Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
CYSTINE DERIVATIVES AS THERAPEUTIC AGENTS FOR MATRIX
METALLOPROTEASE RELATED DISEASES
The invention relates to the use of compounds of formula I as pharmaceuticals
and the
use of such pharmaceuticals as drugs to treat diseases such as tumor growth
and
metastasis, inflammatory diseases like osteo- and rheumatoid arthritis,
osteoporosis,
to multiple sclerosis, periodontitis, restenosis, diseases caused by bacteria
such as
meningitis, sun-induced skin aging and Alzheimers disease.
The family of matrix metalloproteases (MMPs) has become a major target for
drug
design, since these enzymes are involved in tissue remodeling and connective
tissue
is turnover, and thus in several diseases where (i) rapid extracellular matrix
degradation is
taking place, e.g. during congestive heart failure and extravasion of highly
metastatic
tumor cells, or (ii) slow extracellular matrix degradation is occurring, e.g.
artherosclerotic lesion formation and rupture, cartilage matrix loss in
osteoarthritis, bone
matrix degradation in osteoporosis, gingival degradation in periodontal
disease, matrix
2o remodeling and deposition in Alzheimer plaque formation and rheumatoid
arthritis.
The MMP family currently includes seventeen members, thirteen of which are
secreted
from the cells in a soluble form and four members are membrane-bound enzymes.
The
MMPs are zinc dependent and calcium requiring enzymes which are inhibited by
one of
25 the members of the tissue inhibitor of metalloproteinase (TIMP) family.
Synthetic
inhibitors of this class of enzymes have been developed as hydroxamates, N-
carboxyalkyl derivatives, phosphonamidates and phosphinates as well as by
using thiol
groups as ligands for the active-site zinc atom.
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
2
3D-structures of the complexes between the catalytic domains of MMPs and
various
inhibitors have been published as well as the structure of the proenzyme of
MMP-3 with
an N-terminal propeptide of about 80 residues. The propeptide forms a separate
smaller
domain that contains three a-helices and an extended peptide that occupies the
active
site. The catalytic domain in all these structures contains two Zn2+ ions,
i.e. a
"structural" zinc ion and a "catalytic" zinc ion. The "catalytic" zink ion is
coordinated by
the side chains of three histidyl residues of the consensus sequence
HEXX~i~~XGXXH.
The fourth Iigand of the "catalytic" zink in the inhibited enzymes is a
coordinating
group of the inhibitors like the hydroxamate or carboxylate; in the pro-MMP
propeptide
to it is the thiol group of the cysteine residue.
Correspondingly, the thiol-based collagenase inhibitors, proposed so far, are
generally
of peptidic structure containing cysteine or cysteine-like amino acids and
their design
was based on the binding mode of the substrate and more recently, of the
cysteine-
containing propeptide.
Recently Muller et al. (Biol. Chem 378, 1475-1480 (1997)) described a new
class of
MMP inhibitors which were derived from cysteine in a non-peptidic manner.
Some cysteine derivatives are disclosed therein as intermediates to prepare
the final
2o cystin derivatives but no pharmaceutical use of these cystine derivatives
is disclosed or
predicted.
Surprisingly we now have found that similar disulfide compounds, i.e. cystine
derivatives are highly active in vivo. In fact these inhibitors are not active
against matrix
metalloproteases (i. e. Ki > 10~.M). However, activity can be demonstrated in
matrix
metalloprotease related diseases like tumor growth and metastasis. Indeed
these
compounds are better than the inhibitors cited in the paper of Miiller et al.
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
3
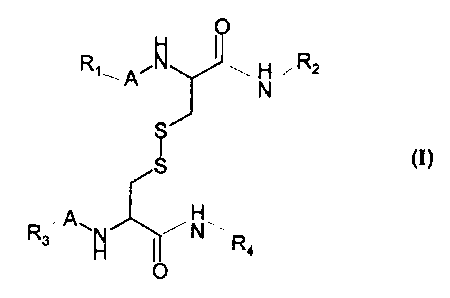
Subject of the present invention are nonpeptidic cystine derivatives of the
general
formula I
Ry A~~ I ~. H ~ R2
N
cn
H
R3~ A\~ I ~N~.. R
4
wherein
R~ and R3 may be the same or different and are selected from hydrogen, an
aromatic or
non-aromatic carbocyclic or heterocyclic ring or a linear or branched
saturated or
unsaturated alkyl group of 1 to 15 carbon atoms which can be interrupted by a
hetero
io atom and which can be substituted by an aromatic or nan-aromatic
carbocyclic or
heterocyclic ring.
