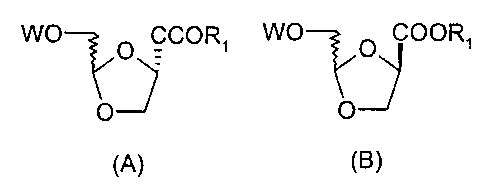Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
STEREOSELECTIVE SYNTHESIS OF NUCLEOSIDE ANALOGUES
FIELD OF THE INVENTION
The present invention relates generally to a novel method
for the preparation of nucleoside analogues and their
precursors and more particularly to a method of preparing
a nucleoside analogue by the use of specific enzymes to
stereoselectively produce dioxolane nucleoside analogues
or their precursors.
BACKGROUND OF THE INVENTION
The pharmacological activity of pharmaceutical compounds
(drugs) depend mainly on their interaction with
biological matrices (drug targets), such as proteins
(receptors and enzymes), nucleic acids (DNA and RNA) and
biomembranes (phospholipids and glycolipids). All these
drug targets have complex three-dimensional structures
which are capable of binding specifically to the drug in
only one of the many possible arrangements in the three-
dimensional space. It is the three-dimensional structure
of the drug target that in part, determines which of the
potential drug is bound within its cavity and with what
affinity.
The spatial arrangement of atoms in an asymetric molecule
is termed chirality. Chirality results in the creation
of stereoisomers. Stereoisomers are compounds with
identical chemical composition and atom connectivity
(i.e. same constitution), but different arrangements of
the atoms in space (i.e. different configurations).
Stereoisomers are classified according to the number of
-1-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
chiral centers in each molecule and the spatial
arrangement of the chiral center.
Chiral centers of organic molecules include chiral carbon
atoms which have four different substituents connected
thereto and arranged in a generally tetrahedral
configuration. Another type of chiral center is a chiral
plane oriented along a rigid C=C bond that has at least
two different substituents connected to the remaining
four bond positions in that arrangement.
The chirality of molecules that are the subject of the
present application refer to chirality created by chiral
atoms and. not chiral bonds. The following discussions
will be limited to chirality created at one or more
chiral carbon atoms which have four different
substituents bound to each of the four different binding
sites of the carbon.
When a molecule has a single chiral carbon, there are two
stereoisomers that are mirror images of each other. This
pair of isomers is termed enantiomers or an enantiomeric
pair. When there are two chiral carbon atoms, there are
four stereoisomers and two pairs of mirror images or
enantiomers. A stereoisomer which is not a mirror image
of another stereoisomer is a diastereoisomer.
One type of stereochemical distinction relates to cyclic
sugars or analogues of cyclic sugars. Cyclic sugars can
be designated as a particular anomer depending upon the
stereochemical configuration.
The term "anomer" designates the spatial arrangement of
cyclic sugars or analogues or derivatives thereof that
_2_
SUBSTITUTE SKEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
have two chiral centers in a five or six member ring.
The anomeric designation defines the relative
configuration of the two chiral centers relative to a
hypothetical plane defined by the ring. The chiral
centers typically have two substituents outside the ring.
One substituent is a H. The other substituent is a
larger moiety such as a hydroxyl, methoxyl, purine or
pyrimidine base, carboxyl, etc.
When the two larger constituents on each chiral center
are on the same side of the plane in the ring, they are
defined as a (3-anomer (cis-isomer). When two larger
moieties are on opposite sides of the plane in the ring,
they are defined as the a-anomer (trans-isomer). An
anomer is a type of diastereoisomer.
Because chirality may affect biological activity or
toxicity, it is important from the point of view of drug
development to evaluate the physiological effect of each
isomer. Frequently, one stereoisomer is considerably
more active than the other. In other situations, the
non-active isomer may inhibit the activity of the more
active form. In some instances, the less preferred
stereoisomer may be equally potent but have greater
toxicity than the preferred stereoisomer. In each of
these instances, the therapeutic effect of a drug can be
increased if the single most preferred stereoisomer is
administered in higher purity.
The current trend in the drug markets reflects a greater
use of single stereoisomer drugs. The sales of single
stereoisomer drugs have increased considerably. In 1995,
sales of single stereoisomer drugs reached $61 billion
-3-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
worldwide. In the year 2000, the annual worldwide sales
are expected to reach $90 billion.
An important class of pharmacological agents relate to
3'-oxa-substituted 2',3'-dideoxynucleoside analogues
("dioxolane nucleoside analogues"). These compounds have
two chiral centers corresponding to the substituted
carbons 2 and 4 of the dioxolane ring (C2 and C4
respectively). Thus each compound can exist as four
different stereoisomers depending on the position of both
substituents with respect to the dioxolane ring.
The stereoisomers of a dioxolane nucleoside analogue are
represented by the following diagrams where the letter B
represents a purine or pyrimidine base or an analogue or
derivative of a purine or pyrimidine base as defined
herewith.
HO O B HO- O B HO O B HO- O B
(2R) (4R) (ZS)~ (4S) (2R)~ (4S) (2S) ~ (4R)
O O O O
~_D ~_L a_D a_L
For the purpose of consistency, the same stereochemical
designation is used when the methyloxyl moiety or the
base moiety is replaced with another substituent group.
The C2 carbon in each of the above formula is the carbon
atom in the ring that connects the methyloxy group to the
dioxolane ring. The C4 carbon is the carbon atom in each
of the above formula that connects the base (B)
substituent to the dioxolane ring.
-4-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
The four stereoisomers represented above correspond to
two pairs of enantiomers. The (3-anomers represent one
set of enantiomers, the ~3-L enantiomer and the ~i-D
enantiomer. The a-anomers represent the other set of
enantiomers, the a-L enantiomer and the a-D enantiomer.
Compounds with D-configuration have an outward directed
methyloxy group when the ring is oriented in the plane of
the page with the oxygen in the first position (03) at
the top of~ the page with the carbon in the two position
(C2) on the left side as illustrated above. This is also
represented by the designation (2R). Compounds having an
L-configuration has inward directed methyloxy group when
the ring is oriented in the plane of the page with the 03
oxygen at the top of the page with the C2 carbon on the
left side as illustrated above. This is also represented
by the designation (2S).
A variety of dioxolane nucleoside analogues have been
identified to have antiviral and anticancer activity.
For example, 9-(~3-D-2-hydroxymethyl-1,3-dioxolan-4-yl)-2-
aminopurine (~3-D-DAPD) and its metabolite 9- ((3-D-2-
hydroxymethyl-1,3-dioxolan-4-yl)-guanine (~i-D-DXG) have
been reported to have potent and selective activity
against human immunodeficiency virus (HIV) and hepatitis
B virus (HBV) (Rajagopalan et al., Antiviral Chem.
Chemother. , 1996, 7 (2) , 65-70) Similarly, 1- ((3-L-2-
hydroxymethyl-1,3-dioxolan-4-yl)-thymine (Dioxolane-
T)(Norbeck et al., Tetrahedron Lett., 1989,30, 6263-66)
possess anti-HIV and anti-HBV activity. 1-((3-L-2-
hydroxymethyl-1,3-dioxolan-4-yl)-cytidine ((3-L-OddC)
(Bednarski et al., Bioorg. Med. Chem. Lett., 1994, 4,
-5-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
2667-72) was discovered to have potent anti-tumor
activity towards human prostate as well as renal
carcinoma (Kadhim et al., Can. Cancer Res., 57(21),4803-
10, 1997). ~i-L-OddC is the first nucleoside analogue
with an L-configuration shown to have anticancer
activity. Since stereoisomers of dioxolane nucleosides
usually have different biological activities and
toxicity, obtaining the pure therapeutically active
isomer becomes crucial.
Chiral synthetic methods have improved over the past
several years with respect to synthetic techniques that
result in single stereoisomer compounds. However, there
is a present need to find novel synthetic methods which
can be widely used to form a particular stereoisomer with
greater efficiency and purity.
For example, for many years a person of ordinary skill in
the art could use enzymes to separate enantiomers of
dioxolane compounds. However, it is not known in the art
how to produce a dioxolane nucleoside analogue using a
step of separating an anomeric mixture of certain
dioxolane precursors using enzymes to produce a
stereochemically pure end product with greater efficiency
and purity.
Because stereochemically pure dioxolane nucleosides are
an important class of compounds due to their known
antiviral and anticancer activity, there is a need for
other inexpensive and efficient stereoselective methods
for their preparation. The present invention satisfies
this and other needs.
-6-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
SUMMARY OF THE INVENTION
The present invention provides a novel process for making
dioxolane nucleoside analogues with a high degree of
steric purity, greater efficiency and higher yields.
Specifically, the present invention provides a process
for making dioxolane nucleoside analogues with a high
degree of steric purity which includes the use of certain
hydrolytic enzymes for separating ~i and a anomers from an
anomeric mixture represented by the following formula A
or formula B:
WO WO
O COORS O COORS
O O
(A) (B>
In the above formula, W is benzyl or benzoyl; R1 is
selected from the group consisting of Cl_6 alkyl and C6_ls
aryl.
The process involves the step of hydrolyzing the mixture
of compounds represented by fc-mula A and formula B with
an enzyme selected from the group consisting of
cholesterol esterase, ESL-001-02, horse liver esterase,
bovine pancreatic protease, a-chymotrypsin, protease from
Streptomyces caespitosis, substilisin from Bacillus
licheniformis, protease from Aspergillus oryzae,
proteinase from Bacilius licheniformis, protease from
Streptomyces griseus, acylase from Aspergillus melleus,
proteinase from Bacillus subtilis, ESL-001-05, pronase
protease from Streptomyces griseus, Lipase from Rhizopus
arrhizus, lipoprotein lipase from Pseudomonas species
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
type B, lipase from Pseudomonas cepacia and bacterial
proteinase. The process stereoselectively hydrolyses
predominantly one anomer to form a product where R1 of
formula A and formula B is replaced with H. The other
anomer remains substantially unhydrolysed. The process
also comprises separating the hydrolyzed product from
unhydrolysed starting material.
The process of one embodiment further includes the step
of stereoselectively replacing the functional group at
the C4 position of the dioxolane (e.g. COOR1) with a
purinyl, pyrimidinyl or analogue or derivative thereof to
produce a dioxolane nucleoside analogue that has a high
degree of steric purity.
According to one embodiment of the invention, the step of
hydrolyzing results in a starting material having an
anomeric purity of at least 80%. According to another
embodiment, the step of hydrolyzing results in a starting
material having an anomeric purity of at least 90%. In
yet another embodiment, the step of hydrolyzing results
in a starting material having an anomeric purity of at
least 95%. In an additional embodiment, the step of
hydrolyzing results in a starting material having an
anomeric purity of at least 98%.
According to one embodiment of the invention, the step of
hydrolyzing results in a product having an anomeric
purity of at least 80%. According to another embodiment,
the step of hydrolyzing results in a product having an
anomeric purity of at least 90%. In yet another
embodiment, the step of hydrolyzing results in a product
having an anomeric purity of at least 95%. In an
_g_
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
additional embodiment, the step of hydrolyzing results in
a product having an anomeric purity of at least 98%.
