Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-1-
METAL O~IDE PARTICLES
FIELD OF THE INVENTION
The invention relates to metal oxide powders.
More particularly, the invention relates to nanoscale
metal oxide particles, such as manganese oxide particles
and lithium manganese oxide particles, produced by laser
pyrolysis. The invention further relates to methods for
producing metal oxide powders with laser pyrolysis and
aerosol precursors. Furthermore, the invention relates
to methods of producing ternary particles, especially
crystalline nanoparticles, by laser pyrolysis.
BACKGROUND OF THE INVENTION
Advances in a variety of fields have created
a demand for many types of new materials. In
particular, a variety of chemical powders can be used in
many different processing contexts, such as the
production of batteries. Specifically, there is
considerable interest in the application of ultrafine or
nanoscale powders that are particularly advantageous for
a variety of applications involving small structures or
high surface area materials. This demand for ultrafine
chemical powders has resulted in the development of
sophisticated techniques, such as laser pyrolysis, for
the production of these powders.
The microminiaturization of electronic
components has created widespread growth in the use of
portable electronic devices such as cellular phones,
pagers, video cameras, facsimile machines, portable
stereophonic equipment, personal organizers and personal
computers. The growing use of portable electronic
equipment has created ever increasing demand for
improved power sources for these devices. Relevant
batteries include primary batteries, i.e., batteries
CA 02350201 2001-05-08
aJVO 00/Z7754 PCT/US99/26343
-2-
designed for use through a single charging cycle, and
secondary batteries, i.e., batteries designed to be
rechargeable. Some batteries designed essentially as
primary batteries may be rechargeable to some extent.
Batteries based on lithium have been the
subj ect of considerable development effort. and are being
sold commercially. Lithium based batteries generally
use electrolytes containing lithium ions. The negative
electrodes for these batteries can include lithium metal
or alloy (lithium batteries), or compositions that
intercalate lithium (lithium ion batteries). Preferred
electroactive materials for incorporation into the
positive electrodes are compositions that intercalate
lithium. The compositions that intercalate lithium, for
use in the positive electrodes, generally are
chalcogenides such as metal oxides that can incorporate
the lithium ions into their lattice.
Manganese can exist in various oxidation
states. Correspondingly, manganese oxides are known to
exist with various stoichiometries. In addition,
manganese oxides with a particular stoichiometry can
have various crystalline lattices, or they can be
amorphous. Thus, manganese oxides exhibit an
extraordinarily rich phase diagram.
Manganese oxides and lithium manganese oxides
with various stoichiometries have been noted as
promising materials for use in positive electrodes for _
lithium based batteries. In particular, appropriate
manganese oxides can intercalate lithium ions into their
crystal structure to form lithium manganese oxides.
Lithium manganese oxides are useful for the production
of lithium based secondary batteries. Because of the
interest in lithium manganese oxides, several approaches
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-3-
have been developed for producing lithium manganese
oxide powders.
SUMMARY OF THE INVENTION
In a first aspect, the invention pertains to
a collection of particles comprising manganese oxide,
the collection of particles having an average diameter
less than about 500 nm, the manganese oxide having a
structure selected from the group consisting of
amorphous manganese oxide, crystalline MnO, crystalline
Mn508 and crystalline Mn203.
In another aspect, the invention pertains to
a method of producing a metal oxide powder, the method
comprising reacting an aerosol within a reaction chamber
to form metal oxide particles, the aerosol comprising a
metal precursor and the metal oxide particles having an
average diameter less than about 500 nm.
In a further aspect, the invention pertains to
a method for altering the stoichiometry of a collection
of manganese oxide particles, the method comprising
heating manganese oxide particles in an oxidizing
environment at a temperature less than about 600°C.
In another aspect, the invention pertains to
a battery having a cathode comprising manganese oxide
particles, said manganese oxide particles having an
average diameter less than about 250 nm.
- In addition, the invention pertains to a
method of producing a composite metal oxide particles,
the method comprising reacting an aerosol to firm a
powder of composite metal oxide particles with an
average diameter less than about one micron, the aerosol
comprising a first metal compound precursor and a second
metal compound precursor. -
In a further aspect, the invention pertains to
a method for producing lithium metal oxide, the method
- CA 02350201 2001-05-08
CVO 00/2~7754 PCT/US99/26343
-4-
comprising pyrolyzing -ar reactant stream in a reaction
chamber, the reactant stream comprising a lithium
precursor, a non-lithium metal precursor, an oxidizing
agent, and an infrared absorber, where the pyrolysis is
driven by heat absorbed from a light beam.
In another aspect, the invention pertains to
a collection of particles comprising lithium manganese
oxide, the collection of particles having an average
diameter less than about 250 nm, wherein the collection
of particles .have a distribution of particle sizes in
which at least about 95 percent of the particles have a
diameter greater than about 40 percent of the average
diameter and less than about 160 percent of the average
diameter.
Furthermore, the invention pertains to a
method of making lithium manganese oxide particles
comprising heating a mixture of manganese monoxide (Mn0)
particles and a lithium compound, the manganese monoxide
particles having an average diameter less than about 250
nm.
Moreover, the invention pertains to a method
of making lithium manganese oxide particles comprising
heating a mixture of particles of a manganese oxide and
a lithium compound, the particles of manganese oxide_
having an average diameter less than about 250 nm, _.
wherein the resulting lithium manganese oxide particles
have a distribution of particle sizes in which at least
about 95 percent of the particles have a diameter
greater than about 40 percent of the average diameter
and less than about 160 percent of the average diameter.
- In a further aspect, the invention features a
battery comprising lithium manganese oxide particles
having an average_diameter less than about 250 nm,
- wherein the lithium manganese oxide particles have a
CA 02350201 2001-05-08
PCT/US99/26343
W O 00/27754
-5-
distribution of particle sizes in which at least about
95 percent of the particles have a diameter greater than
about 40 percent of the average diameter and less than
-about 160 percent of the average diameter.
In another aspect, the invention features a
battery comprising lithium manganese oxide, the battery
having a four volt profile with a cycling stability
within about 20 percent of initial values after 25
cycles.
In addition, the invention features a battery
comprising lithium manganese oxide, the battery having
an initial capacity greater than 120 mAh\g.
Moreover, the invention pertains to a
collection of particles comprising lithium manganese
oxide, the collection of particles having an average
diameter less than about 250 nm, the lithium manganese
oxide comprising Li2Mn409.
In a further aspect, the invention pertains to
a collection of particles comprising lithium manganese
oxide, the collection of particles having an average
diameter less than about 250 nm, the lithium manganese
oxide having a lattice parameter along axis a of no more
than 8.23 angstroms.
Furthermore, the invention pertains to a
method of producing crystalline ternary particles
comprising reacting a reactant stream comprising
precursors including the three atoms of the product
ternary particles, wherein the relative amounts of the
three atoms in the reactant stream and the reaction
conditions are selected to yield the crystalline ternary
particles.
In another aspect, the invention pertains to
a method of producing crystalline lithium manganese
CA 02350201 2001-05-08
~WO 00%27754 PCT/US99/26343
-6-
oxide particles comprising reacting a reactant stream
comprising a manganese precursor and a lithium
precursor, wherein the reaction is driven by energy from
electromagnetic radiation.
In a further aspect, the invention pertains to
a collection of particles comprising a crystalline
multiple metal oxide having an average particle diameter
less than about 500 nm, wherein the lithium manganese
oxide particles have a distribution of particle sizes in
which at least about 95 percent of the particles have a
diameter greater than about 40 percent of the average
diameter and less than about 160 percent of the average
diameter.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a schematic sectional view of a
solid precursor delivery system taken through the center
of the system.
Fig. 2 is a schematic, sectional view of an
embodiment of a laser pyrolysis apparatus, where the
cross section is taken through the middle of the laser
radiation path. The upper insert is a bottom view of
the collection nozzle, and the lower insert is a top
view of the injection nozzle.
Fig. 3 is a schematic, side view of a reactant
delivery apparatus for the delivery of vapor reactants
to the laser pyrolysis apparatus of Fig. 2.
Fig. 4A is a schematic, side view of a
- reactant delivery apparatus far the delivery of an
aerosol reactant to the laser pyrolysis apparatus of
Fig. 2.
Fig. 4B is-a schematic, side view of an
alternative embodiment of a-reactant delivery apparatus
for the delivery of an aerosol reactant to the laser
pyrolysis apparatus of Fig. 2.
CA 02350201 2001-05-08
' PCT/U 599/26343
WO 00/27754
_7_
Fig. 4C is a-schematic, side view of another
alternative embodiment of a reactant delivery apparatus
for the delivery of an aerosol reactant to the laser
pyrolysis apparatus of Fig. 2.
Fig. 5 is a schematic, perspective view of an
elongated reaction chamber for the performance of laser -
pyrolysis, where components of the reaction chamber are
shown as transparent to reveal internal structure.
Fig. 6 is a perspective view of an embodiment
of an elongated reaction chamber for performing laser
pyrolysis.
Fig. 7 is a cut away, siae view v~
reaction chamber of ~'ig . 6 . --
Fig: 8 is a partially sectional, front view of
the reaction chamber of Fig. 6, taken along line 8-8 of
- Fig. 6.
Fig. 9 is a sectional, front view of a
reactant delivery apparatus for the delivery of an
aerosol reactant into the reaction chamber of Fig. 6,
where the cross section is taken through the center of
the reactant delivery apparatus.
Fig. 10 is a fragmentary, sectional front view
of the top portion of the reactant delivery apparatus of
Fig. 9.
Fig. 11 is a top view of the mount o~ zne
reactant delivery apparatus of Fig. 9.
Fig. 12 is a top view of a cap of the aerosol
delivery apparatus of Fig. 9.
Fig . 13 is a sectional view of the cap of Fig .
12 taken along line 13-13.
Fig. 14 is a sectional side view of a spacer
used in the aerosol delivery apparatus of Fig. 9, where
the cross section is taken through the center of the
spacer. -
CA 02350201 2001-05-08
TWO 00%27754 PCT/US99/26343
-8-
Fig. 15 is a~ sectional side view of a shim
used in the aerosol delivery apparatus of Fig. 9, where
the cross section is taken through the center of the
shim.
Fig. 16 is a sectional, side view of an
embodiment of a brushing cap for use in the aerosol
delivery apparatus of Fig. 9, where the cross section is
taken through the center of the brushing cap.
Fig. 17 is a sectional, side view of an
alternative embodiment of a brushing cap for use in the
aerosol delivery apparatus of Fig. 9, where the cross
section is taken through the center of the brushing cap.
Fig. l8 is a sectional, side view of a second
alternative embodiment of a brushing cap for use in the
- aerosol delivery apparatus of Fig. 9, where the cross
section is taken through the center of the brushing cap.
Fig. 19 is a side view of an ultrasonic
aerosol generator having an atomization surface.
Fig. 20 is a sectional, side view of the
ultrasonic aerosol generator of Fig. 19, where the cross
section is taken through the center of the apparatus.
Fig. 21 is a schematic, side view of a liquid
supply system for supplying liquid to the aerosol
generator of Figs. 19 and 20.
Fig. 22A is a schematic, sectional view of an
apparatus for heat treating nanoparticles, in which the
section is taken through_the center of the apparatus.
Fig. 22B is a schematic, sectional view of an
oven for heating nanoparticles, in which the section is
taken through the center of a quartz tube.
Fig. 23 is a schematic, perspective view of a
battery of the invention.
Fig. 24 is an x-ray diffractogram of manganese
-- oxide nanoparticles produced by laser pyrolysis with
CA 02350201 2001-05-08
' PCT/US99/26343
W O 00/27754
-9-
- gaseous reactants according to the parameters specified
in column 1 of Table 1.
Fig. 25 is an x-ray diffractogram of manganese
oxide nanoparticles produced by laser pyrolysis with
gaseous reactants according to the parameters specified
in column 2 of Table 1.
Fig. 26 is an x-ray diffractogram of manganese
oxide nanoparticles produced by laser pyrolysis with
gaseous reactants according to the parameters specified
in column 3 of Table 1.
Fig. 27 is a transmission electron micrograph
of manganese oxide nanoparticles produced by laser
pyrolysis with gaseous reactants according to the
parameters specified in column 2 of Table 1.
Fig. 28 is a plot of particle diameter
_ distribution for the particles shown in the transmission
electron micrograph shown in Fig. 27.
Fig. 29 is an x-ray diffractogram of manganese
oxide nanoparticles produced by laser pyrolysis with an
- aerosol manganese precursor according to the parameters
specified in Table 2.
Fig. 30 is a transmission electron micrograph
of manganese oxide nanoparticles produced by laser
pyrolysis with an aerosol manganese precursor according
to the parameters specified in Table 2.
Fig. 31 is a plot of particle size
distribution for the particles shown in the transmission
electron micrograph of Fig. 30.
Fig. 32 is an x-ray diffractogram of manganese
oxide nanoparticles following a heat treatment of w
particles produced by laser pyrolysis, sample 1 of Table
3.
Fig. 33 is an x-ray diffractogram of manganese
oxide nanoparticles following a -Beat. treatment of
CA 02350201 2001-05-08
WO OO/Z7754 PCT/US99/26343
-10-
particles produced by laser pyrolysis, sample 2A of
Table 3.
Fig. 34 is an x-ray diffractogram of manganese
oxide nanoparticles following a heat .treatment of
particles produced by laser pyrolysis, sample 2B of
Table 3.
Fig. 35 is an x-ray diffractogram of manganese
oxide nanoparticles produced by laser pyrolysis using
with aerosol reactants according to the parameters
specified in column 1 of Table 4.
Fig. 36 is an x-ray diffractogram of manganese
oxide nanoparticles produced by laser pyrolysis using
with aerosol reactants according to the parameters
specified in column 2 of Table 4.
Fig. 37 is an x-ray diffractograrn of
nanoparticles of lithium manganese oxide produced by
laser pyrolysis of a reactant stream with an aerosol.
- Fig. 38 is an x-ray diffractogram of
nanoparticles of lithium manganese oxide made by laser
pyrolysis following heating in an oven.
Fig. 39 is a plot of three x-ray
diffractograms for three samples of lithium manganese
oxide produced by heat treating mixtures of
nanocrystalline manganese oxide and lithium nitrate.
Fig. 40 is a transmission electron micrograph
of manganese oxide nanoparticles used for further
heating processing into lithium manganese oxide.
Fig. 41 is a transmission electron micrograph
of lithium manganese oxide nanoparticles from sample 1.
Fig. 42 is an x-ray diffractogram for a sample
of lithium manganese oxide particles directly produced
by laser pyrolysis.
CA 02350201 2001-05-08
PCT/U S99/26343
WO 00/27754
-11-
_.
Fig. 43 is 3'transmission electron micrograph
of lithium manganese oxide particles corresponding to
the x-ray diffractogram of Fig. 42.
Fig. 44 is a plot of two x-ray diffractograms
of mixed phase materials including silver vanadium oxide
nanoparticles produced directly by laser pyrolysis,
where each plot is produced with materials produced
under slightly different conditions.
Fig . 45A is a transmission electron micrograph
of the materials from the sample corresponding to the
upper diffractogram in Fig. 44.
Fig. 45B is a transmission electron micrograph
of the materials from the sample corresponding to the
lower diffractogram in Fig. 44.
Fig. 46 is = a plot of particle size
distribution for the particles shown in the transmission
electron micrograph of Fig. 45.
Fig. 47 is a schematic, perspective view of
the two electrode arrangement used in the examples.
Fig. 48 is a plot of cell voltage in a range
of four volts for four different positive electrode
active materials.
Fig. 49 is_a plot of cell voltage in a range
of three volts for four different positive electrode
active materials.
Fig. 50 is a plot of capacity as a function of
cycle number for eight different cells produced with _
four different positive electrode active materials.
Fig. 51 is a is a schematic, perspective view
of the three electrode arrangement used for the
following tests.
Fig. 52 is a plot of voltage as a function of
specific capacity for two nanoscale samples and a
commercial lithium manganese oxide.
___-.-_- .- - CA 02350201 2001-05-08
r v
WO 00/27754 PCT/US99/26343
-12-
Fig. 53 is a plot of differential capacity for
the samples used in Fig. 52.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
_ 5 Several approaches are described for the
production of metal oxide nanoparticles. These
approaches provide for the production of metal oxide
particles, such as manganese oxide nanoparticles, with
a wide range of properties. Aerosol based approaches
are described that can make use of low cost precursors
to produce nanoparticles with a high production rate.
Preferred collections of metal oxide particles have an
average diameter less than a micron and a very narrow
distribution of particle diameters. Laser pyrolysis
with or without additional processing is a versatile
approach for the production of a wide range of manganese
oxide materials. The aerosol based approaches described
herein can be used in the production of many other metal
oxide nanoparticles.
