Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
v
CA 02371443 2001-10-25
SPECIFICATION
Title of the Invention
Thermal Recording Material
Field of Technoloey
This invention relates to a thermal recording material comprising a
support and a thermosensitive color-developing layer provided thereon
including a leuco dye which is colorless or light-colored at normal
temperature, an organic acid substance which reacts upon heating with the
leuco dye to develop color and a sensitizes.
Bac~round Technolog3r
Thermal recording materials comprising a support and a thermosensitive
color-developing layer provided thereon including a leuco dye, an organic
acid substance and a sensitizes are used in many fields such as computer
outputs, printers of small-sized electronic calculators, recorders of various
measuring instruments, facsimile, ticket vending machines, thermographic
copying machines and labels.
An increasingly wider use of thermal recording materials has created a
stronger demand for improved performance and reduced pxzce. A large
number of proposals have been made on new leuco dyes, organic acid
substances or sensitizers for improvement of performace, but it is difficult
CA 02371443 2001-10-25
for them to satisfy fully a variety of requirements.
Acid compounds based on diphenyl sulfone have been proposed in JP
(Japanese Patent) 63-46067 B (1988), JP 07-12749 B (1995) and JP 08-
333329 A (1996). However, the use of these compounds, even in combination
with general-purpose sensitizers, was unable to satisfy requirements for
properties such as thermal response and stability of recorded images,
particularly moisture resistance.
Among organic acid substances, 4,4'-dihydroxydiphenyl sulfone is known
to be relatively resistant to plasticizers~ for example, JP 57-11088 A (1982),
JP 58-119893 A (1983) and JP 61-160292 A (1986) disclose that the use of
4,4'-dihydroxydiphenyl sulfone as an organic acid substance improves the
stability of colored images or that of the textur a against plasticizers. On
account of its high melting point and poor compatibility with general-
purpose dyes and sensitizers, however, 4,4'-dihydroxydiphenyl sulfone had a
defect of developing color in low concentration.
JP 08-72406 A (1996) proposes the use of 4,4'-dihydroxydiphenyl sulfone
as an organic acid substance together with an acylacetanilide as a
sensitizer. The use of the two together was able to provide a thermal
recording material with good color-developing sensitivity and plasticizer
resistance on one hand, but with reduced heat resistance of the texture on
the other, and the matexdal was not quite satisfactory in practical use.
Moreover, JP 61-246088 A (1986) proposes the use of a 4-substituted
biphenyl as a sensitizer of good color-developing sensitivity and low fogging
2
CA 02371443 2001-10-25
of the texture however, bisphenol A and bis(3-allyl-4-hydroxyphenyl)
sulfone are solely used here as organic acid substances and there is nothing
to teach how to provide thermal recording materials exhibiting good
stability of images, in particular, good plasticizer resistance.
This invention has been devised in view of the aforementioned problems
and its object is to provide a thermal recording material which exhibits good
color-developing sensitivity and whiteness of the texture and develops little
color in the texture in weathering tests such as wet heat test, plasticizer
resistance test, and heat resistance test. Another object of this invention is
to provide a thermal recording material exhibiting outstanding stability of
recorded images such as storage stability under wet heat conditions and in
contact with plasticizers. Still another object of this invention is to
provide a
thermal recording material with a good balance of the aforementioned
properties.
Disclosure of the Invention
Accordingly, in a thermal recording material compizsing a support and a
thermosensitive color-developing layer provided thereon including a leuco
dye which is colorless or light-colored at normal temperature, an organic
acid substance which reacts upon heating with the leuco dye to develop color
and a sensitizer, this invention relates to a thermal recording material
wherein said thermosensitive color-developing layer includes one kind or
two kinds or more of diphenyl sulfone derivatives selected from a group of 4-
3
CA 02371443 2001-10-25
hydroxydiphenyl sulfone derivatives represented by the following general
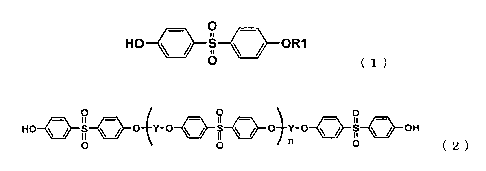
formula (1)
O
HO ~ ~ S ~ ~ OR1
O (1)
(wherein R1 is an alkyl, aralkyl or aryl group), 4,4'-dihydroxydiphenyl
sulfone, 2,4'-dihydroxydiphenyl sulfone and 4-hydroxydipheny sulfone
derivatives represented by the general formula (2)
HO ~ ~ S ~ ~ O Y-O ~ ~ S ~ ~ 0 Y-O ~ ~ S ~ ~ OH
0
o o n O (2)
(wherein Y is a divalent organic group and n is an integer in the range 0-6)
as an organic acid substance and a 4-substituted biphenyl derivative
represented by the following general formula (3)
O
~ C-R2
(3)
(wherein R2 is an alkyl or aryl group) as a sensitizes.
This invention will be described in detail below.
