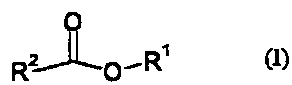Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
INDUCIBLE EXPRESSION SYSTEM FOR USE IN PLANTS
The present invention relates to an expression system for use in plants, in
particular
to an expression system which utilises an exogenous chemical agent as a
control mechanism
and to the use of certain chemicals as said control agent.
Gene expression is controlled by regions upstream (5') of the protein encoding
region,
commonly referred to as the "promoter". A promoter may be constitutive, tissue-
specific,
developmentally-programmed or inducible.
Manipulation of crop plants to improve characteristics (such as productivity
or
1 o quality) requires the expression of foreign or endogenous genes in plant
tissues. Such
genetic manipulation therefore relies on the availability of means to control
gene expression
as required; for example, on the availability and use of suitable promoters
which are effective
in plants. It is advantageous to have the choice of a variety of different
promoters so that the
most suitable promoter may be selected for a particular gene, construct, cell,
tissue, plant or
15 environment. A range of promoters are known to be operative in plants.
Particularly useful promoters in certain instances are promoters which are
inducible
by the application of an exogenous chemical inducer. This allows particular
gene expression
to be controlled at particular stages of plant growth.or development, by the
presence or
absence of a chemical which can applied to the plants or seeds, for example by
spraying or
2o using known seed coating techniques. This is sometimes known as a gene
"switch".
The gene which is under the control of the inducible promoter may be the gene
which
gives rise to the desired characteristic or phenotype itself, or the inducible
promoter may
control expression of a repressor protein which inhibits expression of a
target gene, for
example by interacting with an operator sequence upstream of the target gene
so as to
25 prevent expression of the gene (for example as known in the bacterial tet
and lac
operator/repressor systems). In a further alternative, the gene under the
control of the
inducible promoter may express a protein which interacts with another protein
to inhibit the
activity thereof, as for example in the barnase/barstar system which barnase
will inhibit or
kill cells in the absence of barstar.
3o Gene switches of this type are known in a wide variety of applications.
These
include the production of reversible male sterility, a feature which is highly
desirable
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-2-
in hybrid plant production as described for instance in WO 90/08830. Other
applications of
such promoters include in germplasm protection, where containment of
particular crop
plants, in particular transgenic plants, and the control of volunteers is
necessary and also in
the prevention of pre-harvesting sprouting as described in WO 94/03619.
Many organisms have mechanisms which allow them to metabolise chemicals such
as alcohols or ketones, for example by the production of alcohol dehydrogenase
enzymes.
The promoters of these systems may be useful in gene switches as the promoters
may be
inducible by the presence of the target alcohol or ketone.
One such example can be found in the fungal organism A nidulans which
expresses
1 o the enzyme alcohol dehydrogenase I (ADH 1 ) encoded by the gene alcA only
when it is
grown in the presence of various alcohols and ketones. The induction is
relayed through a
regulator protein encoded by the alcR gene and constitutively expressed. In
the presence of
inducer (alcohol or ketone), the regulator protein activates the expression of
the alcA gene.
The regulator protein also stimulates expression of itself in the presence of
inducer. This
15 means that high levels of the ADHl enzyme are produced under inducing
conditions (ie
when alcohol or ketone are present). Conversely, the alcA gene and its
product, ADH1, are
not expressed in the absence of inducer. Expression of alcA and production of
the enzyme is
also repressed in the presence of glucose.
Thus the alcA gene promoter is an inducible promoter, activated by the alcR
regulator
2o protein in the presence of inducer (ie by the protein/alcohol or
protein/ketone combination).
The alcR and alcA genes (including the respective promoters) have been cloned
and
sequenced (Lockington RA et al, 1985, Gene, 33:137-149; Felenbok B et al,
1988, Gene,
73:385-396; Gwynne et al, 1987, Gene, 51:205-216).
Alcohol dehydrogenase (adh) genes have been investigated in certain plant
species.
25 In maize and other cereals they are switched on by anaerobic conditions.
The promoter
region of adh genes from maize contains a 300 by regulatory element necessary
for
expression under anaerobic conditions. However, no equivalent to the alcR
regulator protein
has been found in any plant. Hence the alcR/alcA type of gene
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-3-
regulator system is not known in plants. Constitutive expression of alcR in
plant cells does
not result in the activation of endogenous adh activity.
WO 93/21334 describes the production of transgenic plants which include such
as
system as a gene switch. This document specifically describes a chemically-
inducible plant
gene expression cassette comprising a first promoter operatively linked to a
regulator
sequence which encodes a regulator protein, and an inducible promoter
operatively linked to
a target gene, the inducible promoter being activated by the regulator protein
in the presence
of an effective exogenous inducer whereby application of the inducer causes
expression of
the target gene. In particular, the alcR/alcA system is utilised in the
constructs. Exogenous
1 o chemical inducers which are applied in this case include those described
by Greaser et al., J.
Biochem. (1984) 225, 449-454) such as butan-2-one (ethyl methyl ketone),
cyclohexanone,
actone, butan-2-ol, 3-oxobutyric acid, propan-2-of and ethanol
For agricultural purposes, alcohols are generally used as the exogenous
chemical
inducer. However, such chemicals are often volatile and therefore difficult to
handle in an
15 agricultural context, as large volumes of chemical may be lost during
spraying.
