Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
METHOD OF STERILIZING
GOVERNMENT SUPPORT
This work was supported, in part, by grants from the National Institutes of
Health NS 14069,
AG08967, AG02132, AG10770 and K08 NS02048-02. The government may have certain
rights in
this work.
FIELD OF THE INVENTION
The present invention relates generally to methods of sterilizing materials
and particularly to
a method of inactivating infectious prions.
BACKGROUND OF THE INVENTION
There are large numbers of known methods of sterilizing materials. Many
methods involve
heating a material to a temperature at which pathogens are killed or
inactivated. Other methods
involve exposing the material to compounds which kill or inactivate pathogens
which are contacted
by the compounds. Still other methods involve irradiating a material with a
sufficient amount of a
particular type of radiation for a period of time sufficient to inactivate,
disrupt or kill pathogens in
the material. These methods are generally directed toward killing bacteria and
inactivating viruses
present in or on the material. Although sterilization methods may be quite
affective in killing
bacteria or inactivating viruses, they do not generally inactivate pathogenic
proteins such as prions
which can be responsible for a number of fatal diseases.
There are a considerable number of diseases associated with a conformationally
altered
protein. For example, Alzheimer's disease is associated with APP, A(3 peptide,
al-antichymotrypin,
tau and non-A~i component. Many of these diseases are neurological diseases.
However, type II
Diabetes is associated with Amylin and Multiple myeloma-plasma cell dyscrasias
is associated with
IgGL-chain. The relationship between the disease onset and the transition from
the normal protein to
the conformationally altered protein has been examined very closely in some
instances such as with
the association between prion diseases and PrPS°.
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
Prion diseases are a group of fatal neurodegenerative disorders that can occur
in hereditary,
sporadic, and infectious forms (Prusiner, S.B. Scrapie prions. Annu. Rev.
Microbiol. 43, 345-374
(1989)). These illnesses occur in humans and a variety of other animals
(Prusiner, S.B. Prions.
Proc. Natl. Acad. Sci. USA 95, 13363-13383 (1998)). Prions are infectious
proteins. The normal,
S cellular form of the prion protein (PrP) designated PrP~ contains three a-
helices and has little /3-
sheet; in contrast, the protein of the prions denoted PrPs° is rich in
(i-sheet structure. The
accumulation of PrPs~ in the central nervous system (CNS) precedes neurologic
dysfunction
accompanied by neuronal vacuolation and astrocytic gliosis.
The spectrum of human prion diseases includes kuru (Gajdusek, D.C., Gibbs,
C.J., Jr. &
Alpers, M. Experimental transmission of a kuru-like syndrome to chimpanzees.
Nature 209, 794-
796 (1966)), Creutzfeldt-Jakob disease (CJD) (Gibbs, C.J., Jr., et al.
Creutzfeldt-Jakob disease
(spongiform encephalopathy): transmission to the chimpanzee. Science 161, 388-
389 (1968)),
Gerstmann-Straussler-Scheinker disease (GSS) and fatal familial insomnia (FFI)
(Goldfarb, L.G., et
al. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease
phenotype determined by
a DNA polymorphism. Science 258, 806-808 (1992); Medori, R., et al. Fatal
familial insomnia: a
second kindred with mutation of prion protein gene at codon 178. Neurology 42,
669-670 (1992)) ,
and a new form of human prion disease, new variant CJD (nvCJD), which has
emerged in Great
Britain and France (Will, R.G. , et al. A new variant of Creutzfeldt-Jakob
disease in the UK.
Lancet 347, 921-925 (1996); Cousens, S.N., Vynnycky, E., Zeidler, M., Will,
R.G. & Smith, P.G.
Predicting the CJD epidemic in humans. Nature 385, 197-198 (1997); Will, R.G.,
et al. Deaths
from variant Creutzfeldt-Jakob disease. Lancet 353, 979 (1999)). Several lines
of evidence have
suggested a link between the nvCJD outbreak and a preceding epidemic of bovine
spongiform
encephalopathy (BSE) (Will, R.G. , et al. A new variant of Creutzfeldt-Jakob
disease in the UK.
Lancet 347, 921-925 (1996); Bruce, M.E., et al. Transmissions to mice indicate
that 'new variant'
CJD is caused by the BSE agent. Nature 389, 498-501 (1997); Hill, A.F., et al.
The same prion
strain causes vCJD and BSE. Nature 389, 448-450 (1997); Lasmezas, C.L, et al.
BSE
transmission to macaques. Nature 381, 743-744 (1996)). Although it is too
early to predict the
number of nvCJD cases that might eventually arise in Great Britain and
elsewhere (Cousens, S.N.,
Vynnycky, E., Zeidler, M., Will, R.G. & Smith, P.G. Predicting the CJD
epidemic in humans.
Nature 385, 197-198 (1997)), it is clear that effective therapeutics for prion
diseases are urgently
needed. Unfortunately, although a number of compounds including amphotericins,
sulfated
polyanions, Congo red dye, and anthracycline antibiotics have been reported as
prospective
therapeutic agents (Ingrosso, L., Ladogana, A. & Pocchiari, M. Congo red
prolongs the incubation
period in scrapie-infected hamsters. J. Virol. 69, 506-508 (1995); Tagliavini,
F., et al.
-2-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
Effectiveness of anthracycline against experimental prion disease in Syrian
hamsters. Science 276,
1119-1122 (1997); Masullo, C., Macchi, G., Xi, Y.G. & Pocchiari, M. Failure to
ameliorate
Creutzfeldt-Jakob disease with amphotericin B therapy. J. Infect. Dis. 165,
784-785 (1992);
Ladogana, A., et al. Sulphate polyanions prolong the incubation period of
scrapie-infected hamsters.
J. Gen. Tirol. 73, 661-665 (1992)), all have demonstrated only modest
potential to impede prion
propagation, and none have been shown to effect the removal of pre-existing
prions from an infected
host.
The PrP gene of mammals expresses a protein which can be the soluble, non-
disease form
PrP~ or be converted to the insoluble, disease form PrPS'. PrP~ is encoded by
a single-copy host
gene [Basler, Oesch et al. (1986) Cell 46:417-428] and when PrP~ is expressed
it is generally found
on the outer surface of neurons. Many lines of evidence indicate that prion
diseases result from the
transformation of the normal form of prion protein (PrP~) into the abnormal
form (PrPs°). There is
no detectable difference in the amino acid sequence of the two forms. However,
PrPs° when
compared with PrP~ has a conformation with higher (i-sheet and lower a-helix
content (Pan, Baldwin
et al. (1993) Proc Natl Acad Sci USA 90:10962-10966; Safar, Roller et al.
(1993) JBiol Chem
268:20276-20284). The presence of the abnormal PrPS° form in the brains
of infected humans or
animals is the only disease-specific diagnostic marker of prion diseases.
PrPS' plays a key role in both transmission and pathogenesis of prion diseases
(spongiform
encephalopathies) and it is a critical factor in neuronal degeneration
(Prusiner (1997) The Molecular
and Genetic Basis of Neurological Disease, 2nd Edition : 103-143). The most
common prion diseases
in animals are scrapie of sheep and goats and bovine spongiform encephalopathy
(BSE) of cattle
(Wilesmith and Wells (1991) Curr Top Microbiol Immunol 172:21-38). Four prion
diseases of
humans have been identified: (1) kuru, (2) Creutzfeldt-Jakob Disease (CJD),
(3)
Gerstmann-Straussler-Scheinker Disease (GSS), and (4) fatal familial insomnia
(FFI) [Gajdusek
(1977) Science 197:943-960; Medori, Tritschler et al. (1992) NEngl JMed
326:444-449]. Initially,
the presentation of the inherited human prion diseases posed a conundrum which
has since been
explained by the cellular genetic origin of PrP.
The assembly and misassembly of normally soluble proteins into
conformationally altered
proteins is thought to be a causative process in a variety of other diseases.
Structural conformational
changes are required for the conversion of a normally soluble and functional
protein into a defined,
insoluble state. Examples of such insoluble protein include: A(3 peptide in
amyloid plaques of
Alzheimer's disease and cerebral amyloid angiopathy (CAA); a-synuclein
deposits in Lewy bodies of
Parkinson's disease, tau in neurofibrillary tangles in frontal temporal
dementia and Pick's disease;
superoxide dismutase in amyotrophic lateral sclerosis; huntingtin in
Huntington's disease; and prions
-3-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
in Creutzfeldt-Jakob disease (CJD): (for reviews, see Glenner et al. (1989) J.
Neurol. Sci. 94:1-
28; Haan et al. (1990) Clin. Neurol. Neurosurg. 92(4):305-310).
Often these highly insoluble proteins form aggregates composed of nonbranching
fibrils with
the common characteristic of a (3-pleated sheet conformation. In the CNS,
amyloid can be present in
cerebral and meningeal blood vessels (cerebrovascular deposits) and in brain
parenchyma (plaques).
Neuropathological studies in human and animal models indicate that cells
proximal to amyloid
deposits are disturbed in their normal functions (Mandybur (1989) Acta
Neuropathol. 78:329-331;
Kawai et al. (1993) Brain Res. 623:142-6; Martin et al. (1994) Am. J. Pathol.
145:1348-1381;
Kalaria et al. (1995) Neuroreport 6:477-80; Masliah et al. (1996) J. Neurosci.
16:5795-5811).
Other studies additionally indicate that amyloid fibrils may actually initiate
neurodegeneration
(Lendon et al. (1997) J. Am. Med. Assoc. 277:825-31; Yankner (1996) Nat. Med.
2:850-2; Selkoe
(1996) J. Biol. Chem. 271:18295-8; Hardy (1997) Trends Neurosci. 20:154-9).
In both AD and CAA, the main amyloid component is the amyloid (3 protein
(A(3). The
A(3 peptide, which is generated from the amyloid (3 precursor protein (APP) by
two putative
secretases, is present at low levels in the normal CNS and blood. Two major
variants, A~i 1~
and A(31_a2, are produced by alternative carboxy-terminal truncation of APP
(Selkoe et a1.(1988)
Proc. Natl. Acad. Sci. USA 85:7341-7345; Selkoe, (1993) Trends Neurosci 16:403-
409). A(3,~2
is the more fibrillogenic and more abundant of the two peptides in amyloid
deposits of both AD
and CAA. In addition to the amyloid deposits in AD cases described above, most
AD cases are
also associated with amyloid deposition in the vascular walls (Hardy (1997),
supra; Haan et al.
(1990), supra; Terry et al., supra; Vinters (1987), supra; Itoh et al. (1993),
supra; Yamada et
al. (1993), supra; Greenberg et al. (1993), supra; Levy et al. (1990), supra).
These vascular
lesions are the hallmark of CAA, which can exist in the absence of AD.
Human transthyretin (TTR) is a normal plasma protein composed of four
identical,
predominantly (3-sheet structured units, and serves as a transporter of
hormone thyroxin. Abnormal
self assembly of TTR into amyloid fibrils causes two forms of human diseases,
namely senile
systemic amyloidosis (SSA) and familial amyloid polyneuropathy (FAP) (Kelly
(1996) Curr Opin
Strut Biol 6(1):11-7). The cause of amyloid formation in FAP are point
mutations in the TTR gene;
the cause of SSA is unknown. The clinical diagnosis is established
histologically by detecting
deposits of amyloid in situ in bioptic material.
To date, little is known about the mechanism of TTR conversion into amyloid in
vivo.
However, several laboratories have demonstrated that amyloid conversion may be
simulated in vitro
by partial denaturation of normal human TTR [McCutchen, Colon et al. (1993)
Biochemistry
32(45):12119-27; McCutchen and Kelly (1993) Biochem Biophys Res Commun 197(2)
415-21].
The mechanism of conformational transition involves monomeric conformational
intermediate which
-4-
WU 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
polymerizes into linear (3-sheet structured amyloid fibrils [Lai, Colon et al.
(1996) Biochemistry
35(20):6470-82]. The process can be mitigated by binding with stabilizing
molecules such as
thyroxin or triiodophenol (Miroy, Lai et al. (1996) Proc Natl Acad Sci USA
93(26):15051-6).
