Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02347915 2001-04-26
WO 00/Z5819 PCT/US99/25558
- 1 -
Enhanced Radiation Thera
Cross-Reference to Related Applications
This application claims priority from U.S. Patent
Application Serial No. 09/183,166, filed on October 29,
1998, and U.S. Provisional Application Serial No.
60/131,418 filed on April 28, 1999, which are both
incorporated herein by reference in their entirety.
Field of the Invention
The invention relates to new methods of enhancing
radiation therapy, e.g., for tumor therapy.
Background of the Invention
Radiation therapy has been used with some success
in treating tumors and other diseases. However, the dose
of effective radiation must be sufficiently limited to
the tumor or other target tissue to avoid injuring the
surrounding tissues and the overall health of the
patient.
Some efforts have been made to enhance the
absorption of radiation by tumors compared to normal
tissues adjacent to the tumor and throughout the body.
For example, it has been shown that the presence of
iodinated x-ray contrast agents within animal tumors
during treatment with an external computed tomographic
(CT) device operating in the orthovoltage range can
somewhat improve treatment response.
Summary of the Invention
The invention is based on the discovery that by
using controlled combinations of (i) specific radiodense
compositions, (ii) specific modes of administration of
these radiodense compositions, and (iii) specific energy
bands and sources of radiation, that the effect of
CA 02347915 2001-04-26
WO 00/25819 PCTNS99/25558
- 2 -
radiation on tumors and other diseased tissues can be
effectively and safely enhanced to provide significantly
improved radiation therapy.
In general, the invention features a method of
treating a target tissue, e.g., a tumor, in a patient by
administering to the patient systemically a radiodense
composition including a small molecule radiodense
material in an amount sufficient to accumulate
selectively within the target tissue compared to non-
target tissue; and inserting a radiation emitting source,
e.g., a probe or radiopharmaceutical, into the target
tissue and irradiating the target tissue from within for
a time and under conditions sufficient to kill cells
within the target tissue. For example, the radiodense
composition can accumulate selectively at the outer edge
of the target tissue, and/or penetrate into the tissue.
In specific embodiments, the radiodense
composition is administered intravenously as a bolus,
followed by an infusion of the same or a different
radiodense composition at a rate that equals the blood
clearance rate of the first radiodense composition. The
radiodense composition can be iohexol, iopamidol,
ioversol, ioxilan, iomeprol, or iodixanol.
In other embodiments, the amount of the radiodense
composition administered is sufficient to increase the
radiation absorption of the outer edge of the target
tissue by at least 10, 50, 100, or 200 Hounsfield units
(HU), or more, e.g., 300, 500, or 1000 HU, the radiation
can have an energy of less than 140 kiloelectron volts,
e.g., about 20 to 80, or 40, kiloelectron volts, or more
than 1.02 megaelectron volts, e.g., 5, 10, or more
megaelectron volts, and the radiodense composition can be
linked to a targeting agent that binds specifically to
the target tissue.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 3 -
In another aspect, the invention features a method
of treating a target tissue, e.g., a tumor or diseased
skin or a lymph node, in a patient by administering to,
e.g., into, the target tissue, e.g., by direct injection
or by painting the composition onto the skin, an amount
of a radiodense composition; and irradiating the target
tissue with an external radiation source emitting
radiation at an energy of less than 140 kiloelectron
volts (Kev), e.g., at 20, 40, or 80 Kev, or more than
1.02 megaelectron volts (Mev) for a time and under
conditions sufficient to kill cells within the target
tissue. For example the radiodense material can be
administered to the target tissue in a stmt implanted
within or adjacent to the target tissue.
In specific embodiments, the amount of the
radiodense composition is sufficient to increase
absorption of radiation in the target tissue by at least
10, 100 or 200 HU, or more, e.g., 500, 750, or 1000 HU or
more. These units can be measured by methods described
herein.
In certain embodiments, the radiodense composition
includes a mixture of a small molecule radiodense
material and a large molecule radiodense material (or
just includes a large or small molecule material), and
the radiodense composition can include iodine, barium,
bismuth, boron, bromine, calcium, gold, silver, iron,
manganese, nickel, gadolinium, dysprosium, tungsten,
tantalum, stainless steel, or nitinol, or a combination
of any one or more of the above. The radiodense
composition can also be a radiodense material present
within a small, lipid soluble molecule, such as ethiodol
(LipiodolT""), which is poppy seed oil in which carbon
atoms are iodinated.
Tn other embodiments, the radiodense composition
has a dwell time within the target tissue of at least 3,
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 4 -
5, 10, 15, 20, or 24 or more hours. Certain compositions
can be designed to have dwell times of several days to
weeks. In specific embodiments, the radiodense
composition can be about 10 nanometers to 10o microns in
size, and can be NI-243, NI-244, NI-212, or a liposome
comprising iohexol (CTP-10, Nycomed, Wayne, PA).
In another aspect, the invention features a method
of treating a diffuse tumor, e.g., a metastatic tumor, in
a patient by administering to the patient systemically a
radiodense composition that includes a small molecule
radiodense material in an amount sufficient to accumulate
selectively within the diffuse tumor tissue compared to
non-tumor tissue; and irradiating the body part of the
patient in which the diffuse tumor is located with
radiation for a time and under conditions sufficient to
kill cells within the diffuse tumor. For example, the
radiodense composition can accumulate selectively at the
outer edge of the tumor and enter and accumulate within
the tumor tissue, and can be administered intravenously
as a bolus, followed by an infusion of the same or a
different radiodense composition at a rate that equals
the blood clearance rate of the radiodense composition.
In other embodiments, the radiation can have the
energy levels described above, and the radiodense
composition can be linked to a targeting agent that binds
specifically to the target tissue, and can be a particle
having ranging in size from, e.g., 30 to 300 nanometers.
Radiodense compositions can be or include small
molecules of radiodense materials, which are less than 1
nanometer in size and diffuse readily in aqueous spaces
of the body. Although these small molecules may not
penetrate biological structures such as most cell
membranes or tight endothelial junctions as found in the
capillaries of brain, retina, or testis, they do
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 5 -
penetrate capillary walls in most other parts of the
body, e.g., the capillaries feeding tumors.
