Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02394611 2008-12-10
30517-176
-1-
Process for the preparation of 1,3-disubstituted 2-nitrolzuanidines
The present invention relates to a novel type of process for the preparation
of 1,3-
disubstituted 2-nitroguanidines.
EP-A-0 483 062 discloses a process for the preparation of 1,3-disubstituted
2-nitroguanidines. They are obtained by hydrolysis of corresponding 2-
nitroimino-
1,3,5-triazacyclohexane derivatives. The hydrolysis is preferably carried out
in the
presence of strong mineral acids or organic acids.
Disadvantages of this process are the long reaction times and the formation of
secon-
dary products, which make it necessary to subject the desired end-products to
a com-
plex cleaning operation.
Moreover, as is known, when working in the presence of aqueous, strong acids,
costly measures must be taken to protect, for example, the reactors, from
corrosion.
Applications JP 03 291 267, JP 10067766 and JP 10147580 relate to similar proc-
esses.
The object of the present invention was to provide an improved process for the
preparation of 1,3-disubstituted 2-nitroguanidines.
CA 02394611 2008-12-10
30517-176
- la -
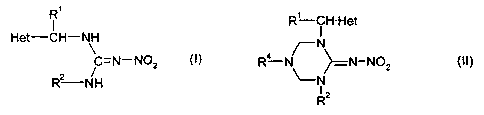
The present invention provides a process for the
preparation of a compound of the formula (I)
Ri
I
Het-CH-NH
~
(1)
C=N-N02
R2-NH
wherein
R' is hydrogen or alkyl,
Rz is hydrogen, alkylp cycloalkyl or -CH2R3,
R3 is alkenyl, alkinyl, aryl or heteroaryl each of
which is optionally substituted,
Het is an unsubstituted or substituted aromatic,
non-aromatic, monocyclic or bicyclic heterocyclic radical,
characterized in that a compound of the formula (II)
R'-CH- Het
I
N
R4-N >__ N-NOz (II)
\-N
R2
wherein
R1, Rz and Het are as defined above, and
R4 is alkyl, cycloalkyl, aryl, arylalkyl or
heterocyclylalkyl, each of which may be unsubstituted or
substituted,
is reacted with anhydrous hydrogen chloride under anhydrous
conditions or with one or more compounds which can generate
CA 02394611 2009-06-12
30517-176
- lb -
hydrogen chloride in the presence of a protic solvent, in
the presence or absence of a diluent.
The present invention provides a process for the
preparation of compounds of the formula (I)
R'
I
Het-CH-NH
C=N-N02 (I)
R2-NH
in which
Le A 34 117-Foreign Countries
-2-
R' is hydrogen or alkyl,
R2 is hydrogen, alkyl, cycloalkyl or -CH2R3,
R3 is alkenyl, alkinyl, or aryl or heteroaryl, each of which is optionally
substituted,
Het is an unsubstituted or substituted aromatic or non-aromatic, monocyc-
lic or bicyclic heterocyclic radical, preferably from the series
~~
~ " `N~
~ ~ ~ N,
N N
N I N I I U I I
0 s O s O
~ N `II N ~
~
(
N` O N'S J L0N k S,N
characterized in that a compound of the formula (II)
R'-CH-Het
I
R4 /_~N-N02 (II)
\-N
N
12
R
in which
Rl, R2 and Het are as defined above, and
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-3-
R4 is alkyl, cycloalkyl, aryl, arylalkyl or heterocyclylalkyl, each of which
may be unsubstituted or substituted,
is reacted with anhydrous hydrogen chloride or with one or more compounds
which can generate hydrogen chloride in the presence or absence of a diluent.
The compounds of the formula (I) can also be in the form of double-bond
isomers as
regards the -N=C(2) bond and in their tautomeric forms (formulae la, Ib):
R~
NH-CH-Het ~
NH-CH-Het
O2N-NH-~ 1a) ~ O2N-NH-C
NH ( N (Ib)
R 2 RZ
Formula (I) is accordingly to be taken to mean that it also includes the
corresponding
double-bond isomers and the formulae (Ia) and (Ib).
