Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
LOW RESISTANCE ELECTRICAL & THERMAL
BOND AND METHOD OF MAKING SAME
BACKGROUND OF THE INVENTION
1. FIELD OF THE INVENTION
The present invention relates to a carbon to metal connection and
methods of making same. More specifically, the present invention relates to a
low resistance electrical and thermal bond between carbon, metal and the
method of making same for a dual graphite battery or energy storage cell.
2. DESCRIPTION OF RELATED ART
Forming a bond between carbon and metal is difficult. If the
application requires the connections to be electrically and thermally
conductive,
the difficulty is compounded. Conventional methods such as soldering tend to
be ineffective due to carbon's lack of a liquid phase and it's inability to be
easily
wetted.
Unlike metal to metal connections, carbon to metal connections in
the prior art are inconsistent in thickness and uniformity, and relatively
high in
resistance. The higher resistance is generally due to the fact that the
connections formed are non-uniform, physical only through pressure contact or
close proximity, and occur between two very dissimilar materials.
1
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
For example, United States Patent No. 5,858,530 to McCullough,
discloses a method for making a carbon to metal connection by electroplating
the carbon with copper (Cu) or other metals. The metallic plating forms a
conductive physical bond between the metal and the carbon. Several problems
with plating are inherent with the process. The process is very time
consuming,
dirty, wasteful, and relatively expensive.
Another problem with the plating method is inconsistency of
results. It is very difficult to control thickness and uniformity of the metal
plating,
especially when handling carbons of varying conductivities. Sample
micrographs frequently show void areas between the plated metal and the
carbon fiber 12, especially after sample agitation. Because of these problems,
metal plating is not a most desirable method for making a metal to carbon
connection.
Other prior art techniques require the carbon-metal contact to
remain out of contact with the electrolyte, or that a noble metal be used
which
does not dissolve in the electrolyte under use conditions. It is desirable to
avoid
these restrictions to provide useful product.
While brazing techniques and compounds are well discussed in
the literature, the carbon/graphite bonding techniques focus mainly on
2
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
carbon/graphite containing ceramics, carbon/graphite composites, carbon rods,
pressed carbon powders, loose carbon powders; and the resulting data focuses
mainly on sheer strength. There is no disclosure of any benefits of brazing in
creating a carbon to metal connection for use in developing a low resistance
electrical and thermal bond. Further, there is no disclosure of the use of a
brazing method or any similar method for creating such a bond.
It would be useful in using electroconductive carbon and graphite
in the form of fibers, bundles of fibers, cloths and foams in batteries, fuel
cells,
electrochemical cells, dual graphite energy storage cells, electrochemical
reactions and the like, to develop methods for making the lowest possible
resistance connections between the carbon/graphite and the metal or metal
alloy collector, wire, etc.
SUMMARY OF THE INVENTION
According to the present invention, there is provided a method of
creating a carbon to metal connection by placing a portion of a carbon
material
onto a metal material at a location where a thermal or electrical contact is
desired and then joining the carbon material and metal material, thereby
creating a uniform bond therebetween. Also provided by the present invention
is
a carbon to metal connection for use in a dual graphite battery including a
carbon material having multiple sides and a metal material joined to the
carbon
3
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
material whereby the connection is made by placing a portion of the carbon
material onto the metal material at the location for the desired connection
and
joining the carbon material and metal material thereby creating a uniform bond
between the carbon material and metal material for use in a dual graphite
energy storage cell or battery.
DESCRIPTION OF THE DRAWINGS
Other advantages of the present invention will be readily appreciated as
the same becomes better understood by reference to the following detailed
description when considered in connection with the accompanying drawings
wherein:
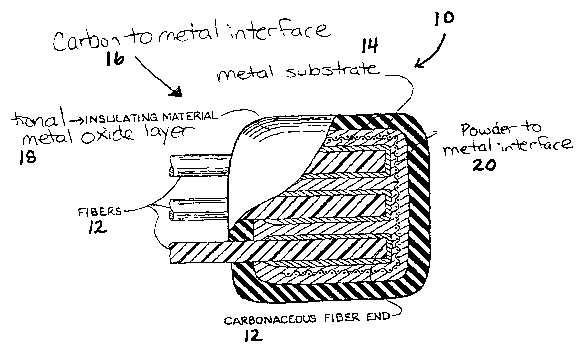
Fig. 1 is a partial cross-sectional view of the encapsulated fiber
ends and metal substrate of the electrode component of the present invention;
Fig. 2 is a partial cross-sectional view of the encapsulated, carbon
inked fiber ends and metal substrate of the electrode component of the present
invention;
Fig. 3 is a 28,OOOx scanning electron microscopy picture showing a
close-up of the bond formation area derived between the carbon material 12,
4
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
metal/allow powder, and the metal/alloy substrate from a plasma arc welding
sample; and
Fig. 4 is a 3,500x scanning electron microscopy picturing showing
the expanded view of the bond formation area derived between a carbon
material 12, metal/alloy powder, and the metal/alloy substrate from a plasma
arc
welding sample.
