Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02349537 2004-O1-15
SONIC OR ULTRASONIC CLEANING DEVICE
Field of the Invention
The present invention generally relates to devices and processes for removing
soils using sonic or ultrasonic waves.
Background of the Invention
Ultrasonic cleaning is a well known cleaning process in industry. For example,
it
is used to clean electronic components after or during immersion in cleaning
solution
such as azeotropic mixtures of flurohydrocarbons. It is also used domestically
to a small
extent in oral hygiene, as in ultrasonic tooth brushes. However, ultrasonic
cleaning has
not found much acceptance domestically beyond this limited application.
Consequently, the need remains for a device which is able to provide
ultrasonic
energy or waves of a useful frequency and power, for a variety of cleaning
applications.
Background Art
US 4,183,011, US 4,103,519, US 4,225,803, US 3,946,599, US 5,770,801, US
5,640,960, US 3,849,195, US 4,168,560, US 4,250,586, US 5,247,716, US
3,937,326,
US 4,069,541, US 4,307,484; JP 10165228, JP 61199829; EP 856,277,
Summary of the invention
The invention meets the needs identified above by providing a device which is
able
to provide sonic or ultrasonic energy or waves of a useful frequency and
power, for a
variety of cleaning applications.
The first embodiment of the present invention is an ultrasonic cleaning device
comprising a housing, said housing comprising a gripping means; a cleaning
head is adapted
to rest on and be moved over surface to be cleaned, wherein said cleaning head
is adapted
to be removably mounted to said housing and the minimum surface area of said
cleaning
head to rest on said surface is greater than about 6.25 cm2; a transducer
means mounted
in said housing for converting electrical energy to sonic or ultrasonic energy
said cleaning head at
a frequency ranging from 100 Hz to 20,000 KHz; and a power supply means for
supplying direct current to said transducer means, wherein said power supply
means is
associated with said device.
CA 02349537 2004-O1-15
2
The second embodiment of the present invention is a cleaning device
coraprising a first housing, said first housing comprising a gripping means; a
cleaning
head adapted to rest on and be moved over surface to be cleaned, and said
cleaning head
is adapted to be removably mounted to said first housing and the minimum
surface area
of said cleaning head to rest on said surface is greater than about 6.25 cm2;
a second
housing, wherein said first housing is associated with said second housing and
said
second housing comprises a transducer means mounted in said second housing for
oscillating said cleaning head at a frequency ranging from 100 Hz to 20,000
KHz; and a
power supply means for supplying direct current to said transducer means,
wherein said
power supply means is associated with said device.
The third embodiment of the present invention is a cleaning product
comprising:
(a) a liquid or gel cleaning composition comprising a cleaning agent; and
(b) a hand held ~ cleaning device comprising a housing, said housing
comprising a gripping means; a cleaning head adapted to rest on and be moved
over surface to be cleaned, wherein said cleaning head is adapted to be
removably mounted to said housing and the minimum surface area of said
cleaning head to rest on said surface is greater than about 6.25 cm2; a
transducer means mounted in said housing for oscillating said cleaning head
at a frequency ranging from 100 Hz to 20,000 KHz; and a power supply means for
supplying direct current to said transducer means, wherein said power supply
means is
associated with said device.
The fourth embodiment of the present invention is a cleaning product
comprising;
(a) a liquid or gel cleaning composition comprising a cleaning agent; and
(b) a; cleaning device comprising a first housing, said first housing
comprising a gripping means; a cleaning head adapted to rest on and be moved
over surface to be cleaned, and said cleaning head is adapted to be removably
mounted to said first housing and the minimum surface area of said cleaning
head to rest on said surface is greater than about 6.25 cm2; a second housing,
wherein said first housing is associated with said second housing and said
CA 02349537 2004-O1-15
3
second housing comprises a transducer means mounted in said second
housing for oscillating said cleaning heat at a frequency ranging from 100 Hz
to
20,000 KHz; and a power supply means for supplying direct current to said
transducer means, wherein said power supply means is associated with said
device.
The fifth embodiment of the present invention is a: cleaning device
comprising a housing, said housing comprising optionally a gripping means, a
retaining
means for removably retaining tableware; a transducer means mounted in said
housing
for oscillating said housing at a frequency ranging from 100 Hz to 20,000 KHz;
and
a power supply means for supplying direct current to said transducer means,
wherein
said power supply means is associated with said device.
The sixth embodiment of the present invention is a cleaning device
comprising a housing, said housing is adapted to be at least partially
immersed in an
aqueous environment, and said housing comprises optionally a gripping means, a
retaining
means for removably retaining tableware; a transducer means mounted in said
housing
for oscillating said aqueous environment at a frequency ranging from 100 Hz to
20,000 KHz; and a power supply means for supplying direct current to said
transducer means, wherein said power supply means is associated with said
device.
By using this device as a source of sonic or ultrasonic energy, stains or
tough soils can be
removed without the use of excessive force, rubbing, pressure or other
manipulation
which causes wear and tear on the stained material or surface. In doing so,
the user does
not need to impart such manual energy to remove the stain, thereby adding to
the
convenience of the user. The invention also encompasses processes by which
such stains
or soils are removed, either from localized regions or from the entire article
to be
cleaned.
The present invention also includes methods of cleaning a hard surface by
contacting the surface with a cleaning composition and then contacting the
surface with
the cleaning head of the device, or the cleaning product, as hereinbefore
described and imparting sonic or ultrasonic energy to said soil,
The present invention also includes methods removing tough food soil from a
hard
surface by contacting the soil with a cleaning composition and then contacting
the soil
CA 02349537 2004-O1-15
4
with the cleaning head of the device or the ultrasonic cleaning product, as
hereinbefore
described and imparting ultrasonic energy to said soil.
As used herein, the phrase "ultrasonic waves" means mechanical pressure or
stress Waves which can propagate through any material media, wherein the
frequency
spectra of these waves can vary from 20,000 cycles/second (Hz) to a few
billion Hz.
All parts, percentages and ratios used herein are expressed as percent weight
unless
otherwise specified.
BRIEF DESCRIPTION OF THE DRAWIrIG
FIGURE 1 is a perspective view of a hand-held, ultrasonic device, with a
cleaning
solution storage means which is adapted to be removably mounted in the device.
Also
shown are a removably mountable cleaning head and an additional cleaning
solution
storage means.
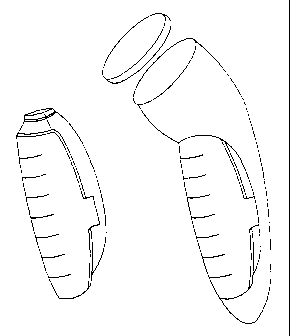
FIGURE 2 is a perspective view of two different hand-held, pen-shaped
ultrasonic
devices, which are used in the invention to impart ultrasonic waves onto a
stain or soil.
FIGURE 3 is a perspective view of a hand-held, pen-shaped ultrasonic device,
which is shown imparting ultrasonic waves onto a soil.
FIGURE 4 is a perspective an ultrasonic device, which are used in the
invention to
impart ultrasonic waves onto a stain or soil. The ultrasonic generator and the
power
source are in a second housing which is associated with the cleaning head
which is in a
first housing.
Detailed Description of the Invention
As it was stated previously, the first embodiment of the present invention is
an
ultrasonic cleaning device comprising a housing, said housing comprising a
griping
means; a cleaning head adapted to rest on and be moved over surface to be
cleaned,
wherein said cleaning head is adapted to be removably mounted to said housing
and the -
minimum surface area of said cleaning head to rest on said surface is greater
than about
G.25 cm2, preferably greater than about 20 cm2; preferably said griping means
is at the
proximal end of said housing and said cleaning head is at the distal end of
said housing; a
transducer means mounted in said housing for oscillating said cleaning head at
a sonic or
ultrasonic frequency; and a power supply means for supplying direct current to
said
CA 02349537 2004-O1-15
transducer means, wherein said power supply means is associated with said
device,
preferably said power supply means is mounted in said housing.
It is also preferred that the cleaning device further comprises at least one,
more
preferably at least two, solution storage means associated with said device,
and said
solution storage means contains at least one, more preferably at least two,
cleaning
composition suitable for cleaning said surface; and at least one, more
preferably at least
two, dispensing means mounted in said housing for supplying said at least one
cleaning
composition from said at least one solution storage means to said surface
prior to or at
the same time as said surface is contacted by said cleaning head. In one
aspect it is
preferred that the solution storage means is adapted to be removably mounted
to said
housing. In another aspect it is preferred that the solution storage means is
mounted in
said housing. One advantage of having two or more storage means is that
incompatible
cleaning ingredients, such as bleach and enzymes, which would ordinarily not.
be possible
to combine in a cleaning composition without the loss of cleaning activity,
can be put in
different storage means. This allows the compositions to gain the cleaning
benefits of
these incompatible ingredients as they only come into contact with one another
either just
before dispensing or when the are applied to the surface. This means that any
loss in
cleaning potential would be minimized.
As it was stated previously, the second embodiment of the present invention is
a
cleaning device comprising a first housing, said first housing comprising a
griping means; a cleaning head adapted to rest on and be moved over surface to
be
cleaned, and said cleaning head is adapted to be removably mounted to said
first housing
and the minimum surface area of said cleaning head to rest on said surface is
greater than
about 6.25 cm2; preferably the griping means is at the proximal end of the
first housing
and the cleaning head is at the distal end of the first housing; a second
housing, wherein
said first housing is associated with said second housing and said second
housing
comprises a transducer means mounted in said second housing for oscillating
said
cleaning head at frequency ranging from 100 Hz to 20,000 KHz; and a power
supply
means for supplying direct current to said transducer means, wherein said
power supply
means is associated with said device, preferably the power supply means is
mounted in
said second housing.
CA 02349537 2004-O1-15
6
It is also preferred that the cleaning device according to the second aspect
further
comprises at least one solution storage means associated with said device, and
said at
least one, more preferably at least two, solution storage means contains at
least one, more
preferably at least two, cleaning composition suitable for cleaning said
surface; and at
least one, more preferably at least two, dispensing means mounted in said
first housing
for supplying said at least one cleaning composition from said at least one
solution
storage means to said surface prior to or at the same time as said surface is
contacted by
said cleaning head. In one aspect it is preferred that the solution storage
means is
adapted to be removably mounted to said first housing. In one aspect it is
preferred that
the solution storage means is adapted to be removably mounted to the second
housing.
In another aspect it is preferred that the solution storage means is mounted
in the first
housing. In another aspect it is preferred that the solution storage means is
mounted in
the second housing. This use of more than one solution storage means has all
the
advantages of using incompatible ingredients as was noted previously above.
In the second aspect it is preferred that the first housing be capable of
being hand
held. In one preferred form the first housing is stored in the second housing
while not in
use. While in use the first housing is used to clean the surface while the
second housing
stores and supplies the cleaning composition(s), power and sonic or ultrasonic
energy
to the first housing to clean the surface.
As it was stated previously, the third and fourth embodiments of the present
invention are ultrasonic cleaning products comprising a liquid cleaning
composition
comprising a cleaning agent; and the hand held ultrasonic cleaning device
according to
the first aspect or the cleaning device according to the second aspect.
Preferably the cleaning agent is present in the liquid cleaning composition in
an
effective amount, more preferably from about 0.0001% to about 99.9%, even more
preferably from about 0.001% to about 55%, even more preferably still from
about
0.005% to about 45% by weight. These cleaning compositions can comprise
additional
cleaning additives and these are exemplified in greater detail hereafter. The
liquid
cleaning composition in the ultrasonic cleaning products can be, for example,
in the
optional at least one solution storage means, in another container in the same
product and
designed to be added to the optional at least one solution storage, in another
container in
CA 02349537 2004-O1-15
7
the same product and directly added to the surface to be cleaned, in another
container' in
the same product and made into an aqueous solution in which the surface is
immersed, in
another container in the same product and applied to by the user from another
container
to the cleaning head either neat or in another container in the same product
and as an
aqueous solution. These are merely some possible examples and not intended to
be
limiting.
It is preferr~ that these cleaning products further comprise instructions
for using the product. One preferred set of instructions comprises the steps
of
(i) applying an effective amount of said liquid cleaning composition to said
surface;
(ii) imparting sonic or ultrasonic waves to said surface using said device;
and
(iii) optionally, rinsing the surface with an aqueous solution.
Another, preferred set of instructions comprises the steps of
(i) using said device to apply an effective amount of said liquid cleaning
composition to said surface concurrently and coterminous with said cleaning
head;
(ii) moving said cleaning head over and maintain contact thereto said surface
and
(iii) optionally, rinsing the surface with an aqueous solution.
These instructions are suitable for incorporation with ultrasonic cleaning
products
based on either the device of the first or second embodiment.
As it was stated previously, the fi$h embodiment of the present invention is a
cleaning device comprising a housing, said housing comprising an optional
griping means, a retaining means for removably retaining tableware; a
transducer means
mounted in said housing for oscillating said housing at a frequency ranging
from 100 Hz
to 20,000 KHz; and a power supply means for supplying direct current to said
transducer
means, wherein said power supply means is associated with said device.
As it was stated previously, the sixth embodiment of the present invention is
a
cleaning device comprising a housing, said housing is adapted to be at least
partially immersed in an aqueous environment, and said housing comprises an
optional
griping means, a retaining means for removably retaining tableware; a
transducer means
mounted in said housing for oscillating said aqueous environment at a,
CA 02349537 2004-O1-15
8
frequency ranging from 100 Hz to 20,000 KHz; and a power supply means for
supplying
direct current to said transducer means, wherein said power supply means is
associated
with said device.
For the devices the power source can be any conventional power
source, such as mains power, rechargeable batteries, disposable batteries,
with
rechargeable battery or rechargeable batteries being preferred.
It is preferred that the surface contacted by the cleaning head is a hard
surface. A
"hard surface" is any surface which is traditionally regarded as hard, that is
tableware,
such as plates, glasses, cutlery, pots and pans, and also includes other
surfaces such as
kitchen counter tops, sinks, glass, windows, enamel surfaces, metal surfaces;
tiles,
bathtubs, floors etc. More preferably, the hard surface is tableware.
The cleaning composition can comprise conventional cleaning additives and
these
are exemplified in greater detail hereafter. The cleaning composition can be
dispensed
from the storage means automatically, or when desired by the device user. The
cleaning
composition can be dispensed from the storage means into the cleaning head and
applied
by the cleaning head directly to the surface. Alternatively, the cleaning
composition, can
be dispensed on to the surface which is not currently in contact with the
cleaning head.
Such as in front of, to either side or behind the direction the cleaning head
is being
moved over the surface. It is preferred that cleaning composition is supplied
to the
surface coterminous with the cleaning head. Furthermore, when the device does
not
contain storage means the cleaning composition can be either applied by the
operator on
to the surface, the stain/soil in need of cleaning or directly on to the
cleaning head. The
cleaning composition can be used neat or as an aqueous solution.
The cleaning head can be of any form suitable for cleaning. For example the
cleaning head could be a sponge, steel wool, scouring pad, foam, or bristles.
However, it
is critical, no matter what the cleaning head be, that the surface area be
greater than about
6.25 cm2. The cleaning head is adapted to be removably mounted, this is for
ease of
replacement, changing head type depending on the surface and/or soil to be
cleanedlremoved, and for overall efficiency and flexibility of use.
The transducer means oscillates at a frequency of from about 100 Hz to about
20,000 kHz, more preferably from about 100 Hz to about 10,000 kHz, mare
preferably
from about 150 Hz to about 2000 kHz, more preferably from about 150 Hz to
about
CA 02349537 2004-O1-15
9
1,000 kHz, more preferably from about 150 Hz to about 100 kHz, more preferably
from
about 200 Hz to about 50 kHz. It is preferred that the average frequency be
from about
1000 Hz to about 100kHz, more preferably from about 10,000 Hz to about 70kHz.
It is
also preferred that the device provides a power output per unit of surface
area of said
cleaning head of at least about 0.02 watts/cmz, mare preferably at least about
0.05
watts/cm2, even more preferably at least about 0.07 watts/cm', even more
preferably still
at least about 0.08 watts/em2.
While it is not essential, it is preferred that the device is adapted to
function while
partially immersed in an aqueous environment, more preferably the device is
adapted to
function while totally immersed in an aqueous environment. Alternatively, it
is preferred
that the device is water resistant, more preferably water proof. That is, when
the device
is made for cleaning in aqueous environment, such as washing dishes, pots
etc., the
device can be either partially or totally immersed without damage to the
device or harm
to the user. While devices that would be only used for cleaning hard surfaces,
such as
floor or tables, would not need to adapted to function while partially
immersed in an
aqueous environment, more preferably the device is adapted to function while
totally
immersed in an aqueous environment, it is highly preferred that the devices at
least be
adapted to function while partially immersed in an aqueous environment.
The present invention also includes a method of method of removing tough food
soil from a hard surface comprising the steps of
(l) contacting said soil with a cleaning composition;
(ii) contacting said soil with said cleaning head of said device according to
either
the first or second aspect and imparting sonic or ultrasonic energy to said
soil; and
(iii)optionally, rinsing said hard surface with an aqueous solution.
Alternatively, step (ii) could be performed first, then (l), with the
possibility of repeating
this order until the soil is removed, or steps(ii), (l) and (iii) in this
order. Steps (l) and (ii)
could be performed at the same time and optionally followed by step (iii).
Another
method that is within the scope of the present invention is one where the soil
is softened
by application of a cleaning composition and the soften soil is the removed by
the
application of sonic or ultrasonic energy to the softened soil.
Cleanine Composition:
CA 02349537 2004-O1-15
The cleaning composition contains cleaning additives. This composition can be
in any conventional form, such as liquid, gel paste, etc. The compositions may
additionally contain aqueous solvents such as water or low molecular weight
alcohols,
such as methanol, or ethanol.
Cleaning additives
The cleaning composition comprises cleaning additives. These cleaning
additives
are for assisting or enhancing cleaning performance, treatment of the
substrate to be
cleaned, or to modify the aesthetics of the detergent composition (e.g.,
perfumes,
colorants, dyes, etc.). Some suitable cleaning additives include builders,
surfactants,
enzymes, bleach activators, bleach catalysts, bleach boosters, bleaches,
alkalinity sources,
antibacterial agent, colorants, perfume, lime soap dispersants, polymeric dye
transfer
inhibiting agents, crystal growth inhibitors, photobleaches, heavy metal ion
sequestrants,
anti-tarnishing agents, anti-microbial agents, anti-oxidants, anti-
redeposition agents, soil
release polymers, electrolytes, pH modifiers, thickeners, abrasives; divalent
ions, metal
ion salts, enzyme stabilizers, corrosion inhibitors, diamines, suds
stabilizing polymers,
solvents, process aids, fabric softening agents, optical brighteners,
hydrotropes. and
mixtures thereof. The following are illustrative examples of such adjunct
materials..
