Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
1
Therapeutic Agents
The present invention relates to the use of compounds for reducing cravings
for food or an addictive substance in mammals particularly human beings.
W095/07274 discloses the use of a compounds of formula I as shown below
as novel compounds useful for treating depression, anxiety, psychoses, tardive
dyskinesia, Parkinson's disease, obesity, hypertension, Tourette's syndrome,
sexual
dysfunction, drug addiction, drug abuse, cognitive disorders, Alzheimer's
disease,
senile dementia, obsessive-compulsive behaviour, panic attacks, eating
disorders,
anorexia, cardiovascular and cerebrovascular disorders, non-insulin dependent
diabetes mellitus, hyperglycaemia, constipation, arrhythmia, disorders of the
neuroendocrine system, stress, prostatic hypertrophy, or spasticity.
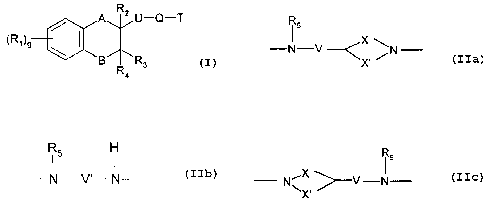
The present invention provides compounds of formula I
A R2 U-Q-T
~R,~9
B R3
Ra
including pharmaceutically acceptable salts thereof in which
A is methylene or -O-;
B is methylene or -O-;
g is 0, 1, 2, 3 or 4;
R, represents a) halo, b) an alkyl group containing 1 to 3 carbon atoms
optionally substituted by one or more halo, c) an alkoxy group containing 1 to
3
carbon atoms optionally substituted by one or more halo, d) an alkylthio group
containing 1 to 3 carbon atoms optionally substituted by one or more halo, e)
hydroxy, f) an acyloxy group containing 1 to 3 carbon atoms, g) hydroxymethyl,
h)
cyano, i) an alkanoyl group containing 1 to 6 carbon atoms, j) an
alkoxycarbonyl
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
2
group containing 2 to 6 carbon atoms, k) a carbamoyl group or carbamoylmethyl
group each optionally N-substituted by one or two alkyl groups each containing
1 to 3
carbon atoms, I) a sulphamoyl or sulphamoylmethyl group each optionally N-
substituted by one or two alkyl groups each containing 1 to 3 carbon atoms, m)
an
amino group optionally substituted by one or two alkyl groups each containing
1 to 3
carbon atoms; or two adjacent R, groups together with the carbon atoms to
which
they are attached form a fused benz ring, the substituents represented by R,
being
the same or different when g is 2, 3 or 4;
Rz is H, an alkyl group containing 1 to 3 carbon atoms, or an alkoxy group
containing 1 to 3 carbon atoms;
R3 and R4, which are the same or different, are H, or an alkyl group
containing
1 to 3 carbon atoms;
U is an alkylene chain containing 1 to 3 carbon atoms, optionally substituted
by one or more alkyl groups each containing 1 to 3 carbon atoms;
Q represents a divalent group of formula Ila, Ilb or Ilc
R5
-N-V ~X %N Ila
X'
Ilb
N V'N
~s
N\ ,>--V-N Ilc
X
in which V is a bond or an alkylene chain containing 1 to 3 carbon atoms
optionally
substituted by one or more alkyl groups each containing 1 to 3 carbon atoms;
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
3
V' is an alkylene chain containing 2 to 6 carbon atoms, optionally substituted
by one or more alkyl groups each containing 1 to 3 carbon atoms;
X is an alkylene chain containing 0 to 2 carbon atoms and X' is an alkylene
chain containing 1 to 4 carbon atoms provided that the total number of carbon
atoms
in X and X' amounts to 3 or 4; RS is H or an alkyl group containing 1 to 3
carbon
atoms; and
T represents an aromatic group optionally containing one or more N atoms
and optionally substituted by one or more substituents selected from halo, an
alkyl
group containing 1 to 3 carbon atoms, an alkoxy group containing 1 to 3 carbon
atoms, or a polyhalogenated alkyl group, for example trifluoromethyl, or T
represents
benzo[b]furanyl or benzodioxanyl with the proviso that T is not 2-pyrimidinyl
when A
is -O- for use in reducing cravings to food or an addictive substance.
In preferred compounds of formula I, A is -O-.
In preferred compounds of formula I, B is -O-.
In more preferred compounds of formula I both A and B are -O-.
