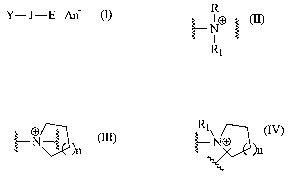Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
QUATERNARY AMMONIUM AND THEIR USE AS ANTI-TUSSIVE AGENT
BACKGROUND OF THE INVENTION
Conventional cough preparations containing an effective anti-tussive agent
such as
codeine have long been used for the symptomatic relief of coughs. However.
codeine has
various side effects which are undesirable.
Accordingly, the present invention relates to compounds and pharmaceutical
compositions having anti-tussive activity, and a method of treating and/or
preventing coughs in
1 o warm-blooded animals in need thereof by administering an effective amount
of the compounds
or the pharmaceutical compositions of the invention.
SUMMARY OF THE INVENTION
The problems of the prior art have been overcome by the present invention.
which
provides compounds and pharmaceutical compositions possessing anti-tussive
activity, and a
method of administering the same to warm-blooded animals, including humans.
The present
invention is related to quaternary ammonium compounds that have been found to
be useful in
the treatment and/or prevention of cough.
2o In one aspect, the present invention concerns the use of certain quaternary
ammonium
compounds as active ingredient in the manufacture of a medicament for use in
the treatment
and/or prevention of cough in warm-blooded animals, including humans.
Another aspect of the present invention provides a method for the treatment
and/or
prevention of cough in warm-blooded animals, including humans. which method
comprises
administering to a warm-blooded animal in need thereof certain quaternary
ammonium
compounds.
Another aspect of the present invention is directed to certain novel
quaternary
3o ammonium compounds that are useful for the treatment and/or prevention of
cough in warm-
blooded animals, including humans.
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
In another aspect. the present invention provides a pharmaceutical composition
for the
treatment and/or prevention of couch, comprising an effective amount of
certain novel
quaternary ammonium compounds and a pharmaceutically acceptable carrier,
diluent or
excipient.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a flow diagram showing the layout of the experimental apparatus
used for
cough determination; and
1 o Figures 2A and 2B are expanded scale recordings of pressure changes
derived from the
differential pressure transducer during characteristic responses exhibited by
a guinea-pig during
exposure to an aerosol of citric acid.
DETAILED DESCRIPTION OF THE INVENTION'
As used herein, the following terms have the following meaning:
"Alkyl" refers to a branched or unbranched hydrocarbon fragment containing the
specified number of carbon atoms and having one point of attachment. Examples
include n-
propyl (a C3 alkyl), isopropyl (also a C3 alkyl) and t-butyl (a C4 alkyl).
"Alkoxyalkyl" refers to an alkylene group substituted with an alkoxy group.
For
example. methyoxyethyl (CH30CH~CH2-) and ethoxymethyl (CH3CH~OCH~-) are both
C3
alkoxyalkyl groups.
"Alkylene" refers to a divalent radical which is a branched or unbranched
hydrocarbon
fragment containing the specified number of carbon atoms and having two points
of attachment.
An example is propylene (-CH2CH2CH2-), a C3 alkylene.
"Aralkyl" refers to an alkylene group wherein one of the points of attachment
is to an
aryl group. An example is the benzyl group (C6HSCH2-), a C~ aralkyl group.
-2-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
"Alkanoyloxy" refers to an ester substituent wherein the ether oxygen is the
point of
attachment to the molecule. Examples include propanoyloxy (CH3CHZC(O)-O-), a
C3
alkanoyloxy and ethanoyloxy (CH3C(O)-O), a CZ alkanoyloxy.
"Alkoxy" refers to an O-atom substituted by an alkyl group, for example
methoxy (-
OCH3), a C1 alkoxy.
"Alkoxycarbonyl" refers to an ester substituent wherein the carbonyl carbon is
the point
of attachment to the molecule. Examples include ethoxycarbonyl (CH3CHzOC=O), a
C3
alkoxycarbonyl, and methoxycarbonyl (CH30C(O)-), a CZ alkoxycarbonyl.
"Aryl" refers to aromatic groups which have at least one ring having a
conjugated pi
electron system and includes carbocyclic aryl, heterocyclic aryl (also known
as heteroaryl
groups) and biaryl groups, all of which may be optionally substituted.
Carbocyclic aryl groups
are generally preferred in the compounds of the present invention, wherein
phenyl and naphthyl
groups are preferred carbocyclic aryl groups.
"Cycloalkyl" refers to a ring, which may be saturated or unsaturated and
monocyclic,
bicyclic or tricyclic formed entirely from carbon atoms. An example is the
cyclopentenyl group
(CSH~-), which is a five carbon unsaturated cycloalkyl group.
"Carbocyclic" refers to a ring which may be either an aryl ring or a
cycloalkyl ring, both
as defined above.
"Thioalkyl" refers to a sulfur atom substituted by an alkyl group, for example
thiomethyl
(CH3S-), a C1 thioalkyl.
"Hydroxyalkyl" refers to a branched or unbranched hydrocarbon fragment bearing
an
hydroxy (-OH) group. Examples include hydroxymethyl (-CH20H, a Clhydroxyalkyl)
and
1-hydroxyethyl (-CHOHCH3, a CZhydroxyalkyl).
"Pharmaceutically acceptable carriers" for therapeutic use are well known in
the
pharmaceutical art, and are described, for example, in Remin ons
Pharmaceutical Sciences,
Mack Publishing Co. (A.R. Gennaro edit. 1985). For example, sterile saline and
-3-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
phosphate-buffered saline at physiological pH may be used. Preservatives,
stabilizers, dyes alnd
even flavoring agents may be provided in the pharmaceutical composition. For
example,
sodium benzoate, sorbic acid and esters of p-hydroxybenzoic acid may be added
as
preservatives. Id. at 1449. In addition, antioxidants and suspending agents
may be used. Id.
"Pharmaceutically acceptable salt" refers to salts of the compounds of the
present
invention derived from the combination of such compounds and an organic or
inorganic acid
(acid addition salts) or an organic or inorganic base (base addition salts).
The compounds of the
present invention may be used in either the free base or salt forms, with both
forms being
1 o considered as being within the scope of the present invention.
The "therapeutically effective amount" of a compound of the present invention
will
depend on the route of administration, the type of warm-blooded animal being
treated, and the
physical characteristics of the specific warm-blooded animal under
consideration. These factors
and their relationship to determining this amount are well known to skilled
practitioners in the
medical arts. This amount and the method of administration can be tailored to
achieve optimal
efficacy but will depend on such factors as weight, diet, concurrent
medication and other factors
which those skilled in the medical arts will recognize.
2o Compositions described herein as "containing a compound of formula (I)"
encompass
compositions that contain more than one compound of formula (I).
The origin of the cough to be treated by the present invention is not
particularly limited,
and can include virtually any respiratory disorder, such as chronic
obstructive pulmonary
disease, tuberculosis, bronchitis, respiratory malignancies, asthma, allergy,
pulmonary fibrosis,
respiratory tract inflammation, emphysema, pneumonia, lung cancer, presence of
foreign bodies,
soar throat, common cold, influenza, respiratory tract infection,
bronchoconstriction, inhalation
of irritants, smoker's cough, chronic non-productive cough, neoplastic cough,
cough due to
angiotension converting enzyme (ACE) inhibitor therapy, etc. Cough may also
occur without a
3 o known cause.
