Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
PEPTIDE (3-TURN MIMETIC COMPOUNDS AND PROCESSES
FOR MAKING THEM
FIELD OF THE INVENTION
The subject invention relates to novel peptide (3-turn mimetic compounds and
processes for
making such compounds.
CROSS REFERENCE
This application claims priority under Title 35, United States Code 119(e)
from Provisional Application Serial No. 60/172,823, filed December 21, 1999.
BACKGROUND
In peptides, the (3-turn is a subset of the reverse turn and is a common
feature of biologically
active peptides and proteins; it is widely thought to act as a molecular
recognition site for many
biological processes. Specific types of (3-turns are classified according to
their geometry.
The b-turn is defined as any tetrapeptide sequence with a 10-membered
intramolecularly
H-bonded ring, in which the Ca to Ca+3 distance varies from 4 to 7~.
R5 i+4
R1 ~ O NH
H N ~2 O'~3 v~ N R4 1+3
LV2 H
i+1 R2 ~N O
O Rs
i+2
Depending on f2, y2, f3 and y3 there are many types of b-turn structures
described in literature. (See:
Gillespie et al., "Conformational Analysis of Dipeptide Mimetics", Bio of ,
Vol. 43, (1997), pp.
191-217; Venkatachalam, Biopolymers, Vol. 6, (1968), pp. 1425-36.
The subject invention compounds are mimetics for ~3-turn peptides. Such
compounds are
useful as probes for the study of molecular recognition events, including
enzyme inhibition, cell-cell
and cell-matrix interactions. One physical consequence of such conformational
constraint of the
1
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
subject compounds is a limiting of the number of accessible conformational
states of the molecules,
leading to a better definition of the bioactive conformation of corresponding
active peptides.
Information regarding ~3-turn peptides and mimetic compounds can also be
found, for
example, in the following references: Ball et al., "(3-Turn Topography",
Tetrahedron, Vol. 49, No.
17 (1993), pp. 3467-3478; Kahn, "Peptide Secondary Structure Mimetics: Recent
Advances and
Future Challenges", SYNLETT, (Nov. 1993), pp. 821-826; Hanessian et al.,
"Design and Synthesis
of Conformationally Constrained Amino Acids as Versatile Scaffolds and Peptide
Mimetics",
Tetrahedron, Vol. 53, No. 38 (1997), pp. 12789-12854.
SUMMARY OF THE INVENTION
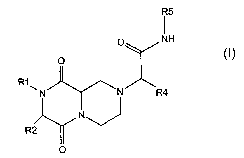
The subject invention includes compounds having the structure:
R5
O NH
O
R1
~N ~ ~N R4
NJ
R2
O
wherein:
(a) R1 is hydrogen or alkyl; and R2 is selected from hydrogen, alkyl, aryl,
heterocyclyl,
carboxy and its esters and amides; or Rl and R2 are attached and are together
alkylene or
heteroalkylene;
(b) R4 is selected from aryl, heteroaryl, and a,(3-unsaturated conjugated aryl
or
heteroaryl; and
(c) R5 is selected from hydrogen, alkyl, aryl, and heterocyclyl;
and an optical isomer, diesteriomer, or enantiomer thereof; a salt, hydrate,
ester, amide, or imide
thereof.
The subject invention also includes libraries of such compounds, and processes
for making
the subject compounds and libraries.
DETAILED DESCRIPTION OF THE INVENTION
As used herein unless specified otherwise, "alkyl" means a hydrocarbon chain
which is
branched, linear or cyclic, saturated or unsaturated (but not aromatic),
substituted or
unsubstituted. The term "alkyl" may be used alone or as part of another word
where it may be
2
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
shortened to "alk" (e.g., in alkoxy, alkylacyl). Preferred linear alkyl have
from one to about
twenty carbon atoms, more preferably from one to about ten carbon atoms, more
preferably still
from one to about six carbon atoms, still more preferably from one to about
four carbon atoms;
most preferred are methyl or ethyl. Preferred cyclic and branched alkyl have
from three to about
twenty carbon atoms, more preferably from three to about ten carbon atoms,
more preferably still
from three to about seven carbon atoms, still more preferably from three to
about five carbon
atoms. Preferred cyclic alkyl have one hydrocarbon ring, but may have two,
three, or more, fused
or spirocycle hydrocarbon rings. Preferred alkyl are unsaturated with from one
to about three
double or triple bonds, preferably double bonds; more preferably they are mono-
unsaturated with
one double bond. Still more preferred alkyl are saturated. Saturated alkyl are
referred to herein
as "alkanyl". Alkyl unsaturated only with one or more double bonds (no triple
bonds) are referred
to herein as "alkenyl". Preferred substituents of alkyl include halo, alkyl,
aryl, heterocycle,
hydroxy, alkoxy, aryloxy, thio, alkylthio, arylthio, amino, alkylamino,
arylamino, amide,
alkylamide, arylamide, formyl, alkylacyl, arylacyl, carboxy and its alkyl and
aryl esters and
amides, nitro, and cyano. Also, unsubstituted alkyl are preferred.