Detergent Builders
The present invention may include an optional builder in the product
composition. The level of detergent saltlbuilder can vary widely depending
upon the end
use of the composition and its desired physical form. When present, the
compositions
will typically comprise at least about 1% detergent builder and more typically
from about
10% to about 80%, even more typically from about 15% to about 50% by weight,
of the
detergent builder. Lower or higher levels, however, are not meant to be
excluded.
Inorganic or P-containing detergent builders include, but are not limited to,
the
allcali metal, ammonium and allcanolammonium salts of polyphosphates
(exemplified by
the tripolyphosphates, pyrophosphates, and glassy polymeric meta-phosphates),
phosphonates, phytic acid, silicates, carbonates (including bicarbonates and
sesquicarbonates), sulphates, and aluminosilicates. However, non-phosphate
salts are
required in some locales. Importantly, the compositions herein function
surprisingly well
even in the presence of the so-called "weak" builders (as compared with
phosphates)
CA 02349537 2004-O1-15
11
such as citrate, or in the so-called "underbuilt" situation that may occur
with zeolite or
layered silicate builders. Mixture of builders are also envisaged.
Examples of silicate builders are the alkali metal silicates, particularly
those
having a Si02:Na20 ratio in the range 1.6:1 to 3.2:1 and layered silicates,
such as the
layered sodium silicates described in U.S. Patent 4,664,839, issued May 12,
1987 to H. P.
ltieck. NaSKS-6 is the trademark for a crystalline layered silicate marketed
by Hoechst
(commonly abbreviated herein as "SKS-6"). Unlike zeolite builders, the Na SKS-
6
silicate builder does not contain aluminum. NaSKS-6 has the delta-Na2Si05
morphology form of layered silicate. It can be prepared by methods such as
those
described in German DE-A-3,41?,649 and DE-A-3,742,043. SKS-6 is a highly
preferred
layered silicate for use herein, but other such layered silicates, such as
those having the
general formula NaMSix02x+l.yH20 wherein M is sodium or hydrogen, x is a
number
from 1.9 to 4, preferably 2, and y is a number from 0 to 20, preferably 0 can
be used
herein. Various other layered silicates from Hoechst include NaSKS-5, NaSKS-7
and
NaSKS-11, as the alpha, beta and gamma forms. As noted above, the delta-
Na2Si05
(NaSKS-6 form) is most preferred for use herein. Other silicates may also be
useful such
as for example magnesium silicate, which can serve as a crispening agent in
granular
formulations, as a stabilizing agent for oxygen bleaches, and as a component
of suds
control systems.
Examples of carbonate salts as builders are the alkaline earth and alkali
metal
carbonates as disclosed in German Patent Application No. 2,321,001 published
on
November 15, 1973.
Aluminosilicate builders may also be added to the present invention as a
detergent salt. Aluminosilicate builders are of great importance in most
currently
marketed heavy duty granular detergent compositions. Aluminosilicate builders
include
those having the empirical formula:
Mz(zA102)y]~xH20
wherein z and y are integers of at least 6, the molar ratio of z to y is in
the range from 1.0
to about 0.5, and x is an integer from about 15 to about 264.
Useful aluminosilicate ion exchange materials are commercially available.
These
aluminosilicates can be crystalline or amorphous in structure and can be
naturally-
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
12
occurring aluminosilicates or synthetically derived. A method for producing
aluminosilicate ion exchange materials is disclosed in U.S. Patent 3,985,669,
Krummel,
et at, issued October 12, 1976. Preferred synthetic crystalline
aluminosilicate ion
exchange materials useful herein are available under the designations Zeolite
A, Zeolite P
(B), Zeolite MAP and Zeolite X. In an especially preferred embodiment, the
crystalline
aluminosilicate ion exchange material has the formula:
Nal2~(A102)12(Si02)12)~xH20
wherein x is from about 20 to about 30, especially about 27. This material is
known as
Zeolite A. Dehydrated zeolites (x = 0 - 10) may also be used herein.
Preferably, the
aluminosilicate has a particle size of about 0.1-10 microns in diameter.
Organic detergent builders suitable for the purposes of the present invention
include, but are not restricted to, a wide variety of polycarboxylate
compounds. As used
herein, "polycarboxylate" refers to compounds having a plurality of
carboxylate groups,
preferably at least 3 carboxylates. Polycarboxylate builder can generally be
added to the
composition in acid form, but can also be added in the form of a neutralized
salt. When
utilized in salt form, alkali metals, such as sodium, potassium, and lithium,
or
alkanolammonium salts are preferred.
Included among the polycarboxylate builders are a variety of categories of
useful
materials. One important category of polycarboxylate builders encompasses the
ether
polycarboxylates, including oxydisuccinate, as disclosed in Berg, U.S. Patent
3,128,287,
issued April 7, 1964, and Lamberti et al, U.S. Patent 3,635,830, issued
January 18, 1972.
See also "TMS/TDS" builders of U.S. Patent 4,663,071, issued to Bush et al, on
May 5,
1987. Suitable ether polycarboxylates also include cyclic compounds,
particularly
alicyclic compounds, such as those described in U.S. Patents 3,923,679;
3,835,163;
4,158,635; 4,120,874 and 4,102,903.
Other useful detergency builders include the ether hydroxypolycarboxylates,
copolymers of malefic anhydride with ethylene or vinyl methyl ether, l, 3, S-
trihydroxy
benzene-2, 4, 6-trisulphonic acid, and carboxymethyloxysuccinic acid, the
various alkali
metal, ammonium and substituted ammonium salts of polyacetic acids such as
ethylenediamine tetraacetic acid and nitrilotriacetic acid, as well as
polycarboxylates such
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
13
as mellitic acid, succinic acid, oxydisuccinic acid, polymaleic acid, benzene
1,3,5-
tricarboxylic acid, carboxymethyloxysuccinic acid, and soluble salts thereof.
Citrate builders, e.g., citric acid and soluble salts thereof (particularly
sodium
salt), are polycarboxylate builders of particular importance. Oxydisuccinates
are also
especially useful in such compositions and combinations.
Also suitable in the detergent compositions of the present invention are the
3,3-
dicarboxy-4-oxa-1,6-hexanedioates and the related compounds disclosed in U.S.
Patent
4,566,984, Bush, issued January 28, 1986. Useful succinic acid builders
include the CS-
C20 alkyl and alkenyl succinic acids and salts thereof. A particularly
preferred
compound of this type is dodecenylsuccinic acid. Specific examples of
succinate
builders include: laurylsuccinate, myristylsuccinate, palmitylsuccinate, 2-
dodecenylsuccinate (preferred), 2-pentadecenylsuccinate, and the like.
Laurylsuccinates
are the preferred builders of this group, and are described in European Patent
Application
86200690.5/0,200,263, published November 5, 1986.
Other suitable polycarboxylates are disclosed in U.S. Patent 4,144,226,
Crutchfield et al, issued March 13, 1979 and in U.S. Patent 3,308,067, Diehl,
issued
March 7, 1967. See also Diehl U.S. Patent 3,723,322.
Fatty acids, e.g., C 12-C 18 monocarboxylic acids, can also be incorporated
into
the compositions alone, or in combination with the aforesaid builders,
especially citrate
and/or the succinate builders, to provide additional builder activity. Such
use of fatty
acids will generally result in a diminution of sudsing, which should be taken
into account
by the formulator.
Surfactants
Surfactants may be included in the compositions of the present invention as
ultrasonic cleaning agent. The surfactant may comprise from about 0.01 %, to
about
99.9%, by weight of the composition depending upon the particular surfactants
used and
the effects desired. More typical levels comprise from about 0.1 % to about
80%, even
more preferably from about 0.5% to about 60%, by weight of the composition.
Examples
of suitable surfactants can be found in McCutcheon's EMULSIFIERS AND
DETERGENTS, North American Edition, 1997, McCutcheon Division, MC Publishing
Company, in U.S. 3,929,678, Dec. 30, 1975 Laughlin, et al, and U.S. 4,259,217,
March
CA 02349537 2004-O1-15
14
31, 1981, Murphy; in the series "Surfactant Science", Marcel Dekker, Inc., New
York
and Basel; in "Handbook of Surfactants", M.R. Porter, Chapman and Hall, 2nd
Ed.,
1994; in "Surfactants in Consumer Products", Ed. J. Falbe, Springer-Verlag,
1987 and
"Surface Active Agents and Detergents" (Vol. I and II by Schwartz, Perry and
Berch) ,
The detersive surfactant can be nonionic, anionic, ampholytic, zwitterionic,
or
cationic. Mixtures of these surfactants can also be used. Preferred detergent
compositions comprise anionic detersive surfactants or mixtures of anionic
surfactants
with other surfactants, especially nonionic surfactants and/or amphoteric
surfactants.
Nonlimiting examples of surfactants useful herein include the conventional C11-
C18 alkyl benzene sulfonates and primary, secondary and random alkyl sulfates,
the C10-
C 18 alkyl alkoxy sulfates, the C 10-C 18 alkyl polyglycosides and their
corresponding
sulfated polyglycosides, CI2-C18 alpha-sulfonated fatty acid esters, C12-C18
alkyl and
alkyl phenol alkoxylates (especially ethoxylates and mixed ethoxy/propoxy), C
12-C 18
betaines and sulfobetaines ("sultaines"), C10-C18 amine oxides, C6 to Cls
branched or
linear alkyl sulfates, C6 to C~8 branched or linear alkyl benzene sulfonates,
C6 to C~$
branched or linear alkyl alkoxy sulfates, and mixtures thereof. and the like.
Other
conventional useful surfactants are listed in standard texts.
Anionic Surfactants -
The anionic surfactants useful in the present invention are preferably
selected from
the group consisting of, linear alkylbenzene sulfonate, alpha olefin
sulfonate, paraffin
sulfonates, alkyl ester sulfonates, alkyl sulfates, alkyl alkoxy sulfate,
alkyl sulfonates,
alkyl alkoxy carboxylate, alkyl alkoxylated sulfates, sarcosinates,
taurinates, and
mixtures thereof, more preferably C6 to CIg branched or linear alkyl sulfates,
C6 to Cps
branched or linear alkyl benzene sulfonates, C6 to Cl$ branched or linear
alkyl alkoxy
sulfates, and mixtures thereof. An effective amount, typically from about 0.5%
to about
90%, preferably about 5% to about 60%, more preferably from about 10 to about
30%,
by weight of anionic detersive surfactant can be used in the present
invention.
Alkyl sulfate surfactants are another type of anionic surfactant of importance
for
use herein. In addition to providing excellent overall cleaning ability when
used in
combination with polyhydroxy fatty acid amides (see below), including good
grease/oil
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
'! 5
cleaning over a wide range of temperatures, wash concentrations, and wash
times,
dissolution of alkyl sulfates can be obtained, as well as improved
formulability in liquid
detergent formulations are water soluble salts or acids of the formula ROS03M
wherein
R preferably is a C 10-024 hydrocarbyl, preferably an alkyl or hydroxyalkyl
having a
C 10-020 alkyl component, more preferably a C 12-C 1 g alkyl or hydroxyalkyl,
and M is H
or a canon, e.g., an alkali (Group IA) metal cation (e.g., sodium, potassium,
lithium),
substituted or unsubstituted ammonium cations such as methyl-, dimethyl-, and
trimethyl
ammonium and quaternary ammonium canons, e.g., tetramethyl-ammonium and
dimethyl piperdinium, and cations derived from alkanolamines such as
ethanolamine,
diethanolamine, triethanolamine, and mixtures thereof, and the like.
Typically, alkyl
chains of C1~_16 are preferred for lower wash temperatures (e.g., below about
50°C) and
C 16-18 amyl chains are preferred for higher wash temperatures (e.g., above
about 50°C).
Alkyl alkoxylated sulfate surfactants are another category of useful anionic
surfactant. These surfactants are water soluble salts or acids typically of
the formula
RO(A)mS03M wherein R is an unsubstituted C l p-024 alkyl or hydroxyalkyl group
having a 010-024 alkyl component, preferably a 012-020 alkyl or hydroxyalkyl,
more
preferably C 12-C 1 g alkyl or hydroxyalkyl, A is an ethoxy or propoxy unit, m
is greater
than zero, typically between about 0.5 and about 6, more preferably between
about 0.5
and about 3, and M is H or a cation which can be, for example, a metal canon
(e.g.,
sodium, potassium, lithium, etc.}, ammonium or substituted-ammonium cation.
Alkyl
ethoxylated sulfates as well as alkyl propoxylated sulfates are contemplated
herein.
Specific examples of substituted ammonium cations include methyl-, dimethyl-,
trimethyl-ammonium and quaternary ammonium cations, such as tetramethyl-
ammonium, dimethyl piperidinium and cations derived from alkanolamines, e.g.
monoethanolamine, diethanolamine, and triethanolamine, and mixtures thereof.
Exemplary surfactants are C 12-C 1 g alkyl polyethoxylate ( 1.0) sulfate, C 12-
C 1 g alkyl
polyethoxylate (2.25) sulfate, 012-Clg alkyl polyethoxylate (3.0) sulfate, and
012-018
alkyl polyethoxylate (4.0} sulfate wherein M is conveniently selected from
sodium and
potassium. Surfactants for use herein can be made from natural or synthetic
alcohol
CA 02349537 2004-O1-15
16
feedstocks. Chain lengths represent average hydrocarbon distributions,
including
branching.
Examples of suitable anionic surfactants are given in "Surface Active Agents
and
Detergents" (Vol. I and II by Schwartz, Perry and Berch). A variety of such
surfactants
are also generally disclosed in U.S. Patent 3,929,678, issued December 30,
1975 to
Laughlin, et al. at Column 23, line 58 through Column 29, line 23.
Another possible surfactant are the so-called Dianionics. These are
surfactants
which have at least two anionic groups present on the surfactant molecule.
Some
suitable dianionic surfactants are further described in wo 98/00498 wo
98/00503;
U.S. Patent No. 5,958,858; WO 98/05742;and WO 98/05749. Other
conventional useful surfactants .are listed in standard texts.
Nonionic Surfactants - One particularly preferred surfactants are nonionic
surfactants.
Nonionic surfactants may be present in amounts from 0.01% to about 40% by
weight,
preferably from about 0.1 % to about 30%, and most preferably from about 0.25%
to
about 20%.
Particularly preferred in the present invention include mixed nonionic
surfactants.
While a wide range of nonionic surfactants may be selected from for purposes
of the
mixed nonionic surfactant systems useful in the present invention
compositions, it is
preferred that the nonionic surfactants comprise both a low cloud point
surfactant as
represented by the ether capped poly(oxyalkylated) alcohol surfactant and high
cloud
point nonionic surfactants) as described as follows. "Cloud point", as used
herein, is a
well known property of nonionic surfactants which is the result of the
surfactant
becoming less soluble with increasing temperature, the temperature at which
the
appearance of a second phase is observable is referred to as the "cloud point"
(See Kirk
Othmer, pp. 360-362, hereinbefore).
As used herein, a "love cloud point" nonionic surfactant is defined as a
nonionic
surfactant system ingredient having a cloud point of less than 30°C,
preferably less than
CA 02349537 2004-O1-15
17
about 20°C, and most preferably less than about 10°C and is
represented by the ether-
capped poly(oxyalkylated) alcohols as described herein.
Of course, other low-cloud point surfactants may be included in conjunction
with
the ether-capped poly(oxyalkylated) surfactants. Such optional low-cloud point
. '
surfactants include nonionic alkoxylated surfactants, especially ethoxylates
derived from
primary alcohol, and polyoxypropylene/polyoxyethylene/polyoxypropylene
(PO/EO/PO)
reverse block polymers. Also, such low cloud point nonionic surfactants
include, for
example, ethoxylated-propoxylated alcohol (e.g., Olin Corporation's Poly-
Tergent~
SLF18) and epoxy-capped poly(oxyalkylated) alcohols (e.g., Olin Corporation's
Poly-
Tergent~ SLF18B series of nonionics, as described, for example, in WO
94/22800,
published October 13, 1994 by Olin Corporation). These nonionic surfactants
can
optionally contain propylene oxide in an amount up to about 15% by weight.
Other
preferred nonionic surfactants can be prepared by the processes described in
U.S. Patent
4,223,163, issued September I6, 1980, BuilIoty,
Optional low cloud point nonionic surfactants additionally comprise a
polyoxyethylene, polyoxypropylene block polymeric compound. Block
polyoxyethylene-
polyoxypropylene polymeric compounds include those based on ethylene glycol,
propylene glycol, glycerol, trixnethylolpropane and ethylenediamine as
initiator reactive
hydrogen compound. Certain of the block polymer surfactant compounds
designated
PLURONIC~, REVERSED PLURO1VIC~, and TETRONIC~ by the BASF-Wyandotte
Corp., Wyandotte, Michigan, are suitable in ADD compositions of the invention.
Preferred examples include REVERSED PLURONIC~ 2582 and TETRONICO 702,
Such surfactants are typically useful herein as low cloud point nonionic
surfactants.
As used herein, a "high cloud point" nonionic surfactant is defined as a
nonionic
surfactant system ingredient having a cloud point of greater than 40°C,
preferably greater
than about 50°C, and more preferably greater than about 60°C.
Preferably the nonionic
surfactant system comprises an ethoxylated surfactant derived from the
reaction of a
monohydroxy alcohol or alkylphenol containing from about 8 to about 20 carbon
atoms,
with from about 6 to about 15 moles of ethylene oxide per mole of alcohol or
alkyl
phenol on an average basis. Such high cloud point nonionic surfactants
include, for
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
18
example, Tergitol 1559 (supplied by Union Carbide), Rhodasurf TMD 8.5
(supplied by
Rhone Poulenc), and Neodol 91-8 (supplied by Shell).
It is also preferred for purposes of the present invention that the high cloud
point
nonionic surfactant further have a hydrophile-lipophile balance ("HLB"; see
Kirk Othmer
hereinbefore) value within the range of from about 9 to about 15, preferably
11 to 1~.
Such materials include, for example, Tergitol I SS9 (supplied by Union
Carbide),
Rhodasurf TMD 8.5 (supplied by Rhone Poulenc), and Neodol 91-8 (supplied by
Shell).
Another preferred high cloud point nonionic surfactant is derived from a
straight or
preferably branched chain or secondary fatty alcohol containing from about 6
to about 20
carbon atoms (C6-C20 alcohol), including secondary alcohols and branched chain
primary alcohols. Preferably, high cloud point nonionic surfactants are
branched or
secondary alcohol ethoxylates, more preferably mixed C9/11 or Cl 1/15 branched
alcohol
ethoxylates, condensed with an average of from about 6 to about 15 moles,
preferably
from about 6 to about 12 moles, and most preferably from about 6 to about 9
moles of
ethylene oxide per mole of alcohol. Preferably the ethoxylated nonionic
surfactant so
derived has a narrow ethoxylate distribution relative to the average.
The preferred nonionic surfactant systems useful herein are mixed high cloud
point and low cloud point nonionic surfactants combined in a weight ratio
preferably
within the range of from about 10:1 to about 1:10.
