Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
1
PHARACEUTICAL COMBINATION OF ETHINYLESTRADIOL AND DROSPIRENONE FOR USE AS A
CONTRACEPTIVE
FIELD OF THE INVENTION
The present invention relates to a pharmaceutical composition comprising
drospirenone
and ethinylestradiol, a method of providing dissolution of drospirenone,
methods of
inhibiting ovulation by administration of drospirenone and the use of
drospirenone and
ethinylestradiol for inhibiting ovulation.
BACKGROUND OF THE INVENTION
Oral contraceptives containing a combination of a gestagen and an estrogen
component
have been used since the 1960's. The earliest contraceptive preparations
consisted of 21
tablets containing the combination of active agents and 7 tablets containing
no active
agent, and the amount of each active agent was the same in each tablet (the so-
called
one-phase preparations). Subsequently, preparations were developed that
consisted of
tablets containing different amounts and ratios of the active agents over the
cycle of
administration (the so-called multiple-phase preparations).
Contraceptive reliability is mainly provided by the gestagen component. The
daily dosage
should be at least the minimum of what is needed for the gestagen in question
to inhibit
ovulation effectively. The estrogen component acts to increase the ovulation
inhibitory
effect of gestagen and to ensure cycle stability. Since the introduction of
oral
contraceptives, the daily dosage of gestagen has been reduced through the
development
of new and more efficient gestagens than were present in the earlier
contraceptive
preparations. It has also been possible to reduce the daily dosage of
estrogen.
6[3,7(3;153,16~i-dimethylene-3-oxo-17a-pregn-4-ene-21,17-carbolactone
(drospirenone) is
known from DE 26 52 761 in which its use as a diuretic compound is disclosed.
In DE 30 22 337, the gestagen-like activity of the compound and its consequent
utility as a
contraceptive agent is described at dosage levels of 0.5-50 mg of drospirenone
per day. It
is also noted that the mechanism of action of the compound is very similar to
that of the
natural corpus luteum hormone progesterone, and that it does not give rise to
increased
blood pressure for which reason it may be administrated to women who have or
are at risk
CONFIRMATION COPY
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
2
of developing increased blood pressure. It is further described that
drospirenone may be
administered together with ethinylestradiol in an amount of 0.03-0.05 mg per
day.
DE 30 51 166 discloses the use of the drospirenone for the treatment of
gynaecological
irregularities and for contraception at a dosage level of 0.5-50 mg per day.
EP 398 460 discloses the use of drospirenone for the treatment of androgen-
induced
disorders, aldosterone-induced disorders and hormonal irregularities as well
as for
contraception at dosage levels of 0.5-50 mg, preferably 1-10 mg per day.
Ethinylestradiol
may be co-administered at a level of 0.02-0.04 mg per day.
US 5,756,490 discloses pharmaceutical combination preparations comprising 23
or 24
dosage units containing a combination of a gestagen and an estrogen and 4-10
dosage
units containing estrogen alone. Drospirenone is mentioned as a possible, but
not
preferred, gestagenic compound and ethinylestradiol is mentioned as a
possible, but not
preferred, estrogenic compound.
SUMMARY OF THE INVENTION
In the course of research leading to the present invention, it has
surprisingly been found
that a hitherto undisclosed minimum dosage level of drospirenone is required
for reliable
contraceptive activity. Similarly, a preferred maximum dosage has been
identified at which
unpleasant side effects, in particular excessive diuresis, may substantially
be avoided.
Accordingly, in a first aspect, the present invention relates to a
pharmaceutical
composition comprising, as a first active agent, 6(3,7(3;153,16(3-dimethylene-
3-oxo-17a-
pregn-4-ene-21,17-carbolactone (drospirenone) in an amount corresponding to a
daily
dosage, on administration of the composition, of from about 2 mg to about 4
mg, and, as a
second active agent, 17a-ethinylestradiol (ethinylestradiol) in an amount
corresponding to
a daily dosage of from about 0.01 mg to about 0.05 mg, together with one or
more
pharmaceutically acceptable carriers or excipients.
Apart form the active substances themselves, it is envisaged that an ester or
prodrug of
drospirenone may be employed in the present composition, e.g. an
oxyiminopregnane
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
3
carbolactone as disclosed in WO 98/24801. Likewise, it is envisaged that
esters or ethers
of ethinylestradiol may be included in the composition.
