Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02352436 2008-08-25
27400-208
- 1 -
Polymorphs of telmisartan, processes for preparing them
and their use in the preparation of a pharmaceutical
composition
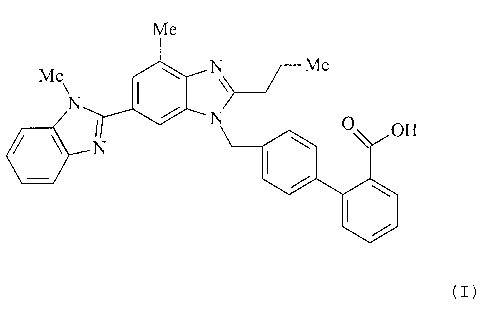
The invention relates to polymorphs of 4'-[2-n-propyl-4-
methyl-6-(1-methylbenzimidazol-2-yl)benzimidazol-l-
ylmethyl]biphenyl-2-carboxylic acid (INN: telmisartan),
particularly the polymorphic form B, mixtures of the
polymorphs, processes for preparing telmisartan containing
form B and the use thereof for preparing a pharmaceutical
composition.
Background of the invention
The compound telmisartan is known from European Patent
EP 502 314 Bl and has the following chemical structure:
Me
I N Me \>
N
N o oH
I ~ \
(I)
Telmisartan and the design physiologically acceptable
salts thereof have valuable pharmacological properties.
Telmisartan is an angiotensin antagonist, particularly an
angiotensin-II-antagonist which by virtue of its
pharmacological properties may be used for example to
treat hypertension and cardiac insufficiency, to treat
ischaemic peripheral circulatory disorders, myocardial
ischaemia (angina), to prevent the progression of cardiac
insufficiency after myocardial infarct, to treat diabetic
neuropathy, glaucoma, gastrointestinal diseases and
bladder diseases. Other possible therapeutic applications
CA 02352436 2001-05-28
- 2 -
can be found in EP 502314 Bl, the contents of which are
hereby referred to.
In the course of the synthesis of telmisartan, the final
step of the synthesis comprises saponifying the tert.butyl
ester (II) according to Diagram 1.
Me Me
Me NMe Me NMe
N N N
~-~ N O O -- JI ~ O OH
Diagram 1:
The corresponding experimental procedure which may be
carried out on a laboratory scale can be found in EP
502314 Bl. Surprisingly, however, it proved to be simple
to transfer the method of synthesis already known to a
large-scale industrial manufacturing process. The
telmisartan synthesised on an industrial scale according
to Diagram 1 is obtained after working up in the form of a
product which has to be subjected to a further
crystallisation step to complete the purification. In this
obligatory crystallisation step the morphology of the end
product which crystallises out leads to unforeseen
problems.
The product which is precipitated as a solid in the form
of long needles is difficult to filter, wash and isolate,
is further characterised by a very long drying time on
account of the presence of solvent and forms large, very
hard fragments during the drying process. Grinding these
fragments produces a dry powder which exhibits a strong
tendency to electrostatic charging and is virtually
impossible to pour.
CA 02352436 2008-08-25
27400-208
- 3 -
The disadvantageous properties of a product as described
above have always proved to be a serious obstacle to the
large-scale manufacture of a compound, as reproducible
manufacture of large amounts of the compound in highly
pure form is only possible with considerable difficulty or
with high additional technical costs.
The aim of the present invention is therefore to prepare
telmisartan in a form which permits the large-scale
synthesis, working up, purification and isolation of
telmisartan in which the above disadvantages are overcome.
Detailed description of the invention
Surprisingly, it has been found that telmisartan may occur
as a solid in different crystalline modifications.
Depending on the nature of the crystallisation process it
may be converted into two different polymorphic forms A
and B.
Polymorph A is a form of telmisartan which is obtainable
according to the prior art, and which gives rise to the
abovementioned problems in large-scale manufacture or
purification, isolation and drying of the product.
The polymorphic form B of telmisartan which was
surprisingly found, however, shows virtually no tendency
to electrostatic charging, is easy to suction filter,
centrifuge, wash and dry and is free-flowing even without
being ground up.
