Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-1 -
SUBSTITUTED ARYLMALONIC ACID DINITRILES AS INTERMEDIATES FOR THE PREPARATION
OF
HERBICIDES
The present invention relates to new substituted arylmalonic acid dinitriles
as intermediates
for a surprisingly advantageous overall process for the preparation of known,
herbicidally
active 3-hydroxy-4-aryl-5-oxopyrazoline derivatives, to a process for the
preparation of
those intermediates and to their use in the preparation of 3-hydroxy-4-aryl-5-
oxopyrazoline
derivatives.
Arylmalonic acid dinitriles and their preparation by means of palladium
complexes are
described, for example, in Chem. Commun. 1984, 932 and JP-A-60 197 650.
Furthermore,
J. Am. Chem. Soc. 121, 1473 (1999) describes the arylation of malonates by
means of
palladium catalysts.
New substituted arylmalonic acid dinitriles have now been found which are
outstandingly
suitable as intermediates for an advantageous process for the preparation of
herbicidal
3-hydroxy-4-aryl-5-oxopyrazoline derivatives.
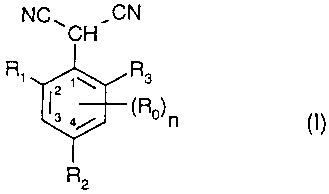
The present invention accordingly relates to compounds of formula I
NC~ ,CN
CH
Rs
R2
wherein
Ro is, each independently of any other, halogen, C,-Csalkyl, C2-Csalkenyl, C2-
Csalkynyl,
C,-Cshaloalkyl, cyano-C,-Cfialkyl, C2-Cshaloalkenyl, cyano-C2-Csalkenyl, C2-
Cshaloalkynyl,
cyano-C2-Csalkynyl, hydroxy, hydroxy-C,-Csalkyl, C,-Csalkoxy, vitro, amino, C,-
Csalkylamino, di(C,-Csalkyl)amino, C,-Csalkylcarbonylamino, C,-
Csalkylsulfonylamino, C,-
Csalkylaminosulfonyl, C,-Csalkylcarbonyl, C,-Csalkylcarbonyl-C,-Csalkyl, C,-C6-
alkoxycarbonyl-C,-Csalkyl, C,-Csalkylcarbonyl-C2-Csalkenyl, C,-
Csalkoxycarbonyl,~ C,-
Csalkoxycarbonyl-C2-Csalkenyl, C,-Csalkylcarbonyl-C2-Csalkynyl, C,-
Csalkoxycarbonyl-C2-
Csalkynyl, cyano, carboxyl, phenyl or an aromatic ring that contains 1 or 2
hetero atoms
selected from the group consisting of nitrogen, oxygen and sulfur, wherein the
latter two
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-2-
aromatic rings may be substituted by C,-C3alkyl, C,-C3haloalkyl, C,-C3alkoxy,
C,-
C3haloalkoxy, halogen, cyano or by vitro; or
Ro, together with the adjacent substituents R,, RZ and R3, forms a saturated
or unsaturated
C3-Cshydrocarbon bridge that may be interrupted by 1 or 2 hetero atoms
selected from the
group consisting of nitrogen, oxygen and sulfur and/or substituted by C,-
C4alkyl;
R,, R2 and R3 are, each independently of the others, hydrogen, halogen, C,-
Csalkyl, C2-C6-
alkenyl, CZ-Csalkynyl, C3-Cscycloalkyl, C,-Cshaloalkyl, C2-Cshaloalkenyl, C,-
Csalkoxy-
carbonyl-C2-Csalkenyl, C,-Csalkylcarbonyl-C2-Csalkenyl, cyano-C2-Csalkenyl,
vitro-C2-C6-
alkenyl, C2-Cshaloalkynyl, C,-Csalkoxycarbonyl-C2-Cfialkynyl, C,-
Csalkylcarbonyl-C2-C6-
alkynyl, cyano-C2-Csalkynyl, vitro-C2-Csalkynyl, C3-Cshalocycloalkyl, hydroxy-
C,-Csalkyl,
C,-Csalkoxy-C,-Csalkyl, C,-Csalkylthio-C,-Csalkyl, cyano, C,-C4alkylcarbonyl,
C,-C6-
alkoxycarbonyl, hydroxy, C,-C,oalkoxy, C3-Csalkenyloxy, C3-Csalkynyloxy, C,-
Cshaloalkoxy,
C3-Cshaloalkenyloxy, C,-Csalkoxy-C,-Csalkoxy, mercapto, C,-Csalkylthio, C,-
Cshaloalkylthio,
C,-Csalkylsulfinyl, C,-Csalkylsulfonyl, vitro, amino, C,-Csalkylamino, di(C,-
Csalkyl)amino or
phenoxy in which the phenyl ring may be substituted by C,-C3alkyl, C,-
C3haloalkyl, C,-C3-
alkoxy, C,-C3haloalkoxy, halogen, cyano or by vitro;
R2 also may be phenyl, naphthyl or a 5- or 6-membered aromatic ring that may
contain 1 or
2 hetero atoms selected from the group consisting of nitrogen, oxygen and
sulfur, wherein
the phenyl ring, the naphthyl ring system and the 5- or 6-membered aromatic
ring may be
substituted by halogen, C3-Cecycloalkyl, hydroxy, mercapto, amino, cyano,
vitro or by
formyl; and/or
the phenyl ring, the naphthyl ring system and the 5- or 6-membered aromatic
ring may be
substituted by C,-Csalkyl, C,-Csalkoxy, hydroxy-C,-Csalkyl, C,-Csalkoxy-C,-
Csalkyl, C,-C6-
alkoxy-C,-Csalkoxy, C,-Csalkylcarbonyl, C,-Csalkylthio, C,-Csalkylsulfinyl, C,-
Csalkylsulfonyl,
mono-C,-Csalkylamino, di(C,-Csalkyl)amino, C,-Csalkylcarbonylamino, C,-
Csalkylcarbonyl-
(C,-Csalkyl)amino, C2-Csalkenyl, C3-Csalkenyloxy, hydroxy-C3-Csalkenyl, C,-
Csalkoxy-C2-C6-
alkenyl, C,-Csalkoxy-C3-Csalkenyloxy, C2-Csalkenylcarbonyl, C2-Csalkenylthio,
C2-Csalkenyl-
sulfinyl, C2-Csalkenylsulfonyl, mono- or di-(CZ-Csalkenyl)amino, C,-Csalkyl(C3-
Csalkenyl)-
amino, C2-Cfialkenylcarbonylamino, CZ-Csalkenylcarbonyl(C,-Csalkyl)amino, C2-
Csalkynyl,
C3-Csalkynyloxy, hydroxy-C3-Csalkynyl, C,-Csalkoxy-C3-Csalkynyl, C,-Csalkoxy-
C4-Cs-
alkynyloxy, C2-Csalkynylcarbonyl, C2-Csalkynylthio, C2-Csalkynylsulfinyl, CZ-
Csalkynylsulfonyl, mono- or di-(C3-Csalkynyl)amino, C,-Csalkyl(C3-
Csalkynyl)amino, C2-
Csalkynylcarbonylamino or by CZ-Csalkynylcarbonyl(C,-Csalkyl)amino; and/or
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-3-
the phenyl ring, the naphthyl ring system and the 5- or 6-membered aromatic
ring may be
substituted by halo-substituted C,-Csalkyl, C,-Csalkoxy, hydroxy-C,-Csalkyl,
C,-C6alkoxy-
C,-Csalkyl, C,-Csalkoxy-C,-Csalkoxy, C,-Csalkylcarbonyl, C,-Cfialkylthio, C,-
Csalkylsulfinyl,
C,-Csalkylsulfonyl, mono-C,-Csalkylamino, di(C,-Cfialkyl)amino, C,-
Csalkylcarbonylamino,
C,-Cfialkylcarbonyl(C,-Csalkyl)amino, C2-C6alkenyl, C3-Csalkenyloxy, hydroxy-
C3-Csalkenyl,
C,-Csalkoxy-C2-Csalkenyl, C,-Csalkoxy-C3-Csalkenyloxy, C2-Csalkenylcarbonyl,
C2-Cs-
alkenylthio, C2-Csalkenylsulfinyl, C2-Csalkenylsulfonyl, mono- or di-(C2-
C6alkenyl)amino,
C,-C6-alkyl(C3-Csalkenyl)amino, C2-Csalkenylcarbonylamino, CZ-
Cfialkenylcarbonyl(C,-C6-
alkyl)amino, C2-Csalkynyl, C3-Csalkynyloxy, hydroxy-C3-Csalkynyl, C,-Csalkoxy-
C3-Csalkynyl,
C,-Csalkoxy-C4-Csalkynyloxy, C2-Csalkynylcarbonyl, C2-Csalkynylthio, C2-
Csalkynylsulfinyl,
C2-Csalkynylsulfonyl, mono- or di-(C3-Csalkynyl)amino, C,-Csalkyl(C3-
Csalkynyl)amino,
C2-Cfialkynylcarbonylamino or C2-Csalkynylcarbonyl(C,-Csalkyl)amino; and/or
the phenyl ring, the naphthyl ring system and the 5- or 6-membered aromatic
ring may be
substituted by a radical of formula COORso, CONRS,, SOzNR53R~ or S020R55,
wherein RSO,
Rs,, Rs2, Rs3, Rsa and R55 are, each independently of the others, C,-Csalkyl,
C2-Csalkenyl or
C3-Csalkynyl or halo-, hydroxy-, alkoxy-, mercapto-, amino-, cyano-, nitro-,
alkylthio-,
alkylsulfinyl- or alkylsulfonyl-substituted C,-Csalkyl, C2-Csalkenyl or C3-
Csalkynyl; and
n is 0, 1 or 2.