In preferred compounds of formula I, g is 0, 1 or 2.
In preferred compounds of formula I, R, represents halo (for example fluoro,
chloro, or bromo), an alkyl group containing 1 to 3 carbon atoms, an alkoxy
group
containing 1 to 3 carbon atoms, hydroxy, or two adjacent R, groups together
with the
carbon atoms to which they are attached form a fused benz ring. In more
preferred
compounds of formula I, R, represents methoxy, fluoro, chloro, hydroxy, or two
adjacent R, groups together with the carbon atoms to which they are attached
form a
fused Benz ring.
In preferred compounds of formula I, RZ is H or an alkyl group containing 1 to
3 carbon atoms. In more preferred compounds of formula I, Rz is H.
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
4
In preferred compounds of formula I, R3 and R4, which are the same or
different, are H or methyl. In more preferred compounds of formulal, R3 and R4
are
both H.
In preferred compounds of formula I, U is methylene.
In preferred compounds of formula I in which Q is a group of formula Ila or
Ilc,
V is methylene or ethylene.
In preferred compounds of formula I, in which Q is a group of formula Ilb, V'
is
an alkylene chain containing 2 to 4 carbon atoms.
In preferred compounds of formula I, RS is H or methyl. In more preferred
compounds of formula I, R5 is H.
In preferred compounds of formula I, T is pyridyl, pyrimidinyl, pyrazinyl,
phenyl, benzo[b]furanyl, 1,4-benzodioxanyl or quinazolinyl all optionally
substituted
by methoxy, trifluoromethyl, or halo (eg fluoro, chloro or bromo). In more
preferred
compounds of formula I, T is 2-pyridyl, 2-pyrimidinyl, 2-pyrazinyl, phenyl,
2,3-
dihydrobenzo[b]furan-7-yl, 1,4-benzodioxan-5-yl or 4-quinazolinyl all
optionally
substituted by methoxy, trifluoromethyl, or halo (eg fluoro, chloro or bromo).
Compounds of formula I may exist as salts with pharmaceutically acceptable
acids. Examples of such salts include hydrochlorides, hydrobromides,
sulphates,
methanesulphonates, nitrates, maleates, acetates, citrates, fumarates,
tartrates [eg
(+)-tartrates, (-)-tartrates or mixtures thereof including racemic mixtures],
succinates,
benzoates and salts with amino acids such as glutamic acid. Compounds of
formula
I and their salts may exist in the form of solvates (for example hydrates).
Compounds of formula I contain one or more chiral centres, and exist in
different optically active forms. When compounds of formula I contain one
chiral
centre, the compounds exist in two enantiomeric forms and the present
invention
includes both enantiomers and mixtures of enantiomers. The enantiomers may be
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
resolved by methods known to those skilled in the art, for example by
formation of
diastereoisomeric salts which may be separated, for example, by
crystallisation;
formation of diastereoisomeric derivatives or complexes which may be
separated, for
example, by crystallisation, gas-liquid or liquid chromatography; selective
reaction of
5 one enantiomer with an enantiomer-specific reagent, for example enzymatic
esterification; or gas-liquid or liquid chromatography in a chiral
environment, for
example on a chiral support for example silica with a bound chiral ligand or
in the
presence of a chiral solvent. It will be appreciated that where the desired
enantiomer
is converted into another chemical entity by one of the separation procedures
described above, a further step is required to liberate the desired
enantiomeric form.
Alternatively, specific enantiomers may be synthesised by asymmetric synthesis
using optically active reagents, substrates, catalysts or solvents, or by
converting one
enantiomer into the other by asymmetric transformation.
When a compound of formula I contains more than one chiral centre it may
exist in diastereoisomeric forms. The diastereoisomeric pairs may be separated
by
methods known to this skilled in the art, for example chromatography or
crystallisation and the individual enantiomers within each pair may be
separated as
described above. The present invention includes each diastereoisomer of
compounds of formula I and mixtures thereof.
Certain compounds of formula I and their salts may exist in more than one
crystal form and the present invention includes each crystal form and mixtures
thereof. Certain compounds of formula I and their salts may also exist in the
form of
solvates, for example hydrates, and the present invention includes each
solvate and
mixtures thereof.