CA 02352436 2008-08-25
27400-208
- 3a -
According to one aspect of the present invention, there is
provided polymorphic crystal modification B (form B) of
telmisartan (formula I),
Me
Me N Me
\ \~
N
I / N OH
N
(I)
characterised by an endothermic maximum at 183 2 C which
occurs during thermal analysis using DSC.
According to another aspect of the present invention, there
is provided process for preparing the form B of telmisartan
described herein or telmisartan described herein, wherein:
a) telmisartan is taken up in a mixture of solvents
consisting of water, formic acid and an organic solvent
miscible therewith, heated, and the resulting solution is
then filtered; b) the organic solvent is distilled off, and
water is optionally added during the distillation in an
amount substantially corresponding to the amount of the
organic solvent distilled off; c) the telmisartan form B is
precipitated out of the remaining solution by the addition
of a base; and d) the product precipitated is centrifuged,
washed and dried.
The following procedure is used according to the invention to
prepare the polymorphic form B of telmisartan.
Crude telmisartan product (crystallized for example from
dimethylformamide, dimethylacetamide or the like) is taken
CA 02352436 2001-05-28
- 4 -
up, in a suitably sized stirring apparatus, optionally
with 1-5 wt.-a, preferably with 3 wt.-o of activated
charcoal in a mixture of solvents consisting of water,
formic acid and a suitable organic solvent and then
dissolved at elevated temperature, preferably at a
temperature of from 50-90 C, most preferably at 60-80 C.
According to the invention it is essential to use the
solvent mixture of formic acid and water with an organic
solvent which must satisfy the following criteria
according to the invention. It must be capable of forming
a solution with the mixture of formic acid and water. It
must be largely chemically inert relative to the mixture
of formic acid and water and it must be capable of being
separated from the mixture of formic acid and water by
distillation. Organic carboxylic acid esters, ketone or
ethers may be used. Acetone, methylethylketone, methyl
acetate, ethyl acetate, ethyl formate, ethyleneglycol
dimethylether or tetrahydrofuran may be mentioned by way
of example. Acetone, methylethylketone, methyl acetate,
ethyl acetate and THF are preferred according to the
invention, while ethyl acetate is particularly preferred.
According to the invention, the mixture of solvents should
be made up of 0.3-0.7 1 of water, 10-15 mol of formic acid
and 0.3-0.9 1 of the organic solvent per mol of
telmisartan. A ratio of 0.4-0.6 1 of water, 11-13 mol of
formic acid and 0.4-0.7 1 of the organic solvent based on
1 mol of telmisartan is preferred. A ratio of about 0.5 1
of water, about 11.5-12 mol of formic acid and about 0.5 1
of the organic solvent based on 1 mol of telmisartan is
particularly preferred.
According to the invention, after the abovementioned
heating, the solution obtained is filtered and washed with
a mixture of the abovementioned organic solvent and formic
acid. The washing solution may contain 0.3-1.0 mol,
preferably 0.4-0.6 mol, most preferably about
CA 02352436 2001-05-28
- 5 -
0.5 mol of formic acid per mol of telmisartan. The
quantity of washing solution will naturally depend on the
quantity of dissolved telmisartan. According to the
invention, 0.1-0.4, preferably 0.15-0.3, most preferably
0.2 1 of the organic solvent are used to each mol of
telmisartan.
After the filter residue has been washed with the washing
solution described above, the organic solvent is distilled
off as much as possible whilst water is added
simultaneously. The temperature is kept in the range from
60-100 C, preferably from 70-100 C. The total quantity of
water added corresponds substantially to the total amount
of solvent distilled off. Almost total distillation of the
organic solvent is desirable according to the invention.
Accordingly, the distillation is continued until water is
also distilled off, partly azeotropically. The organic
solvent distilled off may be used again in subsequent
reactions, if necessary after removal of the aqueous
phase.
In order to precipitate the telmisartan-polymorphic B it
is then cooled to a temperature in the range from 15-60 C,
preferably to 20-30 C, and precipitated with a base. The
quantity of base to be used depends on the amount of
formic acid used. Preferably, 0-2 mol less base is added
than there is formic acid present. Most preferably, 0.3-
1.5 mol less base is added than there is formic acid
present. It is most particularly preferred to add 0.5-1
mol less base than there is formic acid present. Suitable
bases might be either aqueous solutions of potassium
hydroxide, sodium hydroxide, lithium hydroxide or ammonia.
