Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
ANTIFREEZE COOLING SUBSYSTEM
Field Of The Invention
The present invention relates to cooling subsystems for systems which may be
stored at and started from temperatures below the freezing point of water.
More
particularly, the invention relates to glycol-based cooling subsystems and
methods for
obtaining satisfactory conductivity and corrosion characteristics using glycol-
based
coolants. The invention is particularly suitable for solid polymer fuel cell
systems used
in automotive applications and back-up or remote power plants.
Background Of The Invention
Fuel cell systems have been in use for specialty applications (e.g. space
capsules,
sensors) and have been under development for broader applications (e.g.
stationary
power plants, transportation) for many years now. With continued advances,
performance has been improved and costs have been reduced such that many of
these
latter fuel cell systems under development are entering commercial use.
However, in
order to meet the needs of a less specialized market, these fuel cell systems
must be
able to handle a wide range of user conditions, ideally with minimal
additional
complexity to the system. For instance, the ambient temperature and duty cycle
can
vary widely in different applications. lt can be a challenge to meet these
requirements,
particularly when the application involves frequent storage and start-up in
cold
conditions.
A particularly attractive fuel cell is the solid polymer electrolyte fuel
cell. This type
of fuel cell employs an ion conducting membrane as the electrolyte. An
individual
solid polymer fuel cell generally comprises a membrane electrode assembly
(MEA)
containing an ion conducting membrane interposed between a cathode and an
anode.
The ion conducting membrane in the MEA serves as a separator as well as the
electrolyte. Catalyst, for promoting the reactions in the fuel cell, is
located at the
interface between the electrodes and the membrane. Generally, flow field
plates are
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
-2-
positioned adjacent to each electrode for purposes of distributing the fuel
and oxidant
reactants to the appropriate electrodes. The flow field plates also typically
serve as
current collectors, electrode; supports, and separators. Since the operating
voltage of an
individual cell is usually under I volt, most fuel cell systems employ
numerous cells
that are stacked in series to create a higher voltage fuel cell stack.
The electrochemical reactions in a PEM fuel cell proceed more favorably at
higher
temperatures. However, the operating temperature must be limited in order to
prevent
damage to the membrane material. The typical operating temperature of a
hydrogen-
fueled solid polymer fuel ce;ll is under 100 C, which is relatively low
compared to
other types of fuel cells. Since the electrochemical reaction between fuel and
oxidant is
exothermic, temperature regulation generally involves cooling of the solid
polymer fuel
cell stack, hence the temperature regulating subsystem is commonly called the
cooling
subsystem. (However, the cooling subsystem might also desirably serve as a
heating
subsystem during cold start-up in order to bring the fuel cell up to the
desired operating
temperature more quickly.) Solid polymer fuel cell systems are typically
liquid-cooled
rather than air-cooled especially if higher power densities (power output
capability per
unit volume) are desired. T'he reason is that the cooling subsystems typically
must shed
a significant amount of heat at relatively low temperature (circa 80 C) with
respect to
ambient. The use of more e;fficient liquid- as opposed to air-cooling allows
the fuel cell
stack cooling channels to be made smaller and hence a lower overall stack
volume can
be obtained.
In some stationary power applications, a fuel cell system may operate
uninterrupted
for long periods, albeit at varying power levels. However, more commonly
perhaps, a
fuel cell system is subjectecl to frequent on-off cycles and hence it goes
through
numerous cold starts. For outdoor applications in cold climates, this can mean
frequent
shutdowns and storage in sub-zero temperatures. The fuel cell system, and
particularly
the cooling subsystem, must therefore be able to handle repeated storage below
freezing without significant degradation. For example, this requirement
applies to fuel
cell systems for automotive use.
Today's liquid-cooled, i.nternal combustion engine powered automobiles face a
similar requirement. To prevent freezing and hence rupturing of the cooling
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
-3-
subsystems therein, antifreeze is added to the aqueous coolant. The antifreeze
added is
typically ethylene glycol but other antifreeze coolants such as propylene
glycol,
alcohols, and the like can be used. Ethylene glycol transfers heat well, has
superior heat
capacity, and poses less of a fire hazard (e.g., has a flash point greater
than 100 C).