In one embodiment of the present invention, W of formula
A or formula B is benzyl and the enzyme is selected from
the group consisting of cholesterol esterase, ESL-001-02,
horse liver esterase, bovine pancreatic protease, a-
chymotrypsin, protease from Streptomyces caespitosis,
substilisin from Bacillus Iicheniformis. In another
embodiment, the enzyme is a-chymotrypsin. In yet another
embodiment, the enzyme is bovine pancreatic protease.
In one embodiment of the present invention, W of formula
A and formula B is benzoyl and the enzyme is selected
from the group consisting of protease from Aspergillus
oryzae, proteinase from Bacillus licheniformis,
subtilisin from Bacillus licheniformis, protease from
Streptomyces griseus, acylase from Aspergillus melleus,
proteinase from Bacillus subtilis, ESL-001-05, pronase
.protease from Streptomyces griseus, lipase from Rhizopus
arrhizus, lipoprotein lipase from Pseudomonas species
type B, bacterial proteinase, lipase from Pseudomonas
cepacia. In another embodiment, the enzyme is selected
from the group consisting of Aspergillus oryzae protease,
proteinase from Bacillus licheniformis, subtilisin from
Bacillus licheniformis, protease from Streptomyces
griseus, pronase protease from Streptomyces griseus, and
lipase from Rhizopus arrhizus. In yet another embodiment,
the enzyme is selected from the group comprising
Aspergillus oryzae and proteinase from Bacillus
licheniformis.
_9_
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
In one embodiment, the ~i-anomer is the predominant
product. In another embodiment, the a-anomer is the
predominant product. In yet another embodiment, the (3-L-
enantiomer is the predominant product. In an additional
embodiment, the ~3-D-enantiomer is the predominant
product. In yet another embodiment, the a-L-enantiomer
is the predominant product. In an additional embodiment,
the a-D-enantiomer is the predominant product.
In one embodiment, the invention is a process for
stereoselectively preparing a dioxolane nucleoside
analogue by separating (3 and a-anomers from an anomeric
mixture represented by formula A or formula B according
to one of the above embodiments. The process further
includes the step of stereoselectively replacing the
functional group at the C4 position (COOR1) with a purinyl
or pyrimidinyl or analogue or derivative selected from
the group consisting of:
NHR2 O R5 O
N ~ R3 HN R4 N i N HN
t ~ ~ t
O H O H R6 N H R7 N
NHR9 NHR»
Ni _N Ni R10
J
O N O N
H H
In this embodiment, R2, R9 and Rll are independently
selected from the group consisting of hydrogen, C1_s
alkyl, Cl_6 acyl and R8C (O) wherein Ra is hydrogen or C1_s
- 10-
SUBSTITUTE SI3EET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
alkyl. Additionally, R3, R4 and Rlo are each independently
selected from the group consisting of hydrogen, C1_s
alkyl, bromine, chlorine,~fluorine, iodine and CF3; and
RS, R6 and R~ are each independently selected from the
group consisting of hydrogen, bromine, chlorine,
fluorine, iodine, amino, hydroxyl and C3_s
cycloalkylamino. The process results in the production
of a stereochemical isomer of the dioxolane nucleoside
analogue.
According~to one embodiment, the process further includes
the step of stereoselectively replacing the functional
group at the C4 position (COOR1) with a purinyl or
pyrimidinyl or derivative selected from the group
consisting of
NHR2 O R5 O
N~ R3 HN R4 Ni N HN
~ I ~ I '~
O H O H R6 N H R7 N
In this embodiment, R2 is selected from the group
consisting of hydrogen, Cl_6 alkyl, C1_6 acyl and R8C (O)
wherein Re is hydrogen or C1_6 alkyl. Additionally, R3 and
RQ are each independently selected from the group
consisting of hydrogen, C1_6 alkyl, bromine, chlorine,
fluorine , iodine and CF3 ; and RS , R6 and R, are each
independently selected from the group consisting of
hydrogen, bromine, chlorine, fluorine, iodine; amino,
hydroxyl and C3_6 cycloalkylamino. The process results in
the production of a stereochemical isomer of a dioxolane
nucleoside analogue.
-li-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
In another embodiment, the process further includes the
step of stereoselectively replacing the functional group
at the C4 position (COOR1) with a pyrimidinyl or analogue
or derivative selected from the group consisting of:
NHR9 NHR»
N-/ _N ~ R10
N'
O N O~N~N
H H
In this embodiment, R9 and Rll are independently selected
from the group consisting of hydrogen, C1_6 alkyl, C1_s
acyl and R8C (O) . Additionally, Rlo is selected from the
group consisting of hydrogen, C1_6 alkyl, bromine,
chlorine, fluorine, iodine and CF3. The process results in
the production of a stereochemical isomer of a dioxolane
nucleoside analogue.
In another embodiment, the process comprises
stereoselectively preparing a dioxolane nucleoside
analogue by separating (3 and a, anomers from an anomeric
mixture represented by formula A or formula B according
to one of the above embodiments and further comprises
stereoselectively replacing the functional group at the
-12-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
C4 position (COORl) with a moiety selected from the group
consisting of:
NH2 NHZ G
Ni N Ni N Ni N i N
\ I \ I \~ ~N
H2N N N N N H N N N H N. 'N N
H H 2 H 2 H
HOOC NHZ N~ NH2
NH NH N i CH3 N ~ N ~ F
N~ N i N ~ I I I
\ N \ O N O N O~N
I ~ ~ I ~ H H H
HZN N N H N"N N
H 2 H
O O O
HN HN CH3 HN N
O N O N N"N N
H H ~ H
In another embodiment of the present invention, the
process comprises making a dioxolane nucleoside analogue
by separating a compound according to formula A or
formula B. According to this embodiment, the process
includes stereoselectively replacing the R group with a
purinyl or pyrimidinyl moiety or analogue or derivative
thereof by acylating the second mixture to produce an
acylated second mixture. This embodiment also includes
the step of glycosylating the acetylated second mixture
with a purine or pyrimidine base or analogue or
derivative thereof and a Lewis Acid to produce a
dioxolane nucleoside analogue.
-13-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
DETAILED DESCRIPTION OF THE INVENTION
The present invention involves a high yield process of
separating (3 and a anomers from an anomeric mixture of
dioxolane nucleoside analogue precursors which provides
higher yield and greater efficiency. In one embodiment,
this method is used in the production of dioxolane
nucleoside analogues having a high degree of anomeric
purity at lower cost. Additionally, another aspect of
the present invention involves synthesizing starting
material having a higher degree of anomeric purity.
The present invention provides a process of preparing
dioxolane nucleoside analogues having a predominant (3-L-
configuration using enzymes, namely hydrolases. The
procedure improves overall yield and has relatively few
steps, thereby improving overall efficiency. The process
involves the following steps.
A mixture of anomers represented by formula A or formula
B is obtained as described herein in Scheme 1.
WO WO
~ COORS ~ COORS
O
O
CA) CB)
In the above formula, W is benzyl or benzoyl, and R1 is
selected from the group consisting of H, C1_6 alkyl and
~s-~s aryl. The mixture is hydrolyzed with an enzyme
selected from the group consisting of cholesterol
esterase, ESL-001-02, horse liver esterase, bovine
-14-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
pancreatic protease, a-chymotrypsin, protease from
Streptomyces caespitosis, substilisin from Bacillus
licheniformis, protease from Aspergillus oryzae,
proteinase from Bacillus licheniformis, protease from
Streptomyces griseus, acylase from Aspergillus melleus,
proteinase from Bacillus subtilis, ESL-001-05, pronase
protease from Streptomyces griseus, lipase from Rhizopus
arrhizus, lipoprotein lipase from Pseudomonas species
type B, lipase from Pseudomonas cepacia and bacterial
proteinasei. The hydrolyzing step stereoselectively
hydrolyzes the a-anomer of the mixture of either formula
A or formula B. The result is an unhydrolysed ~3-anomer.
The a-anomer can be separated easily from the ~3-anomer.
If an anomeric mixture of the compound of formula A is
selected, the result is the production of the compound of
formula C and formula D:
WO O COOH WO- O COORS
O O
(C) (D)
If an anomeric mixture of the compound of formula B is
selected, the result is the production of the compound of
formula E and formula F:
WO- O COOH WO O COORS
O O
(E) (F)
The mixture (C) / (D) or (E) / (F) is then subjected to
oxidative decarboxylation which replaces the R1 group with
an acyl moiety. It is then glycosylated with a purine or
pyrimidine base or analogue or derivative thereof in the
-15-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
presence of a Lewis Acid. The final step produces a
dioxolane nucleoside analogue in the ~3-L configuration
for the mixture (C)/(D) and a dioxolane nucleoside
analogue in the (3-D configuration for the mixture
(E) / (F) .
At the outset, the following definitions have been
provided as reference. Except as specifically stated
otherwise, the definitions below shall determine the
meaning throughout the specification.
"Nucleoside" is defined as any compound which consists of
a purine or pyrimidine base, linked to a pentose sugar.
"Dioxolane nucleoside analogue" is defined as any
compound containing a dioxolane ring as defined
hereinafter linked to a purine or pyrimidine base or
analogue or derivative thereof. A "dioxolane ring" is
any substituted or unsubstituted five member monocyclic
ring that has an oxygen in the 1 and 3 positions of the
ring as illustrated below:
3
2~0 4
O-'
1 5
Dioxolane
Ring
"Purine or pyrimidine base" is defined as the naturally
occurring purine or pyrimidine bases adenine, guanine,
cytosine, thymine and uracil. A purine or pyrimidine that
is a moiety is a purinyl or pyrimidinyl, respectively.
"Alkyl" is defined as a substituted or unsubstituted,
saturated or unsaturated, straight chain, branched chain
or carbocyclic moiety, wherein the straight chain,
- 16-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
branched chain or carbocyclic moiety can be optionally
interrupted by one or more heteroatoms (such as oxygen,
nitrogen or sulfur). A substituted alkyl is substituted
with a halogen (F, Cl, Br, I), hydroxyl, amino or C6_zo
aryl.
"Aryl" is defined as a carbocyclic moiety which can be
optionally substituted or interrupted by one heteroatom
(such as oxygen, nitrogen or sulfur) and containing at
least one benzenoid-type ring (such as phenyl and
naphthyl).
"Carbocyclic moiety" is defined as a substituted or
unsubstituted, saturated or unsaturated, C3_6 cycloalkyl
wherein a substituted cycloalkyl is substituted with a
C1_6 alkyl, halogen (i.e. F. C1, Br, I), amino, carbonyl
( i . a . COOH ) , or NOz .
A "derivative" of a purine or pyrimidine base refers to
one of the following structures:
H H H
H~.N ' 5 N7 H.,N ~ 4 .H
2I O ~~$ H I~ 5
H..~N N9 .2~ 6.
