Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 1 -
TITLE OF THE INVENTION
Process of obtaining thylakoids from photosynthetic organisms; plant fractions
obtained from the process; pure thylakoids; and methods of use of thylakoids
as
ROS scavengers, photo-protectors, biosensors, biofilters and bioreactors.
FIELD OF THE INVENTION
This invention relates to the isolation and recovery of thylakoids,
which are present substantially in their integral and natural state, at least
a portion
of which is functional or activable. This invention also relates to the
obtention of other
soluble and insoluble plant fractions obtained upon the isolation of
thylakoids. This
invention further relates to the use of thylakoids as ROS scavengers, as
photoprotectors, particularly against U.V. radiations, as well as biosensors,
biofilters
or bioreactors.
BACKGROUND OF THE INVENTION
Antioxidants have become increasingly popular, namely in the
biomedical field, because of their capacity to prevent the formation and
the.noxious
activity of reactive oxygen species (ROS).
Plants and other photosynthetic organisms are particularly well
adapted to resist the effect of ROS, especially to protect vital organelle
photosynthetic membranes called thylakoids against oxidative damages and the
noxious action of U.V. radiations. ,
Sunlight plays a much larger role in our sustenance than we may
expect: all the food we eat and all the fossil fuel we use is a product of
photosynthesis, which is the process that converts energy in sunlight to
chemical
forms of energy that can be used by biological systems. Photosynthesis is
carried out
by many different organisms, ranging from plants to bacteria. The best known
form
of photosynthesis is the one carried out by higher plants and algae, as well
as by
cyanobacteria and their relatives, which are responsible for a major part of
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 2 -
photosynthesis in oceans. All these organisms convert CO2 (carbon dioxide) to
organic material by reducing this gas to carbohydrates in a rather complex set
of
reactions. Electrons for this reduction reaction ultimately come from water,
which is
then converted to oxygen and protons. Energy for this process is provided by
light,
which is absorbed by pigments (primarily chlorophylls and carotenoids).
Chlorophylls
absorb blue and red light and carotenoids absorb blue-green light, but green
and
yellow light are not effectively absorbed by photosynthetic pigments in
plants;
therefore, light of these colors is either reflected by leaves or passes
through the
leaves.
Other photosynthetic organisms, such as cyanobacteria (formerly
known as blue-green algae) and red algae, have additional pigments called
phycobilins that are red or blue and that absorb the colors of visible light
that are not
effectively absorbed by chlorophyll and carotenoids. Yet other organisms, such
as
the purple and green bacteria (which, by the way, look fairly brown under many
growth conditions), contain bacteriochlorophyll that absorbs in the infrared,
in
addition to in the blue part of the spectrum. These bacteria do not evolve
oxygen, but
perform photosynthesis under anaerobic (oxygen-less) conditions. These
bacteria
efficiently use infrared light for photosynthesis. Infrared is light with
wavelengths=
above 700 nm that cannot be seen by the human eye; some bacterial species can
use infrared light with wavelengths of up to 1000 nm. However, most pigments
are
not very effective in absorbing ultraviolet light (<400 nm), which also cannot
be seen
by the human eye. Light with wavelengths below 330 nm becomes increasingly
damaging to cells, but virtually all light at these short wavelengths is
filtered out by
the atmosphere (most prominently the ozone layer) before reaching the earth.
Even
though most plants are capable of producing compounds that absorb ultraviolet
light,
an increased exposure to light around 300 nm has detrimental effects on plant
productivity.
Photosynthetic pigments come in a huge variety: there are many
different types of (bacterio)chlorophyll, carotenoids, and phycobilins,
differing from
CA 02393816 2002-06-27
=
- 3 -
each other in their precise chemical structure. Pigments generally are bound
to
proteins, which provide the pigment molecules with the appropriate orientation
and
positioning with respect to each other. Light energy is absorbed by individual
pigments, but is not used immediately by these pigments for energy conversion.
Instead, the light energy is transferred to chlorophylls that are in a special
protein
environment where the actual energy conversion event occurs: the light energy
is
used to transfer an electron to a neighboring pigment. Pigments and protein
involved
with this actual primary electron transfer event together are called the
reaction
center. A large number of pigment molecules (100-5000), collectively referred
to as
antenna, "harvest" light, capture photons, and transfer the light energy to
the same
reaction center. The purpose is to maintain a high rate of electron transfer
in the
reaction center, even at lower light intensities. The denomination P680 is
assigned
to the chlorophyll pigments of the reaction center PSII, because the pair of
chlorophylls entering it composition absorbs light mostly at a 680 nm
wavelength.
Many antenna pigments transfer their light energy to a single
reaction center by having this energy transfer to another antenna pigment, and
yet
to another, etc., until the energy is "trapped" in the reaction center. Each
step of this
energy transfer must be very efficient to avoid a large loss in the overall
transfer
process, and the association of the various pigments with proteins ensures
that
transfer efficiencies are high by having appropriate pigments close to each
other, and
by having an appropriate molecular geometry of the pigments with respect to
each
other. An exception to the rule of protein-bound pigments are green bacteria
with
very large antenna systems: a large part of these antenna systems consists of
a
"bag" (named chlorosome) of up to several thousand bacterioclhlorophyll
molecules
that interact with each other and that are not in direct contact with protein.
Chlorophyll is used by all photosynthetic organisms as the link between
excitation
energy transfer and electron transfer. Of particular note is the rate with
which these
transfer reactions heed to occur. As the lifetime of the excited state is only
several
nanoseconds (1 nanosecond (ns) is 10-9 s), after absorption of a quantum,
energy
6 1 AMENDED SHEET
ri."111.16.66-04-2002
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 4 -
transfer and charge separation in the reaction center must have occurred
within this
time period. Energy transfer rates between pigments are very rapid, and charge
separation in reaction centers occurs in 3-30 picoseconds (1 picosecond (ps)
is 10-12
s). Subsequent electron transfer steps are significantly slower (200 Ps - 20
ms) but,
nonetheless, the electron transport chain is sufficiently fast that at least a
significant
part of the absorbed sunlight can be used for photosynthesis. The pigments
have a
specific organisation which should be preserved upon isolation and
purification of
thylakoids if the maintenance of the function of the latter is sought.
In many systems the size of the photosynthetic antenna is flexible,
and photosynthetic organisms growing at low light (in the shade, for example)
generally will have a larger number of antenna pigments per reaction center
than
those growing at higher light intensity. However, at high light intensities
(for example,
in full sunlight) the amount of light that is absorbed by plants exceeds the
capacity
of electron transfer initiated by reaction centers. Plants have developed
means to
convert some of the absorbed light energy to heat rather than to use the
absorbed
light necessarily for photosynthesis. However, in particular the first part of
photosynthetic electron transfer in plants is rather sensitive to overly high
rates of
electron transfer, and part of the photosynthetic electron transport chain may
be shut
down when the light intensity is too high; this phenomenon is known as
photoinhibition.
The initial electron transfer (charge separation) reaction in the
photosynthetic reaction center sets into motion a long series of redox
(reduction-
oxidation) reactions, passing the electron along a chain of cofactors and
filling up the
"electron hole" on the chlorophyll, much like in a bucket brigade. All
photosynthetic
organisms that produce oxygen have two types of reaction centers, named
photosystem ll and photosystem I (PS ll and PS I, for short), both of which
are
pigment/protein complexes that are located in specialized membranes called
thylakoids. In eukaryotes (plants and algae), these thylakoids are located in
chloroplasts (organelles in plant cells) and often are found in membrane
stacks
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 5 -
(grana). Prokaryotes (bacteria) do not have chloroplasts or other organelles,
and
photosynthetic pigment-protein complexes either are in the membrane around the
cytoplasm or in invaginations thereof (as is found, for example, in purple
bacteria),
or are in thylakoid membranes that form much more complex structures within
the
cell (as is the case for most cyanobacteria).
All the chlorophyll in oxygenic organisms is located in thylakoids,
and is associated with PS II, PS I, or with antenna proteins feeding energy
into these
photosystems. PS II is the complex where water splitting and oxygen evolution
occurs. Upon oxidation of the reaction center chlorophyll in PS II, an
electron is
pulled from a nearby amino acid (tyrosine) which is part of the surrounding
protein,
which in turn gets an electron from the water-splitting complex. From the PS
ll
reaction center, electrons flow to free electron carrying molecules
(plastoquinone) in
the thylakoid membrane, and from there to another membrane-protein complex,
the
cytochrome b6f complex. The other photosystem, PS I, also catalyzes light-
induced
charge separation in a fashion basically similar to PS II: light is harvested
by an
antenna, and light energy is transferred to a reaction center chlorophyll,
where light-
induced charge separation is initiated. However, in PS I electrons are
transferred
eventually to NADP (nicotinamide adenosine dinucleotide phosphate), the
reduced
form of which can be used for carbon fixation. The oxidized reaction center
chlorophyll eventually receives another electron from the cytochrome b6f
complex.
Therefore, electron transfer through PS ll and PS I results in water oxidation
(producing oxygen) and NADP reduction, with the energy for this process
provided
by light (2 quanta for each electron transported through the whole chain).
Electron flow from water to NADP requires light and is coupled to
generation of a proton gradient across the thylakoid membrane. This proton
gradient
is used for synthesis of ATP (adenosine triphosphate), a high-energy molecule.
ATP
and reduced NADP that resulted from the light reactions are used for CO2
fixation in
a process that is independent of light. CO2 fixation involves a number of
reactions
that is referred to as the Calvin-Benson cycle. The initial CO2 fixation
reaction
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 6 -
involves the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisC0),
which can react with either oxygen (leading to a process named
photorespiration and
not resulting in carbon fixation) or with CO2. The probability with which
RuBisCO
reacts with oxygen vs. with CO2 depends on the relative concentrations of the
two
compounds at the site of the reaction. In all organisms CO2 is by far the
preferred
substrate, but as the CO2 concentration is very much lower than the oxygen
concentration, photorespiration does occur at significant levels. To boost the
local
CO2 concentration and to minimize the oxygen tension, some plants (referred to
as
C4 plants) have set aside some cells within a leaf (named bundle-sheath cells)
to be
involved primarily in CO2 fixation, and others (named mesophyll cells) to
specialize
in the light reactions: ATP, CO2 and reduced NADP in mesophylicells is used
for
synthesis of 4-carbon organic acids (such as malate), which are transported to
bundle sheath cells. Here the organic acids are converted .releasing CO2 and
reduced NADP, which are used for carbon fixation. The resulting 3-carbon acid
is
returned to the mesophyll cells. The bundle sheath cells generally do not have
PS
ll activity, in order to minimize the local oxygen concentration. However,
they retain
PS I, presumably to aid in ATP synthesis.
Thylakoid organization is very sophisticated in order to extract the
energy from light, and to transfer this energy to a proper location, and/or
dissipate
the same. The transfer is rendered possible and efficient by separating
electrical
charges and a high capacity to regenerate a neutral electrical state, ready
for
undertaking again a change in charges (Blankenship et al. 1998).
The electron transfer between the above five main components is
extremely rapid: the transfer from an activated P680 to pheophytin takes less
than
one picosecond. The electron transfer stops when all the pigments return to a
neutral
electrical charge, ready to undertake a new cycle.
Electrons are finally directed to a coupling factor to reduce NADPH,
necessary in ATP synthesis, which will serve in sugar synthesis.
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 7 -
The term "thylakoids" is used hereinbelow and means to cover
organized photosynthetic membrane components obtained from photosynthetic
organisms, eucaryotic and prokarytotic. When the organism has chloroplasts,
the
thylakoids comprise the following membrane constituents: PSII, cytochromes IN
and
f, PSI and the coupling factor. Where thylakoids integrity and functionality
has been
tested from plant material, it has been measured between two reference points:
proximal to PSII and distal to the coupling factor. For certain applications,
thylakoids
do not need to be active although they are apparently integral. Such
thylakoids are
performing and at least as stable as any other antioxidant. Therefore, "active
thylakoids" means thylakoids having the capacity to activate upon hydration,
as
opposed to inactive thylakoids which are integral but which have been actively
or
passively inactivated. In this case, the reaction center is inactive although
thylakoids
structure is substantially preserved. The "inactive" thylakoids are therefore
suitable
antioxidants although they do not have the same dynamism nor do they have the
same capability to regenerate, or the same capacity to respond to ROS as the
active/activable form.
Photosynthesis comprises two fundamental processes that can be
summarized in the two following reactions:
Light
-------------------------- H20 + ADP + Pi +NADP+ -+02+
ATP + NADPH + H+ (1)
CO2 + ATP + NADPH + H+ --------------- (CH20),, + ADP + Pi + NADP+
(2)
During the first reaction in the presence of light, protons are taken
from chloroplast water to produce ATP. The second reaction consists in using
NADPH and ATP in a series of reactions that lead to the reduction of carbon
anhydride in glucides, mainly starch. These two reactions occur
simultaneously;
products formed by process (1) are directed into the reaction of process (2).
Globally,
the photosynthesis results into the production of sugars in the form of starch
and
sucrose and energy under the form of ATP molecules:
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 8 -
Light
6 CO2 + 12 H20 ----------------------------------- ---> C6H1206 + 602 + 2872
KJ
(sugars) (ATP)
Light activation follows a certain pathway in the thylakoids. Light is
first collected by light antenna (LHCII), and the energy is directed to
reaction center
(PSII) and, finally to PSI which also has an independent light collector
(LHCI).
Thylakoids have for functions to collect light and to transfer light energy to
a proper
location for further photosynthesis. The synthesis of ATP and of sugars does
not take
place in the thylakoids but in other chloroplast compartments.
Chlorophylls are the main active pigments. The carotenoids have
more than one role, depending on their location. A first role is as light
collectors,
which results in energy transfer from carotenoids to chlorophylls. A second
role is as
photoprotectors, this time the energy transfer occurring in an opposite
direction
between chlorophylls and carotenoids. Carotenoid singlet state has more energy
that
a singlet chlorophyll while, on the opposite, carotenoid triplet state has
less energy
than triplet chlorophylls. The energy states having a natural tendency to go
from a
high to a low energy level, one will appreciate that the singlet carotenoid
mostly acts
as a light collector passing light energy to a singlet chlorophyll molecule
while the
triplet chlorophyll will readily transfer its energy to the triplet
carotenoid, when the
latter acts as a photoprotector in the reaction center. Carotenoids take
different
configurations upon associating with antenna or reaction center, which
configuration
may be responsible for their energy state upon activation. A "cis"
configuration is
associated with photoprotection in the reaction center. An "all-trans"
configuration is
associated with the light collector function of the antenna.
The transfer of energy is efficient only in conditions in which the
pigments are very close to each other and in a specific organisation. It is
therefore
very important not to disturb the natural organisation of the pigments,
keeping the
membranes in an integral state, if one wants to purify active or fully
activable
thylakoids.
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 9 -
One advantage of recovering intact thylakoids is. found in their
capacity to handle ROS. Such ROS are intended to cover free radicals
(including
super oxides), as well as non-radical oxidants such as singlet oxygen (102)
and
peroxides. A good review of the definition and origin of these species is
found in the
international publication WO 94/13300. The contents of all the references
cited
hereinabove and below are incorporated herein by reference.
Free radicals are atoms, ions, or molecules that contain an
unpaired electron. Free radicals are usually unstable and exhibit short half-
lives.
Elemental oxygen is highly electronegative and readily accepts single electron
transfers from cytochrornes and other reduced cellular components; a portion
of the
02 consumed by cells engaged in aerobic respiration is univalently reduced to
superoxide radical (.02-). Sequential univalent reduction of .02- produces
hydrogen
peroxide (H202), hydroxyl radical (.0H), and water.
Free radicals can originate from many sources, including aerobic
respiration, cytochrome P-450-catalyzed monooxygenation reactions of drugs and
xenobiotics (e.g., trichloromethyl radicals, CCI3. formed from oxidation of
carbon
tetrachloride), and ionizing radiation. For example, when tissues are exposed
to
gamma radiation, most of the energy deposited in the cells is absorbed by
water and
results in scission of the oxygen-hydrogen covalent bonds in water, leaving a
single
electron on hydrogen and one on oxygen creating two radicals H. and .OH. The
hydroxyl radical, .OH, is the most reactive radical known in chemistry. It
reacts with
biomolecules and sets off chain reactions and can interact with the purine or
pyrimidine bases of nucleic acids. Indeed, radiation-induced carcinogenesis
may be
initiated by free radical damage. Also for example, the "oxidative burst" of
activated
neutrophils produces abundant superoxide radical, which is believed to be an
essential factor in producing the cytotoxic effect of activated neutrophils.
Reperfusion
of ischemic tissues also produces large concentrations of oxyradicals,
typically
superoxide. Moreover, superoxide may be produced physiologically by
endothelial
cells for reaction with nitric oxide, a physiological regulator, forming
peroxynitrite,
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 10 -
oNoo- which may decay and give rise to hydroxyl radical, .OH. Additional
sources
of oxyradicals are "leakage" of electrons from disrupted mitochondrial or
endoplasrnic
reticular electron transport chains, prostaglandin synthesis, oxidation of
catecholamines, and platelet activation. Many free radical reactions are
highly
damaging to cellular components; they crosslink proteins, mutagenize DNA, and
peroxidize lipids. Once formed, free radicals can interact to produce other
free
radicals and non-radical oxidants such as singlet oxygen (102) and peroxides.
