Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
COMPOSITIONS AND METHODS FOR REDUCING OR PREVENTING '
FERTILIZATION IN FISH AND BIRDS
FIELD OF THE INVENTION
The present invention relates to a vaccine
composition for the immunocontraception of fish. The present
invention also relates to a vaccine composition for the
immunocontraception of birds.
BACKGROUND OF THE INVENTION
Among vertebrates, mating strategies involve
behaviour, gamete structure and the specificity of recognition
of sperm and egg. Mammalian oocytes are surrounded by an
envelope called the zona pellucida that is composed of three .
glycoproteins in a ratio of 1:2:2 denoted by ZPA, ZPB, and ZPC
(Harris,J.D., Hibler,D.W., Fontenot,G.K., Hsu,K.T.,
Yurewicz,E.C. and Sacco,A.G.(1994) "Cloning and
characterization of zona pellucida genes and cDNA's from a
variety of mammalian species . the ZPA, ZPB and ZPC gene
families DNA sequence." J. Sequencing and Mapping 4:361-393).
The zona pellucida contains species-specific sperm receptors
composed mainly of O-terminal oligosaccharides. Fish eggs have
a teleost equivalent of mammalian zona pellucida wherein the
carbohydrate moiety has some structural similarity to the
carbohydrate moiety of mammalian zona pellucida (Taguchi,T.,
Seko,A., Kitajima,K., Muko,Y., Inoue,S., Knoo,K-H.,
Morris,H.R., Dell,A. and Inoue,Y.(1999) "Structural studies of
a novel type of pentaantennary large glycan unit in the
fertilization-associated carbohydrate-rich glycopeptide
isolated from the fertilized eggs of Oryzias latipes."
J.Biol.Chem 269:8762-8771).
Mouse ZP2 (ZPA) contains a 241-amino acid domain at
the C-terminus with 28~ identity with a white flounder teleost
egg protein (Lyons,C.E., Payette,K.L., Price,J.L. and
1
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Huang,R.C.C.(1993) "Expression and structural analysis of a '
teleost homolog of a mammalian zona pellucida gene."
J.Biol.Chem.268:21351-21358). A 348-amino acid sequence of
mouse ZP1 (ZPB) is 47$ similar (32$ identical) to that of mouse
ZP2 (ZPA) suggesting that this protein domain has been
duplicated in mammals (Epifano,0., Liang,L-F. and Dean, J.
(1996) "Mouse ZP1 encodes a zona pellucida protein homologous
to egg envelope proteins in mammals and fish." J.Biol.Chem.270:
27254-27258). A smaller region of this sequence (275 amino
acids) is 52~ similar (36~ identical) with a white flounder egg
envelope protein that contains 509 amino acids.
Immunization of grey seals with a single
administration vaccine containing soluble zona pellucida
antigens encapsulated in liposomes has been shown to reduce
female fertility by at least 90~ for up to at least six years
(Brown,R.G., Kimmins,W.C., Mezei,M., Parsons,J.L., Pohajdak,B.
and Bowen,W.D. (1996) "Birth control for grey seals." Nature
379:30-31; Brown,R.G., Bowen,W.D., Eddington,J.D.,
Kimmins,W.C., Mezei,M., Parsons,J.L., and Pohajdak,B.(1997)
"Evidence for a long-lasting single administration
contraceptive vaccine in wild grey seals." J.Reproduct.Immunol.
35:43-51~ and US Patent No. 5,736,141). The same vaccine
prevented pregnancy in four rabbits (proven breeders) following
8 coatings (unpublished observations).
An example of the use of liposome encapsulation of
denatured recombinantly produced protein to raise antibodies
against a native protein was shown with Neisseria meningitides
outer membrane protein P1. (Muttilainen,S.,
Idanpaan-Heikkila,I., Wahlstrom,E., Nurminen,M., Makela,P.H.
and Sarvas,M. (1995) "The Neisseria meningitides outer membrane
protein P1 produced in Bacillus subtilis and reconstituted into
phospholipid vesicles elicits antibodies to native P1
epitopes." Microb.Pathog.18:423-436).
Specificity of recognition of sperm and egg is
2
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
essential in any species. However, the mechanism of '
fertilization varies widely, both physiologically and
biochemically, between species. Fertilization in fish differs
from that in mammals in that most teleostean fish spermatozoa
lack an acrosomal structure. Penetration by a spermatozoon of
the fish egg envelope occurs via a discrete micropyle with
closure of the micropyle after penetration of the first
spermatozoon.
Sperm-egg interaction in birds is significantly
different from that in mammals.and different again from fish.
In birds, sperm-egg recognition is initiated by the binding of
spermatozoa to the inner perivitelline layer (IPVL), a
proteinaceous structure surrounding the avian ovum (Bakst,M.R.
and Hawarth,B. (1977) "Hydrolysis of hens perivitelline layer
by cock sperm in vitro." Biol. Reproduct. 17:370-379). There
is no block to polyspermy in avian species but a further
proteinaceous layer, the outer perivitelline layer (OPVL), is
laid down about 15 minutes after the IPVL in chickens and
appears to prevent further penetration of sperm. Therefore, if
spermatozoa can be prevented from entering the avian egg
between the laying down of the IPVL and OPVL, by antibodies
directed against the IPVL, then immunocontraception would be
realized.
There is some similarity between reproduction in
mammals and fish but also many differences. Unlike the
C-terminus, the N-terminus domain of white flounder egg protein
is quite dissimilar to mouse ZP2 (ZPA) and a transmembrane
domain characteristic of all mammalian zona pellucida proteins
is not present in teleost egg protein indicating the divergence
of these species 650 million years ago (Epifano,0., Liang,L-F.
and Dean, J. (1996) "Mouse ZP1 encodes a zona pellucida protein
homologous to egg envelope proteins in mammals and fish."
J.Biol.Chem.270: 27254-27258).