As used herein, "heteroatom" means a nitrogen, oxygen, or sulfur atom.
As used herein, "alkylene" means an alkyl which connects two other moieties,
"heteroalkylene" means an alkylene having one or more heteroatoms in the
connecting chain.
As used herein unless specified otherwise, "aryl" means an aromatic
hydrocarbon ring (or
fused rings) which is substituted or unsubstituted. The term "aryl" may be
used alone or as part of
another word (e.g., in aryloxy, arylacyl). Preferred aryl have from six to
about fourteen,
preferably to about ten, carbon atoms in the aromatic ring(s), and a total of
from about six to
about twenty, preferably to about twelve, carbon atoms. Preferred aryl is
phenyl or naphthyl;
most preferred is phenyl. Preferred substituents of aryl include halo, alkyl,
aryl, heterocycle,
hydroxy, alkoxy, aryloxy, thio, alkylthio, arylthio, amino, alkylamino,
arylamino, amide,
alkylamide, arylamide, formyl, alkylacyl, arylacyl, carboxy and its alkyl and
aryl esters and
amides, nitro, and cyano. Also, unsubstituted aryl are preferred.
As used herein unless specified otherwise, "heterocycle" or "heterocyclyl"
means a
saturated, unsaturated or aromatic cyclic hydrocarbon ring (or fused rings)
with one or more
heteroatoms in the hydrocarbon ring(s). Preferred heterocycles have from one
to about six
heteroatoms in the ring(s), more preferably one or two or three heteroatoms in
the ring(s).
Preferred heterocycles have from three to about fourteen, preferably to about
ten, carbon plus
heteroatoms in the ring(s), more preferably from three to about seven, more
preferably still five or
six, carbon plus heteroatoms in the rings(s); and a total of from three to
about twenty carbon plus
3
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
heteroatoms, more preferably from three to about ten, more preferably still
five or six, carbon plus
heteroatoms. Preferred heterocycles have one ring, but may have two, three, or
more, fused or
spirocycle rings. More preferred heterocycle rings include those which are one
ring with 5 or 6
carbon plus heteroatoms in the ring with no more than three ring heteroatoms,
no more than two
of which are O and S. Still more preferred are such 5- or 6-ring atom
heterocycles with one or
two ring atoms being O or S and the others being C; or with one, two or three
ring atoms being N
and the others being C. Such preferred 5- or 6-ring atom heterocycles are
preferably saturated,
unsaturated with one or two double bonds, or aromatic. Such preferred 5- or 6-
ring atom
heterocycles are preferably a single ring; or fused with a 3- to 6-ring atom
hydrocarbon ring
which is saturated, unsaturated with one double bond, or aromatic (phenyl); or
fused with another
such 5- or 6-ring atom heterocyclic ring. Heterocycles are unsubstituted or
substituted. Preferred
heterocycle substituents are the same as for alkyl.
As used herein unless specified otherwise, "heteroaryl" means an aromatic
heterocycle.
Compounds of the Invention
The subject invention involves compounds having the following structure:
R5
O NH
O
R1
~N ~ ~N R4
N
R2
O (2)
In structure 2, R1 is hydrogen or alkyl. Preferred R1 is alkyl having from 1
to about 12
carbon atoms, more preferably from 1 to about 6 carbon atoms, more preferably
still 1 or 2 carbon
atoms. Preferred alkyl Rl is unsubstituted or substituted; preferred
substituents include aryl,
heterocyclyl, amino, alkylamino, arylamino, hydroxy, alkoxy, aryloxy, thio,
alkylthio, arylthio,
carboxy and its esters and amides; more preferred substituents include phenyl,
naphthyl, and
heterocyclyl having one ring with 5 or 6 ring atoms including I-3 heteroatoms
or two fused rings
with 8-10 ring atoms including 1-4 heteroatoms. More preferred Rl is hydrogen.
