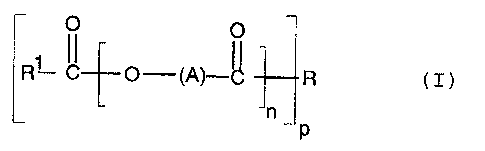Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/0967I
FUEL ADDITTVE AND FUEL COMPOSITION CONTAINING THE
SAME
Field, of the Invention
The invention relates to a fuel additive and fuel
composition containing the same. More in particular, the
invention relates to a fuel additive acting as a
detergent and as a lubricity additive in fuel
compositions, in particular in low-sulphur fuel
compositions, more in particular in low-sulphur diesel
fuel compositions.
Background of the Invention
From EP-A-0,798,364 fuel additives are known, based
on either the salt of a carboxylic acid and an aliphatic
amine, or an amide obtained by dehydration-condensation
between a carboxylic acid and an aliphatic amine. The
additive can be incorporated into a fuel, i.e., a diesel
fuel, and thus reduce the amount of deposit in the
injection nozzle of a compression-ignition diesel
engine, improve lubricity of the diesel fuel, and reduce
wear of the fuel injection pump of the engine. In short,
the additive acts as a detergent and as a lubricity
additive. Fuel additives acting both as detergent and as
lubricity additive are rare. It would therefore be
desirable to extend the range of such additives, or
better still, to provide improved additives acting both
as detergent and lubricity additive.
Summary of the Invention
Accordingly, the invention pa=ovides the use of a
poly(hydroxycarboxylic acid)amide or -ester derivative
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09691
2
of general formula I:
R° C O-(A)-~ R
~n
n (I)
wherein R is the residue of an <~.mine, an aminoalcohol or
a polyol linked to the or each poly(hydroxycarboxylic
acid) via an amide or ester linlcage; R1 is hydrogen or
optionally substituted hydrocarbyl group containing up
to 50 carbon atoms; A is an optionally substituted
hydrocarbyl group; n is from 1-:L00, preferably 1-10 and
p is from 1-5, as a fuel additive acting as a detergent
and as a lubricity additive in f=uel compositions.
Moreover, the invention provides a fuel oil
composition comprising of a major amount of a fuel oil,
and a minor amount of an the additive as well as a
additive concentration for use i.n a fuel oil
composition.
Detailed description of the Invention
As used herein, the term "hv~drocarbvl ~~ rPr~rP~Pnt-~ a
radical formed by removal of ones or more hydrogen atoms
from a carbon atom of a hydrocarbon (not necessarily the
same carbon atoms in case more hydrogen atoms are
removed). In case of Rl useful hydrocarbyls are
aromatic, aliphatic, acyclic or cyclic. Preferably, the
hydrocarbyls are aryl, cycloalkyl, alkyl or alkenyl, in
which case they may be straight-chain or branched-chain.
Representative hydrocarbyls include phenyl, naphthyl,
methyl, ethyl, butyl, pentyl, methylpentyl, hexenyl,
dimethylhexyl, octenyl, cyclooctenyl,
methylcyclooctenyl, dimethylcyclooctyl, ethylhexyl,
octyl, isooctyl, dodecyl; hexadecenyl, eicosyl,
hexacosyl, triacontyl and phenylethyl. The optionally
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
3
substituted Ri is preferably aryl, alkyl or alkenyl
containing up to 50 carbon atoms, especially from 7 to
25 carbon atoms such as heptyl, octyl, undecyl, lauryl,
heptadecyl, heptadenyl, heptadecadienyl, stearyl, oleyl,
or linoleyl. Other examples of R1 include C~_$ cycloalkyl
such as cyclohexyl; polycycloalkyls such as polycyclic
terpenyl groups which are derived from naturally
occurring acids such as abietic acid; aryl such as
phenyl; aralkyl such as benzyl; polyaryl such as
naphthyl, biphenyl, stibenyl and phenylmethylphenyl.
When the hydrocarbyl is substituted, it may contain
a functional group such as carbonyl, carboxyl, vitro,
hydroxy, halo, alkoxy, tertiary amino (no N-H linkages),
oxy, cyano, sulfonyl and sulfoxyl. The majority of the
atoms, other than hydrogen, in substituted hydrocarbyls
are carbon, with the heteroatoms (e. g., oxygen, nitrogen
and sulphur) representing only a minority, about 33~ or
less, of the total non-hydrogen atoms present.
Those skilled in the art will appreciate that
functional groups such as hydroxy, halo, alkoxy, vitro
and cyano in a substituted hydrocarbyl group will
displace one of the hydrogen atoms of the hydrocarbyl,
whilst functional groups such as carbonyl, carboxyl,
tertiary amino (-N-), oxy, sulfonyl and sulfoxyl in a
substituted hydrocarbyl group will displace a -CH- or
-CH2- moiety of the hydrocarbyl..
In "optionally substituted lhydrocarbyl of 1 to 50
carbon atoms", the expression "1 to 50 carbon atoms"
represents the total number of carbon atoms in the
optionally substituted hydrocarbyl group. The same
applies to "optionally substituted hydrocarbyl" of lower
numbers of specified carbon atoms.
CA 02353464 2001-06-O1
WO 00/34418 PCTlEP99/09671
4
The or each radical R1 is preferably unsubstituted
or subtituted by a group selected from hydroxy, halo or
alkoxy group, especially C1-4 all~:oxy. Preferred R1 are
residues of a stearyl, oleyl, 12-hydroxystearyl,
12-hydroxyoleyl, and that derived from naturally
occurring oil such as tall oil fatty acid.
The moiety represented by A may be an aromatic,
aliphatic or cycloaliphatic group. It is preferably an
arylene, alkylene or alkenylene group, especially one
containing from 4 to 25 carbon aaoms with at least 4
carbon atoms between the oxygen atom and carbonyl group.
More preferably it is a saturated alkylene group or an
arylene group. When n is greater' than 1, this moiety may
be the same or different. This moiety may carry other
substituents which do not confer water solubility on the
molecule, such as halogen and C1._4 alkoxy. Preferred
examples of O-A-CO- are 12-oxystearyl, 12-oxyoleyl and
6-axycaproyl. More preferred examples are saturated
groups such as 12-oxystearyl, 6-oxycaproyl.
The amines, aminoalcohols or polyols which react
with poly(hydroxycarboxylic acid) to form products of
formula I are as defined in WO 97/41092. For example,
various amines and their preparations are described in
USP Nos. 3275554, 3438757, 3454555, 3565804, 3755433 and
3822209. Complex amines such as "Starburst" (Trade Mark)
dendrimers may be used, e.g. the compound of formula
[ CH2N ( ( CHz ) zCONH ( CHz ) zNHz ) z l z .
Examples of polyols include ethylene glycol,
glycerol, trimethylolethane, tri:methylolpropane, 1,2-
butanediol, 2,3-hexanediol, 2,4-hexanediol, pinacol,
erythritol, arabitol, sorbitol, mannitol,
pentaerythritol, dipentaerythritol and
tripentaerythritol.