The carbohydrate moiety of teleost egg glycoproteins
3
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
is also dissimilar, for example, rainbow trout egg envelope '
glycoprotein has a unique N-linked glycan containing KDN
(2-keto-3-deoxy-D-glycero-D-galacto-nononic acid) in the second
layer of the vitelline envelope (Tezuka,T., Taguchi,T.,
Kanamori,A., Muto,Y., Kitajima,K., Inoue,Y. and Inoue,S. (1994)
"Identification and structural determination of the
KDN-containing N-linked glycan chains consisting of bi- and
triantennary complex-type units of KDN-glycoprotein previously
isolated from rainbow trout vitelline envelopes."
Biochem.33:6495-6502). This.KDN-glycoprotein is exposed to the
outer surface around the micropyle through which sperm enter
the egg at fertilization. Most fish sperm lack an acrosome and
penetrate the fish egg envelope via a discrete micropyle. The
micropyle forms a guidance system in teleost fertilization that
enhances sperm penetration (Amanze,D. and Iyengar,A. (1990)
"The micropyle: a sperm guidance system in teleost
fertilization." Development 109:995-500). A chemical
attractant may also emanate from the micropyle to enhance the
chance of fertilization.
In spite of the significant structural differences
between fish egg envelope protein and mammalian zona pellucida,
fish egg envelope proteins have been designated the teleost
homolog of zona pellucida (TH-ZP for convenience of reference).
In fish, TH-ZP3 is made in the liver and transported via the
blood to the ovary, while TH-ZP2 is made in the ovary
(Hamazaki,T.S., Nagahama,Y. and Yamagami,K. (1989) "A
glycoprotein from liver constitutes the inner layer of the egg
envelope (zona pellucida interna) of the fish, Oryzias
Iatipes." Dev.Bio1.133:101-110 Murata,K., Sasaki,T.,
Yasumasu,S., Iuchi,I., Enami,J., Yasumasu,I. and Yamagami,K.
(1995) "Cloning of cDNAs for the precursor protein of a
low-molecular weight subunit of the inner layer of the egg
envelope (chorion) of the fish Oryzias latipes."; Chang,Y.S.,
Wang,S.C., Tsao,C.C. and Huang,F.L. (1996) "Molecular cloning,
4
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
"
structural analysis and expression of carp ZP3 gene. '
Mol.Reprod.Dev.44:295-304; Murata,K., Sugiyama,H., Yasumasu,S.,
Iuchi,I., Yasumasu,I. and Yamagami,K. (1997) "Cloning of~cDNA
and estrogen-induced hepatic gene expression for chorigenin H,
a precursor protein of the fish egg envelope (chorion)."
Proc.Natl.Acad.Sci. USA 94:2050-2055; Chang,Y.S., Hsu,C.C.,
Wang,S.C., Tsao,C.C. and Huang,F.L. (1997) "Molecular cloning,
structural analysis and expression of carp ZP2 gene."
Mol.Reprod.Dev.46:258-67).
It is undesirable that transgenic fish escape from
fish farms and mate with fish in the wild. This problem would
be reduced if females were sterile. Such sterile fish could
also redirect their food reserves to increase their body size
rather than roe production. Triploid fish are sterile but
triploid salmon grow poorly (MacKenzie, D. (1996) "Can we make
supersalmon safe?" New Scientist pp 14-15). Triploidy can be
induced in fish by a pulse of pressure that prevents embryos
from ejecting one set of chromosomes.
With respect to birds, population control of certain
species is of great environmental importance. For example,
same Canada geese (Branta canadensis) populations in the USA,
Canada and Europe have increased to a point that threatens
other bird populations and are a nuisance to the enjoyment of
parks, golf courses, etc. Burgeoning populations of snow geese
(Chen caerulescens) are wreaking havoc on precious tundra
habitat (Struzik,E. (1998) "The snow geese dilemma." Equinox
97:50-57) and have resulted in compensation claims in Quebec,
Canada alone of $844,000 in 1996. Some tundra habitats have
been described as 35~ overgrazed, 35$ damaged and 30$ destroyed
by snow geese. In addition, many populations of small birds
such as pigeons (Columba livia) and starlings (Sturnus
vulgaris) cause significant economic loss in many parts of the
world. As a consequence, there is need for management of some
bird populations.
5
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
SUi~IARY OF T8E INVENTION
The present invention provides a single
administration immunocontraceptive for fish.
More specifically, the present invention provides an
immunacontraceptive vaccine composition comprising a teleost
homolog of zona pellucida (TH-ZP), together with a
pharmaceutically acceptable diluent or carrier, for preventing
fertilization in a fish.
In another aspect, the present invention provides a
method for preventing fertilization in a fish comprising
administering an effective amount of the composition of the
invention, comprising a teleost homolog of zona pellucida
(TH-ZP), to the fish.
1S It is preferred that an adjuvant, such as Freund's
complete adjuvant (FCA) or another biologically acceptable
adjuvant, be present to assist in stimulation of an immune
response in fish. It is also preferred that the TH-ZP be
encapsulated into a liposome for administration. Preferably
the liposome is multilamellar and comprises L-a-lecithin
(soybean) and cholesterol, since this will effect slow release
of TH-ZP resulting in an extended period of antibody production
and thereby an extended period of contraception in fish. In
addition, antibodies raised by this immunological procedure
will be directed to the native protein antigens.
The present invention also provides a single
administration immunocontraceptive for birds.
Accordingly, in another aspect, the present invention
provides an immunocontraceptive vaccine composition comprising
an antigen from an inner perivitelline layer (IPVL) of a bird
egg, together with a pharmaceutically acceptable diluent or
carrier, for reducing or preventing fertilization in a bird.
In another aspect, the present invention provides a
method for preventing fertilization in a bird comprising
6
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
administering an effective amount of the composition of the
invention, comprising the antigen from an inner perivitelline
layer (IPVL), to the bird.
It is preferred that an adjuvant, such as Freund's
complete adjuvant (FCA) or another biologically acceptable
adjuvant, be present to assist in stimulation of an immune
response in birds.