This invention describes certain quaternary ammonium compounds and their
utility as
anti-tussive agents. The invention relates to the discovery that quaternary
ammonium
-4-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
compounds of the following formula (I), and pharmaceutically acceptable salts.
esters, amiss,
complexes, chelates, solvates, stereoisomers. stereoisomeric mixtures,
geometric isomers,
crystalline or amorphous forms, metabolites. metabolic precursors or prodrugs
thereof, are
useful in the treatment and/or prevention of cough in warm-blooded animals,
including humans.
Thus, in a first aspect, the present invention is directed to a method for the
treatment
and/or prevention of cough in a warm-blooded animal, which method comprises
administering to a warm-blooded animal in need thereof, a therapeutically
effective amount
of a compound of formula (I), or a pharmaceutically acceptable salt, ester,
amide, complex,
to chelate, solvate, stereoisomer, stereoisomeric mixture, geometric isomer,
crystalline or
amorphous form, metabolite, metabolic precursor or prodrug thereof:
Y-J-E Ari (I)
wherein J is independently selected from a group represented by one of the
following formulae
(II), (III) and (IV):
R
R1
NO~ ~N~~n ~NO ~n
RI _
t s (II) (III) (IV)
such that when J is represented by formulae (II), (III) or (IV), compounds of
the present
invention are represented by the following formulae (V), (VI) or (VII)
respectively:
R Y N-E E-NO
Y-NO E
)n
R1 Y
(V) (VI) (VII)
wherein n is an integer of from 0 to 4; R, Rl and E are independently selected
from
20 -CHZ-RI6 and a group represented by the following formula (VIII):
R2 R4 ~ i 7
C C C X-A
R3 ~R~ Ri 8
p r
(VIII)
-5-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
wherein X is O (oxygen) or S (sulfur); R2, R;, R.~, R;, R16, R, ~ and R18 are
independently selected
from hydrogen, hydroxy, Cl-Cg alkoxv, C1-Cg alkyl, C~-Cg alkoxyalkyl, C~-Cg
hydroxyalkyl and
C~-C12 aralkyl; p is an integer of from 0 to 8, q is an integer of from 0 to 8
and r is an integer of
from 0 to 8; A is selected from C,-C,~ alkyl, a C3-C,3 carbocyclic ring, and
ring systems selected
from formulae (IX), (X), (XI), (XII), (XIII), (XIV), (XV) and (XVI):
R~
\ ~ / \
/ \ H
R9 Rio Rl i Rio R~ t
Rg \ / \ /
(IX) (X) (XI)
\ ~ i
R12
\ Z \ / \
(XII) (XIII)
(XIV)
/ /
Rl~ \ Z>
N
(XV) (XVI)
where R6, R~, Rg, R9, Rlo, R> > and RI2 are independently selected from
bromine, chlorine,
fluorine, carboxy, hydrogen, hydroxy, hydroxymethyl, methanesulfonamido,
nitro, sulfamyl,
trifluoromethyl, C2-C7 alkanoyloxy, Cl-C6 alkyl, C1-C6 alkoxy, C2-C7
alkoxycarbonyl, CI-C6
thioalkyl, aryl and N(R13,R~4) where R13 and RI4 are independently selected
from hydrogen,
acetyl, methanesulfonyl and C1-C6 allcyl, and Z is selected from CH, CH2, O.
S, NH and N-Rl;
2o where Z may be directly bonded to X when Z is CH; and R1; is selected from
hydrogen, C1-C6
alkyl, C3-Cg cyclocalkyl, aryl and benzyl;
Y is independently selected from hydrogen, -CHZ-Rlb and a group represented by
the following
formula (VIII):
-6-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
R~ R4 I 17
C C C X-A
R3 IRs Ri s
p q r
(VIII)
wherein X is O (oxygen) or S (sulfur); R2, R3, R4, R5, R~6, Rl~ and Rlg are
independently selected
from hydrogen, hydroxy, C,-Cgalkoxy, Ci-Cg alkyl, CZ-Cg allcoxyalkyl, Cl-C8
hydroxyalkyl and
C~-C~Z aralkyl; p is an integer of from 0 to 8, q is an integer of from 0 to 8
and r is an integer of
from 0 to 8; A is selected from C;-C i2 alkyl, a C3-C 13 carbocyclic ring, and
ring systems selected
from formulae (IX), (X), (XI), (XII), (XIII), (XIV), (XV) and (XVI):
R7
\ H
\ ~ / \
R9 Rio Ru Rlo Rn
Rg \ / \ /
(IX) (X) (XI)
/ / \ ~ i
Ri2
\ Z \ / \
~ s (XII) (XIII)
(XIV)
/
Riz \ _ ~ wN
Z
(XV) (XVI)
where R6, R7, R8, R9, Rlo, R1~ and R12 are independently selected from
bromine, chlorine,
fluorine, carboxy, hydrogen, hydroxy, hydroxymethyl, methanesulfonamido,
vitro, sulfamyl,
?o trifluoromethyl, C~-C~ alkanoyloxy, C,-C6 alkyl, C1-C6 alkoxy, C2-C~
allcoxycarbonyl, CI-C6
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
thioalkyl, aryl and N(R13,R,4) where R13 and R14 are independently selected
from hydrogen,
acetyl, methanesulfonyl and C~-C6 allyl, and Z is selected from CH, CHI, O, S,
NH and N-R,5
where Z may be directly bonded to X when Z is CH, and R1; is selected from
hydrogen, C~-C6
alkyl, C;-Cg cyclocalkyl, aryl and benzyl; when J is represented by formula
(VII), Y is a group
on any one of the carbon atoms of the nitrogen heterocyclic ring of formula
(VII); An- is the acid
addition salt of a pharmaceutically acceptable acid or the anion from a
pharmaceutically
acceptable salt;
with the provisos that (a) when J is represented by formula (II) then Y is
represented by formula
(VIII); (b) when J is represented by formula (III) then Y is represented by
formula (VIII); (c)
t 0 when J is represented by formula (IV) and Y is not represented by formula
(VIII), Ri and E
cannot both be -CHI-R~6 and (d) p, q and r cannot all be 0.
In one preferred aspect, the present invention concerns a method as described
above in
the first aspect, wherein J is represented by formula (II).
In another preferred aspect, the present invention concerns a method as
described above
in the first aspect, wherein J is represented by formula (III).
In another preferred aspect, the present invention concerns a method as
described above
2o in the first aspect, wherein J is represented by formula (IV).
In another preferred aspect, the present invention concerns a method as
described above
in the first aspect or any one of the preceding preferred aspects, wherein A
is selected from
formulae (IX), (X), (XI), (XII), (XIII), (XIV), (XV) and (XVI).
In yet another preferred aspect, the present invention concerns a method as
described
above in the first aspect or any one of the preceding preferred aspects,
wherein A is selected
from formulae (IX), (X), (XI) and (XII).
3o In another preferred aspect. the present invention concerns a method as
described above
in the first aspect or any one of the preceding preferred aspects, wherein X
is O (oxygen).