_ ' In particular, several alternative approaches
for the formation of nanoscale, crystalline ternary
particles have been discovered. Crystalline, ternary
particles has three types of atoms located at particular
lattice sites within a crystal structure. Ternary
particles of lithium metal oxide, such as lithium
manganese oxide, are of particular interest because of
their usefulness in battery applications.
In a first approach, it has been discovered
that nanoscale manganese oxides provide a suitable
starting material for the formation of nanoscale~lithium
manganese oxides. In particular, lithium manganese
oxides with an average diameter less than a micron can
be formed with a spinel crystal structure by thermal
processing approaches with nanoscale-manganese oxide
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-13-
starting materials. Tie use of a nanoscale starting
material allows for the use of very mild temperatures in
the processing. The resulting nanoscale lithium
manganese oxide spinets provide an excellent material
for the formation of lithium based batteries.
Alternatively, lithium manganese oxide
nanoparticles can be formed by laser pyrolysis.
Amorphous lithium manganese oxide particles produced by
laser pyrolysis can be heated under mild conditions to
anneal the particles producing a spinet crystal
structure. Furthermore, it has been discovered that
crystalline lithium manganese oxide nanoparticles can be
produced directly by laser pyrolysis. The lithium
manganese oxide powders produced by laser pyrolysis can
be subjected to a heat treatment to alter and/or improve
the properties of the particles. Thus, alternative
approaches have been found useful to produce lithium
manganese oxide nanoparticles.
More specifically, in a first approach the
lithium manganese oxide particles are formed by heating
a mixture of nanoscale manganese oxide particles and a
lithium compound. During the heating step the lithium
is incorporated into the manganese oxide lattice. The
manganese oxide particles for thermal lithium.
incorporation can have a variety of stoichiometries
including, surprisingly, MnO. The heating can be
performed either under an oxidizing atmosphere or under
an inert atmosphere. Due to the nanoscale character of
the manganese oxide starting material, the heating can
be performed under surprisingly mild conditions. Under
these mild reaction conditions, lithium manganese oxide
particles are formed that have an average diameter less
than a micron.
CA 02350201 2001-05-08
CVO OOIM754 PCT/US99/26343
-14-
A preferred_approach for the formation of
suitable nanoscale manganese oxide particles for
lithiation involves laser pyrolysis. In particular,
laser pyrolysis is an excellent process for efficiently
producing manganese oxide particles and other metal
oxide particles with a narrow distribution of average
particle diameters.
A basic feature of successful application of
laser pyrolysis for the production of metal oxide
nanoparticles is the generation of a reactant stream
containing a metal precursor compound, a radiation
absorber and a secondary reactant. The secondary
reactant can be an oxygen source. The reactant stream
is pyrolyzed by an intense laser beam. As the reactant
stream leaves the laser beam, the particles are rapidly
quenched.
To perform laser pyrolysis, reactants can be
supplied in vapor form. Alternatively, one or more
reactants can be supplied as an aerosol. The use of an
aerosol provides for the use of a wider range of metal
precursors for laser pyrolysis than are suitable for
vapor delivery only. Thus, less expensive precursors
can be used with aerosol delivery. Suitable control of
the reaction conditions with the aerosol results in
nanoscale particles with a narrow particle size
distribution. The heat-processing of manganese oxide
nanoparticles to form lithium manganese oxide
nanocrystals and batterys formed from these
nanoparticles are described below.
As an alternative to producing lithium
manganese oxide nanoparticles by the thermal processing
of manganese oxide particles; lithium manganese oxide
particles having diameters substantially less than a
micron have been produced directly by laser pyrolysis.
CA 02350201 2001-05-08
PCT/US99/26343
WO 00/27754
-15-
For the direct production of lithium/manganese composite
materials, laser pyrolysis preferably involves an
aerosol based reactant delivery apparatus. Heat
processing of the composite particles results in
crystalline lithium manganese oxide particles with a
spinel crystal structure. The formation of nanoscale,
amorphous lithium manganese oxide directly by laser
pyrolysis is described further below.
Laser pyrolysis experiments described below in
the examples result in the production of amorphous
lithium manganese oxide nanoparticles. In other
experiments, the parameters of the laser pyrolysis
synthesis have been adjusted to yield crystalline
lithium manganese oxide nanoparticles directly by laser
pyrolysis. These are also described below in the
examples. The phase diagram of the materials to be
produce can guide the selection of appropriate laser
pyrolysis conditions. In addition, the.parameters can
be adjusted empirically to arrive at suitable conditions
for the production of desired crystalline ternary
particles. In particular, for the production of spinel
LiMn204, production is favored by high chamber
pressures, high flow rates of oxygen, low laser
intensity for water based aerosols, and relatively
higher laser intensity for aerosols based on water and
- isopropyl alcohol.
The production of a substituted metal oxide
Til_XVXOZ by laser pyrolysis has been described by -Musci
et al., "Laser synthesis of vanadium-titanium oxide
catalysts," J. Mater. Res. Vol. 7(10): 2846-2852
(October 1992), incorporated herein by reference. In
these substituted metal oxides, vanadium substitutes at
a lattice site for a titanium atom. The value of x
could only be increased up to about 0.25 before a
- CA 02350201 2001-05-08
WO 00/7754 PCT/US99/26343
-16-
separate vanadium oxide phase formed. These substituted
metal oxides are not crystalline ternary -compounds since
the metal atoms are not at unique lattice sites. In
crystalline ternary compounds, each atom is located at
a particular lattice site to yield a particular crystal -
structure. Similar substitution compounds'with titanium
substituting for chromium in Cr203 were described by
Schramm et al., U.S. Patent 5,013,706, "Metal Oxide
Powders Or Their Mixtures And Their Use 3n Catalytic
Dehydrogenation Of Hydrocarbons," incorporated herein by
reference.
As noted above, various forms of manganese
oxides and lithium manganese oxides can reversibly
intercalate lithium atoms and/or ions. The manganese
oxide and/or lithium manganese oxide nanoparticles can
be incorporated into a positive electrode film with a
binder such as a polymer. The film preferably includes
additional electrically conductive particles held by the
binder along with the lithium manganese oxide particles .
The positive electrode film can be used in a lithium
battery or a lithium ion battery. The electrolyte for
lithium and lithium ion batteries comprises lithium
ions.
A. Particle Production Using' Laser Pyrolysis _
Laser pyrolysis has been discovered to be a
valuable tool for the production of nanoscale metal
oxide particles, in particular manganese oxide particles
for further processing into lithium manganese oxide or
for the direct production of lithium manganese oxide
particles. In addition, the particles produced by laser
pyrolysis are a convenient material for further
processing to expand the pathways for the production of
desirable metal oxide particles. Thus, using laser
pyrolysis alone or in combination with additional
CA 02350201 2001-05-08
WO 00/2775:1 PCT/US99/26343
-17-
processes, a wide variety of metal oxide particles can
be produced.
Manganese oxide nanoparticles produced by
laser pyrolysis are a preferred starting material for
the lithium incorporation process described herein
involving the mild heating of manganese oxide
nanoparticles with a lithium compound. In addition,
lithium manganese oxide nanoparticles can be produced
directly by laser pyrolysis, where heating can be used
to alter and/or improve the characteristics of the
resulting particles. The lithium manganese oxide
-- nanoparticles formed by laser pyrolysis can be either
amorphous or crystalline.
The reaction conditions determine the
qualities of the particles produced by laser pyrolysis.
The reaction conditions for laser pyrolysis can be
controlled relatively precisely in order to produce
particles with desired properties. The appropriate
reaction conditions to produce a certain type of
particles generally depend on the design of the
particular apparatus. Specific conditions used to
produce manganese oxide particles in two particular
apparatuses and lithium manganese oxide in the first
particular apparatus with two different reactant
delivery systems-are described below in the Examples.
Furthermore, some general observations on the
relationship between reaction conditions and the
resulting particles can be made.
Increasing the laser power results in
increased reaction temperatures in the reaction region
as well as a faster quenching rate. A rapid quenching
rate tends to favor production of high energy phases,
which may not be obtained with processes near thermal
equilibrium. Similarly, increasing the chamber pressure
.... ~_.__...___ -_~=-~:~.~ ~,."...~-~_-----------__.w_.._._._..~--_.-,~....~-
.....
'CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-18-
also tends to favor. the production of higher energy
structures. Also, increasing the concentration of the
reactant serving as the oxygen source in the reactant
stream favors the production of particles with increased
amounts of oxygen.
Reactant flow rate and velocity of the
reactant gas stream are inversely related to particle
size so that increasing the reactant gas flow rate or
velocity tends to result in smaller particle sizes.
Also, the growth dynamics of the particles have a
significant influence on the size of the resulting
particles. In other words, different forms of a product
compound have a tendency to form different size
particles from other phases under relatively similar
conditions. Laser power also influences particle size
with increased laser power favoring larger particle
formation for lower melting materials and smaller
particle formation for higher melting materials.
Laser pyrolysis has been performed generally
with gas phase reactants. The use of exclusively gas
phase reactants is somewhat limiting with respect to the
types of precursor compounds that can be used. Thus,
techniques have been developed to introduce aerosols
containing reactant precursors into laser pyrolysis
chambers. The aerosol atomizers can be broadly
- classified as ultrasonic atomizers, which use an
ultrasonic transducer to form the aerosol, or as
mechanical atomizers, which use energy from one or-more
flowing fluids (liquids, gases, or supercritical fluids)
themselves to form the aerosol. Improved aerosol
delivery apparatuses for reactant systems are described
further in commonly assigned and copending U.S. Patent
Application Serial Number 09/188,670 to Gardner et al.,
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-19-
entitled "Reactant Delivery Apparatuses," filed November
9, 1998, incorporated herein by reference.
Using aerosol delivery apparatuses, solid
precursor compounds can be delivered by dissolving the
compounds in a solvent. Alternatively, powdered
precursor compounds can be dispersed in a liquid\solvent
for aerosol delivery. Liquid precursor compounds can be
delivered as an aerosol from a neat liquid, a multiple
liquid dispersion or a liquid solution, if desired.
Aerosol reactants can be used-to obtain a significant
reactant throughput. The solvent, if any, can be
selected to achieve desired properties of the solution.
Suitable solvents include water, methanol, ethanol,
isopropyl alcohol, other organic solvents and mixtures
-thereof. The solvent should have a desired Level of
purity such that the resulting particles have a desired
purity level. For the production of composite metal
oxide particles by laser pyrolysis, a plurality of metal
compounds can be included in the solution.
Alternatively or additionally, metal precursors can be
delivered into the reaction chamber in the vapor state
in addition to the metal precursors delivered as an
aerosol.
If aerosol precursors are formed with a
solvent present, the solvent is rapidly evaporated by
the laser beam in the reaction chamber such that a gas
phase reaction can take place. Thus, the fundamental
features of the laser pyrolysis reaction is unchanged.
However, the reaction conditions are affected by the
presence of the aerosol. Below, examples are described
for the production of manganese oxide nanoparticles
using gaseous reaction precursors and aerosol precursors
using two different laser pyrolysis reaction chambers.
The production of lithium manganese oxide nanoparticles.
CA 02350201 2001-05-08
1JV0 00/27754 PCT/US99/26343
-20-
by laser pyrolysis in-one of these reaction chambers
using aerosol precursors is also described in the -
Examples. Thus, the parameters associated with aerosol
reactant delivery can be explored based on the
description below.
A number of suitable solid, manganese
precursor compounds can be delivered as an aerosol from
solution. For example, manganese chloride (MnCl2) and
hydrated manganese chloride (MnCl2~ H20) are soluble in
water and alcohols, and manganese nitrate (Mn(N03)Z) is
_- soluble in water and certain organic solvents. Also,
suitable lithium precursors for aerosol delivery from
solution include, for example, lithium chloride (LiCl),
which is somewhat soluble in water, alcohol and some
other organic solvents, and lithium nitrate (LiN03);
which is somewhat soluble in water and alcohol. In
addition, suitable vanadium precursors for aerosol
delivery from solution include, for example, VOC12,
which is soluble in absolute alcohol.
The compounds are dissolved in a solution
preferably with a concentration greater than about 0.5
molar. Generally, the greater the concentration of
precursor in the solution the greater the throughput of
reactant through the reaction chamber. As the
concentration increases, however, the solution can
become more viscous such that the aerosol has droplets
with larger sizes than desired. Thus, selection of
solution concentration can involve a balance of factors
in the selection of a preferred solution concentration.
For the formation of composite metal
particles, the relative amounts of metal precursors in
the solution effects the relative amounts of metals in
the resulting particles. Thus, the desired composition
of the product particles influences the selection of the
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-21-
relative amounts of metal precursors fo= delivery.
While a desired stoichiomeEry may influence the relative
amounts of metals delivered into the reaction chamber,
the relative amounts of metal precursors may alter the
portion of the phase diagram sampled such that a
different material or mixed phase material can be -
produced. Since mixed phase materials may be produced,
the relative amounts of metals in the reactant stream
does not directly translate into particles with a
corresponding stoichiometry.
As noted above, the reaction conditions
determine the type and characteristics of the particles
produced by laser pyrolysis. - Of course, with the
production of -composite metal oxide particles, the
situation is even more complicated because of the added
- complexity of the corresponding phase diagram. There is
an additional parameter, namely, the quantity of
additional metal precursor that effects the resulting
properties of the particle (s) . One can be guided by
known stoichiometries of stable crystalline forms in the
selection of the relative amounts of metal precursors,
although phase diagrams may not be known completely, and
the non-equilibrium conditions in the laser pyrolysis
apparatus may lead to additional uncertainty.
In the production of lithium manganese oxide
that the composition of the metal precursors influences
the crystallinity of the resultant nanoparticles. In
particular, metal chloride precursors favor the
production of amorphous particles while metal nitrates
favor the production of crystalline particles . Based on
kinetic principles, higher quench rates favor amorphous
particle formation while slower quench rates favor
crystalline particle formation. Faster auenches are
CA 02350201 2001-05-08
WO 00/7754 PCT/US99/26343
-22-
accomplished by a fasted reactant stream velocity
through the reaction zone.
Appropriate manganese precursor compounds for
gaseous delivery generally include manganese compounds
with reasonable vapor pressures, i.e., vapor pressures
sufficient to get desired amounts of precursor vapor in
the reactant stream. The vessel holding liquid or solid
precursor compounds can be heated to increase the vapor
pressure of the manganese precursor, if desired.
Suitable solid, manganese precursors with sufficient
vapor pressure of gaseous delivery include, for example,
manganese carbonyl (Mn2 (CO) lo) . A suitable container for
heating and delivering a solid precursor to a laser
pyrolysis apparatus is shown in Fig. 1.
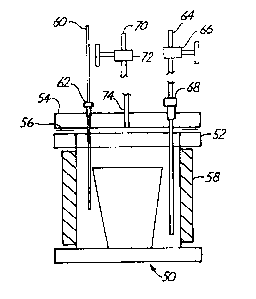
- Referring to Fig. 1, the solid precursor
delivery system 50 for vapor delivery includes a
container 52 and a lid 54. A gasket 56 is located
between container 52 and lid 54. In one preferred
embodiment, container 52 and lid 54 are made from
stainless steel, and gasket 56 is made from copper. In
this embodiment, lid 54 and gasket 56 are bolted to
container 52. Other inert materials, such as Pyrex°,
suitable for the temperatures and pressures applied to
the solid precursor system can be used. Container 52 is
surrounded with a band heater 58, which is used to set
the temperature of the delivery system 50 at desired
values. Suitable band heaters are available from Omega
Engineering Inc. Stamford, Conn. The temperature of the
band heater can be adjusted to yield a desired vapor
pressure of the precursor compound. Additional portions
of the precursor delivery system can be heated to
maintain the precursor in a vapor state after it has
left container 52.
CA 02350201 2001-05-08
WO 00/27754 PCT/US99I26343
-23-
Preferably, a- thermocouple 60 is inserted into
container 52 through lid 54. Thermocouple 60 can be
inserted by way of a Swagelok~ fitting 62 or other
suitable connection. Tubing 64 provides a input flow of
a carrier gas into container 52. Tubing 64 preferably
includes a shut off valve 66 and can be inserted through
lid 54 by way of a Swagelok~ fitting 68 or other
suitable connection. Output tube 70 also preferably
includes a shut off valve 72. Output tube 70 preferably
enters into container 52 through lid 54 at a sealed
connection 74. Tubes 64 and 70 can be made of any
suitable inert material such as stainless steel. A
solid precursor can be placed directly within container
52 or it can be placed within a smaller, open container
within container 52.
Preferred secondary reactants serving as
oxygen source include, for example, O2, CO, CO2, 03 and
mixtures thereof. The secondary reactant compound
should not react significantly with the manganese
precursor and/or lithium precursor prior to entering the
reaction zone since this generally would result in the
formation of large particles.