Singlet oxygen is a particularly noxious compound involved in the
initiation or in the perpetuation of many diseases or disorders. The singlet
oxygen is
also involved in the degradation of protein like chlorophylls. This is why a
photoprotection conferred by the presence of carotenoids becomes important.
Carotenoids protect the chlorophyll life and activity, they further protect
the integrity
of the membranes by preventing protein denaturation. Carotenoids are capable
of
capting the energy of triplet chlorophyll molecules; they become triplet
carotenoid
molecules, which regenerate themselves while dissipating heat thereby avoiding
the
accumulation of a triplet chlorophyll, and minimizing the chances to degrade
the
chlorophyll.
However, in the presence of excess light, damage may occur,
which may originate from the formation of chlorophyll in "triplet state". In a
triplet state
two electrons in the outer shell have identical rather than opposite spin
orientation.
This triplet chlorophyll readily reacts with oxygen, leading to the very
reactive singlet
oxygen, which can damage proteins. To counter this damaging reaction,
carotenoids
are usually present in close vicinity to chlorophylls. Many carotenoids
efficiently
"quench" triplet states of chlorophyll, thus avoiding formation of singlet
oxygen.
Chlorophyll in its free form is very toxic in the light in the presence of
oxygen,
because a close interaction with carotenoids is not always available under
such
circumstances. Therefore, all chlorophyll in a cell in aerobic organisms is
bound to
proteins, generally with carotenoids bound to the same protein.
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
-11 -
A major difficulty in measuring enzyme kinetics at relatively short
time scales (less than 1 ms) is that "traditional" enzyme reactions require a
mixing
of substrate and enzyme, which usually takes a relatively long time. Kinetic
analysis
of light-driven reactions such as photosynthetic electron transport have a
great
advantage in this respect: reactions can be triggered simply by a light pulse,
which
can be even shorter than 1 ps. Moreover, many of the components participating
in
electron transfer have different absorption spectra depending on whether they
are
in the oxidized or reduced form. Using laser spectroscopy methods or more
standard
optical spectroscopy, it is relatively simple to follow the electron around on
a
timescale between 1 ps and several ms. The primary charge separation occurs in
several ps, and reactions become gradually slower as they involve components
that
are further away from the reaction center. Because of the fast speed of early
reactions, the electron and the "electron hole" are physically separated
rapidly by a
large distance (the electron generally has traveled about 2 nm to the other
side of the
membrane within 1 ns after charge separation), so that back reactions (charge
recombinations) are not favorable anymore. Unpaired electrons on reactants
that are
transiently formed during redox reactions involving transfer of a single
electron in
many instances can be detected using electron paramagnetic resonance (EPR) and
derived techniques (including ENDOR, electron nuclear double resonance, and
ESEEM, electron spin echo envelope modulation). Many of these techniques can
be
used to kinetically follow redox reactions, and provide detailed information
regarding
electron spin distributions etc. Therefore, photosynthetic membranes and
reaction
centers have a prominent place as experimental systems in biochemistry and
biophysics.
The anti-oxidative potential of a compound such as chlorophyll is
exarnplified in equation (1)
3Chl* + 302 -> Chi + 102* (1)
Chlorophyll that has been excited into presence of oxygen
becomes in a triplet state (3Chl*), and disactivates to return to a
fundamental state
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 12 -
by producing singlet oxygen (a noxious species) in cells. Plants have found an
efficient means by which they can solve the problem of overproduction of
singlet
oxygen. The plants transfer the chlorophyll energy to another pigment which
has an
inferior energy state. That pigment called carotenoid (equation 2) is abundant
in
plants.
3Chl*+ Car 4 Chi + 3Car* (2)
Although triplet chlorophyll has more energy than a corresponding
carotenoid, the converse is true for the singlet state. As shown in equations
3a and
3b, an activated singlet carotenoid transfers its energy to a chlorophyll
molecule
which becomes activated in a singlet state.
Car + energy - 'Car* (3a)
'Car* + Chi - Car + 'Chi* (3b)
Carotenoids in a triplet state desactivates without forming a noxious
oxygen species. Equation 4 shows that carotenoids inactivate by returning to a
fundamental state and by heat dissipation.
3Car* - Car + Heat (4)
It appears that it is important not to produce ROS to preserve the
properties of the pigments in an extract, but it is also important to remove
those ROS
that may be generated during isolation. For achieving this, we have given
favor to a
way to reverse the equilibrium of equation 1. Consequently, the converse
equation
1 is found in equation 5.
Chi +102* - 3Chl* + 302 (5)
To avoid reversal of equation 5, activated triplet chlorophyll
molecule needs to be in close contact with a carotenoid in its fundamental
state,
which takes the transferred energy and dissipates the latter as heat. This way
the
reversibility of equation 5 is restricted insofar as chlorophyll and
carotenoid pigments
can be found in very close proximity so as to transfer to one another their
energy.
From the above equation 5, it is apparent that, to obtain an extract
that is optimally active, it is preferable to take every possible measures to
maintain
CA 02393816 2002-06-27
. .
- 13 -
both pigments (chlorophyll and carotenoid) in their fundamental state.
isolated
carotenoids, e.g. carotenoids not organized in thylakoid structures, would not
be capable
of an efficient quenching of triplet chlorophyll molecules. The advantage of
having
organized pigments is that the extract will retain the dynamism of natural
thylakoid
membranes, which confers to them the capacity to capture ROS, to transfer the
energy
and to return to a state capable of undertaking new activation cycles again.
This
dynamism and capacity to regenerate is unique to organized pigments. It is
important to
mention that the above reactions are spontaneously produced and this, in
absence of
light This observation is important from a therapeutic point of view, because
internal
administration of a thylakoid extract would preclude the presence of light
Thylakoids having optimized configuration and carotenoid proportions
will retain fun activity especially toward ROS. Such an antioxidant vile be
useful to reduce
the expression of diseases or disorders that involve the production of ROS.
Such
diseases or disorders can be those with an etiology related to inflammation,
cancer and
contact with radiations. Such diseases or disorders comprise those affecting
Skin: such
as bums, solar radiation, psoriasis, dermatitis; Brain: such as trauma,
stroke, Parkinson,
neurotoxins, dementia, Alzheimer; Joints: such as rheumatoid arthritis and
arthrosis;
Gastrointestinal tract such as diabetes, pancreatitis, endotoxin liver injury,
ischemic
bowel; Eye: such as cataractogenesis, retinopathy, degenerative retinal
damage;
Vessels: such as atherosclerosis and vasculitis; Erythrocytes: such as Fanconi
anemia,
malaria; Heart such as coronary thrombosis; Lung: such as asthma, COPD;
Kidney:
such as transplantation, glomerulonephritis; Multiorgan: such as inflammation,
cancer,
ischemia-reflow states, drug toxicity, iron overload, nutritional
deficiencies, alcohol
toxicity, radiation, ageing, amyloid diseases and toxic shock. The literature
related to the
involvement of ROS in some diseases is the following:
Skin:
Bum Youn, 1992
Solar Radiation Golan, 1994
AMENDED SHEET
F-04-2002'
CA 02393816 2002-06-26
WO 01/49305
PCT/CA00/01541
- 14 -
Psoriasis Lange, 1998 a,b
Dermatitis PoIla, 1992
Brain:
Trauma Juurlink, 1998
Stroke El Kossi, 2000
Parkinson Ebadi, 1996
Neurotoxins Foler, 2000
Alzheimer Smith 2000
Joints:
Rheumatoid arthritis Cimen, 2000
Gastrointestinal tract:
Diabetes Gerber, 2000
Pancreatitis Sakorafas, 2000
Endotoxin liver injury McGuire, 1996
lschemic bowel Lai, 2000
Eye:
Cataractogenesis Eaton,1994
Retinopathy of prematurity Hardy, 2000
Degenerative retinal damage Castagne, 2000
Vessels:
Atherosclerosis Singh, 1997
Erythrocytes:
Anemia Anastassopoulou, 2000
Malaria Ginsburg, 1999
Heart:
Coronary thrombosis Chen, 1995
Lung:
Asthma Montuschi, 1999
COPD Montuschi, 2000
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 15 -
Kidney:
Glomerulonephritis: Barros, 2001
Multiorgan:
Transplantation Jonas, 2000
Inflammation El-Kadi, 2000
Cancer Prior, 2000
lschemia Lewen, 2000
Drug toxicity Sinha, 1990
Iron overload Karbownik, 2000
Nutritional deficiencies Olszewski, 1993
Alcohol toxicity Lieber, 1997
Ageing Cadenas, 2000
Radiation Bednarska, 2000
Amyloid diseases Floyd, 1999.
Besides therapeutic applications, it has been found that the
thylakoids of this invention may advantageously replace chloroplasts-derived
compositions of the art that have been tested as biosensors or biofilters or
bioreactors. The art in the field teaches these specific uses, but the
chloroplasts-
derived compositions lack stability and degrade very rapidly, which renders
these
uses unpractical from a commercial point of view. Therefore, a stable and
dynamic
thylakoid extract could advantageously substitute for these non-performing
chloroplasts-derived compositions.
Biosensors :
Detection of toxic products is valuable for evaluating environmental
risks associated with the presence of contaminants. Valid bioassays would
normally
involve living organisms and would fulfil the following minimal conditions:
i) they should be representative of the natural environment,
ii) they should reproducible,
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 16 -
iii) they should be reliable so as to provide no or almost no false
results; and
iv) they should be sensitive.
Toxicity detection should also provide enough flexibility for
analyzing different types of contaminated samples. Toxicity should be ideally
monitored and sensed in real time fashion. Toxicity detection finds
application in at
least three industrial sectors: paper industry, contaminated soil analysis and
agriculture. In all these instances, information is needed on the presence of
contaminant in order to rapidly correct an undesirable situation.
A major problem encountered with the actual technologies to sense
toxic products is in the long delay of obtention of the results of biotests
from 48 to 96
hours, when using organisms like trout or Daphnea magna. A good detecting
device
would be one distinguishing from the available conventional biotests by the
use of
material which would allow measurements of a contaminating potential of an
effluent
in real time and continuously. Although some biodetectors are commercially
sold,
which measure fluorescence generated by plant photosynthetic activity, a
system
that would permit measurement of electrical charges induced by the presence of
light, and modulated by the presence of contaminants would be ideal. This
technology would be much cheaper than the fluorimetric technology. It is
believed
that a technique which would evaluate the photosynthetic activity on a total
thylakoid
material would be preferable over fluorometric methods which measure the
activity
of a specific proteic complex, namely the PSII. A device comprising thylakoid
material
would therefore have the advantage of measuring the toxicity in a larger
spectrum
of action. Such as detecting device would measure the number of electrons
produced with a given light intensity. A current (Epmax) obtained after a few
seconds
should be proportional to the photosynthetic activity of the thylakoids. If
the
photocurrent is plotted against the concentration of contaminants, a typical
sigmoidal
should be obtained, upon which an estimated EC50 should be deduced.
CA 02393816 2002-06-27
= , õ - = =
=
- 17 -
A photocurrent has been already measured by Allen and Crane in 1976.
ft has been found that electron transport constituted a reliable and
representative measure
of global photosynthetic activity and of the physiological health of a plant
From the work
of Allen and Crane, it is conceivable that an extract that would have a great
stability, along
with a dynamism and capacity to regenerate its responsive state to
contaminants and light
would be highly preferable over the known devices. A detector would measure
the number
of electrons produced at a given light intensity. A maximal photo-current
value (IpmEtc)
obtained after a few seconds is proportional to the photosynthetic activity of
the thylakoid
membranes. If one plots Ipmax v. the concentration of a photosynthetic
inhibitor (a
contaminant or a pollutant) present in the photoconversion chamber, a typical
sigmoidal
curve is obtained. The inhibitor potency can be easily evaluated (IC).
A detecting device would comprise: a white light source, a
photoconvetsion chamber receiving two electrodes, a detecting means for
measuring
=
electrical currents induced by light and computer means for collecting and
processing data
(electric currents). A liquid sample comprising a toxic agent, a contaminant
or a pollutant
to be identified or measured, is contacted with a thylakoidh membrane extract.
Once the
mixture introduced in the chamber, a brief illumination is applied (less than
one minute).
The device or apparatus may be conceived to process and analyze a plurality of
samples
simultaneously.
Biofilters/Biareaclors:
Because the photosynthetic apparatus in plants is capable of not only
capturing photons, but also of capturing and accumulating molecules having
affinity for its
components, it is contemplated that the present extract would also have the
same capacity
as the plant itself. Moreover, since some of the captured molecules may be
processed, the
present extract would act as a bioreactor. The molecules susceptible to be
captured are,
for example, herbicides, insecticides, fungicides, urea, ions and heavy metals
as well as
gas Ike 03, CO, H2S, NO, CO2, O. The biofilter of this invention would be
versatible and
would be resistant to temperature variations.
13 AMENDED SHEET
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 18 -
There is no existing practical process in the art teaching how to
recover intact functional thylakoids, capable of retaining activity for a
practical
amount of time.
It is obvious from the above that the plants have a great natural
capacity to manage with threatening situations. The thylakoids are
particularly
adapted to resist and adapt to such extreme situations.
The US Patent 4,698,360 describes a plant extract comprising pro-
,
anthocyanidins useful as free-radical scavengers. The process of making this
extract
comprises the following steps:
a) the obtention of a coarse powder of maritime pine bark;
b) its extraction in boiling water;
c) a separation of liquids from solids;
d) cooling the liquids to ambient temperature;
e) a filtration;
f) a "salting-out" precipitation to remove undesirable matter;
9) extracting active ingredients into ethyl acetate;
h) drying the organic phase;
i) resuspending the solids and reprecipitating the active ingredients
with chloroform; and
j) resuspending the solids before advanced purification.
This reference is concerned with the isolation of a specific type of
active ingredient, and not with the preparation of thylakoids that would
contain a
major portion of its photosynthetic components, in other words wherein
pigments
would not be separated from each another.
This reference is indeed typical of the overall teachings in the
general art which the present invention pertains to. The prior art relates
systematically to the isolation of one or more given plant components, and not
to the
isolation of intact thylakoids comprising a major portion of their
constituents
preserved in an integral and functional state.
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 19 -
Glick et al. (1985) in Planta 164: 487-494 describe the variations in
stoichiometric ratios of photosystems II and I (PSII/PSI) when peas are
submitted to
different types of light. The electron transport capacity of PSI and PSII in
the
presence of indicators such as 2,5-dimethyl-p-benzoquinone and NADP, which are
indicators specific for PSII and PSI, respectively. Although green light is
used, which
is a non-activating light environment, it is not used to condition the plant
in a process
which aims at isolating intact and activable thylakoids. The reference
essentially
relates to the study of the composition of chloroplasts and not the
preservation of
thylakoids activity in function of a given light quality and intensity. The
plants are
rather conditioned in different lights that are depleted or enriched in red
wavelengths.
This reference is not concerned with the fact that the photosynthetic pigments
should
be kept close to each other so as to favorise the energy transfer between
chlorophylls and carotenoids and to favorise free-radicals capture. Thus the
conditions leading to the isolation of photosynthetic pigments in their
natural state in
thylakoids are not specifically taught and met with in this reference.
Mason et al. (1991) in Plant Physiol. 97: 1576-1580 teach a method
for isolating chloroplasts, which makes use of a step of forced passage of a
plant
suspension through a 27-needle at a flow speed of 0.5 ml per second, rather
than
using a dispersion step by homogenization. The plant solution comprises a
buffer
having a pH 7.5 and comprising 0.3 M sorbitol. The preparation that has been
forced
through the needle is centrifuged in a Percoll gradient and the chloroplasts
are
separated from other constituents, including thylakoids. This process is
therefore
different from the present process which aims essentially at the recovery of
thylakoids using quite simple steps and reactants, which present process being
also
easy to scale up. The light conditions are not mentioned in this reference.
Further,
the conditions to keep chloroplasts integral are obviously not the same as
conditions
to disintegrate chloroplasts. In the present process, the chloroplasts are
disintegrated
but thylakoids membranes are recovered substantially intact. This reference
therefore cannot teach the present invention.
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 20 -
The Canadian patent application 2,110,038 describes a process of
stabilizing plant extracts. These extracts are however cell fluids or juices
and not
thylakoid membranes. There is no mention in this reference of withdrawal of
water
as a natural electron donor from the membranes, for the purpose of stabilizing
thylakoids.
In view of the foregoing, no practical process has been taught in
the art, that would lead to the isolation of intact and functional thylakoid
membranes.
There is further no teaching of conditions for stabilizing thylakoid
components. There
is finally no teaching of the use of isolated thylakoid membranes to scavenge
cell
components from ROS.
There is therefore an open challenge in developing a process for
obtaining active thylakoids that remain integral and, optionally activable,
for an
acceptable amount of time and which, upon reactivation are capable of acting
as an
antioxidant by their ROS scavenging activity. Although an increasing body of
literature is available on photosystem components, nobody has published a
practical
process wherein the conditions of isolation and preservation of thylakoid
activity are
taught.
