Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02369244 2002-O1-24
AN INTEGRATED METHOD FOR STEAM-ENHANCED BITUMEN
PRODUCTION USING A PROCESS WASTE STREAM FOR FLUE GAS
DE SULPHURIZATION
Field of the Invention
The invention is in the area of thermal recovery of bitumen from an
underground
reservoir using steam. More specifically, it relates to a method for
integrating bitumen
production with steam generation by burning a portion of the produced high-
sulfur bitumen
and then using a waste stream from a boiler feed water treatment process to
clean the flue gas.
Background of the Invention
In the thermal recovery of bitumen, natural gas is normally used as a fuel to
generate
steam. This fuel has little or no sulfur and hence its use does not require
SOZ removal from
the flue gas. Fuel cost constitutes the majority share of the bitumen lifting
cost at many
production sites including places such as Cold Lake and Athabasca in Alberta,
Canada. With
increasing demand for and a generally upward trend in the price of natural
gas, the need for
an alternative, less expensive fuel is strong.
Many alternative fuel sources have been considered including coal and bitumen
which
may include both whole bitumen and/or the heavier fractions of bitumen. Of
these, whole
bitumen and water emulsions of the whole bitumen and/or the heavier fractions
of bitumen
are the most promising. However, significant problems exist with the use of
bitumen as a fuel
because of the high cost associated with removing SOZ from the flue gas which
results from
burning bitumen.
Furthermore, in a commercial thermal bitumen recovery operation large volumes
of
water are needed to make steam. This water becomes contaminated with bitumen
and
underground minerals during underground injection and subsequent recovery. The
produced
water from bitumen recovery therefore requires treatment before it can be
reused to make
steam. That is, it is a requirement that contaminated water from the
production of bitumen be
treated with chemicals to remove contaminants to enable the water to be re-
used in a steam
generator and that the contaminants are properly disposed of. Accordingly,
past bitumen
recovery operations utilize water treatment facilities which introduce
treatment chemicals to
-1-
CA 02369244 2002-O1-24
the produced water to remove the contaminating hardness ions, silica and/or
clay minerals.
The treatment chemicals enable most contaminants to be removed as precipitated
solids which
are subsequently removed from the facility as waste to a slurry pit.
Accordingly, there is a continuing need to improve the operating cost
structure at a
bitumen production operation of which fuel is a major component. One way this
can be
achieved is by efficiently combining the bitumen burning, the water treatment
and the SOZ
removal processes wherein the waste slurry from treatment of the produced
water, containing
the precipitated contaminants, is used within the SOZ scrubbing facilities in
order to reduce
the S02 removal cost.
A review of the prior art reveals that such a solution has not been provided.
For
example, Canadian Patent No. 1,339,531 discusses a process of producing and
burning a
natural-emulsified liquid fuel in which the alkali metal level in the liquid
fuel is adjusted to
reduce SOz emissions and Canadian Patent No. 2,105,166 relates to scrubbing
SOZ with
mixtures of hydroxides and carbonates of calcium and magnesium in coal-fired
boilers; and
Canadian Patent Application No. 2,335,771 describes a process that uses
hydrovisbreaking
in the reservoir, and fractionates the produced visbroken hydrocarbons into a
heavier fraction,
which is then partially oxidized to generate,hydrogen and fuel gas. In this
latter patent, the
fuel gas is used for steam generation and the steam is then injected with
hydrogen for in situ
hydrovisbreaking.
Summary of the Invention
In accordance with the invention, there is provided an integrated process for
producing
bitumen and removing sulfur dioxide from a flue gas said process comprising:
(a) burning whole bitumen or a heavier fraction thereof to generate steam to
extract bitumen from a bitumen reservoir in a bitumen and produced water
stream, said burning yielding said flue gas;
(b) removing SOZ from said flue gas by contacting said flue gas with a waste
slurry resulting from treatment of water separated from said bitumen and
produced water stream.
-2-
CA 02369244 2002-O1-24
Further still, the invention provides a process for producing bitumen from a
subsurface
reservoir comprising the steps of:
(a) inj ecting steam into a reservoir to recover bitumen;
(b) producing a stream of bitumen and produced water from the reservoir;
(c) separating the produced water and the bitumen;
(d) treating the produced water to form a boiler-feed quality water and a
waste
slurry;
(e) burning at least a portion of the bitumen and using at least a portion of
the
boiler-feed quality water to produce steam and a flue gas; and
(fj contacting the flue gas with the waste slurry to remove sulfur dioxide
from the
flue gas.
In another embodiment, the invention provides a process for scrubbing sulfur
dioxide
from a flue gas comprising contacting the flue gas with a waste slurry from a
water treatment
process, the waste slurry chemically active in removing sulfur dioxide from
the flue gas.