Depending on the concentration, an aqueous mixture of ethylene glycol stops
the
coolant from freezing at temperatures down, for example, to -40 C.
Along with an antifreeze coolant, other additives are used in aqueous
automotive
and other industrial cooling subsystems in order to slow the corrosion of the
metallic
components in the coolant circulation loop of the cooling subsystem. For
instance,
1o silicates are commonly added to automotive coolants in order to protect
aluminum
components in the circulation loop. While corrosion is an issue with any
aqueous
coolant, corrosion can be accelerated by the use of certain antifreeze
coolants. Unlike
water, ethylene glycol and propylene glycol decompose in the presence of
oxygen to
form acidic by-products such as glycolic and lactic acids respectively. The
presence of
these by-products can significantly accelerate corrosion in a coolant
circulation loop.
Further, the rate of decomposition increases with temperature and in the
presence of
transition metals. Thus, the high temperatures (circa 200 C) and metal
constructions of
conventional automotive coolant circulation loops significantly increase the
rate of
glycol decomposition and hence corrosion. For this reason, inhibitors (e.g.
buffers) can
also be added to the glycol--based coolants in order to reduce the
decomposition of the
glycol. Further, cooling subsystems are typically closed (sealed) when
operating at
temperatures above about 60 C in order to avoid rapid oxidation of the glycol.
More
details on this subject can be found in Dow Chemical Company's "Engineering
and
Operating Guide for Ambitrol Inhibited Glycol-based Coolants", Sept 1991.
Historically, glycols, such as ethylene glycol, have been used in alkaline
fuel cell
systems as the fuel itself. Glycol-based coolants have been suggested for use
in the
cooling subsystems of certain fuel cell systems. For instance, U.S. Patent No.
3,507,702 suggests the use of ethylene glycol in the coolant circuit for an
aqueous
alkali electrolyte fuel cell. Therein, the embodiments and discussion pertain
to low
voltage fuel cell stacks (e.g. 30 V or less) and thus there would be no
significant
concern about electrical shock hazards through the coolant fluid. There is no
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
-4-
discussion regarding corrosion, additives/inhibitors, or removal of ions in
the coolant
subsystem. Japanese published patent application number 08-185877 discloses an
antifreeze coolant system employing ethylene glycol wherein pure water for
humidification is obtained via ultrafiltration from the antifreeze coolant.
However, no
means for maintaining the purity of the antifreeze coolant over time appears
to have
been provided.
The coolant subsystem in high voltage fuel cell stacks (above about 50 V) can,
however, present an electrical shock hazard. If the coolant is sufficiently
conductive
and is in electrical contact with and interconnects parts of the fuel cell
stack that are at
1 o different potentials, the coolant fluid can pose a safety problem.
Further, the coolant
also provides a path for the flow of undesirable corrosion currents. These
problems are
discussed and addressed in U.S. Patent No. 3,964,930 wherein various means of
electrical isolation (such as coolant tube coatings) are employed in
combination with a
water-based coolant. A conductivity of less than about 50 S/cm is stated to
be
preferred for the water coolant.
Generally, the electrical conductivity of an aqueous coolant increases with
the
concentration of ions in solution. In some conventional high voltage fuel cell
systems,
shock and corrosion current concerns are dealt with by using substantially
pure de-
ionized water as the coolant. An acceptable level for the conductivity of the
de-ionized
coolant is considered to be of order of 5 S/cm or less.
Substantially pure, de-ionized water is also desirably used in coolant loops
where
there is a possibility of the coolant contaminating or damaging MEA components
(such
as the electrocatalyst and rr-embrane electrolyte) of the fuel cell. Since
pure, de-ionized
water is fundamentally conipatible with the MEA components, fuel cell design
and
construction may be simplified to allow some contact of the coolant with the
MEA
components. Note that, even in constructions that attempt to prevent such
contact (e.g.
constructions having isolated piping or redundant seals), there can still be
reliability
concerns regarding contact resulting from occasional leaks.