R2 and R4 may be the same or different and are selected from hydrogen, a
linear or
branched, saturated or unsaturated alkyl group of 1 to 15 carbon atoms which
can be
interrupted by a hetero atom and which can be substituted by an aromatic or
non-
aromatic carbocyclic or heterocyclic ring and
A is a valency bond or a-CO-, -SOz-, -NHCO-, -NHCS- or -O-CO-group
2o their pharmacologically acceptable salts and optically active forms thereof
and
pharmaceutically acceptable Garners.
With respect to formula I R, or/and Ry represent a branched saturated or
unsaturated
alkyl group of 1 to 1 S carbon atoms selected from methyl, ethyl, propyl, n-
butyl, tert-
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
4
butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl, dodecyl, etc.,
vinyl, etc. as
well as the corresponding alkinyl groups e. g. acetylene.
The carbocyclic aromatic or non aromatic alone or as substituents for said
alkyl groups
are selected from C3-C6 cycloalkyls such as cyclopropyl, cyclobutyl,
cyclopentyl, or
cyclohexyl, or C6-C,4 carbocyclic aromatic substituents such as phenyl,
naphthyl,
fluorenyl, fluorenonyl or anthranyl, or heterocyclic non aromatic substituents
such as
pyrrolidinyl, piperidinyl, piperazinyl, or morpholinyl, or heterocyclic
aromatic
substituents such as pyrrolyl, pyridinyl, furyl, thienyl, thiazolyl,
imidazolyl,
pyrimidinyl, purinyl, indolyl, quinolyl, carbazolyl.
The carbocyclic aromatic or non aromatic ring systems respectively
heterocycles can
optionally be substituted once or several times for example by halogen-, nitro-
,
hydroxy-, C,-C6 alkyl-, C,-C6 alkoxy-, amino-, mercapto-, carboxyl-, cyano-,
benzoyl,
phenoxy or methylsulfonyl groups.
The alkyl group can be interrupted by a heteroatom, preferably by O, N, S.
A preferably denotes the group -CO-, -SOz and -0-CO-.
If A denotes -CO-, the groups R, and R, are preferably selected from the
following
residues:
hydrogen, C,-C6 alkyl, fluorenyl, fluorenonyl, phenyl, benzyl or styryl
whereby the
phenyl rings may be substituted by chloro, methyl, ethyl, methoxy, phenoxy,
benzoyl or
methylsulfonyl.
If A denotes -SOz , R, and R, are preferably selected from the following
residues:
methyl, ethyl, toluolyl or phenyl.
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
If A denotes -O-CO-, R, and R3 are preferably selected from the following
residues:
benzyl or phenyl optionally substituted by halogen.
5 RZ and R4 are preferably selected from the following residues:
phenethyl, phenylinethyl, phenylcyclopropyl, morpholinoethyl,
morpholinopropyl,
cyclohexylethyl, pyridylethyl, imidazolylethyl, indolylethyl or 4-
chlorophenylethyl
whereby the phenyl moities can be substituted by halogen, methyl or methoxy
groups.
to
Subject of the invention are also new compounds of formula I
R~,.. A~N~.w H ~ Rz
N
I1)
H
R3 A\N if N\~. Ra
wherein
R, and R3 may be the same or different and are selected from hydrogen, an
aromatic or
non-aromatic carbocyclic or heterocyclic ring or a linear or branched
saturated or
unsaturated alkyl group of 1 to 15 carbon atoms which can be interrupted by a
hetero
atom and which can be substituted by an aromatic or non-aromatic carbocyclic
or
2o heterocyclic ring.