Surprisingly, the process according to the invention produces, selectively and
in high
yields, the end-products of the formula (I) in pure form after a short
reaction time
under mild reaction conditions.
For example, using 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-
methyl-
1,3,5-triazacyclohexane as starting material, the course of the process
according to
the invention can be shown by the following equation:
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-4-
N N
CH~,--CI CI---\ --CH~IH NHCH3
N S HCl(9) S
CH2 NL- >-N NO Toluol NO
I Z z
CH3
The compounds required as starting materials for the process according to the
inven-
tion are generally defined by the formula (II).
Preferred substituents and ranges of the radicals listed in the formulae
mentioned
above and below are illustrated below:
R1 is preferably hydrogen or Cl-C4-alkyl,
R2 is preferably hydrogen, C1-C6-alkyl, C3-C6-cycloalkyl or -CH2R3,
R3 is preferably C2-C5-alkenyl, C2-C5-alkinyl, phenyl, cyanophenyl, nitro-
phenyl, halogenophenyl having from 1 to 3 halogen atoms, phenyl substituted
by C1-C3-alkyl, Cl-C3-halogenoalkyl having from 1 to 7 halogen atoms, C1-
C3-alkoxy or C1-C3-halogenoalkoxy having from 1 to 7 halogen atoms,
3-pyridyl, 5-thiazolyl, 5-thiazolyl substituted by one to two (preferably one)
substituents from the group consisting of C1-C3-alkyl, C1-C3-halogenoalkyl
having from 1 to 7 halogen atoms, cyclopropyl, halogenocyclopropyl, C2-C3-
alkenyl, C2-C3-alkinyl, C1-C3-alkoxy, C2-C3-halogenoalkenyl having from 1
to 4 halogen atoms, C2-C3-halogenoalkinyl having from 1 to 3 halogen at-
oms, C1-C3-halogenoalkoxy having from 1 to 7 halogen atoms, C1-C3-alkyl-
thio, C1-C3-halogenoalkylthio having from 1 to 7 halogen atoms, allyloxy,
propargyloxy, allylthio, propargylthio, halogenoallyloxy, halogenoallylthio,
halogen, cyano and nitro; or 3-pyridyl substituted by one to four (preferably
one) radicals from the group consisting of C1-C3-halogenoalkyl having from
1 to 7 halogen atoms, cyclopropyl, halogenocyclopropyl, C2-C3-alkenyl, C2-
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-5-
C3-alkinyl, C2-C3-halogenoalkenyl having from 1 to 4 halogen atoms, C2-C3-
halogenoalkinyl having from 1 to 3 halogen atoms, C1-C3-halogenoalkoxy
having from 1 to 7 halogen atoms, C1-C3-alkylthio, C1-C3-halogenoalkylthio
having from 1 to 7 halogen atoms, allyloxy, propargyloxy, allylthio, propar-
gylthio, halogenoallyloxy, halogenoallylthio, cyano, nitro, C1-C3-alkyl, C1-
C3-alkoxy and halogen,
Het is preferably an unsubstituted or substituted aromatic or nonaromatic,
mono-
cyclic or bicyclic heterocyclic radical, preferably from the series
N N
~N I
N)
N N
p g O s O
N II N
~ II
CN S,N
O
which, also depending on the type of heterocycle, can contain one or two sub-
stituents from the group consisting of C1-C3-halogenoalkyl having from 1 to 7
halogen atoms, cyclopropyl, halogenocyclopropyl having from 1 to 3 halogen
atoms, C2-C3-alkenyl, C2-C3-alkinyl, C2-C3-halogenoalkenyl having from 1 to
4 halogen atoms, C2-C3-halogenoalkinyl having from 1 to 3 halogen atoms,
C1-C3-halogenoalkoxy having from 1 to 7 halogen atoms, C1-C3-alkylthio,
Ct-C3-halogenoalkylthio having from 1 to 7 halogen atoms, allyloxy, propar-
gyloxy, allylthio, propargylthio, halogenoallyloxy, halogenoallylthio, cyano,
nitro, Ct-C3-alkyl, C1-C3-alkoxy and halogen.