A leuco dye to be used as a color former in this invention is a substance
which is colorless or light-colored at normal temperature and reacts upon
heating with an organic acid substance to develop color. The following are
4
CA 02371443 2001-10-25
1
examples of such a leuco dye: triarylmethane dyes such as 3,3-bis(p-
dimethylaminophenyl)-6-dimethylaminophthalide, 7'-anilino-3'-
dibutylainino-6'-methylfluoran, 3-(p-dimethylaminophenyl)-3-(1,2-dimethyl-
3-indolyl)phthalide, 3,3-bis(9-ethyl-3-carbazolyl)-5-dimethylaminophthalide
and 3,3-bis(2-phenyl-3-indolyl)-5-dimethylaminophthalide~
diphenylmethane dyes such as 4,4'-bis(dimethylaminobenzhydryl) benzyl
ether thiazine dyes such as benzoyl leucomethylene blue spiro dyes such as
3-methylspirodinaphthopyran~ and fluoran dyes, leucoauramine dyes,
indoline dyes and indigo dyes. These leuco dyes can be used singly or as a
mixture of two kinds or more.
In this invention, one kind or two kinds or more of diphenyl sulfone
derivatives selected from a group of 4-hydroxydiphenyl sulfone derivatives
represented by the aforementioned general formula (1), 4,4'-
dihydroxydiphenyl sulfone, 2,4'-dihydroxydiphenyl sulfone and 4-
hydroxydipheny sulfone derivatives represented by the general formula (2)
are incoporated as an organic acid substance in the thermosensitive color-
developing layer.
In the aforementioned general formula (1), R1 is an alkyl, aralkyl or aryl
group, preferably a lower alkyl group with 1-5 carbon atoms, benzyl group, a
lower alkyl-substituted benzyl group, phenyl group or a lower alkyl-
substituted phenyl group.
Preferable concrete examples of 4-hydroxydiphenyl sulfone derivatives
represented by the aforementioned general formula (1) are Compound 1 and
CA 02371443 2001-10-25
Compound 2 shown below.
[Compound I: melting point 129°C)
O
HO ~ ~ S ~ ~ OCH(CH3)2
O
[Compound 2: melting point 154°C]
O
Ho ~ ~ S ~ ~ OCH2CH2CH3
O
Regarding the method of preparation and preferable examples of 4-
hydroxydiphenyl sulfone derivatives represented by the aforementioned
general formula (2), reference may be made to the method and compounds
described in the aforementioned JP 08-333329 A (1996). The reaction of 4,4'-
dihydroxydiphenyl sulfone with a compound represented by X-Y-X (wherein
X is a halogen such as chlorine and bromine and Y is as defined earlier) can
be utilized for the preparation. It is even possible to prepare preferentially
a
compound of the general formula (1) wherein n = 0 if the amount of X-Y-X is
limited to below 0.5 times that of 4,4'-dihydroxydiphenyl sulfone on a mole
basis. In case the amount of X-Y-X is in the range 0.5-0.9 times in mole, the
reaction yields a mixture of compounds of the general formula (1) wherein n
ranges from 1 to 6 depending on the mole ratio. The unreacted 4,4'-
dihydroxydiphenyl sulfone differs in solubility in an aqueous alkaline
solution from the compounds of the general formula (1) wherein n ranges
6
CA 02371443 2001-10-25
from 0 to 6 and the two can be separated by utilizing this property. The
compounds of the general formula (Z) differing in n can be separated from
one another by the aforementioned procedure or recrystallization, but they
can also be used as a mixture without separation. It is naturally to be
understood that the compounds to be used in this invention are not limited
to those prepared by the aforementioned procedure.
In the general formula (2), Y is an arbitray divalent organic group, for
example, an aliphatic hydrocarbon group, a hydrocarbon group containing
one or more heteroatoms in the main molecular chain, and a hydrocarbon
group containing one or more aromatic rings in the main molecular chain.
However, preferable examples are the following divalent organic groups
cited in the aforementioned JP 08-333329 A (1996): a saturated or
unsaturated divalent hydrocarbon group with 1-12 carbon atoms, a divalent
hydrocarbon group with 1-8 carbon atoms containing an ether linkage or a
group represented by -R- ~ -R- (wherein R is methylene or ethylene group
and ~ is a benzene ring) more preferably, an alkylene group with 1-4
carbon atoms, a group represented by -R'-O-R'- (wherein R' is an alkylene
group with 1-4 carbon atoms) or a group represented by -R- ~ -R- (wherein
R is methylene group and ~ is a benzene ring) n is an integer of 0-6,
preferably n is 0 or 1-3.
Preferable concrete examples of 4-hydroxydiphenyl sulfone derivatives
represented by the general formula (2) are compounds of the following
formula such as Compound 3 (n = 0), Compound 4 (n = 1) and Compound 5
7
CA 02371443 2001-10-25
(n = 2).
_ o _ _ o _
HO ~ ~ S ~ ~ O CHZCHZOCHZCH2-O ~ ~ S ~ ~ O
O O n
_ O _
-CHZCHZOCHZCHZ-O ~ ~ S ~ ~ OH
O
A Biphenyl sulfone derivative to be used as an organic acid substance is
one kind or two kinds or more of compounds selected from a group of 4-
hydroxydiphenyl sulfone derivatives represented by the aforementioned
general formula (1), 4,4'-dihydroxydiphenyl sulfone, 2,4'-dihydroxydiphenyl
sulfone and 4-hydroxydiphenyl sulfone derivatives represented by the
general formula (2). The amount of the organic acid substance is 1-6 parts
by weight, preferably 1.5-2.5 parts by weight, per 1 part by weight of the
leuco dye, although it varies with the kind of leuco dye and sensitizer in
use.