-g_
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
The present invention also provides a method for the treatmen'~and/or
preven~on of
cough in a warm-blooded animal, which method comprises administering to a warm-
blooded
animal in need thereof, a therapeutically effective amount of a compound
having the
following formula, or a pharmaceutically acceptable salt, ester, amide,
complex. chelate,
solvate, stereoisomer, stereoisomeric mixture, crystalline or amorphous form,
metabolite,
metabolic precursor or prodrug thereof:
Rz R4 ~ i 7
~1 C C C X-A
An Rl IR3 IRs R~ s
P q r 2
wherein R and Rl are each -CHZ-R,6; R2, R;, Ra, R;, R~6, Rl~ and R18 are
independently selected
from hydrogen, hydroxy, CI-C8 alkyl. C,-C8 hydroxyalkyl and C~-C12 aralkyl; p
is an integer of
1o from 0 to 3, q is an integer of from 0 to 3 and r is an integer of from 0
to 3; X is O (oxygen); A is
selected from formulae (IX), (X), (XI), (XII), (XIII), (XIV), (XV) and (XVI)
as described above;
and An- is the anion from a pharmaceutically acceptable salt; with the proviso
that p, q and r
cannot all be 0.
The present invention further provides a method for the treatment and/or
prevention of
cough in a warm-blooded animal, which method comprises administering to a warm-
blooded
animal in need thereof, a therapeutically effective amount of a compound
having the
following formula, or a pharmaceutically acceptable salt, ester, amide,
complex, chelate,
solvate, stereoisomer, stereoisomeric mixture, crystalline or amorphous form,
metabolite,
2o metabolic precursor or prodrug thereof:
Rz R4 I i ~
E~1 C C C X-A
I I ( I
Rt R3 Rs R18
p q r An
wherein R, R1 and E are each -CHZ-R16; R2, R3, R4, R5, R,6, Rl~ and RIg are
independently
selected from hydrogen, hydroxy, C,-C8 alkyl, CI-C8 hydroxyalkyl and C~-C12
aralkyl; p
is an integer of from 0 to 3, q is an integer of from 0 to 3 and r is an
integer of from 0 to 3; X is
z5 O (oxygen); A is selected from formulae (IX), (X), (XI), (XII), (XIII),
(XIV), (XV) and (XVI) as
-9-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
described above: and An- is the anion from a pharmaceutically acceptable salt:
with the prov~o
that p, q and r cannot all be 0.
The present invention further provides a method for the treatment and/or
prevention of
cough in a warm-blooded animal, which method comprises administering to a warm-
blooded
animal in need thereof, a therapeutically effective amount of a compound of
formula (I)
which is N,N,N-trimethyl-mexiletine chloride having the following structure,
or a
pharmaceutically acceptable complex, chelate, solvate, stereoisomer,
stereoisomeric mixture,
crystalline or amorphous form, metabolite, metabolic precursor or prodrug
thereof:
O CH3
D~N CH3 Cr
CH3 CH3
to
The present invention further provides a method for the treatment and/or
prevention of
cough in a warm-blooded animal, which method comprises administering to a warm-
blooded
animal in need thereof, a therapeutically effective amount of a compound of
formula (I)
which is N,N-dimethyl-propranolol chloride having the following structure, or
a
pharmaceutically acceptable complex, chelate, solvate, stereoisomer,
stereoisomeric mixture,
crystalline or amorphous form, metabolite, metabolic precursor or prodrug
thereof:
~~, cry cr
,.
cue,
crf; cue;
The present invention further provides a method for the treatment and/or
prevention of
cough in a warm-blooded animal, which method comprises administering to a warm-
blooded
2o animal in need thereof, a therapeutically effective amount of a compound of
formula (I)
which is N,N-dimethyl-pindolol chloride having the following structure, or a
pharmaceutically acceptable complex, chelate, solvate, stereoisomer,
stereoisomeric mixture,
crystalline or amorphous form, metabolite, metabolic precursor or prodrug
thereof:
-10-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
- p~ CH3 Cf
O~N~CH3
OH
CH3 CH3
The present invention also provides for the use of a compound of formula (I)
as
defined in the first aspect as active ingredient in the manufacture of a
medicament for use in
the treatment and/or prevention of cough in a warm-blooded animal.
The present invention further provides for the use of a compound having the
following
formula, or a pharmaceutically acceptable salt, ester, amide, complex,
chelate, solvate,
stereoisomer, stereoisomeric mixture, crystalline or amorphous form,
metabolite, metabolic
1 o precursor or prodrug thereof:
+R R2 R4 I 17
~N C C C X-A
-I
~ R1 R3 R5 Ri s
P q r 2
wherein R and Rl are each -CHI-R16; RZ, R3, R~, R5, R16, R17 and R~8 are
independently selected
from hydrogen, hydroxy, Ci-C8 alkyl, C1-Cg hydroxyalkyl and C~-ClZ aralkyl; p
is an integer of
from 0 to 3, q is an integer of from 0 to 3 and r is an integer of from 0 to
3; X is O (oxygen); A is
selected from formulae (IX), (X), (XI), (XII), (XIII), (XIV), (XV) and (XVI)
as described above;
and An- is the anion from a pharmaceutically acceptable salt; with the proviso
that p, q and r
cannot all be 0, as active ingredient in the manufacture of a medicament for
use in the
treatment and/or prevention of cough in a warm-blooded animal.
2o The present invention further provides for the use of a compound having the
following
formula, or a pharmaceutically acceptable salt, ester, amide, complex,
chelate, solvate,
stereoisomer, stereoisomeric mixture. crystalline or amorphous form,
metabolite, metabolic
-11-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
precursor or prodrug thereof:
+R R2 Ra I i 7
E~l C C C X-A
I I II I
R1 R3 R5 R1 g r Ari
p q
wherein R, RI and E are each -CHI-R,6; RZ, R3, R4, R5, R~6, Rig and R1g are
independently
selected from hydrogen, hydroxy, C,-Cg alkyl, C1-Cg hydroxyalkyl and C~-CI~
aralkyl; p is an
integer of from 0 to 3, q is an integer of from 0 to 3 and r is an integer of
from 0 to 3; X is O
(oxygen); A is selected from formulae (IX), (X), (XI), (XII), (XIII), (XIV),
(XV) and (XVI) as
described above, and An- is the anion from a pharmaceutically acceptable salt;
with the proviso
that p, q and r cannot all be 0, as active ingredient in the manufacture of a
medicament for use
in the treatment and/or prevention of cough in a warm-blooded animal.
The present invention further provides for the use of a compound selected from
N,N,N-trimethyl-mexiletine chloride, N,N-dimethyl-propranolol chloride and N,N-
dimethyl-
pindolol chloride, or a pharmaceutically acceptable complex, chelate, solvate,
stereoisomer,
stereoisomeric mixture, crystalline or amorphous form, metabolite, metabolic
precursor or
prodrug thereof, as active ingredient in the manufacture of a medicament for
use in the
treatment and/or prevention of cough in a warm-blooded animal.