In structure 2, R2 is selected from hydrogen, alkyl, aryl, heterocyclyl,
carboxy and its esters
and amides. Alkyl R2 preferably has from I to about 8 carbon atoms, more
preferably from 1 to
4
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
about 4 carbon atoms, more preferably still 1 or 2 carbon atoms. Alkyl R2 is
preferably
unsubstituted or substituted; preferred substituents include aryl,
heterocyclyl, amino, alkylamino,
arylamino, hydroxy, alkoxy, aryloxy, thio, alkylthio, arylthio, halo, nitro,
cyano, carboxy and its
esters and amides; more preferred substituents include phenyl, naphthyl,
heterocyclyl having one
ring with 5 or 6 ring atoms including I-3 heteroatoms or two fused rings with
8-10 ring atoms
including 1-4 heteroatoms, hydroxy, CI-C6 alkoxy, phenoxy, thio, CI-C6
alkylthio, phenylthio,
carboxy and its CI-C6 esters and amides. Aryl R2 is preferably phenyl or
naphthyl, more
preferably phenyl. Aryl R2 is preferably unsubstituted or substituted;
preferred substituents include
alkyl, aryl, heterocyclyl, amino, alkylamino, arylamino, hydroxy, alkoxy,
aryloxy, thio, alkylthio,
arylthio, halo, nitro, cyano, carboxy and its esters and amides; more
preferred substituents include
CI-C6 alkyl. Heterocycle R2 preferably is one ring having 5 or 6 ring atoms
including 1-3
heteroatoms or two fused rings having 8-10 ring atoms including I-4
heteroatoms. More preferred
heterocycle R2 is heteroaryl. Heterocycle R2 is preferably unsubstituted or
substituted; preferred
substituents include alkyl, aryl, heterocyclyl, amino, alkylamino, arylamino,
hydroxy, alkoxy,
aryloxy, thio, alkylthio, arylthio, halo, nitro, cyano, carboxy and its esters
and amides; more
preferred substituents include C1-C6 alkyl, phenyl, naphthyl, and heterocyclyl
having one ring with
5 or 6 ring atoms including 1-3 heteroatoms or two fused rings with 8-10 ring
atoms including 1-4
heteroatoms.
R2 is preferably selected from known a-amino acid side-chains, especially
those of a-
amino acids which commonly occur in nature.
In structure 2, R1 and R2 may be attached, such attached RI/R2 being alkylene
or
heteroalkylene. Alkylene R1/R2 preferably has from 1 to about 6 carbon atoms,
more preferably
from about 2 to about 4 carbon atoms, more preferably still 3 or 4 carbon
atoms. Heteroalkylene
R1/R2 preferably has from 1 to about 5 carbon atoms, more preferably from 1 to
about 4 carbon
atoms, more preferably still 2 or 3 carbon atoms; and preferably from 1 to
about 3 heteroatoms,
more preferably 1 or 2 heteroatoms, more preferably still 1 heteroatom.
Alkylene and
heteroalkylene R1/R2 are preferably unsubstituted or substituted; preferred
carbon atom substituents
include alkyl, aryl, heterocyclyl, amino, alkylamino, arylamino, hydroxy,
alkoxy, aryloxy, thio,
alkylthio, arylthio, carboxy, and its esters and amides; more preferred carbon
atom substituents
include hydroxy, C1-C6 alkoxy, phenoxy, thio, CI-C6 alkylthio, phenylthio,
carboxy and its Cl-C6
esters and amides; preferred nitrogen atom substituents include Cl-C6 alkyl
(unsubstituted or
substituted).
5
CA 02393759 2002-06-07
WO 01/46197 PCTNS00/34832
In structure 2, R4 is selected from aryl, heteroaryl, and a,(3-unsaturated-
conjugated aryl or
heteroaryl. Aryl R4 is preferably phenyl or naphthyl, more preferably phenyl.
Aryl R4 is preferably
unsubstituted or substituted; preferred substituents include alkyl, aryl,
alkoxy, aryloxy, alkylthio,
arylthio, halo, vitro, cyano, carboxy and its esters and amides; more
preferred substituents include
CI-C6 alkyl, phenyl, CI-C6 alkyoxy, and halo. Heteroaryl R4 preferably is one
ring having 5 or 6
ring atoms including 1-3 heteroatoms or two fused rings having 8-10 ring atoms
including I-4
heteroatoms. Heteroaryl R4 is preferably unsubstituted or substituted;
preferred substituents include
alkyl, aryl, alkoxy, aryloxy, alkylthio, arylthio, halo, vitro, cyano, carboxy
and its esters and amides;
more preferred substituents include CI-C6 alkyl, phenyl, CI-C6 alkoxy, and
halo. Conjugated aryl
and heteroaryl R4 preferably includes styryl. Conjugated aryl and heteroaryl
R4 are preferably
unsubstituted or substituted; preferred substituents include alkyl, alkoxy,
aryloxy, alkylthio,
arylthio, halo, vitro, cyano, carboxy and its esters and amides; more
preferred substituents include
CI-C6 alkyl, phenyl, CI-C6 alkoxy, and halo.