Rz and R4 may be the same or different and are selected from hydrogen, a
linear or
branched, saturated or unsaturated alkyl group of 1 to 1.5 carbon atoms which
can be
interrupted by a hetero atom and which can be substituted by an aromatic or
non-
aromatic carbocyclic or heterocyclic ring and
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
6
A is a valency bond or a-CO-, -SOZ , -NHCO-, -NHCS- or -0-CO-group
with the proviso that if RZ and R4 are benzyl, R,-A and R3 A cannot be formyl,
C,-C,a
alkylcarbonyl, benzoyl, toluenesulfonyl or benzyloxycarbonyl, and
if R,-A and R3 A are benzyloxycarbonyl, RZ and R4 cannot be pyridylmethyl,
phenylethyl, 4-hydroxyphenylethyl, 4-chlorophenylethyl, phenylpropyl or
indolylethyl
1o their pharmacologically acceptable salts and optically active forms
thereof.
The compounds of formula I can be prepared using classical methods of peptide
chemistry.
Acylation of cystine or its carboxy protected derivatives with activated
carboxylic or
sulfonic acids like acid chlorides, active esters like N-hydroxysuccinimid or
hydroxy
benzotriazol esters. These activated esters may be prepared in situ using
activating
agents like carbodiimides or N,N'-carbonyldiimidazole followed by amidation of
the
carboxy group of the cystine using the methods of peptide chemistry.
2o Another method of preparation starts with carboxy protected cystine and
reacting it with
activated acids, isocyanates, isothiocyanates. After the cleavage of the
protecting group
the carboxylic function can be amidated as described above. Useful protecting
groups
are known from peptide chemistry e.g. methyl, ethyl, benzyl or p-
tert.butylesters. Alkyl
esters are cleaved by alkaline hydrolysis, benzyl esters by HBr in acetic
acid. Tert.butyl
esters are cleaved with strong organic acids like trifluoro acetic acid.
The compounds of the present invention are pharmacologically useful in the
treatment
of rheumatoid arthritis and related diseases in which collagenolytic activity
is a
contributing factor, such as, for example, corneal ulceration, osteoporosis,
periodontitis,
3o Paget's disease, gingivitis, tumor invasion, dystrophic epidermolysis,
bullosa, systemic
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
7
ulceration, epidermal ulceration, gastric ulceration, and the like. These
compounds are
particularly useful in the treatment of rheumatoid arthritis (primary chronic
polyarthritis,
PCP), systemic lupus erythematosus (SLE), juvenile rheumatoid arthritis,
Sjiigren's
syndrome (RA + sicca syndrome), polyarteritis nodosa and related vasculities,
e. g.
Wegener's granulomatosis, giant-cell arteritis, Goodpasture's syndrome,
hypersensitiveness angiitis, polymyositis and dermatomyositis, progressive
system
sclerosis, M. Bechterew, Reiter syndrome (arthritis + urethritis +
conjunctivitis), mixed
connective tissue disease (Sharp's syndrome), spondylitis ankylopoetica (M.
Bechterew).
to
The compounds of the present invention may be administered by any suitable
route,
preferably in the form of a pharmaceutical composition adapted to such a route
and in
dose effective for the treatment intended. Therapeutically effective doses of
the
compounds of the present invention required to prevent or arrest the progress
of the
15 medical condition are readily ascertained by one of ordinary skill in the
art.
Accordingly, the invention provides a class of novel pharmaceutical
compositions
comprising one or more compounds of the present invention, in association with
one or
more non-toxic pharmaceutically acceptable carriers and/or adjuvants
(collectively
2o referred to herein as "corner materials") and, if desired, other active
ingredients. the
compounds and compositions may, for example, be administered intravascularly,
intraperitoneally, subcutaneously, intramuscularly or topically.
For all administrations, the pharmaceutical composition may be in the farm of,
for
25 example, a tablet, capsule, suspension or liquid. The pharmaceutical
composition is
preferably made in the form of a dosage unit contained in a particular amount
of the
active ingredient. Examples of such dosage units are tablets or capsules. A
suitable daily
dose for a mammal may vary widely depending on the condition of the patient
and other
factors. However, a dose from about 0.1 to 300 mg/kg body weight, particularly
from
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
8
about 1 to 30 mg/kg body weight may be appropriate. The active ingredient may
also be
administered by injection.
The dose regimen for treating a disease condition with the compounds and/or
compositions of this invention is selected in accordance with a variety of
factors,
including the type, age, weight, sex and medical conditions of the patient.
Severity of
the infection and the role of administration and the particular compound
employed and
thus may vary widely.