Moreover, since free radicals may be responsible for the
degradation of many cell components, it is expected that their capture would
protect
other plant constituents. The present process would therefore produce an
improved
yield of plant components other than thylakoids.
Because there is a demand for powerful antioxidants, particularly
in the pharmaceutical field, a process providing any such antioxidants, as
well as the
antioxidants per se capable of a good potency as well as of a sustained
activity,
would be greatly appreciated. Further, there is a demand for biological
material
useful as sensors or detectors, captors or filters, bioreactors or biological
molecule
producers.
SUMMARY OF THE INVENTION
CA 02393816 2002-06-27
- 21
The present invention aims at providing a simple process for
obtaining an extract having functional thylakoids. The present invention also
provides
a process wherein the thylakoids are purified from other cell components. It
is
another object of this invention to provide a stabilized extract comprising
non-isolated
or isolated thylakoids. The stabilized extract is essentially free of any
electron donor
which would activate the thylakoids. Since the most abundant electron donor is
water, the stabilized extract is therefore preferably water-free. Water can be
chased
by a solvent or by drying, for example. An amphoteric solvent, particularly a
surfactant such as propylene glycol has been tried with success. This type of
solvent
does not disintegrate the membrane structural components, and has the
advantage
of replacing water molecules and of preventing the formation of aggregates
upon
redissolution in an aqueous solution. The stabilized extract has a longer
shelf life with
no substantial loss of activity as long as no electron donor such as water is
added
thereto. The stabilized extract is rehydrated extemporaneously before use to
start the
activation. The activity of extract once activated, lasts much longer than any
other
known antioxidant, which indicates a certain level of regeneration of activity
rather
than immediate and complete exhaustion. Further the antioxidant potency
adapts,
thus increases or decreases, upon the extent of the oxidative insult.
In accordance with the present invention, is provided a method as
defined in claim 1.
This invention provides a method of obtaining an extract obtainable
from photosynthetic organisms comprising thylakoids, the method comprising the
steps of:
a) providing a suspension of organism constituents that contains
thylakoids; and
b) disrupting the constituents while maintaining thylakoids intact in a
medium having a viscosity comprised between 1 to 1.3 centipoise and pH above 2
and below 10; the medium being added in a volume calculated upon the following
equation:
r4 AMENDED SHEET
r2-77172
'aelk
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 22 -
(Volume of medium + plant constituents water content) > 10
Plant constituents dry weight
whereby a first extract essentially constituted of thylakoids, cells
debris/membranes
and a liquid phase is obtained, said thylakoids comprising integral
photosynthetic
pigments.
Preferably, the resultant of the above equation would be comprised
between 25 and 150.
The pH of the medium is preferably comprised between 5 and 8,
more preferably between 7 and 7.5.
The suspension of step a) may be obtained by mechanically
dispersing organism constituents or tissues in said medium.
In a preferred embodiment, step a) is preceded by a step of
submitting said organism to a conditioning parameter selected from light,
osmotic
stress, heat, cold, freezing, dryness, hormones, chemical and biological
inducers.
In a most preferred embodiment, step a) is preceded by a step of
conditioning said organism in a light environment of a wavelength comprised
between about 500 and 600 nm, and step b) is performed under the same light
environment.
The viscosity is partly achieved by adding a sugar. The sugar may
be added in concentration as high as 1.5M and over. Preferably, a sucrose
concentration of about 0.2 to 0.4 M in said solution or a sugar achieving a
viscosity
equivalent to 0.2 to 0.4 M sucrose.
A specific example of a medium used in the above method is: Tris
or acetate or ascorbate buffer (20 mM, pH 7.0 - 7.5), and sorbitol or sucrose
350 mM.
The method of this invention may further comprise the following
step c): separating thylakoids, cell debris/membranes and liquid phase from
each
another, to form a second, third and fourth extracts essentially constituted
by isolated
thylakoids, cell debris and membranes, and liquid phase, respectively.
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 23 -
The step of separating has been particularly performed upon a
difference of sedimentation coefficient of each of thylakoids, cell debris and
membranes, and liquid phase.
A specific example of such separating step comprises centrifuging
the first extract for 10 minutes at 10 000 g in a tube equipped with a filter
in a
superior portion of the tube, the filter having a suitable porosity onto which
cell debris
and membranes deposit while the thylakoids and the liquid phase pass through
the
filter, the thylakoids forming a pellet in an inferior portion of the tube.
Alternatively,
gross purification may be achieved by recovering cell debris and membranes
first by
pressing and/or filtering, for example, followed by a finer purification, e.g.
separating
thylakoids from the liquid phase.
After separation, each first to four extracts may be stabilized by
adding the following step d): eliminating any electron donor from said
extracts so as
to inactivate and stabilize the photosynthetic pigments preferably in the
presence of
sugars (which may protect components against cold). The second and third
extracts
are particularly targeted by this step.
The first contemplated electron donor is water, so the extracts are
=processed to be water free.
Water may be eliminated under vacuum freeze drying or by
exchanging it against a non-denaturing amphoteric solvent or surfactant after
step
c), non-denaturing meaning not capable of dissociating or of damaging the
thylakoid
structural components.
An amphoteric solvent which has been tried with success is
propylene glycol.
It is further another object of this invention to provide products that
result from the above process. A pure thylakoid extract having the capacity to
be
activable is first provided. A stabilized extract is preferred. The above
third extract
being rich in thylakoids and cellulosic material is also within the scope of
this
invention, namely in a stabilized form. The stabilized form for thylakoids may
be dried
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
-24 -
or in a medium composed of an amphoteric solvent such as propylene glycol. The
former is in an insoluble state or suspension; the latter forms a solution.
Thylakoids
comprising extracts are reactivated in the presence of an electron donor. The
first
contemplated electron donor is water. Once activated, the extracts act as
dynamic
scavengers of ROS.
For nutraceutical, cosmeceutical and pharmaceutical applications,
this scavenging activity results in the treatment or the prevention of
diseases or
disorders that are mediated by the formation of ROS, especially those having
an
etiology related to inflammation, cancer, or contact with radiations.
A first scavenging and protecting effect is exploited against
radiations that are in the ultraviolet spectrum. Therefore topical use for the
thylakoids
and topical compositions comprising the thylakoids are within the scope of
this
invention.
It has been found that thylakoids have the capacity to form a
photon-absorbing film or coating on a body surface like skin or mucosa. This
property
appears to be independent from the ROS scavenging activity. The extracts
comprising thylakoids, in an activable form or not, therefore act as a filter
for
radiations, namely in the ultraviolet range. When the extracts further have
functional
thylakoids, they have a dual role as U.V. filter and as a ROS scavenging
compound.
The extracts may be used further in a method or a composition or a device for
detecting, for capturing molecules or for producing or processing molecules
having
affinity for thylakoids or interfering with their activity.
Examples of such molecules are herbicides such as triazines (ex.
atrazine- and diuron-type herbicides), quinones, chlorpromazine, urea,
formaldehyde,
alkylamino cyanoacrylates, trypsin, cyanoacrylate, Tris, adenine derivatives,
disulfiran (metal chelator), acetyl CoA carboxylase, digitonin, heavy metals
(ex., Cu,
Zn, Cd, Pb, Hg ...), SO2, NO2, NH2OH, CO2, CO, 03, 02, H2S, calcium
antagonists
(calmodulin-type), sulfate, sulfite, bisulfite, nitrite, acetate, lactate,
anions such as
NO3-, HCO3, HCO2-, F-, NO2-, HS03-,
CA 02393816 2010-02-08
-25-
DESCRIPTION OF THE INVENTION
This invention will be described hereinbelow, referring to specific
embodiments and the appended figures, the purpose thereof being to illustrate
this
invention rather than to limit its scope.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 represents the relative antioxidant activity of the extract of
the present invention in function of the pH of the exogenous extraction fluid.
Figure 2 shows the relative activity of the extract of the present
invention in function of the sorbitol concentration included in the extraction
fluid.
Figure 3 shows the relative activity of the extract of the present
invention in function of time and of the sugar concentration in the extraction
fluid. Each
sample was kept at -20 C.
Figure 4 represents the relative activity of the extract of the present
invention in function of the nature of the sugar in the extraction fluid.
Figure 5 shows the relative activity of the extract of the present
invention in function of different salts used for extraction.
Figure 6 represents the relative activity of the extract of the present
invention (Free Radical Trapping System (FRTS) non-stabilized) in function of
time and
temperature (wherein Tp is room temperature).
Figure 7 shows the selection of centrifuge conditions for
optimization.
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 26 -
Figure 8 shows the relative activity of the extract of the present
invention in function of the proportion of propylene glycol (propane 1,2 diol)
included
in the resuspension solution.
Figure 9 shows oxidation curves. Curve A represents a lipid
oxidation without any antioxidant; curve B shows lipid oxidation in the
presence of
an anti-oxidant which "allows" some lipid oxidation; curve C represents lipid
oxidation
in the presence of an efficient radical chain breaking antioxidant, such as
vitamin E.
Figure 10 illustrates the oxidation kinetics of lipids PLPC, wherein
B stands for lipids without the extract of this invention, D stands for lipids
in the
presence of Trolox, L stands for lipids in the presence of an active extract
made in
accordance with this invention and T stands for lipids in the presence of
lipids treated
with an inactive extract.
Figure 11 illustrates the protection exerted by the extract of the
present invention (dilution 1:1000) on IMR-32 cells against damages caused by
two
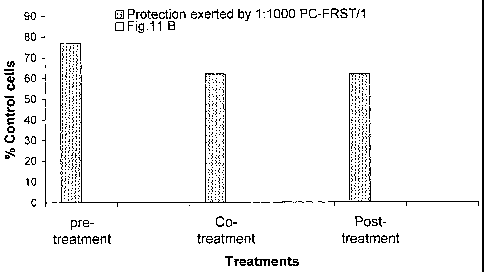
concentrations of TBHP; figure 11a): 25pM; figure 11b): 50 pM.
Figure 12 represents the protection exerted by two different
dilutions of the extract of the present invention on IMR-32 cells after TBHP
treatment;
figure 12a): dilution 1:1000; figure 12b): dilution 1:10000.
Figure 13 illustrates the behaviour of brain hyppocampus slices to
a 120 sec. epoxia and recovery in the presence of a solution comprising the
extract
of the present invention or not (control).
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
-27 -
As used herein, an "antioxidant" is a substance that, when present
in a mixture or structure containing an oxidizable biological substrate,
significantly
delays or prevents oxidation of the biological substrate. Antioxidants can act
by
scavenging biologically important reactive free radicals or other ROS (singlet
oxygen,
.02-, H202, 'OH, HOCI ferryl, peroxyl, peroxynitrite, alkoxyl...), or by
preventing their
formation, or by catalytically converting the free radical or other ROS to a
less
reactive species.
The antioxidant of the present invention is considered as such if,
when added to a cell culture or assay reaction, it produces a detectable
decrease in
the amount of a free radical, such as superoxide, or a nonradical ROS, such as
hydrogen peroxide or singlet oxygen, as compared to a parallel cell culture or
assay
reaction that is not treated with the antioxidant. Suitable concentrations
(i.e.,
efficacious doses) can be determined by various methods, including generating
an
empirical dose-response curve, predicting potency and efficacy of a congener
by
using QSAR methods or molecular modeling, and other methods used in the
pharmaceutical sciences.
The present invention is intended to be used in the medical field to
treat, prevent, or alleviate the symptoms associated with a ROS, associated
disease
or disorder or reduce the expression of such disease or disorder. Such a
disease or
disorder refers to a condition of an individual that results at least in part
from the
production of or exposure to free radicals, particularly oxyradicals, and
other "ROS"
in viva Even though there is only a few if any pathological conditions that
are
monofactorial, there is an increasing body of literature and knowledge related
to the
involvement of ROS in disease etiology. For these reasons, the term "ROS
associated disease" encompasses pathological states that are recognized in the
art
as being conditions wherein damage from ROS is believed to contribute to the
pathology of the disease state, or wherein administration of a free radical
inhibitor
(e.g., desferrioxamine), scavenger (e.g., tocopherol, glutathione), or
catalyst (e.g.,
SOD. catalasel is shown to produce a detectable benefit by decreasina
symptoms.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
-28 -
increasing survival, or providing other detectable clinical benefits in
treating or
preventing the pathological state. For example but not limitation, the disease
states
discussed herein are considered ROS-associated diseases (e.g., ischemic
reperfusion injury, inflammatory diseases, systemic lupus erythematosis,
myocardial
infarction, stroke, traumatic hemorrhage, spinal cord trauma, Crohn's disease,
autoimmune diseases (e.g., rheumatoid arthritis, diabetes), cataract
formation,
uveitis, emphysema, gastric ulcers, oxygen toxicity, neoplasia, undesired cell
apoptosis, radiation sickness. Further, many inflammatory diseases or
disorders will
benefit of the present invention, since it is known that ROS intervene in the
process
of inflammation. For example, the "oxidative burst" of activated neutrophils
produces
abundant superoxide radical, which is believed to be an essential factor in
producting
the cytotoxic effect of actived neutrophils. Further, since neutrophils are
involved in
the early mortality of any grafted or transplanted tissue or cell, an
antioxidant would
increase the early survival of transplanted or grafted cells, which is
critical for the
success of transplantation.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention relates to isolated thylakoids and a method
for isolation of thylakoids, that will constitute a powerful antioxidant
molecule having
a scavenger activity towards ROS. This antioxidant is of a natural origin; it
should
have no toxicity or adverse effect, when employed in a reasonable
concentration.
This antioxidant can also be stabilized, which ensures stability over time,
thus a
reasonable shelf-life. Stabilization is performed by withdrawing electron
donors
(namely water molecules), which make thylakoids to stay in a quiescent form.
Thylakoids are activated by adding an electron donor (namely through
hydration).
Preparation or Conditioning:
A first step undertaken, before going through the steps for
recovering the thylakoids in a crude suspension, may be a conditioning step.
This
conditioning is optional and permits to vary the compositions of the extracts.
To
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 29 -
optimize the levels of pigments in their non-activated state (namely
chlorophyll and
carotenoids), a conditioning step may be performed in the same conditions as
the
working conditions, e.g. under green light or in the dark. Under such
circumstances,
the chlorophylls are preferably in a singlet state while the carotenoids are
preferably
in a fundamental state. This way, when ready to use, the carotenoids will be
activated and ready to take the energy coming from a triplet chlorophyll
(photoprotection).
It is also possible to further protect the thylakoid pigments by
adding other xanthophylls such as violaxanthin in the medium of extraction or
to
increase the number of carotenoids by working under a light having a narrow
range
of wavelengths (465-475 nm).
It is further also possible to enrich the organism, namely a plant,
and its extracts, in some particular constituents by submitting the organism
to a
conditioning step other than light conditioning. Such other conditioning
comprises
osmotic stress, heat, cold, freezing, dryness, hormones, and chemical and
biological
inducers. All these conditioning parameters lead to a response in sensitive
organisms, which then become enriched in said some particular constituents.
As an example of this, a heat treatment would promote the
accumulation of heat shock proteins, that are useful for treating ROS-related
diseases or disorders (namely arthritis). The main objective of the steps of
the
present process is to preserve the integrity of some valuable constituents,
namely
the molecular constituents of thylakoids, and to control the state of the
molecules,
preferably in their fundamental functional state.
Obtention of a crude extract:
When one starts with whole organisms or tissues thereof, such as
plant tissues or whole plants, the first step of the process is a dispersing
step such
as a homogenization step. The plant tissues are, for example, pulverized
mechanically. The mesophylium tissues (leaves or needles) may be cut into
small
pieces with the aid of a rotative knife such as that retrieved in a
homogenizer or a
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 30 -
commercial rotative cutter. Any means leading to the dissociation of the
cellulosic
material to uncover the thylakoids would be suitable.
Besides working under a light source which optimally minimize the
light flux (green light, A = 500-600 nm), the working conditions would ideally
comprise
a working temperature of about 2 to 20 C, preferably less than 4 C, for the
purpose
of increasing the cell density and of preventing any degradation by enzymes.
The
working conditions also include hypertonic conditions using hypertonic agents
such
as sugars. These conditions achieve optimal viscosity and fluidity. A specific
example
of a homogenization buffer is as follows:
Homogenization medium
Volume, Product pH Final
weight Molarity
6 ml Tris Buffer (1M) 7.0 20 mM
50 ml Sorbitol (2 M) 330 mM
1.5 ml MgC12(1 M) 5 mM
243.5 ml H20
300 ml Total
The pH of the solution can vary from above 2 to below 10
preferably from 5 to 8, more preferably maintained at a near neutral value of
7 - 7.5
(figure 1).
Taking spinach as a reference plant, the ratio wet weight of plant
leaf tissues (g)/volume of buffer (ml) is of about 1/3. Thus, the above recipe
is
suitable for extracting thylakoids from 100 g of spinach. The plant is mixed
with the
buffer and homogenized for example, in a domestic blender for about 30
seconds.