Ion exchange resin units and other filters are frequently employed in de-
ionized
water coolant loops of fuel cell systems to continually remove contaminants
and
thereby ensure that the water coolant fluid remains substantially free of
ionic
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
-5-
contaminants. For example;, U.S. Patent No. 5,200,278 discloses a fuel cell
system
having de-ionized liquid water coolant that is also used for membrane
humidification of
inlet reactant streams. The water is preferably de-ionized using ion exchange
resin units
in the loop.
Where tolerance to freezing is required, conventional glycol-based antifreeze
coolants containing additives may be used in high voltage, fuel cell systems.
However,
the coolant subsystem should be reliably isolated electrically and physically
from the
MEAs in the fuel cell stack, so that electrical shock, corrosion shorting, and
contact
with the MEA components are not a concern. The use of glycol without
lo additives/inhibitors might be considered as an alternative to isolating the
cooling
subsystem but it adds to corrosion concerns over those posed by use of water
alone, due
to the decomposition of glycol into acidic by-products. Consequently, it
appears that
the use of glycols has been avoided in the coolant of high voltage fuel cell
systems that
do not have electrically isolated cooling subsystems.
Other antifreeze solvents such as other alcohols and dielectric fluids have
been
contemplated but these may introduce a significant fire hazard (e.g., due to a
lower
flash point) and/or have poorer heat transfer and capacity characteristics.
Instead,
solutions have been developed to cope with subzero conditions using pure water
coolants, e.g. by keeping the system above zero degrees or by removing all
water from
the system prior to shutdown.
Summary Of The Invention
Improved cooling subsystems for liquid-cooled systems (e.g., liquid-cooled
fuel cell
systems used in fuel cell powered vehicles or other applications), and methods
for
providing simplified antifreeze and corrosion protection therein are provided.
A
cooling subsystem for cooling a fuel cell stack includes a liquid coolant and
a
circulation loop for circulating the liquid coolant in thermal contact with
fuel cells in
the stack. The liquid coolant includes a glycol solvent for antifreeze
protection and
may be a glycol/water mixture. The ratio of glycol to water may be selected.
to give the
desired antifreeze protection, for instance about 1: l. However, the glycol-
containing
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
-6-
liquid coolant is characterized by a conductivity less than about 50 S/cm and
this level
is maintained during operation of the fuel cell system by including means for
maintaining the purity of the liquid coolant in the cooling subsystem. A
conductivity of
less than about 5 S/cm is preferred.
A preferred means for maintaining the desired purity of the liquid coolant is
to
incorporate an ion exchange resin unit in the circulation loop of the cooling
subsystem
whereby ionic decomposition products of the glycol solvent are removed from
the
liquid coolant. The ion exchange resin unit may comprise an alkaline anion
resin and,
optionally, an acidic cation resin. A suitable ion exchange resin unit may
employ, for
i o example, an hydroxyl type 2 strong base anion resin.
Use of ion exchange resin units in glycol-based coolant loops can be a
practical
solution for achieving antif'reeze protection under the operating conditions
of certain
fuel cell systems. For instance, in solid polymer fuel cell stacks operating
at less than
about 100 C, the extent of glycol decomposition may be limited such that an
ion
exchange resin unit can provide months of service before needing replacement.
Further, since conductivity levels less than about 5 S/cm can be achieved,
the use of
an ion exchange resin unit in. the coolant loop is suitable for fuel cell
systems operating
at hundreds of volts.
A glycol solvent is defined herein as one which does not contain the
inhibitors
and/or additives commonly present in commercial antifreeze glycol solutions.
