Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
ENERGY EFFICIENT GAS SEPARATION FOR FUEL CELLS
CROSS REFERENCE TO RELATED APPLICATION
This application claims the benefit of Canadian Patent Application No.
2,325,072, filed
October 30, 2000, and U.S. Provisional Application No. 60/323,169, filed
September 17, 2001, the
disclosures of which are incorporated herein by reference.
The present disclosure relates to a fuel cell-based electrical generation
system, which
employs pressure swing adsorption for enhancing the energy efficiency of fuel
cells, particularly high
temperature fuel cells such as molten carbonate and solid oxide fuel cells.
BACKGROUND
Fuel cells provide an environmentally friendly source of electrical current.
One type of high
temperature fuel cell used for generating electrical power, particularly
envisaged for larger scale
stationary power generation, is the molten carbonate fuel cell (MCFC). The
MCFC includes an
anode channel for receiving a flow of hydrogen gas (or a fuel gas which reacts
in the anode channel
to generate hydrogen by steam reforming and water gas shift reactions), a
cathode channel for
receiving a flow of oxygen gas, and a porous matrix containing a molten
carbonate electrolyte which
separates the anode channel from the cathode channel. Oxygen and carbon
dioxide in the cathode
channel react to form carbonate ions, which cross the electrolyte to react
with hydrogen in the anode
channel to generate a flow of electrons. As the hydrogen is consumed, carbon
monoxide is shifted by
steam to generate additional hydrogen. Carbon dioxide and water vapor are
produced in the anode
channel by oxidation of fuel components, and by reduction of carbonate ions
from the electrolyte.
Typical operating temperature of molten carbonate fuel cells is about
600° to about 650°C.
Another type of high temperature fuel cell is the solid oxide fuel cell
(SOFC). The SOFC
includes an anode channel for receiving a flow of hydrogen gas (or a fuel gas
which reacts in the
anode channel to generate hydrogen by steam reforming and water gas shift
reactions), a cathode
channel for receiving a flow of oxygen gas, and a solid electrolyte which is a
ceramic membrane
conductive to oxygen ions and separates the anode channel from the cathode
channel. Oxygen in the
cathode channel dissociates to oxygen ions, which cross the electrolyte to
react with hydrogen in the
anode channel to generate a flow of electrons. As the hydrogen is consumed,
carbon monoxide rnay
be oxidized directly or may be shifted by steam to generate additional
hydrogen. Carbon dioxide and
water vapor are produced in the anode channel by oxidation of fuel components.
Typical operating
temperature of solid oxide fuel cells is about 500° to about
1000°C.
Except in the rare instance that hydrogen (e.g. recovered from refinery or
chemical process
off gases, or else generated from renewable energy by electrolysis of water)
is directly available as
fuel, hydrogen must be generated from fossil fuels by an appropriate fuel
processing system. For
stationary power generation, it is preferred to generate hydrogen from natural
gas by steam reforming
or partial oxidation to produce "syngas" comprising a mixture of hydrogen,
carbon monoxide, carbon
dioxide, steam and some unreacted methane. As hydrogen is consumed in the fuel
cell anode
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
2
channel, much of the carbon monoxide reacts with steam by water gas shift to
generate more
hydrogen and more carbon dioxide. Other carbonaceous feedstocks (e.g. heavier
hydrocarbons, coal,
or biomass) may also be reacted with oxygen and steam to generate syngas by
partial oxidation,
gasification or autothermal reforming. The fuel cell may also be operated on
hydrogen or syngas that
has been generated externally.
A great advantage of MCFC and SOFC systems is that their high operating
temperature
facilitates close thermal integration between the fuel cell and the fuel
processing system. The high
temperature also allows the elimination of noble metal catalysts required by
lower temperature fuel
cells.
Prior art MCFC systems have serious limitations associated with their high
temperature
operation, and with their inherent need to supply carbon dioxide to the
cathode while removing it
from the anode. Prior art SOFC systems face even more challenging temperature
regimes, and are
disadvantaged by the degradation of cell voltages at very high temperatures
under conventional
'
operating conditions.
The lower heat of combustion of a fuel usefully defines the energy (enthalpy
change of the
reaction) that may be generated by oxidizing that fuel. The electrochemical
energy that can be
generated by an ideal fuel cell is however the free energy change of the
reaction, which is smaller
than the enthalpy change. The difference between the enthalpy change and the
free energy change is
the product of the entropy change of the reaction multiplied by the absolute
temperature. This
difference widens at higher temperatures, so higher temperature fuel cell s
inherently convert a lower
fraction of the fuel energy to electrical power at high efficiency, while a
larger fraction of the fuel
energy is available only as heat which must be converted to electrical power
by a thermodynamic
bottoming cycle (e.g. steam or gas turbine plant) at lower efficiency.
Accumulation of reaction products (carbon dioxide and steam) on the fuel cell
anode
opposes the electrochemical reaction, so that the free energy is reduced.
Higher partial pressure of
oxygen and carbon dioxide over the cathode, and higher partial pressure of
hydrogen over the anode,
drive the reaction forward so that the free energy is increased.
Unfortunately, the reaction depletes
the oxygen and carbon dioxide in the cathode channel and depletes hydrogen in
the anode channel
while rapidly increasing the backpressure of carbon dioxide in the anode
channel. Hence the free
energy change is reduced, directly reducing the cell voltage of the fuel
stack. This degrades the
electrical efficiency of the system, while increasing the heat that must be
converted at already lower
efficiency by the thermal bottoming cycle.
The free energy change is simply the product of the electromotive force ("E")
of the cell and
the charge transferred per mole by the reaction ("2F"), where the factor of
two reflects the valency of
the carbonate ion. The following Nernst relation for a MCFC expresses the
above described
sensitivity of the electromotive force to the partial pressures of the
electrochemical reactants in the
anode and cathode channels, where the standard electromotive force ("Eo") is
referred to all
components at standard conditions and with water as vapor.
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
3
E = E - RT In pHZO(anode)'1'coz(anode)
0 0.5
pH 2(anode) 'p02(cathode) 'pC02(cathode)
Prior art MCFC systems do not provide any satisfactory solution for this
problem which
gravely compromises attainable overall efficiency. The challenge is to devise
a method for sustaining
high hydrogen concentration over the anode and high oxygen concentration over
the cathode, while
efficiently transferring hot carbon dioxide from the anode to the cathode.
Despite repeated attempts
to devise an effective carbon dioxide transfer technology that would be
compatible with MCFC
operating conditions, no such attempt has been adequately successful.
The accepted method for supplying carbon dioxide to the MCFC cathode has been
to burn a
fraction of the anode exhaust gas (including unreacted hydrogen and other fuel
components) to
provide carbon dioxide mixed with steam and nitrogen to be mixed with
additional air providing
oxygen to the cathode. This approach has serious limitations. Even more of the
original fuel value is
unavailable for relatively efficient electrochemical power generation, in view
of additional
combustion whose heat can only be absorbed usefully by the thermal bottoming
cycle. Also, the
oxygen/nitrogen ratio of the cathode gas is even more dilute than ambient air,
further reducing cell
voltage and hence transferring more power generation load less efficiently
onto the thermal
bottoming plant.
The following Nernst relation for a SOFC expresses the sensitivity of the
electromotive
force to the partial pressures of the electrochemical reactants in the anode
and cathode channels, with
the simplifying assumption that CO is converted by the water gas shift
reaction. This sensitivity is of
course greatest at the highest working temperatures of SOFC.
E = E - RT In pH20(anode)
0 0.5
ZF pH2(anode)'p02(cathode)
Pressure swing adsorption (PSA) systems are one possibility for providing fuel
gases to a
fuel cell. PSA systems and vacuum pressure swing adsorption systems (VPSA)
separate gas fractions
from a gas mixture by coordinating pressure cycling and flow reversals over an
adsorber or adsorbent
bed which preferentially adsorbs a more readily adsorbed gas component
relative to a less readily
adsorbed gas component of the mixture. The total pressure of the gas mixture
in,the adsorber is
elevated while the gas mixture is flowing through the adsorber from a first
end to a second end
thereof, and is reduced while the gas mixture is flowing through the adsorbent
from the second end
back to the first end. As the PSA cycle is repeated, the less readily adsorbed
component is
concentrated adjacent the second end of the adsorber, while the more readily
adsorbed component is
concentrated adjacent the first end of the adsorber. As a result, a "light"
product (a gas fraction
depleted in the more readily adsorbed component and enriched in the less
readily adsorbed
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
4
component) is delivered from the second end of the adsorber, and a "heavy"
product (a gas fraction
enriched in the more strongly adsorbed component) is exhausted from the first
end of the adsorber.
However, the conventional system for implementing pressure swing adsorption or
vacuum
pressure swing adsorption uses two or more stationary adsorbers in parallel,
with multiple two-way
directional valves at each end of each adsorber to connect the adsorbers in
alternating sequence to
pressure sources and sinks. This system is often cumbersome and expensive to
implement due to the
large size of the adsorbers and the complexity of the valuing required. The
valves would not be
capable of operation at MCFC working temperatures. Further, the conventional
PSA system makes
inefficient use of applied energy because of irreversible gas expansion steps
as adsorbers are
cyclically pressurized and depressurized within the PSA process. Conventional
PSA systems are
bulky and heavy because of their low cycle frequency and consequent large
adsorbent inventory. In
addition, prior art PSA technology may not be capable of operation at such
high temperature. Also,
adsorbents which can separate carbon dioxide in the presence of steam must be
provided for any
anode gas PSA separation working at elevated temperature.