The present invention provides the use of an agriculturally acceptable
hydrolysable
ester in the control of expression of a plant gene, said control being
effected by an inducible
promoter which requires for activation, the presence of an exogenous chemical
which may
comprise an alcohol, wherein hydrolysis of said agriculturally acceptable
ester results in the
2o production of said alcohol.
In particular the agriculturally acceptable ester comprises a compound of
formula (I)
O
R2~0'R~
(I)
in which R' is a lower alkyl, lower alkenyl or lower alkynyl group, and RZ is
a organic group
such that RZCOOH is an agriculturally acceptable acid. Hydrolysis of a
compound of
25 formula (I) yields an alcohol of formula (II)
HO'R~
(II).
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-4-
The term "agriculturally acceptable" as used herein means that the compounds
may
be applied to a particular soil or crop situation without causing unacceptable
levels of soil
damage or phytotoxicity in the crop.
The expression "lower alkyl" as used herein includes C,_6 alkyl groups,
preferably
from C,_4 alkyl groups which may be straight or branched chain. The expression
"lower
alkenyl" and "lower alkynyl" as used herein includes Cz_6 alkenyl and CZ_6
alkynyl groups
respectively, preferably from CZ~ alkenyl or CZ_4 alkynyl groups which may be
straight or
branched chain.
Agriculturally acceptable esters for use in the invention such as those of
formula (I)
to are suitably translocated into the target plant in which the gene control
system is in place
and/or hydrolysed either under enviromental conditions or in the presence of a
suitable
catalytic moiety such as an enzyme or catalytic antibody, at rates which are
appropriate to
provide sufficient quantities of the activating alcohol at the required time
in the necessary
parts of the plant. These may vary depending upon the nature of the plant
species being
treated, the gene being expressed and the timing of the application of the
ester.
Esters such as the compounds of formula (I) are advantageous in that they are
easier
to handle than the corresponding alcohols. It has been found that these
compounds can
produce the desired effect in terms of gene activation.
The compound should be applied at a sufficient period of time prior to the
required
gene activation to allow hydrolysis to occur and this should be reasonable
depending upon
factors such as the growth stage of the plant at which activation is required.
If the rate of
hydrolysis is relatively slow, the time of application may be earlier in order
to ensure that
sufficient hydrolysis has occurred by the time the plant is at the growth
stage at which gene
activation is required. Where this is difficult, more rapidly hydrolysing
esters may be
selected.
Alternatively, more than one ester, with differing rates of hydrolysis may be
applied
in a single treatment. By selecting combinations of esters with different
rates of hydrolysis,
an effective "slow release" of activating alcohol can be achieved so that gene
expression may
be prolonged over the desired period. This means that repeated applications of
chemical may
3o be avoided and "one-shot" treatments are possible.
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-5-
Particular examples of alcohols of formula (II) include methanol, ethanol,
propan-1-
ol, propan-2-ol, butan-2-of or but-3-en-2-ol.
Suitably, the alcohol of formula (II) is a lower alkyl alcohol wherein the
alkyl group
has from 1 to 4 carbon atoms and may be either branched, or linear. Preferred
groups for R'
include ethyl, n-propyl and n-butyl.
A particularly preferred example of a compound of formula (II) is ethanol.
The precise nature of the Rz group is immaterial provided that it gives rise
to an
agriculturally acceptable acid at an appropriate rate in the particular target
plant to which it is
applied. Rates of hydrolysis can be determined using routine methods for
example as
to described by G. Mitchell et al., Pestic. Sci (1995) 44:49-58. and
preferably by testing
against whole plant systems. What is appropriate in any particular instance
will depend upon
a variety of factors including the nature of the gene expression of which is
being controlled,
the particular plant in which the gene is expressed and other external
conditions. The rate of
hydrolysis should be sufficient to allow the desired effect, for example,
reversible male
sterility, to be seen at an appropriate period of time after application of
the chemical inducer.
Rz however may be selected such that the resultant acid of formula (III)
O
RZ~O~H
(III)
has some useful agrochemical effect. In particular, it may itself able to act
as an inducer of
the inducible promoter. For example, it has been found that a number of acids
including 3-
2o hydroxybutyric acid, 2-hydroxybutyric acid, pyruvic acid and 3-oxobutyric
acid can act as an
inducer of the alcR/alcA system (Greaser et al., supra.).
Particular examples of R' include optionally substituted alkyl, optionally
substituted
cycloalkyl, optionally substituted alkenyl, optionally substituted alkynyl,
optionally
substituted aryl or optionally substituted heterocyclyl.
As used herein the term "alkyl" includes straight or branched alkyl chains,
suitably
containing up to 10 carbon atoms, preferably from 1 to 6 carbon atoms. The
terms "alkenyl"
and "alkynyl" includes unsaturated straight or branched chains containing up
to 10 carbon
atoms, preferably from 2 to 6 carbon atoms. The term "aryl" includes phenyl
and naphthyl.