In a further aspect, the invention relates to a method of inhibiting ovulation
in a mammal,
in particular a human, comprising administering, to said mammal, drospirenone
in an
amount in the range of from about 2 mg to about 4 mg of per day, together with
ethinylestradiol in an amount of from about 0.01 mg to about 0.05 mg per day,
said
amounts being effective to inhibit ovulation in said mammal.
In a still further aspect, the invention relates to the use of drospirenone
combined with
ethinylestradiol for preparing a pharmaceutical preparation for the inhibition
of ovulation in
a mammal, in particular a human, the composition comprising an amount of
drospirenone
corresponding to a daily dosage, on administration of the composition, of from
about 2 mg
to about 4 mg, and comprising an amount of ethinylestradiol corresponding to a
daily
dosage, on administration of the composition, of from about 0.01 to about 0.05
mg.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention is further described with reference to the drawings in which
Fig. 1 is a graph showing the in vitro dissolution rate of drospirenone from
tablet cores,
V1-V7 being batches containing micronized drospirenone, and V8 being a batch
containing macrocrystalline drospirenone;
Fig. 2 is a graph showing the in vitro dissolution rate of drospirenone from
tablet cores,
different lines representing different test batches;
Fig. 3 is a graph showing the in vitro dissolution rate of drospirenone from
film-coated
tablets, different lines representing different test batches;
Fig. 4 is a graph showing the in vitro dissolution rate of ethinylestradiol
from tablet cores,
different lines representing different test batches; and
Fig. 5 is a graph showing the in vitro dissolution rate of ethinylestradiol
from film-coated
tablets, different lines representing different test batches.
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
4
DETAILED DISCLOSURE OF THE INVENTION
Drospirenone, which may be prepared substantially as described in, e.g., US
4,129,564 or
WO 98/06738, is a sparingly soluble substance in water and aqueous buffers at
various
pH values. Furthermore, drospirenone is rearranged to an inactive isomer under
acid
conditions and hydrolysed under alkaline conditions. To ensure good
bioavailability of the
compound, it is therefore advantageously provided in a form that promotes
rapid
dissolution thereof.
It has surprisingly been found that when drospirenone is provided in
micronized form (so
that particles of the active substance have a surface area of more than 10,000
cm2/g, and
the following particle size distribution as determined under the microscope:
not more than
2 particles in a given batch with a diameter of more than 30 Vim, and
preferably <_ 20
particles with a diameter of >_ 10 pm and <_ 30 Vim) in a pharmaceutical
composition, rapid
dissolution of the active compound from the composition occurs in vitro
("rapid
dissolution" is defined as the dissolution of at least 70% over about 30
minutes, in
particular at least 80% over about 20 minutes, of drospirenone from a tablet
preparation
containing 3 mg of drospirenone in 900 ml of water at 37°C determined
by the USP XXIII
Paddle Method using a USP dissolution test apparatus 2 at 50 rpm). Instead of
providing
the drospirenone in micronized form, it is possible to dissolve it in a
suitable solvent, e.g.
methanol or ethyl acetate, and spray it onto the surface of inert carrier
particles followed
by incorporation of the particles containing drospirenone on their surface in
the
composition.
Without wishing to be limited to any particular theory, it appears that the in
vitro
dissolution rate of drospirenone is connected to the dissolution rate in vivo
resulting in
rapid absorption of drospirenone in vivo on oral administration of the
compound. This is
an advantage because isomerization of the compound in the gastric environment
and/or
hydrolysis in the intestine is substantially reduced, leading to a high
bioavailability of the
compound.
With respect to ethinylestradiol which is also a sparingly soluble substance,
though less
sensitive to degradation than drospirenone under conditions prevailing in the
gastrointestinal tract, it is also an advantage to provide it in micronized
form or sprayed
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
from a solution, e.g. in ethanol, onto the surface of inert carrier particles.
This has the
added advantage of facilitating a more homogenous distribution of the
ethinylestradiol
throughout the composition which might otherwise be difficult to obtain
because
ethinylestradiol is incorporated in extremely small amounts. When
ethinylestradiol is
5 provided in micronized form, it preferably has the following particle size
distribution as
determined under the microscope: 100% of the particles have a diameter of <_
15.0 pm,
99% of the particles have a diameter of <_ 12.5pm, 95% of the particles have a
diameter of
<_ 10.0 Vim, and 50% of the particles have a diameter of <_ 3.0 pm.