In the above definitions, halogen is to be understood as fluorine, chlorine,
bromine or
iodine, preferably fluorine, chlorine or bromine.
The alkyl groups occurring in the substituent definitions are, for example,
methyl, ethyl,
n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl or tert-butyl, and the
pentyl, hexyl, heptyl,
octyl, nonyl, decyl, undecyl and dodecyl isomers.
Haloalkyl groups preferably have a chain length of from 1 to 6 carbon atoms.
Haloalkyl is,
for example, fluoromethyl, difluoromethyl, difluorochloromethyl,
trifluoromethyl,
chloromethyl, dichloromethyl, dichlorofluoromethyl, trichloromethyl, 2,2,2-
trifluoroethyl, 2-
fluoroethyl, 2-chloroethyl, 2,2-difluoroethyl, 2,2-dichloroethyl, 2,2,2-
trichloroethyl or
pentafluoroethyl, preferably trichloromethyl, difluorochloromethyl,
difluoromethyl,
trifluoromethyl or dichlorofluoromethyl.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-4-
Alkoxy groups preferably have a chain length of from 1 to 6 carbon atoms.
Alkoxy is, for
example, methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec-
butoxy, tert-
butoxy, or a pentyloxy or hexyloxy isomer, preferably methoxy, ethoxy or n-
propoxy.
Haloalkoxy is, for example, fluoromethoxy, difluoromethoxy, trifluoromethoxy,
2,2,2-trifluoro-
ethoxy, 1,1,2,2-tetrafluoroethoxy, 2-fluoroethoxy, 2-chloroethoxy or 2,2,2-
trichloroethoxy.
There may be mentioned as examples of alkenyl radicals vinyl, allyl,
methallyl, 1-
methylvinyl, but-2-en-1-yl, pentenyl and 2-hexenyl; preferably alkenyl
radicals having a
chain length of from 3 to 6 carbon atoms.
There may be mentioned as examples of alkynyl radicals ethynyl, propargyl, 1-
methyl-
propargyl, 3-butynyl, but-2-yn-1-yl, 2-methylbut-3-yn-2-yl, but-3-yn-2-yl, 1-
pentynyl, pent-4-
yn-1-yl and 2-hexynyl; preferably alkynyl radicals having a chain length of
from 3 to 6
carbon atoms.
Suitable haloalkenyl radicals include alkenyl groups substituted one or more
times by
halogen, halogen being in particular bromine. or iodine and especially
fluorine or chlorine,
for example 2- and 3-fluoropropenyl, 2- and 3-chloropropenyl, 2- and 3-
bromopropenyl, 2,2-
difluoro-1-methylvinyl, 2,3,3-trifluoropropenyl, 3,3,3-trifluoropropenyl,
2,3,3-
trichloropropenyl, 4,4,4-trifluorobut-2-en-1-yl and 4,4,4-trichlorobut-2-en-1-
yl. Preferred
alkenyl radicals substituted once, twice or three times by halogen are those
having a chain
length of from 3 to 6 carbon atoms. The alkenyl groups may be substituted by
halogen at
saturated or unsaturated carbon atoms.
Alkoxyalkyl groups have preferably from 1 to 6 carbon atoms. Alkoxyalkyl is,
for example,
methoxymethyl, methoxyethyl, ethoxymethyl, ethoxyethyl, n-propoxymethyl, n-
propoxyethyl,
isopropoxymethyl or isopropoxyethyl.
Haloalkoxy is, for example, fluoromethoxy, difluoromethoxy, trifluoromethoxy,
2,2,2-tri-
fluoroethoxy, 1,1,2,2-tetrafluoroethoxy, 2-fluoroethoxy, 2-chloroethoxy or
2,2,2-trichloro-
ethoxy.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-5-
Alkenyloxy is, for example, allyloxy, methallyloxy or but-2-en-1-yloxy.
Suitable haloalkenyloxy groups include alkenyloxy groups substituted one or
more times by
halogen, halogen being in particular bromine or iodine and especially fluorine
or chlorine,
for example 2- and 3-fluoropropenyloxy, 2- and 3-chloropropenyloxy, 2- and 3-
bromopropenyloxy, 2,3,3-trifluoropropenyloxy, 2,3,3-trichloropropenyloxy,
4,4,4-trifluorobut-
2-en-1-yloxy and 4,4,4-trichlorobut-2-en-1-yloxy.
Alkynyloxy is, for example, propargyloxy or 1-methylpropargyloxy.
Suitable cycloalkyl substituents contain from 3 to 8 carbon atoms and are, for
example,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl or cyclooctyl.
They may be
substituted one or more times by halogen, preferably fluorine, chlorine or
bromine.
Alkylcarbonyl is especially acetyl or propionyl.
Alkoxycarbonyl is, for example, methoxycarbonyl, ethoxycarbonyl, n-
propoxycarbonyl,
isopropoxycarbonyl or a butoxycarbonyl, pentyloxycarbonyl or hexyloxycarbonyl
isomer,
preferably methoxycarbonyl or ethoxycarbonyl.
Alkylthio groups preferably have a chain length of from 1 to 6 carbon atoms.
Alkylthio is, for
example, methylthio, ethylthio, propylthio, butylthio, pentylthio or
hexylthio, or a branched
isomer thereof, but is preferably methylthio or ethylthio.
Haloalkylthio is, for example, 2,2,2-trifluoroethylthio or 2,2,2-
trichloroethylthio.
Alkylsulfinyl is, for example, methylsulfinyl, ethylsulfinyl, n-
propylsulfinyl, isopropylsulfinyl,
n-butylsulfinyl, isobutylsulfinyl, sec-butylsulfinyl or tert-butylsulfinyl,
preferably methylsulfinyl
or ethylsulfinyl.
Alkylsulfonyl is, for example, methylsulfonyl, ethylsulfonyl, n-
propylsulfonyl,
isopropylsulfonyl, n-butylsulfonyl, isobutylsulfonyl, sec-butylsulfonyl or
tert-butylsulfonyl,
preferably methylsulfonyl or ethylsulfonyl.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-6-
Alkylamino is, for example, methylamino, ethylamino, n-propylamino,
isopropylamino or a
butyl-, pentyl- or hexyl-amine isomer.
Dialkylamino is, for example, dimethylamino, methylethylamino, diethylamino, n-
propyl-
methylamino, dibutylamino or diisopropylamino.
Alkylthioalkyl is, for example, methylthiomethyl, methylthioethyl,
ethylthiomethyl, ethylthio-
ethyl, n-propylthiomethyl, n-propylthioethyl, isopropylthiomethyl or
isopropylthioethyl. .
Phenyl and naphthyl in the definition of R2 and phenoxy in the definition of
R,, R2 and R3
may be in substituted form, in which case the substituents may, as desired, be
in the ortho-,
meta- and/or para-position and, in the case of the naphthyl ring system, in
addition in the 5-,
6-, 7- and/or 8-position.
Examples of suitable 5- or 6-membered aromatic rings that contain 1 or 2
hetero atoms
selected from the group consisting of nitrogen, oxygen and sulfur in the
definition of Ro and
R2 are pyrrolidyl, pyridyl, pyrimidyl, triazinyl, thiazolyl, triazolyl,
thiadiazolyl, imidazolyl,
oxazolyl, isoxazolyl, pyrazinyl, furyl, thienyl, pyrazolyl, benzoxazolyl,
benzothiazolyl,
quinoxalyl, indolyl and quinolyl. These heteroaromatic radicals may, in
addition, be
substituted.
Meanings corresponding to those given hereinbefore can also be ascribed to
substituents in
composite definitions, such as, for example, alkoxy-alkoxy, alkyl-
sulfonylamino, alkyl-
aminosulfonyl, phenyl-alkyl, naphthyl-alkyl and heteroaryl-alkyl.
In the definitions for alkylcarbonyl and alkoxycarbonyl, the carbon atom of
the carbonyl is
not included in the upper and lower limits given for the number of carbons in
each particular
case.