CA 02353464 2001-06-O1
WO 00134418 5 PCT/EP99109671
Preferred derivatives of formula I are those wherein
the compound of R(H)p, of which R represents the
residue, has the general formula II:-
HX~ ( CR2R2 ) aC~b~ ( CR3R3 ) cNR4~a~R9 ) s ( CHR3 } eRS~f~ ( CR3R3 ) g ( R6 )
h~ iR~
(II)
wherein X is 0 or NR4; each RZ i:ndependently represents
hydrogen, hydrocarbyl of 1 to 10 carbon atoms or
hydrocarbyl of 1 to 10 carbon atoms substituted by at
least one hydroxy group; each R~ independently
represents hydrogen or hydrocarbyl of 1 to 10 carbon
atoms; each R4 independently represents hydrogen or
hydrocarbyl of 1 to 10 carbon atoms; RS represents a CS_~
cycloalkanediyl-NH- or 1,4-piperazinediyl moiety
optionally substituted by one or more hydrocarbyl groups
of 1 to 10 carbon atoms; each R6 independently
represents NRa or CHRB; R' represents hydrogen,
hydrocarbyl of 1 to 30 carbon atoms or a
-CO (CHOH) t (CR3R3) ~ (NR3 ) k (CR3R3 ) lOH group; R$ represents a
- (CR3R3 } rNR4R' group; R9 represents a CS_~ cycloalkanediyl
moiety optionally substituted by one or more hydrocarbyl
groups of 1 to 10 carbon atoms;
a is 1 to 10; b is 0 to 10; c is 1 to 10; d is 0 to 10;
a is 1 to 10; f is 0 or 1; g is 1 to 10; h is 0 or 1; i
is 0 to 10; j is 1 to 10; k is 0 or 1; 1 is 1 to 10; r
is 1 , to 10; s is 0 or 1, and t is 0 or 1;
and integers b, d, f and i indicate numbers of
associated moieties present, and the various moieties
( CRZRZ } a0 ] . ~ ( CR3R3 } cNR4 ] ~ ~ ( CHR3 ) eR5 ) arid [ ( CR3R3 ) g { R6
) h ]
may be in any linear order.
Preferably in formula II X is O or NR9, each R2
independently represents hydrogen, C1-4 alkyl or C1_4
hydroxyalkyl, each R~ independently represents hydrogen
or C1_4 alkyl, each R4 represents hydrogen or methyl, R5
CA 02353464 2001-06-O1
WO 00/34418 PC'f/EP99/09b71
6
represents a 1,4-piperazinediyl moiety or a
cyclohexanediyl-NH- moiety optionally substituted by up
to three methyl groups, each R6 independently represents
NR$ or CHR$, R' represents hydrogen, methyl or a
-CO (CHOH) t (CHR3 ) ~ (NR3 ) k (CHR3 ) lOH group, R$ represents a
- (CHR3 } rNHR' group, R9 represent: a cyclohexanediyl
moiety optionally substituted by up to three methyl
groups, a is 1 to 5, b is 0 to 5, c is 1 to 6, d is 0 to
5, a is 1 to 5, f is 0 or 1, g is 1 to 5, h is 0 or 1, i
is 0 to 5, j is 1 to 5, k is 0 or 1, 1 is 1 to 5, r is 1
to 5 , s is 0 or 1 and t is 0 or 1 .
Advantageously, X is 0 or NH, each Rz independently
represents hydrogen, methyl or hydroxymethyl, each R3
independently represents hydrogen or methyl, each R4
represents hydrogen or methyl, Fts represents a 1,4-
piperazinediyl moiety or a cyclohexanediyl-NH- moiety
optionally substituted by up to 3 methyl groups, each R6
independently represents NRe or (~HR8, R' represents
hydrogen, methyl , or a CO ( CHOH ) t ( CHR3 ) ~ (NR3 ) k ( CHR3 ) lOH
group, R8 represents a (CHR3)rNHR' group, a is 2 or 3, b
is 0 to 3, c is 2 to 6, d is 0 to 4, a is 3, f is 0 or
1, g is 2 or 3, h is 1, i is 0 or 1, j is 1 to 4, k is 0
or 1, 1 is 1 to 4, r is 1 or 2, s is 0 or 1 and t is 0
or 1.
Examples of preferred such moieties R when p = 1 are
the following:-
-NHCH2CH2N ( CHZCHzNHz ) z : -O ( CHZC ( CHzOH ) z0 ) bH where b is 1 to
3, preferably 1; -NH(CHZCH2NH)dH where d is 1 to 4;
-NHCH2CHzNHCH2CH20H; -NH (CHz) ~NHz, where c is 2 to 6,
3 0 pre f erably 2 to 4 ; -NH ( CHz ) 3NH ( CHz ) zNH ( CHz ) 3NHz ;
-NH ( CHZCH20 ) zCH2CHzNHz ; -NH ( CHZCHzO ) zH;
-NH ( CHz ) 3 ( 1, 4-piperazinediyl ) ( CHz ) 3NHz ;
-NH(1,4-cyclohexanediyl)CHz(1,4-cyclohexanediyl)NHz;
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
7
-NHCH2(1,3,3-trimethyl-5-aminocyclohexyl);
-NH ( CH2CHZCHZNH ) ZH; -NH ( CHZ ) 3CH ( CH2NH2 ) ( CHz ) 4NH2 ;
-NHCHZC ( CH3 ) ZCH2NH2 ; -NH ( CHZ ) 3N ( CH3 ) a ;
-NHCHZCH2N ( CHzCH2NHC0 ( CHZ ) ZCH ( CH3 ) OH ) 2 ;
-NHCHZCH2N ( CHZCH2NHCOCH2N ( CH3 ) CHzCH20H) z .
Examples of preferred such moieties R when p = 2 are
-NH {CH2CHzNH) 3- and -NHCHZCHZN (CH;~CHzNH2 ) CHzCH2NH- .
Most preferably, R(H)p is selected from the group
consisting of glycerol, pentaerythritol,
dipentaerythritol, tripentaerythritol, ethylenediamine,
dietheylenetriamine, triethylenetetramine,
tetraethylenepentamine, pentaeth.ylenehexamine and
tris(2-aminoethyl)amine.
The preparation of poly(hydroxycarboxylic acid) and
its amide or ester derivatives is known and is
described, for instance, in patent documents EP164817,
W095/17473, W096/07689, US5536445, -GB2001083, GB2342746,
GB1373660, US5000792, US4349389.
The poly(hydroxycarboxylic acid) moiety in formula I
20- may be prepared by the interesterification of one or
more hydroxycarboxylic acids together with a non-
hydroxycarboxylic acid which acts as a chain terminator.
The hydroxyl group in the hydroxycarboxylic acid and the
carboxylic group in either carboxylic acid, may be
primary, secondary or tertiary in character. Examples of
suitable hydroxycarboxylic acids are 12-hydroxystearic
acid, 12-hydroxy-9-oleic acid (r:icinoleic acid),
6-hydroxycaproic acid, especially 12-hydroxystearic
acid. Commercial 12-hydroxystearic acid normally
contains up to 15~ wt of stearic acid and other non-
hydroxycarboxylic acids as impurities and can
conveniently be used without further admixture to
produce a polymer of molecular weight about 1000-2000.