DETAILED DESCRIPTION OF THE INVENTION
Generally, a item having therein a carbon to metal connection
made in accordance with the present invention is generally shown at 10 in
Figure 1. The item of the present invention includes a carbon to metal
connection 16. The carbon to metal connection 16 is created by placing a
portion of a carbon material 12 onto a metal material 14 at a location where
it is
desired to have electrical or thermal contact and joining the carbon material
12
and metal material 14 thereby creating a uniform bond between the carbon
material 12 and the metal material 14 for use in the dual graphite energy
storage
cell or battery 10.
By "carbon material 12" as used herein, the term is intended to
include any material made of carbonaceous or graphitic material. Examples of
the carbon materials 12 include, but are not limited to, a single conductive
fiber,
5
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
a multiplicity of conductive fibers, a multiplicity of conductive fibers
formed into a
cloth or mat, a carbon foam, carbon material 12 wherein the fibers are
thermally
fused to each other, and other similar materials known to those of skill in
the art.
By "metal material 14" as used herein the term is intended to
include any metal, metal blend, metal alloy, or combinations thereof having a
reasonable conductivity. Example of the metals include, but are not limited
to,
Ag, AI, Au, Bi, Co, Cr, Cu, Fe, Ga, In, Mg, Mn, Ni, Pb, Sb, Sn, Pt, Pd, Ti,
Zn, and
alloy compounds thereof.
By "joining" as used herein, the term is meant to include methods
which include, but are not limited to, heating, casting, metal sputtering,
vacuum
deposition of metal, hot tinning, reflow soldering, electron beam welding,
chemical vapor deposition, laser welding, inks, and other similar methods
known
to those of skill in the art.
By "coating 18" as used herein, the term is intended to include any
material capable of covering metal material 14 and protecting the metal 14
from
degradation. The coating 18 can include, but is not limited to, an oxidizable
metal, which is then oxidized and a non-conductive polymeric material.
While many techniques are used to make electrical and thermal
connections 16 to carbon/graphite, most are pressure point, physical only
6
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
contacts, and often include the use of a low conductive (or non-conductive)
binder. The physical contacts are frequently non-uniform and can be difficult
to
control. The method of the present invention creates a lower resistance, more
uniform connection 16 to many forms of carbon through a process that is easily
controlled.
Of extreme importance, in any application using carbon/graphite
materials, is that the contact between every individual fiber piece is carried
completely to the exterior of the device, or to the central area of thermal or
electrical collection. Electrically and thermally conductive bonds must,
therefore,
occur between every individual fiber and the metal 14, then, in turn, every
individual fiber to the other fibers in the bundle (tow), every fiber bundle
to every
other fiber bundle in the cloth formation used, and finally to the entire
metal
substrate 14 in order to obtain 100% utilization of all carbon/graphite in the
system where a cloth is used. Therefore, penetration and uniform bond
formation with all portions of the carbon/graphite are required. In the case
of
cloth use, it can be difficult to penetrate the tow bundle in order to capture
all
fibers therein, due simply to the spatial relation of the individual fibers.
The
methods disclosed herein accomplish the 100% penetration/bond
formation/utilization that is difficult with the method disclosed in the prior
art.
The invention herein is applicable to a wide variety of conductive
materials. For example, the invention is applicable to various forms and
grades
7
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
of carbon and graphite particularly graphite fibers, formed from coal tar or
petroleum pitches which are heat treated to graphitize to some degree the
carbonaceous matter. In addition, the method is also applicable to various
polymers which, when heated to above about 800°C, lose their non-carbon
or
substantially lose their non-carbon elements yielding a graphite like material
(a
material having substantial polyaromatic configurations or conjugated double
bond structures) which results in the structure becoming conductive and are in
part at least graphitic in form.
