Une partie des informations de ce site Web a été fournie par des sources externes. Le gouvernement du Canada n'assume aucune responsabilité concernant la précision, l'actualité ou la fiabilité des informations fournies par les sources externes. Les utilisateurs qui désirent employer cette information devraient consulter directement la source des informations. Le contenu fourni par les sources externes n'est pas assujetti aux exigences sur les langues officielles, la protection des renseignements personnels et l'accessibilité.
L'apparition de différences dans le texte et l'image des Revendications et de l'Abrégé dépend du moment auquel le document est publié. Les textes des Revendications et de l'Abrégé sont affichés :
| (12) Brevet: | (11) CA 2366095 |
|---|---|
| (54) Titre français: | ELIMINATION DU CHROME DE LIXIVIATS PRODUITS LORS DE LA LIXIVIATION ACIDE A HAUTE PRESSION DE MINERAIS LATERITIQUES |
| (54) Titre anglais: | CHROMIUM REMOVAL FROM LEACH LIQUORS PRODUCED DURING HIGH PRESSURE ACID LEACHING OF LATERITIC ORES |
| Statut: | Durée expirée - au-delà du délai suivant l'octroi |
| (51) Classification internationale des brevets (CIB): |
|
|---|---|
| (72) Inventeurs : |
|
| (73) Titulaires : |
|
| (71) Demandeurs : |
|
| (74) Agent: | LAVERY, DE BILLY, LLP |
| (74) Co-agent: | |
| (45) Délivré: | 2009-07-21 |
| (22) Date de dépôt: | 2001-12-21 |
| (41) Mise à la disponibilité du public: | 2003-06-21 |
| Requête d'examen: | 2003-11-10 |
| Licence disponible: | S.O. |
| Cédé au domaine public: | S.O. |
| (25) Langue des documents déposés: | Anglais |
| Traité de coopération en matière de brevets (PCT): | Non |
|---|
| (30) Données de priorité de la demande: | S.O. |
|---|
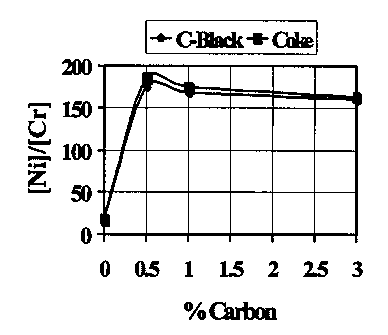
On présente une méthode permettant d'éliminer le chrome produit lors de la lixiviation sous haute pression des minerais de latérite dans un autoclave, cela grâce à l'ajout, dans l'autoclave, de carbone en quantité d'environ 0,5 à 1 % en poids par rapport à la matière première totale à l'état sec.
A method is provided for the removal of chromium from leach liquors produced during high pressure leaching of laterite ores in an autoclave, by addition to the autoclave of carbon in a quantity of about 0.5% - 1% by weight with reference to the total dry feed.
Note : Les revendications sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2024-08-01 : Dans le cadre de la transition vers les Brevets de nouvelle génération (BNG), la base de données sur les brevets canadiens (BDBC) contient désormais un Historique d'événement plus détaillé, qui reproduit le Journal des événements de notre nouvelle solution interne.
Veuillez noter que les événements débutant par « Inactive : » se réfèrent à des événements qui ne sont plus utilisés dans notre nouvelle solution interne.
Pour une meilleure compréhension de l'état de la demande ou brevet qui figure sur cette page, la rubrique Mise en garde , et les descriptions de Brevet , Historique d'événement , Taxes périodiques et Historique des paiements devraient être consultées.