Radiodense compositions can also be or include
large molecules of radiodense materials, which have a
much lower diffusion rate than the small molecules, and
which do not generally penetrate normal blood capillaries
in either transport direction, i.e., from blood to tissue
or from tissue to blood. So considered, these large
molecules are generally larger that about 10 to 20
nanometers, and can be 100 to 400 nanometers in size, and
can be up to several hundred of microns in size, e.g.,
100, 300, 500 or more microns. Large molecules can
include liposomes, esters, polymers, and emulsions as
described herein.
Unless otherwise defined, all technical and
scientific terms used herein have the same meaning as
commonly understood by one of ordinary skill in the art
to which this invention belongs. Although methods and
materials similar or equivalent to those described herein
can be used in the practice or testing of the present
invention, suitable methods and materials are described
below. All publications, patent applications, patents,
and other references mentioned herein are incorporated by
reference in their entirety. In case of conflict, the
present specification, including definitions, will
control. In addition, the materials, methods, and
examples are illustrative only and not intended to be
limiting.
The new methods provide numerous advantages. For
example, the new methods enable shorter therapeutic
regimens and expand treatment options. The new methods
also enable the application of prophylactic radiation of
vascular stents with orthovoltage or megavoltage
equipment, and enable the use of low energy x-rays (e. g.,
20 Kev to 140 Kev) for tumor therapy by increasing the
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 6 -
efficacy of the treatment itself. Thus, the new methods
spare normal tissue from unnecessary radiation.
Furthermore, it is expected that more than half of all
radiation treatments of cancer can be improved by the new
methods.
In addition, after injection of the new radiodense
compositions, the local and any systemic distribution of
the composition can be visualized in the patient using
standard x-ray techniques, and therapy is carried out
only if the resulting distribution is favorable.
Other features and advantages of the invention
will be apparent from the following detailed description,
and from the claims.
Brief Description of the Drawings
Fig, 1 is a schematic diagram of radiation
treatment planning.
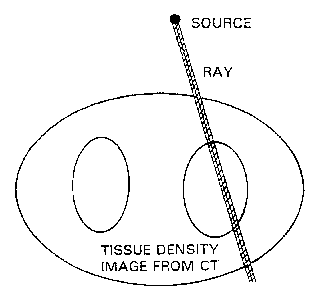
Fig. 2 is a schematic diagram of tissue density
from CT scanning.
Fig. 3 is a graph comparing the change in
radiation absorption in different parts of an
adenocarcinoma injected with a radiodense composition
(Omni-350).
Fig. 4 is a graph comparing the change in
radiation absorption in different parts of a glioma
injected with a radiodense composition (NI-243).
Fig. 5 is a graph comparing the change in
radiation absorption in different parts of an
adenocarcinoma injected with a radiodense composition
(NI-212).
Fig. 6 is a graph showing the effect of radiation
dosage on the survival of tumor cells (V79) in vitro in
the presence of different radiodense compositions.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
Detailed Description
The invention relates to the combination of
specific radiodense compositions, specific modes of
administration of the radiodense compositions, and
specific energies and sources of radiation, to provide a
significant increase in the selective absorption of
radiation in tumors and other diseased tissues to provide
greatly enhanced methods of radiation therapy.
The radiodense compositions act as adjuvants and
enhance, i.e., improve the toxic effect of, radiation
therapy at locations where the composition and the
radiation coexist in the proper dosage range. There is a
nonintuitive relationship between the formulation and
administration regimen of the radiodense composition and
the external or internal radiation source as described in
further detail below.
The physiology of tumors restricts the entry of
certain materials from the blood. The same barrier,
however, restricts the exit of molecules injected into
the tumor. If the injectates contain sufficient high Z
materials, they will change both the attenuation and
absorption of radiation in proportion to their local
change in electron and nuclear density. However, the
injected materials have to be present at the time the
radiation is delivered. For this reason, longer lasting
injectates are desirable to avoid new injections yet
enable repeated, or long duration administrations of
radiation.
The new methods provide for the selective increase
in the level or concentration of radiodense compositions
within tumors by factors of 10 to 20 or from 50 to 3000
HU (as shown in Figs. 3 to 6) as compared to the
surrounding normal tissues, and thus provide a
commensurate increase in the absorption of radiation
within treated tumors. The radiodense compositions
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 8 -
remain within the target tumors following intratumoral
administration for periods of hours, days, or weeks,
depending upon their formulation.
It is common to use computed tomography (CT) to
identify the structures of interest. CT, at its physical
core, plots the geometric distribution of electron
density. It is the interaction of radiation with local
electrons that provides the toxic (cell killing) effect
of orthovoltage radiation. Thus, the CT absorption
differences of the tumor versus the normal tissues
defines where the radiation energy will be absorbed.
Fig. 1 shows the aspects of conventional radiation
therapy targeting, while Fig. 2 shows a schematic CT
where two ovoid regions have increased radiodensity
(higher HU). The difference between the more radiodense
tissues and the less radiodense tissues, again in HU,
directly reflects differences in electron density and the
proportionate differences in radiation absorption at this
incident energy in the orthovoltage range.
As discussed in further details in the Examples
below, Figs. 3-5 show graphs where the relative
radiodensity is plotted versus time for various regions
within experimental tumors. These examples are selected
to show a mixture of small and large molecule radiodense
materials (NI-243 and NI-212), and a small molecule only
(Omni-350).
The clinical drawback of unenhanced CT is that
nearly all soft tissues, normal and tumorous alike, have
similar electron density, and thus the same x-ray
absorption. Conventional 3D radiation treatment planning
is complicated by this lack of any useable differences in
the absorption of radiation between tumors and the
surrounding healthy tissues.
The radiodense compositions described herein can
be tailored to various types of radiation therapy, and
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
_ g _
can be used with standard external radiation sources, or
with new internal radiation sources (such as the
Photoelectron Corp. radiosurgery probe system) as well as
brachytherapy radiopharmaceuticals that emit radiation
from within the tumor.