Specific compounds of formula I are:-
N-(1,4-Benzodioxan-2-ylmethyl)-1-[1-(pyrazin-2-yl)piperid-4-yl]methylamine;
N-(1,4-Benzodioxan-2-ylmethyl)-1-[1-(2-methoxyphenyl)piperid-4-yl]methylamine;
N-(1,4-Benzodioxan-2-ylmethyl)-1-[1-(3-chloropyrid-2-yl)piperid-4-
yl]methylamine;
N-(1,4-Benzodioxan-2-ylmethyl)-1-[1-(quinazolin-4-yl)piperid-4-yl]methylamine;
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
N-(1,4-Benzodioxan-2-ylmethyl)-1-[1-(pyrid-2-yl)piperid-4-yl]methylamine;
_N-(8-Methoxy-1,4-benzodioxan-2-ylmethyl)-1-[1-(2-methoxyphenyl)piperid-4-
yl]methylamine;
N-(1,4-Benzodioxan-2-ylmethyl)-N'-[3-(trifluoromethyl)-2-
pyridyl]ethanediamine;
_N-(8-Methoxy-1,2,3,4-tetrahydronaphth-2-ylmethyl)-1-[1-pyrimidin-2-yl)piperid-
4-
yl]methylamine;
7-{N-[1-(Pyrimidin-2-yl)piperid-4-ylmethyl]aminomethyl}-5,6,7,8-
tetrahydronaphth-1-
ol;
_N-(5-Methoxy-3,4-dihydro-2H-1-benzopyran-3-ylmethyl)-1-[1-(pyrimidin-2-
yl)piperid-
4-yl]methylamine;
N-( 1,4-Benzodioxan-2-ylmethyl)-1-(1-phenylpiperid-4-yl)methylamine;
_N-( 1,4-Benzodioxan-2-ylmethyl)-1-[ 1-( 1,4-benzod ioxan-5-yl) piperid-4-
yl]methylamine;
30
1-[1-(1,4-Benzodioxan-2-ylmethyl)piperid-4-yl]-N-(2-methoxyphenyl)methylamine;
N-(1,4-Benzodioxan-2-ylmethyl)-1-[1-(4-methoxyphenyl)piperid-4-yl]methylamine;
_N-(8-Methoxy-1,4-benzodioxan-2-ylmethyl)-N'-(2-methoxyphenyl)-1,3-
propanediamine;
N-(1,4-Benzodioxan-2-ylmethyl)-1-[1-(3-methoxyphenyl)piperid-4-yl]methylamine;
_N-(6,7-Dichloro-1,4-benzodioxan-2-ylmethyl)-1-[1-(2-methoxyphenyl)piperid-4-
yl]methylamine;
N-(1,4-Benzodioxan-2-ylmethyl)-1-[1-(2-chlorophenyl)piperid-4-yl]methylamine;
_N-(5-Fluoro-1,4-benzod ioxan-2-ylmethyl)-1-[1-(2-methoxyphenyl)piperid-4-
yl]methylamine;
_N-(8-Fluoro-1,4-benzodioxan-2-ylmethyl)-1-[ 1-(2-methoxyphenyl)piperid-4-
yl]methylamine;
1-[1-(2-methoxyphenyl)piperid-4-yl]-N-(naphtho[1,2-b]dioxan-2-
ylmethyl)methylamine;
1-[1-(2,3-Dihydrobenzo[b]furan-7-yl)piperid-4-yl]-N-(8-methoxy-1,4-benzodioxan-
2-
ylmethyl)methylamine;
_N-(6-chloro-1,4-benzodioxan-2-ylmethyl)-1-[1-(2-methoxyphenyl)piperid-4-
yl]methylamine;
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
7
_N-(7-chloro-1,4-benzodioxan-2-ylmethyl)-1-[1-(2-methoxyphenyl)piperid-4-
yl]methylamine;
_N-(8-hydroxy-1,4-benzodioxan-2-ylmethyl)-1-[1-(2-methoxyphenyl)piperid-4-
yl]methylamine;
and pharmaceutically acceptable salts thereof in the form of individual
enantiomers,
racemates, or other mixtures of enantiomers.
Specific enantiomeric forms of compounds of formula I include:
(S)-(-)-_N-( 1,4-Benzodioxan-2-ylmethyl)-1-[ 1-(2-methoxyphenyl)piperid-4-
yl]methylamine;
(RR)-(+)-_N-(1,4-Benzodioxan-2-ylmethyl)-1-[1-(2-methoxyphenyl)piperid-4-
yl]methylamine;
(-)-_N-(1,4-Benzodioxan-2-ylmethyl)-1-[1-(pyrid-2-yl)piperid-4-yl]methylamine
dihydrochloride;
(+)-_N-(1,4-Benzodioxan-2-ylmethyl)-1-(1-(pyrid-2-yl)piperid-4-yl]methylamine
dihydrochloride.