Complete connection to all carbon/graphite is essential to obtain
full utilization of the material in any use. The connection keeps the amount
of
the relatively expensive materials to a minimum, which translates to lower
product costs and waste. For a battery 10, this also translates to the ability
to
obtain higher energy densities by using only the stoichiometric amount of
materials required for the system to function. In the case of a dual graphite
energy storage device, poor utilization of the carbon material 12 leads to
overall
loss in cell capacity.
Carbon/graphite fibers, and their various forms, have the least
amount of resistance in the axial direction, or along the length of the fiber.
Electrical and thermal energy is carried more efficiently along the length of
a
fiber than it is between fibers which are only in direct physical contact with
each
other, even when these fibers are held under pressure or with binders. Binders
8
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
themselves, though often called conductive, are not as conductive as the fiber
itself. Fibers that have only surface contacts with each other, have a large
increased resistance between them due to these factors. For these reasons, it
is preferential to utilize all fibers in a manner which takes advantage of the
low
resistance axial direction. For this reason, continuous fibers are often
preferential to any form of carbon powder, chopped fibers, felt type mats,
mesocarbon microbeads, etc. Accomplishing a contact point with continuous
fibers has its own inherent difficulties, including handling issues due to
fiber
brittleness, etc. However, the methods of the present invention provide an
improved method for fiber to metal contacts in all forms while maintaining low
resistance.
The present invention provides low resistance electrical and
thermal connections 16 that can be prepared between a conductive material
(carbon, graphite, and the like) and a conductive metal 20 or metal alloy by
plasma arc or resistance welding the cloth, or the material or individual
fiber
ends or bundles of fibers ends, to a conductive metal 20 or metal alloy. The
connection 16 can be facilitated by the use of a metallic or metallic alloy
powder
or wire to help wet the fiber material.
In a preferred embodiment of the present invention, the low
resistance electrical and thermal connections 16 are created through the use
of
9
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
heat, casting, metal sputtering, vacuum deposition of metal, hot tinning,
reflow
soldering, electron beam welding, chemical vapor deposition, or laser welding.
A working temperature of greater than 800°C is achieved between
the carbon material 12 and the metal substrate 14 in order to meet necessary
wettability requirements of the carbon material 12 while obtaining a low
resistance chemical and/or physical bond between the two dissimilar materials.
Additionally, a low resistance electrical and thermal connection 16 can be
prepared through the use of a fine granule carbon and solvent based ink or
paint
which is compressed and heat treated while positioned between the carbon
material 12 and the metal substrate 14 thereby allowing a chemical and/or
physical bond to form.
In most end uses it is desirable to have the collector metal 14
insulated against both electrical contact with and/or chemical attack by the
use
environment. The collector metal 14 can be encapsulated in an electrical
insulating material such as a cured resin-hardener blend that is highly cross-
linked and provides a system with a Tg of > 69 ~ 4C, or the metal 14 can be
coated with an oxide or oxidizable metal, all of which withstand chemical
attack
by the use environment. Resin-hardener blends are relatively easy to handle
and cure quickly at reasonable temperatures of between 60°C to
100°C. The
blends can be applied by various means including dipping, roll coating,
pressure
filling, and spraying. The method applied often depends on the exact material
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
used, since working times and desired thickness are known to vary. It is to be
further understood that encapsulation can be dispensed with if the conductive
metal 20/alloy applied is oxidizable to produce a non-conductive coating 18 or
surface which is non-reactive under the conditions of use.
These techniques can be applied to any carbon/graphite form.
Those forms which require the lowest number of connection sites 16 have the
least amount of collector area that must be accommodated in an end use. In
applications where weight and or space is a critical factor, the least amount
of
collector weight and area used by the collector is typically an important
consideration, since it is this collector that generally contributes the most
in
terms of weight, space, and often cost. Specifically in a battery 10, the
reduced
amount of collector translates to improved energy density of the end products,
which in turn provides more possible end uses, and lower costs.
Carbon/graphite powders, chopped fibers, felt type mats,
mesocarbon microbeads, etc., require an individual contact point for every
piece
of carbon/graphite to the collector. This often means that the collector has a
large surface area, and thus uses a great deal of space, adds extra weight and
costs to the end product. Continuous fibers of various forms therefore are
often
the preferred material. For example, a woven cloth contains continuous fibers
that run in two directions which are generally perpendicular to each other.
