Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02364356 2001-12-03
f
L-GVO~/
' - 1 -
PROCESS AND APPARATUS FOR HIGH PRESSURE
GAS QUENCHING IN AN ATMOSPHERIC FURNACE
Field of the Invention
The present invention is directed to a process and
apparatus for recycling and purifying a quenching gas,
such as helium gas, in the presence of a treating gas,
such as carburizing gas, for use with an atmospheric
furnace for treating components.
Background of the Invention
Conventionally, the hardening or treating of
components, such as steel components, generally
requires a heat treatment followed by a rapidly
quenching treatment using a fluid such as oil. The
process using oil can cause safety and environmental
concerns. Exposing oil to a temperature of 900°C could
cause the oil to volatilize and/or oxidize. The
oxidized oil represents a degradation of the oil that
must be filtered out of the quenching bath or removed
by changing the oil. In either case, the oxidized oil
and oil changes represent a waste stream that should be
disposed of or partly recycled. Generally, oil remains
on the treated components removed from the oil quench
bath. Oil tends to drip off the components as they are
handled and moved to the cleaning area. Fires,
slips/falls and other hazards can occur as a result of
using an oil quench process. Spent quenching-oil
coated components may require an additional cleaning
step before they are shipped or machined.
Additionally, quenching with oil may cause the
components to distort significantly. To solve the
CA 02364356 2001-12-03
L-GVOV/
- 2 -
problems posed by the use of oil as a quenching medium,
gas, such as helium, has been used to cool components
after they had been heated in a furnace.
United States Patent No. 5,158,625 discloses a
process for heat treating articles by hardening them in
a recirculating gas medium which is in contact with the
treated articles. The hardening gas is cooled by means
of a heat exchanger, of the type in which helium is
used as hardening gas, and is stored under holding
pressure in a buffer container. At the end of a
hardening operation, a helium load is extracted from
the treatment enclosure, in final phase by means of a
pump until a primary vacuum is obtained. The extracted
helium is brought to purifying pressure by means of a
compressor associated to a mechanical filter, and the
helium under pressure is sent to a purifier in which
impurities are removed, after which it is transferred,
if desired, after recompression in the buffer
container.
United States Patent No. 5,938,866 discloses an
apparatus for the treatment of components by means of a
gas mixture, comprising mainly a first light gas and
minor amounts of a second gas being heavier than the
first gas. The apparatus has a treatment chamber,
where the treatment occurs and a concentration, and
purification device in which the gas mixture is
concentrated and purified to increase the concentration
of the first gas. The treatment chamber comprises an
outlet member provided in an upper part of the
treatment chamber and means being arranged to move the
gas mixture upwardly and out through the outlet member.
CA 02364356 2001-12-03
L-GVOn/
- 3 -
United States Patent No. 4,867,808 discloses a
process for heat treatment of metallic workpieces by
heating in a vacuum furnace followed by quenching in a
coolant gas under above-atmospheric pressure and with
coolant-gas circulation.
United States Patent No. 5,173,524 discloses a
rapid gas quenching process wherein an increased
cooling rate of an article heated to an elevated
temperature is achieved by flowing an inert gas mixture
of helium and another inert gas over the article under
conditions of turbulent flow.
It is an object of the present invention to
provide a process for recycling a quenching gas in an
atmospheric heat-treating furnace system.
It is another object of the present invention to
provide a process for recycling a quenching gas, such
as helium, in an atmospheric carburizing furnace
system.
It is an object of the present invention to
provide an apparatus for the gas treatment of
components in an atmospheric furnace and having means
for the quenching of the components from the
atmospheric furnace using a recycling quenching gas.
It is another object of the present invention to
provide a process and apparatus that effectively
provides means for recycling a quenching gas, such as
helium, in an atmospheric carburizati~n fmrna~a in
which the quenching gas is maintained at a consistent
quenching atmosphere as required for the component to
be processed.