It is preferred that the antigen from the IPVL, e.g.
in an IPVL portion, be encapsulated into a liposome for
administration. Preferably the liposome is multilamellar and
comprises L-a-lecithin (soybean) and cholesterol, to effect
slow release of antigen/IPVL and increase production of
antibodies that bind to the target proteins. This will result
in an extended period of antibody production and thereby an
Z5 extended period of contraception in birds.
As well as FCA, other adjuvants that can be used in
vaccine compositions of the present invention include
non-ulcerative Freund's complete adjuvant, Freund's incomplete
adjuvant, TITERMAX~, MF89, Gerbu, Bacillus Calmette-Guerin,
RIBI (MPL+TDM+CWS), bacterial lipopolysaccharide, sodium
phthalate derivative of bacterial lipopolysaccharide, sodium
phthalate derivative of lipopolysaccharide plus alum,
SUPERCARRIER'r', ADJUPRIMET" and Alum.
In general, any suitable liposome can be used in the
fish or bird vaccine compositions disclosed herein. Anionic
and neutral liposomes are well-known in the art (see, e.g.,
Liposomes: A Practical Approach, RPC New Ed, IRL press (1990),
for a detailed description of methods for making liposomes) and
are useful for delivering a large range of products.
Cationic lipids are also known in the art. Such
lipids include LipofectinTM also known as DOTMA
(N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium
chloride), DOTAP
(1,2-bis(oleyloxy)-3-(trimethylammonio)propane), DDAB
7
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
(dimethyldioctadecylammonium bromide), DOGS '
(dioctadecylamidologlycyl spermine) and cholesterol derivatives
such as DC-Chol (3 beta-(N-(N',N'-dimethyl
aminomethane)-carbamoyl) cholesterol). A description of these
cationic lipids can be found in EP 187,702, WO 90/11092, U.S.
Patent No. 5,283,185, WO 91/15501, WO 95/26356, and U.S. Patent
No. 5, 527, 928.
The route of administration of the vaccine
compositions disclosed herein can be any route used typically
used in the vaccine field. As general guidance, administration
can be via a mucosal surface, e.g., an ocular, intranasal,
pulmonary, oral, intestinal, rectal, vaginal, and urinary tract
surface; or via a parenteral route, e.g., by an intravenous,
subcutaneous, intraperitoneal, intradermal, intraepidermal, or
intramuscular route. The choice of administration route
depends on the formulation that is selected as well as on the
animal to be vaccinated.
Administration is achieved in a single dose or
repeated as necessary at intervals, as can be determined
readily by one skilled in the art. An appropriate dose depends
on various parameters including the recipient (e.g., adult or
infant), the particular vaccine antigen, the route and
frequency of administration and the presence/absence or type of
adjuvant as can be determined by one skilled in the art.
It should be noted that all of the antibody titers
referred to in the specification are measured in comparison
with the antibody titer in a reference serum. The titer in the
reference serum was arbitrarily assigned a value of 100. That
value has no relationship to the absolute titer required to
produce an immunocontraceptive effect. In fact, titers of only
a few percent of those found in the reference serum are
sufficient to produce an immunocontraceptive effect in some
cases. While the reference serum clearly contains sufficient
antibody to effect immunocontraception, it does not represent
8
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
an indication of the minimum antibody titer needed for
immunocontraception.
BRIEF DESCRIPTION OF T8E DRAWINGS
Figure 1 shows a gel chromatography profile of
herring TH-ZP. Fractions 60-75 and 78-80 ml were pooled,
dialyzed and freeze-dried
Figure 2 shows an ion exchange chromatography profile
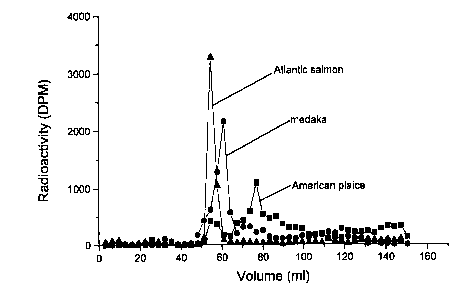
of American plaice, Atlantic salmon and medaka TH-ZP. Each
major peak (64-83 ml, American plaice; 54-70 ml Atlantic
salmon; 54-64 ml medaka) was pooled, dialyzed and freeze dried.
Figure 3 shows the results of an isoelectric
focussing purification of herring TH-ZP. Tubes 12-15
(inclusive) were pooled, dialyzed and freeze dried.
Figure 4 shows the production of anti-TH-ZP
antibodies by rainbow trout immunized with Atlantic salmon
TH-ZP, American plaice TH-ZP, tilapia TH-ZP, medaka TH-ZP and
haddock TH-ZP.
DETAILED DESCRIPTION OF THE INVENTION
1. FISH
Preferred methods of purifying TH-ZP from the eggs of
exemplified fish species are set out below. Rabbits were
conveniently used for production of anti-TH-ZP sera for
screening fractions obtained during purification of TH-ZP. Any
species of fish can be immunized provided the TH-ZP used in the
vaccine is different enough from the targeted fish species to
provoke a good immune response but similar enough that the
antibodies produced cross-react with the targeted species
TH-ZP. In practice, species that are farmed commercially,
including transgenic fish such as salmon, rainbow trout and
tilapia, would be important targets.
Collection of fish eggs.
Atlantic salmon (Salmo salary, American plaice
9
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
(Hippoglossoides platessoides), herring (Clupea harengus) and '
haddock (Melanogramrnus aeglefinus) eggs were obtained from
local commercial suppliers. Tilapia eggs were obtained from a
colony of hybrid tilapia (Oreochronis mossambicus X hornorum)
maintained in the Aquatron, Dalhousie University. Medaka eggs
were harvested daily from a colony of Indian medaka (Oryzias
latipes) and stored at -20°C until extracted. Perch (Perca
flavescens) and smelt (Osmerus mordax) eggs were obtained from
fish caught in Lake Simcoe, Ontario and stored at -20°C until
extracted.
Extraction of TH-ZP.
The method used to extract the teleost homolog of
zona pellucida (TH-ZP) depended on the quantity of eggs
available. Method 1 was used when the wet weight of eggs was
under 100 g. Method 2 was used when the wet weight of eggs was
over 100 g.
