Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02367268 2001-09-18
WO 00/56317 PCT/US00/07202
Weight Loss after Pregnancy
This invention relates to a method of aiding weight loss after pregnancy.
According to the present invention there is provided a method of aiding in
weight loss after pregnancy, in which a therapeutically effective amount of a
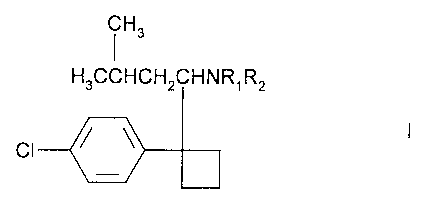
compound of formula I
Hs
H3CCHCHZCHNR~Rz
CI
including enantiomers and pharmaceutically acceptable salts thereof, in which
R,
and RZ are independently H or methyl, is administered in conjunction with a
pharmaceutically acceptable diluent or carrier to a human in need thereof.
A preferred compound of formula I is N,N-dimethyl-1-[1-(4-
chlorophenyl)cyclobutyl]-3-methylbutylamine or a salt thereof, for example the
hydrochloride salt. A preferred form of this hydrochloride is its monohydrate.
The preparation and use of compounds of formula I, such as N,N-
dimethyl-1-[1-(4-chlorophenyl)cyclobutylJ-3-methylbutylamine, N-{1-[1-(4-
chlorophenyl)-cyclobutyl]-3-methylbutyl}-N-methylamine, and 1-[1-(4-
chlorophenyl)-cyclobutyl]-3-methylbutylamine and salts thereof, in the
treatment
of depression is described in British Patent Specification 2098602 and US
Patent
4,522,328. The use of compounds of formula I such as N-N~dimethyl-1-[1-(4-
chlorophenyl)cyclobutyl]-3-methylbutylamine and salts thereof in the treatment
of
Parkinson's disease is described in published PCT application WO 88/06444.
CA 02367268 2001-09-18
WO 00/56317 PCT/US00/07202
The use of N,N-dimethyl-1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine
and
salts thereof in the treatment of cerebral function disorders is described in
US
Patent 4,939,175. The use of N,N-dimethyl-1-[1-(4-chlorophenyl)cyclobutyl]-3-
methylbutylamine hydrochloride in the treatment of obesity is described in
published PCT application W090/06110. A particularly preferred form of this
compound is N,N-dimethyl-1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine
hydrochloride monohydrate (sibutramine hydrochloride) which is described in
European Patent Number 230742. The use of N,N-dimethyl-1-[1-(4-
chlorophenyl)cyclobutyl]-3-methylbutylamine and salts thereof for improving
the
glucose tolerance of humans having Impaired Glucose Tolerance or Non-Insulin
Dependent Diabetes Mellitus is described in published PCT application
W095/20949.
It will be appreciated by those skilled in the art that compounds of formula
I contain a chiral centre. When a compound of formula I contains a single
chiral
centre it may exist in two enantiomeric forms. The present invention includes
the
use of the individual enantiomers and mixtures of the enantiomers. The
enantiomers may be resolved by methods known to those skilled in the art, for
example by formation of diastereoisomeric salts or complexes which may be
separated, for example, by crystallisation; via formation of diastereoisomeric
derivatives which may be separated, for example, by crystallisation, gas-
liquid or
liquid chromatography; selective reaction of one enantiomer with an enantiomer-
specific reagent, for example enzymatic oxidation or reduction, followed by
separation of the modified and unmodified enantiomers; or gas-liquid or liquid
chromatography in a chiral environment, for example on a chiral support, for
example silica with a bound chiral ligand or in the presence of a chiral
solvent. It
will be appreciated that where the desired enantiomer is converted into
another
chemical entity by one of the separation procedures described above, a further
step is required to liberate the desired enantiomeric form. Alternatively,
specific
enantiomers may be synthesised by asymmetric synthesis using optically active
2
CA 02367268 2001-09-18
WO 00/56317 PCT/US00/07202
reagents, substrates, catalysts or solvents, or by converting one enantiomer
to
the other by asymmetric transformation.
Preferred compounds of formula I are N,N-dimethyl-1-[1-(4-chlorophenyl)-
cyclobutyl]-3-methylbutylamine, N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-
methylbutyl}-N- methylamine, and 1-(1-(4-chlorophenyl)cyclobutyl]-3-
methylbutylamine including racemates, individual enantiomers and mixtures
thereof, and pharmaceutically acceptable salts thereof.