In another aspect, the present invention is directed to novel quaternary
ammonium
compounds of the following formula (I), and pharmaceutically acceptable salts,
esters, amides,
2o complexes, chelates, solvates, stereoisomers, stereoisomeric mixtures,
geometric isomers,
crystalline or amorphous forms, metabolites, metabolic precursors or prodrugs
thereof:
Y- J-E Ari (I)
wherein J is independently selected from a group represented by one of the
following formulae
(II), (III) and (IV):
R
RI
NO~ ~N~ ~n ~NO )n
R _
(II) (III) (IV)
-12-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
such that when J is represented by formulae (II), (III) or (IV), compounds of
the present
invention are represented by the following formulae (V), (VI) or (VII)
respectively:
Y-ON-E E-NO
Y-N+ E
)n )n
R1 Y
(V) (VI) (VII)
wherein n is an integer of from 0 to 4; R, R1 and E are independently selected
from -CHZ-Ri6
and a group represented by the following formula (VIII):
R4 ~ t 7
C C C X-A
R3 IRs Ri s
p q r
(VIII)
wherein X is O (oxygen) or S (sulfur); R2, R3, R4, R;, Rib, R17 and R18 are
independently selected
from hydrogen, hydroxy, C,-Cg alkoxy, C,-Cg alkyl, CZ-Cg alkoxyalkyl, Cl-Cg
hydroxyalkyl and
C7-C12 aralkyl; p is an integer of from 0 to 8, q is an integer of from 0 to 8
and r is an integer of
from 0 to 8; A is selected from C5-C~Z alkyl, a C3-C,3 carbocyclic ring, and
ring systems selected
from formulae (IX), (X), (XI), (XII), (XIII), (XIV), (XV) and (XVI):
R7
\ ~ / \
/ \ "
/ ~ Rio \ / Rll Rio Rn
R8 \/ \ /
(IX) (X) (XI)
-13-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
\ ~ i
Rl2
Z \ /
(XII) (XIII)
(XIV)
/ /
R12 \ Z~ ~ w
N
(XV) (XVI)
where R6, R~, R8, R9, Rlo, R1 ~ and R,Z are independently selected from
bromine, chlorine,
fluorine, carboxy, hydrogen, hydroxy, hydroxymethyl, methanesulfonamido,
nitro, sulfamyl,
trifluoromethyl, C2-C~ alkanoyloxy, Cl-C6 alkyl, C1-C6 allcoxy, C2-C7
allcoxycarbonyl, C1-C6
thioalkyl, aryl and N(Rl3,Ri4) where Rl3 and R14 are independently selected
from hydrogen,
acetyl, methanesulfonyl and C1-C6 alkyl, and Z is selected from CH, CH2, O, S,
NH and N-Rls
to where Z may be directly bonded to X when Z is CH; and RIS is selected from
hydrogen, C1-C6
alkyl, C3-Cg cyclocalkyl, aryl and benzyl;
Y is independently selected from hydrogen, -CH2-R,6 and a group represented by
the following
formula (VIII):
R2 R4 I 17
C C C X-A
I I I
R3 Rs Ri s
p q r
(VIII)
wherein X is O (oxygen) or S (sulfur); RZ, R3, R4, RS, R16, RI ~ and Rl8 are
independently selected
from hydrogen, hydroxy, C~-C8 alkoxy, C1-C8 alkyl, C2-Cg alkoxyalkyl, C~-C8
hydroxyalkyl and
2o C7-C12 aralkyl; p is an integer of from 0 to 8, q is an integer of from 0
to 8 and r is an integer of
from 0 to 8; A is selected from C;-C 12 alkyl, a C3-C 13 carbocyclic ring, and
ring systems selected
from formulae (IX), (X), (XI), (XII), (XIII), (XIV), (XV) and (XVI):
-14-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
R~
\ ~ / \ / \
R / ~ Rto \ / Rt t Rto \ / Rt t
s
(IX) (X) (XI)
/ / \ ~ i
Rt2
\ Z \ /
(XIV)
(XII)
(XIII)
Rt2 \ Z~ ~N
(XV) (XVI)
where R6, R7, Rg, R9, Rlo, RI, and Rlz are independently selected from
bromine, chlorine,
fluorine, carboxy, hydrogen, hydroxy, hydroxymethyl, methanesulfonamido,
vitro, sulfamyl,
to trifluoromethyl, CZ-C~ alkanoyloxy, C,-C6 alkyl, C,-C6 allcoxy, CZ-C~
alkoxycarbonyl, Ci-C6
thioalkyl, aryl and N(Rt3,R,4) where R,3 and R14 are independently selected
from hydrogen,
acetyl, methanesulfonyl and C1-C6 alkyl, and Z is selected from CH, CHI, O, S,
NH and N-Rts
where Z may be directly bonded to X when Z is CH, and Ri; is selected from
hydrogen, C,-C6
alkyl, C3-Cg cyclocalkyl, aryl and benzyl; when J is represented by formula
(VII), Y is a group
on any one of the carbon atoms of the nitrogen heterocyclic ring of formula
(VII); An- is the acid
addition salt of a pharmaceutically acceptable acid or the anion from a
pharmaceutically
acceptable salt;
with the provisos that (a) when J is represented by formula (II) then Y and E
are both
represented by formula (VIII); (b) when J is represented by formula (III) then
Y is represented
2o by formula (VIII); (c) when J is represented by formula (IV) and Y is not
represented by
formula (VIII), R1 and E cannot both be -CHZ-R16 and (d) p, q and r cannot all
be 0.
In another aspect, the present invention is directed to novel quaternary
ammonium
compounds of formula (I) as defined in the preceding paragraph wherein J is
represented by
-15-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
formula (II), and pharmaceutically acceptable salts, esters, amides,
complexes, chelates;
solvates, stereoisomers, stereoisomeric mixtures, geometric isomers,
crystalline or amorphous
forms, metabolites, metabolic precursors or prodrugs thereof.
In another aspect, the present invention is directed to novel quaternary
ammonium
compounds of formula (I) as defined in the preceding paragraph wherein J is
represented by
formula (III), and pharmaceutically acceptable salts, esters, amides,
complexes, chelates,
solvates, stereoisomers, stereoisomeric mixtures, geometric isomers,
crystalline or amorphous
forms, metabolites, metabolic precursors or prodrugs thereof.
to
In yet another aspect, the present invention is directed to novel quaternary
ammonium
compounds of formula (I) as defined in the preceding paragraph wherein J is
represented by
formula (IV), and pharmaceutically acceptable salts, esters, amides,
complexes, chelates,
solvates, stereoisomers, stereoisomeric mixtures, geometric isomers,
crystalline or amorphous
15 forms, metabolites, metabolic precursors or prodrugs thereof.
The present invention also provides for a pharmaceutical composition for the
treatment
and/or prevention of cough, comprising an effective amount of a novel
quaternary ammonium
compound of formula (I) as defined in any one of the preceding paragraphs, or
a
2o pharmaceutically acceptable salt, ester, amide, complex, chelate, solvate,
stereoisomer,
stereoisomeric mixture, geometric isomer, crystalline or amorphous form,
metabolite,
metabolic precursor or prodrug thereof and a pharmaceutically acceptable
carrier, diluent or
excipient.