At least one kind of the aforementioned Biphenyl sulfone derivatives may
be used singly or as a mixture with another organic acid substance.
Concrete examples of such another organic acid substance is a phenolic
compound such as bisphenol A and a carboxylic acid such as benzoic acid
and phthalic acid and it is used in an amount less than 50 wt%, preferably
less than 10 wt%, of the total organic acid substances.
In this invention, one kind or two kinds or more of 4-substituted
biphenyl derivatives represented by the aforementioned general formula (3)
are incorporated as a sensitizer in the thermosensitive color-developing
layer. In the general formula (3), R2 is an alkyl or aryl group and,
8
CA 02371443 2001-10-25
preferably, a group to give the drivative in question a melting point in the
range 50-200 °C such as a lower alkyl group with 1-5 carbon atoms,
phenyl
group and a lower alkyl-substituted phenyl group.
Preferable concrete examples of a sensitizer represented by the general
formula (3) for use in this invention are the following:
[Compound 6: melting point 121°C]
O
~ C-CH3
[Compound 7: melting point 96 °C]
O
C-CH2CH3
[Compound 8: melting point 100°C]
O
..
These compounds can be used singly or as a mixture of two kinds or
more. The amount used is normally 1-6 parts by weight, preferably 1.5-2.5
parts by weight, per 1 part by weight of the leuco dye, although the amount
varies with the kind of leuco dye and organic acid substance to be used.
A compound represented by the aforementioned general formula (3) may
be used singly or as a mixture with another sensitizer. Examples of such
9
CA 02371443 2001-10-25
another sensitizer include the following compounds: nitrogen-containing
compounds such as stearamide, palmitamide, linolic acid amide and stearic
acid anilide~ esters such as benzyl 4-hydroxybenzoate, benzyl 4-
benzyloxybenzoate, phenyl 2-naphthoate, benzyl 2-naphthoate, phenyl 1-
hydroxy-2-naphthoate, dibenzyl oxalate, di(4-methylbenzyl) oxalate,
dibenzyl terephthalate, n-butyl isophthalate and phenyl p-toluenesulfonate~
aromatic compounds such as 4-benzylbiphenyl, m-terphenyl, fluorene,
ffuoranthene, 2,6-diisopropylnaphthalene, 3-benzylacenaphthene, 1,2-
bis(3,4-dimethylphenyl)ethane, 1,2-bis(2,4-dimethylphenyl)ethane and 1,2-
bis(2,4,5-trimethylphenyl)ethane~ and ethers and sulfur-containing
compounds such as 2-benzyloxynaphthalene, 1,4-diethoxynaphthalene, 1,2-
diphenoxyethane, 1,2-diphenoxybenzene, 1,4-diphenoxybenzene, 4-(4'-
methylphenoxy)biphenyl, diphenyl sulfone, 4-methyldiphenyl sulfone, 4,4'-
diisopropoxydiphenyl sulfone, 4,4'-diphenoxydiphenyl thioether, benzyl 4-
methylthiophenyl ether and 1,2-bis(phenoxymethyl)benzene. It is
advantageous to add such another sensitizer in an amount less than 50
wt%, preferably less than 10 wt%, of the total sensitzers and maintain the
melting point after mixing in the range 50-200°C.
Moreover, for the purpose of improving the storage stability of the
colored region still further, it is allowable to use epoxy resin and the metal
salt of an organic acid substance such as zinc stearate and zinc salicylate,
although they are slightly less responsive to heat, together with the
aforementioned compounds.
CA 02371443 2001-10-25
In the cases where a high degree of storage stability is required, it is
desirable to incorporate a publicly known preservative in a thermal
recording material of this invention as occasion demands.
Examples of such a preservative include phenolic compounds such as
tris(2,6-dimethyl-4-tert-butyl-3-hydroxybenzyl) isocyanurate, tns(3,5-di-
tert-butyl-4-hydroxybenzyl) isocyanurate, tris[2-[3-(3,5-di-tert-butyl-4-
hydroxyphenyl)propionyloxy]ethyl] isocyanurate, 1,3,5-tris(3,5-dimethyl-4-
hydroxybenzyl)-2,4,6-trimethylbenzene, 1,1,3-tris(2-methyl-4-hydroxy-5-
tert-butylphenyl)butane, 1,1,3-tris(2-methyl-4-hydroxy-5-
cyclohexylphenyl)butane, 4,4'-butylidenebis(2-tert-butyl-5-methyl)phenol,
4,4'-thiobis(2-tert-butyl-5-methyl)phenol and 2,2'-methylenebis(6-tert-butyl-
4-methyl)phenol, epoxy compounds such as 4-benzyloxy-4'-(2-
methylglycidyloxy)diphenyl sulfone and 4,4'-diglycidyloxydiphenyl sulfone,
and sodium 2,2'-methylenebis(4,6-di-tert-butylphenyl) phosphate. Preferable
preservatives are the aforementioned phenolic and epoxy compounds. These
preservatives are normally used in an amount of 0.1-10 parts by weight per
1 part by weight of the leuco dye.