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99109671
8
Where the non-hydroxycarboxylic acid is separately
introduced, the proportion which is required in order to
produce a polymer or oligomer of a given molecular
weight can be determined either by simple experiment or
by calculation.
The interesterification of the hydroxycarboxylic
acid and the non-hydroxycarboxylic acid maybe effected
by heating the starting materials either or not in a
suitable hydrocarbon solvent such as toluene or xylene
20 and azeotroping off the formed water. The reaction may
be carried out at temperature up to 250°C, conveniently
at the reflux temperature of the solvent. Where the
hydroxy group is secondary or tertiary, the temperature
employed should not be so high a.s to lead to dehydration
of the acid molecule. Catalysts for the esterification,
such as p-toluenesulphonic acid, zinc acetate, zirconium
naphthenate or tetrabutyl titana.te, may be included,
with the objective of either increasing the rate of
reaction at a given temperature or of reducing the
temperature required for a given. rate of reaction.
The subsequent amidation with amines, aminoalcohols
or esterification with polyols m.ay be carried out
according to methods known to those skilled in the art,
by heating the poly(hydroxycarboxylic acid) with amines,
aminoalcohols or polyols either or not in a suitable
hydrocarbon solvent such as toluene or xylene and
azeotroping off the formed water, with or without
catalysts such as p-toluenesulphonic acid, zinc acetate,
zirconium naphthenate or tetrabutyl titanate.
In fact, various patent documents disclose
poly(hydroxycarboxylic acid) amide or ester derivatives,
albeit for uses other than in fuels. For instance,
GB1373660 discloses poly(hydroxycarboxylic acid) amide
CA 02353464 2001-06-O1
WO 00/34418 PCT1EP99/09691
9
derivatives with amines such as
3-dimethylaminopropylamine and ethylenediamine for use
as dispersing agent in dispersions of pigments in
organic liquids. GB2001083 discloses
poly(hydroxycarboxylic acid) amide derivatives with
poly(ethyleneimine) (PEI) having a MW greater than 500
for a similar use. In US5000792 too
poly(hydroxycarboxylic acid) amide derivatives with
amines of the formula of NHZ-R'-~N(R")-R"'-NHZ are
disclosed for use as pigment dispersing agent.
W095/17473 discloses poly(hydroxycarboxylic acid) amide
derivatives with amines such as
3-dimethylaminopropylamine, ethylenediamine,
poly(ethyleneimine) (PEI) having a MW greater than 500
and amines of the formula of NHZ-R'-N(R")-R"'-NHZ for
use in a method of preparing a non-aqueous dispersion of
copper phthalocyanine. US4349389 discloses
poly(hydroxycarboxylic acid) am~.de derivatives with
amines such as 3-dimethylaminopropylamine,
poly(ethyleneimine) (PEI) having a MW greater than 500
as dispersing agent in the preparation of a dispersible
inorganic pigment composition. Finally, EP164817
disclose poly(hydroxycarboxylic acid) amide derivatives
with polyamines(ethylenediamine, diethylenetriamine,
etc.), aminoalcohols (diethanolamine, etc.) and ester
derivatives with polyols (glycex-ol, etc.) for use as
surfactant suitable for stabili:aing dispersions of
solids in organic liquids and oil/water emulsions.
None of these patent documer~ts, however, disclosed
the use of poly(hydroxycarboxylic acid) amide or ester
derivatives as fuel additive, acting as a detergent and
as a lubricity additive in fuel compositions, which is
therefore a further embodiment of the present invention.
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
The present invention further provides a fuel oil
composition comprising a major amount of a fuel oil and
a minor amount of a poly(hydroxycarboxylic acid)amide or
ester derivative of formula I as defined above, and an
5 additive concentrate suitable for addition to fuel oils
which comprises a fuel-compatible diluent and a
poly(hydroxycarboxylic acid)amide or ester derivative of
formula I as defined above.
The poly(hydroxycarboxylic acid}amide or ester
10 derivatives of formula I have useful application both in
fuel compositions for spark-ignition engines (gasoline
compositions} and in fuel compositions for compression
ignition engines {diesel fuel compositions).
The "minor amount" referred to above is preferably
less than 10~ w of the composition, more preferably less
than 1~ w and advantageously less than 0.1% w (1000
ppmw) {parts per million by weight) of the composition.
In preferred fuel compositions of the invention, the
poly(hydroxycarboxylic acid)amid.e or ester derivative is
present in an amount in the range 15 to 1000 ppmw of the
fuel composition.
For gasoline compositions, the fuel will be a fuel
boiling in the gasoline boiling range, and it may
consist substantially of hydrocarbons or it may contain
blending components. Alternatively, e.g. in countries
such as Brazil, the fuel may consist substantially of
ethanol.
Suitable liquid hydrocarbon fuels of the gasoline
boiling range are mixtures of hydrocarbon boiling in the
temperature range from about 25°C to about 232°C, and
comprise mixtures of saturated hydrocarbons, olefinic
hydrocarbons and aromatic hydrocarbons. Preferred are
gasoline mixtures having a saturated hydrocarbon content
CA 02353464 2001-06-O1
WO 00/34418 ~ ~ PCT/EP99I09b71
ranging from about 40~ to about 80~ by volume, an
olefinic hydrocarbon content from O~ to about 30~ by
volume and an aromatic hydrocarbon content from about
10~ to about 60~ by volume. The base fuel is derived
from straight run gasoline, polymer gasoline, natural
gasoline, dimer and trimerized olefins, synthetically
produced aromatic hydrocarbon mixtures, from thermally
or catalytically reformed hydrocarbons, or from
catalytically cracked or thermally cracked petroleum
stocks, and mixtures of these. The hydrocarbon
composition and octane level of the base fuel are not
critical. The octane level, (R+M:)/2, will generally be
above about 85 (where R is Research Octane Number and M
is Motor Octane Number).
Any conventional base gasoline can be employed in
the practice of the present invention. For example,
hydrocarbons in the gasoline can be replaced by up to a
substantial amount of conventional alcohols or ethers,
conventionally known for use in fuels. The base
gasolines are desirably substantially free of water
since water could impede a smooth combustion.
Normally, the gasolines to which the invention is
applied may be leaded or unleaded, although are
preferably substantially lead-free, and may contain
minor amounts of one or more blending agents such as
methanol, ethanol, tertiary buta:nol, ethyl tertiary'
butyl ether, methyl tertiary butyl ether, and the like,
at from about 0.1o by volume to about 25o by volume of
the base fuel, although larger amounts (e.g. up to 40~v)
may be utilised. The gasolines can also contain
conventional additives including antioxidants such as
phenolics, e.g. 2,6-di-tert-butylphenol or
phenylenediamines, e.g. N,N'-di-sec-butyl-p-
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
i2
phenylenediamine, dyes, metal deactivators, dehazers
such as polyester-type ethoxylated alkylphenol-
formaldehyde resins. Corrosion inhibitors, such as that
commercially sold by Rhein Chemie, Mannheim, Germany as
"RC 4801", or a polyhydric alcohol ester of a succinic
acid derivative having on at least one of its alpha-
carbon atoms an unsubstituted or substituted aliphatic
hydrocarbon group having from 20 to 500 carbon atoms,
for example, pentaerythritol diester of polyisobutylene-
substituted succinic acid, the polyisobutylene group
having an average molecular weight of about 950, in an
amount from about 1 ppmw to about 1000 ppmw, may also be
present. The fuels can also contain antiknock compounds
such as methyl cyclopentadienylmanganese tricarbonyl,
tetraethyl lead or other lead-containing compounds, and
ortho-azodiphenol as well as co-antiknock compounds such
as benzoyl acetone.