3 ; H 1N H
H '
H H
Purine Pyrimidine
wherein one or more of the pyrimidine H are substituted
with substituents that are known in the art. In the
above illustration, the bonds represented by a broken
line are optional and are present only in cases which
require the bond to complete the valence of the ring
atom. Substitutents bound to the ring members by a
- 17-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
single bond include but are not limited to halogen such
as F, C1, Br, I; an akyl such as lower akyls; aryl; cyano
carbamoyl; amino including primary, secondary and
tertiary amino; and hydroxyl groups. Substituents bound
to the carbon ring atoms by a double bond include but are
not limited to a =O to form a carbonyl moiety in the
ring. It is understood that when the ring is aromatic,
some of the substitutions may form tautomers. The
definition shall include such tautomers.
"Analogue"~of a purine or pyrimidine base refers to any
derivative of purine or pyrimidine bases that is further
modified by substituting one or more carbon in the ring
structure with a nitrogen.
"Stereoselective enzymes" are defined as enzymes which
participate as catalysts in reactions that selectively
yield one specific stereoisomer over other stereoisomers.
"Anomeric purity" is defined as the amount of a
particular anomer of a compound divided by the total
amount of all anomers of that compound present in the
mixture multiplied by 100%.
"Alkoxy" is defined as an alkyl group, wherein the alkyl
group is covalently bonded to an adjacent element through
an oxygen atom (such as methoxy and ethoxy).
"Alkoxycarbonyl", is defined as an alkoxy group attached
to the adjacent group of a carbonyl.
"Acyl" is defined as a radical derived from a carboxylic
acid, substituted (by a halogen, C6_zo aryl or Cl_6 alkyl )
or unsubstituted by replacement of the -OH group. Like
-18-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
the acid to which it is related, an acyl radical may be
aliphatic or aromatic, substituted (by halogen, C1_s
alkoxyalkyl, nitro or Oz) or unsubstituted, and whatever
the structure of the rest of the molecule may be, the
properties of the functional group remain essentially the
same (such as acetyl, propionyl, isobutanoyl, pivaloyl,
hexanoyl, trifluoroacetyl, chloroacetyl and
cyclohexanoyl).
"Alkoxyalkyl" is defined as an alkoxy group attached to
i
the adjacent group by an alkyl group (such as
methoxymethyl).
"Acyloxy" is defined as an acyl group attached to the
adjacent group by an oxygen atom.
"Oxo" is defined as a =O substituent bonded to a carbon
atom.
As noted above, one embodiment of the present invention
is a process for separating (3 and a anomers from an
anomeric mixture represented by the following formula A
or formula B:
WO O COORS WO O COORS
O O
(A) (B)
wherein W is benzyl or benzoyl and R1 is selected from the
group consisting of Cl_6 alkyl and C6_ls aryl.
Ln one embodiment, the process stereoselectively
hydrolyses predominantly the a-anomer to form a product
19-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
where R1 of formula A and formula B is replaced with H.
The (3-anomer remains substantially unhydrolyzed. The
process also comprises separating the hydrolyzed product
from unhydrolyzed starting material.
The process of making a ~3-L dioxolane nucleoside analogue
begins with the preparation of starting materials.
Scheme 1 depicts the manufacture of a mixture that
includes formula A or B.
Scheme 1
o Wo 0
O PTSA O
WOO ~' ~ OR1 OR1
r, 0
Formula lA Formula 1B Formula 1C
W0~ O
~O
OR1
O
Formula 1D
An oxyacetaldehyde represented by formula lA (wherein W
is benzyl or benzoyl) is reacted with 1,3-dioxolane-4-
(4R)carboxylic acid-2,2-dimethyl-methyl ester (formula
1B) in approximately equimolar proportions. The
dioxolane of formula 1B has a chiral center at the C4
carbon. The reaction occurs in a toluene solvent. The
mixture is heated to 58°C. The catalyst, PTSA, is added.
The mixture is heated to a temperature between 64-67°C. A
vacuum is applied at 70 kPa, and the reaction proceeds
for 40 minutes. Traces of solvent are then removed by
high vacuum. The catalyst is removed by filtration using
a 1:1 ratio of Hexane:EtOAc as an eluent. In one
embodiment, the preferred filter is a silica gel pad.
-20-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
The resulting product is a crude oil containing a mixture
of the compounds of formula 1C and 1D, wherein the ratio
is 2:1 of (1C:1D) respectively.
It can be appreciated by a person of skill in the art
that the reaction conditions can be adjusted to optimize
the purity of the stereoisomers. In one embodiment of
the present invention, the reaction of the compound of
formula lA with the compound of formula 1B is done in the
presence of catalyst in an amount between about 1.0 wt%
and 10.0 wt% of the starting material. In another
embodiment the amount of catalyst is between about 2.5
wt% and about 5.5 wt% of the starting materials. Ir yet
another embodiment, the amount of catalyst is between
about 3.0 wt% and about 5.0 wt%. In still another
embodiment, the amount of catalyst is between about 3.5%.
and about 5.5%. In another embodiment, the amount of
catalyst is between about 2.5 wt% and about 7.5 wt%. In
another embodiment the amount of catalyst is about 5.0
wt % .
In an embodiment of the present invention, the reaction
of the compound of formula lA with the compound of
formula 1B is done at a temperature ranging from about
40°C to about 80°C. In another embodiment of the present
invention; the temperature ranges from about 50°C to about
75°C. In still another embodiment, the temperature
ranges from about 60°C to about 70°C. In an additional
embodiment, the temperature ranges from about 65°C to
about 7 9°C .
In an embodiment of the present invention, the reaction
time between the compound of formula lA and the compound
of formula 1B corresponds to a period ranging from about
-21-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
30 minutes to about 2 hours. In yet another embodiment,
the period ranges from about 30 minutes to about 1 hour.
In still another embodiment, the period ranges from about
30 minutes to about 50 minutes.
It will be appreciated by a person of ordinary skill in
the art that the C4 carbon is chiral. Because this
carbon is not involved in the reaction, the chirality is
preserved at that carbon. A starting material can be
selected to have a (4S) or (4R) stereochemistry.
I
According to one embodiment, it is preferable that the
resulting product is an anomeric mixture favoring the ~3-L
configuration over the a-L configuration. To achieve
such a result, the starting material represented by
formula 1B (4S) is selected and shown below:
O
0
oRl
0
Formula 1B (4S)
The reaction proceeds according to the principles
described above. The resulting product, according to one
embodiment, will have an anomeric purity of the (3-L
anomer over the a-L anomer of greater than 55%,
preferably 60% and more preferably 65%.
-22-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
According to one embodiment, the present invention is a
method of separating (3-anomers from a-anomers according
to the following Scheme 2:
Scheme 2
O O O
)~ O
O ,+. /,,,,.. O 1
WO~ OR~WO < OFt~ ~ WO ORS
O O O
Fomnla 2A Formula 2B Formula 2A
O
2) 6chaction in NaHC03 O
~ri~ O ~~~. O
WO ~ ~ ~< ONa+ WO .. ~< OH
H+ / O O
O Formula 2C
O
O O
WO ~ ~ ~< OH WO~ ORS
O
Formula 2C Fomx~la 2A
3) LiOH
4) H+
O
O
WO~ OH
O
According to one embodiment, a mixture of anomers is
obtained as represented by formula 2A or formula 2B. A
mixture represented by formula 2A or formula 2B can be
obtained according to the reaction described above or
according to any method known in the art.
The reaction is prepared as follows: A portion of the
material represented by formula 2A and formula 2B is
weighed into a reaction vessel. For a small scale
reaction, about 0.001 mmol of the mixture of formula 2A
and formula 2B is added to about 46 mL of a 5 mmol
solution of BES buffer (for a final concentration of
-23-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
about 2 mM in substrate). For a preparative scale
reaction, about 0.8 mmol of the mixture is added to about
mL of buffer (for a final concentration of about 80 mM
in substrate). The pH of the buffer, according to one
embodiment, should be between 7.0 and 7.5 and preferably
7.2.
The enzyme is selected from the group consisting of
cholesterol esterase, ESL-001-02, horse liver esterase,
bovine pancreatic protease, a-chymotrypsin, protease from
Streptomyces caespitosis, substilisin from Bacillus
licheniformis, protease from Aspergillus oryzae,
proteinase from Bacillus licheniformis, protease from
Streptomyces griseus, acylase from Aspergillus melleus,
proteinase from Bacillus subtilis, ESL-001-5, pronase
protease from Streptomyces griseus, lipase from Rhizopus
arrhizus, lipoprotein lipase from Pseudomonas species
type B, lipase from Pseudomonas cepacia and bacterial
proteinase.
The commercial sources of the enzymes are readily
available to a person of ordinary skill in the art.
Particularly, some of the materials can be obtained from
the following sources: Bovine cholesterol esterase was
purchased from Genzyme (Cambridge, MA); ESL-001-02 from
Diversa Corp. (San Diego, CA); Horse liver esterase and
subtilisin from Bacillus licheniformis from Fluka Chemie
(Oakville, ON); Bovine pancreas protease type 1, a-
chymotrypsin and Streptomyces caespitosis from Sigma-
Aldrich (Oakville, ON).
In another embodiment, the enzyme is selected from the
group consisting of protease from Aspergillus oryzae,
-24-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
proteinase from Bacillus licheniformis, subtilisin from
Bacillus licheniformis, protease from Streptomyces
griseus, acylase from Aspergillus melleus, proteinase
from Bacillus subtilis, ESL-001-005, pronase protease
from Streptomyces griseus, lipase from Rhizopus arrhizus,
lipoprotein lipase from Pseudomonas species type B,
bacterial proteinase and lipase from Pseudomonas cepacia.
The selection of one of these enzymes is preferred
according to this embodiment when the oxyacetaldehyde
represented by the compound of formula lA in Scheme 1 is
selected to be benzoyloxyacetaldehyde.
According to another embodiment of the invention, the
oxyacetaldehyde represented by the compound of formula lA
is benzoyloxyacetaldehyde. According to this embodiment,
the enzyme is selected from the group consisting of
protease from Aspergillus oryzae, proteinase from
Bacillus licheniformis, subtilisin from Bacillus
licheniformis, pronase protease from Streptomyces
griseus, and lipase from Rhizopus arrhizus. In yet
another embodiment, the enzyme is selected from the group
consisting of protease from Aspergillus oryzae and
proteinase from Bacillus Iicheniformis.
In yet another embodiment, the enzyme is selected from
the group consisting of cholesterol esterase, ESL-001-02,
horse liver esterase, bovine pancreatic protease, a-
chymotrypsin, protease from Streptomyces caespitosis and
substilisin from Bacillus licheniformis. The selection
of one of these enzymes is preferred according to this
embodiment when the oxyacetaldehyde represented by the
compound of formula 1A is benzyloxyacetaldehyde.
The stereospecific enzyme selected is then added to begin
-25-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
the hydrolysis reaction. The enzymatic reaction
hydrolyzes primarily the a-anomer by replacing the R1
group of the a-anomer of the compound of formula 2B with
H to form the compound of formula 2C. The amount of the
enzyme added can be determined according to principles
known by any person of ordinary skill in the art.