Another type of preferred nonionic surfactants are the endcapped alkyl
alkoxylate
surfactants. Suitable endcapped alkyl alkoxylate surfactant are the epoxy-
capped
poly(oxyalkylated) alcohols represented by the formula:
R10[CH2CH(CH3)O]x[CH2CH20]y[CH2CH(OH)R2] (I)
wherein R1 is a linear or branched, aliphatic hydrocarbon radical having from
4 to 18
carbon atoms; R2 is a linear or branched aliphatic hydrocarbon radical having
from 2 to
26 carbon atoms; x is an integer having an average value of from 0.5 to 1.5,
more
preferably 1; and y is an integer having a value of at least 15, more
preferably at least
20.
Preferably, the surfactant of formula I, at least 10 carbon atoms in the
terminal
epoxide unit [CH2CH(OH)R2]. Suitable surfactants of formula I, according to
the
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
19
present invention, are Olin Corporation's POLY-TERGENT~ SLF-18B nonionic
surfactants, as described, for example, in WO 94/22800, published October 13,
1994 by
Olin Corporation.
One preferred ether-capped poly(oxyalkylated) alcohols has the formula:
R1O[CH2CH(R3)O]x[CH2]kCH(OH;I[CH2)jOR2
wherein Rl and R2 are linear or branched, saturated or unsaturated, aliphatic
or aromatic
hydrocarbon radicals having from 1 to 30 carbon atoms; R3 is H, or a linear
aliphatic
hydrocarbon radical having from 1 to 4 carbon atoms; x is an integer having an
average
value from 1 to 30, wherein when x is 2 or greater R3 may be the same or
different and k
and j are integers having an average value of from 1 to 12, and more
preferably 1 to 5.
R 1 and R2 are preferably linear or branched, saturated or unsaturated,
aliphatic or
aromatic hydrocarbon radicals having from 6 to 22 carbon atoms with 8 to 18
carbon
atoms being most preferred. H or a linear aliphatic hydrocarbon radical having
from 1 to
2 carbon atoms is most preferred for R3. Preferably, x is an integer having an
average
value of from 1 to 20, more preferably from 6 to 15.
As described above, when, in the preferred embodiments, and x is greater than
2,
R3 may be the same or different. That is, R3 may vary between any of the
alklyeneoxy
units as described above. For instance, if x is 3, R3may be selected to form
ethlyeneoxy(EO) or propyleneoxy(PO) and may vary in order of (EO)(PO)(EO),
(EO)(EO)(PO); (EO)(EO)(EO); (PO)(EO)(PO); (PO)(PO)(EO) and (PO)(PO)(PO). Of
course, the integer three is chosen for example only and the variation may be
much larger
with a higher integer value for x and include, for example, multiple (E0)
units and a
much small number of (PO) units.
Particularly preferred surfactants as described above include those that have
a low
cloud point of less than 20°C. These low cloud point surfactants may
then be employed
in conjunction with a high cloud point surfactant as described in detail below
for superior
grease cleaning benefits.
Most preferred ether-capped poly(oxyalkylated) alcohol surfactants are those
wherein k is 1 and j is 1 so that the surfactants have the formula:
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
R 1 O[CH~CH(R3)OJXCH2CH(OH)CH20R2
where Rl, RZ and R3 are defined as above and x is an integer with an average
value of
from 1 to 30, preferably from 1 to 20, and even more preferably from 6 to 18.
Most
preferred are surfactants wherein R1 and R~ range from 9 to 14, R3 is H
forming
ethyleneoxy and x ranges from 6 to 15.
The ether-capped poly(oxyalkylated) alcohol surfactants comprise three general
components, namely a linear or branched alcohol, an alkylene oxide and an
alkyl ether
end cap. The alkyl ether end cap and the alcohol serve as a hydrophobic, oil-
soluble
portion of the molecule while the alkylene oxide group forms the hydrophilic,
water-
soluble portion of the molecule.
These surfactants exhibit significant improvements in spotting and filming
characteristics and removal of greasy soils, especially, when used in
conjunction with
high cloud point surfactants, relative to conventional surfactants.
Another suitable class of nonionic surfactants comprises sugar derived
surfactants
such as the polyhydroxy fatty acid amides of the formula:
O
RZCNZ
R~
wherein: R 1 is H, C 1-C4 hydrocarbyl, 2-hydroxy ethyl, 2-hydroxy propyl, or a
mixture
thereof, preferably C1-C4 alkyl, more preferably C1 or C2 alkyl, most
preferably C1
alkyl (i.e., methyl); and R2 is a C5-C31 hydrocarbyl, preferably straight
chain C~-C19
alkyl or alkenyl, more preferably straight chain Cg-C 1 ~ alkyl or alkenyl,
most preferably
straight chain C11-C15 amyl or alkenyl, or mixtures thereof; and Z is a
polyhydroxyhydrocarbyl having a linear hydrocarbyl chain with at least 3
hydroxyls
directly connected to the chain, or an alkoxylated derivative (preferably
ethoxylated or
propoxylated) thereof. Z preferably will be derived from a reducing sugar in a
reductive
amination reaction; more preferably Z will be a glycityl. Suitable reducing
sugars
include glucose, fructose, maltose, lactose, galactose, mannose, and xylose.
As raw
materials, high dextrose com syrup, high fructose corn syrup, and high maltose
corn
syrup can be utilized as well as the individual sugars listed above. These
corn syrups
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
21
may yield a mix of sugar components for Z. It should be understood that it is
by no
means intended to exclude other suitable raw materials. Z preferably will be
selected
from the group consisting of -CH2-(CHOH)n-CH20H, -CH(CH20H)-(CHOH)n-1-
CH20H, -CH2-(CHOH)2(CHOR')(CHOH)-CH20H, and alkoxylated derivatives
thereof, where n is an integer from 3 to 5, inclusive, and R' is H or a cyclic
or aliphatic
monosaccharide. Most preferred are glycityls wherein n is 4, particularly -CH2-
(CHOH)4-CH20H.
R~ can be, for example, N-methyl, N-ethyl, N-propyl, N-isopropyl, N-butyl, N-2-
hydroxy ethyl, or N-2-hydroxy propyl.
R2-CO-N< can be, for example, cocamide, stearamide, oleamide, lauramide,
myristamide, capricamide, palmitamide, tallowamide, etc.
Z can be 1-deoxyglucityl, 2-deoxyfructityl, 1-deoxymaltityl, I-deoxylactityl,
1-
deoxygalactityl, I -deoxymannityl, 1-deoxymaltotriotityl, etc.
Methods for making poIyhydroxy fatty acid amides are known in the art. In
general, they can be made by reacting an alkyl amine with a reducing sugar in
a reductive
amination reaction to form a corresponding N-alkyl polyhydroxyamine, and then
reacting
the N-alkyl polyhydroxyamine with a fatty aliphatic ester or triglyceride in a
condensation/amidation step to form the N-alkyl, N-polyhydroxy fatty acid
amide
product. Processes for making compositions containing polyhydroxy fatty acid
amides
are disclosed, for example, in G.B. Patent Specification 809,060, published
February 18,
1959, by Thomas Hedley & Co., Ltd., U.S. Patent 2,965,576, issued December 20,
1960
to E. R. Wilson, and U.S. Patent 2,703,798, Anthony M. Schwartz, issued March
8,
1955, and U.S. Patent 1,985,424, issued December 25, 1934 to Piggott, each of
which is
incorporated herein by reference.
The preferred alkylpolyglycosides have the formula
R20(CnH2n0)t(glYcosyl~
wherein R2 is selected from the group consisting of alkyl, alkyl-phenyl,
hydroxyalkyl,
hydroxyalkylphenyl, and mixtures thereof in which the alkyl groups contain
from about
to about 18, preferably from about 12 to about 14, carbon atoms; n is 2 or 3,
preferably 2; t is from 0 to about 10, preferably 0; and x is from about 1.3
to about 10,
CA 02349537 2004-O1-15
22
preferably from about 1.3 to about 3, most preferably from about 1.3. to about
2.7. The
glycosyl is preferably derived from glucose. To prepare these compounds, the
alcohol or
alkylpolyethoxy alcohol is formed first and then reacted with glucose, or a
source of
glucose, to form the glucoside (attachment at the I-position). The additional
glycosyl
units can then be attached between their 1-position and the preceding glycosyl
units 2-, 3-
4- and/or 6-position, preferably predominantly the 2-position.
These and other nonionic surfactants are well known in the art, being
described in
more detail in Kirk Othmer's Encyclopedia of Chemical Technology, 3rd Ed.,
Vol. 22,
pp. 360-379, "Surfactants and Detersive Systems"
Further suitable nonionic detergent surfactants are generally disclosed in
U.S. Patent
3,929,678, Laughlin et al., issued December 30, 197, at column I3, line 14
through
column 16; line 6 .
Cationic Surfactants -
Cationic surfactants suitable for use in the compositions of the present
invention
include those having a long-chain hydrocarbyl group. Examples of such cationic
co-
surfactants include the ammonium co-surfactants such as alkyldimethylammonium
halogenides, and those co-surfactants having the formula:
[R2(OR3)y~~R4(OR3)y~2R5N+X_
wherein R2 is an alkyl or alkyl benzyl group having from 8 to 18 carbon atoms
in the
alkyl chain, each R3 is selected from the group consisting of -CH2CH2-, -
CH2CH(CH3)-, -CHZCH(CH20H)-, ' -CH2CH2CH2-, and mixtures thereof; each R4
is selected from the group consisting of C 1-C4 alkyl, C ~ -C4 hydroxyalkyl,
benzyl ring
structures formed by joining the two R4 groups, -CH2CHOH-
CHOHCOR6CHOHCH20H wherein R6 is any hexose or hexose polymer having a
molecular weight less than about 1000, and hydrogen when y is not 0; RS is the
same as
R4 or is an alkyl chain wherein the total number of carbon atoms of R2 plus RS
is not
more than about 18; each y is from 0 to about 10 and the sum of the y values
is from 0 to
about 15; and X is any compatible anion.
CA 02349537 2004-O1-15
23
Examples of other suitable cationic surfactants are described in following
documents,
M.C.
Publishing Co., McCutcheon's, Detergents & Emulsifiers, (North American
edition
1997); Schwartz, et al., Surface Active Agents, Their Chemistry and
Technology, New
York: Interscience Publishers, 1949; U.S. Patent 3,155,591; U. S. Patent
3,929,678; U. S.
Patent 3,959,461 U. S. Patent 4,387,090 and U.S. Patent 4,228,044.
Examples of suitable cationic surfactants are those corresponding to the
general
formula:
R~~N~R3 x
R2/ ~Ra
wherein R1, R2, R3, and R4 are independently selected from an aliphatic group
of from 1
to about 22 carbon atoms or an aromatic, alkoxy, polyoxyalkylcne, alkylamido,
hydroxyalkyl, aryl or alkylaryl group having up to about 22 carbon atoms; and
X is a salt-
fonming anion such as those selected from halogen, (e.g. chloride, bromide),
acetate,
citrate, lactate, glycolate, phosphate nitrate, sulfate, and alkylsulfate
radicals.. The
aliphatic groups can contain, in addition to carbon and hydrogen atoms, ether
linkages,
and other groups such as amino groups. The longer chain aliphatic groups,
e.g., those of
about 12 carbons, or higher, can be saturated or unsaturated. Preferred is
when Rl, R2,
R3, and R4 are independently selected from C1 to about C22 alkyl. Especially
preferred
are cationic materials containing two long alkyl chains and two short alkyl
chains or
those containing one long alkyl chain and three short alkyl chains. The long
alkyl chains
in the compounds described in the previous sentence have from about 12 to
about 22
carbon atoms, preferably from about 16 to about 22 carbon atoms, and the short
alkyl
chains in the compounds described in the previous sentence have from 1 to
about 3
carbon atoms, preferably from 1 to about 2 carbon atoms.
Suitable levels of cationic detersive surfactant herein, when present, are
from
about 0.1 % to about 20%, preferably from about 1 % to about 15%, although
much higher
levels, e.g., up to about 30% or more, may be useful especially in nonionic :
cationic (i.e.,
limited or anionic-free) formulations.
CA 02349537 2001-04-27
WO 00/28874 PCT/US99127201
24
Other Surfactants -
Amphoteric or zwitterionic detersive surfactants when present are usually
useful
at levels in the range from about 0.1 % to about 20% by weight of the
detergent
composition. Often levels will be limited to about 5% or less, especially when
the
amphoteric is costly.
Suitable amphoteric surfactants include the amine oxides corresponding to the
formula:
RR'R"NCO
wherein R is a primary alkyl group containing 6-24 carbons, preferably 10-18
carbons,
and wherein R' and R" are, each, independently, an alkyl group containing 1 to
6 carbon
atoms. The arrow in the formula is a conventional representation of a semi-
polar bond.
Amine oxides are semi-polar surfactants and include water-soluble amine oxides
containing one alkyl moiety of from about 10 to about 18 carbon atoms and 2
moieties
selected from the group consisting of alkyl groups and hydroxyalkyl groups
containing
from about 1 to about 3 carbon atoms; water-soluble phosphine oxides
containing one
alkyl moiety of from about 10 to about 18 carbon atoms and 2 moieties selected
from the
group consisting of alkyl groups and hydroxyalkyl groups containing from about
1 to
about 3 carbon atoms; and water-soluble sulfoxides containing one alkyl moiety
of from
about 10 to about 18 carbon atoms and a moiety selected from the group
consisting of
alkyl and hydroxyalkyl moieties of from about 1 to about 3 carbon atoms.
Preferred amine oxide surfactants having the formula
O
I
R3(OR4)XN(RS)z
wherein R3 is an alkyl, hydroxyalkyl, or alkyl phenyl group or mixtures
thereof
containing from about 8 to about 22 carbon atoms; R4 is an alkylene or
hydroxyalkylene
group containing from about 2 to about 3 carbon atoms or mixtures thereof; x
is from 0
to about 3; and each RS is an alkyl or hydroxyalkyl group containing from
about 1 to
about 3 carbon atoms or a polyethylene oxide group containing from about 1 to
about 3
ethylene oxide groups. The RS groups can be attached to each other, e.g.,
through an
oxygen or nitrogen atom, to form a ring structure.
CA 02349537 2004-O1-15
These amine oxide surfactants in particular include C10-Cig alkyl dimethyl
amine
oxides and Cg-C12 alkoxy ethyl dihydroxy ethyl amine oxides. Preferably the
amine
oxide is present in the composition in an effective amount, more preferably
from about
0.1% to about 20%, even more preferably about 0.1% to about 15%, even more
preferably still from about 0.5% to about 10%,by weight.
Some suitable zwitterionic surfactants which can be used herein comprise the
betaine and betaine-like surfactants wherein the molecule contains both basic
and acidic
groups which form an inner salt giving the molecule both cationic and anionic
hydrophilic groups over a broad range of pH values. Some common examples of
these s
are described in U.S. Pat. Nos. 2,082,275, 2,702,279 and 2,255,082.
One of the preferred zwitterionic compounds have the formula
R2
R1._N-Cg2_R4_y
R3 X
wherein R1 is an alkyl radical containing from 8 to 22 carbon atoms, R2 and R3
contain
from 1 to 3 carbon atoms, R4 is an alkylene chain containing from 1 to 3
carbon atoms,
X is selected from the group consisting of hydrogen and a hydroxyl radical, Y
is selected
from the group consisting of carboxyl and sulfonyl radicals and wherein the
sum of Rl,
R2 and R3 radicals is from 14 to 24 carbon atoms.
Zwitterionic surfactants, as mentioned hereinbefore, contain both a cationic
group
and an anionic group and are in substantial electrical neutrality where the
number of
anionic charges and cationic charges on the surfactant molecule are
substantially the
same. Zwitterionics, which typically contain both a quaternary ammonium group
and an
anionic group selected from sulfonate and carboxylate groups are desirable
since they
maintain their amphoteric character over most of the pH range of interest for
cleaning
hard surfaces. The sulfonate group is the preferred anionic group.
Antimicrobial a ents - an antimicrobial agent is a compound or substance that
kills
microorganisms or prevents or inhibits their growth and reproduction. A
properly
selected antimicrobial agent maintains stability under use and storage
conditions (pH,
temperature, light, etc.), for a required length of time. A desirable property
of the
CA 02349537 2004-O1-15
26
antimicrobial agent is that it is safe and nontoxic in handling, formulation
and use, is
environmentally acceptable and cost effective. Classes of antimicrobial agents
include;
but are not limited to, chlorophenols, aldehydes, biguanides; antibiotics and
biologically
active salts. Some preferable antimicrobial agent in the antimicrobial is
bronopol,
chlorhexidine diacetate, TRICOSAN.TM., hexetidine orparachlorometaxylenol
(PCMX).
More preferably, the antimicrobial agent is TRICOSAN.TM, chlorhexidine
diacetate or
hexetidine.
The antimicrobial agent, when used, is present in a microbiocidally effective
amount, more preferably an from about 0.01% to about 10.0%, more preferably
from
about 0.1% to about 8.0%;even more preferably from about 0.5% to about 2.0%,
by
weight of c the composition.
Bleaching A~ amts
Hydrogen peroxide sources are described in detail in Kirk
Othmer's Encyclopedia of Chemical Technology, 4th Ed (1992, John Wiley &
Sons),
Vol. 4, pp. 271-300 "Bleaching Agents (Survey)", and include the various forms
of
sodium perborate and sodium percarbonate, including various coated and
modified
forms. An "effective amount" of a source of hydrogen peroxide is any amount
capable
of measurably improving stain removal (especially of tea stains) from soiled
dishware
compared to a hydrogen peroxide source-free composition when the soiled
dishware is
washed by the consumer in a domestic automatic dishwasher in the presence of
alkali.
More generally a source of hydrogen peroxide herein is any convenient
compound or mixture which under consumer use conditions provides an effective
amount of hydrogen peroxide. Levels may vary widely and are usually in the
range from
about 0.1% to about 70%, more typically from about 0.5% to about 30%, by
weight of
the compositions herein.
The preferred source of hydrogen peroxide used herein can be any convenient
source, including hydrogen peroxide itself. For example, perborate, e.g.,
sodium
perborate (any hydrate but preferably the mono- or tetra-hydrate), sodium
carbonate
peroxyhydrate or equivalent percarbonate salts, sodium pyrophosphate
peroxyhydrate,
urea peroxyhydrate, or sodium peroxide can be used herein. Also useful are
sources of
TM
available oxygen such as persulfate bleach (e.g., OXONE, manufactured by
DuPont).
CA 02349537 2004-O1-15
27
Sodium perborate monohydrate and sodium percarbonate are particularly
preferred.
Mixtures of any convenient hydrogen peroxide sources can also be used.
A preferred percarbonate bleach comprises dry particles having an average
particle size in the range from about S00 micrometers to about 1,000
micrometers, not
more than about 10% by weight of said particles being smaller than about 200
micrometers and not more than about 10% by weight of said particles being
larger than
about 1,250 micrometers. Optionally, the percarbonate can be coated with a
silicate,
borate or water-soluble surfactants. Percarbonate is available from various
commercial
sources such as FMC, Solvay and Tokai Denka.