While the new methods for enhancing radiation
therapy will be applicable to any solid tumor, it is
envisioned that they are especially useful for treating
prostate, breast, lung, head and neck, brain, and liver
l0 tumors. In addition, the new methods should be useful
for enhancing alternative radiation procedures where the
disease is not cancer, but involves a tissue that can
selectively absorb or bind to the radiodense
compositions.
Radiodense Compositions
The radiodense compositions are designed to
selectively increase the concentration or level of one or
more radiodense (electron dense) agents or materials,
such as iodine, in a target tissue, e.g., a tumor,
immediately upon administration, and to maintain the
elevated level for the time required to deliver one or
more radiation treatments, e.g., several hours, days, or
weeks after the initial administration.
A radiodense composition is a material that
contains more electrons and/or more atomic nuclei per
volume than are contained in the soft tissues of animals
or man. Generally, the material includes elements with a
high Z number, which increases both electron and nuclear
density. Such high Z materials include iodine, barium,
bismuth, boron, bromine, calcium, gold, silver, iron,
manganese, nickel, gadolinium, dysprosium, tungsten,
tantalum, stainless steel, nitinol, or any other material
that absorbs incident radiation.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 10 -
By increasing the level of radiodense compositions
in a tumor, the compositions provide a significant
enhancement of radiation absorption, thereby increasing
the deposition of radiation energy in the tumor and
maximizing the therapeutic effect at the target. As an
added benefit, that radiation that is absorbed within the
tumor is not available to cause damage in the normal
tissues lying beyond the tumor.
The radiation absorbing radiodense composition can
be the element itself, or can include the high Z element
incorporated into other chemical carriers to obtain
useful or improved pharmacokinetics, safety, or cost.
Suitable materials include, for example, small molecule,
water-soluble or lipid-soluble, iodinated agents {Table
1); insoluble iodinated derivatives with sizes from 0.050
to 50 microns; encapsulated agents such as liposomes; and
micelles composed of block co-polymers. Other materials
that absorb radiation and selectively accumulate in a
target tissue are also useful in the new methods. In
some applications, mixtures of radiodense materials may
have a particular utility.
Radiodensity can be calculated from the nature of
the radiodense material. The pharmacokinetics of
electron dense materials are often conveniently
determined with spatially and temporally resolved
computed tomography (CT) where the relative electron
density is expressed in Hounsfield Units (HU). In this
case the local radiodensity can be readily compared to
water or nonenriched soft tissues. A Hounsfield scale
where air is -2000 HU, water is zero HU, and bone is
+4000 HU is used herein.
The level of radiodense enrichment {the radiation
absorption enhancement) of a tissue can be calculated in
HU by:
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 11 -
1. First scanning the region of the body
containing the target tissue with a CT scanner operating
at a set orthovoltage such as 140 KeV;
2. Administering the radiodense composition in
the desired dose, rate, and location;
3. At any time thereafter, repeating the CT scan;
4. Determining the x-ray absorption in HU of each
region of interest;
5. Subtracting the value obtain with the first
scan from any timed value obtained later; and
6. Determining the subtracted value in HU or (nHU
in Figs. 3-5), which is directly related to the increased
electron density in the region of interest at the time of
the post-administration scan.
Since the composition of the radiodense
composition is known, the increment in electron density
of any tissue at any time is directly proportional to the
increment in nuclear density in the same region at the
same time. In fact, the nHU value can be directly
converted into concentration of the added radiodense
composition in mg per volume of tissue by constructing a
standard curve. This can be done by placing samples
containing different known amounts of the radiodense
composition in the scanner and determining the HU for
each concentration; and plotting the ratio between
concentration and HU. The slope of this ratio is the
correction factor that can be used to correct the nHU
values for each tissue directly to concentration of the
administered radiodense composition.
The radiodense compositions can include individual
radiodense materials, e.g., a "small molecule" or "large
molecule" radiodense material {as described herein), or
the compositions can include mixtures of two or more
radiodense materials, e.g., a mixture of different small
or large molecules, or a mixture of small and large
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 12 -
molecules. The combination of small and large molecules
provides a radiodense composition that rapidly increases
the level of the small molecule (and large molecule if
injected into the target) radiodense material in a given
target tissue, e.g., a solid tumor, to a high density,
and then provides a sustained level of the large molecule
radiodense material for several days to weeks.
The small molecule radiodense materials are on the
order of one nanometer or smaller in size, and are
typically water soluble. Thus, these materials quickly
diffuse into and throughout a tumor or the bloodstream to
rapidly increase the level of the radiodense material
within the target tumor. The rapid diffusion is also a
limitation, because these materials diffuse out of the
tumor into the bloodstream within about three to four
hours or so, depending on the specific size and
composition. Thus, small molecule radiodense materials
can be used alone in radiodense compositions only under
certain circumstances as describe herein.
The small molecule radiodense materials are
selectively taken up by tumors compared to healthy
tissues because tumors have greater blood perfusion than
healthy tissue, and because tumors generate cytokines
such as vascular endothelial growth factor that make the
blood vessels that feed the tumor "leaky" to allow for a
faster and greater exchange of materials between the
blood vessels and the tumor compared to normal tissues.
Exemplary small molecule radiodense materials
suitable for use in the new methods are listed in Table
1, and include iohexol (OmnipaqueT"') , Hypaque'"', and
iodixol (VisipaqueT"") . The materials listed in Table 1
are commercially available.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 13 -
Table 1 - Small Molecule Radiodense Materials
Ionic Agents (all concentrations)
Diatrizoates
Hypaque'"", Ang10V7.StTM, MD-60T"', MD-76T"",
Renografin'"", RenocalT"", RenoT"", Renovist'"",
Urovi stT"'
Iothalamates
Conray'"", Angio-Conrayt"", Cysto-Conray'"", Cysto-
Conray TIT"", VascorayT"'
Iodamides
RenovueT""
Ionic-Nonionic Agents
Ioxaglate
Hexabrix'""
Nonionic Monomers
Iohexol
Omnipaquel""
Iopamidol
TsovueT""
Ioversol
Opt i rayT""
Metrizamide
AmipaqueT""
Nonionic Dimers
Iodixanol
VisipaqueT""
Lipid-Soluble Agents
Ethiodol
LipiodolT"'
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 14 -
Biliary Agents
Iodoxamate
Cholovuel'"
Iodipamide
Chlorografin'", TelepaqueT""
In current medical practice, the intratumoral
injection of radiodense adjuvants requires imaging to
visualize the distribution of the radiadense enhancement.