The present invention also includes pharmaceutical compositions containing
a therapeutically effective amount of a compound of formula I or a salt
thereof
together with a pharmaceutically acceptable diluent or carrier.
As used hereinafter, the term "active compound" denotes a compound of
formula I or a salt thereof. In therapeutic use, the active compound may be
administered orally, rectally, parenterally or topically, preferably orally.
Thus the
therapeutic compositions of the present invention may take the form of any of
the
known pharmaceutical compositions for oral, rectal, parenteral or topical
administration. Pharmaceutically acceptable carriers suitable for use in such
compositions are well known in the art of pharmacy. The compositions of the
invention may contain 0.1-99% by weight of active compound. The compositions
of
the invention are generally prepared in unit dosage form. Preferably the unit
dosage
of active ingredient is 1-500 mg. The excipients used in the preparation of
these
compositions are the excipients known in the pharmacist's art.
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
8
Compositions for oral administration are the preferred compositions of the
invention and these are the known pharmaceutical forms for such
administration, for
example tablets, capsules, syrups and aqueous or oil suspensions. The
excipients
used in the preparation of these compositions are the excipients known in the
pharmacist's art. Tablets may be prepared by mixing the active compound with
an
inert diluent such as calcium phosphate in the presence of disintegrating
agents, for
example maize starch, and lubricating agents, for example magnesium stearate,
and
tableting the mixture by known methods. The tablets may be formulated in a
manner
known to those skilled in the art so as to give a sustained release of the
compounds
of the present invention. Such tablets may, if desired, be provided with
enteric
coatings by known methods, for example by the use of cellulose acetate
phthalate.
Similarly, capsules, for example hard or soft gelatin capsules, containing the
active
compound with or without added excipients, may be prepared by conventional
means and, if desired, provided with enteric coatings in a known manner. The
tablets and capsules may conveniently each contain 1 to 500 mg of the active
compound. Other compositions for oral administration include, for example,
aqueous
suspensions containing the active compound in an aqueous medium in the
presence
of a non-toxic suspending agent such as sodium carboxymethyl- cellulose, and
oily
suspensions containing a compound of the present invention in a suitable
vegetable
oil, for example arachis oil.
The active compound may be formulated into granules with or without
additional excipients. The granules may be ingested directly by the patient or
they
may be added to a suitable liquid carrier (for example water) before
ingestion. The
granules may contain disintegrants (for example a pharmaceutically acceptable
effervescent couple formed from an acid and a carbonate or bicarbonate salt)
to
facilitate dispersion in the liquid medium.
Compositions of the invention suitable for rectal administration are the known
pharmaceutical forms for such administration, for example, suppositories with
cocoa
butter or polyethylene glycol bases.
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
9
Compositions of the invention suitable for parenteral administration are the
known pharmaceutical forms for such administration, for example sterile
suspensions
or sterile solutions in a suitable solvent.
Compositions for topical administration may comprise a matrix in which the
pharmacologically active compounds of the present invention are dispersed so
that
the compounds are held in contact with the skin in order to administer the
compounds transdermally. A suitable transdermal composition may be prepared by
mixing the pharmaceutically active compound with a topical vehicle, such as a
mineral oil, petrolatum and/or a wax, for example paraffin wax or beeswax,
together
with a potential transdermal accelerant such as dimethyl sulphoxide or
propylene
glycol. Alternatively the active compounds may be dispersed in a
pharmaceutically
acceptable cream or ointment base. The amount of active compound contained in
a
topical formulation should be such that a therapeutically effective amount of
the
compound is delivered during the period of time for which the topical
formulation is
intended to be on the skin.
The compounds of the present invention may also be administered by
continuous infusion either from an external source, for example by intravenous
infusion or from a source of the compound placed within the body. Internal
sources
include implanted reservoirs containing the compound to be infused which is
continuously released for example by osmosis and implants which may be (a)
liquid
such as a suspension or solution in a pharmaceutically acceptable oil of the
compound to be infused for example in the form of a very sparingly water-
soluble
derivative such as a dodecanoate salt or ester or (b) solid in the form of an
implanted
support, for example of a synthetic resin or waxy material, for the compound
to be
infused. The support may be a single body containing all the compound or a
series
of several bodies each containing part of the compound to be delivered. The
amount
of active compound present in an internal source should be such that a
therapeutically effective amount of the compound is delivered over a long
period of
time.