The term "heterocyclic" includes rings containing up to 10, preferably up to 7
atoms, up to
3o three of which are selected from
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-6-
oxygen, sulphur or nitrogen. These rings may be single rings or may be in the
form of fused
ring systems and these may be aromatic or non-aromatic in nature. The term
"halo" or
"halogen" includes chlorine, fluorine, bromine and iodine. The term "alkoxy"
relates to an
alkyl group as defined above, linked with an oxygen atom.
Suitably RZ is an optionally substituted C,_,o alkyl group which may be linear
or
branched. Preferred alkyl groups RZ are linear and contain 3 to 8 carbon
atoms, in particular
5 carbon atoms.
Suitable optional substitutents for alkyl, alkenyl and alkynyl groups RZ
include
one or more groups selected from halo, vitro, cyano, oxo, optionally
substituted aryl,
optionally substituted heterocyclyl, OR3, C(O)PR3, S(O)",R3, OCOR3, -
NR~C(O)PR3, =NOH,
NRSR6, C(O)NRSR6, C(O)NR3 NR'R6, -CH=NOR3, P(O)R'R8 or P(O)OR'ORB,
NR3CONRSR6, -N=CRSR6, S(O)mNR5R6 or-NR3S(O)mR4, -N=NR3
where each R3, R4, R5, R6, R' and R8 are independently selected from hydrogen,
alkyl,
alkenyl, alkynyl, aryl or heterocyclyl , any of which may be optionally
substituted by a
functional group and in the case of aryl and heterocyclic groups, may also be
substituted by
alkyl, alkenyl or alkynyl groups; or RS and R6 together with the atom to which
they are
attached, may additionally form, together with the atom to which they are
attached, a ring
which may be carbocyclic or heterocyclic; p is 1 or 2 and m is 0, 1, 2 or 3.
As used herein the term "functional group" refers to include halo, cyano,
vitro, oxo,
2o hydroxy, =NOR", C(O)pR", OR", S(O)",R", NR'ZR'3, C(O)NR'ZR'3, OC(O)NR'zR'3,
-
CH=NOR", -NR'zC(O)nR", -NR"CONR'zR'3, -N=CR'ZR'3, S(O)mNR'zR'3 or -
NR'2S(O)mR" where R" , R'Z and R'3 are independently selected from hydrogen or
optionally substituted hydrocarbyl, or R'Z and R'3 together form an optionally
substituted
ring which optionally contains further heteroatoms such as oxygen and nitrogen
or S(O)R'4,
where p is an integer of 1 or 2, m is 0 or an integer of 1-3 and R'4 is
hydrogen or alkyl.
Suitable optional substituents for hydrocarbyl groups R", R''- or R'3 include
halo,
perhaloalkyl such as trifluoromethyl, mercapto, hydroxy, alkoxy, oxo,
heteroaryloxy,
alkenyloxy, alkynyloxy, alkoxyalkoxy, aryloxy (where the aryl group may be
substituted by
halo, vitro, or hydroxy), cyano, vitro, amino, mono- or di-alkyl
3o amino, alkylamido or S(O)pR'4 where m and R'4 are as defined above.
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
7-
Examples of optional substituents on alkyl, alkenyl or alkynyl groups RZ are
one or
more groups selected from oxo; alkoxycarbonyl in particular lower
alkoxycarbonyl; cyano;
halo such as chloro, fluoro or bromo; phenyl optionally subsituted with amino
or mono-or
dialkyl amino or alkyl such as methyl; OR3 where R' is alkyl or heterocyclyl
optionally
substituted by halo or alkyl; S(O)mR" where m is 0 or 2 and R" is alkyl or
phenyl optionally
substituted by alkyl; NRSR6 or C(O)NRSR6 where RS is hydrogen, methyl or
methoxyethyl
and R6 is alkyl such as methyl, phenyl or benzyl optionally substituted with
halo such as
fluoro or chloro, alkyl such as methyl or trifluromethyl or alkoxycarbonyl
where the alkyl
moiety may carry a further alkoxycarbonyl group , or R6 is heterocyclyl such
as thiazinyl
l0 optionally substituted by alkyl and/or acetyl; -NR4C(O)PR3 where p is 2, R3
is alkyl and R4 is
alkyl optionally substituted with alkoxy carbonyl such as ethoxyl carbonyl
alkyl; -
NR3S(O)mRa where R3 is hydrogen, R4 is phenyl optionally substituted by halo
such as
chloro, and m is 2; C(O)NR3 NRSR6 where R3 and RS are hydrogen and R6 is
phenyl
optionally substituted by halo or alkoxy such as methoxy; S(O)mNR5R6 where m
is 2, RS is
15 hydrogen and R6 is alkyl optionally substituted by one or more
alkoxycarbonyl groups;
heterocylclyl such as furyl, pyridyl, pyridinyl or pyrazinyl, triazinyl, any
or which may be
optionally substituted by alkyl, halo, trihalomethyl, phenyl, halophenyl,
cyano or oxo,
Particularly suitable substituents for alkyl, alkenyl or alkynyl groups R'
include
alkoxycarbonyl in particular where the alkoxy group is a lower alkyl group;
alkoxy and in
20 particular two alkoxy groups in the form of a dialkyl acetal; cyano or
optionally substituted
heterocyclyl. Preferred substituents include, but are not limited to lower
alkoxycarbonyl
groups and dialkyl acetals. Alkoxycarbonyl groups and dialkyl acetals are of
particular
interest when the alkyl group of the substituent is the same as R' in the
compound of formula
(I) since on hydrolysis these give rise to more inducer chemical of formula
(II) .