In the cases where a still higher degree of storage stability, p articularly
plasticizer resistance, is required, it is advantageous to provide an overcoat
layer on the thermosensitive color-developing layer of this invention. The
overcoat layer is formed by a suitably selected procedure in order to
manifest the desired performance. For example, a coating fluid composed of
a publicly known resin component such as polyvinyl alcohol) is applied to a
11
. .
CA 02371443 2001-10-25
suitable thickness by a known coating method. However, provision of an
overcoat layer, particularly a somewhat thicker one, tends to lower the
sensitivity and proper selection of the coating conditions is important.
Furthermore, in the cases where long-term weather resistance is
required, it is desirable to add an ultraviolet absorber to the
thermosensitive color-developing layer andlor the overcoat layer in a
thermal recording material of this invention. Examples of ultraviolet
absorbers are the following compounds: 2-hydroxybenzophenone derivatives
such as 2,4-dihydroxybenzophenone, 2-hydroxy-4-methoxybenzophenone, 2-
hydroxy-4-octoxybenzophenone and 5,5'-methylenebis(2-hydroxy-4-
methoxy)benzophenone~ 2-(2-hydroxyphenyl)benzotriazole derivatives such
as 2-(2-hydroxy-5-methylphenyl)benzotriazole, 2-(2-hydroxy-5-tert-
octylphenyl)benzotriazole, 2-(2-hydroxy-3,5-di-tert-butylphenyl)-5-
chlorobenzotriazole, 2-(2-hydroxy-3,5-dicumylphenyl)benzotriazole, 2,2'-
methylenebis(4-tert-octyl-6-benzotriazolyl)phenol and polyethylene glycol 2-
(2-hydroxy-3-tert-butyl-5-carboxyphenyl)benzotriazole ester benzoates such
as phenyl salicylate, resorcinol monobenzoate, 2,4-di-tert-butylphenyl 3,5-di-
tert-butyl-4-hydroxybenzoate and hexadecyl 3,5-di-tert-butyl-4-
hydroxybenzoate~ substituted oxanilides such as 2-ethyl-2'-ethoxyoxani7.ide
and 2-ethoxy-4'-dodecyloxanilide~ cyanoacrylates such as ethyl a -cyano-
a , a -diphenylacrylate and methyl 2-cyano-3-methyl-3-(p-
methoxyphenyl)acrylate~ and derivatives of triazole and triazine such as 2-
(2-hydroxy-4-octoxyphenyl)-s-triazine, 2-(2-hydroxy-4-methoxyphenyl)-4,6-
12
CA 02371443 2001-10-25
diphenyl-s-triazine and 2-(2-hydroxy-4-propoxy-5-methyl)-4,6-bis(2,4-di-tert-
butylphenyl)-s-triazine.
It is allowable to add a variety of additives such as binders and white
pigments to thermal recording materials of this invention depending upon
the end uses. Binders fix the leuco dye and the organic acid substance in the
segregated state from each other while dispersed on fine particles and their
examples are polyvinyl alcohol) (PVA), latex, methylcellulose,
carboxymethylcellulose, poly(acrylic acid), casein, gelatin, starch and their
derivatives. White pigments are added for whiteness of the thermosensitive
color-developing layer, slipperiness of writing devices and sticking and their
examples are calcium carbonate, kaolin, clay, talc and titanium oxide. They
are applied, either mixed or separately, to the support such as paper and
film in the formation of the thermosensitive color-developing layer.
Preferred Embodiments of the Invention
This invention will be described concretely below with reference to
examples and comparative examples.
Example 1
(1) Preparation of LiquidA
A mixture of 11.5 parts by weight of Compound 1 as an organic acid
substance, 11.5 parts by weight of Compound 6 as a sensitizer and 46 parts
by weight of a 5 wt% aqueous solution of PVA was ground in a sand mill to
13
CA 02371443 2001-10-25
prepare Liquid A or a dispersion of a powder with an average particle
diameter of 0.8 ,u m.
(2) Prep aration of Liquid B
A mixture of 5.5 parts by weight of 7'-anilino-3'-dibutylamino-6'-
methylffuoran and 49.5 parts by weight of a 5 wt% aqeuous solution of PVA
was ground in a paint shaker to prepare Liquid B or a suspension of a
powder with an average particle diameter of 0.8 a m.
(3) Preparation of thermal recording paper
A coating fluid was prepared by mixing 20 parts by weight of Liquid A,
parts by weight of Liquid B, 1 part by weight of a dispersion of zinc
stearate (Hydri.n Z-7 available from Chukyo Yushi Co., Ltd.), 4 parts by
weight of an emulsion of para~n wax (Hydrin P-7 available from Chukyo
Yushi Co., Ltd.) and 11.5 parts by weight of a 10 wt% aqueous solution of
PVA and the coating fluid was applied to a paper substrate and dried to give
thermal recording paper with a coating weight of 6 g/m2 after drying.