An effective amount of one or more
poly(hydroxycarboxylic acid)amide or ester derivatives
of formula I are introduced into the combustion zone of
the engine in a variety of ways to prevent build-up of
deposits, or to accomplish the reduction of intake valve
deposits or the modification of existing deposits that
are related to octane requirement. As mentioned, a
preferred method is to add a minor amount of one or more
poly(hydroxycarboxylic acid)amide or ester derivatives
of formula I to the gasoline. For example, one or more
poly(hydroxycarboxylic acid)amide or ester derivatives
of formula I are added directly to the gasoline or are
blended with one or more carriers and/or one or more
hydrocarbon-soluble alkali metal or alkaline earth metal
salts and/or one or more additional detergents before
being added to the gasoline.
CA 02353464 2001-06-O1
WO 00/34418 PC'T/EP99/09671
i3
The amount of poly(hydroxycarboxylic acid)amide or
ester derivative of formula I used will depend on the
particular variation of formula I used, the engine, the
fuel, and the presence or absence of carriers,
additional detergents and diluents.
The carrier, when utilised, may conveniently have an
average molecular weight from about 250 to about 5000.
Suitable carriers, when utilised, include hydrocarbon
based materials such as polyisolbutylenes (PIB's),
1.0 polypropylenes (PP's) and polyalphaolefins (PAO°s),
poly(internal olefins) PIO's, all of which may be
hydrogenated or unhydrogenated but are preferably
hydrogenated, and alkylbenzenes; polyether based
materials including alkylene oxide polymers,
interpolymers, copolymers and derivatives thereof
wherein the terminal hydroxyl groups have been modified
by esterification or etherification such as polybutylene
oxides (poly-BO's), polypropylene oxides (poly-PO's),
polyethylene oxides (poly-EO's),, polyhexadecene oxides
(poly-HO's) and mixtures thereof= (i.e. both (poly-BO) +
(poly-PO), poly-PO-EO, and poly--BO-PO)); and mineral
oils such as those sold by member companies of the Royal
Dutch/Shell group under the designations "HVI", "XHVI",
"EDELEX", "CATENEX", "GRAVER" (Trade Marks), Exxon
Naphthenic 900 sus mineral oil and high viscosity index
oils in general. The carrier is preferably selected from
PIB's, poly-BO's and poly-PO's, poly-PO-EO's with
poly-PO's and poly-PO-EO's being the most preferred.
A particularly prepared carrier fluid comprises a
combination of a polyalphaolefirl having a viscosity at
100°C in the range 2 x 10-6 to 2 x 10-5 m2/s (2 to 20
centistokes) being a hydrogenated oligomer containing 18
to 80 carbon atoms derived from at least one
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
14
alphaolefinic monomer containing from 8 to 16 carbon
atoms, and a polyoxyalkylene compound selected from
glycols, mono- and diethers thereof, having number
average molecular weight (Mn) in the range 400 to 3000,
the weight radio polyalphaolefin: polyoxyalkylene
compound being in the range 1:10 to 10:1.
The polyalphaolefins are primarily trimers,
tetramers and pentamers, and synthesis of such materials
is outlined in Campen et al., "(;rowing use of synlubes",
Hydrocarbon Processing, February 1982, pages 75 to 82.
The polyalphaolefin is preferab~_y derived from an
alphaolefinic monomer containing from 8 to 12 carbon
atoms. Polyalphaolefins derived from decene-1 have been
found to be very effective. The polyalphaolefin
preferably has viscosity at 100°C in the range of 5 x
10-6 to 1 x 10-5 m2 / s ( 6 to 10 centistokes ) .
Polyalphaolefin having a viscosity at 100°C of 8 x 10-6
m2/s (8 centistokes) has been found to be very
effective.
Preferred polyoxyalkylene compounds for use in
combination with these polyalphaolefins are described in
EP-A-588429.
The carrier concentration in. the final fuel
composition is up to about 1000 ppm weight. When a
carrier is present, the preferred concentration is from
about 50 ppm by weight to about 400 ppm by weight, based
on the total weight of the fuel composition. Once the
carrier is blended with one or more compounds of formula
I and any other desired components, the blend is added
directly to the fuel or packaged. for future use.
The hydrocarbon-soluble alkali metal or alkaline
earth metal salt, when utilised, may be one of those
described in WO 87/01126, and the compounds of formula I
CA 02353464 2001-06-O1
WO 00/34418 PCTYEP99/09671
are particularly suitable for incorporation, as
additional component, in fuel compositions as described
in WO 87/01126. Preferred hydrocarbon-soluble alkali
metal or alkaline earth metal salts are, however, alkali
5 metal or alkaline earth metal salts of a succinic acid
derivative. Such a salt of a succinic acid derivative,
when utilised, will have as a substituent on one of its
alpha-carbon atoms an unsubstituted or substituted
aliphatic hydrocarbon group having from 20 to 200 carbon
10 atoms. Alternatively; the succinic acid derivative will
have as a substituent on one of its alpha-carbon atoms
an unsubstituted or substituted hydrocarbon group having
from 20 to 200 carbon atoms which is connected to the
other alpha-carbon atom by means of a hydrocarbon moiety
15 having from 1 to 6 carbon atoms, forming a ring
structure. Suitable such salts are described for example
in EP-A-207560 and in EP-A-491439.
The salts of the succinic acid derivative can be
monobasic or dibasic. Monobasic salts in which the
remaining carboxylic acid group lzas been transformed
into an amide or ester group may also be used. Suitable
alkali metal salts of a partial caster of an alkyl
polyether alcohol with a succinic acid derivative are
described in EP-A-491439.
Suitable metal salts include lithium, sodium,
potassium, rubidium, caesium and calcium salts.
Particularly preferred salts are described in EP-A-
207560.
The aliphatic hydrocarbon substituent(s) of the
succinic acid derivative is suitably derived from a
polyolefin, the monomers of which have 2 to 6 carbon
atoms. Thus, convenient substituents include
polyethylene, polypropylene, polybutylenes,
CA 02353464 2001-06-O1
WO 00/34418 ' 6 PCT/EP99/09671
polypentenes, polyhexenes or mixed polymers.
Particularly preferred is an aliphatic hydrocarbon group
which is derived from polyisobutylene.
The hydrocarbon group may include an alkyl and/or an
alkenyl moiety and may contain substituents. One or more
hydrogen atoms may be replaced b;y another atom, for
example halogen, or by a non-aliphatic organic group,
e.g. an (un)substituted phenyl group, a hydroxy, ether,
ketone, aldehyde or ester. A very suitable substituent
in the hydrocarbon group is at least one other metal
succinate group, yielding a hydrocarbon group having two
or more succinate moieties.