Laser pyrolysis can be performed with a
variety of optical frequencies. Preferred light sources
operate in the infrared portion of the electromagnetic
spectrum. COz lasers are-particularly preferred sources
of light. Infrared absorbers for inclusion in the
molecular stream include, for example, CZH4, NH3, SF6,
SiH4 and 03. 03 can act as both an infrared absorber and
as an oxygen source. The radiation absorber, such as
the infrared absorber, absorbs energy from the radiation
beam and distributes the energy to the other reactants
to drive the pyrolysis.
CA 02350201 2001-05-08
WO 00/7754 PCT/US99/26343
-24-
Preferably, the energy absorbed from the light
beam increases the temperature at a tremendous rate,
many times the rate that heat generally would be
produced even by strongly exothermic reactions under
- 5 controlled condition. While the process generally
involves nonequilibrium conditions, the temperature can
be described approximately based on the energy in the
absorbing region. The laser pyrolysis process is
qualitatively different from the process in a combustion
reactor where an energy source initiates a reaction, but
the reaction is driven by energy given off by an
exothermic reaction. Laser pyrolysis requires
continuous input of laser energy to sustain the chemical
reaction.
An inert shielding gas can be used to reduce
the amount of reactant and product molecules contacting
the reactant chamber components. Appropriate shielding
gases include, for example, Ar, He and Nz.
An appropriate laser pyrolysis apparatus
generally includes a .reaction chamber isolated from the
ambient environment. A reactant inlet connected to a
reactant supply system produces a reactant stream
through the reaction chamber. A laser beam path
intersects the reactant stream at a reaction zone. The
reactant stream continues after the reaction zone to an
outlet, where the reactant stream exits the reaction
chamber and passes into a collection system. Generally,
the laser is located external to the reaction chamber,
and the light beam enters the reaction chamber through
an appropriate window.
- Two laser pyrolysis reaction chambers are
described further below. These laser pyrolysis reaction
chambers can be configured for delivery of gas phase
reactants and/or aerosol reactants.
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-25-
1. First Laser ~~rrol~rsis Reaction Chamber
Referring to Fig. 2, a particular embodiment
100 of a laser pyrolysis apparatus involves a reactant
supply system 102, reaction chamber 104, collection
system 106, laser 108 and shielding gas delivery system
110. Two alternative types of reaction supply systems
can be used with the apparatus of Fig. 2. The first
type of reaction supply system is used to deliver
exclusively gaseous reactants. The second type of
reactant supply system is used to deliver one or more
reactants as an aerosol.
Referring to Fig. 3, a first embodiment 112 of
reactant supply system 102 includes a source 120 of
precursor compound. An optional second precursor source
121 can be used for the production of composite/ternary
particles. For liquid or solid precursors, a carrier
gas from one or more carrier gas sources 122 can be
introduced into precursor source 120 and/or 121 to
facilitate delivery of the precursor as a vapor.
Precursor sources 120 and/or 121 can be a solid
precursor delivery system 50, as shown in Fig. 1. The
carrier gas from source 122 preferably is either an
infrared absorber or an inert gas and is preferably
bubbled through a liquid precursor compound or delivered_
into a solid precursor delivery system. Inert gas used
as a carrier gas can moderate the reaction conditions.
The quantity of precursor vapor in the reaction zone is
roughly proportional to the flow rate of the carrier
gas.
Alternatively, carrier gas can be supplied
directly from infrared absorber source 124 or inert gas
source 126, as appropriate. The secondary reactant can
be supplied from reactant source 128, which can be a gas
cylinder or other suitable container. The gases from
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-26-
the precursor sources 120, 121 are mixed with gases from
reactant source 128, infrared absorber source 124 and
inert gas source 126 by combining the gases in a single
portion of tubing 130. The gases are combined a
sufficient distance from reaction chamber 104 such that
the gases become well mixed prior to their. entrance into
reaction chamber 104.
The combined gas in tube 130 passes through a
duct 132 into rectangular channel 134, which forms part
of an injection nozzle for directing reactants into the
reaction chamber. Portions of reactant supply system
112 can be heated to inhibit the deposition of precursor
compound on the walls of the delivery system.
Referring to Fig. 4A, a second embodiment 150
of the reactant supply system 102 is used to supply an
aerosol to duct 132. Duct 132 connects with rectangular
channel 134 , which forms part of an inj ection nozzle for
directing reactants into the reaction chamber. Reactant
supply system 150 includes a delivery tube 152 that is
connected to duct 132. Venturi tube 154 connects to
delivery tube 152 as a source of the aerosol. Venturi
tube 154 is connected to gas supply tube 156 and liquid
supply tube 158.
Gas supply tube 156 is connected to gas source
160. Gas source 160 can include a plurality of gas
containers that are connected to deliver a selected gas
or gas mixture to gas supply tube 156. The flow of gas __
from gas source 160 to gas supply tube 156 is controlled
by one or more valves 162. Liquid supply tube 158 is
connected to liquid supply 164. Delivery tube 152 also
connects with drain 166 that flows to reservoir 168.
In operation, gas flow through venturi tube
154 creates suction that draws liquid into venturi tube
154 from liquid supply tube 158. The gas-liquid mixture
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-27-
in venturi tube 154 forms an aerosol when venturi tube
154 opens into delivery tube 152. The aerosol is drawn
up into duct 132 by pressure differentials within the
system. Any aerosol that condenses within delivery tube
152 is collected in reservoir 168, which is part of the
closed system.
Referring to Fig. 4B, a third embodiment 170
of the reactant supply system 102 can be used to supply
an aerosol to duct 132. Reactant supply system 170
includes an aerosol generator 172, carrier gas/vapor
supply tube 174 and junction 176. Duct 132, aerosol
generator 172 and supply tube 174 meet within interior
volume 178 of junction 176. Supply tube 174 is oriented
to direct carrier gas along duct 132. Aerosol generator
172 is mounted such that an aerosol 180 is generated in
the volume of chamber 178 between the opening into duct
132 and the outlet from supply tube 174.
Aerosol generator 172 can operate based on a
variety of principles. For example, the aerosol can be
produced with an ultrasonic nozzle, with an
electrostatic spray system, with a pressure-flow or
simplex atomizer, with an effervescent atomizer or with
a gas atomizer where liquid is forced under significant
pressure through a small orifice and sheared into
droplets by a colliding gas stream. Suitable ultrasonic
- nozzles can include piezoelectric transducers.
Ultrasonic nozzles with piezoelectric transducers and
suit-able broadband ultrasonic generators are available
from Sono-Tek Corporation, Milton, NY, such as model
8700-120. Suitable aerosol generators are described
further in copending and commonly assigned, U.S. Patent
Application Serial No. 09/188,670 to Gardner et al_,
entitled "REACTANT DELIVERY APPARATUSES," incorporated
herein by reference. Additional aerosol generators can
CA 02350201 2001-05-08
WO 00/27754
' ' PCT/US99/26343
-28-
be attached to junction 176 through other ports 182 such
that additional aerosols can be generated in interior
- 178 for delivery into the reaction chamber.
Junction 176 includes ports 182 to provide
access from outside junction 176 to interior 178. Thus,
duct 132, aerosol generator 172 and supply tube 174 can
be mounted appropriately. In one embodiment, junction
176 is cubic with six cylindrical ports 182, with one
port 182 extending from each face of junction 176.
Junction 176 can be made from stainless steel or. other
durable, noncorrosive material. A window 181 preferably
is sealed at one port 182 to provide for visual
observation into interior 178. The port 182 extending
from the bottom of junction 176 preferably includes a
drain 183, such that condensed aerosol that is not
delivered through duct 132 can be removed from junction
176.
Carrier gas/vapor supply tube 174 is connected
to gas source 184. Gas source 184 can include a
plurality of gas containers, liquid reactant delivery
apparatuses, and/or a solid reactant delivery
apparatuses, which are connected to deliver a selected
gas or gas mixture to supply tube 174. Thus, carrier
gas/vapor supply tube 174 can be used to deliver a_
variety of desired gases and/or vapors within the
reactant stream including, for example, laser absorbing
gases, reactants, and/or inert gases. The flow of gas
from gas source 184 to supply tube 174 preferably is
controlled by one or more mass flow controllers 186.
Liquid supply tube 188 is connected to aerosol generator
152. Liquid supply tube 188 is connected to liquid
supply 189.
For the production of lithium manganese oxide
-particles, liquid supply 189 can hold a liquid
CA 02350201 2001-05-08
Wb 00/27954 PCT/US99I26343
-29-
comprising both a lithium precursor and a manganese
precursor. Alternatively, for the production of lithium
manganese oxide particles, liquid supply 189 can hold a
liquid comprising manganese precursor while a lithium
precursor is delivered by way of vapor supply tube 174
and gas sources) 184. Similarly, if desired, liquid
supply 189 can hold a liquid comprising lithium
precursor, while a manganese precursor is delivered by
way of vapor_supply tube 174 and gas sources) 184.
Also, two separate aerosol generators 172 can be used to
generate aerosol within junction 176, with one producing
-- an aerosol with 'manganese precursor and the second
producing aerosol with a lithium precursor.
In the embodiment shown in Fig. 4B, aerosol
generator 172 generates an aerosol with momentum roughly
orthogonal to the carrier gas f low from tube 174 to duct
132. Thus, carrier gasjvapor from supply tube 174
directs aerosol precursor generated by aerosol generator
172 into duct 132. In operation, carrier gas flow
directs the aerosol delivered within chamber 178 into
duct 132. In this way, the delivery velocity of the
aerosol is determined effectively by the flow rate of
the carrier gas. -
In alternative preferred embodiments, the
aerosol generator is placed at an upward angle relative
to the horizontal, such that a component of the forward
momentum of the aerosol is directed along duct 132. In
a preferred embodiment, the output directed from the
aerosol generator is placed at about a 45° angle
relative to the normal direction defined by the opening
into duct 132, i.e. the direction of the flow into duct
132 from supply tube 174.
Referring to Fig. 4C, another embodiment 1~
of the reactant supply system 102 can be used to supply
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-30-
an aerosol to duct 13~. Reactant supply system 191
includes an outer nozzle 193 and an inner nozzle 195.
Outer nozzle 193 has an upper channel 197 that leads to
a 5/8 in. by 1/4 in. rectangular outlet 199 at the top
of outer nozzle 193, as shown in the insert in Fig. 4C.
Outer nozzle 193 includes a drain tube 201. in base plate
203. Drain tube 201 is used to remove condensed aerosol
from outer nozzle 193. Inner nozzle 195 is secured to
outer nozzle 193 at fitting 205.
Inner nozzle 195 is a gas atomizer from
Spraying Systems (Wheaton, IL), such as model number
17310-12-lx8jj. The inner nozzle has about a 0.5 inch
diameter and a 12.0 inch length. The top of the nozzle
is a twin orifice internal mix atomizer 207 (0.055 in.
gas orifice and 0.005 in. liquid orifice). Liquid is
fed to the atomizer through tube 209, and gases for
introduction into the reaction chamber are fed to the
atomizer through tube 211. Interaction of the gas with
the liquid assists with droplet formation.
Outer nozzle 193 and inner nozzle 195 are
assembled concentrically. Outer nozzle 193 shapes the
aerosol generated by inner nozzle 195 such that it has
a flat rectangular cross section. In addition, outer
nozzle 193 helps to achieve a uniform aerosol velocity
and a uniform aerosol distribution along the cross
- section. Outer nozzle 193 can be reconfigured for
different reaction chambers . The height of outer nozzle
193 relative to the radiation/laser beam can be adj-usted
to produce spray characteristics that result in desired
particle properties. For the production of lithium
manganese oxide by laser pyrolysis, outer nozzle 193 is -
spaced about 3 inches below the laser beam.
Referring to Fig. 2, shielding gas delivery
system 110 includes inert gas source 190 connected to an
CA 02350201 2001-05-08
V1'VO 00/29754 PCT/US99/26343
-31-
inert gas duct 192. _.lnert gas duct 192 flows into
annular channel 194. A mass flow controller 196
regulates the flow of inert gas into inert gas duct 192.
If reactant delivery system 112 is used, inert gas
source 126 can also function as the inert gas source for
duct 192, if desired.
The reaction chamber 104 includes a main
chamber 200. Reactant supply system 102 connects to the
main chamber 200 at injection nozzle 202. Reaction
chamber 104 can be heated to keep the precursor compound
in the vapor state. The chamber preferably is heated to
a surface temperature above the dew point of the mixture
of reactants and inert components at the pressure in the
apparatus. For many embodiments, the chamber is heated
.to about 120° when a solid precursor is used.
Similarly, for many embodiments, the argon shielding gas
is preferably heated to about 150°C when a solid
precursor is used. The chamber can be examined for
condensation to ensure that precursor is not deposited
on the chamber.
The end of injection nozzle 202 has an annular
opening 204 for the passage of inert shielding gas, and
a reactant inlet 206 for the passage of reactants to
form a reactant stream in the reaction chamber.
Reactant inlet 206 preferably is a slit, as shown in the
lower insert of Fig. 2. Annular opening 204 has, for
example, a diameter of about 1.5 inches and a width
along the radial direction from about 1/8 in to about
1/16 in. The flow of shielding gas through annular
opening 204 helps to prevent the spread of the reactant
gases and product particles throughout reaction chamber
104.
Tubular sections 208, 210 are located on
either side of injection nozzle 202. Tubular sections
CA 02350201 2001-05-08
WO 00/7754 PCT/US99/26343
-32-
208, 210 include ZnSe -windows 212, 214, respectively.
Windows 212, 214 are about 1 inch in diameter. Windows
212, 214 are preferably cylindrical lenses with a focal
length equal to the distance between the center of the
chamber to the surface of the lens to focus the light
beam to a point just-below the center of the nozzle
opening. Windows 212, 214 preferably have an
antireflective coating. Appropriate ZnSe lenses are
available from Laser Power Optics, San -Diego,
California. Tubular sections 208, 210 provide for the
displacement of windows 212, 214 away from main chamber
200 such that windows 212, 214 are less likely to be
contaminated by reactants and/or products. Window 212,
214 are displaced, for example, about 3 cm from the edge
of the main chamber 200.
Windows 212, 214 are sealed with a rubber o-
ring to tubular sections 208, 210 to prevent the flow of
ambient air into reaction chamber 104. Tubular inlets
216, 218 provide for the flow of shielding gas into
tubular sections 208, 210 to reduce the contamination of
windows 212, 214. Tubular inlets 216, 218 are connected
to inert gas source 190 or to a separate inert gas
source. In either- case, flow to inlets 216, 218
preferably is controlled by a mass flow controller 220.
Light source 108 is aligned to generate a
light beam 222 that enters window 212 and exits window
214. Windows 212, 214 define a light path through main
chamber 200 intersecting the flow of reactants at
reaction zone 224. After exiting window 214, light beam
222 strikes power meter 226, which also acts as a beam
dump. An appropriate power meter is available from
Coherent Inc., Santa Clara, CA. Light source 108 can be
a laser or an intense conventional light source such _a.s
an arc lamp. Preferably, light source ~ 08 is-~an
CA 02350201 2001-05-08
WO 00/2'7754 PCT/US99/26343
-33-
infrared laser, especially a CW COZ laser such as an
1800 watt maximum power output laser available from PRC
Corp., Landing, NJ.
Reactants passing through reactant inlet 206
in injection nozzle 202 initiate a reactant stream. The
reactant stream passes through reaction zone 224, where -
reaction involving the metal precursor compounds takes
place. Heating of the gases in reaction zone 224 is
extremely rapid, roughly on the order of 105 degree
C/sec depending on the specific conditions. The
reaction is rapidly quenched upon leaving reaction zone
224, and particles 228 are formed in the reactant
stream. The nonequil~brium natur-a of the process allows
for the production of nanoparticles with a highly
uniform size distribution and structural homogeneity.
- The path of the reactant stream continues to
collection nozzle 230. Collection nozzle 230 is spaced
about 2 cm from injection nozzle 202. The small spacing
between injection noazle 202 and collection nozzle.230
helps reduce the contamination of reaction chamber 104
with reactants and products. Collection nozzle 230 has
a circular opening 232, as shown in the upper insert of
Fig. 2. Circular opening 232 feeds into collection
system 106.
The chamber pressure is monitored with a
pressure gauge attached to the main chamber. The
preferred chamber pressure for the production of the
desired oxides generally ranges from about 80 Torr to
about 650 Torr.
Reaction chamber 104 has two additional
tubular sections not shown. One of the additional
tubular sections projects into the plane of the
sectional view in Fig. 2, and the second additional
tubular section projects out of the plane of the
CA 02350201 2001-05-08
yV0 00/17754
PCT/US99/26343
-34-
sectional view in Fig.- 2. Plhen viewed from above, the
four tubular, sections are distributed roughly,
symmetrically around the center of the chamber. These
additional tubular sections have windows for observing
the inside of the chamber. In this configuration of the
apparatus, the two additional tubular sections are not
used to facilitate production of particles.