(4) Testing method for color development
The thermal recording paper thus prepared was printed and tested for
dynamic color development and at the same time measured for the
concentration of developed color of the texture while printing the specimens
for testing sensitivity and moisture resistance at 24 V and 1.0 ms and those
for testing plasticizer resistance at 27 V and 1.9 ms. The dynamic color
development test was carried out with the use of a printing tester (available
from Ohkura Electric Co., Ltd.) and the concentration of developed color was
14
CA 02371443 2001-10-25
measured by a Macbeth reflection densitometer, Model RD-914.
(5) Testing method for moisture resistance
The thermal recording paper which had been tested for dynamic color
development was stored in a thermo-hygrostat (50 °C, relative humidity
90%) for 24 hours and the concentration of developed color was measured by
a Macbeth reflection densitometer, Model RD-914. The surviving rate was
calculated as follows
Surviving rate = (A-B)/C
wherein A is the concentration of dynamically developed color after the
moisture resistance test, B is the value obtained by subtracting the
concentration of developed color of the texture before the moisture
resistance test from that after the moisture resistance test, and C is the
concentration of dynamically developed color before the moisture resistance
test.
(6) Testing method for plasticizer resistance
The entire printed surface of the thermal recording paper after the
dynamic color development test was covered in close contact with polyvinyl
chloride wrapping film. The paper and the wrapping film were stored in a
dryer (40°C) for 24 hours and the concentration of developed color in
the
printed region was measured by a Macbeth reflection densitometer, Model
RD-914. The surviving rate was calculated as follows
Surviving rate = (D - E)IF
wherein D is the concentration of dynamically developed color after the
CA 02371443 2001-10-25
plasticizer resistance test, E is the value obtained by subtracting the
concentration of developed color of the texture before the plasticizer
resistance test from that after the plasticizer resistance test, and F is the
concentration of dynamically developed color before the plasticizes
resistance test.
Example 2
Thermal recording paper was prepared as in Example 1 except using
Compound 2 in place of Compound 1 in the preparation of Liquid A and it
was tested as in Example 1.
Example 3
Thermal recording paper was prepared as in Example 1 except using
Compound 7 in place of Compound 6 in the preparation of Liquid A and it
was tested as in Example 1.
Example 4
Thermal recording paper was prepared as in Example 1 except using
Compound 8 in place of Compound 6 in the preparation of Liquid A and it
was tested as in Example 1.
Comparative Example 1
Thermal recording paper was prepared as in Example 1 except using
para-benzylbiphenyl in place of Compound 6 in the preparation of Liquid A
and it was tested as in Example 1.
Comparative Example 2
16
CA 02371443 2001-10-25
Thermal recording paper was prepared as in Example 1 except using
bisphenol A in place of Compound 1 in the preparation of Liquid A and it
was tested as in Example 1.
The results of the tests for moisture resistance and plasticizer resistance
are respectively shown in Tables I and 2.
( Table I
Dynamic After
color moisture
resistance
test
develo ment test
24V, 1. Oms
Texture ~ Printed Texture Printed ; Surviving
~
re ' on ~ re ' ~ rate
on ( % )
Ex. 1 0 . 0 ; 1 . 0 0 0 9 0 6 ; 5 8
8 3 . ; . 1
Ex. 2 0 . 0 ; 1 . 0 0 0 8 0 5 ; 5 4
7 5 . ; . 8
Ex. 3 0 . 0 . 1 . 0 0 0 8 0 6 . 6 1
? 2 . . . 3
Ex. 4 0 . 0 ~ 0 . 9 0 0 9 0 6 ~ 6 7
7 9 . ' . 8
Comp. 0 . 0 ; 1 . 0 0 1 2 0 3 ; 2 8
Ex. 1 9 1 . ; . 1
Comp. 0 . 1 ; 0 . 9 0 1 5 0 2 ; 1 7
Ex. 2 0 9 . ; . 2
17
CA 02371443 2001-10-25
[Table 2 ]
Dynamic After
color plasticizer
resistance
test
develo went test
27V, 1. 9ms
Texture ; Printed Texture Printed ; Surviving
;
' re ' on ' re ' ' rate
on ( % )
Ex. 1 0 . 0 ; 1 . 3 0 1 0 0 5 ; 4 1
8 5 . ; . 8
Ex. 2 0 . 0 . 1 . 3 0 0 9 0 6 . 4 4
7 8 . . . 3
Ex. 3 0 . 0 ~ 1 . 3 0 1 0 0 5 ~ 3 8
7 1 . ~ . 3
Ex. ~ 0 . 0 ; 1 . 2 0 0 9 0 4 ; 3 6
7 8 . ' . 8
Comp. 0 . 0 ; 1 . 3 0 1 3 0 3 ; 2 6
Ex. 9 3 . ; . 9
1
Comp. 0 . 1 . 1 . 3 0 1 4 0 2 . 1 2
Ex. 0 0 . . . 0
2
Example 5
Liquid A was prepared as in Example 1 except using 11.5 parts by weight
of 2,4'-dihydroxydiphenyl sulfone in place of Compound 1. Using Liquid A
thus prepared, thermal recording paper was prepared as in Example 1 and
it was tested as in Example 1.
