Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02378334 2001-12-20
WO 01/12292 PCT/US00/22624
ELECTRODEIONIZATION SUBSTRATE, AND
DEVICE FOR ELECTRODEIONIZATION TREATMENT
CONTRACTUAL ORIGIN OF THE INVENTION
The United States Government has rights in this invention under Contract
No. W-31-109-ENG-38 between the U.S. Department of Energy and the
University of Chicago representing Argonne National Laboratory.
BACKGROUND OF THE INVENTION
_1. Field of the Invention
The present invention relates to electrodeionization and more particularly,
the invention relates to an electrodeionization substrate and a device for
treating
fluids via electrodeionization.
2. Background of the Invention
Prior to their ultimate use, feed streams must often be pretreated to
remove unwanted ionic contaminants. Typical clean-up processes include the
use of ion-exchange resins and electrodialysis. In ion-exchange, after the
targeted ions are removed from a feed solution, the ion-exchange resins are
exhausted and have to be regenerated by acids, bases, or salts. Thus the
process produces an equivalent or higher amount of waste salt stream from the
CA 02378334 2001-12-20 O ~ ~ 226 ~t~
f 6'," ~~J '.5 ~~
2
regeneration process.
Electrodialysis is an electrically driven ion-exchange membrane based
process where by using a stack of alternating ration, anion, or bipolar
membranes, ions are removed from a feed solution and purified and
concentrated in a product or concentrate solution. Since the transport of the
ions
are done by electric power, electrodialysis processes do not consume
equivalent
quantities of acids, bases, or salts and do not produce a salt waste stream
When the ion concentration in the feed stream is low, i.e. below 0.5 to 1 %,
~_ electrodialysis processes become unattractive because the low ionic
conductivity
in the dilute feed stream leads to very low flux and high energy consumption.
Electrodeionization (EDI), also known as "electrochemical ion-exchange",
is an advanced ion-exchange technology that combines the advantages of ion-
exchange and electrodialysis. In electrodeionization processes, ion-exchange
resins are sequestered in dilute feed compartments to increase the ionic
conductivity, so that even with an ionically dilute feed, a stable operation
with higher
flux and lower energy consumption than electrodialysis, becomes possible. The
electric power also splits the water (H20) to H+ and OH- ions and the resins
are thus
regenerated while the ions are removed.
EDI technology is increasingly being used to make deionized water for boiler
feed and fiigh purity industrial water applications. There are also many other
- ---
potential uses of such technology for deionization of organic process streams
in the
food processing and chemical industries. These uses will not need the removal
of
ions to low parts per million or parts per billion levels as is required for
high purity
water production. However, these process streams will have multiple types of
ions,
other contaminants and potential foulants, and high concentrations of
organics,
which cannot be lost from the feedstream. Thus, the EDI devices for these
applications must be easily dissembled for frequent cleaning. Also, process
economics require that there is virtually no leakage between the product feed
and
the salt concentrate that is removed.
Many configurations and devices have been patented for electrodeionization.
Almost all of them have ration and anion exchange membranes flanking loose ion-
exchange resins or beads. In order to prevent the escape of these beads, a
wide
variety of and confininglsealing methods are employed. For example, in U.S.
Patent
,~;~~~un~n ~~~r.~t~
CA 02378334 2001-12-20
PGTNS ~ 0 ~ ZZ62~
3
4,804,451 a very complex configuration of a spacer element is described
wherein
the ration and anion membranes are bonded by special adhesives to the spacer
element to form a pocket. The anion and ration exchange resin beads are
confined
within these complex pockets. A very complex assembly of the spacers and
membrane resin pockets are then put together to reduce leakage between the
dilute
and the concentrate compartments.
In U.S. Patent 4,956,071 the membranes are compartmentalized via evenly
spaced ribs to which the membranes are bonded with loose ion exchange resins
filling these pockets. A complex assembly of these compartments are put
together
to prevent leakage between the dilute and the concentrate compartments.
Patents also exist (U.S. Patent Nos. 4,747,929 and 5,681,438) describing
complex spacer construction configurations incorporating attached membranes
and
loose ion exchange resins interspersed between.