The precise mechanisms by which neuritic plaques are formed and the
relationship of plaque
formation to the disease-associated neurodegenerative processes are not well-
defined. The amyloid
fibrils in the brains of Alzheimer's and prion disease patients are known to
result in the inflammatory
activation of certain cells. For example, primary microglial cultures and the
THP-1 monocytic cell
line are stimulated by fibrillar (3-amyloid and prion peptides to activate
identical tyrosine
kinase-dependent inflammatory signal transduction cascades. The signaling
response elicited by
(3-amyloid and prion fibrils leads to the production of neurotoxic products,
which are in part
responsible for the neurodegenerative . C.K. Combs et al, JNeurosci 19:928-39
(1999).
Although research efforts relating to conformationally altered proteins are
advancing efforts
to sterilize materials to avoid infections with such proteins axe not keeping
pace. The present
invention offers a means of sterilizing materials which contain
conformationally altered proteins such
as prions.
BRIEF DESCRIPTION OF THE DRAWING
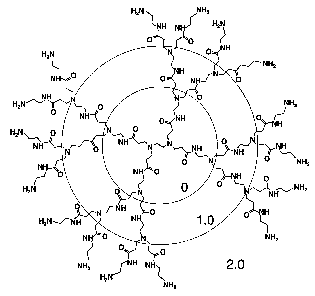
Figure 1 is a schematic drawing of a dendrimer molecule showing the defined
"generations"
of homodisperse structure created using a repetitive divergent growth
technique. The specific
diagram is of PAMAM, generation 2.0 (ethylene diamine core).
SUMMARY OF THE INVENTION
A method is disclosed whereby any type of object can be sterilized by
combining normal
sterilization procedures with the use of a polycationic dendrimer which is
capable of rendering a
conformationally altered protein such as a prion non-infectious. The method is
particularly usefi~l in
sterilizing medical devices such as surgical instruments and catheters which
have been used and
brought into contact with blood or brain tissue. Objects sterilized via the
method are also part of the
invention and include capsules which are made from geletin extracted from
cattle which cattle may
be infected with prions, i.e. have undiagnosed BSE known as "mad cow disease."
The polycationic
dendrimers can be combined with conventional antibacterial and antiviral
agents in aqueous or
alcohol solutions to produce disinfecting agents or surgical scrubs. Branched
polycations for use in
the invention include, but are not limited to, polypropylene imine,
polyethyleneimine (PEI)
poly(4'-aza-4'-methylheptamethylene D-glucaramide), polyamidoamines and
suitable fragments
and/or variants of these compounds.
-5-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
An aspect of the invention is a method of treating objects with a composition
characterized
by its ability to render proteins associated with diseases non-infectious.
An advantage of the invention is that proteins such as prions can be rendered
non-infectious
without the need for extreme conditions such as exposure to heat over long
periods of time, e.g. 1-10
hours at 100°-200°C.
A feature of the invention is that compositions can be useful while containing
only very low
concentrations of polycationic dendrimers, e.g. 1 % to 0.001 %.
Another aspect of the invention is that capsules made with bovine gelatin can
be certified
prion free.
Another aspect of the invention is that drugs produced from cell cultures
treated with
polycationic dendrimers can be certified prion free.
Still another aspect of the invention is that medical devices being reused
after exposure to
blood or brain tissue can be certified prion free.
Still another aspect of the invention is that hospitals, operating rooms and
the devices and
equipment within them can be certified prion free by contacting them with
polycationic dendrimers at
standard temperatures and pressures. A pharmaceutical composition for the
treatment of
insoluble protein deposit formation in an animal, said composition comprising
a therapeutically
effective amount of a branched polycation; and a pharmaceutically acceptable
excipient. In one
embodiment, the branched polycation is a branched polymer, and wherein at
least one branch is
positively charged, and the branched polymer may have multiple charged
branches. The
branches may have the same chemical structure, or the branches may vary in
structure within a
single molecule. Examples of polymers that may be used in such a
pharmaceutical composition
include polypropylene imine, polyethyleneimine (PEI) poly(4'-aza-4'-
methylheptamethylene
D-glucaramide), polyamidoamines and pharmaceutically effective variants or
fragments thereof. In
one embodiment of the pharmaceutical composition, the composition also
contains a second
therapeutic agent, such as an analgesic agent, an antimicrobial agent, anti-
inflammatory agent, an
antioncogenic agent, an antiviral agent, and the like.
The present invention also provides a method of enhancing clearance of a
disease related
conformation of a protein from cells by contacting cells with a branched
polycation for a time
sufficient to enhance the rate of clearance of a disease related conformation
of a protein from the
cells. This branched polycation can be administered in vivo or ex vivo to a
subject including a
human, cow, sheep, deer, dog, cat, goat, chicken and turkey. Examples of such
disease related
proteins include PrPs°, APP, A(3 peptide, a-1-antichymotrypsin. The
method is thus useful for
subjects suffering from disorders such as bovine spongiform encephalopathy,
-6-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
Creutzfeldt-Jacob Disease, fatal familial insomnia, GSS for Gerstmann-
Straussler-Scheinker
Disease, kuru, scrapie, Alzheimer's Disease, Frontal temporal dementia,
Huntington's disease, ALS,
Pick's disease, Parkinson's disease, Diabetes Type II, multiple myeloma,
familial amyloidotic
polyneuropathy, medullary carcinoma of thyroid, chronic renal failure,
congestive heart failure,
senile cardiac and systemic amyloidosis, chronic inflammation,
atherosclerosis, and familial
amyloidosis. The branched polycation can be administered to a subject in an
amount non-toxic to the
subject, for example a dosage of 0.001 mg to 1 mg/kg body weight per day. The
polycation may be
administered in a single dosage form, or it may be repeatedly administered to
the subject. The
branched polycation may also be administered prophylactically to prevent the
formation of the
disease conformation of these proteins.
The invention also provides a food composition for the preventing insoluble
protein deposit
formation in an animal, where the food contains a therapeutically effective
amount of a branched
canon which enhances clearance of conformationally altered protein. The food
can be any food
product, including solid foods such as meat (e.g., beef or lamb) and liquid
foods such as vinegar, oil,
and condiments such as steak sauce and ketchup. The branched polycation is
allowed to contact the
food prior to ingestion for a time sufficient to allow clearance of the
conformationally altered
proteins.
The present invention also provides a method of preventing a farm animal from
acquiring a
disease associated with a conformationally altered protein by feeding the
animals animal feed
containing a branched polycation. The feed containing the branched polycation
may be synthetically
produced, and fed directly to the animals, or the branched polycation may be
introduced to a natural
food source, e.g., the animal feed is grass and the branched polycation is
sprayed on the grass or
introduced to the grass through a plant fertilizer. One aspect of this
embodiment of the invention is a
method of preventing disease caused by ingestion of contaminated food products
by feeding animals
foods containing branched polycations.
The present invention also provides a method of enhancing clearance of a
disease related
conformation of a protein from a meat food product by contacting the meat with
a compound which
enhances clearance of a conformationally altered protein at a pH of 5 or less
for a time sufficient to
allow for destruction of conformationally altered protein.
An advantage of the invention is that conformationally altered protein such as
prions can be
rendered non-infectious with a method which need only consist of applying a
polycationic dendrimer
preferably held at a pH of 5.0 or less.
Another aspect of the invention is soaps, surgical scrubs, detergents and the
like with
polycationic dendrimers therein.
WO 00/72851 CA 02375237 2001-11-26 PCTNS00/14353
An advantage of the invention is that compositions containing polycationic
dendrimers can
be used to inactivate prions which might be present on surgical instruments,
knives and/or other tools
or equipment used by butchers, particularly those used in the butchering of
cows or other animals
which might be infected with prions.
A feature of the invention is that compositions of the invention can be
effective in activating
prions when the polycationic dendrimers are present in very low
concentrations, e.g. 1 % to 0.001
or less.
These and other aspects, advantages, and features of the invention will become
apparent to
those persons skilled in the art upon reading the details of the compounds,
and assay method more
fully described below.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Before the present methods, objects and compositions are described, it is to
be understood
that this invention is not limited to the particular steps, devices or
components described and, as
such, may of course vary. It is also to be understood that the terminology
used herein is for the
purpose of describing particular embodiments only, and is not intended to be
limiting, since the scope
of the present invention will be limited only by the appended claims.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention belongs.
Although any methods and materials similar or equivalent to those described
herein can be used in
the practice or testing of the present invention, the preferred methods and
materials are now
described. All publications mentioned herein are incorporated herein by
reference to disclose and
describe the methods and/or materials in connection with which the
publications are cited.
The publications discussed herein are provided solely for their disclosure
prior to the filing
date of the present application. Nothing herein is to be construed as an
admission that the present
invention is not entitled to antedate such publication by virtue of prior
invention. Further, the dates
of publication provided may be different from the actual publication dates
which may need to be
independently confirmed.
DEFINITIONS
The term "detergent" is used to mean any substance that reduces the surface
tension of
water. The detergent may be a surface active agent which concentrates at oil-
water interfaces, exerts
emulsifying action and thereby aids in removing soils e.g. common sodium soaps
of fatty acids. A
detergent may be anionic, cationic, or monionic depending on their mode of
chemical action.
Detergents include linear alkyl sulfonates (LAS) often aided by "builders." A
LAS is preferably an
_g_
WO 00/72851 CA 02375237 2001-11-26 PCTNS00/14353
alkyl benzene sulfonate ABS which is readily decomposed by microorganisms
(biodegradable). The
LAS is generally a straight chain alkyl comprising 10 to 30 carbon atoms. The
detergent may be in a
liquid or a solid form.
The term "conformationally altered protein" is used here to describe any
protein which has a
three dimensional conformation associated with a disease. The conformationally
altered protein may
cause the disease, be a factor in a symptom of the disease or appear as a
result of other factors. The
conformationally altered protein appears in another conformation which has the
same amino acid
sequence. In general, the conformationally altered protein formed is
"constricted" in conformation as
compared to the other "relaxed" conformation which is not associated with
disease. The following is
a non-limiting list of diseases with associated proteins which assemble two or
more different
conformations wherein at least one conformation is an example of a
conformationally altered protein.
Disease Insoluble Proteins
Alzheimer's Disease APP, A~i peptide,
a 1-antichymotrypsin,
tau, non-A(3 component,
presenillin 1, presenillin
2
apoE
Prion diseases,
Creutzfeldt Jakob
disease, scrapie and
bovine spongiform
encephalopathy prps
ALS SOD and neurofilament
Pick's disease Pick body
Parkinson's disease a-synuclein in Lewy bodies
Frontotemporal dementia tau in fibrils
Diabetes Type II Amylin
Multiple myeloma--
plasma cell dyscrasias IgGL-chain
Familial amyloidotic
polyneuropathy Transthyretin
Medullary carcinoma
of thyroid Procalcitonin
Chronic renal failure ~i2--microglobulin
Congestive heart failure Atrial natriuretic
factor
Senile cardiac and
systemic amyloidosis Transthyretin
4~ Chronic inflammation Serum amyloid A
-9-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
Atherosclerosis ApoAl
Familial amyloidosis Gelsolin
Huntington's disease Huntingtin
The term"acid" is used to describe any compound or group of compounds which
has one or
more characteristics of (a) sour taste; (b) turns litmus dye red; (c) reacts
with certain metals to form
a salt; (d) reacts with certain bases or a.lkalines to form a salt. An acid
comprises hydrogen and in
water undergoes ionization so that H30+ ions are formed - also written as H+
and referred to as
hydronium ions or simply hydrogen ions. Weak acids such as acetic acid or
carbonic acid may be
used as may strong acids such as hydrochloric acid, nitric acid and sulfixric
acid. In compositions of
the invention the acid is preferably present in a concentration so as to
obtain a pH of 5 or less, more
preferably 4 or less and still more preferably 3.5 ~ 1.