Combined cycle power plants with a gas turbine cycle integrated with a fuel
cell system
have been disclosed. In addition, commonly-assigned PCT Published
International Patent
Application No. WO 00/16425 provides examples of how PSA units may be
integrated with gas
turbine power plants, or with fuel cell power plants having a gas turbine
auxiliary engine.
A further need addressed by the disclosed systems and processes is for
mitigation of global
warming driven by cumulative emissions of carbon dioxide from fossil-fuelled
power generation.
The disclosed systems and processes also address the following environmental
needs:
A. concentrated C02 delivered for disposal or sequestration.
B. substantially complete, elimination of NOx emissions by complete
elimination of
combustion in the presence of nitrogen.
C. high overall efficiency to achieve most sustainable use of energy
resources.
SUMMARY OF THE DISCLOSURE
The disclosed MCFC or SOFC based electrical generation systems address the
deficiencies
of the prior art, in general to manipulate reactant concentrations for
enhanced performance and
economics, and in MCFC systems to transfer carbon dioxide from the anode to
the cathode while
enhancing electrical power output.
According to a first embodiment of the disclosed systems and processes, there
is provided an
electrical current generating system that includes at least one fuel cell
operating at a temperature of at
least about 250°C, a hydrogen gas separation system andlor oxygen gas
delivery system that includes
at least one device selected from a compressor or vacuum pump, and a drive
system for the device
that includes means for recovering energy from at least one of the hydrogen
gas separation system,
oxygen gas delivery system, or heat of the fuel cell. According to a second
embodiment of an
electrical current generating system that also includes a high temperature
fuel cell, a gas turbine
system may be coupled to the hydrogen gas separation system or oxygen gas
delivery system,
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
wherein the gas turbine system is powered by energy recovered from at least
one of the hydrogen gas
separation system, oxygen gas delivery system, or heat of the fuel cell. The
hydrogen gas separation
system or the oxygen gas delivery system may include a pressure swing
adsorption module. These
generating systems are particularly useful with molten carbonate fuel cells
and solid oxide fuel cells.
5 The energy recovery means may include a gas turbine and/or a heat exchanger
that receives
a heated and/or pressurized gas stream from the hydrogen gas separation
system, oxygen gas delivery
system, or fuel cell. For example, a fuel cell heat recovery system may be
coupled to the fuel cell and
to the gas turbine system (in this case, a hydrogen gas separation system is
optional). The energy
recovery means translates the recovered energy into a drive force for
operating the compressor and
vacuum pump. For example, a pressure swing adsorption module could establish a
pressure gradient
in a fuel-containing gas stream under conditions sufficient for separating the
fuel-containing gas
stream into a fuel-enriched gas stream and a fuel-depleted gas stream, and at
least one of the fuel-
enriched gas stream or fuel-depleted gas stream is recirculated to a gas
turbine system coupled to a
compressor and/or vacuum pump to capture the recirculation stream's energy.
Another example is a
fuel cell heat recovery system that transfers heat from the fuel cell to a
heat recovery worleing fluid
that can undergo expansion to power the gas turbine system.
The gas turbine system coupled to the PSA may power all compressors and vacuum
pumps
for the 02 PSA, along with vacuum pump and/or heavy reflux compression for the
H2 PSA. This
auxiliary gas turbine cycle allows a heavy reflux vacuum pump and compressor
to be driven by the
turboexpander which expands the products of hydrogen PSA tail gas combustion.
A feature of
certain disclosed embodiments is integration of vacuum pumps) and/or
compressors with the gas
turbine powered directly or indirectly by tail gas combustion or indirectly by
heat exchange to fuel
cell stack waste heat. Thus, neither an electrical generator coupled to the
thermal bottoming cycle
nor an auxiliary power source is required to power all the compressors and
vacuum pumps for the gas
separation systems. The gas turbine system may also be coupled to an auxiliary
device such as an
electrical current generator that could provide power to a vehicle air
conditioning system. Either
single or multiple spool gas turbine configurations may be considered.
Centrifugal or axial machines
may be used as the compressors and pumps. Approaches based on integration of
gas turbines and
fuel cells are particularly favorable for larger power levels. Free spool gas
generators (e.g.
turbochargers) are used in some economically preferred embodiments.
Thus, there are provided advanced MCFC and SOFC systems incorporating a
pressure
swing adsorption (PSA) and integrated gas turbine system to enrich hydrogen
over the anode while
rapidly separating carbon dioxide (to the cathode for MCFC systems). In
certain systems, the
hydrogen PSA system will operate at high temperatures even approaching that of
the MCFC system.
In one variant of the first or second embodiments described above, the
electrical current
generating system comprises a MCFC or SOFC fuel cell, an oxygen gas delivery
system, and/or a
hydrogen gas delivery system. The fuel cell can include an anode channel
having an anode gas inlet
for receiving a supply of hydrogen gas (or a fuel gas which reacts to form
hydrogen in the anode
channel), a cathode channel having a cathode gas inlet and a cathode gas
outlet, and an electrolyte in
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
6
communication with the anode and cathode channel for facilitating ion
transport between the anode
and cathode channel. The hydrogen gas delivery system may include a hydrogen
PSA system,
including a rotary module having a stator and a rotor rotatable relative to
the stator, for enriching
hydrogen to the anode channel and extracting carbon dioxide therefrom. In some
embodiments, the
electrical current generating system also includes a PSA or VPSA system for
enriching oxygen from
air for supply to the cathode channel and/or to the fuel processing system.
The PSA unit for
enriching hydrogen and separating carbon dioxide will be referred to as the
first PSA unit, while a
second PSA or VPSA unit may be provided for oxygen enrichment.
The rotor of a PSA unit for use in the disclosed systems and processes
includes a number of
flow paths for receiving adsorbent material therein for preferentially
adsorbing a first gas component
in response to increasing pressure in the flow paths relative to a second gas
component. The pressure
swing adsorption system also may include compression machinery coupled to the
rotary module for
facilitating gas flow through the flow paths for separating the first gas
component from the second
gas component. The stator includes a first stator valve surface, a second
stator valve surface, and
plurality of function compartments opening into the stator valve surfaces. The
function
compartments include a gas feed compartment, a light reflux exit compartment
and a light reflux
return compartment.
The hydrogen PSA system may itself operate at a working high temperature. For
example,
the operating temperature of the adsorbers in the first or hydrogen PSA unit
may range from
approximately ambient temperature to an elevated temperature up to about
450°C, as may be
facilitated by recuperative or regenerative heat exchange between the first
PSA unit and the fuel cell
anode channel. According to another variation, the operating temperature of
the adsorbers may range
from about the operating temperature of the MCFC stack (e.g., about 600 to
about 650°C) or SOFC
stack (e.g., about 500 to about 1000°C) down to about 450°C, as
may be facilitated by recuperative or
regenerative heat exchange. In particular embodiments, the operating
temperature of the hydrogen
PSA adsorbers may range from ambient to about 800°C, especially about
150°C to about 800°C for
PSA units that contain catalysts and ambient to 200°C for PSA units
that do not contain catalysts.
This PSA unit may be configured to support a temperature gradient along the
length of the flow
channels, so that the temperature at the second end of the adsorbers is higher
than the temperature at
the first end of the adsorbers. As used herein, "operating temperature of the
adsorbers" denotes the
temperature of a gas flowing through the adsorbers and/or the temperature of
the adsorber beds.
According to a third embodiment, there is disclosed an electrical current
generating system
that includes a MCFC or SOFC, and a H2 PSA coupled to the MCFC or SOFC,
wherein the H2 PSA
includes a first adsorbent and at least one second material selected from a
second adsorbent and a
steam reforming catalyst or water gas shift reaction catalyst. The first
adsorbent is chemically
distinct from the second adsorbent. For example, the adsorbent in the
adsorbers of the first or
hydrogen PSA may include a first zone of adsorbent, which is selective at an
elevated operating
temperature (e.g., about 250°C to about 800°C) for carbon
dioxide in preference to water vapor.
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
7
Suitable such adsorbents known in the art include alkali-promoted materials.
Illustrative alkali-
promoted materials include those containing cations of alkali metals such as
Li, Na, K, Cs, Rb, and/or
alkaline earth metals such as Ca, St, and Ba. The materials typically may be
provided as the
hydroxide, carbonate, bicarbonate, acetate, phosphate, nitrate or organic acid
salt compound of the
alkali or alkaline earth metals. Such compounds may be deposited on any
suitable substrate such as
alumina. Examples of specific materials include alumina impregnated with
potassium carbonate and
hydrotalcite promoted with potassium carbonate. For embodiments of the first
PSA unit operating at
temperatures closer to ambient, suitable adsorbents include alumina gel,
activated carbons,
hydrophilic zeolites (e.g. type 13X zeolite and many other zeolites known in
the art), and
hydrophobic zeolites (e.g. type Y zeolite or silicalite).
In high temperature embodiments of the first or hydrogen PSA unit, the
adsorbent in the
same or another zone of the adsorbers may include a component catalytically
active at the operating
temperature of that zone for the steam reforming reaction (e.g. methane fuel
or methanol fuel) and/or
for the water gas shift reaction. The catalytically active component may be a
reduced transition
group metal or mixture of metals, or may be a transition group metal dispersed
in zeolite cages and
reversibly forming a metal carbonyl complex at the operating temperature of
the second zone.