Preference is given to compounds of formula I wherein n is as defined for
formula I; Ro is,
each independently of any other, halogen, C,-Csalkyl, C,-Cshaloalkyl, hydroxy,
C,-Csalkoxy,
nitro, amino, C,-Csalkylamino, di(C,-Csalkyl)amino, C,-Csalkylcarbonylamino,
C,-Csalkyl-
sulfonylamino, C,-Csalkylaminosulfonyl, C,-C4alkylcarbonyl, C,-
Csalkoxycarbonyl or
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
7_
carboxyl; and R,, RZ and R3 are, each independently of the others, hydrogen,
halogen,
C,-Csalkyl, C2-Csalkenyl, C2-Csalkynyl, C3-Cficycloalkyl, C,-Cs-haloalkyl, Cz-
Cshaloalkenyl,
Cz-Cshaloalkynyl, C3-Cshalocycloalkyl, C,-Csalkoxy-C,-Csalkyl, C,-Csalkylthio-
C,-Csalkyl,
cyano, C,-C4alkylcarbonyl, C,-Csalkoxycarbonyl, hydroxy, C,-C,oalkoxy, C3-
Csalkenyloxy,
C3-Csalkynyloxy, C,-Cshaloalkoxy, C3-Cfihaloalkenyloxy, C,-Csalkoxy-C,-
Csalkoxy, mercapto,
C,-Csalkylthio, C,-Cshaloalkylthio, C,-Csalkylsulfinyl, C,-Csalkylsulfonyl,
nitro, amino, C,-
C4alkylamino or di(C,-C4alkyl)amino.
Preference is given also to compounds of formula I wherein R~, Rz and R3 are,
each
independently of the others, hydrogen, halogen, C,-C4alkyl, C,-C4haloalkyl, C2-
C4alkenyl,
C2-C4haloalkenyl, C2-C4alkynyl, C3-Cscycloalkyl, C,-C4alkylcarbonyl, C,-
Csalkoxycarbonyl,
hydroxy, C,-C4alkoxy, C3- or C4-alkenyloxy, C3- or C4-alkynyloxy, C,-
C4haloalkoxy, nitro or
amino.
Preference is given also to compounds of formula I wherein R, is C2-Csalkyl.
Likewise preferred are compounds of formula I wherein n is 0.
Of those, special preference is given to compounds of formula I wherein R, is
C2-C4alkyl,
C,-C4alkoxy, C2-C4alkynyl or C3-Cscycloalkyl and R3 is C,-C4alkyl, C,-
C4alkoxy, C2-C4alkynyl
or C3-Cscycloalkyl.
Likewise preferred are compounds of formula I wherein R, is C2-Csalkynyl.
Preference is given to those compounds of formula I wherein R, and R3 are,
each
independently of the other, C2-Csalkyl, C2-Csalkynyl, C,-C,oalkoxy or C3-
Cscycloalkyl. Of
those, special preference is given to compounds wherein R, is C2-Csalkyl and
R3 is
C2-Csalkyl, C2-Csalkynyl or C,-C,oalkoxy.
Chem. Commun. 1984, 932 and JP-A-60 197 650 describe a palladium-catalysed
synthesis
of arylmalonic dinitriles, in yields of from 56 to..g5 %, by C-C linkage of
aryl halides
- unsubstituted or mono-substituted in the 2- or 4-position - with the malonic
dinitrile anion.
Mention is made, by way of example, of (PPh3)2PdCl2 and Pd(PPh3)4 as palladium
complexes and of tetrahydrofuran as reaction medium, JP-A-60 197 650, which is
drafted
with a broader scope, also mentioning bis(trialkylphosphine)- and
bis(trialkoxy- and
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
_g_
triphenoxy-phosphine)-palladium(II) chloride complexes and, as reaction media,
ethylene
glycol dimethyl ether and dimethylformamide. In both documents, the only aryl
halides
specifically mentioned are unsubstituted or mono-substituted aryl iodides and
a bromo-
benzene activated in the 4-position by a cyano group.
It has now been found, surprisingly, that the C-C linkage of malonic acid
dinitrile with a large
group of mono- or poly-substituted aryl derivatives additionally containing a
leaving group is
feasible in various reaction media in the presence of a wide variety of
palladium(II) or
palladium(0) complexes in high product yields and purities.
The present process is distinguished by:
a) wide variability of reaction media and reaction conditions,
b) high volume concentrations of the reactants (up to 10%),
c) a large number of suitable palladium catalysts,
d) widely - especially in the 2- and 6-positions - substituted phenyl
derivatives as starting
compounds, having various leaving groups, including sterically hindered (low-
reactivity)
leaving groups,
e) easy accessibility of the starting compounds,
f) the use of catalysts that are either commercially available or easily
prepared 'in situ' from
commercially available palladium salts, such as, for example, palladium(II)
chloride solution
(20 %) in concentrated hydrochloric acid with the addition of dimethyl
acetamide (DMA) as
solubility promoter, and corresponding ligands,
g) a simple reaction procedure, such as, for example, reaction of the 'in
situ'-generated
malonic acid dinitrile anion and palladium catalyst with an aryl halide or
arylsulfonate,
h) simple and effective working-up methods that yield the C-C linkage products
of formula I
in a high degree of purity (Example P17),
i) generally very high product yields, and
j) economic and ecological advantages derived from the fact that the process
can be used
as a partial step in a continuous reaction procedure for the preparation of 3-
hydroxy-4-aryl-
5-oxopyrazoline derivatives of formula III (Reaction Scheme 3).
The present preparation process is therefore suitable especially for the large-
scale
preparation of arylmalonic acid dinitrile derivatives of formula I.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
_g_
The process according to the invention for the preparation of compounds of
formula I
comprises reacting a compound of formula II
X
Ri
(II),
wherein Ro, R,, R2, R3 and n are as defined for formula I and X is a leaving
group, with the
malonic acid dinitrile anion in an inert solvent in the presence of a
palladium catalyst. The
malonic acid dinitrile anion is preferably prepared 'in situ' from malonic
acid dinitrile and a
base.
The preparation of compounds of formula I is illustrated in the following
Reaction Scheme 1.
Reaction Scheme 1
NC,C CN
X 1 ) CHZ(CN)~, base e.g. sodium tert-butanolate,
R ~ ~ ~ solvent, e.g. xylene, 0°-100°C R'
(~)
(fin 2) Pd(0) or Pd(II) catalyst e.g. ~ n
PdCl2, P(cyclohexyl)3, 30°-250°C
R2
According to Reaction Scheme 1, the compounds of formula I are obtained from
the
compounds of formula II by adding the latter, in a first reaction step, at a
temperature of
from 0° to 100°C, to a prepared solution of malonic acid
dinitrile in a suitable solvent, for
example an aromatic hydrocarbon, an ether or dimethyl sulfoxide, e.g. xylene
or
tetrahydrofuran, and in the presence of a base, for example an alkali metal
alcoholate, e.g.
sodium tert-butanolate, together with a palladium catalyst, for example a
separately
prepared palladium catalyst, for example,
bis(tricyclohexylphosphine)palladium(II) dichloride
(Pd(PCy3)2CI2). The coupling reaction is started by heating the resulting
reaction solution to
a temperature of from 30° to 250°C, depending on the solvent
used.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-10-
Suitable leaving groups X for the C-C linkage reaction of the compound of
formula II with
the malonic acid dinitrile anion in the presence of palladium catalysts are
halogen,
R,oS(O)ZO- (wherein R,o is C,-C4alkyl, preferably methyl, C,-C4haloalkyl,
preferably
halomethyl or n-C4F9, aryl, preferably phenyl, or phenyl mono- to tri-
substituted by halogen,
methyl or by halomethyl) and mono-, di- or tri-arylmethoxy.
The aryl radicals of the mono-, di- and tri-arylmethoxy groups are preferably
phenyl radicals,
which may be substituted for example by methyl from once to three times, the
substituents
preferably being in the 2-, 4- and/or 6-position of the phenyl ring.
Examples of such leaving groups are methylsulfonyloxy (mesylate),
trifluoromethylsulfonyl-
oxy (triflate), p-tolylsulfonyloxy (tosylate), CF3(CF2)3S(O)20- (nonaflate),
diphenylmethoxy,
di(methylphenyl)methoxy, triphenylmethoxy (trityl) and
tri(methylphenyl)methoxy.
Special preference is given to those leaving groups wherein X is chlorine,
bromine, iodine,
CF3S(O)20- (triflate), CF3(CFZ)3S(O)20- (nonaflate), p-tolyl-S(O)20-
(tosylate), (CsHs)2CH0-,
(CH3-C6H4)ZCHO-, (C6H5)3C0- (trityl) or (CH3-C6H4)3C0-. Of those, preference
is given more
especially to leaving groups wherein X is chlorine, bromine or iodine.
The palladium catalysts suitable for the C-C linkage reaction of the compound
of formula II
with the malonic acid dinitrile anion are generally palladium(II) or
palladium(0) complexes,
such as, for example, palladium(II) dihalides, palladium(II) acetate,
palladium(II) sulfate,
bis(triphenylphosphine)palladium(II) dichloride,
bis(tricyclopentylphosphine)palladium(II)
dichloride, bis(tricyclohexylphosphine)palladium(II) dichloride,
bis(dibenzylideneacetone}-
palladium(0) or tetrakis(triphenylphosphine)palladium(0).