Collection system 106 preferably includes a
curved channel 270 leading from collection nozzle 230.
Because of the small size of the particles, the product
particles follow the flow of the gas around curves.
Collection system 106 includes a filter 272 within the
gas flow to collect the product particles. Due to
curved section 270, the filter is not supported directly
-above the chamber. A variety of materials such as
Teflon, glass fibers and the like can be used for the
filter as long as the material is inert and has a fine
enough mesh to trap the particles. Preferred materials
for the filter include, for example, a glass fiber
filter from ACE Glass Inc. , Vineland, NJ and cylindrical
Nomex~ filters from AF Equipment Co., Sunnyvale, CA.
Pump 274 is used to maintain collection system
106 at a selected pressure. A variety of different
pumps can be used. Appropriate pumps for use as pump
,274 include, for example, Busch Model B0024 pump from
Busch, Inc., Virginia Beach, VA with a pumping capacity
of about 25 cubic feet per minute (cfm) and Leybold
Model SV300 pump from Leybold Vacuum Products, Export,
PA with a pumping capacity of about 195 cfm. It may be
desirable to flow the exhaust of the pump through a
scrubber 276 to remove any remaining reactive chemicals
before venting into the atmosphere. The entire
apparatus 100 can be placed in a fume hood for
ventilation purposes and-for safety considerations.
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-35-
Generally, the laser remains outside of the fume hood
because of its large size.
The apparatus is controlled by a computer.
Generally, the computer controls the light source and
monitors the pressure in the reaction chamber. The
computer can be used to control the flow of reactants
and/or the shielding gas. The pumping rate is
controlled by either a manual needle valve or an
automatic throttle valve inserted between pump 274 and
filter 272. As the chamber pressure increases due to
the accumulation of particles on filter 272, the manual
valve or the throttle valve can be adjusted to maintain
the pumping rate and the corresponding chamber pressure .
The reaction can be continued until sufficient
particles are collected on filter 272 such that pump 274
can no longer maintain the desired pressure in the
reaction chamber 104 against the resistance through
filter 272. When the pressure in reaction chamber 104
can no longer be maintained at the desired value, the
-reaction is stopped, and filter 272 is removed. With
this embodiment, about 1-300 grams of particles can be
collected in a single run before the chamber pressure
can no longer be maintained. A single run generally can
last up to about 10 hours depending on the reactant
delivery system, the type of particle being produced and
the type of filter being used.
The reaction conditions can be controlled
relatively precisely. In particular, the mass flow
controllers are quite accurate. The laser generally has
about 0.5 percent power stability. With either a manual
control or a throttle valve, the chamber pressure can be
controlled to within about 1 percent.
The configuration of the reactant -supply
system 102 and the collection system .106 can be
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-36-
reversed. In this alternative configuration, the
reactants are supplied from the top of the reaction
- chamber, and the product particles are collected from
the bottom of the chamber. In the alternative
configuration, the collection system may not include a
curved section so that the collection filter is mounted
directly below the reaction chamber.
2. Second Laser Pyrolvsis Reaction Chamber
An alternative design of a laser pyrolysis
apparatus has been described in U.S. Patent 5,958,348,
entitled "Efficient Production of Particles by Chemical
Reaction," incorporated herein by reference. This
alternative design is intended to-facilitate production
of commercial_ quantities of particles by laser
pyrolysis. The reaction chamber is elongated along the
- light beam in a dimension perpendicular to the reactant
stream to provide for an increase in the throughput of
reactants and products. The original design _of the
apparatus was based_ on the introduction of purely
gaseous reactants. A particular embodiment for the
introduction of an aerosol into the apparatus is
described below. Additional embodiments for the
introduction of an aerosol with one or more aerosol
generators into an elongated reaction chamber is
described in commonly assigned and copending U. S . Patent
application serial No. 09/188,670 to Gardner et al.,
entitled "Reactant Delivery Apparatuses," filed November
9, 1998, incorporated herein by reference.
In general, the alternative pyrolysis
apparatus includes a reaction chamber designed to reduce
contamination of the chamber walls, to increase the
production capacity and to make efficient use of
resources. To accomplish these objectives, an elongated
reaction chamber is used that provides for an increased
CA 02350201 2001-05-08
VSO 00/29754 PCT/US99/26343
-37-
throughput of reactants and products without a
corresponding increase 'in the dead volume of the
chamber. The dead volume of the chamber can become
contaminated with unreacted compounds and/or reaction
products.
The design of the improved reaction chamber
300 is shown schematically in Fig. 5. A reactant inlet
302 leads to main chamber 304. Reactant inlet 302
conforms generally to the shape of main chamber 304.
The introduction of reactants through reactant inlet
302, for example, for the production of lithium
manganese oxide~particles can be performed by adapting
the discussion above regarding the introduction of
aerosol and/or vapor precursors with the laser pyrolysis
apparatus of Fig. 1, appropriately adapted for the
alternative structure of the reactant inlet. Generally,
the reactant inlet has a length from about 5 mm to about
1 meter when used with an 1800 watt COZ laser. -
Main chamber 304 includes an outlet 306 along
the reactant/product stream for removal of particulate
products, any unreacted gases and inert gases.
Shielding gas inlets 310 are located on both sides of
reactant inlet 302. Shielding gas inlets are used to
form a blanket of inert gases on the sides of the
reactant stream to inhibit contact between the chamber
walls and the reactants-or products.
Tubular sections 320, 322 extend from the main
chamber 304. Tubular sections 320, 322 hold windows
324, 326 to define a light beam path 328 through the
reaction chamber 300. Tubular sections 320, 322 can
include inert gas inlets 330, 332 for the introduction
of inert gas into tubular see-tions 320, 322.
Referring to Figs. 6-8, a specific embodiment
350 of a laser pyrolysis reaction system with an
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-38-
elongated reaction chamber is shown. In this
embodiment, an aerosol reactant delivery apparatus is
adapted for use with the elongated reaction chamber.
Laser pyrolysis reaction system 350 includes reaction
chamber 352, a particle collection system 354, laser 356
and a reactant delivery system (described below).
- Reaction chamber 352 includes reactant inlet 364 at the
bottom of reaction chamber 352 where the reactant
delivery system connects with reaction chamber 352. In
this embodiment, the reactants are delivered from the
bottom of the reaction chamber while the products are
collected from the top of the reaction chamber. The
configuration can be reversed with the reactants
supplied from the top and product collected from the
bottom, if desired.
Shielding gas conduits 365 are located on the
front and back of reactant inlet 364. Inert gas is
delivered to shielding gas conduits 365 through ports
367. The shielding gas conduits direct shielding gas
along the walls of reaction chamber 352 to inhibit
association of reactant gases or products with the
walls.
Reaction chamber 352 is elongated along one
dimension denoted in Fig. 6 by '~w~~. A laser beam path
366 enters the reaction chamber through a window 368
displaced along a tube 370 from main chamber 372 and
traverses the elongated direction of reaction chamber
352. The laser beam passes through tube 374 and exits
window 376. In one preferred embodiment, tubes 370 and
374 displace windows 368 and 376 about 11 inches from --
main chamber 372. The laser beam terminates at beam
dump 378. In operation, the laser beam intersects a
reactant stream generated through reactant inlet 364.
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-39-
The top of _main chamber 372 opens into
particle collection system 354. Particle collection
system 354 includes outlet duct 380 connected to the top
of main chamber 372 to receive the flow from main
chamber 372. Outlet duct 380 carries. the product
particles out of the plane of the reactant stream to a
cylindrical filter 382. Filter 382 has a cap 384 on one
end. The other end of filter 382 is fastened to disc
386. Vent 388 is secured to the center of disc 386 to
provide access to the center of filter 382. Vent 388 is
attached by way of ducts to a pump. Thus, product
particles are trapped on filter 382 by the flow from the
reaction chamber 352 to the pump. Suitable pumps were
described above with respect to the first laser
pyrolysis apparatus in Fig. 2. Suitable filters for use
as filter 382 include, for example, an air cleaner
filter for a Saab 9000 automobile (Purilator part A44-
67), which is wax impregnated paper with Plasticol or
polyurethane end cap 384.
Referring to Fig. 9, an aerosol delivery
apparatus 480 includes an aerosol generator 482, which
is supported by mount 484 and a cap 486. Aerosol
delivery apparatus 480 is secured to reactant inlet 364
of reaction chamber 352 to extend within main chamber
372, shown in Figs. 6-8. Mount 484 is connected to a
- base plate 488. Base plate 488 is fastened to reactant
inlet 364 with bolts 490. An o-ring or the like,
suitably shaped, can be placed within hollow 492 to- form
a seal between base plate 488 and reactant .inlet 364.
Referring to Figs. 10 and 11, mount 484 has a
generally cylindrical shape. Mount 484 includes a lip
506 extending within cylindrical cavity 508. Lip 506
helps support aerosol generator 482. In this
embodiment, lip 506 includes a notch 510, which allows
CA 02350201 2001-05-08
WO 00/27754
PCT/US99/26343
-40-
a portion of aerosol generator 482 to extend past lip
506. Top surface 512 of mount 484 includes a hollow 514
- for holding an o-ring or the like to form a seal with
cap 486 or a spacer, described below. Mount 484 further
includes threads 518 on the outer surface 520.
Referring to Figs. 10, 12 and 13, cap 486
attaches over the top of mount 484. Cap 486 includes
threads 528 that are mated with threads 518 on mount
484. Flange 530 can be used to form a seal with an o-
ring or the like. Surface 532 includes hollow 534.
Hollow 534 can hold an o-ring or the like to form a seal
with aerosol generator 482 or a shim, described further
below.
Tube 536 is in fluid communication with cavity
538. Tube 536 provides for gas flow into cavity 538.
Cavity 538 vents through port 540. Tubes 542 provide
for fluid flow through channels 544 into projecting
tubes 546. In, this embodiment, four projecting tubes
546 project toward the flow stream coming from aerosol
generator 482 and port 540. Four projecting tubes 546
are symmetrically distributed around port 540. More or
less than four projecting tubes 546 can be used, if
desired. Gas can be supplied to tubes 536 and 542
through one or more ports 547 through base plate 488
(Fig. 9) by way of stainless steel tubing or the like.
The use of projecting tubes _ 546 are
particularly useful to mix reactants further within the
reaction chamber away from aerosol generator 482. Using
projecting tubes 546, gases such as reactant gases
and/or radiation absorbing gases can be mixed within
reaction chamber 352 with reactants from aerosol
generator 482 and/or port 540. Laser beam path 548
intersects the reaction stream just above projecting
tubes 546.
CA 02350201 2001-05-08
W O 00/2'1754 PCT/US99/26343
-41-
The position-of aerosol generator 482 relative
to port 540 can affect the properties of the resulting
reactant stream and thereby the properties of the
xeaction product. With an ultrasonic aerosol generator,
the tip of the aerosol generator preferably is located
between positions just slightly below the cap surface to
just slightly above the cap surface.
Spacer 550, shown in Fig. 14, can be placed
between cap 486 and mount 484 to change the posi-Lion of
aerosol generator 482 relative to port 540. Spacer 550
is a cylindrical piece with a hollow 552 along top
surface 554 for holding an o-ring or the like. Top
surface 554 seals against flange 530 of cap 486. Lower
surface 556 of spacer 550 seals against top surface 512
of mount 484. A shim 558, as shown in Fig. 15, is
correspondingly placed between cap 486 and aerosol
generator 482. Top surface 560 of shim 558 engages the
o-ring in hollow 534. Flange 562 engages the aerosol
generator 482.
The flow of reactants into main chamber 372
can be affected by the placement of a cap bushing at the
opening of port 540. More specifically, a cap bushing
can help provide a more confined reactant stream within
main chamber 372. Three embodiments of cap bushings
570, 572, 574 are shown in Figs. 16-18, respectively.
Referring to Fig. 16, cap bushing 570 has a cylindrical
passage 576 and a flat upper surface 578 generally
perpendicular to the central axis of cylindrical passage
576. Referring to Fig. 17, cap bushing 572 has a
conical passage 580 and a flat upper surface 582 _
generally perpendicular to the symmetry axis of conical
passage 580. Referring to Fig. 18, cap bushing 574 has
a conical passage 584 and a top surface with a flat
section 586 and a conical section 588. - Preferred
CA 02350201 2001-05-08
WO 00/7754 PCT/US99/26343
-42-
embodiments of cap bush-ings have a sharp edge between
the internal passage and the top surface.
Reaction chamber 352 and reactant supply
system 480 preferably are constructed from stainless
steel or other corrosion resistant metal. O-rings and
other seals can be made from natural or synthetic rubber
or other polymers.
Referring to Fig. 10, in a preferred
embodiment, aerosol generator 482 includes an ultrasonic
nozzle 600 and nozzle supply 602. Preferred ultrasonic
nozzle 600 is a model 8700-120 from Sono-Tek
Corporation, Milton, NY. Referring to Figs. 19-20,
ultrasonic nozzle 600 includes a nozzle tip 604, a
nozzle body 606, a connector 608 for connection to an
ultrasonic generator, and a liquid connection 610 for
connection to a liquid reservoir directly or by way of
nozzle supply 602. The end of nozzle tip 604 is an
atomization surface 612. The size and shape of
atomization surface 612 can be varied to yield a
desirable spacial distribution of aerosol particles.
Nozzle tip 604 is connected to nozzle body 606
at or near top surface 614. Ultrasonic transducer 616
is located within nozzle body 606 at a suitable position
to- vibrate nozzle tip 604. Generally, ultrasonic
transducer 616 is located toward top surface 614.
- Preferred ultrasonic transducers include, for example,
piezoelectric transducers. Preferably, ultrasonic
transducer 616 includes two or more piezoelectric
transducers 618 coupled to oscillate in phase such that
the amplitudes of the two vibrating piezoelectric
transducers add to create an additive force at atomizing
surface 612.
Ultrasonic transducer 616 is connected to an
ultrasonic generator by way of connector 608. The
CA 02350201 2001-05-08
Vi'O 00!2'7754 PCT/US99/26343
-43-
ultrasonic generator _preferably is a broad band
generator operating over a frequency range from about 20
kHz to about 120 kHz. The electrical signal from the
ultrasonic generator is conveyed from connector 608 to
ultrasonic transducer 616 by way of conductors 620.
Liquid flows from liquid connection 610 to
atomization surface 612 through channel 622, which runs
through nozzle body 606. Referring to Fig. 10, nozzle
supply 602 is connected to liquid connection 610 with a
liquid fitting 630. Nozzle supply 602 includes a needle
valve with pneumatic control. Nozzle supply 602 has a
pneumatic control inlet 632, a needle valve adjustment
634 and a liquid feedstock inlet 636. Pneumatic control
inlet 632 and liquid feedstock inlet 636 are accessed
through central channel 508, which extends through base
plate 488.
Liquid feedstock inlet 636 is connected to a
liquid. supply apparatus 640, shown schematically in Fig.
21. Liquid supply apparatus 640 includes, at least, one
liquid source 642, an outlet tube 644 and a gas supply
tube 646. Tube 644 connects with fitting 648 to liquid
feedstock inlet 636. Similarly, tube 644 is connected
directly or indirectly to liquid source 642. Liquid
source 642 also connects to gas supply tube 646. Gas
supply tube connects to a gas source 666, which can be
a gas cylinder or the like . Flow from gas source 666 to
gas supply tube 646 is controlled by one or more valves
668. Gas under pressure from gas supply tube 646 forces
liquid from liquid source 642 into tube 644.
Proper placement of liquid source 642 can
result in gravity supplying the pressure as an
alternative to using gas pressure. In other
embodiments, mechanical pumps are used to supply a
relatively constant amount of pressure within tube 644.
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-44-
Suitable pumps include,_for example, centrifical pumps
and a plurality of syringe pumps that operate
sequentially.
_. In use, the aerosol generator 482 produces an
aerosol of a liquid supplied to aerosol generator 482.
Aerosol generator 482 can deliver a gas along with the
aerosol. Also, the aerosol can be combined with a gas
supplied through tube 536. Thus, the aerosol and any
gases supplied from aerosol generator 482 and/or tube
536 are directed into reaction chamber 352 near port 540
_- of cap 486. The aerosol and any gases emanating from
aerosol generator 482 and/or tube 536 can be combined
further within reaction chamber 352 with additional
gases from projecting tubes 546. The resulting mixture
of aerosol and gases is subsequently reacted within
reaction chamber 352.
For the performance of laser pyrolysis based
reaction synthesis, the aerosol/gas mixture generally
includes one or more reactants in aerosol form,
optionally, one or more additional reactant gases, a
laser absorbing gas if the reactants and/or solvents)
do not sufficiently absorb the laser radiation, and,
optionally, an inert gas. The gases can be supplied
from a pressurized cylinder or other suitable container.