The plant source may vary, so does the medium volume. The buffer itself may be
any
one suitable for maintaining a near neutral pH. For example, the above Tris
buffer
may be replaced with an acetate or ascorbate buffer. Both substitute buffers
are
acceptable for human consumption and ascorbic further has the advantage of
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 31 -
providing vitamin C to the consumer. The sorbitol has been added to preserve
the
integrity of the membrane (figures 2 and 3) and to insure a viscosity varying
from
about 1 to 1.3 and may be replaced by any other suitable sugar such as
commercial
saccharose, fructose or turbinado in a concentration achieving the same effect
as 0.2
to 1.5 M (preferably 0.2 - 0.4 M) sorbitol. Sucrose 0.2 - 0.4 M would be an
acceptable
less expensive component (Figure 4). Buffer components such as MgC12, NaCI,
ascorbic salt/acid are not believed to be necessary to the present process,
but they
may help recovery more activity or preserving the activity for a long period
(Figure
5).
A near neutral pH was preferably selected for maintaining an
optimal concentration of H+ ions. Sugars and pH are important parameters for
preventing the dissociation of photosynthetic pigments. The density of cell
fluids is
maximized when working in a cool or cold environment, namely below 4 C (see
figure 6, wherein FRTS/1 stands for the present extract). Low temperatures
also may
protect components from enzymatic degradation. All these homogenization
conditions release the membrane structure from its organization in
chloroplasts
without substantially affecting the molecular structural organization of
thylakoids. The
chloroplasts are therefore disorganized without destroying or disintegrating
the
thylakoids. The surface of cell components without any cellulosic protection
is thus
increased.
It was convenient in the present process to use plant tissues
directly in an extraction medium. However, if it becomes advantageous to use
pure
chloroplasts or a preparation enriched in chloroplasts or even preparation of
other
photosynthetic organisms having or not chloroplasts, it is feasible to do so.
Cultured
cells or tissues can also obviously replace whole plants.
Starting from spinach leaves, the yield of thylakoids is fairly good
when one follows the following equation:
a/f3 > 10
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 32 -
a = ratio of wet weight/dry weight; and p = ratio of wet weight/
(Volume of medium + plant constituents water content).
So:
(Volume of medium + plant constituents water content)
_______________________________________________________ >10
Plant constituents dry weight
And more precisely (, preferably)
(Volume of medium + plant constituents water content)
= 25-150
Plant constituents dry weight
It is worthwhile noting that the yield may vary depending on the
volume of buffer that was selected and on the water content of the selected
plant.
For example, pine needles have an endogenous water content that is much less
important than in the case of spinach leaves. For an equal wet weight of plant
material the volume of buffer should be increased for isolating thylakoids
from pine
needles, when compared to the spinach leaves, taking into account all the
parameters of the above equation.
The crude extract obtained alone constitutes a first fraction that can
be used per se, dehydrated, or further fractionnated.
Separation of plant fractions:
The homogenization step is followed by a separation step.
Thylakoids are separated from cell debris and from soluble components, based
on
their different sedimentation coefficients. The sedimentation coefficient of
thylakoids
is superior to that of cell organelles. The thylakoids were centrifuged for 10
minutes
at 10,000 x g in mobile buckets. A centrifuge force of less than 10,000 x g
but
superior to 3,000 g may be used, adjusting the centrifugation time accordingly
(Figure 7). The optimal handiness for the thylakoids pellets was obtained at
10000-
12000 x g for 10 minutes. Any other speed and time achieving equivalent
results may
be adopted. Different speed and time are contemplated in a scaling up process.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2010-02-08
-33-
During sedimentation, the thylakoids pass through a filter corresponding to
this
equation:
0.002 X s 0.2
wherein X is calculated by multiplying the opening per the wire
diameter (all in millimeters). The cell debris and membranes are stopped by
this filter
in a superior portion of a centrifugation tube. Thus, the bottom pellets
comprising the
thylakoids are easily recovered and a pellet may be used immediately or may be
further fractionated or stabilized for any future use. Of course, any other
method of
separation achieving the same purpose of isolating thylakoids could be used.
For
example, on a density gradient like a sucrose gradient could be used. Any
chromatographic or affinity medium and method could be also used. Referring to
the
above specific method, it is conceivable that the gross and fine separation
would not be
achieved in one step in a large scale process. Therefore, a gross purification
could be
made first on a press or a filter and the fine separation of thylakoids and
the liquid
phase would be achieved in a later step.
Stabilization:
The separation step is normally followed by a stabilization step. This
step allows withdrawal of electron donors such as water molecules that are
bound or
non-bound to membranes, and this for eliminating the activator of the PSII
system. The
fractions are recovered and are placed in clean vials. Specifically the first,
second and
third fractions, representing the whole extract, the pellet (thylakoid
fraction) and the cell
debris/membranes fractions, respectively, are lyophilized. The vials are then
submitted
to a vacuum and to a low temperature (about -20 to -50 C), during at least 4
hours.
The fractions so lyophilized remain stable for a long time, until water is
added thereto.
Other stabilizing means could be used. For example, a plurality of surfactants
have
been used to verify their capacity to chase water without destroying the
structure of the
thylakoids. These solvents are the following: TritonTm X-100, PEG, Beta-D-
maltoside,
glycine, glycerine, glycerol, TWEENsTm, SDS, LDS, DMSO, cholate, stearate and
propylene glycol have been used.
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 34 -
Propylene glycol has been preferred and would advantageously
replace lyophilization as a stabilizer. Not only propylene glycol provides an
inactive
quiescent thylakoid preparation (functional and fully activable upon water
addition,
see figure 8), but it also stabilize the thylakoids upon hydration by helping
solubilizing
the same. Upon hydration, thylakoids normally form a suspension; in the
presence
of 100% propylene glycol, thylakoids form a solution having of limit of
solubility of 100
mg/mL solution. This solution may be diluted with water for activation. This
solvent
is also non toxic.
Optional fractionation:
The thylakoid membranes could further be fractionated into sub-
fractions. For example, it could be envisaged to separate reactional centers
or
photosystems, light harvesting complexes, cytochrome complexes, particular
pigments (chlorophylls, carotenoids), plastoquinones, non-photosynthetic
components (cell nuclei), or mitochondria.
Thylakoid integrity and activity:
The extract comprises substantially pure thylakoids (>90%); they
are photosynthetically activable; they are stable; and the extract is
controllable. The
photosynthetic activity has been evaluated with different techniques: the
oxygen
release (Schlodder et al. 1999), the photoreduction of 2,6 dichlorophenol
indophenol
(DCPIP) (Behera et al. 1983) and the fluorescence (Maxwell et al. 2000).
Moreover,
the integrity of the thylakoids has been evaluated with a technique which
measures
a continuous electric current: any disorganization should be detected by any
variation
in this electric current. The current is measured from PSII to the coupling
factor,
which indicates that the thylakoids contain the main subunits listed above and
that
they are functional.
When a green light was used in the working conditions, the
pigments were stabilized in their fundamental state (Fo), thus, permitting the
optimization and synchronization of any desired effect. The stabilization is
possible
because of the withdrawal of the primary electron donor. The stability
measured by
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 35 -
the photosynthetic activity (absent during quiescent state and present upon
activation
with an electron donor) and the concentration in chlorophylls and carotenoids,
persist
for several months after extraction. The ratio chlorophylls/carotenoids is
also
extremely important for the activity of the complex and to maximize the
absorption
and dissipation of energy.
The extracts are easily detectable because of their natural
fluorescence. No toxic product, solvent, detergent or conservation agent has
been
added to the above thylakoids, preserving to the product all its original
nature. The
extracts are indeed edible. Even when propylene glycol is used to stabilize
the
thylakoids, this solvent is harmless because its oxidation yields pyruvic and
acetic
acids. This solvent is currently used as a food emulsifier, which means that
it has
surfactant properties (however, non deleterious to the integrity of the
thylakoids). It
further has an inhibitory activity against fermentation and mold growth.
Therefore,
this solvent may be used at any step in the process, mixed with water during
homogenization, and not mixed with water after step c) (separation step).
The extracts may be presented under a solid form, dry or humid, or
in a liquid form. The extracts are poorly soluble in water although they
rehydrate
easily but they resuspend completely in propylene glycol. Thylakoids are
reactivated
upon rehydration. It is therefore envisageable that a composition comprising
solubilized thylakoids is used; when contacted with an= aqueous medium, the
thylakoids activate.
Byproducts:
Although the thylakoids are the products that have received the
primary attention in the above procedure, the other plant constituents that
are
separated from the thylakoids will also be recovered for their commercial
value. The
liquid phase fraction and the cell debris/membrane fraction may be easily
taken as
starting material to isolate any plant molecule of interest. The latter
fraction may be
reextracted to increase the yield in thylakoids that are recovered per plant
unit. It is
contemplated that the components of other fractions would have a superior
quality
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 36 -
when compared to any corresponding components obtained from the processes of
the prior art. Because the formation of damaging ROS is prevented or because
already formed ROS are captured by the thylakoids prepared in accordance with
the
present process, it is envisageable that any other plant constituents
sensitive to ROS
will also benefit from the present process. Indeed, the other constituents
that would
be normally prone to degrade upon oxidation will be preserved by eliminating a
noxious source of degradation. Thus, any plant constituents such as sugars,
proteins, lipids, vitamins, minerals and hormones can be separately obtained
by
fractionating, for example, the liquid phase obtained from the above process,
which
constituents would have a greater specific activity than usual. In addition to
this, a
proper conditioning step may enrich the extracts in constituents of interest.
After verifying that the extracts were functional, the next step was
to verify their scavenger activity towards ROS.
The use of thylakoids as antioxidants:
Antioxidants are compounds that interact with ROS (such as
oxygen singlet, hydrogen peroxide, superoxide anions and hydroxyl radicals).
In
order to form innocuous degradation products, active oxygen forms degrade or
inactivate other molecules and, potentially cause mutations, cancer or
inflammation.
They may further participate into aging. The antioxidant molecules of the
present
extracts are the following: chlorophylls, carotenoids and vitamins (B, C, E,
K, ...),
cytochromes and anthocyanins. The thylakoids are particularly performing
antioxidants because their physical organization makes the carotenoids capable
of
capturing singlet oxygen in their chlorophyll protective role. The quenching
effect has
both a high efficacy and a relatively long duration because the carotenoids
dissipate
their energy as heat and become ready to accept again the energy coming from
triplet chlorophylls. The thylakoids are therefore outstanding in the field of
antioxidants; because of their capacity to regenerate their functional state,
the
organization of the pigments will permit a sustained anti-oxidative activity.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 37 -
The thylakoids of this invention will hereinbelow be referred to as
FRTS/1 constitute a bioactive molecular complex extracted from plant biomass.
The
functional anti-oxidative activity of FRTS/1 is based on the redox potential
of the
molecular complexes of thylakoids wherein the tridimensional structure and the
natural distance between its different pigments and molecules is preserved.The
antioxidant is an indication of an optimal structure and of an optimal
composition of
matter.
= Quantification of proteins by fluorescamine method
The protein concentration in a stabilized extract is : 0.42-0.65 g/g
of extract.
ANTIOXIDATIVE FUNCTION OF THE THYLAKOIDS
A- Principal reaction pathway in radical-induced lipid oxidation:
Nature employs antioxidants to prevent the oxidation of biomolecules such as
proteins, DNA, lipids, etc. The peroxidation of lipids is a particularly
ubiquitous and
damaging process in living organisms. Peroxyl radicals (ROO) are important
radicals
in biological systems because they are able to initiate lipid peroxidation and
they are
intermediates in many different oxidation processes of biological important
molecules.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2010-02-08
-38-
Initiation
=
ROO + ROOH + (1)
LH L'
Propagation =
=
00.
=
RLW +02 (2)
LOW
=
RL
RL
(3) RL
90H
, LOOH
(Xum = 234 nm)
The peroxidation of unsaturated lipid moieties (reactions 1-3) causes changes
in their
structure which eventually changes the physical properties of biological
membranes.
During the lipid peroxidation a conjugated diene group is formed (see LOOH in
reaction
3) which has an UV/Vis absorption maximum at A,max 234 rim (e = 29,500 n4-1 cm-
1)
and this allows a quantification of the oxidation product formed. To obtain
quantitative
data the lipid peroxidation has to be initiated in a controlled manner
(reaction 1). Often
azo initiators, which produce a well-defined flux of ROO. in aqueous, aerated
solution,
are employed to study lipid oxidation in in vitro experiments. The most
commonly used
water-soluble azo initiator is amidinopropane hydrochloride (AAPH) (sometimes
called
ABAP; reaction 4).
H2N,\ N=N (:12 A H2j4,\ = .240_ H2.N 00. (4)
=331
H2N H2 -N2 H2N H2N
AAPH R00
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 39 -
The decomposition rate of AAPH at 37 C is k= 1.3x10-6 M-1 s-1 and
the efficiency for ROO formation is 50%, i.e. 1 mol AAPH yields 1 mol of ROO'.
This
method of ROO' generation allows the calculation of the exact amount of ROO'
formed during any time period.
The most intensively studied antioxidant is Vitamin E and the most
active compound of the Vitamin E family is a-tocopherol (a-TocH). It is a
radical chain
breaking antioxidant which can trap two peroxyl radicals yielding only non-
radical
products and thereby preventing lipid peroxidation (reactions 5 and 6).
R00* + TocH ROOH + Toc= (5)
ROO. + Toc= non-radical products (6)
The tocopheroxyl radical (Toe) formed in reaction 5 is a relatively
unreactive radical which normally cannot propagate the radical oxidation chain
reaction. As soon as all TocH is consumed the lipid peroxidation occurs as if
no
antioxidant is present.
Carotenoids (Car) are the most likely "antioxidants" in FRTS/1
because chloroplasts utilize carotenoids such as carotenes and xanthophylls
for their
exciton transport chain. There are several pathways for reaction of
carotenoids with
ROS possible and the overall behavior of them ranges from antioxidant activity
all the
way to being effectiveless with respect to lipid peroxidation inhibition. The
experimental results obtained are depending on the reaction conditions
employed.
Possible reactions of carotenoids with ROO' are:
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
-40 -
ROcr + Car [Car-OORI (7)
ICar-000 + ROO' -op- R00-Car-00R (8)
[Car-0011 = + 02 .00-Car-OOR (9)
R00. + Car RO6 + Car" (10)
2 Car"-=)`-- Car + Car2+ (11)
R00 + Car -ill.- ROO H + Car' (12)
Car' + 02 Car00 (13)
R00 + Car Car-00R (14)
Some reactions lead to the formation of non-radical products
(reactions 7+8, 10+11, 12+14) which would result in an overall antioxidant
behavior
of a carotenoid. Other reactions are generating peroxyl radicals (reactions 9
and 13)
which can be involved in the propagation of lipid peroxidation. The overall
behavior
of carotenoids cannot be obviously predicted due to the various possible
reaction
pathways.
Depending on possible reaction pathways of the antioxidant used
the concentration time profiles of the detected LOOH display certain
characteristics.
By using an azo initiator as peroxyl radical source the amount of ROO'
generated
can be calculated and it is possible to determine the amount of lipids
oxidized from
the 234 nm absorption of the conjugated diene moiety. Also the amount of ROO"
trapped during the lag phase can be determined.
A process within the FRTS/1 might "restore" its "original"
antioxidative properties. A possible mechanism for such a behavior might be an
electron transfer rather than a radical trapping process (see reactions 15 and
16),
e.g.:
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 41 -
H+
ROO. + PCPE -011" ROOH + PCPE". (15)
PCPE .4" + S PCPE + S (16)
further
S : Substrate reactions
The concentration/time profiles of the lipid oxidation in the presence
of FRTS/1 will allow examining this hypothesis. From the duration of a
possible lag
phase during lipid peroxidation experiments (Figure 9) the amount of "trapped"
radicals can be calculated. This will allow to draw conclusion to which extend
FRTS/1
is able to inactivate peroxyl radicals. However, it has to be born in mind
that the
described possible regeneration of the antioxidative properties can be only
effective
as long as FRTS/1 is still intact and able to perform its "original" activity.
Overall the
experiments will eventually provide quantitative data for the antioxidative
capacity of
FRTS/1 (see figure 10 for an example of the antioxidative kinetics of FRTS/1
in
comparison of Trolox). When p-carotene was compared to Trolox, the former was
an
antioxidant but with no lag phase typical of antioxidants.
Since lipids are the main components of the cell membrane,
lipoproteins and other membrane structures in living organisms, in the present
study
PLPC-FRTS/1 (1- palmmitoy1-2-linoleoyl-sn-glycero-3-phosphatidylcholine)
micelles
were used as a substrate for the standard oxidation assay. Oxidation of PLPC-
FRTS/1 induced by peroxyl radicals generated from the initiator Azo compound
2,2
Azobis -(2- amidinopropane) dihydrochloride (AAPH) results in oxidation of the
linoleic acid moiety to the corresponding hydroperoxide together with the
formation
of a conjugated diem system with an absorption maximum at 234nm.