U.S. Patent 5,346,924 describes a method for making a non-porous ion
exchange membrane using an ion exchange resin and binders such as
polyethylene of linear low density or high density is described. These non-
porous
membranes are then described in an electrodeionization assembly with loose ion
exchange resins in compartmentalized pockets that are similar to the
previously
described patents.
.- . - ~ Another U.S. Patent 5,308;467 an apparatus that creates an ion
exchange -
material from mono-filaments of ration and anion exchange material by
radiation
grafting is described and this ion exchange material can then be assembled
between ion exchange membranes to make an electrodeionization apparatus.
EDI devices typically are utilized as a final polishing step for already ultra
pure water. As such, fouling of the rather complex compartmentalization and
flow
channels of EDI systems is relatively rare. Indeed, such systems are usually
sealed
upon manufacture inasmuch as the need for disassembly to facilitate cleaning
is nil.
Most of the current configurations develop small leaks from the dilute/feed
compartments to the concentrate compartments. Whereas this is not a
significant
economic penalty for the production of ultra-pure water, such leaks cannot be
tolerated for use with organic feedstreams where such losses from the feed
would
be uneconomical.
~, .;,.-,. W 1r~1 C~L~
CA 02378334 2001-12-20
p 0012262
s ~ ~ ~ 5 S EP 2001
4
In light of the foregoing, none of the current ED devices provide for having
simple assembly and disassembly to facilitate cleaning and reuse. Also, none
of the
current EDI devices provide for virtually leak-free conditions between the
feed
compartment and the concentrate compartment. Such optimal characteristics are
required for EDI devices used to treat process streams high in organic
material
content, such as com syrups, glycerol and others.
Ion exchange beads that are commonly used for EDI applications consist off,
strongly acidic containing sulfonic acid groups, or strongly basic containing
quaternary ammonium groups. Other resins such as those with weakly acidic
(carboxylic acid) or weakly basic (amines) groups are also used when required.
These beads are cross-linked polymers usually styrene - divinyl benzene or
acrylates. The resins can be gel type or macro-reticular type. Usually
equivalent
mixtures of cationic and anionic resins are used in the EDI compartments. For
specialized applications one type of resin or adsorbent beads mixed with ion-
exchange resins may be used.
The complexity of the current EDI devices primarily come from the need to
confine the loose ion exchange or other adsorbent beads between the membranes
while keeping very close contact amongst these beads and between the beads and
the membranes. In one case that gets away from the beads (previously cited
U.S.
Patent 5;308;467) a complex radiation grafting process with mono-filament of
ion-
exchange material is disclosed.
A need exists in the art for a porous immobilized ion-exchange material.
The material should be readily adaptable to current EDI stack configurations.
Also,
a need exists for an EDI device incorporating porous immobilized ion-exchange
material whereby the device minimizes leakage of feed and also minimizes
contamination of feed with any concentrate waste stream formed. The material
and
device should not have ion-exchange particle leakage even at high flow rates,
and
the material should be regenerable in situ. The material also should be
produced
with common substrates.
SUMMARY OF THE INVENTION
It is the object of the present invention to provide a porous ion-exchange
material and a device incorporating the material which overcomes many of the
disadvantages of the prior art.
CA 02378334 2001-12-20
WO 01/12292 PCT/US00/22624
Another object of the present invention is to provide a porous but immobilized
ion-exchange material. A feature of the invention is that standard ion-
exchange
particles are combined with a binder material to immobilize them while also
mainta-
fining the molecular characteristics (such as porosity and internal surface
area) of the
5 particles. An advantage of the invention is conferring a high degree of
ionic con-
ductivity between individual particles of the standard material while also
allowing
high throughput of the treated liquid stream.
Yet another object of the invention is to provide an economical method for
subjecting feed streams to electrodeionization. A feature of the invention is
the
utilization of a porous, immobilized ion-exchange material which provides ion-
conductivities higher than the feed stream. An advantage of the invention is
the
ability for the material to regenerate in situ.