Example 6
Liquid A was prepared as in Example 5 except using Compound 7 in
place of Compound 6. Using Liquid A thus prepared, thermal recording
paper was prepared as in Example 1 and it was tested as in Example 1.
Example 7
Liquid A was prepared as in Example 5 except using Compound 8 in
place of Compound 6. Using Liquid A thus prepared, thermal recording
paper was prepared as i.n Example 1 and it was tested as in Example 1.
18
CA 02371443 2001-10-25
Comparative example 3
Liquid A was prepared as in Example 5 except using para-
benzylbiphenyl in place of Compound 6. Using Liquid A thus prepared,
thermal recording paper was prepared as in Example 1 and it was tested as
in Example 1.
The results of the tests for moisture resistance and plasticizer resistance
are respectively shown in Tables 3 and 4.
(Table 3 ]
Dynamic After
color moisture
resistance
test
develo went test
2 4 V, 1 . Oms
Texture ~ p~ted Texture ~ p~ted ; Surviving
re 'on ; re 'on ; rate
(%)
Ex.S 0. 07 ; 0. 93 0. 10 . 0. 58 ; 59
Ex. 6 0. 0 8 0 . 9 0 0 . 0 0. 5 5 6 0
9
Ex. 7 0 . 0 ' 0. 8 3 0 . 1 ' 0 . ' 6 1
8 0 5 3
Cornp. 0 . 0 ; 0 . 6 0 . 0 ; 0 . ; 6 5
Ex. 7 2 9 4 2
3
19
CA 02371443 2001-10-25
[Table 4 ]
Dynamic color After plasticizer
resistance
test
development test
2 7 V , 1 . 9 ms
Texture ; p~ted Texture p~ted ; Surviving
~
re 'on ~ re 'on ~ rate
(%)
Ex. 0. 0 7 ; 1 . 3 1 0. 1 0 0. 4 ; 3 1
; 3
,
Ex. 0 . 0 8 ; 1 . 2 9 0 . 1 0 0 . ; 3 3
6 ; 4 5
Ex. 0 . 0 8 1 . 3 1 0 . 1 1 0 . 3 1
7 . 4 4
Comp. 0 . 0 7 ' 1 . 2 1 0 . 0 9 0 . ' 2 1
Ex. ' 2 8
3
Example 8
A mixture of 11.5 parts by weight of Compound 3 as an organic acid
substance, 11.5 parts by weight of Compound 6 as a sensitizer and 46 parts
by weight of a 5 wt% aqueous solution of PVA was ground in a sand mill to
prepare Liquid A or a dispersion of a powder with an average particle
diameter of 0.8 ,u m. Using Liquid A this prepared, thermal recording paper
was prepared as in Example 1 and it was tested as in Example 1.
Example 9
Liquid A was prepared as in Example 8 except using a 75:20:5 mixture
by weight of Compound 3, Compound 4 and Compound 5 in place of
Compound 3. Using Liquid A thus prepared, thermal recording paper was
prepared as in Example 1 and it was tested as in Example 1.
Example 10
Liquid A was prepared as in Example 9 except using Compound 7 in
CA 02371443 2001-10-25
place of Compound 6. Using Liquid A thus prepared, thermal recording
paper was prepared as in Example 8 and it was tested as in Example 1.
Example 11
Liquid A was prepared as in Example 9 except using Compound 8 in
place of Compound 6. Using Liquid A thus prepared, thermal recording
paper was prepared as in Example 8 and it was tested as in Example 1.
Comparative Example 4
Liquid A was prepared as in Example 9 except using para-
benzylbiphenyl in place of Compound 6. Using Liquid A thus prepared,
thermal recording paper was prepared as in Example 8 and it was tested as
in Example 1.
The results of the tests for moisture resistance and plasticizes resistance
are respectively shown in Tables 5 and 6.
[ Table 5 ]
Dynamic her moisture
color resistance
test
development
test
2 4 V, 1 . Oms
Texture ~ p~ted Texture p~ted ; Surviving
~
re ' on ; re ' ; rate
on ( % )
Ex.8 0 . 0 . 1 . 0 0 . 1 0 2 ; 1 7
9 3 2 ; . 5
Ex.9 0 . 0 0. 9 6 0. 1 1 0 3 2 5
8 . . 3
Ex. 10 0 . 0 ' 0 . 9 0 . 1 0 3 ' 2 4
8 3 1 ~ . 2
Ex. 11 0. 0 ; 0. 7 0. 1 2 0 2 ; 3 1
8 2 ; . 7
Comp. 0 . 0 ; 0 . 4 0 . 0 0 2 ; 3 4
Ex. 4 8 4 ( 9 ; . 5
21
CA 02371443 2001-10-25
(Table 6 ~
Dynamic After
color plasticizer
resistance
test
develo ment test
2 7 V 1 . 9 ms
,
Texture . printed Texture prated . Surviving
;
' ' .rate
(%)
' re ~ re on
on
Ex. 8 0 . 1 ; 1 . 2 0 . 1 0 9 ; 7 6
0 7 0 ; . 6
Ex. 9 0 . 1 ; 1 . 2 0 . 1 1 1 ; 9 7
0 0 0 ; . 8
Ex. 10 0. 1 . 1 . 1 0 . 1 1 1 . 9 9
1 7 1 ~ . 7
Ex. 11 0 . 1 ' 1 . 1 0 . 1 1 1 ' 9 7
0 2 0 ~ . 1
Comp. 0 . 1 ; 0 . 9 0 . 1 0 9 ; 9 6
Ex. 4 0 8 0 ; . 4
Example 12
(1) Preparation of LiquidA
A mixture of 20 g of 7'-anilino-3'-dibutylamino-6'-methylfl.uoran and 100
g of a 10% aqueous solution of polyvinyl alcohol) was ground thoroughly in
a ball mill to prepare Liquid A or a suspension of a powder with an average
p article diameter of 0.8 a m.