Extraction method 1.
Fish eggs were placed in a Wheaton tissue homogenizer
(30 ml) equipped with a Teflon plunger. The plunger was pushed
to the bottom of the tube and up to the top until microscopical
examination indicated that most eggs were broken. The egg
ghosts were collected on a nylon screen (48 um pore size) and
washed with cold saline to remove cytoplasm. Egg ghosts were
replaced in the tissue homogenizer and agitated with the
plunger to wash any remaining cytoplasm out of the ghosts.
Microscopical examination was used to judge when the egg ghosts
were free of cytoplasm. The egg ghosts were suspended in Tris
buffer (20 mM, pH 8.0) and incubated at 75°C in a water bath for
25 minutes. The suspension was vortexed and centrifuged
(16,000 X g for 15 minutes). The supernatant fluid was
dialyzed, freeze dried and stored at -20°C.
Extraction method 2.
Fish eggs were suspended in saline and the suspension
placed in a Waring blender. The suspension was blended for 30
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
seconds and the egg ghosts collected on a nylon screen (pore
size 500 um). The ghosts were washed with liberal amounts of
cold saline. The egg ghosts were resuspended in saline and
replaced in the Waring blender for 30 seconds. The egg ghosts
were collected on a nylon screen (pore size 209 um) and washed
with cold saline. The egg ghosts were extracted with Tris
buffer as described in method 1.
Detection of TH-ZP.
Proteins in fish egg extracts were labelled with 1°C
by reductive methylation (Jentoft, N. and Dearborn,D.G. (1979)
"Labelling of proteins by reductive methylation using sodium
cyanoborohydride." J.Biol.Chem.259:9359-4365) so that fractions
obtained during purification procedures could be monitored by
determination of radioactivity. Crude extracts (10 mg) were
dissolved in Hepes buffer (20 ml, pH 7.5, 0.1 M) to which
iaC-formaldehyde (10 uCi, 37 mCi/mmol) was added. NaCNBH4 was
added in two equal portions, one at the beginning and one
following 30 minutes incubation at 20°C, to give a final
concentration of 20 mM. After 60 minutes incubation, the
reaction mixture was acidified with acetic acid and dialyzed
overnight. The labelled product was recovered by freeze drying.
To produce TH-ZP that was not radioactive,
purification procedures were repeated with unlabelled egg
extracts. In this case, fractions were monitored for protein
with bicinchoninic acid (Sigma) using bovine serum albumin as a
reference standard.
Fractions were also monitored by ELISA using rabbit
anti-haddock TH-ZP serum during purification of herring, smelt
and perch TH-ZP. Aliquots of fractions from gel
chromatography, ion exchange chromatography and isoelectric
focussing were diluted to contain protein in the range 10-100
ug/ml with sodium carbonate/bicarbonate buffer (Na2C03 0.015 M;
NaHC03,0.035 M: pH 9.6). The diluted fractions (100 uL) were
11
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
placed in wells of a microtiter plate and proteins allowed to .'
absorb at 37°C for 1 hour. Material not absorbed was removed
and the wells coated with gelatin (3 ~ in TBST buffer -
Tris,0.01 M; NaC1,0.15 M; 0.05 ~ Tween 20; pH 8.0) for 10
minutes followed by washing 5 X's with TBST buffer. Rabbit
anti-haddock TH-ZP serum (100 uL, diluted 1:100 with TBST
buffer) was added to each well and the microtiter plate
incubated at 37°C for 1 hour. Unbound antibody and other serum
proteins were removed by washing with TBST buffer (5 X's).
Bound antibody was measured with protein A/alkaline phosphatase
using a Dynatech ELISA plate reader at 405 nm.
Chromatography.
Gel chromatography used TSK-gel (toyopearl HW-65F,
1.5 X 58 cm) eluted with Tris buffer (0.01 M, pH 7.5 containing
0.01 $ NaN) at a flow rate of 15 ml/hr. Crude TH-ZP extracts
were dissolved in Tris buffer (0.01 M, pH 7.5, 5 ml),
centrifuged to remove any insoluble material and aliquots (2
ml) used for gel chromatography. Fractions (3 ml) were
collected and aliquots from each fraction were analyzed for
radioactivity, protein or ELISA using rabbit anti-haddock TH-ZP
serum.
Ion exchange chromatography used Sephacel DEAE (1.5 X
22 cm) eluted with Tris buffer (0.01 M, pH 8) having a linear
gradient from 0 to 0.3 M NaCl in a total volume of 150 ml at a
flow rate of 6.4 ml/hr. Fractions were collected (3.l.or 6.2
ml) and aliquots of each fraction were analyzed for
radioactivity, protein or by ELISA using rabbit anti-haddock
TH-ZP serum.
Isoelectric focussing.
Preparative isoelectric focussing used a Rotofor
(Biorad) at a constant power input of 12 W for 4 hr. The
RotoLytes (Biorad) used were in the range pH 3-9 formed from
combining RotoLytes in the range 2.9-9.1:4.5-6.16.4-7.5 and
7.8-8.9. Twenty fractions were collected and the pH of each
12
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
fraction was adjusted to pH 7-8 with acetic acid or solid '
NaHC03. Aliquots of each fraction were analyzed for TH-ZP by
determination of radioactivity or ELISA using rabbit
anti-haddock TH-ZP or rabbit anti-herring TH-ZP sera.
SDS-PAGE.
SDS-PAGE used gradient gels (Biorad) and kaleidoscope
standards to determine molecular weights. Gels were stained
with coomassie blue or used for Western blotting with rabbit
anti-haddock TH-ZP.
Rabbit anti-TFI-ZP sera.
Rabbit anti-TH-ZP sera were produced by immunizing
one rabbit for each TH-ZP type with a preparation of haddock
TH-ZP that produced a single band (44 kDa) following SDS-PAGE
and coomassie blue staining or a preparation of herring TH-ZP
obtained by gel chromatography and isoelectric focussing that
Western blotting indicated contained a single band (44 kDa).
Immunization of raiabov~r trout.