25 A particular aryl group for Rz is phenyl.
Suitable optional substituents for cycloalkyl, aryl and heterocyclyl groups R2
and for
aryl or heterocyclyl substituents on the above-mentioned alkyl, alkenyl or
alkynyl groups R'-
include halo; haloalkyl; cyano; nitro; amino or mono- or di-alkyl amino;
hydroxy; alkoxy,
th'ioalkyl, alkyl or alkoxycarbonyl wherein the alkyl
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
_g_
moiety of any of these may be optionally substituted with for example one or
more groups
selected from halo, alkoxy, cyano, alkoxycarbonyl, amino, mono- or di-alkyl
amino, aryl or
carboxylate or salts or esters thereof; cycloalkyl; or heterocyclyl.
Particularly suitable substituents for aryl or heterocyclyl groups RZ include
alkoxy in
particular lower alkoxy such as methoxy, alkyl in particular lower alkyl,
alkoxycarbonyl in
particular lower alkoxycarbonyl and halogen.
A particular sub-group of compounds of formula (I) are compounds of formula
(lA)
O
~ i
R~o~O~R Jn
(IA)
where R' is as defined above in relation to formula (I), n is an integer of
from 2 to 4 and R'°
is an alkyl, alkenyl or alkynyl group any of which may be optionally
interposed with a
heteroatom, a cycloalkyl, heterocyclic group or aryl group, or R'° is a
cycloalkyl or aryl
group of valency n.
In particular R'° is an alkyl or aryl group of valency n.
Particularly preferred compounds of formula (I) include:-
Ethyl 2-n-pentyl-3-oxobutanoate (Compound No. 49);
Triethyl 2-carboxyheptan-1,7-dioate (Compound No. 53); and
Ethyl 2,4-dimethoxybenzoate ( Compound No. 60).
Examples of compounds of formula (I) are ethyl esters as shown in Table 1.
2o Table 1
Compd. Formula Compd Formula
No. No.
1 ° 34
i i
~NJ
°
°
J °
SUBSTITUTE SKEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-9
2 O 35 NHz O
NH ~ N
s ~~ / p'\
N
3 H 36 F
\ /N / Ow/ \~O S \
F ~
O / N
O
4 37
O N CI
0
o ~~N /
O H
O
0 38 O
H
\ N~O~ C O~
CI ~ CI
CI
6 O 39 O
HN
\ \ O
7 0 40 Br F
~O~~N O~ F O
O~O 0 F
O
8 ~ ~ ~ 41 O
O
-,
~O
O ~ O ~ F
N
F F
O 42 '
~N / I S / O~
O
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-10
1 O ~ 43 N
0
O H
~N O ~N~N /
~ O O \
0
11 NHZ 44 S
S O ~O \ ~ /N
O~
O
12 H 45
O N / \ S S
O O N ~ O \/
'O
O
13 / / NH2 46 O O
\ ~ O O S
\N N \ _
N_ 0~ ~ ~S ~O
O O
14 O 47 O
O N O // ~~
O O O O
O
15 Oi 48 CI CIO
0
\ N~O~ CI O CI
/ IOI I IO
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00144917 PCT/GB99/04348
16 N 49
II O
o
0
17 O O 50 O
C \\S~
O~ ~O
p \ N
18 0 51
~o ~ ~O
/~o
/ ~O
O O
O
19 H 52
~O ~ N~
O O
O
O O
20 O 53
~\ H / \ ~o °
O N
v v
O~
°
°
O
21 54 0 0
Nw O
° /'°
-o
0
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-12-
22 N 55
O -/ \-.
N / O O
o CI
23 ~o \ 56
N' \% ~ O
i H O
O ~
00~0~
24 / ' 57 _ o
O
oJ\ /~o ~ °\
o \o \
25 58 O
o ~ I / \
/-o -' \
0
26 0 59 _o
° O ~ // ~ ~ ~o
s / o0
t ~ -~ °
27 I ~ ~ 60 o
O
N~N O ~o i
0
O
28 ~ 0 61 o
O S /~ O / \ O
o N-y 1 0
H N \
O O
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-13
29 0 62
~S /
l'~ O O
~O ~ \ ,O /
O/~
O
30 S 63
~o \ N ~ \ ~ o
p E CI II
O O O
form
31 O 64 CI
N I / ~- O N I \
~O~
~N /
O H
CI
32 F F 65 O /N
F / ~N
N ~ ~ / ~/O
~N O~
~O
O
33 66 O
O 'o ~ N \ CI
O /
\ NCH ~ O~/ N I /
/ I
O
Compounds of formula (I) are either known compounds of they can be prepared
from
known compounds using conventional methods.