CA 02364356 2001-12-03
U-LU~bI
- 4 -
Summarv of the Invention
The invention relates to a process for heat
treating components in an atmospheric heat-treating
furnace comprising the steps of:
(a) treating a component in an atmospheric
furnace with a treating gas, such as an endothermic
gas, heated to a desired temperature required to treat
the component;
(b) feeding the heat treated component containing
the treating gas into a quenching chamber;
(c) feeding a quenching gas into the quenching
chamber to contact the treated component and mix with
the treating gas;
(d) feeding the quenching gas and treating gas
mixture of step (c) into a gas recovery chamber where
the treating gas and quenching gas are separated to
provide a purified quenching gas and treating gas;
(e) feeding the purified quenching gas of step
(d) back into the quenching chamber thereby effectively
recycling the quenching gas back to the quenching
chamber; and
(f) removing the cooled treated component from
the gas quenching chamber.
If the furnace is approved for quenching pressure,
then the quenching chamber could be eliminated and
therefore reference to the quenching chamber in steps
(b), (c), (e) and (f) shall mean furnace as recited in
step (a) of the novel process of this invention.
The process of this invention is suitable for
treatment of components manufactured from carbon, alloy
and tool steels. Of particular importance are the
carburizing grades of steel such as AISI grades 5120,
CA 02364356 2001-12-03
- 5 -
8115, 8620 and 9310. A primary use of the novel
process of this invention is for use in atmospheric
carburizing furnaces in which the treating gas can be
at least one gas selected from the group comprising
methane, methane, carbon monoxide, nitrogen, propane
and butane. A common treating gas for carburizing is
endothermic gas which consists of about 20o carbon
monoxide, 40% hydrogen and 40o nitrogen. The treating
gas could be heated to about 750°C and about 1200°C,
preferably about 800°C and about 1000°C. For
carburization treatment of components, the carburizing
gas would be heated between about 850°C and about
1100°C, and preferably about 900°C and about 950°C. The
quenching gas could be at least one gas selected from
the group consisting of helium, preferably as the major
component (>500) and from the group consisting of
nitrogen, argon and carbon monoxide as the minor
component. The preferred quenching gas would be
helium. The quenching gas should be pressurized at
least to 37 psia and preferably between about 74 psia
and about 890 psia, and more preferably between about
147 psia and about 368 psia. The quenched treated
component is generally removed from the quenching
chamber at atmospheric pressure and slightly above
ambient temperature.
The subject invention also relates to an apparatus
for the treatment of components by a gas in an
atmospheric furnace comprising an atmospheric furnace
adapted for receiving treating gas and a component to
be gas treated, the atmospheric furnace coupled to a
quenching chamber which is adapted for receiving the
CA 02364356 2001-12-03
D-20867
- 6 -
treated component from the atmospheric furnace and a
quenching gas; the quenching chamber coupled to a gas
recovery device adapted for receiving spent treating
gas and quenching gas and having means for separating
the gases to provide a purified quenching gas; the gas
recovery device coupled to the quenching chamber and
adapted for transmitting the purified gas into the
quenching chamber; and the apparatus operable such that
quenching gas can be recycled between the quenching
chamber and the recovery device.
Brief Description of the Drawings
Figure 1 is a schematic of a gas quenching system.
Figure 2 is a schematic of a helium/endothermic
gas quenching system of the present invention.
Figure 3 is a schematic of another embodiment of a
helium gas quenching system of the present invention.
Detailed Description of the Invention
Figure 1 shows an equipment orientation that will
allow helium quenching for an atmospheric carburizing
furnace using an endothermic gas or a vacuum
carburizing furnace using a gas such as propane or
methane. At the end of the carburizing step, furnace 1
is opened and the components and furnace atmosphere
enter, via duct 2, heated vacuum chamber 3. Heated
vacuum chamber 3 is sealed from furnace 1 and helium
quenching chamber 5. Upon closure of heated vacuum
chamber 3, the atmosphere is removed via vacuum pump 9.
