Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
QUINAZOLINES AND THEIR USE FOR INHIBITING CYCLIN-
DEPENDENT KINASE ENZYMES
FIELD OF THE INVENTION
This invention relates to quinazolines that inhibit cyclin-dependent kinase
enzymes, and as such are useful to treat cell proliferative diseases and
disorders,
such as cardiovascular diseases (i.e., atherosclerosis, restenosis), and
cancer,
infections (i.e, viral and fungal), autoimmune~diseases, organ transplant
rejections
(i.e., inflammation), gout, kidney disease, and also neurodegenerative
diseases and
disorders.
SUMMARY OF THE RELATED ART
Cyclin-dependent kinases and related serine/threonine protein kinases are
important cellular enzymes that perform essential functions in regulating cell
division and proliferation. The cyclin-dependent kinase catalytic units, of
which 9
have now been described, are activated by regulatory subunits known as
cyclins.
At least 16 mammalian cyclins have been identified (Johnson D.G. and Walker
C.L., Annu. Rev. Pharmacol. Toxicol. 1999;39:295-312). Cyclin B/Cdkl, Cyclin
A/Cdk2, Cyclin E/Cdk2, Cyclin D/Cdk4, Cyclin D/Cdk6, and probably other
heterodimers including Cdk3 and Cdk7 are important regulators of cell cycle
progression. Additional functions of Cyclin/Cdk heterodimers include
regulation
of transcription, DNA repair, differentiation and apoptosis (Morgan D.O.,
Annu.
Rev. Cell. Dev. Biol. 1997;1361-191).
Increased activity or temporally abnormal activation of cyclin-dependent
kinases has been shown to result in the development of human tumors (Sherr
C.J.,
Science 1996;274:1672-1677). Indeed, human tumor development is commonly
associated with alterations in either the Cdk proteins themselves or their
regulators
(Cordon-Cardo C., Am. J. Pathol. 1995;147:545-560; Karp J. E. and Broder S.,
Nat. Med. 1995;1:309-320; Hall M. et al., Adv. Cancer Res. 1996;68:67-108).
These and other observations have prompted an intensive search for small
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-2-
molecule Cdk inhibitors. Strong validation for this approach is provided by
studies
of naturally occurring protein inhibitors of Cdks (Kamb A., Curr. Top.
Microbiol.
Immunol. 1998;227:139-148). Introduction of p16 into lung cancer cell lines
blocked entry into S phase and caused growth inhibition (Jin X. et al., Cancer
Res.
1995;55:3250-3253; Chintaia S.K. et al., Oncogene 1997;15:2049-2057). Other
studies demonstrated a similar effect for p27 (Craig C. et al., Oncogene
1997;14:2283-2289). Ectopic expression of the Cdk inhibitors p21 or p27 in
U937
human leukemia cells resulted in growth arrest and cellular differentiation
(Liu et
al., Genes Dev., 1996;10:142-153). Markers of differentiation also were
observed
in the non-small lung cancer cell line NCI-H358 following expression of
antisense
Cdk2. Expression of antisense cyclin D 1 in human esophageal cancer cells
resulted in growth inhibition (Zhou P. et al., Oncogene, 1995;11:571-580).
Further support for targeting Cdk2 with therapeutic agents is found in the
work of Chen et al., Proc. Natl. Acad. Sci. (USA) 1999;96:4325-4329, who
demonstrated selective killing of transformed U20S cells containing
deregulated
E2F using peptide inhibitors of Cyclin A/Cdk2. Untransformed cell lines were
unaffected by these inhibitors. Thus, Cdk inhibitors potentially could
demonstrate
efficacy in vivo with a useful margin of safety.
In addition to treating cancer, Cdk inhibitors also may have potential
applications in the treatment of cardiovascular disorders such as restenosis
and
atherosclerosis. Vascular smooth muscle proliferation and intimal hyperplasia
following balloon angioplasty are inhibited by over-expression of the cyclin-
dependent kinase inhibitor protein p21 (Chang M.W. et al., J. Clin. Invest.,
1995;96:2260; Yang Z-Y. et al., Proc. Natl. Acad. Sci. (USA) 1996;93:9905.
Moreover, intraluminal exposure of a denuded rat carotid artery to the purine
Cdk2 inhibitor CVT-313 (Ki = 95 nM) resulted in greater than 80% inhibition of
neointima formation (Brooks E.E. et al., J. Biol. Chem. 1997:29207-29211).
Taken together, these observations support the use of small molecule
inhibitors of
cell cycle progression in the treatment of vascular disorders that are due to
aberrant cell proliferation.
The neuronal Cdc2-like kinase, known as CdkS, together with its brain
specific activator protein p35/p25, phosphorylates the neuron-specific
microtubule-associated protein tau (Lew J. and Wang J.H., Trends Biochem. Sci.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-3-
1995;20:33-37). Aberrant expression of CdkS is proposed to contribute to the
neurodegenerative disorder Multiple System Atrophy (Nakamura S. et al.,
J. Neuropathol. Exp. Neurol. 1998;57:690) and tau hyperphosphorylation has
long
been associated with the pathogenesis of Alzheimer's Disease (AD) (Spillantini
M.G. and Giedert M., Trends Neurosci. 1998;21:428-433). In addition to amyloid
plaques, neurofibrillary tangles (NFTs) are a primary marker for AD. The major
component of NFTs are paired helical filament (PHF)-tau, which is a
filamentous
aggregate of hyperphosphorylated tau. The "NFT load" in the brains of
Alzheimer's Disease (AD) patients is strongly correlated with severity of the
disease. This correlation is stronger than that seen for A(3-rich amyloid
plaques.
Abnormal activation of protein kinases (cyclin-dependent kinase 5 (CdkS),
glycogen synthase kinase-3 [3 (GSK-3 (3)), and protein phosphatases (type 2A
and
2B), has been implicated in tau hyperphosphorylation and pathological
activation
of CdkS is likely a major contributor to PHF-tau.
CdkS is an important therapeutic target because its activator protein p35 is
specifically localized in central and peripheral neurons (Tsai J.-H. et al.,
Nature
1984;371:419-423). Indeed the abnormal deposition of amyloid beta peptide
(from
the APP gene) and hyperphosphorylated tau may be combined factors that lead to
early onset AD (Mandelkow E.-M. and Mandelkow E., Trends Cell Biol.
1998;8:425-427). There is growing evidence that abnormally processed tau also
is
associated with other CNS diseases. Recently, mutations in the tau gene on
chromosome 14 have been linked to fronto-temporal dementia with Parkinsonism
(FTDP-17) and progressive supernuclear palsy (PSP) (Spillantini M.G. and
Giedert M., Trends Neurosci. 1998;21:428-433).
Further evidence supporting the role of CdkS in tauopathy and
Alzheimer's disease include the following observations. (1) Increased
CdkS activity has been found in AD brains (Pei J.J. et al., Brain Res.
1998;797:267-77; Lee K.Y. et al., Neurosci Res. 1999;34:21-9). (2) PHF-Tau
found in AD brains has 8 phosphorylation sites that overlap with the sites of
cdk5 phosphorylation (Paudel H.K. et al., J. Biol. Chem. 1993;268:23512-23518;
Baumann K. et al., FEBS Lett. 1993;336:417-424). (3) Synergy among the
different tau protein kinases eg, CdkS-phosphorylated tau is more readily
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-4-
phosphorylated by GSK3 (Sengupta A., et al., Mol. Cell. Biochem. 1997;
167:99-1 OS).
Other diseases in which Cdk inhibitors could find application include those
caused by a variety of infectious agents, including DNA and RNA viruses. For
S example, cyclin-dependent kinases are required for viral replication
following
infection by herpes simplex virus (HSV) (Schang L.M. et al., J. Virol.
1998;72:5626). HSV replication was inhibited by the cyclin-dependent kinase
inhibitors roscovitine and olomoucine but not by a cell cycle inhibitor that
does
not inhibit cyclin-dependent kinase activity. Inactivation of the Cdk
inhibitor
protein p 16 by the viral protein Tax, and inactivation of p27 by viral E 1 A,
have
both been demonstrated to overcome cell cycle suppression and to promote cell
immortalization and the transformed phenotype (Mal A. et al., Nature
1996;380:262-265; Suzuki T. et al., EMBO J. 1996;15:1607-1614; Parker G.A.
et al., Oncogene 1996;13:2541-2549). Cdk2 also has been implicated in the
progression of cytomegalovirus infections (Bresnahan W.A. et al., Virology
1997;231:239-247; Albrecht T. et al., WO 98/39007). Fungal infections may be
expected to be susceptible to Cdk inhibitors on the basis of the known
essential
roles of Cdk homologs in yeast.
It is further possible that various autoimmune disorders may succumb to
treatment with selective Cdk inhibitors. The chronic inflammatory disease
rheumatoid arthritis is characterized by synovial tissue hyperplasia;
inhibition of
synovial tissue proliferation should minimize inflammation and prevent joint
destruction. Consistent with this idea, expression of the Cdk inhibitor
protein
p16 in synovial fibroblasts led to growth inhibition (Taniguchi K. et al.,
Nat. Med.
1999;5:760-767). Similarly, in a rat model of arthritis, joint swelling was
substantially inhibited by treatment with a p16 expressing adenovirus. By
extension, Cdk inhibitors may be effective against other disorders of cell
proliferation including psoriasis (characterized by keratinocyte
hyperproliferation)
and possibly lupus.
Distinct from gene therapy approaches, many research groups are pursuing
small molecule inhibitors of cyclin dependent kinases. Small molecule
inhibitors
of Cdks that have been published to date include purines such as olomoucine
(Vesely et al., Eur. J. Biochem. 1994;224:771-786) and roscovitine (Meijer L.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-5-
et al., Eur. J. Biochem. 1997;243:527-536), butyrolactone, staurosporine and
UCN-O1, suramin, 9-hydroxyellipticine, and several flavonoids including
flavopiridol (Garrett M.D. and Fattaey A., Curr. Opin. Genes & Develop.
1999;9:104-111; Walker D.H., Curr. Top. Microbio. Immunol. 1998:148-165;
Meijer L., Meths. In Enzymol. 1997;223:113-128). Of these inhibitors, most
information is available for flavopiridol.
Flavopiridol is currently in phase II clinical trials for the treatment of
metastatic renal cell carcinoma. It is a relatively nonspecific inhibitor of
Cdks and
produces cell cycle blocks at G1 and G2. Nonetheless, it is a potent inhibitor
of
several breast and lung cancer cell lines (Sedlacek H.H. et al., Int. J.
Oncol.
1996:1143-1168; Kaur et al., J. Natl. Cancer Inst. 1992;84:1736-1740; Bible
K.C.
and Kaufman S.H., Cancer Res. 1996;56:4856-4861) and displays growth
inhibitory activity against tumors in vivo (Patel V. et al., J. Clin. Invest.
1998;102:1674-1681; Drees M. et al., Clin. Cancer Res. 1997;3:273-279;
Wright J. et al., Oncology 1998;12:1018, 1023-1014; Senderowicz A.M. et al.,
J. Clin. Oncol. 1998;16 2988-2999). Flavopiridol analogs have been described
by
Mitotix (International patent application WO 97/16447; US Patent 5,733,920) as
well as by Bristol-Myers Squibb (WO 97/42949).
The purine class of Cdk inhibitors have been extensively studied in vitro,
generally displaying most potency against Cdkl, Cdk2 and CdkS. Analogs of
olomoucine have been synthesized by Meijer and coworkers (Legraverand M.
et al., Bioor. Med. Chem. 1999;7:1281-1293 and references therein), Schultz
and
coworkers (Chang Y-T. et al., Chem. & Biol. 1999;6:361-375), Griffin et al.
(International patent application WO 99/02162) and Mackman et al., (US Patent
5,866,702; WO 98/05335), among others. One of these compounds has been
shown to inhibit neointimal formation in an animal model of restenosis (see
above).
Other classes of small molecule Cdk inhibitors described in the patent
literature include aminothiazoles developed independently by Agouron
(WO 99/21845) and by Bristol-Myers Squibb (WO 99/24416); indolinones, that
have been developed by Sugen (WO 98/50356) and by Glaxo (WO 99/24416);
benzothiadiazines (WO 98/49146); peptides (WO 97/11174);
pyrazolo[3,4-b]pyridines (WO 99/30710); and phenylaminopyrimidines (Novartis,
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-6-
unpublished results). Several of these structural classes of protein kinase
inhibitors
have been reviewed by McMahon et al., Curr. Opin. Drug. Discovery & Develop.
1998;1:131-146). Very little in vitro data and no clinical data are available
for any
of these compounds, and there is still a need for more potent and selective
Cdk
inhibitors with good pharmacokinetic properties that can be employed not just
as
in vitro tools but also in vivo.
We have previously described a class of pyrido[2,3d]pyrimidines that
display selectivity for Cdks versus other protein kinases (WO 98/33798). These
compounds are distinct from the 6-aryl-pyrido[2,3d]pyrimidines (WO 96/15128;
WO 96/34867) which display the opposite selectivity, preferring tyrosine
kinases
over cyclin-dependent kinases. Moreover, they represent a new structural class
when compared to either the pyrimidines and 3,4-dihydropyrimidines of
International Patent application, PCT/US99/10187, filed May 10, 1999, or the
naphthyridones described in international patent application WO 99/09030.
Despite the progress that has been made, the search continues for small
molecular weight compounds that are orally bioavailable and useful for
treating a
wide variety of cell proliferative diseases and disorders, cancer, infections,
autoimmune diseases, gout, kidney disease, and neurodegenerative diseases and
disorders.
SUMMARY OF THE INVENTION
This invention provides quinazolines that are useful for treating cell
proliferative disorders, such as vascular smooth muscle proliferation
associated
with atherosclerosis, postsurgical vascular stenosis and restenosis, and
endometriosis.
This invention also provides quinazolines that are useful for treating
infections, including viral infections such as DNA viruses like herpes and RNA
viruses like HIV, and fungal infections.
This invention further provides quinazolines that are useful for treating
autoimmune diseases such as psoriasis, inflammation like rheumatoid arthritis,
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
_7_
lupus, type 1 diabetes, diabetic nephropathy, multiple sclerosis, and
glomerulonephritis, organ transplant rejection, including host versus graft
disease.
This invention further provides quinazolines that are useful for treating and
neurodegenerative disorders such as Alzheimer's disease.
This invention further provides quinazolines that are useful for treating
cancer.
This invention further provides quinazolines that are useful for treating
gout.
This invention further provides quinazolines that are useful for treating
kidney disease, such as polycystic kidney disease.
The above-identified methods of treatment are preferably carried out by
administering a therapeutically effective amount of a compound of Formulas I
and/or II (set forth below) to a subject in need of treatment. It has been
discovered that a group of 2-arylamino-7-(alkyl)oxy-8-alkylquinazolines and 8-
alkyl-2-arylamino-quinazoline 2,7-diamines are potent inhibitors of
cyclin-dependent kinases (cdks). The compounds are readily synthesized and can
be administered by a variety of routes, including orally and parenterally, and
have
little or no toxicity. The compounds of the invention are members of the class
of
compounds of Formulas I and II, which will be described further below
This invention also provides pharmaceutical formulations comprising a
compound of Formulas I or II together with a pharmaceutically acceptable
carrier,
diluent, or excipient therefor.
Compounds within the scope of the present invention are inhibitors of the
cyclin-dependent kinases such as cdkl, cdk2, cdk4, and cdk5.
DETAILED DESCRIPTION OF THE INVENTION
A new class of compounds have been discovered that are potent inhibitors
of cyclin-dependent kinases (cdks) and are useful agents for treating subjects
suffering from diseases caused by abnormal cell proliferation. Compounds
within
the scope of the present invention are inhibitors of the cyclin-dependent
kinases
such as cdkl, cdk2, cdk4, and cdk5. As inhibitors of cyclin-dependent kinases,
the
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
_g_
compounds of the instant invention are useful in controlling proliferative
diseases
and disorders, cancer, infections, autoimmune diseases, gout, kidney disease,
and
neurodegenerative diseases and disorders.
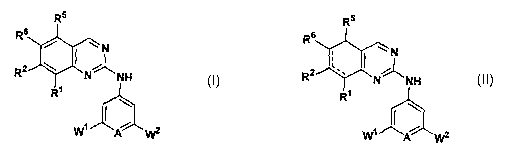
The compounds of the invention comprise those of Formula I:
R5
Rs
~~ N
R2 \ N"NH
R~
W~ A W2
wherein,
R~ is hydrogen, alkyl, alkyl substituted with at least one of amine, halogen,
hydroxy, or alkoxy, cycloalkyl,or heterocycloalkyl;
RZ is OH, alkyloxy, aryloxy, or NR'R~;
A is N, or CW3;
R3 and R4 are independently hydrogen, alkyl, alkenyl, alkynyl, (CH2)"Ar,
cycloalkyl, heterocycloalkyl, or heteroaryl, or R3 and R4 together with the
nitrogen to which they are attached optionally may form a ring having 3 to
7 carbon atoms and said ring optionally contains 1, 2 or 3 heteroatoms
selected from the group consisting of nitrogen, substituted nitrogen,
oxygen and sulfur including S(O) and S(O)Z. Said ring also may be
additionally substituted with up to 3 groups selected from alkyl, haloalkyl,
NRgC(O)R9, C(O)ORg, C(O)Rg, C(O)NR8R9, NRgR9, NRxSO2R9, ORg,
SO,NR:;R'~, or SRB;
W~ and WZ are independently selected from NR3R4, N(O)R3R4, NR3R4RgX, OR3,
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-9-
SR3, hydrogen, halogen, haloalkyl, COR3, COZR3, CONR3R4, S(O)R3,
SOzR3, SOZNR3R4, S03R3, P(O)(OR3)~, aldehyde, nitrile, nitro, alkyl,
T(CHZ)mQRg, C(O)T(CHZ)mQRg, or T(CHZ)mCO2R$ where m is 1-6, T is
O, S, NR3, N(O)R3, NR3R4X, or CR3R4, and Q is O, S, NR9, N(O)R9 or
NR9R'°X, or NR9C(O)T(CHZ)",QR9;
RS is hydrogen, or alkyl;
R6 is hydrogen, alkyl, halogen, haloalkyl, alkynyl, alkenyl, COR3, COZR3,
CONR3R4, SOZNR3R4, SOZR3, S03R3, P(Oj(OR;)~, aldehyde, nitrile, nitro,
OR3, or NR3R4
W3 is NR3R4, N(O)R3R4, NR3R4R8X, OH, OR3, SH, SR3, halogen, COR3, COZR3,
CONR3R4, S(O)R3, SOZR3, SOZNR3R4, SO3R3, (CH~)"P(O)(OR3)z,
NR3SOZR4, aldehyde, nitrile, nitro, alkyl, T(CHZ)mQRB, C(O)T(CHZ)mQRB,
NRBC(O)T(CHZ)",QR'', or T(CHZ)~"CO~R' where m and n are
independently 1-6, T is O, S, NR3, N(O)R3, NR3R4W, or CR3R4, and Q is
O, S, NR9, N(O)R9 or NR9R'°X;
R~, R~, R'° and R" are hydrogen, alkyl, or aryl;
X is a halogen;
or a pharmaceutically acceptable salt, ester, amide or pro-drug thereof.