The terms "sterilizing", "making sterile" and the like are used here to mean
rendering
something non-infectious or rendering something incapable of causing a
disease. Specifically, it
refers to rendering a protein non-infectious or incapable of causing a disease
or the symptoms of a
disease. Still more specifically, it refers to rendering a conformationally
altered protein (e.g. PrPS°
known as prions) incapable of causing a disease or the symptoms of a disease.
By "effective dose" or "amount effective" is meant an amount of a compound
sufficient to
provide the desired sterilizing result. This will vary depending on factors
such as the type of object
or material being sterilized and the amount or concentration of infectious
proteins which might be
present. Polycations of the invention or more specifically polycationic
dendrimer compounds of the
invention could be mixed with a material in an amount in a range 1 to 500 ~g
of dendrimer per ml or
mg of material being sterilized. The concentration is sufficient if the
resulting composition is
effective in decreasing the infectivity of conformationally altered proteins
such that the treated
material over time would not result in infection. Because (1) some materials
will have higher
concentrations of altered protein than others (2) some materials are contacted
more frequently than
others and (3) individual proteins have different degrees of infectivity the
effective dose or
concentration range needed to sterilize can vary considerably. It is also
pointed out that the dose
needed to treat an amount of material may vary somewhat based on the pH the
treatment is carried
out at and the amount of time the compound is maintained in contact with the
material at the desired
low pH (e.g., 5.0 or less) level.
The term "LDSO" as used herein is the dose of an active substance that will
result in 50
percent lethality in all treated experimental animals. Although this usually
refers to invasive
administration, such as oral, pa,renteral, and the like, it may also apply to
toxicity using less invasive
methods of administration, such as topical applications of the active
substance.
-10-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
The term "amine-terminated" includes primary, secondary and tertiary amines.
The terms "PrP protein", "PrP" and like are used interchangeably herein and
shall mean both
the infectious particle form PrPs° known to cause diseases (spongiform
encephalopathies) in humans
and animals and the noninfectious form PrP~ which, under appropriate
conditions is converted to the
infectious PrPs° form.
The terms "prion", "prion protein", "PrPs°protein" and the like are
used interchangeably
herein to refer to the infectious PrPS° form of a PrP protein, and is a
contraction of the words
"protein" and "infection." Particles are comprised largely, if not
exclusively, of PrPs° molecules
encoded by a PrP gene. Prions are distinct from bacteria, viruses and viroids.
Known prions infect
animals to cause scrapie, a transmissible, degenerative disease of the nervous
system of sheep and
goats, as well as bovine spongiform encephalopathy (BSE), or "mad cow
disease", and feline
spongiform encephalopathy of cats. Four prion diseases known to affect humans
are (1) kuru, (2)
Creutzfeldt-Jakob Disease (CJD), (3) Gerstmann-Straussler-Scheinker Disease
(GSS), and (4) fatal
familial insomnia (FFI). As used herein "prion" includes all forms of prions
causing all or any of
these diseases or others in any animals used - and in particular in humans and
domesticated farm
animals.
The term "PrP gene" is used herein to describe genetic material which
expresses proteins
including known polymorphisms and pathogenic mutations. The term "PrP gene"
refers generally to
any gene of any species which encodes any form of a prion protein. Some
commonly known PrP
sequences are described in Gabriel et al., Proc. Natl. Acad. Sci. USA 89:9097-
9101 (1992) and U.S.
Patent No. 5,565,186, incorporated herein by reference to disclose and
describe such sequences. The
PrP gene can be from any animal, including the "host" and "test" animals
described herein and any
and all polymorphisms and mutations thereof, it being recognized that the
terms include other such
PrP genes that are yet to be discovered. The protein expressed by such a gene
can assume either a
PrP~ (non-disease) or PrPs' (disease) form.
The terms "standardized prion preparation", "prion preparation", "preparation"
and the like
are used interchangeably herein to describe a composition (e.g., brain
homogenate) obtained from the
brain tissue of mammals which exhibits signs of prion disease: the mammal may
(1) include a
transgene as described herein; (2) have and ablated endogenous prion protein
gene; (3) have a high
number of prion protein gene from a genetically diverse species; and/or (4) be
a hybrid with an
ablated endogenous prion protein gene and a prion protein gene from a
genetically diverse species.
Different combinations of 1-4 are possible, e.g., 1 and 2. The mammals from
which standardized
pr~ion preparations are obtained exhibit clinical signs of CNS dysfunction as
a result of inoculation
with priors and/or due to developing the disease of their genetically modified
make up, e.g., high
copy number of prior protein genes. Standardized prior preparations and
methods of making such
-11-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
are described and disclosed in U.S. Patent 5,908,969 issued June l, 1999 and
application serial
no. 09/199,523 filed November 25, 1998 both of which are incorporated herein
by reference in
their entirety to disclose and describe standardized prion preparations.
The term "Alzheimer's disease" (abbreviated herein as "AD") as used herein
refers to a
condition associated with formation of neuritic plaques comprising amyloid ~i
protein, primarily
in the hippocampus and cerebral cortex, as well as impairment in both learning
and memory.
"AD" as used herein is meant to encompass both AD as well as AD-type
pathologies.
The term "AD-type pathology" as used herein refers to a combination of CNS
alterations
including, but not limited to, formation of neuritic plaques containing
amyloid ~3 protein in the
hippocampus and cerebral cortex. Such AD-type pathologies can include, but are
not necessarily
limited to, disorders associated with aberrant expression and/or deposition of
APP,
overexpression of APP, expression of aberrant APP gene products, and other
phenomena
associated with AD. Exemplary AD-type pathologies include, but are not
necessarily limited to,
AD-type pathologies associated with Down's syndrome that is associated with
overexpression of
APP.
The term "phenomenon associated with Alzheimer's disease" as used herein
refers to a
structural, molecular, or functional event associated with AD, particularly
such an event that is
readily assessable in an animal model. Such events include, but are not
limited to, amyloid
deposition, neuropathological developments, learning and memory deficits, and
other AD-
associated characteristics.
The term "cerebral amyloid angiopathy" (abbreviated herein as CAA) as used
herein
refers to a condition associated with formation of amyloid deposition within
cerebral vessels
which can be complicated by cerebral parenchymal hemorrhage. CAA is also
associated with
increased risk of stroke as well as development of cerebellar and subarachnoid
hemorrhages
(Vinters (1987) Stroke 18:311-324; Haan et al. (1994) Dementia 5:210-213; Itoh
et al. (1993) J.
Neurol. Sci. 116:135-414). CAA can also be associated with dementia prior to
onset of
hemorrhages. The vascular amyloid deposits associated with CAA can exist in
the absence of
AD, but are more frequently associated with AD.
The term "phenomenon associated with cerebral amyloid angiopathy" as used
herein
refers to a molecular, structural, or functional event associated with CAA,
particularly such an
event that is readily assessable in an animal model. Such events include, but
are not limited to,
amyloid deposition, cerebral parenchymal hemorrhage, and other CAA-associated
characteristics.
The term "(3-amyloid deposit" as used herein refers to a deposit in the brain
composed of
A(3 as well as other substances.
-12-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
Abbreviations used herein include:
CNS for central nervous system;
BSE for bovine spongiform encephalopathy;
CJD for Creutzfeldt-Jakob Disease;
FFI for fatal familial insomnia;
GSS for Gerstmann-Straussler-Scheinker Disease;
AD for Alzheimer's disease;
CAA for cerebral amyloid angiopathy;
Hu for human;
HuPrP for human prion protein;
Mo for mouse;
MoPrP for mouse prion protein;
SHa for a Syrian hamster;
SHaPrP for a Syrian hamster prion protein;
PAMAM for polyamidoamide dendrimers
PEI for polyethyleneimine
PPI for polypropyleneimine
PrPs~ for the scrapie isoform of the prion protein;
PrP~ for the cellular contained common, normal isoform of the prion protein;
PrP 27-30 or PrPs~ 27-30 for the treatment or protease resistant form of
PrPs~;
MoPrPs' for the scrapie isoform of the mouse prion protein;
N2a for an established neuroblastoma cell line used in the present studies;
ScN2a for a chronically scrapie-infected neuroblastoma cell line;
ALS for amyotrophic lateral sclerosis;
HD for Huntington's disease;
FTD for frontotemporal dementia;
SOD for superoxide dismutase
GENERAL ASPECTS OF THE INVENTION
The invention comprises compositions of compounds found to be effective in
rendering
conformationally altered proteins non-infective. The compositions are
preferably low pH solutions
comprised of a non toxic weak acid such as acetic acid having dissolved
therein a branched
polycation. Preferred compositions of the invention are in the form of aqueous
or alcohol solutions
which are comprised of a branched polycation, an antibacterial, an antifungal
and an antiviral
compound. The compositions are coated on, mixed with, injected into or
otherwise brought into
-13-
WD X0/72851 CA 02375237 2001-11-26 PCT/US00/14353
contact with a material to be sterilized. The composition is applied in a
manner so that the branched
polycation is maintained at a low pH (e.g. ~ or less and preferably 3.5 t 1)
in an amount of 1 ~cg or
more polycation per ml or mg of material to be sterilized. The composition is
maintained in the
desired pH range at normal temperature (e.g., 15 ° C to 30 ° C)
for a sufficient period of time (e.g. 1
hour to 1 week) to cause conformationally altered protein present on or in the
material to be
destroyed (e.g. hydrolyzed) or rendered non-infective. Preferred compositions
of the invention are
useful in cleaning and sterilizing and may be comprised of a polycationic
dendrimers, a detergent,
and an acid proving a pH of about 3.5 ~1.
DENDRIMER COMPOUNDS WHICH CLEAR PRIONS
Dendrimers are branched compounds also known as "starburst" or "star" polymers
due
to a characteristic star-like structure (see Figure 1). Dendrimers of the
invention are polymers
with structures built from AB" monomers, with n>_2, and preferably n=2 or 3.
Such dendrimers
are highly branched and have three distinct structural features: 1) a core, 2)
multiple peripheral
end-groups, and 3) branching units that link the two. Dendrimers may be
cationic (full
generation dendrimers) or anionic (half generation dendrimers). For a review
on the general
synthesis, physical properties, and applications of dendrimers, see, e. g. ,
Tomalia et. al, Angew.
Chem. Int. Ed. Engl., 29:138-175, (1990); Y. Kim and C. Zimmerman, Curr Opin
Chem Biol,
2:733-7421 (1997).
In a preferred embodiment, sterilizing compositions of the invention comprise
a cationic
dendrimer preferably dissolved in a low pH solvent such as acetic acid.
Examples of suitable
dendrimers are disclosed in U.S. Pat. Nos. 4,507,466, 4,558,120, 4,568,737,
4,587,329,
4,631,337, 4,694,064, 4,713,975, 4,737,550, 4,871,779, and 4,857,599 to D. A.
Tomalia, et
al., which are hereby incorporated by reference to disclose and describe such
compounds.
Dendrimers typically have tertiary amines which have a pKa of 5.7. The
dendrimers can
optionally be chemically or heat treated to remove some of the tertiary
amines. Other suitable
canons include polypropylene imine, polyethyleneimine (PEI), which has
tertiary amines with a
pKa of 5.9, and poly(4'-aza-4'-methylheptamethylene D-glucaramide), which has
tertiary amines
with a pKa of 6Ø The cationic dendrimer is preferably dissolved in the low
pH solvent such as
vinegar in a concentration of 0.0001 % or more, preferably 0.01 % or more and
more preferably
about 1 % .
Preferably, the dendrimers for use in the invention are polyamidoamines
(hereinafter
"PAMAM"). PAMAM dendrimers are particularly biocompatible, since
polyamidoamine groups
resemble peptide bonds of proteins.
-14-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
Dendrimers are prepared in tiers called generations (see generations 0, l and
2 in Figure
1) and therefore have specific molecular weights. The full generation PAMAM
dendrimers have
amine terminal groups, and are cationic, whereas the half generation
dendrimers are carboxyl
terminated. Full generation PAMAM dendrimers are thus preferred for use in the
present
invention. PAMAM dendrimers may be prepared having different molecular weights
and have
specific values as described in Table 1 below for generations 0 through 10.