During the entire process, heated vacuum chamber 3
remains at the furnace temperature so that the
components do not start to cool. Following the removal
CA 02364356 2001-12-03
L-GVOO/
- 7 -
of atmosphere from heated vacuum chamber 3 the chamber
may or may not be back filled with helium at a
pressure, for example, about 14.7 psia. The components
will move to helium quenching chamber 5 when the
chamber has met the following conditions. Chamber 5 is
empty of the previous load of components, the chamber
has been sealed from the outside atmosphere, and the
outside atmosphere has been removed from chamber 5 via
vacuum pump 9. Once the seal between chambers 3 and 5
is broken the components will move to chamber 5 and the
seal established once again between chambers 3 and 5.
Chamber 5 will then receive helium at the quenching
pressure (e. g. 290 psia). Following the quenching
operation the helium is removed from chamber 5 via duct
10 to helium recovery system 11 and then the components
are moved to the next step in the process, for example,
machining. The spent helium is purified to a desired
level in helium recovery system 11 and the purified
helium is returned to chamber 5 via duct 12.
Atmospheric carburizing processes that quench with
helium find the use of helium very expensive if they do
not recycle the helium or require additional capital
cost for the furnace equipment. The subject invention
recycles helium with carburizing gases present and
maintains a consistent quenching atmosphere as needed
for the components being processed. Figure 2 shows one
embodiment of a novel process of the subject invention.
Several components disclosed in Figure 1 have the same
numerical indicators as components in Figure 2.
Carburized components plus the furnace atmosphere are
moved directly to quenching chamber 3 and then sealed
from furnace 1. Quenching chamber 3 is then
CA 02364356 2001-12-03
U-GUtiE~ /
_ g _
pressurized with quenching gas from the quenching gas
recovery system and the components are quenched. The
quenching gas plus furnace atmosphere is then removed
from quenching chamber 3 via quenching gas recovery
system 7. The quenched components are then moved on to
the next step in the process (e.g. machining 5).
Figure 2 shows the difference between the
embodiment described by Figure 1 above and the subject
invention. Subject invention results in reduced
equipment cost and process complexity. Both require
the use of a quenching chamber. However, previous
recycle systems were not feasible to remove the
carburizing gases.
Table 1 shows a typical carburization gas
composition that would enter the quenching chamber when
an evacuation of the chamber is not performed. In
addition to water a significant amount of carbon
dioxide, carbon monoxide, methane, hydrogen and
nitrogen enter into the system. With the addition of
the quenching gas to 20 bar, the carburization gas will
represent 50 of the total gas in the quenching chamber.
TABLE 1
0 of Mass in Quenching
Compound Carburization Gas Chamber (lbs)
COZ 1 0 . 0 8
CH4 1 0 . 0 3
H20 2 0 . 07
CO 19 0.98
Hz 38 0.28
Nz 39 2 . 02
Purifying the spent quenching gas would not be
possible without oxygen since oxygen must be added to
CA 02364356 2001-12-03
L-GVDV/
g _
remove hydrogen. The subject invention could use a
catalyst followed by a molecular sieve to purify the
entire quenching gas stream and return pure helium to
the quenching chamber.
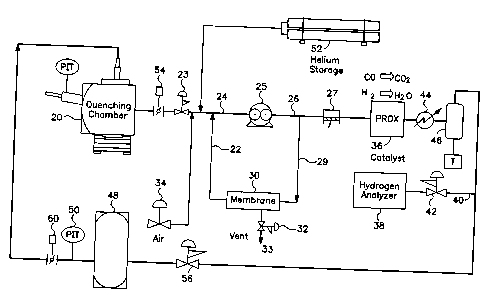
Figure 3 shows a gas recovery system in which
quenching gas flows from quenching chamber 20 via duct
24 to the suction side of oil flooded screw compressor
25. The pressure of the suction side of oil flooded
screw compressor 25 is controlled to a maximum by
pressure regulator 23. Oil flooded screw compressor 25
will discharge the quenching gas at 150 psig or higher.