According to another embodiment, about 500 ~,L was added
to begin the reaction. The rate and degree of hydrolysis
was monitored by a pH-stat according to principles known
in the art;. ,As the compound of formula 2B is hydrolyzed,
the pH of the mixture decreases. Thus, the change in pH
as monitored by a pH stat corresponds to the completeness
of the reaction.
If the reaction time is allowed to proceed longer than
the optimal reaction time, the (3-anomer may be converted
resulting in lower anomeric purity of the final product:
If the reaction time is too short, less than optimal
amount of the a-anomer is converted resulting in a lower
anomeric purity of the remaining unhydrolyzed reactant.
According to one embodiment, the reaction is allowed to
proceed until 43% completion. It will be appreciated by
a person of ordinary skill in the art that the exact
degree of completion may change depending upon the
reactant used, the enzyme used and other principles known
to a person of ordinary skill in the art.
As noted, the ester starting material is separated from
the hydrolyzed acid product when the desired endpoint is
reached. The ester starting material and the hydrolysed
product are separated by increasing the pH of the
solution to more than pH 7.0 with bicarbonate solution
and extracting with ethyl acetate (for example, 3 x 20
-26-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
mL). The unhydrolyzed starting material is extracted in
the ethyl acetate and the hydrolyzed product remains in
salt form in the aqueous solution. The pH of the
solution is then adjusted to pH 2. The hydrolyzed
product is further extracted with ethyl acetate (for
example, 3 x 20 mL). The reactants and the products are
dried with MgS04, filtered and concentrated in-vacuo.
Alternatively, the unhydrolyzed product can be hydrolyzed
by procedures known in the art such as reaction with LiOH
followed by acidification.
Because of the enzyme selectivity, the anomeric purity of
the hydrolyzed and separated (3-anomer is considerable.
Furthermore, the anomeric purity of the unhydrolyzed and
separated a-anomer is also considerable. In one
embodiment, the anomeric purity of the respective
separated a-anomer and/or the ~3-anomer is greater than
80%. In another embodiment, the anomeric purity of the
respective separated a-anomer and/or the (3-anomer is
greater than 90%. In another embodiment, the anomeric
purity of the respective separated a-anomer and/or the
anomer is greater than 95%. In another embodiment, the
anomeric purity of the respective separated a-anomer
and/or the (3-anomer is greater than 98%.
In another embodiment, the procedure of Scheme 2 is
followed except the anomeric mixture represented by
formula 2A or 2B is replaced with an anomeric mixture
represented by formula 2D and 2E, respectively.
_27_
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
O O O
/,,,, O ,,, ,,,
WO I~<~~~ ORS WO~~ ORS
O O
Formula 2D Formula 2E
According to this embodiment the a-anomer represented by
formula 2E is hydrolyzed. The result is the separation of
the hydrolyzed a-anomer represented by formula 2F from
the unhydrolyzed (3-anomer represented by formula 2D.
O
O ,,,,
WO~ O OH
Formula 2F
In another embodiment, the procedure of Scheme 2 is
followed except a mixture represented by formula 2A and
2B is replaced with a mixture of four stereoisomers
represented by formula 2G.
O
O
WO~ ORS
O
Formula 2G
According to this embodiment, the a-anomer containing
both D and L enantiomers is hydrolyzed. The result is
the separation of the hydrolyzed a-anomer containing both
D and L enantiomers from the unhydrolyzed ~3-anomer
containing both D and L enantiomers.
-28-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
After hydrolysis, purification and oxidative
decarboxylation, the resulting dioxolane ring can be
linked with a purine or pyrimidine base or analogue or
derivative. There are several examples known by skilled
artisan on how to link a purine or pyrimidine base or
analogue or derivative to the dioxolane ring. For
example, PCT Publ. No. WO/97/21706 by Mansour et al.
describes one method of stereoselectively attaching the
purine or pyrimidine base or analogue or derivative to a
dioxolane ring. WO/97/21706 is incorporated herein fully
by reference.
According to the process disclosed in WO/97/21706 the
starting material is an acylated dioxolane ring. The
starting material of the procedure disclosed in
WO/97/21706 can be obtained by oxidative decarboxylation
of a product of Scheme 2 discussed above. Oxidative
decarboxylation destroys the stereochemistry of the C4
carbon while preserving the stereochemistry of the C2
carbon.
As noted, the oxidative decarboxylation step occurs after
the hydrolysis step of Scheme 2. A compound having the
desired stereochemistry on the C2 carbon is selected.
For each mmol of compound that is processed, it is
dissolved in between about 2.5 and about 4.0 mL of
acetonitrile. In another embodiment, between about 3.0
and about 3.5 mL of acetonitrile was added for each mmol
of compound. In yet another embodiment, between about
3.3 and about 3.4 mL of acetonitrile was added for each
mmol of compound.
For each mmol of compound, between about 0.08 and about
0.12 mL of pyridine was added. In another embodiment,
-29-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
between about 0.09 and about 0.11 mL of pyridine was
added for each mmol of compound. In yet another
embodiment, approximately O.1 mL of pyridine was added
for each mmol of compound.
To this mixture, between 1.1 and 1.5 mmoles of Pb(OAc)9
was added for each mmol of compound. In another
embodiment, between about 1.2 mmoles and about 1.4 mmoles
of Pb(OAc)4 is added for each mmol of compound. In yet
another embodiment, about 1.3 mmoles of Pb(OAc)4 is added
for each mmol of compound.
Thereafter, the mixture was stirred for 18 hours at room
temperature. Then, the mixture was poured into a
saturated solution of NaHC03. Between approximately 2.0
and 3.0 mL of NaHC03 were used for each mmol of compound.
In one embodiment, between about 2.5 mL and about 2.7 mL,
and more preferably about 2.6 mL of NaHC03 was used for
each mmol of compound. The solution was then stirred for
an additional 30 minutes. The organic layer was
separated from the aqueous layer by four extractions of
ethyl acetate. Extracts were combined, dried on
anhydrous Na2S04 and evaporated under a vacuum.
Optionally, the crude can be further purified by
chromatography on silica gel using a gradient of 0-15%
ethyl acetate in hexane.
-30-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
In one embodiment of the present invention, the oxidative
decarboxylation step is followed by glycosylation. The
glycosylation is represented by the following Scheme 3.
SCHEME 3
iodosilane O
WO ~", O
,...~O~OAc ---TWO ~",.....~~I
O
Formula 3A Formula 3B
P
"B ' Deprotection O " B
O ~ HO
O
Formula 3C Formula 3D
The first step in the glycosylation procedure is to
obtain a compound with the desired stereospecificity at
the C2 carbon. According to one embodiment, a compound
having an S stereochemistry at the C2 carbon, as
represented by the compound of formula 3A is preferred.
The result is that a higher ratio of the (3-L anomer is in
the product 3C. According to another embodiment,
compound having an R stereochemistry at the C2 carbon is
preferred. The result is a product that has a higher
ratio of the (3-D anomer in the final product.
The compound of formula 3A is reacted with an iodosilane
to produce the compound of formula 3B. In one
embodiment, the iodosilane is iodotrimethylsilane.
In another embodiment, the iodosilane is diiodosilane.
Important to the reaction is that it occurs at low
temperatures. According to one embodiment, the
temperature is preferably between 0 °C and -78°C prior to
-31 -
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
glycosylation with silylated pyrimidine or purine base or
analogue or derivative thereof.
The iodo intermediate represented by formula 3B is then
dissolved. in dichloromethane and is cooled down to
between 0° C and -78° C. A purine or pyrimidine base or
analogue or derivative thereof is then selected.
According to one embodiment, the purine or pyrimidine
base or analogue or derivative thereof is selected from
the following group:
NHR2 O R5 O
N ~ R3 HN R4 N i N HN
~ I ~ I '~
O H ~ H R6 N H R7 N
NHR9 NHR~1
N-/ _N i R10
N
O' _N
H O H
Wherein:
R2, R9 and R11 are each independently selected from the
group consisting of hydrogen,Cl_6 alkyl, C1_6 acyl and
R8C (O) wherein Re is hydrogen or C1_6 alkyl ;
R3, R4 and Rl° are each independently selected from the
group consisting of hydrogen, C1_6 alkyl, bromine,
chlorine, fluorine, iodine and CF3; and
R5, R6 and R~ are each independently selected from the
group consisting of hydrogen, bromine, chlorine,
fluorine, iodine, amino, hydroxyl and C3_s
cycloalkylamino.
-32-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
According to one embodiment, the purine or pyrimidine
base or derivative is selected from the group consisting
of
NHR2 O R5 O
N ~ R3 HN R4 N i ~ HN ~N
~ I ~ I ~
O H O H R6 N H R7 N
In this embodiment, R2 is selected from the group
consisting of hydrogen, C1_6 alkyl, C1_6 acyl and RBC (O)
wherein RB is hydrogen or C1_6 alkyl. Additionally, R3 and
R4 are each independently selected from the group
consisting of hydrogen, C1_6 alkyl, bromine, chlorine,
fluorine, iodine and CF3; and R5, R6 and R~ are each
independently selected from the group consisting of
hydrogen, bromine, chlorine, fluorine, iodine, amino,
hydroxyl and C3_6 cycloalkylamino.
In another embodiment, the purine or pyrimidine base or
analogue or derivative thereof is selected from the group
consisting of:
NHR9 NHR»
N i 'N R10
N~
O H O~N~N
H
In this embodiment, R9 and R11 are independently selected
from the group consisting of hydrogen, C1_6 alkyl, C1_s
acyl and R8C (0) . Additionally, Rlp is selected from the
-33-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
group consisting of hydrogen, C1_6 alkyl, bromine,
chlorine, fluorine, iodine and CF3.
The purine or pyrimidine or analogue or derivative
thereof is persylated by a sylating agent and ammonium
sulphate followed by evaporation of HMDS to form a
persylated purine or pyrimidine base or analogue or
derivative thereof herein referred to as the persylated
base and designated as P in Scheme 3. According to one
embodiment, the sylating agent is selected from the group
consisting of 1,1,1,3,3,3-hexamethyldisilazane,
trimethylsilyl triflate, t-butyldimethylsilyl triflate or
trimethylsilyl chloride. In one embodiment, the sylating
agent is 1,1,1,3,3,3,-hexamethyldisilazane.
The persylated base P was dissolved in 30 mL of
dichloromethane and was added to the iodo intermediate
represented by formula 3B. The reaction mixture was
maintained at between 0 and -78°C for 1.5 hours then
poured onto aqueous sodium bicarbonate and extracted with
dichloromethane (2x25 mL). The organic phase was dried
over sodium sulphate to obtain the compound of formula
3C. As used in Scheme 3, the B represents a moiety of
the purine or pyrimidine base or analogue or derivative
thereof which was persylated in the above step to form P.