TABLE A
LIST OF PAMAM DENDRIMERS AND THEIR
MOLECULAR WEIGHTS (Ethylene Diamine core, amine terminated),
GENERATION TERMINAL GROUPS MO L. WT. /mole
0 4 517
1 8 1430
2 216 3256
3 32 6909
4 64 14,215
5 128 28,795
6 256 58,048
7 512 116,493
8 1024 233,383
9 2048 467,162
10 4096 934,720
As shown in Table A, the number of terminal amine groups for PAMAM dendrimers
generations 0 through 10 range from 4 to 4,096, with molecular weights of from
517 to 934,720.
PAMAM dendrimers are available commercially from Aldrich or Dendritech.
Polyethyleneimine
or polypropylene dendrimers or quaternized forms of amine-terminated
dendrimers may be
prepared as described by Tomalia et. al, Angew, Chem. Int. Ed. Engl., 29:138-
175 (1990)
incorporated by reference to describe and disclose methods of making
dendrimers.
STERILIZING COMPOSITIONS
Examples provided here show that highly-branched polycations, e.g. dendrimer
compounds, affect the extent and distribution of PrPs° protein deposits
in scrapie-infected cells.
The presence of dendrimers in a low pH environment and at relatively low, non-
cytotoxic levels
results in a significant reduction in detectable PrP S~ in cells and brain
homogenates. Thus, the
present invention encompasses compositions for reducing, inhibiting, or
otherwise mitigating the
degree of infectivity of a protein. A composition of the invention is
comprised of any compound
-15-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
capable of destroying conformationally altered proteins when in a low pH
environment, (e.g. a
polycationic dendrimer) in solution, suspension or mixture.
STERILIZING FORMULATIONS
Sterilizing compositions of the invention preferably contain highly branched
polycations,
e.g. polycationic dendrimer, in a concentration from 0.0001 to 10% of the
formulation. The
following methods and excipients are merely exemplary and are in no way
limiting.
In addition to including the compound such as a highly branched cationic
compound in
the formulation it is important to maintain that compound in a low pH
environment. Any number
of known acids or mixtures of acids could be used with the invention. Non-
limiting examples of
commercially available products which could be supplemented with the cationic
compounds are
described below. In these formulations the percentage amount of each
ingredient can vary. In
general a solvent ingredient (e.g. water or alcohol) is present in amounts of
40% to 100% and
the last listed ingredient is present in a range of 0.5 % to 5 % . The other
ingredients are present
in an amount in a range of 1 % to 60 % and more generally 5 % to 20 % . In
each case the
polycationic compounds of the invention are added in amounts of about 0.01 %
to 5 % and
preferably 0.1 % to 2 % and more preferably about 1 % . The amount added is an
amount needed
to obtain the desired effect.
FORMULATION 1
Component wt %
acid 90 - 99.99
polycationic dendrimer0.01 - 10
FORMULATION 2
Component wt %
water 10 - 99
acid 1- 20
polycationic dendrimer0.01 - 10
FORMU LATION 3
Component wt %
water 10 - 98
acid 1 - 20
detergent 1 - 20
polycationic dendrimer0.01 - 5
-16-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
FORMULATION 4
Component wt %
water 10 - 98
acetic acid 1 - 20
linear alkyl sulfonate 1 - 20
polycationic dendrimer 0.01 - 5
FORMU LATION 5
Component wt %
water 1 - 98
alcohol 0 - 98
acid 1 - 20
detergent 1 - 20
polycationic dendrimer0.1 - 5
FORMULATION 6
Component wt %
water 1 - 99
acid 1 - 20
antibacterial 0.1 - 5
detergent 1 - 20
polycationic dendrimer0.1 - 5
FORMULATION 7
Component wt %
water 3 - 98.889
antimicrobial active 0.001- 5
agent
anionic surfactant 1 - 80
protein donating 0.1 - 12
agent
polycationic dendrimer 0.01 - 5
FORMU LATION 8
Component wt
Polycationic Dendrimer0.5
Ethanol 74.0
Benzalkonium chloride0.2
CAE 0.02
Glycerine 1.0
Chain silicone 0.5
Triglyceride 0.5
Lactic acid 10.0
Purified water 13.28
-17-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
FORMULA TION 9
Component wt
Polycationic Dendrimer1.0
Ethanol 75.0
Benzalkonium chloride0.2
CAE 0.02
Glycerine 1.0
Cyclic silicone 0.2
Triglyceride 0.3
Acetic Acid 20.0
Purified water 2.28
FORMULA TION 10
Component wt
Polycationic Dendrimer0.25
Ethanol 74.0
Chlorhexedine gluconate0.75
Benzalkonium chloride0.2
CAE 0.02
Glycerine 2.0
Chain silicone 0.2
Cyclic silicone 0.2
Triglyceride 0.3
Acetic Acid 20.0
Purified water 2.08
FORMU LATION 11
Component wt
Polycationic Dendrimer0.1
Ethanol 75.0
Chlorhexedine gluconate0.9
Benzalkonium chloride0.2
CAE 0.02
Glycerine 1.0
Chain silicone 0.5
Cyclic silicone 0.5
Triglyceride 0.3
Lactic acid 14.0
Purified water 7.98
-18-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
FORMU LATION 12
Component wt
Polycationic Dendrimer0.01
Ethanol 75.0
Benzalkonium chloride0.2
CAE 0.02
Glycerine 2.0
Chain silicone 0.99
Cyclic silicone 2.0
Triglyceride 3.0
Lactic acid 9
Purified water 7.78
FORMU LATION 13
Component wt
Polycationic Dendrimer1
Ethanol 75.0
Chlorhexedine gluconate0.2
Benzalkonium chloride0.2
CAE 0.02
Glycerine 0.8
Chain silicone 0.2
Cyclic silicone 0.2
Triglyceride .38
Acetic acid 10
Purified water 12
FORMU LATION 14
Component wt
Polycationic Dendrimer0.001
Ethanol 75.99
Chlorhexedine gluconate0.2
CAE 0.02
Glycerine 1.0
Chain silicone 0.2
Triglyceride 0.3
Lactic acid 14
Purified water 8.28
-19-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
FORMULAT ION 15
Component wt
Polycationic Dendrimer1
Ethanol 75.0
Benzalkonium chloride0.2
CAE 0.02
l, 3-butylene glycol 1.0
Metylphenyl polysiloxane0.2
Isopropyl myristate 0.3
(IPM)
~ Purified water ~ 22.28
By using the disclosure provided here and other information such as taught in
U.S.
Patents 5,767,054; 6,007,831; 5,830,488; 5,968,539; 5,416,075; 5,296,158; and
patents and
publications cited therein those skilled in the art can produce countless
other formulations of the
invention. Further, such formulations can be used as described in such
publications and can be
packaged in any suitable container or dispenser device, e.g. taught in
5,992,698.
Formulations of the invention used with a cell culture have the advantage that
they are
non-toxic. For example, parenteral administration of a solution of the
formulations of the
invention is preferably nontoxic at a dosage of 0.1 mg/mouse, which is an LD
So of less than one
at 40 mg/Kg. Various nutrient formulations and/or injectable formulations of
the type known to
those skilled in the art can be used to prepare formulations for treating cell
cultures.
Those skilled in the art will understand that in some situations it may be
desirable to further
reduce the pH environment to obtain the desired results. This can be
accomplished by adding any
desired acid. If desired, the pH can be raised to a normal level after
treatment is complete, i.e. after a
sufficient amount of any conformationally altered protein present are
destroyed.
Compounds effective in sterilizing compositions containing conformationally
altered proteins
are determined via a cell culture assay and an organ homogenate assay each of
which is described
below in detail.
ScN2a CELL BASED ASSAY
Efforts were made to optimize the transfection of ScN2a cells with pSPOX
expression
plasmids (Scott, M.R., Kohler, R., Foster, D. & Prusiner, S.B. Chimeric prion
protein expression in
cultured cells and transgenic mice. Protein Sci. l, 986-997 (1992)). In
connection with those effects
an evaluation was made of a transfection protocol that used SuperFect reagent
(QIAGEN~). It was
found that epitope-tagged (MHM2) PrPs° (Scott, M.R., Kohler, R.,
Foster, D. & Prusiner, S.B.
Chimeric prion protein expression in cultured cells and transgenic mice.
Protein Sci. 1, 986-997
-20-
WU 00/72851 CA 02375237 2001-11-26 pCT/US00/14353
(1992)) could not be detected in ScN2a cells following SuperFect-mediated
transfection, whereas
MHM2 PrPs' was efficiently formed when a cationic liposome method for DNA
delivery was used.
Close scrutiny revealed that, prior to protease digestion, SuperFect
transfected samples expressed
MHM2 bands, which are not seen in the background pattern of an untransfected
sample. The 3F4
monoclonal antibody does not react with MoPrP but does exhibit high background
staining on
Western blots of mouse ScN2a cells. Increased immunostaining in the 20-30 kDa
region was
observed compared to the non-transfected sample. These observations led us to
conclude that
MHM2 PrP was successfully expressed using SuperFect transfection reagent, but
that conversion of
MHM2 PrP~ to protease-resistant MHM2 PrPs° was inhibited by
SuperFect.
To investigate this apparent inhibition, a Western blot was reprobed with 8073
polyclonal
antiserum to detect endogenous MoPrPs°, the presence of which is
diagnostic for prion infection in
ScN2a cells (Butler, D.A., et al. Scrapie-infected murine neuroblastoma cells
produce protease-
resistant prion proteins. J. Virol. 62, 1558-1564 (1988)). Surprisingly, it
was found that the
SuperFect treated ScN2a cells no longer contained detectable quantities of
MoPrPs° - also confirmed
in Western blots. To investigate the mechanism by which SuperFect reduced the
level of pre-existing
PrPs° in chronically infected ScN2a cells, measurements were made of
endogenous PrPs' in ScN2a
cells exposed to various concentrations of SuperFect in the absence of plasmid
DNA. The results
showed that treatment with SuperFect (a branched polycation) caused the
disappearance of PrPs°
from ScN2a cells in a dose-dependent manner. The concentration of SuperFect
required to eliminate
>95% of pre-existing PrPs° with a three hour exposure was found to be
about 150 ~cg/ml. Duration
of treatment also influenced the ability of SuperFect to remove PrPs°
from ScN2a cells: exposure to
150 /.cg/ml SuperFect for 10 min did not affect PrPs° levels, whereas
7.5 /,cg/ml SuperFect eliminated
all detectable PrPs' with a t'/Z= 8 h.
SuperFect is a mixture of branched polyamines derived from heat-induced
degradation of a
PAMAM dendrimer (Tang, M.X., Redemann, C.T. & Szoka, F.C.J. In vitro gene
delivery by
degraded polyamidoamine dendrimers. Bioconjug. Chem. 7, 703-714 (1996)).
Knowing this
structure the ability of several other branched and unbranched polymers to
eliminate PrPS° from
ScN2a cells (Table 1). The branched polymers investigated include various
preparations of PEI, as
well as intact PAMAM and PPI dendrimers. Dendrimers are manufactured by a
repetitive divergent
growth technique, allowing the synthesis of successive, well-defined
"generations" of homodisperse
structures (Figure 1). The potency of both PAMAM and PPI dendrimers in
eliminating PrPs~ from
ScN2a cells increased as the generation level increased. The most potent
compounds with respect to
eliminating PrPs° were PAMAM generation 4.0 and PPI generation 4.0,
whereas PAMAM
-21-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
generation 1.0 showed very little ability to eliminate PrPS° (Table 1).
Similarly, a high MW fraction
of PEI was more potent than low MW PEI.
From the foregoing data, it is clear that for all three branched polyamines
tested, increasing
molecular size corresponded to an increased potency for eliminating
PrPs° . To determine whether
this trend was directly attributable to increased surface density of amino
groups on the larger
molecules, PAMAM-OH generation 4.0 was tested. This is a dendrimer that
resembles PAMAM
generation 4.0 except that hydroxyls replace amino groups on its surface.