Compounds of formula (I) may be hydrolysed in the target plant either
chemically, or
enzymatically by a naturally occurring enzyme in the target plant or by an
enzyme
introduced by genetic engineering into the plant and expressed within the
plant, or by an
appropriate catalytic antibody, or catalytically active portion of a
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
- 14
catalytic antibody introduced by genetic engineering into the plant and
expressed within the
plant.
Suitable enzymes include, but are not limited to, esterases and lipases.
Suitable catalytic antibodies may be generated by standard techniques from
analogues
of a tetrahedral ester hydrolysis transition state, e.g. as for the hydrolysis
of the pro-drug
ester of chloramphenicol, when appropriate phosphonates were used, Ole K et
al., 1998, J.
Mol. Biol., 281:501-51 l, and for the detoxification of cocaine by methyl
ester hydrolysis,
Mets B et al., 1998, Proc. Nat. Acad. Sci. USA, 95:10176-10181.
Metabolism of the compound of invention has been investigated further and the
1 o results of these investigations are reported in the Examples hereinafte.
Without being limited
to mechanistic considerations, it is believed for example that a
representative compound of
the invention, Compound 53, is metabolised in accordance with the following
scheme:
0 off_ ~ Jo~
O O~~OH O~~OH
00~~~0-~ O ~ O~. ~ OH- _ O~
-. -~ O~'~OH
OH O OH O O OH
Od\~O-~
O ~ O OH
15 Triester Mono Acids Di Acids Tri acid
A product of this metabolism is ethanol which can act as a chemical inducer as
described above.
In a further aspect, the invention provides a method for controlling
expression of a
2o target gene in a plant, wherein said plant is transformed with a chemically-
inducible plant
gene expression cassette comprising a first promoter operatively linked to a
regulator
sequence which encodes a regulator protein, and an inducible promoter
operatively linked to
a target gene, the inducible promoter being activated by the regulator protein
in the presence
of an alcohol such as a compound of formula (II) as defined above, said method
comprising
25 applying to said plant an ester which hydrolyses to form said alcohol such
as a compound of
formula (I) as defined above, so as to cause expression of the target gene.
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-15-
Suitably the regulator sequence encodes the alcR protein as described above
and the
inducible promoter is the alcA promoter sequence.
Where necessary or desired, the plant may also be transformed so that it
expresses or
overexpresses an enzyme or catalytic antibody or catalytically active fragment
thereof,
which hydrolyses the compound of formula (I) to form a compound of formula
(II).
Enzymes which are inactive in the absence of enzymes or other moieties which
must
be engineered into the plant may be preferable in some circumstances since
they will be
effective only in the target transformed seed.
The nucleic acid sequences which encode the hydrolytic enzyme, antibody or
1 o antibody fragment may be included in the construct containing the
regulator protein and/or
the target gene operatively linked to the inducible promoter or they may be
present on a
separate construct which is used to co-transform the plant. Such systems
however are novel.
Thus in a further aspect there is provided a plant gene expression system
comprising
(i) a first promoter operatively linked to a regulator sequence which encodes
a
15 regulator protein,
(ii) an inducible promoter operatively linked to a target gene, the inducible
promoter
being activated by the regulator protein in the presence of an effective
exogenous inducer of
formula (I) as defined above, whereby application of the inducer causes
expression of the
target gene; and
20 (iii) a sequence which encodes a protein which effects hydrolysis of an
ester such as a
compound of formula (I) to the corresponding alcohol under the control of a
further promoter
which allows its expression in plant tissue.
The target gene may comprise any gene which is required to be introduced into
a
plant in order to modify the characteristics thereof as outlined above. The
target gene may
25 be an endogenous plant gene or a foreign gene, and may be a single gene or
a series of genes.
The target gene sequence encodes at least part of a functional protein or an
antisense
sequence.
Any transformation method suitable for the target plant or plant cells may be
employed, including infection by A~robacterium tumefaciens containing
recombinant
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-16-
Ti plasmids, electroporation, microinjection of cells and protoplasts,
microprojectile
transformation and pollen tube transformation. The transformed cells may then
in suitable
cases be regenerated into whole plants in which the new nuclear material is
stably
incorporated into the genome. Both transformed monocot and dicot plants may be
obtained
in this way.
Examples of genetically modified plants which may be produced include field
crops,
cereals, fruit and vegetables such as: canola, sunflower, tobacco, sugarbeet,
cotton, soya,
maize, wheat, barley, rice, sorghum, tomatoes, mangoes, peaches, apples,
pears, strawberries,
bananas, melons, potatoes, carrot, lettuce, cabbage, onion.
1 o The invention further provides a plant cell containing a gene expression
system
according to the invention. The gene expression system may be stably
incorporated in the
plant's genome by transformation. The invention also provides a plant tissue
or a plant
comprising such cells, and plants or seeds derived therefrom.
Preferred examples of compounds of formula (I) used in this method are those
15 described above.
Agriculturally acceptable esters of the invention such as compounds of formula
(I)
are suitably applied in the form of an agriculturally acceptable composition,
in combination
with a diluent or carrier, Such compositions form a further aspect of the
invention.
The concentration of the agriculturally acceptable ester in the formulation is
2o preferably at a concentration of about 5% wt/wt or less. It is preferably
at a concentration
between about 2% and 5% wt/wt.