While not preferred for the compositions of the present invention which
comprise
detersive enzymes, the present invention compositions may also comprise as the
bleaching agent a chlorine-type bleaching material. Such agents are well known
in the
art, and include for example sodium dichloroisocyanurate ("NaDCC").
Organic Peroxides, especiall~iacyl Peroxides
These are extensively illustrated in Kirk Othmer, Encyclopedia of Chemical
Technology, Vol. 17, John Wiley and Sons, 1982 at pages 27-90 and especially
at pages
63-72. _ If a diacyl peroxide is used, it will preferably
be one which exerts minimal adverse impact on spotting/filming. Preferred
diacyl
peroxides include dibenzoyl peroxide.
Metal-containing Bleach Catalysts
The present invention compositions and methods utilize metal-containing bleach
catalysts that are effective for use in ADD compositions. Preferred are
manganese and
cobalt-containing bleach catalysts.
One type of metal-containing bleach catalyst is a catalyst system comprising a
transition metal cation of defined bleach catalytic activity, such as copper,
iron, titanium,
ruthenium tungsten, molybdenum, or manganese cations, an auxiliary metal
cation
having little or no bleach catalytic activity, such as zinc or aluminum
cations, and a
sequestrate having defined stability constants for the catalytic and auxiliary
metal cations,
particularly ethylenediaminetetraacetic acid, ethylenediaminetetra
(methylenephosphonic
acid) and water-soluble salts thereof. Such catalysts are disclosed in U.S.
Pat. 4,430,243.
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
28
Other types of bleach catalysts include the manganese-based complexes
disclosed
in U.S. Pat. 5,246,621 and LT.S. Pat. 5,244,594. Preferred examples of theses
catalysts
include MnN2(u-O)3(1,4,7-trimethyl-1,4,7-triazacyclononane)2-(PF6)2
("MnTACN"),
Mn~2(u-O)1(u-OAc)2(1,4,7-trimethyl-1,4,7-triazacyclononane)2-(C104)2, MnN4(u-
O)6(I,4,7-triazacyclononane)4-(C104)2, Mn~MnN4(u-O)1(u-OAc)2(I,4,7-trimethyI-
I,4,7-triazacyclononane)2-(C104)3, and mixtures thereof. See also European
patent
application publication no. 549,272. Other ligands suitable for use herein
include 1,5,9-
trimethyl-1,5,9-triazacyclododecane, 2-methyl-1,4,7-triazacyclononane, 2-
methyl-1,4,7-
triazacyclononane, and mixtures thereof.
The bleach catalysts useful in automatic dishwashing compositions and
concentrated powder detergent compositions rnay also be selected as
appropriate for the
present invention. For examples of suitable bleach catalysts see U.S. Pat.
4,246,612 and
U.S. Pat. 5,227,084.
Other bleach catalysts are described, for example, in European patent
application,
publication no. 408,131 (cobalt complex catalysts), European patent
applications,
publication nos. 384,503, and 306,089 (metallo-porphyrin catalysts), U.S.
4,728,455
(manganese/multidentate ligand catalyst), U.S. 4,711,748 and European patent
application, publication no. 224,952, (absorbed manganese on aluminosilicate
catalyst),
U.S. 4,601,845 (aluminosilicate support with manganese and zinc or magnesium
salt),
U.S. 4,626,373 (manganese/ligand catalyst), U.S. 4,119,557 (ferric complex
catalyst),
German Pat. specification 2,054,019 (cobalt chelant catalyst) Canadian 866,191
(transition metal-containing salts), U.S. 4,430,243 (chelants with manganese
cations and
non-catalytic metal cations), and U.S. 4,728,455 (manganese gIuconate
catalysts).
Preferred are cobalt catalysts which have the formula:
[Co(NH3)n(M')mI Yy
wherein n is an integer from 3 to S (preferably 4 or 5; most preferably 5); M'
is a
labile coordinating moiety, preferably selected from the group consisting of
chlorine,
bromine, hydroxide, water, and (when m is greater than 1) combinations
thereof; m is an
integer from 1 to 3 (preferably 1 or 2; most preferably 1); m+n = 6; and Y is
an
appropriately selected counteranion present in a number y, which is an integer
from 1 to
CA 02349537 2001-04-27
WO OOI28874 PCT/US99/27201
29
3 (preferably 2 to 3; most preferably 2 when Y is a -1 charged anion), to
obtain a charge-
balanced salt.
The preferred cobalt catalyst of this type useful herein are cobalt pentaamine
chloride salts having the formula [Co(NH3)SCl] Yy, and especially
[Co(NH3)SCI]C12.
More preferred are the present invention compositions which utilize cobalt
(Ill)
bleach catalysts having the formula:
[Co~3)n(M)m(B)b) TY
wherein cobalt is in the +3 oxidation state; n is 4 or 5 (preferably 5); M is
one or more
ligands coordinated to the cobalt by one site; m is 0, 1 or 2 (preferably 1);
B is a ligand
coordinated to the cobalt by two sites; b is 0 or 1 (preferably 0), and when
b=0, then m+n
= 6, and when b=l, then m=0 and n=4; and T is one or more appropriately
selected
counteranions present in a number y, where y is an integer to obtain a charge-
balanced
salt (preferably y is 1 to 3; most preferably 2 when T is a -1 charged anion);
and wherein
further said catalyst has a base hydrolysis rate constant of less than 0.23 M-
1 s-1 (25°C).
Preferred T are selected from the group consisting of chloride, iodide, I3-,
formate, nitrate, nitrite, sulfate, sulfite, citrate, acetate, carbonate,
bromide, PFb , BF4 ,
B(Ph)4-, phosphate, phosphite, silicate, tosylate, methanesulfonate, and
combinations
thereof. Optionally, T can be protonated if more than one anionic group exists
in T, e.g.,
HP042-, HC03-, H2P04 , etc. Further, T may be selected from the group
consisting of
non-traditional inorganic anions such as anionic surfactants (e.g., linear
alkylbenzene
sulfonates (LAS), alkyl sulfates {AS), alkylethoxysulfonates (AES), etc.)
and/or anionic
polymers (e.g., polyacrylates, polymethacrylates, etc.).
The M moieties include, but are not limited to, for example, F-, S04 2, NCS-,
SCN-, 5203-2, NH3, P043-, and carboxylates (which preferably are mono-
carboxylates,
but more than one carboxylate may be present in the moiety as long as the
binding to the
cobalt is by only one carboxylate per moiety, in which case the other
carboxylate in the
M moiety may be protonated or in its salt form). Optionally, M can be
protonated if
more than one anionic group exists in M (e.g., HP042-, HC03-, H2P04-,
CA 02349537 2001-04-27
WO 00/28874 PCT/US99127201
HOC(O)CH~C(O)O-, etc.) Preferred M moieties are substituted and unsubstituted
C1-
C30 carboxylic acids having the formulas:
RC(O)O-
wherein R is preferably selected from the group consisting of hydrogen and C 1-
C30
(preferably C 1-C 1 g) unsubstituted and substituted alkyl, C6-C3p (preferably
C6-C 1 g)
unsubstituted and substituted aryl, and C3-C30 (preferably CS-Clg)
unsubstituted and
substituted heteroaryl, wherein substituents are selected from the group
consisting of -
~~3~ -~~4+~ -C(O)OR', -OR', -C(O)NR'2, wherein R' is selected from the group
consisting of hydrogen and C I -C6 moieties. Such substituted R therefore
include the
moieties -(CH~)nOH and -(CH2)nNR'4+, wherein n is an integer from 1 to about
16,
preferably from about 2 to about 10, and most preferably from about 2 to about
5.
Most preferred M are carboxylic acids having the formula above wherein R is
selected from the group consisting of hydrogen, methyl, ethyl, propyl,
straight or
branched C4-C12 alkyl, and benzyl. Most preferred R is methyl. Preferred
carboxylic
acid M moieties include formic, benzoic, octanoic, nonanoic, decanoic,
dodecanoic,
malonic, malefic, succinic, adipic, phthalic, 2-ethylhexanoic, naphthenoic,
oleic, palmitic,
triflate, tamate, stearic, butyric, citric, acrylic, aspartic, fumaric,
lauric, linoleic, lactic,
malic, and especially acetic acid.
The B moieties include carbonate, di- and higher carboxylates (e.g., oxalate,
malonate, malic, succinate, maleate), picolinic acid, and alpha and beta amino
acids (e.g.,
glycine, alanine, beta-alanine, phenylalanine).
Cobalt bleach catalysts useful herein are known, being described for example
along with their base hydrolysis rates, in M. L. Tobe, "Base Hydrolysis of
Transition-
Metal Complexes", Adv. Inor~. Bioinor~ Mech., (1983}, 2, pages 1-94. For
example,
Table 1 at page 17, provides the base hydrolysis rates (designated therein as
kOH) for
cobalt pentaamine catalysts complexed with oxalate (kOH= 2.5 x 10-4 M-1 s-1
(25°C)),
NCS- (kOH= 5.0 x 10-4 M-1 s-1 (25°C)), formate (kpH= 5.8 x 10-4 M-1 s-1
(25°C)),
and acetate (kpH= 9.6 x 10-4 M-1 s-1 (25°C)). The most preferred cobalt
catalyst useful
CA 02349537 2004-O1-15
31
herein are cobalt pentaamine acetate salts having the formula [Co(NH3)SOAc]
Ty,
wherein OAc represents an acetate moiety, and especially cobalt pentaamine
acetate
chloride, [Co(NH3)SOAc]C12; as well as [Co(NH3)SOAc](OAc)2;
[C°~3)SOAc](PF6)2~ [Co~3)SOAc](S04)~ [Co~3)SOAc]{BF4)2~ and
[Co(NH3)SOAc](N03)2~
Cobalt catalysts according to the present invention made be produced according
to the synthetic xoutes disclosed in U.S. Patent Nos. 5,559,261, 5,581,005,
and
5,597,936.
These catalysts may be coprocessed with adjunct materials so as to reduce the
color impact if desired for the aesthetics of the product, or to be included
in enzyme-
containing particles as exemplified hereinafter, or the compositions may be
manufactured
to contain catalyst "speckles".
As a practical matter, and not by way of limitation, the cleaning compositions
and
cleaning processes herein can be adjusted to provide on the order of at least
one part per
hundred million of the active bleach catalyst species in the aqueous washing
medium,
and will preferably provide from about 0.01 ppm to about 25 ppm, more
preferably from
about 0.05 ppm to about 10 ppm, and most preferably from about 0.1 ppm to
about 5
ppm, of the bleach catalyst species in the wash liquor. In order to obtain
such levels in
the wash liquor compositions herein will comprise from about 0.0005% to about
0.2%,
more preferably from about 0.004% to about 0.08%, of bleach catalyst by weight
of the
cleaning compositions.
Preferred bleach catalysts, along with methods of there use can be
additionally
found in U.S. Patents 5,705,464, 5,804,542, 5,798,326, 5,703,030 and
5,599,781.
Bleach Actiyators
Preferably, when composition contains a peroxygen bleach component the
composition is formulated with an activator (peracid precursor). Preferred
activators are
selected from the group consisting of tetraacetyl ethylene diamine (TAED),
benzoylcaprolactam (BzCL), 4-nitrobenzoylcaprolactam, 3-
chlorobenzoylcaprolactam,
benzoyloxybenzenesulphonate (BOBS), nonanoyloxybenzencsulphonate (NOBS),
phenyl
CA 02349537 2004-O1-15
32
benzoate (PhBz), decanoyloxybenzenesulphonate (C10-OBS), benzoylvalerolactam
(BZVL), octanoyloxybenzenesulphonate (Cg-OBS), perhydrolyzable esters and
mixhnes
thereof, most preferably benzoylcaprolactam, NOBS, TAED and
benzoylvalerolactam.
Particularly preferred bleach activators in the pH range from about 8 to about
9.5 are
those selected having an OBS or VL leaving group.
Preferred bleach activators are those described in U.S. Patent 5,130,045,
Mitchell
et al, and 4;412,934, Chung et al, and in w0 94/28103; EP 0699229; wp
94/27970;
WO 94/28104 and WO 94/28106.
The mole ratio of peroxygen bleaching compound (as Av0) to bleach activator in
the present invention generally ranges from at least 1:1, preferably from
about 20:1 to
about 1:1, more preferably from about 10:1 to about 3:1.
Quaternary substituted bleach activators may also be included. The present
detergent compositions preferably comprise a quaternary substituted bleach
activator
(QSBA) or a quaternary substituted peracid (QSP); more preferably, the former.
Preferred QSBA structures are further described in a , s. Patent -Nos .
5,686,015; 5,460,747; 5,584,888 and 5,578,136.
Levels of bleach activators herein may vary widely, e.g., from about 0.01% to
about
90%, by weight of the composition, although lower levels, e.g., more
preferably from
about 0.1% to about 30%, even more preferably from about 0.1% to about 20%,
even
more preferably from about 0.5% to about 10%, even more still preferably from
about
1 % to about 8%; by weight of the composition are more typically used.
Preferred hydrophilic bleach activators include N,N,N'N'-tetraacetyl ethylene
diamine (TAED) or any of its close relatives including the triacetyl or other
unsymmetrical derivatives. TAED and the acetylated carbohydrates such as
glucose
pentaaeetate and tetraacetyl xylose are preferred hydrophilic bleach
activators.
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
33
Depending on the application, acetyl triethyl citrate, a liquid, also has some
utility, as
does phenyl benzoate.
Preferred hydrophobic bleach activators include substituted amide types
described
in detail hereinafter, such as activators related to NAPAA, and activators
related to
certain imidoperacid bleaches, for example as described in U.S. Patent
5,061,807, issued
October 29, 1991 and assigned to Hoechst Aktiengesellschaft of Frankfurt,
Germany.
Other suitable bleach activators include sodium-4-benzoyloxy benzene sulfonate
(SBOBS); sodium-1-methyl-2-benzoyloxy benzene-4-sulphonate; sodium-4-methyl-3-
benzoyloxy benzoate (SPCC); trimethyl ammonium toluyloxy-benzene sulfonate; or
sodium 3,5,5-trimethyl hexanoyloxybenzene sulfonate (STHOBS).
Bleach activators may be used in any amount, typically up to 20%, preferably
from O.I-10% by weight, of the composition, though higher levels, 40% or more,
are
acceptable, for example in highly concentrated bleach additive product forms
or forms
intended for appliance automated dosing.
Highly preferred bleach activators useful herein are amide-substituted and
have
either of the formulae:
O O O O
II II II i1
R~-C-N-R2-C-L, R~-N-C-R2-C-L
I I
R5 R5
or mixtures thereof, wherein R1 is alkyl, aryl, or alkaryl containing from
about 1 to
about 14 carbon atoms including both hydrophilic types (short R1) and
hydrophobic
types (R1 is especially from 6, preferably about 8, to about 12), R2 is
alkylene, arylene
or alkarylene containing from about 1 to about 14 carbon atoms, RS is H, or an
alkyl,
aryl, or alkaryl containing from about 1 to about 10 carbon atoms, and L is a
leaving
group which is herein before defined.
Preferred bleach activators also include those of the above general formula
wherein L is selected from the group consisting of:
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
34
Y R3 R3Y
-O ~ -O ~ Y , and -O
wherein R3 is as defined above and Y is -S03 M+ or -C02 M+ wherein M is as
defined above.
Preferred examples of bleach activators of the above formulae include:
(6-octanamidocaproyl)oxybenzenesulfonate,
(6-nonanamidocaproyl)oxybenzenesulfonate,
(6-decanamidocaproyl)oxybenzenesulfonate, and mixtures thereof.
Other useful activators, disclosed in LT.S. 4,966,723, are benzoxazin-type,
such
as a C6H4 ring to which is fused in the 1,2-positions a moiety --C(O)OC(R1)=N-
. A
highly preferred activator of the benzoxazin-type is:
O
II
O
I
'C
N
Acyl lactam activators are very useful herein, especially the acyl
caprolactams
(see for example WO 94-28102 A) and acyl valerolactams (see U.S. 5,503,639) of
the
formulae:
O OC
C N ~ wN~
R~
and
wherein R6 is H, alkyl, aryl, alkoxyaryl, an alkaryl group containing from 1
to about 12
carbon atoms, or substituted phenyl containing from about 6 to about 18
carbons. See
also U.S. 4,545,784 which discloses acyl caprolactams, including benzoyl
caprolactam
adsorbed into sodium perborate.
Nonlimiting examples of additional activators useful herein are to be found in
U.S. 4,915,854, U.S. 4,412,934 and 4,634,551.
CA 02349537 2004-O1-15
Additional activators useful herein include those of U.S. 5,545,349. EXamples
include esters of an organic acid and ethylene glycol, diethylene glycol or
glycerin, or the
acid imide of an organic acid and ethylenediamine; wherein the organic acid is
selected
from methoxyacetic acid, 2-methoxypropionic acid, p-methoxybenzoic acid,
ethaxyacetic
acid, 2-ethoxypropionic acid, p-ethoxybenzoic acid, propoxyacetic acid, 2-
propoxypropionic acid, p-propoxybenzoic acid, butoxyacetic acid, 2-
butoxypropionic
acid, p-butoxybenzoic acid, 2-methoxyethoxyacetic acid,2-methoxy-1-
methylethoxyacetic acid, 2-methoxy-2-methyIethoxyacetic acid, 2-
ethoxyethoxyacetic
acid, 2-(2-ethoxyethoxy~ropionic acid, p-(2-ethoxyethoxy)benzoic acid, 2-
ethoxy-1-
methylethoxyacetic acid, 2-ethoxy-2-methylethoxyacetic acid, 2-
propoxyethoxyacetic
acid, 2-propoxy-1-methylethoxyaceticacid, 2-propoxy-2-methylethoxyacetic acid,
2-
butoxyethoxyacetic acid ,2-butoxy-1-methylethoxyacetic acid, 2-butoxy-2-
methylethoxyacetic acid, 2-(2-methoxyethoxy)ethoxyacetic acid, 2-(2-methoxy-1-
methylethoxy)ethoxyacetic acid, 2-(2-methoxy-2-methylethoxy)ethoxyacetic acid
and
2-(2-ethoxyethoxy)ethoxyacetic acid.
Useful herein as oxygen bleaches are the inorganic peroxides such as Na202,
superoxides such as K02, organic hydroperoxides such as cumene hydroperoxide
and t
butyl hydroperoxide, and the inorganic peroxoacids and their salts such as the
peroxosulfuric acid salts, especially the potassium salts of peroxodisulfuric
acid and,
more preferably, of peroxomonosulfuric acid including the commercial triple-
salt form
TM
sold as OXONE by DuPont and also any equivalent commercially available forms
such
TM TM
as CUROX from Akzo or CAROAT from Degussa. Certain organic peroxides, such as
dibenzoyl peroxide, may be useful, especially as additives rather than as
primary oxygen
bleach.
Mixed oxygen bleach systems are generally useful, as are mixtures of any
oxygen
bleaches with the known bleach activators, organic catalysts, enzymatic
catalysts and
mixtures thereof; moreover such mixtures may further include brighteners,
photobleaches
and dye transfer inhibitors of types well-known in the art.