Unlike PAMAM
generation 4.0, PAMAM-OH generation 4.0 did not cause a reduction of
PrPs° levels even at the
highest concentration tested ( 10 mg/ml), establishing that the amino groups
are required for the
elimination of PrPs' by PAMAM (Table 1).
In an effort to assess the contribution of the branched architecture to the
clearing ability of
polyamines for PrPs°, the linear molecules poly-(L)lysine and linear
PEI were also tested. Both of
these linear compounds were less potent than a preparation of branched PEI
with similar average
molecular weight (Table 1), establishing that a branched molecular
architecture optimizes the ability
of polyamines to eliminate PrPs°, presumably because the branched
structures achieve a higher
density of surface amino groups.
Kinetics of PrPSc elimination by polyamines.
The preceding results demonstrate the potent ability of branched polyamines to
clear PrPs°
from ScN2a cells within a few hours of treatment. The utility of these
compounds to act as
therapeutics for treatment of prion disease was tested by determining whether
they were cytotoxic for
ScN2a cells, using as criteria cell growth, morphology, and viability as
measured by trypan blue
staining. None of the compounds was cytotoxic to ScN2a cells after exposure
for one week at
concentrations up to 7.5 ~g/ml. To determine whether branched polyamines can
cure ScN2a cells of
2~ scrapie infection without affecting cell viability, the kinetics of prion
clearance was examined in the
presence of a non-cytotoxic concentration (7.5 ~g/ml) of three different
branched polyamines. ScN2a
cells were exposed to SuperFect, PEI, or PAMAM generation 4.0 for varying
periods of time. The
kinetics of PrPs' elimination were assessed by Western blotting. All three
compounds caused a
substantial reduction in PrPS' levels after 8-16 h of treatment, and of the
three compounds, PEI
appeared to remove PrPs' most quickly, with a t'/2= 4 h.
Curing neuroblastoma cells of scrapie infection.
The above results show that it is possible to reverse the accumulation of
PrPS° in ScN2a
cells under non-cytotoxic conditions. It was also found that extended exposure
to even lower levels
-22-
WO 00/72851 CA 02375237 2001-11-26 pCT/US00/14353
of the branched polyamines ( 1.5 ~g/ml) was sufficient to eliminate
PrPS°. Based on these findings,
this protocol was used to determine whether the severe reduction in PrPs~
levels following exposure
to branched polyamines would persist after removal of the compounds. Following
the exposure of
ScN2a cells to a 1.5 ~g/ml SuperFect for 1 week, PrPs~ was reduced to <1 % of
the baseline level,
but then increased back to ~5% of the baseline level after 3 additional weeks
in culture in the absence
of polyamine. In contrast, following exposure to 1.5 ~g/ml of either PEI or
PAMAM generation 4.0
for 1 week, PrPSc was completely eliminated and did not return even after 3
weeks in culture without
polyamines. A more intensive course of treatment with 1.8 ~cg/ml SuperFect for
9 d also cured
ScN2a cells of scrapie infection fully, manifested by the absence of
PrPs° 1 month after removal of
SuperFect.
Evidence for polyamines acting within an acidic compartment.
The above results showed the potent activity of branched polyamines in rapidly
clearing
scrapie prions from cultured ScN2a cells. Based on these results the mechanism
by which these
compounds act was investigated. All of the compounds which effect removal of
PrPSc from ScN2a
cells are known to traffic through endosomes (Boussif, O., et al. A versatile
vector for gene and
oligonucleotide transfer into cells in culture and in vivo: polyethyleneimine.
Proc. Natl. Acad. Sci.
U.S.A. 92, 7297-7301 (1995); Haensler, J. & Szoka, F.C.J. Polyamidoamine
cascade polymers
mediate efficient transfection of cells in culture. Bioconjug. Chem. 4, 372-
379 (1993)). Since PrP~
is converted into PrPs° in caveolae-like domains (CLDs) or rafts
(Gorodinsky, A. & Hams, D.A.
Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant
complexes without
caveolin. J. Cell Biol. 129, 619-627 (1995); Taraboulos, A., et al.
Cholesterol depletion and
modification of COOH-terminal targeting sequence of the prion protein inhibits
formation of the
scrapie isoform. J. Cell Biol. 129, 121-132 (1995); Vey, M., et al.
Subcellular colocalization of
the cellular and scrapie prion proteins in caveolae-like membranous domains.
Proc. Natl. Acad. Sci.
USA 93, 14945-14949 (1996); Kaneko, K., et al. COOH-terminal sequence ofthe
cellular prion
protein directs subcellular trafficking and controls conversion into the
scrapie isoform. Proc. Natl.
Acad. Sci. USA 94, 2333-2338 (1997)) and is then internalized through the
endocytic pathway
(Caughey, B., Raymond, G.J., Ernst, D. & Race, R.E. N terminal truncation of
the scrapie-
associated form of PrP by lysosomal protease(s): implications regarding the
site of conversion of PrP
to the protease-resistant state. J. I~irol. 65, 6597-6603 (1991); Borchelt,
D.R., Taraboulos, A. &
Prusiner, S.B. Evidence for synthesis of scrapie prion proteins in the
endocytic pathway. J. Biol.
Chem. 267, 16188-16199 (1992)), it was deduced that polyamines act upon PrPs'
in endosomes or
lysosomes. This deduction was investigated by determining the effect of
pretreatment with the
-23-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
lysosomotropic agents chloroquine and NH4C1 on the ability of polyamines to
eliminate PrPs'. These
lysosomotropic agents alkalinize endosomes and have no effect on PrP'' levels
when administered to
ScN2a cells (Taraboulos, A., Raeber, A.J., Borchelt, D.R., Serban, D. &
Prusiner, S.B. Synthesis
and trafficking of prion proteins in cultured cells. Mol. Biol. Cell 3, 851-
863 (1992)). Experimental
results obtained shows that 100 ~cM chloroquine, but not 30 ~cM NH4C1, blocked
the ability of PEI to
eliminate PrPs'. Similar results were obtained with SuperFect and PAMAM,
generation 4Ø
Although the failure of NH4C1 to affect PrPs' levels is not easily explained,
the ability of chloroquine
to attenuate the ability of branched polyamines to remove PrPs' is consistent
with the notion that
these agents act in endosomes or lysosomes.
ORGAN HOMOGENATE ASSAY
The above results with cell cultures prompted investigating the possibility
that in an acidic
environment branched polyamines, either by indirectly interacting with PrPs'
or with another cellular
component, could cause PrPs' to become susceptible to hydrolases present in
the
endosome/lysozome. An in vitro degradation assay was developed to evaluate the
effect of pH on the
ability of polyamines to render PrPs' sensitive to protease. Crude homogenates
of scrapie-infected
mouse brain were exposed to a broad range of pH values in the presence or
absence of SuperFect
and then treated with proteinase K prior to Western blotting. Whereas PrPs'
remained resistant to
protease hydrolysis throughout the pH range (3.6-9.6) in the absence of
Superfect, addition of the
branched polyamine at pH 4.0 or below caused PrPS' to become almost completely
degraded by
protease.
Polyamine addition showed a dramatic effect on clearance in vitro which was
optimized at
pH 4 or less. These results show that polyamines act on PrPs' in an acidic
compartment. To
establish that the in vitro degradation assay is a valid approximation of the
mechanism by which
branched polyamines enhance the clearance of PrPs' from cultured cells, a
structure activity analysis
was performed with several of the compounds tested in culture cells. An
excellent correlation was
found between the clearance of PrPs' in cultured ScN2a cells (Table 1) and the
ability to render
PrPS' susceptible to protease at acidic pH in vitro. Notably, PAMAM-OH
generation 4.0 failed to
render PrPs' susceptible to protease, whereas PAMAM generation 4.0 and PPI,
generation 4.0
exhibited an even stronger activity than Superfect in vitro, as expected from
their observed potency
in cultured ScN2a cells (Table 1).
-24-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
MECHANISM OF ACTION
The results discussed here show that certain branched polyamines cause the
rapid
elimination of PrPS° from ScN2a cells in a dose- and time-dependent
manner. These compounds
demonstrate a potent ability to remove prions from cultured cells at
concentrations that are
completely non-cytotoxic. The cells may be maintained indefinitely in culture
in the presence of
therapeutic levels of branched polyamines. Furthermore, when ScN2a cells were
exposed to these
compounds for ~ 1 week, PrPs' was reduced to undetectable levels and remained
so for at least one
month after removal of the polyamine.
Clarification of the exact mechanism of PrPS~ elimination by branched
polyamines is an
important objective. Although a number of possible scenarios exist, several
possibilities may be
excluded already. One possibility that was eliminated was that polyamines act
by induction of
chaperones such as heat shock proteins that mediate prion protein refolding
because the above results
show that it was possible to reproduce the phenomenon in vitro. Furthermore
polyamines seem to
offer advantages over other putative therapeutics that would seek to promote
refolding: at very high
concentrations, dimethyl sulfoxide (DMSO) and glycerol act as direct "chemical
chaperones" and
inhibit the formation of new PrPs° (Tatzelt, J., Prusiner, S.B. &
Welch, W.J. Chemical chaperones
interfere with the formation of scrapie prion protein. EMBO J. 15, 6363-6373
(1996)), but these
compounds cannot reduce pre-existing PrPS° levels. Furthermore,
polyamines inhibit PrPs° formation
at much lower concentrations than these agents. The ability of polyamines to
effect the rapid
clearance of PrPS' also contrasts with the activity of other potential prion
therapeutics. Sulfated
polyanions may inhibit PrPs° accumulation in ScN2a cells by directly
binding to PrP~ (Gabizon, R.,
Meiner, Z., Halimi, M. & Ben-Sasson, S.A. Heparin-like molecules bind
differentially to prion-
proteins and change their intracellular metabolic fate. J. Cell. Physiol. 157,
(1993); Caughey, B.,
Brown, K., Raymond, G.J., Katzenstein, G.E. & Thresher, W. Binding of the
protease-sensitive
form of PrP (prion protein) to sulfated glycosaminoglycan and Congo red. J.
Ifirol. 68, 2135-2141
(1994)), but because branched polyamines are able to clear pre-existing
PrPs°, their mechanism of
action cannot simply involve binding to PrP~ and inhibiting de novo synthesis.
Another possible mechanism which can be excluded is endosomal rupture. The
branched
polyamines which were effective in clearing PrPs° from ScN2a cells in
our experiments, PEI,
SuperFect and PAMAM, are also potent lysosomotropic, osmotic agents which can
swell in acidic
environments and rupture endosomes (Boussif, O., et al. A versatile vector for
gene and
oligonucleotide transfer into cells in culture and in vivo: polyethyleneimine.
Proc. Natl. Acad. Sci.
U.S.A. 92, 7297-7301 (1995); Haensler, J. & Szoka, F.C.J. Polyamidoamine
cascade polymers
mediate efficient transfection of cells in culture. Bioconjug. Chem. 4, 372-
379 (1993)). This might
-25-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
suggest that branched polyamines clear PrPs° from ScN2a cells by
rupturing endosomes and
exposing PrPs' to cytosolic degradation processes. However, it is known that
the lysosomotropic,
endosome-rupturing agents NH4C1, chloroquine, and monensin do not interfere
with the formation of
PrPs° in ScN2a cells (Taraboulos, A., Raeber, A.J., Borchelt, D.R.,
Serban, D. & Prusiner, S.B.
Synthesis and trafficking of prion proteins in cultured cells. Mol. Biol. Cell
3, 851-863 (1992)).
Furthermore, the results also show that chloroquine interferes with the
ability of branched
polyamines to clear PrPs° and that polyamines can clear PrPs' in vitro
at acidic pH in the absence of
cell membranes. Together, these observations rule out endosome rupture as the
mechanism by which
branched polyamines remove PrPS°.