Because carbon dioxide is preferentially adsorbed relative to steam, while
enriched hydrogen is
continually removed to the anode channel, the concentrations of carbon dioxide
and hydrogen over
the catalytically active component are maintained at a reduced level by the
PSA process so as to shift
the reaction equilibria favorably for the steam reforming and/or wafer gas
shift reactions to proceed
within the adsorbers of the first PSA unit. The conversion of carbon monoxide
and reformable fuel
components is driven toward completion to generate carbon dioxide and
additional hydrogen. This is
an example of a PSA reactor or "sorption enhanced reactor", enhancing the
simple gas separation
effect to further generate enriched hydrogen while removing the carbon dioxide
and driving the water
gas shift reaction substantially to completion while achieving adequate
purification of the hydrogen.
Industrial H2 PSA is normally conducted at considerably elevated pressures (>
10 bars) to
achieve simultaneous high purity and high recovery (~ 80%-85%). Fuel cell
systems operating with
pressurized methanol reformers or in integration with gas turbine cycles may
operate at relatively
high pressures. Molten carbonate fuel cells operate at pressures from
atmospheric up to about at most
10 bars, with lower pressures strongly preferred at present as required to
achieve extended stack life.
Solid oxide fuel cells may be designed to operate at any pressure, with
working pressures of about 5-
20 bars being preferred in the present invention.
The pressure of the light product gas exiting from the hydrogen PSA and oxygen
PSA may
vary widely in the disclosed systems and processes. Compressors or other
pressure-increasing
mechanisms may be employed to boost the light product gas pressure if
necessary prior to
introduction into the fuel cell. At very low feed pressures (e.g., 2-3 bars),
the first PSA may utilize
supplemental compression to achieve higher recovery of hydrogen and
simultaneously higher
concentration of carbon dioxide. Alternative approaches include vacuum pumping
to widen the
working pressure ratio, or alternatively "heavy reflux" which is recompression
and recycle to the
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
PSA feed of a fraction of its exhaust stream at full pressure. Vacuum and
heavy reflux options may
be combined by using an oversized vacuum pump. .
The disclosed systems and processes can improve overall efficiency of fuel
cell systems to
reduce the proportionate amount of carbon dioxide formed, while enabling
delivery as and when
desired of that carbon dioxide in highly concentrated form for most convenient
sequestration from the
atmosphere, e.g. by underground disposal in depleted natural gas reservoirs or
for enhanced oil
recovery from petroleum reservoirs. In addition, exported power may be
delivered only from the fuel
cell stack, thus there is no export of power from a thermal bottoming cycle,
or generators and
associated gear boxes on the thermal bottoming turbines which are thus reduced
to simple
turbochargers. Instead, according to certain embodiments, the system utilizes
high grade waste heat
from the fuel cell stack to drive free rotor turbochargers as required for
feed air compression, vacuum
pumping of exhaust nitrogen-enriched air, and heavy reflux compression of
carbon dioxide enriched
anode tail gas; with the stack waste heat matched to these auxiliary loads so
as to facilitate operation
at high current density.
The fuel cell stack can run at relatively high current density (e.g., about
200 to about 400
mA/cm2) to generate the required amount of waste heat for the auxiliary
compression loads, since the
disclosed PSA systems have dramatically raised open circuit voltages (e.g.,
about 0.75 to about 0.95
volts). The necessary size of the fuel cell stack size per kW can be reduced
greatly at high current
density. Equivalently, the same size fuel cell stack can achieve the full
power output formerly
achieved by the stack plus the thermal bottoming generator, which can be
eliminated in certain
disclosed embodiments.
The foregoing features and advantages will become more apparent from the
following
detailed description of several embodiments that proceeds with reference to
the accompanying
figures.
BRIEF DESCRIPTION OF THE DRAWINGS
Certain embodiments are described below with reference to the following
figures:
FIG. 1 shows an axial section of a rotary PSA module.
FIGS. 2 through SB show transverse sections of the module of FIG. 1.
FIGS. 6 through 9 show simplified schematics of alternative MCFC cell plants
embodiments.
FIGS. 10 through 14 show simplified schematics of alternative SOFC cell plants
embodiments.
~35 Detailed Description of SEVERAL Embodiments
FIGS. 1-5
An oxygen-enrichment rotary PSA module is described below in connection with
FIGS. 1-
SB, but the same or similar rotary PSA module configuration could be used for
hydrogen enrichment
(i.e., separation) in the disclosed electrical current generating systems. As
used herein, a "rotary
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
9
PSA" includes, but is not limited to, either a PSA wherein an array of
adsorbers rotates relative to a
fixed valve face or stator or a PSA wherein the valve face or stator rotates
relative to an array of
adsorbers.
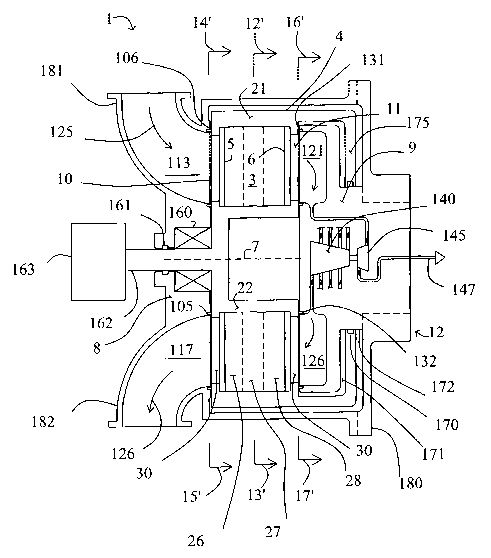
FIG. 1 shows a rotary PSA module 1, which includes a number "N" of adsorbers 3
in
adsorber housing body 4. Each adsorber has a first end 5 and a second end 6,
with a flow path
therebetween contacting a nitrogen-selective adsorbent (for oxygen
enrichment). The adsorbers are
deployed in an axisymmetric array about axis 7 of the adsorber housing body.
The housing body 4 is
in relative rotary motion about axis 7 with first and second functional bodies
8 and 9, being engaged
across a first valve face 10 with the first functional body 8 to which feed
gas mixture is supplied and
from which the heavy product is withdrawn, and acxoss a second valve face 11
with the second
functional body 9 from which the light product is withdrawn.
In embodiments as particularly depicted in FIGS. 1-5, the adsorber housing 4
rotates and
shall henceforth be referred to as the adsorber rotor 4, while the first and
second functional bodies are
stationary and together constitute a stator assembly 12 of the module. The
first functional body shall
henceforth be referred to as the first valve stator 8, and the second
functional body shall henceforth be
referred to as the second valve stator 9. In other embodiments, the adsorber
housing 4 may be
stationary, while the first and second functional bodies are rotary
distributor valve rotors.
In the embodiment shown in FIGS. I-5, the flow path through the adsorbers is
parallel to
axis 7, so that the flow direction is axial, while the first and second valve
faces are shown as flat
annular discs normal to axis 7. However, more generally the flow direction in
the adsorbers may be
axial or radial, and the first and second valve faces may be any figure of
revolution centred on axis 7.
The steps of the process and the functional compartments to be defined will be
in the same angular
relationship regardless of a radial or axial flow direction in the adsorbers.
FIGS. 2-5 are cross-sections of module 1 in the planes defined by arrows 12'-
13', 14'-15',
and I6'-1T. Arrow 20 in each section shows the direction of rotation of the
rotor 4.
FIG. 2 shows section 12'-13' across FIG.1, which crosses the adsorber rotor.
Here, "N"=72.
The adsorbers 3 are mounted between outer wall 21 and inner wall 22 of
adsorber wheel 208. Each
adsorber comprises a rectangular flat pack 3 of adsorbent sheets 23, with
spacers 24 between the
sheets to define flow channels here in the axial direction. Separators 25 are
provided between the
adsorbers to fill void space and prevent leakage between the adsorbers.
As shown in FIG. 1, the adsorbers 3 may include a plurality of distinct zones
between the
first end 5 and the second end 6 of the flow channels, here shown as three
zones respectively a first
zone 26 adjacent the first end 5, a second zone 27 in the middle of the
adsorbers, and a third zone 28
adjacent the second end 6. As an alternative to distinct zones of adsorbents,
the different adsorbents
may be provided in layers or mixtures that include varying gradients of
adsorbent concentrations
along the gas flow path. The transition from one adsorbent to another may also
be a blended mixture
of the two adsorbents rather than a distinct transition. A further option is
to provide a mixture of the
different adsorbents that may or may not be homogeneous.
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
In the case of a H2 PSA operating at ambient temperature up to about
250°C, the first zone
may contain an adsorbent or desiccant selected for removing very strongly
adsorbed components of
the feed gas mixture, such as water or methanol vapor, and some carbon
dioxide. The second zone
may contain an adsorbent typically selected for bulk separation of impurities
at relatively high
concentration, and the third zone may contain an adsorbent typically selected
for polishing removal
of impurities at relatively low concentration.