25 The compounds of the present invention may be prepared by direct
quaternisation of the
corresponding amino precursors with an appropriate alkyl halide. For example,
N,N,N-
trimethyl-mexiletine chloride is synthesized by treatment of mexiletine
(commercially available
from e.g. Sigma-Aldrich) with methyl chloride. Similarly N,N,N-trimethyl-
mexiletine iodide is
synthesized by treatment of mexiletine (commercially available from e.g. Sigma-
Aldrich) with
3o methyl iodide. Quaternary propranolol such as N,N-dimethyl-propranolol
chloride and N,N-
dimethyl-propranolol iodide can be similarly prepared from propranolol
(commercially available
from e.g. Sigma-Aldrich) and methyl chloride or methyl iodide respectively.
Conditions for
these preparations and other closely related analogs were described in e.g.
U.S. 4,048,335. In an
- 16-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
analogous approach, quaternary pindolol can be synthesized by treatment of
pindolol
(commercially available from e.g. Sigma-Aldrich) with the appropriate alkyl
halide. N,N-
dimethyl-pindolol chloride can thus be synthesized by reaction of pindolol
with methyl chloride.
Alternatively, the compounds of the present invention may be prepared by
analogy with
other known synthetic methodology such as reaction of a halide (e.g. chlorine)
derivative of
formula (VIII) with an appropriate tertiary amine in a solvent such as
methanol in the presence
of a catalyst (e.g., potassium iodide) to form the corresponding quaternary
ammonium product.
The chlorinated substrate can react as well with a secondary or primary amine
to provide the
1 o respective tertiary or secondary amine, which is then further reacted with
a halide derivative to
form eventually a quaternary ammonium product.
The synthetic procedures described herein, especially when taken with the
general
knowledge in the art, provide sufficient guidance to those of ordinary skill
in the art to perform
the synthesis, isolation, and purification of the compounds of the present
invention.
It is recognized that there is at least one chiral center in the compounds
used within the
scope of the present invention and thus such compounds will exist as various
stereoisomeric
forms. Applicants intend to include all the various stereoisomers within the
scope of the
2o invention. Though the compounds may be prepared as racemates and can
conveniently be used
as such, individual enantiomers also can be isolated or preferentially
synthesized by known
techniques if desired. Such racemates and individual enantiomers and mixtures
thereof are
intended to be included within the scope of the present invention. Pure
enantiomeric forms if
produced may be isolated by preparative chiral HPLC. The free base may be
converted if
desired, to the monohydrochloride salt by known methodologies, and
subsequently, if desired, to
other acid addition salts by reaction with inorganic or organic salts. Acid
addition salts can also
be prepared metathetically by reacting one acid addition salt with an acid
that is stronger than
that of the anion of the initial salt.
3o The present invention also encompasses the pharmaceutically acceptable
salts, esters,
amides, complexes, chelates, solvates, crystalline or amorphous forms,
metabolites, metabolic
precursors or prodrugs of the compounds of formulae (I). Pharmaceutically
acceptable esters and
amides can be prepared by reacting, respectively, a hydroxy or amino
functional group with a
-17-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
pharmaceutically acceptable organic acid, such as identified below. A prodrug
is a drug~which
has been chemically modified and may be biologically inactive at its site of
action, but which is
degraded or modified by one or more enzymatic or other in vivo processes to
the parent
bioactive form. Generally, a prodrug has a different pharmakokinetic profile
than the parent drug
such that, for example, it is more easily absorbed across the mucosal
epithelium, it has better salt
formation or solubility and/or it has better systemic stability (e. g., an
increased plasma half life).
Those skilled in the art recognize that chemical modifications of a parent
drug to yield a
prodrug include: (1) terminal ester or amide derivatives which are susceptible
to being cleaved
1o by esterases or lipases; (2) terminal peptides which may be recognized by
specific or nonspecific
proteases; or (3) a derivative that causes the prodrug to accumulate at a site
of action through
membrane selection, and combinations of the above techniques. Conventional
procedures for the
selection and preparation of prodrug derivatives are described in H.
Bundgaard, Design of
Prodrugs, (1985). Those skilled in the art are well-versed in the preparation
of prodrugs and are
well-aware of its meaning.
In another embodiment, the present invention provides compositions which
include a
compound of the present invention as described above in admixture or otherwise
in association
with one or more inert carriers, excipients and diluents, as well as optional
ingredients if desired.
2o Inert Garners include any material which does not degrade or otherwise
covalently react with a
compound of the invention. Thus, the present invention provides a
pharmaceutical or veterinary
composition (hereinafter, simply referred to as a pharmaceutical composition)
containing a
compound of the present invention as described above, in admixture with a
pharmaceutically
acceptable carrier, excipient or diluent. The invention further provides a
pharmaceutical
composition containing an effective amount of a compound of the present
invention as described
above, in association with a pharmaceutically acceptable Garner.
The pharmaceutical compositions of the present invention may be in any form
which
allows for the composition to be administered to a patient. For example, the
composition may
3o be in the form of a solid, liquid or gas (aerosol). Typical routes of
administration include,
without limitation, oral, topical, parenteral, sublingual, rectal, vaginal,
and intranasal. The term
parenteral as used herein includes subcutaneous injections, intravenous,
intramuscular, epidural,
intrasternal injection or infusion techniques. Pharmaceutical composition of
the invention are
-18-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
formulated so as to allow the active ingredients contained therein to 1~-
bioavailable upyln
administration of the composition to a patient. Compositions that will be
administered to a
patient take the form of one or more dosage units, where for example, a
tablet, capsule or cachet
may be a single dosage unit, and a container of a compound of the present
invention in aerosol
form may hold a plurality of dosage units.
Materials used in preparing the pharmaceutical compositions should be
pharmaceutically
pure and non-toxic in the amounts used. The inventive compositions may include
one or more
compounds (active ingredients) known for a particularly desirable effect. It
will be evident to
1 o those of ordinary skill in the art that the optimal dosage of the active
ingredients) in the
pharmaceutical composition will depend on a variety of factors. Relevant
factors include,
without limitation, the type of subject (e.g., human), the particular form of
the active ingredient,
the manner of administration and the composition employed.
In general, the pharmaceutical composition includes a compound of the present
invention as described herein, in admixture with one or more Garners. The
carriers) may be
particulate, so that the compositions are, for example, in tablet or powder
form. The carriers)
may be liquid, with the compositions being, for example, an oral syrup or
injectable liquid. In
addition, the Garner(s) may be gaseous, so as to provide an aerosol
composition useful in, e.g.,
2o inhalatory administration.
When intended for oral administration, the composition is preferably in either
solid or
liquid form, where semi-solid, semi-liquid, suspension and gel forms are
included within the
forms considered herein as either solid or liquid.
As a solid composition for oral administration, the composition may be
formulated into a
powder, granule, compressed tablet, pill, capsule, cachet, chewing gum, wafer,
lozenges, or the
like form. Such a solid composition will typically contain one or more inert
diluents or edible
Garners. In addition, one or more of the following adjuvants may be present:
binders such as
3o syrups, acacia, sorbitol, polyvinylpyrrolidone, carboxymethylcellulose,
ethyl cellulose,
microcrystalline cellulose, gum tragacanth or gelatin, and mixtures thereof;
excipients such as
starch, lactose or dextrins, disintegrating agents such as alginic acid,
sodium alginate, Primogel,
corn starch and the like; lubricants such as magnesium stearate or Sterotex;
fillers such as
-19-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
lactose, mannitols, starch, calcium phosphate, sorbitol, methylcellulose; and
mixtures tlfereof;
lubricants such as magnesium stearate, high molecular weight polymers such as
polyethylene
glycol, high molecular weight fatty acids such as stearic acid, silica,
wetting agents such as
sodium lauryl sulfate. glidants such as colloidal silicon dioxide; sweetening
agents such as
sucrose or saccharin, a flavoring agent such as peppermint, methyl salicylate
or orange
flavoring, and a coloring agent.