In the
absence of such additives and/or inhibitors, decomposition of the glycol
solvent is the
main source of impurities in the cooling subsystem leading to high coolant
liquid
conductivities. If the liquid coolant is in electrical contact with fuel cells
in the fuel cell
stack, high coolant conductivities result in shock and corrosion current
problems in
high voltage fuel cell systems. Herein, high voltage refers to systems
comprising fuel
cell stacks operating above about 50 volts.
The glycol solvent employed may be selected from the more common glycols, such
as ethylene glycol, propylene glycol, polyethylene glycol, and polypropylene
glycol.
Ethylene glycol has been found to be more compatible with the membrane
electrode
assemblies of solid polyme;r fuel cells and is therefore a preferred choice.
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
-7-
The circulation loop offthe cooling subsystem may comprise aluminum hardware
which is exposed to the liquid coolant. In order to reduce decomposition of
the glycol
solvent, the coolant circulation loop in the cooling subsystem is preferably
essentially
isolated from air.
Brief Description Of The Drawings
Figure 1 a is a schematic diagram of an embodiment of a solid polymer fuel
cell system
which includes a sealed coolant subsystem comprising a glycol-based coolant
and an
io ion exchange resin unit.
Figure 1 b is a schematic diagram of an embodiment of a solid polymer fuel
cell system
for a fuel cell powered vehicle which includes a sealed coolant subsystem
comprising a
glycol-based coolant and an ion exchange resin unit.
Figure 1 c is a schematic diagram of an alternative embodiment of a solid
polymer fuel
cell system for a fuel cell powered vehicle which includes a sealed coolant
subsystem
comprising a glycol-based coolant and an ion exchange resin unit.
Figure 2 shows the pH versus time of the comparative and test glycol/water
mixtures
exposed to metal pieces in Example 1.
Figure 3 shows the pH versus time for the glycol/water mixture of Example 2
wherein
an ion exchange resin unit is employed to maintain the purity of the mixture.
Figure 4 shows the conductivity versus time for the glycol/water mixture as
above
wherein an ion exchange resin unit is employed to maintain the purity of the
mixture.
Figure 5 shows the voltage versus current density for the test fuel cell and
the
comparative fuel cell of Example 3 wherein the membrane electrode assembly of
the
test fuel cell was exposed to an ethylene glycol/water mixture.
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
-8-
Figure 6 shows the voltage versus current density for the test fuel cells and
the
comparative fuel cell of Example 3 wherein the membrane electrode assemblies
of the
test fuel cells were exposed to various glycol solvents.
Detailed Description Of The Preferred Embodiments
A fuel cell system of the invention is liquid-cooled and includes a fuel cell
stack, a
liquid coolant, and a circulation loop for circulating the liquid coolant in
thermal
contact with fuel cells in the stack. The liquid coolant comprises a glycol
solvent for
antifreeze protection. Preferably, a glycol/water mixture is used as the
liquid coolant.
The ratio of glycol to water is selected to provide the desired level of
protection against
freezing (a 1:1 ratio provides protection down to about -40 C and is not
flammable).
Simplified protection against electrical shock and corrosion current problems
in high
voltage fuel cell stacks is provided by suitable means for maintaining the
purity and
hence the conductivity of'the liquid coolant below about 50 S/cm.
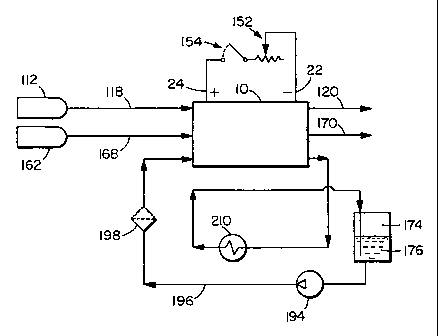
Figure la shows a schematic of a preferred fuel cell system comprising a high
voltage solid polymer fuel cell stack 10, a glycol/water liquid coolant 176, a
circulation
loop 196, and an ion exchange resin unit 198 in the circulation loop 196. The
circulation loop 196 also includes a circulation pump 194 and a heat exchanger
210. In
this schematic drawing, a supply of glycol/water liquid coolant 176 is
provided in
reservoir 174.