In the case of a H2 PSA operating at about 250°C to about 800°C,
the first zone may contain
an adsorbent that preferentially adsorbs C02 relative to water vapor as
described above. The second
zone may contain an adsorbent (e.g., zeolite, Cu(I)-containing material, or
Ag(I)-containing material)
IO that preferentially adsorbs CO relative to water vapor. The third zone may
contain a desiccant for
removing water vapor such as alumina gel. According to one version, the C02-
selective adsorbent
and the CO-selective adsorbent may be included or mixed together in a single
zone rather than in two
distinct zones.
The reforming and/or water gas shift reaction catalysts) described above may
be included in
any part of the adsorber bed, but typically are included in the section prior
to removal of the water
vapor since water vapor is a reactant for the reforming and water gas shift
reactions. In the
temperature range of about 600°C to about 1000°C, nickel
supported on alumina is an effective
catalyst for steam reforming of methane and the water gas shift reaction. In
the temperature range of
about 350°C to about 600°C, iron/chromia catalysts are effective
for the water gas shift reaction. In
the temperature range of about 200°C to about 300°C, copperlzinc
oxide catalysts are effective for the
water gas shift reaction.
In those embodiments in which the H2 PSA is performing the exothermic water
gas shift
reaction, any excess heat may be removed from the PSA by providing, for
example, heat exchange
means in a wall of the PSA or in the adsorber beds. In those embodiments in
which the H2 PSA is
performing the endothermic reforming reaction, any required additional heat
may be delivered to the
PSA by providing, for example, heat exchange means in a wall of the PSA or in
the adsorber beds or
by integrating a burner with the PSA.
The adsorbent sheets comprise a reinforcement material (e.g., glass fibre,
metal foil or wire
mesh) to which the adsorbent material is attached with a suitable binder. For
air separation to
produce enriched oxygen, alumina gel may be used in the first zone to remove
water vapor, while
typical nitrogen-effective adsorbents in the second and third zones are X, A
or chabazite type
zeolites, typically exchanged with lithium, calcium, strontium, magnesium
and/or other cations, and
with optimized silicon/aluminium ratios as well known in the art. The zeolite
crystals are bound with
silica, clay and other binders, or self bound, within the adsorbent sheet
matrix. The nitrogen-
selective zeolite adsorbents tend to be effective in the temperature range
from ambient up to about
100oC.
Satisfactory adsorbent sheets have been made by coating a slurry of zeolite
crystals with
binder constituents onto the reinforcement material, with successful examples
including nonwoven
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
11
fibreglass scrims, woven metal fabrics, and expanded aluminium foils. Spacers
are provided by
printing or embossing the adsorbent sheet with a raised pattern, or by placing
a fabricated spacer
between adjacent pairs of adsorbent sheets. Alternative satisfactory spacers
have been provided as
woven metal screens, non-woven fibreglass scrims, and metal foils with etched
flow channels in a
photolithographic pattern. Adsorbers of the layered adsorbent sheet material
may be formed by
stacking flat or curved sheets; or by forming a spiral roll, with the flow
channels between the sheets
extending from the first end of the adsorber to the second end thereof; to
fill the volume of the
adsorber housing of the desired shape. Examples of methods and structures with
packed, spirally
wound adsorbents are disclosed in commonly-owned, co-pending U.S. Provisional
Application No.
60/285,527, filed April 20, 2001, and incorporated herein by reference.
Typical experimental sheet thicknesses have been 150 microns, with spacer
heights in the
range of 100 to 150 microns, and adsorber flow channel length approximately 20
cm. Using X type
zeolites, excellent performance has been achieved in oxygen separation from
air at PSA cycle
frequencies in the range of 1 to at least 150 cycles per minute, particularly
at least 25 cycles per
minute.
FIG. 3 shows the porting of rotor 4 in the first and second valve faces
respectively in the
planes defined by arrows 14'-15', and 16'-1T. An adsorber port 30 provides
fluid communication
directly from the first or second end of each adsorber to respectively the
first or second valve face.
FIG. 4 shows the first stator valve face 100 of the first stator 8 in the
first valve face 10, in
the plane defined by arrows 14'-15'. Fluid connections are shown to a feed
compressor 101 inducting
feed air from inlet filter 102, and to an exhauster 103 delivering nitrogen-
enriched second product to
a second product delivery conduit 104. Compressor 101 and exhauster 103 are
shown coupled to a
drive motor 105.
Arrow 20 indicates the direction of rotation by the adsorber rotor. In the
annular valve face
between circumferential seals 106 and 107, the open area of first stator valve
face 100 ported to the
feed and exhaust compartments is indicated by clear angular segments 111-116
corresponding to the
first functional ports communicating directly to functional compartments
identified by the same
reference numerals 111-116. The substantially closed area of valve face 100
between functional
compartments is indicated by hatched sectors 118 and 119, which are slippers
with, zero clearance, or
preferably a narrow clearance to reduce friction and wear wifhout excessive
leakage. Typical closed
sector 118 provides a transition for an adsorber, between being open to
compartment 114 and open to
compartment 115. A gradual opening is provided by a tapering clearance channel
between the slipper
and the sealing face, so as to achieve gentle pressure equalization of an
adsorber being opened to a
new compartment. Much wider closed sectors (e.g. 119) are provided to
substantially close flow to
or from one end of the adsorbers when pressurization or blowdown is being
performed from the other
end.
The feed compressor provides feed gas to feed pressurization compartments 111
and 112,
and to feed production compartment 113. Compartments 111 and 112 have
successively increasing
working pressures, while compartment 113 is at the higher working pressure of
the PSA cycle.
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
12
Compressor 101 may thus be a multistage or split stream compressor system
delivering the
appropriate volume of feed flow to each compartment so as to achieve the
pressurization of adsorbers
through the intermediate pressure levels of compartments 111 and 112, and then
the final
pressurization and production through compartment 113. A split stream
compressor system may be
provided in series as a multistage compressor with interstage delivery ports;
or as a plurality of
compressors in parallel, each delivering feed gas to the working pressure of a
compartment 111 to
113. Alternatively, compressor 101 may deliver all the feed gas to the higher
pressure, with throttling
of some of that gas to supply feed pressurization compartments 111 and 112 at
their respective
intermediate pressures.
Similar, exhauster 103 exhausts heavy product gas from countercurrent blowdown
compartments 114 and 115 at the successively decreasing working pressures of
those compartments,
and finally from exhaust compartment 116 which is at the lower pressure of the
cycle. Similarly to
compressor 101, exhauster 103 may be provided as a multistage or split stream
machine, with stages
in series or in parallel to accept each flow at the appropriate intermediate
pressure descending to the
lower pressure.
In the example embodiment of FIG. 4A, the lower pressure is ambient pressure,
so exhaust
compartment 116 exhaust directly to heavy product delivery conduit 104.
Exhauster 103 thus
provides pressure letdown with energy recovery to assist motor 105 from the
countercurrrent
blowdown compartments 114 and 115. For simplicity, exhauster 103 may be
replaced by throttling
orifices as countercurrent blowdown pressure letdown means from compartments
114 and 115.
In some embodiments, the lower pressure of the PSA cycle is subatmospheric.
Exhauster
103 is then provided as a vacuum pump, as shown in FIG. 4B. Again, the vacuum
pump may be
multistage or split stream, with separate stages in series or in parallel, to
accept countercurrent
blowdown streams exiting their compartments at working pressures greater than
the lower pressure
which is the deepest vacuum pressure. In FIG. 4B, the early countercurrent
blowdown stream from
compartment 114 is released at ambient pressure directly to heavy product
delivery conduit 104. If
for simplicity a single stage vacuum pump were used, the countercurrent
blowdown stream from
compartment 115 would be throttled down to the lower pressure over an orifice
to join the stream
from compartment 116 at the inlet of the vacuum pump. A vacuum pump can allow
the PSA to
operate at lower pressures that may be advantageous when the PSA is coupled to
a fuel cell operating
at lower pressures such as a MCFC operating at ambient pressure. Vacuum PSA
operation favors
high oxygen yield or fractional recovery, and hence high-energy efficiency, in
air separation.
FIGS. 5A and SB shows the second stator valve face, at section 16'-1T of FIG.
1. Open
ports of the valve face are second valve function ports communicating directly
to a light product
delivery compartment 121; a number of light reflux exit compartments 122, 123,
124 and 125; and
the same number of light reflux return compartments 126, 127, 128 and 129
within the second stator.
The second valve function ports are in the annular ring defined by
circumferential seals 131 and 132.
Each pair of light reflux exit and return compartments provides a stage of
light reflux pressure
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
13
letdown, respectively for the PSA process functions of supply to backfill,
full or partial pressure
equalization, and cocurrent blowdown to purge.
Illustrating the option of light reflux pressure letdown with energy recovery,
a split stream
light reflex expander 140 is shown in FIGS. 1 and SA to provide pressure let-
down of four light
reflex stages with energy recovery. The light reflex expander provides
pressure let-down for each of
four light reflex stages, respectively between light reflex exit and return
compartments 122 and 129,
123 and 128, 124 and 127, and 125 and 126 as illustrated. The light reflex
expander 140 may power
a light product booster compressor 145 by drive shaft 146, which delivers the
oxygen enriched light
product to oxygen delivery conduit 147 and compressed to a delivery pressure
above the higher
pressure of the PSA cycle. Illustrating the option of light reflex pressure
letdown with energy
recovery, a split stream light reflex expander 140 is provided to provide
pressure letdown of four
light reflex stages with energy recovery. The light reflex expander serves as
pressure let-down
means for each of four light reflex stages, respectively between light reflex
exit and return
compartments 122 and 129, 123 and 128, 124 and 127, and 125 and 126 as
illustrated.