The compounds of the invention further comprise those of Formula II:
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-10-
R5
Rs
~N
i ~
RZ N- 'NH
R~
W~ A W2
wherein,
the dotted line represents a bond at the CS-C6, C6-C~, or C~-C~ position.
R' is hydrogen, alkyl, alkyl substituted with at least one of amine, halogen,
hydroxy, or alkoxy, cycloalkyl,or heterocycloalkyl;
RZ is OH, alkyloxy, aryloxy, or NR3R'~;
A is N, or CW3;
R3 and R4 are independently hydrogen, alkyl, alkenyl, alkynyl, (CHZ)"Ar,
cycloalkyl, heterocycloalkyl, or heteroaryl, or R3 and R4 together with the
nitrogen to which they are attached optionally may form a ring having 3 to
7 carbon atoms and said ring optionally contains 1, 2 or 3 heteroatoms
selected from the group consisting of nitrogen, substituted nitrogen,
oxygen and sulfur including S(O) and S(O)2. Said ring also may be
additionally substituted with up to 3 groups selected from alkyl, haloalkyl,
NR~C(O)R9, C(O)ORg, C(O)R8, C(O)NRgR9, NR~R~, NRgSO2R9, OR8 ,
SO~NR'R~, or SRg;
W1 and WZ are independently selected from NR3R4, N(O)R3R4, NR3R'~RgX, OR3,
SR3, hydrogen, halogen, haloalkyl, COR3, COZR3, CONR3R4, S(O)R3,
SOZR3, SOZNR3R4, S03R3, P(O)(OR')~, aldehyde, nitrite, nitro, alkyl,
T(CHZ)",QRB, C(O)T(CHZ)mQRg, or T(CHZ)mCOZRB where m is 1-6, T is
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
O, S, NR3, N(O)R3, NR3R4X, or CR3R4, and Q is O, S, NR9, N(O)R9 or
NR9R'°X, or NR9C(O)T(CHZ)",QR9;
RS is hydrogen, or alkyl;
R6 is hydrogen, alkyl, halogen, haloalkyl, alkynyl, alkenyl, COR3, COZR3,
CONR3R4, SOZNR3R4, SOZR3, S03R3, P(O)(OR~)~, aldehyde, nitrile, nitro,
OR3, or NR3R4
W3 is NR3R4, N(O)R3R4, NR3R4RBX, OH, OR3, SH, SR3, halo, COR3, COZR3,
CONR3R4, S(O)R3, SOZR3, SOZNR3R4, SO3R3, (CHz)~P(O)(OR3)Z,
NR3SOZR4, aldehyde, nitrite, nitro, alkyl, T(CHZ)mQRB, C(O)T(CH2)mQRB,
NRBC(O)T(CHZ)mQR", or T(CHZ)",COZR3 where n and m independently
are 1-6; T is O, S, NR3, N(O)R3, NR3R4W, or CR3R4; and Q is O, S, NR9,
N(O)R9 or NR9R~°X;
RB, R9, R'° and R" are hydrogen, alkyl, or aryl;
X is a halogen;
or a pharmaceutically acceptable salt, ester, amide or pro-drug thereof.
Preferred examples of W3 include piperidine; piperidine substituted with
up to 2 groups consisting of alkyl, haloalkyl, OR9, (CHZ)~OR9, COZR9,
CONR9R'°, NR9R'°; 3-aminopyrrolidine, 3-
acylaminopyrrolidine; 3-
alkylaminopyrrolidine, 3-aminoalkylpyrrolidine; pyrrolidine substituted with
up to
3 groups selected from alkyl, haloalkyl, NRBC(O)R9, C(O)NRBR9, NRBR~, ORB;
piperazine; N acylpiperazine; N aminoacylpiperazine; piperazine substituted
with
up to five groups selected from alkyl, haloalkyl, ORB, (CHZ)"ORB, COZRB,
NRBC(O)R9, C(O)NRBR9, NRBR9, SOZR, SOZNRBRv; piperazine substituted on
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-12-
nitrogen with a group selected from alkyl, haloalkyl, ORB, (CHZ)"ORB, COZRB,
CONRBR9, NRBR~, SOZR, SOZNRBR9; homopiperazine; N acylhomopiperazine; N
alkylhomopiperazine; N haloalkylhomopiperazine; tetrahydro-1,4-pyrimidone.
Especially preferred groups include N acetylpiperazine, 3,5-
dimethylpiperazine,
3,3,4-trimethylpiperazinyl, N methylpiperazine, homopiperazine, N
acetylhomopiperazine, N tent-butylacetylpiperazine, N pivaloylpiperazine, N-
leucylpiperazine, N isoleucylpiperazine; N valylpiperazine, N-
prolylpiperazine,
N alanylpiperazine, and N glycylpiperazine.
A preferred group of compounds of Formulas I and II have the above
formulas wherein R1 is alkyl.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein R1 is isopropyl.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein R' is cycloalkyl.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein R' is cyclopentyl.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein RZ is hydroxy.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein RZ is alkyloxy.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein RZ is methoxy.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein RZ is NR3R4.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-13-
Another preferred group of compounds of Formulas I and II have the
above formulas wherein A is CW3.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein W3 is piperidine.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein W3 is substituted piperidine.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein W3 is pyrolidine.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein W3 is substituted pyrolidine.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein W3 is piperazine
Another preferred group of compounds of Formulas I and II have the
above formulas wherein W3 is substututed piperazine.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein W3 is hydrogen.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein W2 and W3 are hydrogen.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein RS is hydrogen.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein RS is alkyl.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein R6 is hydrogen.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein R6 is alkyl.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-14-
Another preferred group of compounds of Formulas I and II have the
above formulas wherein W1, W2, and W3 are hydrogen.
Another preferred group of compounds of Formulas I and II have the
above formulas wherein RS and R6 are hydrogen.
A particularly preferred group of compounds of Formulas I and II have the
above formulas wherein W1, W2, and W3 are hydrogen, and R1 is alkyl.
Another particularly preferred group of compounds of Formulas I and II
have the above formulas wherein W1, W2, and W3 are hydrogen, and R1 is
cycloalkyl.
A further particularly preferred group of compounds of Formulas I and II
have the above formulas wherein W3 is piperidine or substituted piperidine,
and
R1 is alkyl or cycloalkyl.
A further particularly preferred group of compounds of Formulas I and II
have the above formulas wherein W3 is pyrrolidine or substituted pyrrolidine,
and
R1 is alkyl or cycloalkyl.
A further particularly preferred group of compounds of Formulas I and II
have the above formulas wherein W3 is piperazine or substituted piperazine,
and
R1 is alkyl or cycloalkyl.
A further particularly preferred group of compounds of Formulas I and II
have the above formulas wherein W3 is piperidine or substituted piperidine, RZ
is
alkyloxy, and R1 is alkyl or cycloalkyl.
A further particularly preferred group of compounds of Formulas I and II
have the above formulas wherein W3 is pyrrolidine or substituted pyrrolidine,
RZ
is alkyloxy, and R1 is alkyl or cycloalkyl.
A further particularly preferred group of compounds of Formulas I and II
have the above formulas wherein W3 is piperazine or substituted piperazine, RZ
is
alkyloxy, and R1 is alkyl or cycloalkyl.
The following compounds illustrate specific embodiments provided by the
present invention, and the compounds listed below are among the preferred
embodiments:
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-15-
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-phenyl-amine;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-phenyl-amine;
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-(4-piperidin-1-yl-phenyl)-amine;
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-(4-pyrrolidin-1-yl-phenyl)-
amine;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(4-piperidin-1-yl-pheny1)-
amore;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(4-pyrrolidin-1-yl-phenyl)-
amore;
I 0 8-Isopropyl-2-phenylamino-quinazolin-7-ol;
4-[4-(8-Isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-piperazine- I -
carboxylic acid tert-butyl ester;
4-[4-(8-Cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
piperazine-I-carboxylic acid tert-butyl ester;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(4-piperazin-1-yl-pheny1)-
amine;
8-Cyclopentyl-2-(4-piperazin-I-yl-phenylamino)-quinazolin-7-ol;
2-Amino-1-{4-[4-(8-cyclopentyl-7-methoxy-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-ethanone;
8-Isopropyl-2-(4-piperazin-1-yl-phenylamino)-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-(4-piperazin-1-yl-phenyl)-amine;
2-Amino-1-{4-[4-(8-cyclopentyl-7-hydroxy-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl }-ethanone;
2-Amino-I-{4-[4-(8-isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl}-ethanone;
2-Amino-1-{ 4-[4-(7-hydroxy-8-isopropyl-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl}-ethanone;
2-Amino-1-{4-[4-(8-cyclopentyl-7-methoxy-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl } -4-methyl-pentan- I -one;
2-Amino-1-{ 4-[4-(8-cyclopentyl-7-hydroxy-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl }-4-methyl-pentan-1-one;
2-Amino-1-{4-[4-(8-isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl }-4-methyl-pentan- I -one;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-16-
2-Amino-1-{4-[4-(7-hydroxy-8-isopropyl-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl } -4-methyl-pentan-1-one;
N-(4-{4-[4-(8-cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl } -4-oxo-butyl)-acetamide;
N-(4-{4-[4-(8-Cyclopentyl-7-hydroxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl } -4-oxo-butyl)-acetamide;
N-(4-{4-[4-(8-isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl } -4-oxo-butyl)-acetamide;
N-(4-{4-[4-(7-Hydroxy-8-isopropyl-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl}-4-oxo-butyl)-acetamide;
8-Isopropyl-2-(pyridin-4-ylamino)-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-pyridin-4-yl-amine;
(8-cyclopentyl-7-methoxy-quinazolin-2-yl)-pyridin-4-yl-amine;
8-cyclopentyl-2-(pyridin-4-ylamino)-quinazolin-7-ol;
1 S (8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-[4-(5-methyl-hexahydro-
pyrrolo[3,4-c]pyrrol-2-yl)-phenyl]-amine;
8-Cyclopentyl-2-[4-(5-methyl-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-
phenylamino]-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-[4-(5-methyl-hexahydro-
pyrrolo[3,4-c]pyrrol-2-yl)-phenyl]-amine;
8-Isopropyl-2-[4-(5-methyl-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-
phenylamino]-quinazolin-7-ol;
[4-(3-Amino-pyrrolidin-1-yl)-phenyl]-(8-cyclopentyl-7-methoxy-
quinazolin-2-yl)-amine;
2-[4-(3-Amino-pyrrolidin-1-yl)-phenylamino]-8-cyclopentyl-quinazolin-
7-0l;
[4-(3-Amino-pyrrolidin-1-yl)-phenyl]-(8-isopropyl-7-methoxy-quinazolin-
2-yl)-amine;
2-[4-(3-Amino-pyrrolidin-1-yl)-phenylamino]-8-isopropyl-quinazolin-
7-0l;
(4-Fluoro-3-trifluoromethyl-phenyl)-(8-isopropyl-7-methoxy-quinazolin-
2-yl)-amine;
2-(4-Fluoro-3-trifluoromethyl-phenylamino)-8-isopropyl-quinazolin-7-ol;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-17-
(4-Fluoro-3-trifluoromethyl-phenyl)-(8-cyclopentyl-7-methoxy-
quinazolin-2-yl)-amine;
2-(4-Fluoro-3-trifluoromethyl-phenylamino)-8-cyclopentyl-quinazolin-
7-0l;
N-{ 1-[4-(8-Cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
pyrrolidin-3-yl}-acetamide;
N-{ 1-[4-(8-Cyclopentyl-7-hydroxy-quinazolin-2-ylamino)-phenyl]-
pyrrolidin-3-yl } -acetamide;
N-{ 1-[4-(8-Isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
pyrrolidin-3-yl}-acetamide;
N-{ 1-[4-(8-Isopropyl-7-hydroxy-quinazolin-2-ylamino)-phenyl]-
pyrrolidin-3-yl}-acetamide;
1-{4-[4-(8-Isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-piperazin-
1-yl } -ethanone;
1-{4-[4-(7-Hydroxy-8-isopropyl-quinazolin-2-ylamino)-phenyl]-piperazin-
1-yl }-ethanone;
{4-[4-(8-Cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl}-pyrrolidin-2-yl-methanone;
{4-[4-(8-Cyclopentyl-7-Hydroxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl}-pyrrolidin-2-yl-methanone;
1-{4-[4-(8-Cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl }-ethanone;
1-{4-[4-(8-Cyclopentyl-7-hydroxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl} -ethanone;
{4-[4-(8-Isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-piperazin-1-
y1 }-pyrrolidin-2-yl-methanone;
{4-[4-(7-Hydroxy-8-isopropyl-quinazolin-2-ylamino)-phenyl]-piperazin-1-
y1 }-pyrrolidin-2-yl-methanone;
2-Amino-1-{4-[4-(8-isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl}-propan-1-one;
2-Amino-1- { 4-[4-(7-hydroxy-8-isopropyl-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl }-propan-1-one;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-18-
2-Amino-1- {4-[4-(8-cyclopentyl-7-methoxy-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-propan-1-one;
2-Amino-1-{4-[4-(8-cyclopentyl-7-hydroxy-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-propan-1-one;
1- {4-[4-(8-Cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
piperazin-I-yl}-propan-1-one;
1-{4-[4-(8-Cyclopentyl-7-hydroxy-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl}-propan-I-one;
1-{4-[4-(8-Isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-piperazin-
1-yl }-propan-1-one;
1-{4-[4-(7-Hydroxy-8-isopropyl-quinazolin-2-ylamino)-phenyl]-piperazin-
1-yl}-propan-1-one;
8-Isopropyl-2-phenylamino-5,6-dihydro-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-phenyl-amine;
8-Isopropyl-2-phenylamino-5,8-dihydro-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-phenyl-amine;
8-Cyclopentyl-2-phenylamino-5,6-dihydro-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-phenyl-amine;
8-Cyclopentyl-2-phenylamino-5,8-dihydro-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-phenyl-amine;
8-Isopropyl-2-(4-pyrrolidin-1-yl-phenylamino)-5,6-dihydro-quinazolin-
7-0l;
(8-Isopropyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-(4-pyrrolidin-I-yl-
phenyl)-amine;
8-Isopropyl-2-(4-pyrrolidin-1-yl-phenylamino)-5,8-dihydro-quinazolin-
7-0l;
(8-Isopropyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-(4-pyrrolidin-1-yl-
phenyl)-amine;
8-Cyclopentyl-2-(4-pyrrolidin-1-yl-phenylamino)-5,6-dihydro-quinazolin-
7-0l;
(8-Cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-(4-pyrrolidin- I -
yl-phenyl)-amine;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-19-
8-Cyclopentyl-2-(4-pyrrolidin-1-yl-phenylamino)-5,8-dihydro-quinazolin-
7-0l;
(8-Cyclopentyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-(4-pyrrolidin-1-
yl-phenyl)-amine;
8-Isopropyl-2-(4-piperazin-1-yl-phenylamino)-5,6-dihydro-quinazolin-
7-0l;
(8-Isopropyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-(4-piperazin-1-yl-
phenyl)-amine;
8-Isopropyl-2-(4-piperazin-1-yl-phenylamino)-5,8-dihydro-quinazolin-
7-0l;
(8-Isopropyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-(4-piperazin-1-yl-
phenyl)-amine;
8-Cyclopentyl-2-(4-piperazin-1-yl-phenylamino)-5,6-dihydro-quinazolin-
7-0l;
(8-Cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-(4-piperazin-1-yl-
phenyl)-amine;
8-Cyclopentyl-2-(4-piperazin-1-yl-phenylamino)-5, 8-dihydro-quinazolin-
7-0l;
(8-Cyclopentyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-(4-piperazin-1-yl-
phenyl)-amine;
2-(4-Fluoro-3-methyl-phenylamino)-8-isopropyl-5,6-dihydro-quinazolin-
7-0l;
(4-Fluoro-3-methyl-phenyl)-(8-isopropyl-7-methoxy-5,6-dihydro-
quinazolin-2-yl)-amine;
2-(4-Fluoro-3-methyl-phenylamino)-8-isopropyl-5,8-dihydro-quinazolin-
7-0l;
(4-Fluoro-3-methyl-phenyl)-(8-isopropyl-7-methoxy-5,8-dihydro-
quinazolin-2-yl)-amine;
8-Cyclopentyl-2-(4-fluoro-3-methyl-phenylamino)-5,6-dihydro-
quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-(4-fluoro-3-
methyl-phenyl)-amine;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-20-
8-Cyclopentyl-2-(4-fluoro-3-methyl-phenylamino)-5,8-dihydro-
quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-(4-fluoro-3-
methyl-phenyl)-amine;
8-Isopropyl-2-(pyridin-4-ylamino)-5,6-dihydro-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-pyridin-4-yl-amine;