When the composition is in the form of a capsule, e.g., a gelatin capsule, it
may contain,
in addition to materials of the above type, a liquid carrier such as
polyethylene glycol or a fatty
t o oil.
The composition may be in the form of a liquid, e.g., an elixir, syrup,
solution, aqueous
or oily emulsion or suspension, or even dry powders which may be reconstituted
with water
and/or other liquid media prior to use. The liquid may be for oral
administration or for delivery
by injection, as two examples. When intended for oral administration,
preferred compositions
contain, in addition to the present compounds, one or more of a sweetening
agent, thickening
agent, preservative (e.g., alkyl p-hydoxybenzoate), dye/colorant and flavor
enhancer (flavorant).
In a composition intended to be administered by injection, one or more of a
surfactant,
preservative (e.g., alkyl p-hydroxybenzoate), wetting agent, dispersing agent,
suspending agent
(e.g., sorbitol, glucose, or other sugar syrups), buffer, stabilizer and
isotonic agent may be
included. The emulsifying agent may be selected from lecithin or sorbitol
monooleate.
The liquid pharmaceutical compositions of the invention, whether they be
solutions,
suspensions or other like form, may include one or more of the following
adjuvants: sterile
diluents such as water for injection, saline solution, preferably
physiological saline, Ringer's
solution, isotonic sodium chloride, fixed oils such as synthetic mono or
digylcerides which may
serve as the solvent or suspending medium, polyethylene glycols, glycerin,
propylene glycol or
other solvents; antibacterial agents such as benzyl alcohol or methyl paraben;
antioxidants such
as ascorbic acid or sodium bisulfate; chelating agents such as
ethylenediaminetetraacetic acid;
3o buffers such as acetates, citrates or phosphates and agents for the
adjustment of tonicity such as
sodium chloride or dextrose. The parenteral preparation can be enclosed in
ampoules,
disposable syringes or multiple dose vials made of glass or plastic.
Physiological saline is a
preferred adjuvant. An injectable pharmaceutical composition is preferably
sterile.
-20-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
A liquid compositions intended for either parenteral or oral administration
should
contain an amount of the inventive compound such that a suitable dosage will
be obtained.
Typically, this amount is at least 0.01 % of a compound of the invention in
the composition.
When intended for oral administration, this amount may be varied to be between
0.1 and about
70% of the weight of the composition. Preferred oral compositions contain
between about 4%
and about 50% of the active compound of the present invention. Preferred
compositions and
preparations according to the present invention are prepared so that a
parenteral dosage unit
contains between 0.01 to 10% by weight of active compound.
The pharmaceutical composition may be intended for topical administration, in
which
case the carrier may suitably comprise a solution, emulsion, ointment, cream
or gel base. The
base, for example, may comprise one or more of the following: petrolatum,
lanolin, polyethylene
glycols, bee wax, mineral oil, diluents such as water and alcohol, and
emulsifiers and stabilizers.
Thickening agents may be present in a pharmaceutical composition for topical
administration. If
intended for transdermal administration, the composition may include a
transdermal patch or
iontophoresis device. Topical formulations may contain a concentration of the
inventive
compound of from about 0.1 to about 25% w/v (weight per unit volume).
2o The composition may be intended for rectal administration, in the form,
e.g., of a
suppository which will melt in the rectum and release the drug. The
composition for rectal
administration may contain an oleaginous base as a suitable nonirritating
excipient. Such bases
include, without limitation, lanolin, cocoa butter and polyethylene glycol.
Low-melting waxes
are preferred for the preparation of a suppository, where mixtures of fatty
acid glycerides and/or
cocoa butter are suitable waxes. The waxes may be melted, and the compound of
the present
invention is dispersed homogeneously therein by stirring. The molten
homogeneous mixture is
then poured into convenient sized molds, allowed to cool and thereby solidify.
The composition may include various materials which modify the physical form
of a
solid or liquid dosage unit. For example, the composition may include
materials that form a
coating shell around the active ingredients. The materials which form the
coating shell are
typically inert, and may be selected from, for example, sugar, shellac, and
other enteric coating
agents. Alternatively, the active ingredients may be encased in a gelatin
capsule or cachet.
-21 -
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
The composition in solid or liquid form may include an agent which binds to
the
compound of the present invention and thereby assists in the delivery of the
active components.
Suitable agents which may act in this capacity include a monoclonal or
polyclonal antibody, a
protein or a liposome.
The pharmaceutical composition of the present invention may consist of gaseous
dosage
units, e.g., it may be in the form of an aerosol. The term aerosol is used to
denote a variety of
systems ranging from those of colloidal nature to systems consisting of
pressurized packages.
1o Delivery may be by a liquefied or compressed gas or by a suitable pump
system which dispenses
the active ingredients. Aerosols of compounds of the invention may be
delivered in single
phase, bi-phasic, or tri-phasic systems in order to deliver the active
ingredient(s). Delivery of
the aerosol includes the necessary container, activators, valves,
subcontainers, and the like,
which together may form a kit. Preferred aerosols may be determined by one
skilled in the art,
without undue experimentation. The pharmaceutical compositions may be prepared
by
methodology well known in the pharmaceutical art. The compounds of the present
invention
may be in the form of a solvate in a pharmaceutically acceptable solvent such
as water or
physiological saline.
2o Suitable pharmaceutically acceptable salts include acid addition salts of
acids such as
hydrochloric, hydrobromic, benzenesulfonic (besylate), benzoic,
camphorsulfonic,
ethanesulfonic, fumaric, gluconic, glutamic, isethionic, malefic, malic,
mandelic,
methanesulfonic, mucic, nitric, pamoic, pantothenic, succinic, p-
toluenesulfonic, phosphoric,
sulphuric, citric, tartaric, lactic and acetic acid, although the preferred
acid addition salt is the
hydrochloride salt.
As indicated above, the magnitude of the therapeutic or prophylactic dose of
the
compounds of the present invention in the treatment and/or prevention of cough
will depend
upon the severity and nature of the condition being treated and the route of
administration. The
3o dose and the frequency of the dosing will also vary according to age, body
weight and response
of the individual patient. In general, the total daily dose range for the
compounds of the present
invention for the treatment or prevention of cough is from about 0.1 to about
800 mg in single or
repeated doses.
-22-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
Any suitable route of administration as described above may be employed to
provide an
effective dosage of the compounds of the present invention, although
administration by
inhalation is preferred, most preferably in aerosol form. Suitable forms of
administration
include, but are not limited to, inhalation (delivered by, e.g., metered-dose
inhaler, jet nebulizer,
ultrasonic nebulizer, dry powder inhaler. etc.), nasal sprays, nebulization,
oral administration
such as via tablets, capsules, lozenges, syrups, sprays, suspensions, elixirs,
gargles, and other
liquid preparations, aerosol foams, parental administration, and sublingal
administration.