For therapeutic purposes, the compounds of the invention are ordinarily
combined with
one ore more adjuvants appropriate to the indicated route of administation. If
per os, the
compounds may be admixed with lactose, sucrose, starch powder, cellulose
esters of
alkanoic acids, cellulose alkyl ester, talc, stearic acid, magnesium stearat,
magnesium
oxide, sodium and calcium salts of phosphoric and sulphuric acids, gelatine,
acacia,
sodium alginate, polyvinyl-pyrrolidone and/or polyvinyl alcohol, and thus
tabletted or
encapsulated for convenient administration. Alternatively, the compounds rnay
be
dissolved in water, polyethylene glycol, propylene glycol, ethanol, corn oil,
cotton seed
oil, peanut oil, sesam oil, benzyl alcohol, sodium chloride and/or various
buffers. Other
adjuvants and modes of administration are well and widely known in the
pharmaceutical
2o art. Appropriate dosages in any given instance, of course, depend upon the
nature and
severity of the condition treated, the route of administration and the species
of mammal
involved, including its size and any individual idiosyncracies.
Representative carriers, dilutions and adjuvants include, for example, water,
lactose,
gelatine starch, magnesium stearate, talc, vegetable oils, gums, polyalkylene
glycols,
petroleum gelly, etc. The pharmaceutical compositions may be made up in a
solid form,
such as granules, powders or suppositories, or in liquid form, such as
solutions,
suspensions or emulsions. The pharmaceutical compositions may be subjected to
conventional pharmaceutical adjuvants, such as preservatives, stabilizers,
wetting
agents, emulsifiers, buffers, etc.
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
9
For use in the treatment of rheumatoid arthritis, the compounds of this
invention can be
administered by any convenient route, preferably in th.e form of a
pharmaceutical
composition adapted to such route and in a dose effective for the intended
treatment. In
the treatment of arthritis, administration may be conveniently be by the oral
route or by
injection infra-articularly into the affected joint.
As indicated, the dose administered and the treatment regimen will be
dependent, for
example, on the disease, the severity thereof, on the patient being treated
and his
to response to treatment and, therefore, may be widely varied.
The following compounds are synthezised in analogy to Miiller et al. (Biol.
Chem. 378,
1475-1480 (1997)).They are new and subject of the invention:
1 ) 2-Formylamino-3-(2-formylamino-2-phenethylcarbamoyl-ethyldisulfanyl)-N
phenethyl-propionamide
2) 2-Acetylamino-3-(2-acetylamino-2-phenethylcarbamoyl-ethyldisulfanyl)-N
phenethyl-propionamide
3) 2-Propanoylamino-3-(2-propanoylamino-2-phenethylcarbamoyl-
ethyldisulfanyl}-N phenethyl-propionamide
4) 2-Hexanoylamino-3-(2-hexanoylamino-2-phenethylcarbamoyl-ethyldisulfanyl)-
N phenethyl-propionamide
5) 2-Phenacetylamino-3-(2-pherlacetylamino-2-phenethylcarbamoyl-
ethyldisulfanyl)-N phenethyl-propionamide
CA 02348946 2001-05-02
WO 00/27378 PCTlEP99/08460
6) 2-Cinnamoylamino-3-(2-cinnamoylamino-2-phenethylcarbamoyl-
ethyldisulfanyl)-N phenethyl-propionamide
7) 2-Benzoylamino-3-(2-benzoylamino-2-phenethylcarbamoyl-ethyldisulfanyl)-N
5 phenethyl-propionamide
8) 2-(4-Chlor-benzoyl)amino-3-(2-(4-chlor-benzoyl)amino-2-phenethylcarbamoyl-
ethyldisulfanyl)-N phenethyl-propionamide
10 9) 2-(4-Methyl-benzoyl)amino-3-(2-(4-methyl-benzoyl)amino-2-