Three rainbow trout for each TH-ZP preparation were
immunized by a single intramuscular injection (18 gauge, 2.5
in. needle) with TH-ZP (50 ug) encapsulated in liposomes
containing phospholipon 90G (Nattermann Phospholipid, Cologne,
Germany, 0.04 g) and cholesterol (0.004 g) in saline (0.3 ml).
A single dose of the vaccine contained liposomes (0.3 ml, 50 ug
TH-ZP) emulsified in Freund's complete adjuvant (FCA, 0.3 ml).
Mean body masses of rainbow trout at the time of vaccination
were in the range 1.4-1.8 kg. Six rainbow trout were not
immunized and served as controls.
Determination of rainbow trout anti-TH-ZP antibody tit~rs.
Rainbow trout were anesthetized with MS 222 and blood
samples taken from the caudal vein before immunization and
1,3,5,6 and 8 months post-immunization.
Anti-TH-ZP antibody titers were measured by ELISA
using a 96-well microtiter plate. To each well, TH-ZP (1 ug)
in sodium carbonate/bicarbonate buffer (100 uL) was allowed to
13
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
adsorb at 37°C for 1 hour. TH-ZP not adsorbed was removed. -
Plates were coated with gelatin as previously described.
Rainbow trout serum samples were added in doubling dilutions
using TBST from 1/25 to 1/3200 and incubated at 20°C for 1.5
hours. Unbound antibody and other serum proteins were removed
by washing with TBST (5 X's). Mouse monoclonal IgM
anti-Chinook salmon antibody (100 uL, 1/100 dilution in TBST)
was added to all wells. Although the mouse MAb was raised
against Chinook salmon antibody, the mouse MAb bound strongly
to rainbow trout antibody reflecting the close phylogenetic
relationship between the two salmonid species. The plate was
incubated for 1.5 hours at 20°C. Unbound antibody was removed
by washing with TBST (5 X's). Bound mouse monoclonal antibody
was measured with goat anti-mouse IgM-alkaline phosphatase
solution (100 uL, diluted 1:1000 with TBST from liquid stock,
Sigma) using a Dynatech ELISA plate reader at 405 nm. One row
in each plate did not receive serum (antibody) and served as a
blank. Another row in each plate received doubling dilutions
of a reference serum. The reference serum was anti-medaka
TH-ZP serum that has a titer of 6,400. Production of
antibodies by rainbow trout is expressed relative to this serum
to avoid interassay variability.
Ova production.
Rainbow trout normally spawn in the spring, however,
the rainbow trout used in this study were from St. Peter's fish
hatchery, Nova Scotia, Canada and spawn in the autumn
(Herbinger,C.M., Doyle,R.W., Pitman,E.R., Paquet,D., Mesa,K.A.,
Morris,D.B., Wright,J.M. and Cook, D. (1995) "DNA fingerprint
based analysis of paternal and maternal effects on offspring
growth and survival in communally reared rainbow trout.
Aquaculture 137:245-256). To measure ova production, treated
and control rainbow trout (three fish in each treatment group
were weighed then the ova were removed and weighed. Rainbow
trout immunized with smelt TH-ZP, herring TH-ZP and perch TH-ZP
14
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
and three control fish were processed on November 7 (six months
post-immunization). Since the ova weighed less than expected,
the remaining rainbow trout were maintained for another month
(eight months post-immunization) before being processed.
S Extraction of TH-ZP.
Extraction of haddock roe (1.1 kg wet weight) yielded
a crude preparation of haddock TH-ZP that weighed 949 mg and
extraction of herring roe (230 g wet weight) yielded a crude
preparation of herring TH-ZP that weighed 152 mg. Yields of
crude TH-ZP preparations from other fish roe were similar.
Reductive methylation of TH-ZP.
Reductive methylation of crude preparations of TH-ZP
yielded material having radioactivity in the range 545-9770
DPM/mg protein.
Purification of TH-ZP.
Gel chromatography of most crude extracts of TH-ZP
yielded one broad peak as detected by ELISA using rabbit
anti-haddock TH-ZP serum or by measurement of protein or
radioactivity. Fractions containing the major peak were
pooled, dialyzed and freeze dried. Based on recovery of
protein or radioactivity, yields were lower than expected (for
example, 20 mg of 61 mg placed on the gel) suggesting that gel
chromatography removed non-protein components, but did little
to resolve proteins. In some cases, two peaks were obtained
(Figure 1). In these cases, each peak was pooled, dialyzed,
and freeze dried.
The freeze dried material was chromatographed on
Sephacel-DEAF (Figure 2). Fractions were analysed by ELISA or
for radioactivity, pooled, dialyzed and freeze dried. SDS-PAGE
was used to assess purity of preparations. American plaice,
haddock, medaka and tilapia TH-ZP preparations contained a
single protein band (44 kDa) and were not further purified.
The expected molecular weight was 45 kDa based on a polypeptide
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
chain of 509 amino acids (Lyons et a1.,1993). The Atlantic '
salmon TH-ZP preparation contained a major band at 90 kDa and
minor bands at 29 and 87 kDa.
Herring, smelt and perch TH-ZP preparations were
further purified by preparative isoelectric focussing (Figure
3). Following isoelectric focussing, coomassie blue staining
of a SDS-PAGE gel detected a wide band (90-120 kDa) in the
herring, perch and smelt TH-ZP preparations. A Western blot
using rabbit anti-haddock TH-ZP serum detected only one band
(44 kDa) in the herring preparation suggesting coomassie blue
staining material on either side of the major band was present
in minor quantities and was unrelated to TH-ZP. Western
blotting of SDS-PAGE gels detected two bands in the smelt
preparation (70 and 90 kDa) and two major bands in the perch
preparation (29 and 70 kDa). Although the preparations of
Atlantic salmon TH-ZP, smelt TH-ZP and perch TH-ZP contained
more than one protein and the molecular weights of these
proteins differed from the expected value of 45 kDa, detection
by rabbit anti-haddock TH-ZP serum in Western blots suggests
that the material in the Atlantic salmon, perch and smelt
preparations was related to TH-ZP. Therefore, these
preparations as well as the other TH-ZP preparations were used
to immunize rainbow trout.