The solid polymer fuel cell system of Figure 1 a may be conventional in other
respects. Reactant streams, fuel 112 and oxidant 162, are supplied to fuel
cell stack 10
via inlets 118 and 168 respectively. tlsually, for gaseous reactants, one or
both of the
reactant streams are humidified before being supplied to the fuel cell stack
to prevent
the membrane electrolyte from drying out. The fuel and oxidant exhaust streams
exit
the stack through outlets 120 and 170 respectively. If, for example,
substantially pure
hydrogen is used as the fuel, the fuel exhaust can be recirculated so fuel is
not wasted.
Useable electric power is obtained via the depicted external circuit
comprising negative
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
-9-
and positive bus plates 22 and 24 respectively, variable load 152 and
contactor switch
154.
The ion exchange resin unit 198 contains at least an anion exchange resin to
remove
ionic decomposition products of the glycol solvent. Suitable anion exchange
resins
include an hydroxyl type 2 strong base anion resin. Cation exchange resin
units and/or
other filtration units (e.g. charcoal filters) may optionally be incorporated
as well to
eliminate other impurities that may accumulate in the coolant loop.
An embodiment of a fuel cell system for a fuel cell powered vehicle is shown
in the
schematic diagram of Figur.e lb. Therein, coolant is pumped through
circulation loop
lo 11 by pump 3. As shown, circulation loop 11 branches into three parallel
lines leading
to a heat exchanger 7, ion exchange resin unit 8, and fuel cell 9. The coolant
is then
directed into a single line again to thermostatic valve 2. Depending on the
coolant
temperature, the coolant can be directed by thermostatic valve 2 to radiator I
for
cooling or can be directed to by-pass radiator 1 and go directly to pump 3.
Ion
exchange resin unit 8 may be placed at other locations in circulation loop 11.
It is also
possible to employ more than one ion exchange unit in the circulation loop.
An alternative embodinient of a fuel cell system comprising two coolant
circulation
loops for a fuel cell powered vehicle is shown in the schematic diagram of
Figure 1 c.
In a like manner to Figure 1. b, high purity glycol/water coolant (e.g.,
having a
conductivity below about 50 S/cm) is pumped through first circulation loop I
by pump
6. As shown, first circulation loop I branches into three parallel lines
leading to a heat
exchanger 7, ion exchange :resin unit 8, and fuel cell 9. The coolant is then
directed
into a single line to another heat exchanger 5 in which heat is exchanged
between the
coolant in first circulation loop I and the coolant in second circulation loop
II.
Circulation loop II however does not contain an ion exchange resin unit and
the coolant
therein may comprise a mixture of water and commercial antifreeze solution
containing
inhibitors and/or other additives. Coolant is pumped through second
circulation loop II
by pump 3 and, as shown, branches into two parallel lines leading to heat
exchangers 4
and 5. Again, depending on the coolant temperature, the coolant can be
directed by
thermostatic valve 2 to radiator 1 for cooling or can be directed to by-pass
radiator 1
and go directly to pump 3.
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
- 10-
The glycol solvent employed can be one of the more common glycols, such as
ethylene glycol, propylene glycol, polyethylene glycol, and polypropylene
glycol.
Ethylene glycol is a preferred antifreeze solvent in other applications for
reasons of
viscosity, heat exchanger efficiency, and freezing point depression. Further,
it seems to
be reasonably compatible with certain membrane electrode assemblies and thus
is a
preferred solvent in case of'subsystem leaks that might result in contact of
the coolant
with a membrane electrode assembly. Optionally, a mixture comprising more than
one
glycol solvent might be used in the liquid coolant.