Multiple reactants can be mixed in the liquid phase and
delivered as the aerosol.
Alternative aerosol generators can be used
with the elongated reaction chamber. In addition, one
or more aerosol generators can be configured with the
elongated reaction chamber in a variety of ways.
Heat Processing -
1. Particle Conditioning
As noted above, properties of the metal oxide
particles can be modified by further processing.
CA 02350201 2001-05-08
W'O 00/27754 PCT/US99/26343
-45-
Suitable starting material for the heat treatment
include metal oxide particles, such as, lithium
manganese oxide particles, produced by laser pyrolysis.
In addition, particles used as starting material can
have been subjected to one or more prior heating steps
under different conditions. For the heat processing of -
metal oxide particles formed by laser pyrolysis, the
additional heat processing can improve the
crystallinity, remove contaminants, such as elemental
carbon, and possibly alter the stoichiometry, for
example, by incorporation of additional oxygen or of
atoms from other gaseous species. The use of
sufficiently mild conditions, i~e., temperatures well
below the melting point of the particles, results in the
processing of the particles without significantly
- sintering the particles into larger particles.
The starting materials generally can be
particles of any size and shape, although nanoscale
particles are preferred starting materials. The
nanoscale particles have an average diameter of less
than about 1000 nm and preferably from about 5 nm to
about 500 nm, and more preferably from about 5 nm to
about 150 nm. Suitable nanoscale starting materials
have been produced by laser pyrolysis.
The metal oxide particles are preferably
heated in an oven or the like to provide generally
uniform heating. The processing conditions generally
are mild, such that significant amounts of particle
sintering does not occur. The temperature of heating
preferably is low relative to the melting point of both
the starting material and the product material.
For certain target product particles,
additional heating does not lead to further variation in
the particle composition once equilibrium has been
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-46-
reached. The atmosphere for the heating process can be
an oxidizing atmosphere or an inert atmosphere. In
particular, for conversion of amorphous particles to
crystalline particles or from one crystalline structure
to a different crystalline structure of essentially the
same stoichiometry, the atmosphere generally can be
inert . The atmosphere over the particles can be static,
or gases can be flowed through the system.
Appropriate oxidizing gases include, for
example, O2, 03, CO, COz, and combinations thereof . The
OZ can be supplied as air. Oxidizing gases optionally
can be mixed with inert gases such as Ar, He and Nz .
When inert gas is mixed with the oxidizing gas, the gas
mixture can include from about 1 percent oxidizing gas
-to about 99 percent oxidizing gas, and more preferably
from about 5 percent oxidizing gas to about 99 percent
oxidizing gas. Alternatively, either essentially pure
oxidizing gas or pure inert gas can be used, as desired.
The precise conditions can be altered to vary
the type of metal oxide particles that are produced.
For example, the temperature, time of heating, heating
and cooling rates, the gases and the exposure conditions
with respect to the gases can all be changed, as
desired. Generally, while heating under an oxidizing
atmosphere, the longer the heating period the more
oxygen that is incorporated into the material, prior to
reaching equilibrium. Once equilibrium conditions are
reached, the overall conditions determine the
crystalline phase of the powders.
A variety of ovens or the like can be used to
perform the heating. An example of an apparatus 660 to
perform this processing is displayed in Fig. 22A.
Apparatus 660 includes a jar 662, which can be made from
__ glass or other inert material, into which the particles
CA 02350201 2001-05-08
V110 00/27754 PCT/US99/26343
-47-
are placed. Suitable g~.~ss reactor jars are available
from Ace Glass (Vineland, NJ). The top of glass jar 662
is sealed to a glass cap 664, with a Teflon° gasket 666
between jar 662 and.cap 664. Cap 664 can be held in
place with one or more clamps. Cap 664 includes a
plurality of ports 668, each with a Teflon~ bushing. A
multiblade stainless steel stirrer 670 preferably is
inserted through a central port 668 in cap 664. Stirrer
670 is connected to a suitable motor.
One or more tubes 672 are inserted through
ports 668 for the delivery of gases into j ar 662 . Tubes
672 can be made from stainless steel or other inert
material. Diffusers 674 can be included at the tips of
tubes 672 to disburse the gas within jar 662. A
heater/furnace 676 generally is placed around jar 662.
Suitable resistance heaters are available from Glas-col
(Terre Haute, IN) . One port- preferably includes a T-
connection 678. The temperature-within jar 662 can be
measured with a thermocouple 678 inserted through T-
connection 678. T-connection 678 can be further
connected to a vent 680. Vent 680 provides for the
venting of gas circulated through jar 662. Preferably
vent 680 is vented to a fume hood or alternative
ventilation equipment.
Preferably, desired gases are flowed through
jar 662. Tubes 672 generally are connected to an
oxidizing gas source and/or an inert gas source.
Oxidizing gas, inert gas or a combination thereof to
produce the desired atmosphere are placed within jar 662
from the appropriate gas source(s). Various flow rates -=
_ can be used. The flow rate preferably is between about
1 standard cubic centimeters per minute (sccm) to about
1000 sccm and more preferably from about 10 sccm to
about 500 sccm. The flow rate gener-aily is constant
CA 02350201 2001-05-08
WO 00127754 PCT/US99/26343
-48-
through the processing step, although the.flow rate and'
the composition of the gas can be varied systematically
over time during processing, if desired. Alternatively,
a static gas atmosphere can be used.
For the processing of manganese oxide and
lithium magnanese oxide nanoparticles, for.example, the -
temperatures preferably range from about 50°C to about
600°C and more preferably from about 50°C to about
550°C, and even more preferably from about 60°C to about
400°C. The heating preferably is continued for greater
than about 5 minutes, and generally is continued for
from about 2 hours to about 120 hours, preferably from
about 2 hours to about 25 hours. Some empirical
adjustment may -be required to produce the conditions
appropriate for yielding a desired material. The use of
- mild conditions avoids interparticle sintering resulting
in larger particle sizes. Some controlled sintering of
the particles can be performed at somewhat higher
temperatures to produce slightly larger, average
particle diameters.
The conditions to convert crystalline VOz to
orthorhombic V205 and 2-D crystalline V205, and amorphous
VZOS to orthorhombic Vz05 and 2-D crystalline V205 are
describe in copending and commonly assigned U.S. Patent
application serial number 08/897,903, to Bi et al.,
entitled ~~Processing of Vanadium Oxide Particles With
Heat,~~ incorporated herein by reference.
2. Thermal Production of Lithium Manganese Oxide
In an alternative approach to the formation of
lithium manganese oxide nanoparticles, it has been
discovered that heat processing can be used to form
nanoscale lithium manganese oxides. In a preferred
approach to the thermal formation of lithium manganese -
oxide; manganese oxide nanoscale particles first are
CA 02350201 2001-05-08
WO 00/2?754 PCT/US99/26343
-49-
mixed with a lithium compound. The resulting mixture is
heated in an oven to form a lithium manganese oxide.
The heating resulting in lithium incorporation into the
manganese oxide lattice can be performed in an oxidizing
environment or an inert environment. In either type of
environment, the heating step generally results in
alteration of the oxygen-to-manganese ratio, lithium-to-
manganese ratio, lithium-to-oxygen ratio or a
combination thereof.
. The use of sufficiently mild conditions, i.e.,
temperatures well below the melting point of the
manganese oxide particles, results in lithium
incorporation into the manganese oxide particles without
significantly sintering the particles into larger
particles. The manganese oxide particles used for the
lithiation process preferably are nanoscale manganese
oxide particles. It has been discovered that spinel
lithium manganese oxides can be formed from manganese
oxides with an oxidation state less than +4. In
particular, manganese oxides with an oxidation states
from +2 (Mn0) to +4 (Mn02) can be used to form lithium
manganese oxide spinels. Suitable manganese oxide
nanoparticles can have a stoichiometry of, for example,
MnO, Mn304; Mn2O3, Mn508, MnOz, and corresponding mixed
phase materials.
Suitable lithium compounds include for
example, lithium nitrate__(LiN03), lithium chloride
(LiCl) , Li2C03, LiOH, LiOH~H20, Li2C209, LiHC204,
LiHC204 ~ H20, Li3C6Hs0, - 4H20, LiCOOH ~ HZO, and LiCZH302 ~ Hz0 .
Lithium incorporation into manganese oxide nanoparticles
with some of these lithium compounds may require oxygen
in the atmosphere during the heat processing.
Appropriate oxidizing gases include, for example, Oz,
03, CO, COZ and combinations thereof. The reactant gas
CA 02350201 2001-05-08
WO 00/27754
PCT/US99/26343
-50-
can be diluted with inert gases such as Ar, He and Nz_
For example, air and/or clean, dry air can be used as a
source of oxygen and inert gas. Alternatively, the gas
atmosphere can be exclusively inert gas. Lithium
manganese oxides have been produced with either an inert
atmosphere or an oxidizing atmosphere, as.described in
- the Examples below.
In addition, the heat processing can result in
an alteration of the crystal lattice and/or removal of
adsorbed compounds on the particles to improve the
quality of the particles. The processing generally of
metal oxide nanoscale particles in an oven is discussed
further in copending and commonly assigned, U.S. Patent
Application Ser. No. 08/897,903, filed July 21, 1997,
entitled ~~Processing of Vanadium Oxide Particles With
Heat,~~ incorporated herein by reference. In particular,
heat processing under mild conditions can be used to
alter the crystal structure of lithium manganese oxide
nanoparticles formed by laser pyrolysis. Specifically,
amorphous lithium manganese oxide can be annealed to
crystalline, cubic spinel, lithium manganese oxide
without sintering the particles into larger particles.
A variety of apparatuses can be used to
perform the heat processing for lithium incorporation
and/or annealing of a sample. For example, the heating
apparatus shown in Fig. 22A, as described above, can be
used to perform heat processing for lithium_
incorporation. Another embodiment of an apparatus 700
_ to perform this processing is displayed in Fig. 22B.
Apparatus 700 includes a tube 702 into which the
particles are placed. Tube 702 is connected to a _
reactant- gas source 704 and inert gas source 706.
Reactant gas, inert gas or a combination thereof are
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-51-
placed within tube -702 to produce the desired
atmosphere.
Preferably, the desired gases are flowed
- through tube 702. Tube 702 is located within oven or
furnace 708. Oven 708 maintains the relevant portions
of the tube at a relatively constant temperature,
although the temperature can be varied systematically
through the processing step, if desired. Temperature in
oven 708 generally is measured with a thermocouple 710.
Vial 712 prevents loss of the particles due to gas flow.
Vial 712 generally is oriented with the open end
directed toward the direction of the source of the gas
flow. To form lithium manganese oxide in the heating
step, a mixture of manganese oxide particles and
particles of lithium compound can be placed in tube 702
within a vial 712. In alternatively embodiments,
lithium manganese oxide particles produced by laser
pyrolysis are placed into vial 712 for heating in tube
702.
The precise conditions including type of
oxidizing gas (if any), concentration of oxidizing gas,
pressure or flow rate of gas, temperature and processing
time can be selected to produce the desired type of
product material. The temperatures generally are mild, _
i.e., significantly below the melting point of the w
material. The use of mild conditions avoids
interparticle sintering resulting in larger particle
sizes. Some controlled sintering of the particles can
be performed in oven 708 at somewhat higher temperatures
to produce slightly larger, average particle diameters.
- For lithium incorporation into manganese
oxide, the temperature preferably ranges from about 60°C
to about 600°C and more preferably from about 100°C to
about 550°C. The particles preferably are heated for
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-52-
about 5 minutes to ab~olit_ 300 hours . For the heat
processing (annealing) of lithium manganese oxide
produced by laser pyrolysis, the temperature preferably
ranges from about 50°C to about 600°C and more
preferably from about 50°C to about 550°C. The lithium
manganese particles preferably are heated for about 5
minutes to about 100 hours. Some empirical adjustment
may be required to produce the conditions appropriate
for yielding a desired material.
C. Particle Properties
A collection of particles of interest,
comprising, for example, either manganese oxides or
lithium manganese oxides, generally has an average
diameter for the primary particles of less than about
500 nm, preferably from about 5 nm to about 100 nm, more
preferably from about 5 nm to about 50 nm. The primary
particles usually have a roughly spherical gross
appearance. Upon closer examination, crystalline
particles generally have facets corresponding to the
underlying crystal lattice. Nevertheless, crystalline
primary particles tend to exhibit growth that is roughly
equal in the three physical dimensions to give a gross
spherical appearance.- In preferred embodiments, 95
percent of the primary particles, and preferably 99
percent, have ratios of the dimension along the major
axis to the dimension along the minor axis less than
about 2. Diameter measurements on particles with
asymmetries are based on an average of length
measurements along the principle axes of the particle.
Because of their small size, the primary
particles tend to form loose agglomerates due to van der -
Waals and other electromagnetic forces between nearby
particles. Nevertheless, the manometer scale of the
primary particles is clearly observable in transmission
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-53-
electron micrographs of~the particles. The particles
generally have a surface area corresponding to particles
on a nanometer scale as observed in the micrographs.
Furthermore, the particles can manifest unique
properties due to their small size and large surface
area per weight of material. For example, vanadium
oxide nanoparticles generally exhibit surprisingly high
energy densities in lithium batteries, as described in
U.S. Patent 5,952,125, entitled "Batteries With
Electroactive Nanoparticles," incorporated herein by
reference.
The primary particles preferably have a high
degree of uniformity in size. Laser pyrolysis, as
described above, generally results.in primary particles
having a very narrow range of particle diameters.
Furthermore, heat processing under mild conditions does
not alter the very narrow range of particle diameters.
With aerosol delivery, the distribution of particle
diameters is particularly sensitive to the reaction
conditions. Nevertheless, if the reaction conditions
are properly controlled, a very narrow distribution of
particle diameters can be obtained with an aerosol
delivery system, as described above. As determined from
examination of transmission electron micrographs, the
primary particles generally have a distribution in sizes
- such that at least about 95 percent, and preferably 99
percent, of the primary particles have a diameter
greater than about 40 percent of the average diameter
and less than about 160 percent of the average diameter.
Preferably, the primary particles have a distribution of
diameters such that at least about 95 percent, and
preferably 99 percent, of the.primary particles have a
diameter greater than about 60 percent of the average
- CA 02350201 2001-05-08
CVO 00/,2??54 PCT/US99/26343
-54-
diameter and less than about 140 percent of the average
diameter.
Furthermore, in preferred embodiments no
primary particles have an average diameter greater than
about 4 times the average diameter and preferably 3
times the average diameter, and more preferably 2 times
the average diameter. In other words, the particle size
distribution effectively does not have a tail indicative
of a small number of particles with significantly larger
sizes. This is a result of the small reaction region
and corresponding rapid quench of the particles. An
effective cut off in the tail of the size distribution
indicates that there are less than about 1 particle in
106 have a diameter greater than a specified cut off
value above the average diameter. Narrow size
distributions, lack of a tail in the distributions and
the roughly spherical morphology can be exploited in a
variety of applications.
In addition, the nanoparticles generally have
a very high purity level. The crystalline manganese
oxide and lithium manganese oxide nanoparticles produced
by the above described methods are expected to have a
purity greater than the reactants because the crystal
formation process tends to exclude contaminants from the
lattice. Furthermore, crystalline manganese oxide
particles produced by laser pyrolysis have a high degree
of crystallinity. Similarly, the crystalline lithium
manganese oxide nanoparticles produced by heat
processing have a high degree of crystallinity.
Impurities on the surface of the particles may be
removed by heating the particles to achieve not only .
high crystalline purity but high purity overall.
Manganese oxides are known to exist in a wide
__ range of oxidation states from +2 to +4. The most
CA 02350201 2001-05-08
VNO 00/2'754 PCT/US99/26343
-55-
common stoichiometries for manganese oxides include MnO,
Mn304 , Mn203 , Mn508 , and Mn02 . Mn0 and Mn508 have only a
single known crystalline phase. In particular, Mn0 has
a cubic crystal structure while MnsOB has a monoclinic
crystal structure. Several of the manganese oxides can
exist in alternative crystal structures. For example,
Mn,Os has either a tetragonal or orthorhombic crystal
structure. Mnz03 has either a cubic or a hexagonal
crystal structure. Also, Mn02 has either a cubic,
orthorhombic or tetragonal crystal structure.
Lithium manganese oxides have a complex phase
diagram that reflects some of the complexity of the
manganese oxide phase diagram. High lithium content
spinel phases of lithium manganese oxide can have a
stoichiometry over the range f rom Lil,xMnz-x04 , where -
Osxs0.33. In addition, oxygen rich (deficient, y
negative? defect spinel phases exist with
stoichiometries of LiMn204,y, where -0.4sys0.5.