Production of anti-TH-ZP antibodies by rainbow trout.
Rainbow trout immunized with medaka TH-ZP produced
the most antibody (Figure 4). Production of anti-TH-ZP
antibodies by rainbow trout immunized with TH-ZP from American
plaice, haddock, and tilapia was similar. Antibody titers
increased during the 8 months post-immunization period but the
increase was not linear titers were highest 6 or 8 months
post-immunization. Titers of sera from rainbow trout immunized
Atlantic salmon TH-ZP were low. Anti-Atlantic salmon TH-ZP
titers were highest 3 months post-immunization but declined
thereafter.
16
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Measurement of crossreactivity using rainbow trout
anti-sera collected six months post-immunization confirmed the
low anti-Atlantic salmon TH-ZP titers and indicated that
rainbow trout produced the highest titer when immunized with
medaka TH-ZP (TABLE 1). Crossreactivity generally reflected
phylogenetic relationship among the fish species investigated,
that is, anti-TH-ZP sera crossreact strongest with TH-ZP from
fish that are phylogenetically related. This relationship was
not true of sera from trout immunized with TH-ZP from smelt,
herring and perch (TABLE 2).. These sera were obtained from
rainbow trout early in roe production when TH-ZP 3 is being
transported from the liver to the ovary via the blood. If
contact between antibodies and TH-ZP 3 is high, this could
result in removal of anti-TH-ZP antibodies binding to rainbow
trout TH-ZP 3. This proposal is supported by the observation
that rainbow trout anti-herring TH-ZP antibodies bound best to
herring and perch TH-ZP, the two TH-ZP types least related
phylogenetically to rainbow trout TH-ZP (TABLE 3). In
contrast, rainbow trout anti-haddock TH-ZP serum crossreacted
with TH-ZP from teleosts in the order predicted by phylogenetic
relationships. One possible explanation is that when rainbow
trout anti-haddock TH-ZP serum was being collected, the
terminal stage in roe production was reached and less TH-ZP 3
was present in blood to remove anti-TH-ZP antibodies
crossreacting with rainbow trout TH-ZP. As a consequence,
anti-TH-ZP antibodies recognizing medaka, tilapia, American
plaice and Atlantic salmon TH-ZP were able to accumulate in the
blood.
Rabbit anti-haddock TH-ZP and anti-herring TH-ZP sera
crossreacted best with TH-ZP from fish closest phylogenetically
to either haddock TH-ZP or herring TH-ZP. Rainbow trout
anti-haddock TH-ZP serum demonstrated a similar relationship
suggesting that rainbow trout can make antibodies with the same
discriminating specificity as mammals. Perch TH-ZP was the
17
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
exception. Rabbit anti-haddock TH-ZP and rabbit anti-herring=
TH-ZP sera recognized perch TH-ZP better than would be
predicted from phylogenetic considerations. Interestingly,
rainbow trout anti-herring TH-ZP serum also recognized perch
TH-ZP better than expected based on phylogeny.
Ova production.
When ova production was measured, many of the rainbow
trout were found to be males. This was unexpected since
presumably this study started with all female rainbow trout.
As a consequence, sample sizes are not large enough to permit
statistical analysis (TABLE 4).
In spite of the above problem, the results suggest
that immunization of rainbow trout with salmon TH-ZP, haddock
TH-ZP, herring TH-ZP or perch TH-ZP reduces roe production.
American plaice, haddock, medaka, tilapia and herring
TH-ZP preparations contained a single protein having the
expected molecular weight of 45 kDa. Atlantic salmon, smelt
and perch TH-ZP preparations contained more than one protein
with molecular weights that differed than the expected value of
45 kDa. Western blots suggested that these proteins were
related to TH-ZP from other teleosts and therefore these
proteins were included in the present study.
Of the TH-ZP preparations studied, immunization of
rainbow trout with salmon, haddock, herring or perch TH-ZP
reduced roe production. The difficulty of determining the sex
of experimental animals caused too few females to be included
in each experimental group, consequently, statistical analysis
of the results was not possible.
Relating anti-TH-ZP antibody titers and
crossreactivity to roe production was difficult as the quantity
of roe produced is a significant portion of fish weight.
Consequently, significant quantities of antibodies could be
bound to TH-ZP 3 as TH-ZP 3 was being transported from the
liver to the ovary and consequently would not be available
18
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
during measurements of titers and determination of
crossreactivity. This potential difficulty would be greatest
when roe production was maximal.
19
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
TABLE 1. Anti-TH-ZP titers in rainbow trout six months '
postimmunization.
Titer ($ of reference serum)1
TH-ZP used in ELISA assay
Vaccine Tilapia Atlantic Haddock American Medaka
TH-ZP salmon plaice
Tilapia 33 5 19 11 44
Atlantic 8 3 3 2 18
salmon
Haddock 19 2 67 9 52
American 11 6 7 85 26
plaice
Medaka 23 8 16 7 100
1 Each titer is an average of measurements using sera from three
rainbow trout immunized with the same antigen. The average
titer of sera from rainbow trout immunized against medaka TH-ZP
was arbitrarily set at 100.
CA 02356452 2001-06-21
WO 00/37100 ~ PCT/CA99/01225
TABLE 2. Anti-TH-ZP titers in rainbow trout three months
postimmunization.
Titer (~ of reference serum)1
Vaccine TH-ZP used in ELISA assay
TH-ZP smelt herring perch
smelt 3 1 0
herring 8 58 30
perch 5 14 65
1 Titer is expressed relative to a rainbow trout anti-medaka
TH-ZP sera to avoid interassay variability.
21
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
TABLE 3. Crossreactivity of rainbow trout anti-herring and
anti-haddock TH-ZP and rabbit anti-herring and anti-haddock
TH-ZP sera.