In an especially advantageous variant of the process according to the
invention, the
palladium catalyst can also be prepared 'in situ' from palladium(II) or
palladium(0)
compounds as a result of complexing with the desired ligands, for example by
placing the
palladium(II) salt to be complexed, e.g. palladium(II) dichloride (PdCl2) or
palladium(II)
acetate (Pd(OAc)2), together with the desired ligand e.g. triphenylphosphine
(PPh3) or
tricyclohexylphosphine (PCy3) together with the selected solvent, malonic acid
dinitrile and
base. Palladium(II) dichloride, as a reasonably priced palladium salt, can
advantageously
also be used in the form of a 20 % PdCl2-solution in concentrated hydrochloric
acid and in
the presence of dimethyl acetamide (DMA) as solubility promoter (Examples P4
and P17),
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-11 -
whereas the more expensive palladium(II) diacetate is substantially soluble,
for example, in
tetrahydrofuran. The desired ligand is advantageously added to the reaction
medium in a
molar excess of up to 10, based on the palladium salt. Heating the reaction
medium then
causes the palladium(II) or palladium(0) complex desired for the C-C coupling
reaction to
form 'in situ', which then starts the C-C coupling reaction.
Examples of suitable ligands for palladium(II) and palladium(0) complexes are
trimethyl-
phosphine, triethylphosphine, tris(tert-butyl)phosphine,
tricyclopentylphosphine, tricyclo-
hexylphosphine (PCy3), tri(methylcyclohexyl)phosphine,
methyl(tetramethylene)phosphine,
tert-butyl(pentamethylene)phosphine, triphenylphosphine (PPh3),
tri(methylphenyl)-
phosphine, 1,2-{diphenylphosphino)cyclohexane, 1,2-
(diphenylphosphino)cyclopentane,
2,2'-(diphenylphosphino)biphenyl, 1,2-bis(diphenylphosphino)ethane, 1,3-
bis(diphenyl-
phosphino)propane, 1,4-bis(diphenylphosphino)butane, 3,4-
bis(diphenylphosphino)-
pyrrolidine, 2,2'-{diphenylphosphino)-bisnaphthalene (BINAP), 1,1'-
bis(diphenylphosphino)-
ferrocene, 1,1'-bis(di-tert-butylphosphino)ferrocene, diphenyl ether bis-
diphenylphosphine
i PCYz
( I i ~ I - dpephos), and R,o \ , wherein
PPh2
PPh2 PPhz / ~ P(Ri~)2
R,o is hydrogen or dimethylamino, and R" is cyclohexyl or tert-butyl. These
latter electron-
rich and sterically bulky diphenyl derivatives are especially suitable
phosphine ligands for
the preparation of the present specific palladium catalysts, the so-called
Buchwald
catalysts. These, in turn, are especially suitable for the C-C linkage
according to the
invention of malonic acid dinitrile with polysubstituted aryl derivatives in
combination with
alkali metal hydrides or alkali metal phosphates as the base and toluene or
xylenes as
solvent.
The said ligands and palladium complexes are known and are described in a
number of
references in the literature, such as, for example, J. Am. Chem. Soc. 121,
4369-4378
(1999), EP-A-0 564 406, EP-A-0 646 590 and 'Palladium Reagents and Catalysts',
Editor
J. Tsuji, John Wiley & Sons, 1995.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-12-
Palladium(0) complexes with mono- and bi-dentate, tertiary or di-tertiary
amines,
phosphines and arsines as ligands are generally used. The N-, P- and/or As
atoms of those
ligands may be substituted by identical or different, straight-chain or
branched aliphatic
radicals containing from 1 to 18 carbon atoms, preferably from 1 to 12 carbon
atoms and
especially from 1 to 8 carbon atoms.
Also suitable are unsubstituted or C,-C4alkyi-substituted CS-C,cycloalkyl,
unsubstituted or
C,-C4alkyl-substituted C6-C,oaryl radicals, especially phenyl and alkylphenyl
radicals, and
the benzyl radical, which is unsubstituted or substituted by C,-C4alkyl.
Two of the aliphatic radicals bound to the hetero atoms N, P and/or As may
together form
an unsubstituted or C,-C4alkyl-substituted C4- or CS-hydrocarbon bridge,
thereby forming,
together with the hetero atom, a 5- or 6-membered heterocyclic ring.
In the case of bidentate ligands, two of the N, P and/or As atoms are
(bivalently) linked by
way of an aliphatic, unsubstituted or C,-C4alkyl-substituted CZ-Cshydrocarbon
chain. The
aliphatic, bivalent hydrocarbon chain can optionally be interrupted by 1 or 2
hetero atoms,
such as, for example O, N and/or S and/or may be part of a ring or ring
system.
Preference is given to phosphine ligands, especially basic and sterically
bulky phosphine
ligands, such as, for example, tricyclohexyl- or tri-tert-butyl-phosphine,
because the
concentration of the corresponding palladium complexes can then be
significantly reduced
(by a factor of from 3 to 10) without loss of their catalytic activity.
The palladium catalysts are used in an amount of from 0.001 to 50 mol %,
preferably in an
amount of from 0.01 to 10 mol % and especially in an amount of from 0.1 to 3
mol %, based
on the compound of formula II.
Suitable solvents for the formation of the malonic acid dinitrile anion (Step
1 in Reaction
Scheme 1 ) and for the palladium-catalysed C-C linkage reaction with the
compound of
formula II (Step 2 in Reaction Scheme 1 ) are aliphatic, cycloaliphatic or
aromatic hydro-
carbons, such as, for example, pentane, hexane, petroleum ether, cyclohexane,
methyl-
cyclohexane, benzene, toluene and xylenes, aliphatic halohydrocarbons, such
as, for
example, methylene chloride,, chloroform and di- or tetrachlorethane,
nitrites, such as, for
example, acetonitrile, propionitrile and benzonitrile, ethers, such as, for
example, diethyl
ether, dibutyl ether, tert-butyl methyl ether, ethylene glycol dimethyl ether,
ethylene glycol
diethyl ether, diethylene glycol dimethyl ether, tetrahydrofuran and dioxane,
alcohols, such
as, for example, methanol, ethanol, propanol, butanol, ethylene glycol,
diethylene glycol,
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-13-
ethylene glycol monomethyl or monoethyl ether and diethylene glycol monomethyl
or
monoethyl ether, ketones, such as, for example, acetone and methyl isobutyl
ketone, esters
or lactones, such as, for example, ethyl or methyl acetate and valerolactone,
N-substituted
lactams, such as, for example, N-methylpyrrolidone (NMP), amides, such as, for
example,
N,N-dimethylformamide (DMF) and dimethyl acetamide (DMA), acyclic ureas, such
as, for
example, N,N'-dimethylethylene urea (DMEU), sulfoxides, such as, for example,
dimethyl
sulfoxide or mixtures of these solvents. Of these, special preference is given
to aromatic
hydrocarbons, ethers and dimethyl sulfoxide.
Suitable bases for the preparation of the malonic acid dinitrile anion are
weakly nucleophilic
bases, such as, for example, tri-alkali metal phosphates, alkali metal and
alkaline earth
metal hydrides, alkali metal and alkaline earth metal amides and alkali metal
alcoholates, for
example tripotassium phosphate, potassium or sodium tert-butanolate, lithium
diisopropyl-
amide (LDA) and potassium or sodium hydride. The said bases are preferably
used in an
excess of from 2 to 10 equivalents, based on malonic acid dinitrile.
Base/solvent combinations especially suitable for producing the malonic acid
dinitrile anions
(Step 1 in Reaction Scheme 1 ) are, for example, alkali metal alcoholates in
aliphatic,
cycloaliphatic or aromatic hydrocarbons, for example sodium tert-butanolate in
xylene.
Combinations of palladium catalysts and leaving groups X in compounds of
formula II that
are especially suitable for the C-C linkage reaction (Step 2 in Reaction
Scheme 1 ) are
palladium(II)-bis(tricycloalkylphosphine) dihalides and halogens, for example
palladium(II)-
bis(tricyclohexylphosphine) dichloride and bromide.
Advantageously, the formation of the malonic acid dinitrile anion is carried
out at reaction
temperatures of from 0° to 100°C, preferably at temperatures of
from 20° to 80°C, and the
reaction thereof with the compound of formula II in the presence of the
palladium catalyst is
carried out at reaction temperatures of from 30° to 250°C,
preferably from 50° to 200°C and
especially from 80° to 160°C, depending on the reaction medium
and reaction pressure
used.
The C-C coupling reaction of the malonic acid dinitrile anion with a compound
of formula II
may optionally be carried out at an elevated pressure of from 1.1 to 10 bar.
That process in
a closed system at an elevated pressure is suitable especially for reactions
at temperatures
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-14-
above the boiling point of the solvent used, for example at temperatures of
140°C in the
case of toluene.