Briefly, the invention provides a porous immobilized ion-exchange material
comprising ion-exchange resins having cation-exchange moieties and anion-
exchange moieties; and a means for immobilizing the moieties relative to each
other
while conferring ion-conductivity and liquid permeability to the material.
Also provided is an electrodeionization device comprising a cation-exchange
membrane; an anion-exchange membrane juxtaposed co-planarly to said cation
exchange membrane; porous ion-exchange material positioned intermediate said
cation-exchange membrane and said anion exchange membrane to form a
compartment, whereby the material comprises anion-exchange entities and cation
exchange entities immobilized relative to each other; and a means for applying
an
electrical potential to said compartment.
The invention also provides for a method for subjecting a fluid to
electrodeionization, the method comprising supplying a porous, ion-exchange
material wherein the material comprises anion exchange entities and cation
exchange entities immobilized relative to each other; applying an electrical
potential
across the material; contacting the fluid to the substrate so as to facilitate
removal
of ionic contaminants from the fluid; and simultaneous with step c,
regenerating the
resin, in situ.
BRIEF DESCRIPTION OF THE DRAWINGS
CA 02378334 2001-12-20
WO 01/12292 PCT/US00/22624
6
These and other objects and advantages of the present invention will
become readily apparent upon consideration of the following detailed
description
and attached drawings, wherein:
FIG. 1 is a schematic depiction of an EDI process incorporating the invented
porous immobilized ion-exchange material, in accordance with features of the
present invention;
FIG. 2 is a schematic depiction of an exemplary porous, immobilized ion-
exchange material, in accordance with features of the present invention;
FIG. 3A is a depiction of an exemplary porous immobilized ion-exchange
material in communication with a stack gasket, in accordance with features of
the
present invention;
FIG 3B is an expanded view of a portion of FIG 3A, taken along line 3-3; and
FIG. 4 is a flow diagram of an EDI device incorporating an exemplary porous
immobilized ion-exchange material, in accordance with features of the present
invention.
DETAILED DESCRIPTION OF THE INVENTION
A porous, immobilized ion-exchange material and an EDI device
incorporating the material is provided. The device is unique for removing
ionic
contaminants from a feed stream without loss from the treated feed stream.
Typical
impurities removed by the invented substrate and method include the chloride
and
sulfate salts of sodium and potassium, various organic acids, proteins, and
color
bodies. A very suitable application for the invented material and device is in
the
purification of such fluids as corn sweetener syrups.
A general overview of an improved electrodeionization device is designated
as numeral 10 in FIG. 1. A salient feature of the device is a unique porous,
immobilized ion-exchange material 12 which facilitates rapid deployment of
ionic
contaminants out of a diluate conduit, 14.
The material, discussed infra, is removably positioned between (i.e.,
intermediate) a cation exchange membrane 16 and an anion exchange membrane
18, the entire triad therefore comprising a "cell compartment." A means for
facilitating ion transport through this compartment is employed. For example,
an
electrical potential imparted via opposing electrodes 20, 22 (cathode and
anode,
CA 02378334 2001-12-20
0 0 / 22b2,~
~(,L1S 6 SEP Z00~
respectively) provides the gradient to facilitate ion transfer out of the
diluate conduit
14, and into the respective concentrate conduits 15.
An exemplary formulation of the material is that of making it into wafers of
uniform thickness. This wafer configuration confers the following three
advantages
to EDI processes: First, the inventors found that in very dilute solutions,
where most
of the deionization takes place, the ionic conductance of the wafer is higher
than
that of the solution itself. This means that the wafers will aid in the EDI
process
efficiency and increase throughput when compared to trying to deionize the
solution
itself.
Second, when the wafers are incorporated in a standard ED stack
configuration, no leakage between the compartments occurred, even when the
flow
rates were 5 to 10 fold higher than that used in typical EDI processes.
Third, the wafer configuration facilitated the demineralization of dilute salt
in
water and dilute salt in a concentrated carbohydrate solution.