phenethylcarbamoyl-ethyldisulfanyl)-N phenethyl-propionamide
10) 2-(4-Methoxy-benzoyl)amino-3-(2-(4-methoxy-benzoyl)amino-2-
phenethylcarbamoyl-ethyldisulfanyl)-N phenethyl-propionamide
11 ) 2-Methylsulfonylamino-3-(2-methylsulfonylamino-2-phenethylcarbamoyl-
ethyldisulfanyl)-N phenethyl-propionamide
12) 2-Ethylsulfonylamino-3-(2-ethylsulfonylamino-2-phenethylcarbamoyl-
2o ethyldisulfanyl)-N phenethyl-propionamide
13) 2-Benzylsulfonylamino-3-(2-benzylsulfonylamino-2-phenethylcarbamoyl-
ethyldisulfanyl)-N phenethyl-propionamide
14) 2-Benzenesulfonylamino-3-(2-benzenesulfonylamino-2-phenethylcarbamoyl-
ethyldisulfanyl)-N phenethyl-propionamide
15) 2-Toluolsulfonylamino-3-(2-toluolsulfonylamino-2-phenethylcarbamoyl-
ethyldisulfanyl)-N phenethyl-propionamide
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
11
16) 2-Formylamino-3-(2-formylamino-2-phenylmethylcarbamoyl-ethyldisulfanyl)-
N phenylmethyl-propionamide
17) 2-Formylamino-3-(2-formylamino-2-(2-phenylcyclopropyl)-carbamoyl-
ethyldisulfanyl)-N (2-phenylcyclopropyl)-propionamide
18) 2-Formylamino-3-(2-formylamino-2-morpholinoethylcarbamoyl-
ethyldisulfanyl)-N morpholinoethyl-propionamide
1o 19) 2-Formylamino-3-(2-formylamino-2-cyclohexylethylcarbamoyl
ethyldisulfanyl)-N cyclohexylethyl-propionamide
CA 02348946 2001-05-02
WO 00/27378 PCT/EP99/08460
12
Experimental part
Example 1
2-(4-Chlor-benzoyl)amino-3-(2-(4-chlor-benzoyl)amino-2-phenethylcarbamo 1-
ethyldisulfanyl)-N-phenethyl-propionamide
1) N,N'-Di-tert.butoxycarbonyl-L-cystin-bis-phenethylamide
1o N,N'-Di tert.butyloxycarbonyl-L-cystin (2.2 g) is dissolved in
tetrahydrofurane (120
ml) and treated with N-hydroxybenzotriazole (1.35 g), O-(benzotriazole-1-yl)-
N,N,N',N'-tetramethyluronium-tetrafluoroborat (4.27 g), di-isopropylethylamine
(3.37
ml) and phenethylamine (1.38 ml). The mixture is stirred at room temperature
for 4
hours and left over night without stirnng. The reaction mixture was
concentrated in
15 vacuo. The residue was dissolved in dichloromethane and the organic phase
washed two
times with NaHS04 solution. A solid precipitated from the biphasic solvent
mixture.
The precipitate was filtered and the organic phase washed three times with
NaHC03
solution and water. The filtrate was concentrated and the residue combined
with the
previously isolated precipitate to yield 3.18 g (98 %) of the title compound.
2o Rf silica gel = 0.66 (dichloromethane/methanol 95:5), m/e [M+H] = 647
1. L-Cystin-bis-phenethylamide
The product obtained by the above procedure (3.18 g) was dissolved in
dichloromethane
25 (30 ml) and trifluoro-acetic acid (8.16 ml). The mixture was kept at room
temperature
overnight , concentrated and neutralized with a solution of NaHC03 in water.
The
precipitate was filtered and washed with water to yield 1.22 g of the title
compound.
Rf silica gel = 0.4 (dichloromethane/methanol 9:1)
30 3. 2-(4-Chlor-benzoyl)amino-3-(2-(4-chlor-benzoyl)amino-2-
phenethylcarbamoyl-
ethyldisulfanyl)-N-phenethyl-propionamide
4-Chlorobenzoic acid (156.5 mg) was dissolved in tetrahydrofurane (10 rnl) and
treated
with 1-hydroxybenzotriazole (135 mg), O-(benzotriazole-1-y1)-N,N,N',N'-
35 tetramethyluronium-tetrafluoroborat (389 mg}, di- isopropylethylamine (342
pl) and L-
cystin-bis-phenethylamide (223 mg) as a solution in 10 ml tetrahydrofurane.
The
reaction mixture was stirred for 24 hours. The precipitate was filtered washed
with
tetrahydrofurane and dried to yield 280 mg (77%) of the title compound.