Without committing to any particular mechanism of action it appears likely
that branched
polyamines require the acidic environment of intact endosomes or lyzosomes to
destroy PrPS~. The
structure-activity profile of polymers tested reveals that the most active
compounds possess densely
packed, regularly-spaced amino groups, suggesting that these compounds may
bind to a ligand which
has periodically-spaced negative charges. Several scenarios remain possible.
(1) Branched
polyamines may bind directly to PrPs° arranged as an amyloid with
exposed negatively-charged
moieties and induce a conformational change under acidic conditions. (2)
Treatment of PrP 27-30
with acid decreases turbidity and increases a-helical content, suggesting that
such conditions might
dissociate PrPs~ into monomers (Safar, J., Roller, P.P., Gajdusek, D.C. &
Gibbs, C.J., Jr. Scrapie
amyloid (prion) protein has the conformational characteristics of an
aggregated molten globule
folding intermediate). It is therefore possible that polyamines bind to an
equilibrium unfolding
intermediate of PrPs' present under acidic conditions. (3) Alternatively,
polyamines might sequester
a cryptic, negatively charged component bound to PrPS' that is essential for
protease resistance, but
which is only released when PrPS' undergoes an acid-induced conformational
change. Such a
component might act as a chaperone for PrPs° inside endosomes or
lysosomes. (4) Finally, another
possibility is that polyamines activate an endosomal or lysosomal factor which
can induce a
conformational change in PrPs'. Clearly, more work will be required to
determine the precise
mechanism by which branched polyamines destroy PrPs'
GENERAL APPLICABILITY OF ASSAY
The in vitro assay described here is generally applicable in the search for
compounds that
effectively clear conformationally altered proteins present in food thereby
preventing a number of
degenerative diseases, where the accumulation of proteins seems to mediate the
pathogenesis of these
illnesses. By simulating lysosomes, where proteases hydrolyze proteins under
acidic conditions, the
-26-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
in vitro brain homogenate assay is able to rapidly evaluate the efficacy of a
variety of polyamines to
induce degradation of PrPS'.
The in vitro assay which used scrapie infected brain homogenate to test for
compounds
which clear PrPs' could be modified to assay for compounds which would clear
any conformationally
altered protein. The assay is carried out by homogenizing the organ or tissue
where the
conformationally altered protein is present in the highest concentration. The
pH of the homogenate is
then reduced to less than 5.0 and preferably 4.0 or less. For example
pancreatic tissue can be
homogenized to produce an assay to test for compounds which clear amylin which
is associated with
type II Diabetes. Homogenized kidney could be used to test for compounds which
clear (3z -
microglobulin and homogenized heart or vascular tissue used to test for
compounds which clear atrial
natriuretic factor. Those skilled in the art will recognize other organs and
tissue types which can be
homogenized to test for other compounds which clear other conformationally
altered proteins.
Besides using the in vitro assay to screen for potential drugs, the compounds
found via the
assay such as branched polyamines provide a new tool for exploring the
conversion of a protein to
conformationally altered protein, e.g. PrP~ into PrPS°. The mechanism
by which branched
polyamines render PrPs° susceptible to proteolysis, remains to be
established. Whether the
interaction of branched polyamines with PrPS' is reversible is unknown. In
addition, we do not know
whether branched polyamines are able to solubilize PrPs' without irreversibly
denaturing the protein.
Whatever the mechanism by which branched polyamines interact with PrPs~, it is
likely to be
different from that found with chaotropes as well as denaturing detergents and
solvents (Prusiner,
S.B., Groth, D., Serban, A., Stahl, N. & Gabizon, R. Attempts to restore
scrapie prion infectivity
after exposure to protein denaturants. Proc. Natl. Acad. Sci. USA 90, 2793-
2797 (1993)).
Using the assays described and disclosed here certain specific branched
polyamines have
been found which mediate the clearance of PrPS' from cultured cells under non-
cytotoxic conditions.
These compounds offer the intriguing possibility of being added to a wide
range of low pH food
products to neutralize conformational altered proteins present. Since the
compounds found act by
stimulating normal cellular pathways of protein degradation to destroy
PrPs°, this class of
compounds would also likely be of value in the treatment of other degenerative
and hereditary
disorders where abnormally folded, wild-type or mutant proteins accumulate.
Such an approach
may find merit in developing an effective therapeutics for one or more of the
common, degenerative
illnesses including Alzheimer's disease, Parkinson's disease, amyotrophic
lateral sclerosis,
frontotemporal dementia, adult onset diabetes mellitus and the amyloidoses
(Beyreuther, K. &
Masters, C.L. Serpents on the road to dementia and death. Accumulating
evidence from several
studies points to the normal function of presenilin l and suggests how the
mutant protein contributes
-27-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
to deposition of amyloid plaques in Alzheimer's disease. Nature Medicine 3,
723-725 (1997);
Masters, C.L. & Beyreuther, K. Alzheimer's disease. BMJ316, 446-448 (1998);
Selkoe, D.J. The
cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's
disease. Trends in Cell
Biol. 8, 447-453 (1998); Selkoe, D.J. Translating cell biology into
therapeutic advances in
Alzheimer's disease. Nature 399, A23-31 (1999); Wong, P.C., et al. An adverse
property of a
familial ALS-linked SOD1 mutation causes motor neuron disease characterized by
vacuolar
degeneration ofmitochondria. Neuron 14, 1105-1116 (1995); Spillantini, M.G.,
Crowther, R.A.,
fakes, R., Hasegawa, M. & Goedert, M. a-Synuclein in filamentous inclusions of
Lewy bodies from
Parkinson's disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA
95, 6469-6473
(1998); Hutton, M., et al. Association of missense and 5'-splice-site
mutations in tau with the
inherited dementia FTDP-17. Nature 393, 702-705 (1998); Stone, M.J.
Amyloidosis: a final
common pathway for protein deposition in tissues. Blood 75 , 531-545 (1990)).
Whether branched
polyamines might also prove efficacious in a variety of inherited disorders
where the accumulation of
abnormal proteins is a hallmark of the illness remains to be established;
these genetic maladies
include heritable forms of prion disease, Alzheimer's disease, Parkinson's
disease, amyotrophic
lateral sclerosis, frontotemporal dementia, Pick's disease and amyloidosis, as
well as the triplet repeat
diseases including Huntington's disease, spinal cerebellar ataxias and
myotonic dystrophy (Fu, Y.-H.,
et al. An unstable triplet repeat in a gene related to myotonic muscular
dystrophy. Science 255,
1256-1259 (1992); Group, T.H.s.D.C.R. A novel gene containing a trinucleotide
repeat that is
expanded and unstable on Huntington's disease chromosomes. Cell 72, 971-983
(1993)).
Compounds identified via assays of the invention such as branched polyamines
will find utility in
preventing or delaying the onset of these genetic diseases where earners can
often be identified
decades in advance of detectable neurologic or systemic dysfunction.
The invention is based on the discovery that several dendritic polycations,
including the
starburst dendrimers SuperfectT"' (QIAGEN~, Valencia, CA), polyamidoamide
(PAMAM), and the
hyperbranched polycation polyethyleneimine (PEI), were surprisingly found to
eliminate PrPs° from
cultured scrapie-infected neuroblastoma cells. These highly-branched,
polycationic compounds
provide a novel class of therapeutic agents to combat prion diseases and other
degenerative disease
including the amyloidoses. The removal of PrPs° is dependent on both
the concentration of dendritic
polymer and length of exposure. Dendritic polymers were able to cleax
PrPS° at concentrations
which were not cytotoxic. Repeated exposures to heat-degraded starburst PAMAM
dendrimer or
PEI caused a dramatic reduction in PrPs° levels which persisted for a
month even after removal of the
compound. Dendritic polycations did not appear to destroy purified PrPS' in
vitro, and therefore may
act through a generalized mechanism. Dendritic polycations represent a class
of compounds which
-28-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
can be used as therapeutic agents in prion diseases and other disorders
involving insoluble protein
deposits, such as the amyloidoses.
EXAMPLES
The following examples are put forth so as to provide those of ordinary skill
in the art with a
complete disclosure and description of how to make and use the present
invention, and are not
intended to limit the scope of what the inventors regard as their invention
nor are they intended to
represent that the experiments below are all or the only experiments
performed. Efforts have been
made to ensure accuracy with respect to numbers used (e.g. amounts,
temperature, etc.) but some
experimental errors and deviations should be accounted for. Unless indicated
otherwise, parts are
parts by weight, molecular weight is weight average molecular weight,
temperature is in degrees
Centigrade, and pressure is at or near atmospheric.
METHODS AND MATERIALS
Chemicals. High molecular weight PEI was purchased from Fluka. DOTAP cationic
lipid
was purchased from Boehringer Mannheim and SuperFect transfection reagent was
purchased from
QIAGEN~. All other compounds were purchased from Sigma-Aldrich. All test
compounds were
dissolved in water at stock concentration of 3 mg/ml and filtered through a
Millipore 0.22 m m filter.
Cultured cells. Stock cultures of ScN2a cells were maintained in MEM with 10%
FBS,
10% Glutamax (Gibco BRL), 100 U penicillin, and 100 mg/ml streptomycin
(supplemented DME).
hrunediately prior to addition of test compounds, the dishes were washed twice
with fresh
supplemented DME media. After exposure to test compounds, dishes were drained
of media and
cells were harvested by lysis in 0.25-1 ml 20 mM Tris pH 8.0 containing 100 mM
NaCl, 0.5%
NP-40, and 0.5% sodium deoxycholate to obtain a total protein concentration of
1 mg/ml measured
by the BCA assay. Nuclei were removed from the lysate by centrifugation at
2000 rpm for 5 min.
For samples not treated with proteinase K, 40 ~1 of whole lysate (representing
40 ~g total protein)
was mixed with an equal volume of 2x SDS reducing sample buffer. For
proteinase K digestion, 20
~g/ml proteinase K (Boehringer Mannheim) (total protein:enzyme ratio = 50:1)
was added, and the
sample was incubated for 1 h at 37°C. Proteolytic digestion was
terminated by the addition of
Pefabloc to a final concentration of 5 mM. One ml samples were centrifuged at
100,000 x g for 1 h
at 4°C, the supernatants were discarded, and the pellets were
resuspended in 80 /.c1 of reducing SDS
sample buffer for SDS-PAGE.
-29-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
Brain homogenates. Brain homogenates from RML scrapie-affected CD-1 mice (10%
(w/v) in sterile water) were prepared by repeated extrusion through syringe
needles of successively
smaller size, from 18 to 22 gauge. Nuclei and debris were removed by
centrifugation at 1000 x g for
min. The bicinchnoninic acid (BCA) protein assay (Pierce) was used to
determine protein
5 concentration. Homogenates were adjusted to 1 mg/ml protein in 1 % NP-40.
For reactions, 0.5 ml
homogenate was incubated with 25 ml 1.0 M buffer (sodium acetate for pH 3-6
and Tris acetate for
pH 7-10) plus or minus 10 ml of polyamine stock solution (3 mg/ml) for 2 h at
37 ° C with constant
shaking. The final pH value of each sample was measured directly with a
calibrated pH electrode
(Radiometer Copenhagen). Following incubation, each sample was neutralized
with an equal volume
0.2 M HEPES pH 7.5 containing 0.3 M NaCI and 4% Sarkosyl. Proteinase K was
added to achieve
a final concentration of 20 ~cg/ml, and samples were incubated for 1 h at
37°C. Proteolytic digestion
was terminated by the addition of Pefabloc to a final concentration of 5 ~M.
Ten ~cl of digested
brain homogenate was mixed with equal volume 2x SDS sample buffer and analyzed
by SDS-PAGE
followed by Western blotting.
Western blotting. Following electrophoresis, Western blotting was performed as
previously
described (Scott, M., et al. Transgenic mice expressing hamster prion protein
produce species-
specific scrapie infectivity and amyloid plaques. Cell 59, 847-857 (1989)).