CA 02394611 2002-06-18
CA 02394611 2008-12-10
30517-176
-6-
R4 is preferably C1-Clp-alkyl, C3-C6-cycloalkyl, C1-Clp-alkyl substituted by
from 1 to 6 radicals from the group consisting of halogen, hydroxyl, CI-C4-
alkoxy, C1-C4-halogenoalkoxy having from 1 to 9 halogen atoms, di-(C1-C4-
alkyl)-amino and Cl-C5-alkoxycarbonyl, C3-C6-cycloalkyl substituted by
from 1 to 4 radicals from the series C1-C4-alkyl and halogen, phenyl, benzyl,
or phenyl or benzyl substituted by from I to 3 ring substituents from the
group consisting of halogen, Ct-C4-alkyl, C1-C4-halogenoalkyl having from I
to 9 halogen atoms, Ct-C4-alkoxy, C1-C4-halogenoalkoxy having from 1 to 9
halogen atoms, C1-C4-alkylthio, nitro or cyano, or heterocyclylmethyl where
heterocyclyl is an unsaturated or saturated 5- or 6-membered heterocycle
having one or two (preferably one) heteroatoms from the series nitrogen,
oxygen and sulphur (in particular furyl, tetrahydrofuryl, thienyl or pyridyl
R1 is particularly preferably hydrogen, methyl, ethyl, n- or i-propyl,
R2 is particularly preferably hydrogen, methyl, ethyl, n-propyl, i-propyl or n-
butyl, cyclopropyl, cyclopentyl, cyclohexyl or -CH2R3,
R3 is particularly preferably C2-C5-alkenyl, C2-C5-alkinyl, phenyl,
cyanophenyl,
nitrophenyl, halogenophenyl having from 1 to 3 halogen atoms, phenyl sub-
stituted by C1-C3-alkyl, C1-C3-halogenoalkyl having from 1 to 7 halogen at-
oms, CI-C3-alkoxy or C1-C3-halogenoalkoxy having from I to 7 halogen at-
oms, 3-pyridyl, 5-thiazolyl, or 5-thiazolyl substituted by one or two (prefer-
ably one) substituents from the group consisting of C1-C3-alkyl, C1-C3-halo-
genoalkyl having from 1 to 7 halogen atoms, cyclopropyl, halogenocyclo-
propyl, C2-C3-alkenyl, C2-C3-alkinyl, Cl-C3-alkoxy, C2-C3-halogenoalkenyl
having from 1 to 4 halogen atoms, C2-C3-halogenoalkinyl having from 1 to 3
halogen atoms, C1-C3-halogenoalkoxy having from 1 to 7 halogen atoms,
Ci-C3-alkylthio, Cl-C3-halogenoalkylthio having from 1 to 7 halogen atoms,
allyloxy, propargyloxy, allylthio, propargylthio, halogenoallyloxy, halo-
genoallylthio, halogen, cyano or nitro; or 3-pyridyl substituted by one to two
Le A 34 117-Foreign Countries
-7-
(preferably one) radicals from the group consisting of C1-C3-halogenoalkyl
having from 1 to 7 halogen atoms, cyclopropyl, halogenocyclopropyl, C2-C3-
alkenyl, C2-C3-alkinyl, C2-C3-halogenoalkenyl having from 1 to 4 halogen at-
oms, C2-C3-halogenoalkinyl having from 1 to 3 halogen atoms, C1-C3-halo-
genoalkoxy having from 1 to 7 halogen atoms, C1-C3-alkylthio, C1-C3-halo-
genoalkylthio having from 1 to 7 halogen atoms, allyloxy, propargyloxy,
allylthio, propargylthio, halogenoallyloxy, halogenoallylthio, cyano, nitro,
C1-C3-alkyl, Cl-C3-alkoxy or halogen.
Het is particularly preferably an unsubstituted or mono- or disubstituted
(prefer-
ably monosubstituted) heterocyclic radical from the series
~N
nN~ NN N~ / N /
N
i ~ I kt0 s o s o
ix"
N` N~S L0N S1N
O
in particular from the series
kg
~ (E::] where the substituents are chosen from the series fluorine, chlorine,
bromine,
methyl, ethyl, methoxy and ethoxy.