In structure 2, RS is selected from hydrogen, alkyl, aryl, and heterocyclyl.
Alkyl RS
preferably has from 1 to about 10 carbon atoms, more preferably from I to
about 4 carbon atoms,
more preferably still I or 2 carbon atoms. Alkyl RS is preferably
unsubstituted or substituted;
preferred substituents include alkyl, aryl, heterocyclyl, amino, alkylamino,
arylamino, hydroxy,
alkoxy, aryloxy, thio, alkylthio, arylthio, formyl, alkylacyl, arylacyl, halo,
vitro, cyano, carboxy and
its esters and amides; more preferred substituents include phenyl, naphthyl,
and heterocyclyl having
one ring with 5 or 6 ring atoms including I-3 heteroatoms or two fused rings
with 8-10 ring atoms
including I-4 heteroatoms. Aryl RS is preferably phenyl or naphthyl, more
preferably phenyl. Aryl
RS is preferably unsubstituted or substituted; preferred substituents include
alkyl, aryl, heterocyclyl,
amino, alkylamino, arylamino, hydroxy, alkoxy, aryloxy, thio, alkylthio,
arylthio, formyl, alkylacyl,
arylacyl, halo, vitro, cyano, carboxy and its esters and amides; more
preferred substituents include
CI-C6 alkyl, phenyl, and heterocyclyl having one ring with 5 or 6 ring atoms
including 1-3
heteroatoms or two fused rings with 8-10 ring atoms including 1-4 heteroatoms.
Heterocycle RS is
preferably one ring having 5 or 6 ring atoms including I-3 heteroatoms or two
fused rings having 8-
10 ring atoms including I-4 heteroatoms. More preferred heterocycle RS is
heteroaryl. Heterocycle
RS is preferably unsubstituted or substituted; preferred substituents include
alkyl, aryl, heterocyclyl,
amino, alkylamino, arylamino, hydroxy, alkoxy, aryloxy, thio, alkylthio,
arylthio, formyl, alkylacyl,
arylacyl, halo, vitro, cyano, carboxy amid its esters and amides; more
preferred substituents include
CI-C6 alkyl, phenyl, and heterocyclyl having one ring with 5 or 6 ring atoms
including 1-3
heteroatoms or two fused rings with 8-10 ring atoms including 1-4 heteroatoms.
6
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
The subject invention includes optical isomers, diasteromers, and enantiomers
of the
compounds of structure 2. The subject invention includes salts, hydrates,
esters, amides, and imides
of such compounds.
As used herein, a "salt" is a cationic salt formed at any acidic group (e.g.,
carboxy group),
or an anionic salt formed at any basic group (e.g., amino group) on a compound
of structure 2.
Many salts are known. Preferred cationic salts include the alkali metal salts,
such as sodium and
potassium, alkaline earth metal salts, such as magnesium and calcium, and
organic salts, such as
ammonium. Preferred anionic salts include halides, sulfonates, carboxylates,
phosphates, and the
like. Salts of addition may provide an optical center where once there was
none.
The compounds of the subject invention, and salts thereof, may have one or
more chiral
centers. The invention includes all optical isomers of the compounds of
structure 2 and salts
thereof, including diasteriomers and enatiomers.
The subject invention also includes libraries of compounds having structure 2.
Such
libraries can be mixtures of compounds of structure 2 or collections of
individual compounds of
structure 2.
Processes for Making the Compounds
Another aspect of the subject invention is processes for making compounds of
structure 2,
as generally depicted in Scheme 1.
Scheme 1
O O
HO~N~B°c a, b O~NH
Fmoc'NJ
Fmoc' N
3 4
R5
O NH O O NH
O
f-h R~~N~N R4
O~ N R4 ~
HNJ R2~NJ
~ ~O
5 2
7
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
Orthogonally protected, resin bound piperazinic acid is used. Deprotection
reaction is followed by
functionalization of the b-nitrogen atom via the Petasis reaction. Subsequent
amide bond formation
leads to the desired inclusion of R4 and RS substituents. Unblocking of the a-
nitrogen, followed by
Boc-N protected a-amino acid coupling, deprotection and cyclizative cleavage,
introduces the R2
and R1 substituents, and leads to bicyclic product 2.