(2) Preparation of Liquid B
A mixture of 20 g of 4,4'-dihydroxydiphenyl sulfone as an organic acid
substance and 100 g of a 10% aqueous solution of polyvinyl alcohol) was
ground thoroughly in a ball mill to prepare Liquid B or a suspension of a
powder with an average particle diameter of 0.8 a m.
(3) Preparation of Liquid C
22
CA 02371443 2001-10-25
A mixture of 20 g of Compound 6 as a sensitizes and 100 g of a 10%
aqueous solution of polyvinyl alcohol) was ground thoroughly in a ball mill
to prepare Liquid C or a suspension of a powder with an average particle
diameter of 0.8 a m.
(4) Preparation of Liquid D
A mixture of 10 g of 1,1,3-tris(2-methyl-4-hydroxy-5-
cyclohexylphenyl)butane as a preservative and 100 g of a 10% aqueous
solution of polyvinyl alcohol) was ground thoroughly in a ball mill to
prepare Liquid D or a suspension of a powder with an average particle
diameter of 0.8 a m.
(5) Preparation of Liquid E
A mixture of 10 g of zinc stearate, 1 g of dimethylolurea, 1 g of calcium
carbonate and 100 g of a 10% aqueous solution of polyvinyl alcohol) was
ground thoroughly in a ball mill to prepare Liquid E or a suspension of a
powder with an average particle diameter of 0.8 ,u m.
(6) Preparation of Liquid F
Liquid F was prepared in the same manner as Liquid E except further
adding 10 g of 2,4-dihydroxybenzophenone as an ultraviolet absorber.
(7) Preparation of thermal recording paper
To 200 g of a 1:2:2:1 mixture by weight of the aforementioned Liquids A,
B, C and E was added 50 g of calcium carbonate and the mixture was
. , thoroughly dispersed. The resulting coating fluid was applied to a paper
substrate and dried to give thermal recording paper with a coating weight of
23
CA 02371443 2001-10-25
6 g/m2 after drying. The results of various tests are shown in Table 7.
(8) Color development test
The dynamic color development test (24 V, 1.0 ms) was performed on the
thermal recording papers thus prepared and the concentration of developed
color was measured for the printed region and the texture. The dynamic
color development test was performed with the use of a printing tester
(available from Ohkura Electric Co., Ltd.) and the concentration of
developed color was measured by a Macbeth reflection densitometer, Model
RD-914.
(9) Test for moisture resistance
The thermal recording paper which had been tested for dynamic color
development was stored in a thermo-hygrostat (50 °C, relative humidity
90%) for 24 hours and the concentration of developed color in the printed
region and texture was measured by a Macbeth reflection densitometer,
Model RD-914.
(10) Test for plasticizer resistance
The whole surface of the printed region of the thermal recording paper
which had been tested for dynamic color development was covered in close
contact with polyvinyl chloride) wrapping film. The test specimen was
stored in a dryer (40 °C) for 24 hours and the concentration of
developed
color of the printed region and texture was measured by a Macbeth
reflection densitometer, Model RD-914.
(11) Test for heat resistance
24
CA 02371443 2001-10-25
The thermal recording paper which had been tested for dynamic color
development was stored in a constant-temperature dryer (80 °C) for 24
hours and the concentration of developed color of the printed region and
texture was measured by a Macbeth reflection densitometer, Model RD-914.
(12) Test for light resistance
The thermal recording paper which had been tested for dynamic color
development was irradiated with light in a fadeometer for 12 hours and the
concentration of ~ developed color of the printed region and texture was
measured by a Macbeth reflection densitometer, Model RD-914.
Example 13
Thermal recording paper was prepared as in Example 12 except using
Compound 7 in place of Compound 6 in the preparation of Liquid C and it
was tested as in Example 12.
Example 14
Thermal recording paper was prepared as in Example 12 except using
Compound 8 in place of Compound 6 in the preparation of Liquid C and it
was tested as in Example 12.
Example 15
Thermal recording paper was prepared as in Example 12 except using a
1:1 mixture of 4,4'-dihydroxydiphenyl sulfone and 4-isopropoxy-4'-
hydroxydiphenyl sulfone as an organic acid substance in the preparation of
Liquid B and it was tested as in Example 12.