Experimentation has shown that the decomposition rate of the glycol and the
corrosion of aluminum harclware exposed to the coolant at the typical
operating
temperatures of solid polynier fuel cells does not overwhelm a conventional
ion
exchange resin unit. Thus, aluminum components, e.g. a radiator, may be used
in the
circulation loop. Other metals or plastic components might be used but they
must be
screened in order to determine if they either accelerate glycol decomposition
or corrode
to produce soluble ionic impurities in the pure coolant (e.g. some stainless
steel
components will accelerate decomposition but will not themselves corrode).
However,
in order to reduce the decornposition of the glycol solvent, the circulation
loop in the
cooling subsystem should be sealed so that the coolant is not freely exposed
to air.
Also, the use of dissimilar rnetals in the coolant loop may lead to galvanic
corrosion
and this is to be avoided where possible.
In this way, the conductivity of the glycol-containing coolant can be kept
acceptably low (below about 5 S/cm) for reasonable lengths of time (months).
Further, the pH of the coolant can also be kept desirably neutral. Thus, use
of an ion
exchange unit in the cooling subsystem represents a simplified method of
protecting
against electrical shock and corrosion current using an antifreeze coolant.
However, other means for maintaining the necessary purity of the coolant might
be
employed instead. For instance, means for suitably reducing the rate of
decomposition
might be used instead of means for removing the ionic impurities after
decomposition
takes place. An alternative might therefore involve scavenging oxygen in the
circulating coolant thereby retarding the decomposition rate (e.g. by bubbling
the fuel
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
-11-
exhaust stream through a coolant reservoir or using a de-oxidizing resin such
as
PuroliteTM A3 l OLC that removes dissolved oxygen).
The following examples have been included to illustrate different embodiments
and
aspects of the invention but these should not be construed as limiting in any
way.
Example 1
Three flasks containing a 1:1 mixture of reagent grade ethylene glycol and de-
ionized water were prepared. The first flask was left as is. Pieces of both
aluminum
and steel were placed in each of the second and third flasks. The third flask
also
contained a sodium phosphate pH buffer. The flasks were then stored at 80 C in
air,
and pH and AC conductivity measurements (at I KHz) were taken at periodic
intervals.
Figure 2 shows the pH of the three water/glycol mixtures versus time. The pH
in both
unbuffered mixtures fell significantly over a period of about 10 days. The
decrease was
somewhat faster for the mixture in the second flask presumably due to the
presence of
the metal pieces. The conductivity of both unbuffered mixtures (the first and
second
flasks) stayed in the range from about 10-20 S/cm for the monitoring period.
The pH
of the buffered mixture in the third flask remained substantially neutral (pH
about 7)
over the same period, but its conductivity was about 1000 S/cm due to the
presence of
the buffer.
This example illustrates that the pH of an unbuffered de-ionized
water/ethylene
glycol mixture may be unacceptable (too acidic) in a fuel cell cooling system
in just a
few days. A conventionally buffered solution may maintain an acceptable pH but
has
an unacceptably high conductivity (>>50 S/cm).
Example 2
Approximately 10 litres of a 1:1 mixture of commercial automotive antifreeze
(containing ethylene glycol and inhibitors) and de-ionized water was prepared
and the
conductivity was measured to be over 1000 S/cm. The mixture was then
circulated in
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
- 12-
a circulation loop comprising an ion exchange resin unit to remove ions
present in the
commercial antifreeze. The ion exchange resin unit contained 500 mL of a
strong base
type 2 anion resin. Also, the unit contained 500 mL of a sulfonic acid type
cation resin
and 500 mL of activated carbon. The hardware which contacted the coolant
mixture in
the circulation loop included an aluminum radiator, 316 stainless steel
fittings, and
either PTFE or UltemTM (product of GE Plastics) plastics.
After a period, the ion exchange cartridge was presumed to be saturated and
was
replaced. Circulation continued with the circulation loop isolated from air at
80 C.
Again, pH and conductivity measurements were taken at periodic intervals.
Figures 3
1o and 4 show the pH and conductivity of the mixture respectively versus time.