Preparation Detail of Porous
Immobilized Ion-Exchange Material
Finely dispersed latex emulsions that are micron sized elastomer emulsions
are commonly used for coating surfaces. Recently fluorinated latex elastomers
such
as those sold under the trade name Fluorolast by Lauren Chemicals of New
Philadelphia, Ohio, are available in aqueous medium with the fluoroelastomer
emulsion particles in 0.5 to 2 microns in size. Generally these are used for
coating
concrete surfaces or metal surfaces to provide very durable and chemical
resistant
coatings because the fluoropolymers are chemically resistant to acids and
alkali and
are stable to moderately high temperatures up to 200 °C. Typically
these elastomer
dispersions are mixed with a catalytic curing agent which have aminoalkyl
functionalities such as oligomeric aminoalkyl siloxanes. The mixture is then
sprayed
or spread on the surface to be coated. Upon drying, the elastomer particles
adhere
to the surfaces as well get cross linked to each other and form a very durable
non-
porous coating.
Surprisingly and unexpectedly, the inventors have found that these latex
emulsions are suitable for making ion exchange materials with a high degree of
porosity; in other words, when applied to ion-exchange resin beads, the latex
was
found not to coat the outer surfaces of the resin beads and render them
unsuitable
CA 02378334 2001-12-20 _
l "6 SEP 2~,~1
s
for ion-exchange or contaminant removal. The novel findings of this invention
show
that these aqueous elastomer latex emulsions when properly used can indeed
produce a porous, immobilized, ion-exchange material from ion exchange resin
beads which have the following highly desirable properties:
- The molecular sized pores and the molecular porosity and the internal
surface area of the resin and the beads are maintained.
- The external surface is not substantially coated or blinded to impede the
passage of the ions and other impurities.
- The material is very porous so that the liquid stream easily flows through
it with little pressure drop or channeling.
- The resin bead particles are in close contact so that the overall ion
transport properties or mass transport properties are not substantially
reduced when compared to the packed bed.
- The immobilized matrix can handle the resins shrinking and swelling and
does not fall apart.
- The material has good mechanical properties it can be moved, handled,
cut, squeezed and stretched.
The following examples are intended merely to illustrate the utility of the
invented
substrate and method. As such, the specific constituents of the formulations
in the
examples should not be construed to relegate the scope of the invention
thereto.
Example 1
The following is a general protocol for producing porous immobilized ion
exchange material using a water-borne fluoroelastomer. In this example, the
fluoroelastomer FLUOROLASI'~ was used to bind the mixed ration and anion
resins
to form a thick wafer. The mixed resins used were the strong acid gel-type
ration
(C100E) and strong base gel-type anion (A444) resins supplied by Purolite Inc.
Two
different types of wafer were made using different resins that varied in
particle size
e~.~.._
CA 02378334 2001-12-20
WO 01/12292 PCT/US00/22624
9
and particle size distribution. The following general procedure was used to
make
a wafer molded to fit inside a hollowed out and shaped rubber gasket. Such a
wafer
filled gasket could then be used in to make an EDI stack.
1. A concentration in the range of 35 - 70% w/w of fluoroelastomer in emulsion
was
mixed with 2-10% w/w of the aminoxyl siloxane catalyst/curing agent.
2. The mixed cation and anion resins were packed, in wet form, into the rubber
mold gasket. This was approximately 6 millimeter thick and shaped to have
inlet
and outlet ports that would become suitable for use in an EDI stack.
3. A perforated supporting plate covered with one sheet of a nylon screen and
additional perforated wax paper on the top ofthe screen was placed beneath the
resin and mold gasket.
4. The free water trapped between the wet resin beads was removed by letting
it
drain out or by vacuuming.
5. The Fluoroelastomer solution was allowed to cure for approximately 15-60
minutes in air at room temperature before applying (pouring) onto the molded
resin bed.
6. After a second application of the fluoroelastomer solution, the excess was
drained away and then the resin and mold was put into the oven at temperatures
ranging between 25 to 50 °C for 24 -72 hours to cure and dry.
7. After the curing and drying the resin wafer would shrink in size. Water was
added and the resin wafer swelled to fit tightly into the rubber mold gasket.