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-8-
R4 is particularly preferably Ct-Ca-alkyl optionally substituted by halogen
(in
particular fluorine or chlorine), or C3-C6-cycloalkyl, phenyl, phenyl-C1-C4-
alkyl or heterocyclylmethyl, each of which may be substituted by halogen (in
particular fluorine or chlorine) or C1-C4-alkyl, where heterocyclyl [lacuna]
an
unsaturated or saturated 5- or 6-membered heterocycle having one or more
heteroatoms from the series nitrogen, oxygen and sulphur (in particular thi-
enyl, pyridyl, furyl or tetrahydrofuryl).
Rl is very particularly preferably hydrogen, methyl or ethyl,
R2 is very particularly preferably hydrogen, methyl, ethyl, n-propyl, i-
propyl,
n-butyl, cyclopropyl, cyclopentyl, cyclohexyl or a radical -CH2R3,
R3 is very particularly preferably C2-C3-alkenyl, C2-C3-alkinyl, phenyl,
cyanophenyl, nitrophenyl, halogenophenyl having from 1 to 3 halogen atoms,
phenyl substituted by C1-C3-alkyl, C1-C3-halogenoalkyl having from 1 to 7
halogen atoms, Ci-C3-alkoxy or Cl-C3-halogenoalkoxy having from 1 to 7
halogen atoms, 3-pyridyl, 5-thiazolyl, 5-thiazoyl or 3-pyridyl substituted in
each case by one or two (preferably one) substituents from the group con-
sisting of Cl-C3-alkyl, C1-C3-halogenoalkyl having from 1 to 7 halogen at-
oms, C1-C3-alkoxy, C1-C3-halogenoalkoxy having from 1 to 7 halogen atoms,
Cl-C3-alkylthio, C1-C3-halogenoalkylthio having from 1 to 7 halogen atoms,
halogen, cyano or nitro,
R4 is very particularly preferably C1-C4-alkyl, cyclopropyl, cyclopentyl or
cyclo-
hexyl C1-C4-alkyl substituted by halogen, C3-C6-cycloalkyl substituted in
each case by 1 or 2 radicals from the series methyl, ethyl, fluorine and chlo-
rine, is phenyl, benzyl or phenyl, benzyl, furylmethyl, tetrahydrofurylmethyl,
thienylmethyl or pyridylmethyl in each case substituted by 1 or 2 ring sub-
stituents from the group consisting of methyl, ethyl, fluorine and chlorine.
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-9-
Het is very particularly preferably thiazolyl, pyridyl or tetrahydrofuranyl,
each of
which may be unsubstituted or mono- or disubstituted (in particular mono-
substituted), the substituents being chosen from the series fluorine,
chlorine,
methyl and methoxy,
In the definitions, unless stated otherwise, halogen (atoms) is F, Cl, Br, I,
preferably
F, Cl, Br, particularly preferably F, Cl.
Rl is very particularly preferably hydrogen, methyl or ethyl, especially hydro-
gen,
R2 is very particularly preferably hydrogen, methyl, ethyl, n-propyl,
cyclopropyl,
cyclopentyl, allyl, propargyl or p-chlorobenzyl, especially methyl.
R4 is very particularly preferably methyl, ethyl, n-propyl, cyclopropyl, cyclo-
pentyl, cyclohexyl, phenyl, benzyl or tetrahydrofurylmethyl,
Het is very particularly preferably one of the radicals
I~ CI or O~ =
s '
C
Particularly preferred starting materials for the process according to the
invention are
compounds of the formula (IIa)
N
CH2 \ ~ `CI
I s
R4 N-NO2 (Ila)
N\-N
CH3
in which
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-10-
R4 is methyl, ethyl, cyclopropyl, cyclopentyl, benzyl or
tetrahydrofurylmethyl,
where, of these, methyl, benzyl and tetrahydrofurylmethyl are in turn pre-
ferred.
