Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02355260 2001-06-13
WO 00/36189 PCTNS99/301Z4
-1 -
DOUBLE-DIP Pd/Sn CROSSLINKER
The invention concerns a process for the direct metallizing of the surface of
a plastic object, using these steps:
roughening the surface of the plastic object by pickling;
activating the surface with the aid of a colloidal or ionogen aqueous solution
of a first precious metal, which colloidal or ionogenic solution also contains
a second
base metal, whereby an activating coat containing the first precious and
second base
metal is formed on the surface; and
providing electron conductivity on the activating coat with the aid of a
preferably alkaline treatment solution, with which the second base metal is at
least
partially dissolved out of the activating coat and an electron-conductive
substance is
adsorbed in the activation coat;
whereby the electron-conductive activation layer is then metallized.
The invention thus concerns a process for the direct galvanic metallizing of a
plastic surface. Of course, it is part of the invention that several plastic
objects can
be metallized at the same time with the process under the invention. Plastics
metallized in this way are used for decorative purposes, for example in the
health
sector or in automobile manufacturing. Metallizing of plastics is also
performed as
part of the coating of electronic components for the purpose of
electromagnetic
shielding. In principle, different plastics can be galvanically metallized.
For example,
the surface of plastic such as acrylonitrile-butadiene-styrene (ABS) and,
where
necessary, blends of ABS and polycarbonates (PC) can be metallized for
decorative
purposes.
The known process of the type named at the beginning, from which the
invention starts (DE 195 10 855 C2), is used for the partial or selective
electrolytic
metallizing of the surfaces of acrylonitrile-butadiene-styrene copolymer
substrates.
After the surface of the plastic is roughened by pickling with chromic
sulfuric acid,
chrome (VI) is reduced to chrome (III) as part of the process. This is foNowed
by
activation in a colloidal acid solution of palladium, which contains
additional tin
compounds. On the base of the activation coat created, sufficient electron
conductivity is produced for the subsequent metallization. For this, a
tinlcopper
exchange is performed. The treatment solution used for this contains copper
ions,
which are bound by a biologically degradable complex forming substance. The
tin/copper exchange is based on a charge exchange, in which tin (II) is
oxidized by
CA 02355260 2001-06-13
the copper ions to tin (IV) and whereby the copper ions themselves are reduced
to
metallic copper and deposited on palladium clusters on the surface of the
plastic. As
a result of this deposition of copper, the palladium clusters which formed
during
activation with the colloidal solution become electron-conductive. This known
process is characterized by a number of disadvantages. First, only limited
quantities
of palladium/tin can be applied to the roughened plastic surface with this
process.
There are corresponding limits to the metallization of these plastic surfaces.
In
addition, only ABS or ABS blends can normally be metallized with some degree
of
acceptability with this process. The metallization of ABS blends in particular
leaves
much to be desired. The metallization can often not be performed with any
degree
of reproducibility, and frequently certain areas on the surface of the plastic
object to
be metallized are not covered with an adequate metal coat. If plastic objects
composed of different plastics are to be metallized with the known process,
only ABS
or ABS blends are metallized as a rule.
By contrast, the invention is based on the technical problem of describing a
process of the type named at the beginning, with which different plastics can
be
metallized safely and reproducibly, and in which the plastics are given a
metal coat
which meets all requirements.
To solve this technical problem, the invention defines a process as described
above which is characterized by the repetition of at least once of the
sequence of
steps "activation of the surface according to step 1.2) and making the
activation layer
electron-conductive according to step 1.3)" before the metallizing of the
electron
conductive activation coat. It is part of the invention that steps 1.2) and
1.3) are
repeated several times before the metallizing of the electron-conductive
activation
coat.
The invention is based on the finding that metallizing of plastic objects that
is
especially safe and effective can be achieved if the theory according to
patent claim
1 can be realized. By repeatedly performing steps 1.2) and 1.3), the volume of
adsorbed precious metal/base metal on the surface of the plastic can be
increased
substantially - surprisingly as part of the activation. Unexpectedly, metal
coats with
outstanding quality can be applied to the plastic objects as a result.