Since the light reflex and light product have approximately the same purity,
expander 140
and light product compressor 145 may be hermetically enclosed in a single
housing which may
conveniently be integrated with the second stator as shown in FIG. 1. This
configuration of a
"turbocompressor" booster without a separate drive motor is advantageous, as a
useful pressure boost
can be achieved without an external motor and corresponding shaft seals, and
can also be very
compact when designed to operate at high shaft speeds.
FIG. 5B shows the simpler alternative of using a throttle orifice 150 as the
pressure letdown
means for each of the light reflex stages.
Turning back to FIG. 1, compressed feed gas is supplied to compartment 113 as
indicated by
arrow 125, while heavy product is exhausted from compartment 117 as indicated
by arrow 126. The
rotor is supported by bearing 160 with shaft seal 161 on rotor drive shaft 162
in the first stator 8,
which is integrally assembled with the first and second valve stators. The
adsorber rotor is driven by
motor 163 as rotor drive means.
A buffer seal 170 is provided to provide more positive sealing of a buffer
chamber 171
between seals 131 and 171. In order to further minimize leakage and to reduce
seal frictional torque,
buffer seal 171 seals on a sealing face 172 at a much smaller diameter than
the diameter of
circumferential seal 131. Buffer seal 170 seals between a rotor extension 175
of adsorber rotor 4 and
the sealing face 172 on the second valve stator 9, with rotor extension 175
enveloping the rear portion
of second valve stator 9 to form buffer chamber 171. A stator-housing member
180 is provided as
structural connection between first valve stator 8 and second valve stator 9.
Direct porting of
adsorbers to the stator face is an alternative to providing such seals and is
described in commonly
owned, co-pending U.S. Provisional Application No. 60/301,723, filed June 28,
2001, and
incorporated herein by reference.
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
14
In the following system figures of this disclosure, simplified diagrams will
represent a PSA
apparatus or module. These highly simplified diagrams will indicate just a
single feed conduit 181 to,
and a single heavy product conduit 182 from, the first valve face 10; and the
light product delivery
conduit 147 and a single representative light reflux stage 184 with pressure
let-down means
communicating to the second valve face 11.
FIGS. 6-14 disclose various energy recovery systems using different heat
recovery working
fluids. In one variant, the oxygen PSA compressor is integrated with an
indirectly-heated gas turbine
bottoming cycle using air as the working fluid. At least a portion of the air
is provided to an oxygen
enrichment PSA at suitable feed pressures for the PSA process; and the
remainder of the air is
compressed to a higher pressure as a gas turbine cycle working fluid
indirectly heated by the fuel cell
stack through heat exchangers coupled to the cathode and/or
anode flow loops.
In other embodiments, the thermal bottoming working fluid is an anode loop gas
in a gas
turbine or Brayton cycle. If the hydrogen enrichment PSA is operated near
ambient temperature, a
recuperative heat exchanger is used to achieve high thermodynamic efficiency
of the thermal
bottoming cycle. Alternatively, if the hydrogen enrichment PSA is operated
with its second end at an
elevated temperature approaching that of the fuel cell stack while its first
end is maintained at a heat
rejection temperature near ambient, it may be used as a thermal rotary
regenerator for the gas turbine
cycle using anode gas as working fluid.
Hydrogen may be used as the fuel for SOFC power plants. With the anode gas as
a thermal
bottoming cycle working fluid, hydrogen containing a substantial fraction of
steam (e.g. about 25% to
about 50% steam in hydrogen) may be the working fluid for expansion, while the
working fluid for
compression is hydrogen from which fuel cell product water has been
substantially removed by
condensation. A radial flow expander may be used for the hydrogen/steam
mixture exiting the fuel
cell anode. Because of the low molecular weight of relatively dry hydrogen
being compressed after
condensation, alternative suitable compressors include high-speed centrifugal,
multistage centrifugal,
and positive displacement (e.g. twin screw) compressors.
For small power plants, the thermal bottoming cycle may use a separate working
fluid from
the cathode or anode gases, such as steam in a Rankine cycle or hydrogen in a
Stirling cycle. For
small SOFC fuel cells powered by hydrogen, the use of a Stirling engine for
thermal bottoming is
particularly attractive because the engine working fluid may be replenished
from the hydrogen fuel
supply. The need for completely leak-tight Stirling engine seals for working
fluid containment is thus
relaxed in the present application.
Because the present disclosed systems and processes use oxygen enrichment and
hydrogen
enrichment by PSA to elevate the voltage and/or current density delivered by
the fuel cell stack, the
fractional amount of fuel heating value delivered as high grade waste heat to
a thermal bottoming
cycle is greatly reduced over the prior art. Accordingly, the thermal
bottoming working fluid flow
rates and heat exchange duties are correspondingly reduced. The power rating
of the thermal
bottoming cycle is reduced in proportion to increased power delivered directly
by the fuel cell stack.
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
The net mechanical power delivered by the thermal bottoming cycle is applied
predominantly or
exclusively to the compression loads associated with the PSA auxiliaries.
FIGS. 6-9
5 Each of FIGS. 6-9 is a simplified schematic of an example of a molten
carbonate fuel cell
power plant 200, including the fuel cell 202, a high temperature PSA unit 204
co-operating with a
combustor 206 to transfer carbon dioxide from the anode side to the cathode
side of the fuel cell, and
an integrated gas turbine unit 208 for gas compression and expansion. The PSA
unit 204 increases
hydrogen concentration and reduces carbon dioxide concentration over the
cathode, thus increasing
10 cell voltage. This directly increases fuel cell stack efficiency and
electrical output, while also
reducing the heat generated by the fuel cell so that the fraction of plant
power output to be recovered
less efficiently by a thermal bottoming cycle is reduced. The systems shown in
FIGS. 6-9 are only
examples and other systems with different arrangements of devices and
conduits, or with additional
or fewer devices and conduits could also be used.
15 Molten carbonate fuel cell stack 202 includes the molten carbonate
electrolyte 210 supported
on a porous ceramic matrix, interposed between anode channel 212 and cathode
channel 214. The
anode channel has an inlet 216 and an outlet 218, while the cathode channel
214 has an inlet 220 and
an outlet 222.
The embodiment of FIG. 6 illustrates two alternatives for feed gas supply in
combination.
More typically, either of these feed gas supply alternatives might be used
separately in any given
MCFC installation. These alternatives correspond to the suitability of the
feed gas for direct
admission to the fuel cell anode, or for admission only after treatment by the
first PSA unit. For the
case of natural gas being the fuel, these alternatives also correspond to the
fuel processing options or
combinations of (1) "internal reforming" within the fuel cell stack, (2)
"sorption enhanced
reforming" within the first PSA unit, or (3) "external reforming" outside the
immediate MCFC
system as here described.
Endothermic reforming reactions are CH4 + H20 -> CO + 3H2
and CH4 + 2H20 --> C02 + 4H2,
with exothermic water gas shift CO + H20 ~ C02 + H2 ,
supplemented by partial combustion in the case of autothermal reforming
CH4 + %z02 --> CO + 2H2
A first feed gas supply inlet 230 communicates to anode inlet 214, introducing
a first feed
gas already compressed and preheated to the MCFC working conditions. The first
feed gas might be
hydrogen, syngas generated by an external fuel processor (e.g. coal gasifier
or steam methane
reformer), or natural gas for internal reforming within the anode channel 212
which would then be
modified as known in the art to contain a suitable steam reforming catalysts
such as nickel supported
on alumina.
A second feed gas supply inlet 240 communicates to a feed production
compartment in first
rotary valve face 10 of the first PSA unit 204, again introducing feed gas
already compressed and
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
16
preheated to the first PSA unit higher pressure and working temperature. The
carbon dioxide and
steam enriched heavy product stream is released from the blowdown and exhaust
compartments in
first rotary valve face 10 into conduit 242 at the lower pressure of the first
unit PSA cycle. The
higher pressure of the first PSA is slightly above the working pressure of the
MCFC, while the lower
pressure may be atmospheric or subatmospheric. If the MCFC working pressure is
selected to be
near atmospheric, the first PSA would be a vacuum PSA with the lower cycle
pressure in the range of
about 0.1 to 0.5 bars absolute.
The heavy product stream from conduit 242 is compressed back up to the higher
pressure of
first PSA by carbon dioxide compressor 244, which delivers the compressed
heavy product stream to
conduit 246 which branches to heavy reflex conduit 247 communicating to a feed
production
compartment in first rotary valve face 10 of the first PSA unit 204, and to
gas turbine combustor 206.
Alternatively, if the heavy product stream in conduit 242 is at sub-
atmospheric pressure, then device
244 could be a vacuum pump for extracting the heavy product stream.
Enriched hydrogen light product gas from first PSA 204 is delivered by conduit
250 from
the second rotary valve face 11 of the first PSA unit to anode inlet 216.
Three stages of light reflex
are shown, in which separate streams of light product gas at successively
declining pressures are
withdrawn from the second rotary valve face 11 for pressure letdown in
respective stages of light
reflex expander 140, and then returned to the second rotary valve face for
purging and
repressurization of the adsorbers. After passing through the anode channel
212, anode gas depleted
in hydrogen and enriched in carbon dioxide and steam is withdrawn from anode
exit 218 through
conduit 255 for treatment by first PSA unit 204 to recover hydrogen, carbon
dioxide, and methane
fuel components, while removing carbon dioxide and at least a portion of the
steam.