The compound of formula 3C was removed by filtration and
the solvent was evaporated in-vacuo to produce a crude
mixture. The product represented by formula 3C has
predominantly a 4S configuration at the C4 carbon with an
anomeric purity of 80%. When the starting material is a
compound represented by formula 3A, the product forms
predominantly the (3-L enantiomer having an anomeric
purity of 800.
-34-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Next, the compound of formula 3C is deprotected to
produce the compound of formula 3D. This can be
accomplished by dissolving a compound represented by
formula 3C in ethanol and then adding cyclohexene and
palladium oxide. The deprotection step can also be done
by other methods which are well known by those skilled in
the art. The reaction mixture is refluxed for 7 hours.
It is then cooled and filtered to remove solids. The
solvents are removed from the filtrate by vacuum
distillation. The product represented by formula 3D is
purified by flash chromatography on silica-gel (5% MEOH
in ethylacetate). The deprotection step can also be done
by other methods that are well known by a person skilled
in the art.
In another embodiment, compounds of Scheme 1 may be
prepared by an alternative process which is shown below
in Scheme 4.
Scheme 4
O O
H~ ~..r 1 ) NaN02IH2S04/H20 HO O~-~ 2) R10H
HO n~A HO
Conpound 4B
O
HO OR,~ O
~ORi "~ WO ~ 3) C2H4G2/PTSA (7.5%) O
HO ~ ~ WO~ W
Conpound 4C ~P°~nd 4D O
Cortpound 4E
Between about 1.0-1.4 eq of sulfuric acid was added in
portions to a large excess of water while stirred at a
temperature between 0-5°C. By way of example and not by
limitation, if 9.06 mol of D-Serine represents 1
-35-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
equivalent of reactant, then between about 9.5-13.3 mol
of sulfuric acid is added to 7.3 L of water. In another
embodiment, between about 1.1-1.3 eq of sulfuric acid was
added to an excess of water. In a further embodiment,
1.2 eq of sulfuric acid was added to an excess of water.
About 1 equivalent of D-Serine was added in one portion
under vigorous stirring. Then, between about 1.0 and 1.4
eq. of aqueous sodium nitrite was added dropwise. The
temperature was kept between 0-5°C during the addition
time (about seven hours). The reaction vessel was
stirred overnight at room temperature. The water was
removed by vacuum and the residue (D-glyceric acid) co-
evaporated with toluene (3X1L). The residue was then
stirred with about 6L of an alcohol solvent for about 30
minutes. According to one embodiment, the alcohol is of
the formula R10H wherein R1 is a C1_4 alkyl. According to
another embodiment, the alcohol is methanol or ethanol.
The resulting solid was removed by filtration. The clear
solution was stirred at room temperature for 30-40 hours,
the alcohol removed by vacuum to yield a D-glycerate in
the form of a yellow viscous syrup. The D-glycerate is
then reacted with between about 0.9-1.1 eq of a dialkyl
acetal at a temperature of about 85-95°C. Examples of
suitable dialkyl acetals include benzoyloxyacetaldehyde
dialkyl acetal and benzyloxyacetaldehyde dialkyl acetal.
Examples of suitable alkyls for the dialkyl acetal is
methyl and ethyl.
Then, between about 1 wt% and about 10 wto of PTSA is
added. According to another embodiment, about 5 wto PTSA
is added. In another embodiment, about 0.02 eq. of solid
PTSA is added. The reaction mixture is kept under vacuum
at a temperature between 85-95°C for 2-3 hours. The
-36-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
mixture is then cooled to room temperature, diluted with
ethylacetate (250 mL) and poured onto saturated sodium
bicarbonate solution (250 mL) under stirring. The
organic phase is separated and the aqueous phase
concentrated, purified on a silica gel column eluting
with 5-10% ethylacetate/hexanes to yield the desired
dioxolane as a light yellow oil (about 59%) with (3/a
ratio of 2:1 or higher.
Alternatively, the reactants of step 3 of Scheme 4 can be
substituted by corresponding reactants of Scheme 1. For
example, the D-glycerate represented by Formula 4C is
replaced with an 1,3 dioxolane-4-(4R)carboxylic acid-2,2-
dimethyl alkyl ester represented by Formula 1B according
to one embodiment. Additionally or alternatively, the
dialkyl acetal represented by Formula 4D is replaced with
an oxyaldehyde represented by Formula lA. These
substitutions do not require changing the reaction
conditions substantially disclosed above for the third
step of Scheme 4.
In a further embodiment of the present invention, the
starting material of Scheme 4 is L-Serine which produces
an end product having an S-configuration at the C4 carbon
of the dioxolane ring. Alternatively, the L-glycerate of
Step 3 can be replaced with an 1,3 dioxolane-4-
(4S)carboxylic acid-2,2-dimethyl alkyl ester to produce
an end product having predominantly an S-configuration at
the C4 carbon of the resulting dioxolane ring.
-37-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 1. Enzyme catalyzed hydrolytic resolution of the
dioxolane methyl ester
A 2:1 ((3:a) anomeric mixture of (2- (S) -benzyloxymethyl) -
4-carboxylic acid-1,3-dioxolane methyl ester) (136.5 mg,
0.541mmo1) was weighed into a reaction vessel and BES
buffer(6,263 mL of a 5 mM solution, pH 7.2) was added.
The substrate remained as insoluble droplets. a-
Chymotrypsin (500 ~,L of a 5mg/mL BES buffer, pH 7.2
solution, X0.019 units by 4-nitrophenylacetate assay) was
added to begin the reaction and the rate and degree of
hydrolysis was monitored by a pH-stat which maintained
the pH at 7 by automatic titration with 0.0981 mmol NaOH.
The reaction was terminated at 43% conversion for high
anomeric purity as determined by Sih's equations for
recycling (Chen. C.S.; Fujimoto, Y.; Girdaukas, G.; Sih,
C.J., J.AM. Chem. Soc. 1982, 104, 7294-99), by extracting
the remaining starting material ester with ethyl acetate
(3x20mL). The aqueous layer was adjusted to pH 2 and the
product acid extracted with ethyl acetate (3x20mL).
Both extracts were dried with MgS09, filtered and
concentrated in-vacuo. By this method, we obtained the
(2-(S)-benzyloxymethyl)-4-(S)-carboxylic acid-1,3-
dioxolane methyl ester).
Example 2. Purity of ~3-Anomer by NMR
Analysis was performed on a Varian Gemini 200 MHz NMR
spectrometer in CDC13. The a-ester shows a triplet at 8
5.33 (3J = 4.6 Hz) and the (3-ester shows a triplet upfield
at 8 5.23 (3 J = 4. 6 Hz). The a-acid shows a triplet at
8 5.33 (3J = 3 .6 Hz) , while the (3-acid shows a broad
-38-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
singlet upfield at 8 5.19. We did not observe any
epimerization of the substrate or product acid during
work-up. By NMR analysis, the purity of the (3-anomer is
determined to have about 95% anomeric purity or higher.
Example 3: Purity of the a-Anomer
The product acid is obtained from example 1 after it was
dried with MgS04, filtered and concentrated in-vacuo. It
is analyzed for purity by NMR. The a-anomer is isolated
with high anomeric purity.
Example 4: Enzymatic Resolution of ~3-anomer with Protease
from Asperc~illus oryzae.
The procedure of Examples 1-2 were followed using
protease from Aspergillus oryzae as the enzyme to
separate a 2 : 1 ( ~3 : a ) anomeri c mixture of ( 2 - ( S ) -
benzoyloxymethyl)-4-carboxylic acid-1,3-dioxolane methyl
ester). The result is a ~3-anomer that has high anomeric
purity.
Example 5: Enzymatic Resolution of a-anomer with Protease
from AspercTillus oryzae.
The product acid is obtained from Example 4 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
-39-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 6: Enzymatic Resolution of ~i-anomer with
Proteinase from Bacillus licheniformis
The procedure of Examples 1-2 were followed using
proteinase from bacillus licheniformis as the enzyme to
separate a 2:1 ((3:a) anomeric mixture of (2- (S) -
benzoyloxymethyl)-4-carboxylic acid-1,3-dioxalane methyl
ester). The result is a ~3-anomer that has high anomeric
purity.
Example 7: Enzymatic Resolution of a-anomer with
Proteinase from Bacillus licheniformis.
The product acid is obtained from Example 6 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 8: Enzymatic Resolution of ~3-anomer with
Subtilisin from Bacillus Zicheniformis.
The procedure of Examples 1-2 were followed using
subtilisin from bacillus licheniformis as the enzyme to
separate a 2 : 1 ( (3 : a ) anomeric mixture of ( 2 - ( S ) -
benzoyloxymethyl)-4-carboxylic acid-1,3-dioxolane methyl
ester). The result is a (3-anomer that has high anomeric
purity.
-40-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 9: Ezizymatic Resolution of a-anomer with
Subtilisin from Bacillus licheniformis.
The product acid is obtained from Example 8 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 10: Enzymatic Resolution of (3-anomer with
Protease from Streptomyces Qriseus
The procedure of Examples 1-2 were followed using
protease from streptomyces griseus as the enzyme to
separate a 2:1 (~i:a) anomeric mixture of (2- (S) -
benzoyloxymethyl)-4-carboxylic acid-1,3-dioxolane methyl
ester). The result is a (3-anomer that has high anomeric
purity.
Example 11: Enzymatic Resolution of a-anomer with
Protease from Streptomyces crriseus
The product acid is obtained from Example 10 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 12: Enzymatic Resolution of (3-anomer with Acylase
from Asperctillus melleus.
The procedure of Examples 1-2 were followed using acylase
from aspergillus melleus as the enzyme to separate a 2:1
((3:a) anomeric mixture of (2- (S) -benzoyloxymethyl) -4-
carboxylic acid-1,3-dioxolane methyl ester). The result
is a ~i-anomer that has high anomeric purity.
-41 -
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 13: Enzymatic Resolution of a-anomer with Acylase
from Aspercrillus melleus.
The product acid is obtained from Example 12 after it is
dried with MgS04, filtered and concentrated in-vacuo.
The a-anomer is isolated with high anomeric purity.
Example 14: Enzymatic Resolution of a-anomer with
Proteinase.from Bacillus subtilis.
The procedure of Examples 1-2 were followed using
proteinase from bacillus subtilis as the enzyme to
separate a 2 : 1 ( ~i : a ) anomeri c mixture of ( 2 - ( S ) -
benzoyloxymethyl)-4-carboxylic acid-1,3-dioxolane methyl
ester). The result is a ~3-anomer that has high anomeric
purity.
Example 15: Enzymatic Resolution of a-anomer with
Proteinase from Bacillus subtilis.
The product acid is obtained from Example 14 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 16: Enzymatic Resolution of (3-anomer with ESL-
001-05
The procedure of Examples 1-2 were followed using diversa
clonezymes #5 as the enzyme to separate a 2:1 (~i:a)
anomeric mixture of (2-(S)-benzoyloxymethyl)-4-carboxylic
- 42 -
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
acid-1,3-dioxolane methyl ester). The result is a (3-
anomer that has high anomeric purity.