Particularly preferred starting materials for the process according to the
invention are
compounds of the fonnula (Hb) and (IIc)
H2 CI
R4 _~=N-NO2 (Ilb)
~N
1
CH3
a
1CH2
R4-N_'~=N-NO2 (Ilc)
N
1
CH3
in which
R4 is as defined for the compounds of the formula (IIa).
The end-products of the process according to the invention are, when the
compound
of the formula (IIa) is used, the following compound
N
CI~~CH2 NH NHCH
S y 3
N
NOz
CA 02394611 2002-06-18
= Le A 34 117-Foreign Countries
-11-
when the compound of the formula (IIb) is used, the following compound
CI LX)_CHi-NH NHCH3
y
N
\ ~
NO2
and when the compound of the formula (IIc) is used, the following compound
CHZ NH NHCH
O y
3 N
\
NO2
The radical definitions and explanations given in general terms above or
listed in the
preferred ranges can be combined with one another as desired, i.e. also
between the
respective ranges and preferred ranges. They apply to the end-products and
also to
the precursors and intermediates.
The term alkyl also means here the branched isomers, e.g. t-butyl for C4-
alkyl.
Preference is given to using those compounds of the formula (II) which have a
com-
bination of the preferred meanings given above in the process according to the
in-
vention.
Particular preference is given to using those compounds of the formula (II)
which
have a combination of the particularly preferred meanings given above in the
process
according to the invention.
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-12-
Very particular preference is given to using those compounds of the formula
(II)
which have a combination of the very particularly preferred meanings given
above in
the process according to the invention.
The starting materials of the formula (II) are known or can be prepared by
known
processes (cf. EP-A-0 483 062, JP-03 291 267, EP-A-0 483 055, EP-A-0 428 941,
EP-A-0 386 565, WO 98/42690).
The process according to the invention is carried out by reacting the
compounds of
the formula (II) with anhydrous hydrogen chloride or with compounds which are
able
to generate hydrogen chloride with protic solvents, in particular with
alcohols or
carboxylic acids.
The compounds which can generate hydrogen chloride include, for example, acid
chlorides in particular compounds of the formula (III) (Group A):
~O
R5-C~ (III)
CI
in which
R5 alkyl, cycloalkyl, aryl, arylalkyl or heterocyclylalkyl, each of which may
be
substituted.
RS is preferably C1-Clo-alkyl, C3-Clo-cycloalkyl, aryl-C1-C4-alkyl, in
particular
phenyl-C1-C4-alkyl, each of which may be mono- or polysubstituted, where
suitable substituents are OH, SH, halogen, C1-C6-alkoxy, C1-C6-alkylthio,
Cl-C6-alkyl and aryl, in particular phenyl, or is heterocyclylmethyl, where
heterocyclyl is an unsaturated or saturated 5- or 6-membered heterocycle
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-13-
having one or two (preferably one) heteroatoms from the series nitrogen,
oxygen, sulphur, in particular furyl, tetrahydrofiuyl, thienyl or pyridyl.
Here, halogen (atoms) is preferably F, Cl, Br, I, in particular F, Cl, Br and
especially
F, Cl.
R5 is particularly preferably Cl-C4-alkyl, C3-C6-cycloalkyl, benzyl or phen-
ylethyl, each of which may be monosubstituted to pentasubstituted (prefer-
ably monosubstituted to trisubstituted, particularly preferably monosubsti-
tuted or disubstituted), where suitable substituents are OH, Cl, Br, F, C1-C4-
alkoxy, C1-C4-alkylthio, Cl-C4-alkyl and aryl, in particular phenyl; or hetero-
cyclylmethyl, where heterocyclyl is, in particular furyl, tetrahydrofuryl, thi-
enyl or pyridyl.
R5 is very particularly preferably the respective radical R4 of the compound
of
the formula (II) to be reacted or one of the radicals from the series benzyl,
HO-CH2-CH2-, n-hexyl or cyclohexyl.
The compounds of the formula (III) are known and are available commercially or
can
be readily prepared by known methods.