CA 02371443 2001-10-25
Example 16
Thermal recording paper was prepared as in Example 12 except using
Liquid D in place of Liquid E in the preparation of a coating fluid and it was
tested as in Example 12.
Example 17
Liquid E was applied to the thermal recording paper obtained in
Example 12 until the amount of coating after drying became 3 g/m2 and
dried to give thermal recording paper with an overcoat layer. The paper
with the overcoat was tested as in Example 12.
Example 18
Thermal recording paper was prepared as in Example 17 except using
liquid D in place of Liquid E in the preparation of a coating fluid,
thereafter
overcoated as in Example 17, and it was tested as in Example 17.
Example 19
Thermal recording paper was prepared as in Example 18, overcoated as
in Example 18 except using liquid F in place of Liquid E, and tested as in
Example 18.
Example 20
Thermal recording paper was prepared as in Example 12 except using
2,4'-dihydroxydiphenyl sulfone as an organic acid substance in the
preparation of Liquid B and using Liquid D in place of Liquid E in the
preparation of a coating fluid and it was tested as in Example 12.
Example 21
26
CA 02371443 2001-10-25
Thermal recording paper was prepared as in Example 12 except using
2,4'-dihydroxydiphenyl sulfone as an organic acid substance in the
preparation of Liquid B, then coated with Liquid E until the weight of
coating became 3 g/m2 after drying, and dried to give thermal recording
paper with an overcoat layer. The paper with the overcoat layer was tested
as in Example 12.
Example 22
Thermal recording paper was prepared as in Example 21 except using
Liquid D in place of Liquid E in the preparation of a coating fluid, then
overcoated as in Example 21, and tested as in Example 21.
Example 23
Thermal recording paper was prepared as in Example 22, overcoated as
in Example 22 except using Liquid F in place of Liquid E, and tested as in
Example 22.
Comparative Example 5
Thermal recording paper was prepared as in Example 12 except using
para-benzylbiphenyl in place of Compound 6 in the preparation of Liquid C
and tested as in Example 12.
Comparative Example 6
Thermal recording paper was prepared as in Example 12 except using
acetoacetanilide in place of Compound 6 in the preparation of Liquid C and
tested as in Example 12.
27
CA 02371443 2001-10-25
The test results are summarized and shown in Table 7.
[Table 7
Dynamic
color
Moisture Plasticizes H eat Light
development
test resistance resistance resistance resistance
test test test test
Texture/Printed Te.cture/Printed Texture/Printed Texture/Printed
Texture/Printed
re re region re region
ion 'on ion
Ex. 0.08 1.010.09 0.62 0.10 0.77 0.12. 1.080.13 0.98
l2 . . .
Ex. 0.07 0.970.09 0.55 0.09 0.68 0.08~ 1.040.12 0.90
l3 ~ ~ ~ ~
,
Ex. 0.07 0.910.08 0.56 0.09 0.60 0.08; 1.000. 0.88
l4 ; ; 15
;
Ex. 0.09 1. 0. 0.82 0.09 0.79 0. ; 1. 0. 1.05
15 ; 10 10 ; 15 13 18
;
Ex. 0.08 1.020.08 0.71 0.09 0.78 0.15. 1.030.14 0.98
l6 . v . .
Ex. 0.08 0.960.08 0.92 0. 0.93 0. ' 0.980. 0.94
17 ' 10 13 13
' '
Ex. 0.07 0.990.09 0.95 0.09 0.96 0. ; 1.020. 0.97
18 ; ; 18 12
;
Ex. 0.08 0.980. 0.97 0. 0.92 0. . 1.000.09 0.93
19 ; 10 11 12 ;
;
Ex.200.08 1.090.09 0.83 0.09 0.85 0.131.01 0.12 1.04
. . .
, . ,
Ex.210.08 1.070.09 1.05 0. 1.00 0. ' 1.020. 1.01
' 10 14 13
' '
Ex.220.09 1.050.09 1.03 0. 1.02 0.13; 1.030. 1.01
; 10 13
; ;
Ex.230.09 1.040.09 1.01 0. 1.01 0. ; 1.000.09 1.01
; 10 13 ;
;
Ex.240.10 0.980.15 0.48 0.13 0.78 0.88. 1.020.18 0.90
. .
, . ,
CompØ07 0.420.08 0.22 0.09 0.26 0. ~ 0.480. 0.38
~ ; 12 10
~
Ex.S ' ' ' '
CompØ 0.980. 0.48 0.13 0.78 0.88; 1.020. 0.90
10 15 ; 18
; ;
Ex. . . , ,
6
Industrial Applicability
A combination of an organic acid substance and a sensitizes of this
invention gives a thermal recording material which is well balanced in
properties and exhibits outstanding color-developing sensitivity, whiteness
28
CA 02371443 2001-10-25
of the texture after printing and also after long-term storage and good
storage stability of images in exposure to moist heat or in contact with
plasticizers. In consequence, this thermal recording material performs
excellently in storage of images such as printed pictures, letters and
symbols and is useful for recording media such as receipts, tickets, highway
cards, baggage tags and barcode labels. Because of its good resistance to
plasticizers, it is also useful for labels on food packages.
29