Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
-1-
IMPROVED PROCESS FOR DEASPHALTING RESIDUA
BY REACTIVE RECYCLE OF HIGH BOILING MATERIAL
FIELD OF THE INVENTION
The present invention relates to an improved process for
deasphalting a residua feedstock by use of a short vapor residence time
process
unit comprised of a horizontal moving bed of fluidized and/or stirred hot
particles. The vapor phase product stream from said process unit is passed to
a
soaker drum where a high boiling fraction is separated and recycled to the
process unit after undergoing reactions causing molecular weight growth. This
reactive recycle using the soaker drum results in substantially improved
qualities
of the liquid products compared with what is achieved by once-through residua
deasphalting process alternatives.
BACKGROUND OF THE INVENTION
In a typical refinery, crude oils are subjected to atmospheric
distillation to separate lighter materials such as gas oils, kerosenes,
gasolines,
straight run naphtha, etc. from the heavier materials. The residue from the
atmospheric distillation step is then distilled at a pressure below
atmospheric
pressure. This later distillation step produces a vacuum gas oil distillate
and a
vacuum reduced residual oil which often contains relatively high levels of
asphaltene molecules. These asphaltene molecules usually contain most of the
Conradson Carbon residue and metal components of the resid. They also contain
relatively high levels of heteroatoms, such as sulfur and nitrogen. Such feeds
have little commercial value, primarily because they cannot be used as a fuel
oil
because of ever stricter environmental regulations. They also have little
value as
feedstocks for refinery processes, such as fluid catalytic cracking, because
they
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
-2-
produce excessive amounts of gas and coke. Also, their high metals content
leads to catalyst deactivation. Thus, there is a great need in petroleum
refining
to upgrade residual feeds to more valuable cleaner and lighter feeds.
There are a number of techniques used for recovering the lighter
components from various asphaltic petroleum residual feeds. Many such
processes involve the extraction of the lighter components with a deasphalting
solvent such as propane, and thereafter separating and recovering the lighter
Lomponents from the solvent. In U.S. Patent No. 2,950,244, a process for the
extraction of petroleum residue containing asphalt is disclosed. The solvent
utilized is a liquefied normally gaseous solvent, such a propane, which is
maintained at a temperature between about 100°F and 200°F and at
a pressure
sufficient to maintain the solvent in a liquid phase.
Variations of the deasphalting process using propane, or similar
short chain aliphatics as solvents, are taught in U.S. Patent No. 2,669,538 to
Yuraski et al.; U.S. Patent No. 3,516,928 to King et al. issued June 23, 1970;
U.S. Patent No. 4,017,383 to Beavon, issued April 12, 1977; and U.S. Patent
No.
4,201,660 to Szosel, issued May 6, 1980. King et al. additionally suggest that
carbon dioxide and ammonia, under certain circumstances are equivalent
solvents to the lower alkanes, alkenes, and their halogenated derivatives.
While propane is often used in conventional solvent deasphalting
operations, other solvents have been suggested. For example, in U.S. Patent
No.
4,054,512, an asphalt-containing mineral oil is deasphalted by contacting the
oil
with liquid hydrogen sulfide. The use of liquid neopentane, at a temperature
between 0°F and 250°F, taught in U.S. Patent No. 3,334,043.
Also, in U.S.
Patent No. 2,337,448, heavy residual oil is deasphalted by a solvent selected
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
-3-
from the group consisting of ethane, ethylene, propane, propylene, butane,
butylene, isobutane, and mixtures thereof.
U.S. Patent No. 4,191,639 to Audeh et al teaches a process
whereirn. hydrocarbon oils, such as residual petroleum oils, are deasphalted
and
demetallized by contact with a liquid mixture of at least two of the
components
selected from hydrogen sulfide, carbon dioxide, and propane.
Also, US Patent 5,714,056 teaches a process for deasphalting
residua in a short vapor contact time thermal process unit comprised of a
horizontal moving bed of fluidized hot particles. This is a once through
process
whereby the removal of feed contaminants is limited to what can be achieved in
a single pass. There is no suggestion of separating a high boiling fraction
from
the vapor product fraction and recycling it to the reaction zone.