There then follows an often time-consuming determination
of the radiation treatment plan and transfer of the
patient to the site where therapeutic radiation is
administered. Since the effect of some of the new
methods is related to retained radiatian dose, it is
important that clearance and redistribution of the
injected materials be slow in relation to the interval
between administration of the radiodense material and
administration of radiation. In addition, radiation
delivery is not instantaneous, further increasing the
need for materials with slow clearance and
redistribution.
As a result, for intratumoral injection or for
single bolus systemic injections, the pharmacokinetics of
the standard small molecule radiodense materials listed
in Table 1 above axe too rapid and provide significant
practical limits to prior technologies. For these
agents, a bolus injection should be followed by an
infusion regimen to sustain the radiodense enrichment of
the target tissue compared to non-target, healthy tissues
during irradiation. Of course, the efficacy of the
prescribed regimen should be individually determined, for
example, by quantitative CT methods as described herein.
On the other hand, the large molecule radiodense
materials are designed to have a much longer dwell time
in the target tissue. Accordingly, these large molecules
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 15 -
are less water soluble and diffuse much more slowly than
the small molecules, and are too large to pass easily
into or out of the bloodstream or from the tumor into the
blood. As a result, they provide a dwell time of one or
more days to weeks once injected into a tumor. These
large molecule radiodense materials are on the order of
50 or 100 to 400 nanometers, or even larger in size, and
can be up to several tens or hundreds of microns, in
size.
Examples of the large molecule radiodense
materials include water-insoluble esters of diatrizoic
acids. The esters of these diatrizoic acids are combined
into large, solid conglomerates that are then milled or
ground into small uniformly sized particles of, e.g., 100
to 300 nm.
Particularly suitable large molecules include WIN
8883 [ethyl-bis(3,5-acetylamino)-2,4,6-triiodobenzoate;
Sterling] which is a water-insoluble nanoparticulate of
diatrizoic acid ester about 300 nm in size. Other useful
large molecule radiodense materials include NC 70146 [1-
(ethoxycarbonyl)pentyl-bis (3,5-acetylamino)-2,4,6-
triiodobenzoate; Nycomed, Wayne, PA], NC 67722 [6-
(ethoxycarbonyl)hexyl-bis (3,5-acetylamino)-2,4,6-
triiodobenzoate; Nycomed]; and NC 1290:1
[(ethoxycarbonyl)methyl-bis (3,5-acetylamino)-2,4,6-
triiodobenzoate]. All of these compositions are
insoluble in water and are milled to the desired particle
size. They differ in their ease of hydrolysis in the
body and in their metabolism. Other large molecule
radiodense materials include gadolinium oxide, gadolinium
oxalate, manganese doped hydroxyapatite.
Additional large molecule radiodense materials
include liposomes that encapsulate or entrap radiopaque
agents, or that include radiopaque agents in the external
phase (i.e., continuous solution phase). For example,
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 16 -
the radiopaque agent CTP-10 (iohexol) can be encapsulated
within a liposome using standard techniques. The size of
the liposomes can be about 10 to 400 manometers. The
radiodense material can be water-soluble and present at a
high concentration within the liposome and in equal
concentration in the membrane of the liposomes.
Examples of radiodense compositions that include
both small and large molecule radiodense materials are
NI-212 (Nycomed), NI-223, and NI-244 (Nycomed). NI-212
is an insoluble trii.odinated ester (listed above) and
contains water soluble iohexol (OmnipaqueT""). NI-244 is
an insoluble triiodinated ester (67722, listed above)
with its first soluble metabolite sodium 6-[3,5-
bis(acetylamino)-2,4,6 triiodophenyl)carbonyloxy]
hexanoate (small molecule, NC 68056) which is the
corresponding carboxylic acid derived from the loss of
the ethyl ester.
Modes of Administering Radiodense Compositions
The radiodense compositions can be administered
directly into a solid tumor, administered systemically to
contact the surface and permeate into the interior of a
solid tumor from the outer surface, or administered
systemically to permeate rapidly into small, diffuse,
e.g., metastatic, tumors. The mode of administration
depends upon the type of tumor, the nature of the
radiodense composition, and the type and source of the
radiation to be employed for therapy.
When treating a solid tumor using an external
source of orthovoltage or megavoltage radiation, such as
a CT scanner, it is advisable to administer the
radiodense compositions intratumorally, e.g., by direct
injection (e.g., using a small gauge needle of the
appropriate length). To the extent that the treatment
will require long or repeated exposures, a large molecule
CA 02347915 2001-04-26
WO 00/25$19 PCT/US99l2555$
- 17 -
radiodense material with a long dwell time should be
included. However, the addition of a small molecule
radiodense material can give a "boost" to that region of
the tumor accessed by the diffusion of the small molecule
and very significant radiation enhancement can be
achieved shortly after intratumoral administration of a
mixed small and large molecule composition. As tumor
size increases, it may be desirable to deposit the
radiodense composition at several central locations in
the tumor.
This allows the radiodense composition to diffuse
and migrate within the tumor from the inside towards the
outside, with the large molecule material remaining at or
near the site of injection, and the small molecule
material moving outwards from the site of injection,
creating a gradually decreasing electron density
(concentration gradient of the radiodense material)
within in the tumor from the highest in the center to the
lowest at the edges of the tumor. Thus, the combination
radiodense compositions are ideal for use with an
external radiation source, which provides an energy
profile within the tumor that is the most intense at the
outer edge of the tumor, and which gradually decreases in
intensity towards the center of the tumor.