The aliphatic hydrocarbon group should contain 20 to
200, preferably 35-150, carbon atoms. When a polyolefin
is used as substituent the chain length is conveniently
expressed as the number average molecular weight. The
number average molecular weight of the substituent, e.g.
determined by osmometry, is advantageously from 400 to
2000.
The succinic acid derivative may have more than one
Czo-Zoo aliphatic hydrocarbon group attached to one or
both alpha-carbon atoms, but preferably it has one Czo_zoo
aliphatic hydrocarbon group on one of its alpha-carbon
atoms and on the other alpha-carbon atom either no
substituent or a hydrocarbon of only a short chain
length, e.g. Ci_6 group. The latter group can be linked
with the CZO-Zoo hydrocarbon group forming a ring
structure.
The gasoline compositions of the present invention
may also contain one or more additional detergents. When
additional detergents are utilised, the gasoline
composition will comprise a mixture of a major amount of
hydrocarbons in the gasoline boiling range as described
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
hereinbefore, a minor amount of one or more compounds of
formula I as described hereinbefore and a minor amount
of an additional detergent selected from the group
consisting of polyalkenyl amines, e.g.
polybutyleneamines, such as "KEROCOM"
polyisobutyleneamine, available ex BASF, Mannish amines,
polyalkenyl succinimides, poly(axyalkylene)amines,
poly(oxyalkylene} carbamates, poly(alkenyl)-N-
substituted carbamates, and mixtures thereof. As noted
above, a carrier as described hereinbefore may also be
included. The "minor amount" is preferably less than
about 10~ by weight of the total fuel composition, more
preferably less than about 1~ by weight of the total
fuel composition and yet more preferably less than about
0.1~ by weight of the total fuel. composition.
The polyalkenyl amine detergents utilised comprise
at least one monovalent hydrocarbon group having at
least 50 carbon atoms and at least one monovalent
hydrocarbon group having at most: five carbon atoms bound
directly to separate nitrogen atoms of a diamine.
Preferred polyalkenyl amines arm polyisobutenyl amines.
Polyisobutenyl amines are known in the art and
representative examples are disclosed in various US
Patents including US-A-3,753,670, US-A-3,756,793, US-A-
3,574,576 and US-A-3,438,757. Particularly preferred
polyisobutenyl amines for use in the present fuel
composition include N-polyisobut:enyl-N', N'-dimethyl-
1,3-diaminopropane (PIB-DAP), OGA-472 (a polyisobutenyl
ethylene diamine available commercially from Oronite),
N-polyisobutenyl diethylene triamine {PIB-DETA} and N
polyisobutenyl triethylene tetramine (PIB-TETA).
The Mannish amine detergents utilised comprise a
condensation product of a high molecular weight alkyl-
CA 02353464 2001-06-O1
WO 00/34418 PC'd'1EP99/09671
18
substituted hydroxyaromatic compound, an amine which
contains an amino group having at least one active
hydrogen atom (preferably a polyamine), and an aldehyde.
Such Mannich amines are known in the art and are
disclosed in IJS-A-4,231,759. Preferably, the Mannich
amine is an alkyl substituted Mannich amine.
The polyalkenyl succinimide detergents comprise the
reaction product of a dibasic acid anhydride with either
a polyoxyalkylene diamine, a hydrocarbyl polyamine or
mixtures of both. Typically the succinimide is
substituted with the polyalkeny7_ group but the
polyalkenyl group may be found on the polyoxyalkylene
diamine or the hydrocarbyl polyamine. Polyalkenyl
succinimides are also known in the art and
representative examples are disclosed in various patent
references including US-A-3,443,918, EP-A-208560, DE-OLS
3,22&,404, US-A-4,234,435, US-A-4,810,261, US-A-
4,852,993, US-A-4,958,321, US-A-4,985,047, US-A-
5,061,291 and US-A-5,147,414.
Particularly effective succinimide detergents are
those obtained by reacting at least one amine, with a
polyalkenyl derivative of a monoethylenically
unsaturated C4_1o dicarboxylic acid material in which the
ratio of dicarboxylic acid moieties per polyalkenyl
chain is not greater than 1.2:1 and the number average
molecular weight (M") of the pol~~ralkenyl chain is in the
range from 1600 to 5000, e.g. as described in EP-A-
587250.
Amines employed in the preparation of said
succinimide detergents are preferably C~_3o; more
preferably C1_~_8, and especially Cg_12, amines containing
1 to 8 nitrogen atoms. Such amines may be branched or
unbranched, saturated aliphatic, primary or secondary
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
19
amines, containing 1 to 8 nitrogens, preferably mono- or
diamines, such as ethylamine, butylamine, sec.
butylamine, diethylamine and 3-dimethylamino-1-
propylamine, but including higher polyamines such as
alkylene polyamines, wherein pairs of nitrogen atoms are
joined by alkylene groups of 2 to 4 carbon atoms.
Poly(oxyalkylene)amines are described, for example,
in US Patents Nos. 4,985,047 and 4,332,595, in EP-A-440
248, EP-A-310 875, EP-A-208 978, WO 85/01956 and WO
97/41092.
The poly(oxyalkylene) carbarriate detergents comprise
an amine moiety and a poly(oxyal.kylene} moiety linked
together through a carbamate linkage, i.e.,
--O-C(0}-N_- {IX)
These poly(oxyalkylene) carbamates are known in the
art and representative examples are disclosed for
example in US-A-4,191,537, US-A-4,160,648, US-A-
4,236,020, US-A-4,270,930, US-A-4,288,612 and US-A-
4,881,945. Particularly preferred poly(oxyalkylene)
carbamates for use in the present fuel composition
include OGA-480 (a poly{oxyalkylene) carbamate which is
available commercially from Oronite}.
The poly(alkenyl)-N-substituted carbamate detergents
utilised are of the formula:
R°~-A-C (=O) -OR""
(X)
in which R° is a poly(alkenyl) chain; R"" is a
hydrocarbyl or substituted hydrocarbyl group; and A is
an N-substituted amino group. Poly{alkenyl)-N-
substituted carbamates are known in the art and are
disclosed in US-A-4,936,868 and in WO 97/41092.
The one or more additional detergents are added
directly to the fuel boiling in the gasoline boiling
CA 02353464 2001-06-O1
WO 00/3441$ PCT/EP99/09671
range, blended with one or more carriers, blended with
one or more acid derivatives of formula I, or blended
with one or more acid derivatives of formula I and one
or more carriers before being added to the fuel.
5 The concentration of the one or more additional
detergents in the final fuel composition is generally up
to about 1000 ppmw for each additional detergent. When
one or more additional detergents are utilised, the
preferred concentration for each additional detergent is
10 from about 10 ppmw to about 400 ppmw, based on the total
weight of the fuel composition, even more preferably
from about 25 ppmw to about 250 ppmw, based on the total
weight of the fuel composition.
Additive components can be added separately to the
15 gasoline or can be blended with one or more diluents,
forming an additive concentrate, and added to the
gasoline together. Suitable gasoline-compatible diluents
are hydrocarbons and mixtures of hydrocarbons with
alcohols or ethers, such as methanol, ethanol, propanol,
20 2-butoxyethanol, methyl tert-butyl ether, or higher
alcohols such as "Dobanol 91", (grade Mark) available
from member companies of the Royal Dutch/Shel1 group.