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
In some formulations it may be beneficial to use the compounds of the
present invention in the form of particles of very small size, for example as
obtained
by fluid energy milling.
5 In the compositions of the present invention the active compound may, if
desired, be associated with other compatible pharmacologically active
ingredients.
The use of compounds of the present invention in the manufacture of
pharmaceutical compositions is illustrated by the following description. In
this
10 description the term "active compound" denotes any compound of the
invention but
particularly any compound which is the final product of one of the preceding
Examples.
a) Capsules
In the preparation of capsules, 10 parts by weight of active compound and
240 parts by weight of lactose are de-aggregated and blended. The mixture is
filled
into hard gelatin capsules, each capsule containing a unit dose of part of a
unit dose
of active compound.
b) Tablets
Tablets are prepared from the following ingredients.
Parts by weight
Active compound 10
Lactose 190
Maize starch 22
Polyvinylpyrrolidone 10
Magnesium stearate 3
The active compound, the lactose and some of the starch are de-aggregated,
blended and the resulting mixture is granulated with a solution of the
polyvinyl-
pyrrolidone in ethanol. The dry granulate is blended with the magnesium
stearate
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
11
and the rest of the starch. The mixture is then compressed in a tabletting
machine to
give tablets each containing a unit dose or a part of a unit dose of active
compound.
Enteric coated tablets
Tablets are prepared by the method described in (b) above. The tablets are
enteric coated in a conventional manner using a solution of 20% cellulose
acetate
phthalate and 3% diethyl phthalate in ethanol:dichloromethane (1:1).
d) Suppositories
In the preparation of suppositories, 100 parts by weight of active compound is
incorporated in 1300 parts by weight of triglyceride suppository base and the
mixture
formed into suppositories each containing a therapeutically effective amount
of active
ingredient.
The pharmaceutical compositions containing a therapeutically effective amount
of a compound of formula I or III may be used to treat drug misuse or other
addictive
disorders. Whilst the precise amount of active compound administered in such
treatment will depend on a number of factors, for example the age of the
patient, the
severity of the condition and the past medical history, and always lies within
the
sound discretion of the administering physician, the amount of active compound
administered per day is in the range 1 to 1000 mg preferably 5 to 500 mg given
in
single or divided doses at one or more times during the day.
In another aspect the present invention provides a method of treating drug
misuse or other addictive disorders which comprises the administration of a
therapeutically effective amount of a compound of formula I to a patient in
need
thereof.
The present invention provides a method of reducing cravings to food or an
addictive substance in a mammal comprising administering an effective amount
of a
compound of formula I to a mammal in need thereof.
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
12
Suitably the addictive substance is cocaine, amphetamine, nicotine, opiates,
tobacco or alcohol. The addictive substance may also be MDMA (ecstasy), a
cannabinoid, LSD, MDA or PCP. .The term opiates includes heroin and morphine.
In yet another aspect, the present invention provides the use of a compound of
formula I or III in the manufacture of a medicament for use in the treatment
ofdrug
misuse or other addictive disorders.
Conditions which may be advantageously treated with the compounds of the
present invention include disorders arising from drug misuse including drug
withdrawal symptoms, aiding in the cessation of smoking, aiding in the
prevention of
relapse after cessation of drug use and similar use in the treatment of other
addictive
disorders such as compulsive gambling, compulsive shopping disorder and
compulsive sexual disorder.
In another aspect the present invention provides a method of treating
addictive-drug-induced psychoses comprising administering a therapeutically
effective amount of a compound of formula I to a mammal, particularly a human
being, in need thereof.
Addictive drugs which may cause psychoses include benzodiazepines,
cannabinoids, LSD, MDMA, MDA, PCP, opiates including heroin and morphine,
amphetamine, cocaine and alcohol.
The pharmacological activity of the compounds of the present invention may
be demonstrated by one or more of the following tests.
STUDY 1 METHODS
Subjects: The subjects are four male rhesus monkeys (Macaca mulatta),
weighing 5.7-8.1 kg and maintained on a diet of 3-4 monkey biscuits and one
piece
of fresh fruit per day. During the week, all food is delivered after the
experimental
session, whereas at weekends, food is delivered between 9 a.m. and noon. Water
is
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
13
freely available at all times. The monkeys are housed in a humidity and
temperature
controlled room with a 12 h light-dark cycle (lights on from 7 a.m. to 7
p.m.).