Furthermore, no particle
is larger than 20 Vim, and <_ 10 particles have a diameter of >_ 15 pm and <_
20 pm.
To obtain a more rapid rate of dissolution, it is preferred to include
carriers or excipients
which act to promote dissolution of both active substances. Examples of such
carriers and
excipients include substances that are readily soluble in water such as
cellulose
derivatives, carboxymethylcellulose, hydroxypropyl cellulose,
hydroxypropylmethyl
cellulose, gelled starch, gelatin or polyvinylpyrrolidone. In particular, it
appears as though
polyvinylpyrrolidone might be particularly helpful to promote dissolution.
The composition of the invention preferably comprises drospirenone in an
amount
corresponding to a daily dosage of from about 2.5 mg to about 3.5 mg, in
particular about
3 mg. The amount of ethinylestradiol preferably corresponds to a daily dosage
of from
about 0.015 mg to about 0.04 mg, in particular from about 0.015 mg to about
0.03 mg.
More particularly, the present composition comprises an amount of drospirenone
corresponding to a daily dosage of from about 3.0 to about 3.5 mg and
ethinylestradiol in
an amount corresponding to from about 0.015 to about 0.03 mg.
Apart from its ability to inhibit ovulation, the composition of the invention
has been found
to possess pronounced anti-androgenic properties and may therefore be used in
the
prevention or treatment of androgen-induced disorders, in particular acne.
Such use may
be independent from or concomitant with the use as a contraceptive disclosed
above.
Furthermore, since drospirenone is an aldosterone antagonist, it has diuretic
properties
and is therefore suitable for counteracting the water-retentive properties of
ethinylestradiol.
In a particular embodiment, the invention relates to a pharmaceutical
preparation
consisting of a number of separately packaged and individually removable daily
dosage
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
6
units placed in a packaging unit and intended for oral administration for a
period of at least
21 consecutive days, wherein each of said daily dosage units comprises a
combination of
drospirenone in an amount of from about 2 mg to about 4 mg and
ethinylestradiol in an
amount from about 0.01 to about 0.05 mg.
In one embodiment, the preparation further comprises 7 or less said daily
dosage units
containing no active agent. Alternatively, it is possible to include, in the
dosage regimen, a
period of 7 days or less during which no dosage units are ingested. For
compliance
reasons, however, it may be preferred to include an appropriate number of
blanks in the
preparation, in which case the total number of daily dosage units in the
preparation is at
least 28. The inclusion of blanks, or the pill-free days, will then trigger
withdrawal
bleeding.
The preparation may be a one-phase composition, i.e. a preparation wherein the
amounts
of either active agent remains constant for the entire at least 21-day period,
or the
amounts of either or both active agents may be varied over the at least 21-day
period to
generate a multiple-phase preparation, e.g. a two- or three-phase preparation,
substantially as disclosed in, e.g., EP 148 724. In case of multiple-phase
preparation, it
may be possible to include a natural estrogen such as estradiol, e.g. in an
amount of from
about 0.5 mg to about 4 mg per day, instead of ethinylestradiol.
In suitable embodiments of the present preparation, the number of daily dosage
units
comprising the combination of drospirenone and ethinylestradiol may be 21, 22,
23 or 24,
and the number of daily dosage units containing no active agent may then be 7,
6, 5 or 4,
as the case may be. In a further embodiment of the present preparation, the
number of
daily dosage units comprising the combination of drospirenone and
ethinylestradiol is 28,
or a multiple of 28 such as 2-4, in particular 2 or 3, times 28.
In an alternative embodiment, the invention relates to a contraceptive
preparation
consisting of a number of separately packaged and individually removable daily
dosage
units placed in a packaging unit and intended for oral administration for a
period of at least
28 consecutive days, wherein at least 21 of said daily dosage units comprises
a
combination of drospirenone in an amount of from about 2 mg to about 4 mg and
ethinylestradiol in an amount from about 0.01 to about 0.05 mg, and wherein 7
or less of
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
7
said daily dosage units contain ethinylestradiol alone in an amount from about
0.01 to
about 0.05 mg.