As a result, the radiodense composition enhances
the absorption (and thus killing power') of the radiation
most in the center of the tumor, where the radiation
intensity is lowest, and gradually decreases the
enhancement towards the outer surface of the tumor, where
the least enhancement is required (because the radiation
intensity is the highest). This provides the optimal
radiation dosage (absorption) throughout the tumor, and
can be tailored to specific sizes and types of tumors by
adjusting the ratio of small to large molecule materials,
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 18 -
the radiation dosage, and the timing and length of
radiation administration.
Of course, a sufficient amount of the radiodense
composition is administered into the tumor to ensure a
certain minimal level of enhancement at the surface or
outer edge of the tumor to allow the tumor to selectively
absorb a greater amount of radiation than neighboring
healthy tissue.
On the other hand, when treating a solid tumor
with an internal radiation source, the radiation is most
intense in the immediate vicinity of the source, e.g., in
the center of the tumor if the source is inserted into
the center. Therefore, the radiation requires the most
enhancement at the surface or edge of the tumor, and the
concentration of the radiodense composition should
gradually decrease to the lowest level at the center of
the tumor. Suitable internal radiation sources include
small radioprobes, such as a radiosurgery probe
manufactured by Photoelectron Corp. (Lexington, MA), and
brachytherapy implants of solid or liquid radioactive
materials. Such implants can include solid or
encapsulated radiopharmaceuticals such as P-32, Sc-47,
Co-60, Cu-67, Sr-89, Y-90, Rh-105, I-131, I-125, Sm-153,
Lu-177, Re-188, Ir-1.94, Au-199, Ra-226, Rn-222, and Am-
241.
In this setting, e.g., when using a radiosurgery
probe operating in the low kiloelectron voltage range,
the radiodense composition should include mostly small
molecules that are administered systemically to diffuse
into the tumor, which ensures a gradually decreasing
concentration gradient of the composition from the outer
surface or edge to the center of the tumor, and thus a
gradually decreasing level of enhancement of the
radiation absorption from the outer surface to the
center, which corresponds inversely to the radiation
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 19 -
intensity profile emitted by the source centered in the
tumor. Again, this provides the optimal radiation dosage
throughout the tumor, and can be tailored to specific
sizes and types of tumors and radiation sources. Due to
their safety, nonionic small molecule radiodense
materials such as iohexol, iopamidol, ioversol, ioxilan,
and iodixanol are suitable.
4~Ihen the small molecules are administered
systemically adjustments should be made for the rate at
which the radiodense materials diffuse out of or are
cleared from the bloodstream (e. g., into the target such
as a solid tumor). For example, the radiodense
compositions can be given as an intravenous bolus with a
subsequent infusion equal to the blood clearance rate of
the composition to sustain the desired concentration.
The bolus dose should be sufficient to increase the edge
region of leakage by 10 to 200 Hounsfield units, and the
bolus and/or infusion must sustain the edge enhancement
for the duration of treatment, e.g., 0.5 to 3 hours.
For other internal radiation sources, the
radiodense compositions can be administered as above,
taking into consideration the radiation dosimetry for the
particular source. For example, the energy level emitted
by the specific radiopharmaceutical should be determined,
and the radiodense composition chosen accordingly. For
example, I-125 and Am-241 emit in the orthovoltage range,
while Ra-226, Rn-222, and Y-90 emit in the megavoltage
range. The radiodense materials described herein all
increase both electron density and nuclear density
because they contain high Z materials. Thus, they can be
used with radiation sources that emit in the orthovoltage
and megavoltage ranges, as well as the midrange, as
described in further detail below.
In yet another scenario, if the tumor to be
treated is a diffuse and/or metastatic tumor, then the
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 20 -
radiodense composition should include only small molecule
radiodense materials, and should be administered
systemically to provide radiodense enhancement due to the
leakiness of such tumors and their large extracellular
space that can be loaded with the radiation enhancer.
The radiation is then administered to the part of the
body known to harbor the metastatic tumor or to the body
region at risk of metastasis in the case of
"prophylactic" radiation therapy.
In general, when the radiodense compositions are
administered systemically, tumors that are more "leaky"
will require either a lower concentration of the
radiodense composition, and/or a lower radiation dose,
while less leaky tumors will require a higher systemic
concentration of the radiodense composition, or a longer
duration in the bloodstream (e.g., maintained by
infusion) to allow sufficient accumulation within the
tumor. The specific therapeutic regimen of radiation and
systemic administration of a particular radiodense
composition can be determined using modern imaging and
temporal and spatially quantitative methods (such as
functional computed tomography) and radiation simulation.
Certain radiodense compositions are designed to be
targeted to specific parts of the body, e.g., by
naturally accumulating selectively in the kidneys, lymph
nodes, and/or liver. These compositions are of a size
and material, and are administered in a way, that induces
the selective accumulation.
For example, radiation treatment of disease,
usually neoplasia, in the lymph nodes can be
significantly enhanced using radiodense materials that
are naturally accumulated selectively in the lymph nodes
(to both neoplastic and healthy tissue) by the body.
Large molecule radiodense materials with a particle size
of about 30 or 50 to 300 nanometers (with or without a
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 21 -
small radiodense water soluble molecule) can be
administered percutaneously to the interstitial site that
supplies the lymph to the target lymph node, e.g., by
using methods describe in, e.g., U.S. Patents Nos.
5,111,706 and 5,496,536. The target lymph node
accumulates the radiodense material selectively with
radiation absorption enhanced by 100 HU to often more
than 400 HU. When then exposed to either external or
internal radiation, the radiodense material in the target
lymph node will enhance radiation damage to the node and
its contents while allowing lower radiation doses to the
surrounding, uninvolved tissues.
This method has special utility for the sentinel
nodes of great clinical interest in breast cancer and
melanoma, where the location of these nodes can be
determined by diagnostic lymphography, and therapy then
directed at the small body region containing the target
node or nodes. Other deep nodes such as those in the
thorax, neck and abdomen could be similarly enhance with
radiodense adjuvants and treated with radiation therapy
with less damage to nearby structures. Diffuse processes
involving lymph nodes such as lymphoma and Hodgkins
disease, can also be targeted with the same methods and
materials. Large radiodense molecules of the type
describe above also naturally and automatically target
organs rich in macrophages, such as the liver and spleen,
following systemic administration. Thus, the new methods
are especially effective when used to treat tumors that
are located in one of these naturally targeted body
locations or organs, if the radiodense compositions are
designed to take advantage of this natural targeting
mechanism.