In step a, hydroxymethylpolystyrene resin is reacted with N-protected
piperazinic acid (3)
to bind it to the resin. This reaction is preferably carried out, after
swelling the resin in DCM, in the
presence of Ph3P and DEAD which act as Mitsunobu reagents, in THF solvent. The
resin product is
preferably filtered and washed several times using one or more of THF, DCM,
and MeOH to purify
the product.
In step b, the protecting group is removed from the ~3-nitrogen of the resin-
bound
piperazinic acid to produce 4. This is preferably accomplished by using a
solution of TFA and
DCM, preferably about 40% TFA, preferably after swelling the resin reactant in
DCM. The resin
product is preferably filtered and washed several times using DCM and/or MeOH,
neutralized, and
filtered and washed more to purify it.
In step c, piperazinic ester resin 4 is reacted with R4-boronic acid and
glyoxylic acid. This
reaction is preferably carried out in DCM solvent, preferably after swelling
the resin reactant in
DCM. The resin product is preferably filtered and washed several times using
DCM and MeOH, to
purify it. This reaction step and purification are preferably repeated to
increase the yield of the
desired product.
In step d, the resin product from step c is reacted with amine RS-NH2. Prior
to addition of
the amine in this step, the resin reactant is preferable swelled using DMF,
contacted with HOBt
which acts as an activating agent, followed by contact with DIC which acts as
a coupling agent, and
is filtered and washed with DMF. After reaction with the amine, the resin
product is preferably
filtered and washed with DMF.
In step e, the resin product from step d is contacted with piperidine which
acts as a
deprotecting agent. This step is preferably carried out in a solution of
piperidine in DMF, the
solution being from about 1 S % to about 30 % piperidine. Resulting resin
product 5 is preferably
filtered and washed several times in DMF. This step is preferably repeated to
increase the yield of
the desired product.
In step f, resin product 5 is reacted with a N-protected a-amino acid. This
reaction is
preferably carried out in the presence of PyBOP which acts as a coupling
agent, and then a base
DiPEA. Prior to adding the amino acid, the resin reactant is preferably
swelled using DMF. The
8
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
resin product is preferably filtered and washed several times with DMF. This
step is preferably
repeated.
In step g, the protecting group of the a-amino acid of step f is removed. This
is preferably
done using a solution of TFA in DCM, preferably about 25% TFA. The resulting
resin product is
preferably filtered and washed several times with DCM and MeOH.
In step h, the resin product from step g is cleaved from the resin and
cyclized to produce
product 2. This step is preferably carried out in a solution of from about 5%
to about 20% AcOH in
iPrOH, at an elevated temperature of from about 30 °C to about 80
°C for a period of from about 20
h to about 80 h. The cleaved resin is filtered off and washed several times,
preferably using MeOH.
The filtrate and washings are preferably combined, concentrated, and dried to
give 2 as a solid. The
solid product is preferably purified by co-evaporating it several times with
chloroform, and then
drying under vacuum.
The following non-limiting examples illustrate, in more detail, processes of
the subject
invention.
Example 1
a) Hydroxymethylpolystyrene resin ( 1.0 g, 1.44 mmol/g, Advanced Chemtech) is
swelled in anhydrous dichloromethane (DCM) (6 mL). Triphenylphosphine (Ph3P)
(1.13
g, 4.32 mmole) is dissolved in this slurry and the heterogeneous reaction is
cooled to 0 °C
under nitrogen. To this gently stirring slurry is added a tetrahydrofuran
(THF) (30 mL)
solution of Na-Fmoc-Np-Boc-2-carboxypiperazine 3 (1.95 g, 4.32 mmol) and
diethylazodicarboxylate (DEAD) (751 mL, 4.32 mmole) over a period of 30
minutes. The
reaction is allowed to stir for 72 h, at which point the resin is filtered and
washed with
THF (3X), DCM (3X), MeOH (3X), then multiple, successive and alternating DCM
and
MeOH washes (standard manner).
b) Piperazinic resin ester from step a ( 1.4 g) is swelled in DCM, filtered
and treated
with a 40% solution of trifluoroacetic acid (TFA) in DCM for 1 h. The resin is
filtered
and washed with DCM (3X), MeOH (3X), then alternating DCM/MEOH as in (a). The
resin bound amine TFA salt is then neutralized with a 10% solution of
diisopropylethylamine (DiPEA) in DCM and re-washed in the standard manner.
c) The resin ester 4 from step b is swelled in DCM (10 mL) and to this is
added
glyoxylic acid (265g, 2.88 mmol) and a boronic acid (2.88 mmol) as a solution
in MeOH
(15 mL). The resulting slurry is agitated for 5 h, before filtering the resin
and washing
with MeOH (3X). The above procedure is repeated again for 16 h, after which
the resin is
again filtered and washed in the standard manner.