Because of the very small amount of (readily decomposable) palladium catalyst
used for the
C-C linkage reaction, it is advantageously introduced into the reaction
mixture under an
inert gas atmosphere and at the very end of the sequence of the reagent
addition (Step 2 in
Reaction Scheme 1 ) (Example P17).
The compounds of formula II wherein X is, for example, halogen are known or
can be
prepared by known methods, such as, for example, the Sandmeyer reaction, from
the
corresponding substituted anilines of formula VIII
NH2
Ri ~ Rs
i ~R~~n
(VIII),
Rz
wherein Ro, R,, R2, R3 and n are as defined for formula I, via the
corresponding diazonium
salts.
The compounds of formula II wherein X is, for example, R,oS(O)20- or mono-, di-
or tri-
arylmethoxy can be prepared by standard methods from the corresponding phenols
of
formula IX
OH
R~ ~ Ra
i ~R~~" (IX),
R2
wherein Ro, R,, Rz, R3 and n are as defined hereinbefore.
The substituted anilines of formula VIII either are known or can be prepared
by known
methods, for example as described in EP-A-0 362 667 via alkylation of anilines
using
olefins.
Similarly, the substituted phenols of formula IX either are known or can be
prepared, for
example, from the corresponding anilines of formula VIII or diazonium salts
thereof by so-
called Phenolic Boiling.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-15-
The following Reaction Scheme 2 illustrates the possible methods of preparing
the
compounds of formula II.
Reaction Scheme 2
NH2 X
R R
R~ ~ ~ R3 Sandmeyer e.g. '
(R°)n NaN02, HBr ( 48 %), T ~ / (R°)n
Rz Rz
VIII II: X=halogen e.g. Br
1 ) NaNOz, H30+
2) hydrolysis (Phenolic
Boiling), T
OH X
R1 I \ R(R°) R~oS(O)2CI or C6HSCH2Br, R~ I \ (R )
/ n solvent, base / ° n
R2 R2
IX II: X= R~oS(O)20, mono-, di- or
tri-arylmethoxy
The present substituted aryl dinitriles of formula I are in particular used as
intermediates for
the preparation of substituted 3-hydroxy-4-aryl-5-oxopyrazoline derivatives of
formula III
R°)n R~ O
Rs
R2 \ / \3 2 N (III),
N
R3 O~ Ra
wherein R°, R,, R2, R3 and n are as defined for formula I, and R4 and
RS are, each
independently of the other, hydrogen, C1-Cl2alkyl, C,-C,2haloalkyl, C2-
Cealkenyl, C2-C$-
alkynyl, C,-C,°alkoxy-C~-Cealkyl, poly-C,-C,°alkoxy-C,-Cealkyl,
C,-C,°alkylthio-C,-CBalkyl,
C3-Cecycloalkyl, C3-Cehalocycloalkyl, 4- to 8-membered heterocyclyl, phenyl, a-
or
~i-naphthyl, phenyl-C,-Csalkyl, a- or ~i-naphthyl-C,-Csalkyl, 5- or 6-membered
heteroaryl or
5- or 6-membered heteroaryl-C,-Csalkyl, wherein the aromatic and
heteroaromatic rings
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-16-
may be substituted by halogen, C,-Csalkyl, C,-Cshaloalkyl, C,-Cfialkoxy, C,-
Cshaloalkoxy,
nitro or by cyano, or
R4 and R5, together with the nitrogen atoms to which they are bonded, form a
saturated or
unsaturated, 5- to 8-membered heterocyclic ring that 1 ) may be interrupted by
oxygen,
sulfur or by -NR,- and/or substituted by halogen, C,-C,oalkyl, C,-
C,ohaloalkyl, hydroxy, C,-
Csalkoxy, C,-Csalkoxy-C,-Csalkoxy, C,-Cshaloalkoxy, mercapto, C,-Csalkylthio,
C3-C,cyclo-
alkyl, heteroaryl, heteroaryl-C,-Csalkyl, phenyl, phenyl-C,-Csalkyl or by
benzyloxy, wherein
the phenyl rings of the last 3 substituents may in turn be substituted by
halogen, C,-Csalkyl,
C,-Cshaloalkyl, C,-Csalkoxy, C,-Cshaloalkoxy or by nitro, and/or 2) may
contain a fused or
spiro-bound alkylene or alkenylene chain having from 2 to 6 carbon atoms that
is optionally
interrupted by oxygen or by sulfur, or at least one ring atom of the saturated
or unsaturated
heterocyclic ring bridges that alkylene or alkenylene chain; R, is hydrogen,
C,-C4alkyl,
C,-Csalkylcarbonyl, C,-Csalkylsulfonyl, C3-Csalkenyl or C3-Csalkynyl; and G is
hydrogen, a
metal ion equivalent or an ammonium, sulfonium or phosphonium ion, by either
a) hydrolysing a compound of formula I
NC~CH~CN
R1 ~ Rs
i ~R°~n
wherein Ro, R,, R2, R3 and n are as defined hereinbefore, to form a compound
of formula IV
HOOC~ ~COOH
CH
~ Rs
i ~R~~n (IV),
and then, by known standard procedures, in a manner known per se, either
a,) esterifying that compound with an alcohol of formula VII
R6-OH (VII),
wherein R6 is C,-Csalkyl, C,-Cshaloalkyl or benzyl, to form the arylmalonic
acid ester of
formula V
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-17-
RsOOC~ ~COORs
CH
R1 ~ Rs
~Ro~n (V),
R2
wherein Ro, R,, R2, R3, R6 and n are as defined hereinbefore, or
a2) converting that compound, using an acid halide, into the
halocarbonylketene of
formula X
O
CO-Hal
R1 R3
(X),
~Ro~n
R2
wherein Ro, R,, RZ, R3 and n are as defined hereinbefore and Hal is halogen,
or
b) subjecting a compound of formula I
NC~CH~CN
R~ ~ Rs
(I),
R2
wherein Ro, R,, RZ, R3 and n are as defined hereinbefore, to alcoholysis
directly with a
compound of formula VII
R6-OH (VII),
wherein R6 is as defined hereinbefore, to form a compound of formula V
R600C~ ~COORs
CH
Rs
~Ro~n (V),
R2
wherein Ro, R,, Rz, R3, Rs and n are as defined hereinbefore,
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-18-
and then reacting that compound of formula V or a compound of formula X
(variant a
followed by a2) with a compound of formula VI, Vla or Vlb
R
/ 5
HN M)~ HN ' ~H'~) Ma) a' HN ~ 2(Fi~Hal) (Vlb) ,
.Ra .R4 .Ra
wherein R4 and RS are as defined hereinbefore and H~Hal is a hydrogen halide,
in an inert
organic solvent, optionally in the presence of a base, and then optionally
converting the
resulting compound of formula III wherein G is a metal ion equivalent or an
ammonium
cation, by salt conversion into the corresponding salt of formula III wherein
G is a sulfonium
or phosphonium cation, or by treatment with a Bronsted acid into the
corresponding
compound of formula III wherein G is hydrogen.
Meanings corresponding to those given for compounds of formula I can be
ascribed to the
halogen, alkyl, haloalkyl, alkenyl, alkynyl, alkoxyalkyl, alkylthioalkyl,
cycloalkyl and
halocycloalkyl radicals present in the radicals R4 and RS in compounds of
formula III.
Polyalkoxy-alkyl is, for example, methoxymethoxy-methyl, ethoxymethoxy-methyl,
ethoxy-
ethoxy-methyl, n-propoxyethoxy-methyl, isopropoxyethoxy-methyl, methoxymethoxy-
ethyl,
ethoxymethoxy-ethyl, ethoxyethoxy-ethyl, n-propoxyethoxy-methyl, n-
propoxyethoxy-ethyl,
isopropoxyethoxy-methyl, isopropoxyethoxy-ethyl or (ethoxy)3-ethyl.
Phenyl and naphthyl may be in substituted form, in which case the substituents
may, as
desired, be in the ortho-, meta- and/or para-position and, in the case of the
naphthyl ring
system, in addition in the 5-, 6-, 7- and/or 8-position. Preferred positions
for the substituents
are the ortho- and para-position to the ring attachment point. Phenyl and
naphthyl
substituents are, for example, C,-C4alkyl, halogen, C,-Cshaloalkyl, C,-
Csalkoxy, C,-Cshalo-
alkoxy, nitro, cyano, amino, C,-C4alkylamino and di(C,-C4alkyl)amino.
Heterocyclyl radicals in the definition of R4 and RS are preferably 4- to 8-
membered rings
that contain 1 or 2 hetero atoms, such as, for example, N, S and/or O. They
are usually
saturated.