Other useful peracids and bleach activators herein are in the ' family of
imidoperaeids and imido bleach activators. These include
phthaloylimidoperoxycaproic acid and related arylimido-substituted and
CA 02349537 2004-O1-15
36
acyloxynitrogen derivatives, For listings of such compounds, preparations and
their
incorporation into Laundry compositions including both granules and liquids,
See U.S:
5,487,818; U.S. 5,470,988, U.S. 5,466,825; U.S. 5,419,846; U.S. 5,415,796;
U.S:
5,391,324; U,S. 5,328,634; U.S. 5,310,934; U.S: 5,279,757; U.S. 5,246,620;
U.S.
5;245,0?S; U.S. 5,294,362; U.S. 5,423,998; U.S. 5,208,340; U.S. 5,132,431 and
U.S.
5,08738.
Additional bleach activators are those described in U.S. Patent 5,130,045,
Mitchell et al, and 4,412,934, Chung et al, and w0 94/28103; EP 0599229;
WO 94/27970; WO 94/28104 and WO 94/28106.
Quaternary substituted bleach activators may also be included. The present
detergent compositions preferably comprise a quaternary substituted bleach
activator
(QSBA) or a quaternary substituted peracid (QSP); more preferably, the former.
Preferred QSBA structures are further described in copending U.S. Patent Nos.
5,460,747, 5,584,888 and 5,578,136.
Useful diperoxyacids include, for example, 1,12-diperoxydodecanedioic acid
(DPDA); 1,9-diperoxyazelaic acid; diperoxybrassilic acid; diperoxysebasic acid
and
diperoxyisophthaIic acid; 2-decyldiperoxybutane-1,4-dioic acid; and 4,4'-
sulphonylbisperoxybenzoic acid. Owing to stnzctures in which two relatively
hydrophilic groups are disposed at the ends of the molecule, diperoxyacids
have
sometimes been classified separately from the hydrophilic and hydrophobic
monoperacids, for example as "hydrotropic". Some of the diperacids are
hydrophobic
in a quite literal sense, especially when they have a long-chain moiety
separating the
peroxyacid moieties.
ReducingBleaches
Another class of useful bleaches are the so called reducing bleaches. These
are
bleaches which "reduce" the soil, in the electrochemical sense, instead of
oxidizing the
soil as conventional bleaches do. Examples of suitable reducing bleaches can
be found
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
37
in These are extensively illustrated in Kirk Othmer, Encyclopedia of Chemical
Technology, Vol. 17, John Wiley and Sons, 1982.
Enzymatic sources of hydrogen peroxide
On a different track from the oxygen bleaching agents illustrated hereinabove,
another suitable hydrogen peroxide generating system is a combination of a C 1
-C4
alkanol oxidase and a C 1 -C4 alkanol, especially a combination of methanol
oxidase
(MOX) and ethanol. Such combinations are disclosed in WO 94/03003. Other
enzymatic
materials related to bleaching, such as peroxidases, haloperoxidases,
oxidases,
superoxide dismutases, catalases and their enhancers or, more commonly,
inhibitors, may
be used as optional ingredients in the instant compositions.
Oxygen transfer agents and precursors
Also useful herein are any of the known organic bleach catalysts, oxygen
transfer
agents or precursors therefor. These include the compounds themselves and/or
their
precursors, for example any suitable ketone for production of dioxiranes
and/or any of
the hetero-atom containing analogs of dioxirane precursors or dioxiranes, such
as
sulfonimines R1R2C=NS02R3, see EP 446 982 A, published 1991 and
sulfonyloxaziridines, for example:
O
R RZC NSOZR3
see EP 446,981 A, published 1991. Preferred examples of such materials include
hydrophilic or hydrophobic ketones, used especially in conjunction with
monoperoxysulfates to produce dioxiranes in situ, and/or the imines described
in U.S.
5,576,282 and references described therein. Oxygen bleaches preferably used in
conjunction with such oxygen transfer agents or precursors include
percarboxylic acids
and salts, percarbonic acids and salts, peroxymonosulfuric acid and salts, and
mixtures
thereof. See also U.S. 5,360,568; U.S. 5,360,569; and U.S. 5,370,826. In a
highly
preferred embodiment, the invention relates to a detergent composition which
incorporates a transition-metal bleach catalyst in accordance with the
invention, and
organic bleach catalyst such as one named hereinabove, a primary oxidant such
as a
hydrogen peroxide source, a hydrophilic bleach activator, and at least one
additional
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
38
detergent, hard-surface cleaner or automatic dishwashing adjunct. Preferred
among such
compositions are those which further include a precursor for a hydrophobic
oxygen
bleach such.
Composition pH
Compositions of the invention will have a pH range of from about 2 to about
13,
preferably, pH is alkaline, more preferably from about 7 to about 12.5, more
preferably
from about 8 to about 12, even more preferably from about 9 to about 11.5. If
a
composition with a pH greater than 7 is to be more effective, it preferably
should contain
a buffering agent capable of providing a generally more alkaline pH in the
composition
and in dilute solutions, i.e., about 0.1% to 0.4% by weight aqueous solution,
of the
composition. The pKa value of this buffering agent should be about 0.5 to I.0
pH units
below the desired pH value of the composition (determined as described above).
Preferably, the pKa of the buffering agent should be from about 7 to about 10.
Under
these conditions the buffering agent most effectively controls the pH while
using the least
amount thereof.
The buffering agent may be an active detergent in its own right, or it may be
a low
molecular weight, organic or inorganic material that is used in this
composition solely for
maintaining an alkaline pH. Preferred buffering agents for compositions of
this
invention are nitrogen-containing materials. Some examples are amino acids
such as
lysine or lower alcohol amines like mono-, di-, and tri-ethanolamine. Other
preferred
nitrogen-containing buffering agents are Tri(hydroxymethyl)amino methane
(HOCH2)3CNH3 (TRIS), 2-amino-2-ethyl-1,3-propanediol, 2-amino-2-methyl-
propanol,
2-amino-2-methyl-1,3-propanol, disodium glutamate, N-methyl diethanolamide,
1,3-
diamino-propanol N,N'-tetra-methyl-1,3-diamino-2-propanol, N,N-bis(2-
hydroxyethyl)glycine (bicine) and N-tris (hydroxymethyl)methyl glycine
(tricine).
Mixtures of any of the above are also acceptable. Useful inorganic
buffers/alkalinity
sources include the alkali metal carbonates and alkali metal phosphates, e.g.,
sodium
carbonate, sodium polyphosphate. For additional buffers see McCutcheon's
EMULSIFIERS AND DETERGENTS, North American Edition, 1997, McCutcheon
Division, MC Publishing Company Kirk and WO 95/07971 both of which are
incorporated herein by reference.
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
39
The buffering agent, if used, is present in the compositions of the invention
herein
at a level of from about 0.1% to 15%, preferably from about 1% to 10%, most
preferably
from about 2% to 8%, by weight of the composition.
Diamines -It is preferred that the diamines used in the present invention are
substantially free from impurities. That is, by "substantially free" it is
meant that the
diamines are over 95% pure, i.e., preferably 97%, more preferably 99%, still
more
preferably 99.5%, free of impurities. Examples of impurities which may be
present in
commercially supplied diamines include 2-Methyl-1,3-diaminobutane and
alkylhydropyrimidine. Further, it is believed that the diamines should be free
of
oxidation reactants to avoid diamine degradation and ammonia formation.
It is further preferred that the compositions of the present invention be
"malodor"
free. That is, that the odor of the headspace does not generate a negative
olfactory
response from the consumer. This can be achieved in many ways, including the
use of
perfumes to mask any undesirable odors, the use of stabilizers, such as
antioxidants,
chelants etc., and/or the use of diamines which are substantially free of
impurities. It is
believed, without wanting to being limited by theory, that it is the
impurities present in
the diamines that are the cause of most of the malodors in the compositions of
the present
invention. These impurities can form during the preparation and storage of the
diamines.
They can also form during the preparation and storage of the inventive
composition. The
use of stabilizers such as antioxidants and chelants inhibit and/or prevent
the formation
of these impurities in the composition from the time of preparation to
ultimate use by the
consumer and beyond. Hence, it is most preferred to remove, suppress and/or
prevent the
formation of these malodors by the addition of perfumes, stabilizers and/or
the use of
diamines which are substantially free from impurities.
Preferred organic diamines are those in which pKl and pK2 are in the range of
about 8.0 to about 11.5, preferably in the range of about 8.4 to about 11,
even more
preferably from about 8.6 to about 10.75. Preferred materials for performance
and
supply considerations are 1,3-bis(methylamine)-cyclohexane, 1,3 propane
diamine
(pKl=10.5; pK2=8.8), 1,6 hexane diamine (pKl=11; pK2=10), 1,3 pentane diamine
(Dytek EP) (pKl=10.5; pK2=8.9), 2-methyl 1,5 pentane diamine (Dytek A)
(pKl=11.2;
pK2=10.0). Other preferred materials are the primary~primary diamines with
alkylene
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
spacers ranging from C4 to C8. In general, it is believed that primary
diamines are
preferred over secondary and tertiary diamines.
Definition of pKl and pK2 - As used herein, "pKal" and "pKa2" are quantities
of
a type collectively known to those skilled in the art as "pKa" pKa is used
herein in the
same manner as is commonly known to people skilled in the art of chemistry.
Values
referenced herein can be obtained from literature, such as from "Critical
Stability
Constants: Volume 2, Amines" by Smith and Martel, Plenum Press, NY and London,
1975. Additional information on pKa's can be obtained from relevant company
literature, such as information supplied by Dupont, a supplier of diamines.
As a working definition herein, the pKa of the diamines is specified in an all-
aqueous solution at ?SoC and for an ionic strength between 0.1 to 0.5 M. The
pKa is an
equilibrium constant which can change with temperature and ionic strength;
thus, values
reported. in the literature are sometimes not in agreement depending on the
measurement
method and conditions. To eliminate ambiguity, the relevant conditions and/or
references used for pKa's of this invention are as defined herein or in
"Critical Stability
Constants: Volume 2, Amines". One typical method of measurement is the
potentiometric titration of the acid with sodium hydroxide and determination
of the pKa
by suitable methods as described and referenced in "The Chemist's Ready
Reference
Handbook" by Shugar and Dean, McGraw Hill, NY, 1990.
It has been determined that substituents and structural modifications that
lower
pKl and pK2 to below about 8.0 are undesirable and cause losses in
performance. This
can include substitutions that lead to ethoxylated diamines, hydroxy ethyl
substituted
diamines, diamines with oxygen in the beta (and less so gamma) position to the
nitrogen
in the spacer group (e.g., Jeffamine EDR 148). In addition, materials based on
ethylene
diamine are unsuitable.
The diamines useful herein can be defined by the following structure:
R2wN.CX,A~C~.N~Ra
i
R3 Rs
wherein R2_S are independently selected from H, methyl, -CH3CH2, and ethylene
oxides; CX and C~ are independently selected from methylene groups or branched
alkyl
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
41
groups where x+y is from about 3 to about 6; and A is optionally present and
is selected
from electron donating or withdrawing moieties chosen to adjust the diamine
pKa's to the
desired range. If A is present, then x and y must both be 1 or greater.
Alternatively the preferred diamines can be those with a molecular weight less
than or equal to 400 glmol. It is preferred that these diamines have the
fommla:
R6 R~'
~N-X-N
R6~ ~R6
wherein each R6 is independently selected from the group consisting of
hydrogen, Cl-C4
linear or branched alkyl, alkyleneoxy having the formula:
-(R~O~RB
wherein R~ is C2-C4 linear or branched alkylene, and mixtures thereof; R8 is
hydrogen,
C1-C4 alkyl, and mixtures thereof; m is from 1 to about 10; X is a unit
selected from:
i) C3-Cl0 linear alkylene, C3-Cl0 branched alkylene, C3-Cl0 cyclic
alkylene, C3-C l 0 branched cyclic alkylene, an alkyleneoxyalkylene
having the formula:
-.~7p~7--
wherein R~ and m are the same as defined herein above;
ii) C3-Clp linear, C3-Cl0 branched linear, C3-Cl0 cyclic, C3-Cl0 branched
cyclic alkylene, C6-Cl0 arylene, wherein said unit comprises one or more
electron donating or electron withdrawing moieties which provide said
diamine with a pKa greater than about 8; and
iii) mixtures of (i) and (ii)
provided said diamine has a pKa of at least about 8.
Examples of some preferred diamines include the following:
~N~NHZ
Dimethyl aminopropyl amine: ~ ;
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/?7201
42
HZN
1,6-Hexane Diamine: NHZ
HpN~NH2 ,
1,3 propane diamine - ,
HZN NHz
2-methyl 1,5 pentane diamine - ;
H2N
I
1,3-pentanediamine, available under the tradename Dytek EP NH2
H2N\ ~ /NHZ
1-methyl-diaminopropane - ;
HzN~0~0~/NHz
Jeffamine EDR 148 - ;
NH2
CH~CHZNH2
Isophorone diamine - '
CH~NH~
CH,NH2
1,3=bis(methylamine)-cyclohexane ;
and mixtures thereof.
Solvents.
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
43
Optionally, the compositions of the present invention may further comprise one
or
more solvents. These solvents may be used in conjunction with an aqueous
liquid carrier
or they may be used without any aqueous liquid carrier being present. Solvents
are
broadly defined as compounds that are liquid at temperatures of 20°C-
25°C and which
are not considered to be surfactants. One of the distinguishing features is
that solvents
tend to exist as discrete entities rather than as broad mixtures of compounds.
Some
solvents which are useful in the hard surface cleaning compositions of the
present
invention contain from 1 carbon atom to 35 carbon atoms, and contain
contiguous linear,
branched or cyclic hydrocarbon moieties of no more than 8 carbon atoms.
Examples of
suitable solvents for the present invention include, methanol, ethanol,
propanol,
isopropanol, 2-methyl pyrrolidinone, benzyl alcohol and morpholine n-oxide.
Preferred
among these solvents are methanol and isopropanol.
The compositions used herein may optionally contain an alcohol having a
hydrocarbon chain comprising 8 to 18 carbon atoms, preferably 12 to 16. The
hydrocarbon chain can be branched or linear, and can be mono, di or
polyalcohols. The
compositions used herein can optionally comprise from 0.1% to 3% by weight of
the
total composition of such alcohol, or mixtures thereof, preferably from 0.1 %
to 1 %.
The solvents which can be used herein include all those known to the those
skilled in the art of hard-surfaces cleaner compositions. Suitable solvents
for use herein
include ethers and diethers having from 4 to 14 carbon atoms, preferably from
6 to 12
carbon atoms, and more preferably from 8 to 10 carbon atoms. Also other
suitable
solvents are glycols or alkoxylated glycols, alkoxylated aromatic alcohols,
aromatic
alcohols, aliphatic branched alcohols, alkoxylated aliphatic branched
alcohols,
alkoxylated linear C1-CS alcohols, linear C1-CS alcohols, C8-C14 alkyl and
cycloalkyl
hydrocarbons and halohydrocarbons, C6-C16 glycol ethers and mixtures thereof.
Suitable glycols which can be used herein are according to the formula HO-
CR1R2-OH wherein RI and R2 are independently H or a C2-C10 saturated or
unsaturated aliphatic hydrocarbon chain and/or cyclic. Suitable glycols to be
used herein
are dodecaneglycol and/or propanediol.
Suitable alkoxyiated glycols which can be used herein are according to the
formula R-(A)n-Rl-OH wherein R is H, OH, a linear saturated or unsaturated
alkyl of
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
44
from 1 to 20 carbon atoms, preferably from 2 to 15 arid more preferably from 2
to 10,
wherein Rl is H or a linear saturated or unsaturated alkyl of from 1 to 20
carbon atoms,
preferably from 2 to 1 ~ and more preferably from 2 to 10, and A is an alkoxy
group
preferably ethoxy, methoxy, and/or propoxy and n is from 1 to 5, preferably 1
to 2.
Suitable alkoxylated glycols to be used herein are methoxy octadecanol andlor
ethoxyethoxyethanol.
Suitable alkoxylated aromatic alcohols which can be used herein are according
to
the formula R (A)n-OH wherein R is an alkyl substituted or non-alkyl
substituted aryl
group of from 1 to 20 carbon atoms, preferably from 2 to 15 and more
preferably from 2
to 10, wherein A is an alkoxy group preferably butoxy, propoxy and/or ethoxy,
and n is
an integer of from 1 to 5, preferably 1 to 2. Suitable alkoxylated aromatic
alcohols are
benzoxyethanol and/or benzoxypropanol.
Suitable aromatic alcohols which can be used herein are according to the
formula
R-OH wherein R is an alkyl substituted or non-alkyl substituted aryl group of
from 1 to
20 carbon atoms, preferably from 1 to 15 and more preferably from 1 to 10. For
example
a suitable aromatic alcohol to be used herein is benzyl alcohol.
Suitable aliphatic branched alcohols which can be used herein are according to
the formula R-OH wherein R is a branched saturated or unsaturated alkyl group
of from 1
to 20 carbon atoms, preferably from 2 to 15 and more preferably from 5 to 12.
Particularly suitable aliphatic branched alcohols to be used herein include 2-
ethylbutanol
and/or 2-methylbutanol.
Suitable alkoxylated aliphatic branched alcohols which can be used herein are
according to the formula R (A)n-OH wherein R is a branched saturated or
unsaturated
alkyl group of from 1 to 20 carbon atoms, preferably from 2 to 15 and more
preferably
from S to 12, wherein A is an alkoxy group preferably butoxy, propoxy and/or
ethoxy,
and n is an integer of from 1 to 5, preferably 1 to 2. Suitable alkoxylated
aliphatic
branched alcohols include 1-methylpropoxyethanol and/or 2-methylbutoxyethanol.
Suitable alkoxylated linear C1-CS alcohols which can be used herein are
according to the formula R (A)n-OH wherein R is a linear saturated or
unsaturated alkyl
group of from 1 to 5 carbon atoms, preferably from 2 to 4, wherein A is an
alkoxy group
preferably butoxy, propoxy and/or ethoxy, and n is an integer of from 1 to 5,
preferably 1
CA 02349537 2004-O1-15
to 2. Suitable alkoxylated aliphatic linear C1-CS alcohols are butoxy propoxy
propanol
(n-BPP), butoxyethanol, butoxypropanol, ethoxyethanol or mixtures thereof.
Butoxy
prapoxy propanol is commercially available under the trade name n-BPP~ from
Dow
chemical.
Suitable linear C1-CS alcohols which can be used herein are according to the
formula R-OH wherein R is a linear saturated or unsaturated alkyl group of
from 1 to 5
carbon atoms, preferably from 2 to 4. Suitable linear C1-CS alcohols are
methanol,
ethanol, propanol or mixtures thereof.