9
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
d) The resin ester from step c is swelled in DMF (5 ml) and to this is added
hydroxybenzotriazole (HOBt) (1.10 g, 7.2 mmol) followed by
diisopropylcarbodiimide
(DIC) (907 mg, 7.2 mmol). The reaction is agitated for 3h, after which the
resin is
washed with dimethylformamide (DMF) (4X). The resin is swelled again in DMF
(10
ml), and to this slurry is added an amine (7.2 mmol). The reaction is allowed
to agitate
for 15 h. The resin is filtered and washed with DMF (3X) followed by the
standard
manner wash.
e) The resin ester from step d is treated with 25% piperidine in DMF for 20
min.
The resin is filtered and rinsed with DMF (2X) before repeating. The resin
product is
filtered and washed in the standard manner.
f) The resin product 5 from step a is swelled in DMF. To this is added a boc-a-
amino acid (Boc-AA) (7.2 mmole), benzotriazol-1-yl-oxy-tris-pyrrolidino-
phosphonium
hexafluorophosphate (PyBOP) (3.74 g, 7.2 mmole) followed by DiPEA ( 1.11 g,
8.64
mmole). The reaction is agitated for 5 h, filtered and rinsed with DMF (3X) in
the
standard manner.
g) The resin product from step f is treated with 25% TFA/DCM for 1 h. The
resin is
filtered and washed.
h) The resin product from step g is taken up in 10% acetic acid in isopropanol
(AcOH/iPrOH) and heated for 16 h at 50 °C. The resin is filtered off
and washed several
times using MeOH. The filtrate and washings are combined and concentrated to
give an
off white solid. The solid as co-evaporated several times using chloroform
before being
dried under vacuum for 15 h.
Examples 2-7
The following non-limiting exemplary compounds are made using the process of
Example 1 by reacting the indicated boronic acids, amines, and boc-a-amino
acids
therein:
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
~NH2
O O NH
O O NH H~N~N S
H \ ~,, N J I /
N~N
I O
,.~,'~N~ ~J
H02C O 2a HZN 2b
N
I O NH I
O
O O NH H~N~N I W
S ''~~' ~ N ~ ~ F
\ N~N I O
~,,,~ N J / \
W
O
2c 2d
I~
~N I~
O NJ
O
O O O NH
H.N~N
,,,,, N ~ / N ~ N W
o N
O ",~~ J
I
~I O w
2e 2f
Example Boronic Acid Amine Amino Acid Product
phenylboronic acid isoamylamine Boc-Asp (O-tBu)- 2a
OH
2-thiopheneboronic mono-Boc-butyl- Boc-Lys(Boc)-OH 2b
acid 1,4-diamine
Exam,~le Boronic Acid Amine Amino Acid Product
benzothiophene-2- 4-aminomethyl- Boc-Tic-OH 2c
bornonic acid pyridine
11
CA 02393759 2002-06-07
WO 01/46197 PCT/US00/34832
S 4-fluoroboronic acid phenethylamine Boc-Phe-OH 2d
6 2-furylboronic acid benzylamine Boc-Phe-OH 2e
7 2-naphthylboronic aniline Boc-Hyp(OBn)-OH 2f
acid
The above processes of the subject invention are carried out using solid-
support resin.
This makes reaction separation and purification of intermediates and final
product amenable to
automation. Libraries of compounds of structure 2 are readily prepared using
the subject
invention processes. Automation of the preparation of such libraries is
achieved using equipment
known to the skilled combinatorial chemist. Such equipment can be used to make
libraries which
are mixtures of compounds of structure 2 or collections of individual
compounds of structure 2
each in an isolated well.
While particular embodiments of the subject invention have been described, it
will be
obvious to those skilled in the arts that various changes and modifications of
the subject
invention can be made without departing from the spirit and scope of the
invention. It is intended
to cover, in the appended claims, all such modifications that are within the
scope of this
invention.
12