Apparatus: Each monkey is housed individually in a well-ventilated,
stainless steel chamber (56 x 71 x 69 cm) which includes an operant panel (28
x 28
cm) mounted on the front wall. Three response keys are arranged in a
horizontal
row 3.2 cm from the top of the operant panel. Each key can be transilluminated
by
red or green stimulus lights (Superbright LEDs). An externally mounted pellet
dispenser delivers 1 g fruit-flavoured food pellets to a food receptacle
beneath the
operant response panel. A computer, located in a separate room, controls the
operant panels and data collection.
Discrimination Training: Discrimination training is conducted 5 days per week
during daily sessions composed of multiple cycles. Each cycle consists of a 15
min
time-out period followed by a 5 min response period. During the time-out, all
stimulus lights are off, and responding has no scheduled consequences. During
the
response period, the right and left response keys are transilluminated red or
green,
and monkeys can earn up to 10 food pellets by responding under a FR 30
schedule
of food presentation. For one monkey, the left key is illuminated green and
the right
key is illuminated red, the colours of the response-keys are reversed for the
other
three monkeys. The centre key is not illuminated at any time and responding on
it
has no scheduled consequences. If all available food pellets are delivered
before the
end of the 5 min response period, the stimulus lights are turned off and
responding
has no scheduled consequences for the remainder of the 5 min period.
On training days, monkeys are given either saline or 0.40 mg/kg cocaine, i.m.,
10 min before the response period. Following the administration of saline,
responding on only the green key (the saline-appropriate key) produces food,
whereas following administration of 0.40 mg/kg cocaine, only responding on the
red
key (the drug-appropriate key) produces food. Responses on the inappropriate
key
reset the FR requirement on the appropriate key. Sessions consist of 1 toy
cycles
and, if cocaine is administered, this occurs only during the last cycle. Thus,
training
days consist of 0 to 5 saline cycles followed by 0 or 1 cocaine cycle.
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
14
During each response period, 3 dependent variables are determined:
1 ) Percent injection-appropriate responding prior to delivery of the first
reinforcer.
2) Percent injection-appropriate responding for the entire response period
3) Response Rate.
Monkeys meeting the following criteria during the training day immediately
proceeding the test day and in at least 6 of 7 consecutive training sessions
before
this are used for discrimination testing:
1 ) the percent injection-appropriate responding prior to delivery of the
first
reinforcer is >_ 80% for all cycles;
2) the percent injection-appropriate responding for the entire cycle is >_ 90%
for
all cycles;
3) Response rates during saline training cycles are >0.5 responses per second.
If responding did not meet criterion levels of discrimination performance,
then training
is continued until criterion levels of performance are obtained for at least
two
consecutive days.
Discrimination Testing: Test sessions are identical to training sessions
except
that responding on either key produces food, and the test compound is
administered
using a Pretreatment Protocol. In this protocol, a cumulative dose-effect
curve for
cocaine (0.013-1.3 mg/kg) is determined either alone or following pretreatment
with
the test compound, which is administered 20 min before the first dose of
cocaine.
Mean data from saline and drug cycles during the training day immediately
proceeding the initial test day serve as the control data for the subsequent
test day.
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
Data Analysis: The Percent Cocaine-Appropriate Responding and the
Response Rate are plotted as a function of the dose of cocaine (log scale).
Where
possible, the EDSO value for cocaine is determined by drawing a line between
the
5 points above and below 50% cocaine-appropriate responding, and then using
linear
regression to interpolate the dose that would produce 50% cocaine-appropriate
responding. EDSO values for cocaine administered alone and following
pretreatment
with the test compound are then compared.
10 Drugs: Cocaine hydrochloride is dissolved in sterile saline. The test
compound is dissolved in 1 % lactic acid in distilled water.
RESULTS
Control mean saline-appropriate responding = 99.8% (~ 0.2) and 100%
appropriate responding are obtained during cocaine cycles.