Preferably the diluent is an aromatic hydrocarbon
solvent such as toluene, xylene, mixtures thereof or
mixtures of toluene or xylene wii~h an alcohol.
Additionally preferred diluents include "Shellsol AB",
"Shellsol R", {Trade Marks) and low aromatic white
spirit (LAWS), which are available from member companies
of the Royal Dutch/Shel1 group.
For diesel fuel compositions, the fuel will be a
diesel oil, which may be a hydrocarbon fuel (a middle
distillate fuel oil), which may be a conventional fuel
or a low-sulphur fuel having a sulphur concentration
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
21
below 500 ppmw, preferably below 50 ppmw, advantageously
below 10 ppmw. Diesel fuels typically have initial
distillation temperature about 160°C and 90~ point of
290-360°C, depending on fuel grade and use. Vegetable
oils may also be used as diesel fuels per se or in
blends with hydrocarbon fuels.
Low-sulphur fuels will typically require a lubricity
additive to reduce fuel pump wear.
Additive concentrates suitable for incorporating in
diesel fuel compositions will contain the
poly(hydroxycarboxylic acid)amide or -ester derivative
of formula I and may contain a fuel-compatible diluent,
which may be a non-polar solvent. such as toluene,
xylene, white spirits and those sold by member companies
of the Royal Dutch/Shell Group under the Trade Mark
"SHELLSOL", and/or a polar solvent such as esters and ,
in particular, alcohols, e.g. hexanol, 2-ethylhexanol,
decanol, isotridecanol and alcohol mixtures such as
those sold by member companies of the Royal Dutch/Shell
Group under the Trade Mark "LINEVOL", especially
"LINEVOL" 79 alcohol which is a mixture of C~_9 primary
alcohols, or the C1a-14 alcohol mixture commercially
available from Sidobre Sinnova, France under the Trade
Mark "SIPOL".
Additive concentrates and diesel fuel compositions
prepared therefrom may additionally contain additional
additives such as corrosion inhibitors, flow improvers,
low molecular weight amine co-detergents,
polyisobutylene succinimides as defined in WO 98/42808,
dehazers, e.g. alkoxylated phenol formaldehyde polymers
such as those commercially available as "NALCO" (Trade
Mark) 7D07 (ex Nalco), and "TOLAD" (Trade Mark) 2683 (ex
Petrolite; anti-foaming agents (e. g. the polyether-
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
22
modified polysiloxanes commercially available as
"TEGOPREN" (Trade Mark) 5851, Q 25907 {ex Dow Corning)
or "RHODORSIL" (Trade Mark) (ex Rhone Poulenc));
ignition improvers (e. g. 2-ethylhexyl nitrate,
cyclohexyl nitrate, di-tertiary-butyl peroxide and those
disclosed in US-A-4,208,290 at Column 2, line 27 to
Column 3, line 21); anti-rust agents {e. g. that
commercially sold by Rhein Chemie, Mannheim, Germany as
"RC 4801", or polyhydric alcohol esters of a succinic
acid derivative, the succinic acid derivative having on
at least one of its alpha-carbon atoms an unsubstituted
or substituted aliphatic hydrocarbon group containing
from 20 to 500 carbon atoms, e.g. the pentaerythritol
diester of polyisobutylene-substituted succinic acid),
re-odourants, biocidal additives, anti-wear additives;
anti-oxidants (e. g. phenolics such as 2,6-di-tert-
butylphenol; or phenylenediamines such as N,N'-di-sec-
butyl-p-phenylenediamine), metal deactivators and
lubricity agents (e.g. those commercially available as
EC831 (ex Paramins) or "HITEC" (Trade Mark) 580 (ex
Ethyl Corporation), or those described in W098/01516 or
W098/16596).
Preferred low molecular weight amine co-detergents
are Clo_zo alkylamines. Aliphatic primary monoamines,
particularly linear aliphatic primary monoamines, having
10 to 20 carbon atoms are particularly preferred. The
alkylamine preferably has 10 to 18, e.g. 12 to 18, more
preferably 12 to 16 carbon atoms. Dodecylamine is
particularly preferred. Another particularly preferred
group are the polyisobutylene su.ccinimides disclosed in
WO 98/42808.
Unless otherwise stated, the (active matter)
concentration of each additive in the diesel fuel is
CA 02353464 2001-06-O1
WO 00134x18 PCT/EP99109691
23
preferably up to 1 percent by weight more preferably in
the range from 5 to 1000 ppmw (parts per million by
weight of the diesel fuel). The (active matter}
concentration of the compound of formula I in the diesel
fuel is preferably 50 to 1000 ppmw.
The (active matter) concentration of the dehazer in
the diesel fuel is preferably in the range from 1 to 20,
more preferably from 1 to 15, still more preferably from
1 to 10 and advantageously from 1 to 5 ppmw. The (active
matter) concentrations of other additives (with the
exception of the ignition improver and the lubricity
agent) are each preferably in the range from 0 to 20,
more preferably from 0 to 10 and advantageously from 0
to 5 ppmw. The (active matter) concentration of the
ignition improver in the diesel fuel is preferably in
the range from 0 to 600 and more preferably from 0 to
500 ppmw. If an ignition improver is incorporated into
the diesel fuel, it is conveniently used in an amount of
100 to 500 ppmw. If a lubricity agent is incorporated
into the diesel fuel, it is conveniently used in an
amount of 100 to 500 ppmw.
The diesel oil itself may be an additised (additive-
containing} oil or an unadditised (additive-free) oil.
If the diesel oil is an additised oil, it will contain
minor amounts of one or more additives, e.g. one or more
additives selected from anti-static agents, pipeline
drag reducers, flow improvers (e. g. ethylene/vinyl
acetate copolymers or acrylate/maleic anhydride
copolymers) and wax anti-settling agents (e. g. those
commercially available under the=_ Trade Marks "PARAFLOW"
(e. g. "PARAFLOW" 450; ex Paramins}, "OCTEL" (e. g.
"OCTEL" W 5000; ex Octel) and "DODIFLOW" (e. g.
"DODIFLOW" V 3958; ex Hoechst).
CA 02353464 2001-06-O1
WO 00/34418 2~, PCT/EP99/09671
The present invention still further provides a
method of operating an internal combustion engine (e. g.
a spark-ignition engine or a compression-ignition
engine) which comprises introducing into the combustion
chambers of said engine a fuel composition (e.g. a
gasoline composition or diesel fuel composition, as
appropriate) as defined above.
Use of poly(hydroxycarboxylic acid)amide or -ester
derivatives of formula I as additives in fuels for
internal combustion engines may result in attaining one
or more of a number of effects such as inlet system
cleanliness (intake valves, fuel injectors,
carburettors), combustion chamber cleanliness (in each
case either or both of keep clean and clean-up effects),
anti-corrosion (including anti-rust) and reduction or
elimination of valve-stick.
The invention will be further understood from the
following illustrative examples in which Examples 1 to 6
relate to the preparation of poly(hydroxycarboxylic
acid)amide or -ester derivatives of formula I.