The radiodense compositions can also include known
targeting agents that will home within the body to
selected targets, such as tumors or other diseased
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 22 -
tissue. For example antibodies, e.g., monoclonal
antibodies, that bind specifically to tumor surface
antigens can be linked (e. g., by covalent, non-covalent,
ionic, or by nonionic bonds) to the radiodense materials.
Alternatively, numerous binding pairs, e.g.,
streptavidin/biotin, are known that can be used in the
present methods. For example, biotin can be linked to a
monoclonal antibody that binds specifically to the
surface of a tumor. Streptavidin is linked to a
radiodense composition, and the complex is administered
systemically. The very high binding specificity between
biotin and streptavidin provides a very selective and
powerful targeting mechanism for the radiodense
composition.
In addition, there a various polymers and long-
chain compounds that. are known to accumulate selectively
in the kidneys, lymph nodes, and/or liver. These
compounds can also be linked to the radiodense
compositions to provide a targeting to tumors in these
organs. For example, compounds that bind specifically to
mannose receptors on liver cells, can be used to target
the radiodense compositions when treating liver cancer.
The new methods can be used to treat tissues other
than neoplastic tissues. For example, the methods can be
used to treat excessive local cell proliferation in other
clinical circumstances where such proliferation is
harmful. Radiation damage is known to be cell cycle
dependent and non-neoplastic cells that are proliferating
have increased susceptibility to damage by radiation.
However, damage to non-target cells must, and can be
avoided using the new methods.
Such a circumstance occurs following vascular
intervention such as balloon angioplasty or bypass
surgery using natural or artificial grafts. As a
response to the local trauma, cells proliferate beginning
CA 02347915 2001-04-26
WO 00!25819 PCT/US99/25558
- 23 -
a few days following intervention and may proliferate to
the point where the volume of proliferated cells can
jeopardize the vascular lumen. A partial solution to
this problem has been to place a metallic stmt across
the traumatized vascular surface, but cell proliferation
can still occur with migration through the stent.
However, according to the new methods, if the stent
includes a radiodense material as described herein, and
is located immediately adjacent to the zone where
proliferation will subsequently occur, the radiodense
material will enhance absorption of radiation in the
target region. Thus, when the stent is irradiated (with
an external or internal source), restenosis will be
reduced and adjacent non-target tissues will be spared.
The new methods can also be used to treat external
tissues, such as the skin in diseases like acne or
psoriasis, or skin tumors like melanomas. In these
cases, the radiodense,compositions are ~~painted~~ on the
target tissue (e. g., mixed with an agent that enhances
skin permeability, such as DMSO) prior to radiation
therapy.
All of the radiodense compositions described
herein can be administered either alone or together with
conventional oncology therapeutic drugs. Moreover, it
should be clear from the foregoing that the new methods
require knowledge of the pharmacokinetics of the
radiodense material in the composition in addition to the
radiation dosimetry usually considered for the radiation
regimen.
The new methods also provide an important safety
benefit, in that radiation will not be administered to a
patient if the radiodense compositions do not diffuse
throughout the tumor, or leak out of the tumor (when
injected directly), or accumulate in the tumor (when
administered systemically) as planned. This is possible,
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 24 -
because the distribution of the radiodense compositions
can be determined with quantitative imaging prior to
therapy with, for example, CT. Thus, the amount of
radiation enhancement, its duration, and the expected
response can be accurately modeled. For example, if a
radiodense composition is administered systemically, the
tumor can be imaged at short intervals to determine
exactly when a sufficient concentration of the
composition has accumulated within the tumor. This
regimen can be repeated when the radiation is to be
administered. In the case of diffuse and/or metastatic
tumors, only one specific portion of the tumor needs to
be imaged, as all the other portions of the tumor will
accumulate the radiodense composition in a similar
manner.
Radiation Dosages and Methods of Irradiation
Radiation is useful for killing tumor cells by
inducing irreparable damage to the genome; normal cells
are equally damaged but may have a slight repair
advantage. Rarely is such radiation absorbed as a single
and total exchange of energy between the beam and a locus
on a gene. Instead, radiation absorption is usually
through multiple interactions with tissue, often
scattered over some distance. The radiation source can
be either external or internal. For radiation therapy,
the useful energy ranges are from 20 Kev to tens of Mev.
The dominant mechanism of absorption of radiation
is fairly well understood and varies with the incident
energy ranges as shown in Table 2. There are three
general energy ranges listed in this table known as
"orthovoltage," "midrange," and "megavoltage." Although
the three ranges overlap somewhat, it is convenient to
use these ranges for discussion with respect to the new
methods.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 25 -
The lowest energy (orthovoltage range) is
efficiently absorbed by resident electrons in proportion
to their abundance. Some K-shell electrons are more
efficient at selected energies, but this effect is not
prominent with normal tissue composition. As Table 2
shows, absorption of this energy range decreases with the
inverse of incident energy cubed, but increases with
atomic number cubed (and electron density). Orthovoltage
has poor penetration depths and lots of scatter.
The midrange of radiation energy is mostly limited
to radioactive materials and brachytherapy applications.
The megavoltage range is currently the most useful
due to enhanced depth of penetration and little increased
absorption with moderate Z number radiodense materials
such as present in bane (calcium phosphate salts).
However, above 1.02 Mev, the dominant mechanism of
absorption is pair production due to interaction with the
atomic nucleus. As Table 2 shows, this interaction
increases with energy, Z number, and nuclear density.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 26 -
Table 2
Enercry Description Dominant Broad
Mechanism Patterns
20 to 250 Orthovoltage photoelectron 1/E3
Kev "bound Z3 times Z
electrons" # electrons
times density
200 Kev Midrange Compton and 1/E
to 1 Mev coherent Free density
electrons matters, Z
does not
21.02 Mev Megavoltage Pair Z x Z x E
production nuclear
atomic cross-section
nucleus & density
increases
with energy
Table 3 below Shows hypothetical calculations of
the relative absorption of radiation (targeting ratio)
where the tumor target has been increased by 1000 HU with
a radiodense material that increases the tissue density
from 1.004 to 1.203 such as might occur with a large
molecule radiodense material. This simulation shows the
large increments of radiation absorption enhancement in
the target in the orthovoltage and megavoltage range.