The
supporting plate along with the polymer screen and the wax paper were
removed.
This material was examined under an optical microscope at a magnification
ranging from 100 to 400. A schematic drawing of the structure is shown in
Figure
CA 02378334 2001-12-20
v !~ s s E~ 2oor
>< o
1. The resin beads 24 are connected to each other by strands 26 of elastomer
binding polymerwith such strands (noted herein as dendrites) binding through
only
a small fraction of the resin beads surface area. The material was very porous
as
evidenced by free passage of water that was dropped on top or on the side of
the
resin wafer. The material was firm and could be taken out of the mold gasket,
cut
and shaped with a sharp knife. The material could be squeezed and stretched
like
an elastomeric material.
The ionic electrical resistance of this material was measured to determine
its is ionic conductivity. Low resistance, i.e. high ionic conductivity,
indicates that
the transport to the ion exchange sites inside the resin beads is not blinded.
It also
indicates that the material is porous, allowing the bulk solution to flow 28
while
simultaneously confirming that the ion exchange beads are not separated far
from
each other by other ionically non conduction materials.
The ion electric resistance with and without a 6mm thick wafer made of the
material were measured in solutions of different NaCI concentrations. A four-
point
ac impedance measurement (LCZ 3321, Keithley Instruments, Inc., Cleveland, OH)
was used to determine the ion electric resistance of the resin wafer. As
indicated
in Table 1, the high ionic resistance of the solution was lowered by the
presence of
resin wafer when the NaCI concentrations were less than 500 ppm. This means
- that this material has good ionic conductivity and would aid in the
transport of ions
from dilute solutions.
This property makes this material very suitable for electrodeionization
applications, because in most applications electrodeionization (EDI)
technology
needs to reduce the ion concentration to less than 50 ppm.
Table 1: Ionic Conductivity Measurement Data
Resin Wafer Solution Ratio of S.R.
Solution Specific Specific WaferISol
NaCI Resistance Resistance
ohm-cm ohm-cm
500 ppm 893.8 653.9 1.37
100 ppm 1363.0 3033.7 0.45
50 ppm 998.0 4448.3 0.22
20 ppm 1114.4 8543.1 0.13
CA 02378334 2001-12-20
WO 01/12292 PCT/US00/22624
11
Example 2
Wafers were manufactured using 15 to 20 weight percent polyethylene as
binding material. The ion exchange resins used in one wafer were the macro-
reticular type of strong acid cation exchange resin (Purolite C-155),
available from
Purolite, Inc., Bala Cynwyd, PA. and the gel type of strong base anion
exchange
resin (Purolite A-444). The resins and the polyethylene were mixed, heated and
molded to produce a porous wafer. Each wafer is 6.0 mm thick. The average
thickness variations of different spots on one single wafer is less than 0.5%
(i.e.,
0.05 mm).
Table 2 Additional Ion-Conductivity Measurements of Porous Immobilized lon-
Exchange material.
Resin Wafer Solution Ratio of S.R.
Solution Specific Specific Wafer/Sol
NaCI ohm-cm Resistance
(ohm-cm)
100 ppm 3054 2267 1.35
50 ppm 2878 3707 0.78
ppm 2379 8625 0.28
The ion electric resistances of the solution with and without resin wafer were
measured in different NaCI concentrations. A four-points ac impedance
measurement (LCZ 3321, Keithley Instruments, Inc., Cleveland, Ohio) was used
to
20 acquire the ion electric resistance of the resin wafer. As indicated in
Table 2, the
high electric resistances of the solution were improved by the presence of
resin
wafer when the NaCI concentrations were less than 50 ppm. Most of the use of
electrodeionization (EDI) technology need to reduce the ion concentration to
less
than 50 ppm. Therefore, the resin wafer can be very suitable for use in the
EDI
process.