1 o The compounds of the present invention can include pharmaceutically
acceptable
carriers and other conventional additives, including aqueous based Garners, co-
solvents such as
ethyl alcohol, propylene glycol and glycerin, fillers, lubricants, wetting
agents, flavoring agents,
coloring agents, emulsifying, suspending or dispersing agents, suspending
agents. etc. For
aerosol delivery of the compounds of the present invention, pharmaceutically
acceptable
diluents, Garners, and/or propellants may be included in the formulations for
use in appropriate
devices. These are prepared by procedures well known to those skilled in the
art (see e.g.
Medication Teaching Manual, 5th Ed., Bethesda, MD, American Society of
Hospital
Pharmacists, 1991 ).
2o The compositions of the present invention may optionally include other
known
therapeutic agents, including decongestants such as pseudoephedrine HCI,
phenylephrine HCl
and ephedrine HCI, non-steroidal anti-inflammatory drugs such as
acetaminophen, aspirin,
phenacetin, ibuprofen and ketoprofen, expectorants such as glyceryl
guaiacolate, tenpin hydrate
and ammonium chloride, antihistamines such as chlorpheniramine maleate,
doxylamine
succinate, brompheniramine maleate and diphenhydramine hydrochloride, and
anesthetic
compounds such as phenol.
The following examples are offered by way of illustration and not by way of
limitation.
- 23 -
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
L V A l~ilDT L' 1
Synthesis of 1-(2.6-Dimethyl phenoxv)-N.N,N-trimethylpropan-2-aminium iodide
(N,N.N-trimethyl-mexiletine iodide)
Mexiletine hydrochloride (1.03 g, 4.76 mmol) was pardoned between
dichloromethane
(20 mL) and saturated aqueous NaHC03 (5 mL). The aqueous layer was collected
and extracted
with dichloromethane (2 x 5 mL). The organic layers were combined and dried
over anhydrous
Na2S04. To the filtrate was added methyl iodide (2.1 mL, 34.3 mmol) and Na2C03
(1.61 g, 15.2
mmol). The reaction mixture was stirred at room temperature for 162 hours,
filtered, and the
filtrate was concentrated in vacuo to give a brown solid. Recrystallization
from a mixture of
ethanol and ether ( 1:4, v/v, 25 mL) gave the product as yellow crystals (
1.28 g, 77% yield). 13C
NMR (75 MHz, DMSO-db) b 12.1 (CH3), 16.4 (CH;), 51.4 (CH3), 68.9 (CH), 69.3
(CHZ), 124.4
(CH), 129.0 (CH), 130.3 (C), 154.6 (C).
EXAMPLE 2
Synthesis of 2-Hvdroxy-N isopropyl-N.N dimethvl-3-(1-naphthyloxy)propan-1-
aminium iodide
~N,N-dimethvl-propranolol iodide)
Propanolol hydrochloride (2.81 g, 9.49 mmol) was dissolved in H20 ( 15 mL),
saturated
2o aqueous NaHC03 (45 mL), and extracted with dichloromethane (3 x 60 mL). The
organic
layers were combined, dried over anhydrous Na2S04, and concentrated in vacuo
to 50 mL. To
the residual solution was added methyl iodide (2.8 mL, 45.1 mmol) and KZC03
(4.1 g, 29.7
mmol). The reaction mixture was stirred for 63 hours at room temperature and
then refluxed for
67 hours. The solvent was then concentrated in vacuo to give a white solid
which was dissolved
in H20 (220 mL). The solution was extracted with ether (3 x 220 mL) and again
with
dichloromethane (3 x 220 mL). The combined dichloromethane extracts were dried
over
anhydrous Na2S04 and the solvent removed in vacuo to give the crude product.
Recrystallization from a mixture of ethanol and ether (3:2, v/v, 25mL)
provided the product as
yellow crystals (1.2 g, 30% yield). 13C NMR (75MHz, CDC13 ) 8 16.8 (CH3), 16.9
(CH3), 49.3
(CH3), 49.8 (CH3), 65.7 (CH), 65.8 (CH2), 68.2 (CH), 71.6 (CHZ), 106.3 (CH),
121.9 (CH),
122.8 (CH), 126.4 (CH), 126.6 (CH), 126.9 (CH), 127.5 (CH), 128.5(CH), 135.9
(C), 155.2 (C).
-24-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
EXAMPLE 3
Synthesis of 2-Hydroxv-3-(1H-indol-4=yloxy)-N-isoprowl-N.N-dimeth~propan-1-
aminium
iodide
(N,N-dimethvl-pindolol iodide)
To a solution of pindolol (0.280 g, 1.13 mmol) in THF (60 mL) was added methyl
iodide
(0.71 mL, 11.32 mmol). The resultant solution was stirred for 6 hours then
refluxed for 40
hours. The precipitate was collected and washed with THF (5 x 5 mL) to give
the title
compound (0.10 g). The filtrate was then concentrated in vacuo to 30 ml,
methyl iodide (0.50
1 o mL, 8.03 mmol) was added, and the mixture was refluxed for 16 hours. The
resultant precipitate
was collected and washed with THF (3 x 5 ml) to yield a second crop (0.02 g)
(29% overall
yield). 13C NMR (75MHz, DMSO ) 8 15.9 (CH3), 16.1 (CH3), 48.0 (CH3), 48.3
(CH3), 63.8
(CH), 64.2 (CH2), 65.5 (CH), 69.8 (CHI), 98.4 (CH), 100.0 (CH), 105.3 (CH),
118.2 (C), 121.6
(CH), 123.6 (CH), 137.2 (C), 151.3 (C).
EXAMPLE 4
The following method is one of the general methods available to determine the
antitussive activity of the compounds of the present invention.
Male albino Dunkin-Hartley strain guinea-pigs (weight 300-400g) can be
obtained from
various commercial suppliers.
The method is a modification of that described by Adcock J.J., Schneider C.
and Smith
T.W., "Effects of Morphine and a Novel Opioid Pentapeptide BW443C, on Cough,
Nociception
and Ventilation in the Unanaesthetized Guinea-pig", Br. J. Pharmacol., 93, 93-
100 (1988).
Individual conscious guinea-pigs are placed unrestrained into a sealed purpose
built perspex
exposure chamber (3,000 cm3 volume) and allowed to acclimatize prior to
aerosol
administration. The layout of the experimental apparatus used is shown in
Figure 1.
3o Cylinder air is introduced into the exposure chamber at a flow rate of 1
liter/min,
maintained by a needle valve and monitored by a rotameter. From the rotameter
the air passes
through the cup of an ultrasonic nebulizer (DeVilbis UltraNeb 2000) which is
used to generate
aerosols of drug or citric acid at 0.15 ml/min. A Fleisch pneumotachograph,
connected to a
- 25 -
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
differential pressure transducer (Grass model PTS) is attached to the outflow
from the exposure
chamber and provides a measurement of airflow from the chamber. The
differential pressure
transducer is connected to a Grass polygraph from which a hard copy record can
be produced.