Due to the enhancement of tissue density, there is a
measurable benefit even in the midrange.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 27 -
Table 3
Energy (Mev) Unenhanced Enhanced Targeting
ratio
0.1 0.1566 1.753 12.2
0.15 0.1375 0.646 5.7
0.20 0.1245 0.339 3.73
0.5 0.0874 0.0966 2.10
1.0 0.0639 0.0589 1.92
5.0 0.0279 0.0360 2.29
10.0 0.0210 0.0394 2.88
20.0 0.0178 0.0467 3.62
50.0 0.0179 0.0597 4.51
100.0 0.0179 0.06893 4.87
Examples
The invention is further described in the
following examples, which do not limit the scope of the
invention described in the claims.
Example 1 - Formulation of a Larcte Molecule Radiodense
Composition
Formulations suitable for use in the new methods
can be prepared from an insoluble iodinated material that
is subsequently milled to provide the desired size as
described, e.g., in U.S. Patents Nos. 5,322,679 and
5,318,767. For example, NC 67722 (6-
ethoxycarbonyl)hexyl-bis(3,5-acetylamino-2,4,6
triiodobenzoate; Nycomed, Wayne, PA) can be the starting
insoluble iodinated material. The 67722 suspension can
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 28 -
be wet milled using water soluble iodinated agents such
as iohexol as an auxiliary wetting agent. Milling is
continued until the desired particle size of 67722 is
obtained, e.g., about 100 to 300 nm. The resulting
mixture can then be diluted with a presterilized stock
solution of Pluronic F98 (BASF, Parsippany, NJ) and
glycerol and the final pH adjusted.
If the water soluble wetting agent is NC 69056, a
final composition of 15% (wt/vol) NC 67722, 3% NC 68056,
3% F98, and 1.75% glycerol is obtained with an iodine
concentration of 180 mg I/ml. This formulation,
designated NI-244, is suitable for lymph node and
intratumoral applications where the average particle size
is 97 nanometers as determine with a light scattering
device (Horiba, model 910).
Other starting materials can be used to prepare
other radiodense compositions using similar methods.
These compositions can be coated with surfactants, and
can range in size from about 0.05 to 50 microns.
Insoluble materials that have been used to prepare
radiodense compositions include NC 12901, NC 70146, and
WIN 8883 as identified above. The insoluble iodinated
agents can also be prepare without the water soluble
iodinated agent to provide a composition containing only
large radiodense molecules.
Example 2 - Large Molecule Radiodense Compositions with
Added Therapeutic Ingredients
Numerous patents describe nanoparticulates of
drugs. See, e.g., U.S. Patent No. 5,145,684. As solids,
these materials are somewhat more dense than soft tissues
and may be targets for radiation therapy, thereby
combining local drug efficacy with radiodense properties
useful for radiation therapy. However, since the
starting materials are often insoluble particulates, they
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 29 -
can be mixed with the insoluble iodinated materials to
generate a composition that is imageabl.e, has local drug
efficacy, and serves as a target for radiation therapy in
the new methods.
Example 3 - Combinations of Small and Large Molecule
Radiodense Materials
Various radiodense compositions have been prepared
that include both small molecule radiodense materials and
large molecule radiodense materials. For example, NI-243
combines Win 8883 with water soluble iohexol; NI-212
combines NC 72144 (ethyl 3,5-dihexyl-2,4,6-
triiodophenoxyacetate) with iohexol; and NI-244 combines
NC 67722 with NC 68506 in ratios of approximately 5 parts
large molecule to 1 part small molecule (wt/vol). These
ratios are selected depending upon their intended
purpose. In particular, it is easy to add more water
soluble small molecule such as iohexol if it is intended
to create a high, relatively short-lived, enhancement of
a tumor through intratumoral injection.
Other radiodense compositions that have similar
characteristics include liposomal compositions containing
equal amounts of water soluble small radiodense materials
inside and outside the liposome. The size of the
liposome can be varied as well as the composition of the
lipid membrane. The encapsulated material can be one of
several known water soluble, small molecule radiodense
materials such as iohexol, iopamidol, iomeprol,
iodixanol, and ioversol.
Other radiodense compositions are micellar block
co-polymers such as those described in U.S. Patent No.
5,567,410. As described above, these micellar
compositions can be enriched by the addition of water
soluble small molecule radiodense materials.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 30 -
Example 4 -- Dwell Times of Radiodense Compositions in
Vivo
Immunologically tolerant mice were implanted with
several different human neoplasms. When the tumors
reached a size of 1-2 cm, radiodense compositions were
injected intratumora:Lly via a percutaneous route using a
27 gauge needle. The mice and their tumors where then
serially imaged with computed tomography to determine the
local pharmacokinetics of the injectates by measuring the
x-ray attenuation (in HU) of regions of interest.
Fig. 3 is a plot of the temporal change of x-ray
attenuation in a mouse bearing a human adenocarcinomas
(LS174T). The radiodense material was a water soluble,
small molecule radiodense material (Omnipaque'"" with a
concentration of 350 mg I/ml as iohexol). A peak
contrast of nearly 3000 HU was attained in the center of
the tumor, with smaller degrees of contrast enhancement
surrounding the injected area. This small molecule was
rapidly cleared with values of only 2000 HU at the peak
location 60 minutes later.
Fig. 4 shows a similar experiment in which the
human tumor was a glioma (U87-VC2) and the intratumor
injectate was NI-243. In this example, the small
molecule created a peak contrast of about 1300 HU, with a
rapid washout over 60 minutes. The large molecule
sustained a concentration of about 500 HU for more than 1
day.