By including an appropriate number of dosage units comprising ethinylestradiol
alone,
high contraceptive reliability, low follicular development and satisfactory
cycle control with
little or no intermenstrual bleeding may be obtained.
In this case, too, the preparation may be one in which the amounts of either
active agent
remains constant for the entire at least 21-day period (i.e. a two-phase
preparation), or the
amounts of either or both active agents may be varied over the at least 21-day
period to
generate a multiple-phase preparation, e.g. a three- or four-phase
preparation,
substantially as disclosed in, e.g., EP 148 724. In case of multiple-phase
preparation, it
may be possible to include a natural estrogen such as estradiol, e.g. in an
amount of from
about 0.5 mg to about 4 mg per day, instead of ethinylestradiol.
In suitable embodiments of the present preparation, the number of daily dosage
units
comprising the combination of drospirenone and ethinylestradiol may be 21, 22,
23 or 24,
and the number of daily dosage units containing ethinylestradiol alone may
then be 7, 6, 5
or 4, as the case may be.
In one embodiment of the present method of inhibiting ovulation, the method
comprises
administering, to said mammal, on each day of at least 21 consecutive days, a
daily
dosage unit comprising a combination of drospirenone in an amount of from
about 2 mg to
about 4 mg and ethinylestradiol in an amount from about 0.01 to about 0.05 mg,
followed
by administering, on each day of 7 or less consecutive days, a daily dosage
unit
containing no active agent, or alternatively, administering no dosage units
for 7 days or
less.
In suitable embodiments of this method, the daily dosage units comprising the
combination of drospirenone and ethinylestradiol may be administered for 21,
22, 23 or 24
consecutive days, and the daily dosage units containing no active agent may
then be
administered for 7, 6, 5 or 4 consecutive days, as appropriate. Furthermore,
the daily
dosage units comprising the combination of drospirenone and ethinylestradiol
may be
administered for 28 consecutive days. In a variant of this embodiment, the
daily dosage
units comprising the combination of drospirenone and ethinylestradiol are
administered for
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
8
2-4, preferably 2 or 3, times 28 consecutive days, followed by administration
of the daily
dosage units comprising the combination of drospirenone and ethinylestradiol
for 21, 22,
23 or 24 consecutive days and subsequently administration of the daily dosage
units
containing no active agent, or administration of no daily dosage units, for 7,
6, 5 or 4
consecutive days.
Alternatively, the present method comprises administering, on each day of at
least 21
consecutive days, a daily dosage unit comprising a combination of drospirenone
in an
amount of from about 2 mg to about 4 mg and ethinylestradiol in an amount from
about
0.01 to about 0.05 mg, followed by administering, on each day of 7 or less
consecutive
days, a daily dosage unit containing ethinylestradiol alone in an amount of
from about
0.01 mg to about 0.05 mg.
In this alternative method, the daily dosage units comprising the combination
of
drospirenone and ethinylestradiol may suitably be administered for 21, 22, 23
or 24
consecutive days, and wherein the daily dosage units comprising
ethinylestradiol alone
may then be administered for 7, 6, 5 or 4 consecutive days, as appropriate.
In~a further
embodiment of the method, the daily dosage units comprising the combination of
drospirenone and ethinylestradiol are administered for 2-4, preferably 2 or 3,
times 28
consecutive days, followed by administration of the daily dosage units
comprising the
combination of drospirenone and ethinylestradiol for 21 consecutive days and
subsequently administration of the daily dosage units comprising
ethinylestradiol alone for
7 consecutive days.
For use according to the invention, the pharmaceutical preparation may
suitably be in the
form of a number of separately packaged and individually removable daily
dosage units
placed in a packaging unit and intended for oral administration for a period
of at least 21
consecutive days, wherein each of said daily dosage units each comprises a
combination
of drospirenone in an amount of from about 2 mg to about 4 mg and
ethinylestradiol in an
amount from about 0.01 to about 0.05 mg.
As indicated above, the preparation may further comprise 7 or less daily
dosage units
containing no active agent (or may contain 7 or less empty "places", e.g. in
the form of
empty blisters in a blister pack, marking the days on which no daily dosage
units are
administered).