Surprisingly, a
variety of different plastics can be metallized effectively and safely with
the process
under the invention. Besides ABS, ABS blends, particularly ABS/PC blends, can
be
metallized reproducibly and the resulting metal coats meet all requirements.
As part
of the invention, large plastic objects, for example large ABS/PC parts such
as
6M~Alll~n cuGCr
CA 02355260 2001-06-13
WO 00/36189 PCT/US99/30124
-3
automotive radiator grills, can be metallized without difficulty. The surfaces
of plastic
objects consisting of a number of different plastics can be metallized without
difficulty
and, if desired, completely.
In another type of implementation of the invention, roughening the surface of
the plastic object is carried out by pickling with chromic sulfuric acid. For
practical
terms, a solution containing 400 g/l chromic acid and 400 g/I sulfuric acid is
used.
Pickling can be carried out solely with chromic acid. It is part of the
invention that
after pickling with chromic sulfuric acid or chromic acid, chrome (VI) is
removed as
completely as possible from the surface of the roughened or etched surface.
Preferably, intensive rinsing is performed first after the roughening of the
surface. In
accordance with a preferred type of implementation of the invention, chrome
(VI) is
reduced to chrome (III) after the pickling with chromic sulfuric acid or
chromic acid.
It is within the scope of the invention that this chrome reduction takes place
after the
aforementioned rinsing. Potassium bisulfide or potassium hyposulfite can be
used,
for example, for the reduction of chrome (VI) to chrome (III). Preferably, at
least one
rinsing takes places following the chrome reduction. In another version of the
invention, roughening of the plastic surtace is carried out by pickling with a
permanganate solution. In yet another version, the roughening is achieved by
plasma
pickling.
It is within the scope of the invention that the surface of the plastic object
is
pretreated with acid by immersion before the activation of the surface in
accordance
with step 1.2). The acid is preferably a mineral acid, hydrochloric acid is
the most
preferred. For practical purposes, the pretreatment is performed by immersion
in a
concentrate of hydrochloric acid, preferably 30% by volume hydrochloric acid.
According to the very much preferred type of implementation within the scope
of the invention, which is particularly important as part of the invention,
the steps
"pretreatment by immersion in an acid, activation of the surface in accordance
with
step 1.2) and providing electron conductivity in accordance with step 1.3)"
are
repeated at least once before metallizing the electron-conducting activation
coat. In
other words, following the roughening of the surface in accordance with 1.1 ),
the
steps "immersion -- activation - providing electron conductivity" are
performed in
succession and then this sequence of steps "immersion -- activation --
providing
electron conductivity" is repeated at least once. As part of the inventive
process, this
sequence of steps can be repeated several times, i.e. more than twice, before
metaliizing the electron-conducting activation coat. Following roughening in
CA 02355260 2001-06-13
WO 00/36189
PCTNS99/30124
-4-
accordance with 1.1 }, the "immersion - activation -- providing electron
conductivity"
steps can then be performed and subsequently only the sequence "activation --
providing electron conductivity" is repeated at least once.
It is within scope of the invention that two different metallic elements are
used
in the colloidal or ionogenic aqueous solution to the activate the surface,
namely a
precious metal and a second base metal that is different from the first metal.
In one
version of the invention, the precious metal is palladium and the base metal
is tin.
For practical terms, a tin (II) stabilized palladium colloidal solution is
used for
activation. Palladium and tin are adsorbed from this solution on the
pretreated
surface of the plastic object. Preferably, a hydrochloric acid palladium-tin
solution is
used to activate the surface. In accordance with the version described
previously, the
activation coat consists of a palladium-tin coating. Preferably, an acidic,
most
preferably a hydrochloric acid, colloidal aqueous solution is used in the
inventive
process. It is within the scope of the invention that rinsing is performed
after the
activation of the surface.