EDSO values for cocaine are calculated. Administration of cocaine alone
produces a dose-dependent increase in cocaine-appropriate responding in all
four
monkeys. Complete substitution is obtained at the training dose of cocaine
(0.4
mg/kg) in all monkeys, and a higher dose of 1.3 mg/kg usually decreases
response
rates. Pretreatment with 0.01 mg/kg of the test compound produces a rightward
shift
in the cocaine dose-effect curve and a 3-fold increase in the cocaine EDSO
value in
monkey 2, but it has no effect on the cocaine discrimination dose-effect curve
in the
other three monkeys. A higher dose of 0.032 mg/kg of the test compound
produces
rightward shifts in the cocaine dose-effect curves in all four monkeys. The
test
compound (0.01 and 0.032 mg/kg) also eliminated responding during the first
one to
three cycles of the cumulative cocaine dose-effect curve determination (i.e.
in
combination with 0.013 and 0.04 mg/kg cocaine). However, monkeys responded
after administration of higher cocaine doses, thereby permitting evaluation of
the
effects on cocaine discrimination. Interestingly, response rates following
administration of the highest dose of cocaine (1.3 mg/kg) are often higher
following
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
16
test compound pretreatment than for cocaine alone, suggesting that the test
compound attenuated the rate-decreasing effects of high cocaine doses.
These studies can establish that the test compound antagonises the
discriminative stimulus effects and possibly also the rate decreasing effects
of
cocaine at doses that also produce effects on response rates by comparing EDso
values (mg/kg) for cocaine administered either alone or after pretreatment
with test
compound.
STUDY 2 METHODS
Subjects: The subjects are four male rhesus monkeys (Macaca mulatta). Each
monkey is maintained on a diet of 3 monkey biscuits and one piece of fresh
fruit per
day in addition to fruit-flavoured pellets delivered during operant sessions
(see
below). Water is freely available at all times. The monkeys are housed in a
humidity
and temperature controlled room with a 12 hr light-dark cycle (lights on from
7 a.m. to
7 p.m.).
Monkeys are surgically implanted with double-lumen silicone rubber catheters
(inside diameter 0.7 mm, outside diameter 2.0 mm) to facilitate concurrent
delivery of
cocaine and treatment compounds. Catheters are implanted in the jugular or
femoral
vein and exteriorized in the midscapular region. All surgical procedures are
performed under aseptic conditions. Monkeys are sedated with ketamine (5
mg/kg,
s.c.), and anaesthesia is induced with sodium thiopental (10 mg/kg, i.v).
Monkeys
receive 0.05 mg/kg atropine, to reduce salivation. Following insertion of a
tracheal
tube, anaesthesia is maintained with isoflurane (1-1.5% in oxygen). After
surgery,
monkeys are administered aspirin or acetaminophen (80-160 mg/day; p.o.) for 3
days and Procaine Penicillin 0 (300,000 units/day, i.m.) every day for 5 days.
The i.v.
catheter is protected by a tether system consisting of a custom-fitted nylon
vest
connected to a flexible stainless steel cable and fluid swivel (t-omir
Biomedical;
Malone, NY), which permits the monkeys to move freely. Catheter patency is
periodically evaluated by i.v. administration of the short-acting barbiturate
methohexital (3 mg/kg i.v.) or ketamine (2-3 mg/kg i.v.). The catheter is
considered
Data Analysis: The Percent
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
17
patent if i.v. administration of methohexital or ketamine produces loss of
muscle tone
within 10 seconds after its administration.
Apparatus: Each monkey is housed individually in a well-ventilated
stainless steel chamber (64 x 64 x 79 cm which includes an operant panel (28 x
28
cm) mounted on the front wall. Three response keys (6.4 x 6.4 cm) are arranged
in a
horizontal row 3.2 cm from the top of the operant panel. Each key can be
transilluminated by red or green stimulus lights (Superbright LEDs). An
externally
mounted pellet dispenser delivers 1 g fruit-flavoured food pellets to a food
receptacle
beneath the operant response panel. Two syringe pumps are mounted above each
cage for delivery of saline or drug solutions through the intravenous
catheters.
Operant panels and data collection are controlled by a computer through a MED-
PC
interface.
Training: As shown in the diagram below, food and i.v. drug or saline
injections are available during three alternating components: a 5 min food
component, a 100-min drug component, and a second 5 min food component. Both
food and i.v. injections are available under a FR 30 schedule of
reinforcement.
During the two food components, the response key is transilluminated red.
During
the drug component, the response key is transilluminated green. Following the
delivery of each food pellet or drug injection, there is a 10 sec timeout
period, during
which the stimulus light illuminating the centre response key is turned off
and
responding has no scheduled consequences. The food and drug components are
separated by 5-min timeout periods when the response key is dark, and
responding
has no scheduled consequences. The entire food/drug/food session lasts 120
min.