Heteroaryl radicals in the definition of R4 and RS are usually 5- or 6-
membered aromatic
heterocycles that preferably contain from 1 to 3 hetero atoms, such as N, S
and/or O.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-19-
Examples of suitable heterocyclyl and heteroaryl radicals are pyrrolidyl,
piperidyl, pyranyl,
dioxanyl, azetidyl, oxetanyl, pyridyl, pyrimidyl, triazinyl, thiazolyl,
triazolyl, thiadiazolyl,
imidazolyl, oxazolyl, isoxazolyl, pyrazinyl, furyl, thienyl, morpholyl,
piperazinyl, pyrazolyl,
benzoxazolyl, benzothiazolyl, quinoxalyl, indolyl and quinolyl. These
heterocycles and
heteroaromatic radicals may, in addition, be substituted, for example by
halogen, C,-Csalkyl,
C,-Csalkoxy, C,-Cshaloalkyl, C,-Cshalogenalkoxy, nitro or by cyano.
Metal ion equivalents, such as, for example, alkali metal or alkaline earth
metal ions, and
ammonium ions, for the substituent G in the compound of formula III are, for
example, the
cations of sodium, potassium, magnesium, calcium and ammonium, such as, for
example,
triethylammonium and methylammonium. Sulfonium cations include, for example,
tri(C,-C4-
alkyl)sulfonium cations and can be obtained, for example, by converting the
corresponding
alkali metal salts, e.g. with the aid of a cation exchanger.
The substituent definition according to which "R4 and R5, together with the
nitrogen atoms to
which they are bonded, form a saturated or unsaturated, 5- to 8-membered
heterocyclic ring
that 1 ) may be interrupted by oxygen, sulfur or by -NR,- and/or substituted
by halogen,
C,-C,oalkyl, C,-C,ohaloalkyl, hydroxy, C,-Csalkoxy, C,-Csalkoxy-C,-Csalkoxy,
C,-Cshalo-
alkoxy, mercapto, C1-Csalkylthio, C3-C,cycloalkyl, heteroaryl, heteroaryl-C,-
Csalkyl, phenyl,
phenyl-C,-Csalkyl or by benzyloxy, wherein the phenyl rings of the last 3
substituents may in
turn be substituted by halogen, C,-Csalkyl, C,-Cshaloalkyl, C,-Csalkoxy, C,-
Cshaloalkoxy or
by nitro, and/or 2) may contain a fused or spiro-bound alkylene or alkenylene
chain having
from 2 to 6 carbon atoms that is optionally interrupted by oxygen or by
sulfur, or at least one
ring atom of the saturated or unsaturated heterocyclic ring bridges that
alkylene or
alkenylene chain" signifies, for example, the following heterocyclic ring
systems in the
compounds of formula III:
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-20-
I
O~
~O
O
o ~I C ~I
o ~.
y ~ O ,~ or
In the above polycyclic ring systems, the abbreviated representation ~ denotes
O R~
the group N / ~ ~ R2 ,
\ ~R°~n
OG R3
The 5- to 8-membered heterocyclic rings that the substituents R4 and RS
together may form
and the fused or spiro-bound alkylene or alkenylene chains having from 2 to 6
carbon
atoms may, accordingly, be interrupted once or twice by hetero atoms.
The use of compounds of formula I in the preparation of 3-hydroxy-4-aryl-5-
oxopyrazoline
derivatives of formula III is illustrated in Reaction Scheme 3.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-21 -
Reaction Scheme 3
CN HOOC H COOH O
NC , C , . C i ~ \ CO-Hal
R~ \ R3 a) hydrolysis e.g. R~ \ R3 a2)acid i,aiides e.g. R~ \ Rs
(Ro)n Hzg04 aq.,~ ~ / (Ro)n soci2 ~ / (R~n
Rz Rz Rz
I IV X (Hal=CI)
solvent,
b) 1. alcoholysis e.g. a~) esterification e.g. base, HN - Rs
R60H, H+ , T + _ R I ~ (H~HaI)
cat. R60H, H HN s HN
VII Vll cat. I or R4
2. OHz HN . R Vla
VI 4 or HN ~ Rs
I ~2(H~HaI)
R600C , C ~ COOR6 HN . R
Vlb
R~ / R3 R1 O
(Ro) solvent, base, - N , Rs
\ n HN ' Rs HN ~ Rs Rz
or i ~(H~HaI)
Rz HN , R4 HN , R R30G . R4
a (Ro) n
V VI Vla
III (G=H, alkali, alkaline-earth metal
or HN ~ Rs or ammonium cation)
i ~2(H.HaI)
HN~R
4
Vlb
The hydrolysis of the arylmalonic dinitriles of formula I to form the
arylmalonic acids of
formula IV (Reaction Scheme 3, Route a) is carried out according to known
standard
procedures, such as, for example, by heating at about 50°C for several
hours in dilute
sulfuric acid.
The subsequent esterification of the resulting arylmalonic acid of formula IV
to form the
arylmalonic ester of formula V is carried out according to known standard
procedures, for
example by reaction with an excess of an alcohol of formula VII in the
presence of catalytic
amounts of acid (Reaction Scheme 3, Route a1).
Alternatively, the arylmalonic acids of formula IV can also be converted into
the correspond-
ing halocarbonylketenes of formula X, wherein Ro, R~, Rz, R3 and n are as
defined herein-
before and Hal is halogen, preferably chlorine or bromine, analogously to WO
97/02243 ,
using an acid halide, such as, for example, oxalyl chloride, thionyl chloride,
thionyl bromide,
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-22-
phosphorus trichloride, phosphorus pentachloride or phosgene, optionally in
the presence
of a catalyst, such as, for example, diethylformamide or triphenylphosphine
and optionally in
the presence of a base, such as, for example, pyridine or triethylamine, at
temperatures of
from -20°C to 200°C (Reaction Scheme 3, Route a2).
The arylmalonic dinitriles of formula I can also be converted directly into
the arylmalonic
acid esters of formula V via alcoholysis (Pinner reaction) with an alcohol of
formula VII in
the presence of catalytic amounts of acid, optionally at an elevated
temperature, and
subsequent working-up in an aqueous medium (Reaction Scheme 3, Route b). Such
alcoholysis reactions are described, for example, in Chem. Rev. 61, 179 (1961
).
The condensation of the arylmalonic acid esters of formula V with a hydrazine
derivative of
formula VI or a salt thereof of formula Vla or Vlb is carried out in a manner
analogous to
that described, for example, in WO 92/16510 or WO 97/02243 in an organic
solvent, such
as, for example, xylene, optionally in the presence of a base, such as, for
example,
triethylamine, and yields the desired target compound of formula III (G = H)
or a salt thereof
(G = alkali metal or alkaline earth metal ion equivalent or ammonium ion)
depending on the
working-up method and on the base used in the condensation reaction. The
corresponding
sulfonium and phosphonium salts can be produced by means of salt conversion,
for
example using a cation exchanger.
The condensation reaction of the compounds of formula V with compounds of
formula VI
can also be carried out in the absence of a base, whereas the same
condensation reaction
with compounds of formula Vla or Vlb (instead of a compound of formula VI) is
advantageously carried out in the presence of a base (in an equimolar amount).
The condensation of the halocarbonylketenes of formula X with a hydrazine
derivative of
formula VI or a salt thereof of formula Vla or Vlb to form compounds of
formula III is carried
out in a manner analogous to that described, for example, in WO 97/02243,
optionally in an
organic solvent, such as, for example, toluene or xylene and in the presence
of a base,
such as an alkaline earth metal carbonate, pyridine or triethylamine, at
temperatures of from
-20°C to 250°C.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-23-
If the starting materials employed are not enantiomerically pure, the
compounds of
formula III obtained in the above-described process are generally in the form
of racemates
or diastereoisomeric mixtures which, if desired, can be separated on the basis
of their
physicochemical properties according to known methods, such as, for example,
fractional
crystallisation following salt formation with optically pure bases, acids or
metal complexes,
or by chromatographic procedures, such as, for example, high-pressure liquid
chromatography (HPLC) on acetyl cellulose.
Depending on the substituents Ro to RS and G, the compounds of formula III may
be in the
form of geometric and/or optical isomers and isomeric mixtures (atropisomers)
or as
tautomers and tautomeric mixtures.
The compounds of formulae VI, Vla and Vlb either are known or can be prepared
analog-
ously to known methods as described, for example, in WO 95/00521 and WO
99/47525.
The alcohols of formula VII are known.
The Examples that follow further illustrate the invention without limiting it.
Preparation Examples:
Example P1: Preparation of 2.6-diethyl-4-methylbromobenzene
A solution of 8.83 g (0.128 mol) of sodium nitrite in 200 ml of water is added
dropwise, at
4°C, within a period of 4 hours, to a suspension of 20 g (0.1225 mol)
of 2,6-diethyl-4-
methylaniline in 500 ml of 48 % hydrobromic acid and the brown solution is
then heated to
80°C. After stirring for one hour at that temperature, the reaction
mixture is cooled to 20°C,
diluted with 1 litre of water and extracted 3 times with hexane. The combined
organic
phases are washed twice with brine, dried over sodium sulfate and concentrated
in vacuo at
60°C. 27.5 g of crude product are obtained, purification of which by
silica gel
chromatography (500 g of silica gel; eluant: hexane) yields 19.89 g (71 % of
theory) of the
desired target compound in the form of a colourless oil.'H-NMR (CDC13): 6.89
ppm (s, 2H);
2.75 ppm (q, 4H); 2.27 ppm (s, 3H); 1.22 ppm (t, 6H).