While solvent deasphalting has met with commercial success, there
is nevertheless a continuing need in the art for deasphalting processes which
result in higher liquid yields and improved liquid product quality than
solvent
deasphalting. There is also a need in the art for improved processes capable
of
deasphalting an asphalt-containing residual feedstock without the use of a
solvent.
SUMMARY OF THE INVENTION
In accordance with the present invention there is provided a
process for deasphalting an asphalt-containing feedstock in a deasphalting
process unit comprised of
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
-4-
(i) a heating zone wherein solids containing carbonaceous deposits are
received from a stripping zone and heated in the presence of a heating gas
which
may contain oxygen for partial combustion purposes;
(ii) a short vapor residence time reaction zone containing a horizontal
moving bed of fluidized and/or stirred hot solids recycled from the heating
zone
and feed, which reaction zone is operated at a temperature from about
450°C to
about 700°C and operated under conditions such that the solids
residence time
and the vapor residence time are independently controlled, which vapor
residence time is less than about 5 seconds, and which solids residence is
from
about 5 to about 60 seconds; and
(iii) a stripping zone through which solids having carbonaceous deposits
thereon are passed from the reaction zone and wherein lower boiling additional
hydrocarbon and volatiles are recovered with a stripping gas;
which process comprises:
(a) feeding the residua feedstock to the short vapor residence time
reaction zone wherein it contacts the hot solids thereby resulting in high
Conradson Carbon components and metal-containing components being
deposited onto said hot solids, and a vaporized fraction;
(b) separating the vaporized fraction from the solids; and
(c) passing the solids to said stripping zone where they are
contacted with a stripping gas, thereby removing volatile components
therefrom;
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
-5-
(d) passing the stripped solids to a heating zone where they are
heated to an effective temperature that will maintain the operating
temperature of
the reaction zone;
(e) recycling hot solids from the heating zone to the reaction zone
where they are contacted with fresh feedstock;
(f) passing the vaporized fraction from step (b) above to a soaker
drum where it is quenched to produce a vapor fraction boiling less than about
450-600°C and a high boiling fraction condensate having an initial
boiling point
in the range of about 450-600°C;
(g) providing sufficient residence time and reactor severity in the
soaker drum to permit molecular weight growth reactions to occur;
(h) recycling said high boiling fraction to the short vapor contact
time reaction zone; and
(i) recovering the vapor fraction having a lower concentration of
contaminants from step (h).
In a preferred embodiment of the present invention, steam, C4
minus gas, or both, is injected into the soaker drum to maintain the solids in
suspension and to strip out lower boiling range products.
In another preferred embodiment of the present invention the
soaker drum is operated at increased pressure and temperature to reduce
reaction
time and therefore the soaker drum size.
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
-6-
In a preferred embodiment of the present invention, the particles of
the short contact time reaction zone are fluidized and/or stirred with the aid
of a
mechanical means.
In another preferred embodiment of the present invention, steam,
C4 minus gas, or both are injected into the vaporized fraction upstream of the
soaker drum to reduce the partial pressure of the CS plus hydrocarbon to
condense the high boiling fraction as per step (f) at a temperature which is
lower
than its initial boiling point of about 450-600°C.
In another prefeiTed embodiment of the present invention poly-
merization initiators are present in the soaker drum to increase reaction
rates.
In-yet another preferred embodiment of the present invention the
soaker drum can include a mechanical mixing device providing the advantages of
self cleaning to minimize coke deposits and to achieve substantially plug flow
reaction conditions of the liquid phase.
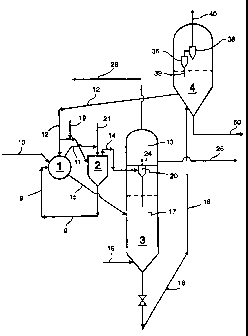
BRIEF DESCRIPTION OF THE FIGURE
The sole figure hereof is a schematic flow plan of a non-limiting
preferred embodiment of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
Residua feedstocks which are upgraded in accordance with the
present invention are those petroleum fractions boiling above about
380°C,
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
_ '7 _
preferably above about 540°C, more preferably above about 560°C.