| Description | Date |
|---|---|
| Inactive : Périmé (brevet - nouvelle loi) | 2021-12-21 |
| Représentant commun nommé | 2019-10-30 |
| Représentant commun nommé | 2019-10-30 |
| Demande visant la révocation de la nomination d'un agent | 2018-09-14 |
| Demande visant la nomination d'un agent | 2018-09-14 |
| Inactive : Regroupement d'agents | 2018-09-01 |
| Inactive : Regroupement d'agents | 2018-08-30 |
| Lettre envoyée | 2015-06-30 |
| Lettre envoyée | 2015-06-30 |
| Lettre envoyée | 2015-06-30 |
| Lettre envoyée | 2015-06-30 |
| Accordé par délivrance | 2009-07-21 |
| Inactive : Page couverture publiée | 2009-07-20 |
| Préoctroi | 2009-05-01 |
| Inactive : Taxe finale reçue | 2009-05-01 |
| Un avis d'acceptation est envoyé | 2009-04-02 |
| Lettre envoyée | 2009-04-02 |
| Un avis d'acceptation est envoyé | 2009-04-02 |
| Inactive : CIB enlevée | 2009-03-30 |
| Inactive : CIB en 1re position | 2009-03-30 |
| Inactive : CIB enlevée | 2009-03-30 |
| Inactive : CIB enlevée | 2009-03-30 |
| Inactive : CIB enlevée | 2009-03-30 |
| Inactive : CIB enlevée | 2009-03-30 |
| Inactive : CIB enlevée | 2009-03-30 |
| Inactive : CIB attribuée | 2009-03-30 |
| Inactive : CIB enlevée | 2009-03-30 |
| Inactive : CIB enlevée | 2009-03-30 |
| Inactive : CIB enlevée | 2009-03-30 |
| Inactive : Approuvée aux fins d'acceptation (AFA) | 2008-12-03 |
| Modification reçue - modification volontaire | 2008-04-16 |
| Modification reçue - modification volontaire | 2007-12-11 |
| Inactive : Dem. de l'examinateur art.29 Règles | 2007-06-15 |
| Modification reçue - modification volontaire | 2006-04-21 |
| Inactive : CIB de MCD | 2006-03-12 |
| Inactive : CIB de MCD | 2006-03-12 |
| Inactive : CIB de MCD | 2006-03-12 |
| Inactive : CIB de MCD | 2006-03-12 |
| Inactive : CIB de MCD | 2006-03-12 |
| Inactive : CIB de MCD | 2006-03-12 |
| Inactive : CIB de MCD | 2006-03-12 |
| Inactive : CIB de MCD | 2006-03-12 |
| Lettre envoyée | 2004-02-26 |
| Exigences relatives à la révocation de la nomination d'un agent - jugée conforme | 2004-02-12 |
| Inactive : Lettre officielle | 2004-02-12 |
| Inactive : Lettre officielle | 2004-02-12 |
| Exigences relatives à la nomination d'un agent - jugée conforme | 2004-02-12 |
| Demande visant la nomination d'un agent | 2004-02-04 |
| Exigences de rétablissement - réputé conforme pour tous les motifs d'abandon | 2004-02-04 |
| Demande visant la révocation de la nomination d'un agent | 2004-02-04 |
| Inactive : Lettre officielle | 2004-01-16 |
| Réputée abandonnée - omission de répondre à un avis sur les taxes pour le maintien en état | 2003-12-22 |
| Lettre envoyée | 2003-12-19 |
| Toutes les exigences pour l'examen - jugée conforme | 2003-11-10 |
| Exigences pour une requête d'examen - jugée conforme | 2003-11-10 |
| Requête d'examen reçue | 2003-11-10 |
| Demande publiée (accessible au public) | 2003-06-21 |
| Inactive : Page couverture publiée | 2003-06-20 |
| Inactive : CIB attribuée | 2002-03-20 |
| Inactive : CIB attribuée | 2002-03-20 |
| Inactive : CIB en 1re position | 2002-03-20 |
| Inactive : Certificat de dépôt - Sans RE (Anglais) | 2002-01-29 |
| Exigences de dépôt - jugé conforme | 2002-01-29 |
| Lettre envoyée | 2002-01-29 |
| Demande reçue - nationale ordinaire | 2002-01-29 |
| Date d'abandonnement | Raison | Date de rétablissement |
|---|---|---|
| 2003-12-22 |
Le dernier paiement a été reçu le 2008-12-01
Avis : Si le paiement en totalité n'a pas été reçu au plus tard à la date indiquée, une taxe supplémentaire peut être imposée, soit une des taxes suivantes :
Veuillez vous référer à la page web des taxes sur les brevets de l'OPIC pour voir tous les montants actuels des taxes.
Les titulaires actuels et antérieures au dossier sont affichés en ordre alphabétique.
| Titulaires actuels au dossier |
|---|
| GLENCORE CANADA CORPORATION |
| Titulaires antérieures au dossier |
|---|
| CARLTON A. POTTER |
| DEBBIE MARSHALL |
| MARC BOISSONEAULT |
| MAXINE HOFFMAN |
| MOHAMED BUARZAIGA |