Example P2: Preparation of 2.6-diethyl-4-methyliodobenzene
100 g of 4-methyl-2,6-diethylaniline and then 480 g of ice are added to a
solution of 91.4 ml
of sulfuric acid in 370 ml of water. To the resulting reaction solution there
are added drop-
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-24-
wise, at from 0 to 5°C, a solution of 44.6 g of sodium nitrite in 110
ml of water within a
period of 45 minutes and, subsequently, a solution of 136.4 g of potassium
iodide in 150 ml
of water. After stirring for 15 hours at 20°C, the reaction mixture is
extracted 3 times with a
total of 3 litres of tert-butyl methyl ether (TBME); the combined organic
phases are washed
once with 8 % hydrochloric acid and water, dried and concentrated. 137.75 g of
crude
product are obtained, purification of which by distillation (boiling point
125°C/5 mbar) yields
57.8 g of the desired target compound in the form of a colourless liquid.'H-
NMR (CDC13):
6.90 ppm (s, 2H); 2.75 ppm (q, 4H); 2.30 ppm (s, 3H); 1.23 ppm (t, 6H).
Example P3: Preparation of 4-methYlphenylmalonic acid dinitrile
0.18 g of malonic acid dinitrile is dissolved in 15 ml of xylene and 0.72 g of
sodium tert-
butanolate is slowly added dropwise to the resulting solution. The yellow
suspension formed
is stirred at 20°C for 1 hour. 0.43 g of 4-bromotoluene and 0.035 g of
bis(triphenyl-
phosphine)palladium(II) dichloride (Pd(PPh3)2CI2) are then added and further
stirring is
carried out overnight at an external temperature of 150°C. For working-
up, 25 ml of water
and 25 ml of 1 N hydrochloric acid are then added to the reaction mixture and
extraction with
diethyl ether is carried out. The combined ether phases are dried over sodium
sulfate and
concentrated. 0.72 g of crude product is obtained; its content of desired
title compound is
determined as 54 % by HPLC (high-pressure liquid chromatography on Nucleosil;
eluant:
acetonitrile/water + 0.1 % trifluoroacetic acid (TFA)), resulting in a yield
of pure compound
of 97 % of theory.
Example P4: Preparation of 4-meth~phenylmalonic acid dinitrile
0.18 g of malonic acid dinitrile is dissolved in 13 ml of xylene, and 0.72 g
of sodium tert-
butanolate is slowly added dropwise to the resulting solution. The yellow
suspension formed
is stirred at 20°C for 1 hour. 0.43 g of 4-bromotoluene and 0.2 ml of a
concentrated
hydrochloric acid solution of 20 % palladium(II) dichloride (PdCl2) in DMA
(about 0.01 mol),
and 65 g of triphenylphosphine (PPh3) in 2 ml of xylene are then added. The
yellow
suspension is stirred overnight at an external temperature of 150°C.
For working-up, 25 ml
of water and 25 ml of 1 N hydrochloric acid are then added to the reaction
mixture and
extraction with diethyl ether is carried out. The combined ether phases are
dried over
sodium sulfate and concentrated. 0.91 g of crude product is obtained; its
content of desired
title compound is determined as 40.5 % by HPLC (Nucleosil; eluant:
acetonitrile/water +
0.1 % trifluoroacetic acid (TFA)), resulting in a yield of pure compound of 97
% of theory.
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-25-
Example P5: Preparation of 2.4.6-trimethylphenylmalonic acid dinitrile
13.2 g of malonic acid dinitrile are dissolved in 500 ml of tetrahydrofuran,
and 12 g of
sodium hydride (NaH, 60 %) are slowly added to the resulting solution. The
yellow
suspension formed is stirred at 20°C for 1 hour. 25 g of 2;4,6-mesityl
iodide and 0.738 g of
palladium(II)-bis(tricyclohexylphosphine) dichloride (Pd(PCy3)2C12) are then
added and the
yellow suspension is further stirred overnight at an external temperature of
80°C. For
working-up, 500 ml of water and 500 ml of 1 N hydrochloric acid are then added
to the
reaction mixture and extraction with diethyl ether is carried out. The
combined ether phases
are dried over sodium sulfate and concentrated. 32.2 g of crude product are
obtained.
Purification is carried out by silica gel chromatography (eluant: ethyl
acetate/hexane 1/10)
and yields 16 g (86 % of theory) of the desired title compound.
Example P6: Preparation of 2,6-dieth I-4-methylphenylmalonic acid dinitrile
0.66 g of malonic acid dinitrile is dissolved in 30 ml of tetrahydrofuran, and
0.6 g of sodium
hydride (60 %) is slowly added to the resulting solution. The yellow
suspension formed is
stirred at 20°C for 1 hour. 1.46 g of 2,6-diethyl-4-methyliodobenzene
and 0.11 g of
palladium(II)-bis(tricyclohexylphosphine) dichloride (Pd(PCy3)2C12) ) are then
added and the
yellow suspension is further stirred overnight at an external temperature of
80°C. For
working-up, 25 ml of water and 25 ml of 1 N hydrochloric acid are then added
to the reaction
mixture and extraction with diethyl ether is carried out. The combined ether
phases are
dried over sodium sulfate and concentrated. 1.9 g of crude product are
obtained.
Purification is carried out by silica gel chromatography (eluant: ethyl
acetate/hexane 1/10)
and yields 0.62 g (58 % of theory) of the desired title compound.'H-NMR
(CDC13): 7.05 ppm
(s, 2H); 5.32 ppm (s, 1 H); 2.85 ppm (q, 4H); 2.37 ppm (s, 3H); 1.35 ppm (t,
6H).
Example P7: Preparation of 2.6-diethyl-4-methylphenylmalonic acid dinitrile
0.66 g of malonic acid dinitrile is dissolved in 30 ml of dimethyl sulfoxide
(DMSO), and 0.6 g
of sodium hydride (60 %) is slowly added to the resulting solution. The yellow
suspension
formed is stirred at 20°C for 1 hour. 1.18 g of 2,6-diethyl-4-
methylbromobenzene and 0.07 g
of palladium(II)-bis(tricyclohexylphosphine) dichloride (Pd(PCy3)2CI2) are
then added and the
yellow suspension is further stirred overnight at an external temperature of
150°C. For
working-up, 25 ml of water and 25 ml of 1 N hydrochloric acid are then added
to the reaction
mixture and extraction with diethyl ether is carried out. The combined ether
phases are
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
- 26 -
dried over sodium sulfate and concentrated. 1.5 g of crude product are
obtained.
Purification is carried out by silica gel chromatography (eluant: ethyl
acetate/hexane 1/10)
and yields 0.88 g (83 % of theory) of the desired title compound.
Example P8: Preparation of 2.6-diethyl-4-meth~lphenylmalonic acid dinitrile
0.18 g of malonic acid dinitrile is dissolved in 15 ml of xylene, and 0.72 g
of sodium tert-
butanolate is added to the resulting solution. The yellow suspension formed is
stirred at
20°C for 1 hour. 0.59 g of 2,6-diethyl-4-methylbromobenzene and 0.035 g
of bis(triphenyl-
phosphine)palladium(II) dichloride (Pd(PPh3)2C12) are then added and the
yellow suspension
is further stirred overnight at an external temperature of 150°C. For
working-up, 25 ml of
water and 25 ml of 1 N hydrochloric acid are then added to the reaction
mixture and
extraction with diethyl ether is carried out. The combined ether phases are
dried over
sodium sulfate and concentrated. 0.92 g of crude product is obtained.
Purification is carried
out by silica gel chromatography (eluant: ethyl acetate/hexane 1/10) and
yields 0.47 g
(89 % of theory) of the desired title compound.
Example P9: Preparation of 4-bromo-2.6-diethylaniline
27 ml (0.5 mol) of bromine in 50 ml of glacial acetic acid are added dropwise,
at 10°C, to a
solution of 74.6 g (0.5 mol) of 2,6-diethylaniline in 200 ml of glacial acetic
acid. Stirring is
carried out at 20°C for 1 hour to complete the reaction; the reaction
mixture is poured into
an ice/water mixture, is rendered alkaline with sodium hydroxide solution and
is extracted
twice with ethyl acetate. The combined organic phases are washed with sodium
thiosulfate
solution and with brine, dried over sodium sulfate and concentrated. 112.4 g
of crude oil are
obtained, purification of which by distillation (boiling point 129-131
°C/1 mbar) yields 92 g
(81 % of theory) of the desired target compound in the form of an oil.
'H-NMR (CDCI3): 7.07 ppm (s, 2H); 3.60 ppm (broad signal, 2H); 2.50 ppm (q,
4H);
1.25 ppm (t, 6H).