Preparation of PLPC(1-palmmitoy1-2-linoleoyl-sn-glycero-3-
phosphatidylcholine)
Micelles:
170pL of a 25 mg/rnl_ solution of PLPC-FRTS/1 in CHC13 (Avanti
Polar Lipids) was evaporated to dryness under a stream of N2. Phosphate
buffered
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
-42 -
saline (PBS) (281pL) which had been previously treated with Chelexoto remove
metal ions was added to the PLPC-FRTS/1 and the mixture was vortexed 2 min.
Aqueous Chelex treated sodium cholate 109pL, 30 mg/mL, (Aldrich Chemical
Company Inc., Milwaukee, WI, U.S.A) was added to the mixture and vortexed 2
min.
The mixture was passed 20 times through a polycarbonate membrane (pore size
100
nm) in order to homogenize the size of the micelles.
FRTS/1 was dissolved in CHCI3 (12mg/mL) and I ml of this solution
was mixed with 6mL of PLPC-FRTS/1 (25 mg/mL) to obtain a final concentration
of
PC-FRTS/1 of 6mg/ml. Micelles were prepared as described above. Azo initiator
(AAPH) was used at a concentration of 5mg/mL in PBS.
Standardization:
Initial experiments were performed using FRTS/1 in a dehydrated
form, to standardize the concentration of micelles, azo initiators and
wavelengths.
Optimal absorbance was at 234nm.
Two preliminary experiments were run with 100p1 micelle solution
prepared from PLPC-FRTS/1 and PLPC-FRTS/1 + FRTS/1 in 3mL of PBS with two
dilutions (10p1 and 20 pl) of 5mg/mL solution of azo-initiator for 10 h at 37
C at
wavelength 234nm on a Cary 3 UV-Visible spectrophotometer from Varian. The
background absorption due to the PC-FRTS/1 at 234nm was too high under these
reaction conditions.
Experiments:
Based on the results obtained from the above experiments, it was
decided to use further diluted solutions of the FRTS/1 (final concentrations
of FRTS/1
were 6.7 pg). As negative control, the FRTS/1 was incorporated into micelles
made
from 1,2- Dimyristoyl-sn-glycero-3-phosphatydylcholine (DMPC) a compound which
is resistant to oxidation mediated by peroxyl radicals derived from AAPH.
Micelles
were prepared from 15.937mg of DMPC-FRTS/1 (stock solution 25mg/m1) in
0.6375mL of PBS + 1.053 mL of PBS+ 0.408 mL of Sodium cholate + 0.2435mL of
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
-43 -
FRTS/1 solution in CHCI3 (2mg/mL) as described above. The following
experiments
were therefore conducted:
= 3mL of PBS + 100p1 of PLPC-FRTS/1 micelles + 10p1 of azo-initiator
= 3mL of PBS + 100p1 of PLPC-FRTS/1 micelles + 20p1 of azo-initiator
= 3 mL of PBS + 100p1 of DMPC + FRTS/1 + 10pL of azo-initiator
= 3mL of PBS+ 100 pl of DMPC + FRTS/1 20pL of azo-initiator
= 3 mL of PBS+ 10p1 of azo-initiator
= 3 mL of PBS+ 20p1 of azo-initiator
The reactions were monitored on a spectrophotometer at 37 C for
10 h.
Conclusion
These results show that the maximum OD (at 234 nm wavelength)
of PLPC-FRTS/1 micelles containing FRTS/1 at 0.3 mg/mL was 0.25 after 180 min
while OD of PLPC-FRTS/1 micelles without FRTS/1 was 3.2 after the same period
of time. The results indicate that the FRTS/1 undoubtedly demonstrate
antioxidative
properties.
ANTIOXIDANT PROPERTIES OF FRTS/1 SOLUTION IN COMPARISON OF
VITAMIN E
Lower concentrations of antioxidants was used to compare the
antioxidative properties of FRTS/1 and Trolox, a water soluble analog of
Vitamin E
(0.3mg/mL in PBS). The following samples were run on spectrophotometer for 10
h
at 37 C.
1. 3000pL of PBS + 10pL of azo-initiator.
2. 3000pL of PBS + 100pL of PLPC micelles + 10pL of azo-initiator.
3. 2998pL of PBS +2pL of antioxidant (0.5mg/mL aqueous solution) +100pL of
PLPC micelles + 10pLof azo-initiator
4. 2995pL of PBS +5pL of antioxidant (0.5mg/mL aqueous solution) + 100pL of
PLPC micelles + 10pLof azo-initiator
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2010-02-08
-44-
5. 2990pL of PBS +10pL of antioxidant (0.5mg/mL aqueous solution) + 100pL
of
PLPC micelles + 10pL of azo-initiator
6. 2980pL of PBS + 20pL of antioxidant (0.5mg/mL aqueous solution) + 100pL
of
PLPC micelles + 10pL of azo-initiator
7. 2990pL of PBS +10pL of Trolox + 100pL of PLPC micelles + 10pL of azo-
initiator
8. 2980pL of PBS + 204 of Trolox + 100pL of PLPC micelles + 10pL of azo-
initiator
9. 2970pL of PBS + 30pL of Trolox + 100pL of PLPC micelles + 10pL of azo-
Figure 10 shows a mare sustained activity for PRISM than for
Trolox.
Protection mechanism:
Radical Oxygen Species (ROS) readily interact with cellular
macromolecules and structures, resulting in membrane permeability changes,
activation of proteases and nucleases, and altered gene expression. It is well
known
that these cellular changes induced by ROS lead to apoptotic cell death. We
attempted:
To evaluate the antioxidative properties of FRTS/1; and
- To determine the action mode of FRTS/1 as an antioxidant.
IMR-32 cells constitute a good model for evaluating the antioxidant
potency of our extract. These cells are neuroblastoma cells that are sensitive
to an
oxidative stress which provokes apoptosis.
Selection of cell culture
Human neuroblastoma cell line (IMR-32), which is known to respond
to oxidative stress by apoptosis, was used as an in vitro cell model. IMR32
cells are
particularly sensitive to ROS and other toxicants because their p53 gene
product is
Selection of ROS inducer
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 45 -
Tert-butyl hydroperoxide (TBHP) was used as an oxidative stress-
inducing agent. TBHP does not have any neuron specificity in contrast to
oxidative
stress induced by MPTP in dopaminergic neurons. This will allow comparisons if
studies relative to more generalized oxidative stress conditions (like the
ones found
in many neurodegenerative diseases) have to be performed on different cells
phenotypes.
A. Determination of optimal dosage of TBHP to induce apoptosis on IMR-32 cells
culture
Experiments were done using 1000 IMR-32 cells per well, for I hour
of incubation. TBHP 50, 75 and 100 pM produced 78%, 87% and 87% apoptosis.
TBHP 50 pM was selected to induce apoptosis in the other experiments.
B. Dosage of FRTS/1
Different dilutions were used on IMR-32 cells along with TBHP. A
mother solution 1:10 was constituted, starting from the lyophilized thylakoid
fraction,
in propylene glycol. Unless otherwise specified, the mentioned dilutions are
dilutions
of this mother solution. The protection conferred by FRTS/1 was 28%, 35% and
75%
at dilutions 1:10, 1:100 and 1:1000, respectively. The latter dilution was
adopted for
further experimentation.
1. Cell Culture
IMR-32 Cells were grown in MEM supplemented with 10% FBS at
37 C in a humidified atmosphere of 95% air and 5% CO2. The cells were seeded
at
a density of 1X104 cells/T25 Falcon tissue culture flasks and subcultured
twice
weekly. 48h old cultures were used in all the experiments. 1000 cells were
plated per
well in the first experiments, the number was increased to 3000 afterwards as
indicated in the tables.
2. Oxidative Stress in vitro Protocol
1000 or 3000 cells/ well/ 100 uL were seeded in 96 well (Linbro flat bottom)
plates
in all the wells except well No. 12 of all the rows. After 24 h the cells were
washed
2 times with 250 ul of PBS (pH 7.2) and 32 wells each were treated
respectively with
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
-46 -
100 and 200 uM solutions of TBHP (70% aqueous solution from Sigma Chemical
Company) for 1 h. After 1 h the cells were gently washed 2 times with PBS
before
adding fresh growth medium. After 24 h the cell survival was assayed by a
sensitive
fluorimetric assay based on DNA binding fluorescent dye Hoechst by the
following
procedure. The test measures the total DNA of the population, a measurement
which
closely correlates with cell number. The medium was aspirated by gentle
suction.
Cells were rinsed with 250u1 PBS. PBS was aspirated with gentle suction. The
rinse
step was repeated. 100 uL lysis buffer (0.02% SDS in 1 X SSC) was added in
every
well except the ones for DNA standard and Blank in row 12. The plate was
incubated
at 37 C for 1 h with occasional swirling. 100 uL of 4Oug/mL of DNA was added
to
the DNA wells and 100 uL of 1 X SSC buffer was added to the wells that were
treated as Blank. 100 uL of 40 ug/ml of Hoechst 33258 in 1 X SSC buffer was
added
to every well, and the plate was covered with Aluminum foil to protect it from
light.
The plate was agitated gently for 5 minutes and fluorescence was read at
excitation
wavelength 355nm and emission wavelength 460nm.
3. Calculations
CT untreated =100%: survival after TBHP: 42%, dead cells: 100-
42=58%; population size after PC: 88% difference from CT: 100-88=12% Expected
population size after TBHP +PC: 100-58-12= 30%. Recovered survival: 60%
Survival
gain in %: 60-30=30 Protection exerted: 30:60 x 100=50%
4. Estimation of Cytotoxicity by LDH Assay:
For this experiment IMR-32 cells were grown in MEM medium
without I-Glutamine, Phenolphtaleine and sodium pyruvate 3000 IMR-32 cell/well
seeded in 96 well plates. 24 hours after the oxidative stress, the cells were
washed
2 times with PBS and two rows each were treated with 1:1000 and 1: 10000 PC-
FRTS/1 for 1 hat 37 C. After 1 hour the cells were washed with PBS two times
and
PBS was replaced with the growth medium. Two rows each were treated with
1:1000
and 1:10000 PC-FRTS/1 while two rows each we treated with 25 and 50uM TBHP
respectively. Two rows were left untreated as Control. The LDH activity was
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 47 -
measured by Lactate Dehydrogenase Assay Kit provided by Sigma Diagnostics. The
colorimetric assay measures the residual pyruvate (substrate of the enzymatic
reaction). The basal activity present in the medium alone (in the absence of
Cells)
was considered 0% and was systematically subtracted from each experimental
value. Control untreated cells in two rows were lysed with Triton X-1 00
(0.02%) and
were treated as samples with 100% LDH release.
Initial two experiments were done to standardize the procedure,
number of cells used wave length for optimum absorbance, optimum pyruvate
substrate to be used. It was established that 3000 cells/well, 0.4m1 of
pyruvate,
spectrophotometer readings at 440 nm were standards.
FRTS/1 was used as pre-treatment, co-treatment and post-
treatment with the oxidative insult.
= Pre-treatment.
The cells were pre-treated with FRTS/1 for 2h before exposure to
TBHP doses.
TBHP
25uM 50uM
Cell damages TBHP /CT 58% 70%
FRTS/1 1:1000 decrease 12% 12%
Total expected decrease 70% 82%
Expected survival 30% 18%
Survival TBHP + PC/ CT 60% 53%
Protection exerted by PC 60% 77%
= Co-treatment
The cells were treated simultaneously with FRTS/1 and TBHP for
1 hour.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
-48 -
TBHP 25uM 50uM
Cell damages TBHP /CT 39% 67%
FRTS0 1:1000 decrease 7% 7%
Total expected decrease 46% 74%
Expected survival 54% 26%
Survival TBHP + PC/ CT 83% 62%
Protection exerted by PC 35% 62%
= Post treatment
The cells were treated with 3 doses of FRTS/1, 1 hour after to have
been exposed to TBHP doses.
TBHP 25uM 50uM
Cell damages TBHP /CT 38% 66%
FRTS/1 decrease 7% 7%
Total expected decrease 46% 74%
Expected survival 54% 26%
Survival TBHP + PC-CT 71% 72%
Protection exerted by PC 23% 62%
The Cell damages TBHPICT represents the damages caused
by the oxidant calculated as a percentage from the survival of untreated
controls.
The f(FRTS/1 decreased refers to the difference in population size in controls
and
FRTS/1 exposed cells. The Total expected decreases)) sums the difference in
population size due to TBHP and FRTS/1 exposure individually. The Expected
survival)) is 100-total expected decrease. The survival TBHP+PC/CT is the
actual
survival in the presence of FRTS/1 diluted 1:1000. The protection exerted by
PC))
is calculated as reported above.
In the pre-treatment experiment, the effect of FRTS/1 is enhanced
when compared to protection exerted by a post treatment (compare 50% to 23%
and
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 49 -
77% to 62%). It strongly suggests that the protective effect of PC-FRTS/1 is
exerted
through its antioxidant properties. At low dose (25uM), the protection against
the
damages caused by the oxidant doubles.
Clearly, the efficacy of PC-FRTS/1 is confirmed as a preventive
treatment due to its antioxidant properties.
The antioxidant properties of FRTS/1 are confirmed in the co-
treatment experiment. The efficiency of the product is similar to the one
reported for
post-treatment at the highest dose of oxidant and intermediate to the one
obtained
following pre- and post-treatment, respectively.
FRTS/1 exerts its protection against apoptosis caused by TBHP
during the course of TBHP damages.
The strong antioxidant properties of FRTS/1 are confirmed via its
protective effect on ROS generated in the IMR-32 cells by the oxidant TBHP.
Under
our standardized conditions, the protective effect averages 62%.
The antioxidant effect occurs on pre, co- and post- treatment.
(Figure 11 a and b)
The more the oxidative damages caused by TBHP on the IMR32
cells (as TBHP dosage increases), the more the protection exerted by FRTS/1.
This
is independent from the concentration of FRTS/1 since it shows at 1/1000 as
well as
at 1/10,000 dilutions (illustrated in Figure 12A and 12B respectively and).
These
results show the dynamism of the present extract.
The fact that the protection increases with increasing damages
indicates that the mechanism of action of FRTS/1 may differ from that of
conventional
antioxidants as indicated in the studies relative to the chemistry of the
reaction:
1) the protective effect exerted by FRTS/1 lasts longer
2) The vitamin E and its analogs are used up at given concentrations, while
they
exert their antioxidant effect. It does not seem to be the case with FRTS/1 as
illustrated by the above chemical reactions shown in above.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 50 -
The correlation between extent of oxidative damages and
protection by PC-FRST/1 is a unique property shown by this antioxidant.
Increasing the cell density from 1000 to 3000, obviously decreases
the dose of product that each cell receives. This is a well-known effect in
toxicology
and pharmacology. The protection exerted by FRTS/1 remains excellent even when
the number of cells to be protected triple. The effect is reproducible.
= Estimation of apoptosis in IMR32 cells by Lactic Deshydrogenase (LDH)
assay:
The classical LDH assay measures the release of the LDH enzyme
by apoptotic cells. The assay used in the present study measures the residual
level
of enzyme substrate (pyruvate) using a colorimetric reaction. The more the
substrate
in the medium, the less enzyme released. Medium cells was taken as 0% enzyme
activity released, and lysed cells in the medium as 100% released. The table
values
are calculated from these two parameters.
Level of apoptosis measured by release of LDH by the damaged cells:
PC-FRTS/1 doses 1:1000 1:10000
TBHP concentrations 25 uM 50 uM 25 uM 50 uM
LDH release/TBHP 64% 92% 64% 92%
LDH release /PC 32% 32% 0% 0%
Expected LDH releases 96% 124% 64% 92%
Observed LDH TBHP + 49% 73% 30% 66%
FRTS/1 Release
The total release of LDH by TBHP plus FRTS/1 individual
exposures was compared to the observed LDH activity (last row).
The protective effect of FRTS/1 is obvious. A post treatment by
FRTS/1 protects effectively the IMR-32 cells against apoptosis induced by the
ROS
generated by TBHP.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 51 -
Conclusion
FRTS/1 compound exhibits potent antioxidant properties as
assessed by chemical assays.
These highly efficient antioxidant protective effects are also
exhibited in a biological in vitro assay:
= FRTS/1 antioxidant properties demonstrated chemically are confirmed
biologically;
= FRTS/1 exhibits highly protective effects against ROS damages causing
apoptosis in IMR-32 cells following TBHP exposure;
= FRTS/1 presents the unique property to be dynamic so to exert higher
protective
effect as oxidative damages increase (the higher the damages by ROS, the
higher
the protection by FRTS/1);
= FRTS/1 exhibits a long lasting (hours) anti-oxidative effect which shows
a great
level of stability and/or a capacity to regenerate, which is unique to this
antioxidant;
= FRTS/1 is efficient at doses that are not toxic.
= Interaction between cells:
To be an effective therapeutic medication, the present extract must
fulfil at least some characteristics. Amongst these, the extract not be toxic,
immunogenic or hinder the normal tissue function, particularly the oxygen and
carbon
dioxide erythrocyte transport.