The
woven cloth has fiber ends exposed on four sides. The woven cloth then
11
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
requires at least two edges of collection to utilize all carbon/graphite
materials in
the cloth, and thus takes more space and weight for current collection than
for
instance a unidirectional cloth, but less than is required for a graphite felt
which
would have one entire side of the material coated as a collector.
Unidirectional
cloths, or braids such as biaxial or triaxial, contain continuous fibers that
run in
essentially one direction. The fibers start and then end with only two edges
of
exposed fiber ends, these then require only one edge of collection. A
carbon/graphite foam, or mat of thermally bonded fibers, requires only one
point
of collection to attach all carbon/graphite together, since the material is
fused
together creating essentially one continuous fiber.
The thickness of the metallic collection material is also critical to
overall battery energy density. In dual graphite energy storage systems the
metallic collection material is not directly involved in the battery
processes,
except to carry electrical energy to and from the device. The metallic
collection
material is then considered an inactive weight material. When an inactive
material is reduced in weight and/or space, the cell energy density is
increased;
therefore, the less collector material, the higher the energy density. For
this
reason, the less of the metallic materials used in the end product the better.
The
only factors which then limit the low end of collector amount are the fiber
utilization and the electrical and/or thermal carrying requirements. A
complete
diffusion layer between the carbon fiber 12 and the metal 14 must be
maintained
to minimize the resistance. There must be complete coverage of fiber with
metal
12
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
14 in the desired collector location and the metal 14 should be distributed in
equal thickness over the fiber.
The methods of the present invention overcome the problems of
the prior art by creating a method which is more time efficient, has greater
ease
of control, the flexible fibers are able to stay within a set geometry during
processing, and preferably there is no hazardous materials handling during the
process.
Due to the various collector forms and uses, corrosion resistance
of the metals employed as collectors have been approached in many ways.
This is especially true in a battery system 10 where the environmental
conditions
are often very harsh, and in the case of lithium ion batteries, this often
influences
the active materials chosen for the electrochemical cells. Due to the reduced
area of collector in a dual graphite energy storage system using the various
forms of continuous fibers, the corrosion resistance of the collector
materials is a
less complicated matter. The collector is relatively easy to encapsulate or
treat
for corrosion resistance without fear of interfering in the fiber to collector
bond.
The metal collector 14 can be alternatively coated with an oxide or
oxidizable metal, all of which withstand chemical attack by the use
environment.
It is to be further understood that encapsulation of any type can be dispensed
with if the conductive metal 20/alloy applied as the collector is oxidizable
to
13
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
produce a non-conductive coating 18 or surface which is non-reactive under the
conditions of use.
Although particular embodiments of the present invention have
been described in the foregoing description, it will be understood by those
skilled
in the art that the invention is capable of numerous modifications,
substitutions
and rearrangements without departing from the spirit or essential attributes
of
the invention.
The above discussion provides a factual basis for the use of the
carbon to metal connection 16. The method used with and the utility of the
present invention can be shown by the following non-limiting examples and
accompanying figures.
EXAMPLES
EXAMPLE 1
Plasma arc welding of a unidirectional carbon fiber 12 cloth to a
metal substrate 14 to obtain a low resistance bond between the two materials
was performed using the following steps. The metal substrate 14, which in this
instance is Ti Grade 1 Cp with a thickness of 0.01 Oinch, is positioned in an
automatic moving table that keeps all materials and weld tips under a constant
14
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
atmosphere of Argon (Ar) during a weld. The desired carbon fiber cloth 12
piece
size is cut from a unidirectional cloth of approximately 60MS1 carbon graphite
fiber, and is positioned on top of the Ti metal ribbon (1.9 x 22cm) using a
series
of positioning sets in the moving table apparatus. The carbon fiber
unidirectional cloth 12 sits essentially perpendicular to the length of the
metal
ribbon (or foil strip). The weld tip torch distance from the fiber is set,
according
to the equipment scale only (not a true distance), between 1-12/32 & 1-
13/32inches. Shield gas of Argon (Ar) runs along side the weld torch to ensure
inert atmosphere at the weld point. This reduces the amount of rvetal
oxidation
that occurs during the weld. A working gas of Ar is also required for the arc
weld
to occur.