Various abbreviations are employed in the examples
as follows:-
"AV" denotes acid value, and this was determined
using a "Metrohm 670" (trademark) potentiametric
titrometer according to a method based upon ASTM D 664-
89 with modified solvent system (the product is first
dissolved in a toluene/methyl ethyl ketone 60/40
weight/weight mixture, and then diluted with a tert-
butanol/water toluene 38.8/2.9/58.2 weight/weight/weight
mixture);
"TBN" denotes total basic nitrogen, and this was
determined using a "Metrohm 670" (Trade Mark)
potentiometric titrometer according to a method based
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99109671
upon ASTM D 2895 with modified solvent system (75~ w
toluene, 12.5% w acetonitrile, 1.2.5% w acetic acid);
"meqg-1" denotes milliequivalents per gram.
In the examples and tests which follow, all parts
5 and percentages are by weight unless stated otherwise,
and temperatures are in degrees Celsius.
Commercial 12-hydroxystearic acid (referred to in
the examples as 'HSa', ex Oleotec) used in the following
examples contains 1% palmitic acid, 10% stearic acid
10 (referred to in the examples as 'Sa'), 4% 12-ketostearic
acid as impurities. Commercial ricinoleic acid (Nouracid
CS80 ex Akzo Nobel) has an acid value of 175 mg KOH/g
and hydroxyl value of 150 mg KOH/g.
Example 1
15 400 g (1330 mmol) of HSa and 5 g (2& mmol) of
p-toluenesulphonic acid ('pTSA') were heated to reflux
in 500 ml of xylene ('Xy')overnight and the water
produced was removed via a Dean-Stark trap. After
cooling, the solution was washed three times with 100 m1
20 water, dried over magnesium sulphate whereupon the
solvent was removed under vacuum. The product gave an AV
of 0.74 meqg-1 and a calculated molecular weight of
1400.
100 g (74.3 mmol) of poly(hydroxystearic acid) so
25 produced and 15.5 (66.7 mmol, 0.9 eq) of
pentaethylenehexamine ('PEHA') were heated to reflux in
150 ml Xy while removing water formed for a further
three hours. After cooling, the solvent was removed
under vacuum to give 115 g product with a TBN of 3.24%.
GPC analysis using polystyrene as standard gave a Mn of
1585 and a polydispersity of 2.1. 60 g of this product
were dissolved in 500 ml of toluene and washed twice
with 250 ml of water, dried over magnesium sulphate. The
CA 02353464 2001-06-O1
WO 00/34418 2s PCT/EP99/09671
solvent was removed under vacuum to give 56 g of product
with a TBN of 2.04%. This product was used in engine
testing.
Examplas 2 - 16
Derivatives were made in a similar manner as in
Example 1. Minor variations include the presence or
absence of pTSA, and the presence or absence of a
solvent. In case no solvent is used, then the reaction
temperature is set to 200 °C. Reagents and properties of
the products are set out in Table 1. The product of
Example 13 is an ester, based on glycerol. The products
of Examples 15 and 16 are amide derivatives of
poly{ricinoleic acid).
Comparative example 1 Preparation of oleylamine amide
derivative of oleic acid according to Oronite's patent
EP-A-798,364
70g (223 mmol) of oleic acid(90~ purity, Aldrich)
and 74.68 {223 mmol) of oleylamine(80~ purity, Aldrich)
were heated with stirring at 120 °C for 3hrs and 150 °C
for a few minutes under high vacuum. A quantitative
yield of the product was obtained. IR(cm-1): 1633, 1539.
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
27
3
M
TW-iN r O 01 O a1 '[f
R.N N .-iN .--t N '-1~ U
U
Lf1ofl0 O lfl tU
N ~ l0
y O M 01 O N k, W
h M N ~ ~p ~
-Ir-Iv-~Ir1 v-i .--1r-i
x a~
~ w
v mnv~ ~rm n m o M ,-,000~o ~r en w .-i~
H O M Ln N a000 tnl0t000 00l0a1 M O ~0 Gh .Ll
1~ O
N .-iN M r-IN N M r M O r--I.--I ,~ ,-~~ . b
O
W ..aO
O O
~ U U U
b' .-i
(31Q101 O O N 01a1O 01 Q1~ ~ 01O O W p 3 -~
+~ ~
O O O ~-iN .-fO O N O O O O O r-1.-1~ ~O
H W H H LL W C4x x 0.,W x ~ LL x N U
W W W W W W W W W W ~ W W r-IW W ~ N
W H H H H H H H W CL C1H W C7H !1 N
-i -
r ~-ilYj-~-i
~, N 3
O O OoM u7 O l0~ ~ pp pp ~ ~ N
c tf1t1~OJ O O 01 r-Il0ts'fN M l0~ tnN N W '"~ O
M M Op O C c~ U'7r C'~ O O O COV' ~ ~ ~ ,-
.-i~ of r ,-a~ ~ rn,-io~ ,-i,-a~ u~.-i,-i~ N
.1.~ ?a
H rtfO ~-IU
N ~ ,~~ ~2,U
crc~rl rlh l0N 01~f'Yr C V~.-IO O .-I ~C~ .S-~
~n ~ W ~ N
FCr r o h ~ ~oo ~00 0~~ o~r-r r
o o ,--i,-io 0 0 ~ o ~ 0 0 0 ,-io o ~ " u~
N ~
,
~ s~~ ~ o
a~ N N 3 ~ 'O
J,0 0 0 0 0 ~ o G ~ o
~ .~ ,.~~ O
o r o 0 o O ~nO O W ,G H
~C O
Gn .-iM u7u'7~ ~ M G ~ ~ W
H
.. ~ ~ -d N
'CS C b O
H l~ . M M C ~ ~ ~ ~ Lo N b ~ V7 ~ri
~r~ x
O O O O N O ~ a -~~-.~
ClnN h N .--iri r-i ri 1,~ O g
G ~ ~ ~ N G
3
-~~ ,d,
O 3
N_ rtf I O ZfN
rtf ~ M l0~Oco N d' ~ O .c0
o o ~c'1 M ~ ~ ~ .s-1r-i
t!~ O O r O .-iN .-t O (aO U
-.~ .~..~ M ~ _~~ ~ ~
f(fM O r a o ~ 3 ~ "'~'c5ro
M r r N r ~r.,~N .,~ o ra x O
U)M (n O1 ~C1 (~(n lfl O '1 ~ ~ ~ ~''~
M l0t9 M lfll0tJ1M O f0 "~ ~ O .,
.-i~0 .-1 l000(0f(f r-i!tfODr~ ~ O .C~,1~1 N U
- W ~ N Z
N
N H CON .ClM
O
o ,~N M ~rm
W .-iN M C u7tO r of01.-i.-1rir-ir-iW -I
SUBSTfTUTE SHEET (RULE 26)
CA 02353464 2001-06-O1
WO 00/34418 2$ PCT/EP99/09671
The products of Examples 1-4, 7-13, 15-16 and
Comparative example 1 were subjected to at least one of
the following tests in liquid fuels:
(i) Intake Valve Deposits using 1.2L Opel kadett
Engine
(ii) Injector fouling using 1.9L VW Passat (Indirect)
Injection Diesel Engine
(iii) XI1D-9AL engine test (according to CEC PF-023-X-96
(iv) HFFR testing.