The extract should not stick to erythrocytes, although they should
disperse in the recipient body to target a tissue or organ to be treated. We
have
verified if macrophages, which are first line defence cells do not destroy the
present
extract. Macrophagic mode of destruction is normally production of free
radicals
which destroy big particles before phagocytosis. Upon phagocytosis,
macrophages
produce cytokines which are molecules signaling the presence of any intruder
or the
malfunctioning of a tissue or cell. Cytokines are molecules sent to other
cells,
signalling the presence of intruders or of a malfunctioning of a tissue. The
cells
responding to cytokines are cells like fibroblasts, endothelial cells,
macrophages,
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 52 -
lymphocytes, neutrophils and eosinophils. These cells are involved in the
process of
destruction and/or reconstruction of tissues and organs. These processes
involve
inflammation. If the response is disorganized or if the intruder is
continuously present
in the organism, the latter suffers of a chronical diseases such as
rheumatism,
cutaneous irritation, conjunctivitis, alveolitis, asthma, and even cancer. The
interaction between cells and FRTS/1 were performed with the FACS technique.
This
apparatus detect fluorescence of cells (autofluorescence) and also all
fluorescent
molecules fixed around. FRTS/1 is a autofluorescent complex, so we can
quantify
interactions without any modification.
Conclusion: FRTS/1 adhere to macrophages (commercial lines:
NR-8383 by ATCC) at 370 C with comparison with mastocytes (positive control,
RCMC provided by ATCC). Macrophages phagocyte FRTS/1 (1/3) after 2 hours,
which demonstrates that FRTS/1 stays for a rather long period in blood flow.
Slow
phagocytosis is not a bad news, per se, since another type of beneficial
effect could
be observed consequent to the cytokin activation ("phase II" effect). Since
macrophages phagocyte the extract and since IMR-32 cells appear to allow
entrance
to the extract into the cytoplasm, it is believed that the extract may enter
the cells by
endocytosis.
OXIDATIVE EX VIVO MODELS:
Liver perfusion model and brain perfusion model are good
experimental models responsive to an oxidative stress. They were used to
demonstrate the protective effect of the extract towards vital organs.
LIVER PERFUSION MODEL:
A plurality of hepatic functions have been evaluated. The method
used to perfuse the liver was described by Drouin et al. 2000 and by Lavoie et
al.
2000. Glucose, lactate, ALT and LDH were determined by spectrophotometry,
while
bile production was simply measured by volumetry. When compared to a control
vehicle, there was no deleterious effect observed with the perfusion of the
extract.
On the contrary, when the potency of the extract to reduce the expression of a
stress
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 53 -
created by ishemia followed by re-perfusion (I/R), the results were that the
extract
had a protective effect against oxidative damages induced in the liver.
In this model, the liver is perfused in situ with a controled
extracorporeal circulation, which isolates the liver while preserving its
vascular bed
intact and saving its structural and functional integrity. This model is well
documented
(Ross 1972) and allows the study of oxidative stress as well as of cell damage
(Bailey 2000).
Effect on liver viability is undertaken to evaluate the effect FRTS/1
on liver viability. More specifically, the effect of FRTS/1 on hepatic
functions during
in situ perfusion is evaluated.
The livers of Sprague-Dawley rats are perfused in situ with a single-
pass system in a standard Krebs-Henseleit (K-H) solution. [pH 7.4, 02:CO2
(95%:5%)] The K-H solution is composed of: NaCI (118 mM), KCI (4.8 mM), KH2PO4
(1.2 mM), MgSO4=7H20, CaCl2 (1.5 mM), NaHCO3 (25 mM) and albumin (2% w/v).
Under anesthesia (pentobarbital; 50 mg = kg-1 body weight), a
laparatomy is performed to expose the portal vein, the inferior vena cava and
the
biliary channel for canulation. The portal canulation is used as the entrance
of the
perfusate into the liver and that of the vena cava is used for recovering the
perfusate
at the liver exit. The nervus vagus is sectioned to isolate the liver from any
vasomotor
influence. The total surgical procedure is completed within 15 minutes. The
time
interval between the insertion of a canula into the portal vein and the
beginning of the
circulation is not more than 3 minutes. The time lapsed between the cardiac
arrest
caused by the thoracic cage opening and the beginning of the perfusion is not
beyond one minute.
The total duration of the perfusion was 60 minutes. During the first
minutes, a wash out was performed. During that period, the K-H solution (37
C),
was supplemented with glucose (8 mM), lactate (0.5 mM), alanine (0.2 mM) and
glycerol (0.02 mM) was circulated in the liver in an open circuit. Thereafter,
the liver
was exposed to vehicles only (control), or to FRTS/vehicles (treated FRTS/1
group),
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 54 -
for another 30 minutes. The vehicles comprise either saline (1 m1/800m1
perfusate),
or 1,3-propanediol (24 m1/800m1 perfusate). FRTS/1 was added to either
propanediol
(0.06 mg FRTS/ml propanedio1:24 m1/800 ml perfusate) or to saline (2 mg
FRTS/ml
saline: 1 m1/800m1 perfusate). The perfusate flow rate was kept constant at 6
ml/minute/100g body weight). A small sample of the perfusate was taken at the
entrance and at the exit of the liver, for determining the production of the
utilization
of metabolites.
The concentrations of glucose, lactate, ALT and LDH in the
perfusate were determined by photospectronnetry using commercial procedure
disclosed by Sigma-Aldrich Canada n. 17-UV, No. 735, No. 59-UV and No. DH1240-
UV, respectively). The extraction or the production of a substrate by the
liver is
measured by the differences between the entrance and the exit of a metabolite,
multiplied by the perfusate flow rate.
Since the nature of the vehicle did not influence the measured
parameters, the results have been combined.
The bile production was similar in both control and treated groups
(0.55 0.10 v. 0.62 0.12 mg/min/g liver, in the control and treated group,
respectively). During reperfusion, bile production diminushed when compared to
the
pre-ischemia levels in both treated and control groups. Bile production
returned to
normal levels within 10 minutes after reperfusion.
At the entrance, the glucose concentration was similar in both
groups (7.04 0.40 v. 7.24 0.07 mM in control and treated group,
respectively).1n
both groups, the liver has slight tendency to use glucose. The exposure to
FRTS/1
has no effect on glucose capture (0.32 0.21 v. 0.39 0.25 pM/min/g liver in
control
and treated group, respectively). The concentration of lactate at the entrance
was
also similar in both groups (0.60 0.33 v. 0.50 0.10 mM in control and
treated
group, respectively); Upon exposure to FRTS, there is a slight tendency in the
treated livers to produce lactate (-0.01 0.1 v. 0.40 0.005 pM/min/g liver
in the
control and treated group, respectively).
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 55 -
Perfusing the liver by itself does not provoke any release of ALT or
LDH in the control groups (-0.07 0.14 and 1.79 0.47 pM/min/g,
respectively).
Treatment with FRTS/1 does not appear to provoke a release of ALT or LDH by
the
liver (0.57 0.21 and 2.36 1.10 pM/min/g), although there is a slight
tendency to
increase. When liver were partly perfused before the use of FRTS, there was a
progressive increase of the LDH production by the liver (35.57 8.96
pM/min/g).
These preliminary results lead to believe that FRTS/1 has no
remarkable effect on the viability of perfused liver. More specifically,
treatment with
FRTS/1 do not appear to modify the liver functions during a 30 minute
perfusion
duration, as evaluated by the utilization of glucose, the production of
lactate and of
bile. There is no apparent structural damage to the hepatocytes, since there
is no
increase of ALT and LDH.
The above was slightly modified to study the effect of ischemia and
reperfusion.The total duration of the perfusion was 105 minutes. The 31st
minute
constituted a wash out period. During that period, a K-H solution (pH 7.4 and
in the
presence of 02 :CO2 95% :5%) was added to glucose 8mM, lactate 0.5mM, alanine
0.2mM and glycerol 0.2mM. The solution was circulated in an open circuit.
Then, the
liver was exposed to the extract or to the vehicle: 1,3-propanediol for 15
minutes.
The perfusing rate was kept constant at 6 ml per minute per 100 g of body
weight
(Drouin et al. 2000, Lavoie et al. 2000). The perfusion was stopped for 30
minutes.
During this arrest, ischemia developed. Then circulation was re-established
for a
duration of 30 minutes. The perfusing liquids were taken at the entrance and
at the
exit of the tested livers, for measuring the production or the use of the
evaluated
biological markers.
Upon re-perfusion, the bioproduction diminishes compared to the
pre-ischemia levels in both control and treated livers. These levels return to
normal
within 10 minutes after the beginning of the re-perfusion. Control and treated
livers
release glucose in identical way (10.4 0.7 pM=minute-I=g-1). Glucose
production
was however slightly superior after 80 minutes in the treated group, compared
to the
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2010-02-08
-56-
control organs. Lactate accumulated during ischemia in higher levels in
treated livers
(5.9 0.5 pM=minute=g"1). when compared to the control organs (4.5 0.1
pM=minute"
Pre-treatment with the extract decreases the release of ALT (1.09
0.44 mU=minute-l=evs . 2.44 0.79 mUsminuteig-i, respectively) during re-
perfusion.
Pre-treatment with the extract appears to diminish the impact of ischemia for
the first
30 minutes of re-perfusion. Ischemia without any pre-treatment with the
extract,
provokes an increase of LDH (108.7 27.3 mU=minute'1=g'1) during re-
perfusion. Pre-
treatment with the extract does not influence LDH in increase (115.9 60.8
mt.I=minute"
Potassium and sodium plasmatic concentrations were also
measured in both groups. At the beginning of the re-perfusion, the release of
potassium in treated group is superior to the control (0.43 0.01
mli=minute"l=g-lvs 5.4
0.1 mU=minute=g-1, in control and treated groups, respectively). In the
perfusate, at
the entrance, the plasmatic concentration of potassium and sodium were similar
in both
groups. (K+ = 5.6 0.3 mM and 5.4 0.1 mM in control and treated groups,
respectively; Na + = 142.2 8.6 mM and 137.2 15.2 mM, in control and
treated
groups, respectively).
At the beginning of the re-perfusion, the release of sodium was also
superior in treated groups when compared to the controls (1.2 1.1
pM=minute'1=g-1
and 16.5 3.9 pM=rninute"1=g-1, respectively).
lschemia followed by re-perfusion is characterized with circulatory
and metabolic disturbance, and with tissue damage provoked by free-radicals
(Lee
2000). This particular model is currently used to evaluate the damage provoked
by
free radicals in tissues or organs subject to transplantation (Smrekova 2000,
Cohen
2000). Structural and functional disturbance is occasioned by I/R and are
reflected by
an increase in the release of enzymes (Bailey 2000, Vollmar 1994), a decrease
of bile
production (Vol!mar 1994) and a depletion of ATP reserve (Hwang 1999).
Ischemia
may lead to the
CA 02393816 2010-02-08
-57-
production of singlet oxygen, peroxide, and superoxide (02-*, H202, OH
following the
release of metal ions like iron and cupper (Halliwell 1999). This model is
therefore a
good one for characterizing a protective effect, if any, which would be
present in the
present extract following a radical insult.
Metabolic changes insuring protection of hepatic cells are affected
by pre-treatment with the extract. A radical attack consequent to I/R provoke
decrease
in ATP cell content, thereby modifying the energetic state of the cell
(Peralta 2000,
Mallet 1990). Tamarina et al. (1984) suggest that ischemia damages the
glycolytic
system of a cell, which renders difficult the lactate production. A healthy
cell (or a cell
under the action of a protecting agent) would protect itself from such a
stress by
increasing its ATP production via the glycolitic pathway. Sano of al. (1995)
proposed
that glycolytic activation reduces I/R-induced free-radical formation. Also
phosphoenolpyruvate prevents the ATP decrease consequent to I/R (Saiki 1999).
Hwang of a/. (1999) suggest that a decrease of the ratio NADf/NADH is
essential to the
resistance against free radicals to minimize cell damages. Pre-treating the
cells with
the present extract increases lactate production during the ischemic period,
which
apparently reflects the activation of a defense mechanism against the decrease
in ATP
provoked by ischemia. Kowalski 1992 and Groussard 2000 suggest that lactate
could
play a protective role in ischemia. Lactate could buffer superoxide (OH.),
generating
pyruvate which also buffers peroxide and superoxide, while decomposing into
acetate
and CO2 (Herz 1997).
The potassium exit of a cell pre-treated with the present extract also
supports a protective effect of the extract on a cell. Membrane component
peroxidation
may damage potassic channels (Halliwell 1999). The potassium release may be a
beneficial adaptation against a metabolic stress (Wang et at 1996). Potassium
channel
opening would permit capturing substrates for the intracellular ATP
generation.
Potassium releasing would also inhibit HCO3" transport, contributing to the
acidification
of the cytoplasm, which is also protective
CA 02393816 2010-02-08
-58-
to the cell (Currin 1991). Potassium release appears early in a perfusate and
precedes
the ALT release, and is proportional to the ATP decrease.
Sodium accumulation in the cell plays a major role in cell damage
induced by I/R (Carini 2000, vanEchteld 1991 and Xia 1996). This increase may
be
due to: 1) a dysfunctional Na + /K+ pump, which is due to a decrease in ATP
and to an
increase of inorganic phosphate, or 2) a stimulation of the Na/H anti-carrier,
due to the
acidification of the cytoplasm. Any reversal of such a situation could
decrease the
impact of a metabolic stress induced by sodium accumulation (Fiegen 1997). The
sodium release may therefore be a defense mechanism against damages. Both
decrease of potassium and sodium have been observed with livers perfused with
present extract, which supports the protective effect of the extract in
hepatic cells.
Sodium release is superior in the treated group when compared to the control
group,
10 minutes after re-perfusion, which suggests a late recovery in aTP contents
in the
controls.
The present extract stimulates the cellular mechanisms associated
with cell protection against a radical attack.
The ex-vivo effect of the present extract in brain:
Numerous brain pathologies involve an increase production of free
radicals. The latter are believed to contribute to the neurodegenerating
process,
namely pursuant to cerebral vascular accidents, or during the development of
Alzheimer disease. It is believed that anti-radical components may be useful
for
reducing the expression of neurodegenerative diseases, or for their prevention
or their
treatment.
Hyppocampus region of the brain is vulnerable to neurotoxic effect
of free radicals, namely during a cerebral anoxia. It has been demonstrated in
vitro
that neuronal transmission is attenuated during anoxia, because of the over
expression
of antioxidant molecules. Therefore, the effect of the present extract on the
hyppocampal neuronal transmission was evaluated. Electrophysiological
responses
were registered in this brain structure. Particularly, the recovery of
CA 02393816 2010-02-08
-59-
neuronal potentialisation would be studied. Hyppocampal slices of 450 pm
thickness
were obtained from rat brains and were transferred to electrophysiological
chambers.
The slices were maintained during 60 to 90 minutes in an oxygenated
physiological
solution, comprising or not different concentration or not different
concentrations of the
extract. After this resting period, electrical stimulation were applied every
25 second in
hyppocampal afferences (Schaffer, regions of CA1 region). These
electrical
stimulations evolved synaptic responses. The initial slope of post-excitatory
potentials
(EPSP) was calculated to quantify the synaptic transmission efficacy pursuant
anoxia.
The study the neuronal potentialization, a high frequency stimulation train
was applied
to the neuronal circuits after anoxia. As a positive control, the response to
glutamate
with or without ischemia may be measured and the presence of the present
extract
should restore the response to glutamate.
TOXICITY OF FRTS/1
The toxicity has been evaluated in two different models: the in situ
liver perfusion and in situ brain perfusion. These organs did not show any
sign of
toxicity due to the presence of the extract.
ADVERSE REACTIONS
The present extract is non-immunogenic.
The immune response provoked by the present extract has been
evaluated by injecting 125 pg of the extract intraperiteonally in mice three
times at 7
day intervals. After a week spent following the last injection, blood was
harvested for
immune serum obtention. The mice were anesthetized and blood was taken by
cardiac
puncture. Blood samples were put immediately on ice. Blood clotting was
allowed to
proceed on ice and samples were centrifuged 12 hours after harvesting. The
centrifuging conditions were the following: 10 minutes at 2500 g, which
provided about
500 pl of serum. The extract was adsorbed on a microplate to provide a fixed
antigen
preparation. 200 pl of the extract (5pg/m1) and 200 pl of ELISA buffer were
poured in
the wells of a 96-well plate. The ELISA buffer was made of 100 mM of sodium
carbonate buffer (pH 9.6). After incubation of the antigen at 4 C overnight,
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 60 -
the non-adsorbed antigen was eliminated three washing steps with a sodium
phosphate buffer 100 mM / NaCI, 100 mM (pH 7.4). The free adsorption sites
were
blocked in a 60 minute-incubation at room temperature with a solution of
caseine 3%
in the sodium phosphate / NaCI buffer. Excess of caseine was washed three
times.
The serum samples providing the antibodies, if any, were prepared as follows:
serial
dilutions in the sodium phosphate / NaCI buffer supplemented with 0.05 %Tween
2OTM were made and 25 pl of these dilutions were added to the wells. The
microplate
was further incubated for 1 h at 37 C, which step was followed by 5 washes
with the
sodium phosphate / NaCI / Tween buffer.