Non-
limiting examples of such fractions include vacuum resids, atmospheric resids,
heavy and reduced petroleum crude oil; pitch; waste oils; asphalt; bitumen;
solvent deasphalter residue; and tar sand oil. It is understood that such
resids
may also contain minor amounts of lower boiling material. These feedstocks
cannot be fed in substantial quantities to refinery process units, such as FCC
units, because they are typically high in Conradson Carbon and they usually
contain an undesirable amount of metal-containing components. Conradson
Carbon residues will deposit on the FCC cracking catalyst and cause excessive
deactivation. Metals, such as nickel and vanadium will also deactivate the
catalyst by acting as catalyst poisons. Such feeds will typically have a
Conradson Carbon content of at least 5 wt.%, generally from about 5 to 50
wt.%.
As to Conradson Carbon residue, see ASTM Test D189-165.
Residuum feedstocks are upgraded in accordance with the present
invention in a short vapor residence time process unit which is comprised of a
heating zone, a short vapor residence time horizontal fluidized and/or stiiTed
bed
reaction zone and a stripping zone. Reference is now made to the sole figure
hereof wherein a residual feedstock which is high in Conradson Carbon and/or
metal-components is fed via line 10 to one or more short vapor residence time
reaction zone 1 which contains a horizontal moving bed of fluidized and/or
stirred hot solids. It is preferred that the solids in the short vapor
residence time
reactor are fluidized andJor stirred with assistance of mechanical means. The
reactor may be stripped by use of a stripping gas, such as steam, or C4 minus
gas, or by the vapors resulting from the vaporization of a fraction of the
feed-
stock. It is preferred that the mechanical means be a self cleaning mechanical
mixing system characterized as having a relatively high radial mixing e~ciency
with only minor amounts of axial backmixing. Such a mixing system acts like a
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
-g-
plug flow system with a flow pattern which ensures that the residence time is
nearly equal for all particles. The most preferred mechanical mixer is the
mixer
referred to by Lurgi AG of Germany as the LR-Mixer or LR-Flash Coker which
was originally designed for processing oil shale, coal, and tar sands. The LR-
Mixer consists of two or more horizontally oriented rotating screws which mix
the feed and hot solids while stirring and transporting the mixture through
the
reactor. Although it is preferred that the solid particles be coke particles,
they
may be any other suitable refractory particulate material. Non-limiting
examples
of such other suitable refractory materials include those selected from the
group
consisting of silica, alumina, zirconia, magnesia, mullite, synthetically
prepared
or naturally occurring material such as pumice, clay, kieselguhr, diatomaceous
earth, bauxite, and the like. It is within the scope of the present invention
that
the solids be inert or have catalytic properties. The solids will preferably
have
an average particle size of about 40 microns to 2,000 microns, more preferably
from about 200 microns to about 1000 microns.
The feedstock is contacted with the hot solids at a temperature
from about 450°C to about 700°C, preferably from about
500°C to 600°C, more
preferably from about 520°C to 600°C. When this happens, a
substantial portion
of the high Conradson Carbon and metal-containing components will deposit on
the hot solid particles in the form of high molecular weight carbon and metal
moieties. The remaining portion will be vaporized on contact with the hot
solids. The residence time of vapor products in reaction zone 1 will be an
effective amount of time so that substantial secondary cracking does not
occur.
This amount of time will typically be less than about 5 seconds, preferably
less
than about 2 seconds. The residence time of solids in the reaction zone will
be
from about 5 to 60 seconds, preferably from about 10 to 30 seconds. One novel
aspect of the present invention is that the residence time of the solids and
the
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
-9-
residence time of the vapor products, in the reaction zone, are independently
controlled. Most fluidized bed processes are designed so that the solids
residence time, and the vapor residence time cannot be independently
controlled,
especially at relatively short vapor residence times. It is preferred that the
short
vapor residence time process unit be operated so that the ratio of solids to
feed
be from about 30 to 1 to 3 to 1, preferably about 5 to 1. It is to be
understood
that the precise ratio of solids to feed will primarily depend on the heat
balance
requirement of the short vapor residence time reaction zone and the
temperature
of the solids. Associating the oil to solids ratio with heat balance
requirements is
within the skill of those having ordinary skill in the art, and thus will not
be
elaborated herein any further. A minor amount of the feedstock will deposit on
the solids in the form of combustible carbonaceous material. Metal components
will also deposit on the solids. Consequently, the vaporized portion will be
substantially lower in both Conradson Carbon and metals when compared to the
original feed.