The output from the polygraph is directed to a computerized data acquisition
system (PolyNe-
Mah) for real time recording of data. A tie-clip microphone is placed in the
exposure chamber
and connected via a pre-amplifier to a loudspeaker output to provide the
observer with an audio
monitor of responses.
Cough responses are induced by exposure to an aerosol of citric acid (1M) for
10
1 o minutes. Animals are continuously monitored by trained observer, and the
number of coughs
are counted during a 1 ~ minute period from commencement of the citric acid
aerosol
administration. Three characteristic responses can be produced by exposure to
citric acid:
cough, sneeze and "wet dog" shake.
The three types of response are differentiated primarily by sound and visual
observation.
Confirmation of the numbers of multiple coughs is determined by reference to
the change in
flow rate displayed by the Poh-Ne-Mah system monitor. Printouts demonstrating
the pressure
changes characteristic of the different response to irntant are shown in
Figures 2A and 2B. Data
records for individual guinea-pigs on the Poh-Ne-Mah system are stored on an
optical disk.
2o Each cough is marked on the Grass polygraph paper trace, and from these
record numbers,
frequency and time of onset of coughs are determined. The cough response is
defined by a
characteristic coughing sound and behavior, associated with a marked biphasic
pressure change.
The biphasic pressure changes associated with a sneeze are not of as great a
magnitude as those
associated with a cough, the secondary rise in pressure also being far less
than during a cough
(Figure 2B). The sound of a sneeze differed from that of a cough, and sneezing
is associated
with nose rubbing activity. The third response, a "wet dog" shake, produces a
rise in pressure
only (Figure 2A) and lacked the definitive sound of a cough or sneeze.
Quantities of drugs are weighed out and dissolved in a vehicle. Equal volumes
are
3o aliquotted into sample tubes before being passed, together with another
sample tube containing
the same volume of vehicle, to an independent observer for coding. Pre-
treatments are matched
by concentration together with a vehicle control group. Two to five guinea-
pigs are randomly
allocated to each treatment group. Animals are pre-treated with either vehicle
(e.g. distilled
-26-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
water, 0.9% sterile saline, Tween or 1 to 25% ethanol depending on the
solubility of the
compound), reference compound (e.g. mexiletine, propranolol or pindolol) or
test drugs for 5
minutes immediately prior to citric acid aerosol exposure. Test drugs and
reference compound
are administered as aerosols at concentrations selected from 0.1, 1.0, 2.0,
5.0 and 10.0 mg/ml.
The sequence of pre-treatment administration is determined according to a 4x4
Latin Square
design.
Data can be presented as the mean ~SEM number of coughs produced by individual
guinea-pigs within each group during the 15 minute observation period or
mean~SEM latency
of cough and are analyzed using one way analysis of variance to compare mean
responses
between matched groups of animals (doses) and between unmatched groups
(treatments)
followed by the Tukey-Kramer multiple comparison test where appropriate.
In one set of experiments using the general protocol described above, the
antitussive
activity of N,N-dimethyl-propranolol iodide was tested. Results showed that
pre-treatment of
guinea pigs with aerosols of N,N-dimethyl-propranolol iodide at 10.0 mg/ml
immediately
before exposure to citric acid (1M) inhibited cough responses by 50-70%
compared with vehicle
(saline) pre-treated guinea pigs over the 15 minute observation period.
Similarly, other
quaternary ammonium compounds of the present invention can be evaluated by
this method.
EXAMPLE 5
In another experiment similar to that described above in Example 4, the
duration of the
antitussive effects of the compounds of the present invention against citric
acid-induced cough
responses can be investigated in conscious guinea pigs. Quaternary ammonium
compounds of
the present invention, reference compounds or vehicle are tested by
administering as aerosol pre-
treatments (0.1, 1.0, 2.0, 5.0 or 10.0 mg/ml, 5 minute duration) at 5 minutes,
30 minutes, 1 hour,
2 hours and 4 hours prior to induction of cough responses by citric acid
aerosol. Data and results
are analyzed as described in Example 4.
3 o EXAMPLE 6
The antitussive effects of a 5 minute pre-treatment with aerosolized compounds
of the
present invention and reference compound (e.g. mexiletine, propranolol or
pindolol) on
-27-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
capsaicin aerosol-induced cough can be investigated in conscious guinea-pigs
using a m,~thod
similar to that described in Example 4. Data and results are analyzed as
described in Example 4.
Ti'Y A MpT ~' '7
Therapeutic treatment with the compounds of the present invention can also be
determined by a similar method as described in Example 4. The antitussive
effects of
compounds of the present invention and reference compound (e.g. mexiletine,
propranolol or
pindolol) that are administered after induction of cough responses by exposure
to citric acid
aerosol are investigated in conscious guinea pigs. Vehicle or test agents are
administered as
1 o aerosols ( 10, 5, 2, 1.0 or 0.1 mg/ml; 5 minute duration) 2 minutes after
exposure to citric acid
aerosol began. Cough responses are recorded during a 15 minute observation
period (t=0 to
t=15 minutes) from initiation of the citric acid exposure. Data and results
are analyzed as
described in Example 4.
EXAMPLE 7
Investigation of antitussive activity of aerosolized test compound on citric
acid-induced
cough responses in conscious rabbits
Protocol
2o Twenty-two male New Zealand white rabbits are randomly allocated to either
of two
groups of 11 rabbits.
Pairs of rabbits (control versus test) are placed in individual exposure
chambers with an
airflow of 5 liter/min through the chambers.
Each rabbit is exposed to ozone (3 ppm) for 1 hour.
The rabbits are then immediately exposed to aerosols of either vehicle
(chamber 1 ) or
test compound (10 mg/ml, chamber 2) at a nebulization rate of 0.9 ml/min.
Cough responses are induced with citric acid aerosol (1.6 M).
Coughs are counted during the 10 minute exposure to citric acid.
-28-
SUBSTITUTE SHEET (RULE 26)
CA 02393699 2002-06-07
WO 01/44167 PCT/CA00/01508
All rabbits are exposed to ozone before vehicle or test drug pre-treatment.
Data and results are analyzed as described in Example 4.
All publications and patent applications mentioned in this specification are
herein
incorporated by reference to the same extent as if each individual publication
or patent
application was specifically and individually incorporated by reference.
1o The invention includes all embodiments and variations substantially as
hereinbefore
described and with reference to the examples and drawings. From the foregoing,
it will be
appreciated that. although specific embodiments of the invention have been
described herein for
purposes of illustration, various modifications may be made without deviating
from the spirit
and scope of the invention. Accordingly, the invention is not limited by the
disclosed
embodiments or examples. Many adaptations and modifications may be made within
the
scope of the invention in accordance with the common general knowledge of
those skilled in
this art. Such modifications include the substitution of known equivalents for
any aspect of
the invention in order to achieve the same result in substantially the same
way. Numeric
ranges are inclusive of the numbers defining the range. In the specification,
the word
"comprising" is used as an open-ended term, substantially equivalent to the
phrase "including,
but not limited to", and the word "comprises" has a corresponding meaning.
Citation of
references herein shall not be construed as an admission that such references
are prior art to
the present invention.
-29-
SUBSTITUTE SHEET (RULE 26)