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
9
Alternatively, the pharmaceutical preparation may be in the form of a number
of
separately packaged and individually removable daily dosage units placed in a
packaging
unit and intended for oral administration for a period of at least 28
consecutive days,
wherein at least 21 of said daily dosage units each comprises a combination of
drospirenone in an amount of from about 2 mg to about 4 mg and
ethinylestradiol in an
amount of from about 0.01 to about 0.05 mg, said packaging unit further
comprising 7 or
less daily dosage units comprising ethinylestradiol alone in an amount of from
about 0.01
mg to about 0.05 mg.
The composition of the invention may be formulated in any manner known in the
pharmaceutical art. In particular, as indicated above, the composition may be
formulated
by a method comprising providing drospirenone and, if desired,
ethinylestradiol in
micronized form in said unit dosage form, or sprayed from a solution onto
particles of an
inert carrier in admixture with one or more pharmaceutically acceptable
excipients that
promote dissolution of the drospirenone and ethinylestradiol so as to promote
rapid
dissolution of drospirenone and preferably ethinylestradiol on oral
administration.
Examples of suitable excipients include fillers, e.g. sugars such as lactose,
glucose or
sucrose, sugar alcohols such as mannitol, sorbitol or xylitol, starch such as
wheat, corn or
potato starch, modified starch or sodium starch glycolate, lubricants such as
talc,
magnesium stearate, calcium stearate, colloidal silica or stearic acid, and
binders such as
polyvinylpyrrolidone, cellulose derivatives, carboxymethyl cellulose,
hydroxypropyl
cellulose, hydroxypropylmethyl cellulose, methyl cellulose or gelatin, for
making oral
dosage forms such as tablets, pills or capsules.
Tablets may conveniently be coated with a suitable film-forming agent, e.g.
hydroxypropylmethyl cellulose, hydroxypropyl cellulose or ethyl cellulose, to
which a
suitable excipient may optionally be added, e.g. a softener such as glycerol,
propylene
glycol, diethylphthalate or glycerol triacetate, filler such as sucrose,
sorbitol, xylitol,
glucose or lactose, a colorant such as titanium hydroxide, etc.
The present composition may also be formulated in liquid form, e.g. as a
solution,
suspension or emulsion, together with conventional diluents or excipients in a
manner
known per se in the pharmaceutical art.
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
A packaging unit comprising the daily dosage units described above may be
prepared in a
manner analogous to that of making other oral contraceptives. This may for
instance be a
conventional blister pack or any other form known for this purpose, for
instance a pack
comprising the appropriate number of dosage units (in this case at least 21,
or for
5 particular applications, 28 or a multiple of 28) in a sealed blister pack
with a cardboard,
paperboard, foil or plastic backing and enclosed in a suitable cover. Each
blister container
may conveniently be numbered or otherwise marked, e.g. starting with the first
of the at
least 21 dosage units that contain the combination of drospirenone and
ethinylestradiol,
optionally followed by 7 or less empty blisters or by the 7 or less dosage
units that contain
10 no active agent or that only contain ethinylestradiol (although the
numbering may also
start with the first of the 7 or less dosage units that only contain
ethinylestradiol).
It is also envisaged that the present composition may be in the form of a
parenteral
formulation such as a subcutaneous implant or transdermal formulation. For
making
implants, the active agents may suitably be formulated together with one or
more
polymers that are gradually eroded or degraded when in use, e.g. silicone
polymers;
ethylene vinylacetate, polyethylene or polypropylene.
Where transdermal formulations are concerned, they may be prepared in the form
of
matrices or membranes or as fluid or viscous formulations in oil or hydrogels.
For
transdermal patches, an adhesive which is compatible with the skin should be
included,
such as polyacrylate, a silicone adhesive or polyisobutylene, as well as a
foil made of, e.g.
polyethylene, polypropylene, ethylene vinylacetate, polyvinylchloride,
polyvinylidene
chloride or polyester, and a removable protective foil made from, e.g.,
polyester or paper
coated with silicone or a fluoropolymer. For the preparation of transdermal
solutions or
gels, water or organic solvents or mixtures thereof may be used. Transdermal
gels may
furthermore contain one or more suitable gelling agents or thickeners such as
silicone,
tragacanth, starch or starch derivatives, cellulose or cellulose derivatives
or polacrylic
acids or derivatives thereof. Transdermal formulations may also suitably
contain one or
more substances that enhance absorption though the skin, such as bile salts or
derivatives thereof and/or phospholipids. Suitable transdermal formulations
may, for
instance, be made in a manner analogous to that described in WO 94/04157 for 3-
ketodesogestrel. Alternatively, transdermal formulations may be prepared
according to a
method disclosed in, e.g., BW Barry, "Dermatological Formulations,
Percutaneous
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
11
Absorption", Marcel Dekker Inc., New York - Basel, 1983, or YW Chien,
"Transdermal
Controlled Systemic Medications", Marcel Dekker Inc., New York - Basel, 1987.