Example 17: Enzymatic Resolution of a-anomer with ESL-
001-05
The product acid is obtained from Example 16 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 18: Enzymatic Resolution of ~i-anomer with Pronase
protease from Streptomyces griseus.
The procedure of Examples 1-2 were followed using pronase
from streptomyces as the enzyme to separate a 2:1 (~3:a)
anomeric mixture of (2-(S)-benzoyloxymethyl)-4-carboxylic
acid-1,3-dioxolane methyl ester). The result is a ~3-
anomer that has high anomeric purity.
Example 19: Enzymatic Resolution of a-anomer with Pronase
protease from Streptomyces Qriseus.
The product acid is obtained from Example 18 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 20: Enzymatic Resolution of ~i-anomer with Lipase
from Rhizopus arrhizus.
The procedure of Examples 1-2 were followed using Lipase
from rhizopus arrhizus as the enzyme to separate a 2:1
((3:a) anomeric mixture of (2- (S) -benzoyloxymethyl) -4-
-43-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
carboxylic acid-1,3-dioxolane methyl ester). The result
is a ~3-anomer that has high anomeric purity.
Example 21: Enzymatic Resolution of a-anomer with Lipase
from Rhizopus arrhizus.
The product acid is obtained from Example 20 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 22: Enzymatic Resolution of ~~3-anomer with
Lipoprotein Lipase from Pseudomonas Species Type B.
The procedure of Examples 1-2 were followed using
lipoprotein lipase from pseudomonas sp. type B as the
enzyme to separate a 2:1 ((3:a) anomeric mixture of (2-
(S)-benzoyloxymethyl)-4-carboxylic acid-1,3-dioxolane
methyl ester). The result is a (3-anomer that has high
purity.
Example 23: Enzymatic Resolution of a-anomer with
Lipoprotein lipase from Pseudomonas Species Type B.
The product acid is obtained from Example 22 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 24: Enzymatic Resolution of (3-anomer with
Bacterial Proteinase.
The procedure of Examples 1-2 were followed using
bacterial proteinase as the enzyme to separate a 2:1
-44-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
(~i:a) anomeric mixture of (2- (S) -benzoyloxymethyl) -4-
carboxylic acid-1,3-dioxolane methyl ester). The result
is a ~3-anomer that has high anomeric purity.
Example 25: Enzymatic Resolution of a-anomer with
Bacterial Proteinase.
The product acid is obtained from Example 24 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer i;s isolated with high anomeric purity.
Example 26: Enzymatic Resolution of Q-anomer with Lipase
from Pseudomonas cepacia.
The procedure of Examples 1-2 were followed using lipase
from pseudomonas cepacia as the enzyme to separate a 2:1
((3:a) anomeric mixture of (2- (S) -benzoyloxymethyl) -4-
carboxylic acid-1,3-diaxolane methyl ester). The result
is a (3-anomer that has high anomeric purity.
Example 27: Enzymatic Resolution of a-anomer with Lipase
from Pseudomonas cepacia.
The product acid is obtained from Example 26 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 28: Enzymatic Resolution of ~i-anomer with
Cholesterol esterase.
The procedure of Examples 1-2 were followed using
cholesterol esterase as the enzyme to separate a 2:1
(~i:a) anomeric mixture of (2- (S) -benzyloxymethyl) -4-
-45-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
carboxylic acid-1,3-diaxolane methyl ester). The result
is a ~3-anomer that has high anomeric purity.
Example 29: Enzymatic Resolution of a-anomer with
Cholesterol esterase.
The product acid is obtained from Example 28 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 30: Enzymatic Resolution of ~3-anomer with
ESL-001-02.
The procedure of Examples 1-2 were followed using ESL-
001-02 as the enzyme to separate a 2:1 ((3:a) anomeric
mixture of (2-(S)-benzyloxymethyl)-4-carboxylic acid-1,3-
dioxolane methyl ester). The result is a (3-anomer that
has high anomeric purity.
Example 31: Enzymatic Resolution of a-anomer with
ESL-001-02.
The product acid is obtained from Example 30 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 32: Enzymatic Resolution of ~i-anomer with Horse
Liver Esterase.
The procedure of Examples 1-2 were followed using horse
liver esterase as the enzyme to separate a 2:1 ((3:a)
anomeric mixture of (2-(S)-benzyloxymethyl)-4-carboxylic
-46-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
acid-1,3-diaxolane methyl ester). The result is a (3-
anomer that has high anomeric purity.
Example 33~ Enzymatic Resolution of a-anomer with Horse
Liver Esterase.
The product acid is obtained from Example 32 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 34~ Enzymatic Resolution of Q-anomer with Bovine
Pancreatic Protease.
The procedure of Examples 1-2 were followed using bovine
pancreatic protease as the enzyme to separate a 2:1 ((3:a)
anomeric mixture of (2-(S)-benzyloxymethyl)-4-carboxylic
acid-1,3-dioxolane methyl ester). The result is a (3-
anomer that has high anomeric purity.
Example 35~ Enzymatic Resolution of a-anomer with Bovine
Pancreatic Protease.
The product acid is obtained from Example 34 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 36~ Enzymatic Resolution of (3-anomer Protease
from Streptomyces caespitosus.
The procedure of Examples 1-2 were followed using
protease from streptomyces caespitosis as the enzyme to
separate a 2 : 1 ( (3 : a ) anomeri c mixture of ( 2 - ( S ) -
-47-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
benzyloxymethyl)-4-carboxylic acid-1,3-dioxolane methyl
ester). The result is a ~i-anomer that has high anomeric
purity.
Example 37: Enzymatic Resolution of a-anomer with
Protease from Streptomyces caespitosus
The product acid is obtained from Example 36 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
Example 38: Enzymatic Resolution of ~-anomer with
Substilisin from Bacillus licheniformis.
The procedure of Examples 1-2 were followed using
substilisin from bacillus licheniformis as the enzyme to
separate a 2:1 ((3:a) anomeric mixture of (2- (S) -
benzyloxymethyl)-4-carboxylic acid-1,3-dioxolane
methylester). The result is a ~i-anomer that has high
anomeric purity.
Example 39: Enzymatic Resolution of a-anomer with
Substilisin from Bacillus Iicheniformis.
The product acid is obtained from Example 38 after it is
dried with MgS04, filtered and concentrated in-vacuo. The
a-anomer is isolated with high anomeric purity.
-48-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 40: Preparation of 2-(S)-Benzyloxymethyl-4-(R)
iodo-1,3-dioxolane and 2-(S)-Benzyloxymethyl-4-(S)-iodo
1,3-dioxolane (Compound 40)
/,,,,,, O I
Bn0
O
Compound 40
A mixture .consisting of 2S-benzyloxymethyl-4S acetoxy-
1,3-dioxolane and 2S-benzyloxymethyl-4R-acetoxy-1,3-
dioxolane in 1:2 ratio (6g; 23.8 mmol) was dried by
azeotropic distillation with toluene in-vacuo. After
removal of toluene, the residual oil was dissolved in dry
dichloromethane (60 mL) and iodotrimethylsilane (3.55 mL;
1.05 eq.) was added at -78°C, under vigorous stirring.
The dry-ice/acetone bath was removed after addition and
the mixture was allowed to warm up to room temperature
(15 min.). The product was 2S-benzyloxymethyl-4R-iodo-
1,3-dioxolane and 2S-benzyloxymethyl-4S-iodo-1,3-
dioxolane.
It would be understood by a person of ordinary skill in
the art that if the starting mixture was chosen
consisting of 2R-benzyloxymethyl-4S acetoxy-1,3-dioxolane
and 2R-benzyloxymethyl-4R-acetoxy-1,3-dioxolane. The
resulting product is 2R-benzyloxymethyl-4R-iodo-1,3-
dioxolane and 2R-benzyloxymethyl-4S-iodo-1,3-dioxolane.
Furthermore, the starting material having a benzoyl
substituent group instead of a benzyl would result in a
product having a benzoyl substituent and not a benzyl.
-49-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 41: Synthesis of 2-(S)-Benzyloxymethyl-1,3-
dioxolan-4-(S)-yl)-2-oxo-4-aminoacetyl-pyrimidine
(Compound 41).
NHAc
~~ N
B n0 ~',,,I,~, O ,, N O
O
Compound 41
The previously prepared iodo intermediate (Compound 40)
in dichloromethane, was cooled down to -78° C. Persylated
N-acetyl cytosine (l.l eq) formed by reflux in
1,1,1,3,3,3-hexamethyl disilazane (HMDS) and ammonium
sulphate followed by evaporation of HMDS was dissolved in
30 mL of dichloromethane and was added to the iodo
intermediate. The reaction mixture was maintained at
-78°C for 1.5 hours then poured onto aqueous sodium
bicarbonate and extracted with dichloromethane (2x25mL).
The organic phase was dried over sodium sulphate, the
solid was removed by filtration and the solvent was
evaporated in-vacuo to produce 8.1 g of a crude mixture.
~i-L-4'-benzyl-2'-deoxy-3'-oxacytidine and its a-L isomer
were formed in a ratio of 5:1 respectively. This crude
mixture was separated by chromatography on silica-gel
(5% methanol in ethylacetate) to generate the pure (3-L ((3)
isomer (4.48 g). Alternatively, recrystallization of the
mixture from ethanol produces 4.92 g of pure (3 isomer and
3.18 g of a mixture of ~3 and a-isomers in a ratio of 1:1.
-50-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 42: 2-(S)-Benzyloxymethyl-1,3-dioxolan-4-(S)-yl)-
2-oxo-4-amino-pyrimidine (Compound 42).
NH2
~~ N
/,,,,,,I' O N O
BnO
O
Compound 42
The protected (3-L isomer (4.4 g) (Compound 41) was
suspended in saturated methanolic ammonia (250 mL) and
stirred at room temperature for 18 hours in a closed
vessel. The solvents were then removed in-vacuo to
afford the deacetylated nucleoside in pure form.
Example 43: 2-(S)-hydroxymethyl-1,3-dioxolan-4-(S)-yl)-2-
oxo-4-amino=pyrimidine (Compound 43).
NH2
~~ N
O N O
HO ~,,,',L.~
O
Compound 43
~3-L-4'-Benzyl-2'-deoxy-3'-oxacytidine (Compound 42) was
dissolved in EtOH (200 mL) followed by addition of
cyclohexene (6 mL) and palladium oxide (0.8 g). The
reaction mixture was refluxed for 7 hours then it was
cooled and filtered to remove solids. The solvents were
removed from the filtrate by vacuum distillation. The
-51-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
crude product was purified by flash chromatography on
silica-gel (5% MeOH in EtOAc) to yield a white solid
(2.33 g; 86% overall yield) . aDZZ - -46.7° (c = 0.285;
MeOH) m.p. - 192 - 194°C.
The following examples 44-46 illustrate a method of
preparing the starting material of example 1 (2-(S)-
benzyloxymethyl-4-carboxylic acid-1,3-dioxolane methyl
ester) .