The individual enantiomers can be prepared by enantioselective synthesis
from optically active precursors, or by resolving the racemic compound which
can
be prepared as described above. Enantiomers of secondary amines of the
formula I can also be prepared by preparing the racemate of the corresponding
primary amine, resolving the latter into the individual enantiomers, and then
converting the optically pure primary amine enantiomer into the required
secondary amine by methods described in British Patent Specification 2098602.
Specific examples of compounds of formula I are:
(+)-N-[1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N-methylamine;
(-)-N-{1-[1-(4-chlorophenyl)cyclobutyl-3-methylbutyl}-N-methylamine;
(+)-1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine;
(-)-1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine;
(+)-N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N-N-dimethylamine;
(-)-N-{1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl}-N-N-dimethylamine.
The hydrochloride salts are preferred in each case, but the free bases
and other pharmaceutically acceptable salts are also suitable.
The compound of formula I may be administered in any of the known
pharmaceutical dosage forms. The amount of the compound to be administered
3
CA 02367268 2001-09-18
WO 00/56317 PCT/US00/07202
will depend on a number of factors including the age of the patient, the
severity of
the condition and the past medical history of the patient and always lies
within the
sound discretion of the administering physician but it is generally envisaged
that
the dosage of the compound to be administered will be in the range 0.1 to 50
mg
preferably 1 to 30 mg per day given in one or more doses.
Oral dosage forms are the preferred compositions for use in the present
invention and these are the known pharmaceutical forms for such
administration,
for example tablets, capsules, granules, syrups and aqueous or oil
suspensions.
The excipients used in the preparation of these compositions are the
excipients
known in the pharmacist's art. Tablets may be prepared from a mixture of the
active compound with fillers, for example calcium phosphate; disintegrating
agents, for example maize starch; lubricating agents, for example magnesium
stearate; binders, for example microcrystalline cellulose or
polyvinylpyrrolidone
and other optional ingredients known in the art to permit tableting the
mixture by
known methods. The tablets may, if desired, be coated using known methods
and excipients which may include enteric coating using for example
hydroxypropylmethylcellulose phthalate. The tablets may be formulated in a
manner known to those skilled in the art so as to give a sustained release of
the
compounds of the present invention. Such tablets may, if desired, be provided
with enteric coatings by known methods, for example by the use of cellulose
acetate phthalate. Similarly, capsules, for example hard or soft gelatin
capsules,
containing the active compound with or without added excipients, may be
prepared by known methods and, if desired, provided with enteric coatings in a
known manner. The contents of the capsule may be formulated using known
methods so as to give sustained release of the active compound. The tablets
and capsules may conveniently each contain 1 to 50 mg of the active compound.
Other dosage forms for oral administration include, for example, aqueous
suspensions containing the active compound in an aqueous medium in the
presence of a non-toxic suspending agent such as sodium carboxy-
4
CA 02367268 2001-09-18
WO 00/56317 PCT/US00/07202
methylcellulose, and oily suspensions containing a compound of the present
invention in a suitable vegetable oil, for example arachis oil. The active
compound may be formulated into granules with or without additional
excipients.
The granules may be ingested directly by the patient or they may be added to a
suitable liquid carrier (for example, water) before ingestion. The granules
may
contain disintegrants, eg an effervescent couple formed from an acid and a
carbonate or bicarbonate salt to facilitate dispersion in the liquid medium.
The therapeutically active compounds of formula 1 may be formulated into
a composition which the patient retains in his mouth so that the active
compound
is administered through the mucosa of the mouth.
Dosage forms suitable for rectal administration are the known
pharmaceutical forms for such administration, for example, suppositories with
cocoa butter or polyethylene glycol bases.
Dosage forms suitable for parenteral administration are the known
pharmaceutical forms for such administration, for example sterile suspensions
or
sterile solutions in a suitable solvent.
Dosage forms for topical administration may comprise a matrix in which
the pharmacologically active compounds of the present invention are dispersed
so that the compounds are held in contact with the skin in order to administer
the
compounds transdermally. A suitable transdermal composition may be prepared
by mixing the pharmaceutically active compound with a topical vehicle, such as
a
mineral oil, petrolatum and/or a wax, e.g. paraffin wax or beeswax, together
with
a potential transdermal accelerant such as dimethyl sulphoxide or propylene
glycol. Alternatively the active compounds may be dispersed in a
pharmaceutically acceptable cream, gel or ointment base. The amount of active
compound contained in a topical formulation should be such that a
therapeutically
5
CA 02367268 2001-09-18
WO 00/56317 PCT/US00/07202
effective amount of the compound is delivered during the period of time for
which
the topical formulation is intended to be on the skin.