The presence of an antibody was revealed by the formation of an
anti-Ig-peroxidase complex. 25 pl of the enzyme dilution in the sodium
phosphate /
NaCI / Tween buffer is added to each well followed by a one hour-incubation at
37
C. The enzyme dilution varied between 1:750 and 1-3000, depending on the
conjugate (These are anti-Ig goat anti-sera, IgM, IgG, Ig1, Ig2 and Ig3,
labelled with
peroxidase). The labelling step was followed with 5 washings with the buffer.
Substrate hydrogen peroxide 0.015% and the chromogene ABTS (2,2 azino-
diethylbenzthiazoline-6-sulfonate) 0.05% dissolved in a phosphate citrate
buffer 100
mM (pH 4.0) were added to the wells. Another 30 minute-incubation at room
temperature and in the dark was allowed to proceed. The action of the enzyme
released a colored substance which can be read spectrophotometrically at an
absorbance wavelength of 405 nm. The serum level of antibody specific to the
extract should be proportional to the color intensity. The results that have
been
obtained indicate that the extract is non-immunogenic to the recipient
individuals.
These results indicate that the extract is not toxic to individuals,
since it is neither hepatotoxic nor immunogenic.
COMPOSITIONS AND DOSAGE REGIMENS:
Due to the stability and the potency of the present extract and the
fact that it is non-toxic to animals, it is believed that dosage rates
extending from 1
ng per kg of body weight to 1 g per kg of body weight per day could be
administered
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2010-02-08
-61-
to individuals in need for such administration (a dose in a lower pg range may
be
preferred). The dosage depends on the agressivity sought for the treatment of
a
disease. The dosages may also depend on the formulations and their route of
administration. For example, a topical composition will not comprise the same
dose as
an intravenous composition or an enteral composition.
TOPICAL COMPOSITIONS FOR TREATING SKIN OR MUCOSAL DISEASES:
AI lerpy/Asthma:
Brown Norway rats are high IgE producers. There is a well
established model of allergen-induced airway hyperresponsiveness in Brown
Norway
rats that reflects many features of human allergic asthma, including both
early and late
(70% of animals) phase reactions, increase in antigen-specific IgE following
active
immunization, airway inflammation, and increased bronchial responsiveness to
several
stimuli following allergen challenge.
Measurement of pulmonary responses. Brown Norway sets are
sensitized by intraperitoneal injection of 1 ml of 1 mg ovalbumin/100 mg
Al(OH)3 in
saline as previously described. Twenty one days later, animals are
anesthetized and
intubated as previously described with the end of the endotracheal tube
connected to
the PlexiglasTM box. A water-filled oesophageal catheter attached to a
pressure
transducer is used to determine changes in pleural pressure. Airflow is
measured by a
pneumotachograph coupled to a differential transducer attached to the
Plexiglas box.
Air flow, volume and transpulmonary pressure, pulmonary resistance (RL) are
determined at different times to identify both the early and late phase
reactions.
Sensitized Brown Norway rats are challenged with saline or ovalbumin (2% in
saline)
using the 'Wright" nebuliser from Roxon Medi-Tech Lte (Montreal, PQ) using
compressed air with a pressure giving an output of 0.1-0.2 ml/min passed into
the
Plexiglas box. Pulmonary resistance is measured every 5 minutes for the first
hour and
every 15 minutes for the next 10 hours. Pre-, co- and post-treatment with the
extracts
administered i.p. or by inhalation (about 1 to 100 pg) are tested in this
model to provide
improvement.
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 62 -
PROTECTION AGAINST UV RADIATION
The ability of FRST/1 to prevent or reduce the UV-induced skin
damages in hairless mice was investigated. As it is known that most of the
skin
cancers are induced by exposure to UV radiation, there is a need to identify
new
potent natural compounds that could prevent the adverse effects of UV
radiation.
Animals
Hairless albino (SKH/1) mice will be purchased from Charles River
laboratories (Wilmington, MA).
All mice are 6 weeks old at the beginning of the irradiation period.
Mice are housed and maintained under standard conditions (23 10C, 42 6%
relative
humidity, 12:12-h light-dark cycle) at the Animal Facility of IBS. Lights are
automatically switched on daily at 7 AM and switched off daily at 7 PM. Mice
are fed
Purina chow diet (24% protein, 4% fat, and 4.5% fiber) and water ad libitum.
For
irradiation, mice are placed in plastic cages and are allowed to move freely
within the
cages during irradiation.
The animals were acclimated for one week prior to treatment and
divided into randomly into 5 groups as follows.
= Group I: Control non-irradiated, non-treated (n=5);
= Group II: non- UV-irradiated animals treated with a preparation of
topical
ointment containing FRST/1 (n=5).
= Groups Ill: UV irradiated animals treated with the preparation of topical
ointment without FRST/1 (n=5)
= Group IV: animals receiving topical application of the cream containing
FRST/1 during UV irradiation (n=5).
= Group V: non- treated UV- irradiated animals (n=5).
Treatments consist in:
I. Weighing all animals to assess their health on the day of the
treatment and
once every second day thereafter.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 63 -
2. Performing a dorsal topical application of one type of cream (known to
be non-
toxic to animals and human). The cream contains or not FRST/1. The extract
is present at a 1:10,000 dilution (starting from the lyophilized thylakoid
fraction). One gram of cream is dispensed just before (and remain during and
after UV irradiation) on the back of 3 groups of animals. Among these, one
group of 5 animals receives the cream and no irradiation. One group receives
the cream without FRST/1 and is exposed to UVB and one group receives the
FRST/1 cream and UVB. The UVB irradiated groups (n=5) are exposed to
sunlamps for 10 min once. One group (n=5) is exposed to UV sunlamps
without cream treatment.
3. A single 10-min UVB irradiation by sunlamps located at 60 cm from the
animal
backs is performed. The two groups of 5 animals tested for cream protection
(with and without FRST) and a single group of 5 animals without protection
are submitted to the single dose of UVB.
4. After treatment, all animals are kept in cages and photographed from the
top
of the cage on the day of UV treatment and 3 to 4 days later, without
manipulation.
5. After one week, the animals are sacrificed before removing a piece of
their
dorsal skin for further studies.
Westinghouse FS40 sunlamps, an IL-1400 radiometer, and a UVB
photometer are used. The spectral irradiance for the UV lamps is 280-400nm,
80%
of which are in UVB region and 20% in UVA region. The peak intensity of the
light
source are 297 nm. The fluence at 60 cm from the dorsal surface of the mice
are
0.48-0.50mJ/cm2/s. The mice are placed in plastic cages without lid as
mentioned
above.
Negative control mice (Groups I, II, ) are treated in an identical way,
but UV lamp is not be switched on. Group II receives the ointment without
FRST/1
topically.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2010-02-08
-64-
The mice from Group III, IV and V are given a single exposure of a
total of 200 mJ UV light / cm 2 (acute dosage) for 10 min. In Group IV the
animals
receive topical application of a FRST/1 preparation immediately before, during
and
after UV exposure. This approach has been adopted to take into account minor
differences in UV absorption characteristics (optical density differences) in
the 290-320
nm range that might optically influence the UV fight irradiation condition.
The mice are kept and weighed every second day one week before
and one week after UV irradiation. At the end of the experiment, the mice are
sacrificed and the following parameters are compared in all the groups.
1. Body weight
2. Epidermal observation and photographs
3. Following sacrifice of the mice, a piece of the dorsal skin is
surgically removed
and used for further analyses.
4. Comparison of Cytokeratin patterns of expression by quantitative Western
blot.
Solar radiation is the major environmental factor that affects the
structure and function of human skin. Long term cutaneous photodamage as a
consequence of cumulative UV radiation injury often leads to photoaging and
skin
cancer in fair-skinned individuals. Studies involving photobiological effects
of ultraviolet
radiation reveal that the ultraviolet B (UVB) component (290-320nm) in
particular is
erythemogenic, carcinogenic and induces skin photoaging changes preceded by
direct
damage to DNA, RNA, proteins (including enzymes), cell membrane and other cell
organelles.
There is a clear distinction between chronological aging and
photoaging. Photoaging is used to describe the clinical and histological
damages
produced by chronic exposure of the skin to sunlight or solar-
CA 02393816 2010-02-08
-65-
simulated UV radiation. Histologically, these changes are manifested in the
form of
marked changes in elasticity, glycosaminoglycans, and disordered collagen,
together
with an increase in the number of mast cells and inflammatory cells. This
phenomenon
of photoaging has clinically been recognized as irreversible, although recent
therapeutic approaches have helped to minimize the over expression of
intrinsic aging
and photoaging changes. The use of sunscreens on a regular basis has been
reported
to help prevent actinic damage to connective tissue. The use of both
sunscreens and
antioxidants (e.g., green tea, Vitamin C, Vitamin E) appears to have an
inhibitory effect
on UVB-induced acute skin damage that contributes to both photoaging and
photocarcinogenesis.
The photoprotective ability or antioxidant effect of FRST/1 against
acute UV radiation exposure has been evaluated in the hairless albino mouse
model.
The skin of hairless albino mouse (SKH-1) has been recognized as
a useful and relevant experimental model for studying and understanding
effects of UV
radiation and photoaging of human skin. The visually and microscopically
recognizable
responses of the epidermis and of the dermis to UVB radiation and absence of
hair
make the skin of hairless mice particularly useful in studying and evaluating
the
damaging effects of UV radiation. This mouse model has also been used to study
and
examine the immunological alterations and carcinogenesis induced by UVB
radiation
both locally in the skin and systematically.
Results:
Right after irradiation, all the non-treated irradiated mice showed
signs of skin irritation and itchiness. They were otherwise healthy and
active, although
they did not gain weight. No symptoms of irritation or redness was observed
CA 02393816 2002-06-27
- 66 -
on irradiated mice when pre-treated as well as when post-treated with FRST/1
and
mice gained in weight in 80% of cases. Therefore, topical compositions, either
solar
screen lotion, cream, ointment, oil, gel or spray, are objects of this
invention.
Skin cytokeratins analysis:
The cytokeratins 1 and 10 are representative of mature skin. A
decrease in these keratins is an indication of epidermis regeneration
following lesion.
The cytokeratins 5 and 8 are representatives of suprabasal and
basal layers and are supposed to be expressed in actively proliferating
epidermis.
The presence of these cytokeratins was evaluated with specific
antibodies.
The extracts from mice skin were individually extracted and the skin
of mice was analyzed individually per group. The same amount of total
extracted
cytoplasmic proteins was applied per well: 35 ug to enable comparison among
animals and treatments.
Conclusions:
FRST/1 protected mice showed a pattern similar to that of controls
untreated non-irradiated, while all other treatments showed a drastic decrease
in high
molecular weight keratins. Especially, cytokeratin 10 remained very well
expressed
in the skin of FRST/1 mice, which is a good indication of a protective effect.
At a very
low dose, the extract was active topically. The dose may be increased at will
since
the dose of the extract is not limited by any toxicity.
Although the present invention has been described hereinabove by
way of preferred embodiments thereof, it can be modified, without departing
from the
spirit and nature of the subject invention as defined in the appended claims.
=
AMENDED SHEET
=3007
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 67 -
.. ,,.,. ---.....,
r, -,v---n- = 41i,1.101' ,g,<2,..t--.- -,-
, , Qgat.,..
- -------m:.-------.4e7-44 1..---U.,,,7,,k*,*.,4iF,;--6--r.`_;=1----1-
4*,:nc.tirgil===:_4,-_. , ----,
s':+V.,,, cg.,*.i-,-.1;7,õ..,,-,- ,7-. - -
, -1.,õ-.-9141t=-=10.47-1A.Rt*- - ----
193
--. õ=-'7:¨..,=b =,_=:"... - - ,,--,-,...-.. :._,,v, ,..õ,
1,12
0
-.4,, . ,..õ, (),84
__ . _________________ roõ-,,-.1s1r.õ..., 7,..,....--ww4v.K.Azg-,
100
t'i-- 14,--- = . ---01,4õõ,,.,,*, 1,11
--A. .4õ..-- -,..c.4,-v...-,..Ar ,f.v.,-...==
300
I.,',',.6,f.õ.C. ,i ,iitritil,--tir.zt-t77-7..õ.w,,W 1,1
4-,.... vp,.. = i --p--?,.. - -0,:tr-i-spk_rol-AV4
193
õ=:.4.2::::;,-..-*..- sf-,,e, , '141::- ' - - '-1-1,-,-, ,
i
054
. -..10-.-
- 7 - y..??,=Z ' VI- = -, ,` Av-1 1:',....,..","::',-.-- 0.4.1r*".
VS ..-erakfnlai..4
' '''' R"*. "."LY "Ittri. 33' '1N , di.4.;" . , = a'''' ^",f `'S.
..=,,w4. Vf p g I F. ' ..÷44.-1:. ,r. A aii0k .,3.,....,..;1
0,4 193
-IVV=41"ttrf4-tttq
-,1, -5.,164g..:*A494-;-4,i ,
*,4;,,,....-i. I, ' "tri-- ---Aw'..t.,..,...4w1/4-1R-, = '---,
193
4.õ.. 0,83
7-.,µ 51.
Y -- :=,õ_.,v2.:,,.. -,,-.,,,, A ,-= -¨...,
1-74------- ,,..,-., r- :-.ZI,..%-n,-444
-4,- VoiA. -:õi ,,,,,-- .,..,-- = .4, Itetyr' ,,:,''
c==-.=-
i,,i,õtiti;ri r .t S. $, = - _,:._ , N - '=;:õ_46.-1.-_,..,,e-
--E__),-.4-,,-- ....,, ,=...-..,. ...w....õ
¨ A------ ¨, 4,4-ea- 'Tz, õ ,:t.=,. -o--
3::1-,--..,,,,x*..:=- , '=-.,.õ-.-õ*.-=,,=-_ = ,:t. :;-.1:1-0 = - ...,:.-1-::
1,11., 7-,.-'4....-; ,=*-./___-:,..,,,.... ---w,,,,p241-4µ,
,_...:,..õ..3
*
19.3
0
õIA.- x..,,A",' 4.04.5',..At-g-rk 0,95
4..is,v,,,,,..= 7,..,FAU V, 34.--q3'& 4A*..7*1:Pk,er.tiv-
193
0,29
...,,-.4
,
.!:, i- 4it,4..vt,,i4rifti
a.4-1-1:k 11 PA , 7'
0,93 193
- --..--,-40-_,,,,,õ . = tojp:$.=,-,-õ,,,
,, ...õ,. .,,..õ. ;7-. = i !-- 'V t4,. J.,
,:4,,,,,,,,,t,...a.4
' Ift:-.1`I'M = - tV'..v,:4:VAVA..151 10a
14ii 7,,,ii.-, . , , VA** gt
097 193
.õ ' 4- 14-. w.3.:-.,õ:1...õ=,..---.,..4r.õ..õ:-.:24
,
=..49=An,m.õ'..144t,,,v 0,8 0
-,-,...ingro0-.,::Ave--,,
100
t.:,
4.
1,5
,kei--e4..,..,c-4,-.bµh-4,-,7-4-;v1;7-
a-.,p.,,.........õ,,,.=.=-v- õ...,,,,...t.õ,zo..=s= 0,95 300
,ff-,--,-,-"4:r ,-,=-kit....----.-z,--7,--&m..rivin..4.k,=74.74.:it
193
0,71
....,..10,&-.th,79r ¨ .4.:1.-.4-4-;:s',.
0
4.--- ' ....,õ_:-.7- 0,--mvAlir,,,, 2, 0 ,02
tõ' : ; : , 3 ,7,11'..õ4õ,:r.,=-,-_-;,,-- _ 10._,WWõet,-1,--, Wik..-Ait
õ1õfert.-1--11121,41et;k:ir...õ 0,37 100
4.,-,11k=*ffl¨wa,---=
0,67 300
,, 1.`-., .AArs....L_.-,- , ----,7.6 --=,-- ..4:-,r0
" = A.7--f------1 " : .-',"=,,,.. 4.Q.-*,,,,,f,
'ifir- Tftz?.,443,4
=-,-',-, ; ' ' '-; - ,i,r;-; '--:/- , ..-,%õ_.A.4,--Nik.:- vi.3.-,:.,
400
-0.---wta-pa.,..--=
0,7
.1.4-,.... -..; - ....õ_ --,-,,, -e=_L-1. ......---...;
,
Table 1.: Relative activity
einxofug
function of the
species and the
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 68 -
BIBLIOGRAPHY
Eidelman, D. H., S. Bellofiore, and J. G. Martin. 1988. Late airway responses
to
antigen challenge in sensitized inbred rats. Am. Rev. Respir. Dis. 137:1033-
1037.
Waserman, S., R. Olivenstein, P. M. Renzi, L. J. Xu, and J. G. Martin. 1992.
The
relationship between late asthmatic responses and antigen-specific
imrnunoglobulin.
J. Allergy Clin. Immunol. 90:661-669.
Renzi, P. M., R. Olivenstein, and J. G. Martin. 1993. Inflammatory cell
populations
in the airways and parenchyma after antigen challenge in the rat. Am. Rev.
Respir.
Dis. 147:967-974.