Fig. 5 shows a third experiment of this kind where
the mouse was implanted with an adenocarcinoma (LS174T)
and the intratumor injectate was NI-212. A smaller
volume was administered and the temporal graph shows a
high peak contrast that disappears with about the same
clearance rate as the iohexol above, but the large
molecule concentration (in the peak area) was sustained
at 200 HU for about 3 days.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 31 -
Example 5 - In Vitro Evidence of Radiation Enhancement
The effect of the new radiodense compositions on
cancer cells was also studied in vitro. Individual V79
Chinese Hamster Ovary cancer cells were suspended in
nutrient medium in test tubes and mixed with a sufficient
amount of one of several new radiodense compositions to
increase the radiodensity of the cell suspension. The
three radiodense compositions were Omni-350, which is a
small molecule radiodense material (iohexol with an
iodine concentration of 350 mg I/ml, diluted to provide
400 HU in the nutrient medium); WIN 8883, which is a
large molecule radiodense material (also diluted to
provide 400 HU in the nutrient medium); and iodix
(iodixanol, VISIPAQUET"') which is a small molecule, water
soluble nonionic dimer that is clinically available and
has shown to be very sate.
As shown in Fig. 6, all three radiodense materials
significantly reduced the radiation energy required to
kill a particular percentage of cells. For example, at a
radiation dosage of ~.0 Gray (Gy), about 1 x 10-1 cancer
cells survived in the presence of nutrient medium alone,
whereas only between 1 x 10-2 and 9 x 10-2 cancer cells
survived in the presence of the radiodense materials
(with iodix and Omni being the more effective). At a
radiation energy level of 12 Gy, about 8 x 10-1 cells
survived in the nutrient medium alone, while only 1 x 10-3
to 1 x 10-' survived in the presence of the radiodense
materials.
These results indicate that the presence of the
radiodense materials significantly enhances the killing
effect of radiation.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 32 -
Example 6 - In Vivo Study of Diffusion of Small and Large
Radiodense Materials After Intratumoral Infection
About 200 ml of either a small molecule
radiodense material (iohexol) or a large molecule
radiodense material (WIN 8883) was injected directly into
separate VX2 tumors growing in the thigh of a rabbit. To
measure the diffusion time of the two materials in the
tumor interstitium, the rabbit was euthanized and the
corpus imaged using CT over the next 24 hours to measure
the spatial and temporal distribution of each material.
As shown in Table 4 below, there was very little
expansion of the volume of the large molecule radiodense
material over 21 hours. On the other hand, the volume of
the small molecule radiodense material expanded rapidly
beyond the initial injection locus over the same 21 hour
time period. Table 4 below shows the volume of each
material over time in units of total number of
intratumoral voxels containing at least 200 HU.
Table 4
Win 8883 Volume Omni 350 Volume
Initial 10420 12722
1.5 Hrs 10898 23912
4.5 Hrs 11826 22104
21.0 Hrs 11682 44720
These results show that the large molecule
radiodense material is indeed trapped within the tumor,
and remains active to enhance radiation absorption, for
an extended period of time of at least 21 hours.
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 33 -
Example 7 - In Vivo Evidence of Radiation Enhancement
Three rabbits with VX2 tumors growing in the thigh
were used to evaluate the radiation therapy effect of the
new radiodense compositions and radiation administered
internally. In the first rabbit, iohexol was injected
systemically as a bolus in a volume of 3 ml/kg, resulting
in a peak enhancement of the leaky edge of the tumor by
over 50 HU. Radiation of 30 Gy was administered over 45
minutes using a delivery energy of 40 Kev from the probe
tip, which was centered in the tumor. The next day, the
rabbit was euthanized following the administration of
Evans Blue to demarcate the leaky vasculature of the
treated tumor as well as an untreated, control tumor in
the opposite thigh. Histological analysis of the treated
tumor revealed about 95°s necrosis of viable tumor cells
in the treated leg, compared with the control. All of
the viable cells were located in the edge of the treated
tumor and this edge of viable cells was much smaller than
the rim of viable cells in the untreated tumor.
Two additional rabbits were treated with a nearly
identical protocol, except that the same radiodense
material was given as an intravenous bolus plus a
sustaining infusion to keep the leaky portions of the
tumor enhanced for the entire duration of the radiation
treatment. In these two experiments, no evidence of
viable tumor was seen on serial computed tomography
studies over the next several days. Based on other
experiments, it is known that this follow-up interval of
several days is sufficient to identity incompletely
treated VX2 tumor due to its rapid growth.
Example 8 - In Vivo Pharmacokinetics and Distribution of
Radiodense Materials
In nude Swiss mice bearing MeWo tumors on each
flank, one tumor was randomly selected for direct
CA 02347915 2001-04-26
WO 00/25819 PCT/US99/25558
- 34 -
injection of one of a group of various radiodense
materials (100 ~.1 each). A CT image of the distribution
of the agent was obtained. Each mouse was then
transported to an orthovoltage treatment facility a few
minutes away. Both flank tumors were treated with 10 Gy
radiation (17 minutes at 120 kev). The treated mice were
transported back to the CT suite and another scan taken
to document the pharmacokinetics and distribution of the
radiodense formulation.
The three materials tested were: iohexal, NI-244,
and CTP-10. Upon return to the CT suite, approximately
45 minutes later, the intratumoral iohexol had markedly
diminished, while the NI-244 and CTP-1- formulations
decreased less than 30~.
Histologic examination of tumors removed 2-5 days
later, showed that the control MeWo tumors were
unaffected (no radiation necrosis) at the dose of 10 Gy.
However, test injected tumors showed enhanced necrosis in
direct proportion to the retained radiodense enhancement
with necrosis severity ordered as follows: CTP-10 > NI-
244 > iohexol.
These experiments illustrate the practical
importance of slow pharmacokinetics where therapeutic
radiation is delivered following an interval of time
after a radiodense composition is administration.
Other Embodiments
It is to be understood that while the invention
has been described in conjunction with the detailed
description thereof, the foregoing description is
intended to illustrate and not limit the scope of the
invention, which is defined by the scope of the appended
claims. Other aspects, advantages, and modifications are
within the scope of the following claims.