TLC: Rf silica gel = 0.7 (dichloromethane/methanol 95:5}
CA 02348946 2001-05-24
13 51 ~8ioa
Example 2
The compounds in the following table were synthesized using the procedure from
example 1
Number Chemical Name TLC Rf value
Silica gel
1 2-(Butanoyl)amino-3-(2-(butanoyl)amino-2-0.5
phenethylcarbamoyl-ethyldisplfanyl)-N-phenethyl-dichlormethaae/methano195;5
propionamide
2 2-(4-Methylbenzoyl)amino-3-(2-(4-methyl-0.7
benzoyl)amino-2-phenethylcarbamoyl- dichlormethane/methanol
95 ; 5
ethyldisulfanyl)-N phenethyl-propionamide
3 2-((3-Benzoyl)-benzoyl)aminq-3-(2-(3-benzoyl)-0.7
benzoyl)amino-2-phenethylcarbamoyl- dichlormethane/methanol
95:5
ethyldisulfanyl~N-phenetliyl-propionamide
4 2-((Fluoren-1-yl-carbonyl)amino-3-(2-(3-benZOyl)-0.75
benzoyl)amino-2-phenethylcatbamoyl- dichlorniethane/methanol
95:5
ethyldisulfanyl)-N-phenethyl-propionamide
S 2-((Fluorenon-1-yl-carbonyl)anuno-3-(2-(3-0.5
benzoyl)-benzoyl)amino-2-phenethylcarbamoyl-dichlortnethanelmethanol95:5
ethyldisulfanyl)-N-phenethyl-propionamide
6 2-(Peatanoyl)amino-3-(2-(pen'tanoyl)amino-2-0.65
phenethylcarbamoyl-ethyldisu'lfanyl)-N-phenethyl-dichlormethane/methanol95:5
propionamide
7 2-(4-Ethyl-biphenyl-1-yl-carbonyl)amino-3-(2-(4-0.65
Ethyl-biphenyl-I-carbonyl)arr~ino-2-phenethyl-dichlormethane/methano195:5
carbamoyl-ethyldisulfanyl)-N=phenethyl-
propionamide
8 2-(3,4-Dichloro-benzoyl)amirio-3-(2-(3,4-dichloro-0.8
benzoyl)amino-2-plienethylca~bamoyl- dichlormethanelmethanol
95:5
ethyldisulfanyl)-N-phenethyl-propionamide
9 2-(4-Phenoxy-benzoyl)amino-',3-(2-(4-phenoxy-0.8
benzoyl)amino-2-phenethylcarbamoyl- dichlormethane/methanol
95;5
ethyldisulfanyl)-N- henethyl-pro ionamide
2-(4-Toluenesulfonyl)amino-3,-(2-(4- 0.56
toluenesulfonyl)amino-2-pheriethylcarbamoyl-dichlormethane/methano195:5
ethyldisulfanyl)-N-phenethyl-propionamide
11 2-(Propionyl)amino-3-(2-(pm~ionyl)amino-2-0.4
phenethylcarbamoyl-ethyldisulfanylrN-phenethyl-dichlormethane/methano195:5
propionamide
12 2-(4-Methylsulfonyl-benzoyl)amino-3-(2-(4-methyl-0,31
sulfonyl-benzoyl)amino-2-phenethylcarbamoyl-dichlonnethane/methano195:5
ethyldisulfanyl)-N-phenethyl-propionamide
' CA 02348946 2001-05-24
14 S 178/OA
13 2-(4-Chlom-phenylacetyl)amino-3-(2-(4-Chloro-0.8
phenyl-acetyl)amino-2-phenet~ylcarbamoyl-dichlormethane/methanol
ethyldisulfanyl)-N-phenethyl-propionarnide90:10
14 2-(4-Methyl-cinnamoyl)aminQ-3-(2-(4-methyl-0.3
cinnamoyl)amino-2-phenethylcarbamoyl-dicblonmeihane/methanol
95: S
ethyldisulfanyl)-N-phenethyl-propionamide
IS 2-(4-Methyl-phenylaeetyl)am'mo-3-(2-(4-methyl-0.37
phenyl-acetyl)amino-2-phenet~ylcarbamoyl-dichlormethane/methano195:5
ethyldisulfanyl)-N-phenethyl-propionamide
16 2-(4-Methoxy-benzoyl)amino ~ 3-(2-(4-methoxy-0.65
benzoyl)amino-2-phenethylca~bamoyl- dichlorrnetbane/methanol
95:5