Samples were boiled for
5 min and cleared by centrifugation for 1 min at 14,000 rpm in aBeckman
ultrafuge. SDS-PAGE
was carned out in 1.5 mm, 12% polyacrylamide gels(Laemmli, U.K. Cleavage of
structural proteins
during the assembly of the head of bacteriophage T-4. Nature 227, 680-685
(1970)). Membranes
were blocked with 5% non-fat milk protein in PBST (calcium- and magnesium-free
PBS plus 0.1%
Tween 20) for 1 h at room temperature. Blocked membranes were incubated with
primary 8073
polyclonal antibody (to detect MoPrP) (Serban, D., Taraboulos, A., DeArmond,
S.J. & Prusiner,
S.B. Rapid detection of Creutzfeldt-Jakob disease and scrapie prion proteins.
Neurology 40, 110-
117 (1990)) or 3F4 monoclonal antibody (to detect MHM2 PrP) (Kascsak, R.J., et
al. Mouse
polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J.
I~irol. 61, 3688-3693
(1987)) at 1:5000 dilution in PBST overnight at 4°C. Following
incubation with primary antibody,
membranes were washed 3 x 10 min in PBST, incubated with horseradish
peroxidase-labeled
secondary antibody (Amersham Life Sciences) diluted 1:5000 in PBST for 30 to
60 min at 4°C and
washed again for 3 x 10 min in PBST. After chemiluminescent development with
ECL reagent
(Amersham) for 1 min, blots were sealed in plastic covers and exposed to ECL
Hypermax film
(Amersham). Films were processed automatically in a Konica film processor.
-30-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
EXAMPLE 1A
Branched polyamines inhibit formation of
nascent PrPs' and induce clearance of pre-existing PrPs'
Western blots were probed with 3F4 monoclonal antibody which recognizes newly
expressed
MHM2 PrP. ScN2a cells were exposed to SuperFect for 3 h and harvested 3 d
after removal of
SuperFect. Gells were run on both undigested, control sample and a sample
subjected to limited
proteolysis. The samples were run in separate lanes 1-6 with a control and
limited proteolysis
sample for each of the 6 lanes as follows: Lane 1: DOTAP-mediated
transfection. Lane 2: 30 ~cg/ml
SuperFect, 5 ~cg pSPOX MHM2. Lane 3: 75 ~cg/ml SuperFect, 5 ~g pSPOX MHM2.
Lane 4: 150
~cg/ml SuperFect, 5 ~g pSOX MHM2. Lane 5: 150 ~g/ml SuperFect, 10 ~g pSPOX
MHM2.
Lane 6: No addition of either transfection reagent or DNA. Forty ~1 of
undigested brain homogenate
was used in these studies while those samples subjected to limited digestion
with proteinase K were
concentrated 25-fold prior to SDS-PAGE. One ml of the digest were centrifuged
at 100,000 x g for
1 h at 4 ° C and the pellets suspended in 80 ~l of SDS sample buffer
prior to SDS-PAGE followed by
Western blotting. Apparent molecular weights based on migration of protein
standards are 34.2,
28.3, and 19.9 kDa.
All of the control lanes 1-6 show multiple bands as expected. However, of the
samples
subjected to limited proteolytic only lane 1 shows bands. Unexpectedly, all of
the partially digested
sample lanes 2-5 show no bands and as expected no bands in the partially
digested lane 6. These
results show the effect of using SuperFect in clearing PrPs'.
EXAMPLE 1B
The blot described above was stripped of antibody, exposed to labeled 8073 and
redeveloped. The antibody 3F4 used in Example 1 binds to PrP~ but not to
PrPs'. However, 8073
binds to PrPs' and PrP~. Lanes 1, 2 and 3 show decreasing amounts of PrPs' and
lanes 4 and 5 show
no detectable PrPS'.
EXAMPLE 2A
Gels were run on undigested controls 1-4 and as above, samples subjected to
limited
proteolysis. The lanes were as follows: Lane l: No SuperFect. Lane 2: 30
/.cg/ml SuperFect. Lane
3: 75 /.cg/ml SuperFect. Lane 4: 150 /.cg/ml SuperFect. ScN2a cells were
exposed to SuperFect for
3 h and harvested 3 d after removal of SuperFect. Apparent molecular weights
based on migration
of protein standards are 33.9, 28.8, and 20.5 kDa. In that each sample was
tested after the same
-31-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
time period the results show the dose-dependent effect of SuperFect on
PrPs° removal. Lanes 1, 2
and 3 show decreasing amounts of PrPs° and lane 4 shows no detectable
PrPs'.
EXAMPLE 2B
To determine the time-dependent effect of SuperFect three different panels
with four lanes
each were prepared and run as follows: ScN2a cells were exposed to 7.5 ~cg/ml:
SuperFect (lanes
1-4), PEI (average molecular weight ~60,000)(lanes 5-8), or PAMAM, generation
4.0 (lanes 9-12).
Time of exposure times for each polyamine: 0 hours (lanes 1, 5, and 9), 4
hours (lanes 2, 6, and 10),
8 hours (lanes 3, 7, and 11), 16 hours (lanes 4, 8, and 12). All samples were
subjected to limited
proteolysis to measure PrPs~. Apparent molecular weights based on migration of
protein standards
are 38, 26, and 15 kDa. Lanes of each of the three panels show decreasing
amounts of PrPs°.
EXAMPLE 3
In this example four panels A,B, C and D were created with panels having three
double
(control and test) lanes each. ScN2a cells were exposed to 1.5 ~g/ml: (A)
SuperFect, (B) PEI
(average molecular weight 60,000), (C) PAMAM, generation 4.0, or (D) no
addition. Cells were
harvested: Lane 1, before addition; Lane 2, immediately following 1 week
continuous exposure to
test compounds; and Lane 3, three weeks after removal of test compounds. Minus
(-) symbol
denotes undigested, control sample and plus (+) symbol designates sample
subjected to limited
proteolysis. Apparent molecular weights based on migration of protein
standards are 33.9, 28.8, and
20.5 kDa. Test lanes 3 in panel A showed slight PrPs' after three weeks and
test lanes 3 in panels B
and C showed no detectable PrPs~ whereas PrPs° was present in all lanes
in panel D.
EXAMPLE 4A
Four separate gels were run to demonstrate the effect of adding chloroquine
would have on
PrPs° levels. The lanes 1 control and 3 where chloroquine was added
show clear bands for PrPs°
whereas lanes 2 and 4 with no chloroquine show barely detectable amounts of
PrPs°. The four lanes
were prepared as follows: ScN2a cells were treated Lane 1: Control media. Lane
2: 7.5 ~g/ml PEI
(average molecular weight 60,000). Lane 3: PEI plus 100 ~cM chloroquine. Lane
4: PEI plus 30
~cM NH4Cl. Chloroquine and NH4Cl were added 1 h prior to addition of PEI.
Cells were harvested
16 hours after addition of PEI. All samples shown were subjected to limited
proteolysis to measure
PrPs'. Apparent molecular weights based on migration of protein standards are
3 8, 26, and 15 kDa.
-32-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
EXAMPLE 4B
Eight lanes with SuperFect (+SF) and eight lanes without SuperFect (-SF) were
prepared.
Lanes 1-8 of each group had an adjusted pH of 3.6" 4, 5, 6, 7, 8, 9 and 9.6.
In vitro mixture of
crude mouse brain homogenates with SuperFect under a range of pH conditions
was performed as
described in methods (measured final pH of each sample denoted above the
lanes). Addition of 60
~g/ml SuperFect denoted as "+SF" and control with no addition as "-SF". All
samples shown were
subjected to limited proteolysis to measure PrPs'. Apparent molecular weights
based on migration of
protein standards are 30 and 27 kDa. All lanes ofthe -SF group showed PrPS'
present. Lanes 3-8 of
the +SF group showed PrPs'. However, lanes l and 2 with respective pH levels
of 3.6 and 4.0
showed very slight detectable PrPs'. The results show that the ability of a
blanched polycation such
as SuperFect to clear PrPs' is pH dependent.
EXAMPLE 5
Sixteen different lanes were prepared as described. Lanes 1 and 2 were control
lanes and
each of lanes 3-16 contained a different compound as tested in Table 1. The
test compounds were all
polyamines. Thus, the results show removal of PrPs' from brain homogenate in
vitro by various
polyamines. Samples were incubated with polyamines at pH 3.6 and processed as
described in
Methods. Each polyamine was tested at 60 ~cg/ml concentration. Lanes 1 and 2:
control. Lane 3:
poly-(L)lysine. Lane 4: PAMAM, generation 0Ø Lane 5: PAMAM, generation 1Ø
Lane 6:
PAMAM, generation 2Ø Lane 7: PAMAM, generation 3Ø Lane 8: PAMAM,
generation 4Ø
Lane 9: PAMAM-OH, generation 4Ø Lane 10: PPI, generation 2Ø Lane 11: PPI,
generation 4Ø
Lane 12: linear PEI. Lane 13: high MW PEI. Lane 14: low MW PEI. Lane 15:
average MW PEI.
Lane 16: SuperFect. All samples shown were subjected to limited proteolysis to
measure PrPs'.
Apparent molecular weights based on migration of protein standards are 30 and
27 kDa.Table 1.
Removal of PrPs~ by polymer compounds. IC50 = approximate concentration of
polymer required to
reduce PrPs' to 50% of control levels in ScN2a cells after exposure for 16
hours. All compounds
were tested at 5 different concentrations. PrPS' levels were measured by
densitometry of Western
blot signals.
-33-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
TABLE 1
(Note that Table 1 includes information
on the characteristics of compounds used but that
the list does not correspond directly to lanes 1-16)
Molecular Primary NH2 ICSp (ng/xnl)
Weight groups
PAMAM generation 0.0 517 4 >10,000
PAMAM generation 1.0 1,430 8 >10,000
PAMAM generation 2.0 3,256 16 2,000
PAMAM generation 3.0 6,909 32 400
PAMAM generation 4.0 14,215 64 80
PAMAM-OH generation 4.0 14,279 0 >10,000
PPI generation 2.0 773 8 2,000
PPI generation 4.0 3,514 32 80
Low MW PEI 25,000 2,000
Average MW PEI 60,000 400
High MW PEI 800,000 80
Linear PEI 60,000 2,000
poly-(L)lysine 60,000 >500 10,000
SuperFect 400
Lanes 7, 8, 11 and 13 showed the best results, i.e. best ability to clear
PrPs' under these
conditions. Specifically, PAMAM generation 4.0 in lane 8 showed the best
ability to clear PrPS'
under these conditions whereas PAMAM-OH generation 4.0 showed almost no
detectable ability to
clear PrPs' and was comparable to the control.
EXAMPLE 6
Transfection of PrPs' Expressing Cells with Dendrimer Compounds
Cells of neuronal origin expressing PrPs' were examined for the ability of
compounds to
suppress PrPs° formation.
Transfection Studies
Stock cultures of N2a and ScN2a cells were maintained in MEM with 10% FBS, 10%
Glutamax (Gibco BRL), 100 U penicillin, and 100 ~cg/ml streptomycin. Cells
from a single
confluent 100 mm dish were trypsinized and split into 10 separate 60 mm dishes
containing DME
plus 10% FBS, 10% Glutamax, 100 U penicillin, and 100 ~cg/ml streptomycin
(supplemented DME)
one day prior to transfection. Immediately prior to transfection, the dishes
were washed twice with 4
ml supplemented DME media and then drained.
-34-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
For DOTAP-mediated transfection, 15 ~g pSPOX MHM2 was resuspended in 150 ~cl
sterile
Hepes Buffered Saline (HBS) on the day of transfection. The DNA solution was
then mixed with an
equal volume of 333 ~cg/ml DOTAP (Boehringer Mannheim) in HBS in Falcon 2059
tubes and
incubated at room temperature for 10 minutes to allow formation of DNA/lipid
complexes.
Supplemented DME (2.5 ml) was added to the mixture, and this was then pipetted
onto drained cell
monolayers. The following day, the medium containing DNA/lipid was removed and
replaced with
fresh supplemented DME. Cells were harvested three days later.