Furthermore, the lithium manganese oxide can be lithium
deficient corresponding to stoichiometries of Lil_ZMn204,
Oszs0.2. Overall, the spinels and defect spinels cover
stoichiometries of Lihx-ZMn2-x04.y~ where Osxs0.33, -
0.4sys0.5 and Oszs0.2. Other states of lithium
manganese oxides are known, such as Li2Mn03, Lia_33MnOz,
Li9MnsOlz, tetragonal LixMn204, l.8sxs2 . 2, LiMn02, LizMn02,
and ~-Mn02. ~-Mn02 is formed by chemically extracting
lithium from LiMn204 with acid, a . g . , 1M HzS04 or HN03 .
a-Mn02 has a structure of LixMn204, 0.05<x<0.20,
depending on the extraction conditions.
D. Battery Application of Lithium Mancranese
Oxides _
Referring to Fig. 23, battery 750 has an
negative electrode 752, a positive electrode 754 and
separator 756 between negative electrode 752 and
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-56-
positive electrode 754: A single battery can include
multiple positive electrodes and/or negative electrodes. -
Electrolyte can be supplied in a variety of ways as
described further below. Battery :750 preferably
includes current collectors 758, 760 associated with
negative electrode 752 and positive electrode 754,
respectively. Multiple current collectors can be
associated with each electrode if desired.
Lithium has been used in reduction/oxidation
reactions in batteries because it is the lightest metal
and because it is the most electropositive metal.
- Certain forms of lithium manganese oxide are known to
incorporate additional lithium ions into its structure
through intercalation or similar mechanisms such as
topochemical absorption. Intercalation of lithium~ions
into suitable forms of a lithiated lithium manganese
oxide lattice forms LiXMnOy.
In lithium manganese oxide spinels, a portion
of the lithium is at tetrahedral spinel lattice sites.
Changes in lithium incorporation into the lattice can
involve variations in the amount of lithium at the
tetrahedral sites from about 0.1 to about 1.0 per two
manganese atoms. At- low enough lithium concentration,
the spinel crystal structure collapses. Alternatively,
additional lithium can occupy octahedral intercalation
sites within the spinel lattice once the tetrahedral
sites are essentially full.
Lithium intercalates into the lithium
manganese oxide lattice during discharge of the battery.
Upon discharge, the positive electrode acts as a cathode
and the negative electrode acts as an anode. The
lithium leaves the lattice upon recharging, i.e., when
a voltage is applied to the cell such that electric
current flows into the positive electrode clue to the
CA 02350201 2001-05-08
WO OO/Z7754 PCT/US99/26343
-57-
application of an exCernal EMF to the battery.
Appropriate lithium manganese oxides can be an effective
- electroactive material for a positive electrode in
either a lithium or lithium ion battery.
There are several forms of lithium manganese
oxide spinels suitable for use as a positive electrode -
active material within a lithium based battery. The
stoichiometric spinel, LiMn204, is a normal spinel
consisting of an oxygen close-packed lattice with
lithium occupying one-eighth of the tetrahedral
positions and manganese occupying one-half of the
octahedral positions. If lithium is removed from this
material within an el~ctrochemica3 cell, the voltage of
the cell is geserally_ above 3.5V and typically above
3.8V, and can extend to about 4.4V br higher. Such a
- voltage profile is referred to as a 4 volt profile and
the capacity derived from the cell is referred to as a
4 volt capacity. A material possessing an appreciable
amount of 4 volt capacity is referred to as a 4 volt
material.
If there is excess lithium, a lithium
substituted spinel, Lil,~'In2_y04, is formed where the
excess lithium occupy the manganese sites. For values
of y less than about 0.33, lithium can still be
extracted, and a cell containing this material would
exhibit a 4 volt profile . As y is increased, the amount
of extractable lithium decreases with a concomitant
decrease in the 4 volt capacity.
For values around 0.33 with a stoichiometry of
Li1.33Mn1.s~Da or Li4Mn5012, the material becomes a 3 volt
material since only a minor amount of 4 volt capacity
remains. Such a profile is referred to as a 3 volt
profile and the capacity derived from the cell is
referred to as a 3 volt capacity. A material possessing
CA 02350201 2001-05-08
,WO 00/,27754 PCT/US99/26343
-58-
an appreciable amount of~3 volt capacity is referred to
as a 3 volt material.
If there are cationic vacancies in the spinel,
a defect spinel is formed with the general formula Lil_
aMn2_za0s . A common form is a defect spinel with z=0 . 11
yielding Lio.89Mn1..,eO4 or LizMn409 . This . material is
primarily a 3 volt material. Additionally, this
material is a lower-temperature material that usually is
synthesized in an oxygen-rich environment. Upon heating
in an oxygen atmosphere to a high temperature or in an
inert environment, LizMn409 converts into LiMn204.
Positive electrode 754 includes electroactive
nanoparticles such as lithium manganese oxide
nanoparticles held together with a binder such as a
polymeric binder. Nanoparticles for use in positive
electrode 754 generally can have any shape, e.g.,
roughly spherical nanoparticles or elongated
nanoparticles. In addition to lithium manganese oxide,
positive electrode 754 can include other electroactive
nanoparticles such as Ti02 nanoparticles, vanadium oxide
nanoparticles, and/or manganese oxide nanoparticles.
The production of Ti02 nanoparticles has been described,
see U.S. Patent Ser. No. 4,705,762, incorporated herein
by reference. The use of vanadium oxide nanoparticles
in lithium based batteries is described in U.S. Patent
5,952,125, entitled "Batteries With Electroactive
Nanoparticles," incorporated herein by reference.
While some electroactive materials are
reasonable electrical conductors, a positive electrode
generally includes electrically conductive particles in
addition to the electroactive nanoparticles. These _ _
supplementary, electrically conductive particles
generally are also held by the binder. Suitable
- electrically conductive particles include conductive
CA 02350201 2001-05-08
WO 00/23754 PCT/US99/26343
-59-
carbon particles such as~carbon black, metal particles
such as silver particles, stainless steel fibers and the
like.
High loadings of particles can be achieved in
the binder. Particles preferably make up. greater than
about 80 percent by weight of the positive electrode,
- and more preferably greater than about 90 percent by
weight. The binder can be any of various suitable
polymers such as polyvinylidene fluoride, polyethylene
oxide, polyethylene, polypropylene, polytetrafluoro
ethylene, polyacrylates, ethylene-(propylene-diene
monomer) copolymer (EPDM) and mixtures and copolymers
thereof .
Negative electrode 752 can be constructed from
a variety of materials that are suitable for use with
lithium ion electrolytes. In the case of lithium
batteries, the negative electrode can include lithium
metal or lithium alloy metal either in the form of a
foil, grid or metal particles in a binder.
~ Lithium ion batteries use particles of an
composition that can intercalate lithium. The particles
are held with a binder in the negative electrode.
Suitable intercalation compounds include, for example,
graphite, synthetic graphite, coke, mesocarbons, doped
carbons, fullerenes, niobium pentoxide, tin alloys,
Sn02, and mixtures and composites thereof.
Current collectors 758, 760 facilitate flow of
electricity from battery 750. Current collectors 758,
760 are electrically conductive and generally made of
metal such as nickel, iron, stainless steel, aluminum
and copper and can be metal foil or preferably a metal
grid. Current collector 758, 760 can be on the surface
of their associated electrode or embedded within their
associated electrode.
CA 02350201 2001-05-08
WO 00/2??54
PCT/US99/26343
-60-
The separator element 756 is electrically
insulating and provides for passage of at least some -
types of ions. Ionic transmission through the separator
provides for electrical neutrality in the different
sections of the cell. The separator generally prevents
electroactive compounds in the positive electrode from
contacting electroactive compounds in the negative
electrode.
A variety of materials can be used for the
separator. For example, the separator can be formed
from glass fibers that form a porous matrix. Preferred
separators are formed from polymers such as those
suitable for use as binders. Polymer separators can be
porous to provide for_ionic conduction. Alternatively,
polymer separators can be solid electrolytes formed from
polymers such as polyethylene oxide. Solid electrolytes
incorporate electrolyte into the polymer matrix to
provide for ionic conduction without the need for_ liquid
solvent.
Electrolytes for lithium batteries or lithium
ion batteries can include any of a variety of lithium
salts. Preferred lithium salts have inert anions and
are nontoxic. Suitable lithium salts include, for _
example, lithium hexafluorophosphate, lithium
hexafluoroarsenate,lithium bis(trifluoromethyl sulfonyl
imide), lithium trifluoromethane sulfonate, lithium
tris(trifluoromethyl sulfonyl) methide, lithium
tetrafluoroborate, lithium perchlorate, lithium
tetrachloroaluminate, lithium chloride and lithium
perfluorobutane.
If a liquid solvent is used to dissolve the
electrolyte, the solvent preferably is inert and does
not dissolve the electroactive materials. Generally
appropriate solvents include,_ for example, propylene
CA 02350201 2001-05-08
VtlO 00/27754 PCT/US99/26343
-61-
carbonate, dimethyl carbonate, diethyl carbonate, 2-
methyl tetrahydrofuran, dioxolane, tetrahydrofuran, 1,
2-dimethoxyethane, ethylene carbonate,~y-butyrolactone,
dimethyl sulfoxide, acetonitrile, formamide,
dimethylformamide and nitromethane.
The shape of the battery components can be
adjusted to be suitable for the desired final product,
for example, a coin battery, a rectangular construction
or a cylindrical battery. The battery generally
includes a casing with appropriate portions in
electrical contact with current collectors and/or
electrodes of the battery. If a liquid electrolyte is
used, the casing should prevent the leakage of the
electrolyte. The casing can help to maintain the
battery elements in close proximity to each other to
reduce resistance within the battery. A plurality of
battery cells can be placed in a single case with the
cells connected either in series or in parallel.-
PARTICLE SYNTHESIS EXAMPLES
Examgle 1 Manganese Oxide Particle Synthesis, Gas
Phase Reactants
The synthesis of manganese oxide particles
described in this example was performed by laser
pyrolysis. The particles were produced using
essentially the laser pyrolysis apparatus of Fig. 2,
described above, using the reactant delivery apparatus _
of Fig. 3 along with the solid precursor delivery system
shown schematically in Fig. 1.
The manganese carbonyl (Strem Chemical, Inc.,
Newburyport, MA) precursor vapor was carried into the
reaction chamber by flowing -Ar gas through the solid
precursor delivery system containing the Mn2(CO)lo. The
precursor was heated to a temperature as indicated in
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-62-
Table 1. CZHS gas was _used as a laser absorbing gas,
and Argon was used as an inert gas. The reaction gas
mixture containing Mn2 (CO) lo, Ar, OZ and CZH4 was
introduced into the reactant gas nozzle for injection
into the reaction chamber. The reactant gas nozzle had
an opening with dimensions of 5/8 in. x 1/16 in.
- Additional parameters of the laser pyrolysis synthesis
relating to the particles of Example 1 are specified in
Table 1.
TABLE 1
1 2 3
Crystalline Manganosite ManganositeManganosite
&
Phase
unidentified
Crystal Cubic Cubic Cubic
1 5
Structure
Pressure (Torr)180 320 430
- Argon F.R.- 700 700 700
Window (SCCM)
Argon F.R.- 1.71 1.99 1.99
2 0
Shielding (SLM)
Ethylene (SCCM)492 517 517
Carrier Gas 507 507 627
(Argon) SCCM
Oxygen (SCCM) 348 400 420
2 5 Laser Output 260 108 206
(Watts)
Precursor 140 140 150
Temperature
C
30 sccm = standard cubic centimeters per minute
slm = standard liters per minute
Argon - Win. - argon flow through inlets 216, 218
Argon - Sld. - argon flow through annular channel 142.
35 The production rate of manganese. oxide
w particles was typically about 1 g/hr. To evaluate the
atomic arrangement, the samples were examined by x-ray
diffraction using the Cu (Ka) radiation line on a Siemens
D500 x-ray diffractometer. X-ray diffractograms for a
CA 02350201 2001-05-08
WO 00/2'7754 PCT/US99/26343
-63-
sample produced under the conditions specified in the
three columns of Table 1 is shown in Figs. 24-26,
respectively. Under the set of conditions specified in
Table 1, the particles had an x-ray diffractogram
corresponding to manganosite (cubic) MnO. The particles
produced under the conditions in the third column of
Table 1 also had a peak at 65° produced by the aluminum
samples holder. The sample holder is occasionally seen
in the diffractogram. The diffractograms may also have
peaks indicating the presence of small amounts of
amorphous carbon, which can form as a coating on the
particles. The amorphous carbon can be removed by
gentle heating in an oxygen environment. Such coating
of amorphous carbon are described further in copending
and commonly assigned U.S. Patent Application serial
number 09/136,483 to Kumar et al., entitled "Aluminum
Oxide Particles," incorporated herein by reference.
Transmission electron microscopy (TEM) was
used to determine particle sizes and morphology. A TEM
photograph of the particles produced under the
conditions in the second column of Table 1 are shown in
Fig. 27. An examination of a portion of the TEM
micrograph yielded an average particle size of about 9
nm. The corresponding particle size distribution is_
shown in Fig. 28. The approximate size distribution was
determined by manually measuring diameters of the
particles distinctly visible in the micrograph of Fig.
27. Only those particles having clear particle
boundaries were measured to avoid regions distorted or
out of focus in the micrograph. Measurements so
obtained should be more accurate and are not biased
since a single view cannot show a clear view of all
particles. It is significant that the particles span a
rather narrow range of sizes.
CA 02350201 2001-05-08
WO 00!27754 PCT/US99/26343
-64-
Example 2 - Mancranese Oxide Particle Synthesis - Aerosol
Metal Precursors, First Laser Pyrol~rsis Apparatus
The synthesis of manganese oxide particles
described in this example was performed by laser
pyrolysis. The particles were produced using
essentially the laser pyrolysis apparatus of Fig. 2,
described above, using the reactant delivery apparatus
of Fig. 4A.
The manganese chloride (Alfa Aesar, Inc . , Ward
Hill, MA) precursor vapor was carried into the reaction
chamber as an aerosol of an aqueous solution formed with
deionized water. C2H4 gas was used as a laser absorbing
gas, and Argon was used as an inert gas. The reactant
mixture containing MnCl2, Ar, OZ and C2H4 was introduced
into the reactant nozzle for injection into the reaction
chamber. The reactant nozzle had an opening with
dimensions of 5/8 in. x 1/16 in. Additional parameters
of the laser pyrolysis synthesis relating to the
particles of Example 2 are specified in Table 2.
TABLE 2
i
Crystalline Phase Amorphous + Manganosite
(Mn0)
Crystal Structure Amorphous + Cubic
Pressure (Torr) 350
2 5 Argon F.R.-Window (SCCM) - 700
Argon F.R.-Shielding (SLM) 6.8
Ethylene (SLM) 1.27
Carrier Gas (Argon) SLM 6.35
Oxygen (SCCM) 883
3 0 Laser Output (Watts) 660
Precursor Manganese Chloride solution
in water
Precursor Molarity 2 M
CA 02350201 2001-05-08
WO OO/Z7754 PCT/US99/26343
-65-
~~ Precursor Temperature °C ~ ~ Room Temperature
sccm = standard cubic centimeters per minute
slm = standard liters per minute
Argon - Win. - argon flow through inlets 216, 218
Argon - Sld. - argon flow through annular. channel 142.
The production rate of manganese oxide
particles was typically about 1 g/hr. To evaluate the
atomic arrangement, the samples were examined by x-ray
diffraction using the Cu(Ka) radiation line on a Siemens
D500 x-ray diffractometer. X-ray diffractograms for a
sample produced under the conditions specified in Table
2 is shown in Fig. 29. The particles again had an x-ray
diffractogram corresponding to manganosite (cubic) MnO,
although the peaks in the x-ray diffractogram were very
weak indicating that the particles were substantially
amorphous. Based on these results, variations in the
reaction conditions should result in either amorphous
Mn0 or more highly crystalline MnO.
Transmission electron microscopy (TEM) was
used to determine particle sizes and morphology. A TEM
micrograph for the particles produced under the
conditions of Table 2 is displayed in Fig. 30. ~ The
corresponding particle size distribution is shown in
Fig. 31. The particle size distribution was obtained
following the procedure described in Example 1.
Exam le 3 - Man anese Oxide Particles Heat Treated
Samples -
Samples of manganese oxide nanoparticles
produced by laser pyrolysis according to the conditions
specified in the second column of Table 1 and in Table
2 were heated in an oven under oxidizing conditions.
Three samples were heat treated. Two separate samples
were heat processed starting with the nanoparticles
CA 02350201 2001-05-08
dV0 OOf27754 PCT/US99/26343
-66-
produced under the conditions in Table 2. The oven was
essentially as described above with respect to Fig. 5. -
Between about 100 and about 300 mg of nanoparticles were
placed in an open 1 cc vial within the quartz tube
projecting through the oven. Oxygen gas was flowed
through a 1.0 in diameter quartz tube. Other parameters
of the heat processing are specified in Table 3.