8-Isopropyl-2-(pyridin-4-ylamino)-5,8-dihydro-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-pyridin-4-yl-amine;
8-Cyclopentyl-2-(pyridin-4-ylamino)-5,6-dihydro-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-pyridin-4-y1-
amine;
8-Cyclopentyl-2-(pyridin-4-ylamino)-5,8-dihydro-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-pyridin-4-y1-
amore;
2-[4-(3-Amino-pyrrolidin-1-yl)-phenylamino]-8-isopropyl-5,6-dihydro-
quinazolin-7-ol;
[4-(3-Amino-pyrrolidin-1-yl)-phenyl]-(8-isopropyl-7-methoxy-5,6-
dihydro-quinazolin-2-yl)-amine;
2-[4-(3-Amino-pyrrolidin-1-yl)-phenylamino]-8-isopropyl-5,8-dihydro-
quinazolin-7-ol;
[4-(3-Amino-pyrrolidin-1-yl)-phenyl]-(8-isopropyl-7-methoxy-5,8-
dihydro-quinazolin-2-yl)-amine;
2-[4-(3-Amino-pyrrolidin-1-yl)-phenylamino]-8-cyclopentyl-5,6-dihydro-
quinazolin-7-ol;
[4-(3-Amino-pyrrolidin- I -yl)-phenyl]-(8-cyclopentyl-7-methoxy-5,6-
dihydro-quinazolin-2-yl)-amine;
2-[4-(3-Amino-pyrrolidin-1-yl)-phenylamino]-8-cyclopentyl-5,8-dihydro-
quinazolin-7-ol;
[4-(3-Amino-pyrrolidin-1-yl)-phenyl]-(8-cyclopentyl-7-methoxy-5,8-
dihydro-quinazolin-2-yl)-amine;
8-Isopropyl-2-[4-(5-methyl-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-
phenylamino]-5,6-dihydro-quinazolin-7-ol;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-21-
(8-Isopropyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-[4-(5-methyl-
hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-phenyl]-amine;
8-Isopropyl-2-[4-(5-methyl-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-
phenylamino]-5,8-dihydro-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-[4-(5-methyl-
hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-phenyl]-amine;
8-Cyclopentyl-2-[4-(S-methyl-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-
phenylamino]-5,6-dihydro-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-yl)-[4-(5-methy1-
hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-phenyl]-amine;
8-Cyclopentyl-2-[4-(5-methyl-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-
phenylamino]-5,8-dihydro-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-5,8-dihydro-quinazolin-2-yl)-[4-(5-methy1-
hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-phenyl]-amine;
1-{ 4-[4-(7-Hydroxy-8-isopropyl-5,6-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl }-ethanone;
1-{4-[4-(8-Isopropyl-7-methoxy-5,6-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl } -ethanone;
1- {4-[4-(7-Hydroxy-8-isopropyl-5,8-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-ethanone;
1-{4-[4-(8-Isopropyl-7-methoxy-5,8-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl } -ethanone;
1-{ 4-[4-(8-Cyclopentyl-7-hydroxy-5,6-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl } -ethanone;
1-{4-[4-(8-Cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-ethanone;
1-{4-[4-(8-Cyclopentyl-7-hydroxy-5,8-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-ethanone;
1-{ 4-[4-(8-Cyclopentyl-7-methoxy-5,8-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-ethanone;
1-{4-[4-(7-Hydroxy-8-isopropyl-5,6-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl } -propan-1-one;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-22-
1-{4-[4-(8-Isopropyl-7-methoxy-5,6-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-propan-1-one;
1-{4-[4-(7-Hydroxy-8-isopropyl-5,8-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-propan-1-one;
1-{4-[4-(8-Isopropyl-7-methoxy-5,8-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl } -propan-1-one;
1-{4-[4-(8-Cyclopentyl-7-hydroxy-5,6-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl } -propan-1-one;
1-{ 4-[4-(8-Cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-propan-1-one;
1-{4-[4-(8-Cyclopentyl-7-hydroxy-5,8-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl } -propan-1-one;
1-{4-[4-(8-Cyclopentyl-7-methoxy-5, 8-dihydro-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl }-propan-1-one;
2-Amino-1-{4-[4-(7-hydroxy-8-isopropyl-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl }-3-methyl-butan-1-one;
2-Amino-1-{4-[4-(8-isopropyl-7-methoxy-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl } -3-methyl-butan-1-one;
2-Amino-1-{4-[4-(7-hydroxy-8-isopropyl-5,8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl}-3-methyl-butan-1-one;
2-Amino-1-{4-[4-(8-isopropyl-7-methoxy-5,8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl } -3-methyl-butan-1-one;
2-Amino-1- { 4-[4-(8-cyclopentyl-7-hydroxy-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl }-3-methyl-butan-1-one;
2-Amino-1-{4-[4-(8-cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl } -3-methyl-butan-1-one;
2-Amino-1-{4-[4-(8-cyclopentyl-7-hydroxy-5,8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl } -3-methyl-butan-1-one;
2-Amino-1-{4-[4-(8-cyclopentyl-7-methoxy-5,8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl}-3-methyl-butan-1-one;
2-Amino-1-{4-[4-(7-hydroxy-8-isopropyl-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl }-4-methyl-pentan-1-one;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-23-
2-Amino-1-{4-[4-(8-isopropyl-7-methoxy-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl } -4-methyl-pentan-1-one;
2-Amino-1-{ 4-[4-(7-hydroxy-8-isopropyl-5,8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl}-4-methyl-pentan-1-one;
2-Amino-1-{4-[4-(8-isopropyl-7-methoxy-5, 8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl } -4-methyl-pentan-1-one;
2-Amino-1-{4-[4-(8-cyclopentyl-7-hydroxy-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl}-4-methyl-pentan-1-one;
2-Amino-1-{4-[4-(8-cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl}-4-methyl-pentan-1-one;
2-Amino-1-{4-[4-(8-cyclopentyl-7-hydroxy-5,8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl } -4-methyl-pentan-1-one;
2-Amino-1-{4-[4-(8-cyclopentyl-7-methoxy-5, 8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl } -4-methyl-pentan-1-one;
2-Amino-1-{4-[4-(7-hydroxy-8-isopropyl-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl }-ethanone;
2-Amino-1-{4-[4-(8-isopropyl-7-methoxy-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl}-ethanone;
2-Amino-1-{4-[4-(7-hydroxy-8-isopropyl-5,8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl}-ethanone;
2-Amino-1-{4-[4-(8-isopropyl-7-methoxy-5,8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl}-ethanone;
2-Amino-1-{4-[4-(8-cyclopentyl-7-hydroxy-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl }-ethanone;
2-Amino-1-{4-[4-(8-cyclopentyl-7-methoxy-5,6-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl }-ethanone;
2-Amino-1-{4-[4-(8-cyclopentyl-7-hydroxy-5,8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl }-ethanone;
2-Amino-1-{4-[4-(8-cyclopentyl-7-methoxy-5,8-dihydro-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl}-ethanone;
(4-Fluoro-3-methyl-phenyl)-(8-isopropyl-7-methoxy-quinazolin-2-yl)-
amore;
2-(4-Fluoro-3-methyl-phenylamino)-8-isopropyl-quinazolin-7-ol;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-24-
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(4-fluoro-3-methyl-phenyl)-
amore;
8-Cyclopentyl-2-(4-fluoro-3-methyl-phenylamino)-quinazolin-7-ol;
(3-Chloro-4-fluoro-phenyl)-(8-isopropyl-7-methoxy-quinazolin-2-yl)-
amine;
2-(3-Chloro-4-fluoro-phenylamino)-8-isopropyl-quinazolin-7-ol;
(3-Chloro-4-fluoro-phenyl)-(8-cyclopentyl-7-methoxy-quinazolin-2-yl)-
amore;
2-(3-Chloro-4-fluoro-phenylamino)-8-cyclopentyl-quinazolin-7-ol;
(3,4-Difluoro-phenyl)-(8-isopropyl-7-methoxy-quinazolin-2-yl)-amine;
2-(3,4-Difluoro-phenylamino)-8-isopropyl-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(3,4-difluoro-phenyl)-amine;
8-Cyclopentyl-2-(3,4-difluoro-phenylamino)-quinazolin-7-ol;
(3-Fluoro-4-piperazin-1-yl-phenyl)-(8-isopropyl-7-methoxy-quinazolin-2-
yl)-amine;
2-(3-Fluoro-4-piperazin-1-yl-phenylamino)-8-isopropyl-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(3-fluoro-4-piperazin-1-yl-
phenyl)-amine;
8-Cyclopentyl-2-(3-fluoro-4-piperazin-1-yl-phenylamino)-quinazolin-7-ol;
[4-(3-Amino-pyrrolidin-1-yl)-3-fluoro-phenyl]-(8-isopropyl-7-methoxy-
quinazolin-2-yl)-amine;
2-[4-(3-Amino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-8-isopropyl-
quinazolin-7-ol;
[4-(3-Amino-pyrrolidin-1-yl)-3-fluoro-phenyl]-(8-cyclopentyl-7-methoxy-
quinazolin-2-yl)-amine;
2-[4-(3-Amino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-8-cyclopenty1-
quinazolin-7-ol;
{ 3-Fluoro-4-[4-(3-morpholin-4-yl-propyl)-piperidin-1-yl]-phenyl } -(8-
isopropyl-7-methoxy-quinazolin-2-yl)-amine;
2-{3-Fluoro-4-[4-(3-morpholin-4-yl-propyl)-piperidin-1-yl]-
phenylamino}-8-isopropyl-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-{3-fluoro-4-[4-(3-morpholin-
4-yl-propyl)-piperidin=1-yl]-phenyl }-amine;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-25-
8-Cyclopentyl-2-{ 3-fluoro-4-[4-(3-morpholin-4-yl-propyl)-piperidin-1-yl]-
phenylamino}-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-{4-[4-(3-morpholin-4-yl-
propyl)-piperidin-1-yl]-phenyl }-amine;
8-Isopropyl-2- {4-[4-(3-morpholin-4-yl-propyl)-piperidin-1-yl]-
phenylamino}-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-{4-[4-(3-morpholin-4-yl-
propyl)-piperidin-1-yl]-phenyl } -amine;
8-Cyclopentyl-2-{4-[4-(3-morpholin-4-yl-propyl)-piperidin-1-yl]-
phenylamino}-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-{4-[4-(3-piperazin-1-yl-propyl)-
piperidin-1-yl]-phenyl } -amine;
8-Isopropyl-2-{ 4-[4-(3-piperazin-1-yl-propyl)-piperidin-1-yl]-
phenylamino}-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-{4-[4-(3-piperazin-1-yl-
propyl)-piperidin-1-yl]-phenyl}-amine;
8-Cyclopentyl-2-{4-[4-(3-piperazin-1-yl-propyl)-piperidin-1-yl]-
phenylamino}-quinazolin-7-ol;
{ 3-Chloro-4-[4-(3-piperazin-1-yl-propyl)-piperidin-1-yl]-phenyl } -(8-
isopropyl-7-methoxy-quinazolin-2-yl)-amine;
2- { 3-Chloro-4-[4-(3-piperazin-1-yl-propyl)-piperidin-1-yl]-phenylamino} -
8-isopropyl-quinazolin-7-ol;
{ 3-Chloro-4-[4-(3-piperazin-1-yl-propyl)-piperidin-1-yl]-phenyl }-(8-
cyclopentyl-7-methoxy-quinazolin-2-yl)-amine;
2-{ 3-Chloro-4-[4-(3-piperazin-1-yl-propyl)-piperidin-1-yl]-phenylamino }
8-cyclopentyl-quinazolin-7-ol;
(3-Fluoro-4-{4-[3-( 1 H-tetrazol-S-yl)-propyl]-piperidin-1-yl }-phenyl)-(8-
isopropyl-7-methoxy-quinazolin-2-yl)-amine;
2-(3-Fluoro-4-{4-[3-( 1 H-tetrazol-5-yl)-propyl]-piperidin-1-yl }-
phenylamino)-8-isopropyl-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(3-fluoro-4-{ 4-[3-( 1 H-
tetrazol-5-yl)-propyl]-piperidin-1-yl} -phenyl)-amine;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-26-
8-Cyclopentyl-2-(3-fluoro-4-{4-[3-( 1 H-tetrazol-5-yl)-propyl]-piperidin-1-
y1 }-phenylamino)-quinazolin-7-ol;
(4-{ 4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl } -3-chloro-
phenyl)-(8-isopropyl-7-methoxy-quinazolin-2-yl)-amine;
2-(4-{ 4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl }-3-chloro-
phenylamino)-8-isopropyl-quinazolin-7-ol;
(4- {4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl }-3-chloro-
phenyl)-(8-cyclopentyl-7-methoxy-quinazolin-2-yl)-amine;
2-(4- {4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl }-3-chloro-
phenylamino)-8-cyclopentyl-quinazolin-7-ol;
(4- { 4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl } -phenyl)-(8-
isopropyl-7-methoxy-quinazolin-2-yl)-amine;
2-(4-{ 4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl } -
phenylamino)-8-isopropyl-quinazolin-7-ol;
(4-{ 4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl } -phenyl)-(8-
cyclopentyl-7-methoxy-quinazolin-2-yl)-amine;
2-(4-{ 4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl }-
phenylamino)-8-cyclopentyl-quinazolin-7-ol;
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-{4-[4-(4-methoxy-phenyl)-
piperazin-1-yl]-phenyl}-amine;
8-Isopropyl-2-{ 4-[4-(4-methoxy-phenyl)-piperazin-1-yl]-phenylamino }-
quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-{4-[4-(4-methoxy-phenyl)-
piperazin-1-yl]-phenyl } -amine;
8-Cyclopentyl-2-{4-[4-(4-methoxy-phenyl)-piperazin-1-yl]-phenylamino }-
quinazolin-7-ol;
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-[4-(3,3,4-trimethyl-piperazin-1-
yl)-phenyl]-amine;
8-Isopropyl-2-[4-(5-methyl-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-
phenylamino]-quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-[4-(5-methyl-hexahydro-
pyrrolo[3,4-c]pyrrol-2-yl)-phenyl]-amine;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-27-
8-Cyclopentyl-2-[4-(5-methyl-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-
phenylamino]-quinazolin-7-ol;
8-Isopropyl-2-[4-(3,3,4-trimethyl-piperazin-1-yl)-phenylamino]-
quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-[4-(3,3,4-trimethyl-piperazin-
1-yl)-phenyl]-amine;
8-Cyclopentyl-2-[4-(3,3,4-trimethyl-piperazin-1-yl)-phenylamino]-
quinazolin-7-ol;
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-[4-(5-methyl-hexahydro-
pyrrolo[3,4-c]pyrrol-2-yl)-phenyl]-amine;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(4-perhydro-1,4-diazepin-1-
yl-phenyl)-amine;
8-Cyclopentyl-2-(4-perhydro-1,4-diazepin-1-yl-phenylamino)-quinazolin-
7-0l;
2-{4-[4-(8-Cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-2,6-
dimethyl-piperazin-1-yl } -ethanol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-[4-(2-methylamino-ethoxy)-
phenyl]-amine;
1-{4-[4-(8-Cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyl]-
perhydro-1,4-diazepin-1-yl}-ethanone;
8-Cyclopentyl-2-[4-(3,3-dimethyl-piperazin-1-yl)-phenylamino]-
quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-[4-(3,3-dimethyl-piperazin-1-
yl)-phenyl]-amine;
1-{4-[4-(8-Cyclopentyl-7-hydroxy-quinazolin-2-ylamino)-phenyl]-
perhydro-1,4-diazepin-1-yl }-ethanone;
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(4-{4-[3-(1 H-tetrazol-5-yl)-
propyl]-piperidin-1-yl}-phenyl)-amine;
8-Cyclopentyl-2-(4-{4-[3-( 1 H-tetrazol-5-yl)-propyl]-piperidin-1-yl }-
phenylamino)-quinazolin-7-ol;
8-Cyclopentyl-N2-(4-piperazin-1-yl-phenyl)-quinazoline-2,7-diamine;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-2 8-
8-Cyclopentyl-N2-[4-(4-methyl-piperazin-1-yl)-phenyl]-quinazoline-2,7-
diamine;
1-{4-[4-(7-Amino-8-cyclopentyl-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl } -ethanone;
8-Cyclopentyl-N2-(4-perhydro-1,4-diazepin-1-yl-phenyl)-quinazoline-2,7-
diamine;
8-Cyclopentyl-N2-[4-(3,3-dimethyl-piperazin-1-yl)-phenyl]-quinazoline-
2,7-diamine;
8-Cyclopentyl-N7-methyl-N2-(4-piperazin-1-yl-phenyl)-quinazoline-2,7-
diamine;
8-Cyclopentyl-N7-methyl-N2-[4-(4-methyl-piperazin-1-yl)-phenyl]-
quinazoline-2,7-diamine;
1-{4-[4-(8-Cyclopentyl-7-methylamino-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl } -ethanone;
8-Cyclopentyl-N7-methyl-N2-(4-perhydro-1,4-diazepin-1-yl-phenyl)-
quinazoline-2,7-diamine;
8-Cyclopentyl-N2-[4-(3,3-dimethyl-piperazin-1-yl)-phenyl]-N7-methyl-
quinazoline-2,7-diamine;
8-Cyclopentyl-N7,N7-dimethyl-N2-(4-piperazin-1-yl-phenyl)-quinazoline-
2,7-diamine;
8-Cyclopentyl-N7,N7-dimethyl-N2-[4-(4-methyl-piperazin-1-yl)-phenyl]-
quinazoline-2,7-diamine;
1-{4-[4-(8-Cyclopentyl-7-dimethylamino-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl}-ethanone;
8-Cyclopentyl-N7,N7-dimethyl-N2-(4-perhydro-1,4-diazepin-1-yl-
phenyl)-quinazoline-2,7-diamine;
8-Cyclopentyl-N2-[4-(3,3-dimethyl-piperazin-1-yl)-phenyl]-N7,N7-
dimethyl-quinazoline-2,7-diamine;
8-Cyclopentyl-N2-(4- {4-[3-( 1 H-tetrazol-5-yl)-propyl]-piperidin-1-yl } -
phenyl)-quinazoline-2,7-diamine;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-29-
8-Cyclopentyl-N7-methyl-N2-(4-{4-[3-( 1 H-tetrazol-S-yl)-propyl]-
piperidin-1-yl }-phenyl)-quinazoline-2,7-diamine;
8-Cyclopentyl-N2-{4-[4-(3-piperazin-1-yl-propyl)-piperidin-1-yl]-
phenyl}-quinazoline-2,7-diamine;