The compounds which are able to generate hydrogen chloride also include (Group
B):
reactive nonmetal and metal chlorides, and also reactive nonmetal and metal
oxy-
chlorides, preferably boron trichloride, aluminium trichloride, silicon
tetrachloride,
oxalyl chloride, trichlorosilane, phosphorus trichloride, phosphorus
pentachloride,
phosphorus oxychloride, sulphur dichloride, titanium tetrachloride, titanium
trichlo-
ride, vanadium trichloride, vanadium(V) oxytrichloride, thionyl chloride and
sul-
phuryl chloride, particularly preferably aluminium chloride, phosphorus
trichloride,
phosphorus pentachloride, phosphorus oxychloride, thionyl chloride, sulphuryl
chlo-
CA 02394611 2002-06-18
CA 02394611 2008-12-10
30517-176
-14-
ride and oxalyl chloride, very particularly preferably thionyl chloride,
sulphuryl chlo-
ride, phosphorus oxychloride and oxalyl chloride.
The compounds of group B are known compounds which are available commercially
as such or can be prepared in a known manner.
The process according to the invention is optionally carried out in the
presence of a
diluent.
Suitable diluents when using hydrogen chloride are organic solvents, polar
protic
solvents, such as methanol, ethanol, n-propanol, i-propanol, n-butanol or i-
butanol,
and also polar aprotic solvents, for example acetone, acetonitrile and acetic
ester (e.g.
ethyl acetate), ethers and cyclic ethers, such as diethyl ether, diisobutyl
ether, THF,
dioxane, or nonpolar, aprotic solvents, such as hydrocarbons, for example
benzene,
toluene or xylene, halogenated hydrocarbons, such as methylene chloride,
chloro-
form, tetrachloromethane, chlorobenzene or o-dichlorobenzene.
It is also possible to use mixtures of said diluents.
Suitable diluents when using compounds of group (A) or (B) are polar, protic
sol-
vents, for example alcohols or carboxylic acids.
Particularly suitable are alcohols, in particular methanol, ethanol, n-
propanol, i-
propanol, n-butanol, i-butanol.
It may be advantageous to add another diluent to the reaction mixture.
Suitable sol-
vents are ethers, for example dibutyl ether, THF, dioxane, glycoldimethylether
or
diglycoldimethylether, and also hydrocarbons, such as benzene, toluene or
xylene,
halogenated hydrocarbons, such as methylene chloride, chloroform, tetra-
chloromethane, chlorobenzene or o-dichlorobenzene, nitriles, such as
acetonitrile,
Le A 34 117-Foreign Countries
-15-
carboxylic esters, such as ethyl acetate or also ketones, such as acetone or
methyl
isopropyl ketone.
Mixtures of said diluents may also be used.
The process according to the invention is generally carried out at
temperatures be-
tween 0 C and 200 C, preferably between 40 C and 150 C.
The process is preferably carried out under atmospheric pressure and,
particularly in
the case of low-boiling diluents, it can optionally also be carried out under
increased
pressure.
The anhydrous hydrogen chloride and the compounds of the formula (III) or of
group
B are generally used in a molar ratio of from 0.5:1 to 10:1, preferably 1:1 to
5:1,
based on the starting compound of the formula (II).
The reaction is generally carried out by heating the starting material of the
formula
(II), and the hydrogen chloride or the compound of the formula (III) or (IV),
option-
ally in a diluent and optionally in a solvent, to the desired temperature. It
is also pos-
sible to meter in successively the hydrogen chloride or the compound of the
formula
(III) or of group B over the course of the reaction.
To work-up, after cooling, water is optionally added, and the end-product,
optionally
after evaporating the mixture, is isolated, for example by filtration or
extraction.
The reaction is preferably carried out in a diluent from which, when the
reaction
mixture is cooled, the end product can be directly crystallized out and
isolated in a
simple manner, for example by filtration. Suitable diluents for this purpose
are alco-
hols, in particular methanol, ethanol, propanol, i-propanol, isobutanol, n-
butanol, sec-
butanol.