Table 3
TemperatureTime Oxygen Crystalline
Flow Rate Phase
1 Sample 1 480 C 3 hrs 200 cc/minMn50g
0
Sample 2A 480 5 hrs 300 cc/minMn30" MnZO,
Sample 2B 300 20 350 Mn304
hrs cc/min
Sample 1 - Sample prepared from particles produced
according to the parameters in the second column of
Table 1.
Samples 2A & 2B - Samples prepared from particles
produced according to the parameters of Table 2.
The crystal structure of the resulting heat
treated particles were determined by x-ray diffraction.
The x-ray diffractogram for samples 1, 2A and 2B of
Table 3 are shown in Figs. 32-34, respectively. The x-
ray diffractogram shown in Fig. 32 indicates that the
manganese oxide in Sample 1 was converted to a form with
a stoichiometry of MnsOg. The x-ray diffractogram of
Sample 2A shown in Fig. 33 indicates the presence of
Mn304, with additional peaks in the spectrum at 23° and
33 ° corresponding to a minor amount of MnZ03 . The x-ray
diffractogram of Sample 2B in Fig. 34 indicates that the
manganese oxide was converted to Mn304. It is not clear
why the Mn0 samples upon heat treatment resulted in
different stoichiometries of manganese oxide. The
different results may be due to the different properties
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-67-
of the starting materials_or the different amounts of
heating times.
Example 4 - Manganese Oxide Particle Synthesis - Aerosol
Metal Precursors, Second Laser Pyrolysis Apparatus
The synthesis of manganese oxide particles
described in this example was performed by laser
pyrolysis. The particles were produced.using a laser
pyrolysis apparatus essentially as shown in Figs. 6-13,
described above and the ultrasonic nozzle essentially as
shown in Figs. 19-20. No cap bushing was used. A
spacer 550 and shim 558 was used to raise the level of
the ultrasonic nozzle to approximately the top of the
cap. The solution delivered by the aerosol delivery
apparatus contained 2 molar MnN03~ H20 (Strem Chemical,
Inc., Newburyport, MA) in solvent formed from 495m1 of
99% isopropyl alcohol and 5m1 of 38% aqueous HC1.
Isopropyl alcohol acts as a infrared absorber. Oxygen
was mixed with the aerosol by delivery through tube 536.
Projecting tubes 546 in Fig. 10 were not present. The
top of cap 486 was about 0.85 inches from the center
line of the laser beam. Additional parameters for two
runs are presented in Table 4.
- Table 4
w 1 2
Crystalline Phase Mn0 + Mn0 +
Mn309 Mn304
Pressure (Torr) 300 200
Argon Window (SLM) 25 7.5
Argon Shielding (SLM) 40 70
Oxygen (SLM) 5 5
Laser Power (input) 1500 1800
(watts)
CA 02350201 2001-05-08
' WO 00/27754 PCT/US99/26343
-68-
Laser Power (output) 1300 1300 -
(watts)
Absorbed Laser Power 200 500
(Watts)
Mass of Powder 3.4 5.0
Recovered
Run Duration (min.) about 30 <30
Ultrasonic Transducer 2.3 4.6
Power (Watts)
sccm = standard cubic centimeters per minute
slm = standard liters per minute
Argon - Win. - argon flow through inlets 330, 332
Argon - Sld. = argon flow through shielding gas conduits
365.
Laser Power (input) - Laser power input into reaction
chamber.
Laser Power (output) - Laser power exiting the reaction
chamber into the beam dump.
Powder manganese oxide was made at a rate of
roughly 20 g/hr. The conditions specified in column 1
of Table 4 resulted in brown powder while the parameters
specified in the.second column of Table 4 resulted in
yellow powder.
To evaluate the atomic arrangement, _the
samples were examined by x-ray diffraction using the
Cu(Kcr) radiation line on a Siemens D500 x-ray
diffractometer. X-ray diffractograms for a sample
produced under the conditions specified in column 1 and
column 2 of Table 4 is shown in Figs. 35 and 36,
respectively. The particles produced under the
conditions in columns 1 and 2 of Table 4 had x-ray
diffractograms indicating the presence of both
manganosite (cubic) MnO~_and hausmannite Mn304. .
Example 5 - Lithium Mancranese oxide Particles by Laser
Pyrolvsis - Aerosol Metal Precursor
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/Z6343
-69-
The synthesis' of manganese oxide/lithium
manganese oxide particles described in this example was
performed by laser pyrolysis. The particles were
produced using essentially the laser pyrolysis apparatus
of Fig. 2, described above, using the reactant delivery
apparatus of Fig. 4A.
The manganese chloride (Alfa Aesar, Inc. , Ward
Hill, MA) precursor and lithium chloride (Alfa Aesar,
Inc.) precursor were dissolved into deionized water.
The aqueous solution had a concentration of 4 molar LiCl
and 4 molar MnCl2. The aqueous solution with the two
metal precursors was carried into the reaction chamber
as an aerosol. C2H4 gas was used as a laser absorbing
gas, and Argon was used as an inert gas . 02, Ar and C2H4
were delivered into the gas supply tube of the reactant
supply system. The reactant mixture containing MnClz,
LiCl, Ar, 02 and C2H4 was introduced into the reactant
nozzle for injection into the reaction chamber. The
reactant nozzle had an opening with dimensions of 5/8
in. x 1/16 in. Additional parameters of the laser
pyrolysis synthesis relating to the particles of Example
1 are specified in Table 5.
TABLE 5
i
2 5 Crystal Structure Amorphous
Pressure (Torr) 450
Argon-Window (SCCM) 700
Argon-Shielding (SLM) 5.6
Ethylene (SLM) 1.27
3 0 Argon (SLM) 1.46
Oxygen (SLM) 1.07 -
Laser Output (Watts) 590
_ Li Precursor - 4 M Lithium Chloride
CA 02350201 2001-05-08
''WO 00127754 PCT/US99/26343
-70-
Mn Precursor 4 M Manganese Chloride
Precursor Temperature C Room Temperature
sccm = standard cubic centimeters per minute
slm = standard liters per minute
Argon - Win. - argon flow through inlets 216, 218
Argon - Sld. - argon flow through annular channel 142.
Argon = Argon directly mixed with the aerosol
The production rate of manganese oxide/
lithium manganese oxide particles was typically about 1
g/hr. To evaluate the atomic arrangement, the samples
were examined by x-ray diffraction using the Cu(Ka)
radiation line on a Siemens D500 x-ray diffractometer.
X-ray diffractograms for a sample produced under the
conditions specified in Table 5 is shown in Fig. 37.
The x-ray diffractogram shown in Fig. 37 indicates that
the sample was amorphous. In particular, a broad peak
from about 27° to about 35° corresponds to the amorphous
lithium manganese oxide. A sharp peak at about 15° is
due to the presence of a trace amount of manganese
chloride contamination. A sharp peak at 53° is due to
a trace amount of an unidentified contaminant.
Example 6 - Heat Treatment of Lithium Mancranese Oxide
Particles Produced by Laser PyrolYsis
A sample of manganese oxide/lithium manganese
oxide nanoparticles produced by laser pyrolysis
according to the conditions specified in the Example 5
were heated in an oven under oxidizing conditions. The
oven was essentially as described above with respect to
Fig. 22. Between about 100 and about 300 mg of
nanoparticles were placed in an open 1 cc vial within
the quartz tube projecting through the oven. Oxygen gas -
was flowed through a 1.0 inch diameter quartz tube at a
f low rate of 308 cc/min. The oven was heated to about
400°C. The particles were heated for about 16 hours.
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-71-
The crystal structure of the resulting heat
treated particles were determined by x-ray diffraction.
The x-ray diffractogram for heated sample is shown in
Fig. 38. The x-ray diffractogram shown in Fig. 38
indicates that the collection of particles involved
mixed phase material with major components of ~LiMn20~
( about 6 0 % by volume ) and Mn30q ( about 3 0 % by vo lume )
and a minor component of Mn203 ( about 10 % by volume ) .
The LiMn20~ compound has a cubic spinel crystal
structure. It is possible that the sample included
additional amorphous phases of materials. In
particular, based on the amount of lithium introduced in
the reactant stream, the sample presumably contains
additional lithium that is not identified in the
crystalline phases.
- Example 7 - Lithium Incorporation Into Manctanese Oxid_e_
Particles '
Manganese oxide particles produced as
described in Example 4 were further treated to form
- lithium manganese oxide. The manganese oxide particles
used were a mixture of particles formed under the
conditions for the synthesis specified in columns 1 and
2 of Table 4. About 2.0 grams of nanocrystalline
manganese oxide was mixed with about 1.2 grams of
lithium nitrate, LiN03 (Alfa Aesar, Inc a, Ward Hill,
MA). The mixtures were heated in an oven under either
pure Oz or under pure Ar. The oven was essentially as
described above with respect to Fig. 22B. The mixture
- of nanocrystalline manganese oxide and lithium nitrate
were placed in an alumina boat within the quartz tube --
projecting through the oven. The selected gas was
flowed through a 1.0- inch diameter quartz tube at a flow
rate of about 40 cc/min. The oven was heated to about
400°C. The particles were heated for about 16 hours.
CA 02350201 2001-05-08
'WO 00!27754 PCT/US99/26343
-~2- _
Three samples were treated. The first sample
_- had a weight ratio of 1.84 parts nanocrystalline MnO to -
1 part LiN03. The second sample had a weight ratio of
1.66 parts nanocrystalline Mn0 to 1 part LiN03. Samples
1 and 2 were heat treated under a flow of oxygen gas.
Sample 3 had a weight ratio of 1.63 parts -
nanocrystalline Mn0 to 1 part LiN03. Sample 3 was heat
treated under a flow of argon gas.
To evaluate the crystal structure of the
materials following heat treatment, the samples were
examined by x-ray diffraction using the Cu (Ka) radiation
line on a Siemens D5000 x-ray diffractometer. The x
ray diffraction spectra for samples 1-3 are depicted in
Fig. 39. The spectrum for sample 1 has peaks
corresponding to unreacted manganese oxide. By
decreasing the manganese oxide to lithium nitrate ratio
from 1.84 to 1.66, complete reaction of the manganese
- oxide was observed. The lithium manganese oxide of
sample 2 had a lattice parameter of approximately 8.17
A, which is similar to that of defect spine! Li2Mn409.
The lithium manganese oxide of sample 3 had a lattice
parameter of approximately 8.23 A, which is similar to
the lattice parameter of LiMnz09. _
Transmission electron microscopy (TEM) was
used to determine particle sizes and morphology of the
lithium manganese oxide and the manganese oxide starting
materials. A TEM micrograph for the manganese oxide
nanoparticle starting material is shown in Fig. 40. A
TEM micrograph for the lithium manganese oxide of sample
1 is shown in-.Fig. 41. -Note that the particle size did
not change significantly, if at all, during the heating
process. Due to the lack of optimization of the aerosol
conditions, the particles of manganese oxide displayed
in Fig. 40 have a broader particle size distribution
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-73-
than found for manganese_oxide in Examples 1 and 2
above. Since the incorporation of lithium into the
manganese oxide nanoparticles does not significantly
change the size of the particles, lithium manganese
oxide nanoparticles with a narrow size distribution can
be made using the manganese oxide nanoparticles
described above.
Example 8 - Direct Laser Pyrolysis Synthesis of
Crystalline Lithium Mancranese Oxide With an Aerosol
The synthesis of crystalline lithium manganese
oxide particles described in this example was performed
by laser pyrolysis. The particles were produced using
essentially the laser pyrolysis apparatus of Fig. 2,
described above, using the reactant delivery apparatus
of Fig. 4B or 4C.
Two solutions were formed with manganese
nitrate (Mn (N03) z, Alfa Aesar, Inc . , Ward Hill, MA)
precursor, lithium nitrate (Alfa Aesar, Inc.) precursor
and urea (CH4Nz0) . The first solution was used to form
sample 3 of Table 6. The first solution was an aqueous
solution with a concentration of 3 molar LiN03 and 4
molar Mn (N03) z . The solvent for the second solution was
a 50:50 percent by weight mixture of isopropyl alcohol
and deionized water. The second solution had a
concentration of 2 molar LiN03, 2 molar Mn (N03) 2, and 3 . 6
molar urea. The second-solution was used to form the
first and second samples of Table 6. _
The selected solution with the two metal
precursors was carried into the reaction chamber as an
aerosol. CZH4 gas was used as a laser absorbing gas,
and Argon was used as an inert gas . 02, Ar and C2H4 were
delivered into the gas supply tube of the reactant
supply system. The reactant mixture containing
Mn(N03)Z, LiN03, Ar, OZ and CZH4 was introduced into the
CA 02350201 2001-05-08
CVO 00/27754 PCT/US99/26343
-74-
reactant nozzle for delivery into the reaction chamber.
The reactant nozzle had an opening with dimensions of ,
5/8 in. x 1/4 in. The first two samples were formed
with a reactant delivery system essentially as shown in
Fig. 4B. The third sample was prepared with a reactant
delivery system essentially as shown 'in Fig. 4C.
Additional parameters of the laser pyrolysis synthesis
relating are specified in the first two columns of Table
6.
TABLE 6
1 -- 2 _" 3 -.
Crystal Structure LiMnzO~ LiMnzO, LiMnzO,
(major) (major) (major)
+ Mn~O, + Mn~O, + Mn30,
Pressure (Torr) 600 600 600
Argon-Window (SLM) 2.24 2.24 2.24
1 5 Argon-Shielding (SLM) 9.86 9.86 9.86
Ethylene (SLM) 0.80 0.80 1.24
Argon (SLM) 3.61 3.60 4.17
Oxygen (SLM) 0.97 0.99 1.46
Laser Input (Watts) 650 970 380
2 0 Laser Output (Watts) 540 830 320
Production Rate (gm/hr) 1.8 1.3 17.0
Precursor Temperature C Room Room Room
Temp. Temp. Temp.
slm = standard liters per minute
25 Argon - Win. - argon flow through inlets 216, 218
Argon - Sld. - argon flow through annular channel 142.
Argon = Argon directly mixed with the aerosol
To evaluate the atomic arrangement, the
- samples were examined by x-ray diffraction using the
30 Cu(Ka) radiation line on a Siemens D500 x-ray
diffractometer. X-ray diffractograms for samples
produced under the conditions of columns 1 and,2
specified in Table 6 are shown in Fig. 42. This is a
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-75-
representative diffractogram, although some samples had
relatively small peaks due to Mn304 contamination. X-
ray diffraction peaks characteristic of spinel lithium
manganese oxide are clearly visible in the
diffractogram. Small differences in stoichiometry
within the spinel structure are difficult to elucidate
from the x-ray diffractogram. In addition, the x-ray
diffractogram peaks are broad, which may be due to the
smal l particle size or inhomogeneous broadening
resulting from either having a mixed phase material or
variations in stoichiometry. Nevertheless, the
diffractogram is consistent with the sample containing
a mixture of LiMn204 and Li4Mn5012 or an intermediate
stoichiometry material. These conclusions are confirmed
by electrochemical evaluation described below. In any
case, the crystalline lithium manganese oxide seems to
comprise a majority (greater than about 50%) of the
material in one form or another.
Transmission electron microscopy (TEM) was
used to determine particle sizes and morphology of the
as synthesized, crystalline lithium manganese oxide. A
TEM micrograph for the lithium manganese oxide of the
sample produced under the conditions of column 2 of
Table 6 is shown in Fig. 43. The corresponding particle
size distribution is shown in Fig. 44. The particle
size distribution was obtained following the procedure
described in Example 1. The average particle diameter
is about 40 nm. The particle size distribution shows a
relatively broad particle size distribution relative to
particle size distributions generally obtainable by
laser pyrolysis. Reactant delivery with the reactant
delivery apparatus of Fig. 4C has a higher reactant
throughput and a correspondingly larger production rate
compared with the aerosol delivery apparatuses of Figs.
CA 02350201 2001-05-08
,WO 00/27754
PCT/US99/26343
-76-
4A and 4B . The aerosol produced by the Apparatus of
Fig. 4C evidently is not as uniform as the aerosols
produced by the other two apparatuses. The particle
size distribution using the reactant delivery apparatus
of Fig. 4C can be narrowed by using a lower pressure
from about 200-300 Torr and by increasing the 02 flow to
obtain the desired phase of product.
Example 9 - Silver Vanadium Oxide Nanopartic~les
The synthesis of silver vanadium oxide
nanoparticles described in this example was performed by
laser pyrolysis. The particles were produced using
essentially the laser pyrolysis apparatus of Fig. 2,
described above, using the reactant delivery apparatus
of Fig. 4B or 4C.
Two solutions were prepared for delivery into
the reaction chamber as an aerosol. Both solutions were
produced with comparable vanadium precursor solutions.