In further embodiments, the water treatment process is operatively connected
to a
bitumen recovery operation and/or the waste slurry comprises at least one of
calcium
hydroxide, magnesium hydroxide, calcium carbonate and a magnesium silicate
mineral and
one or more alkaline compounds. It is also preferred that the total solids
concentration in the
waste slurry is 4-20% by weight and more preferably 5-15% by weight.
In another preferred embodiment, the process is integrated with a bitumen-
fired steam
generation process and the flue gas originates from the bitumen-fired steam
generation
process.
Brief Description of the Drawings
Preferred embodiments of the present invention will now be described with
reference
to the attached figures, wherein:
Figure 1 is a schematic diagram of the presently practiced process of bitumen
production in accordance with the prior art;
Figure 2 is a configuration for a high sulfur fuel bitumen production
operation; and
Figure 3 is a schematic diagram of an integrated bitumen production, waste-
water
-3-
CA 02369244 2002-O1-24
treatment and SOZ scrubbing process in accordance with the invention.
Detailed Description of the Invention
As shown in Figure 1, in a conventional commercial bitumen recovery operation
l,
steam 2 is generated from water 3 and treated water 3a by burning a natural
gas fuel 4 in a
steam generator or boiler 16. The steam 2 is injected into an underground
reservoir 6 to reduce
the viscosity of the underground bitumen by heat thereby enabling the bitumen
to be
recovered as a bitumen/water mixture 7~ The steam 2 and condensed water also
react with the
naturally occurring minerals in the reservoir 6 and pick up hardness ions,
silica, and clay
minerals which contaminate the water portion of the bitumen/water mixture 7.
Separation of
the mixture in a separator 8 provides product bitumen 9 and produced water 9a.
The produced
water 9a is treated in a water treatment facility 7a with chemicals 7c which
remove the
contaminants as a sludge/slurry 7b enabling re-use of the treated water 3 in
the production of
steam 2. The flue gas 16a does not require scrubbing.
Figure 2 shows a variation 1 a of the conventional bitumen recovery operation
in which
the natural gas fuel can be substituted for by a high sulfur fuel such as
bitumen or an emulsion
of bitumen or its heavier fractions 4a. Unlike burning natural gas, the
burning of a high sulfur
fuel produces a high sulfur flue gas 16a which requires scrubbing of SOZ in a
conventional
scrubber 5. Scrubber 5, using techniques known in the art, removes SOZ by
reacting the SOz
with scrubbing chemicals 5a which will produce a scrubber slurry Sb which is
disposed in a
slurry pit. The cost of the chemicals 5a is such that the process is not
always cost-competitive
with burning natural gas, depending on the price of natural gas.
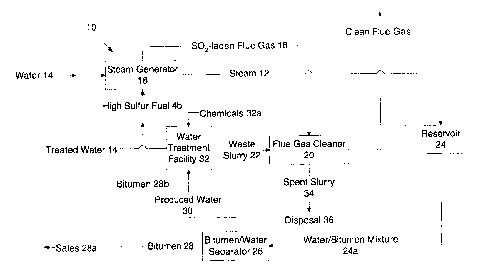
In accordance with the invention, an integrated process 10 of water treatment
and
scrubbing is shown in Figure 3 for a high sulfur fuel fired steam generation
and bitumen
recovery process. As shown, and as in a conventional bitumen recovery
operation described
above, steam 12 is generated from water 14 and treated water 14a by burning a
fuel (including
whole bitumen or heavy fractions thereof) in a steam generator 16 for
injection into the
reservoir 24. Recovered bitumen and produced water 24a are separated from each
other in a
bitumen-water separator 26 to produce bitumen 28 and produced water 30. The
produced
water 30 is treated in a water treatment facility 32 to remove contaminants
including hardness
ions, silica and clay through the addition of chemicals 32a. A portion of the
bitumen 28b is
-4-
CA 02369244 2002-O1-24
preferably used as fuel for the steam generator 16 and a portion 28a is
directed to sales.
The produced water 30 is treated by techniques known in the art. Within the
water
treatment facility 32, chemicals including calcium hydroxide, magnesium
hydroxide, sodium
hydroxide, sodium carbonate, and a flocculant are added to the water, as
necessary, to react
with or provide adsorption sites for the hardness ions, silica and clay
minerals thereby forming
a waste slurry 22 which has both a liquid and a precipitated solids component.
The waste slurry 22 generally comprises unreacted chemicals and reacted
product. The
typical solid concentration range in the waste slurry is 4 to 20% by weight
with the
corresponding water concentration range as 96 to 80% by weight. A preferred
concentration
range of the solids is 5 to 15% by weight with the corresponding water
concentration range
as 95 to 85% by weight.