Suitable carriers or diluents will be apparent to the skilled person and will
vary
depending upon the particular nature of the compounds of formula (I) employed.
For
instance, where the compound of formula (I) is an oil, it may require the
presence of an
25 emulsifier in order to allow it to be sprayed in aqueous solution.
Emulsifiers are well known
in the art, and a particular example is partially hydrolysed polyvinyl acetate
(PVA) or
TweenTM
Organic solvents or diluents such as acetone may also be present.
Thus a preferred composition comprises an agriculturally acceptable ester such
as a
3o compound of formula (I), an emulsifier such as PVA and a diluent such as
water. The
relative amounts of the components will be determined to a large extent by
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-17-
the mutual miscibility of the various components. Suitably however, the
emulsifier will be
present in the composition in amounts of from 1-5%w/w, preferably at about
2.5%w/w.
Thus examples of formulations of the invention include the following:
Formulation 1
1.5% Compound of the invention (e.g. Compound 53)
2.5% PVA
Balance water
Formulation 2
l0 1.5% Compound of the invention (e.g. Compound 53)
5% acetone
0.05% Tween-20TM
Balance water
Formulation 3
3.0% Compound of the invention (e.g. Compound 53)
2.5% PVA
Balance water
Formulation 4
3.0% Compound of the invention (e.g. Compound 53)
5% acetone/H20
0.05% Tween-20TM
Balance water
Alternative compositions which may be employed are similar to those described
in
our copending British Patent application No. 9902236Ø In particular, the
compositions will
comprise the components:
(a) an agriculturally acceptable ester such as a compound of formula (I);
(b) a polyethoxylated C,°-Cz° alcohol or a trisiloxane
polyethoxylate and
(c) a diluent.
The diluent (c) may be, for example, water.
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-18
Component (b) of the formulation described above is preferably, a
polyethoxylated
oleyl, lauryl, stearyl or cetyl alcohol. It is more preferably a
polyoxyethylene-oleyl alcohol
having a mean molar ethylene oxide content in the range of 0 to 3 ~ and more
preferably in
the range of 2 to 20. It is most preferably a polyoxyethylene-(2)-oleyl
alcohol, a
polyoxyethylene-( 10)-oleyl alcohol or a polyoxyethylene-(20)-oleyl alcohol.
Component (b)
is, however, preferably a polyoxyethylene-(20)-oleyl alcohol (the number in
brackets
indicates the mean ethylene oxide content per molecule). Such products are
commercially
available as BRIJ 92T"", BRIJ 97T"" and BRIJ 98T"".
Preferably, component (b) of the formulation is at a concentration of about
0.5%
to wt/wt or less. It is preferably at a concentration between about 0.2% wv'wt
and 0.5% wt/wt.
In an alternative embodiment, the formulation includes as component (b), a
hydrogen
or methyl end-capped trisiloxane polyethoxylate In particular, component (b)
is a methyl
end-capped trisiloxane polyethoxylate. The methyl end-capped trisiloxane
polyethoxylate
preferably has a mean molar ethylene oxide content of between 4 and 12 per
molecule and is
15 most preferably 8 per molecule. Such products are commercially available as
SILWET 77T"'
(SILWET is a trademark of Witco).
Preferably, the methyl end-capped trisiloxane polyethoxylate is at a
concentration of
about 0.5% wt/wt or less. It is preferably at a concentration between about
0.2% and 0.5%
wt/wt.
2o Component (c) of the formulation is preferably at a concentration between
about 90%
and 98% wtlwt.
Other additives which may be included in the formulations include dispersants,
antibacterial compounds, wetter compounds and anti-evaporants may also be
added.
The invention will now be particularly described by way of example.
25 Example 1
Testing chemicals on soil grown plants
Following a preliminary screen on hydroponiv solution, the compounds were used
in a test
carried out on 4 week old alccat tobacco homozygous line 30 plants in 1'/2"
pots in the
glasshouse. Leaf samples were removed from the plants as an "uninduced"
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
- 19
control. The leaf pieces were ground in 200u1 of 250mM Tris pH8.0, centrifuged
and the
supernatant recovered and stored at 4°C overnight. The compounds were
dissolved in 50%
acetone 50% dH20 with 0.05% tween-20 as a 200mg/ml solution. This was then
diluted 1/10
for a 2% solution. unless otherwise stated, Smls of the solution was applied
to the soil of
each of 2 plants to be replica treatments as a root drench. After 22 and 44
hours leaf
samples were removed, extracted as described above and the supernatants stored
at 4°C. The
samples were analysed for cat protein quantification by using a Boehringer
Mannheim CAT
ELISA kit and the total protein level determined by a Bradford determination.
The results
are shown in Table 2 where a CAT value of 1 equates to the detection of 0-
SOOOng/, 2
1o equates to the detection of 5001-10,000 ng/g, 3 equates to 10,001-
15,OOOng/g .