Other suitable solvents include, but are not limited to, butyl diglycol ether
(BDGE), butyltriglycol ether, ter amilic alcohol and the like. Particularly
preferred
solvents which can be used herein are butoxy propoxy propanol, butyl diglycol
ether,
benzyl alcohol, butoxypropanol, ethanol, methanol, isopropanol and mixtures
thereof.
Typically, the compositions used in the methods of the present invention
preferably comprise up to 20% by weight of the total composition of a solvent
or
mixtures thereof, more preferably from 0.5% to 10%, even more preferably from
3% to
10%. and even more preferably still from 1 % to 8%, by weight.
Other suitable solvents for use herein include propylene glycol derivatives
such as
n-butoxypropanol or n- butoxypropoxypropanol, water-soluble CARBITO R solvents
or water-soluble CELLOSOLVE R solvents; water-soluble CARBITOL R solvents are
compounds of the 2-(2-alkoxyethoxy)ethanol class wherein the alkoxy group is
derived
from ethyl, propyl or butyl; a preferred water-soluble carbitol is 2-(2-
butoxyethoxy)ethanol also known as butyl carbitol. Water-soluble CELLOSOLVE R
solvents are compounds of the 2-alkoxyethoxy ethanol class, with 2-
butoxyethoxyethanol
being preferred. Other suitable solvents include benzyl alcohol, and diols
such as 2-
ethyl-1, 3-hexanediol and 2,2,4-trimethyl-1,3-pentanediol and mixtures
thereof. Some
preferred solvents for use herein are n-butoxypropoxypropanol, BUTYL CARBITOL
and mixtures thereof.
The solvents can also be selected from the group of compounds comprising ether
derivatives of mono-, di- and tri-ethylene glycol, propylene glycol, butylene
glycol ethers,
and mixtures thereof. The molecular weights of these solvents are preferably
less than
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
46
350, more preferably between 100 and 300, even more preferably between 115 and
250.
Examples of preferred solvents include, for example, mono-ethylene glycol n-
hexyl
ether, mono-propylene glycol n-butyl ether, and tri-propylene glycol methyl
ether.
Ethylene glycol and propylene glycol ethers are commercially available from
the Dow
Chemical Company under the tradename "Dowanol" and from the Arco Chemical
Company under the tradename "Arcosolv". Other preferred solvents including
mono-
and di-ethylene glycol n-hexyl ether are available from the Union Carbide
company.
Hydrophobic Solvent
In order to improve cleaning in liquid compositions, one can use a hydrophobic
solvent that has cleaning activity. The hydrophobic solvents which may be
employed in
the hard surface cleaning compositions herein can be any of the well-known
"degreasing"
solvents commonly used in, for example, the dry cleaning industry, in the hard
surface
cleaner industry and the metalworking industry.
A useful definition of such solvents can be derived from the solubility
parameters
as set forth in "The Hoy," a publication of Union Carbide, incorporated herein
by
reference. The most useful parameter appears to be the hydrogen bonding
parameter
which is calculated by the formula:
1/2
_a - _l
YH =YT a
wherein yH is the hydrogen bonding parameter, a is the aggregation number,
(Log a = 3.39066 Tb/Tc - 0.15848 - Log M), and
d
yT is the solubility parameter which is obtained from the formula:
112
(JHps _ R'I~d
~fT=
M
where OH25 is the heat of vaporization at 25°C, R is the gas constant
(1.987
cal/mole/deg), T is the absolute temperature in oK, Tb is the boiling point in
oK, Tc is
the critical temperature in °K, d is the density in g/ml, and M is the
molecular weight.
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/2720t
47
For the compositions herein, hydrogen bonding parameters are preferably less
than
7.7, more preferably from 2 to 7, or 7.7, and even more preferably from 3 to
6. Solvents
with lower numbers become increasingly difficult to solubilize in the
compositions and
have a greater tendency to cause a haze on glass. Higher numbers require more
solvent
to provide good greasy/oily soil cleaning.
Hydrophobic solvents are typically used, when present, at a level of from 0.5%
to
30%, preferably from 2% to 15%, more preferably from 3% to 8%. Dilute
compositions
typically have solvents at a level of from 1% to 10%, preferably from 3% to
6%.
Concentrated compositions contain from 10% to 30%, preferably from 10% to 20%
of
solvent.
Many of such solvents comprise hydrocarbon or halogenated hydrocarbon moieties
of the alkyl or cycloalkyl type, and have a boiling point well above room
temperature,
i.e., above 20°C.
One highly preferred solvent is limonene, which not only has good grease
removal
but also a pleasant odor properties.
The formulator of compositions of the present type will be guided in the
selection
of solvent partly by the need to provide good grease-cutting properties, and
partly by
aesthetic considerations. For example, kerosene hydrocarbons function quite
well for
grease cutting in the present compositions, but can be malodorous. Kerosene
must be
exceptionally clean before it can be used, even in commercial situations. For
home use,
where malodors would not be tolerated, the formulator would be more likely to
select
solvents which have a relatively pleasant odor, or odors which can be
reasonably
modified by perfiuning.
The C6-Cg alkyl aromatic solvents, especially the C6-Cg alkyl benzenes,
preferably octyl benzene, exhibit excellent grease removal properties and have
a low,
pleasant odor. Likewise, the olefin solvents having a boiling point of at
least 100°C,
especially alpha-olefins, preferably 1-decene or 1-dodecene, are excellent
grease removal
solvents.
Generically, glycol ethers useful herein have the formula R11 O-(R120-)m1H
wherein each R11 is an alkyl group which contains from 3 to 8 carbon atoms,
each R12
it
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
48
is either ethylene or propylene, and m 1 is a number from 1 to 3. The most
preferred
glycol ethers are selected from the group consisting of
monopropyleneglycolmonopropyl
ether, dipropyleneglycolmonobutyl ether, monopropyleneglycolmonobutyl ether,
ethyleneglycolmonohexyl ether, ethyleneglycolmonobutyl ether,
diethyleneglycolmonohexyl ether, monoethyleneglycolmonohexyl ether,
monoethyleneglycolmonobutyl ether, and mixtures thereof.
A particularly preferred type of solvent for these hard surface cleaner
compositions
comprises diols having from 6 to 16 carbon atoms in their molecular structure.
Preferred
diol solvents have a solubility in water of from 0.1 to 20 g/100 g of water at
20°C. The
diol solvents in addition to good grease cutting ability, impart to the
compositions an
enhanced ability to remove calcium soap soils from surfaces such as bathtub
and shower
stall walls. These soils are particularly difficult to remove, especially for
compositions
which do not contain an abrasive. Other solvents such as benzyl alcohol, n-
hexanol, and
phthalic acid esters of C 1 _,~ alcohols can also be used.
Solvents such as pine oil, orange terpene, benzyl alcohol, n-hexanol, phthalic
acid
esters of C1-4 alcohols, butoxy propanol, Butyl Carbitol~ and 1(2-n-butoxy-1-
methylethoxy)propane-2-of (also called butoxy propoxy propanol or dipropylene
glycol
monobutyl ether), hexyl diglycol (Hexyl Carbitol~), butyl triglycol, diols
such as 2,2,4-
trimethyl-1,3-pentanediol, and mixtures thereof, can be used. The butoxy-
propanol
solvent should have no more than 20%, preferably no more than 10%, more
preferably no
more than 7%, of the secondary isomer in which the butoxy group is attached to
the
secondary atom of the propanol for improved odor.
The level of hydrophobic solvent is preferably, when present, from 1% to 15%,
more
preferably from 2% to 12%, even more preferably from 5% to ZO%.
Hydrotropes
The compositions used in the methods of the present invention may optionally
comprise one or more materials which are hydrotropes. Hydrotropes suitable for
use in
the compositions herein include the C1-C3 alkyl aryl sulfonates, C6-C12
alkanols, C1-
C6 carboxylic sulfates and sulfonates, urea, C1-C6 hydrocarboxylates, C1-C4
carboxylates, C2-C4 organic diacids and mixtures of these hydrotrope
materials. The
CA 02349537 2001-04-27
rte.
WO 00/28874 PCT/US99/27201
49
composition of the present invention preferably comprises from 0.5% to 8%, by
weight
of the liquid detergent composition of a hydrotrope selected from alkali metal
and
calcium xylene and toluene sulfonates.
Suitable C1-C3 alkyl aryl sulfonates include sodium, potassium, calcium and
ammonium xylene sulfonates; sodium, potassium, calcium and ammonium toluene
sulfonates; sodium, potassium, calcium and ammonium cumene sulfonates; and
sodium,
potassium, calcium and ammonium substituted or unsubstituted naphthalene
sulfonates
and mixtures thereof.
Suitable C1-Cg carboxylic sulfate or sulfonate salts are any water soluble
salts or
organic compounds comprising 1 to 8 carbon atoms (exclusive of substituent
groups),
which are substituted with sulfate or sulfonate and have at least one
carboxylic group.
The substituted organic compound may be cyclic, acylic or aromatic, i.e.
benzene
derivatives. Preferred alkyl compounds have from 1 to 4 carbon atoms
substituted with
sulfate or sulfonate and have from 1 to 2 carboxylic groups. Examples of this
type of
hydrotrope include sulfosuccinate salts, sulfophthalic salts, sulfoacetic
salts, m-
sulfobenzoic acid salts and diester sulfosuccinates, preferably the sodium or
potassium
salts as disclosed in U.S. 3,915,903.
Suitable C1-C4 hydrocarboxylates and C1-C4 carboxylates for use herein include
acetates and propionates and citrates. Suitable C2-C4 diacids for use herein
include
succinic, glutaric and adipic acids.
Other compounds which deliver hydrotropic effects suitable for use herein as a
hydrotrope include C6-C12 alkanols and urea.
Preferred hydrotropes for use herein are sodium, potassium, calcium and
ammonium cumene sulfonate; sodium, potassium, calcium and ammonium xylene
sulfonate; sodium, potassium, calcium and ammonium toluene sulfonate and
mixtures
thereof. Most preferred are sodium cumene sulfonate and calcium xylene
sulfonate and
mixtures thereof. These preferred hydrotrope materials can be present in the
composition
to the extent of from 0.5% to 8% by weight.
Polymeric Suds Stabilizers
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
The compositions of the present invention may also contain a polymeric suds
stabilizer. The compositions preferably comprise at least an effective amount
of the
polymeric suds stabilizers described herein, more preferably from about 0.01 %
to about
10%, even more preferably from about 0.05% to about 5%, even more preferably
still
preferably from about 0.1% to about 2% by weight, of said composition. What is
meant
herein by "an effective amount polymeric suds stabilizers " is that the suds
volume and
suds duration produced by the presently described compositions are sustained
for an
increased amount of time relative to a composition which does not comprise one
or more
of the polymeric suds stabilizer described herein. Additionally, the polymeric
suds
stabilizer can be present as the free base or as a salt. Typical counter ions
include,
citrate, maleate, sulfate, chloride, etc.
One preferred polymeric suds stabilizer are polymers comprising at least one
monomeric unit of the formula:
Rz
R'
R3 I J
A-WZ-L O
wherein each of R~,.RZ and R3 are independently selected from the group
consisting of
hydrogen, C~ to C6 alkyl, and mixtures thereof, preferably hydrogen, C~ to C3
alkyl, more
preferably, hydrogen or methyl. L is selected from the group consisting of a
bond, O,
NRb, SR~Rg and mixtures thereof, preferably, O, NR6, wherein R6 is selected
from the
group consisting of hydrogen, C 1 to Cg alkyl and mixtures thereof,
preferably, hydrogen,
C ~ to C3, and mixtures thereof, more preferably hydrogen, methyl; each of R'
and R8 are
independently hydrogen, O, C~ to C8 alkyl and mixtures thereof, preferably,
hydrogen, C1
to C3, and mixtures thereof, more preferably hydrogen or methyl. By "O", an
oxygen
linked via a double bond is meant, such as a carbonyl group. Furthermore this
means
that when either or both R~Rg is "O", SR~Rg can have the following structures:
O R8 O
I
II II
-S- -S- -S-
R~ O O
or
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
51
Alternatively, SR'Rg form a heterocyclic ring containing from 4 to 7 carbon
atoms,
optionally containing additional hetero atoms and optionally substituted. For
example
SR'Rg can be:
\S/ \S/ \S/ \S/ \S/ \S/ \S/
or
NH , N NR
> >
O
However, it is preferred that SR'R8, when present, is not a heterocycle.
When L is a bond it means that there is a direct link, or a bond, between the
carbonyl carbon atom to Z, when z is not zero. For example:
J
CH3
CH ~N (CH2CH2O)3 O O~N- CH
( 22
3
When L is a bond and z is zero, it means L is a bond from the carbonyl atom to
A. For
example:
J
NCO
O ~N O
HN N
> >
Z is selected from the group consisting of: -{CHZ)-, (CH2-CH=CH)-, -(CHZ-
CHOH)-, (CHZ-CHIVR6)-, -(CHz-CHR~4-O)- and mixtures thereof, preferably -(CHZ)-
.
R~4 is selected from the group consisting of hydrogen, C, to C6 alkyl and
mixtures
thereof, preferably hydrogen, methyl, ethyl and mixtures thereof; z is an
integer selected
from about 0 to about 12, preferably about 2 to about 10, more preferably
about 2 to
about 6.
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/2710t
52
A is NR'~R'. Wherein each of R'' and R5 are is independently selected from the
group consisting of hydrogen, C1-Cg linear or branched alkyl, alkyleneoxy
having the
formula:
-(R 1 Op~yR 11
wherein R10 is C2-C4 linear or branched alkylene, and mixtures thereof; R11 is
hydrogen, C1-C4 alkyl, and mixtures thereof; y is from 1 to about 10.
Preferably R'' and
R' are independently, hydrogen, C, to C.~ alkyl. Alternatively, NR''RS can
form a
heterocyclic ring containing from 4 to 7 carbon atoms, optionally containing
additional
hetero atoms, optionally fused to a benzene ring, and optionally substituted
by C, to C
hydrocarbyl. Examples of suitable heterocycles, both substituted and
unsubstituted, are
indolyl, isoindolinyl imidazolyl, imidazolinyl, piperidinyl pyrazolyl,
pyrazolinyl,
pyridinyl, piperazinyl, pyrrolidinyl, pvrrolidinyl, guanidino, amidino,
quinidinyl,
thiazolinyl, morpholine and mixtures thereof, with morpholino and piperazinyl
being
preferred. Furthermore the polymeric suds stabilizer has a molecular weight of
from
about 1,000 to about 2,000,000 preferably from about 5,000 to about 1,000,000,
more
preferably from about 10,000 to about 750,000, more preferably from about
20,000 to
about 500,000, even more preferably from about 35,000 to about 300,000
daltons. The
molecular weight of the polymeric suds boosters, can be determined via
conventional gel
permeation chromatography.
While, it is preferred that the polymeric suds stabilizers be selected from
homopolymer,
copolymers and terpolymers, other polymers (or multimers) of the at least one
monomeric unit, the polymeric suds stabilizers can also be envisioned via
polymerization
of the at least one monomeric unit with a wider selection of monomers. That
is, all the
polymeric suds stabilizers can be a homopolymers, copolymers, terpolymers,
etc. of the
at least one monomeric unit, or the polymeric suds stabilizer can be
copolymers,
terpolymers, etc. containing one, two or more of the at least one monomeric
unit and one,
two or more monomeric units other than the at least one monomeric unit. In the
copolymer, terpolymer, etc., the distribution of the monomers can be either
random or
repeating.
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
53
Some preferred suds stabilizing polymers are homopolymers, copolymers or
terpolymers which comprise at least one monomeric units, selected from:
CH3 CH3CH2\ -
I N
CH ~N~O~O CH CH2 ~O O H2N~ O
3 3 O
-~O
O N N
N O
~~ NH , ~ or
An example of a preferred homopolymer is 2-dimethylaminoethyl methacrylate
(DMAM) having the formula:
cH3 J
CH3~N~0 O
Some preferred copolymers include:
copolymers of
CH3 J
CH ~N~p OCH3~N O
3 I
CH3
cH3 ~ ~ J
N~ O
CH3 O HO O
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
54
CH3 .J
CH3~ ~ O O HO O
and
An example of a preferred copolymer is the (DMA)/(DMAM) copolymer having
the general formula:
CHI
CH3~N O N~ O
I CH3~ O
CH3
wherein the ratio of (DMA) to (DMAM) is about 1 to about 10, preferably about
1 to
about 5, more preferably about 1 to about 3
An example of a preferred copolymer is the (DMAM)/(DMA) copolymer having
the general formula:
CH3 _j
CH ~N~O OCH3~N O
3 I
CH3
wherein the ratio of (DMAM) to (DMA) is about 1 to about 5, preferably about 1
to
about 3.
Another prefered suds stabilizing polymer are the proteinaceous suds
stabilizers.
These can be peptides, polypeptides, amino acid containing copolymers, and
mixtures
thereof. Any suitable amino acid can be used to form the backbone of the
peptides,
polypeptides, or amino acid containing copolymers of the present invention
provided at
least 10% to about 40% of said amino acids which comprise the peptides are
capable of
being protonated at a pH of from 7 to about 11.5.
In general, the amino acids suitable for use in forming the proteinaceous suds
stabilizers of the present invention have from 2 to 22 carbon atoms, said
amino acids
having the formula:
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
R~ R R2 O
I I I II
H2N (C)x-C-(C)y-C-OH
R~ Rl R'
wherein R and R1 are each independently hydrogen, C1-C6 linear or branched
alkyl, C1-
C6 substituted alkyl, and mixtures thereof. The indices x and y are each
independently
fromOto2.
An example of a more preferred amino acid according to the present invention
is
the amino acid lysine having the formula:
NH2
O
(I
H2N- i -C-OH
H
wherein R is a substituted C 1 alkyl moiety, said substituent is 4-imidazolyl.
One type of suitable proteinaceous suds stabilizer is comprised entirely of
amino
acids. Said polyamino acid compounds may be naturally occurring peptides,
polypeptides, enzymes, and the like, provided said compounds have an
isoelectric point
of from about 7 to about 11.5 and a molecular weight greater than or equal to
about 1500
daltons. An example of a polyamino acid which is suitable as a proteinaceous
suds
stabilizer according to the present invention is the enzyme lysozyme.
Another preferred polymeric suds stabilizers are homopolymers or copolymers
wherein the monomers which comprise said homopolymers or copolymers contain a
moiety capable of being protonated at a pH of from about 4 to about 12, or a
moiety
capable of being de-protonated at a pH of from about 4 to about 12, of a
mixture of both
types of moieties.
A preferred class of zwitterionic polymer suitable for use as a suds volume
and
suds duration enhancer has the formula:
CA 02349537 2001-04-27
WO 00128874 PCT/US99/27201
56
R1 R2
I I
(R)x-'(C~y-(CH)z
n
wherein R is C 1-C 1 ~ linear alkylene, C 1-C 12 branched alkylene, and
mixtures
thereof; preferably C1-C4 linear alkylene, C3-C4 branched alkylene; more
preferably
methylene and 1,2-propylene. R~ and R'' are defined herein after. The index x
is from 0
to 6; y is 0 or 1; z is 0 or 1. The index n has the value such that the
zwitterionic polymers
of the present invention have an average molecular weight of from about 1,000
to about
2,000,000 preferably from about 5,000 to about 1,000,000, mare preferably from
about
10,000 to about 750,000, more preferably from about 20,000 to about 500,000,
even
more preferably from about 35,000 to about 300,000 daltons. The molecular
weight of
the polymeric suds boosters, can be determined via conventional gel permeation
chromatography.