The discharge of oil flooded screw compressor 25 will
pass through oil removal equipment (not shown) in duct
26 and then through the suction side of diaphragm
compressor 27. The discharge of compressor 27 is at a
higher pressure such as 575 psig (~40 bar absolute)
Between the oil removal equipment and compressor
27, approximately 600 of the total flow through
diaphragm compressor 27 of the quenching gas will take
side branch 29 and pass through membrane 30. The
membrane will discard methane, carbon monoxide, carbon
dioxide and nitrogen through valve 32. The membrane
permeate will return to the suction side of oil flooded
screw compressor 25 via duct 22 and duct 24. The
stream compositions for the feed, permeate and
raffinate are given below in Table 2. The membrane
feed as shown in Table 2 represents the steady state
composition of the gas in the quenching chamber (i.e.
quenching gas plus endo gas). The permeate at
approximately 94o pure helium will mix with the
unpurified gas and pass through the catalyst and water
removal. The composition of gas in the receiver before
CA 02364356 2001-12-03
U-GVCSt~ /
- 10 -
equalization with the quenching chamber is
approximately 95o pure helium. Upon equalization with
the quenching chamber, the endo gases as shown in Table
1, lowers the helium purity to approximately 900.
Oxygen was not shown in the simulation below but would
be present because air inlet valve 34 feeds the suction
of compressor 25. Oxygen is completely consumed in the
conversion of hydrogen to water and carbon monoxide to
carbon dioxide. The presence of oxygen in the membrane
is expected to have an insignificant impact on the
helium recovery and final steady state gas composition.
TABLE 2
CALCULATED PROCESS PARAMETERS
For Membrane 19
FEED RAFT PERM
F,MMSCFD (60F) 1 0.0504 0.9496
PRESS, psia 150.00 150.00 6.00
TEMP, F 108.00 108.00 108.00
Molec. Weight 6.35 27.06 5.26
Viscos, cp 0.0205 0.0182 0.0204
CONCENTRATIONS, o1%
M
HELIUM 89.8000 10.0000 94.0354
NITROGEN 3.8000 61.3748 0.7442
HYDROGEN 2.1000 0.4062 2.1899
CARBON MONOXIDE 1.0000 15.6039 0.2249
WATER 0.2000 0.0008 0.2106
CARBON DIOXIDE 3.0000 10.9495 2.5781
METHANE 0.1000 1.6648 0.0169
Percent recovery of helium in stream No. 22 = 99.44
Percent recovery of nitrogen in stream No. 33 = 81.40
Percent recovery of hydrogen in stream No. 33 = 0.97
Percent recovery of carbon monoxide in stream No. 33 =
78.64
Percent recovery of water in stream No. 33 = 0.02
CA 02364356 2001-12-03
V-GVOV/
- 11 -
Percent recovery of carbon dioxide in stream No. 33 =
18.40
Percent recovery of methane in stream No. 33 = 83.91
The hot gas from diaphragm compressor 27 passes
through catalyst bed 36 to convert some of the hydrogen
to water and carbon monoxide to carbon dioxide. Oxygen
is provided for the reaction by air inlet valve 34 at
the suction side of oil flooded screw compressor 25.
Valve 34 allows the air to enter the quenching gas
recovery system and is controlled by a signal from
hydrogen analyzer 38. When the level of hydrogen is
over a predetermined set point, hydrogen analyzer 38
will send a signal to valve 34 to let in air. Analyzer
38 maintains an excess of hydrogen in the system. The
combination of catalyst and excess hydrogen will cause
the removal of oxygen to the PPM level such as <10 PPM.