8-Cyclopentyl-N7-methyl-N2-{4-[4-(3-piperazin-1-yl-propyl)-piperidin-1-
yl]-phenyl } -quinazoline-2,7-diamine;
8-Cyclopentyl-N7,N7-dimethyl-N2-{4-[4-(3-piperazin-1-yl-propyl)-
piperidin-1-yl]-phenyl}-quinazoline-2,7-diamine;
(8-Cyclopentyl-7-methoxy-5-methyl-quinazolin-2-yl)-(4-perhydro-1,4-
diazepin-1-yl-phenyl)-amine;
8-Cyclopentyl-5-methyl-2-(4-perhydro-1,4-diazepin-1-yl-phenylamino)-
quinazolin-7-ol;
2-{4-[4-(8-Cyclopentyl-7-methoxy-5-methyl-quinazolin-2-ylamino)-
phenyl]-2,6-dimethyl-piperazin-1-yl }-ethanol;
(8-Cyclopentyl-7-methoxy-5-methyl-quinazolin-2-yl)-[4-(2-methylamino-
ethoxy)-phenyl]-amine;
1-{ 4-[4-(8-Cyclopentyl-7-methoxy-5-methyl-quinazolin-2-ylamino)-
phenyl]-perhydro-1,4-diazepin-1-yl }-ethanone;
8-Cyclopentyl-2-[4-(3,3-dimethyl-piperazin-1-yl)-phenylamino]-5-methyl-
quinazolin-7-ol;
(8-Cyclopentyl-7-methoxy-5-methyl-quinazolin-2-yl)-[4-(3,3-dimethyl-
piperazin-1-yl)-phenyl]-amine;
1-{4-[4-(8-Cyclopentyl-7-hydroxy-5-methyl-quinazolin-2-ylamino)-
phenyl]-perhydro-1,4-diazepin-1-yl}-ethanone;
(8-Cyclopentyl-7-methoxy-5-methyl-quinazolin-2-yl)-(4- { 4-[3-( 1 H-
tetrazol-5-yl)-propyl]-piperidin-1-yl }-phenyl)-amine;
8-Cyclopentyl-5-methyl-2-(4-{ 4-[3-( 1 H-tetrazol-5-yl)-propyl]-piperidin-1-
yl}-phenylamino)-quinazolin-7-ol;
1- {4-[4-(8-Cyclopentyl-7-hydroxy-5-methyl-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl}-ethanone;
1-{ 4-[4-(8-Cyclopentyl-7-methoxy-5-methyl-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl } -ethanone;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-30-
8-Cyclopentyl-5-methyl-2-(4-piperazin-1-yl-phenylamino)-quinazolin-
7-0l;
(8-Cyclopentyl-7-methoxy-5-methyl-quinazolin-2-yl)-(4-piperazin-1-yl-
phenyl)-amine;
(8-Cyclopentyl-7-methoxy-5-methyl-quinazolin-2-yl)-{4-[4-(3-piperazin-
1-yl-propyl)-piperidin-1-yl]-phenyl }-amine;
8-Cyclopentyl-5-methyl-2- { 4-[4-(3-piperazin-1-yl-propyl)-piperidin-1-yl]-
phenylamino}-quinazolin-7-ol;
8-Cyclopentyl-5-methyl-N2-(4-piperazin-1-yl-phenyl)-quinazoline-2,7-
diamine;
8-Cyclopentyl-5-methyl-N2-[4-(4-methyl-piperazin-1-yl)-phenyl]-
quinazoline-2,7-diamine;
1-{4-[4-(7-Amino-8-cyclopentyl-5-methyl-quinazolin-2-ylamino)-phenyl]-
piperazin-1-yl}-ethanone;
8-Cyclopentyl-5-methyl-N2-(4-perhydro-1,4-diazepin-1-yl-phenyl)-
quinazoline-2,7-diamine;
8-Cyclopentyl-N2-[4-(3,3-dimethyl-piperazin-1-yl)-phenyl]-S-methy1-
quinazoline-2,7-diamine;
8-Cyclopentyl-S,N7-dimethyl-N2-(4-piperazin-1-yl-phenyl)-quinazoline-
2,7-diamine;
8-Cyclopentyl-S,N7-dimethyl-N2-[4-(4-methyl-piperazin-1-yl)-phenyl]-
quinazoline-2,7-diamine;
1-{ 4-[4-(8-Cyclopentyl-5-methyl-7-methylamino-quinazolin-2-ylamino)-
phenyl]-piperazin-1-yl } -ethanone;
8-Cyclopentyl-S,N7-dimethyl-N2-(4-perhydro-1,4-diazepin-1-yl-phenyl)-
quinazoline-2,7-diamine;
8-Cyclopentyl-N2-[4-(3,3-dimethyl-piperazin-1-yl)-phenyl]-S,N7-
dimethyl-quinazoline-2,7-diamine;
8-Cyclopentyl-S,N7,N7-trimethyl-N2-(4-piperazin-1-yl-phenyl)-
quinazoline-2,7-diamine;
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-31-
8-Cyclopentyl-S,N7,N7-trimethyl-N2-[4-(4-methyl-piperazin-1-yl)-
phenyl]-quinazoline-2,7-diamine;
1-{4-[4-(8-Cyclopentyl-7-dimethylamino-5-methyl-quinazolin-2-
ylamino)-phenyl]-piperazin-1-yl } -ethanone;
8-Cyclopentyl-S,N7,N7-trimethyl-N2-(4-perhydro-1,4-diazepin-1-yl-
phenyl)-quinazoline-2,7-diamine;
8-Cyclopentyl-N2-[4-(3,3-dimethyl-piperazin-1-yl)-phenyl]-S,N7,N7-
trimethyl-quinazoline-2,7-diamine;
8-Cyclopentyl-5-methyl-N2-(4-{4-[3-( 1 H-tetrazol-5-yl)-propyl]-piperidin-
1-yl }-phenyl)-quinazoline-2,7-diamine;
8-Cyclopentyl-S,N7-dimethyl-N2-(4-{4-[3-( 1 H-tetrazol-5-yl)-propyl]-
piperidin-1-yl }-phenyl)-quinazoline-2,7-diamine;
8-Cyclopentyl-5-methyl-N2-{ 4-[4-(3-piperazin-1-yl-propyl)-piperidin-1-
yl]-phenyl}-quinazoline-2,7-diamine;
8-Cyclopentyl-S,N7-dimethyl-N2-{4-[4-(3-piperazin-1-yl-propyl)-
piperidin-1-yl]-phenyl}-quinazoline-2,7-diamine; and
8-Cyclopentyl-S,N7,N7-trimethyl-N2-{ 4-[4-(3-piperazin-1-yl-propyl)-
piperidin-1-yl]-phenyl} -quinazoline-2,7-diamine.
Additional preferred embodiments of the present invention include those
in Examples 1-10, 85, 54, and 57, infra.
Compounds of Formula I wherein R2 is alkyl, RS and R6 are hydrogen are
especially useful as intermediates leading to compounds that display good
inhibitory activity against cyclin-dependent kinases. Other especially useful
intermediates leading to compounds that display good inhibitory activity
against
cyclin-dependent kinases are compounds of Formula III
R5
Rs
W N III
R2 \ N~X
R1
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-32-
wherein R1, R2, R5, and R6 are as defined above and X is oxygen or halogen.
Particularly useful intermediates are those wherein R2 is alkyloxy, RS is
hydrogen
or alkyl, R6 is hydrogen, and Rg is oxygen or chlorine or a pharmaceutically
acceptable salt, ester, amide, or pro-drug thereof.
The present invention also discloses processes for preparing compounds of
Formula I, II and III. These processes are described by the nonlimiting
examples
set forth below.
Unless otherwise expressly stated, the following definitions are adhered to
throughout this disclosure.
"Alkyl" means a straight or branched hydrocarbon radical having from
1 to 10 carbon atoms (unless stated otherwise) and includes, for example,
methyl,
ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, n-
pentyl, iso-
pentyl, n-hexyl, and the like. "Halo" includes fluoro, chloro, bromo, and
iodo.
"Alkenyl" means straight and branched hydrocarbon radicals having from
2 to 6 carbon atoms and one double bond and includes ethenyl, 3-buten-1-yl,
2-ethenylbutyl, 3-hexen-1-yl, and the like.
"Alkynyl" means straight and branched hydrocarbon radicals having from
2 to 6 carbon atoms and one triple bond and includes ethynyl, 3-butyn-1-yl,
propynyl, 2-butyn-1-yl, 3-pentyn-1-yl, and the like.
"Cycloalkyl" means a monocyclic or polycyclic hydrocarbyl group such as
cyclopropyl, cycloheptyl, cyclooctyl, cyclodecyl, cyclobutyl, adamantyl,
norpinanyl, decalinyl, norbornyl, cyclohexyl, and cyclopentyl. Such groups can
be
substituted with groups such as hydroxy, keto, and the like. Also included are
rings in which 1 to 3 heteroatoms replace carbons. Such groups are termed
"heterocyclyl", which means a cycloalkyl group also bearing at least one
heteroatom selected from O, S, or NR2, examples being substituted or
unsubstituted oxiranyl, pyrrolidinyl, piperidyl, tetrahydropyran, piperazinyl,
acylpiperazinyl, pyrrolidinyl, and morpholine.
"Alkoxy" refers to the alkyl groups mentioned above bound through
oxygen, examples of which include methoxy, ethoxy, isopropoxy, tert-butoxy,
and
the like. In addition, alkoxy refers to polyethers such as -O-(CH2)2-O-CH3,
and
the like.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-33-
"Alkanoyl" groups are alkyl linked through a carbonyl, ie, Cl-CS-C(O)-.
Such groups include formyl, acetyl, propionyl, butyryl, and isobutyryl.
"Acyl" means an alkyl or aryl (Ar) group bonded through a carbonyl
group, ie, R-C(O)-. For example, acyl includes a Cl-C6 alkanoyl, including
substituted alkanoyl, wherein the alkyl portion can be substituted by NR4R5 or
a
carboxylic or heterocyclic group. Typical acyl groups include acetyl, benzoyl,
and
the like.
The alkyl, alkenyl, alkoxy, and alkynyl groups described above are
optionally substituted, preferably by 1 to 3 groups selected from NR4R5,
phenyl,
substituted phenyl, thio C 1-C6 alkyl, C 1-C6 alkoxy, hydroxy, carboxy, C 1-C6
alkoxycarbonyl, halo, nitrile, cycloalkyl, and a 5- or 6-membered carbocyclic
ring
or heterocyclic ring having 1 or 2 heteroatoms selected from nitrogen,
substituted
nitrogen, oxygen, and sulfur. "Substituted nitrogen" means nitrogen bearing
C 1-C6 alkyl or (CH2)nPh where n is 1, 2, or 3. Perhalo and polyhalo
substitution
is also embraced.
Examples of substituted alkyl groups include 2-aminoethyl,
pentachloroethyl, trifluoromethyl, 2-diethylaminoethyl, 2-dimethylaminopropyl,
ethoxycarbonylmethyl, 3-phenylbutyl, methanylsulfanylmethyl, methoxymethyl,
3-hydroxypentyl, 2-carboxybutyl, 4-chlorobutyl, 3-cyclopropylpropyl,
pentafluoroethyl, 3-morpholinopropyl, piperazinylmethyl, and
2-(4-methylpiperazinyl)ethyl.
Examples of substituted alkynyl groups include 2-methoxyethynyl,
2-ethylsulfanyethynyl, 4-(1-piperazinyl)-3-(butynyl), 3-phenyl-5-hexynyl,
3-diethylamino-3-butynyl, 4-chloro-3-butynyl, 4-cyclobutyl-4-hexenyl, and the
like.
Typical substituted alkoxy groups include aminomethoxy,
trifluoromethoxy, 2-diethylaminoethoxy, 2-ethoxycarbonylethoxy,
3-hydroxypropoxy, 6-carboxhexyloxy, and the like.
Further, examples of substituted alkyl, alkenyl, and alkynyl groups include
dimethylaminomethyl, carboxymethyl, 4-dimethylamino-3-buten-1-yl,
5-ethylmethylamino-3-pentyn-1-yl, 4-morpholinobutyl,
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-34-
4-tetrahydropyrinidylbutyl, 3-imidazolidin-1-ylpropyl, 4-tetrahydrothiazol-3-
yl-
butyl, phenylmethyl, 3-chlorophenylmethyl, and the like.
The terms "Ar" and "aryl" refer to unsubstituted and substituted aromatic
groups. Heteroaryl groups have from 4 to 9 ring atoms, from 1 to 4 of which
are
independently selected from the group consisting of O, S, and N. Preferred
heteroaryl groups have 1 or 2 heteroatoms in a 5- or 6-membered aromatic ring.
Mono and bicyclic aromatic ring systems are included in the definition of aryl
and
heteroaryl. Typical aryl and heteroaryl groups include phenyl, 3-chlorophenyl,
2,6-dibromophenyl, pyridyl, 3-methylpyridyl, benzothienyl, 2,4,6-
tribromophenyl,
4-ethylbenzothienyl, furanyl, 3,4-diethylfuranyl, naphthyl, 4,7-
dichloronaphthyl,
pyrrole, pyrazole, imidazole, thiazole, and the like.
Preferred Ar groups are phenyl and phenyl substituted by 1, 2, or 3 groups
independently selected from the group consisting of alkyl, alkoxy, thio,
thioalkyl,
hydroxy, -COOR7, amino of the formula -NR4R5, and T(CH2)mQR4 or
T(CH2)mC02R4 wherein m is 1 to 6, T is O, S, NR4, N(O)R4, NR4R6Y, or
CR4R5, Q is O, S, NRS, N(O)R5, or NRSR6Y wherein R4 and RS are as
described above, and R7 is alkyl or substituted alkyl, for example, methyl,
trichloroethyl, diphenylmethyl, and the like. The alkyl and alkoxy groups can
be
substituted as defined above. For example, typical groups are carboxyalkyl,
alkoxycarbonylalkyl, hydroxyalkyl, hydroxyalkoxy, and alkoxyalkyl.
The symbol "-" means a bond.
The term "patient" means all animals including humans. Examples of
patients include humans, cows, dogs, cats, goats, sheep, and pigs.
The compounds of the present invention can exist in unsolvated forms as
well as solvated forms, including hydrated forms. In general, the solvated
forms,
including hydrated forms, are equivalent to unsolvated forms and are intended
to
be encompassed within the scope of the present invention.
The compounds of Formulas I, II, and III are capable of further forming
both pharmaceutically acceptable formulations comprising salts, esters,
amides,
and prodrugs. As used herein, the term "pharmaceutically acceptable salts,
esters,
amides, and prodrugs" refers to those carboxylate salts, amino acid addition
salts,
esters, amides, and prodrugs of the compounds of the present invention which
are,
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-35-
within the scope of sound medical judgment, suitable for use in contact with
the
tissues of patients without undue toxicity, irritation, allergic response, and
the like,
commensurate with a reasonable benefit/risk ratio, and effective for their
intended
use, as well as the zwitterionic forms, where possible, of the compounds of
the
invention. The term "salts" refers to the relatively non-toxic, inorganic and
organic acid addition salts of compounds of the present invention. These salts
can
be prepared in situ during the final isolation and purification of the
compounds or
by separately reacting the purified compound in its free base form with a
suitable
organic or inorganic acid and isolating the salt thus formed and including,
but not
limited to, acid addition and/or base salts, solvents and N-oxides of a
compound
of Formulas I, II, and III. This invention also provides pharmaceutical
formulations comprising a compound of Formulas I, II, or III together with a
pharmaceutically acceptable carrier, diluent, or excipient therefor. All of
these
forms are within the present invention.
Pharmaceutically acceptable acid addition salts of the compounds of
Formulas I, II and III include salts derived form inorganic acids such as
hydrochloric, nitric, phosphoric, sulfuric, hydrobromic, hydriodic,
phosphorus,
and the like, as well as the salts derived from organic acids, such as
aliphatic
mono- and dicarboxylic acids, phenyl-substituted alkanoic acids, hydroxy
alkanoic acids, alkanedioic acids, aromatic acids, aliphatic and aromatic
sulfonic
acids, etc. Such salts thus include sulfate, pyrosulfate, bisulfate, sulfite,
bisulfate,
nitrate, phosphate, monohydrogenphosphate, dihydrogenphosphate,
metaphosphate, pyrophosphate, chloride, bromide, iodide, acetate, propionate,
caprylate, isobutyrate, oxalate, malonate, succinate, suberate, sebacate,
fumarate,
maleate, mandelate, benzoate, chlorobenzoate, methylbenzoate, dinitrobenzoate,
phthalate, benzenesulfonate, toluenesulfonate, phenylacetate, citrate,
lactate,
maleate, tartrate, methanesulfonate, and the like. Also contemplated are the
salts
of amino acids such as arginate, gluconate, galacturonate, and the like; see,
for
example, Berge, et al., "Pharmaceutical Salts," .I. of Pharmaceutical Science,
1977;66:1-19.
The acid addition salts of the basic compounds are prepared by contacting
the free base form with a sufficient amount of the desired acid to produce the
salt
in the conventional manner. The free base form may be regenerated by
contacting
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-3 6-
the salt form with a base and isolating the free base in the conventional
manner.
The free base forms differ from their respective salt forms somewhat in
certain
physical properties such as solubility in polar solvents, but otherwise the
salts are
equivalent to their respective free base for purposes of the present
invention.
Pharmaceutically acceptable base addition salts are formed with metals or
amines, such as alkali and alkaline earth metal hydroxides, or of organic
amines.
Examples of metals used as cations are sodium, potassium, magnesium, calcium,
and the like. Examples of suitable amines are N,N'-dibenzylethylenediamine,
chloroprocaine, choline, diethanolamine, ethylenediamine, N-methylglucamine,
and procaine; see, for example, Berge, et al., supra.
The base addition salts of acidic compounds are prepared by contacting the
free acid form with a sufficient amount of the desired base to produce the
salt in
the conventional manner. The free acid form may be regenerated by contacting
the
salt form with an acid and isolating the free acid in a conventional manner.
The
free acid forms differ from their respective salt forms somewhat in certain
physical properties such as solubility in polar solvents, but otherwise the
salts are
equivalent to their respective free acid for purposes of the present
invention.
Examples of pharmaceutically acceptable, non-toxic esters of the
compounds of this invention include C1-C6alkyl esters wherein the alkyl group
is
a straight or branched chain. Acceptable esters also include CS-C~cycloalkyl
esters as well as arylalkyl esters such as, but not limited to benzyl. C I -
C4alkyl
esters are preferred. Esters of the compounds of the present invention may be
prepared according to conventional methods.