In this example the metal powder used is Ti/Cu (50/50 by weight).
The metal powder is fed into the weld tip during the weld process so that the
feed rate (i.e. g/sec) deposits an appropriate amount of powder for the table
speed. As the weld process is started, the table is moved automatically
(electronically controlled), so that the tip movement rate allows for a good
weld.
Table rate is dependent upon powder feed rate to avoid "puddles" of powder
being welded in one spot and to avoid too little powder being used to obtain a
good weld. The powder, the metal substrate 14, and at least the carbon fiber
surface 12 are bonded together during the high temperature plasma arc weld.
This is confirmed through SEM, high resolution microscopy, and the electrical
continuity was verified as the resistance was found to be within the expected
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
limits of the resistance of the graphite fiber. After the welding was
complete, the
electrode was allowed to sit in air to oxidize the metal surfaces 14 and make
them more stable in an energy storage device environment.
EXAMPLE 2
Resistance welding of a unidirectional carbon fiber cloth 12 to a
metal substrate 14 to obtain a bond between the two materials was performed
using the following steps. The metal substrate 14, which in this instance is
Cu
foil with a thickness of 0.010inch, is positioned in a metal brake to fold the
metal
ribbon 90 degrees at center. The desired carbon fiber cloth piece 12 is cut
from
a unidirectional cloth of approximately 60MS1 carbon graphite fiber. The cloth
end fibers 12 are submersed in a powder bath to help increase the penetration
of the bonding into the bulk of the carbon fiber tows 12. The powder used in
this
case is Ti/Cu/Ni (60/15/25 by weight). The fiber 12 is positioned in the bend
of
the metal ribbon so that the carbon fiber unidirectional cloth 12 is
essentially
surrounded by the metal ribbon (or foil strip), and is tucked into the folded
metal
14. The folded metal containing the powder and fiber is then mechanically
pressed together for a superficial bond. All materials are then placed under a
constant atmosphere of Argon (Ar) before and during the resistance weld. The
powder, the metal substrate 14, and at least the carbon fiber surface 12 are
bonded together during the high temperature weld; this is confirmed through
SEM, high resolution microscopy, and the electrical continuity was verified as
16
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
the resistance was found to be within the expected limits of the resistance of
the
graphite fiber.
EXAMPLE 3
Carbon ink deposition between a unidirectional carbon fiber cloth
12 and a metal substrate 14 to obtain a bond between the materials was
performed using the following steps. The metal substrate 14, which in this
instance is AI with a thickness of 0.010inch, is positioned in a metal brake
to fold
the metal ribbon in half. The desired carbon fiber cloth piece 12 is cut from
a
unidirectional cloth of approximately 60MS1 carbon graphite fiber. The cloth
end
fibers 12 are submersed in a carbon ink bath to help increase the penetration
of
the paint into the bulk of the carbon fiber tows 12. The ink used in this case
is
Engelhard Corp. carbon ink "LT1 A6162-XA". The fiber is positioned in the bend
of the AI metal ribbon so that the carbon fiber unidirectional cloth 12 is
essentially surrounded by the metal ribbon (or foil strip), and is tucked into
the
folded metal. The folded metal containing the ink and fiber is then
mechanically
pressed together for a superficial bond. All materials are then placed in a
constant temperature oven. The powder, the metal substrate 14, and at least
the
carbon fiber surface 12 are bonded together during the high temperature
treatment; this is confirmed through SEM, high resolution microscopy, and the
electrical continuity was verified as the resistance was found to be within
the
expected limits of the resistance of the graphite fiber.
17
CA 02368680 2001-09-24
WO 01/54856 PCT/USO1/02634
Throughout this application, various publications, including United
States patents, are referenced by author and year and patents by number. Full
citations for the publications are listed below. The disclosures of these
publications and patents in their entireties are hereby incorporated by
reference
into this application in order to more fully describe the state of the art to
which
this invention pertains.
The invention has been described in an illustrative manner, and it
is to be understood that the terminology which has been used is intended to be
in the nature of words of description rather than of limitation.
Obviously, many modifications and variations of the present
invention are possible in light of the above teachings. It is, therefore, to
be
understood that within the scope of the appended claims, the invention can be
practiced otherwise than as specifically described.
18