Past 1 gasoline engine inlet system cleanliness
(i) Intake Valve Deposits using 2.2L Opel Kadett Engine)
Opel Kadett engine tests were carried out as
described in the section of 'Intake valve and combustion
chamber deposits using 1.2L Opel Kadett engine on page
57 of W097/41092.
The test materials of a number of the Examples
together with 250 ppmw of a carrier have been tested in
a laboratory multicylinder engine to evaluate their
intake valve deposit control performance. This engine
was a 1.2L twin carburettor four cylinder spark-ignition
engine manufactured by General Motors' Ope1 subsidiary
and is used in the published inlet system cleanliness
test CEC F-04-A-87. It has 79 mm bore, 61 mm stroke and
is operated under a prescribed load and speed schedule
representative of typical driving conditions asset
forth in in the following table:
Step Time, sec Load, Nm Speed, r/min
1 30 0 1200
2 60 35 :3000
3 60 29 :1300
4 120 32 1850
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/0967i
29
In modifying the procedure, th.e air inlet was
maintained at 25°C (+ or - 2 degrees) and no extra oil
injection down the valve guides wa.s used. The
lubricating oil in the sump was "SHELL" "HELIX" 10 w/40
lubricating oil (API SG quality). The test duration was
40 hours including 2 hour shutdowns after the first and
second 12 hour running periods. A twin carburettor set
up was used to enable two additives to be tested
simultaneously. Consequently, intake valve and
combustion chamber deposit weights are average values
from 2 cylinders.
Comparative A is base gasoline which has a RON 97of
and a MON of 86.1, contains 36.70 v of aromatics with
8.9% v of olefins (IP156:92) and h.as a final boiling
point of 201C (IS03405:88).
Table 2 Opel Kadett results
Fuel example [additive] in fuel Intake valve deposits
(ppmw) (mg/valve)
Comparative 0 220.5
A
Example 1 250 3
Example 2 250 5
Example 3 250 13
Example 11 250 68
It will be noted that gasoline compositions
containing test materials of present invention gave much
lower intake valve deposits than base gasoline.
Part 2 as diesel engine injector nozzle cleanliness
detergents
SUBSTITUTE SHEET (RULE 26~
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
Base fuels used in the following experiments had the
following physical properties:
Base fuel B C
Density (kg/1) at 15C (ASTM D 4052) 0.8398 0.8431
Sulphur (ppmw) (IP336) 500 400
Distillation, degree C (ASTM D 86)
IBP 162 165
10% 203 208
20% 220 225
50% 272 269
90% 341 328
95% 356 341
FBP 384 365
Total Aromatics content (%w) 26.1 35.7
In the following tests additive concentrates were
5 used comprising a standard co-additive mixture (composed
of an anti-rust agent, a dehazer, an antifoaming agent,
a solvent, optionally a mineral oil, and an ignition
improver) and the additive produced in the Examples 1-4,
7-10, 12, 13, 15 or 16.
10 Tests were performed by pouring the additive
concentrate directly into the fuel.
(ii) Injector fouling using 1.91a ZTGO Passat (Indirect)
Injection Diesel Engine.
Passat engine tests were carried out as described on
15 page 25 of W098/42808. Steady-state injector nozzle
fouling tests were performed according to the following
method, employing a four cylinder VW Passat AAZ 1.9 TD
(turbo diesel) IDI (indirect injection) diesel engine of
1896 cc displacement, equipped with a Bosch fuel
20 injection system employing injector nozzles of type DNO
SD 308.
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
31
In this test method, the same injector nozzles were
used for engine warm-up as for the steady-state deposit
accumulation stage. New nozzles, cleaned with n-heptane,
were used for each test.
The engine was warmed up at 1500 rpm engine speed
and 25 Nm dynamometer load for 20 minutes. The engine
speed was then raised to 2000 rpm and the dynamometer
load was increased to 90 Nm over 15 seconds, and the
engine was run at that speed and load for 3 hours.
A fouling index was generated from measurements of
air flow through the injector nozzles, assessments being
made on the new nozzles, before the test (Flow Clean),
and afterwards on the fouled nozzles (Flow Fouled). Air
flow was measured in a Ricardo air-flow rig according to
ISO 4010, measurements being recorded at needle lifts of
0.1, 0.2 and 0.3 mm, with a vacuum pressure 600 mBar
(60,000 Pa).
Build up of deposits in the nozzles causes a
reduction in measured air flow, and degree of nozzle
fouling (F) was calculated as follows:
F = Flow Clean - Flow Fouled x 100
Flow Clean
A fouling number for one nozzle was calculated by
averaging the three values of F obtained at the three
different needle lifts. The fouling index (FI) was
obtained by averaging the fouling numbers from all four
nozzles. Test were performed on low-sulphur fuel
formulations as described above, containing 499 ppmw of
the poly(hydroxycarboxylic acid) amine or -ester
derivatives.
CA 02353464 2001-06-O1
WO 00/34418 PCT/EP99/09671
32
Results of this test are given in Table 3 as
follows:-
additive made in Example [additive] in fuel (ppmw)FI (%)
Comparative B * 0 45
1 499 13
2 499
3 499 g
15 499 31
26 499 30
.i. n n c __
~.~...yuu vt a lut~tl'll.y cluQlLlVe as CilsCl0SeC1 In W
9s~az5m .
Tt will be noted that diesel compositions containing
test materials of present invention, especially those
from saturated hydroxycarboxyiic acid (example l, 2, 3)
gave much lower injector fouling than base diesel as
measured by Fouling Index (FT%).
(iii) XUD-9AI, engine tests .
Results of this test axe given in Table 4 as
follows:-
Additive made in Example [additive] in fuel(ppmw) RF (o)
Comparative B * 0 11
Comparative C * 0 10
2 250 34
4 250 21
130 2g
$ 130 3$
130 16
10 130 35
12 130 39
13 130 39
Comparative Example 1 130 1$
SUBSTITUTE SHEET (RULE 26j
CA 02353464 2001-06-O1
WO 00/34418 33 PCT/EP99109671
* 225ppm of a lubricity additive as disclosed in WO
98/01516.
It will be noted that diesel compositions containing
test materials of present invention, examples 2, 4, 7, 8,
9, 10, 12 and 13 gave much improved injector cleanliness
as measured in Residual Flow (RFo) than base diesel or
the additive of comparative example 1.
(iv) HFRR results
HFRR testing was carried out as described in the
section of 'further fuel testing examples' on page 14 of
WO 98/01516. The results are compiled in Table 1.
Comparative D is Comparative H in Table 2 of WO 98/01516.
Additive made [additive] in fuel Aver wear scar
in Example (ppmw) diameter (microns)
Comparative D 0 622
Example 4 150 362
Example 14 130 307
Comparative 150 404
example 1
It will be noted that diesel compositions containing
test materials of present invention, examples 4 and 14
gave surprisingly enhanced lubricity as measured in HFRR
than base diesel.
SUBSTITUTE SHEET (RULE 26)