Bellofiore, S., and J. G. Martin. 1988. Antigen challenge of sensitized rats
increases
airway responsiveness to methacholine. J. Appl. Physiol. 65:1642-1646.
Elwood, W., J. 0. LOtvall, P. J. Barnes, and K. F. Chung. 1991.
Characterization of
allergen-induced bronchial hyperresponsiveness and airway inflammation in
actively
sensitized Brown-Norway rats. J. Allergy Clin. Immunol. 88:951-960.
Renzi, P. M., J. P. Yang, T. Diamantstein, and J. C. Martin. 1996. Effects of
depletion
of cells bearing the interleukin-2 receptor on immunoglobulin production and
allergic
airway responses in the rat. Am. J. Respir. Crit. Care Med. 153:1214-1221.
Bailey SM, Reinke LA. Effect of low flow ischemia-reperfusion injury on liver
function.
Life Sci 66(11) :1033-44,2000.
Bruck R, Haddad P, Graf J, Boyer JL. Regulatory volume decrease stimulates
bile
flow, bile acid excretion, and exocytosis in isolated perfused rat liver. Am J
Physiol
262:0806-12,1992.
Caraceni P, Gasbarrini A, Van Thiel DH, Borle AB. Oxygen free radical
formation by
rat hepatocytes during postanoxic reoxygenation: scavenging effect of albumin.
Am
J Physiol 266:G451-8,1994.
Carini R, De Cesaris MG, Splendore R, Bagnati M, Bellomo G, Albano E.
Alteration
of Na(+) homeostasis in hepatocytes reoxygenation injury. Biochem Biophys Acta
1500(3):297-305,2000.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 69 -
Cohen AJ, Burczynski FJ, Rosser BG, Lipschitz J, Minuk GY. The effects of
various
organ preservation solutions on hepatocyte membrane potentials, intracellular
calcium concentrations, and outcome following liver transplantation. Am J Surg
179(2):154-60,2000.
Currin RI, Gores GJ, Thurman RG, Lemasters JJ. Protection by acidotic pH
against
anoxic cell killing in perfused rat liver: evidence for a pH paradox. FASEB J
5(2):207-
10,1991.
Drouin R, Milot M, Robert G, Massicotte D, Peronnet F, Lavoie C. Hepatic
Glucagon
Sensitivity Induced by Endurance Training: Effect mediated by Increased
Glycogenolysis? MSSE: 32: S224 # 1061,2000.
Erlinger S. Review article: new insights into the mechanisms of hepatic
transport and
bile secretion. J Gastroenterol Hepatol 11(6):575-9,1996.
Fiegen RJ, Rauen U, Hartman M, Decking UK, de Groot H. Decrease of ischemia
injury to the isolated perfused rat liver by loop diuretics. Hepatology
25(6):1425-
31,1997.
Groussard C, Morel I, Chevanne M, Monnier M, Cillard J, Delamarche A. Free
radical
scavenging and antioxidant effects of lactate ion: an in vitro study. J Appl
Physiol
89:169-175,2000.
Halliwell B, Gutteridge JM. Free radicals in biology and medicine. Third
edition,
Oxford Science Publication,1999.
Hashimoto K, Nishizaki T, Yoshizumi T, Uchiyama H, Okano S, lkegami T, Yanaga
K, Sugimachi K. Benfecial effect of FR167653 on colf ischemia/reperfusion
injury in
rat liver transplantation. Transplantation 70(9):1318-22,2000.
Herz H, Blake DR, Grootveld M. Multicomponent investigations of the hydrogen
peroxide- and hydroxyl radical-scavenging antioxidant capacities of biofluids:
the role
of exogenous pyruvate and lactate. Free Radic Res 26: 19-35,1997.
Hwang K, Jeong DW, Lee JW, Kim IH, Chang HI, Kim HJ, Kim IY. Alteration of the
NAD+/NADH ratio in CHO cells by stable transfection with human cytosolic
glycerol-
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 70 -
3-phosphate dehydrogenase: resistance to oxidative stress. Mol Cells 9(4):429-
35,1999.
Jaechke H. Glutathione disulfide as index of oxidant stress in rat liver
during hypoxia.
Am J Physiol 258(21):G499-G505,1990.
Kowalski DP, Aw TY, Park Y, Jones DP. Postanoxic oxidative injury in rat
hepatocytes: lactate-dependent protection against tert-butylhydroperoxide.
Free
Radical Biol Med 12(3): 205-12,1992.
Lavoie C, Drouin R., Milot M,Robert G, Massicotte D,POronnet F. Hepatic
Glucagon
Sensitivity Induced by Endurance Training: Effect mediated by Increased
Gluconeogenesis? MSEE 32: S224 # 1062,2000
Lee SM, Park MJ, Cho TS, Clemens MG. Hepatic injury and lipid peroxidation
during
ischemia and reperfusion. Shock 13(4):279-84,2000.
Mallet RT, Hartman DA, Bunger R. Glucose requirement for postischemic recovery
of perfused working heart. Eur J Biochem 188(2):481-93,1990.
Peralta C, Bartrons R, Riera L, Manzano A, Xaus C, Gelpi E, Rosello-Catafau J.
Hepatic preconditioning preserves energy metabolism during sustained ischemia.
Am
J Physiol Gastrointest Liver Physiol 279(1):G163-71,2000.
Peralta C, Leon OS, Xaus C, Prats N, Jalil EC, Planell ES, Puig-Parellada P,
Gelpi
E, Rosello-Catafau J. Protective effect of ozone treatment on the injury
associated
with hepatic ischemia-reperfusion: antioxidant-prooxidant balance. Free
Radical Res
31(3):191-6,1999.
Ross BD. Perfusion techniques in biochemistry. Oxford University Press,
Oxford, UK,
1972.
Saiki S, Yamaguchi K, Chijiiwa K, Shimizu S, Hamasaki N, Tanaka M.
Phosphoenolpyruvate prevents the decline in hepatic ATP and energy charge
after
ischemia and reperfusion injury in rats. J Surg Res 73(1):59-65,1997.
Sano W, Watanabe F, Tamai H, Furuya E, Mino E. Beneficial effect of fructose-
1,6-
bisphosphate on mitochondrial function during ischemia-reperfusion of rat
liver.
Gastroenterology 108(6):1785-92,1995.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 71 -
Smrekova R, Vajdova K, Kukan M, ulina 0, Lutterova M, Wsolova L, Horecky J. A
rapid, simple, and cost-effective method for screening liver preservation
solutions in
the rat. Transplantation 70(3):430-6,2000.
StrubeIt 0, Younes M, Li Y. Protection by albumin against ischemia- and
hypoxia-
induced hepatic injury. Pharmacol Toxicol 75(5): 280-4,1994.
Tamarina NZ, Chumakov VN, Lystsova GV. [Effect of NAD and ADP on glycolysis
in the kidneys and liver of rats during ischemia]. WMJ 56(1):46-52,1984.
van Echteld CJ, KirkeIs JH, Eijgelshoven MH, van der Meer P, Ruigrok TJ.
Intracellular sodium during ischemia and calcium-free perfusion: a 23Na NMR
study.
J Mol Cell Cardiol 23(3):297-307,1991.
Vollmar B., Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory
perfusion failure is a dterminant of liver dysfunction in warm ischemia-
reperfusion.
Am J Pathol 145(6):1421-31,1994.
Wang Y, Sostman A, Roman R, Stribling S, Vignas S. Hannun Y, Raymond J, Fitz
JG. Metabolic stress opens K(+) channels in hepatoma cells through a Ca(2+)-
and
protein kinase Ca-dependent mechanism. J Biol Chem 271(30):18107-113,1996.
Xia ZF, Horton JW, Zhao PY, Babcock EE, Sherry AD, Malloy CR Effects of
ischemia
on intracellular sodium and phsophates in the in vivo rat liver. J Appl
Physiol
81(3):1395-403,1996.
Youn YK, Lalonde C, Demling R, Oxidants and the pathophysiology of burn and
smoke inhalation injury, Free Radic Biol Med, 1992;12(5):p. 409-15
Golan TD, Dan S, Haim H, Varda G, Sol K, Solar ultraviolet radiation induces
enhanced accumulation of oxygen radicals in murine SLE-derived splenocytes in
vitro, Lupus 1994;3(2):p. 103-6
Lange RW, Germolec DR, Foley JF, Luster MI, Antioxidants attenuate anthralin-
induced skin inflammation in BALB/c mice: role of specific proinflammatory
cytokines.
J Leukoc Biol 1998a;64(2)p. 170-6
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 72 -
Lange RW, Hayden PJ, Chignell CF, Luster MI, Anthralin stimulates keratinocyte-
derived proinflammatory cytokines via generation of reactive oxygen species,
Inflamm Res 1998b Apr;47(4):p. 174-81.
PoIla BS, Ezekowitz RA, Leung DY, Monocytes from patients with atopic
dermatitis
are primed for superoxide production, J Allergy Clin Immunol 1992 89(2):p.545-
51
Juurlink BH, Paterson PG, Review of oxidative stress in brain and spinal cord
injury:
suggestions for pharmacological and nutritional management strategies, J
Spinal
Cord Med 1998;21(4): p.309-34
El Kossi MM, Zakhary MM, Oxidative stress in the context of acute
cerebrovascular
stroke, Stroke 2000; 31(8): p. 1889-92
Ebadi M, Srinivasan SK, Baxi MD, Oxidative stress and antioxidant therapy in
Parkinson's disease, Prog Neurobiol 1996 Jan;48(1)p. 1-19
Foler P. Riederer P. Influence of neurotoxins and oxidative stress on the
onset and
progression of Parkinson's disease. J Neurol. 2000 Apr;247 Suppl 2:1182-94.
Review.
Smith MA, Rottkamp CA, Nunornura A, Raina AK, Perry G, Oxidative stress in
Alzheimer's disease, Biochim Biophys Acta 2000 Jul 26;1502(1):p.139-44.
Cimen MY, Cimen OB, Kacmaz M, Ozturk HS, Yorgacioglu R, Durak I.
Oxidant/antioxidant status of the erythrocytes from patients with rheumatoid
arthritis.
Clin Rheumatol. 2000;19(4):275-7.
Gerber RT, Holemans K, O'brien-Coker 1, Mallet Al, Van Bree R, Van Assche FA,
Poston L, Increase of the isoprostane 8-isoprostaglandin f2alpha in maternal
and
fetal blood of rats with streptozotocin-induced diabetes: evidence of lipid
peroxidation.Am J Obstet Gynecol. 2000 Oct;183(4):1035-40.
Sakorafas GH, Tsiotos GG, Sarr MG. Ischemia/Reperfusion-Induced pancreatitis.
Dig
Surg. 2000;17(1):3-14. Review.
McGuire GM, Liu P, Jaeschke H. Neutrophil-induced lung damage after hepatic
ischemia and endotoxemia. Free Radic Biol Med. 1996;20(2):189-97.
Lai HS, Chen WJ, Chiang LY. Free radical scavenging activity of fullerenol on
the
ischemia-reperfusion intestine in dogs. World J Surg. 2000 Apr;24(4):450-4.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 73 -
Eaton JW, UV-mediated cataractogenesis: a radical Perspective, Doc Ophthalmol
1994-95;88(3-4):p. 233-42
Hardy P. Dumont I. Bhattacharya M. Hou X. Lachapelle P. Varma DR, Chemtob S.
Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: a
basis
for ischemic retinopathy. Cardiovasc Res. 2000 18;47(3):489-509.
Castagne V, Clarke PG. Neuroprotective effects of a new glutathione peroxidase
mimetic on neurons of the chick embryo's retina. J Neurosci Res. 2000 Feb
15; 59(4):497-503
Singh RB, Singh NK, Rastogi SS, Wander GS, Aslam M, Onouchi Z, Kummerow FA,
Nangia S. Antioxidant effects of lovastatin and vitamin E on experimental
atherosclerosis in rabbits. Cardiovasc Drugs Ther. 1997,11(4):575-80
Anastassopoulou J, Anifantakis B. Anifantakis ZA, Dovas A, Theophanides T. The
role of free radical reactions with haemoglobin and thalassaemia. J lnorg
Biochem.
2000; 79(1-4):327-9.
Ginsburg H. Golenser J. Redox metabolism in glucose-6-phosphate dehydrogenase
deficient erythrocytes and its relation to antimalarial chemotherapy.
Parassitologia.
1999 41(1-3):309-11.
Chen LY, Nichols WW, Hendricks J, Mehta JL. Myocardial neutrophil
infiltration, lipid
peroxidation, and antioxidant activity after coronary artery thrombosis and
thrombolysis. Am Heart J. 1995;129(2):211-8
Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ.
Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate
of
asthma patients. Am J Respir Crit Care Med. 1999;160(1):216-20
Montuschi P, Collins JV, Ciabattoni G, Lazzeri N, Corradi M, Kharitonov Sa,
Barnes
PJ. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in
patients
with COPD and healthy smokers. Am J Respir Crit Care Med. 2000;162(3 Pt
1):1175-7
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
-74 -
Barros LF, Stutzin A, Calixto A, Catalan M, Castro J, Hetz C, hermosilla
Nonselective
cation channels as effectors of free radical-induced rat liver cell
necrosis.Hepatology.
2001;33(1):114-122.
Jonas CR, Puckett AB, Jones DP, Griffith DP, Szeszycki EE, Bergman GE, Furr
CE,
Tyre C, Carlson JL, Galloway JR, Blumberg JB, Ziegler TR. Plasma antioxidant
status after high-dose chemotherapy: a randomized trial of parenteral
nutrition in
bone marrow transplantation patients. Am J Clin Nutr. 2000,72(1):181-9
El-Kadi AO, Bleau AM, Dumont I, Maurice H, du Souich P. Role of reactive
oxygen
intermediates in the decrease of hepatic cytochrome P450 activity by serum of
humans and rabbits with an acute inflammatory reaction. Drug Metab Dispos.
2000;
28(9):1112-20.
Prior RL, Prior RL, Cao G. Analysis of botanicals and dietary supplements for
antioxidant capacity: a review. J AOAC Int. 2000;83(4):950-6.
Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma.
2000,17(10):871-90.
Sinha BK, Mimnaugh EG. Free radicals and anticancer drug resistance: oxygen
free
radicals in the mechanisms of drug cytotoxicity and resistance by certain
tumors.Free
Radic Biol Med. 1990;8(6):567-81.
Karbownik M, Reiter RJ, Garci JJ, Tan D. Melatonin reduces phenylhydrazine-
induced oxidative damage to cellular membranes: evidence for the involvement
of
ironint J Biochem Cell Biol. 2000,32(10):1045-54.
Olszewski AJ, McCully KS. Homocysteine metabolism and the oxidative
modification
of proteins and lipids. Free Radic Biol Med. 1993 Jun;14(6):683-93.
Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;
3;257(1):59-84.
Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress,
and
aging. Free Radic Biol Med. 2000,29(3-4):222-30
Bednarska K, Wachowicz B, Buczynski A. UV-B-induced generation of free
radicals
in blood platelets. J Photochem Photobiol B. 2000;55(2-3):109-12.
SUBSTITUTE SHEET (RULE 26)
CA 02393816 2002-06-26
WO 01/49305 PCT/CA00/01541
- 75 -
Floyd RA. Antioxidants, oxidative stress, and degenerative neurological
disorders.
Proc Soc Exp Biol Med. 1999;222(3):236-45.
Blankenship RE, and Hartman H. The origin and evolution of oxygenic
photosynthesis.Trends Biochem Sci. 1998, 23(3):94-97.
Allen MJ & Crane AE. (1976). Null potential voltammetly - An approach to the
study
of plant photosystems. Bioelectrochem. Bioenerg. 3, 84-91.
Barber J. Kuhlbrandt W. Photosystem II. Curr. Opin Struct Biol. 1999, 9, 469-
475.
Schlodder E, Witt HT, Stoichiometry of proton release from the catalytic
center in
photosynthetic water oxidation. Reexamination by a glass electrode study at ph
5.5-
7.2. J Biol Chem 1999;274 (43): 30387-92
Behera BK, and Misra BN. Analysis of the effect of industrial effluent on
pigments,
proteins, nucleic acids, and the 2,6-dichlorophenol indophenol Hill reaction
of rice
seedlings. Environ Res. 1983 Aug;31(2):381-9.
Maxwell K. and Johnson GN. Chlorophyll fluorescence -- a practical guide. J
Exp Bot.
2000 Apr,51(345):659-68. Review.
Furling D., Ghribi 0., Lahsaini A. Mirault ME, Massicotte G. Impairment of
synaptic
transmission by transient hypoxia in hippocampal slices: improved recovery in
glutathione peroxidase transgenic mice.Proc Natl Acad Sci U S A. 2000
11;97(8):4351-6.
Engvall, E. (1980). Enzyme Immunoassay ELISA and EMIT. Methods in
Enzymology 70 , 419-439.
Starch M.J., Lohmann-Matthes, M.L. (1984). A new rapid method for Ig class and
...
J. Immunol. Methods 68, 304-309.
Claassen E, Kors,N., VanRooijen N. (1987). Immunology 60 , 509-515.
SUBSTITUTE SHEET (RULE 26)