Examr~le 44: Preparation of D-c~rlyceric acid
O O
HZN OH H~ OH
HO HO
Portions of sulfuric acid (297 mL; 11.14 mol; 1.23 eq)
was added to a large excess of water (7,300 mL) under
stirring and cooling (0-5°C). D-Serine (952 8;9.06 moll
eq) was added in one portion under vigorous stirring,
followed by dropwise addition of aqueous sodium nitrite
(769 8;11.14 mol; 1.23 eq in 3,060 mL water).
Temperature was kept between 0-5°C during the addition
time (seven hours). The reaction vessel was stirred
overnight at room temperature and the reaction monitored
by TLC (ninhydrin). In order to complete the reaction,
additional sulfuric acid (115 mL; 4.31 mol; 0.47 eq) and
aqueous sodium nitrite (255 g; 3.69 mol; 0.4 eq in 1,100
mL water) was added, keeping the reaction vessel
temperature between 0-5°C. The reaction vessel was then
kept under stirring at room temperature for another 18
hours. Nitrogen was bubbled through the solution for one
hour and the water removed by vacuum, keeping the
-52-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
reaction vessel temperature between 28-30°C. The residue
(D-glyceric acid) was co-evaporated with toluene (3X1L).
Example 45: Preparation of D-methyl c~lycerate
O O
HO HO
-OH OMe
HO HO
D-glyceric acid was stirred with methanol (6L) for 30
minutes and the solid removed by filtration. The clear
solution was stirred at room temperature for 35-38 hours
and the reaction monitored by TLC (DCM/MeOH 8:2 Rf=0.63).
Methanol was removed by vacuum to yield a yellow viscous
syrup ( 1, 10 0 g ) .
Example 46: Preparation of 2-(R,S)-benzoyloxymethyl-4-R-
methylcarboxylate-1,3-dioxolane.
O O
HO OMe BzO~OMe ",,"_ ,O
BzO~ ~'''~ OMe
OMe ~ O
HO
A mixture of benzoyloxyacetaldehyde dimethyl acetal (146
g, 95%, 0.66 mole, 1 eq) and D-methyl glycerate (99 g,
0.82 mole, 1.25 eq) was heated to 90oC, followed by the
addition of solid PTSA (2.75 g, 0.145 moles, 0.022 eq).
The reaction mixture was kept under vacuum (water
aspirator) at 90-95°C for 2.5 hours (TLC,
Hexanes/Ethylacetate 1:1, Rf-0.47). The reaction mixture
was cooled down to room temperature, diluted with
ethylacetate (250 mL) and poured onto saturated NaHC03
solution (250 mL) under stirring. The organic phase was
-53-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
separated and the aqueous phase was extracted one with
ethylacetate (150 mL). The combined organic phase was
concentrated and purified on a silica gel column eluting
with 5-10% ethylacetate/hexanes to yield 112.4 g of the
desired product as a light yellow oil (59%) with (3/a
ratio of 2.1:1.
The following example 47 illustrates a large scale
version of the enzymatic separation of Example 1.
Example 47: Large scale preparation of j3 2-(R)-
benzoyloxymethyl-1,3-dioxolane-4-(R)-methylcarboxylate.
O O
O O
BzO~ OMe BzO~ OMe
O O
A mixture of ~i/a 2-benzoyloxymethyl-4-methylcarboxylate-
1,3-dioxolane (20 g; 75.12 mmol) was suspended in
acetonitrile (40 mL) and phosphate buffer (160 mL) at
30°C. a-Chymotrypsin was added (7.5 mL) in one portion
followed by dropwise addition of a 1N solution of NaOH
(total volume 46 mL) over six hours to hydrolyze the a
isomer. The pH was constantly monitored and maintained
between 7.1 and 7.2 while the temperature was kept at
30°C. After six hours, the mixture was extracted with
EtOAc (1X80 mL). Then, the organic layer was separated,
and the aqueous layer was extracted with EtOAc (2X50mL).
Combined organic layers were washed with saturated NaHC03
(20 mL), dried over anhydrous NazS04 and the solvent
removed under vacuum to leave 12.63 g of a clear oil
(85.6%; (3-isomer with less than 2.24% of the a-isomer by
HPLC ) .
-54-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
It is understood by a person of ordinary skill in the art
that this example can be followed substituting
chymotrypsin with any of the enzymes used in examples 4
through 39 to result in a large scale production of the
(3-isomer of 2-benzoyloxymethyl-4-methylcarboxylate-1,3-
dioxolane.
Example 48. Preparation of ~i 2-(R)-benzoyloxymethyl-1,3-
dioxolane-4-(R)-carboxylic acid.
O O
O O
BzO~ OMe BzO~ OH
O O
(3 2-(R)-benzoyloxymethyl-1,3-dioxolane-4-( R)-
methylcarboxylate-1,3-dioxolane (15.327 g; 57.57 mmol) is
dissolved in THF (60 mL) then water (15 mL) was added
under stirring. The internal temperature was set to 20°C.
Then, a solution of LiOH (2.41 g; 57.57 mmol) in water
(15 mL) was added dropwise over 7 minutes. The reaction
mixture was stirred at 22°C for an additional 40 minutes.
THF was removed under vacuum, and the residue diluted
with water (70 mL). The resulting solution was extracted
with dichloromethane (2X35mL). The aqueous phase was
acidified by 30o H2SO4 (9.5 mL) under tight pH-meter
control (initial pH:8.36 to 3.02) then extracted with DCM
(4X60mL). The organic phases were combined and the
solvent removed under vacuum to furnish a light green
syrup (14.26 g) which was kept under vacuum overnight.
-55-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 49: Preparation of j3 2-(R)-benzoyloxymethyl-4-
(R,S)-methylcarboxylate-1,3-dioxolane.
O COOH O OAc
BzO~~ ~ BzO
O O
Lead tetraacetate (944, 8 g; 2,024 mole; 1,2 eq) was
added portion-wise to an acetonitrile (6.8 L) solution of
the acid (425,5 g; 1,687 mole; 1,0 eq) and pyridine (193
mL) in an;ice bath. The reaction vessel was allowed to
warm up to room temperature and stirred. The reaction
was checked by TLC (hexanes:ethyl acetate 6:4). It was
filtered through a small pad of celite (about 1 inch).
Then, the filtrate was poured onto 5 L of saturated
aqueous sodium bicarbonate solution (reaction mixture
turned brown), and the pH as adjusted to 8 by adding
solid sodium bicarbonate. The filtrate was again
filtered through a small pad of celite (about 1 inch) to
remove the black lead salts to yield a pale yellow
mixture. The organic phase was separated and the aqueous
phase was extracted with ethylacetate (4X2L). The
combined organic phase was concentrated, and the oil
obtained was co-evaporated with toluene (3X2L) to yield a
brown syrup.
This syrup (374 g) was further purified by filtering
through a small pad of silica gel (1 g crude; 2 g
silica), eluting with 3.5 L of the solvent mixture (ethyl
acetate:hexanes 8:2) to yield 332,3 g (740) of pure
product. This last filtration step is optional.
- 56 -
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 50: Preparation of 9-(2-(R)-benzyloxymethyl-1,3-
dioxolan-4-yl)-6-chloro-2-amino purine (Compound 50a) and
9-(2-(R)-benzyloxymethyl-1,3-dioxolan-4-vl -6-iodo-2-
amino purine (Compound 50b
N
O OAc O N CI(I)
BzO~~ ~ BzO
O O N~ N
NH2
Compound 50a(b)
TMSI (28.2 mL; 198.12 mol eq) was added dropwise to a
dichloromethane (750 mL) solution of the sugar (2-(R)-
benzyloxymethyl-4-carboxyl-1,3-dioxolane)(52.75 g; 198.12
mmol; 1 eq) at -15°C. After 2.5 hr at -15°C, silylated 2-
amino-6-chloropurine (62 g; 198 mmol; 1 eq) was added to
the reaction mixture as a solid. The stirring was
continued at the same temperature for another 2.5 hr.
The reaction mixture was allowed to warm up slowly to
room temperature followed by continued stirring for 40 hr
at room temperature. Then, the mixture was poured onto
aq NaHC03 solution (1 L). It was stirred for 20 min with
Na2Sz03 and filtered through a small pad celite. Then,
the organic phase was separated and the aqueous phase was
extracted with dichloromethane (1 X 200 mL). The
combined organic phases were concentrated to get 87 g of
the crude. Column purification of the crude on silica
gel (450 g), eluting with ethylacetate/hexane (6:4)
yielded 67.7 g (810; 1:1 chloro/iodo mixture) of the
coupled product with (3/a ratio 2.3:1.
Alternatively, if the desired final product is the same
compound but with opposite stereochemistry (i.e. a 2:1
mixture of (3:a stereoisomers in the L-configuration).
-57-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
The procedure discussed above is followed. However, the
sugar 2-(R)-benzyloxymethyl-4-carboxyl-1,3-dioxolane is
replaced with 2-(S)-benzyloxymethyl-4-carboxyl-1,3-
dioxolane.
Example 51: Preparation of 9- (2- (R) -benzyloxymeth~rl-1, 3-
dioxolan-4-yl)-6-(N-cyclopropyl)amino-2-amino purine
Compound 51).
N
O N CI(I) O ~=N
NH
/ I ~ BzO~ N /
O N I
N\/ O N~ N
~NH2
NH2
Compound 51
A solution of the starting material (6.3 g; 14.95 mmol; 1
eq; average f.w.=421.52; C1:I/1:1) in ethanol (100 mL)
was refluxed at 75-80°C with cyclopropylamine (3.1 mL;
44.84 mmol; 3 eq) for 20 hrs and cooled to room
temperature. The reaction mixture was concentrated,
dissolved in dichloromethane (25 mL) and poured onto
saturated aqueous sodium bicarbonate solution. After 10
min. of stirring, the organic phase was separated, and
the aqueous phase was extracted with dichloromethane
(2X15 mL). Then, the combined organic phase was
concentrated to get a quantitative yield of the crude,
which was then purified by column chromatography (silica
gel, ethylacetate:MeOH 98.5:2.5 and 95:5) to yield 5.3 g
( 8 9 % ) of the product as a (3/a mixture .
-58-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 52: Preparation of 9-(2-(R)-hydroxymethyl-1,3-
dioxolan-4-yl)-6-(N-cyclopropyl)amino-2-amino purine
(Compound 52).
/-N N
O N NH O N NH
BzO~~ ~ I HO~~ ~ I
N w N ~' O
N~N
NH2 NHZ
Compound 52
The starting material (3.3 g) was stirred with ammonia in
MeOH (80 mL; 2M) for 20 hrs. Nitrogen was bubbled
through the reaction mixture to remove the excess
ammonia. Then, the solution was concentrated to yield
the crude as a (3/a mixture ((3/a. = 2.3:1) . The ~3/a
isomers were separated by chromatography on silica gel
using DCM/MeOH as eluent to yield 1.18 g (70% ~3 isomer).
-59-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 53: Preparation of 9-(2-(R)-hydroxymethyl-1 3-
dioxolan-4-yl-6-(N-2-cyclopropyl-2-aminomethoxyl)-2-amino
purine (Compound 53).