In addition to the food/drug/food session described above, monkeys are also
given the opportunity to self-administer additional food pellets during
supplementary
food sessions. During these sessions, food is available under a FR30/Timeout
10
sec schedule, and a maximum of 25 pellets per session can be earned. These
food
sessions provide additional enrichment opportunities for the monkeys and
behavioural information relevant for the evaluation of prolonged treatment
drug
effects.
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/OS'735
18
During training, the solution available for self-administration during the
drug
component is alternated between 0.032 mg/kg/inj cocaine (the maintenance dose
of
cocaine) and saline. Each period of cocaine or saline availability usually
lasts from 3
to 10 days. Monkeys are trained until they met the following criteria for
stable
cocaine self-administration: 1 ) three consecutive days during which the
response
rate during the drug component of each session differs by no more than 20%
from
the mean drug component response rate and there is no upward or downward
trend;
and 2) rapid saline extinction as indicated by a decrease in drug component
response rates on the first day of saline substitution.
Evaluation of Test Compound: The effects of the test compound (0.0032-
0.10 mg/kg) on cocaine self-administration and food-maintained behaviour are
evaluated using the standard pretreatment test procedure. In this procedure,
the test
compound is administered i.m. 20-min prior to a test session during which a
test unit
dose of cocaine is available during the drug component. Two series of studies
are
described here. In the first, the unit dose of cocaine is 0.0032 mg/kg/inj (at
or near
the peak of each monkey's cocaine self-administration dose-effect curve) and
the
effects of pretreatment with each dose of test compound are determined in
single
sessions for all monkeys. In the second series of studies, the effects of
pretreatment
with each of two doses of the test compound (0.003 and 0.01 mg/kg) on the
entire
cocaine dose-effect function are determined. In these studies, the dose of
cocaine is
systematically varied for single test sessions after pretreatment with each
dose of the
test compound. Both the dose of cocaine and the pretreatment dose of the test
compound are varied across test sessions in an irregular order among monkeys.
At the conclusion of each pretreatment test in either series of studies,
training
conditions (availability of saline or the maintenance dose of cocaine) are
reinstated.
Test sessions generally are conducted on Tuesdays and Fridays, and either
saline or
the maintenance dose of cocaine is available during training sessions for the
remainder of the week. On occasion, another dose of cocaine is substituted for
the
maintenance dose to insure that the position of the cocaine dose-effect
function in
individual monkeys is stable. In addition, test days are occasionally omitted
to allow
several days of saline substitution.
CA 02378389 2002-O1-04
WO 01/02391 PCT/EP00/05735
19
Data Analysis: The dependent variables are the response rates during each
food and drug component. The response rate is calculated as (total # responses
(component duration - S timeouts)]. Control response rates for each food and
drug
component during availability of each unit dose of cocaine are defined as the
response rate obtained when that unit dose of cocaine is available and no
pretreatment is administered. The EDS° value for the test compound
during each
food or drug component is defined as the dose of the test compound that
decreases
rates of cocaine or food self-administration to 50% of control response rates.
The
ED5° values are determined where possible by linear regression from the
linear
portion of the test compound dose-effect curve.
For subsequent studies, in which the unit dose of cocaine is varied and the
pretreatment dose of the test compound is held constant, response rates are
graphed as a function of the unit dose of cocaine. Control cocaine dose-effect
curves are determined in the absence of pretreatment and are visually compared
to
cocaine dose-effect curves determined following pretreatment with the test
compound.
Drugs: Cocaine hydrochloride is dissolved in saline. A stock solution of 10
mg/ml of the test compound is prepared using a vehicle of 1 % lactic acid in
distilled
water, and dilutions are made with distilled water. Aseptic precautions are
taken in
every phase of cocaine solution preparation and dispensing. Cocaine solutions
are
filter-sterilised using a 0.22 micron Millipore Filter and stored in sterile,
pyrogen-free
vials. Sterility of the entire fluid path for drug solutions is maintained
throughout the
study. Each unit dose of cocaine is delivered i.v. in an injection volume of
0.1 ml.
Doses of the test compound are delivered i.m. in a volume of 0.2-3.0 ml.
These studies can establish that treatment with the test compound diminishes
cocaine self-administration and food-maintained behaviour.