The SOZ laden flue gas 18 from the generator 16 is directed to a flue gas
cleaner or
scrubber 20 where, in accordance with the invention, it is contacted with the
waste slurry 22
from the water treatment facility 32 thus utilizing both the reacted and
unreacted chemicals
of the waste slurry 22 within the scrubbing process. In most circumstances, no
additional
chemicals need to be added. After treatment of the SOz-laden flue gas 18, the
spent slurry 34
is sent to disposal 36.
The precise composition of the chemicals added to the produced water in the
water
treatment facility may be varied as is known by those skilled in the art for
effecting water
treatment and will depend on the particular contaminants that may result from
a particular
reservoir. In order to provide effective scrubbing capabilities, it is
preferred that the waste
slurry composition include calcium hydroxide, magnesium hydroxide, calcium
carbonate and
a magnesium silicate mineral, some of which may be present in the water
produced from the
reservoir before treatment. A representative composition of this slurry is
shown in the
examples.
Accordingly, the system provides an effective way of combining the use of
bitumen
as a fuel and scrubbing SOz gas from combustion with chemicals contained in
the slurry
generated by treating the produced water from bitumen extraction.
As a result, an advantage of the invention is that the chemicals for SOZ
scrubbing are
continuously generated in the water treatment facility. Moreover, no
additional slurry pit is
required as per other conventional flue gas desulfizrization processes.
Further still, the fuel for
-5-
CA 02369244 2002-O1-24
the process is generated internally.
In another embodiment where bitumen is used as a fuel, part of the total
produced
bitumen 28 may be used in the generator 16 and the remainder shipped to market
28a. In one
embodiment, further separation of the bitumen 28 is provided to produce
heavier and lighter
bitumen fractions where the heavier fraction 28b is used as a fuel in the
generator 16 and the
lighter fraction is shipped to market. As a fuel, the heavier fraction 28b is
may be emulsified
in water and used as an emulsion fuel in the generator 16.
Moreover, the utilization of the waste stream for SOZ scrubbing offers a cost-
effective
method for utilizing sulfur-rich fuel that is cheaper than natural gas. The
fuel is also internally
generated as whole or a fraction of the produced bitumen; in the latter case
also improving the
viscosity and gravity of the remaining marketable bitumen.
The invention is illustrated by the following example.
EXAMPLE
Two litres of waste slurry from the Imperial Oil water treatment facility at
Cold Lake,
Alberta were separated into a water portion and a solids portion for analysis.
The water portion of the waste slurry had the following representative
properties and
composition as shown in Table 1:
Table 1- Water Composition of Waste Slurry
Properties/Composition Preferred Range General Range
pH at 22C 9.5 to 10 9 to 11
Total Dissolved Solids 10000 to 12000 ppm 8000 to 14000
ppm
Total Hardness 80 to 160 ppm 70 to 180 ppm
Tannin and Lignin 60 to 65 ppm 50 to 80 ppm
Alkalinity Phen. 8.3 300 to 400 ppm 250 to 500 ppm
as CaC03
Alkalinity Total as 500 to 650 ppm 400 to 800 ppm
CaC03
Carbonate Ions 200 to 300 ppm 150 to 400 ppm
Hydroxyl Ions 40 to 70 ppm 30 to 100 ppm
Chloride Dissolved 5000 to 7000 ppm 4000 to 10000
ppm
Total Organic Carbon 140 to 160 ppm 120 to 200 ppm
Silica 10 to 50 ppm 5 to 100 ppm
Sulfate 60 to 100 ppm 50 to 120 ppm
Magnesium Ions 10 to 30 ppm 5 to 40 ppm
Calcium Ions 14 to 15 ppm 10 to 40 ppm
Potassium Ions 150 to 180 ppm 130 to 200 ppm
-6_
CA 02369244 2002-O1-24
Sodium Ions 3500 to 4500 ppm 3000 to 5000 ppm
Sulfur Dissolved 30 to 40 ppm 25 to 50 ppm
The slurry may also contain a non-ionic flocculating agent, such as NALCO
86070.
The solid portion of the waste slurry contained the following metal ions as
shown in
Table 2A:
Table 2A- Solids Portion Composition of Waste Slurry
Properties/CompositionPreferred Range General Range
Mg 10 to 20 wt% 5 to 25 wt%
Ca 20 to 25 wt% 15 to 35 wt%
Si 5 to 6 wt% 4 to 8 wt%
Na 0:45 to 0.80 wt% 0.4 to 1.0 wt%
A1 0:04 to 0.08 wt% 0.03 to 0.10
wt%
K 0:04 to 0.05 wt% 0.03 to 0.06
wt%
Fe 0.15 to 0.25 wt% 0.10 to 0.40
wt%
Sr 1500 to 2400 ppm 1200 to 3000
ppm
Ba 500 to 700 ppm 400 to 1000 ppm
By mineralogical analysis, the solid portion of the waste slurry contains the
minerals
shown in Table 2B and their typical proportions. These minerals were
identified by X-ray
diffraction and scanning electron microscope and their relative amounts
determined using
elemental analysis and mineral quantification software. The adsorbed water in
the solid was
not measured which explains why the sum of the mineral proportions is less
than 100%. Some
of these minerals were formed in the water treatment process, while others
were either leached
out from the reservoir sand or formed in the reservoir following steam inj
ection.