Titer (~ of homologous serum)1
TH-ZP
used in anti-herring TH-.ZP anti-haddock TH-ZP
ELISA
rainbow trout rabbit rainbow trout rabbit
herring 100 100 ND 81
haddock 2 45 100 100
medaka 4 3 77 7
tilapia 1 0 28 4
American 1 0 13 2
plaice
Atlantic 0 41 3 12
salmon
smelt 2 11 ND 140
perch 43 93 ND 68
1 Titers of anti-TH-ZP sera are expressed as a percentage of the
homologous anti-serum. ND = not determined.
22
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
TABLE 4. Effect of immunization of rainbow trout with TH-ZP on
ova production.
Trial 1 Trial 2
vaccine ova production vaccine ova production
Ag (~ of fresh weight) Ag ($ of fresh weight)
Control 1.87 Control 14.2
1.63
Atlantic salmon 6.7
smelt 1.54 93
herring 0.93 haddock 6.7
1.28 10.5
perch 0.98 American plaice 11.7
medaka 12.2
8.8
tilapia 14.7
10.8
14.1
23
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
2. BIRDS
Preferred methods of purifying the inner
perivitelline layer (IPVL) from eggs of exemplified bird
species are set out below. Any bird species can be immunized
provided the correct balance between foreignness of IPVL to
provoke a good immune response and relatedness to foster good
crossreactivity is chosen. This requires matching the target
species with the bird species from which the IPVL is obtained
and used as antigen in the vaccine. In practice, a bird
species in need of population. control such as the Canada goose
(Branta canadensis) would be chosen.
Antigen.
The inner perivitelline layer (IPVL) of chicken, duck
and goose eggs was isolated from laid eggs as described by
Robertson, L., Brown,H.L., Staines,H.J. and Wishart,G.J. (1997)
"Characterization and application of an avian in vitro
spermatozoa-egg interaction assay using the inner perivitelline
layer from laid chicken eggs." J. Reproduct. Fertil.
110:205-211. Goose or duck IPVL (0.2 mg/ml) was suspended in
Tris (pH 9.0, 0.01 M) buffered saline at 25°C for 30 minutes.
The resulting suspension was encapsulated in liposomes as
described below.
Vaccine.
A single dose of the vaccine contained either goose
or duck IPVL (50 pg) suspended in Tris buffered saline (250 pL).
Duck or goose IPVL was encapsulated in multilamellar liposomes
and suspended in Freund's complete adjuvant (FCA; 0.25 ml) as
previously described (Brown et al., 1997). The placebo vaccine
contained all the above except IPVL. Brown Leghorn chickens
(1.3 - 1.9 kg; 54 weeks old) were immunized by injection into
the breast (22 gauge,. 1.5 in. needle).
Titers.
Blood samples were taken from the wing vein and
allowed to clot. Sera were recovered from the blood samples by
24
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
centrifugation. Anti-goose IPVL and anti-duck IPVL titers were-
measured by ELISA using affinity purified rabbit anti-chicken
IgG (Sigma C-2288) and protein A/alkaline phosphatase. All
anti-goose IPVL and anti-duck IPVL titers are expressed
relative to the titer of the 1.5 month post-immunization
anti-sera of chickens 1179 and 1049, respectively.
Egg Fertility.
All hens were artificially inseminated twice, three
days apart, with pooled semen from nine Barred Rock roosters
immediately following semen collection. Beginning one day after
the second insemination, eggs were collected daily over a 14
day period and stored at 7°C until incubation. All eggs were
incubated at 37.5°C, 60 ~ relative humidity and turned three
times daily until hatching. The eggs were candled at days 7 and
14, and infertile eggs evaluated by breakout. Fertile eggs were
incubated until hatching to assess the effect of vaccination on
chick development.
Results and Discussion.
Immunization of chickens with goose and duck IPVL
produced antibodies that persisted during the period when eggs
were collected from immunized hens (Table 5}. The fertility of
eggs from chickens that received the placebo vaccine was high
(Table 6). A slight reduction in fertility occurred during
development of the chicken embryo from fertilization until
hatching. The fertility of eggs from chickens that were
immunized with goose IPVL also showed a slight reduction in
fertility during incubation (Table 7). There was no significant
difference in fertility between eggs from chickens that
received the placebo vaccine and eggs from chickens that were
immunized with goose IPVL following 14 days incubation (Xz
=0.203: P = 0.6). The fertility of eggs from chickens that were
immunized with duck IPVL was significantly less than the
fertility of eggs from chickens that received the placebo
vaccine (Table 8: XZ = 4.63: P = < 0.005). These results
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
demonstrate that immunization of chickens with duck IPVL can -
significantly reduce the fertility of chicken eggs.
Immunization of chickens with goose IPVL and duck
IPVL did not significantly affect fertility after the eggs were
laid based on analysis of the decline in fertility from day 0
to day 14 among fertile eggs (X2 = 0.871; 2 df; P = >0.5).
Therefore, the differences in fertility between treatments are
due to causes arising before laying.
The percent of chicken anti-goose IPVL and anti-duck
IPVL antibodies that bound to chicken IPVL varied from 1.6 -7.3
~ for anti-goose IPVL and 0-5.2 ~ for anti-duck IPVL (Table 9).
Therefore, the quantity of anti-goose IPVL and anti-duck IPVL
antibody binding to chicken IPVL is low.
PAGE of IPVL from chicken, goose and duck eggs
followed-by Western analysis using chicken anti-goose IPVL
identified proteins having molecular weights of 48,000 and
45,000 as the main antigens in chicken IPVL. Selection of IPVL
from bird eggs that crossreacted more strongly with chicken
IPVL would improve the reduction in fertility.
It is expected that goose IPVL and duck IPVL will be
similar to IPVL from snow and Canada geese, which are one
possible target species. Therefore, effectiveness in fertility
reduction when this vaccine is administered to snow and Canada
geese is expected.
26
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
TABLE 5. Production of anti-goose IPVL and anti-duck IPVL
antibodies by chickens immunized against goose IPVL and duck
IPVL using liposome delivery.