Anionic Units - R1 is a unit capable of having a negative charge at a pH of
from about 4
to about 12. Preferred R 1 has the formula:
(I-)i (S)j-R3
wherein L is a linking unit independently selected from the following:
O O O O
-O-C-NR'-, -C-O- ~ -O-C- , -O-C-O- , -O- ,~d
mixtures thereof, wherein R' is independently hydrogen, C 1-C4 alkyl, and
mixtures
thereof; preferably hydrogen or alternatively R' and S can form a heterocycle
of 4 to 7
carbon atoms, optionally containing other hetero atoms and optionally
substituted.
Preferably the linking group L can be introduced into the molecule as part of
the original
monomer backbone, for example, a polymer having L units of the formula:
O
II
-C-O
can suitably have this moiety introduced into the polymer via a carboxylate
containing
monomer, for example, a monomer having the general formula:
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
57
CO~H R2
I - I
~)x-(CH)y (CH)z
When the index i is 0, L is absent.
For anionic units S is a "spacing unit" wherein each S unit is independently
selected from C 1-C 12 linear alkylene, C 1-C 12 branched alkylene, C 3-C 12
linear
alkenylene, C3-C 1 ~ branched alkenylene, C3-C 12 hydroxyalkylene, C4-C 1 ~
dihydroxyalkylene, C6-C 10 arylene, Cg-C 12 dialkylarylene, -(R50)kR5-, -
(RSO)kR6(ORS)k-, -CH2CH(OR~)CH2-, and mixtures thereof; wherein RS is C2-C4
linear alkylene, C3-C4 branched alkylene, and mixtures thereof, preferably
ethylene, 1,2-
propylene, and mixtures thereof, more preferably ethylene; R6 is C2-C12 linear
alkylene,
and mixtures thereof, preferably ethylene; R~ is hydrogen, C1-C4 alkyl, and
mixtures
thereof, preferably hydrogen. The index k is from 1 to about 20.
R3 is independently selected from hydrogen, -C02M, -S03M, -OS03M, -
CH2P(O)(OM)2, -OP(O)(OM)2, units having the formula:
-CRgR9R10
wherein each Rg, R9, and R10 is independently selected from the group
consisting of
hydrogen, -(CH2)mRll, and mixtures thereof, wherein R11 is -C02H, -S03M,
OS03M, -CH(C02H)CH2C02H, -CH2P(O)(OH)2, -OP(O)(OH)2, and mixtures thereof,
preferably -C02H, -CH(C02H)CH2C02H, and mixtures thereof, more preferably -
C02H; provided that one Rg, R9, or R10 is not a hydrogen atom, preferably two
R8, R9,
or R10 units are hydrogen. M is hydrogen or a salt forming cation, preferably
hydrogen.
The index m has the value from 0 to 10.
Cationic Units - R2 is a unit capable of having a positive charge at a pH of
from about 4
to about 12. Preferred R2 has the formula:
-(Ll )i'-(S)j'-R4
wherein L1 is a linking unit independently selected from the following:
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
58
-O-O- -O-O- -O-O
C-O- ,
O R R O R O R
-C-N- , -'-~1-C- , -N-C-N- ,
, , . ,
R i R O R R O
-N-C-N- , -O-C-N-, -N-C-O- ,
R' R' R'
-N=C- , -C=N- , -N- , -O- ,
and mixtures thereof; wherein R' is independently hydrogen, C1-C4 alkyl, and
mixtures
thereof; preferably hydrogen or alternatively R' and S can form a heterocycle
of 4 to 7
carbon atoms, optionally containing other hetero atoms and optionally
substituted.
When the index i' is equal to 0, L1 is absent.
For cationic units S is a "spacing unit" wherein each S unit is independently
selected from C 1-C 1 ~ linear alkylene, C 1-C 12 branched alkylene, C3-C 12
linear
alkenylene, C3-C 1 ~ branched alkenylene, C3-C 12 hydroxyalkylene, C4-C 12
dihydroxyalkylene, C6-C 1 p arylene, Cg-C 12 dialkylarylene, -(R50)kRS-,
(R50)kR6(ORS)k-, -CH2CH(OR~)CH2-, and mixtures thereof; wherein RS is C~-C4
linear alkylene, C3-C4 branched alkylene, and mixtures thereof, preferably
ethylene, 1,2-
propylene, and mixtures thereof, more preferably ethylene; R6 is C2-C 12
linear alkylene,
and mixtures thereof, preferably ethylene; R~ is hydrogen, C1-C4 alkyl, and
mixtures
thereof, preferably hydrogen. The index k is from 1 to about 20.
R4 is independently selected from amino, alkylamino carboxamide, 3-imidazolyl,
4-imidazolyl, 2-imidazolinyl, 4-imidazolinyl, 2-piperidinyl, 3-piperidinyl, 4-
piperidinyl,
1-pyrazolyl, 3-pyrazoyl, 4-pyrazoyl, 5-pyrazoyl, 1-pyrazolinyl, 3-pyrazolinyl,
4-
pyrazolinyl, 5-pyrazolinyl, 2-pyridinyl, 3-pyridinyl, 4-pyridinyl,
piperazinyl, 2-
pyrrolidinyl, 3-pyrrolidinyl, guanidine, amidino, and mixtures thereof,
preferably
dialkylamino having the formula:
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
59
-N~l 1 )~
wherein each R11 is independently hydrogen, C1-C4 alkyl, and mixtures thereof,
preferably hydrogen or methyl or alternatively the two RI 1 can form a
heterocycle of 4 to
8 carbon atoms, optionally containing other hetero atoms and optionally
substituted.
An example of a preferred zwitterionic polymer according to the present
invention has the formula:
X C02
CH~-CH-CH-CH
I
O=C
I n
NH
CH2CH2CH2N+H(CH3)2
wherein X is C6, n has a value such that the average molecular weight is from
about
5,000 to about 1,000,000 daltons.
Further preferred zwitterionic polymers according to the present invention are
polymers comprising monomers wherein each monomer has only cationic units or
anionic units, said polymers have the formula:
R1 R2
I I
~)x-(C~y ~)x~(C~z
n1 n2
wherein R, R1, x, y, and z are the same as defined herein above; n1 + n2 = n
such that n
has a value wherein the resulting zwitterionic polymer has a molecular weight
of form
about 5,000 to about 1,000,000 daltons.
An example of a polymer having monomers with only an anionic unit or a
cationic unit has the formula:
CO2
I
CH2-CH H2-CH
n1 C=O n2
NH
CH2CH2CHZN+H(CH3)z
CA 02349537 2001-04-27
WO 00/28874 PCTIUS99/27ZOt
wherein the sum of nI and n2 provide a polymer with an average molecular
weight of
from about 5,000 to about 750,000 daltons.
Another preferred zwitterionic polymer according to the present invention are
polymers which have limited crosslinking, said polymers having the formula:
R~ R' R1
(R)x-(C~y-~CH)z ~R)x-(C~v (CH
n 1 ri
L~
R1~
(.~)),
Ll
R~ I R( R'-
~)x-(CHIy-(CH) ~)xWCH)y-(CH)z
n" n2
wherein R, Rl, L1, S, j', x, y, and z are the same as defined herein above; n'
is equal to
n", and the value n' + n" is less than or equal to 5% of the value of n1 + n2
= n; n
provides a polymer with an average molecular weight of from about 1,000 to
about
2,000,000 daltons. R I 2 is nitrogen, C 1-C 1 ~ linear alkylene amino alkylene
having the
formula:
_R13_N_R13_
L1, and mixtures thereof, wherein each R13 is independently L1 or ethylene.
The zwitterionic polymers of the present invention may comprise any
combination of monomer units, for example, several different monomers having
various
R1 and R2 groups can be combined to form a suitable suds stabilizer.
Alternatively the
same R1 unit may be used with a selection of different R2 units and vice
versa.
Furthermore another preferred type of polymeric suds stabilizers are polymers
which contain units capable of having a cationic charge at a pH of from about
4 to about
12, provided that the suds stabilizer has an average cationic charge density
from about
0.0005 to about 0.05 units per 100 daitons molecular weight at a pH of from
about 4 to
about 12. Additionally, the polymeric suds stabilizer can be present as the
free base or as
a salt. Typical counter ions include, citrate, maleate, sulfate, chloride,
etc.
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/2720t
61
For the purposes of the present invention the term "cationic unit" is defined
as "a
moiety which when incorporated into the structure of the suds stabilizers of
the present
invention, is capable of maintaining a cationic charge within the pH range of
from about
4 to about 12. The cationic unit is not required to be protonated at every pH
value within
the range of about 4 to about 12." Non-limiting examples of units which
comprise a
cationic moiety include lysine, ornithine, the monomeric unit having the
formula:
CH3
I
CHI-CHZ-CH-CH
O=C
I
NH
CH2CH2CH~N+H(CH3)2.
the monomeric unit having the formula:
H CH3
~N+
CH3' ~ O O
the monomeric unit having the formula:
CH3
l
CH2-CH2-CH-CH
O=C
I
NH
CH2CH~CH2N+(CH3)3
the monomeric unit having the formula:
CH3 C02H
CHZ-CH-CH-CH
O=C
I
NH
~HZCH2CH~N+H(CH3)2
and the monomeric unit having the formula:
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/?720t
62
CH3 C02H
i I
-~ CHZ-CH-CH-CH
O=C
NH
CH,CH~CH2HN+(CH3)3
the latter of which also comprises a moiety capable of having an anionic
charge at a pH
of about 4 to about 12.
For the purposes of the present invention the term "anionic unit" is defined
as "a
moiety which when incorporated into the structure of the suds stabilizers of
the present
invention, is capable of maintaining an anionic charge within the pH range of
from about
4 to about 12. The anionic unit is not required to be de-protonated at every
pH value
within the range of about 4 to about 12." Nan-limiting examples of units which
comprise
a anionic moiety include, acrylic acid, methacrylic acid, glutamic acid,
aspartic acid, the
monomeric unit having the formula:
CO~
I
CHZ-CHI -CH -CH2
and the monomeric unit having the formula:
CH3 C02
CHI-CH-CH-CH
I
O=C
I
NH
I
CH~CH2CH~N(CH3)2
the latter of which also comprises a moiety capable of having a cationic
charge at a pH of
about 4 to about 12. This latter unit is defined herein as "a unit capable of
having an
anionic and a cationic charge at a pH of from about 4 to about 12."
For the purposes of the present invention the term "non-charged unit" is
defined
as "a moiety which when incorporated into the structure of the suds
stabilizers of the
present invention, has no charge within the pH range of from about 4 to about
12." Non-
limiting examples of units which are "non-charged units" are styrene,
ethylene,
propylene, butylene, 1,2-phenylene, esters, amides, ketones, ethers, and the
like.
The units which comprise the polymers of the present invention may, as single
units or monomers, have any pKa value.
CA 02349537 2004-O1-15
63
The formulator may combine any suitable monomers or units to form a polymeric
suds stabilizer, for example, amino acids may be combined with polyacrylate
units.
Further information on these and other suitable suds stabilizing polymers, and
processes for their preparation are further described in wo 99/27058; wo
99/27054;
WO 99/27054 and WO 99/27057.
Enzymes - Detergent compositions of the present invention may further comprise
one or more enzymes which provide cleaning performance benefits. Said enzymes
include enzymes selected from cellulases, hemicellulases, peroxidases,
proteases, gluco-
amylases, amylases, lipases, cutinases, pectinases, xylanases, reductases,
oxidases,
phenoloxidases, lipoxygenases, ligninases, pullulanases, tannases,
pentosanases,
malanases,13-glucanases, arabinosidases or mixtures thereof. A preferred
combination is
a detergent composition having a cocktail of conventional applicable enzymes
like
protease, amylase, lipase, cutinase and/or cellulase. Enzymes when present in
the
compositions, at from about 0.0001% to about 5% of active enzyme by weight of
the
detergent composition.
Proteol is Enzyme - The proteolytic enzyme can be of animal, vegetable or
microorganism (preferred) origin. The proteases for use in the detergent
compositions
herein include (but are not limited to) trypsin, subtilisin, chymotrypsin and
elastase-type
proteases. Preferred for use herein are subtilisin-type proteolytic enzymes.
Particularly
preferred is bacterial serine proteolytic enzyme obtained from Bacillus
subtilis and/or
Bacillus licheniformis.
Suitable proteolytic enzymes include Novo Industri A/S Alcalase~ (preferred),
Esperase~~ Savinase~ (Copenhagen, Denmark), Gist-brocades' Maxatase~, Maxacal~
and Maxapem 15~ (protein engineered Maxacal~) (Delft, Netherlands), and
subtilisin
BPN and BPN'(preferred), which are commercially available. Preferred
proteolytic
enzymes are also modified bacterial serine proteases, such as those made by
Genencor
International, Inc. (San Francisco, California) which are described in
European Patent
251,446B, granted December 28, 1994 (particularly pages 17, 24 and 98) and
which are
also called herein "Protease B". U.S. Patent 5,030,378, Vcnegas, issued July
9, 1991,
CA 02349537 2004-O1-15
64
refers to a modified bacterial serine proteolytic enzyme (Genencor
International) which is
called "Protease A" herein (same as BPN'). In particular see columns 2 and 3
of U.S.
Patent 5,030,378 for a complete description, including amino sequence, of
Protease A
and its variants. Other proteases are sold under the tradenames: Primase,
Durazym,
Opticlean and Optimase. Preferred proteolytic enzymes, then, are selected from
the
group consisting of Alcalase ~ (Novo Industri A/S), BPN', Protease A and
Protease B
(Genencor), and mixtures thereof. Protease B is most preferred.
Of particular interest for use herein are the proteases described in U.S.
Patent No.
5,470,733.
Also proteases described in wo 95/10591, ' can be
included in the detergent composition of the invention.
Another preferred protease, referred to as "Protease D" is a carbonyl
hydrolase
variant having an amino acid sequence not found in nature; which is derived
from a
precursor carbonyl hydrolase by substituting a different amino acid for a
plurality of
amino acid residues at a position in said carbonyl hydrolase equivalent to
position +76,
preferably also in combination with one or more amino acid residue positions
equivalent
to those selected from the group consisting of +99, +101, +103, +104, +107,
+123, +27,
+105, +109, +126, +128, +135, +156, +166, +195, +197, +204, +206, +210, +216,
+217,
+218, +222, +260, +26~, and/or +274 according to the numbering of Bacillus
amyloliquefaciens subtilisin, as described in WO 95/10615 published April 20,
1995 by
Genencor International (A. Baeck . et al. entitled "Protease-Containing
Cleaning
Compositions"havingU.S. patent No. 5,679,630).
Useful proteases are also described in PCT publications: WO 95/30010 published
November 9, 1995 by The Procter & Gamble Company; WO 95/30011 published
November 9, 1995 by The Procter & Gamble Company; WO 95129979 published
November 9, 1995 by The Procter & Gamble Company.
Protease enzyme may be incorporated into the compositions in accordance with
the
invention at a level of from 0.0001 % to 2% active enzyme by weight of the
composition.
Amylase - Amylases (a, and/or l3) can be included for removal of
carbohydrate-based stains. Suitable amylases are Termamyl~ (Novo Nordisk),
Fungamyl~ and BAN~ (Novo Nordisk). The enzymes may be of any suitable origin,
CA 02349537 2004-O1-15
such as vegetable, animal, bacterial, fungal and yeast origin. Amylase enzymes
are
normally incorporated in the detergent composition at levels from 0.0001 % to
2%,
preferably from about 0.0001% to about 0.5%, more preferably from about
0.0005% to
about 0.1 %, even more preferably from about 0.001 % to about 0.05 % of active
enzyme
by weight of the detergent composition.
Amylase enzymes also include those described in W095/26397 and in co-
pending application by Novo Nordisk wo96/238731A1. Other specific amylase
enzymes for use in the detergent compositions of the present invention
therefore include
(a) a-amylases characterised by having a specific activity at least 25% higher
than the
specific activity of Termamyl~ at a temperature range of 25°C to
55°C and at a pH value
in the range of 8 to 10, measured by the Phadebas~ a-amylase activity assay.
Such
Phadebas~ a-amylase activity assay is described at pages 9-10, W095/25397.
(b) a-amylases according (a) comprising the amino sequence shown in the SEQ )D
listings in the above cited reference. or an a-amylase being at least 80%
homologous
with the amino acid sequence shown in the SEQ ID listing.
(c) a-amylases according (a) obtained from an alkalophilic Bacillus species,
comprising
the following amino sequence in the N-terminal : His-His-Asn-Gly-Thr-Asn-Gly-
Thr-
Met-Met-Gln-Tyr-Phe-Glu-Trp-Tyr-Leu-Pro-Asn-Asp.
A polypeptide is considered to be X% homologous to the parent amylase if a
comparison of the respective amino acid sequences, performed via algorithms,
such as
the one described by Lipman and Pearson in Science 227, 1985, p. 1435, reveals
an
identity of X%
(d) a-amylases according (a-c) wherein the a-amylase is obtainable from an
alkalophilic
Bacillus species; and in particular, from any of the strains NCIB 12289, NCIB
12512,
NCIB 12513 and DSM 935.
In the context of the present invention, the term "obtainable from" is
intended not only to
indicate an amylase produced by a Bacillus strain but also an amylase encoded
by a DNA
sequence isolated from such a Bacillus strain and produced in an host organism
transformed with said DNA sequence.
CA 02349537 2001-04-27
WO 00/28874 PCTNS99/27201
66
(e)a-amylase showing positive immunological cross-reactivity with antibodies
raised
against an a-amylase having an amino acid sequence corresponding respectively
to those
a-amylases in (a-d).
(f) Variants of the following parent a-amylases which (i) have one of the
amino acid
sequences shown in corresponding respectively to those a-amylases in (a-e), or
(ii)
displays at least 80% homology with one or more of said amino acid sequences,
and/or
displays immunological cross-reactivity with an antibody raised against an a-
amylase
having one of said amino acid sequences, and/or is encoded by a DNA sequence
which
hybridizes with the same probe as a DNA sequence encoding an a-amylase having
one of
said amino acid sequence; in which variants
1. at least one amino acid residue of said parent a-amylase has been deleted;
and/or
2. at least one amino acid residue of said parent a-amylase has been replaced
by a
different amino acid residue; and/or
3. at least one amino acid residue has been inserted relative to said parent a-
amylase;
said variant having an a-amylase activity and exhibiting at least one of the
following properties relative to said parent a-amylase : increased
thermostability,
increased stability towards oxidation, reduced Ca ion dependency, increased
stability and/or a-amylolytic activity at neutral to relatively high pH
values,
increased a-amylolytic activity at relatively high temperature and increase or
decrease of the isoelectric point (pI) so as to better match the pI value for
a-
amylase variant to the pH of the medium.