Table 2B- Mineral Analysis of Solid Portion of Waste Slurry
Minerals
Name Formula wt%
Quartz Si02 4.6 - 6.0 wt%
K-spar KA1Si308 0.3 - 0.4 wt%
Albite NaA1Si30g 0.1 - 0.4 wt%
Calcite CaC03 55.5 - 59.3 wt%
_7_
CA 02369244 2002-O1-24
Magnesite MgC03 1.5 3.6 wt%
-
Siderite FeC03 0.4 0.5 wt%
-
Halite NaCl 1.2 1.9 wt%
-
Serpentine Mg6Si40,(OH) $ 8.2 9.2 wt%
-
Brucite Mg(OH) 2 9.8 11.5
- wt%
Sepiolite Mg4Si60,5(OH)2.6H20 7.6 8.2 wt%
-
Organic Carbon 1.0 2.3 wt%
-
Total 95.8 - 97.9
wt%
Assuming a water concentration of 85-95% by weight, the whole waste slurry
would
have a composition representative of that shown in Table 3.
Table 3-Whole Waste Slurry Composition
Properties/Composition Preferred Range General Range
Solid 5 to 15 wt % 4 to 20 wt%
pH at 22C 9.5 to 10 9 to 11
Total Dissolved Solids 8500 to 11400 ppm 6400 to 13440
ppm
Total Hardness 68 to 152 ppm 56 to 173 ppm
Tannin and Lignin 51 to 62 ppm 40 to 77 ppm
Alkalinity Phen. 8.3 255 to 380 ppm 200 to 480 ppm
as CaC03
Alkalinity Total as 425 to 618 ppm 320 to 768 ppm
CaC03
Carbonate Ions 170 to 285 ppm 120 to 384 ppm
Hydroxyl Ions 34 to 67 ppm 24 to 96 ppm
Chloride Ions 4250 to 6650 ppm 3200 to 9600 ppm
Total Organic Carbon 119 to 152 ppm 96 to 192 ppm
Dissolved Silica 3 to 5 ppm 0 to 48 ppm
Sulfate Ions 51 to 95 ppm 40 to 115 ppm
Magnesium Ions 9 to 29 ppm 4 to 38 ppm
Calcium Ions 12 to 14 ppm 8 to 38 ppm
Potassium Ions 128 to 171 ppm 104 to 192 ppm
Sodium Ions 3000 to 4275 ppm 2400 to 4800 ppm
Sulfur Dissolved 26 to 38 ppm 20 to 48 ppm
Mg in solid 0.5 to 3 wt% 0.2 to 4 wt%
Ca in solid 1 to 4 wt% 0.6 to 7 wt%
Si in solid 0.3 to 0.9 wt% 0.16 to 1.6 wt
A1 in solid 0.002 to 0.012 wt% 0.0012 to 0.02
wt
K in solid 0.002 to 0.0075 0.0012 to 0.012
wt% wt%
Fe in solid 0.0075 to 0.0375 0.004 to 0.08%
wt % wt%
Sr in solid 75 to 360 ppm 48 to 600 ppm
Ba in solid 25 to 105 ppm 16 to 200 ppm
_g_
CA 02369244 2002-O1-24
Pure SOZ from a gas cylinder was bubbled through the waste slurry at room
temperature. The SOZ volume was measured with a Dry Test meter. Using a Drager
tube, the
SOZ in the effluent gas was monitored and recorded as a :function of the
volume of SOZ
bubbled through. As shown in Table 4, the SOZ concentration in the effluent
gas was only 6
ppm after 20 litres of SOZ at standard temperature and pressure conditions
(STP) was bubbled
through. At this point, the test was terminated. The pH of the slurry dropped
from 9.9 to 4.3
at the end of the test.
Table 4- SOz Concentration in Effluent Gas
Volume SOZ Bubbled (L@STP) SOZ Concentration in Effluent Gas (ppm)
8 Trace
16 Trace
18 1
20 6
This test shows that two litres of Cold Lake waste slurry is capable of
removing 20
litres of pure SOZ at STP.
The above-described embodiments of the invention are intended to be examples
of the
present invention. Alterations, modifications and variations may be effected
to the particular
embodiments by those of skill in the art, without departing from the scope of
the invention
which is defined solely by the claims appended hereto.
-9-