Example 3
The desalting performance of an electrodeionization stack using the resin
wafer was tested. The resin wafers was packed using an electrodialysis stack
(TS-
2, Tokoyama Inc.) Six thin rubber gaskets were stacked together to provide
proper
CA 02378334 2001-12-20
WO 01/12292 PCT/US00/22624
12
flow path as shown in Figure 2. Silicone caulking adhesive (Dow Coring Inc.)
was
applied to both sides of the wafer which contacted with the gaskets. This was
to
prevent the flow by-pass via the sides of the wafer. Four pairs of dilute and
concentrate compartments packed with the resin wafers were assembled. The
stack was first tested for a flow leakage. With a flow rate of 0.45 gallon per
minute
(GPM) circulated in the dilute and concentrate compartments, the pressure drop
was less than 1.5 psi. No external as well internal leakage was found. This
shows
that the EDI device can be assembled using commonly used electrodialysis stack
and equipment. It also indicates that good flow rate with low pressure drop
and
operation without leakage between the cell compartments can be achieved.
Example 4
After the leak test, the desalting performance was carried out. The
electrodeionization (i.e., desalting) was operated in a continuous process
with 500
mg/L of NaCI as the feed solution. Four liters of 5000 mg/L NaCI solution was
circulated in the concentrate compartment. Three wt.% Na2S04 was used as the
electrolytic rinse solution.
Table 3 shows the desalting capability of the resin wafer stack. A NaCI
removal efficiency of 70% in the feed stream was achieved using this prototype
resin wafer stack with a single pass through.
Table 3
Feed Product Product Product Removal Rate Removal
flow
Rate (NaCI) ConductivitypH
ml/min m /L mS/cm Value m /hour/cmz
188.0 208.5 0.600 3.44 4.122 57.8%
156.0 184.6 0.531 3.51 3.707 62.6%
132.0 185.2 0.501 3.57 3.131 62.5%
105.0 148.9 0.427 3.59 2.783 69.9%
Example 5
The electrodeionization of NaCI concentration from dextrose syrup was
carried out. Using the same operating parameters as example 3 except the feed
solution was replaced with 500 mg/L of NaCI in 32 wt. % of glucose solution.
The
presence of high concentration of glucose significantly decreased the
conductivity
of the feed solution. Table 4 shows the results. Even at such low conductivity
feed
CA 02378334 2001-12-20
WO 01/12292 PCT/US00/22624
13
stream, a removal efficiency of 62% was achieved with a single pass through
using
this EDI device. Very notably, 99.9 percent of the dextrose was recovered and
the
loss to the concentrate was less than 0.1 percent.
Table 4
Feed FlowProduct Product Product Removal Removal
Rate
Rate (NaCI) ConductivitypH Efficiency
ml/min (mg/L) mS/cm Value m /hour/cm2
63.0 271.6 0.235 3.38 1.148 46.6%
50.0 258.6 0.221 3.42 0.961 49.2%
48.0 234.1 0.203 3.45 1.013 54.0%
35.0 194.9 0.176 3.48 0.844 61.7%
Example 6
Wafers were manufactured using an average of 30 weight percent (range of
25 to 35 weight percent) of a binding material, such as polyethylene.
Strong acid cation exchange resin of the gel type and strongly basic anion
exchange resin of the gel type were used in making these wafers. Exemplary
acid
ration exchange resins, and basic anion exchange resins include , Purolite C-
155),
and Purolite A 444), respectively, both available from Purolite, Inc. Bala
Cynwyd,
PA.
These rigid molded wafers were 3 mm in thickness and had a porosity of
approximately 35% free liquid space. Water and other solutions flowed freely
through the wafer with little resistance and pressure drop. For example, at a
flow
rate of a dextrose (30 wt%) solution at 30 ml/min.cm2 across the width of the
wafer,
a pressure drop of only 6 pounds per square inch was required. These wafers
had
very good ionic conductivities and were used for desalination of salt solution
in water
and deionization of carbohydrate containing solutions.
The wafers were cut and fitted into rubber gaskets and sandwiched between
ration and anion exchange membranes in a typical electrodialysis stack (e.g.,
TS-2,
Tokuyama, Inc.) as described in the earlier examples. The stack assembly was
then
connected to the flow pumps and power supply and operated for the deionization
tests.