As part of the invention, providing electron conductivity on the activation
coat
means sufficient electron conductivity of the activation coat for the
subsequent
metallizing. That, according to step 1.3), the second base metal is at least
partially
dissolved out of the activation coat and an electron-conductive substance is
adsorbed
in the activation coat, means, according to one version of the invention, that
the
second base metal, for example tin, is partially or completely dissolved out
of the
activation coat and replaced with an electron-conducting substance, for
example
another metal. That an electron-conducting substance is adsorbed in the
activation
coat also means, according to another version of the invention, that the
second base
metal is dissolved at least partially out of the activation coat and an
electron-
conducting substance is formed in the activation coat. So it is within the
scope of the
invention that as the result of treatment with the preferably alkaline
solution, a
reaction with the second base metal takes place and an electron-conducting
substance, respectively an electron-conducting compound, is formed in the
activation
coat. Through adsorption of the electron-conducting substance, a type of cross-
linking of the activation coat with the electron-conducting substance takes
places. In
one version of the invention, the second base metal is replace with a third
metal to
establish the electron conductivity of the activation coat. It is within the
scope of the
invention that the second base metal in the activation coat has an oxidation
level >0.
This second metal occurring in an "oxidized form" is replaced in practical
terms by the
CA 02355260 2001-06-13
WO 00/36189
PCTNS99/30124
_5_
third metal, which, preferably in an elementary metallic form, ensures the
electron
conductivity of the activation coat. For practical terms, the second base
metal is
replaced by a third metal, which is precious compared with the second one. The
third
metal is preferably copper. In this version, the treatment solution contains
copper
ions, which are preferably bonded by a complex forming substance. The base
metal
bonded to the plastic surface, preferably tin, is exchanged for copper in the
treatment
solution. In practical terms, tin (II) ions are oxidized by the copper ions to
tin (IV).
The copper ions themselves are reduced to metallic copper and bonded to the
plastic
surface. This gives the surface adequate electrical conductivity, so that
direct
metallizing of the plastic can be carried out afterwards. The electron-
conducting
substance, which partially or completely replaces the second base metal from
the
activation coat, does have to be a metal as part of the invention, but not
absolutely.
For the purpose of establishing the electron conductivity of the activation
coat, a
preferably alkaline treatment solution of electron conducting compounds of
elements
from the 6th and/or 7th main group of the periodic system, or mixtures of
them, is
used in another type of implementation of the invention. It is within the
scope of the
invention that, in this version, the electron conducting substance in the
activation coat
is formed by chemical reaction, for example with a second base metal.
When sufficient electron conductivity has been established in the activation
coat, direct metallizing of the surface of the plastic object can take place.
It is within
the scope of the invention that direct electrolytic metallizing is carried
out. In one
version of the invention, a copper coat is applied to the electron conducting
activation
coat by means of electrolytic metallizing. In another type of the invention, a
nickel
coat is applied to the electron conducting activation coat by means of
electrolytic
metallizing. It is part of the invention that other metal coats, for example a
chrome
coat, can be applied to the electron conducting activation coat as part of
metallizing.
It is evident that not just one metal coat can be applied, but a number of
metal coats
can be applied in succession.
Substantial and surprising benefits are achieved as a result of repeating
steps
1.2) and 1.3) of the invention before metallizing, or preferably, by repeating
the steps
"immersion using an acid -- activation of the surface -- providing electron
conductivity." As was already demonstrated above, considerably greater
quantities
of precious and base metal, for example palladium/tin, can -- surprisingly -
be
deposited on the surtace of plastic objects as an activation coat, in
comparison to the
known process explained at the beginning. Surprisingly, this is possible with
many
CA 02355260 2001-06-13
WO 00/36189
-6-
PCTNS99/301Z4
different plastics, even with ABS blends in particular. The process of the
invention
also makes it possible to save considerably quantities of the precious metal,
for
example palladium. If the aforementioned steps are repeated twice as described
in
the invention, a colloidal or ionogenic aqueous solution can be used, which,
in
comparison to the known process explained at the beginning, contains only half
the
concentration of the precious metal, for example of palladium. So large
quantities of
the normally very expensive precious metal can be saved, with great benefit.
Nevertheless, equal amounts or ever greater amounts of the metals, palladium
and
tin for example, can be applied to the. surface as the activation layer. On
the other
hand, the invention shows that with the same concentration of the precious
metal in
the colloidal or ionogenic solution, only half the activation time is
required, if the
appropriate steps in the process are repeated twice as described in the
invention and
the same amount of precious metal is applied to the surface as in the known
process.