For SuperfectT"'-mediated transfections/exposures, SuperfectT~~ with or
without DNA was
added to 1 ml supplemented DME in a Falcon 2059 tube to achieve the specific
concentrations
needed for each experiment. This mixture was pipetted up and down twice and
then onto drained cell
monolayers. After exposure for the indicated times, the medium containing
SuperfectT"~ was
removed and replaced with fresh supplemented DME. Cells were harvested at
specified times after
removal of SuperfectT~~.
Exposures to PPI (DAB-Am-8, Polypropylenimine octaamine Dendrimer, Generation
2.0
Aldrich 46,072-9), Intact PAMAM (Starburst (PAMAM)Dendrimer, Generation 4.
Aldrich 41,244-9), PEI (Sigma), poly-(L)lysine (Sigma), and poly-(D) lysine
(Sigma) were
performed as described above for SuperfectT"'.
Isolation of Protein from Treated Cells
Cells were harvested by lysis in 1.2 ml of 20 mM Tris pH 8.0 containing 100 mM
NaCI,
0.5% NP-40, and 0.5% sodium deoxycholate. Nuclei were removed from the lysate
by
centrifugation at 2000 rpm for 5 min. This lysate typically had a protein
concentration of 0.5 mg/ml
measured by the BCA assay. For samples not treated with proteinase K, 40 ~l of
whole lysate
(representing 20 ~g total protein) was mixed with 40 ~l of 2x SDS sample
buffer. For proteinase K
digestion, 1 ml of lysate was incubated with 20 ~g/ml proteinase K (total
protein:enzyme ratio =
25:1) for 1 hr at 37°C. Proteolytic digestion was terminated by the
addition of 8 ~1 of 0.5M PMSF
in absolute ethanol. Samples were then centrifuged for 75 min in a Beckman TLA-
45 rotor at
100,000 x g at 4 ° C. The pellet was resuspended by repeated pipetting
in 80 ~1 of 1X SDS sample
buffer. The entire sample (representing 0.5 mg total protein before digestion)
was loaded for
SDS-PAGE.
-35-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
Western Blot Analysis
Immunoreactive PrP bands from the DOTAP-mediated transfection were detected
before and
after digestion with proteinase K with monoclonal antibody 3F4. The construct
used to express
PrPs° in the ScN2a cells is MHM2 a chimeric construct that differs from
wild-type (wt) MoPrP at
positions 108 and 111 (Scott et al., (1992) Protein Sci. 1:986-997).
Substitution at these positions
with the corresponding residues ( 109 and 112 respectively) from the Syrian
hamster (SHa) PrP
sequence creates an epitope for 3F4 (Kascsak et al., (1987) J. ljirol. 61:3688-
3693), which does not
recognize endogenous wt MoPrP in ScN2a cells and hence facilitates specific
detection of the
transgene by Western blot.
Following electrophoresis, Western blotting was performed as previously
described (Scott et
al., (1989) Cell 59:847-857). Samples were boiled for 5 minutes and cleared by
centrifixgation for 1
minute at 14,000 rpm in a Beckman ultrafuge. SDS-PAGE was carried out in 1.5
mm, 12%
polyacrylamide gels (Laemtnli (1970) Nature 227:661-665). Membranes were
blocked with 5%
nonfat milk protein in PBST (calcium- and magnesium-free PBS plus 0.1% Tween
20) for 1 hour at
room temperature. Blocked membranes were incubated with primary 8073
polyclonal or 3F4
monoclonal antibody at a 1:5000 dilution in PBST overnight at 4 °C.
Following incubation with primary antibody, membranes were washed 3 x 10
minutes in
PBST, incubated with horseradish peroxidase-labeled secondary antibody
(Amersham Life Sciences)
diluted 1:5000 in PBST for 25 minutes at room temperature and washed again for
3x 10 minutes in
PBST. After ehemiluminescent development with ECL reagent (Amersham) for 1
minute, blots were
sealed in plastic covers and exposed to ECL Hypermax filin (Amersham). Films
were processed
automatically in a Konica film processor.
In contrast to DOTAP transfected cells, ScN2a cells transfected with varying
concentrations
of SuperfectT"~ and DNA did not appear to contain protease-resistant MHM2.
Close scrutiny
revealed that, prior to protease digestion, SuperfectTM transfected samples
express MHM2 bands
which are not seen in the background pattern of the control sample. These
observations indicate that
MHM2 PrP was successfully expressed using SuperfectT"' transfection reagent,
but conversion of
MHM2 PrP~ to protease-resistant MHM2 PrPs° was inhibited by
SuperfectT"'.
To examine whether SuperfectT"' had affected levels of preexisting PrPs' in
ScN2a cells, the
Western blot probed with 3F4 antibody was reprobed with polyclonal antibody
8073, which is able
to recognize endogenous MoPrP. Remarkably, SuperfectTM caused the
disappearance of preexisting
MoPrPs° from ScN2a cells in a dose-dependent manner. After treatment
with SuperfectT"~, PrPs'
could not be detected in the nuclear fraction, pellet, supernatant, or media.
The concentration of
SuperfectT"" required to fi>Zly remove preexisting PrPs° with a three
hour exposure was 300 pg/ml,
-36-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
whereas 30 ~g/ml was sufficient to interfere with the formation of new MHM2
PrPs' within the same
time frame.
Length of exposure dramatically influenced the ability of SuperfectT"' to
remove PrPs' from
ScN2a cells. Whereas a 3 hour exposure to 150 ~g/ml SuperfectT"" significantly
lowered PrPs' levels
in ScN2a cells, exposure for 10 min to the same dose of SuperfectT"' did not
affect PrPs' levels.
When ScN2a cells were exposed to 2 pg/ml SuperfectT"' continuously for 1 week,
PrPs' disappeared
completely.
The conditions tested did not appear to be toxic for the cells. Neither 150
~g/ml SuperfectT""
for 3 hrs nor 2 ~g/ml SuperfectT"' continuously for 1 week caused any obvious
changes in cell
morphology, viability, or growth as judged by phase contrast microscopy.
EXAMPLE 7
Elimination of PrPs' by repeated exposures to SuperfectT'"
The duration in the reduction in PrPs' levels after exposure to SuperfectT"'
was examined,
and it was shown that this reduction could persist for extended periods after
removal of SuperfectT"'
Following the exposure of ScN2a cells to a single dose of 150 ~g/ml
SuperfectT"" for 3 hrs, PrPs'
levels remained low for one week, but returned to near baseline levels after 3
weeks in culture
without SuperfectT~A
In contrast, when ScN2a cells were exposed to 4 separate doses of SuperfectT"~
over the
course of 16 days, very little PrPs' could be detected 4 weeks after the final
exposure to SuperfectT"'
This result offers hope that prolonged exposure to SuperfectT"' may lead to
long term cure of scrapie
infection in cultured cells.
EXAMPLE 8
SuperfectT"' does not destroy PrPs' directly
The dendrimer SuperfectT"' was used to determine if it could exert a similar
inhibitory effect
on PrPs' in either crude brain homogenates or purified PrP 27-30 rods.
Brain homogenates from normal and scrapie-affected Syrian hamsters (10% (w/v)
in sterile PBS)
were prepared by repeated extrusion through syringe needles of successively
smaller size, from 18 to
22 gauge. Nuclei and debris were removed by centrifugation at 1000 x g for 10
min. The
bicinchnoninic acid (BCA) protein assay (Pierce) was used to determine protein
concentration.
Homogenates were adjusted to 10 mg/ml protein with PBS and 50 p1 was added to
450 p1 of lysis
buffer containing 100 mM NaCI, 1 mM EDTA, 0.55% sodium deoxycholate, 0.55%
Triton X-100,
and 50 mM Tris-HCl pH 7.5. This mixture was then incubated with 0-300 ~g/ml
SuperfectT"' for 3
-37-
WU 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
hrs at 37 °C and then centrifuged for 10 min at 14,000 rpm in a Beckman
Ultrafuge. The pellet was
resuspended in 450 ~l lysis buffer without SuperfectT"'. Proteinase K
(Boehringer Mannheim) was
added to achieve a final concentration of 20 ~g/ml, and thus the ratio of
total protein/enzyme was
50:1. Samples were incubated for 1 h at 37 °C. Proteolytic digestion
was terminated by the addition
of 8 ~1 of 0.5 M PMSF in ethanol. Samples were then centrifuged for 75 min in
a Beckman TLA-45
rotor at 100,000 x g at 4 °C. Undigested samples (10 1t1) were mixed
with an equal volume of 2x
SDS sample buffer. For digested samples, the pellet was resuspended by
repeated pipetting in 100 ~l
lx SDS sample buffer. Twenty ~1 (equivalent to 100 ~g of total protein prior
to proteinase K
digestion) of each sample was loaded for SDS-PAGE.
PrP 27-30 rods were purified from scrapie-affected Syrian hamster brains and
previously
described (Prusiner et al., (1983) Cell 35:349-358). Purified rods (3.5 ~g/ml)
were incubated with
or without 900 ~g/ml SuperfectrM in 100 ~l supplemented DME. After 16 hrs at
37 °C, the
suspension was centrifuged at 100,000 x g at 4 °C. The pellet was
resuspended in 500 ~1 of buffer
containing 1 mg/ml BSA, 100 mM NaCI, 1 mM EDTA, 0.55% sodium deoxycholate,
0.55% Triton
X-100, and 50 mM Tris-HCl pH 7.5. Proteinase K was added to achieve a final
concentration of 20
pg/ml. Samples were incubated for 1 h at 37 ° C. Proteolytic digestion
was terminated by the
addition of 8 p.1 of 0.5 M Pefabloc (Boehringer Mannheim). Samples were then
centrifuged for 75
min at 100,000 x g at 4 °C. Undigested samples (50 p1) were mixed with
an equal volume of 2x SDS
sample buffer. For digested samples, the pellet was resuspended by repeated
pipetting in 100 p1 lx
SDS sample buffer. Forty p1 of each sample was loaded for SDS-PAGE.
When SuperfectT~~ was mixed with either crude homogenates of scrapie-affected
Syrian
hamsters or with purified Syrian hamster PrP 27-30, there was no significant
change in the level of
proteinase K-resistant PrPS°. These results suggest that the removal of
PrPS' from ScN2a cells by
SuperfectTM depends on the presence of intact cellular machinery.
EXAMPLE 9
Clearance of PrPS' levels by other dendritic polycations
The SuperfectT"~ compound is a high molecular weight component of heat-
degraded
PAMAM Starburst dendrimers, which is a cationic, highly-branched, monodisperse
polymers (Tang
et al., (1996) Bioconjugate Chem. 7:703-714). To identify other potentially
usefi~l anti prion
therapeutic agents, we screened three other dendritic polycations and two
linear cationic polymers for
their ability to clear PrPs' from ScN2a cells. Among the dendritic
macromolecules tested,
polyetheleneimine (PEI) was the most potent, removing the majority of PrPs'
from ScN2a cells after
3 hrs when used at a concentration of 10 ~g/ml. Intact PAMAM displayed a
potency comparable to
-38-
WO 00/72851 CA 02375237 2001-11-26 PCT/US00/14353
Superfectr"', removing approximately half of the detectable PrPs~ when used at
a concentration of 50
pg/ml. In contrast, the dendrimer polypropyleneimine (PPI), poly-(L)lysine,
and the linear
polycation poly-(D)lysine failed to reduce PrPs° levels at
concentrations between 10-50 ~g/ml.
These results demonstrate that a branched polymeric architecture is required
to clear PrPs'.
Furthermore, exposure of ScN2a cells to either PEI or intact PAMAM for one
week at a
concentration of 1.5 ~cg/ml completely removes PrPs~, effectively curing the
cells of scrapie infection.
While the present invention has been described with reference to the specific
embodiments
thereof, it should be understood by those skilled in the art that various
changes may be made and
equivalents may be substituted without departing from the true spirit and
scope of the invention. In
addition, many modifications may be made to adapt a particular situation,
material, composition of
matter, process, process step or steps, to the objective, spirit and scope of
the present invention. All
such modifications are intended to be within the scope of the claims appended
hereto.
-3 9-