Table 2
Compound No CAT score
O hours 22 hours 44 hours
1 0 1 0
(2.Smls)2 0 1 0
3 0 1 2
(2.Smls)4 0 0 1
5 0 0 3
8 1 1 1
9 1 0 2
2 0 1
12 1 0 2
13 0 0 0
14 0 1 0
0 1 1
16 1 1 6
17 1 2 1
18 0 1 1
19 0 1 1
0 0 1
21 0 0 1
22 0 0 1
23 0 0 1
24 1 0 1
0 0 1
(2.Smls)26 0 0 0
28 0 0 1
0 2 2
32 0 0 2
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-20
33 1 1 1
(2.Smls)35 0 0 1
36 0 0 1
38 0 0 3
39 0 0 1
40 0 0 0
(2.Smls)41 1 0 1
42 2 1 1
43 0 0 2
44 1 0 0
45 1 0 2
46 2 0 1
47 3 0 2
Smls formulation0 0 0
2.Smls formulation0 0 0
S% ethanol 1 8 10
5% butan-1-of 1 1 3
dH20 1 0 0
Example 2
Further treatment of soil gown t~lants
In this experiment, compounds were dissolved in 50% acetone 50% dH20 with
0.05% tween-
20 as a 200mg/ml solution. These were diluted to a 1.5% and 0.1% solution.
Leaf samples
were removed from the seedlings as an "uninduced" control. The leaf pieces
were ground in
200u1 of 250mM Tris pH8.0, centrifuged and the supernatant recovered and
stored at 4°C
overnight. Smls of the solution was applied to the soil of each of 2 plants as
replica
to treatments as a root drench. After 22 and 65 hours leaf samples were
removed from each of
two plants treated, extracted as described in Example l and the supernatants
stored at 4°C.
The samples were analysed for cat protein quantification by using a Boehringer
Mannheim
CAT ELISA kit and the total protein level determined by a Bradford
determination. The
compounds tested and results are shown in Table 3, where the CAT scores
represent the
I5 values set out in respect of Table 2.
Table 3
Compound No. CAT protein
Score
1.5% root 0 hrs 22 hrs 65 hrs
drench
17 0 3 1
50 0 2 1
51 0 1 3
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-21
51 0 1 3
52 0 2 1
53 0 3 g
54 0 1 2
55 0 1 1
56 0 2 1
57 0 2 5
58 0 1 0
59 0 1 1
61 0 1 1
62 0 1 2
63 0 1 1
64 0 1 1
65 0 0 1
ethanol 0 3 6
butan-1-of 0 3 5
formulation 0 0 0
dH20 0 0 0
Example 3
Determination of the time course of expression of the switch after induction
In order to determine the time course of expression of the alc switch after
induction from the
esters several compounds were applied to the soil or leaves of 5 week old
alccat homozygous
line 30 plants in 1'/z" pots in the glasshouse. The compounds were dissolved
in 50% acetone
50% dH20 with 0.05% tween-20 as either a 150mg/ml or 50mg/ml solution. They
were then
diluted 1/10 for a 1.5% or 0.5% solution. Leaf samples were removed from the
seedlings as
an "uninduced" control. The leaf pieces were frozen in dry ice/ethanol and
stored at -70°C.
5mls of the solution was applied as either a root drench or a leaf spray to up
to 8 plants.
Leaf samples were removed at various time points and frozen in dry ice/ethanol
and stored
at -70°C. When all the samples were harvested and stored they were
extracted in 200u1 of
250mM Tris pH8.0, centrifuged and the supernatant recovered and stored at
4°C overnight.
The samples were analysed for cat protein quantification by using a Boehringer
Mannheim
CAT Elisa kit and the total protein level determined by a Bradford
determination. The
compounds tested and results are shown on Tables 4, 5, 6, 7 8, 9 and 10. The
CAT units are
as set out above in Example 1 with each successive number representing an
incremental
S,OOOng/g range. Results shown in shaded form were obtained in separate but
similar trials.