After metallizing, metal coats with optimal surface qualities are achieved
using the
process of the invention. There are almost no failures in coverage. Larger
plastic
surfaces can be metatlized easily. It is even possible to metallize ABS blends
effectively and safely.
The invention is explained in greater detail below, using drawings. The
schematic representations show:
Fig. 1, which illustrates the time-dependent adsorption of palladium/tin on
the
surface of a plastic object using the known state-of the-art process, wherein
the x-axis
represents time expressed in minutes, and the y-axis represents the
paliadium/tin
adsorption expressed in mg/dm2; and
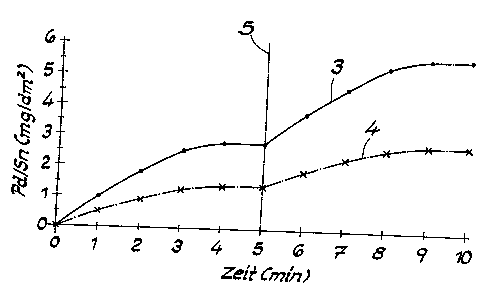
Fig. 2, which illustrates the time-dependent adsorption of palladiumltin on
the
surface of a plastic object using the process of the invention, wherein the x-
axis
represents time expressed in minutes, and the y-axis represents the
palladium/tin
adsorption expressed in mg/dmz.
Fig. 1 shows the adsorption of paUadiumltin that is achieved by using the
known process explained at the beginning on the surtace of a plastic object.
Curve
1 shows the adsorption on the surtace of an ABS plastic object, and Curve 2
shows
the adsorption on the surtace of an ABS/PC plastic object. The plastic objects
were
first pickled in a solution of 400 g/I of chromic acid and 400 g/l of sulfuric
acid, and
then a reduction of chrome (VI) to chrome (III) was carried out. Then came a
pre-
immersion in a 300 ml/l concentration of hydrochloric acid. Activation
followed
afterwards in a tin (II) stabilized palladium colloidal solution. It can be
seen in Fig. 1
CA 02355260 2001-06-13
WO 00/36189 PCT/US99/30124
-7-
that the palladium/tin adsorption increases at the greatest rate in the first
3 minutes.
After 5 minutes no additional palladium/tin is deposited on the surface of the
plastic
object. Even when higher concentrations of the colloidal aqueous solution are
chosen, greater adsorption of palladium/tin cannot be achieved. It can also be
seen
in Fig. 1 that only a slight, unsatisfactory adsorption of palladium/tin takes
place on
the surface of the ABS/PC object. Following activation, the surface of the
plastic
object was treated with a solution in accordance with step 1.3). Then
electrolytic
copper plating or electrolytic nickel plating was carried out.
Fig. 2 shows the adsorption of palladium/tin on the surface of plastic objects
using the process from the invention. Curve 3 shows the adsorption of
palladium/tin
on an object made of ABS, and Curve 4 shows the adsorption on an object made
of
ABS/PC. With the process from the invention, the procedure was initially
basically the
same as with the known process described previously. However, after the first
time
the treatment was carried out according to step 1.3), steps "pre-immersion in
the
hydrochloric acid -- activation in the palladium solution -- treatment in
accordance with
1.3" were repeated once. The dividing line 5 in Fig. 2 shows the end of the
first
sequence of steps and the beginning of the second sequence of steps. In Fig.
2, it
can be seen clearly that the result with the process from the invention is
that about
twice the amount of palladium/tin is adsorbed in comparison with the known
process,
both on the object made of ABS as well on the object made of ABS/PC. This has
to
come as a surprise to the expert. With the process from the invention,
unexpectedly
large amounts of palladium/tin can also be adsorbed on ABS/PC blends and other
ABS blends, permitting surprisingly effective and safe subsequent metallizing.
If the
aforementioned steps are repeated more than twice, the content of adsorbed
palladium/tin can be increased still further. Unexpected benefits are achieved
in the
result with the process from the invention.