It is also possible to use suitable organic bases such as
triethylamine, diisopropylethylamine or DBU
(diazabicycloundecene). Particularly preferred bases are
the abovementioned aqueous solutions of the potassium
CA 02352436 2001-05-28
- 6 -
hydroxide, sodium hydroxide, lithium hydroxide or ammonia,
the aqueous solutions of ammonia being particularly
important.
The product precipitated is centrifuged, washed with water
and normally dried in vacuo at 120-125 C.
A sample taken directly after centrifuging and dried in a
thin layer in a circulating air drier in the laboratory
typically shows a content of 95-99% of crystalline form B.
After centrifugation, the product begins to change
partially into form A, depending on the temperature, pH,
retention time, and water content, towards the end of the
drying. Therefore, in working mixtures, ratios of form A
to form B of at best about 10:90 are obtained after
drying, but ratios of 60:40 may also be obtained.
However, even a content of form B as low as this
guarantees that the product will have the positive
qualities required for large-scale production (e.g. a low
tendency to electrostatic charging, a low tendency to
clumping, free-flowing characteristics etc.). What is
essential to the invention in the crystallisation process
mentioned above is that initially only form B is produced,
with its characteristic macroscopic crystalline form. This
macroscopic crystalline form is largely retained under the
drying conditions, in spite of partial microscopic
rearrangement into form A.
Other highly advantageous aspects of the procedure
according to the invention are the high space/time yield
of the present process and the high yield of pure
telmisartan product, which can be isolated in virtually
quantitative amounts.
The telmisartan of form A which can be obtained by the
manufacturing process known from the prior art differs
from the telmisartan obtainable according to the
invention, which is characterised in that it contains some
CA 02352436 2008-08-25
27400-208
- 7 -
polymorphic form B, in the advantageous qualities of the
product already mentioned hereinbefore. Other
distinguishing features will be described hereinafter.
Telmisartan of form A crystallises as long, fine or thin
needles which cling together in a felt-like manner. The
crystal modification of telmisartan of form B produces
very compact cubic to spherical crystals which trickle
like sand or silica gel.
The two polymorph forms A and B of telmisartan differ
considerably in their melting point. Form B melts at
183+/-2 C (determined by DSC), form A at 269+/-2 C
(determined by DSC). After melting, the lower-melting form
B of telmisartan crystallises out again as form A. This
results, for example, in the endothermic maximum at 183+/-
2 C determined by DSC being followed by a characteristic
exothermic maximum which reflects the crystallisation of
the melt of form B into the high-melting form A. The DSC
diagrams (DSC=Differential Scanning Calorimetry) obtained
TM
with a Mettler DSC-20, TA8000 system are shown in Figure
1.
The polymorphs A and B also differ in their IR spectrum.
On the basis of this difference, the IR spectroscopy may
optionally be used for quantitative determination of the
ratio of the two crystal modifications in the end product
after drying. Pure polymorph A has a characteristic band
at 815cm-1 in the IR spectrum. In polymorph B this
oscillation is_shifted to 830cm -1. Since these two
characteristic bands of polymorphs A and B are
sufficiently far apart, they are particularly suitable for
the abovementioned quantitative determination of the ratio
of the two crystal modifications.
The IR-spectroscopic characterisation of the two
polymorphic forms A and B was carried out using the
CA 02352436 2008-08-25
27400-208
- 8 -
Nicolet FTIR Spectrometer Magna - IR 550 in KBr (2.S mol
per 300 mg KBr; Nicolet software package OMNIC, version
1.20).
The Examples which follow serve to illustrate purification
and crystallisation processes carried out by way of
example in order to prepare the polymorphic form B of
telmisartan. They should be regarded purely as possible
procedures described by way of example, without
restricting the invention to their contents.