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-22
Table 4
Hours Compound 51 CAT score
0 0.5% 49 Spray 0
0.5% 49 S p
0.5%49S 0
26 0.5% 49 S 0
48 0.5% 49 S 0
98 0.5% 49 S 0
144 0.5% 49 S 0
193 0.5% 49 S 0
0 1.5% 49 S 0
5 1.5% 49 S 0
10 1.5% 49 S 0
26 1.5% 49 S 0
48 1.5% 49 S 1
98 1.5% 49 S 0
144 1.5% 49 S 0
193 1.5% 49 S 0
0 0.5% 49 Root drench 0
5 0.5%49R 0
10 0.5%49R 1
26 0.5% 49 R 2
48 0.5% 49 R 1
98 0.5% 49 R 4
144 0.5%49R 4
193 0.5% 49 R 0
0 1.5% 49 R 0
10 1.5% 49 R 1
22 hours 1.5% root 1
26 1.5% 49 R 1
48 1.5% 49 R 3
65 hours 1.5% root 3
98 1.5% 49 R 4
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
- 23
Table 5
Hours Compound No 57 CAT score
t=0 .5%30 Spray 0
t=5 .5%30 S 0
t=10 .5%30 S 0
t=26 .5%30 S 1
t=48 .5%30 S 1
t=0 1.5% 30S 0
t=5 1.5% 30S 0
t=10 1.5% 30S 0
t=26 1.5% 30S 0
t=48 1.5% 30S 0
t=0 .5%30 Root drench 0
t=5 0.5%30 R 0
t=10 .5%30 R 1
t=26 .5%30 R 2
t=48 .5%30 R 2
t=98 .5%30 R 4
t=0 1.5% 30R 0
t=10 1.5% 30R 1
22 hours 1.5% root 2
t=26 1.5% 30R 1
t=48 1.5% 30R 2
65 hours 1.5% root 5
t=98 1.5% 30R 3
SUBSTITUTE SHEET (RULE Z6)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-24-
Table 6
Hours Compound No 53 CAT Score
0 0.5%51 Spray 0
0.5%51 S 0
0.5%51 S 1
26 0.5%51 S 1
48 0.5%51 S 1
98 0.5%51 S 1
144 0.5%51 S 1
193 0.5%51 S 0
0 1.5% 51 S 0
5 1.5% 51 S 1
10 1.5% 51 S 2
26 1.5% 51 S 1
48 1.5% 51 S 1
98 1.5% 51 S 1
144 1.5% 51 S 0
193 1.5% 51 S 1
0 0.5%51 Root drench 0
5 0.5%51 R 1
10 0.5%51 R 1
26 0.5%51 R 1
48 0.5%51 R 1
98 0.5%51 R 3
144 0.5%51 R 2
193 0.5%51 R 0
0 1.5% 51 R 0
10 1.5% 51 R 1
22 hours 1.5% root 4
26 1.5% 51 R 4
48 1.5% 51 R 8
65 hours 1.5% root 8
98 1.5% 51 R 5
SUBSTITUTE SI~iEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
- 25 -
Table 7
Hours Compound No CAT Score
60
t=0 _ 0
0.5% 57 Root drench
t=10 0.5% 57 R 1
t=26 0.5% 57 R 1
t=48 0.5% 57 R 2
t=98 0.5% 57 R 5
t=144 0.5% 57 R g
t=0 1.5%57 R 0
t=10 1.5%57 R 1
22 hours 1.5% root 1
t=26 1.5%57 R 1
t=48 1.5%57 R 1
65 hours 1.5% root 1
t=98 1.5%57 R 4
Table 8
Hours Compo 5 CAT score
_
t=0 0.5%50Spray _
0
t=10 0.5%SOS 0
t=26 0.5%50S 0
t=48 0.5%SOS 1
t=98 0.5%50S 0
t=144 0.5%50S 0
t=193 0.5%50S 0
t=0 1.5% 50S 0
t=10 1.5% 50S 0
t=26 1.5% 50S 0
t=48 1.5% 50S 0
t=98 1.5% 50S 0
t=144 1.5% 50S 0
t=193 1.5% 50S 0
t=0 0.5%50Root drench 0
t=10 0.5%50R 1
t=26 0.5%50R 1
t=48 0.5%50R 2
t=98 0.5%50R 1
t=144 0.5%50R 1
t=193 0.5%50R 1
t=0 1.5% 50R 0
t=10 1.5% SOR 0
22 hours 1.5% root 2
t=26 1.5% 50R 1
t=48 1.5% 50R 2
65 hours 1.5% root 1
t=98 1.5% 50R 1
SUBSTITUTE SKEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-26-
Table 9
Hours Ethanol CAT score
0 Etoh 0.5% Spray 0
S Etoh 0.5% S 2
Etoh 0.5% S 2
26 Etoh 0.5% S 2
48 Etoh 0.5% S 1
98 Etoh 0.5% S 1
144 Etoh 0.5% S 1
193 Etoh 0.5% S 1
0 Etoh 1.5% S 0
5 Etoh 1.5% S 2
10 Etoh 1.5% S 4
26 Etoh 1.5% S 5
48 Etoh 1.5% S 4
98 Etoh 1.5% S 2
144 Etoh 1.5% S 2
193 Etoh 1.5% S 1
0 Etoh 0.5% Root drench 0
5 Etoh 0.5% R 2
10 Etoh 0.5% R 4
26 Etoh 0.5% R 6
48 Etoh 0.5% R 4
98 Etoh 0.5% R 4
144 Etoh 0.5% R 4
193 Etoh 0.5% R 3
0 Etoh 1.5% R 0
10 Etoh 1.5% R 4
26 Etoh 1.5% R 9
48 Etoh 1.5% R 7
98 Etoh 1.5% R 6
SUBSTITUTE SHEET (RULE 26)
CA 02362551 2001-07-12
WO 00/44917 PCT/GB99/04348
-27-
Table 10
Hours dH20 CAT Score
0 dH20 Root drench 0
dH20 R p
dH20 R p
26 dH20 R p
48 dH20 R 0
98 dH20 R 0
144 dH20 R p
193 dH20 R 0
Formulation
hours
0 Spray 0
5 S 0
10 S 0
26 S 0
48 S ~ 0
98 S 0
144 S 0
193 S 0
0 Root drench 0
5 R 0
10 R 0
26 R 0
48 R 1
98 R 1
144 R 1
193 R 0
These results show that the time course of the induction can be altered by
using esters in
accordance with the invention, as compared to alcohols. Thus the appropriate
selection of
ester would lead to a favoured induction profile.
SUBSTITUTE SHEET (RULE 26)