HOOC
N .~'~H
Ni N
I '>
H2N O N
HO~
O
Compound 53
A solution of (2R)-2-benzoyloxymethyl-4-(2'-amino-6'-
cyclopropylamino-purine-9'-yl)-1,3-dioxolane (480 mg) in
30 ml of saturated methanolic ammonia was stirred at room
temperature for 18 h. The mixture was evaporated to
dryness in vacuo. The residue was dissolved in 20 ml of
water, washed twice with 10 ml of methylene chloride and
lyophilized to give 283 mg of white solid in 80% yield.
The resulting product had a mixture of (3:a anomers having
a ratio of about 2:1.
Alternatively, if the desired final product is the same
compound but with opposite stereochemistry (i.e. a 2:1
mixture of (3:a stereoisomers in the L-configuration).
The procedure discussed above is followed. However, when
following the steps of Example 50, the sugar 2-(R)-
benzyloxymethyl-4-carboxyl-1,3-dioxolane is replaced with
2-(S)-benzyloxymethyl-4-carboxyl-1,3-dioxolane.
-60-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 54: Preparation of 9-(2-(S)-hydroxymethyl-1,3-
dioxolan-4-vl)-2-amino purine (Compound 54).
Ni N
H2N N N
/,,,,,, /O
H (O
O
Compound 54
The procedure of Example 50 was performed. Thereafter
6.3 g of Compound 50 was subject to hydrogenation
conditions under 50 psi of hydrogen over 10% Pd/c in 300
mL of ethanol containing 100 mL of triethylamine. After
3 hours of shaking, the catalyst was removed by
filtration. Then the sovent was evaporated to yield a
solid which was recrystallised to from ethanol-ether to
give about 4 g of Compound 54 having about a 2:1 mixture
of ~i:a stereoisomers in the L-configuration.
Alternatively, if the desired final product is the same
compound but with opposite stereochemistry (i.e. about a
2:1 mixture of (3:a stereoisomers in the D-configuration).
The procedure discussed above is followed. However, when
following the steps of Example 50, the sugar 2-(S)-
benzyloxymethyl-4-carboxyl-1,3-dioxolane is replaced with
2-(R)-benzyloxymethyl-4-carboxyl-1,3-dioxolane.
-61-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 55: Preparation of 9-(2-(S) hydroxymethyl-1,3-
dioxolan-4-yl)-6-amino purine (Compound 55).
NH2
Ni N
N N
/,,,,,, /O
H (O
O
Compound 55
The procedures set forth in Examples 50 and 51 were
performed. However, when following the steps of Example
50, the 1 equivalent of the silated 2-amino-6-
chloropurine is replaced with 1 equivalent of silated 6-
aminopurine. The result is a yield of 9-(2-(S)-
hydroxymethyl-1,3-dioxolan-4-yl)-6-amino purine having a
(3:a ratio of about 2:1.
Alternatively, if the desired final product is the same
compound but with opposite stereochemistry (i.e. a 2:1
mixture of (3:a stereoisomers in the D-configuration).
The procedure discussed above is followed. However, when
following the steps of Example 50, the sugar 2-(S)-
benzyloxymethyl-4-carboxyl-1,3-dioxolane is replaced with
2-(R)-benzyloxymethyl-4-carboxyl-1,3-dioxolane.
-62-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 56: Preparation of 9-(2-(S) hydroxymethyl-1,3-
dioxolan-4-yl)-6,2-diamino purine (Compound 56)
NH2
Ni N
H2N N O N
/,,,,..
HO
O
Compound 56
The procedure of Example 50 was performed. Thereafter, 6
g of Compound 50 was dissolved in 0.9 L of methanol
saturated at 0°C with dry ammonia and the solution is
heated in a steel bomb to 105°C to 110°C for 16 hours.
The solution was evaporated to dryness and the residue
purified by chromatography on silica gel using
chloroform-methanol (4:1) as the eluent to give about 3g
of crude Compound 56. The product can be recrystallised
from methanol-ether to yield purified Compound 56 having
a (3:a ratio of about 2:1.
Alternatively, if the desired final product is the same
compound but with opposite stereochemistry (i.e. a 2:1
mixture of (3:a stereoisomers in the D-configuration).
The procedure discussed above is followed. However, when
following the steps of Example 50, the sugar 2-(S)-
benzyloxymethyl-4-carboxyl-1,3-dioxolane is replaced with
2-(R)-benzyloxymethyl-4-carboxyl-1,3-dioxolane.
-63-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 57: Preparation of 9-(2-(S) hydroxymethyl-1,3-
dioxolan-4-yl)-6-oxo-2-amino purine (Compound 57)
O
HN N
I '>
H2N ENO N
/,,,,.,
HO
O
Compound 57
The procedure of Example 50 was performed. Thereafter,
about 6 g of Compound 50 was dissolved in a mixture of
200 mL of methanol, 50 mL of water and 10 g of NaoH. The
solution was heated under reflux for 5 hours after which
time it was diluted with 300 mL of water and excess
pyridinium sulfonate resin. The slurry was filtered, the
resin washed with water and the combined aqueous
filtrates were evaporated to dryness in vacuo to leave a
residue which was taken up in 50% aqueous methanol. The
solution was treated with activated charcoal, filtered
and the filtrate evaporated to dryness in vacuo to give a
solic residue that was recrystallized from ethanol water
to yield pure compound 57 having a (3:a ratio of about
2:1.
Alternatively, if the desired final product is the same
compound but with opposite stereochemistry (i.e. a 2:1
mixture of (3:a stereoisomers in the D-configuration).
The procedure discussed above is followed. However, when
following the steps of Example 50, the sugar 2-(S)-
benzyloxymethyl-4-carboxyl-1,3-dioxolane is replaced with
2-(R)-benzyloxymethyl-4-carboxyl-1,3-dioxolane.
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 58: Preparation of 9-(2-(S) hydroxymethyl-1,3-
dioxolan-4-yl)-2-oxo-4-amino-5-methyl pyrimidine
(Compound 58).
NH2
N ~ CH3
O N
O
/,,,,..
HO
O
Compound 58
The procedure of Example 50 was performed followed by the
procedure of Example 52. However, when following the
steps of Example 50, the 1 equivalent of the silated 2-
amino-6-chloropurine is replaced with 1 equivalent of
silated 2-oxo-4-amino-5-methyl-pyrimidine. The result is
a yield of 9-(2-(S)-hydroxymethyl-1,3-dioxolan-4-yl)-2-
oxo-4-amino-5-methyl pyrimidine having a ~i:a ratio of
about 2:1.
Alternatively, if the desired final product is the same
compound but with opposite stereochemistry (i.e. a 2:1
mixture of ~3:a, stereoisomers in the D-configuration).
The procedure discussed above is followed. However, when
following the steps of Example 50, the sugar 2-(S)-
benzyloxymethyl-4-carboxyl-1,3-dioxolane is replaced with
2-(R)-benzyloxymethyl-4-carboxyl-1,3-dioxolane.
-65-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 59: Preparation of 9-(2-(S) hydroxymethyl 1 3
dioxolan-4-yl)-2-oxo-4-amino-5-fluoro pyrimidine
(Compound 59).
NH2
N~ F
00 N
/,,,,..
HO
O
Compound 59
The procedure of Example 50 was performed followed by the
procedure of Example 52. However, when following the
steps of Example 50, the 1 equivalent of the silated 2-
amino-6-chloropurine is replaced with 1 equivalent of
silated 2-oxo-4-amino-5-fluoro-pyrimidine. The result is
a yield of 9-(2-(S) hydroxymethyl-1,3-dioxolan-4-yl)-2-
oxo-4-amino-5-fluoro pyrimidine having a (3:a ratio of
about 2:1.
Alternatively, if the desired final product is the same
compound but with opposite stereochemistry (i.e. a 2:1
mixture of /3:a stereoisomers in the D-configuration).
The procedure discussed above is followed. However, when
following the steps of Example 50, the sugar 2-(S)-
benzyloxymethyl-4-carboxyl-1,3-dioxolane is replaced with
2-(R)-benzyloxymethyl-4-carboxyl-1,3-dioxolane.
-66-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 60: Preparation of 9-(2-(S) hvdroxymethvl-1,3
dioxolan-4-yl)-2,4-dioxo pyrimidine (Compound 60)
O
HN
O' _N
/,,,,,, /O
H CO
O
Compound 60
The procedure of Example 50 was performed followed by the
procedure of Example 52. However, when following the
steps of Example 50, the 1 equivalent of the silated 2-
amino-6-chloropurine is replaced with 1 equivalent of
silated 2,4-dioxo pyrimidine. The result is a yield of
9-(2-(S)-hydroxymethyl-1,3-dioxolan-4-yl)-2,4-dioxo
pyrimidine having a ~i:a ratio of about 2:1.
Alternatively, if the desired final product is the same
compound but with opposite stereochemistry (i.e. a 2:1
mixture of ~3:a stereoisomers in the D-configuration. The
above formula is followed. However, when following the
steps of Example 50, the sugar 2-(S)-benzyloxymethyl-4-
carboxyl-1,3-dioxolane is replaced with 2-(R)-
benzyloxymethyl-4-carboxyl-1,3-dioxolane.
-67-
SUBSTITUTE SHEET (RULE 26)
CA 02362570 2001-08-09
WO 00/47759 PCT/CA00/00144
Example 61: Preparation of 9-(2-(S) hydroxymethyl 1,3
dioxolan-4-yl)-2,4-dioxo-5-methyl pyrimidine (Compound
61 .
O
CH3
HN
O "N
O
/,,,,..
HO
O
Compound 61
The procedure of Example 50 was performed followed by the
procedure of Example 52. However, when following the
steps of Example 50, the 1 equivalent of the silated 2-
amino-6-chloropurine is replaced with 1 equivalent of
silated 2,4-dioxo-5-methyl pyrimidine. The result is a
yield of 9-(2-(S) hydroxymethyl-1,3-dioxolan-4-yl)-2,4-
dioxo-5-methyl pyrimidine having a ~3:a ratio of about
2:1.
Alternatively, if the desired final product is the same
compound but with opposite stereochemistry (i.e. a 2:1
mixture of ~3:a stereoisomers in the D-configuration).
The procedure discussed above is followed. However, when
following the steps of Example 50, the sugar 2-(S)-
benzyloxymethyl-4-carboxyl-1,3-dioxolane is replaced with
2-(R)-benzyloxymethyl-4-carboxyl-1,3-dioxolane.
Some modifications and variations of the present
invention including but not limited to selection of
enzymes with high degree of sequence homology and
optimization of reaction conditions will be obvious to a
-68-
SUBSTITUTE SHEET (RULE Z6)
CA 02362570 2001-08-09
WO 00!47759 PCT/CA00/00144
person of ordinary skill in the art from the foregoing
detailed description of the invention. Such
modifications and variations are intended to fall within
the scope of one or more embodiments of the present
invention as defined by the following claims.
-69-
SUBSTITUTE SHEET (RULE 26)