Examples of pharmaceutically acceptable, non-toxic amides of the
compounds of this invention include amides derived from ammonia, primary
Cl-C6alkyl amines and secondary Cl-C6dialkyl amines wherein the alkyl groups
are straight or branched chain. In the case of secondary amines the amine may
also be in the form of a 5- or 6-membered heterocycle containing one nitrogen
atom. Amides derived from ammonia, Cl-C3alkyl primary amines, and
Cl-C2dialkyl secondary amines are preferred. Amides of the compounds of the
invention may be prepared according to conventional methods.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-3 7-
The term "pro-drug" refers to compounds that are rapidly transformed
in vivo to yield the parent compound of the above formulae, for example, by
hydrolysis in blood. A thorough discussion is provided in T. Higuchi and V.
Stella,
"Pro-drugs as Novel Delivery Systems," Vol 14 of the A.C.S. Symposium Series,
and in Bioreversible Carriers in Drug Design, ed. Edward B. Roche, American
Pharmaceutical Association and Pergamon Press, 1987, both of which are
incorporated herein by reference.
A therapeutically effective amount is an amount of a compound of
Formulas I, II, or III, that when administered to a patient, ameliorates a
symptom
of the disease.
The term "cardiovascular disorders and diseases" includes, but is not
limited to atherosclerosis, psoriasis, and restenosis. Those skilled in the
art are
easily able to identify patients having atherosclerosis, psoriasis,
restenosis, or at
risk of having atherosclerosis or restenosis. For example, patients who are at
risk
of having restenosis include, but are not limited to, patients having
undergone
balloon angioplasty or other surgical vascular procedures.
The term "cancer" includes, but is not limited to, the following cancers:
breast; ovary; cervix; prostate; testis; esophagus; glioblastoma;
neuroblastoma;
stomach; skin, keratoacanthoma; lung, epidermoid carcinoma, large cell
carcinoma, adenocarcinoma; bone; colon, adenocarcinoma, adenoma; pancreas,
adenocarcinoma; thyroid, follicular carcinoma, undifferentiated carcinoma,
papillary carcinoma; seminoma; melanoma; sarcoma; bladder carcinoma; liver
carcinoma and biliary passages; kidney carcinoma; myeloid disorders; lymphoid
disorders, Hodgkin's, hairy cells; buccal cavity and pharynx (oral), lip,
tongue,
mouth, pharynx; small intestine; colon-rectum, large intestine, rectum; brain
and
central nervous system; and leukemia.
The term autoimmune disease includes, but is not limited to inflammation,
such as rheumatoid arthritis, and organ graft rejections.
The term kidney disease includes, but is not limited to kidney disease, such
as polycystic kidney disease.
Compounds within the scope of the present invention effectively inhibit
vascular smooth muscle cell proliferation and migration. The method entails
inhibiting vascular smooth muscle proliferation, and/or migration by
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-3 8-
administering an effective amount of a compound of Formulas I, II, or III to a
subject in need of treatment.
The compounds of the present invention are useful for treating cancer (for
example, leukemia and cancer of the lung, breast, prostate, and skin such as
melanoma), cardiovascular disorders and diseases, infections, autoimmune
diseases, gout, kidney diseases, and neurodegenerative diseases and disorders.
To
utilize a compound of the present invention to treat these indications, a
patient
having the indication is administered a therapeutically effective amount of a
pharmaceutically acceptable composition comprising an invention compound.
The term "neurological disease and disorders" includes, but is not limited
to, Alzheimer's disease. Compounds within the scope of the present invention
effectively inhibit the degradation of neurons. The method entails inhibiting
neural degeneration by administering an effective amount of a compound of
Formulas I, II, or III to a subject in need of treatment.
The compounds of the present invention can be formulated and
administered in a wide variety of oral and parenteral dosage forms, including
transdermal and rectal administration. It will be recognized to those skilled
in the
art that the following dosage forms may comprise as the active component,
either
a compound of Formulas I, II, or III or a corresponding pharmaceutically
acceptable salt or solvate of a compound of Formulas I, II, or III.
A further embodiment of this invention is a pharmaceutical formulation
comprising a compound of Formulas I, II, or III together with a
pharmaceutically
acceptable carrier, diluent, or excipient therefor. For preparing
pharmaceutical
compositions with the compounds of the present invention, pharmacuetically
acceptable carriers can be either a solid or liquid. Solid form preparations
include
powders, tablets, pills, capsules, cachets, suppositories, and dispensible
granules.
A solid carrier can be one or more substances which may also act as diluents,
flavoring agents, binders, preservatives, tablet disintegrating agents, or an
encapsulating material.
In powders, the carrier is a finely divided solid such as talc or starch which
is in a mixture with the finely divided active component. In tablets, the
active
component is mixed with the carrier having the necessary binding properties in
suitable proportions and compacted in the shape and size desired.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-3 9-
The formulations of this invention preferably contain from about S% to
about 70% or more of the active compound. Suitable carriers include magnesium
carbonate, magnesium stearate, talc, sugar, lactose, pectin, dextrin, starch,
gelatin,
tragacanth, methylcellulose, sodium carboxymethylcellulose, a low melting wax,
cocoa butter, and the like. A preferred form for oral use are capsules, which
include the formulation of the active compound with encapsulating material as
a
carrier providing a capsule in which the active component with or without
other
carriers, is surrounded by a carrier, which is thus in association with it.
Similarly,
cachets and lozenges are included. Tablets, powders, capsules, pills, cachets,
and
lozenges can be used as solid dosage forms suitable for oral administration.
For preparing suppositories, a low melting wax, such as a mixture of fatty
acid glycerides or cocoa butter, is first melted and the active component is
dispersed homogenously therein, as by stirring. The molten homogenous mixture
is then poured into convenient size molds, allowed to cool, and thereby to
solidify.
Liquid form preparations include solutions, suspensions, and emulsions
such as water or water/propylene glycol solutions. For parenteral injection,
liquid
preparations can be formulated in solution in aqueous polyethylene glycol
solution, isotonic saline, 5% aqueous glucose, and the like. Aqueous solutions
suitable for oral use can be prepared by dissolving the active component in
water
and adding suitable colorants, flavors, stabilizing and thickening agents as
desired.
Aqueous suspensions suitable for oral use can be made by dispersing the finely
divided active component in water and mixing with a viscous material, such as
natural or synthetic gums, resins, methylcellulose, sodium
carboxymethylcellulose, or other well-known suspending agents.
Also included are solid form preparations that are intended to be
converted, shortly before use, to liquid form preparations for oral
administration.
Such liquid forms include solutions, suspensions, and emulsions. These
preparations may contain, in addition to the active component, colorants,
flavors,
stabilizers, buffers, artificial and natural sweeteners, dispersants,
thickeners,
solubilizing agents, and the like. Waxes, polymers, microparticles~ and the
like
can be utilized to prepare sustained-release dosage forms. Also, osmotic pumps
can be employed to deliver the active compound uniformally over a prolonged
period.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-40-
The pharmaceutical preparations of the invention are preferably in unit
dosage form. In such form, the preparation is subdivided into unit doses
containing appropriate quantities of the active component. The unit dosage
form
can be a packaged preparation, the package containing discrete quantities of
preparation, such as packeted tablets, capsules, and powders in vials or
ampules.
Also, the unit dosage form can be a capsule, tablet, cachet, or lozenge
itself, or it
can be the appropriate number of any of these in packaged form.
The therapeutically effective dose of a compound of Formulas I, II, or III
will generally be from about 1 mg to about 100 mg/kg of body weight per day.
Typical adult doses will be about 50 mg to about 800 mg per day. The quantity
of
active component in a unit dose preparation may be varied or adjusted from
about
0.1 mg to about 500 mg, preferably about 0.5 mg to 100 mg according to the
particular application and the potency of the active component. The
composition
can, if desired, also contain other compatible therapeutic agents. A subject
in need
of treatment with a compound of Formulas I, II, or III is administered a
dosage of
about 1 to about 500 mg per day, either singly or in multiple doses over a 24-
hour
period.
The compounds of the present invention are capable of binding to and
inhibiting the activity of proteins having the ability to phosphorylate other
proteins, such as cdks. Cdks form complexes with cyclins, and these complexes
phosphorylate key proteins allowing cells to proceed through the cell cycle
(Meijer L., Progress in Cell Cycle Research, 1995;1:351-363). The compounds of
this invention inhibit this phosphorylation and therefore can be used as anti-
proliferative agents for the treatment of cancer and/or restenosis and other
proliferative diseases, and as anti-neural degenerative agents for the
treatment of
neural degenerative disorders such as Alzheimer's disease.
Because of their inhibitory activity against cdks and other kinases, the
compounds of the present invention are also useful research tools for studying
the
mechanism of action of those kinases, both in vitro and in vivo.
While the forms of the invention herein constitute presently preferred
embodiments, many others are possible. It is not intended herein to mention
all of
the possible equivalent forms or ramifications of the invention. It is
understood
that the terms used herein are merely descriptive rather than limiting, and
those
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-41-
skilled in the art will realize that various changes may be made without
departing
from the spirit or scope of the invention.
Compounds of Formulas I, II, and III may be prepared according to the
syntheses outlined in Scheme 1, infra. Although this scheme often indicates
exact
structures, those with ordinary skill in the art will appreciate that the
methods
apply widely to analogous compounds of Formulas I, II, and III, given
appropriate
consideration to protection and deprotection or reactive functional groups by
methods standard to the art of organic chemistry. For example, hydroxy groups,
in
order to prevent unwanted side reactions, generally need to be converted to
ethers
or esters during chemical reactions at other sites in the molecule. The
hydroxy
protecting group is readily removed to provide the free hydroxy group. Amino
groups and carboxylic acid groups are similarly derivatized to protect them
against unwanted side reactions. Typical protecting groups and methods for
attaching and cleaving them are described fully by Greene and Wuts in
Protective
Groups in Organic Synthesis, John Wiley and Sons, New York, (2nd Ed., 1991),
and McOmie, Protective Groups in Organic Chemistry, Plenum Press, New York,
1973.
Scheme 1 describes a typical method for the preparation of the quinazoline
compounds of the invention.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-42
Scheme 1
Li/NH3 > I I nBuLi ~
CH30 OCH3 CH30 OCH3 R -X CH30 I OCH3
R
(2) (3)
aq HCI NaOCH3/THF NaH/EtOCHO
' I ' ' w Benzene '
CH30 Rt O CH30 R1 O
(4) (5)
~ -OH Guanidine carbonate ~ ~N 10% Pd-C
DMF ' ~ I ~ nitrobenzene'
CH30 Rl O CH30 'Ri N NH2
(6)
I ~ N HBF4 ~ I ~ N POC13 ~ I °N
> ~ >
CH30 \ N NH2 NaN02 CH30 \ H O CH30 \ N CI
R1 Rl R1
(8) (9) (10)
R2-NH ~ I ~N NaSEt ~ ~ ~N
2
N~NH ~ HO \ N~NH
CH30 Rt R2 RI R2
(11) (12)
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-43
Schemes 2 and 3
Scheme 2
NIIH
R2 ~-/ H~NH2
i OH (17) , I ~ N aq HCI
CH30 \ O CH30 ~ N~NH
R' R'
(7) ~~Rz
N BnNHz, H+ ~ N Pd/C i ~ N
O I N~NH N ~~ I N~NH nitrobenzene HN ~ I N~NH
R' / Bn R' , Bn R'
Rz ~Rz ~Rz
(13) (14) (15)
Hz, Pd/C ~ I ~ N
HZN \ N~NH
R'
Rz
(16)
Scheme 3
(~) / vN
N
z z NH
R Booty NHBoC R ~ (ref: Bernatowivz et al.
NHz ~ / N"NHz Tetrahedron Gett. 1993, 34, 3389)
H
(2) TFA
(17)
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-44
EXAMPLES
The following examples are for illustrative purposes only and are not
intended, nor should they be construed as limiting the invention in any
manner.
Those skilled in the art will appreciate that variations and modifications can
be
made without violating the spirit or scope of the invention.
EXAMPLE 1
1,5-Dimethox ~~-cyclohexa-1,4-dime
A solution containing 1,3-dimethoxybenzene (10 g), dry THF (15 mL) and tBuOH
(15 mL) was added to distilled ammonia (ca 250 mL). To the reaction mixture
was added lithium wire (1.5 g) in small portions, and the deep blue colored
solution was stirred for 3 hours. The reaction mixture was decolorized by
dropwise addition of methanol. Ammonia was evaporated at room temperature.
To the residue, ammonium chloride solution was added, then the product was
extracted with hexane (3 x 120 mL). The combined extracts were washed with
water and dried over anhydrous sodium sulfate. Removal of the solvent under
reduced pressure gave the title compound as a clear liquid (9.5 g). 1 H NMR
(CDCl3): cS 2.7-2.9 (m, 4H, 2 x CH2), 3.58 (s, 6H, 2 x OCH3), 4.66 (t, 2H,
olefmic).
EXAMPLE 2
6-Isopropyl-1,5-dimethoxy-cyclohexa-1,4-dime
To a solution of 1,5-dimethoxy-cyclohexa-1,4-dime (7 g, 50 mmol) in dry THF
(140 mL) was added dropwise nBuLi solution (2.5 M, 30 mL) in hexane at -
20°C.
The orange colored reaction mixture was stirred for 0.5 hour at -
20°C, then
isopropyl iodide (17 g, 100 mmol) was added dropwise. The reaction mixture was
stirred for 1 hour, while the temperature was raised to 0°C. Excess
nBuLi was
destroyed cautiously with methanol. Cold water (140 mL) was added to the
reaction mixture, then the product was extracted with ethyl acetate (3 x 70
mL).
The combined organic extracts were washed with brine, dried over anhydrous
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-45-
sodium sulfate, and concentrated to give the title compound as a light yellow
liquid (8.5 g, 93.4%). 1H NMR CDCl3): 8 0.90 (d, 6H, 2 x CH3), 2.1 (m, 1H,
CH), 2.76 (m, 3H, CH2 and CH), 3.53 (s, 6H, 2 x OCH3), 4.73 (t, 2H, olelinic).
EXAMPLE 3
2-Isopropyl-3-methox~cyclohex-3-enone
To a solution of 6-isopropyl-1,5-dimethoxy-cyclohexa-1,4-dime (7 g) in
methanol
(50 mL) was added 2.5% hydrochloric acid (10 mL) drop wise at 0°C. The
reaction mixture was stirred for 45 minutes, then diluted with ice-cold water
(30 mL). The product was extracted with ether (3 x 70 mL). The combined
organic extracts were washed with brine and dried over anhydrous sodium
sulfate.
Removal of the solvent in vacuo yielded the title compound as a light yellow
oil
(6 g, 93%).
EXAMPLE 4
2-Isopropyl-3-methoxy-cyclohex-2-enone
To a solution of 2-isopropyl-3-methoxy-cyclohex-3-enone (6 g) in dry THF
(120 mL), sodium methoxide (1.5 g) was added in portions at room temperature.
After stirring at room temperature for 1.5 hours, the reaction mixture was
cooled
in an ice bath and neutralized with 10% sodium hydrogen phosphate solution.
The
organic layer was separated, then the aqueous layer was extracted with ethyl
acetate (2 x 50 mL). The combined organic extracts were washed with brine,
then
. dried over anhydrous sodium sulfate. The solvent was removed under reduced
pressure, and the resulting product was purified on a column of silica gel
(Hex:EtOAc) to give the title compound as a yellow liquid (5.4 g, 90%). 1H NMR
(CDCl3): 8 1.09 (d, 6H, 2 x CH3), 1.95 (m, 2H, CH2), 2.31 (t, 2H, CH2), 2.54
(t,
2H, CH2), 3.2 (m, 1H, CH), 3.78 (s, 3H, OCH3).
EXAMPLE 5
6-Hydroxymethylene-2-isopropyl-3-methoxy-cyclohex-2-enone
To a suspension of sodium hydride (3.6 g, 60% suspension, 90 mmol) in benzene
( 100 mL), a mixture of ethyl formate ( 12.5 g) and 2-isopropyl-3-methoxy-
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-46-
cyclohex-2-enone (5 g, 29.7 mmol) in benzene was added dropwise at S°C.
The
reaction mixture was stirred at ice temperature for 1 hour, and at room
temperature for 18 hours. The reaction mixture was cooled in an ice bath, and
10% sodium hydrogen phosphate solution (200 mL) was added dropwise. The
organic layer was separated, and the aqueous layer was extracted with ethyl
acetate (2 x 50 mL). The combined organic layers were washed with brine and
dried over anhydrous sodium sulfate. The solvent was removed in vacuo, and the
resulting product was purified on a column of silica gel (Hex:EtOAc) to give
the
title compound as a thick viscous liquid (3.5 g, 60%). 1H NMR (CDC13): 8
1.15 (d, 6H, 2 x CH3), 2.35-2.6 (m, 4H, 2 x CH2), 3.15 (m, 1H, CH), 3.8 (s,
3H,
OCH3), 7.17 (d, 1H, =CHOH).
EXAMPLE 6
8-Isopropyl-7-methoxy-guinazolin-2-ylamine
A mixture of 6-hydroxymethylene-2-isopropyl-3-methoxy-cyclohex-2-enone
(3.5 g) and guanidine carbonate (7 g) in DMF (40 mL) was heated at
150°C for
4 hours. The solvent was removed under reduced pressure, then ice-cold water
was added to the residue. The product was extracted with ethyl acetate
(3 x 50 mL). The combined organic layers were washed with water, then brine,
and dried over anhydrous sodium sulfate. Solvent was removed under reduced
pressure to give a crude mixture of dihydroquinazolines (4 g). The
dihydroquinazolines were aromatized to the title compound by heating in
nitrobenzene (70 mL) at 150°C in the presence of 10% Pd-C (400 mg) for
3 days.
The catalyst was removed by filtration, then the filtrate was concentrated,
and the
resulting product was purified on a column of silica gel (Hex:EtOAc) to give
the
title compound as an off white solid (2.1 g, 54.2%), mp 112-113°C; 1H
NMR
(CDCl3): 8 1.38 (d, 6H, 2 x CH3), 3.95 (s, 3H, OCH3), 4.24 (m, 1H, CH),
5.0 (br s, 2H, NH2), 7.02 (d, 1 H, Ar-H, J = 9 Hz), 7.55 (d, 1 H, Ar-H, J = 9
Hz),
8.87 (s, 1 H, Ar-H); MS m/z 218.05; C 12H 1 SN30 (M++1 ).