To produce the first vanadium precursor solution, a 10.0
g sample of vanadium (III) oxide (V203) from Aldrich
Chemical (Milwaukee, WI) was suspended in 120 ml of
deionized water. A 30 ml quantity of 70% by weight
aqueous nitric acid (HNO,) solution was added dropwise
to the vanadium (III) oxide suspension with vigorous
stirring: Caution was taken because the reaction with
nitric acid is exothermic and liberates a brown gas
suspected to be N02. The resulting vanadium precursor
solution (about 150 m1) was a dark blue solution. The _
second vanadium precursor solution involved the scale-up
of the first precursor solution by a factor of three in
all ingredients.
To produce a first silver solution, a solution _'
of silver carbonate (Ag2C03) from Aldrich Chemical
(Milwaukee, WI) was prepared by suspending 9.2 g of
silver carbonate in a 100 ml volume of deionized water.
CA 02350201 2001-05-08
VVO 00/2'7754 PCT/US99/26343
_77_
A 10 ml quantity of 70%-by weight aqueous nitric acid
(HN03) was added dropwise with vigorous stirring. A
clear colorless solution resulted upon completion of the
addition of nitric acid. To produce a ffirst metal
mixture solution for aerosol delivery, the silver
solution was added to the first vanadium precursor
solution with constant stirring. The resulting dark
blue first metal mixture solution had a molar ratio of
vanadium to silver of about 2:1.
To produce a second silver solution, 34.0 g of
silver nitrate (AgN03) from Aldrich Chemical (Milwaukee,
WI) was dissolved in a 300 ml volume of deionized water.
To prepare a second solution of metal mixtures for
aerosol delivery, the silver nitrate solution was added
to the second vanadium precursor solution with constant
stirring. The resulting dark blue second metal mixture
solution also had a molar ratio of vanadium to silver of
about 2:1.
The selected aqueous solution with the
vanadium and silver precursors was carried into the
reaction chamber as an aerosol. CZH4 gas was used as a
laser absorbing gas, and Argon was used as an inert gas .
O2, Ar and C2H4 were delivered into the gas supply tube
of- the reactant supply system. The reactant mixture
containing vanadium oxide, silver nitrate, Ar, Oz and
C2H4 was introduced into the reactant nozzle for
injection into the reaction chamber. The reactant
nozzle had an opening with dimensions of 5/8 in.-x 1/4
in. Additional parameters of the laser pyrolysis
synthesis relating to the particle synthesis are
specified in Table 7. Sample 1 was prepared using the
reactant delivery system essentially as shown in Fig. 4B
while sample 2 was prepared using the reactant delivery
system essentially as shown in Fig. 4C.
CA 02350201 2001-05-08
WO 00/27754
PCT/US99/26343
_78_
10
slm = standard liters per minute
Argon - Win. - argon flow through inlets 216, 218
Argon - Sld. - argon flow through annular channel 142.
Argon = Argon directly mixed with the aerosol
To evaluate the atomic arrangement, the
samples were examined by x-ray diffraction using the
Cu(Ka) radiation line on a Siemens D500 x-ray
diffractometer. X-ray diffractograms for samples 1
(lower curve) and 2 (upper curve) produced under the
conditions specified in Table 7 are shown in Fig. 45.
The samples had peaks corresponding to VOZ, elemental
silver and peaks that did not correspond- to known
materials. A significant crystalline phase for these
samples had peaks at 28 equal to about 30-31°, 32, 33
and 35. This phase is thought to be a previously
unidentified silver vanadium oxide phase. This
crystalline silver vanadium oxide phase is observed in
samples prepared by mixing vanadium oxide nanoparticles
and silver nitrate under conditions where the samples
- TABLE ~ _
CA 02350201 2001-05-08
WO 00/37754 PCT/US99/26343
_79_
are heated for an insufficient time period to produce
AgZV~011. Specific capacity measurements of sample 1 in
a coin cell are consistent with this interpretation.
Powders of samples produced under the
conditions specified in Table 7 were further analyzed
using transmission electron microscopy. The TEM
micrographs are shown in Figs. 46A (first column of
Table 7) and 46B (second column of Table 7). The TEM
micrograph has a particles falling within different size
distributions. This is characteristic of mixed phase
materials made by laser pyrolysis, where each material
generally has a very narrow particle size distribution.
The portion of silver vanadium oxide in the mixed phase
material should be increased by an increase in oxygen
flow, a decrease in laser power and an increase in
pressure. The production of silver vanadium oxide
particle by laser pyrolysis is described further in U.S.
Patent Application Serial No. 09/311,506 to Reitz et
al., entitled "Metal Vanadium Oxide Particles,"
incorporated herein by reference.
BATTERY EXAMPLES
In addition, lithium manganese oxide based
lithium batteries were evaluated to determine the charge
capacity and energy density of the lithium manganese
oxide powders used as active materials in the positive
electrodes. The batteries tested in examples 9-11 were
all produced following a common procedure. The lithium
manganese oxide powders (LMO) were mixed with a
conductive acetylene black powder (AB) (Catalog number
55, Chevron Corp.) at a ratio of 80:10. The powder
mixture was ground with a mortar and pestle to
thoroughly mix the powders.
A few drops of polytetrafluoroethylene (PTFE)
solution were added to the homogeneous powder mixture.
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
_80_ _
The 10 percent PTFE solution included PTFE (Aldrich
Chemical Co., Milwaukee, WI) solution in water. The
final ratio of LMO:AB:PTFE was 80:10:10. A small amount
of methyl alcohol (Aldrich Chemical Co., Milwaukee, WI)
was added to the mixture. In addition, isopropyl
alcohol (Aldrich Chemical Co., Milwaukee,~WI) was added
to cover the mixture.
The slurry was put in a blended to mix
thoroughly, and the solution was passed through a vacuum
filter to remove the solvents. The resulting powder
mixture was needed and rolled to a 5-mil thickness. An
aluminum mesh (Dekler, Branford CT) was placed on the
mixture, and further rolled down to achieve a final
thickness with the mesh of 5 mils. The mixture with the
aluminum mesh was baked in a vacuum oven for two hours
at 250°C to remove residual solvent and to melt the
PTFE. After removal from the oven, the electrodes were
punched to l6mm and pressed at 50001bs of pressure . The
punched out electrodes were placed again in the vacuum
oven overnight at 120°C to remove residual moisture.
After removal from the oven, the electrodes were
immediately placed in a glove box (Vacuum Atmosphere
Co., Hawthorne, CA) under an argon atmosphere. In the
glove box, the electrodes were weighted and measured for
thickness.
The samples were tested in an cell 800 with an
airtight two-electrode configuration shown in Fig. 47.
T_he casing 802 for the sample battery was obtained from
Hohsen Co., Osaka, Japan. The casing included a top
portion 804 and a bottom portion 806, which are secured
with four screws 808. The two other screws not shown in
Fig. 44 are behind the two screws shown. Lithium metal
(Alfa/Aesar, Ward Hill, MA) was used as a negative
electrode 812. Negative electrode 812 was placed within
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-81-
the bottom portion 806.._ A separator 814, Celgard~ 2400
(Hoechst Celanese, Charlotte, NC), was placed above the
lithium metal. A Teflon° ring 816 was placed above
separator 814. A positive electrode 818 was placed mesh
side up within Teflon ring 816. An aluminum pellet 820
was placed above positive electrode 818, and electrolyte
was added. The electrolyte from EM Industries
(Hawthorne, NY) was 1M LiPFs in 1:1 ethylene carbonate/
dimethyl carbonate. A Teflon o-ring is located between
top portion 804 and bottom portion 806 to electrically
insulate the two electrodes. Similarly, screws 808 are
placed within a Teflon sleeve to electrically insulate
screws 808 from top portion 804 and bottom portion 806.
Electrical contact between the battery tester and cell
- 800 is made by way of top portion 804 and bottom portion
806.
The samples were tested with a discharge/
charge rate at a constant current of 0.5 mA/cm2, and
cycled between 2.5V to 4.4V, or 2.2V to 3.3V, or 3.5V to
4.4V at 25°C. The measurements were controlled by an
Arbin Battery Testing System, Model BT4023, from Arbin
Instruments, College Station, TX. The charging/
discharging profiles were recorded, and the discharge
capacity of the active material was obtained.
The energy density is evaluated by the
integral over the discharge time of the voltage
multiplied by the current divided by the mass of the
active material. The current during testing was lmA,
corresponding to a current density of 0.5 mA/cm2. The
active material mass ranged from about 30 to about 50
mg.
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
-82-
Example 10 - Four Va1-t Cyclina Behavior Thermal
Synthesis of Lithium Mancranese Oxide
This example explores the four volt cycling
behavior of four different lithium manganese oxide
materials. Cells were produced following the procedure
outlined above with material produced as sample 2 and
sample 3 of Example 7. For comparison, cells were also
produced using standard LiMnZ04 and Li2Mn409. Commercial
LiMn204 was purchased from Alfa Aesar, a Johnson-Matthey
Company, Ward Hill, MA. Li2Mn409 (standard Li2Mn909) was
synthesized using a standard procedure based on heating
a mixture of manganese carbonate and lithium carbonate
at about 400°C for about 60 hours. The cycling behavior
in the four volt, range was examined by charging the
material to a voltage of 4.40 volts and allowing the
material to discharge. The resulting discharge curve is
shown in Fig. 48. In Fig. 48, the cycling behavior of
cells made with nanoparticles from Example 7 are
labelled sample 2 and sample 3, appropriately, and the
cycling behavior of cells made with the commercial or
standard powders are labelled by their stoichiometry
with the notation ~~Commercial~~ . Note that the cell with
sample 2 had a discharge curve similar to the LizMn409
commercial material, and the cell with sample 3 had a
discharge curve similar to LiMn2O9 commercial material.
Examt~le 11 - Three Volt Cycling Behavior Thermal
Synthesis of Lithium Manganese Oxide
This example explores the three volt cycling
behavior of four different lithium manganese oxide
materials. Cells were produced following the procedure
outlined above with material produced as sample 2 and '
sample 3 of Example 7. For comparison, cells were also
produced using commercial LiMnz09 and Li2Mn409. T_he
cycling behavior in the three volt range was -examined by
CA 02350201 2001-05-08
NJO 00/37754 PCT/US99/26343
-83-
charging the material -to a voltage of 3.30 volts and
allowing the material to discharge. The resulting
- discharge curve is shown in Fig. 49. In Fig. 49, the
cycling behavior of cells made with nanoparticles from
Example 7 are labelled sample 2 and sample 3,
appropriately, and the cycling behavior of cells made -
with the commercial powders are labelled by their
stoichiometry with the notation "Commercial". Note that
the cell with sample 2 had an insertion potential
similar to the Li2Mn409 commercial material, and the cell
with sample 3 had a insertion potential similar to
LiMn204 commercial material.
Example 12 - CvclinQ Properties -. Thermal Svnthesis of
Lithium Manganese Oxide
The cycling behavior of cells made as
- described above was explored further for four different
positive electrode materials. Cells were produced
_ following the procedure outlined above with material
produced as sample 2_and sample 3 of Example 7. For
comparison, cells were also produced using commercial
LiMn204 and Li2Mn409. Cells with positive electrode
materials from sample 2 and commercial LiMn204 were
produced and run in duplicates. Only one cell was
produced with the sample 3 materials, while three cells
with commercial Li2Mn409 were produced and ruri. For each
cycle, the capacity in mAh/g were evaluated. The cells
were cycled between about 3.3 volts and 2.0 volts. The
results are plotted in Fig. 50.
The cycling results show that the cells
produced with_ the nanoparticles have greater cycling
stability. The improved cycling stability may be due to
the nanoparticles being more structurally resistant to
the repetitive volume expansion - and contraction
accompanying the lithium insertion and extraction.
CA 02350201 2001-05-08
WO 00/27754 PCT/US99/26343
_ 84 _ . _
Thus, this data indicates an advantage of nano-scale
lithium manganese oxide particles as a lithium based
rechargeable battery active material.
Example 13 - Beaker Cell Testing of Lithium Mancranese
Oxide Directly Produced by Laser Pyrolysis Synthesis
The properties of crystalline lithium
manganese oxide nanoparticles directly synthesized by
laser pyrolysis was examined using a beaker cell test.
Test cells were produced with two samples of
IO nanomaterials. The first sample was produced under the
conditions indicated in column 1 in Table 6 of Example
8, and the second sample was produced under the
conditions indicated in column 2 in Table 6. Both
samples had a majority component of LiMnZ04 and a
minority phase of Mn304. .
To produce the batteries for beaker cell
testing, the lithium manganese oxide powders were mixed
with a conductive acetylene black powder (Catalog number
55, Chevron Corp.) at a ratio of 60:30. The powder
mixture was ground with a mortar and palette to
thoroughly mix the powders.
A few drops of polyvinylidene fluoride (PVDF)
solution were added to the homogeneous powder mixture.
The IO percent PVDF solution included PVDF (type 714,
Elf Atochem North America, Inc., Philadelphia, PA)
dissolved in 1-methyl-2-pyrroidinone (Aldrich Chemical
Co . , Mi lwaukee , WI ) . The f final rat io of LiXMnYOZ : AB : PVDF
was 60:30:10. The resulting slurry was spread onto a
preweighed aluminum metal mesh. The mesh with the
slurry was baked in a vacuum oven overnight at 120°C to
remove the solvent and residual moisture. After removal _
from the oven, the electrodes were immediately placed in
a glove box (Vacuum Atmosphere Co., Hawthorne, CA) under
,_- an argon atmosphere and weighted again.
CA 02350201 2001-05-08
CVO 00/7754 PCT/US99/26343
-85-
All discharge/charge experiments were
conducted in the glove box. The water and oxygen
concentrations in the glove box were measured to be less
than 1 ppm and 1.5 ppm, respectively. The samples were
tested in a three electrode configuration, as shown in
Fig. 51. In the test set up, cathode 832 on aluminum
mesh 834 is place in container 836. Container 836 holds
liquid electrolyte 838. Counter electrode 840 and
reference electrode 842 are also placed into container
836. Lithium metal was used as both counter electrode
and reference electrode. The electrodes are connected
to a battery testing system 844.
No separator is needed for this testing
configuration since the electrodes are physically
separated. Alternatively, the liquid electrolyte can be
viewed as the separator. The liquid electrolyte (from
- Merck & Co., Inc.) was 1M LiC109 in propylene carbonate.
Charge and discharge experiments were
conducted at an approximately constant current
equivalent to about 5 mA per gram of oxide within the
electrode. Each electrode contained about 10 mg of
nanoparticles. Thus, the currents were about 0.05 mA.
If the material were pure lithium manganese oxide, this
charge/discharge rate corresponds to a rate of C/30
(i.e., a rate such that the cathode would be fully
discharged in 30 hours). The cells were initially
charged from their open-circuit voltage up to 4.4 volts
and then discharged down to 2.0 volts.
The measurements were controlled by an Arbin
Battery Testing System, Model BT4023, from Arbin
Instruments, College Station, TX. The charging/
discharging profiles were recorded, and the specific
capacity was obtained. The specific capacity was
evaluated as the discharge capacity divided by the mass
CA 02350201 2001-05-08
WO 00/27?54 PCT/US99/26343
-86-
of the active material._ Also, the differential capacity
(bx/SV} was determined by taking the derivative of the
- discharge capacity with respect to voltage. Therefore,
the differential capacity is the inverse slope of the
charge and discharge profile with respect to voltage.
Peaks in the plot of differential capacity versus
voltage indicate voltages where lithium inserts into the
host material. In a lithium metal cell, the cell
voltage is approximately proportional to the chemical
potential of Li' in the host material. Therefore, the
differential capacity can be used to characterize and/or
identify the material and its structure.
In Fig. 52~ the discharge curves for samples
1 and 2 are compared with the discharge curve for bulk
commercial LiMn209. While the laser pyrolysis
synthesized lithium manganese oxide had a significantly
lower specific capacity, the nanoparticles exhibited
significant specific capacities. The differential
capacity of the nanoparticles and the bulk/commercial
materials, shown in Fig. 53, had similar peak position
and shape. This indicates that the electrochemically
active phases of all three materials have nearly
identical insertion profiles. Thus, the crystal
structure of the three materials was the same. The
lower specific capacities of the nanomaterials can be
attributed to phases that are not electrochemically
active in this test, including the manganese oxide
material and lithium that has not been incorporated into
lithium manganese oxide.
The embodiments described above are intended
to be illustrative and not limiting. Additional
embodiments are within the claims below. Although the
present invention has been described~with reference to
preferred embodiments, workers skilled in the art will
CA 02350201 2001-05-08
1~N0 00/2?754 PCT/US99/26343
_87_
recognize that changes-Fray be made in form and detail
without departing from the spirit- and scope of the
invention.