Example 1
205.6 kg of recrystallised telmisartan (recrystallised
from dimethylformamide or dimethylacetamide), 6.2 kg of
activated charcoal, 205.6 1 of water, 211.6 kg of formic
acid (99-100%) and 205.6 1 of ethyl acetate are placed in
a 1200 1 stirring apparatus. The mixture is stirred for
about 1 h at 70 - 80 C and then filtered into another
1200 1 stirring apparatus and washed with a mixture of
82.2 1 of ethyl acetate and 9.2 kg of formic acid (99-
1000). About 308 1 of solvent are distilled off at 80 -
100 C whilst simultaneously 308 1 of water are added. The
mixture is then cooled to 20 - 30 C and precipitated by
the metered addition of 313 kg of 25 % ammonia solution.
The product precipitated is centrifuged, washed with water
and dried at 120-125 C.
Yield : 200 kg telmisartan (97.3 a of theory)
Example 2
185 kg of recrystallised telmisartan (recrystallised from
dimethylformamide or dimethylacetamide), 5.6 kg of
activated charcoal, 185 1 of water, 190.4 kg of formic
acid (99-100%) and 185 1 of tetrahydrofuran are placed in
a 1200 1 stirring apparatus. The mixture is stirred for
. CA 02352436 2001-05-28
- 9 -
about 1 h at 60 - 70 C and then filtered into another
1200 1 stirring apparatus and washed with a mixture of 74
1 of tetrahydrofuran and 8.3 kg of formic acid (99-1000).
About 278 1 of solvent are distilled off at 70 - 100 C
whilst simultaneously 278 1 of water are added. The
mixture is then cooled to 20 - 30 C and precipitated by
the metered addition of 281.5 kg of 25 % ammonia solution.
The product precipitated is centrifuged, washed with water
and dried at 120-125 C.
Yield : 180 kg telmisartan (97.3 % of theory)
f ^' Example 3
185 kg of recrystallised telmisartan (recrystallised from
dimethylformamide or dimethylacetamide), 5.6 kg of
activated charcoal, 185 1 of water, 190.4 kg of formic
acid (99-100%) and 185 1 of methylethylketone are placed
in a 1200 1 stirring apparatus. The mixture is stirred for
about 1 h at 60 - 70 C and then filtered into another
1200 1 stirring apparatus and washed with a mixture of 74
1 of methylethylketone and 8.3 kg of formic acid (99-
1000). About 278 1 of solvent are distilled off at 80 -
100 C whilst simultaneously 278 1 of water are added. The
mixture is then cooled to 20 - 30 C and precipitated by
the metered addition of 281.5 kg of 25 % ammonia solution.
The product precipitated is centrifuged, washed with water
and dried at 120-125 C.
Yield : 178 kg of telmisartan (96.2 % of theory)
Comparison Example
150 kg of telmisartan (recrystallised from
dimethylformamide or dimethylacetamide), 7.5 kg of
activated charcoal, 750 1 of ethanol and 30 kg of 25%
aqueous ammonia solution are placed in a 1200 1 stirring
apparatus. The mixture is stirred for about 1 h and then
filtered into another 1200 1 stirring apparatus and washed
with 150 1 of ethanol. The mixture is heated to 70 -
CA 02352436 2001-05-28
- 10 -
80 C, 35 kg of glacial acetic acid are added and the
mixture is stirred for a further 1.5 - 2 h at 75 - 80 C.
The mixture is then cooled to 0 - 10 C and stirred for a
further 2 h. The product precipitated is centrifuged,
washed with 300 1 of ethanol and with 300 1 of water and
dried at 70 - 90 C.
Yield : 135 kg of telmisartan (90 % of theory) pure form A
In the preparation process according to the invention, as
a result of the partial conversion of the polymorphic form
B into the polymorphic form A during the drying process,
telmisartan occurs as a pure substance in a mixture of two
polymorphic forms. However, this does not affect the
properties of the pharmaceutical composition, as in the
course of the manufacture of telmisartan tablets, for
example, the mixture of the polymorphic forms A and B is
dissolved in 0.1 N NaOH solution and converted by spray
drying into a homogeneous and totally amorphous granulate
which is then subjected to the other tablet making steps.
For more detailed information on the use of the products
according to the invention for preparing a pharmaceutical
composition, cf. EP 502314 B1, the contents of which are
hereby referred to.