Anode channel 212, conduit 255, the PSA unit 204, and conduit 250 comprise an
anode loop
in which hydrogen is recirculated and replenished for substantially complete
utilization of the
hydrogen and other fuel components, while carbon dioxide is continually
removed by PSA 204. A
pressure booster means may be useful to overcome flow pressure drop around the
anode loop. In
FIG. 6, the pressure booster means is the PSA unit 204, with no mechanical
pressure booster being
required. The anode exit gas in conduit 255 is at a moderately lower pressure
than the feed gas in
feed conduit 240 and the heavy reflex gas in conduit 247. Hence the anode
exhaust gas is introduced
to a feed repressurization compartment in first rotary valve face 10. After
the anode exhaust gas has
entered the adsorbers 3, it is there compressed back up to the higher pressure
by feed gas and heavy
reflex gas entering the adsorbers from conduits 240 and 247.
Optionally, the heavy reflex step and conduit 247 may be eliminated, which
will increase the
fraction of fuel gas components (hydrogen, carbon monoxide and methane)
delivered to combustor
206. With a relatively high-pressure ratio between the higher and lower
pressures in the first PSA,
relatively high recovery of the fuel gas components in the light product gas
(for recycle to the fuel
cell anode) will be achieved. With a sufficiently large heavy reflex stream,
and corresponding power
consumption in heavy reflex compression, fuel gas components may be
substantially removed from
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
17
the heavy product of carbon dioxide and/or water vapour so that combustor 206
might be eliminated
or replaced with a small catalytic combustor.
A first heat exchanger 256 may be provided for the feed, heavy reflux and
exhaust conduits
communicating to the first valve face 10, so as to establish a first
temperature at the first end of the
adsorbers. A second heat exchanger 257 may be provided for the light product,
light reflux exit and
light reflux return conduits communicating to the second valve face 11, so as
to establish a second
temperature at the second end of the adsorbers. A third heat exchanger 258 may
be provided to
transfer heat from the anode exit conduit 255 to the light reflux exit
conduits communicating to the
inlets of the light reflux expander stages 140, so that high grade heat from
the fuel cell stack is
recovered at least in part in the expander 140.
Gas turbine assembly 208 includes compressor 260 and turbine 262, coupled to a
motorlgenerator 264 by shaft 266 and to heavy product compressor 244 and light
reflux expander 140
by shaft 267. Ambient air is introduced to compressor 260 by infeed conduit
270, and is there
compressed to working pressure for delivery by conduit 272 to combustor 206.
Combustor 206 burns
residual fuel values (including some hydrogen and unconverted carbon monoxide
and fuel) in the
carbon dioxide rich heavy product stream. A catalyst may be provided in
combustor 206 to ensure
stable combustion with high inert concentrations, or supplemental fuel may be
added thereto.
According to the embodiment shown in FIG. 6, the hot gas (i.e., the combustion
product) exiting
combustor 206 by conduit 280 is cooled in recuperative heat exchanger 285 to
approximately the
MCFC operating temperature for admission as cathode gas to cathode inlet 220.
The cathode gas
contains carbon dioxide and residual oxygen, diluted by steam and nitrogen.
After circulation
through cathode channel 214 in which some oxygen and carbon dioxide are
consumed, the depleted
cathode gas is conveyed from cathode exit 222 by conduit 290 back to
recuperator 285 for reheat to
an elevated turbine entry temperature for admission by conduit 291 to turbine
262. After expansion
through turbine 262, the exhaust cathode gas is discharged through conduit 292
where further heat
exchange would preferably take place to obtain most efficient heat recovery,
e.g. for preheating the
feed gas to inlets 230 and 240. Thus, turbine 262 drives turbine assembly 208.
According to another embodiment (not shown), a portion of the hot gas (i.e.,
the combustion
product) exiting combustor 206 may be diverted directly to turbine 262 rather
than passing through
the cathode channel 214. A further variant would involve providing a second
heavy product gas
stream from PSA 204 into a second combustor and then introducing the hot
combustion product
directly into turbine 262.
Also shown in FIG. 6 is the removal of water from the heavy product in conduit
242, either
prior to compression by carbon dioxide compressor 244 as shown in FIG. 6, or
after compression if a
vacuum pump is used as compressor 244 as shown in FIG. 7. A condenser 320 may
be provided in
conduit 242 for water removal and for cooling the heavy product gas so as to
reduce the compression
power required by compressor 244. Liquid water is removed by drain 321. The
condensation
temperature may be established by cooler 322. A fourth heat exchanger 325 may
be provided for
recuperative heat exchange between conduits 242 and 246.
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
18
Several alternative features and improvements are shown in FIG. 7. In this
figure, a
thermally integrated reformer is shown. Already compressed fuel and water (or
steam) are admitted
from infeed conduit 300, passing through an exhaust recuperator 302 for
recovering heat from
expanded cathode exhaust in conduit 292, and then passing through recuperator
285 to reach an
elevated reforming temperature (e.g. 800° to 1200°C) for
admission to catalytic reforming reactor
310. The endothermic reforming reaction reduces the temperature of the
delivered syngas to about
the MCFC temperature, and this syngas is delivered by conduit 240 to a feed
production compartment
in the first rotary valve face 10 of PSA unit 204.
A further feature in FIG. 7 is the provision of a mechanical pressure booster
for the anode
loop, as booster compressor 330 which is powered directly by light reflux
expander 140 through shaft
267. Recompressed anode gas from conduit 255 is boosted back to the higher
pressure by booster
330, and is delivered by conduit 331 to a production feed compartment in the
first rotary valve face
10. A portion of the anode exhaust gas.in conduit 255 may still be delivered
directly to a feed
pressurization compartment by conduit 333. In this example, the sole power
source for booster 330 is
expander 140, which is now separated from gas turbine assembly 208.
In FIGS. 8 and 9, further embodiments are shown incorporating an oxygen VPSA
in order to
boost the oxygen and carbon dioxide partial pressures in the cathode channel,
so as to increase the
cell electromotive force and thus reduce the thermal bottoming load while
enhancing overall plant
efficiency. In FIGS. 8 and 9 as in FIGS. 6 and 7, various details of
recuperative heat recovery and
water condensation from the heavy product are shown in simplified schematic
form.
The oxygen PSA or VPSA unit 400 includes a rotary module 401 with nitrogen-
selective
adsorbent in adsorbers 403, a first rotary valve face 410 and a second rotary
valve face 411. The first
rotary valve face 410 receives compressed feed air at a feed production
compartment from feed air
compressor 260 via conduit 420, and discharges exhaust nitrogen enriched air
from an exhaust
compartment via conduit 422 to an optional vacuum pump 424 (to be included for
VPSA or excluded
for simple PSA) for discharge to atmosphere or any other use for moderately
enriched nitrogen. The
second rotary valve face 411 delivers enriched light product oxygen at e.g.
90% purity by non-return
valve 430 in conduit 431 to oxygen compressor 432 which delivers the oxygen at
a pressure of at
least the MCFC working pressure to conduit 434 and thence combustor 206. Light
reflux pressure
letdown throttles 436 are also provided for light reflux stages in the second
rotary valve face 411.
According to a variation of the embodiments shown in FIGS. 8 and 9, the anode
exhaust gas
exiting anode outlet 218 could be introduced directly into a combustor 206
without first passing
through a hydrogen PSA unit. The anode exhaust gas then could be burned with
the enriched oxygen
stream produced by the oxygen PSA unit 400.
Oxygen enrichment of the air provided to combustor 206 may substantially
reduce the inert
load of nitrogen and argon in the cathode channel, thus enhancing
electrochemical energy conversion
performance as discussed above. The working fluid for the gas turbine expander
262 is thus largely
concentrated carbon dioxide with only small amounts of atmospheric gases.
Moreover, oxygen
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
19
enrichment may provide more complete combustion without a catalyst or with a
smaller amount of
catalyst and it may substantially eliminate the production of NOX emissions.
FIG. 9 shows the additional feature that a portion of the enriched oxygen from
PSA 400 is
used for fuel processing, either within the plant as here shown, or externally
as in the example that
coal gasification is used to generate syngas feed. Here, a portion of the
compressed oxygen in
conduit 434 is conveyed by conduit 440 to reformer 310, which here is an
autothermal reformer for
e.g., steam reforming natural gas.
FIGS. 10-14
FIG. 10 shows a simplified schematic of an example of an SOFC system
embodiment 450 to
which fuel gas (which may be natural gas, ~syngas or hydrogen) is provided by
fuel inlet 230.
Embodiment 450 includes an oxygen VPSA whose compression machinery is
primarily powered by a
regenerative gas turbine cycle using the anode gas as working fluid to recover
cell stack waste heat as
a thermal bottoming cycle to power system auxiliary compression loads.
Alternatively, enriched
oxygen may be delivered by a positive pressure PSA process as illustrated in
FIG. 4A. Components
and reference numerals generally follow the description as given above for
FIGS 8 and 9. The
systems shown in FIGS. 10-14 are only examples and other systems with
different arrangements of
devices and conduits, or with additional or less devices and conduits could
also be used.