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-47
EXAMPLE 7
8-Isopropyl-7-methox -~ 1 H-quinazolin-2-one
To a solution of 2-amino-7-methoxy-8-(2-propyl)quinazoline (1.3 g) in
tetrafluoroboric acid (50 mL), sodium nitrite solution (2.6 g in 10 mL of
water)
was added dropwise at 0°C. The reaction mixture was stirred at
0°C for 1 hour
and at room temperature for 18 hours. The reaction mixture was cooled in an
ice
bath and neutralized with 30% ammonia solution. The product was extracted with
ethyl acetate (3 x SO mL), combined organic extract was washed with brine and
dried over anhydrous sodium sulfate. The solvent was removed under reduced
pressure to give the title compound as an off-white solid (1.2 g, 92.3%), mp
188-190°C (decom); 1H NMR (CDC13): 8 1.32 (d, 6H, 2 x CH3), 3.44 (m,
1H,
CH), 3.97 (s, 3H, OCH3), 6.88 (d, 1H, Ar-H, J=8.8 Hz), 7.59 (d, 1H, Ar-H,
8.8Hz), 9.0 (s, 1 H, Ar-H), 9.1 (brs, 1 H, NH).
EXAMPLE 8
2-Chloro-8-isopropyl-7-methoxy-quinazoline
7-Methoxy-8-(2-propyl)quinazol-2(1H)-one (1.2 g) was dissolved in 25 mL of
phosphorus oxychloride, and the resulting reaction mixture was heated at
110°C
for 2 hours. Excess POCl3 was removed under reduced pressure. To the residue
ice-cold water was added, and the product was extracted with ethyl acetate
(3 x 50 mL). The combined organic extract was washed with brine, dried over
anhydrous sodium sulfate, and concentrated to give the title compound. This
was
purified on a column of silica gel (Hexane:Ethyl acetate), off white solid,
(780 mg, 60%), mp 135-137°C; 1H NMR (CDC13): 8 1.41 (d, 6H, 2 x CH3),
4.02 (s, 3H, OCH3), 4.33 (m, 1H, CH), 7.38 (d, 1H, Ar-H, J = 9 Hz), 7.8 (d,
1H,
Ar-H, J = 9 Hz), 9.1 (s, 1 H, Ar-H).
EXAMPLE 9
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-phenyl-amine
A mixture of 2-chloro-7-methoxy-8-(2-propyl)quinazoline (100 mg, 0.42 mmol)
and aniline (120 mg, 1.29 mmol) in acetonitrile (3 mL) was heated at
80°C for
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-48-
18 hours in a sealed tube. The solvent was removed under reduced pressure, to
the
residue 5% sodium carbonate solution was added. The product was extracted with
ethyl acetate (3 x 25 mL). The combined organic extract was washed with brine,
dried (Na2S04), and concentrated. The product was purified on a column of
silica
gel to give the title compound as an off white solid. (95 mg, 76.3%), mp
172-173°C; 1H NHR (CDCl3): ~ 1.46 (d, 6H, 2 x CH3), 3.97 (s, 3H, OCH3),
4.33 (m, 1H, CH), 7.0-7.1 (m, 2H, Ar-H), 7.38 (t, 2H, Ar-H, 7.58 (d, 1H, Ar-
H),
7.85 m, 2H, Ar-H), 8.93 (s, 1H, Ar-H); MS m/z C18H19N30 294.21 (M++1);
Analysis: Calcd.: C, 73.7; H, 6.53; N, 14.32. Found: C, 73.8; H, 6.83; N,
14.59.
EXAMPLE 10
6-Cyclopentyl-1,5-dimethoxy-cyclohexa-1,4-dime
To a solution of 2,5-dihydro-1,3-dimethoxybenzene (9.88 g, 70 mmol) in dry THF
(150 mL) was added dropwise nBuLi solution (2.5 M, 42.4 mL) in hexane at
-20°C. The orange colored reaction mixture was stirred for 0.5 hour at -
20°C, then
cyclopentyl bromide (20.9 g, 140 mmol) was added dropwise. The reaction
mixture was stirred for 1 hour at -20°C to 0°C. Excess nBuLi was
destroyed
cautiously with methanol. To the reaction mixture, 140 mL of cold water was
added. The product was extracted with ethyl acetate (3 x 80 mL). The combined
organic extract was washed with brine, dried over anhydrous sodium sulfate,
and
concentrated to give the title compound as light yellow liquid (14.12 g,
96.8%).
1H NMR: 8 1.0-2.4 (Complex, 9H), 2.7-2.94 (m, 3H), 3.52 (s, 6H, 2 x OCH3),
4.70 (t, 2H).
EXAMPLE 11
2-Cyclopentyl-3-methoxy-cyclohex-3-enone
To a solution of 2-cyclopentyl-2,5-dihydro-1,3-dimethoxybenzene ( 5 g, 27
mmol)
in methanol (50 mL) was added 2.5% hydrochloric acid (10 mL) dropwise at
0°C.
The reaction mixture was stirred for 45 minutes and diluted with 30 mL of ice-
cold water. The, product was extracted with ether (3 x 100 mL). The combined
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-49-
organic extract was washed with brine and dried (Na2S04). Removal of the
solvent in vacuo yielded the title compound as light yellow oil (4.3 g,
94.7%).
EXAMPLE 12
2-Cyclopentyl-3-methoxy-cyclohex-2-enone
To a solution of 2-cyclopentyl-3-methoxycyclohex-3-en-1-one ( 4.3 g) in dry
THF
( 120 mL), sodium methoxide ( 1.1 g) was added in portions at room
temperature.
After stirring the reaction mixture at room temperature for 1.5 hours, cooled
in an
ice bath, and neutralized with sodium hydrogen phosphate solution. The organic
layer was separated, aqueous layer was extracted with ethyl acetate (2 x 50
mL).
The combined organic extract was washed with brine and dried over anhydrous
sodium sulfate. The solvent was removed under reduced pressure, and the
resulting product was purified on a column of silica gel (Hex:EtOAc) to give
the
title compound as yellow liquid (2.82 g, 65.5%). 1 H NMR (CDC13): b
1.25-1.8 (m, 8H, 4 x CH2), 1.96 (m, 2H, CH2), 2.36 (t, 2H,CH2), 2.56 (t, 2H,
CH2), 3.22 (m, 1H, CH), 3.79 (s, 3H, OCH3).
EXAMPLE 13
2-Cyclopentyl-6-hydroxymethylene-3-methoxy-cyclohex-2-enone
To a suspension of sodium hydride (2.04 g, 60% suspension, 51 mmol) in benzene
(100 mL), a mixture of ethyl formate (6.7 mL) and 2-cyclopentyl-
3-methoxycyclohex-3-en-1-one (2.82 g, 17 mmol) in benzene was added dropwise
at 5°C. The reaction mixture was stirred at ice temperature for 1 hour
and 18 hours
at room temperature. The reaction mixture was cooled in ice bath, and 200 mL
of
10% sodium hydrogen phosphate solution was added dropwise. The organic layer
was separated, and aqueous layer was extracted with ethyl acetate (2 x 50 mL).
Combined organic extract was washed with brine and dried over anhydrous
sodium sulfate. Solvent was removed in vacuo and the resulting product was
purified on a column of silica gel (Hex:EtOAc) to give the title compound as a
viscous liquid (2 g, 60%).
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-50
EXAMPLE 14
8-Cyclopentyl-7-methoxy-.quinazolin-2-ylamine
A mixture of 6-formyl-2-cyclopentyl-3-methoxycyclohex-2-en-1-one (2.0 g,
10.2 mmol) and guanidine carbonate (4.6 g) in DMF (30 mL) was heated at
150°C
for 4 hours. The solvent was removed under reduced pressure and to the residue
ice-cold water was added. The product was extracted with ethyl acetate
(3 x 50 mL). The combined organic extract was washed with water and brine and
dried over anhydrous sodium sulfate. Solvent was removed under reduced
pressure to give 2.0 g of crude dihydroquinazoline. The dihydroquinazoline was
aromatized to the title compound by heating in nitrobenzene at 150°C in
the'
presence of 10% Pd-C. To a solution of dihydroquinazoline (2.0 g, 8.2 mmol) in
nitrobenzene (20 mL), 200 mg of 10% Pd-C was added, and the reaction mixture
was heated at 150°C for 3 days. The Pd-C was removed by filtration. The
filtrate
was concentrated, and the resulting product was purified on a column of silica
gel
(Hex:EtOAc) to give the title compound as an off white solid (1 g, 50% yield),
mp
181-183°C; 1H NMR (CDC13): 8 1.6-2.2 (complex, 8H, 4 x CH2), 3.9 (s,
3H,
OCH3), 4.27 (t, 1H, CH), 5.02 (brs, 2H, NH2), 7.02 (d, 1H, Ar-H, J = 9 Hz),
7.54 (d, 1H, Ar-H, J = 9 Hz), 8.87 (s, 1H, Ar-H); MS: m/z 244.07, C14H17N30
(M++1 ).
EXAMPLE 15
8-Cyclopentyl-7-methoxy-1 H-quinazolin-2-one
To a solution of 2-amino-8-cylopentyl-7-methoxyquinazoline (1.38 g,) in
tetrafluoroboric acid (30 mL), sodium nitrite solution (1.16 g in 10 mL of
water)
was added dropwise at 0°C. The reaction mixture was stirred at
0°C for 1 hour
and at room temperature for 18 hours. The reaction mixture was cooled in a ice
bath and neutralized with 30% ammonia solution. The product was extracted with
ethyl acetate (3 x 50 mL), combined organic extract was washed with brine and
dried over anhydrous sodium sulfate. The solvent was removed under reduced
pressure to give the title compound as a orange solid (1.0 g, 73%), mp 201-
204°C;
1H NMR (CDC13): 8 1.7-2.0 (complex, 8H, 4 x CH2), 3.43 (m, 1H, CH), 3.96 (s,
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-51-
3H, OCH3), 6.89 (d, 1H, Ar-H, J = 8.8 Hz), 7.59 (d, 1H, Ar-H, J = 8.8 Hz),
8.92 (brs, 1 H, NH), 9.03 (s, 1 H, Ar-H).
EXAMPLE 16
2-Chloro-8-cyclopentyl-7-methoxy-guinazoline
7-Methoxy-8-(2-propyl)quinazol-2(1H)-one (0.95 g) was dissolved in 40 mL of
phosphorus oxychloride, and the resulting reaction mixture was heated at
110°C
for 4 hours. Excess POCl3 was removed under reduced pressure. To the residue
ice-cold water was added, and the product was extracted with ethyl acetate
(3 x 50 mL). The combined organic extract was washed with brine, dried over
anhydrous sodium sulfate, and concentrated to give the chloro compound. This
was purified on a column of silica gel (Hexane:Ethyl acetate), beige solid,
(710 mg, 69% yield), mp 250°C (decomp); 1H NMR (CDC13): 8 1.7-2.0
(complex, 8H, 4 x CH2), 4.17 (s, 3H, OCH3), 4.27 (m, 1H, CH), 7.65 (d, 1H,
Ar-H, J = 9 Hz ), 8.30 (d, 1 H, Ar-H), 9.71 (s, 1 H, Ar-H).
EXAMPLE 17
(8-Cyclopentyl-7-methox~quinazolin-2-yl)-phenyl-amine
A mixture of 2-chloro-7-methoxy-8-(2-propyl)quinazoline (200 mg, 0.76 mmol)
and aniline (212 mg, 2.28 mmol) in acetonitrile (3 mL) was heated at
90°C for
60 hours in a sealed tube. The solvent was removed under reduced pressure, to
the
residue 5% sodium carbonate solution was added. The product was extracted with
ethyl acetate (3 x 40 mL). The combined organic extract was washed with brine,
dried (Na2S04), and concentrated. The product was purified on a column of
silica
gel to give the title compound as a yellow solid (90 mg, 37%), mp 187-
188°C;
1 H NMR (CDCl3) 8: 1.72-2.30 (complex, 8H, 4 x CH2), 3.96 (s, 3H, OCH3),
4.33 (m, 1 H, CH), 7.23-7.41 (m, 2H, Ar-H), 7.58 (d, 1 H, Ar-H), 7.83 (d, 2H,
Ar-H), 8.93 (s, 1H, Ar-H); MS: m/z 319.81, C2pH21N3O; Analysis: Calcd.:
C, 75.21; H, 6.63; N, 13.16. Found: C, 75.03; H, 6.73; N, 13.05.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-52-
EXAMPLE 18
General procedure for coupling_anilines
A mixture of 2-chloroquinazoline (0.03 mmol) and the appropriate aniline
(0.09 mmol) in acetonitrile (5 mL) was heated in a sealed tube for 24 to 72
hours
at 110°C. Solvent was removed under reduced pressure, and the residue
was
suspended in ethyl acetate and treated with 5% Na2C03 solution. The organic
layer was separated, and the aqueous layer was extracted with ethyl acetate
(2 x 20 mL). The combined organic extracts were washed with brine and dried
over Na2S04. The product was purified by chromatography on silica gel.
EXAMPLE 19
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-phenyl-amine
mp.172-173 °C; 1H NHR (CDC13) 8 1.46 (d, 6H, 2xCH3), 3.97 (s, 3H,
OCH3),
4.33 (m, 1H, CH), 7.0-7.1 (m, 2H, Ar-H), 7.38 (t, 2H, Ar-H, 7.58 (d, 1H, Ar-
H),
7.85 m, 2H, Ar-H), 8.93 (s, 1H, Ar-H); MS: m/z C18H~9N30 294.21 (M++1);
Analysis: Calcd.: C 73.7, H 6.53, N 14.32; Found: C 73.8, H 6.83, N 14.59
EXAMPLE 20
1- { 4-~4-(8-Cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyll-piperazin-1-
y1 ~ -ethanone
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-53-
Yellow solid, yield 83%, mp 247-249 °C;~H NMR (CDC13) b: 1.6- 2.3
(complex,
8H, 4XCH2), 2.18 (s, 3H, COCH3), 3.13 (m, 4H), 3.63 (m, 2H), 3.78 (m, 2H),
3.96 (s, 3H, OCH3), 4.30 (m, 1H, CH), 6.96 (d, 2H, Ar-H, J=9 Hz), 7.05 (d, 1H,
Ar-H, J=8.8 Hz), 7.11 (s, 1H, NH), 7.56 (d, 1H, Ar-H, J=8.8 Hz), 7.72 (d, 2H,
Ar-
H, J=9Hz), 8.90 (s, 1 H, Ar-H); MS (ES): m/z 446.29, C26H3,NSO2, (MH+)
Analysis: Calcd.: C, 70.09; H, 7.01, N, 15.72. Found: C, 69.38; H, 7.43; N,
15.06
EXAMPLE 21
(8-Isopropyl-7-methoxy-guinazolin-2-yl)-(4-piperidin-1-yl-phenyl)-amine
Yellow solid, Yield 54.5%, mp 166-167°C; 1H NMR (CDC13): 8 1.44
(d, 6H,
2 x CH3), 1.6 (m, 2H, CH2), 1.74 (m, 4H, 2 x CH2), 3.13 (t, 4H, 2 x CH2),
3.96 (s, 3H, OCH3), 4.30 (m, 1H, CH), 6.9-7.1 (2d and s merged, 3 Ar-H, NH),
7.54 (d, 1 H, Ar-H), 7.7 (d, 2H, Ar-H). 8.88 (s, 1 H, Ar-H); MS (ES): m/z
376.97,
C23H28N40 (M+); Analysis: Calcd.: C, 73.37; H, 7.5; N, 14.88. Found: C, 73.18;
H, 7.69; N, 14.89.
EXAMPLE 22
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-(4-pyrrolidin-1-ylphenyl)-amine
Orange solid, 64.7%, mp 188-190°C; 1H NMR (CDCl3): 8 1.43 (d, 6H, 2
x CH3),
2.01 (m, 4H, 2 x CH2), 3.30 (t, 4H, 2 x CH2), 3.95 (s, 3H, OCH3), 4.29 (m, 1H
CH), 6.61 (d, 2H, Ar-H, J = 8.9 Hz), 6.9-7.1 (d and s merged, 2H, 1 Ar-H, NH),
7.52 (d, 1 H, Ar-H, J = 8.9 Hz), 7.63 (d, 2H, Ar-H, J = 8.9 Hz), 8.85 (s, 1 H,
Ar-H);
MS (ES): m/z 363.02, C22H26N40 (M++1); Analysis: Calcd., C, 72.90; H, 7.23;
N, 15.46. Found: C, 73.35; H, 7.67; N, 14.74.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-54-
EXAMPLE 23
(8-Cyclopentyl-7-methoxy-g uinazolin-2-~)-(4-piperidin-1-yl-phenyl)-amine
Yellow solid, 28% yield, mp 212-214°C; I H NMR (CDCl3): 8 1.55-
2.28
(complex, 14H, 7 x CH2), 3.08-3.14 (m, 4H, 2 x CH2), 3.95 (s, 3H, OCH3), 4.29
(m, 1H, CH), 6.94-7.08 (complex, 4H, 3Ar-H, NH), 7.52-7.57 (m, 1H, Ar-H),
7.65-7.72 (m, 2H, Ar-H), 8.88 (s, 1H, Ar-H); MS (ES): m/z 403.17, C25H30N40
(M++1); Analysis: Calcd.: C, 74.60; H, 7.51; N, 13.92. Found: C, 74.83, H,
7.57,
N, 13.82.
EXAMPLE 24
(8-Cyclopentyl-7-methoxy-guinazolin-2-yl)-(4-pyrrolidin-1-yl-phenyl)-amine
Yellow solid, yield 42.7%, mp 240-242°C; 1H NMR (CDC13): 8 1.68-
2.29
(complex, 12H, 6 x CH2), 3.30 (m, 4H, 2 x CH2), 3.94 (s, 3H, OCH3), 4.28 (m,
1 H, CH), 6.59 (d, 2H, Ar-H, J = 8.9 Hz), 6.95 (s, 1 H, NH), 7.0 (d, 1 H, Ar-
H,
J = 8.9 Hz, ), 7.52 (d, 1H, Ar-H, J = 8.9 Hz), 7.60 (d, 2H, Ar-H, J = 8.9 Hz),
8.86 (s, 1H, Ar-H); MS (ES): m/z 388.98, C24H28N40 (M+); Analysis: Calcd.:
C, 74.20; H, 7.26; N, 14.42. Found: C, 73.94, H, 7.34, N, 14.36.