The therapeutically active compound of formula I may be formulated into
a composition which is dispersed as an aerosol into the patients oral or nasal
cavity. Such aerosols may be administered from a pump pack or from a
pressurised pack containing a volatile propellant.
The therapeutically active compounds of formula I used in the method of
the present invention may also be administered by continuous infusion either
from an external source, for example by intravenous infusion or from a source
of
the compound placed within the body. Internal sources include implanted
reservoirs containing the compound to be infused which is continuously
released
for example by osmosis and implants which may be (a) liquid such as an oily
suspension of the compound to be infused for example in the form of a very
sparingly water-soluble derivative such as a dodecanoate salt or a lipophilic
ester
or (b) solid in the form of an implanted support, for example of a synthetic
resin or
waxy material, for the compound to be infused. The support may be a single
body containing all the compound or a series of several bodies each containing
part of the compound to be delivered. The amount of active compound present in
an internal source should be such that a therapeutically effective amount of
the
compound is delivered over a long period of time.
In some formulations it may be beneficial to use the compounds of the
present invention in the form of particles of very small size, for example as
obtained by fluid energy milling.
In the compositions of the present invention the active compound may, if
desired, be associated with other compatible pharmacologically active
ingredients.
6
CA 02367268 2001-09-18
WO 00/56317 PCT/US00/07202
The invention further provides the use of compounds of formula I in the
manufacture of a medicament for aiding in weight loss after pregnancy.
In another aspect, the invention further provides a pharmaceutical
composition for aiding in weight loss after pregnancy, comprising a compound
of
formula I in conjunction with a pharmaceutically acceptable diluent or
carrier.
Pregnancy can result in excessive weight gain and retention. A certain
amount of weight gain during pregnancy is desirable. However weight gain
beyond the desired amount is predominantly maternal adipose tissue. It is this
fat
tissue that, in large measure, accounts for the postpartum retention weight
gained during pregnancy. This retention reflects a postpartum energy balance
that does not lead to catabolism of the gained adipose tissues. Administration
of
a compound of Formula I helps to change the energy balance.
Monoamine reuptake inhibitors have been used to treat certain of the
disorders described in the present invention. However, these compounds are
known to suffer from a number of disadvantages. Firstly such compounds are
not effective in all patients. Secondly where the compounds are effective they
may not provide a complete cure of the disorder. Thirdly, there are many
undesirable side-effects known with this type of compound. Such side-effects
include nausea, sexual dysfunction, tight headedness, somnolence, sweating,
tremor, dry mouth, asthenia, insomnia, diarrhoea, headache, vomiting, anxiety,
drowsiness, dizziness, fever, rash or allergic reactions, arthralgia, myalgia,
convulsions, hypomania and mania.
Sibutramine (Formula I, R~ = CH3 , R2 = CH3) has a pharmacological
profile which is unique amongst monoamine reuptake inhibitors. Through its
pharmacologically active metabolites, (metabolite 1, R~ = H, R2 = CH3 in
Formula
I and metabolite 2, R~ = H, R2 = H in Formula I) sibutramine inhibits the
reuptake
7
CA 02367268 2001-09-18
WO 00/56317 PCT/US00/07202
of all three monoamines differentiating it from serotonin (5-HT)-selective
reuptake
inhibitors, e.g. fluoxetine, noradenaline-selective reuptake inhibitors, e.g.
desipramine, dopamine-selective reuptake inhibitors, e.g. bupropion, and
serotonin-noradenaline reuptake inhibitors, e.g. venlafaxine (Table 1). It is
this
unique combination of pharmacological actions which renders sibutramine, and
the other compounds of formula I, efficacious in aiding weight loss after
pregnancy.
The assays below are pertormed in a similar manner to those described
in W098/41528.
TABLE
Comparison of the in vitro monoamine reuptake inhibition profiles of Examples
1
and 2, and various reference monoamine reuptake inhibitors in rat brain tissue
Ki (nM)
[3H]Noradenaline[3H]5-HT [3H]Dopamine
Example 1 3 18 24
Example 2 5 26 31
Bupropion 2590 18312 409
Desipramine 2 200 4853
Fluoxetine 320 11 2025
Venlafaxine 196 26 2594
The results are the means of ~3 separate determinations
Example 1 R~ = H, R2 = CH3 in Formula I
8
CA 02367268 2001-09-18
WO 00/56317 PCT/US00/07202
Example 2 R~ = H, R2 = H in Formula I
The efficacy of compounds of formula I in treating postpartum retention
of weight is demonstrable through clinical trials in a relevant population
set.
The invention has been described with reference to various specific
embodiments. However, many variations and modifications may be made while
remaining within the scope and spirit of the invention.
9