Solid oxide fuel cell stack 502 includes a solid oxide electrolyte membrane
510 interposed
between anode channel 512 and cathode channel 514. The anode channel has an
inlet 516 and an
outlet 518 connected by anode loop 519, while the cathode channel 514 has an
inlet 520 and an outlet
522. If the fuel is natural gas, it is internally reformed within the anode
channel 512, while a suitable
steam concentration is maintained in anode loop 519 so as to prevent carbon
deposition.
The heavy product gas from the first PSA is in part exhausted by conduit 455
branching
from conduit 242 and conveying the anode loop exhaust to combustor 206.
Cathode tail gas may be
used as oxidant in combustor 206, and is conveyed from cathode outlet 522 by
conduit 457 to the
combustor. Flue gas from combustor 206 is discharged by exhaust conduit 459
after heat recovery in
heat exchanger 460, superheating the light reflux gas before entry to the
stages of light reflux
expander 140. The working fluid in expander 140 is a mixture of steam and
hydrogen if hydrogen is
the fuel, also including carbon dioxide if methane or syngas is the fuel,
introduced by fuel feed inlet
230.
The adsorber working temperature of the first PSA may be close to ambient
temperature, in
which case heat exchangers 256 and 257 will be heavily loaded recuperators.
Alternatively, the first
PSA may operate at elevated temperature, in which case the second temperature
adjacent the second
valve face is preferably elevated relative to the first temperature adjacent
the first valve face, so that
the adsorber rotor functions as a thermal rotary regenerator.
In one embodiment, the first zone 26 of the adsorbers operates in the
temperature range from
substantially ambient to about 300oC using, for example, alumina, zeolite 13X,
or an at Least
moderately hydrophobic zeolite such as zeolite Y as the adsorbent. The second
zone 27 of the
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
adsorbers may operate in the temperature range from about 300oC to about 500oC
using, for
example, alumina or a promoted hydrotalcite adsorbent. The third zone 28 of
the adsorbers may
operate in the temperature range from about 5300oC to about 800oC using, for
example, alumina or
ultrastable Y zeolite hydrotalcite adsorbent. Alternatively, the third zone 28
may contain (instead of
5 adsorbent) a substantially nonadsorptive ceramic or metal material selected
for utility in the high
temperature zone of a rotary regenerator.
FIG. 11 shows a simplified schematic of another embodiment 475 of a SOFC fuel
cell
system, for which the fuel is hydrogen. This embodiment is particularly useful
for smaller scale
installations for which high efficiency is required. In embodiment 475, a
Stirling engine 480 is used
10 as the thermal bottoming system to recover waste heat. Engine 480 has a hot
end 481 in which
expansion of a Stirling cycle working fluid is performed to take up heat from
a thermally insulated
jacket 482 enclosing the fuel cell stack. Engine 480 has a cool end 483 in
which compression of the
Stirling cycle working fluid is performed to reject heat at substantially
ambient temperature from
cooler 484. Compressed hydrogen may be used as the Stirling cycle working
fluid.
15 The Stirling engine may have a crank mechanism 485 to drive shaft 486
coupled to anode
gas recirculation blower 490, the oxygen PSA feed blower 260, an optional PSA
vacuum pump 424,
and an optional generator 264. Alternatively, a free piston Stirling engine
mechanism may be used to
drive all or some of the above compression loads directly without a shaft
coupling.
FIG. 12 shows a simplified schematic of an example of an SOFC system
embodiment 500 to
20 which externally generated and purified hydrogen is provided by fuel inlet
230. Embodiment 500
illustrates an oxygen VPSA whose compression machinery is primarily powered by
free rotor gas
turbines (turbochargers) recovering fuel cell stack waste heat as a thermal
bottoming cycle used only
to power system auxiliary compression loads. Enriched oxygen may alternatively
be delivered by a
positive pressure PSA process as illustrated in FIG. 4A.
Solid oxide fuel cell stack 502 includes a solid oxide electrolyte membrane
510 interposed
between anode channel 512 and cathode channel 514. The anode channel has an
inlet 516 and an
outlet 518 connected by anode loop 519, while the cathode channel 514 has an
inlet 520 and an outlet
522 connected by cathode loop 523. The anode and cathode loops pass through a
heat exchanger 525
to reject stack waste heat at substantially the fuel cell working temperature.
Recirculation blowers (or
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
21
delivers additional compressed air as heat recovery working fluid by conduit
540 to the first end 541
of a first thermal recuperator 542 which also has a second end 543 at a
temperature approaching the
working temperature of the fuel cell stack. The heat recovery working fluid is
heated in recuperator
542 and then by heat exchanger 525 before being delivered to the inlet 549 of
a first expander turbine
550. After expansion in first turbine 550, the heat recovery working fluid is
conveyed by conduit 551
to be reheated in heat exchanger 525 before being delivered to the inlet 559
of a second expander
turbine 560. After expansion to substantially atmospheric pressure in second
turbine 560, the heat
recovery working fluid is conveyed by conduit 561 through recuperator 542
where its remaining
sensible heat is recovered for preheating air in conduit 540 and enriched
oxygen in conduit 567, and
then the spent heat recovery working fluid is discharged by conduit 565.
In the example of FIG. 12, first turbine 550 is used to drive feed compressor
101 in a
turbocharger 570, and second turbine 560 is used to drive vacuum pump 103 in a
turbocharger 572.
It will be evident that this use of the first and second turbines could be
reversed, and also that an
electrical generator may also be connected to either turbine or to a third
turbine. Also, the turbines
may be supplied with the heat recovery working fluid in parallel rather than
in series. Operation in
series with reheat is thermodynamically more efficient. Intercooling may also
be provided between
stages of the feed compressor 101.
Enriched oxygen from the VPSA unit 401 is delivered through non-return valve
430 to an
oxygen compressor 145 to boost the pressure of the enriched oxygen to
substantially the working
pressure of the cathode loop channel 514. According to the working pressure
selected, compressor
145 may include several stages, and the stages may be powered by any suitable
motor or other drive
means. FIG. 12 shows a light reflux expander turbine 140 as the power source
for oxygen
compressor 145 as shown in FIG. 5A. This arrangement achieves highest energy
efficiency by
recovering energy from the pressure letdown of the light reflux gas, and has
the advantage that the
oxygen compressor 145 is driven by an oxygen expander 140 in a free rotor
assembly which may be
hermetically enclosed. For high working pressures (e.g. > 5 bars) it may be
necessary to provide
additional oxygen compression stages with a power source different or
supplementary to light reflux
expansion.
As the enriched oxygen delivered by simple VPSA systems typically contains
about 5%
argon and some minor amount of nitrogen impurity, it may be useful to remove a
purge stream from
the cathode loop 523 by a purge conduit 580. Purge conduit 580 passes through
recuperator 542 for
recovery of sensible heat energy from the purge stream, and includes a
throttle valve 581 or other
means for pressure letdown before reaching the purge discharge port 582. If
desired, all or a portion
of the purge may be discharged to ambient, or alternatively all or a portion
of the purge may be
recycled from port 582 to a feed pressurization compartment of the VPSA unit
401 in order to retain
enriched oxygen and also for recovery of compression energy in the VPSA
process. The fractional
amount of the purge stream to be recycled into the VPSA unit will depend on
optimisation analysis to
determine the allowable accumulation of recycled argon impurity within the
cathode loop. With
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
22
purge recycle, moderately concentrated argon may be recovered as a
commercially useful by product
of the power plant 500.
A second thermal recuperator 590 may be provided for preheating hydrogen fuel
delivered
to the anode side at substantially the anode channel working pressure by fuel
inlet 230. First end 591
of recuperator 590 may be at substantially ambient temperature (or at a
temperature at which
hydrogen is stored). Second end 592 of recuperator 590 is at substantially the
stack working
temperature. In order to prevent undesirable accumulation of water vapor as
the product of the fuel
cell reaction in the anode channel, a fraction of recirculated anode gas is
diverted through a
condensation loop including a cooling conduit 593 through recuperator 590 to
condenser 595 and a
reheating conduit 596 through recuperator 590 back to the anode inlet 516. A
cooling coil 597 and a
liquid water discharge throttle valve 598 are included in condenser 595.
It will be evident from consideration of FIG. 12 that the oxygen VPSA unit and
the
associated compression machinery provided therein as free rotor "turbocharger"
machines for fuel
cell stack waste heat recovery may also be applied to MCFC systems, subject to
a stream of
concentrated C02 also being supplied to the cathode loop so that two moles of
C02 are available for
each mole of 02 consumed in the MCFC cathode reaction.
FIGS. 13 and 14 show SOFC embodiments 600 with steam reformed natural gas
fuelling.
Desulphurized natural gas is introduced at substantially the fuel cell working
pressure to inlet 601,
and thence by conduit 602 to first end 603 of reformer thermal recuperator
604, which preheats the
natural gas feed as it flows to the second end 605 of the reformer
recuperator. Second end 605 is at
an elevated temperature approaching the fuel cell stack working temperature.
The preheated natural
gas flows by conduit 610 from the second end 605 of the reformer recuperator
to inlet 619 of
reformer reactor 620. The natural gas reacts with steam in reactor 620 to
produce syngas containing
hydrogen, carbon monoxide and carbon dioxide; and some of the carbon monoxide
may further react
with steam to produce more hydrogen.