When the replacement cartridge was initially installed, the mixture had a
undesirably low pH of aboirt 5.5 and an undesirably high conductivity of about
300
S/cm. However, shortly thereafter the mixture was sufficiently purified such
that a
pH of about 7 and a conductivity of less than 5 S/cm were obtained. These
levels
were maintained for over 60 days whereupon the pH of the mixture began to
fall,
presumably as a result of saturation of the ion exchange cartridge with
decomposition
products from the ethylene glycol. (Note that Fig. 3 shows an apparent
temporary drop
in pH after about 900 hours which was due to pH meter error.)
This example shows that an acceptable pH and conductivity can be obtained and
maintained using an ethylene glycol/water liquid coolant in combination with
an ion
exchange resin unit. Although the ion exchange unit may need periodic
replacement or
servicing, the frequency is of order of every few months, not hours, and is
thus
practical.
Example 3
A liquid-cooled fuel cell system equipped with a glycol-based cooling
subsystem
and ion exchange resin unit in the coolant circulation loop was operated for
about 1000
hours at 80 C. The comporients present in the circulation loop included a
radiator, heat
exchangers, circulation pump and housing, and coolant lines. The coolant
comprised
50% ethylene glycol and 50% water by volume. The ion exchange resin unit
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
- 13-
comprised a mixed-bed of a strongly acidic cation exchange resin and a
strongly
alkaline anion exchange resin. After 1000 hours of operation, the components
in the
circulation loop were examined and showed no significant corrosion. The
conductivity
of the coolant after 1000 hours of operation was also measured and was found
to be less
than 10 S/cm.
This example confirms that such a liquid-cooled fuel cell system can be
operated
for a substantial period of time without suffering significant corrosion and
without
needing replacement or servicing of the ion exchange unit.
Example 4
Various individual PEM fuel cells were constructed and operated for purposes
of
evaluating the effect of glycol exposure on the MEA therein. The MEAs
comprised
electrodes made of platinum loaded carbon fibre paper and a NafionTM 112
membrane
electrolyte. Pressurized anci humidified air and hydrogen gases were used as
the
reactants and the test fuel cells operated at about 80 C.
In one trial, a 0.38:0.62 mixture of reagent grade ethylene glycol and de-
ionized
water was prepared and manually painted on the cathode of a test fuel cell
before
construction, thereby exposing the cathode to ethylene glycol. A similar fuel
cell was
also constructed without treating with ethylene glycol for comparative
purposes. The
two fuel cells were then tested for power output. Figure 5 shows the voltage
versus
current density performance characteristics for the ethylene glycol treated
test cell and
the untreated comparative cell. There was no significant difference in the
performance
characteristics.
In another trial, various unmixed reagent grade glycol solvents (i.e. ethylene
glycol,
propylene glycol, polyethylene glycol, and polypropylene glycol) were painted
on the
electrodes of a series of test fuel cells prior to construction. Again, an
untreated
comparative fuel cell and the test fuel cells were tested. Figure 6 shows the
voltage
versus current density performance characteristics for these fuel cells. Here,
the fuel
cell treated with unmixed ethylene glycol performed slightly worse than the
untreated
comparative fuel cell. The fuel cells treated with propylene glycol and
polyethylene
CA 02344856 2001-03-20
WO 00/17951 PCT/CA99/00850
-14-
glycol showed progressively worse performance respectively. The fuel cell
treated with
polypropylene glycol would not operate at 100 amps per square foot (ASF) and
thus its
performance is not shown.
This example shows that fuel cell performance can still be acceptable even if
the
MEA is exposed to a glyccil solvent employed in the cooling subsystem. Of the
glycols
tested, ethylene glycol seems most compatible with the MEA and is thus
preferred.
While particular elements, embodiments and applications of the present
invention
have been shown and described, it will be understood, of course, that the
invention is
1o not limited thereto since modifications may be made by those skilled in the
art without
departing from the spirit and scope of the present disclosure, particularly in
light of the
foregoing teachings.