EXAMPLE 25
4-~4-(8-Isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyll-piperazine-
1-carboxylic acid tert-butyl ester
Yellow solid, yield 64.4%, mp 173-175°C; 1H NMR (CDCl3): 8 1.44
(d, 6H,
2 x CH3), 1.49 (s, 9H, 3 x CH3), 3.1 (t, 4H, 2 x CH2), 3.6 (t, 4H, 2 x CH2),
3.96 (s, 3H, OCH3), 4.3 (m, 1H, CH), 6.98 (d, 2H, Ar-H), 7.04 (d, IH, Ar-H),
7.13 (s, 1 H, NH), 7.56 (d, 1 H, Ar-H), 7.74 (d, 2H, Ar-H), 8.89 (s, 1 H, Ar-
H);
MS (ES): m/z 478.11, C27H35N503 (M++1); Analysis: Calcd. C, 67.9; H, 7.39,
N, 14.66. Found: C, 68.06; H, 7.61; N, 14.16.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-55-
EXAMPLE 26
4-~4-(8-Cyclopentyl-7-methoxy-guinazolin-2-ylamino)-phenyl-piperazine-
1-carboxylic acid tert-butyl ester
Yellow solid, yield 54%, mp 183-184°C; 1H NMR (CDCl3): 8 1.49 (s,
9H,
3 x CH3), 1.68-2.29 (complex, 8H, 4 x CH2), 3.10 (t, 4H, 2 x CH2), 3.61(t, 4H,
2 x CH2), 3.96 (s, 3H, OCH3), 4.29 (m, 1H, CH), 6.96 (d, 2H, Ar-H, J = 8.9
Hz),
7.04 (d, 1 H, J = 8.9 Hz), 7.09 (s, 1 H, NH), 7.56 (d, 1 H, Ar-H, J = 8.9 Hz),
7.71 (d,
2H, Ar-H, J = 8.9 Hz), 8.89 (s, 1H, Ar-H); MS (ES): m/z 504.35,
C29H37N503 (M++1); Analysis: Calcd.: C, 69.16; H, 7.4; N, 13.91. Found:
C, 68.84, H,7.73, N, 13.58.
EXAMPLE 27
(8-Cyclopentyl-7-methoxy-guinazolin-2-yl)-(4-piperazin-1-yl-phenyl)-amine
Yellow solid, yield 77%, mp 218-219°C; 1H NMR (CDCl3): 8 1.68-2.29
(complex, 8H, 4 x CH2), 3.11 (m, 8H, 4 x CH2), 3.96 (s, 3H, OCH3), 4.30 (m,
1 H, CH), 6.97 (d, 2H, Ar-H, J = 8.9 Hz), 7.04 (d, 1 H, J = 8.8 Hz), 7.06 (s,
1 H,
NH), 7.55 (d, 1H, Ar-04.12, C24H29N50 (M++1); Analysis: Calcd.: C, 71.44;
H, 7.24; N, 17.36. Found: C, 70.33; H, 7.86; N, 16.73.
EXAMPLE 28
1-i4-(4-(8-Isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl-piperazin- ~1}-
ethanone
Light yellow solid, yield 79%, mp 197-198°C; 1H NMR (CDCl3): ~
1.44
(d, 6H, 2 x CH3), 2.16 (s, 3H, COCH3) 3.15 (m, 4H), 3.65 (m, 2H), 3.80 (m,
2H),
3.97 (s, 3H, OCH3), 4.30 (m, 1H, CH), 6.98 (d, 2H, Ar-H), 7.04 (d, 1H, Ar-H),
7.13 (s, 1H, NH), 7.56 (d, 1H, Ar-H), 7.75 (d, 2H, Ar-H), 8.89 (s, 1 H, Ar-H);
MS (ES): m/z 420.0 C24H29N502 (M++1); Analysis: Calcd.: C, 68.71; H, 6.97;
N, 16.69. Found: C, 69.07; H, 7.42; N, 16.24.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-56-
EXAMPLE 29
8-Isopropyl-2-phenylamino-guinazolin-7-of
A mixture of 1 (150 mg, 0.51 mmol) and sodium thioethoxide (214 mg,
2.55 mmol) in dimethylformamide (5 mL) was heated at 150°C for 5 hours.
The
solvent was removed under reduced pressure. To the residue, ice-cold water
(30 mL) was added, and the mixture was acidified with acetic acid (0.5 mL).
The
product was extracted with ethyl acetate (3 x 30 mL). The combined organic
extracts were washed with brine, dried over anhydrous sodium sulfate, and
concentrated. The product was purified on a column of silica gel (EtOAc:MeOH)
to give a light yellow solid, yield 70%, mp 103-104°C; 1H NMR(CDC13): 8
1.52
(d, 6H, 2 x CH3), 4.30 (m, 1H, CH), 5.7 brs, 1H, OH), 6.83 (d, 1H, Ar-H,
J = 8.7 Hz), 7.06 (m, 1H, Ar-H), 7.3-7.5 (s and m merged, 3H, 2 Ar-H, NH),
7.49 (d, 1 H, Ar-H, 8.7 Hz), 7.83 (m, 2H, Ar-H), 8.89 (s, 1 H, Ar-H); MS (ES):
m/z
279.37, C17H17N30 (M+). Analysis: Calcd.: C, 73.10; H, 6.13; N, 15.04. Found:
C, 73.34; H, 6.37, N, 14.72.
EXAMPLE 30
General procedure for deprotection of an N Boc group
To a solution of N Boc-protected compound (0.2 mmol) in
dichloromethane (3 mL) was added trifluoroacetic acid dropwise. The reaction
mixture was stirred at room temperature for 3 hours. The solvent was removed
under reduced pressure, and to the residue was added ice-cold water (30 mL).
This
mixture was basified with 5% Na2C03 solution, then the product was extracted
with chloroform (3 x 30 mL). The combined organic extracts were washed with
brine and dried over anhydrous sodium sulfate. The solvent was removed under
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-57-
reduced pressure, and the product was purified on a column of florisil
(EtOAc:MeOH).
EXAMPLE 31
(8-Isopropyl-7-methoxy-auinazoline-2-yl)-(4-piperaziny-1-yl-phenyl)-amine
Amorphous solid, yield 84.4%. 1H NMR (CDC13): 8 1.44 (d, 6H, 2 x CH3),
3.0-3.2 (m, 8H, 4 x CH2), 3.96 (s, 3H, OCH3), 4.3 (m, 1H, CH), 6.9-7.1 (2d
merged, 3H, Ar-H), 7.2 (s, 1H, NH), 7.55 (d, 1H, Ar-H), 7.73 (d, 2H, Ar-H),
8.88 (s, 1H, Ar-H); MS (ES): m/z 378.15, C22H27N50 (M++1).
EXAMPLE 32
2-Amino-1-{4-f4-(8-isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl~-
piperazin-1-yl~-ethanone
Yellow solid, yield 85%; mp 183-185°C; 1H NMR (CDC13): 8 1.44 (d,
6H,
2 x CH3), 3.15 (m, 4H), 3.52 (s, 2H), 3.55 (m, 2H, CH2), 3.83 (m, 2H), 3.97
(s,
3H, OCH3), 4.30 (m, 1H, CH), 6.98 (d, 2H, Ar-H, 8.9 Hz), 7.05 (d, 1H, Ar-H,
8.9 Hz), 7.14 (s, 1H, NH), 7.56 (d, 1H, Ar-H, J = 8.9 Hz), 7.76 (d, 2H, Ar-H,
J = 8.9 Hz), 8.89 (s, 1 H, Ar-H); MS (ES): m/z 434.66 C24H30N602 (M+).
EXAMPLE 33
1- f 4-~4-(8-Isopropyl-7-methoxy-guinazolin-2-ylamino)-phenyl-piperazin-1-yl ~
-
PthannnP
Light yellow solid, yield 79%, m.p. 197-198 °C. 'H NMR (CDC13) 8: 1.44
(d, 6H,
2XCH3), 2.16 (s, 3H, COCH3) 3.15 (m, 4H), 3.65 (m, 2H), 3.80 (m, 2H), 3.97 (s,
3H, OCH3), 4.30 (m, 1H, CH), 6.98 (d, 2H, Ar-H), 7.04 (d, 1H, Ar-H), 7.13 (s,
1H, NH), 7.56 (d, 1H, Ar-H), 7.75 (d, 2H, Ar-H), 8.89 (s, 1H, Ar-H).
MS (ES): m/z 420.0 C24Ha9Ns02 (M++1). Analysis: Calcd. C, 68.71; H, 6.97; N,
16.69. Found: C, 69.07; H, 7.42; N, 16.24.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-58-
EXAMPLE 34
8-Isopropyl-7-methoxy-quinazolin-2-yl)-(4-piperazin-1-yl-phenyl)-amine
Amorphous solid, Yield 84.4%. 'H NMR (CDC13) 8: 1.44 (d, 6H, 2XCH3), 3.0-3.2
(m, 8H, 4XCH2), 3.96 (s, 3H, OCH3), 4.3 (m, 1H CH), 6.9-7.1 (2d merged, 3H,
Ar-H), 7.2 (s, 1 H, NH), 7.55 (d, 1 H, Ar-H), 7.73 (d, 2H, Ar-H), 8.88 (s, 1
H, Ar-
H).MS (ES): m/z 378.15, C22HZ~N50 (M++1).
EXAMPLE 35
2-Amino-1- f 4-~4-(8-isopropyl-7-methoxy-quinazolin-2-ylamino)-phenyl~-
piperazin-1-yl ~ -ethanone
Yellow solid, yield 85%; m.p. 183-185 °C; 'H NMR (CDC13) b: 1.44
(d, 6H,
2XCH3), 3.15 (m, 4H), 3.52 (s, 2H), 3.55 (m, 2H, CHZ), 3.83 (m, 2H), 3.97 (s,
3H,
OCH3), 4.30 (m, 1H, CH), 6.98 (d, 2H, Ar-H, 8.9 Hz), 7.05 (d, 1H, Ar-H, 8.9
Hz),
7.14 (s, 1H, NH), 7.56 (d, 1H, Ar-H, J=8.9 Hz), 7.76 (d, 2H, Ar-H, J=8.9 Hz),
8.89 (s, 1H, Ar-H). MS (ES): m/z 434.66 C24HsoN602 (M+)
EXAMPLE 36
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-(4'-methoxy-b~henyl-4-yl)-amine
Solid, yield 69%, m.p. 198-199 °C; 'H NMR (CDCl3) 8: 1.45 (d, 6H,
2XCH3),
3.2-3.4 (m, 8H, 4xCH2), 3.78 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.31 (m, 1H,
CH), 6.8-7.2 (complex, 8H, 7 Ar-H, NH), 7.56 (d, 1 H, Ar-H), 7.75 (d, 2H, Ar-
H),
8.89 (s, 1H, Ar-H).MS (ES): m/z 484.22 C29H33NsOa (M++1). Analysis: Calcd. C,
72.02; H, 6.88; N, 14.48. Found: C, 71.58; H, 7.30; N, 14.29.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-59-
EXAMPLE 37
~4-(3-Amino-pyrrolidin-1-yl)-phenyls-(8-isopropyl-7-methoxy guinazolin-2-yl)-
amino
Yellow solid, yield 82.3%, mp 125-127 °C, ~H NMR (CDC13) 8: 1.43
(d, 6H,
2xCH3), 1.84 (m, 1H), 2.3 (m, 1H), 3.08 (m, 1H), 3.3-3.6 (complex, 3H), 3.76
(m,
1H), 3.95 (s, 3H, OCH3), 4.30 (s, 1H), 6.59 (d, 2H, Ar-H, J=8.7 Hz), 6.99 (d,
1H,
Ar-H, J= 8.8 Hz), 7.02 (s, 1H, NH), 7.52 (d, 1H, Ar-H, J=8.8 Hz), 7.64 (d, 2H,
Ar-
H, J=8.7 Hz), 8.86 (s, 1H, Ar-H). MS (ES) m/z 378.15 (MH+) CZZHZ~N50.
EXAMPLE 38
(3-Fluoro-4-morpholin-4-yl-phenyl)-(8-isopropyl-7-methoxy-quinazolin-2-yl)-
amino
Yellow solid, yield 65.7% , mp 202-203 °C; ~H NMR (DMSO-d6) b: 1.41
(d, 6H,
2xCH3), 2.96 (t, 4H, 2xCHz), 3.74 (t, 4H, 2XCH2), 3.95 (s, 3H, OCH3), 4.25 (m,
1 H, CH), 7.01 (m, 1 H, Ar-H), 7.24 (d, 1 H, Ar-H, J=8.9 Hz), 7.5 5 (m, 1 H,
Ar-H),
7.78 (d, 1 H, Ar-H, J=8.9 Hz), 8.04. (m, 1 H, Ar-H), 9.12 (s, 1 H, Ar-H), 9.79
(s,
1H, NH). MS(ES), 397.35 (MH+), CZZHzsFNa02. Analysis: Calcd.: C, 66.65; H,
6.36; N, 14.13; Found: C, 66.76; H, 6.34; N, 13,96.
EXAMPLE 39
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-(4-morpholin-4-Y-phenyl)-amine
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-60-
Yellow solid, yield 81.8%, mp. 187-189 °C; 'H NMR (CDCl3) b: 1.44
(d, 6H,
2xCH3), 3.1 S (t, 4H, 2XCH2), 3.89 (t, 4H, 2xCH2), 3.96 (s, 3I-I, OCH3), 4.31
(m,
1 H, CH), 6.97 (d, 2H, Ar-H, J=8.9 Hz), 7.04 (d, 1 H, Ar-H, J=8.9 Hz), 7.12
(s, 1 H,
NH), 7.56 (d, 1 H, Ar-H, J=8.9 Hz), 7.75 (d, 2H, Ar-H, J=8.9 Hz), 8.89 (s, 1
H, Ar-
H). MS (ES): m/z 379.18 (MH+), CZZH26N40z. Analysis: Calcd.: C, 69.82; H,
6.92; N, 14.80. Found: C, 69.95; H, 7.06; N, 14.59.
EXAMPLE 40
2-Amino-1-f 4-~4-(8-cyclopentyl-7-methoxy-guinazolin-2-ylamino)-phenyl~-
piperazin-1-yl~-ethanone
Yellow solid, yield 60%, mp 202 °C; 'H NMR (CDC13) 8: 1.6-2.3
(complex, 8H,
4xCH2), 3.1 (t, 4H, 2xCH2), 3.52 (s, 2H, CHZ), 3.56 (m, 2H), 3.83 (m, 2H),
3.96
(s, 3H, OCH3), 4.3 (m, 1H, CH), 6.96 (d, 2H, Ar-H, J=9 Hz), 7.05 (d, 1H, Ar-H,
8.9 Hz), 7.1 (s, 1 H, NH), 7.57 (d, 1 H, Ar-H, J=8.9 Hz), 7.72 (d, 2H, Ar-H,
J=9
Hz), 8.9 (s, 1 H, Ar-H).
MS (ES): m/z 461.19 (MH+) C26H32N6O2.
EXAMPLE 41
L4-(3-Amino-pyrrolidin-1-yl)-phenyll-(8-cyclopentyl-7-methoxy-guinazolin-2-yl)-
amore
Yellow solid, yield 83%, m.p. 220-221 °C; 'H NMR (CDCl3) 8: 1.6-2.4
(complex,
10H), 3.04 (m, 1H), 3.3-3.6 (m, 3H), 3.7 (m, 1H), 3.95 (s, 3H, OCH3), 4.30 (m,
1 H, CH), 6.58 (d, 2H, Ar-H, J=8.9 Hz), 6.98 (s, 1 H, NH), 7.0 (d, 1 H, Ar-H,
J=8.8
Hz), 7.53 (d, 1 H, Ar-H, J=8.8 Hz), 7.61 (d, 2H, Ar-H, J=8.9 Hz), 8.86 (s, 1
H, Ar-
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-61-
H). MS (ES): m/z 404.22 (MH+) C24H29NSO. Analysis: Calcd.: Found: C, 71.44;
H, 7.24; N, 17.36. Found: C, 70.75; H, 7.44; N, 17.1.
EXAMPLE 42
2-Amino-1-~4-f4-(8-cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyl~-
piRerazin-1-yl ~-4-methyl-pentan-1-one
Yellow solid, yield 77%, m.p. 130-132 °C; 'H NMR (CDC13) 8: 0.97
(m, 6H,
2xCH3), 1.39 (m, 2H), 1.6-2.1 (m, 7H), 2.2 (m, 2H), 3.16 (m, 4H), 3.64 (m,
2H),
3.8 (m, 3H), 3.81 (s, 3H, OCH3), 4.3 (m, 1H, CH), 6.96 (d, 2H, Ar-H, J=8.9
Hz),
7.05 (d, 1 H, Ar-H, J=8.8 Hz), 7.1 (s, 1 H, NH), 7.57 (d, 1 H, Ar-H, J=8.8
Hz), 7.72
(d, 2H, Ar-H, J=8.9 Hz), 8.9 (s, 1H, Ar-H). MS (ES): m/z 517.28 (MH+)
C30H40N602~ ~alysis: Calcd.: C, 69.14; H, 7.8; N, 16.27. Found: C, 69.08; H,
8.22; N, 15.96.
EXAMPLE 43
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(3-fluoro-4-morpholin-4-yl-phenyl)-
amore
Yellow solid, yield 50%, m.p. 252-253 °C; 'H NMR (DMSO-d~) 8: 1.6-
2.2
(complex, 8H, 4xCH2), 2.95 (t, 4H, 2xCH2), 3.74 (t, 4H, 2xCHz), 3.95 (s, 3H,
OCH3), 4.24 (m, 1 H, CH), 7.05 (m, 1 H, Ar-H), 7.2 (d, 1 H, Ar-H, J=8.9 Hz),
7.44
(m, 1 H, Ar-H), 7.78 (d, 1 H, Ar-H, J=8.9 Hz), 8.07 (m, 1 H, Ar-H), 9.12 (s, 1
H, Ar-
H), 9.78 (s, 1H, NH). MS (ES): m/z 423.19 (MH+) C24HZ~FN40z.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-62-
EXAMPLE 44
(8-Cyclopentyl-7-methoxy-guinazolin-2-yl)-(4-morpholin-4-yl-phenyl)-amine
Yellow solid, yield 85%, m.p. 243-244 °C; 'H NMR (DMSO-d6) 8: 1.6-
2.3 (m,
8H, 4xCHz), 3.05 (t, 4H, 2xCHz), 3.75 (t, 4H, 2xCHz), 3.93 (s, 3H, OCH3), 4.24
(m, 1 H, CH), 6.91 (d, 2H, Ar-H, J=9 Hz), 7.19 (d, 1 H, Ar-H, J=8.9 Hz), 7.7-
7.9
(2d merged, 3H, Ar-H), 9.06 (s, 1H, Ar-H), 9.47 (s, 1H, NH). MS (ES): m/z
405.2 (MH+) Cz4HzsNaOz.