The present invention is further described in the following examples which are
not in any
way intended to limit the scope of the invention as claimed.
EXPERIMENTAL
Example 1
Preparation of tablets containing drospirenone and ethinylestradiol
Tablet cores of the following composition
micronized drospirenone 3.00 mg
micronized ethinylestradiol 0.03 mg
lactose monohydrate 48.17 mg
corn starch 14.40 mg
modified starch 9.60 mg
polyvinylpyrrolidone 25,000 4.00 mg
magnesium stearate 0.80 mg
were prepared by charging a fluidized bed granulator with 31.68 kg corn
starch, 21.12 kg
modified starch, 6.60 micronized drospirenone, 0.066 kg micronized
ethinylestradiol and
105.974 kg lactose monohydrate and activating the fluidized bed. An aqueous
solution of
8.80 kg polyvinylpyrrolidone 25,000 in 46.20 kg purified water was sprayed
continuously
onto the fluidized bed while drying by heating the air stream of the fluidized
bed. At the
end of the process 1.76 kg magnesium stearate was sucked into the granulator
and mixed
with the granules by maintaining the fluidized bed. The resulting granulate
was pressed
into tablet cores by compression using a rotary tablet press.
2.22464 kg of hydroxypropylmethylcellulose and 0.44528 macrogol 6000 were
dissolved
in 14.67 kg purified water. 0.44528 kg talc, 1.22430 kg titanium dioxide and
0.06050 kg
ferric oxide pigment were suspended in 10.26 kg purified water with stirring
and
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
12
homogenized. The solution and suspension were combined and used to coat the
tablet
cores by continuous application of the coating suspension in a coater.
Example 2
Dissolution of drospirenone from tablets
The rate of dissolution of drospirenone from the tablets prepared in Example 1
was
determined by the USP XXIII Paddle Method using a USP Dissolution Test
Apparatus 2
including 6 covered glass vessels and 6 paddles. Tablets were placed in 900 ml
water at
a temperature of 37°C (~ 0.5°C) and stirred at 50 rpm.
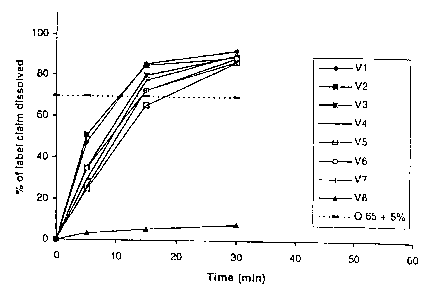
The results appear from Figs. 1, 2 and 4. From Fig. 1, it appears that the
batch numbered
V8 containing macrocrystalline drospirenone (but otherwise identical to the
tablets
prepared in Example 1 ) exhibited an extremely slow dissolution rate of
drospirenone,
whereas all batches containing micronized drospirenone exhibited a dissolution
rate of
more than 70% within 30 minutes.
Fig. 2 and Fig. 4 shows the results of dissolution of drospirenone from tablet
cores and
film-coated tablets, respectively. In both cases more than 70% of the active
agent is
dissolved within 30 minutes. Thus, the film coating did not significantly
influence the rate
of dissolution.
Example 3
Dissolution rate of ethinylestradiol from tablets in vitro
The rate of dissolution of ethinylestradiol from tablets prepared as described
in Example 1
was determined according to the USP Paddle Method as described in Example 2
for
drospirenone. The results appear from Figs. 3 and 5 showing the dissolution
rates from
tablet cores and film-coated tablets, respectively. In both cases, more than
70% of the
active agent was dissolved within 30 minutes. Thus, the film coating did not
significantly
influence the rate of dissolution.