The vaporized fraction is passed via line 11 to soaker drum 2
which is maintained at effective conditions so that the highest boiling
materials
are condensed out. Typically these conditions will include controlled
quenching
of the vapor fraction just below the dewpoint. The condensate is maintained in
the soaker drum for an effective amount of time and reaction severity to
initiate
polymerization to coke precursors. A quench stream can also be passed into
soaker drum via line 21. The quench stream will typically be an oil stream
ranging from naphtha (CS/150°C) to residuum stream (550°C+).
Preferred
quench streams are fractionator bottoms having a boiling range of 300°C
to
700°C. Coke or polymerization initiators which include those selected
from the
group consisting of elemental sulfur, peroxides, and spent cracking catalysts
can
be added to the soaker drum via line 19. Steam, C4 minus, air, or a mixture
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
- 10-
thereof, can also be used in the soaker drum to increase reaction rates, strip
lower boiling components, and to keep the solids suspended in a slurry. In
addition, the soaker drum may be continuously cleaned by mechanical means to
minimize coke deposits.
The soaker drum is operated at effective temperatures and
residence times to initiate coking reactions, but not to the extent that coke
deposits significantly build-up in the soaker drum. Preferred conditions
include
temperatures from about 350°C to about 520°C, preferably from
about 400°C to
about 450°C and residence times of from about 1 to 60 minutes,
preferably from
about 5 to 30 minutes, depending on the feed properties and desired feed
decontamination rates. Proper use of the soaker drum will selectively condense
only the highest boiling vapor products and produce a pre-polymerized heavy
oil
which is recycled to reaction zone 1 via line 9. This will result in an
increased
metals rejection rate from about 90% for once-through to 95% or greater with
extinction recycle. Use of the soaker drum also enables increased rejection of
other feed contaminants, such as Conradson Carbon, sulfur, and nitrogen. Thus,
the resulting liquid product quality is substantially improved and of higher
value
as feed to refinery conversion processes.
The vapor fraction from soaker drum 2 is passed via line 14 to
cyclone 20 where most of the entrained solids, or dust, are removed. The
dedusted vapors are then passed to quench zone 13 via line 24 where the vapors
are reduced to temperatures below which substantial thermal cracking occurs.
This temperature will preferably be below about 450°C, more
preferably below
about 340°C. Solids, having carbonaceous material deposited thereon,
are
passed from reaction zone 1 via line 15 to the bed of solids 17 in stripper 3.
The
solids pass downwardly through the stripper and past a stripping zone at the
CA 02370591 2001-10-15
WO 00/63320 PCT/US00/09607
-11-
bottom section where any remaining volatiles, or vaporizable material, are
stripped from the solids with use of a stripping gas, preferably steam,
introduced
into the stripping zone via line 16. Stripped vapor products pass upwardly in
stripper vessel 3 to quench zone 13 where a light product is removed overhead
via line 28. The light product will typically be a 550°C minus product
stream.
A 550°C plus stream will also be collected from the quench zone via
line 26.
The stripped solids are passed via line 18 to heater 4 which contains a
heating
zone. The heating zone is operated in an oxidizing gas environment, preferably
air, at an ei~ective temperature. That is, at a temperature that will meet the
heat
requirements of the reaction zone. The heating zone will typically be operated
at
a temperature of about 40°C to 200°C, preferably from about
50°C to 175°C,
more preferably from about 50°C to 120°C in excess of the
operating tempera-
ture of reaction zone 1. It is understood that preheated air can be introduced
into
the heater. While some carbonaceous residue will be burned from the solids in
the heating zone, it is preferred that only partial combustion take place so
that
the solids, after passing through the heater, will have value as a fuel.
Excess
solids can be removed from the process unit via line 50. Flue gas is removed
overhead from heater 4 via line 40. The flue gas is passed through a cyclone
system 36, 39, and 38 to remove most solid fines. Dedusted flue gas will be
passed to a co-boiler for waste heat recovery (not shown), scrubbed to remove
contaminants and particulates, and passed to atmosphere. The hot inert solids
are then recycled via lines 12 to thermal zone 1.