EXAMPLE 45
~ 1-f 4-(8-Cyclopentyl-7-methoxy-quinazolin-2-ylamino)-phenyl-pyrrolidin-3-yl
~ -
carbamic acid tert-butyl ester
Yellow solid, yield 65%, m.p. 157 °C (decomp); 1H NMR (CDC13) 8: 1.57
(s, 9H,
3XCH3), 1.6-2.4 (complex, 10H), 3.1-3.65 (m, 4H), 3.96 (s, 3H, OCH3), 4.2-4.4
(m, 2H), 4.75 (s, 1 H, CONH), 6.6 (d, 2H, Ar-H, J=8.9 Hz), 7.06 (s, 1 H, NH),
7.08
(d, 1 H, Ar-H, J=8.9 Hz), 7.54 (d, 1 H, Ar-H, J=8.9 Hz), 7.63 (d, 2H, Ar-H,
J=8.9
Hz), 8.88 (s, 1H, Ar-H). MS (ES): m/z 504.24 (MH+) Cz9H3~N5O3~
EXAMPLE 46
(4-Fluoro-3-methyl-phenyl)-(8-isopropyl-7-methoxy-guinazolin-2-yl)-amine
Off white solid, yield 80%, mp 169-171 °C; ~H NMR (CDC13) 8: 1.46
(d, 6H,
2xCH3), 2.33 (d, 3H, Ar-CH3, J=1.7 Hz), 3.97 (s, 3H, OCH3), 4.3 (m, 1 H, CH),
. 6.99 (m, 1 H, Ar-H), 7.06 (d, 1 H, Ar-H, J=8.9 Hz), 7.18 s, 1 H, NH), 7.4
(m, 1 H,
Ar-H), 7.58 (d, 1 H, Ar-H, J=8.9 Hz), 7.90 (m, 1 H, Ar-H), 8.91 (s, 1 H, Ar-
H).
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-63-
MS(ES) m/z 326.24 (MH+). Analysis: Calcd for C,9HZOFN30.1/3H20; C, 68.86,
H, 6.29, N, 12.68. Found: C, 69.21, H, 6.26, N, 12.59.
EXAMPLE 47
(8-Isopropyl-7-methoxy-quinazolin-2-yl)-(4-~peridin-1-yl-phen~)-amine
Yellow solid, yield 72%, mp 160-161 °C; 'H NMR (CDC13) 8: 1.46 (d,
6H,
2xCH3), 1.6 (m, 2H, CH2), 1.77 (m, 4H, 2xCH2), 3.0 (t, 4H, 2xCH2), 3.97 (s,
3H,
OCH3), 4.31 (m, 1 H, CH), 6.96 (m, 1 H, Ar-H), 7.06 (d, 1 H, Ar-H, J=8.8 Hz),
7.17
(s, 1 H, NH), 7.25 (m, 1 H, Ar-H), 7.58 (d, 1 H, Ar-H, J=8.8 Hz), 7.93 (m, 1
H, Ar-
H), 8.9 (s, 1H, Ar-H). MS (ES): m/z 395.48 (MH+) C23HZ~FN40. Analysis Calcd
C, 70.03, H, 6.9, N, 14.2; Found: C, 69.44; H,7.01; N, 14.06.
EXAMPLE 48
(8-Cyclopentyl-7-methoxy-guinazolin-2-yl)-(4-fluoro-3-meth ~~l-phenyl)-amine
Off white solid, yield 56%, mp 208-209 °C; 'H NMR (CDC13) 8: 1.6-
2.2
(complex, 8H, 4XCHZ), 2.32 (d, 3H, CH3, J=1.8 Hz), 3.97 (s, 3H, OCH3), 4.36
(m,
1 S 1 H, CH), 6.98 (m, 1 H, Ar-H), 7.07 (d, 1 H, Ar-H, J=8.9 Hz), 7.13 (s, 1
H, NH),
7.44 (m, 1 H, Ar-H), 7.5 8 (d, 1 H, Ar-H, J=8.9 Hz), 7.82 (m, 1 H, Ar-H), 8.91
(s,
1H, Ar-H). MS (ES): m/z 352.29 (MH+) CZ,HZZFN30. Analysis: Calcd. for
CZiH22FNs0.1/2 H20: C, 69.98; H, 6.43; N, 11.66. Found: C, 69.62; H, 6.09; N,
11.51.
EXAMPLE 49
(8-cyclopentyl-7-methoxy-quinazolin-2-yl)-(3-Fluoro-4-piperidin-1-yl-phenyl)-
amore
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-64-
Yellow solid, yield 56%, mp 200-201 °C; 'H NMR (CDC13) 8: 1.4-2.3
(complex,
14H, 7xCHz), 3.0 (t, 4H, 2xCHz), 3.96 (s, 3H, OCH3), 4.32 (m, 1H, CH), 6.95
(m,
1 H, Ar-H), 7.06 (d, 1 H, Ar-H, J=8.8 Hz), 7.16 (m, 1 H, Ar-H), 7.19 (s, 1 H,
NH),
7.57 (d, 1H, Ar-H, J=8.8 Hz), 7.96 (m, 1H, Ar-H), 8.9 (s, 1H, Ar-H). MS (ES):
m/z 421.20 (MH+). Cz5Hz9FNa0. Analysis: Calcd. For Cz5Hz9FNa0.1/2 HzO; C,
69.91; H, 7.04; N, 13.04. Found: C, 69.67; H, 6.84; N, 12.79.
EXAMPLE 50
(8-Cyclopentyl-7-methoxy-quinazolin-2-yl)-(3-fluoro-4-piperazin-1 yl-phenyl)-
amore
Yellow solid, yield 82%, mp 228-229 °C; 'H NMR (CDC13) 8: 1.7-2.3.
(complex,
8H, 4xCHz), 3.06 (s, 8H, 4xCHz), 3.97 (s, 3H, OCH3), 4.3 (m, 1H, CH), 6.94 (m,
1 H, Ar-H), 7.08 (d, 1 H, Ar-H, J=9 Hz), 7.1-7.2 (s and m merged, 2H, NH, Ar-
H),
7.58 (d, 1H, Ar-H, J=9 Hz), 7.99 (m, 1H, Ar-H, 8.91 (s, 1H, Ar-H). MS (ES):
m/z
422.24 (MH+) Cz4HzgFN50. Analysis calcd for Cz4HzgFN50.H20; C, 65.58; H,
6.88; N, 15.93. Found: C, 65.44; H, 6.2; N, 15.57.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-65-
ExampleCdkB Cdk2A Cdk2E Cdk4
Number IC50 IC50 IC50 IC50
19 1.038 0.253 1.075 0.125
20 >5 0.050 0.265 0.002
21 >5 0.410 3.100 0.052
22 >5 1.597 >5 0.560
23 >5 0.384 >5 0.011
24 >5 7.690 >5 1.750
25 10.200 1.622 10.000 0.063
26 4.200 0.646 5.000 0.051
27 0.132 0.028 0.250 0.001
29 0.813 0.250 0.550 0.325
33 na na 0.900 0.025
34 0.506 0.185 1.200 0.016
35 0.800 0.191 1.100 0.010
36 na na >5 0.225
37 1.300 0.296 2.550 0.011
38 1.233 0.451 2.750 na
39 1.970 0.665 4.400 0.052
40 0.270 0.095 0.450 0.002
41 0.398 0.203 0.950 0.116
42 0.333 0.103 0.500 na
43 >5 0.966 >5 0.070
44 1.590 0.383 0.650 0.018
45 > 1.7 2.395 6.900 0.275
46 1.201 0.246 1.200 1.500
47 1.070 0.264 4.100 0.075
48 1.170 0.574 2.200 2.400
49 >5 na 8.000 0.085
50 0.214 0.062 0.300 0.004
Table 1. ICS values in ~M
As noted above, the compounds of this invention are potent inhibitors of
cyclin-dependent kinases, and accordingly, are useful in treating and
preventing
neurodegenerative disorders, atherosclerosis, and other cell proliferative
disorders
like cancer. The compounds have exhibited excellent inhibitory activity
against a
wide variety of cyclin-dependent kinases, all in assay systems routinely
utilized to
measure such activity. A typical assay, for instance, measures inhibitory
activity
against the cyclin D dependent kinase 4 enzyme (cdk4/D). The invention
compounds of Formula I exhibited IC50 values ranging generally from 0.011 yM
to 1.75 p.M. The cdk4 assay was carried out as follows.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-66-
Cyclin-Dependent Kinase 4 (cdk4) Assay
Enzyme assays for IC50 determinations (Table 1 above) and kinetic
evaluation were performed in 96 well filter plates (Millipore MADVN6550). The
total volume was 0.1 mL containing a final concentration of 20 mM TRIS
(tris[hydroxymethyl]aminomethane), at pH 7.4, 50 mM NaCI, 1 mM
dithiothreitol, 10 mM MgCl2, 25 pM ATP containing 0.25 ~Ci of [32P]ATP,
20 ng of cdk4, 1 pg of retinoblastoma, and appropriate dilutions of a compound
of
the present invention. All components except the ATP were added to the wells,
and the plate was placed on a plate mixer for 2 minutes. The reaction was
started
by adding [32P]ATP and the plate was incubated at 25°C for 15 minutes.
The
reaction was terminated by addition of 0.1 mL of 20% trichloroacetic acid
(TCA).
The plate was kept at 4°C for at least 1 hour to allow the substrate to
precipitate.
The wells were then washed five times with 0.2 mL of 10% TCA and 32p
incorporation was determined with a beta plate counter (Wallac Inc.,
Gaithersburg, MD).
The IC50 determinations and kinetic evaluation were performed in a
96-well filter plate (Millipore MADVN6550) in a total volume of 0.1 mL of
mM TRIS (tris[hydroxymethyl]aminomethane), at pH 7.4, 50 mM NaCI, 1 mM
dithiothreitol, 10 mM MgCl2, 12 mM ATP containing 0.25 pCi of [32P]ATP,
20 20 ng of enzyme (either cdk2/cyclinE, cdk2/A, or cdc2/cyclinB), 1 pg
retinoblastoma, and appropriate dilutions of the particular invention
compound.
All components except the ATP were added to the wells, and the plate was
placed
on a plate mixer for 2 minutes. The reaction was begun by addition of
[32P]ATP,
and the plate was incubated at 25°C for 15 minutes. The reaction was
terminated
by addition of 0.1 mL of 20% TCA. The plate was kept at 4°C for at
least 1 hour
to allow the substrate to precipitate. The wells were then washed five times
with
0.2 mL of 10% TCA and 32P incorporation determined with a beta plate counter
(Wallac Inc., Gaithersburg, MD).
The ICSp values were determined using enzyme assays performed in a
total volume of 0.1 mL of a solution comprising 25 mM Hepes, pH 7.4, 5 mM
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-67-
MgCL2, 2 mM MnCL2, 50 pM sodium vanadate, 5 to 10 ng of cdk4, and 200 yM
of a substrate peptide Ala-Glu-Gly-Ser-Ala-Tyr472_Glu-Glu-Val-NH2, derived
from the amino acid [Try472 has been shown to be 1 of 4 tyrosines in PLC-y
that
are phosphorylated by the EGF receptor tyrosine kinase (Wahl M.L, et al., J.
Biol.
Chem., 1990;265:3944-3948), and the peptides derived from the enzyme sequence
surrounding this site are excellent substrates for the enzyme], 10 pM ATP
containing 1 ~Ci of [32]ATP and incubated for 10 minutes at room temperature.
The reaction was terminated by the addition of 2 mL of 75 mM phosphoric acid,
and the contents of the reaction passed through a 2.5 em phosphocellulose
filter
disc to bind the peptide. The filter was washed five times with 75 mM
phosphoric
acid, placed in a vial along with S mL of scintillation fluid (Ready gel,
Beckman),
and counted on a scintillation counter.
The compounds of Formula I have also exhibited good inhibitor activity
against other cyclin-dependent kinase enzymes such as cdk2/cyclinE,
cdk2/cyclinA, and cdkl/cyclinB.
When measured against cdk2/E, the invention compounds exhibited IC50
values ranging generally from about 0.004 to greater than ~ pM. Against
cdk2/A,
the compounds exhibited ICAO values ranging from about 0.028 to greater than
5 ~1VI, and against cdk2/B, generally from about 0.214 to greater than5 ~l\M.
The
assays were carried out as described above, and specific data is given in
Table 1
above.
CdkS/n25 Proline-directed Protein Kinase Assav Protocol
Source of enzyme: recombinant baculovirus-infected insect cell sf9 - expressed
recombinant CdkS-p25 complex
Purpose: To evaluate the ability of test agents to inhibit CdkS/p25
phosphorylation
of Histone H1.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-68-
Method: Baculovirus-insect cell His-tagged CdkS/Glu-tagged p25 (or GST-p25)
enzyme complex is diluted to a concentration of 50 ng/20 yL in Enzyme Dilution
Buffer (EDB - 50 mM Tris-HCI [pH 8.0], 10 mM NaCI, 10 mM MgCl2, and
1 mM DTT). A 20 pL sample of test agent (diluted in EDB) is then combined
S with 20 pL of the of the final CdkS/p25 enzyme preparation and allowed to
stand
for 5 minutes at room temperature. Twenty-five microliters of substrate
solution
containing 115 pL/mL Histone Hl, 30 pM ATP (vanadate-free), and 30 yCi/mL
Y_33p ATP (Amersham) in EDB is then added to the test agent/enzyme
preparation and shaken at 30°C for 45 minutes. A 50 yL sample of the
final
preparation is added to 100 mL of 150 mM phosphoric acid on ice for 30 minutes
to facilitate precipitation. The precipitate is then filtered through a 96
well
phosphocellucose filter plate and subsequently rinsed 3 times with 75 mM
phosphoric acid. Each well then receives 20 pL of scintillation cocktail, and
the
plates are counted for beta emissions using a Trilux Counter (33P filter
protocol).
Test samples are compared to Control (no test agent present; as 0% inhibition)
and
Baseline level (no enzyme, no test agent; as 100% inhibition) beta emissions
to
determine the percent inhibition of Histone Hl phosphorylation.
The invention compounds can be formulated in conventional manners to
provide convenient dosage forms for delivery to mammals by various routes,
including oral, parenteral (i.e., subcutaneous, intravenous, and
intramuscular),
transdermal, e.g. slow release skin patch or cream, as well as by slow release
delivery devices such as osmotic pumps, suppositories, and buccal seals. The
following examples further illustrate how the compounds are readily
formulated.
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-69
EXAMPLE 51
50 mg Tablet Formulation
Per Per 10,000
Tablet Tablets
0.050 Example 20 500 g
g
0.080 Lactose 800 g
g
0.010 corn starch (for mix) 100 g
g
0.008 corn starch (for paste) 80 g
g
0.148 1480 g
g
0.002 magnesium stearate (1%) 20 g
g
v.l..rv ~ 1JVV
The quinazoline, lactose, and corn starch (for mix) are blended to
uniformity. The corn starch (for paste) is suspended in 600 mL of water and
heated with stirring to form a paste. This paste is used to granulate the
mixed
powders. The wet granules are passed through a No. 8 hand screen and dried at
80°C. The dry granules are then passed through a No. 16 screen. The
mixture is
lubricated with 1 % magnesium stearate and compressed into tablets in a
conventional tableting machine. The tablets are useful for treating cancers
such as
breast, prostate, lung, ovarian, colon, pancreatic, melanoma, esophageal,
brain,
Kaposi's sarcoma, and lymphomas, or neurodegenerative disorders such as
Alzheimer's.
EXAMPLE 52
Preparation of Oral Suspension
Ingredient Amount
Example 20 500 mg
Sorbitol solution (70% N.F.) 40 mL
Sodium benzoate 150 mg
Saccharin 10 mg
Cherry flavor 50 mg
Distilled water qs 100 mL
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-70-
The sorbitol solution is added to 40 mL of distilled water, and the
quinazoline is suspended therein. The saccharin, sodium benzoate, and
flavoring
are added and dissolved. The volume is adjusted to 100 mL with distilled
water.
Each milliliter of syrup contains 5 mg of invention compound.
EXAMPLE 53
Preparation of Parenteral Solution
In a solution of 700 mL of propylene glycol and 200 mL of water for
injection is suspended 20.0 g of Example 20 with stirring. After suspension is
complete, the pH is adjusted to 5.5 with hydrochloric acid, and the volume is
made up to 1000 mL with water for injection. The formulation is sterilized,
filled
into 5.0 mL ampoules, each containing 2.0 mL (representing 40 mg of invention
compound) and sealed under nitrogen.
EXAMPLE 54
Suppositories
A mixture of 400 mg of Example 20, and 600 mg of theobroma oil is
stirred at 60°C to uniformity. The mixture is cooled and allowed to
harden in a
tapered mold to provide a 1 g suppository.
EXAMPLE 55
Slow Release Formulation
Five hundred milligrams of Example 20 is converted to a hydrochloride
salt and placed into an Oros osmotic pump for controlled release for treatment
of
atherosclerosis.
EXAMPLE 56
Skin Patch Formulation
Fifty milligrams of Example 20 is admixed with 50 mg of propylene
glycol monolaurate in a polydimethylsiloxane adhesive. The mixture is layered
onto an elastic film made with an adhesive formulation of polybutene,
polyisobutylene, and propylene glycol monolaurate. The layers are placed
between 2 layers of polyurethane film. A release liner is attached to the
adhesive
CA 02386955 2002-04-09
WO 01/38315 PCT/US00/30376
-71-
surface, and is removed prior to application to a skin surface. The propylene
glycol monolaurate serves as a permeation-enhancing agent.