The syngas generated in reactor 620 is delivered from exit 621 thereof by
conduit 622 back
through the reformer recuperator (or a portion thereof) to cool the syngas
down to the working
temperature of the first PSA unit (for carbon dioxide extraction from the
hydrogen anode fuel), and is
thence delivered by conduit 623 to a feed compartment of the first H2 PSA unit
204.
As discussed above, the working temperature of the first PSA unit 204 may be
close to that
of the fuel cell stack or the reformer reactor. For example, the working
temperature of the H2 PSA
unit may be within about 100 to about 200°C of fuel cell stack or the
reformer reactor. If the working
temperature of the first PSA unit is high enough for the methane steam
reforming reaction (e.g., at
least about 600°C) and a suitable catalyst is included within the
adsorbers thereof, the steam
reforming reaction may be conducted as sorption enhanced reaction within the
PSA unit in an
adsorber zone approaching or exceeding about 600°C. At somewhat lower
temperatures of the first
PSA unit (e.g., at least about 200°C to about 300°C), water gas
shift may be conducted by sorption
enhanced reaction over a suitable catalyst within the adsorbers. At still
lower temperatures down to
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
23
ambient, the first PSA unit may be operated with conventional adsorbents for
adsorbing C(72 from
hydrogen.
Enriched hydrogen product from the first PSA unit is delivered as light
product by conduit
630 to anode loop conduit 632, and thence after pressure boost by anode
recirculation blower 526 to
the anode inlet 516 of the fuel cell stack. Anode gas is withdrawn from anode
exit 518 into conduit
640, which passes through reformer reactor heater 642 and thence to loop
conduit 632.
Enriched carbon dioxide from the first PSA unit is withdrawn as heavy product
at lower
pressure by conduit 242 to the inlet of carbon dioxide compressor (or vacuum
pump) 244 which
serves as a heavy reflux compressor, and compresses the enriched carbon
dioxide stream back to
substantially the upper pressure of the first PSA unit cycle. A portion of the
C02 is recycled back to
the PSA unit by conduit 247 to a heavy reflux feed compartment of the first
PSA unit. The balance
of the compressed C02 is withdrawn by conduit 650 for disposal in the depicted
case of a SOFC
plant.
In the opposite case of a MCFC plant which may also be represented by FIG. 13,
this C02
steam would be transferred by conduit 651 (shown as a dashed line in FIG. 13)
for mixing into the
enriched oxygen stream between non-return valve 430 and enriched oxygen
compressor 145 so as to
provide a suitable MCFC cathode oxidant stream with two moles of C02 for each
mole of 02
consumed.
The carbon dioxide compressor or heavy reflux compressor 244 is shown in FIGS.
13 and
14 as powered by a third expander turbine 670 in a free rotor "turbocharger"
assembly 672. In FIG.
13, the third turbine 670 is shown in parallel operation with first turbine
550, so that the inlet conduit
675 to turbine 670 is connected to conduit 540 which is the inlet to turbine
550, and exhaust conduit
676 from turbine 670 is connected to conduit 551 which is the exhaust conduit
from turbine 550.
In FIG. 14, all three turbines are operated in series for staged expansion of
the heat recovery
working fluid air. Conduit 540 admits heated air to the inlet of turbine 550,
then conduit 677 admits
the partially expanded air to the inlet of turbine 670, and conduit 678 admits
the further expanded air
to heat exchanger 525 for reheat and thence by conduit 551 to the inlet of
turbine 560 for final
expansion to atmospheric pressure. Desirably, conduit 677 would also be looped
though heat
exchanger 525 for reheat so that the inlet to each turbine stage is heated to
the highest available
temperature.
Superheating or reheating in FIGS. 13 and 14 may also be provided by an anode
tail gas (or
first PSA exhaust gas) combustor, which is not shown in these simplified
schematics. The anode tail
gas burner will not generate any NOx emissions if the oxidant is highly
enriched oxygen generated by
the oxygen PSA or VPSA unit 401. Since anode tail gas will be mostly C02 with
very little heating
value of fuel components, enriched oxygen is desirably used as the oxidant, to
avoid or minimize the
need for a catalyst that would be needed for combustion of such extremely low
BTU gas in air.
In FIG. 13, the fuel gas in the anode channel includes hydrogen and will
probably also
include carbon monoxide as a fuel component, so that water vapor and carbon
dioxide are continually
formed as reaction products. A slipstream of anode gas is continually
withdrawn from adjacent the
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
24
anode exit 518 by conduit 680, and cooled through reformer recuperator 604 to
the appropriate
temperature for admission to a feed compartment for the first PSA unit by
conduit 681. In this
embodiment, the first PSA unit thus receives three feed streams in order of
ascending C02
concentration: (1) the anode gas slip stream in conduit 680, (2) steam
reforming reactor syngas in
conduit 622, and (3) heavy reflux concentrated C02 from conduit 247. Within
the PSA process, each
adsorber should receive those three feed streams in the same order (from
conduit 681, then conduit
623, then conduit 247), so as to maintain the correct sequence of ascending
C02 concentration. Care
must be taken with water vapor management in the embodiment of FIG. 13, so as
to maintain an
adequate steam/carbon ratio in the reformer and in the anode channel to
prevent any carbon
deposition and consequent catalyst deactivation. Water vapor must be supplied
with or into the
natural gas feed gas. It may be necessary to use a somewhat hydrophobic
adsorbent in the first PSA
unit, or alternatively to inject. supplemental water vapor into the fuel cell
anode channel. In this
embodiment, the separation is less stringent, since CO need not be separated
while C02 is being
extracted and concentrated.
In FIG. 14, the fuel gas in the anode channel is envisaged as purified
hydrogen that has been
separated by the first PSA unit, here designed and operated to remove CO and
CH4 impurities as well
as C02. [Again, a tail gas burner may be used for combustion of residual fuel
components in the
PSA heavy reflux CO2 enriched product stream, with the useful heat applied to
preheating or
reheating applications for waste heat recovery into expander turbines.] The
first PSA unit of FIG. 14
receives two feed streams, the steam methane reformer reactor syngas from
conduit 623, followed by
the compressed heavy reflux from conduit 247, and has no recycle from the
anode loop to which it
delivered purified hydrogen. In this case, no CO2 is formed in the anode
channel, whose only
reaction product is water vapor. Water vapor could be extracted from the anode
loop by recuperative
heat exchange to a condenser as shown in FIG. 12, but in FIG. 14 water vapor
is extracted by a rotary
desiccant humidity exchanger 690 coupled between conduits 610 and 640.
Humidity exchanger 690
includes a desiccant wheel 691 engaged at first and second ends with valve
faces 692 and 693. The
humidity exchanger transfers anode product water vapor from anode exit conduit
640 to steam
reforming reactor feed conduit 610, so as to remove water vapor from the anode
loop while providing
all of the water vapor required for steam methane reforming.
In FIG. 14, conduit 640 carries humid anode gas through valve face 692 into
one side of the
desiccant wheel from which dried anode gas is delivered through valve face 693
to conduit 640'
connecting to anode loop conduit 632. Conduit 610 delivers humidified steam
reformer feed gas
through valve face 692 from the other side of the desiccant wheel to which dry
preheated natural gas
was feed through valve face 693 from conduit 610'. The driving force for
humidity transfer may be
augmented by either establishing a higher temperature in conduit 610' relative
to a lower temperature
in conduit 640, or by establishing a higher pressure in conduits 640 and 640'
relative to a lower
pressure in conduits 610' and 610.
It will be evident that there may be many other alternatives and variations of
the disclosed
systems and processes.
CA 02394924 2002-06-19
WO 02/37590 PCT/CA01/01522
For example, the disclosed systems and process can be used in connection with
various fuel cells,
feed gases and PSA units such as the following possibilities:
A. Direct MCFC or SOFC running on natural gas, PSA units on both anode and
cathode.
5 B. MCFC or SOFC running on syngas generated e.g. by oxygen-blown coal
gasification, PSA units on both anode and cathode.
C. Indirect SOFC running on hydrogen reformed from natural gas, PSA units on
reformer (C02 rejection), anode (H20 rejection which alternatively could be
done by condensation)
and cathode (nitrogen rejection).
10 D. SOFC running on hydrogen from any source, PSA units on anode (H2O
rejection
which might alternatively be done by condensation) and cathode (nitrogen
rejection).
Estimated efficiencies based on fuel lower heating value are in the rough
range of 60% for
the MCFC embodiments, 70% for fossil fueled SOFC and 80% for hydrogen fueled
SOFC at
commercially attractive current densities.
15 For MCFC systems, the disclosed systems and process can avoid accumulation
of C02 on
the anode where C02 is generated by the reactions of CH4 and CO and well as by
carbonate transport
through the electrolyte, while also avoiding accumulation of inert nitrogen on
the cathode.
A few potential advantages of certain disclosed SOFC embodiments are:
1. the problem of reduced cell voltage at extremely high temperature may be
20 overcome by manipulating partial pressures;
2. the C02 mass flow from anode per unit of fuel may be only about 20% as
large as
the C02 mass flow in an MCFC anode into which most of the C02 is delivered
from the electrolyte,
hence the heavy reflux compressor or vacuum pump may be much smaller and will
need less power;
and
25 3. higher grade waste heat improves efficiency of heat recovery
turbochargers.
Having illustrated and described the principles of our disclosure with
reference to several
embodiments, it should be apparent to those of ordinary skill in the art that
the invention may be
modified in arrangement and detail without departing from such principles.