For salt solution desalination, a solution of 500 parts per million NaCI in
water
CA 02378334 2001-12-20
WO 01/12292 PCT/US00/22624
14
was fed and a voltage of approximately 5 to 6 volts was applied across the
stack.
Greater than 99% desalination was readily achieved and under certain
conditions
99.8% desalination was achieved in one pass flow through this EDI device.
For carbohydrate solution desalination a solution containing approximately
30% (w/w) dextrose in water with 500 ppm equivalent of NaCI ions was fed and a
voltage of approximately 10 volts was applied across the stack. Desalination
ranging
between 90 to 95% was readily achieved in one pass flow through the EDI
device.
Wafer Material
Application Detail
The porous immobilized ion-exchange material can be shaped into a
myriad of configurations, depending on the EDI device utilized. For typical
stack
configurations, which incorporate a plurality of diluant and concentrate
compartments, wafers of the material, having relatively uniform thicknesses of
between approximately 2 and 6 millimeters, are preferable. The wafers are
suitably porous with between 20 percent and 60 percent porosity so that a
liquid
will flow through it with minimal resistance and the resin beads should be
uniformly dispersed in close proximity to each other. "Porosity" is construed
herein as the macroscopic void space that can be filled by a liquid.
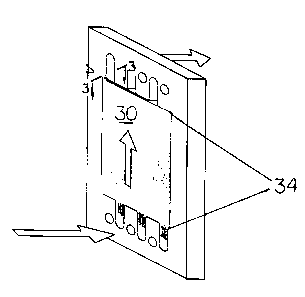
As depicted in FIG 3, a wafer 30 is cut into a form that is used in typical
electrodialysis stacks. The wafer 30 is generally secured in an opening 34
defined by the rubber gasket, via adhesive (such as the silicone caulking 36
depicted in 3B), or via friction which occurs upon swelling of the wafer once
wet.
The swelling phenomenon is due to the expansion of the resin particles in the
wafer.
A schematic depiction of a stack assembly with diluting/feed
compartments, the concentrate compartments, and .the invented porous,
immobilized ion-exchange material is designated as numeral 100 in Figure 4.
But for the juxtaposition of the invented wafer 30 between the anion exchange
membranes and cation exchange membranes, the assembly 100 is a flow
diagram of a typical electrodialysis stack assembly. The diluate compartment
41 is formed from the juxtaposition of the wafer 30 intermediate the cation
CA 02378334 2001-12-20
. PC'~'lUS 0 0 ~ 2 2 b 2 ~4
lP~,~E,A.,/~S ~ s SEP 2001
~s
exchange membrane 16 and anion exchange membrane 18, upstream from the
concentrating compartment, 43.
Note that the concentrating compartment 43 also can be fitted with a
wafer so that there are no force imbalances on the membranes. This assures
that the wafers are evenly pressed on the membranes, thereby preventing flow
channeling between the membranes and the wafers while also facilitating
sealing between the compartments.
In operation, an untreated feedstream 46 enters the stack assembly 100.
Upon contact with the wafer 30, the feedstream permeates upwardly, in the
direction of the arrows, while simultaneously being subjected to the effect of
the
anionic exchange membrane 18 and cationic exchange membrane 16. As the
feedstream permeates upwardly through the wafer 30, anions and cations are
pulled off of the ion-exchange resin particles as a result of an electrical
potential
(not shown) applied to the stack. This facilitates regeneration of the wafer
constituents in situ.
At the top of the stack, diluate liquid (i.e., feed stream liquid without
ionic
contaminant) enters diluate conduits 38 and removed via diluate exit ports 40.
Concomitant with the diluate removal, concentrated salt solution is removed
via
transport through separate concentrate conduits 42 and ultimately from the
. 20 . stack assembly through concentrate exit ports 44.
Concentrate ports 36 direct extracted ionic contaminants out of the stack
and in a direction opposite the flow of the treated diluant.
While the invention has been described with reference to details of the
illustrated embodiment, these details are not intended to limit the scope of
the
invention as defined in the appended claims.
~UVIENDFr; ~;~~~r