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-16-
It is also possible to work up the reaction mixture without water by, when the
reac-
tion is complete, distilling off the diluent where appropriate and the solvent
where
appropriate and extracting the residue which remains with a suitable
extractant. Suit-
able extractants are, in principle, all solvents which are inert with respect
to the end-
products and in which the end-products are sufficiently soluble.
Examples thereof include aliphatic hydrocarbons, such as n-pentane, n-hexane,
cyclohexane, halogenated aliphatic hydrocarbons, such as methylene chloride or
chloroform, aromatic hydrocarbons, such as benzene, toluene or xylene,
halogenated
aromatic hydrocarbons, such as chlorobenzene or o-dichlorobenzene or else
ethers,
such as, for example, methyl tert-butyl ether.
The end-products crystallize out, optionally after evaporating off the
extractant, and
can be isolated by filtration, or the extractant is completely or virtually
completely
removed and, if necessary, the residue is purified, for example by
recrystallization.
The compounds of the formula (I) prepared according to the invention are
useful ac-
tive ingredients in pest control. In particular, the compounds of the formula
(I) are
suitable for controlling insects and arachnids, which are encountered in
useful and
ornamental plants in agriculture, in particular, cotton, vegetable and fruit
plantations,
in forests, in the protection of stored products and materials and in the
hygiene sec-
tor, in particular on pets and useful animals (see e.g. EP-A-0 376 279, EP-A-
0 375 907, EP-A-0 383 091).
CA 02394611 2002-06-18
= Le A 34 117-Foreign Countries
-17-
Examnles
Example 1
Preparation of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-3-methylguanidine
N H H
CI--~:~'N N-CH3
S 11
\N+/O
O
a) 19.2 g of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-methyl-
1,3,5-triazacyclohexane
:~~N rN)
CIN-CH3
S Y
N+~O
I
O-
are dissolved in 100 ml of anhydrous toluene, and, at 65 C, dry HCl gas is
introduced. The mixture is then stirred for five hours at 65 C and the product
is isolated by filtration. The crystals are then dried.
Yield: 16.2 g, purity (HI'LC): 75%, selectivity: 99%
The crude product is stirred with 50 ml of butanol at 50 C, and the solid is
filtered off at 25 C and dried.
Yield: 10.5 g, purity (HPLC): 98%
CA 02394611 2002-06-18
=' Le A 34 117-Foreign Countries
-18-
According to its chromatographic and spectroscopic data, the product is iden-
tical to an authentic sample of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-3-
methylguanidine obtained by another route.
b) 19.2 g of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-methyl-
1,3,5-triazacyclohexane are dissolved in 100 ml of anhydrous acetone, and, at
20 C, dry HCl gas is introduced. The mixture is then stirred for five hours at
20 C and the product is isolated by filtration. The crystals are then dried.
Yield: 19.8 g, purity (HPLC): 62%, selectivity: 99%
c) 19.2 g of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-methyl-
1,3,5-triazacyclohexane are dissolved in 100 ml of anhydrous ethyl acetate,
and, at 65 C, dry HCl gas is introduced. The mixture is then stirred for five
hours at 65 C and the product is isolated by filtration. The crystals are then
dried.
Yield: 16.5 g, purity (HPLC): 75%, selectivity: 99%
d) 19.2 g of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-methyl-
1,3,5-triazacyclohexane are dissolved in 100 ml of anhydrous methanol, and,
at 20 C, dry HCl gas is introduced. The mixture is then stirred for one hour
at
20 C and the product is isolated by filtration. The crystals are then dried.
Yield: 14.2 g, purity (IPLC): 79%, selectivity: 91%
e) 19.2 g of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-methyl-
1,3,5-triazacyclohexane are dissolved in 100 ml of anhydrous methanol. At
40 C, 5.1 g of acetyl chloride are added dropwise over 20 minutes, and the
mixture is then stirred for three and a half hours at 40 C. The resulting sus-
pension is cooled to 0-5 C and the product is isolated by filtration. The crys-
tals are then dried.
