKA - neurokinin A; HOBt - 1-
CA 02339638 2001-02-05
13-07-2000 EP 009905459
hydroxybenzotriazole; rt = retention time; THF = tetrahydrofuran. The
numbering
of the substituents on the succinic group indicated as -Suc(1-NH2~ is realised
with R4 = NH2 and X3 and X4 = CONR.
Biological Activity
s The compounds described in the present invention act as antagonists on the
NK2
receptor of tachykinins
The biological activity was tested in three different functional tests in
vitro using
rabbit pulmonary arteria (RPA), hamster trachea (HT) and rat urinary bladder
(RUB) according to the methods described by Maggi C.A. et al. Br. J.
Pharmacol.
io 1990, 100, 588, D'Orleans-Juste P. et al. Eur. J. Pharmacol. 1986, 125, 37
a
Maggi C.A. et al. J. Pharmacol. Exp. Ther. 246, 308, 1988. The affinity of the
compounds for the human NK2 receptor was evaluated in a test of binding using
membranes of CHO (Chinese hamster ovary) cells transfected with the NK-2
receptor of human ileum and the radioligand ['~'I]NKA (Amersham, specific
is activity 2000 Ci/mmol) at the concentration of 100 pM in studies of
competition.
The examined compounds were tested in a range of concentration comprised
between 0.01 nM and lOmM. After incubation (30 min., 20°C) the samples
were
ftltered and the radioactivity was determined using a gama-counter.
The data collected by functional studies are expressed as pA2 (Arunlakshana O.
2o and Schild H.O., Br. J. Pharmacol. Chemother. 1959, 14, 45) and those
deriving
from studies of binding are expressed as pKi (-log Ki calculated with the
program
LIGAND: Munson P.J. et al. Anal. Biochem. 1980, 107, 220).
The compounds of the invention showed good activity in all the above said
tests
with values of pAz up to 9.5 and values of pKi up to 10.6
AMENDED SHEET
CA 02339638 2001-02-05
WO 00/08046 PCT/EP99/05459
37
Activity Table
Compound pKi pA2
s (EXAMPLE) RPA HT RUB
W09834949; ex 27 8.5 7.8 8.5
W09834949; ex 34 8.6 7.8 8.5 8.0
W09834949; ex 35 8.6 8.4 8.5
io W09834949; ex 36 8.7 7.9
W09834949; ex 37 8.8 8.2
W09834949; ex 39 8.8
W09834949; ex 40 7.9 7.6 7.5
W09834949; ex 44 8.2 7.8 7.9
i ex. 1 10.2 9.2 9.1
s
ex. 3 9.7 8.8 9.0
ex. 5 10.6 9.0 9.1
ex. 7 9.8 8.8
ex. 14 9.0
2o ex. 16 10.3 9.5
ex. 31 9.2 8.7
ex. 32 9.3 9.0
ex. 34 9.5 9.0
ex. 38 9.9 9.1
2s ex.39 9.3 9.2
ex. 40 9.7 8.9
ex. 48 9.2 9.0