The hydrogen analyzer is located in duct 40 after valve
42. Following catalyst 36, the gas stream is cooled in
heat exchanger 44 and passed through separator 46 to
remove entrained water. The entrained water passes to
a trap and is discharged from the system. The trap may
operate by a float or a timer (T). The trap seals the
quenching gas recovery system from outside air and does
not allow quenching gas to escape from the quenching
gas recovery system. The quenching gas will fill
quenching gas ballast tank 48 from valve 56 until the
pressure reaches, for example, 590 psig as measured by
PIT 50. Not all of the gas in the quenching chamber is
removed to the quenching gas recovery system and some
quenching gas is lost during purification with membrane
30. Replacing the~lost quenching gas with helium is
done at the suction side of oil flooded screw
CA 02364356 2001-12-03
U-LUtSb l
- 12 -
compressor 25. When the suction pressure of oil
flooded screw compressor 25 falls below a predetermined
set point, then make up helium will flow from helium
storage 52 through a control valve (not shown).
Once quenching gas ballast tank 48 reaches a
predetermined set point pressure, then the quenching
gas recovery system has finished and shuts down. When
the quenching gas recovery system shuts down, butterfly
valve 54 closes. Air/nitrogen or other gas back fills
the quenching chamber and the components are removed.
The empty chamber is closed and purged with nitrogen or
other gas. A new load of hot components is then placed
in quenching chamber 20 and quenching gas ballast tank
48 is equalized with quenching chamber 20 through
butterfly valve 60. The next cycle begins.
For the preferred embodiment of the subject
invention, quenching gas pressure requirements of
approximately 10 bar or less would use only one
compressor. For a helium purity of approximately 90%
and carbon monoxide conversion, the compressor could
circulate 60% of the recovered gas in the quenching
chamber through the compressor and through the
membrane. Therefore, the compressor could remove 875
CF of quenching gas from the quenching chamber. From
the discharge of the compressor, 525 CF could pass
through the membrane back to the suction side of the
compressor. For a cycle time of 15 minutes, the
compressor would move 1400 CF or 5600 SCFH. Thus,
compressor 27 is significantly smaller at 3500 SCFH. A
smaller compressor 27 saves on capital cost and
operating cost over the prior art. A water separator
could be used to remove entrained water (Figure 3 #46).
CA 02364356 2001-12-03
U-GVOt~!
- 13 -
Heater exchanger 44 could be augmented with a chiller
for lower volumes of water in the quenching gas. The
amount of water in the quenching gas should remain
constant as a saturated gas, at the temperature and
pressure of the stream, entering ballast tank 48.
Modifications can be made to the quenching gas
recovery system of the subject invention as follows:
1. Quenching chamber 20 would not be required if
the furnace chamber is approved for quenching
pressures. The quenching gas recovery system would
remain the same.
2. A separate vacuum pump could be used in a
side process connected to duct 24 before valve 23 to
evacuate quenching chamber 20 so that a greater
percentage of quenching gas is recovered. The vacuum
pump would be turned on after the quenching chamber
reached atmospheric pressure.
3. The oil flooded screw and diaphragm
compressors could be replaced with other style
compressors and/or combined into one compressor.
4. The purification side stream that flows
through the membrane could take place anywhere after
the discharge of compressor 27.
5. Purification of the side stream could replace
membrane 30 with molecular sieve or a purge.
6. Purification of the side stream could use
molecular sieve or another membrane on the raffinate
stream of membrane 30 to increase helium recovery.
Also, the raffinate could be placed in a separate
receiver and serve as purge gas for the quenching
chamber.
CA 02364356 2001-12-03
L - G V U V
- 14 -
7. Molecular sieve or another membrane could be
added to the permeate of membrane 30 for additional
purification.
8. Valve 34 could inlet pure oxygen instead of
air.
9. Heat exchanger 44 could be augmented with a
chiller to further reduce the amount of water in the
quenching gas.
10. The system could run continuously if a line
and valve were placed between the quenching gas ballast
tank 48 and the suction side of oil flooded screw
compressor 25, thus, allowing the system to run
continuously would increase the helium content of the
quenching gas or a smaller compressor 25 to get the
same helium content in the quenching gas.