Yield: 11.1 g, purity (HPLC): 99%
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-19-
f) 19.2 g of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-methyl-
1,3,5-triazacyclohexane are dissolved in 100 ml of anhydrous methanol. At
25 C, 7.7 g of thionyl chloride are added dropwise over 15 min, and the
mixture is then stirred for three hours at this temperature. The resulting sus-
pension is cooled to 0-5 C and the product is isolated by filtration. The crys-
tals are then dried.
Yield 10.8 g, purity (HPLC): 98%
g) 19.2 g of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-methyl-
1,3,5-triazacyclohexane are dissolved in 100 ml of anhydrous methanol. At
25 C, 8.3 g of oxalyl chloride are added dropwise over 25 min, and the mix-
ture is then stirred for five hours at 40 C. The resulting suspension is
cooled
to 0-5 C and the product is isolated by filtration. The crystals are then
dried.
Yield 10.4 g, purity (HPLC): 98%
h) 19.2 g of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-methyl-
1,3,5-triazacyclohexane are dissolved in 100 ml of anhydrous methanol. At
C, 6.3 g of methyl chloroformate are added dropwise over 10 min, and the
mixture is then stirred for five hours at 40 C. The resulting suspension is
20 cooled to 0-5 C and the product is isolated by filtration. The crystals are
then
dried.
Yield 10.9 g, purity (BPLC): 98%
i) 19.2 g of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-methyl-
25 1,3,5-triazacyclohexane are dissolved in 100 ml of anhydrous methanol. At
25 C, 7.3 g of chloroacetyl chloride are added dropwise over 10 min, and the
mixture is then stirred for five hours at 40 C. The resulting suspension is
cooled to 0-5 C and the product is isolated by filtration. The crystals are
then
dried.
Yield 10.9 g, purity (HPLC): 98%
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-20-
j) 19.2 g of 1-(2-chlorothiazol-5-ylmethyl)-2-nitro-imino-5-benzyl-3-methyl-
1,3,5-triazacyclohexane are dissolved in 100 ml of anhydrous methanol. At
25 C, 10 g of phosphorus oxychloride are added dropwise over 30 min, and
the mixture is then stirred for one hour at 40 C. The resulting suspension is
cooled to 0-5 C and the product is isolated by filtration. The crystals are
then
dried.
Yield: 9.1 g, purity: 97.1 %
Using a similar method, it is also possible to obtain the compounds of the
formula (1)
given in the table below:
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-21-
Table
Example Het Rl R2
No.
2 I CI Ni H H
3 N,, H -CH3
CI
4 CI N H -C2H5
CI N H -C3H7(n)
6 CI N H --<
7 CI N H -C4H9(n)
8 CI Ni H -CH(CH3)2
-CH ~ \\
9 H 2
-
CI N
_CH2 ( \
H i
CI N N
_CH2 ~ \
H
i
11
CI N N CI
CA 02394611 2002-06-18
. Le A 34 117-Foreign Countries
-22-
Table (continuation)
Example Het R1 R2
No.
-CH2 ~ ~ CI
12 H
CI N
13 ~ CI N -CH3 -CH3
14 ( i CI N -CH3 -C2H5
15 CI -CH3 ---<
16 I i CI N -CH3 -C3H7(n)
17 CI N -C2H5 -CH3
18 I i CI N -C2H5 -C2H5
19 CI -C2H5
20 CI ~ H H
~N
21 CI____J_ I H CH3
~~N
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-23-
Table (continuation)
Example Het R1 R2
No.
22 CI~ H -C2H5
N
23 CI-LNf H
24 C 1 H CH2
25 S I H -CH2 ~~ ci
CI--~N
26 CI-__~, ~ CH3 CH3
27 ci - ` ~ C2H5 CH3
~N
28 CI~ ~ CH3 C2H5
N
29 ci ~ CH3
~N
30 CI 1 H -CH2-CH=CH2
CA 02394611 2002-06-18
Le A 34 117-Foreign Countries
-24-
Table (continuation)
Example Het Rl R2
No.
N
31 ~ / H -CH2-C=CH
CI
CI
32 S H -CH2-CH=CH2
CI-~ I
33 ~ H -CH2-C CH
S
O
34 Y H CH3
O
35 CH3 CH3
CA 02394611 2002-06-18