Said variants are described in the patent application PCT/DK96/00056.
Other amylases suitable herein include, for example, a-amylases described in
GB
1,296,839 to Novo; RAPIDASE~, International Bio-Synthetics, Inc. and
TERMAMYL~, Novo. FUNGAMYL~ from Novo is especially useful. Engineering of
enzymes for improved stability, e.g., oxidative stability, is known. See, for
example J.
Biological Chem., Vol. 260, No. 11, Tune 1985, pp. 6518-6521. Certain
preferred
embodiments of the present compositions can make use of amylases having
improved
CA 02349537 2004-O1-15
67
stability in detergents such as automatic dishwashing types, especially
improved
oxidative stability as measured against a reference-point of TERMAMYL~ in
commercial use in 1993. These preferred amylases herein share the
characteristic of
being "stability-enhanced" amylases, characterized, at a minimum, by a
measurable
improvement in one or more of: oxidative stability, e.g., to hydrogen
peroxide/tetraacetylethylenediamine in buffered solution at pH 9-10; thermal
stability,
e.g., at common wash temperatures such as about 60oC; or alkaline stability,
e.g., at a pH
from about 8 to about 11, measured versus the above-identified reference-point
amylase.
Stability can be measured using any of the art-disclosed technical tests. See,
for example,
references disclosed in WO 9402597. Stability-enhanced amylases can be
obtained from
Novo or from Genencor International. One class of highly preferred amylases
herein
have the commonality of being derived using site-directed mutagenesis from one
or more
of the Bacillus amylases, especially the Bacillus a-amylases, regardless of
whether one,
two or multiple amylase strains are the immediate precursors. Oxidative
stability-
enhanced amylases vs. the above-identified reference amylase are preferred for
use,
especially in bleaching, more preferably oxygen bleaching, as distinct from
chlorine
bleaching, detergent compositions herein. Such preferred amylases include (a)
an
amylase according to WO 9402597, Novo, Feb. 3, 1994, as
further illustrated by a mutant in which substitution is made, using alanine
or threonine,
preferably threonine, of the methionine residue located in position 197 of the
B.
licheniformis alpha-amylase, known as TERMAMYL~, or the homologous position
variation of a similar parent amylase, such as B, amyloliquefaciens, B.
subtilis, or B.
stearothermophilus; (b) stability-enhanced amylases as described by Genencor
International in a paper entitled "Oxidatively Resistant alpha-Amylases"
presented at the
207th American Chemical Society National Meeting, March 13-17 1994, by C.
Mitchinson. Therein it was noted that bleaches in automatic dishwashing
detergents
inactivate alpha-amylases but that improved oxidative stability amylases have
been made
by Genencor from B. licheniformis NCIB8061. Methionine (Met) was identified as
the
most likely residue to be modified. Met was substituted, one at a time, in
positions 8, 15,
197, 256, 304, 366 and 438 leading to specific mutants, particularly important
being
M197L and M197T with the M197T variant being the most stable expressed
variant.
CA 02349537 2004-O1-15
68
Stability was measured in CASCADE~ and SUNLIGHT~; (c) particularly preferred
amylases herein include amylase variants having additional modification in the
immediate parent as described in WO 9510603 A and are available from the
assignee,
Novo, as DUR.AMYL~. Other particularly preferred oxidative stability enhanced
amylase include those described in WO 9418314 to Genencor International and WO
9402597 to Novo. Any other oxidative stability-enhanced amylase can be used,
for
example as derived by site-directed mutagenesis from known chimeric, hybrid or
simple
mutant parent forms of available amylases. Other preferred enzyme
modifications are
accessible. See WO 9509909 A to Novo.
Various carbohydrase enzymes which impart antimicrobial activity may also be
included in the present invention. Such enzymes include endoglycosidase, Type
II
endoglycosidase and glucosidase as disclosed in U.S. Patent Nos. 5,041,236,
5,395,541,
5,238,843 and 5,356,803.
Of course, other enzymes having antimicrobial activity may be employed as well
including peroxidases, oxidases and various other enzymes.
It is also possible to include an enzyme stabilization system into the
compositions
of the present invention when any enzyme is present in the composition.
Perfumes - Perfumes and perfumery ingredients useful in the present
compositions and processes comprise a wide variety of natural and synthetic
chemical
ingredients, including, but not limited to, aldehydes, ketones, esters, and
the like. Also
included are various natural extracts and essences which can comprise complex
mixtures
of ingredients, such as orange oil, lemon oil, rose extract, lavender, musk,
patchouli,
balsamic essence, sandalwood oil, pine oil, cedar, and the like. Finished
perfumes can
comprise extremely complex mixtures of such ingredients. Finished perfumes
typically
comprise from about 0.01 % to about 2%, by weight, of the detergent
compositions
herein, and individual perfumery ingredients can comprise from about 0.0001 %
to about
90% of a finished perfume composition.
Non-limiting examples of perfume ingredients useful herein include: 7-acetyl-
1,2,3,4,5,6,7,8-octahydro-1,1,6,7-tetramethyl naphthalene; ionone methyl;
ionone
gamma methyl; methyl cedrylone; methyl dihydrojasrnonate; methyl 1,6,10-
trimethyl-
2,5,9-cyclododecatrien-1-yl ketone; 7-acetyl-1,1,3,4,4,6-hexamethyl tetralin;
4-acetyl-6-
CA 02349537 2001-04-27
WO 00/28874 PCTNS99/27201
69
tent-butyl-1,1-dimethyl indane; para-hydroxy-phenyl-butanone; benzophenone;
methyl
beta-naphthyl ketone; 6-acetyl-1,1,2,3,3,5-hexamethyl indane; 5-acetyl-3-
isopropyl-
1,1,2,6-tetramethyl indane; 1-dodecanal, 4-(4-hydroxy-4-methylpentyl)-3-
cyclohexene-
1-carboxaldehyde; 7-hydroxy-3,7-dimethyl ocatanal; 10-undecen-1-al; iso-
hexenyl
cyclohexyl carboxaldehyde; formyl tricyclodecane; condensation products of
hydroxycitronellal and methyl anthranilate, condensation products of
hydroxycitronellal
and indol, condensation products of phenyl acetaldehyde and indol; 2-methyl-3-
(para-
tert-butylphenyl)-propionaldehyde; ethyl vanillin; heliotropin; hexyl cinnamic
aldehyde;
amyl cinnamic aldehyde; 2-methyl-2-{para-iso-propylphenyl)-propionaldehyde;
coumarin; decalactone gamma; cyclopentadecanolide; 16-hydroxy-9-hexadecenoic
acid
lactone; 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-gamma-2-benzo-
pyrane; beta-naphthol methyl ether; ambroxane; dodecahydro-3a,6,6,9a-
tetramethyl-
naphtho[2,1b)furan; cedrol, 5-(2,2,3-trimethylcyclopent-3-enyl)-3-methylpentan-
2-ol; 2-
ethyl-4-(2,2,3-trimethyl-3-cyclopenten-1-yl)-2-buten-1-ol; caryophyllene
alcohol;
tricyclodecenyl propionate; tricyclodecenyl acetate; benzyl salicylate; cedryl
acetate; and
para-(tert-butyl) cyclohexyl acetate.
Particularly preferred perfume materials are those that provide the largest
odor
improvements in finished product compositions containing cellulases. These
perfumes
include but are not limited to: hexyl cinnamic aldehyde; 2-methyl-3-(para-tert-
butylphenyl)-propionaldehyde; 7-acetyl-1,2,3,4,5,6,7,8-octahydro-1,1,6,7-
tetramethyl
naphthalene; benzyl salicylate; 7-acetyl-1,1,3,4,4,6-hexamethyl tetralin; para-
tent-butyl
cyclohexyl acetate; methyl dihydro jasmonate; beta-napthol methyl ether;
methyl beta-
naphthyl ketone; 2-methyl-2-(para-iso-propylphenyl)-propionaldehyde;
1,3,4,6,7,8-
hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta-gamma-2-benzopyrane; dodecahydro-
3a,6,6,9a-tetramethylnaphtho[2,1b)furan; anisaldehyde; coumarin; cedrol;
vanillin;
cyclopentadecanolide; tricyclodecenyl acetate; and tricyclodecenyl propionate.
Other perfume materials include essential oils, resinoids, and resins from a
variety of sources including, but not limited to: Peru balsam, Olibanum
resinoid, styrax,
Iabdanum resin, nutmeg, cassia oil, benzoin resin, coriander and lavandin.
Still other
perfume chemicals include phenyl ethyl alcohol, terpineol, Iinalool, linaIyl
acetate,
CA 02349537 2004-O1-15
geraniol, nerol, 2-(1,1-dimethylethyl)-cyclohexanol acetate, benzyl acetate,
and eugenol.
Carriers such as diethylphthalate can be used in the finished perfume
compositions.
Dispersant Polymers
The compositions used in the methods of the present invention may also
optionally contain from about 0.1% to about 20%, more preferably from about
0.5% to
about 10% by weight of the composition of a dispersant polymer. Dispersant
polymers
are compounds which act as soil suspending agents in the aqueous wash liquor.
That is,
they act to suspend the soils in solution and prevent the soils from re-
depositing on the
surfaces of fabrics or dishes. This allows soils to be removed with the wash
liquor.
Dispersant polymers are well-known and conventional and are available from
BASF
Corp. and Rohm & Haas. Typical examples include polyethoxylated amines and
acrylic
acid/maleic acid copolymers.
Soil Release A ents
The compositions according to the present invention may optionally comprise
one
or more soil release agents. Polymeric soil release agents are characterized
by having
both hydrophilic segments, to hydrophilize the surface of hydrophobic fibers,
such as
polyester and nylon, and hydrophobic segments, to deposit upon hydrophobic
fibers and
remain adhered thereto through completion of the laundry cycle and , thus,
serve as an
anchor for the hydrophilic segments. This can enable stains occuring
subsequent to
treatment with the soil release agent to be more easily cleaned in later
washing
procedures.
if utilized, soil release agents will generally comprise from about 0.01% to
about
10% preferably from about 0.1 % to about 5%, more preferably from about 0.2%
to about
3% by weight, of the composition.
The following describe soil release polymers
suitable for us in the present invention. U.S. 5,691,298 Gosselink et al.,
issued
November 25, 1997; U.S. 5,599,782 Pan et al., issued February 4, 1997; U.S.
5,415,807
Gosselink et al., issued May 16, 1995; U.S. 5,182,043 Mon:all et al., issued
January 26,
1993; U.S. 4,956,447 Gosselink et al., issued September 11, 1990; U:S.
4,976,879
Maldonado et al. issued December 11, 1990; U.S. 4,968,451 Scheibel et al.,
issued
November 6, 1990; U.S. 4,925,577 Borcher, Sr. et al., issued May 15, 1990;
U.S.
CA 02349537 2004-O1-15
71
4,861,512 Gosselink, issued August 29, 1989; U.S. 4,877,896 Maldonado et al.,
issued
October 31, 1989; U.S. 4,771,730 Gosselink et al., issued October 27, 1987;
U.S.
711,730 Gosselink et al., issued December 8, 1987; U.S. 4,721,580 Gosselink
issued
January 26, 1988; U.S. 4,000,093 Nicol et al., issued December 28, 1976; U.S.
3,959,230
Hayes, issued May 25, 1976; U.S. 3,893,929 Basadur, issued July 8, 1975; and
European
Patent Application 0 219 048, published April 22, 1987 by Kud et al.
Further suitable soil release agents are described in U.S. 4,201,824 Voilland
et
al.; U.S. 4,240,918 Lagasse et al.; U.S. 4,525,524 Tung et al.; U.S. 4,579,681
Ruppert et
al.; U.S. 4,220,918; U.S. 4,787,989; EP 279,134 A, 1988 to Rhone-Poulenc
Chemie; EP
457,205 A to BASF (1991); and DE 2,335,044 to Unilever N.V., 1974,
Bri; ht
Any optical brighteners or other brightening or whitening agents known in the
art
can be present at levels typically from about 0.05% to about 1.2%, by weight,
in the
compositions used herein. Commercial optical brighteners which may be useful
in the
present invention can be classified into subgroups, which include, but are not
necessarily
limited to, derivatives of stilbene, pyrazoline, coumarin, carboxylic acid,
methinecyanines, dibenzothiphene-5,5-dioxide, azoles, 5- and 6-membered-ring
heterocycles, and other miscellaneous agents. Examples of such brighteners are
disclosed in "The Production and Application of Fluorescent Brightening
Agents", M.
Zahradnik, Published by John Wiley & Sons, New York (1982).
Specific examples of optical brighteners which are useful in the present
compositions are those identified in U.S. Patent 4,790,856, issued to Wixon on
December 13, 1988. These brighteners include the PHORWHITE series of
brighteners
from Verona. Other brighteners disclosed in this reference include: Tinopal
UNPA,
Tinopal CBS and Tinopal SBM; available from Ciba-Geigy; Artic White CC and
Artic
White CWD, available from Hilton-Davis, located in Italy; the 2-(4-stryl-
phenyl)-2H-
napthol[1,2-d]triazoles; 4,4'-bis- (1,2,3-triazol-2-yl)-stil- benes; 4,4'-
bis(stryl)bisphenyls;
and the aminoeoumarins. Specific examples of these brighteners include 4-
methyl-7-
diethyl- amino coumarin; 1,2-bis(-venzimidazol-2-yl)ethylene; 1,3-diphenyl-
phrazolines;
2,5-bis(benzoxazol-2-yl)thiophene; 2-stryl-napth-[1,2-d]oxazole; and 2-
(stilbene-4-yl)-
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
72
2H-naphtho- [ 1,2-d]triazole. See also U.S. Patent 3,646,015, issued February
29, 1972 to
Hamilton. Anionic brighteners are preferred herein.
Other Ingredients - The compositions can further preferably comprise one or
more
detersive adjuncts selected from the following: polysaccharides, abrasives,
bactericides,
tarnish inhibitors, dyes, buffers, antifungal or mildew control agents, insect
repellents,
perfumes, thickeners, processing aids, anti-corrosive aids, stabilizers and
antioxidants. A
wide variety of other ingredients useful in detergent compositions can be
included in the
compositions herein, including other active ingredients, carriers,
antioxidants, processing
aids, dyes or pigments, solvents for liquid formulations, etc.
Usual ingredients can include one or more materials for assisting or enhancing
cleaning performance, treatment of the substrate to be cleaned, or to modify
the
aesthetics of the composition. Usual detersive adjuncts of detergent
compositions
include the ingredients set forth in U.S. Pat. No. 3,936,537, Baskerville et
al. Adjuncts
which can also be used in the compositions employed in the present invention,
in their
conventional art-established levels for use (generally from 0% to about 20% of
the
detergent ingredients, preferably from about 0.5% to about 10%), include other
active
ingredients such as enzyme stabilizers, color speckles, anti-tarnish and/or
anti-corrosion
agents, dyes, fillers, optical brighteners, germicides, alkalinity sources,
anti-oxidants,
enzyme stabilizing agents, perfumes, dyes, solubilizing agents, clay soil
removal/anti-
redeposition agents, carriers, processing aids, pigments, solvents for liquid
formulations,
fabric softeners, static control agents, etc. Dye transfer inhibiting agents,
including
polyamine N-oxides such as polyvinylpyridine N-oxide can be used. Dye-transfer-
inhibiting agents are further illustrated by polyvinylpyrrolidone and
copolymers of N-
vinyl imidazole and N-vinyl pyrrolidone. If desired, soluble magnesium salts
such as
MgCl2, MgS04, and the like, can be added at levels of, typically, 0.1 %-2%, to
enhance
grease removal performance.
Various detersive ingredients employed in the present compositions optionally
can be further stabilized by absorbing said ingredients onto a porous
hydrophobic
substrate, then coating said substrate with a hydrophobic coating. Preferably,
the
detersive ingredient is admixed with a surfactant before being absorbed into
the porous
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
73
substrate. In use, the detersive ingredient is released from the substrate
into the aqueous
washing liquor, where it performs its intended detersive function.
To illustrate this technique in more detail, a porous hydrophobic silica
(trademark
SIPERNAT D10, DeGussa) is admixed with a proteolytic enzyme solution
containing
3%-5% of C13-15 ethoxylated alcohol (E0 7) nonionic surfactant. Typically, the
enzyme/surfactant solution is 2.5 X the weight of silica. The resulting powder
is
dispersed with stirring in silicone oil (various silicone oil viscosities in
the range of 500-
12,500 can be used). The resulting silicone oil dispersion is emulsified or
otherwise
added to the final detergent matrix. By this means, ingredients such as the
aforementioned enzymes, bleaches, bleach activators, bleach catalysts,
photoactivators,
dyes, fluorescers, fabric conditioners and hydrolyzable surfactants can be
"protected" for
use in detergent compositions.
An antioxidant can be optionally added to the detergent compositions of the
present invention. They can be any conventional antioxidant used in detergent
compositions, such as 2,6-di-tert-butyl-4-methylphenol (BHT), carbamate,
ascorbate,
thiosulfate, monoethanolamine(MEA), diethanolamine, triethanolamine, etc. It
is
preferred that the antioxidant, when present, be present in the composition
from about
0.001 % to about 25/0, preferably from about 0.01 % to about 10%, more
preferably from
about 0.05% to about 5%, by weight.
The compositions of this invention can be in any form, including liquid,
tablet,
paste, gel, microemulsion or tricritical composition. Highly preferred
embodiments are
in liquid or gel form. Liquid detergent compositions can contain water and
other
solvents as carriers. Low molecular weight primary or secondary alcohols
exemplified
by methanol, ethanol, propanol, and isopropanol are suitable. Monohydric
alcohols are
preferred for solubilizing surfactant, but polyols such as those containing
from 2 to about
6 carbon atoms and from 2 to about 6 hydroxy groups (e.g., 1,3-propanediol,
ethylene
glycol, glycerine, and 1,2-propanediol) can also be used. The compositions may
contain
from S% to 90%, typically 10% to 50% of such carriers.
An example of the procedure for making liquid compositions herein is as
follows:
- To the free water and citrate are added and dissolved. To this solution
amine oxide,
betaine, ethanol, hydrotrope and nonionic surfactant are added. If free water
isn't
CA 02349537 2001-04-27
WO 00/28874 PCT/US99/27201
74
available, the citrate are added to the above mix then stirred until
dissolved. At this
point, an acid is added to neutralize the formulation. It is preferred that
the acid be
chosen from organic acids such as malefic and citric, however, inorganic
mineral acids
may be employed as well. In preferred embodiments these acids are added to the
formulation followed by diamine addition. AExS is added last.
Compositions of the invention will have a pH range of from about 2 to about
13,
preferably, pH is alkaline, more preferably from about 7 to about 12.5, more
preferably
from about 8 to about 12, even more preferably from about 9 to about 11.5.