11. More than one quenching chamber can be used
in one quenching gas recovery system. Equipment can be
sized based on the number of quenching chambers and the
controls are adjusted so that the quenching gas in each
quenching chamber can reach the desired gas composition
and pressure.
12. Minimizing the amount of oxygen present in
quenching chamber 20 during the quenching step would
require a purge gas through the quenching chamber
before the introduction of hot components. The purge
gas could be nitrogen, argon, helium or used
endothermic gas from the carburizing process.
13. To accomplish an oxygen free quenching
chamber, a separate chamber could be added that
receives the components from the quenching chamber.
The additional chamber could have a purge of nitrogen,
argon, or helium.
CA 02364356 2001-12-03
U-GV~SbI
- 15 -
14. Ballast tank 48 could provide purge gas to
quenching chamber 20. The quenching gas recovery
system would be set up to run continuously. However,
after the components are removed from quenching chamber
20, valves 60 and 54 would open and allow gas to purge
quenching chamber 20 for a period of time. At the end
of the purge, valve 56 would close first and then valve
22 would close, leaving the chamber at near atmospheric
pressure. Then the next cycle would start with the
addition of hot components to quenching chamber 20.
15. The flow of gas through duct 29 could be
reduced resulting in lower helium purity as the
quenching gas. Helium purity of 400 or greater may be
used depending on the desired cooling curves in the
quenching chamber.
16. Oxygen or air can be introduced to the
quenching gas recovery system after compressor 27.
Introduction of additional gas after the compressor
would reduce the flow through valve 34 since the
membrane would discard none of the oxygen. This option
would have the most value when pure oxygen was being
used to oxidize hydrogen and carbon dioxide.
17. Catalyst temperature and type can be adjusted
to minimize or practically eliminate the conversion of
carbon monoxide to carbon dioxide. To maintain a
helium purity of 90o requires only 400 of the stream
through duct 29 when carbon monoxide is not oxidized.
A 40% flow in duct 29 represents a 33% decrease over
the preferred method as described above. Table 3 shows
the feed, raffinate and permeate compositions when
carbon monoxide is not converted to carbon dioxide.
The membrane is approximately four times as efficient
CA 02364356 2001-12-03
L - G V V V /
- 16 -
at discharging carbon monoxide as it is carbon dioxide.
Another advantage is that less oxygen consumption is
required for quenching gas recovery system oxidation.
This option would be the preferred method if reduction
in the quenching chamber of carbon dioxide to carbon
monoxide is possible and undesirable.
TABLE 3
CALCULATED PROCESS PARAMETERS
For Membrane 30 Without CO Conversion
FEED RAFT PERM
F,MMSCFD (60F) 1 0.07797 0.922
PRESS, psia 150.00 150.00 ~ 6.00
TEMP, F 108.00 108.00 108.00
Molec. weight 6.18 25.55 4.54
Viscos, cp 0.0206 0.0185 0.0203
CONCENTRATIONS, olo
M
HELIUM 88.8000 10.0000 95.4633
NITROGEN 5.6000 58.9144 1.0917
HYDROGEN 2.1000 0.4003 2.2437
CARBON MONOXIDE 2.8000 28.5080 0.6261
WATER 0.2000 0.0007 0.2169
CARBON DIOXIDE 0.4000 1.0937 0.3413
METHANE 0.1000 1.0829 0.0169
Percent recovery of helium in stream No. 22 = 99.12
Percent recovery of nitrogen in stream No. 33 = 82.02
Percent recovery of hydrogen in stream No. 33 = 1.49
Percent recovery of carbon monoxide in stream No. 33 =
79.38
Percent recovery of water in stream No. 33 = 0.03
Percent recovery of carbon dioxide in stream No. 33 =
31.32
Percent recovery of methane in stream No. 33 = 84.43
The invention is not limited to the embodiment
shown and it will be appreciated that it is intended to
CA 02364356 2001-12-03
U-LUtib
- 17 -
cover all modifications and equipment within the scope
of the appended claims.