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
13
Example 4
Bioavailability of drospirenone and ethinylestradiol from tablets containing 3
mg of
drospirenone and 0.03 mg ethinylestradiol
42 healthy women between 18 and 35 years of age were included in an open-label
crossover study after their informed consent had been obtained. The aim of the
study was
to investigate the relative bioavailability of drospirenone and
ethinylestradiol from a tablet
formulation containing 3 mg drospirenone and 0.03 mg ethinylestradiol with
reference to
an oral suspension containing 6 mg of drospirenone and 0.06 mg
ethinylestradiol per vial.
The bioavailability was determined using serum concentrations of each active
agent as
parameters. Compared to the oral suspension, the relative bioavailability of
drospirenone
and ethinylestradiol from the tablets is 107% and 117%, respectively. It was
therefore
concluded that both drospirenone and ethinylestradiol are completely released
from the
tablets in vivo.
The absolute bioavailability of drospirenone was determined in two studies to
be
76%~13% after oral administration of 2 mg drospirenone to 8 young healthy
women and
85%~24% after oral administration of a microcrystalline suspension containing
3.13 mg
drospirenone to 6 postmenopausal women.
The oral bioavaliability of ethinylestradiol was determined in several
studies, and mean
values of from 36% to 59% were reported in the literature, indicating a first-
pass effect.
Example 5
Contraceptive efficacy of formulations containing drospirenone and
ethinylestradiol
An open-label, randomized trial with 52 female volunteers aged 20-35 years
whose
informed consent had been obtained included 1 pre-treatment cycle, 3 treatment
cycles
with two different tablet containing 2 mg and 3 mg drospirenone, respectively,
but
otherwise corresponding to the tablets prepared in Example 1, and a follow-up
phase. A
wash-out phase of 1 month preceded the treatment.
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
14
At defined time points, selected central and peripheral parameters were
investigated: LH,
FSH, 17~i-estradiol, progesterone, cervical score, "spinnbarkeit", fern
phenomenon.
Ovarian function was checked by ultrasound. In addition, SHBG, CBG,
prolactine, total
testosterone, androstenedione, DHEA-S and selected metabolic parameters (serum
glucose, triglycerides, cholesterol, HDL, LDL) were examined. Blood pressure,
heart rate,
body weight and cycle control were documented.
The results of the study showed that both LH and FSH were clearly suppressed
with both
trial preparations. Accordingly, the secretion of estradiol and progesterone
were greatly
reduced over all three treatment cycles with the exception of 3 volunteers
receiving the 2
mg drospirenone preparation. This result was, in principle, confirmed by the
accompanying ultrasound examinations. Follicular ripening occurred in several
cases with
both trial preparations. Although three ovulations were diagnosed with the
preparation
containing 2 mg drospirenone (one of which was described as "equivocal" and
the other
as a "tablet-taking error"), no differences were demonstrable statistically (p
> 0.05)
between the two trial preparations as regards the hormones LH, FSH, estradiol
and
progesterone, and the parameter "ovulation during the treatment cycles". In
keeping with
the hormones, cervical function was greatly limited and the "spinnbarkeit" and
crystallisability of the cervical mucus was greatly reduced with both trial
preparations.
Prolactin increased minimally and SHBG and CBG distinctly with both
preparations.
Triglycerides and HDL levels increased with both trial preparations, while LDL
levels
decreased. Total cholesterol was largely unchanged in both treatment groups.
Oral
glucose tolerance remained virtually unchanged or was slightly decreased.
Testosterone,
androstenedione and DHEA-S decreased minimally.
The subjective and objective tolerance was good with both treatments. This was
also the
case for cycle control with the exception of the first cycle with 2 mg
drospirenone. Blood
pressure, heart rate and body weight remained constant in the majority of
cases or
showed a slight tendency to decrease.
After three months' treatment, it was concluded:
The two trial preparations were equally good as regards the subjective and
objective
tolerance.
CA 02382426 2002-02-26
WO 01/15701 PCT/IB00/01213
No negative metabolic effects were observed with either preparation. HDL was
influenced
positively in the sense of an increase.
The results confirmed the results of earlier studies that the 2 mg
drospirenone preparation
was in the threshold region of ovulation inhibition, whereas the 3 mg
drospirenone
5 preparation had a demonstrable ovulation-inhibiting effect in all cases
examined.