Anti-IPVL titres
Chicken (~ of reference serum)
ID Treatment)
Post-immunization (days)
0 45 72
1009 Placebo 0 0 0
946 , 0 ~ 0 0
1080 0 0 0
1075 0 0 0
1055 0 0 0
1030 0 0 0
1179 Goose IPVL 0 100 62
1012 0 89 57
694 0 81 44
1081 0 79 -
639 0 89 82
690 0 98 80
1019 0 92 79
1074 0 79 70
1049 Duck IPVL 0 100 76
1093 0 98 83
1031 0 67 43
650 0 97 98
695 0 81 80
1032 0 53 76
1026 0 39 39
637 0 25 14
Reference sera were from chickens 1179 and
1049 at
one and
one-half months post- immunization duck IPVL
for goose
and
respectively.
27
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Table 6. Fertility of eggs from chickens immunized with a
placebo vaccine.
Chicken ID Post-immunization Fertility status post-laying
(days) (days)
0 1 7 14 hatch
1009 58 F F F F F
60 F F F F F
62 F F F F F
63 .F F F F F
65 F I I I I
68 F F F F F
69 F F F F F
946 56 F F F F F
59 F F F F F
61 F F F F F
62 F F F F F
63 F F F F F
64 F F F F F
66 F F F F F
68 F F F F F
70 F F F F F
72 F F F F F
1080 56 F F F F F
57 F F F F F
59 F F F F F
61 I I I I I
62 F F F F F
63 F F F F F
64 F F F F F
66 F F F F F
69 F F F F F
70 F F F F F
72 F F F F -
28
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Table 6 (continued) '
1075 57 F I I I I
58 F F F F F
59 F I I I I
61 F F F F -
62 F I I I I
63 F F F I I
66 F I I I I
67 F F F F F
1069 . F F F F F
70 F F F F
72 F F F F -
1056 57 F F F F F
58 F F F F F
15~59 F F F F F
60 F F F F F
62 F F F F F
63 F F F F F
64 F F F F F
2066 F F F F F
67 F F F F F
70 F F F F F
72 F F F F F
25 Percent fertile 98 ~~ ~~ ~~o u~
Time post-immunization that the egg was laid.
F = fertile; I = infertile; - - not determined
29
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Table 7. Fertility of eggs from chickens immunized with goose
IPVL.
Chicken ID Post-immunization Fertility status post-laying
(days) (days)
0 1 7 14 hatch
1179 57 F F F F F
59 F F F F F
60 F I I I I
62 F I I I I
63 F F F F I
65 F F F F F
68 F F F F F
70 F F F F F
72 F F F F F
1012 56 F F F F F
57 F F F F F
59 F F F I I
60 F F F F F
62 F F F F F
63 I I I I I
65 F F F F F
66 F F F F F
68 F F F F F
69 F I I I I
70 F F F F F
694 57 I I I I I
58 F F F F F
59 F F I I I
61 F F F F F
62 F F F F F
63 F F F F F
65 F F F F F
66 F F F F F
30
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Table (continued) '
7
68 F F F F F
69 F F F F F
70 F F F F F
690 56 F F F F F
58 F F F F F
59 F F F F F
61 F I I I I
62 F F F F F
63 . F F F F F
65 F F F I I
66 F F F F F
68 F F F F F
72 F F F F F
1019 59 I I I I I
66 I I I I I
68 F F F F I
72 F F F F F
1074 57 F F F F F
59 F F F F F
60 F F F F F
61 F F F F F
62 F F F F F
63 F F F F F
65 F F F F F
66 F F F F F
67 F F F F F
68 F F F F F
69 F F F F F
71 F F F F F
72 F I I I I
Percent fertile 93 84 83 79 74
31
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Table 7 (continued)
1 Time post-immunization that the egg was laid.
F = fertile; I = infertile; - - not determined
32
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Table 8. Fertility of eggs from chickens immunized with duck
IPVL.
Chicken ID Post-immunization Fertility status post-laying
(days) (days)
0 1 7 14 hatch
1049 56 F I I I I
58 F F F F F
59 F F F F F
60 . F F F F -
62 F F F I I
63 F F F F F
64 F F F F F
65 F I I I I
66 F F F F F
68 I I I I -
69 F F F F F
72 F I I I I
1031 56 I I I I I
S8 F F F F F
59 F I I I I
61 I I I I I
62 F F F I I
64 F I I I I
66 F F F F F
68 F I I I I
70 I I I I I
72 I T I I I
1093 56 F F F F F
58 F F F F F
59 F F F F F
60 F F F F F
62 F I I I I
63 F F F F F
33
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Table (continued)
8
65 F F F F F
66 F F F F F
68 F F F F F
69 I I I I I
72 I I I I I
695 56 F F F F F
57 F I I I I
59 F F F F F
60 . F F F F F
63 F F F F F
64 F F F F F
65 F F F F F
67 F F F F F
69 F I I I I
72 F F F F F
1032 57 I I I I I
60 F F F F F
70 F F F F F
72 F I I I I
1026 56 F F F F F
57 F F F I I
59 F F F F F
61 I I I I I
63 F I I I I
64 F F F I I
66 F F F F F
67 F F F F F
69 F F F F F
70 F F F F F
72 F F F F F
637 57 I I I I I
58 F F F F F
60 I I I I I
34
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Table 8 (continued)
61 F F F I I
62 F F F F F
63 F F F F F
64 F F F F F
65 F F F F F
66 F F F F F
69 I I I I I
72 I I I I I
72 . I I I I I
Percent fertile 80 64 64 56 48
Time post-immunization that the egg was laid.
F = fertile; I = infertile; - - not determined
CA 02356452 2001-06-21
WO 00/37100 PCT/CA99/01225
Table 9. Binding of chicken anti-goose IPVL and chicken
anti-duck IPVL seral to chicken IPVL.
Chicken Binding to chicken IPVL
ID (as ~ of binding to homologous antigen)
1179 1.6
1012 3.5
639 7.3
690 6.3
1049 5.2
1093 3.6
650 1.0
695 3.8
1032 0.0
Chicken anti-goose IPVL and anti-duck IPVL sera were
collected 72 days post-immunization.
36