ethyldisulfanyl)-N-phenethyl-propionamide
17 2-(4-Methoxy-cinnamoyl)ami$~o-3-(2-(4-methoxy-0.67
cinnamoyl)-amino-2-phenethylcarbamoyl-dichlormethane/methanol
95 ; S
ethyldisulfanyl)-N-phenethyl-propionamide
18 2-(4-Chloro-cinnamoyl)amino-3-(2-(4-chloro-0.5 8
cinnamoyl)-amino-2-pheneth~lcarbamoyl-dichlormethaneimethanol
95:5
ethyldisuLfanyl)-N-phenethyl-propionamide
19 2-(Acetyl)amino-3-(2-(acetyl)ramino-2-phenetlxyl-0.25
carbamoyl-ethyldisulfanyl)-N=phenethyl-dichlormethane/methanol
95:5
propionamide
Example 3
2-Benzyloxycarbonyl)amino-3-(2-(benzyloxycarbonyl)amino-2-hexylcarbamoyl-
ethyldisulfanyl)-N-hexyl-propionaunide
Di-benzyloaycarbonyl-L-cystine (508 mg~ was dissolved in tetrahydrofurane (25
ml)
1o and treated with 1-hydroxybenzotriazole (270 mg), O-(Benzotriazole-1-yl)-
N,N,N',N'-
tetramethyluronium-tetrafluoroborat (777~mg) and di-isopropylethylamine (0.68
ml}.
The mixture was stirred for 10 min and n~hexylamine (0.29 ml) was added. After
stirring overnight the reaction mixture wa,~ concentrated . T'he residue was
dissolved in
ethyl acetate, washed two times with NaI~S04 solution , NaHCO, solution and
water.
The organic phase was dried and concentrated. The residue was triturated with
isohexane to yield 550 mg (77%) of the title compound.
Rt (silica gel) = 0.3 (dichloromethane/me'thanol 97:3)
CA 02348946 2001-05-24
15 5178~oA
Example 4
The compounds in the following table were synthesized using the procedure of
example 1
Number Chemical name TLC Rf value
Silica gel
1 2-(Benzyloxy-carbonyl)amino-3-(2-(benzyloxy-0.72
carbonyl~amino-.2-(4-fluoro-phenethyl-)carbamoyl-dichloromethane/methanol
ethyldisulfanyl)-N-(4-fluoro-phenethyl-)97:3
propionamide
2 2-(Benzyloxy-carbonyl)amino-3-(2-(benzyloxy-0.3
carbonyl)-amino-2-(3,4-dim,et$oxy-phenethyl)dichloromethane/methanol
carbamoyl-ethyldisulfanyl)-N=(3,4-dimethoxy-97:3
phenethyl-~ropionamide
3 2-(Benzyloxy-carbonyl)amino-3-(2-(benzyloxy-0.4
carbonyl)-amino-2-(morpholino-pmpyl) dichloromethane/methanol
carbamoyl-
ethyldisulfanyl)-N-(morpholmo-propyl-)95:5
propionamide
4 2-(Beazyloxy-carbonyl)amino-3-(2-(benzyloxy-0.6
carbonyl)-amino-2-(4-imidazolyl-ethyl)-carbamoyl-dichloromethanelmethanol
ethyldisulfanyl)-N-(4-imida2olyl-ethyl-)80:20
propionamide
5 2-(Benzyloxy-carbonyl)amino-3-(2-(benzyloxy-0.77
carbonyl)-amino-2-(4-chlom-phenethyl)-carbamoyl-dichloromethane/methanol
ethyldisulfanyl)-N-(4-chloro-phenethyl~97:3
propionamide
6 2-(Benzyloxy-carbonyl)amino-3-(2-(benzyloxy-0.6
carbonyl)-amino-2-(3-indolyl-ethyl)-carbamoyl-dichloromethane/methanol/am
ethyldisulfanyl)-N-(3-indolyl-ethyl)-propionamidemonia cone. 16:4;0.1
7 2-(Benzyloxy-carbonyl)amino-3-(2-(benzyloxy-O.A~S
carbonyl)-amino-2-phenethyl-catbamoyl-dichloromethane/methanol
ethyldisulfanyl)-N-phenethyl-propionamide98:2