Example P10: Preparation of 4-phenyl-2.6-diethylaniline
912 mg (0.004 mol) of 4-bromo-2,6-diethylaniline, 732 mg (0.006 mol) of
phenylboric acid
and 1820 mg (0.012 mold of caesium fluoride (CsF) are placed in 20 ml of
degassed
dioxane, and a solution of 18 mg (0.00008 mol) of Pd(OAc)2 and 47 mg (0.00012
mol) of
(2'-dicyclohexylphosphanylbiphenyl-2-yl)dimethylamine in 1 ml of dioxane is
added; stirring
is carried out at 20°C for 16 hours. The reaction mixture is poured
into dilute sodium
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-27-
hydroxide solution and is extracted twice with ethyl acetate. The organic
phases are
washed with brine, dried over sodium sulfate and concentrated. 1.0 g of crude
oil is
obtained, which is chromatographed over silica gel, resulting in a yield of
pure compound of
742 mg (82 % of theory) of an oil.
'.H-NMR (CDC13): 7.55 ppm (m, 2H); 7.39 ppm (m, 2H); 7.25 ppm (m, 1 H); 7.21
ppm (s, 2H);
3.70 ppm (broad signal, 2H); 2.60 ppm (q, 4H); 1.28 ppm (t, 6H).
Example P11: Preparation of 1.4-dibromo-2,6-diethylbenzene
5.7 g (0.025 mol) of 4-bromo-2,6-diethylaniline are placed in 10 ml of water
and 10 ml of
48 % hydrobromic acid, and 5.25 ml (0.02625 mol) of a 5 molar sodium nitrite
solution are
added dropwise at about 0°C. Stirring is carried out in an ice bath for
30 minutes and then
at 100°C for 45 minutes. The reaction mixture is diluted with water and
extracted twice with
ethyl acetate. The organic phases are washed with brine, dried over sodium
sulfate and
concentrated. 6.48 g of a crude oil are obtained, yielding, after
chromatography over silica
gel, 3.62 g (50 % of theory) of the desired product in the form of an oil.
'H-NMR (CDCI3): 7.20 ppm (s, 2H); 2.75 ppm (q, 4H); 1.22 ppm (t, 6H).
Example P12: Preparation of 4-(2-pYridyl)-1-bromo-2.6-diethylbenzene
790 mg (0.005 mol) of 2-bromopyridine are placed in 7 ml of tetrahydrofuran at
-70°C, and
6.7 ml (0.010 mol) of a 1.5 molar solution of tert-butyllithium in pentane are
added dropwise.
Stirring is carried out in a COZ bath for 15 minutes; a solution of 1.12 g
(0.005 mol) of zinc
bromide in 8 ml of tetrahydrofuran is then added dropwise and the batch is
stirred without
further cooling. Then, at 20°C, 1.46 g (0.005 mol) of 1,4-dibromo-2,6-
diethylbenzene and
288 mg (0.00025 mol) of tetrakis(triphenylphenylphosphine)palladium(0) are
added and
then the batch is stirred at a bath temperature of 60°C for 1.5 hours.
The reaction mixture is
poured onto saturated ammonium chloride solution and extracted twice with
ethyl acetate.
The combined organic phases are washed with brine, dried over sodium sulfate
and
concentrated. 1.79 g of crude oil are obtained, yielding, after chromatography
over silica
gel, 700 mg (48 % of theory) of the desired title compound in the form of an
oil.
'H-NMR (CDCI3): 7.74 ppm (m, 2H); 7.70 ppm (s, 2H); 7.23 ppm (m, 1 H); 2.87
ppm (q, 4H);
1.30 ppm (t, 6H).
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-28-
Example P13: Preparation of 4-phenyl-1-bromo-2 6-diethylbenzene (4-bromo-3,5-
diethyl-
biphenyl)
609 mg (0.0027 mol) of 4-phenyl-2,6-diethylaniline are emulsified in 2 ml of
water and, at
0°C, 2 ml of hydrogen bromide solution (48 %) are added. 0.568 ml
(0.002842 mol) of
molar sodium nitrite solution is then added thereto and stirring is carried
out in an ice bath
for 30 minutes and then at 100°C for 45 minutes. The reaction mixture
is cooled, diluted
with ice-water and extracted twice with ethyl acetate. The combined organic
phases are
washed with water and brine, dried over sodium sulfate and concentrated. 730
mg of a
crude product are obtained, yielding, after chromatography over silica gel,
the desired target
compound, having a melting point of 72-74°C, in a yield of 347 mg (44 %
of theory).
' H-NMR (CDCI3): 7.58 ppm (m, 2H); 7.43 ppm (m, 2H); 7.35 ppm (m, 1 H); 7.28
ppm (s, 2H);
2.87 ppm (q, 4H); 1.28 ppm (t, 6H).
Example P14: Preparation of 2.6-diethyl-4-phenylphenylmalonic acid dinitrile
84 mg (0.00128 mol) of malonic acid dinitrile are dissolved in 7 ml of
degassed xylene,
84 mg (0.00349 mol) of sodium tert-butanolate are added and stirring is
carried out at 20°C
for 30 minutes. 336 mg (0.00116 mol) of 4-phenyl-1-bromo-2,6-diethylbenzene
and 16.3 mg
(0.0000232 mol) of bis(triphenylphosphine)palladium(II) dichloride are then
added and
stirring is carried out at 150°C for 17 hours. The reaction mixture is
poured into ice-water
acidified with hydrochloric acid and is extracted twice with ethyl acetate.
The combined
organic phases are washed with water and brine, dried over sodium sulfate and
concentrated. The desired title compound is obtained in a yield of 315 mg (99
% of theory).
'H-NMR (CDCI3): 7.35-7.50 ppm (m, 3H); 7.40 ppm (s, 2H); 5.35 ppm (s, 1H);
2.93 ppm (q,
4H); 1.39 ppm (t, 6H).
Analogously to the Examples hereinbefore, the following compounds (Examples
P15 and
P16) are also obtained:
2.6-diethyl-4- 2-pyridyl~phenylmalonic acid dinitrile, 'H-NMR (CDCI3): 8.72
ppm (m, 1 H);
7.82 ppm (s, 2H); 7.77 ppm (m, 2H); 7.28 ppm (m, 1 H); 5.37 ppm (s, 1 H); 2.95
ppm (q, 4H);
1.41 ppm (t, 6H), and
2.6-dimethyl-4- 4-pyridYl)-phenylmalonic acid dinitrile,'H-NMR (CDC13): 8.70
ppm (d, 2H);
7.47 ppm (d, 2H); 7.40 ppm (s, 2H); 5.39 ppm (s, 1 H); 2.64 ppm (s, 6H).
CA 02368827 2001-11-09
WO 00/78712 PCT/EP00/05477
-29-
Example P17: Preparation of 2.6-diethyl-4-methyl-phenyl-malonic acid dinitrile
198.8 g (2.06 mol) of sodium tert-butanolate and 600 ml of xylene are placed
in a 2.5 litre
sulfonation flask having an internal thermometer, argon gas connection and
reflux
condenser or distillation head and then, at 60°C, a melt of 50.4 g
(0.76 mol) of malonic acid
dinitrile is added to the resulting solution, the reaction temperature
increasing to 103°C.
About 50 ml of the tert-butanol formed are distilled off at 80°C within
a period of 70 minutes
under a gentle stream of argon gas and then 496 g of 2,6-diethyl-4-
methylbromobenzene
(31.4 % in xylene) are added at the same temperature. The reaction solution is
then heated
at 130°C for 2 hours.
At the same time, 1.98 g of tricyclohexylphosphine are placed in a separate
100 ml round-
bottom flask under argon gas; a mixture of 35 ml of dry xylene and 27 ml of
N,N-dimethyl
acetamide (DMA) is introduced by syringe and the resulting solution is
degassed. 0.73 g of
a 20 % palladium(II) chloride solution (conc. hydrochloric acid) is then added
by syringe and
the resulting yellow suspension is stirred at 20°C for 1 hour. The
catalyst solution prepared
in that manner is introduced into the above reaction solution at 105°C
by syringe and the
suspension formed is then stirred at from 120 to 130°C for 2 hours. A
sample analysed by
gas chromatography shows 100 % reaction without by-products or starting
materials.
For working-up, the reaction mixture is cooled to 40°C and 800 ml of
ice/water mixture are
added. The aqueous phase (about 1.4 litres) is separated off and 365 ml of
water/xylene
mixture are distilled off using a rotary evaporator. The aqueous phase is then
cooled further
to 15°C and subsequently 142 g of 32 % hydrochloric acid solution are
added so that the
pH-value is from 5 to 5.5. The crystalline crude product precipitates out and
can readily be
filtered off and then washed with 250 ml of water. The resulting 163 g (112 %
of theory) of
crude product are dried overnight in a vacuum drying cabinet at 60°C,
yielding 143.8 g
(99 % of theory) of the desired title compound having a purity of 98.8 %.